Abstract
VEGF-driven tumor angiogenesis has been validated as a central target in several tumor types deserving of continuous and further considerations to improve the efficacy and selectivity of the current therapeutic paradigms. Epsins, a family of endocytic clathrin adaptors, have been implicated in regulating endothelial cell VEGFR2 signaling, where its inactivation leads to nonproductive leaky neo-angiogenesis and, therefore, impedes tumor development and progression. Targeting endothelial epsins is of special significance due to its lack of affecting other angiogenic-signaling pathways or disrupting normal quiescent vessels, suggesting a selective modulation of tumor angiogenesis. This review highlights seminal findings on the critical role of endothelial epsins in tumor angiogenesis and their underlying molecular events, as well as strategies to prohibit the normal function of endogenous endothelial epsins that capitalize on these newly understood mechanisms.
Keywords: Tumor angiogenesis, Endocytosis, VEGFR2, Signaling, Epsins, Target
Tumor angiogenesis, therapeutic approaches, and targets
Recent advances have established tumor angiogenesis, an intricate process involving the coordination between multiple signaling pathways and cell types, as an important target in tumor biology and clinical settings [1–3].
Solid tumor growth, largely driven by the secretion of VEGF, requires neo blood vessel formation through the expansion of the host tumor vasculature; and these newly formed vessels can also potentiate tumor dissemination and progression. One such cancer treatment approach is to block this angiogenic process by inhibiting VEGF signaling [1, 4], where anti-VEGF therapies, such as bevacizumab (also known by the trade name Avastin, a humanized monoclonal anti-VEGF-A antibody), have been proven to be effective on a wide variety of cancers [5]. VEGF family members act through the receptor tyrosine kinases VEGFR1-3 and receptor tyrosine kinase inhibitors, such as sorafenib and sunitinib, two FDA-approved drugs, have also been shown to exhibit potent anti-tumoral effects [2, 6]. However, an imperative issue in the field is that not all patients benefit from therapies involving the blockade of VEGF signaling. For patients who respond, the compensation of other signaling pathways (e.g., FGF, PlGF et al.) often results in resistance due to metabolic and proteomic changes in the tumor cells that permit tumor cell adaptation [7–9].
A better understanding of the molecular events governing tumor angiogenesis can provide alternative or complementary strategies to combat tumor formation. VEGF dynamically regulates the tumor endothelial expression of a Notch receptor ligand, Delta-like ligand 4 (Dll4), where its deletion or blockade results in excessive angiogenesis and paradoxically reduced tumor growth. As a result, the deregulating vascularity is nonfunctional, as shown by poor perfusion [10, 11]. Inhibitors or antibodies targeting the Notch receptors show capability to partially block tumor growth through the generation of poorly functioning tumor vasculature in other preclinical studies [12, 13]. Currently, it is of major interest to investigate such approaches for angiogenic-signaling pathways to be rallying for unstrained and dysfunctional tumor angiogenesis. Particularly, epsins have emerged as new targets for the development of anti-angiogenic strategies.
Epsins as regulators and targets for anti-angiogenic cancer therapy
Epsins: endocytic adaptor proteins that select specific cargos for endocytic internalization through clathrin-coated pits or vesicles are reported to have additional functions in the regulation of GTPases involved in actin remodeling [14–17]. Structurally, epsins contain a conserved NH2-terminal homology (ENTH) domain anchoring it to the plasma membrane, ubiquitin-interacting motifs (UIM) providing specificity with which epsins interact with the ubiquitinated cargo, and an unstructured carboxyl-terminal tail for bringing the cargo to coated vesicle formation sites for subsequent internalization [16, 18, 19].
Mammals express three different epsin isoforms. Besides epsin 3, which is primarily distributed in the stomach and epidermis, epsins 1 and 2 are ubiquitously expressed [16, 20]. Global deletion of epsins 1 and 2 (DKO) in mice leads to prenatal lethality at embryonic day 10 with profound vascular defects, but single deletion of either isoforms does not [21]. Embryos constitutively lacking endothelial epsins 1 and 2 (EC-DKO) phenocopied the vascular defects with increased vascular density and disorganized vascular networks as displayed in global DKO embryos, as well as exhibiting delayed lethality [22]. This indicates that endothelial epsins are indispensable in regulating angiogenesis.
VEGF and Notch-signaling pathways also have fundamental roles in embryonic angiogenesis. Including their roles in postnatal angiogenesis, progress has been made towards developing target-based therapies specifically for tumor angiogenesis [2, 23]. A recent study reports the postnatal investigation of blood vessel formation in adult mice with tamoxifen-inducible, endothelial specific deletion of epsins (EC-iDKO). Under normal physiological conditions, the EC-iDKO mice exhibit almost unaffected morphology and functioning of pre-existing host blood vessels. In contrast, using both subcutaneously implanted tumors and spontaneous tumor models, vasculature in EC-iDKO tumors are found to be enlarged, highly disorganized with negligible mural cell coverage, and poorly perfused—resulting in impaired tumorigenesis. The study also identifies the epsin UIM as a central element in epsin function [24], and these intriguing findings suggest that targeting endothelial epsins may provide a novel and alternative therapeutic target for anti-angiogenic therapy. However, some barriers still remain. For instance, is it possible to specifically target endothelial epsins and circumvent a systemic effect that perturbs epsins’ function in other cell types? Is it feasible to disturb epsin function? If yes, would it be effective to curb established tumors?
A follow-up study makes a plausible attempt and utilizes a chemically synthesized, tumor endothelial cell-homing, UIM-containing peptide (named epsin mimetic peptide) to competitively and specifically prohibit endogenous epsin function in tumor vasculature. The peptide is sophisticatedly equipped with iRGD at the UIM C-terminus to facilitate peptide homing to and internalization by tumor endothelial cells; and with a plasma membrane-anchoring-peptide from the Lyn kinase H4 domain, to enrich plasma membrane localization of the UIM peptide conjugate [25–27]. The designed epsin mimetic peptide also disrupts functional angiogenesis and generates remarkable inhibition of tumor growth in U87 and GL261 (glioblastoma), LLC (lung), B16 (melanoma), and TRAMP (prostate) preclinical cancer models. The viability of endothelial epsins as a therapeutic target, in particular for the resulting effects of aberrant, nonproductive leaky tumor vessels, is further evaluated in the study. For example, one could speculate that the epsin mimetic peptide disrupts the tumor vasculature, and may, therefore, cause brain swelling and fluid buildup in orthotopic gliobastoma models. However, this phenomenon is not observed, and the treatment is equally as effective at inhibiting tumor growth as currently available anti-VEGF therapies. One could also speculate that these vessels may decrease tumor perfusion and would not allow efficient delivery of chemotherapy to the tumor. However, the results in subcutaneous allografts of lung cancer cell models find that the treatment sustains tumor growth when combined with cytotoxic chemotherapeutics (e.g., doxorubicin, Taxol, OKN-007). These vessels in metastasis models could be hypothesized to provide a route of tumor dissemination because of their leakiness despite the study demonstrating that the treatment impedes cancer metastasis [28]. The indicated findings provide strong in vivo evidence for endothelial epsins as druggable targets.
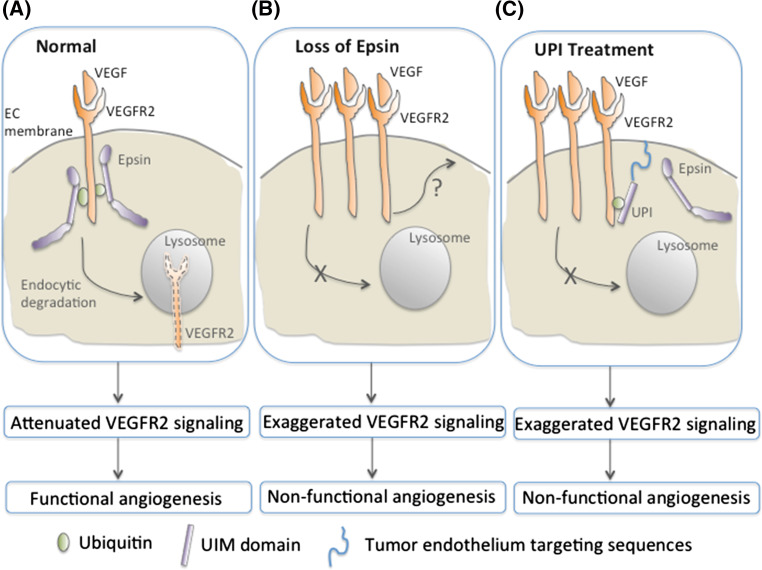
Mechanistically, the vascular dysfunction responsible for the previously described cancer suppression phenotype is a result of impaired VEGFR2 internalization, degradation, and failure to attenuate VEGF signaling. VEGF stimulates VEGFR2 internalization and degradation in part by inducing VEGFR2 ubiquitination [29]. It is suggested that via their UIMs, epsins recognize ubiquitinated VEGFR2 at the plasma membrane and recruits it for clathrin-dependent endocytosis and subsequent degradation within the endosome-lysosome pathway to downregulate VEGF signaling (Fig. 1a) [24, 29, 30]. In this capacity, loss of endothelial epsins or epsin mimetic peptide treatment leads to the accumulation of VEGFR2 cell surface and augmentation of VEGRF2 signaling, including phosphorylation of VEGFR2, PLC-γ, Akt, and ERK—which induces nonproductive leaky angiogenesis and inhibits tumor progression (Fig. 1b–c) [24, 28].
Fig. 1.
Proposed models of modulation of VEGFR2 signaling by endothelial epsins. a VEGF promotes epsin binding to ubiquitinated VEGFR2, where this interaction is required for endocytic degradation of VEGFR2 and is relied on epsin UIMs. Endothelial epsins function as unique attenuators of VEGFR2 signaling and produces functional tumor angiogenesis. b Loss of endothelial epsins impairs endocytic degradation of VEGFR2, which results in excessive VEGFR2 signaling and produces nonfunctional tumor angiogenesis. c UPI is a tumor-endothelium-targeting chimeric peptide for inhibiting endogenous tumor endothelial epsins by competitively binding ubiquitinated VEGFR2. This epsin mimetic peptide stabilizes VEGFR2 protein to enhance VEGFR2 signaling and produce excessive, but nonfunctional tumor vessels
Several open questions regarding the proposed mechanism still remain. Does VEGFR2 signaling initiated at the plasma membrane differ from what is classically proposed of originating from early endosomes [31]? How is the duration of hyperactivated VEGFR2 signaling achieved? Does the accumulation of cell surface VEGFR2 and changes in the dynamic and distribution of other closely related, membrane-bound components reciprocally influence each other? Perhaps, could inhibition of the epsin- and clathrin-dependent endocytic pathway shift VEGFR2 trafficking to another pathway? Given that VEGFR2 endocytosis and trafficking have been implicated as important regulatory mechanisms in promoting VEGFR2 signaling, it is reasonable to speculate a mechanism of VEGFR2 differential regulation sorting for signaling endosomes or degradative lysosomes (presumably by different endocytic adaptors), so that the disturbance of epsin function specifically disturbs VEGFR2 destined for degradation, while advancing VEGFR2 rapidly recycles back to the plasma membrane through signaling endosomes (Fig. 1b). This would lead to an increase of VEGFR2 cell surface localization and enhanced VEGFR2 signaling originating from either the plasma membrane, signaling endosomes, or both [28, 31–35].
The original understanding suggests that the overexpression of VEGF induces tortuous neo-vessel formation in promoting tumor development and progression, where VEGFR2 is the key mediator of VEGF-stimulated tumor angiogenesis. Therefore, a central idea of normalizing tumor vasculature using anti-angiogenic therapies is to reduce VEGF/VEGFR2 signaling [36]. Overstimulation of the VEGF/VEGFR2 signaling, however, results in more tortuous and dysfunctional leaky vessels that also inhibit tumor growth and metastasis. Taken together, these results suggest a model in which a moderate level of VEGF signaling is required to sustain tumor growth and the VEGF signaling extent fine-tuned by a feedback mechanism, whereby VEGF activation of VEGFR2 induces epsins-mediated VEGFR2 internalization and degradation. Future studies may need to address at least the two following questions: (1) when exactly will pro-angiogenic effects (due to a differential effect of VEGFR2 activation) be tumor promoting rather than tumor suppressive, and how could it be controlled it in the therapy [37]? And because it still remains elusive if the resistance is accompanied by alterations of the VEGF-mediated VEGFR2 ubiquitination pathway: (2) Is targeting endothelial epsins beneficial for patients with tumors that are already resistant to anti-VEGF therapies [38]?
Modulation of VEGFR2 but not other signaling pathways by endothelial epsins
Epsins also have a fundamental role in regulating Notch, as evidenced by impaired Notch signaling in E9 DKO embryos due to epsin loss [21]. Notch-signaling activation can be simplified into a two-step mechanism: removal and trans-endocytosis of the Notch receptor extracellular domain upon Dll4 binding (in signal sending cells); and release of the Notch receptor intracellular domain (in signal receiving cells). The trans-endocytosis is dependent on the ubiquitination of Dll4, and it has been postulated to form a Notch extracellular domain/Dll4/epsins triple complex from the facilitation of the epsin UIM-binding domain [39]. Although profound vascular defects found in DKO embryos are reminiscent of defects caused by loss of Notch genes in the embryo proper, placenta, and yolk-sac, the phenotype is significantly more severe than any single or double Notch deletion—suggesting a major contribution of alternative signaling pathways other than Notch [40–42]. In both EC-iDKO models and motif mimetic of epsin treatments, the contribution of Notch is excluded, because: (1) transgenic expression of the active form of Notch fails to rescue the increased vascular leak and retarded tumor growth phenotype and (2) endothelial epsins attenuate VEGFR2 signaling regardless of the presence or absence of functional Notch [24, 28]. These findings are compatible with the notion that VEGF acts upstream of Notch signaling [43, 44]. In addition, the embryonic lethality of the global DKO embryos may be due, in large part, to epsins’ role in regulating Notch signaling from other cell types.
Besides Notch, loss of endothelial epsins or epsin mimetic peptide treatment does not affect other angiogenic receptors and/or their downstream targets, including PDGFR, FGFR, EGFR, TGFβR, and VEGFR1/3 [24, 28]. The exclusion of the contribution from other signaling pathways raises an intriguing question: Are epsins unique adaptor proteins for VEGFR2 endocytosis and signaling in endothelial cells? Two additional lines of evidence are in agreement with this idea: (1) deficiency of epsins 1 and 2 in either primary endothelial cells or mouse embryonic fibroblasts do not seem to impair housekeeping clathrin-mediated endocytosis, such as that of transferrin receptors [21, 24] and (2) genetic reduction of VEGFR2 in endothelial cells decreases elevated VEGF signaling, and rescues aberrant angiogenesis caused by epsin deficiencies [22].
In primary endothelial cells, the deletion mutation of epsin UIM is sufficient to abolish binding of epsins and VEGFR2 [24]. The binding between epsins with ubiquitinated cell surface receptors, including that of activated VEGFR2, is attributed to the interaction between UIM and ubiquitin, which is highly conserved, but with low affinity and minimal specificity [19, 45–47]. A recent study unveiled a molecular binding mechanism underlying selective interactions between epsins and VEGFR2 via specific protein–protein interactions. Residues E183, E184, and E185 within the epsin UIM, along with residues H891 and S1021 within the VEGFR2 kinase domain, were identified to achieve this specificity. VEGF stimulation reportedly induces VEGFR2 ubiquitination via recruitment of the Casitas B-lineage Lymphoma E3 ubiquitin-protein ligase (c-Cbl) [29]. Interestingly, c-Cbl-dependent ubiquitination of epsin increases in response to VEGF stimulation to further promote the interaction between epsin and VEGFR2. The two residues identified as novel interfaces for binding to epsin 1, H891, and S1021 show potential in interacting with ubiquitin [48]. In this scenario, residues E183, E184, and E185 determine the classical function of UIM to bind with VEGFR2, while the novel ubiquitin-interacting interface of the VEGFR2 kinase domain binds with ubiquitinated epsins to thereby reinforce interactions between the two. Remarkably, the study also demonstrates in vitro and in vivo activities of peptide-based angiogenesis modulators targeting this interaction [48].
Perspectives
Although most studies in angiogenesis research focus on endothelial cell function within the tumor vasculature, it is important to recognize that the synergistic interplay among tumor cells, lymphatic endothelial cells, vascular supporting cells (e.g., pericytes), and myeloid cells in the tumor microenvironment determines the activities of cancer therapy in terms of efficiency and precision. Epsins are reportedly overexpressed in several types of cancer, such as prostate, colon, breast, lung, and skin cancers [49]. A recent study using a genetic mouse model reveals that loss of intestinal epithelial epsins protect against colon cancer by significantly reducing the stability of the crucial Wnt signaling effector, dishevelled, and impairing Wnt signaling [50]. Further studies are needed to delineate the intrinsic roles of epsins. It has been recently proposed that VEGF may have a direct effect on tumor cell growth [51]. VEGFR2 is also reported on many types of tumor cells, thus it would be an intriguing question if the epsin mimetic peptide could inhibit VEGF-driven tumor cell growth when specifically targeting tumor cells [52]. Besides blood vessels, the lymphatic route provides an important, yet under-appreciated role for facilitating tumor development and progression. The finding that temporal and spatial regulations of epsin abundance, as well as the VEGFR3 signaling required for lymphatic valve formation and function provides a hint that epsins may also affect tumor-associated lymphangiogenesis [53]. The increasing understanding of additional extrinsic roles of epsins will further contribute together in maximizing tumor inhibition. To date, the potential roles of epsins in pericytes and immune cells still remain largely unexplored. Given that VEGF action has emerged as a potent immunoinhibitory factor as evidenced by its modulation of expression of inhibitory checkpoints on T cells in tumors (also mediated by VEGFR2 on T cells), whether the epsin mimetic peptide could also enhance adaptive anti-tumor immunity is of special interest [54, 55].
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197108122850711. [DOI] [PubMed] [Google Scholar]
- 2.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y, Arbiser J, D’Amato RJ, D’Amore PA, DE Ingber, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, Dvorak H, Langer R. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3:114rv3. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 6.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Horsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 7.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 9.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 10.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 11.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 12.Wu Y, Cain-Hom C, Choy L, Hagenbeek TJ, de Leon GP, Chen Y, Finkle D, Venook R, Wu X, Ridgway J, Schahin-Reed D, Dow GJ, Shelton A, Stawicki S, Watts RJ, Zhang J, Choy R, Howard P, Kadyk L, Yan M, Zha J, Callahan CA, Hymowitz SG, Siebel CW. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 13.Funahashi Y, Hernandez SL, Das I, Ahn A, Huang J, Vorontchikhina M, Sharma A, Kanamaru E, Borisenko V, Desilva DM, Suzuki A, Wang X, Shawber CJ, Kandel JJ, Yamashiro DJ, Kitajewski J. A notch1 ectodomain construct inhibits endothelial notch signaling, tumor growth, and angiogenesis. Cancer Res. 2008;68:4727–4735. doi: 10.1158/0008-5472.CAN-07-6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wendland B. Epsins: adaptors in endocytosis? Nat Rev Mol Cell Biol. 2002;3:971–977. doi: 10.1038/nrm970. [DOI] [PubMed] [Google Scholar]
- 15.Tessneer KL, Cai X, Pasula S, Dong Y, Liu X, Chang B, McManus J, Hahn S, Yu L, Chen H. Epsin family of endocytic adaptor proteins as oncogenic regulators of cancer progression. J Cancer Res Updates. 2013;2:144–150. doi: 10.6000/1929-2279.2013.02.03.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenthal JA, Chen H, Slepnev VI, Pellegrini L, Salcini AE, Di Fiore PP, De Camilli P. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J Biol Chem. 1999;274:33959–33965. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 17.Messa M, Fernandez-Busnadiego R, Sun EW, Chen H, Czapla H, Wrasman K, Wu Y, Ko G, Ross T, Wendland B, De Camilli P. Epsin deficiency impairs endocytosis by stalling the actin-dependent invagination of endocytic clathrin-coated pits. Elife. 2014;3:e03311. doi: 10.7554/eLife.03311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–797. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci USA. 2005;102:2766–2771. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko G, Paradise S, Chen H, Graham M, Vecchi M, Bianchi F, Cremona O, Di Fiore PP, De Camilli P. Selective high-level expression of epsin 3 in gastric parietal cells, where it is localized at endocytic sites of apical canaliculi. Proc Natl Acad Sci USA. 2010;107:21511–21516. doi: 10.1073/pnas.1016390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, Perucco E, Collesi C, Min W, Zeiss C, De Camilli P, Cremona O. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc Natl Acad Sci USA. 2009;106:13838–13843. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tessneer KL, Pasula S, Cai X, Dong Y, McManus J, Liu X, Yu L, Hahn S, Chang B, Chen Y, Griffin C, Xia L, Adams RH, Chen H. Genetic reduction of vascular endothelial growth factor receptor 2 rescues aberrant angiogenesis caused by epsin deficiency. Arterioscler Thromb Vasc Biol. 2014;34:331–337. doi: 10.1161/ATVBAHA.113.302586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol. 2011;12:551–564. doi: 10.1038/nrm3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pasula S, Cai X, Dong Y, Messa M, McManus J, Chang B, Liu X, Zhu H, Mansat RS, Yoon SJ, Hahn S, Keeling J, Saunders D, Ko G, Knight J, Newton G, Luscinskas F, Sun X, Towner R, Lupu F, Xia L, Cremona O, De Camilli P, Min W, Chen H. Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling. J Clin Invest. 2012;122:4424–4438. doi: 10.1172/JCI64537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510–520. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science. 2010;328:1031–1035. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kovarova M, Tolar P, Arudchandran R, Draberova L, Rivera J, Draber P. Structure-function analysis of Lyn kinase association with lipid rafts and initiation of early signaling events after Fc epsilon receptor I aggregation. Mol Cell Biol. 2001;21:8318–8328. doi: 10.1128/MCB.21.24.8318-8328.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong Y, Wu H, Rahman HN, Liu Y, Pasula S, Tessneer KL, Cai X, Liu X, Chang B, McManus J, Hahn S, Dong J, Brophy ML, Yu L, Song K, Silasi-Mansat R, Saunders D, Njoku C, Song H, Mehta-D’Souza P, Towner R, Lupu F, McEver RP, Xia L, Boerboom D, Srinivasan RS, Chen H. Motif mimetic of epsin perturbs tumor growth and metastasis. J Clin Invest. 2015;125:4349–4364. doi: 10.1172/JCI80349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duval M, Bedard-Goulet S, Delisle C, Gratton JP. Vascular endothelial growth factor-dependent down-regulation of Flk-1/KDR involves Cbl-mediated ubiquitination. Consequences on nitric oxide production from endothelial cells. J Biol Chem. 2003;278:20091–20097. doi: 10.1074/jbc.M301410200. [DOI] [PubMed] [Google Scholar]
- 30.Ewan LC, Jopling HM, Jia H, Mittar S, Bagherzadeh A, Howell GJ, Walker JH, Zachary IC, Ponnambalam S. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic. 2006;7:1270–1282. doi: 10.1111/j.1600-0854.2006.00462.x. [DOI] [PubMed] [Google Scholar]
- 31.Simons M. An inside view: VEGF receptor trafficking and signaling. Physiology. 2012;27:213–222. doi: 10.1152/physiol.00016.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 33.Traub LM. Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection. J Cell Biol. 2003;163:203–208. doi: 10.1083/jcb.200309175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, Itoh N, Hirose T, Breier G, Vestweber D, Cooper JA, Ohno S, Kaibuchi K, Adams RH. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol. 2013;15:249–260. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palfy M, Remenyi A, Korcsmaros T. Endosomal crosstalk: meeting points for signaling pathways. Trends Cell Biol. 2012;22:447–456. doi: 10.1016/j.tcb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, Jain RK. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgess DJ. Angiogenesis: a happy medium? Nat Rev Cancer. 2013;13:4–5. doi: 10.1038/nrc3426. [DOI] [PubMed] [Google Scholar]
- 38.Klauber-Demore N. Are epsins a therapeutic target for tumor angiogenesis? J Clin Invest. 2012;122:4341–4343. doi: 10.1172/JCI66171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C. elegans . Development. 2004;131:5807–5815. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- 40.Gale NW, Dominguez MG, Noguera I, Pan L, Hughes V, Valenzuela DM, Murphy AJ, Adams NC, Lin HC, Holash J, Thurston G, Yancopoulos GD. Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc Natl Acad Sci USA. 2004;101:15949–15954. doi: 10.1073/pnas.0407290101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- 42.Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 43.Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedito R, Rocha SF, Woeste M, Zamykal M, Radtke F, Casanovas O, Duarte A, Pytowski B, Adams RH. Notch-dependent VEGFR3 upregulation allows angiogenesis without VEGF-VEGFR2 signalling. Nature. 2012;484:110–114. doi: 10.1038/nature10908. [DOI] [PubMed] [Google Scholar]
- 45.Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, Chen H, De Camilli P, Di Fiore PP. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–455. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 46.Hicke L. Gettin’ down with ubiquitin: turning off cell-surface receptors, transporters and channels. Trends Cell Biol. 1999;9:107–112. doi: 10.1016/S0962-8924(98)01491-3. [DOI] [PubMed] [Google Scholar]
- 47.Hofmann K, Falquet L. A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci. 2001;26:347–350. doi: 10.1016/S0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- 48.Rahman HN, Wu H, Dong Y, Pasula S, Wen A, Sun Y, Brophy ML, Tessneer KL, Cai X, McManus J, Chang B, Kwak S, Rahman NS, Xu W, Fernandes C, McDaniel JM, Xia L, Smith L, Srinivasan RS, Chen H. Selective targeting of a novel Epsin-VEGFR2 interaction promotes VEGF-mediated angiogenesis. Circ Res. 2016;118:957–969. doi: 10.1161/CIRCRESAHA.115.307679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tessneer KL, Pasula S, Cai X, Dong Y, Liu X, Yu L, Hahn S, McManus J, Chen Y, Chang B, Chen H. Endocytic adaptor protein epsin is elevated in prostate cancer and required for cancer progression. ISRN Oncol. 2013;2013:420597. doi: 10.1155/2013/420597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang B, Tessneer KL, McManus J, Liu X, Hahn S, Pasula S, Wu H, Song H, Chen Y, Cai X, Dong Y, Brophy ML, Rahman R, Ma JX, Xia L, Chen H. Epsin is required for dishevelled stability and Wnt signalling activation in colon cancer development. Nat Commun. 2015;6:6380. doi: 10.1038/ncomms7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tam BY, Wei K, Rudge JS, Hoffman J, Holash J, Park SK, Yuan J, Hefner C, Chartier C, Lee JS, Jiang S, Nayak NR, Kuypers FA, Ma L, Sundram U, Wu G, Garcia JA, Schrier SL, Maher JJ, Johnson RS, Yancopoulos GD, Mulligan RC, Kuo CJ. VEGF modulates erythropoiesis through regulation of adult hepatic erythropoietin synthesis. Nat Med. 2006;12:793–800. doi: 10.1038/nm1428. [DOI] [PubMed] [Google Scholar]
- 52.Chatterjee S, Heukamp LC, Siobal M, Schottle J, Wieczorek C, Peifer M, Frasca D, Koker M, Konig K, Meder L, Rauh D, Buettner R, Wolf J, Brekken RA, Neumaier B, Christofori G, Thomas RK, Ullrich RT. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123:1732–1740. doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X, Pasula S, Song H, Tessneer KL, Dong Y, Hahn S, Yago T, Brophy ML, Chang B, Cai X, Wu H, McManus J, Ichise H, Georgescu C, Wren JD, Griffin C, Xia L, Srinivasan RS, Chen H. Temporal and spatial regulation of epsin abundance and VEGFR3 signaling are required for lymphatic valve formation and function. Sci Signal. 2014;7:ra97. doi: 10.1126/scisignal.2005413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavalas NG, Tsiatas M, Tsitsilonis O, Politi E, Ioannou K, Ziogas AC, Rodolakis A, Vlahos G, Thomakos N, Haidopoulos D, Terpos E, Antsaklis A, Dimopoulos MA, Bamias A. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br J Cancer. 2012;107:1869–1875. doi: 10.1038/bjc.2012.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139–148. doi: 10.1084/jem.20140559. [DOI] [PMC free article] [PubMed] [Google Scholar]