Abstract
Background
This study sought to determine if preoperatively measured high-sensitivity cardiac troponin T (hs-cTnT) and N-terminal pro-brain natriuretic peptide (NT-proBNP) improve cardiac risk prediction in patients undergoing major non-cardiac surgery when compared to standard risk indices.
Methods
In this ancillary study to the Vitamins in Nitrous Oxide (VINO) trial, patients were included who had preoperative hs-cTnT and NT-proBNP measured (n=572). Study outcome was the incidence of postoperative myocardial infarction (MI) within the first three postoperative days. hs-cTn was considered elevated if >14 ng/L and NT-proBNP if >300 ng/L. Additional cutoff values were investigated based on ROC statistics. Biomarker risk prediction was compared to Lee’s Revised Cardiac Risk Index (RCRI) using standard methods and net reclassification index (NRI).
Results
The addition of hs-cTnT (>14 ng/L) and NT-proBNP (>300 ng/L) to RCRI significantly improved the prediction of postoperative MI (event rate 30/572 (5.2%), AUC ROC increased from 0.590 to 0.716 with a 0.66 NRI [95% CI 0.32 – 0.99] p<0.001). Using 108 ng/L as cutoff for NT-proBNP improved sensitivity compared to 300 ng/L (0.87 vs. 0.53). Sensitivity, specificity, positive and negative predictive value for hs-cTnT were 0.70, 0.60, 0.09 and 0.97, and 0.53, 0.68, 0.08, 0.96 for NT-proBNP.
Conclusions
The addition of cardiac biomarkers hs-cTnT and NT-proBNP to RCRI improves prediction of adverse cardiac events in the immediate postoperative period after major non-cardiac surgery. The high negative predictive value of preoperative hs-cTnT and NT-proBNP suggest usefulness as a “rule-out” test to confirm low risk of postoperative MI.
Adverse cardiac events, including acute myocardial infarction, are serious and frequent complications after non-cardiac surgery and portend an adverse prognosis.(1–3) The reliable identification of patients at risk for such events prior to surgery is an important goal of perioperative medicine, as it may allow targeted interventions. However, how to achieve accurate preoperative prediction of postoperative cardiac events is rudimentary at best.(4,5) Most practitioners rely on simple scores and risk indices such as Lee’s Revised Cardiac Risk Index (RCRI)(6) or the American Society of Anesthesiologists (ASA) physical status(7), whose six and five levels, respectively, do not provide an adequate level of discrimination among patients.
Cardiac biomarkers, such as high-sensitivity cardiac troponin T (hs-cTnT) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are used in cardiology and general medical practice for risk prediction and case management.(8–13) We have recently reported that hs-cTnT improves preoperative risk prediction.(14) We now sought to investigate whether NT-proBNP(15–21) and hs-cTnT augment the accuracy of standard risk indices such as RCRI and ASA physical status to predict postoperative MI. Accordingly, we conducted a nested cohort study within the completed Vitamins in Nitrous Oxide (VINO) Trial. The primary purpose of VINO was to investigate the effects of nitrous oxide plus B-vitamins on perioperative cardiac events.(22)
Methods
Study Design and Population
This was an ancillary nested cohort study of patients enrolled in the VINO Trial (Clinicaltrials.gov number NCT00655980). Hypotheses tested in this ancillary study were post-hoc and not designed a priori. VINO was a double-blind, randomized, placebo-controlled, single-center trial; patients were enrolled between March 2008 and December 2011. A detailed description of the trial methods and main results have been published elsewhere.(22) VINO enrolled 625 adult patients with either known coronary artery disease or multiple risk factors for coronary artery disease who were scheduled for major non-cardiac surgery under general anesthesia. Patients were randomly assigned to receive nitrous oxide and B-vitamins (250 patients) or nitrous oxide and placebo (250 patients). A concurrent reference group who received neither nitrous oxide nor B-vitamins was also enrolled (125 patients). The trial results were negative, i.e. B-vitamins had no effect on cardiac events.
Inclusion criteria for this ancillary study were the availability of a preoperative hs-cTnT and NT-proBNP value (572 patients fulfilled this criterion) plus at least one postoperative value for each biomarker.
The study was approved by the Washington University in St. Louis institutional review board, and all patients provided written, informed consent.
Biomarker Assays
Blood and 12-lead electrocardiograms were obtained at five pre-defined time points: preoperative (baseline), which was within 2 hours before surgery; within 30 minutes after arrival in the post-anesthesia care unit; and on the mornings of postoperative days 1, 2 and 3. Samples were collected in lithium heparin tubes and immediately put on ice and centrifuged within 30 minutes after collection. Plasma was then transferred into cryogenic tubes and stored at −70°C. Biomarker measurements were performed in batches (samples had no more than two freeze-thaw cycles) and were performed by study personnel unaware of clinical outcomes.
hs-cTnT and NT-proBNP concentrations were measured on a Roche Elecsys 2010 analyzer (for hs-cTnT: limit of detection: 5.0 ng/L; 99th percentile: 14 ng/L; a 10% CV at 13 ng/L; NT-proBNP: limit of detection: 1.0 ng/L; <5% CV at concentrations > 70 ng/L).(23) Standard cTnI concentrations were measured with a contemporary assay on a Siemens Dimension RxL analyzer (99th percentile URL is 0.07 µg/L).). Please note that concentrations for the hs-cTn assays are designated in ng/L to distinguish from contemporary cTn assays.
Outcomes
The outcome of this study was postoperative MI within the first three days after surgery. MI was defined according to the Universal Definition (rising pattern of cTnI with at least one elevation > 99th percentile plus new ECG changes indicative of myocardial ischemia and/or clinical symptoms).(24) New Q-waves, ST-segment depression or T-wave inversion ≥ 0.1mV, or ST-elevation ≥ 0.2 mV in at least two contiguous leads were considered indicative of myocardial ischemia. ECGs were read and analyzed by a physician blinded to biomarker results.
Statistical Analysis
All cTn and NT-proBNP values are reported as medians plus interquartile ranges due to skewness of the data. The estimated glomerular filtration rate (eGFR) was calculated according to the CKD-EPI creatinine formula.(25) Preoperative hs-cTnT and NT-proBNP were assessed as both continuous as well as categorical variables. 14 ng/L (99th percentile URL) was the cutoff value used for hs-cTnT. Because sex-specific cutoff values for hs-cTnT were not helpful in our previous analysis, they were not used in this analysis.(14)
For NT-proBNP we initially used continuous data, probed 300 ng/L as the cutoff value as proposed in the literature, and determined the optimal cutoff value based on Youden's J statistic (J = Sensitivity + Specificity – 1) on the ROC curve value that maximizes J.(15,17,21)
Univariate and multiple logistic regression, unadjusted or adjusted for age, sex, eGFR and a history of coronary artery disease were used to assess the association of preoperative RCRI, ASA status, hs-cTnT and NT-proBNP with postoperative MI (RCRI and ASA status were only adjusted for age and sex). Wald’s test was used to determine the contribution of individual covariates. The ability of Lee’s RCRI index and each biomarker to predict postoperative cardiac events was determined by the area under the Receiver Operator Characteristic (ROC) curve.
The biomarker AUC ROC values were compared to Lee’s index AUC using the methods of Delong.(26) The ability of the biomarkers to improve upon Lee’s RCRI was evaluated by calculating the category-free Net Reclassification Improvement (NRI).(27) The category-free NRI measures the correctness of patient reclassification after adding the biomarker as a predictor of outcome in addition to Lee’s index. A correct reclassification occurs when the predicted probability of Lee’s RCRI + additional biomarker(s) is greater than Lee’s RCRI alone among patients with outcome events and/or when the predicted probability is less than Lee’s RCRI alone among patients without outcome events. The NRI is determined as the net improvement among events plus the net improvement among non-events, where net improvement is the difference between those correctly vs. those incorrectly reclassified. NRI values range from −2 to 2, with positive values indicating overall improvement when adding the biomarker.
Statistical analyses were performed on SAS v9.4 as well as JMP 12.2.0 (SAS Institute Inc., Cary, NC). Graphs were constructed on GraphPad Prism 6.01 (GraphPad Software Inc., La Jolla, CA).
Results
The study population consisted of 572 patients from the VINO trial in whom preoperative hs-cTnT and NT-proBNP were measured (original VINO sample size: n=625). All patients had several cardiac risk factors and more than half had previously been diagnosed with coronary artery disease; the distribution within the Revised Cardiac Risk Index (RCRI) and ASA physical status are listed in Table 1.
Table 1.
Preoperative Characteristics of the Study Population
| Preoperative Biomarker Status | |||||
|---|---|---|---|---|---|
| hs-cTnT < 14 ng/L NT-proBNP < 300 ng/L |
hs-cTnT > 14 ng/L NT-proBNP < 300 ng/L |
hs-cTnT < 14 ng/L NT-proBNP > 300 ng/L |
hs-cTnT > 14 ng/L NT-proBNP > 300 ng/L |
Total | |
| n= 279 (48.8%) | n= 102 (17.8%) | n=53 (9.3%) |
n=138 (24.1%) | n=572 (100%) |
|
| Mean age – yr (SD) | 60.1 (9.4) | 65.8 (8.5) | 66.2 (8.6) | 70.5 (10.1) | 64.9 (10.7) |
| Male Sex, n (%) | 153 (54.8) | 76 (74.5) | 32 (60.4) | 94 (68.1) | 355 (62.1) |
| Race, n (%) | |||||
| White | 221 (79.2) | 83 (82.2) | 45 (84.9) | 112 (81.8) | 461 (80.1) |
| Black | 56 (20.1) | 18 (17.8) | 8 (15.1) | 25 (18.2) | 107 (18.8) |
| Other | 2 (0.7) | 0 | 0 | 0 | 2 (0.4) |
| Smoking history, n (%) | 218 (78.1) | 71 (69.6) | 47 (88.7) | 94 (69.1) | 430 (75.4) |
| Current smoker, n (%) | 90 (32.3) | 22 (21.5) | 22 (41.5) | 32 (23.2) | 166 (29.0) |
| Pack-years (median, IQR) | 37.5 (20; 50) | 32 (19; 60) | 40 (25; 55.5) | 40 (20; 60) | 40 (20;60) |
| Diabetes, n (%) | 83 (29.9) | 40 (39.6) | 13 (24.5) | 71 (51.8) | 207 (36.8) |
| Insulin dependent, n (%) | 24 (29.3) | 16 (40.0) | 4 (30.8) | 38 (53.5) | 82 (14.3) |
| Hypertension, n (%) | 208 (74.8) | 90 (88.2) | 48 (90.6) | 116 (84.1) | 462 (80.1) |
| Hypercholesterolemia, n (%) | 176 (63.1) | 66 (64.7) | 34 (64.2) | 97 (71.3) | 373 (65.4) |
| Chronic renal failure, n (%) | 17 (6.2) | 8 (7.9) | 3 (5.7) | 31 (22.6) | 59 (10.4) |
| On hemodialysis, n (%) | 1 (0.4) | 1 (0.4) | 0 | 4 (2.9) | 6 (1.0) |
| eGFR (median, IQR) | 90 (75;101) | 79 (62; 94) | 75 (57; 90) | 60 (46; 82) | 80 (61; 95) |
| COPD, n (%) | 35 (12.5) | 11 (10.8) | 12 (22.6) | 19 (13.8) | 77 (13.5) |
| Coronary artery disease, n (%) | 126 (45.3) | 60 (58.8) | 31 (58.5) | 105 (76.1) | 322 (56.4) |
| Previous MI, n (%) | 57 (20.4) | 27 (26.5) | 20 (37.7) | 50 (36.8) | 154 (27.0) |
| Previous PCI/stent, n (%) | 82 (29.7) | 34 (33.7) | 15 (28.3) | 62 (45.9) | 193 (34.2) |
| Previous CABG, n (%) | 28 (10.1) | 18 (17.6) | 9 (17.0) | 44 (31.9) | 99 (17.4) |
| Congestive heart failure, n (%) | 21 (7.5) | 8 (7.8) | 8 (15.1) | 32 (23.4) | 69 (12.1) |
| Peripheral vascular disease, n (%) |
84 (30.2) | 26 (26.0) | 16 (30.2) | 63 (46.0) | 189 (33.3) |
| Carotid disease, n (%) | 17 (6.2) | 13 (12.9) | 4 (7.5) | 14 (10.2) | 48 (8.5) |
| Stroke/TIA, n (%) | 34 (12.2) | 11 (10.8) | 12 (22.6) | 23 (16.8) | 80 (14.0) |
| Atrial fibrillation, n (%) | 18 (6.5) | 6 (5.9) | 8 (15.4) | 36 (26.3) | 68 (11.9) |
| Lee’s revised cardiac risk index | |||||
| I | 104 (37.5) | 32 (31.4) | 15 (28.8) | 24 (17.4) | 175 (30.8) |
| II | 121 (43.7) | 50 (49.0) | 23 (44.2) | 56 (40.6) | 250 (43.9) |
| III | 48 (17.3) | 17 (16.7) | 12 (23.1) | 39 (28.3) | 116 (20.4) |
| IV | 4 (1.4) | 3 (2.9) | 2 (3.8) | 19 (13.8) | 28 (4.9) |
| ASA status, n (%) | |||||
| II | 61 (21.9) | 18 (17.8) | 5 (9.4) | 8 (5.8) | 92 (16.1) |
| III | 211 (75.9) | 79 (78.2) | 47 (88.7) | 119 (86.2) | 456 (80.0) |
| IV | 6 (2.2) | 4 (4.0) | 1 (1.9) | 11 (8.0) | 22 (3.9) |
| hs-cTnT ng/L (median, IQR) | 8.6 (6.3; 10.5) | 18.2 (15.7; 22.4) | 10.0 (7.7; 11.8) | 23.7 (18.6; 34.8) | 12.0 (8.3; 19.3) |
| NT-proBNP ng/L (median, IQR) | 66 (35; 112) | 122 (70; 179) | 479 (360; 718) | 936 (493; 1926) | 140 (60; 421) |
Prior to surgery, hs-cTnT was detectable in 563/572 patients (98.5%) with 240 patients having elevated hscTnT ≥14 ng/L (42%), while contemporary cTnI was detectable in only 74/569 patients (13%). Baseline NT-proBNP was detectable in all patients, with 191 having elevated NT-proBNP >300 ng/L (33%). At baseline, hs-cTnT and NT-proBNP were positively correlated (Spearman’s rho= 0.54).
Prediction of Perioperative Myocardial Injury and Infarction
Within the first three postoperative days 30/572 patients (5.2%) developed an acute MI. Postoperative myocardial infarction was more frequent among patients with RCRI level 4 and ASA physical status IV and in patients with isolated or dual preoperative cardiac biomarker elevation (Table 2).
Table 2.
Postoperative Study Outcomes
| Myocardial Infarction (n=30) |
Unadjusted Odds Ratio (95% CI) |
|
|---|---|---|
| Lee’s RCRI, n (%) | ||
| I (n=175) | 5 (2.9%) | 1 (ref.) |
| II (n=250) | 15 (6.0%) | 2.18 (0.83 – 6.80) |
| III (n=116) | 4 (3.5%) | 1.23 (0.30 – 4.73) |
| IV (n=28) | 5 (17.9%) | 7.40 (1.92 – 28.52) |
| Missing (n=3) | 1 | |
| ASA status, n (%) | ||
| II (n=92) | 2 (2.2%) | 1 (ref.) |
| III (n=456) | 22 (4.9%) | 2.29 (0.66 – 14.46) |
| IV (n=22) | 5 (22.7%) | 13.24 (2.62 – 97.87) |
| Missing (n=2) | 1 | |
| Preoperative Biomarker Profile, n (%) | ||
| hs-cTnT <14 ng/L/NT-proBNP <300 ng/L (n=279) |
6 (2.2%) | 1 (ref.) |
|
hs-cTnT >14 ng/L/NT-proBNP <300 ng/L (n=102) |
8 (7.8%) | 3.87 (1.31 – 12.04) |
| hs-cTnT <14 ng/L/NT-proBNP >300 ng/L (n=53) |
3 (5.7%) | 2.73 (0.56 – 10.71) |
|
hs-cTnT >14 ng/L/NT-proBNP >300 ng/L (n=138) |
13 (9.6%) | 4.81 (1.85 – 13.96) |
RCRI – Revised Cardiac Risk Index; ASA – American Society of Anesthesiologists
Lee’s RCRI, ASA-physical status, as well as preoperative hs-cTnT and NT-proBNP concentrations were individually associated with postoperative MI (Table 3a). After adjusting for age, sex, eGFR and pre-existing coronary artery disease, elevated hs-cTnT (≥14 ng/L) prior to surgery was associated with an adjusted odds ratio (aOR) for acute MI of 2.26, 95% CI 0.93 – 5.83, p=0.07), while elevated NT-proBNP (>300 ng/L) was associated with an aOR of 1.55 (95% CI 0.66 – 3.36, p=0.31). In a sensitivity analysis (Table 3b) comparing the association of individual predictors in patients with or without known coronary artery disease, elevated hs-cTnT prior to surgery was associated with an aOR of 6.04 (95% CI 0.94, 38.90, p=0.06) for postoperative MI, whereas NT-proBNP had no discernible effect. In patients with known CAD, elevated hs-cTnT and NT-proBNP prior to surgery were associated with aORs of 1.55 (95% CI 0.54, 4.43, p-0.41), and 1.84 (95% CI 0.70, 4.87, p= 0.22) for postoperative MI.
Table 3.
| a. Association of Predictors with Postoperative Myocardial Infarction | |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multiple Regression Analysis | ||||||
| Outcome | Variable | OR | 95% CI | p-value | aOR | 95% CI | p-value |
| Postoperative MI | Lee’s RCRI (overall) | 1.56 | (1.02, 2.37) | 0.04 | 1.53 | (1.00, 2.33) | 0.05 |
| ASA physical status (overall) | 4.26 | (1.67, 10.81) | 0.003 | 4.17 | (1.60, 10.64) | 0.003 | |
| hs-cTnT baseline (continuous) | 1.02 | (1.01, 1.03) | 0.01 | 0.99 | (0.98, 1.00) | 0.13 | |
| hsTnT baseline > 14 ng/L(yes vs. no) | 3.58 | (1.61, 7.97) | 0.001 | 2.26 | (0.93, 5.83) | 0.07 | |
| NT-pro BNP baseline (continuous) | 1.00 | (1.00, 1.00) | 0.03 | 1.00 | (1.00, 1.00) | 0.34 | |
| NT-pro BNP baseline >300 ng/L | 2.42 | (1.16, 5.08) | 0.02 | 1.55 | (0.66, 3.63) | 0.31 | |
| b. Sensitivity Analysis Comparing Individual Predictors in Patients with or without known Coronary Artery Diseas | ||||||
|---|---|---|---|---|---|---|
| No CAD | CAD | |||||
| Variable | aOR | 95% CI | p-value | aOR | 95% CI | p-value |
| Lee’s RCRI | 1.0 | (0.24, 4.10) | 1.00 | 1.07 | (0.59, 1.97) | 0.82 |
| hsTnT baseline > 14 ng/L | 6.04 | (0.94, 38.90) | 0.06 | 1.55 | (0.54, 4.43) | 0.41 |
| NT-pro BNP baseline >300 ng/L | 0.56 | (0.05, 6.31) | 0.64 | 1.84 | (0.70, 4.87) | 0.22 |
RCRI – Revised cardiac risk index
The multiple regression model adjusted for age, sex, eGFR, coronary artery disease in Table 3a and for age, sex, eGFR in Table 3b.
Of note, among the 74 patients who had a detectable contemporary cTnI concentration prior to surgery, 7 (10%) developed acute MI (10%; aOR 2.07; 95% CI 0.79 – 4.81, p=0.13). Using ROC curve analyses, the optimal NT-proBNP concentration cutoff (which maximizes the sum of sensitivity + 1-Specificity) for prediction of acute MI was 108 ng/L.
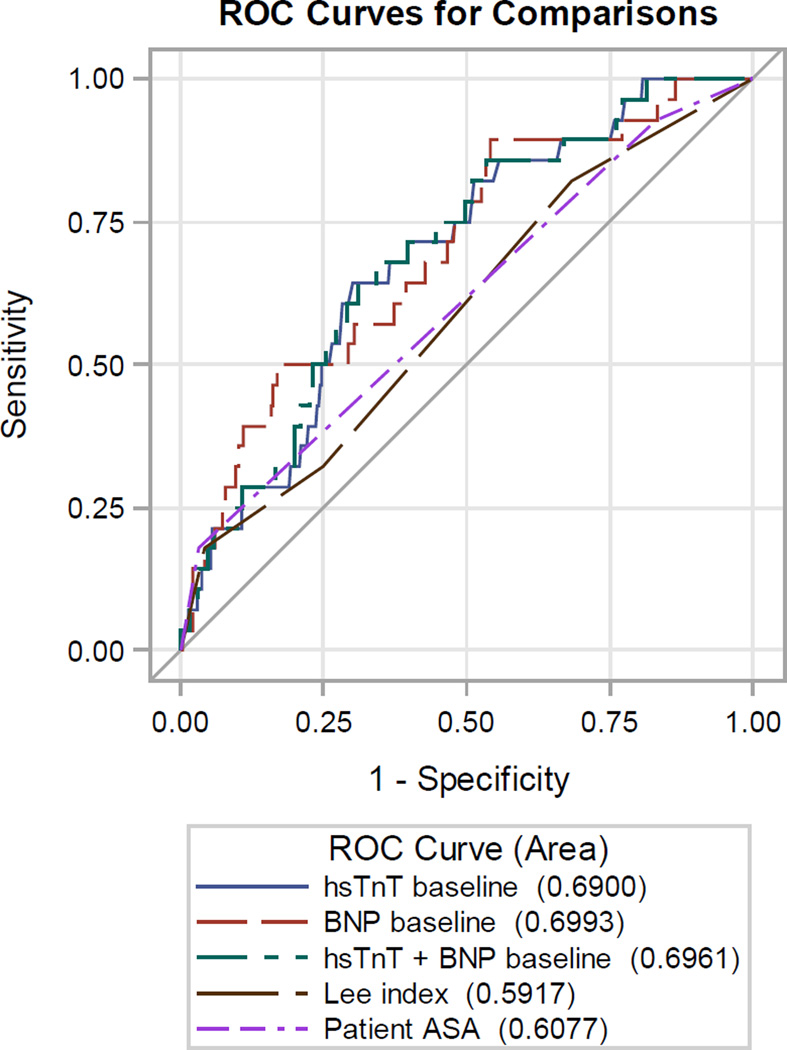
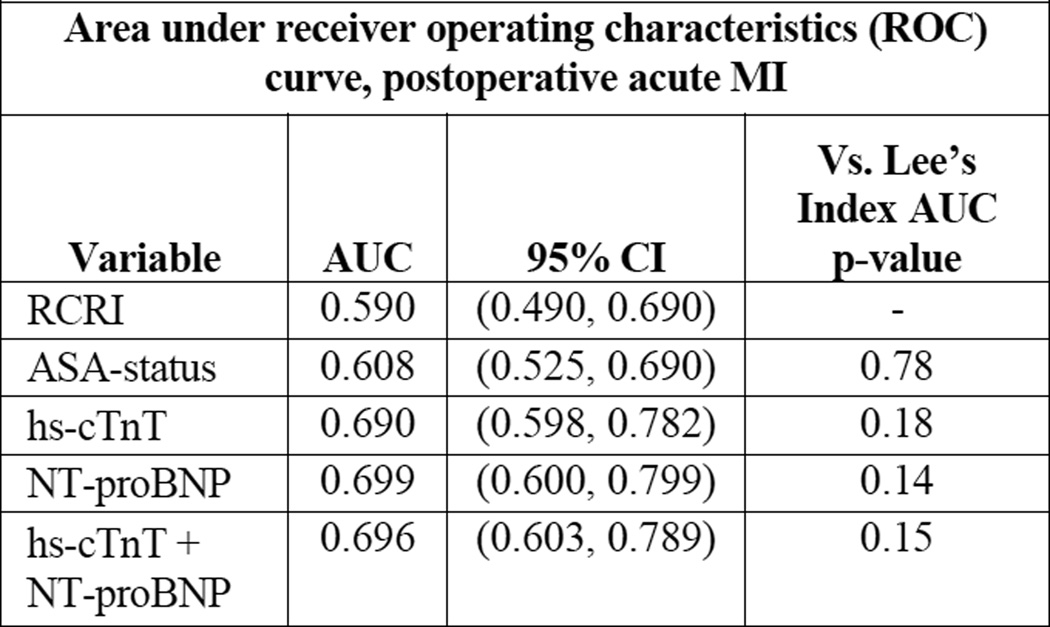
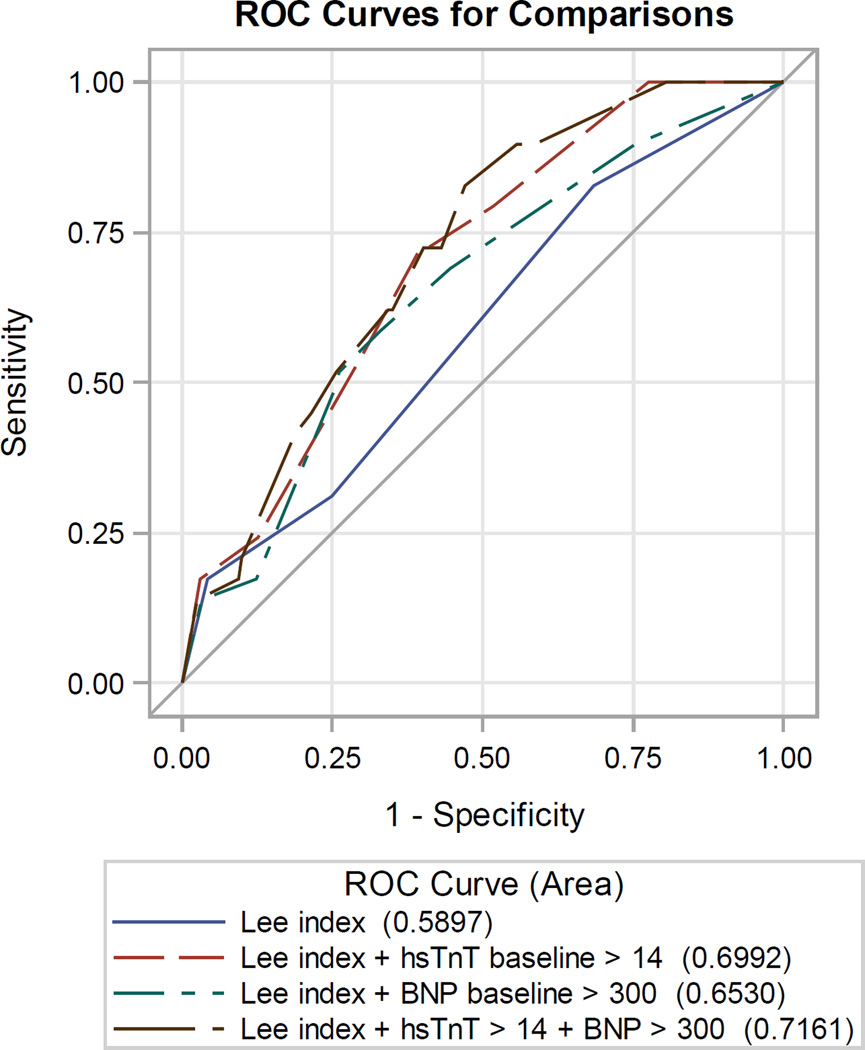
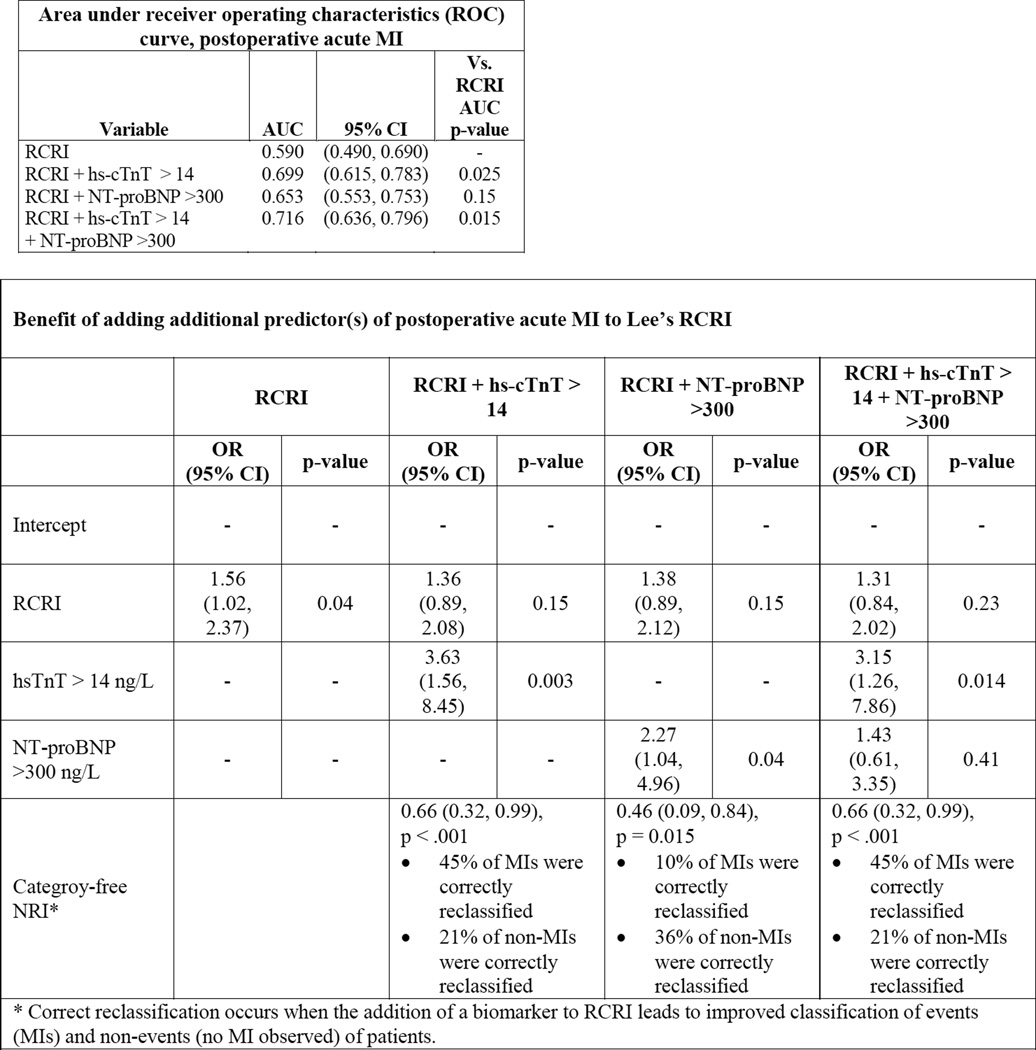
Lee’s RCRI and ASA physical status had mediocre discriminatory ability in correctly predicting postoperative MI: the area under the curve (AUC) of the receiver operator characteristics (ROC) curve was 0.590 and 0.608 for acute MI, respectively (Figure 1). Compared to RCRI, hs-cTnT and NT-proBNP on a continuous scale each improved discrimination: 0.690 and 0.699 for acute MI. The addition of hs-cTnT (cutoff 14 ng/L) and NT-proBNP (cutoff 300 ng/L) to RCRI significantly improved the prediction of postoperative MI (Figure 2), the area under the ROC increased from 0.590 to 0.716 when both biomarkers were added to RCRI (p=0.02) with a 0.66 improved event classification (NRI 0.66, 95% CI 0.32 – 0.99, p<0.001).
Figure 1.
| Area under receiver operating characteristics (ROC) curve, postoperative acute MI | |||
|---|---|---|---|
| Variable | AUC | 95% CI | Vs. Lee’s Index AUC p-value |
| RCRI | 0.590 | (0.490, 0.690) | - |
| ASA-status | 0.608 | (0.525, 0.690) | 0.78 |
| hs-cTnT | 0.690 | (0.598, 0.782) | 0.18 |
| NT-proBNP | 0.699 | (0.600, 0.799) | 0.14 |
| hs-cTnT + NT-proBNP |
0.696 | (0.603, 0.789) | 0.15 |
Figure 2.
| Area under receiver operating characteristics (ROC) curve, postoperative acute MI | |||
|---|---|---|---|
| Variable | AUC | 95% CI | Vs. RCRI AUC p-value |
| RCRI | 0.590 | (0.490, 0.690) | - |
| RCRI + hs-cTnT > 14 | 0.699 | (0.615, 0.783) | 0.025 |
| RCRI + NT-proBNP >300 | 0.653 | (0.553, 0.753) | 0.15 |
| RCRI + hs-cTnT > 14 + NT-proBNP >300 |
0.716 | (0.636, 0.796) | 0.015 |
| Benefit of adding additional predictor(s) of postoperative acute MI to Lee’s RCRI | ||||||||
|---|---|---|---|---|---|---|---|---|
| RCRI | RCRI + hs-cTnT > 14 |
RCRI + NT-proBNP >300 |
RCRI + hs-cTnT > 14 + NT-proBNP >300 |
|||||
| OR (95% CI) |
p-value | OR (95% CI) |
p-value | OR (95% CI) |
p-value | OR (95% CI) |
p-value | |
| Intercept | - | - | - | - | - | - | - | - |
| RCRI | 1.56 (1.02, 2.37) |
0.04 | 1.36 (0.89, 2.08) |
0.15 | 1.38 (0.89, 2.12) |
0.15 | 1.31 (0.84, 2.02) |
0.23 |
| hsTnT > 14 ng/L | - | - | 3.63 (1.56, 8.45) |
0.003 | - | - | 3.15 (1.26, 7.86) |
0.014 |
| NT-proBNP >300 ng/L |
- | - | - | - | 2.27 (1.04, 4.96) |
0.04 | 1.43 (0.61, 3.35) |
0.41 |
| Categroy-free NRI* |
0.66 (0.32, 0.99), p <.001 • 45% of MIs were correctly reclassified • 21% of non-MIs were correctly reclassified |
0.46 (0.09, 0.84), p = 0.015 • 10% of MIs were correctly reclassified • 36% of non-MIs were correctly reclassified |
0.66 (0.32, 0.99), p <.001 • 45% of MIs were correctly reclassified • 21% of non-MIs were correctly reclassified |
|||||
Sensitivity, specificity, positive and negative predictive value to predict postoperative MI for hs-cTnT were 0.70, 0.60, 0.09 and 0.97, and 0.53, 0.68, 0.08, 0.96 for NT-proBNP.
Using the empirically obtained “optimal” cutoff value of 108 ng/L for NT-proBNP markedly improved sensitivity compared to 300 ng/L (0.87 vs. 0.53), while also improving the net reclassification index from 0.66 to 0.71 (95% CI 0.37 – 1.04) for postoperative MI.
Discussion
The goal of this study was to determine whether cardiac biomarkers hs-cTnT and NT-proBNP can improve preoperative cardiac risk prediction compared to standard risk indices such as RCRI and ASA physical status. In our high-risk population, classical risk indices (i.e., Lee’s RCRI and ASA physical status) had mediocre ability to predict postoperative MI. Preoperatively measured cardiac biomarkers hs-cTnT and NT-proBNP outperformed Lee’s RCRI or ASA physical status, either alone or when added to the risk indices. A joint elevation of both biomarkers indicated patients with the highest risk for postoperative cardiac morbidity (4–5--fold increase). While both biomarkers hs-cTnT and NT-proBNP were significant predictors of adverse cardiac events, the stronger discriminator was hs-cTnT. Employing a lower NT-proBNP cutoff value of 108 ng/L determined from our data increased sensitivity compared to a 300 ng/L cutoff.
BNP and NT-proBNP have been used for many years to diagnose and stratify patients with acute and chronic heart failure.(28) In perioperative medicine, several studies have shown that preoperative BNP and NT-proBNP values are associated with postoperative cardiac events after major non-cardiac surgery.(15–18,20,21,29–32) High-sensitivity cardiac troponin assays now allow the detection of more subtle episodes of cardiac injury.(9,11) Baseline hs-cTn is a strong predictor of cardiac morbidity and mortality in the general adult population.(12,33,34) Several perioperative studies, including one from this cohort, have shown that baseline hs-cTnT alone can predict postoperative myocardial injury and infarction as well as long-term mortality.(14,19,35) We observed that the 99th percentile of the upper reference limit of the hs-cTnT assay (14 ng/L) appeared to be a good cutoff to identify the patients at highest risk for subsequent postoperative cardiac morbidity and mortality.
We enrolled a high-risk patient population: many patients either suffered from coronary artery disease or were at high risk for CAD from a combination of several risk factors (diabetes, hypertension, renal disease, stroke, etc.). It should therefore come as no surprise most patients had either an elevated NT-proBNP or hs-cTnT value prior to surgery. At the outset of this study it was unclear if both cardiac biomarkers would identify the same high-risk patients, i.e. if both cardiac biomarkers would be jointly elevated. While we observed a modest correlation of 0.54, many patients had either an isolated hs-cTnT or NT-proBNP elevation, which indicates predominantly distinct patient sub-populations.
Despite the significant improvement in postoperative cardiac risk prediction by cardiac biomarkers compared to risk indices, the overall level of discrimination still is modest, which is in line with prior evidence from other studies.(36,37) In our population, hs-cTnT had a sensitivity of 70% and a specificity of 60% for acute postoperative MI. The low positive predictive value (20%), but very high negative predictive value (>90%) indicates the potential utility of preoperative cardiac biomarkers as “rule out” markers, i.e., patients with a normal biomarker value have a very low risk of developing postoperative cardiac events. However, the negative predictive value of a test is influenced by the low prevalence of postoperative MI. The pattern of low positive, but high negative predictive value may, however, change when hs-cTn assays are used for postoperative event detection, which should result in a larger number of events.(38)
An interesting inconsistency, however, relates to the fact that a high negative predictive value of a test with strong “rule-out” features would be expected to mostly correct the non-events. Our study showed that hs-cTnT and NT-proBNP had corrective effects for both events and non-events and it is unclear why. A possible explanation may lie in the fact that the negative predictive value, like other epidemiological test metrics such as sensitivity and specificity, is determined in isolation, i.e. for each test or biomarker individually. The net reclassification index, however, is asking if the addition of a biomarker to RCRI – when we already know the RCRI – can improve risk prediction beyond the RCRI. Thus, these may be two separate questions and explain the inconsistency.
Our study has several limitations. First, the study population comprised a targeted group of high-risk patients which may not be representative of a general surgical population. In a general surgical population, one would expect a higher number of healthy patients with fewer cardiac risk factors and therefore fewer patients with an elevated hs-cTnT or NT-proBNP. On the one hand this would probably result in less efficient and more expensive screening; on the other hand, if elevated hs-cTnT or NT-proBNP levels were found, it may improve identification of increased cardiovascular risk in these patients. Second, although both biomarkers were associated with postoperative cardiac morbidity, they could not identify all patients who experienced these outcomes. Third, despite enrolling a high-risk patient population, event rates were low and thus the precision of our findings modest. In addition, we used a standard non-high sensitivity cardiac troponin assay to define events. Without doubt, this assay reduced the number of events detected postoperatively and thus may have exaggerated or diminished the ability of biomarkers to predict events. Fourth, based on our prior research, we decided not to use sex-specific cutoffs for hs-cTnT,(14) but future work may find that using sex-specific cutoffs may improve risk prediction.(39) The sample size of our study limited the robustness of the findings and several associations became statistically non-significant after adjustment for several covariates, indicating limited statistical power. Lastly, our study used a contemporary, non-high-sensitivity cTn assay, the current standard of care in the United States, but not a high-sensitivity cTn assay to diagnose study outcomes. As we show in a related analysis, the use of hscTnT more than doubles the diagnosis of postoperative MI. High-sensitivity cTn assays have become the standard-of-care in many countries worldwide, but these assay have not yet been cleared by the FDA.
An important consideration is in regards to the RCRI. The RCRI was originally devised to predict MACE (major adverse cardiac events), including myocardial infarction, pulmonary edema, ventricular fibrillation or primary cardiac arrest, and complete heart block. Like most subsequent studies, our study did not assess pulmonary edema, ventricular fibrillation, or complete heart block which jointly comprised more than half of the observed events in the original RCRI derivation.(6) Secondly, neither RCRI nor ASA physical status were designed to measure postoperative cardiac troponin elevation, a condition that has recently been termed MINS (myocardial injury after non-cardiac surgery)(40) and which has independently been associated with adverse long-term outcomes.(41–45)
In conclusion, the addition of cardiac biomarkers hs-cTnT and NT-proBNP to RCRI improved preoperative prediction of adverse cardiac events after major non-cardiac surgery. Employing a lower NT-proBNP cutoff value of 108 ng/L provides increased sensitivity and improved risk prediction compared to a 300 ng/L cutoff. Recently, experts presented a compelling case for a new revision of the RCRI.(46,47) Perhaps the inclusion of preoperative cardiac biomarkers may further improve the identification of patients at risk for adverse postoperative cardiac outcomes.
Table 4.
Sensitivity, Specificity, Negative and Positive Predictive Value of hs-cTnT and NT-proBNP
| MI | No MI | Odds Ratio (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
Likelihood Ratio |
|
|---|---|---|---|---|---|---|---|---|
| hs-cTnT > 14 ng/L | 21 | 217 | 3.47 (1.56 – 6.98) |
0.70 (0.51 – 0.85) |
0.60 (0.56 – 0.64) |
0.09 (0.06 – 0.13) |
0.97 (0.95 – 0.99) |
1.74 |
| hs-cTnT < 14 ng/L | 9 | 323 | ||||||
| NT-proBNP >300 ng/L | 16 | 173 | 2.42 (1.16 – 5.09) |
0.53 (0.34 – 0.72) |
0.68 (0.64 – 0.72) |
0.08 (0.05 – 0.13) |
0.96 (0.94 – 0.98) |
1.67 |
| NT-proBNP <300 ng/L | 14 | 367 | ||||||
| NT-proBNP >108 ng/L | 26 | 293 | 5.48 (1.89 – 15.90) |
0.87 (0.69– 0.96) |
0.46 (0.41 – 0.50) |
0.08 (0.05 – 0.11) |
0.98 (0.96 – 1.00) |
1.60 |
| NT-proBNP <108 ng/L | 4 | 247 |
Acknowledgments
| Nagele: | Research Support: Roche Diagnostics US; Abbott Diagnostics. |
| Scott: | Research Support - Siemens Healthcare Diagnostic; Abbott Diagnostics, Instrumentation Laboratories; Consulting - Instrumentation Laboratories; Becton-Dickinson |
| Jaffe: | Consultation: Beckman, Ortho, Abbott, Alere, Critical Diagnostics, Roche, Radiometer, Siemens, ET Healthcare, Lpath, Novartis, Amgen and theHeart.org |
| Apple: | Industry Grant/Research Support through Minneapolis Medical Research Foundation, no salary, that involve cardiac troponin: Abbott Diagnostics, Siemens, Ortho-Clinical Diagnostics, Roche Diagnostics, Alere, Trinity BioTech, Beckman Coulter; Paid Consultant: Philips Healthcare Incubator, and Metanomics Health GmbH. |
Funding/Support:
The parent VINO trial was funded by a grant from the National Institute for General Medical Sciences (K23 GM087534) and a grant to Washington University Institute of Clinical and Translational Sciences (UL1RR024992), the Foundation for Anesthesia Education and Research (FAER), and the Division of Clinical and Translational Research, Department of Anesthesiology, Washington University. Roche Diagnostics (Indianapolis, IN) provided the hs-cTnT and NT-proBNP assays and covered the costs of running these assays.
Footnotes
Dr. Michael Kopec was awarded the First Prize of the 2013 American Society of Anesthesiologists Resident Research Essay Contest for research contributing to this manuscript.
Disclosures:
Kopec, Brown J, Brown F, Duma, Helwani, Novak, Gage, Gibson, Miller: No conflicts of interest.
Contributor Information
Michael Kopec, Division of Clinical and Translational Research, Department of Anesthesiology, Washington University School of Medicine in St. Louis
Andreas Duma, Division of Clinical and Translational Research, Department of Anesthesiology, Washington University School of Medicine in St. Louis
Mohammad A. Helwani, Division of Clinical and Translational Research, Department of Anesthesiology, Washington University School of Medicine in St. Louis
Jamie Brown, Division of Clinical and Translational Research, Department of Anesthesiology, Washington University School of Medicine in St. Louis
Frank Brown, Division of Clinical and Translational Research, Department of Anesthesiology, Washington University School of Medicine in St. Louis
Brian F. Gage, Department of Internal Medicine, Washington University School of Medicine in St. Louis
David W. Gibson, Department of Internal Medicine, Washington University School of Medicine in St. Louis
J. Philip Miller, Department of Internal Medicine, Washington University School of Medicine in St. Louis Division of Biostatistics, Washington University School of Medicine in St. Louis.
Eric Novak, Department of Internal Medicine, Washington University School of Medicine in St. Louis
Allan S. Jaffe, Cardiovascular Division, Department of Internal Medicine and Division of Core Clinical Laboratory Services, Department of Laboratory Medicine and Pathology, Rochester, Minnesota, Mayo Clinic and Medical School
Fred S. Apple, Department of Laboratory Medicine & Pathology, Minneapolis, Minnesota, Hennepin County Medical Center and University of Minnesota School of Medicine
Mitchell G. Scott, Department of Pathology & Immunology, Washington University School of Medicine in St. Louis
Peter Nagele, Division of Clinical and Translational Research, Department of Anesthesiology, Washington University School of Medicine in St. Louis
References
- 1.Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009;119:2936–2944. doi: 10.1161/CIRCULATIONAHA.108.828228. [DOI] [PubMed] [Google Scholar]
- 2.Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, Leslie K, Rao-Melacini P, Chrolavicius S, Yang H, Macdonald C, Avezum A, Lanthier L, Hu W, Yusuf S Investigators P. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Annals of internal medicine. 2011;154:523–528. doi: 10.7326/0003-4819-154-8-201104190-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 4.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 5.Nagele P, Liggett SB. Genetic variation, beta-blockers, and perioperative myocardial infarction. Anesthesiology. 2011;115:1316–1327. doi: 10.1097/ALN.0b013e3182315eb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, Sugarbaker DJ, Donaldson MC, Poss R, Ho KK, Ludwig LE, Pedan A, Goldman L. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100:1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 7.Cullen DJ, Apolone G, Greenfield S, Guadagnoli E, Cleary P. ASA Physical Status and age predict morbidity after three surgical procedures. Ann Surg. 1994;220:3–9. doi: 10.1097/00000658-199407000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clinical chemistry. 2012;58:1574–1581. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 9.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA : the journal of the American Medical Association. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA : the journal of the American Medical Association. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E Prevention of Events with Angiotensin Converting Enzyme Inhibition Trial Investigators. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neeland IJ, Drazner MH, Berry JD, Ayers CR, deFilippi C, Seliger SL, Nambi V, McGuire DK, Omland T, de Lemos JA. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. 2013;61:187–195. doi: 10.1016/j.jacc.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagele P, Brown F, Gage BF, Gibson DW, Miller JP, Jaffe AS, Apple FS, Scott MG. High-sensitivity cardiac troponin T in prediction and diagnosis of myocardial infarction and long-term mortality after noncardiac surgery. American heart journal. 2013;166:325 e1–332 e1. doi: 10.1016/j.ahj.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahla E, Baumann A, Rehak P, Watzinger N, Vicenzi MN, Maier R, Tiesenhausen K, Metzler H, Toller W. N-terminal pro-brain natriuretic peptide identifies patients at high risk for adverse cardiac outcome after vascular surgery. Anesthesiology. 2007;106:1088–1095. doi: 10.1097/01.anes.0000267591.34626.b0. [DOI] [PubMed] [Google Scholar]
- 16.Ryding AD, Kumar S, Worthington AM, Burgess D. Prognostic value of brain natriuretic peptide in noncardiac surgery: a meta-analysis. Anesthesiology. 2009;111:311–319. doi: 10.1097/ALN.0b013e3181aaeb11. [DOI] [PubMed] [Google Scholar]
- 17.Cuthbertson BH, Croal BL, Rae D, Harrild K, Gibson PH, Prescott GJ, Kengne AP, Hillis GS. N-terminal pro-B-type natriuretic peptide concentrations and long-term outcome after cardiac surgery: a prospective cohort study. British journal of anaesthesia. 2013;110:214–221. doi: 10.1093/bja/aes379. [DOI] [PubMed] [Google Scholar]
- 18.Farzi S, Stojakovic T, Marko T, Sankin C, Rehak P, Gumpert R, Baumann A, Hofler B, Metzler H, Mahla E. Role of N-terminal pro B-type natriuretic peptide in identifying patients at high risk for adverse outcome after emergent non-cardiac surgery. British journal of anaesthesia. 2013;110:554–560. doi: 10.1093/bja/aes454. [DOI] [PubMed] [Google Scholar]
- 19.Weber M, Luchner A, Seeberger M, Mueller C, Liebetrau C, Schlitt A, Apostolovic S, Jankovic R, Bankovic D, Jovic M, Mitrovic V, Nef H, Mollmann H, Hamm CW. Incremental value of high-sensitive troponin T in addition to the revised cardiac index for peri-operative risk stratification in non-cardiac surgery. Eur Heart J. 2013;34:853–862. doi: 10.1093/eurheartj/ehs445. [DOI] [PubMed] [Google Scholar]
- 20.Rodseth RN, Biccard BM, Le Manach Y, Sessler DI, Lurati Buse GA, Thabane L, Schutt RC, Bolliger D, Cagini L, Cardinale D, Chong CP, Chu R, Cnotliwy M, Di Somma S, Fahrner R, Lim WK, Mahla E, Manikandan R, Puma F, Pyun WB, Radovic M, Rajagopalan S, Suttie S, Vanniyasingam T, van Gaal WJ, Waliszek M, Devereaux PJ. The prognostic value of pre-operative and post-operative B-type natriuretic peptides in patients undergoing noncardiac surgery: B-type natriuretic peptide and N-terminal fragment of pro-B-type natriuretic peptide: a systematic review and individual patient data meta-analysis. J Am Coll Cardiol. 2014;63:170–180. doi: 10.1016/j.jacc.2013.08.1630. [DOI] [PubMed] [Google Scholar]
- 21.Potgieter D, Simmers D, Ryan L, Biccard BM, Lurati-Buse GA, Cardinale DM, Chong CP, Cnotliwy M, Farzi SI, Jankovic RJ, Lim WK, Mahla E, Manikandan R, Oscarsson A, Phy MP, Rajagopalan S, Van Gaal WJ, Waliszek M, Rodseth RN. N-terminal pro-B-type Natriuretic Peptides' Prognostic Utility Is Overestimated in Meta-analyses Using Study-specific Optimal Diagnostic Thresholds. Anesthesiology. 2015;123:264–271. doi: 10.1097/ALN.0000000000000728. [DOI] [PubMed] [Google Scholar]
- 22.Nagele P, Brown F, Francis A, Scott MG, Gage BF, Miller JP, Team VS. Influence of nitrous oxide anesthesia, B-vitamins, and MTHFR gene polymorphisms on perioperative cardiac events: the vitamins in nitrous oxide (VINO) randomized trial. Anesthesiology. 2013;119:19–28. doi: 10.1097/ALN.0b013e31829761e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clinical chemistry. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, White HD, Joint Task Force for the Redefinition of Myocardial Infarction. Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Annals of internal medicine. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 27.Kerr KF, Wang Z, Janes H, McClelland RL, Psaty BM, Pepe MS. Net reclassification indices for evaluating risk prediction instruments: a critical review. Epidemiology. 2014;25:114–121. doi: 10.1097/EDE.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts E, Ludman AJ, Dworzynski K, Al-Mohammad A, Cowie MR, McMurray JJ, Mant J Nice Guideline Development Group for Acute Heart Failure. The diagnostic accuracy of the natriuretic peptides in heart failure: systematic review and diagnostic meta-analysis in the acute care setting. BMJ. 2015;350:h910. doi: 10.1136/bmj.h910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodseth RN, Lurati Buse GA, Bolliger D, Burkhart CS, Cuthbertson BH, Gibson SC, Mahla E, Leibowitz DW, Biccard BM. The predictive ability of pre-operative B-type natriuretic peptide in vascular patients for major adverse cardiac events: an individual patient data meta-analysis. J Am Coll Cardiol. 2011;58:522–529. doi: 10.1016/j.jacc.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Payne CJ, Gibson SC, Bryce G, Jardine AG, Berry C, Kingsmore DB. B-type natriuretic peptide predicts long-term survival after major non-cardiac surgery. British journal of anaesthesia. 2011;107:144–149. doi: 10.1093/bja/aer119. [DOI] [PubMed] [Google Scholar]
- 31.Karthikeyan G, Moncur RA, Levine O, Heels-Ansdell D, Chan MT, Alonso-Coello P, Yusuf S, Sessler D, Villar JC, Berwanger O, McQueen M, Mathew A, Hill S, Gibson S, Berry C, Yeh HM, Devereaux PJ. Is a pre-operative brain natriuretic peptide or N-terminal pro-B-type natriuretic peptide measurement an independent predictor of adverse cardiovascular outcomes within 30 days of noncardiac surgery? A systematic review and meta-analysis of observational studies. J Am Coll Cardiol. 2009;54:1599–1606. doi: 10.1016/j.jacc.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Fellahi JL, Hanouz JL, Le Manach Y, Gue X, Monier E, Guillou L, Riou B. Simultaneous measurement of cardiac troponin I, B-type natriuretic peptide, and C-reactive protein for the prediction of long-term cardiac outcome after cardiac surgery. Anesthesiology. 2009;111:250–257. doi: 10.1097/ALN.0b013e3181a1f720. [DOI] [PubMed] [Google Scholar]
- 33.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E, Investigators P. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1249. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Nordenskjold AM, Ahlstrom H, Eggers KM, Frobert O, Jaffe AS, Venge P, Lindahl B. Short- and long-term individual variation in cardiac troponin in patients with stable coronary artery disease. Clinical chemistry. 2013;59:401–409. doi: 10.1373/clinchem.2012.191700. [DOI] [PubMed] [Google Scholar]
- 35.Biccard BM, Devereaux PJ, Rodseth RN. Cardiac biomarkers in the prediction of risk in the non-cardiac surgery setting. Anaesthesia. 2014;69:484–493. doi: 10.1111/anae.12635. [DOI] [PubMed] [Google Scholar]
- 36.Davis C, Tait G, Carroll J, Wijeysundera DN, Beattie WS. The Revised Cardiac Risk Index in the new millennium: a single-centre prospective cohort re-evaluation of the original variables in 9,519 consecutive elective surgical patients. Can J Anaesth. 2013;60:855–863. doi: 10.1007/s12630-013-9988-5. [DOI] [PubMed] [Google Scholar]
- 37.Ford MK, Beattie WS, Wijeysundera DN. Systematic review: prediction of perioperative cardiac complications and mortality by the revised cardiac risk index. Annals of internal medicine. 2010;152:26–35. doi: 10.7326/0003-4819-152-1-201001050-00007. [DOI] [PubMed] [Google Scholar]
- 38.Nagele P. The Case for a Revised Definition of Myocardial Infarction—Resolving the Ambiguity of Type 2 Myocardial Infarction. JAMA Cardiology. 2016;1:247. doi: 10.1001/jamacardio.2016.0511. [DOI] [PubMed] [Google Scholar]
- 39.Dallmeier D, Denkinger M, Peter R, Rapp K, Jaffe AS, Koenig W, Rothenbacher D, Acti FESG. Sex-specific associations of established and emerging cardiac biomarkers with all-cause mortality in older adults: the ActiFE study. Clinical chemistry. 2015;61:389–399. doi: 10.1373/clinchem.2014.230839. [DOI] [PubMed] [Google Scholar]
- 40.Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, Guyatt G, Cruz P, Graham M, Wang CY, Berwanger O, Pearse RM, Biccard BM, Abraham V, Malaga G, Hillis GS, Rodseth RN, Cook D, Polanczyk CA, Szczeklik W, Sessler DI, Sheth T, Ackland GL, Leuwer M, Garg AX, Lemanach Y, Pettit S, Heels-Ansdell D, Luratibuse G, Walsh M, Sapsford R, Schunemann HJ, Kurz A, Thomas S, Mrkobrada M, Thabane L, Gerstein H, Paniagua P, Nagele P, Raina P, Yusuf S, Devereaux PJ, Devereaux PJ, Sessler DI, Walsh M, Guyatt G, McQueen MJ, Bhandari M, Cook D, Bosch J, Buckley N, Yusuf S, Chow CK, Hillis GS, Halliwell R, Li S, Lee VW, Mooney J, Polanczyk CA, Furtado MV, Berwanger O, Suzumura E, Santucci E, Leite K, Santo JA, Jardim CA, Cavalcanti AB, Guimaraes HP, Jacka MJ, Graham M, McAlister F, McMurtry S, Townsend D, Pannu N, Bagshaw S, Bessissow A, Bhandari M, Duceppe E, Eikelboom J, Ganame J, Hankinson J, Hill S, Jolly S, Lamy A, Ling E, Magloire P, Pare G, Reddy D, Szalay D, Tittley J, Weitz J, Whitlock R, Darvish-Kazim S, Debeer J, Kavsak P, Kearon C, Mizera R, O'Donnell M, McQueen M, Pinthus J, Ribas S, Simunovic M, Tandon V, Vanhelder T, Winemaker M, Gerstein H, McDonald S, O'Bryne P, Patel A, Paul J, Punthakee Z, Raymer K, Salehian O, Spencer F, Walter S, Worster A, Adili A, Clase C, Cook D, Crowther M, Douketis J, Gangji A, Jackson P, Lim W, Lovrics P, Mazzadi S, Orovan W, Rudkowski J, Soth M, Tiboni M, Acedillo R, Garg A, Hildebrand A, Lam N, Macneil D, Mrkobrada M, Roshanov PS, Srinathan SK, Ramsey C, John PS, Thorlacius L, Siddiqui FS, Grocott HP, McKay A, Lee TW, Amadeo R, Funk D, McDonald H, Zacharias J, Villar JC, Cortes OL, Chaparro MS, Vasquez S, Castaneda A, Ferreira S, Coriat P, Monneret D, Goarin JP, Esteve CI, Royer C, Daas G, Chan MT, Choi GY, Gin T, Lit LC, Xavier D, Sigamani A, Faruqui A, Dhanpal R, Almeida S, Cherian J, Furruqh S, Abraham V, Afzal L, George P, Mala S, Schunemann H, Muti P, Vizza E, Wang CY, Ong GS, Mansor M, Tan AS, Shariffuddin II, Vasanthan V, Hashim NH, Undok AW, Ki U, Lai HY, Ahmad WA, Razack AH, Malaga G, Valderrama-Victoria V, Loza-Herrera JD, De Los Angeles Lazo M, Rotta-Rotta A, Szczeklik W, Sokolowska B, Musial J, Gorka J, Iwaszczuk P, Kozka M, Chwala M, Raczek M, Mrowiecki T, Kaczmarek B, Biccard B, Cassimjee H, Gopalan D, Kisten T, Mugabi A, Naidoo P, Naidoo R, Rodseth R, Skinner D, Torborg A, Paniagua P, Urrutia G, Maestre ML, Santalo M, Gonzalez R, Font A, Martinez C, Pelaez X, De Antonio M, Villamor JM, Garcia JA, Ferre MJ, Popova E, Alonso-Coello P, Garutti I, Cruz P, Fernandez C, Palencia M, Diaz S, Del Castillo T, Varela A, de Miguel A, Munoz M, Pineiro P, Cusati G, Del Barrio M, Membrillo MJ, Orozco D, Reyes F, Sapsford RJ, Barth J, Scott J, Hall A, Howell S, Lobley M, Woods J, Howard S, Fletcher J, Dewhirst N, Williams C, Rushton A, Welters I, Leuwer M, Pearse R, Ackland G, Khan A, Niebrzegowska E, Benton S, Wragg A, Archbold A, Smith A, McAlees E, Ramballi C, Macdonald N, Januszewska M, Stephens R, Reyes A, Paredes LG, Sultan P, Cain D, Whittle J, Del Arroyo AG, Sessler DI, Kurz A, Sun Z, Finnegan PS, Egan C, Honar H, Shahinyan A, Panjasawatwong K, Fu AY, Wang S, Reineks E, Nagele P, Blood J, Kalin M, Gibson D, Wildes T. Vascular events In noncardiac Surgery patIents cOhort evaluatioN Writing Group oboTVeInSpceI, Appendix 1. The Vascular events In noncardiac Surgery patIents cOhort evaluatio NSIWG, Appendix 2. The Vascular events In noncardiac Surgery patIents cOhort evaluatio NOC, Vascular events In noncardiac Surgery patIents cOhort evaluatio NVSI. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564–578. doi: 10.1097/ALN.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 41.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I. Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, Srinathan S, Biccard BM, Chow CK, Abraham V, Tiboni M, Pettit S, Szczeklik W, Lurati Buse G, Botto F, Guyatt G, Heels-Ansdell D, Sessler DI, Thorlund K, Garg AX, Mrkobrada M, Thomas S, Rodseth RN, Pearse RM, Thabane L, McQueen MJ, VanHelder T, Bhandari M, Bosch J, Kurz A, Polanczyk C, Malaga G, Nagele P, Le Manach Y, Leuwer M, Yusuf S. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA : the journal of the American Medical Association. 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 42.van Waes JA, Nathoe HM, de Graaff JC, Kemperman H, de Borst GJ, Peelen LM, van Klei WA Cardiac Health After Surgery Investigators. Myocardial injury after noncardiac surgery and its association with short-term mortality. Circulation. 2013;127:2264–2271. doi: 10.1161/CIRCULATIONAHA.113.002128. [DOI] [PubMed] [Google Scholar]
- 43.Levy M, Heels-Ansdell D, Hiralal R, Bhandari M, Guyatt G, Yusuf S, Cook D, Villar JC, McQueen M, McFalls E, Filipovic M, Schunemann H, Sear J, Foex P, Lim W, Landesberg G, Godet G, Poldermans D, Bursi F, Kertai MD, Bhatnagar N, Devereaux PJ. Prognostic value of troponin and creatine kinase muscle and brain isoenzyme measurement after noncardiac surgery: a systematic review and meta-analysis. Anesthesiology. 2011;114:796–806. doi: 10.1097/ALN.0b013e31820ad503. [DOI] [PubMed] [Google Scholar]
- 44.Landesberg G, Shatz V, Akopnik I, Wolf YG, Mayer M, Berlatzky Y, Weissman C, Mosseri M. Association of cardiac troponin, CK-MB, and postoperative myocardial ischemia with long-term survival after major vascular surgery. J Am Coll Cardiol. 2003;42:1547–1554. doi: 10.1016/j.jacc.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 45.Beattie WS, Karkouti K, Tait G, Steel A, Yip P, McCluskey S, Farkouh M, Wijeysundera DN. Use of clinically based troponin underestimates the cardiac injury in non-cardiac surgery: a single-centre cohort study in 51,701 consecutive patients. Can J Anaesth. 2012;59:1013–1022. doi: 10.1007/s12630-012-9782-9. [DOI] [PubMed] [Google Scholar]
- 46.London MJ. From Durban to Boston, a "modest proposal" to improve perioperative cardiovascular risk stratification. Anesthesia and analgesia. 2015;120:515–518. doi: 10.1213/ANE.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 47.Biccard B. Proposed research plan for the derivation of a new Cardiac Risk Index. Anesthesia and analgesia. 2015;120:543–553. doi: 10.1213/ANE.0000000000000598. [DOI] [PubMed] [Google Scholar]