Abstract
Dopamine D2 receptors (D2Rs) consistently emerge as a critical substrate for the etiology of some major psychiatric disorders. Indeed, a central theory of substance use disorders (SUDs) postulates that a reduction in D2R levels in the striatum is a determining factor that confers vulnerability to abuse substances. A large number of clinical and preclinical studies strongly support this link between SUDs and D2Rs; however, identifying the mechanism by which low D2Rs facilitate SUDs has been hindered by the complexity of circuit connectivity, the heterogeneity of D2R expression and the multifaceted constellation of phenotypes observed in SUD patient. Animal models are well‐suited for understanding the mechanisms because they allow access to the circuitry and the genetic tools that enable a dissection of the D2R heterogeneity. This review discusses recent findings on the functional role of D2Rs and highlights the distinctive contributions of D2Rs expressed on specific neuronal subpopulations to the behavioral responses to stimulant drugs. A circuit‐wide restructuring of local and long‐range inhibitory connectivity within the basal ganglia is observed in response to manipulation of striatal D2R levels and is accompanied by multiple alterations in dopamine‐dependent behaviors. Collectively, these new findings provide compelling evidence for a critical role of striatal D2Rs in shaping basal ganglia connectivity; even among neurons that do not express D2Rs. These findings from animal models have deep clinical implications for SUD patients with low levels D2R availability where a similar restructuring of basal ganglia circuitry is expected to take place.
Keywords: Addiction, basal ganglia, cocaine, D2 receptors, dopamine, G‐protein coupled receptors, medium spiny neurons, striatum, substance use disorders, synaptic transmission
Individuals who abuse stimulant drugs, such as cocaine and amphetamine, and those that abuse alcohol display low binding potential for a D2‐like agonist in the striatal nucleus of the caudate, putamen and the nucleus accumbens (Volkow et al. 1993, 1996, 2001). Obese patients also have low availability of striatal D2Rs when tested using positron emission tomography (PET; Volkow et al. 2008). Conversely, high levels of D2Rs in the forebrain have been reported in individuals suffering from schizophrenia (Wong et al. 1986). Psychomotor dysfunction and alterations in inhibitory control and motivation are three common symptoms of these disorders. Motivation and motor function are regulated by dopamine through activation of D2Rs and as such, these receptors have surfaced as a likely mediator of these symptoms. Here, we will present evidence that D2Rs mediate the cellular and behavioral response to stimulant drugs and discuss how varying levels of D2Rs in the basal ganglia affect connectivity and circuit function to create vulnerability to stimulant abuse and dependence.
Link between stimulant abuse and D2Rs
An influential theory of addiction is that low levels of striatal dopamine D2Rs predispose individuals to develop SUDs, specifically towards stimulant drugs such as cocaine and amphetamine. This hypothesis originated from PET imaging studies, which showed that cocaine users had lower levels of D2R availability in the striatum compared to healthy controls and also lower glucose metabolism in the orbitofrontal and cingulate cortices, indicative of decreased activity in these cortical areas (Volkow et al. 1993, 2001). Furthermore, the ability of a mixed D1/D2 receptor agonist to suppress plasma human growth hormone and prolactin levels is severely blunted in cocaine abusers relative to non‐abusing controls, providing additional neuroendocrine evidence for dysregulated dopamine and dopamine receptor functioning in cocaine abusers (Hollander et al. 1990). Low D2R availability is found in the striatum of cocaine abusers during early withdrawal and also after detoxification and protracted withdrawal, suggesting that this is not a temporary response to cocaine abstinence but rather a long‐lasting alteration relative to healthy controls (Volkow et al. 1990, 1993).
Multiple pre‐clinical studies have been conducted to address whether low D2R availability is a cause or a consequence of stimulant abuse. The results and conclusions point to evidence that both mechanisms are in play. For example, studies conducted in non‐human primates and rodents showed that chronic self‐administration of stimulant drugs leads to decreased striatal D2R availability, suggesting a consequential role (Besson et al. 2013; Conrad et al. 2010; Moore et al. 1998; Nader et al. 2002). However, other studies in non‐human primates found that dominant monkeys display higher levels of striatal D2Rs than subordinate monkeys and that they are more resistant to compulsive cocaine seeking and taking (Morgan et al. 2002). Evidence for a causative role of D2Rs also comes from rodent work showing that outbred rats with low levels of D2Rs or mice with a genetic deletion of D2Rs demonstrate increased cocaine self‐administration, while virally induced upregulation of D2Rs can lead to decreases in cocaine self‐administration in rats (Caine et al. 2002; Dalley et al. 2007; Edwards et al. 2007; Thanos et al. 2008). Further, in an elegant study in non‐human primates, Nader and colleagues assessed D2R availability before and during cocaine self‐administration. They found that initial D2R availability is negatively correlated with the rate of cocaine self‐administration, and also that chronic cocaine self‐administration further reduced the levels of D2Rs, which remained low even during protracted abstinence (Nader et al. 2006). Thus, it appears that striatal D2R availability is both a cause and a consequence of stimulant abuse behaviors.
Impulsivity is a trait linked to poor inhibitory control and is thought to be a root cause of the vulnerability to developing addiction. High impulsivity, assessed in rodents using the five‐choice serial reaction time task (5‐CSRT), predicted high rates of cocaine self‐administration (Dalley et al. 2011; Jentsch & Taylor 1999; Verdejo‐Garcia et al. 2008). Further, high impulsivity was associated with lower D2R availability (Besson et al. 2010, 2013; Caprioli et al. 2015; Dalley et al. 2007). Abnormalities in fronto‐striatal circuitry were associated with poor self‐control in human stimulant abusers as well as in their non‐stimulant abusing biological siblings, supporting the idea that these traits and neuro‐circuitry alterations predate the drug taking (Ersche et al. 2012).
Taken together, a unified hypothesis is emerging from the literature in which low function of striatal D2Rs causes deficits in inhibitory control and behavioral disinhibition, which contribute to the development of impulsivity and compulsive stimulant use and dependence. The current challenge is determining how low levels of D2R function leads to deficits in behavioral inhibition, to impulsivity, and to stimulant abuse. New findings discussed in this review suggest that decreased function of striatal D2Rs causes a reorganization of striatal connectivity that involves a strengthening of the lateral inhibition between medium spiny neurons (MSNs), which in turn, affects the behavioral response to stimulant drugs. Thus, behavioral or pharmaceutical interventions that enhance D2R availability or activate D2Rs should continue to be considered as novel treatments for stimulant abuse.
Heterogeneity of D2Rs throughout the basal ganglia
Expression, isoforms and subcellular localization
D2Rs are expressed by many different cell‐types throughout the basal ganglia. The highest levels are found in the dorsal striatum, nucleus accumbens and olfactory cortex and tubercles, but they are also expressed in the cortex, septum, amygdala, hippocampus, hypothalamus, ventral tegmental area/substantia nigra, pituitary and retina (Beaulieu & Gainetdinov 2011). Within the striatum, D2Rs are expressed on at least three different neuronal types: on indirect‐pathway medium spiny neurons (iMSNs) which are a subpopulation of striatal GABA projections neurons highly abundant (∼48 % of neurons), on cholinergic striatal interneurons that make less than 2% of striatal neurons, and on the afferents to the striatum from midbrain dopamine neurons (Fig. 1; Bello et al. 2011; Delle Donne et al. 1996, 1997; Li et al. 2012; Sesack et al. 1994) There are also reports of D2Rs in a subset of GABA interneurons and on glutamate afferents to the striatum from cortical neurons (Bamford et al. 2004; Centonze et al. 2003; Higley & Sabatini 2010; Maurice et al. 2004; Surmeier et al. 2011)
Figure 1.
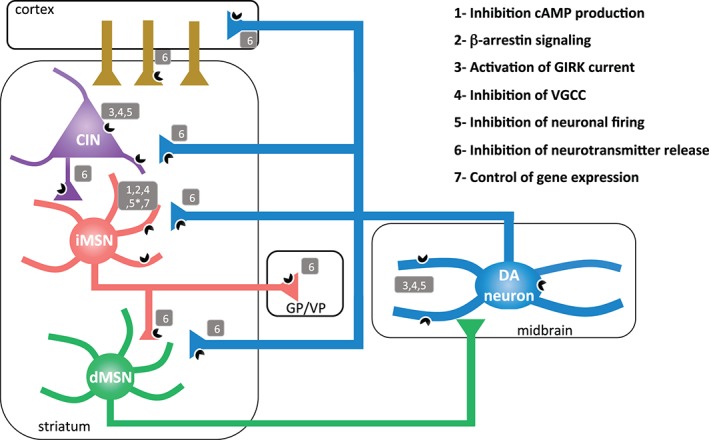
Dopamine D2Rs have heterogeneous cellular localization and functions within the basal ganglia. Schematic diagram depicting D2R localization to indirect‐pathway medium spiny neurons (iMSNs, coral), dopamine terminals emanating from the midbrain dopamine neurons (DA, blue), cholinergic interneurons (CIN, purple) and glutamatergic cortical inputs (brown). Grey boxes containing numbers 1‐7 correspond to the different cellular effects that have been attributed, thus far, to D2R activation and signaling on that specific cell type. * indicates disparate findings in the literature. dMSN, direct pathway medium spiny neuron; GP, globus pallidus; VP, ventral pallidum.
There are two isoforms of D2Rs, D2L and D2S for ‘long’ and ‘short’, which are generated by alternative splicing. Their selective expression patterns provide an additional layer of complexity to the already heterogeneous pattern of D2R expression. There is an appreciable consensus that the D2S isoform possesses properties more typical of the D2 autoreceptor, the receptors expressed in neurons that release dopamine (Gantz et al. 2015b; Khan et al. 1998; Lindgren et al. 2003; Usiello et al. 2000). The D2L isoform, on the other hand, is predominantly found on striatal projection neurons (Centonze et al. 2002; Lindgren et al. 2003; Usiello et al. 2000); however, it should be noted that this isoform is also expressed in dopamine neurons (Jomphe et al. 2006; Khan et al. 1998; Neve et al. 2013). Functionally, different roles have been linked to the D2L and D2S isoforms. For example, the cataleptic effects of the D2‐like antagonist haloperidol are absent in mice lacking the D2L isoform (Usiello et al. 2000). The extra 29‐amino acid domain in the third intracellular loop of the D2L, which is absent in D2S, is thought to serve as an interaction site for G‐proteins or confer unique signaling properties (Picetti et al. 1997). Other studies suggest this domain grants some resistance to receptor desensitization and internalization (Gantz et al. 2015b; Ito et al. 1999; Itokawa et al. 1996; Liu et al. 1992; Thibault et al. 2011).
Global deletion of D2Rs as well as systemic or striatal‐specific treatment with D2‐like agonists and antagonists have revealed an important role for D2Rs in controlling the behavioral response to stimulants (Baker et al. 1996; Britton et al. 1991; Caine et al. 2002; Chausmer & Katz 2001; Chausmer et al. 2002; Spealman et al. 1999). However, the heterogeneity of D2R expression, be it via localization to different neuronal types or the expression of different isoforms, should be considered when interpreting these data as it might help reconcile the seemingly disparate findings of genetic and pharmacological manipulations (Fig. 2).
Figure 2.
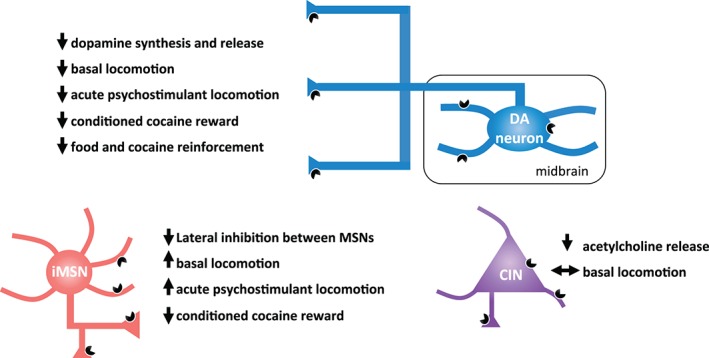
Behavioral and cellular consequences of activation of D2Rs localized to different cell‐types. Schematic diagram depicting the different behavioral and cellular functions of D2R activation and signaling at different location and cell‐types within the basal ganglia. D2Rs on dopamine terminals are critical for exerting inhibitory control over dopamine synthesis and release as well as the psychomotor, rewarding and reinforcing properties of cocaine. D2Rs on iMSNs are critical for constraining GABAergic transmission to promote basal locomotion and acute psychomotor activation in response to cocaine. D2Rs on cholinergic interneurons regulate acetylcholine release without affecting basal locomotion (double‐ended arrow).
Molecular effectors and cellular response to D2Rs
D2Rs belong to a family of Gαi/o‐coupled receptors that also includes the D3 and D4 receptors. Multiple effectors and signaling pathways have been identified as mediators of the cellular response to D2R activation. It is important to be aware that the expression of these effectors varies among the different neuronal types expressing D2Rs, and as such, the cellular and behavioral responses to D2R activation are also heterogeneous throughout the brain.
The canonical molecular and cellular effects downstream of D2R activation have been attributed to the G protein‐dependent inhibition of adenylyl cyclase and subsequent reduction of intracellular levels of cyclic adenosine monophosphate (cAMP). D2R‐mediated regulation of cAMP production suppresses the activity of protein kinase A (PKA) and of the dopamine‐ and cAMP‐regulated phosphoprotein‐32 (Fig. 1, #1; DARPP‐32, Beaulieu & Gainetdinov 2011; Kebabian & Calne 1979; Kebabian & Greengard 1971). D2R agonists also activate extracellular signal regulated kinases (ERK) primarily through a G protein‐dependent mechanism (Lan et al. 2009; Peterson et al. 2015b; Fig. 1, #1). Through membrane delimited protein interactions, activation of D2Rs can also inhibit voltage‐gated calcium channels (VGCC) to decrease intracellular calcium concentration (Fig. 1, #4), and activate G‐protein gated inward rectifying potassium (GIRK) channels to hyperpolarize the membrane potential of neurons (Fig. 1, #3; Kuzhikandathil et al. 1998; Lavine et al. 2002; Missale et al. 1998; Nishi et al. 1997). Dissociation of Gβγ subunits activates GIRK channels with a time constant on the order of hundreds of milliseconds (Logothetis et al. 1987; Wickman et al. 1994). As a result of this signaling, neurons with high expression of GIRK channels, such as in midbrain dopamine neurons, show a marked hyperpolarization and fast inhibition of action potential firing upon D2R activation (Anzalone et al. 2012; Beckstead et al. 2004; Bello et al. 2011; Paladini et al. 2003). In contrast, D2R activation in striatal MSNs and cholinergic interneurons (CINs) has been shown to result in an inhibition of L‐ and N‐type voltage‐gated calcium channel activity (Hernandez‐Lopez et al. 2000; Yan et al. 1997), as well as in a reduction of the magnitude of calcium transients in the dendrites of striatal MSNs, measured using 2‐photon laser microscopy (Day et al. 2008; Mizuno et al. 2007). This D2R mediated inhibition of calcium influx is thought to mediate the potent D2R‐mediated inhibition of neurotransmitter release from dopamine terminals and GABAergic terminals from iMSNs (Adrover et al. 2014; Bello et al. 2011; Dobbs et al. 2016; Jones et al. 1999; Fig. 1, #6).
A G‐protein independent mechanism has also been reported to contribute to the cellular and behavioral effects of D2R activation. Non‐selective dopamine receptor agonists and psychostimulants regulate Akt and glycogen synthase kinase‐3 (GSK3) signaling pathways via the activation of beta‐arrestin signaling molecules (for a full review see (Beaulieu et al. 2011; Beaulieu et al. 2009). The beta‐arrestin pathway has traditionally been associated with receptor desensitization and internalization (Shenoy & Lefkowitz 2011). However, it is now known that beta‐arrestin dependent activation of MAP kinases can regulate the trafficking, insertion and conductance of ion channels important for intrinsic excitability and plasticity (Thomas & Huganir 2004). Biased D2R ligands (Allen et al. 2011; Free et al. 2014), mutant mouse lines (Beaulieu et al. 2005), and evolutionary trace analysis (Peterson et al. 2015a) have also revealed significant contributions of the beta‐arrestin‐dependent signaling pathways in mediating the functional consequences of D2R activation (Fig. 1, #2). For example, a recently generated biased D2R ligand devoid of Gi/o protein signaling but with partial agonism for the beta‐arrestin pathway mitigated schizophrenia‐like behaviors in a rodent model, such as hyperlocomotion, impaired pre‐pulse inhibition and novel object recognition, and enhanced conditioned avoidance, while inducing a much lower level of catalepsy than haloperidol (Park et al. 2016).
Changes in gene expression are reported upon chronic manipulations of D2R activation levels. Low D2R activation causes an enhancement of prepro‐enkephalin mRNA and a decrease of Substance P mRNA in the striatum (Fig. 1, #7; Baik et al. 1995; Gerfen et al. 1990; Romano et al. 1987). Further, changes in the excitability of striatal neurons have been reported following chronic overexpression and down‐regulation of D2Rs (Cazorla et al. 2012; Lemos et al. 2016). These excitability changes, as well as the decreases in dendritic branching seen after D2R overexpression (Cazorla et al. 2012), likely involve changes in gene expression.
The large variety of signaling pathways and effectors engaged by D2Rs provide ample opportunity for cell‐type specificity in the cellular response to dopamine, which goes beyond the receptors subtypes. At the same time, the diversity sometimes complicates the interpretation of pharmacological experiments and precludes us from making generalizations and extrapolations based on the D2R effect in one neuronal type to another.
Cell‐type specific cellular and synaptic consequences of D2R activation
The next section focuses on the cellular functions of D2Rs expressed on midbrain dopamine neurons, striatal GABAergic MSNs, and to lesser extent, striatal CINs. Dissecting out the role of the striatal D2Rs expressed in the different cells‐types within the striatum is critical for ultimately revealing the mechanism by which low D2R levels can facilitate SUDs. Indeed, it is possible that only D2Rs expressed within one cell type are responsible for triggering the vulnerability. Alternatively, it is possible that low levels of D2R expression at each different cell‐type makes small contributions that summate or even synergize to confer vulnerability for SUDs.
D2 autoreceptors mediate feedback inhibition over dopamine levels at synapses
D2Rs expressed on dopamine neurons are often referred to as D2 autoreceptors because they are activated by dopamine released from the same neuron, or neighboring neurons, as discussed below. In midbrain dopamine neurons of the ventral tegmental area and the substantia nigra pars compacta, D2 autoreceptors suppress both the firing of action potentials and dopamine release from these neurons. Decades of research by the group of JT Williams and others have shown that D2 autoreceptor activation inhibits dopamine neuron excitability by increasing potassium conductances and hyperpolarizing the membrane potential (Fig. 1, #3,5; Beckstead et al. 2004; Lacey et al. 1987; Luscher & Slesinger 2010; Mercuri et al. 1997). Further, local electrical stimulation delivered with a pattern that mimics the burst firing of dopamine neurons (5 pulse, 40 Hz, 0.5 milliseconds duration) can evoke slow inhibitory post‐synaptic currents (IPSCs) that are mediated by activation of D2 autoreceptors in midbrain dopamine neurons (Fig. 1,#3; Beckstead et al. 2004, 2007; Ford et al. 2006). This local stimulation protocol in the midbrain was shown to generate dopamine transients that directly precede the slow D2R‐mediated IPSCs in dopamine neurons (Ford et al. 2009). Thus, synchronized burst firing of action potentials triggers dopamine release, presumably from somatodendritic compartments of neighboring neurons, that in turns activates D2 autoreceptors to evoke a slow hyperpolarization that prevents further firing for a few seconds. Further confirmation came from experiments using targeted deletion of D2 autoreceptors to dopamine neurons, which resulted in a loss of the evoked D2R‐IPSCs and of the D2R‐like agonist mediated suppression of firing of dopamine neurons (Fig. 1, #3,5; Anzalone et al. 2012; Bello et al. 2011). It is indeed thought that D2 autoreceptors mediate critical feedback inhibition of this circuit by limiting dopamine neuron firing and neurotransmitter release (as discussed below).
Bath application of cocaine enhances evoked D2R‐IPSCs by blocking the dopamine transporter and increasing the extracellular dopamine concentration upon release (Beckstead et al. 2004). A recent study by Gantz et al. also revealed the existence of spontaneous D2R‐IPSCs, which were observed in the absence of stimulation and were insensitive to the sodium channel blocker TTX. These miniature D2R‐IPSCs are thought to result from the spontaneous release of single vesicles of dopamine from DA neurons (Gantz et al. 2015a). Interestingly, a single non‐contingent administration of cocaine (20 mg/kg) enhanced both evoked and spontaneous D2R‐IPSCs suggesting that the magnitude of the D2 autoreceptor response is plastic and can be regulated by stimulants (Gantz et al. 2015a).
D2 autoreceptors are also localized to pre‐synaptic terminals in the striatum from dopamine neuron projections. These presynaptic D2 autoreceptors exert a potent and reliable inhibition of dopamine release that can be measured using fast scan cyclic voltammetry (IC50 for quinpirole of ∼30 nm in rodents; (Fig. 1, #6 Adrover et al. 2014; Bello et al. 2011; Ding et al. 2010; Groves & Wilson 1980; Kennedy et al. 1992; Lemos et al. 2016; Phillips et al. 2003). It remains unclear whether this effect is due to suppression of calcium channel activation or activation of GIRKs on the terminals. Presynaptic D2R‐mediated inhibition is engaged by endogenous dopamine during trains of stimulus pulses that mimic the phasic firing pattern of dopamine neurons. Indeed, both sulpiride pre‐treatment and selective deletion of D2Rs from dopamine neurons enhance the peak and area of dopamine transients evoked by trains of electrical stimuli (Fig. 1, #6; Bello et al. 2011; Lemos et al. 2016). Another condition during which D2 autoreceptors are engaged is during exposure to cocaine. By blocking dopamine transporters and enhancing extracellular dopamine levels around the release sites, cocaine engages D2Rs on the presynaptic terminals of dopamine neurons and further inhibits dopamine release (Adrover et al. 2014; Bello et al. 2011; Holroyd et al. 2015). In the absence of D2 autoreceptors, cocaine application leads to an even larger amount of extracellular dopamine (Holroyd et al. 2015). Furthermore, the deletion also results in increased TH activation and suggests increased dopamine synthesis (Anzalone et al. 2012; Bello et al. 2011). Collectively these recent findings generated from cell‐specific Drd2 knockout mice demonstrate a larger extracellular concentration of dopamine at synaptic sites in the absence of D2R‐mediated inhibitory regulation.
D2R regulation of cholinergic interneuron activity
There is strong evidence that D2Rs are localized to CINs within the striatum (Maurice et al. 2004). Less understood is the functional role of D2Rs on CINs and very little known regarding the effect of cocaine on these cells. Microdialysis studies have demonstrated that D2Rs can suppress the release of acetylcholine (Fig. 1, #6; DeBoer & Abercrombie 1996; DeBoer et al. 1996). In an in vitro slice preparation, agonist for D2/3 receptors can reduce the firing rate by inhibiting NaV channels (Fig. 1, #5; Maurice et al. 2004). CINs have both tonic and phasic firing modes. Following thalamocortical stimulation, CINs can shift into a burst‐pause mode, in which the pause following the burst is blocked by D2/3 antagonist (Fig. 1, #5; Ding et al. 2010). Furthermore, selective optogenetic stimulation of dopamine fibers generates a D2R‐dependent pause in CIN firing rate and produces a ‘burst‐pause’ activity mode in CINs located in the NAc shell (Fig. 1, #3,4,5; Chuhma et al. 2014). Finally, deletion of D2Rs from CINs did not produce changes in tonic firing rate or intrinsic membrane properties, but significantly reduced the ‘pause’ component when the cell was stimulated and shifted into a ‘burst‐pause’ mode (Fig. 1, #5; Kharkwal et al. 2016a). Collectively, these data suggest that D2Rs in CINs play a more prominent role in regulating phasic activity of these interneurons.
Acute cocaine application appears to prolong the pause in CINs, presumably by increasing D2R activity, although this has not been unequivocally shown (Ding et al. 2010). Another study showed that in vitro cocaine application increased firing of CINs (Witten et al. 2010). Further studies are needed to clarify the actions of cocaine in CIN activity and the cellular and synaptic mechanisms involved.
In addition to releasing acetylcholine, activity of CINs can evoke dopamine release via activation of nicotinic acetylcholine receptors present on dopamine axons (Cachope et al. 2012; Threlfell et al. 2012). It was recently demonstrated that selective deletion of D2Rs from CINs leads to a 10% reduction in D2R mediated inhibition of dopamine overflow indicating a small contribution of D2Rs on CINs to the overall D2R‐mediated inhibition of dopamine release (Kharkwal et al. 2016a) . Further, activation of D2Rs on CINs are thought to be required for striatal long‐term depression (LTD) of glutamatergic synapses on MSNs by reducing ACh release from CINs and subsequently limiting M1 muscarinic receptor activation on MSNs (Wang et al. 2006). These are two examples by which D2Rs on CINs can indirectly affect the output of striatal neurons and the dopamine modulation. While there has been some progress in understanding the role of D2Rs on CINs, more research is needed to improve our understanding of the role of these D2Rs in shaping striatal connectivity and the cellular and behavioral responses to cocaine.
D2Rs in medium spiny neurons suppress lateral inhibition between striatal neurons
The highest level of D2R expression is found in striatal GABAergic MSNs, which project to the globus pallidus (GP) and ventral pallidum (VP) to form the indirect‐pathway output of the striatum. These D2Rs are found in the somatodendritic compartments as well as in the synaptic terminals from these indirect‐pathway MSNs (iMSNs) in rodents (Delle Donne et al. 1996, 1997). Decades of pharmacological studies in combination with the current knowledge of the circuitry support the idea that activation of D2Rs inhibits the output of iMSNs to foster firing in the GP/VP and mediate locomotion. By extrapolating from what it is known of D2 autoreceptors, the most common sense hypothesis is that D2Rs in iMSNs activate K+ conductances to decrease excitability and inhibit neuronal firing. However, the direct effects of D2R activation in iMSNs have been inconsistent and sometimes hard to detect, in large part because these neurons are intermingled with identically looking MSNs that express D1Rs instead of D2Rs. There are reports that D2R‐like agonists induce membrane hyperpolarization in iMSNs in rat brain slice preparations, suggestive of D2R coupling through GIRK (Fig. 1, #5; Ferguson et al. 2011; Lalchandani et al. 2013; Orefice et al. 2013; Surmeier et al. 2011). However, these effects are not reproduced in mouse preparations where iMSNs can be visually identified, as D2R‐like agonists fail to produce any significant reduction of intrinsic excitability of iMSNs (Fig. 1, #5; Dobbs et al. 2016; Lemos et al. 2016). Furthermore, expression of GIRK channels has yet to be confirmed in iMSNs.
D2Rs in iMSNs have been shown to decrease calcium currents and subsequent SK channel activity during the up‐states of these neurons (Fig. 1, #4; Hernandez‐Lopez et al. 2000; Tritsch & Sabatini 2012). By inhibiting calcium signals, D2Rs in iMSNs can also affect the phosphorylation and function of other channels and receptors important in regulating excitability under specific conditions and also can affect gene expression, which could lead to long‐term changes in excitability that have been reported following D2R activation and D2R overexpression or knockdown (Cazorla et al. 2012; Kourrich & Thomas 2009; Lemos et al. 2016).
Possibly one of the most reliable and fast consequences of D2R activation in iMSNs is the suppression of inhibitory synaptic transmission from these neurons within the striatum. Axons from iMSNs form collateral projections that extend within the striatum and form inhibitory GABA synapses on neighboring MSNs, in addition to their long‐range projections to GP and VP (Smith et al. 1998; Wilson & Groves 1980). The D2R‐like agonist quinpirole inhibits GABA synaptic transmission by about 50% from iMSNs to neighboring MSNs in the dorsal striatum, shown using paired recordings (Tecuapetla et al. 2009), and following synchronized optogenetic stimulation of iMSN axon collaterals in the nucleus accumbens (Dobbs et al. 2016; Fig. 1, #6). Quinpirole also reliably inhibits the amplitude of IPSCs recorded from pairs of MSNs in slices and primary cultures of striatal neurons, while it inhibits only a third of the synaptic connections between pairs of fast‐spiking interneurons and MSNs (Fig. 1, #6; Cazorla et al. 2014; Kohnomi et al. 2012). In all cases, the inhibition was blocked by a D2R antagonist and it was absent in mice lacking D2Rs only in iMSNs (Dobbs et al. 2016). The fast and potent suppression of synaptic transmission suggests that D2Rs are localized to presynaptic terminals where they most likely inhibit calcium channels and reduce neurotransmitter release (Fig. 1, #6; Salgado et al. 2005). Simultaneous stimulation of GABA inputs from multiple iMSNs reduced action potential firing in D1R‐expressing direct‐pathway MSNs (dMSNs), indicating that this lateral inhibition is indeed potent enough to control the output of the dMSNs. By suppressing the lateral inhibition onto dMSNs, D2Rs in iMSNs disinhibit the firing of action potentials in dMSNs, thus gating basal ganglia output (Dobbs et al. 2016). Surprisingly, recent data revealed that D2R‐like agonists are much less efficacious at suppressing transmission from iMSN long‐range projections to VP neurons (Dobbs et al. 2016), suggesting either differential targeting/trafficking or coupling efficacy of D2Rs on local axon collaterals vs. long‐range axonal projections. Taking the old and new findings together, a clearer picture begins to emerge, in which activation of D2Rs in iMSNs leads to a reduction of indirect‐pathway output. However, the mechanisms involved appear to be less related to changes in intrinsic excitability, but rather downstream of action potential firing, and mediated by a potent inhibition of neurotransmitter release at axon collaterals within the striatum. At the circuit level, the most prominent function of these D2Rs is to suppress the lateral inhibition between striatal neurons and as consequence, D2Rs in iMSNs have a unique and critical role in controlling striatal output of both the indirect and direct pathways.
Expression levels of D2R in MSNs have profound impact on basal ganglia circuit function
Individuals with SUDs appear to have a chronic reduction of D2R function. In animal models, alterations in the levels of D2R activation not only affect how the striatum acutely responds to dopamine or stimulants, but also triggers a profound re‐organization of basal ganglia circuitry, which could be the key for understanding the vulnerability to drug abuse and changes in patients with chronic substance use disorder. Significant cellular changes and restructuring of synaptic strength and connectivity in the basal ganglia were reported following either up‐ or down‐regulation of striatal D2Rs, which seems to be the root cause of hyperactivity and bradykinesia, respectively (Fig. 3). Kellendonk and colleagues reported that generalized D2R overexpression in the striatum results in down‐regulation of Kir 2.1/2.3 channels, which are potassium channels that contribute to the passive conductance of MSNs, and result in higher excitability of MSNs (Cazorla et al. 2012). In addition, D2R overexpression reduces dendritic arborization in MSNs and causes an overall reduction in striatal volume. In a subsequent study, investigators used Cre‐dependent expression of a dominant negative Kir2.1/2.3 channel to reduce Kir function in a cell‐specific manner targeted to iMSNs. This manipulation enhanced MSN excitability as well as led to an increase in bridging collaterals from dMSNs to the GPe, (Cazorla et al. 2014), an anatomical and functional connection which is normally relatively weak. The enriched density of bridging collaterals resulted in enhanced GABAergic transmission onto GPe neuron's activity upon direct‐pathway activation, indicating that the bridging collaterals form functional inhibitory synapses. Both non‐specific and iMSN‐specific D2R‐overexpression caused a restructuring of striatal circuit connections as well as behavioral changes defined as hyper‐locomotion and increased water consumption (polydipsia) (Gallo et al. 2015). There is also evidence that transient D2R overexpression in the striatum decreases alcohol consumption (Surmeier & Kitai 1997), however, it this has not been confirmed yet with iMSN‐specific overexpression of D2Rs (Gallo et al. 2015).
Figure 3.
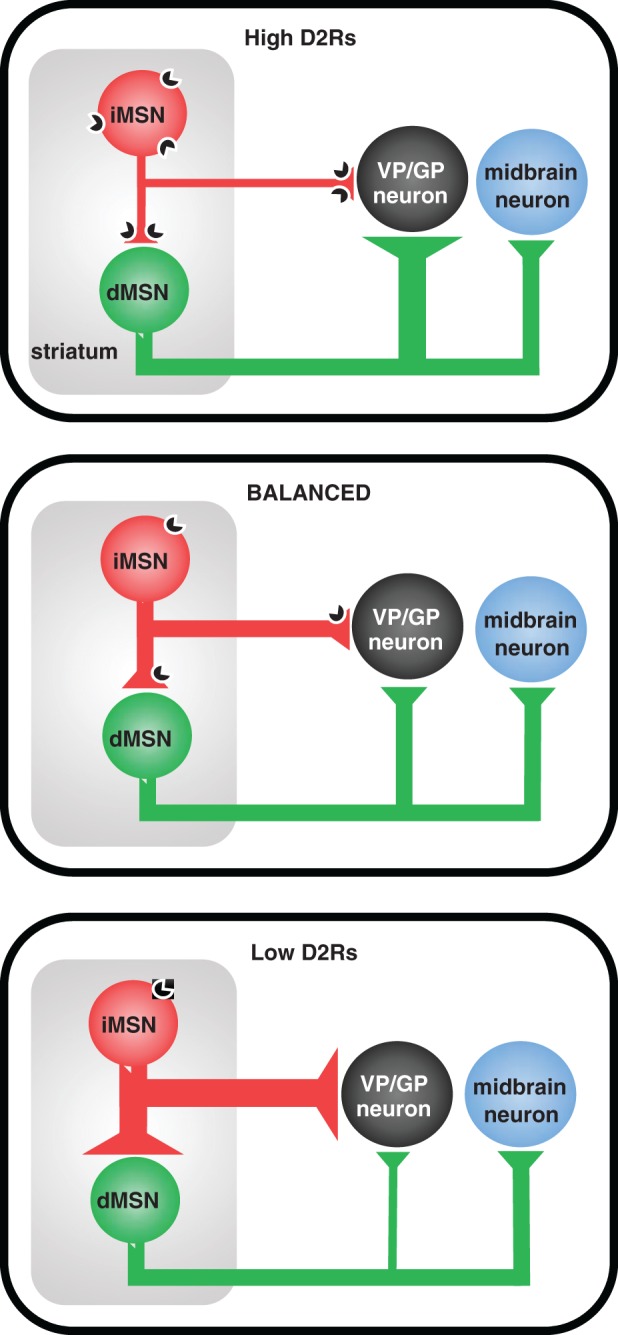
Alterations in D2R levels can lead to aberrant GABA transmission resulting in an imbalance in indirect and direct pathway output. Schematic diagram depicting the hypothesized role of D2R expression levels (top: high, middle: balanced, bottom: low) in iMSNs in regulating the functional balance of iMSN and dMSN output.
In a recent complimentary study from our group, targeted deletion of D2Rs from iMSNs caused a reduction in the in vivo firing of MSNs and pallidal neurons in awake, behaving mice. A reduction in the intrinsic excitability was also observed in iMSNs with low expression of D2R. However, the most significant changes were observed in neurons that did not even express D2Rs: a large increase of GABAergic synaptic transmission was observed in D1R‐expressing dMSNs and in globus pallidus neurons, the two major synaptic targets of iMSNs (Lemos et al. 2016). In dMSNs, there were larger GABAA receptor mediated tonic currents, measured as gabazine‐sensitive holding current, as well as enhanced fast synaptic transmission (higher frequency and amplitude of miniature IPSC). In the GPe, low levels of D2Rs in iMSNs produced a robust increase in the frequency with no change in amplitude of GABAA receptor mediated mIPSC, suggestive of enhanced release probability following D2R deletion. There are several sources of GABAergic input onto dMSNs and GPe neurons; iMSNs being only one of them. Thus, striatal interneurons (e.g. fast‐spiking (FSI) and low‐threshold spiking (LTS)), as well as dopamine terminals and GPe feedback connections, can also contribute to the enhanced inhibition observed in the striatum and GP upon D2R deletion (Gittis et al. 2011; Glajch et al. 2016; Tritsch et al. 2012). It has been suggested that MSNs form synaptic connection onto distal dendrites of other MSNs, while FSIs form synaptic connections onto the somas of MSN, based on amplitude and decay time data of evoked IPSC (Tepper et al. 2004). Thus, it is might be possible to use mIPSC amplitude information to segregate inputs from different GABAergic sources. However, targeted deletion of D2Rs from iMSNs produces an enhancement in mIPSC frequency across all amplitudes suggesting a strengthening of iMSN to MSN connectivity as well as FSI to MSN connectivity as is seen in dopamine‐depletion models(Gittis et al. 2011). Interestingly, acute activation of Gi‐signaling using DREADDs reversed the increase in GABA‐mediated tonic current and restored levels to normal with no change in mIPSC frequency or amplitude. These data suggested that other sources of GABA transmission, in addition to iMSNs, are also strengthened upon downregulation of D2Rs in iMSNs. Moreover, it indicates that, at least within the striatum, iMSN collateral transmission onto neighboring dMSNs serves to constrain excitability by modulating the size of the GABA tonic current.
Selective deletion of D2Rs in iMSNs produces a robust and reliable decrease in locomotor activity (Anzalone et al. 2012; Kharkwal et al. 2016b; Lemos et al. 2016). To test whether the motor deficit produced by downregulation of D2Rs in iMSNs was due to the increased GABAergic transmission within the striatum, a sub‐threshold dose of the GABA‐A antagonist picrotoxin was infused into the striatum. While the sub‐threshold dose of picrotoxin had no effect on locomotor activity in control mice, it increased locomotion in mice lacking D2Rs in iMSNS (Lemos et al. 2016). Further, chemogenetic tools were used to selectively inhibit GABA transmission from iMSNs in the dorsal and ventral striatum, which also led to higher locomotion and confirmed that the enhanced GABA transmission from iMSNs is a main driver of the motor deficit observed in mice lacking D2Rs. In summary, D2Rs localized to iMSNs acutely inhibit GABA and inhibitory synaptic transmission to regulate the output of the indirect‐pathway and, at the same time, through the collateral axon connections within the striatum, D2Rs also control the excitability of dMSNs and the strength of connectivity across the entire basal ganglia circuit. Alteration of D2R levels or function results in system‐wide restructuring of connectivity that impact the basal ganglia output and behavior and are likely to contribute to the vulnerability to develop SUD.
Regulation of behavioral response to cocaine by D2Rs
Use of conventional pharmacology or global D2R deletion has provided strong evidence for the various roles D2Rs play in behavioral responses to cocaine. However, in some instances these studies have yielded conflicting results. In the following section, it will become clear that in some instances D2R activation at dopamine terminals and D2R activation at iMSN terminals produce opposite behavioral effects, while in other instances they seem to act cooperatively. Being aware of the cell‐specific functions of D2Rs can help reconcile some of the controversies that presently exist in the field.
D2 autoreceptors limit the behavioral responses to cocaine
Given the acute in vitro effects of cocaine on D2 autoreceptor function, both at somatodendritic and synaptic bouton compartments, it stands to reason that downregulation of D2 autoreceptors would have an effect on behavioral responses to cocaine. Mice lacking D2 autoreceptors (autoDrd2KO) have increased basal locomotion and are more sensitive to the acute locomotor stimulatory effect of cocaine (Anzalone et al. 2012; Bello et al. 2011; Kharkwal et al. 2016b). In addition, these mice showed conditioned place preference for a low dose of cocaine that did not produce preference in littermate controls, indicating a heightened sensitivity to the rewarding properties of cocaine (Bello et al. 2011). The reinforcing properties of cocaine and the incentive salience of cocaine predictive cues were also enhanced when D2 autoreceptor mediated feedback inhibition was absent (Holroyd et al. 2015). Additionally, a higher percentage of autoDrd2KO mice met the acquisition criteria for cue‐induced cocaine self‐administration and showed impaired extinction of cocaine seeking, specifically when drug‐paired cues were present (Holroyd et al. 2015). These findings are particularly relevant to patient studies, in which individuals suffering from stimulant addiction have a difficult time suppressing drug‐seeking behavior in the presence of drug‐associated cues (Childress et al. 1993, 1994). Acute down‐regulation of the Drd2 gene in adult rats using shRNAs caused a similar increase in the locomotor response to cocaine and increase in the progressive ratio for cocaine (de Jong et al. 2015). Neither acute nor long‐term decrease in D2 autoreceptors affected the rate of operant responding for cocaine, daily cocaine intake, nor changed the ability to extinguish cocaine seeking behavior when drug‐associated cues were not present (Holroyd et al. 2015). Thus, the behavioral alterations induced by low levels of D2 autoreceptor activation are very specific and sometimes subtle. However, they point to a critical role of D2 autoreceptors in titrating the dopamine concentration at synapses, a mechanism that is especially important when cocaine is on board and the feedback inhibition is engaged. Deficiency in the feedback inhibition then causes larger dopamine transients indicative of a higher concentration of extracellular dopamine, which in turn strengthens learning and the association between cues and drugs.
Motivation for food was also increased under high effort conditions (FR100) following prolonged and acute down‐regulation of D2 autoreceptors (Bello et al. 2011; de Jong et al. 2015). However, when trained under low effort (FR1), no significant changes in progressive ratios for food reward were detected (Holroyd et al. 2015).
Thus, reduced D2 autoreceptor signaling on dopamine neurons contributes to a context‐dependent increase in the rewarding and reinforcing properties of cocaine in rodents. This could lead to higher vulnerability to engage in cocaine taking behavior and stronger salience of drug paired cues, which could enhance craving and increase the likelihood of relapse. Most PET studies in human and non‐human primate have focused on quantifying D2R levels in the caudate/putamen (striatum) because it has the highest density of D2Rs. The assumption has been that decreases in D2R availability seen in abusers is due to low D2Rs in MSNs. However, D2 autoreceptors localized to presynaptic dopamine terminals are likely to contribute to the total D2R availability and also to the vulnerability to abuse and dependence. Indeed, two recent studies in humans found that low levels of midbrain D2R availability are associated with increased craving for amphetamine as well as increased impulsivity and novelty seeking traits (Buckholtz et al. 2010; Zald et al. 2008).
D2Rs in MSNs contribute to the acute stimulant response to cocaine
New evidence indicates that cocaine relies on D2Rs in iMSNs, likely localized to axon collaterals within the nucleus accumbens, to elicit its canonical acute increase in locomotion. In the in vitro slice preparation, cocaine, like a D2/3 agonist, suppresses the GABAergic synaptic transmission from iMSNs to dMSNs by activation of D2Rs expressed in iMSNs. Mice lacking these receptors lose the suppression of collateral transmission and show a severely depressed cocaine‐induced locomotion, demonstrating that D2Rs in iMSNs are required for the acute locomotor response to cocaine. At the same time, chemogenetic rescue experiments indicated that Gi‐signaling in iMSNs, which normally occurs following D2R activation, restores the suppression of collateral transmission between MSNs and is sufficient to induce a mild locomotor response that can be further enhanced when paired with cocaine. Thus, a parsimonious interpretation of these results is that activation of D2Rs in iMSNs acts to suppress the inhibition onto dMSNs to allow for direct activation of these neurons by dopamine (likely via D1Rs). Thus, D2Rs in iMSNs act more as a gate that permits cocaine‐induced locomotion rather than mediating the psychomotor response per se (Dobbs et al. 2016). Another interesting finding is that, despite the blunted locomotor response to cocaine, mice with targeted deletion of D2Rs to iMSNs show locomotor sensitization upon repeated cocaine exposure and also cocaine conditioned place preference (see section below). These new findings are in agreement with previous data showing that administration of the D2R‐like antagonist sulpiride into the nucleus accumbens attenuates cocaine‐induced locomotion (Baker et al. 1996; Neisewander et al. 1995). Similarly, global D2R deletion abolishes the acute locomotor response to cocaine (Chausmer & Katz 2001; Chausmer et al. 2002; De Mei et al. 2009; Sim et al. 2013; Welter et al. 2007).
Thus, cocaine‐induced locomotion is mediated by an increase in striatal dopamine acting on two synergistic mechanisms: (1) via direct activation of D1Rs in dMSNs, and (2) via indirect activation of D2Rs in iMSNs, which effectively ‘lifts the break’ onto neighboring dMSNs. Furthermore, the attenuated acute cocaine locomotor response in mice lacking D2Rs in iMSNs does not appear to arise from developmental alterations from early life deletion of D2Rs since mice with a D2R deletion introduced in adulthood and restricted to the nucleus accumbens also exhibit a blunted locomotor response to acute cocaine.
D2Rs regulate cocaine induced behavioral plasticity and reinforcement
The role of striatal D2Rs in reward learning, behavioral plasticity and self‐administration has been controversial in that there have been several conflicting findings within the literature. Locomotor sensitization, defined as enhanced locomotor response to repeated stimulant challenge, has been proposed as a behavioral read‐out of cocaine‐induced plasticity at the circuit level. Interestingly, in contrast to the effect on the acute locomotor response to cocaine, targeted deletion of D2Rs in iMSNs does not impair locomotor sensitization to cocaine (Anzalone et al. 2012; Dobbs et al. 2016), demonstrating a dissociation of the role of D2Rs in iMSNs between the acute locomotor response to cocaine and the long‐term plasticity that occurs following repeated cocaine exposures. Sensitization is also observed when D2R deletion is restricted to the ventral striatum and induced in adulthood, suggesting that developmental compensation following early‐life D2R deletion is not driving this phenotype (Dobbs et al. 2016). These findings are consistent with the global D2R knockout mice, which similarly show no effect of D2R deletion on cocaine locomotor sensitization (Sim et al. 2013). More transient manipulations of D2R‐like Gi‐signaling using chemogenetic and optogenetic tools have lead to opposite results and suggest that D2Rs can regulate sensitization. For example, selective activation of hM4Di in iMSNs, the Gi‐coupled Designer Receptor Exclusively activated by Designer Drugs (DREADDs), facilitated the development of amphetamine locomotor sensitization in rats (Ferguson et al. 2011). Conversely, optogenetic activation of D2R‐containing iMSNs suppressed the development and expression of cocaine locomotor sensitization (Lobo et al. 2010). There are several factors that could possibly account for the difference in the results, such as the stimulant drug used (amphetamine vs. cocaine), the species (mouse vs. rat), and the different signaling and cellular effects of activating D2Rs, hM4Di, and optogenetic stimulation. However, the most relevant distinction between these studies is likely the transient vs. long‐term nature of these perturbations. It is becoming apparent that long‐term perturbations (weeks) of D2R levels can produce different phenotypes, and sometimes even opposite results. Unpublished work indicates that mice lacking D2Rs in iMSNs also display a hypersensitive behavioral response to D1R‐like agonists and upregulation of D1R signaling, which might prime these mice for cocaine locomotor sensitization.
The conditioned rewarding properties of stimulants have been classically assessed using the conditioned place preference procedure, which involves pairing non‐contingent stimulant administration with distinct contexts and cues (Mucha et al. 1982). The activity and expression of D2Rs have been implicated in regulating the conditioned rewarding and reinforcing effects of psychostimulants. For example, global D2R knockout mice have normal cocaine place preference (Sim et al. 2013; Welter et al. 2007). Selective deletion of D2 autoreceptors enhances the acquisition of cocaine conditioned place preference and increases striatal dopamine release (Bello et al. 2011). Additionally, mice with a selective D2R deletion from striatal iMSNs acquire cocaine conditioned place preference faster as littermate controls (Dobbs et al. 2016).
Studies utilizing pharmacological and global genetic manipulations also suggest a complex role for D2Rs in mediating the reinforcing effects of cocaine. The effect of D2R‐like antagonists on self‐administration varies depending on the dose of D2R antagonist and cocaine used. Generally, D2R‐like antagonists dose‐dependently facilitate self‐administration of cocaine, particularly at high doses of cocaine. However, high‐doses of D2R‐like antagonist produce catalepsy and therefore, inhibit cocaine self‐administration as well as many other behavioral outputs (Britton et al. 1991; Caine & Koob 1994; Caine et al. 2002; Hubner & Moreton 1991; Woolverton 1986). Similarly, global D2R deletion enhances self‐administration of high‐dose cocaine (Caine et al. 2002). It should be noted that at low doses of cocaine, D2 antagonism or deletion produces the opposite or no effect of self‐administration. In light of what it is now known about the function of D2Rs localized to different cell‐types in the striatum, it is reasonable to interpret these biphasic effects of D2R antagonist/deletion as the result of targeting D2 autoreceptors (shown to be engaged when cocaine is on board) at low doses and D2Rs in iMSNs and CINs causing reduced movement (bradykinesia) and catalepsy at higher doses.
The role of D2Rs in reinstatement to cocaine seeking
Relapse to stimulant abuse has been modeled in animals using reinstatement paradigms, which involves acutely exposing an animal to a drug prime, stressor or drug‐paired cue following drug conditioning and extinction (Epstein et al. 2006). Multiple acute manipulations result in the reinstatement to drug‐seeking behavior and it is possible the pathways underlying each manipulation's effects on reinstatement are different. In the case of D2R regulation of reinstatement behavior, it has been shown that global D2R knockout mice have normal drug‐induced reinstatement of cocaine seeking, but do not exhibit stress‐induced cocaine seeking (Sim et al. 2013). This is in contrast to the findings that D2R‐like antagonists classically attenuate drug‐primed reinstatement of cocaine seeking (Khroyan et al. 2000; Spealman et al. 1999). Both D2R‐like agonists and the chemogenetic activation of Gi‐signaling specifically in iMSNs enhanced motivation for cocaine seeking as measured using drug‐primed reinstatement and progressive ratio, respectively (Bock et al. 2013; Fuchs et al. 2002; Self et al. 1996). Moreover, the administration of D2R‐like agonists alone reinstate cocaine‐seeking behavior (De Vries et al. 1999; Fuchs et al. 2002; Khroyan et al. 2000; Self et al. 1996; Spealman et al. 1999). These disparate findings again highlight potential differences between chronic reduction/ablation of D2Rs compared to acute pharmacological manipulations of D2R activity.
The general picture that emerges is that low D2R activity facilitates cocaine self‐administration but inhibits reinstatement; however, in order to fully parse out the effect of D2R in regulating cocaine taking, seeking and relapse, manipulations that can be targeted specifically to each cell‐type expressing D2Rs in the striatum are required. In particular, an important next step is to assess the roles of D2Rs in iMSNs on the self‐administration of psychostimulants. For example, studies using mice with targeted deletion of D2Rs to iMSNs could directly test the current theory linking low D2Rs to stimulant abuse. In this case, the use heterozygote mice will be the most relevant since the partial reduction of D2R levels better resemble the down‐regulation levels of striatal D2R availability observed in human abusers (Volkow et al. 1993, 2001).
Summary and final conclusions
The heterogeneity of D2R expression and function throughout the CNS and within striatum is profound and likely to reflect the involvement of D2Rs in regulating multiple diverse brain functions. At the same time, experimentally these factors have lead to paradoxical results and limited the interpretation of findings from studies using pharmacological manipulations or global genetic D2R deletion. Novel transgenic approaches targeting D2R deletion or overexpression to specific cell‐types have greatly aided our understanding of how D2Rs regulate striatal function and behavioral output. These more precise manipulations have also revealed a remarkable complexity of the circuit connectivity and new biological sources of variability, such as the difference between acute and long‐term perturbations of D2R level function that seem to trigger different behavioral outcomes.
It is important to highlight a gap of knowledge with regards to the behavioral function of D2Rs localized to CIN and to glutamatergic inputs to the striatum. More studies are also needed in regards to their role in the response to stimulant drugs. A recent study in which D2Rs were selectively deleted from CINs showed no effect on basal locomotion, yet selective disruption of catalepsy induced by D2R‐like antagonists and impaired locomotor response to D1R‐like agonist (Kharkwal et al. 2016a).
At this point, more is known about the physiological and behavioral functions of D2 autoreceptors localized to dopamine neurons and terminals than D2Rs in iMSNs (heteroreceptors). Both from a physiological and a behavioral perspective, there are clear similarities and differences in the function of D2Rs localized to these two disparate cell‐types. On the one hand, both the autoreceptors and the heteroreceptors act to inhibit synaptic transmission by reducing neurotransmitter release (dopamine and GABA, respectively) from presynaptic terminals. On the other hand, there are differences in the ability of D2Rs to acutely modulate the intrinsic excitability of these neurons: potent inhibition in midbrain dopamine neurons vs. weak/unreliable effect in GABAergic MSNs. Also, D2R mediated changes in gene expression are reported in iMSNs but less in dopamine neurons so far. Interestingly, D2 autoreceptors and D2Rs localized to iMSNs have opposite roles in regulating the locomotor response to acute dopamine elevation or cocaine exposure, with D2 autoreceptors suppressing and D2Rs on iMSNs facilitating both behaviors.
When assessing behavioral responses to cocaine that require more chronic cocaine exposure, D2 autoreceptors and D2Rs in iMSNs appear to have a more synergistic role in constraining the rewarding and reinforcing properties of cocaine. Interestingly, D2 autoreceptors appear to have an important and newly discovered role in limiting cocaine reinforcement and seeking that has been previously underappreciated.
Long‐term reduction of D2R expression levels, that likely occurs in the SUD patient population, not only impacts subsequent responses to stimulant drugs and the cues associated with them, but also cause fundamental changes in the connectivity and synaptic strength of the striatal circuit in ways that impact basic brain function and behavior. We then propose that the long‐term cellular changes and the synaptic reorganization generate a vulnerability to acquire stimulant use as well as abuse and relapse. These finding are likely to have clinical implications and further our understanding of the factors and mechanisms that drive the vulnerability to develop stimulant drug use and abuse in humans.
Acknowledgments
The authors declare no conflict of interest. Funding was provided by the Intramural Research Programs of NIAAA and NINDS at the National Institutes of Health (AA000421) to V.A.A. and PRAT Fellowship from NIMGS to J.C.L.
REFERENCES
- Adrover, M.F. , Shin, J.H. & Alvarez, V.A. (2014) Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine. J Neurosci 34, 3183–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, J.A. , Yost, J.M. , Setola, V. , Chen, X. , Sassano, M.F. , Chen, M. , Peterson, S. , Yadav, P.N. , Huang, X.P. , Feng, B. , Jensen, N.H. , Che, X. , Bai, X. , Frye, S.V. , Wetsel, W.C. , Caron, M.G. , Javitch, J.A. , Roth, B.L. & Jin, J. (2011) Discovery of beta‐arrestin‐biased dopamine D2 ligands for probing signal transduction pathways essential for antipsychotic efficacy. Proc Natl Acad Sci USA 108, 18488–18493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone, A. , Lizardi‐Ortiz, J.E. , Ramos, M. , De Mei, C. , Hopf, F.W. , Iaccarino, C. , Halbout, B. , Jacobsen, J. , Kinoshita, C. , Welter, M. , Caron, M.G. , Bonci, A. , Sulzer, D. & Borrelli, E. (2012) Dual control of dopamine synthesis and release by presynaptic and postsynaptic dopamine D2 receptors. J Neurosci 32, 9023–9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik, J.H. , Picetti, R. , Saiardi, A. , Thiriet, G. , Dierich, A. , Depaulis, A. , Le Meur, M. & Borrelli, E. (1995) Parkinsonian‐like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377, 424–428. [DOI] [PubMed] [Google Scholar]
- Baker, D.A. , Khroyan, T.V. , O'Dell, L.E. , Fuchs, R.A. & Neisewander, J.L. (1996) Differential effects of intra‐accumbens sulpiride on cocaine‐induced locomotion and conditioned place preference. J Pharmacol Exp Ther 279, 392–401. [PubMed] [Google Scholar]
- Bamford, N.S. , Robinson, S. , Palmiter, R.D. , Joyce, J.A. , Moore, C. & Meshul, C.K. (2004) Dopamine modulates release from corticostriatal terminals. J Neurosci 24, 9541–9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu, J.M. & Gainetdinov, R.R. (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63, 182–217. [DOI] [PubMed] [Google Scholar]
- Beaulieu, J.M. , Sotnikova, T.D. , Marion, S. , Lefkowitz, R.J. , Gainetdinov, R.R. & Caron, M.G. (2005) An Akt/beta‐arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122, 261–273. [DOI] [PubMed] [Google Scholar]
- Beaulieu, J.M. , Gainetdinov, R.R. & Caron, M.G. (2009) Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol 49, 327–347. [DOI] [PubMed] [Google Scholar]
- Beaulieu, J.M. , Del'guidice, T. , Sotnikova, T.D. , Lemasson, M. & Gainetdinov, R.R. (2011) Beyond cAMP: the regulation of Akt and GSK3 by dopamine receptors. Front Mol Neurosci 4, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead, M.J. , Grandy, D.K. , Wickman, K. & Williams, J.T. (2004) Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42, 939–946. [DOI] [PubMed] [Google Scholar]
- Beckstead, M.J. , Ford, C.P. , Phillips, P.E. & Williams, J.T. (2007) Presynaptic regulation of dendrodendritic dopamine transmission. Eur J Neurosci 26, 1479–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello, E.P. , Mateo, Y. , Gelman, D.M. , Noain, D. , Shin, J.H. , Low, M.J. , Alvarez, V.A. , Lovinger, D.M. & Rubinstein, M. (2011) Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci 14, 1033–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson, M. , Belin, D. , McNamara, R. , Theobald, D.E. , Castel, A. , Beckett, V.L. , Crittenden, B.M. , Newman, A.H. , Everitt, B.J. , Robbins, T.W. & Dalley, J.W. (2010) Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology 35, 560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson, M. , Pelloux, Y. , Dilleen, R. , Theobald, D.E. , Lyon, A. , Belin‐Rauscent, A. , Robbins, T.W. , Dalley, J.W. , Everitt, B.J. & Belin, D. (2013) Cocaine modulation of frontostriatal expression of Zif268, D2, and 5‐HT2c receptors in high and low impulsive rats. Neuropsychopharmacology 38, 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock, R. , Shin, J.H. , Kaplan, A.R. , Dobi, A. , Markey, E. , Kramer, P.F. , Gremel, C.M. , Christensen, C.H. , Adrover, M.F. & Alvarez, V.A. (2013) Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci 16, 632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton, D.R. , Curzon, P. , Mackenzie, R.G. , Kebabian, J.W. , Williams, J.E. & Kerkman, D. (1991) Evidence for involvement of both D1 and D2 receptors in maintaining cocaine self‐administration. Pharmacol Biochem Behav 39, 911–915. [DOI] [PubMed] [Google Scholar]
- Buckholtz, J.W. , Treadway, M.T. , Cowan, R.L. , Woodward, N.D. , Li, R. , Ansari, M.S. , Baldwin, R.M. , Schwartzman, A.N. , Shelby, E.S. , Smith, C.E. , Kessler, R.M. & Zald, D.H. (2010) Dopaminergic network differences in human impulsivity. Science 329, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cachope, R. , Mateo, Y. , Mathur, B.N. , Irving, J. , Wang, H.L. , Morales, M. , Lovinger, D.M. & Cheer, J.F. (2012) Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell Rep 2, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine, S.B. & Koob, G.F. (1994) Effects of dopamine D‐1 and D‐2 antagonists on cocaine self‐administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270, 209–218. [PubMed] [Google Scholar]
- Caine, S.B. , Negus, S.S. , Mello, N.K. , Patel, S. , Bristow, L. , Kulagowski, J. , Vallone, D. , Saiardi, A. & Borrelli, E. (2002) Role of dopamine D2‐like receptors in cocaine self‐administration: studies with D2 receptor mutant mice and novel D2 receptor antagonists. J Neurosci 22, 2977–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli, D. , Jupp, B. , Hong, Y.T. , Sawiak, S.J. , Ferrari, V. , Wharton, L. , Williamson, D.J. , McNabb, C. , Berry, D. , Aigbirhio, F.I. , Robbins, T.W. , Fryer, T.D. & Dalley, J.W. (2015) Dissociable rate‐dependent effects of oral methylphenidate on impulsivity and D2/3 receptor availability in the striatum. J Neurosci 35, 3747–3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla, M. , Shegda, M. , Ramesh, B. , Harrison, N.L. & Kellendonk, C. (2012) Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci 32, 2398–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazorla, M. , de Carvalho, F.D. , Chohan, M.O. , Shegda, M. , Chuhma, N. , Rayport, S. , Ahmari, S.E. , Moore, H. & Kellendonk, C. (2014) Dopamine D2 receptors regulate the anatomical and functional balance of basal ganglia circuitry. Neuron 81, 153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze, D. , Usiello, A. , Gubellini, P. , Pisani, A. , Borrelli, E. , Bernardi, G. & Calabresi, P. (2002) Dopamine D2 receptor‐mediated inhibition of dopaminergic neurons in mice lacking D2L receptors. Neuropsychopharmacology 27, 723–726. [DOI] [PubMed] [Google Scholar]
- Centonze, D. , Grande, C. , Usiello, A. , Gubellini, P. , Erbs, E. , Martin, A.B. , Pisani, A. , Tognazzi, N. , Bernardi, G. , Moratalla, R. , Borrelli, E. & Calabresi, P. (2003) Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci 23, 6245–6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chausmer, A.L. & Katz, J.L. (2001) The role of D2‐like dopamine receptors in the locomotor stimulant effects of cocaine in mice. Psychopharmacology (Berl) 155, 69–77. [DOI] [PubMed] [Google Scholar]
- Chausmer, A.L. , Elmer, G.I. , Rubinstein, M. , Low, M.J. , Grandy, D.K. & Katz, J.L. (2002) Cocaine‐induced locomotor activity and cocaine discrimination in dopamine D2 receptor mutant mice. Psychopharmacology (Berl) 163, 54–61. [DOI] [PubMed] [Google Scholar]
- Childress, A.R. , Hole, A.V. , Ehrman, R.N. , Robbins, S.J. , McLellan, A.T. & O'Brien, C.P. (1993) Cue reactivity and cue reactivity interventions in drug dependence. NIDA Res Monogr 137, 73–95. [PubMed] [Google Scholar]
- Childress, A.R. , Ehrman, R. , McLellan, A.T. , MacRae, J. , Natale, M. & O'Brien, C.P. (1994) Can induced moods trigger drug‐related responses in opiate abuse patients? J Subst Abuse Treat 11, 17–23. [DOI] [PubMed] [Google Scholar]
- Chuhma, N. , Mingote, S. , Moore, H. & Rayport, S. (2014) Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron 81, 901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad, K.L. , Ford, K. , Marinelli, M. & Wolf, M.E. (2010) Dopamine receptor expression and distribution dynamically change in the rat nucleus accumbens after withdrawal from cocaine self‐administration. Neuroscience 169, 182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley, J.W. , Fryer, T.D. , Brichard, L. , Robinson, E.S. , Theobald, D.E. , Lääne, K. , Peña, Y. , Murphy, E.R. , Shah, Y. , Probst, K. , Abakumova, I. , Aigbirhio, F.I. , Richards, H.K. , Hong, Y. , Baron, J.C. , Everitt, B.J. & Robbins, T.W. (2007) Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science 315, 1267–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley, J.W. , Everitt, B.J. & Robbins, T.W. (2011) Impulsivity, compulsivity, and top‐down cognitive control. Neuron 69, 680–694. [DOI] [PubMed] [Google Scholar]
- Day, M. , Wokosin, D. , Plotkin, J.L. , Tian, X. & Surmeier, D.J. (2008) Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci 28, 11603–11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mei, C. , Ramos, M. , Iitaka, C. & Borrelli, E. (2009) Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol 9, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries, T.J. , Schoffelmeer, A.N. , Binnekade, R. & Vanderschuren, L.J. (1999) Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin following long‐term withdrawal of IV drug self‐administration. Psychopharmacology (Berl) 143, 254–260. [DOI] [PubMed] [Google Scholar]
- DeBoer, P. & Abercrombie, E.D. (1996) Physiological release of striatal acetylcholine in vivo: modulation by D1 and D2 dopamine receptor subtypes. J Pharmacol Exp Ther 277, 775–783. [PubMed] [Google Scholar]
- DeBoer, P. , Heeringa, M.J. & Abercrombie, E.D. (1996) Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur J Pharmacol 317, 257–262. [DOI] [PubMed] [Google Scholar]
- Delle Donne, K.T. , Sesack, S.R. & Pickel, V.M. (1996) Ultrastructural immunocytochemical localization of neurotensin and the dopamine D2 receptor in the rat nucleus accumbens. J Comp Neurol 371, 552–566. [DOI] [PubMed] [Google Scholar]
- Delle Donne, K.T. , Sesack, S.R. & Pickel, V.M. (1997) Ultrastructural immunocytochemical localization of the dopamine D2 receptor within GABAergic neurons of the rat striatum. Brain Res 746, 239–255. [DOI] [PubMed] [Google Scholar]
- Ding, J.B. , Guzman, J.N. , Peterson, J.D. , Goldberg, J.A. & Surmeier, D.J. (2010) Thalamic gating of corticostriatal signaling by cholinergic interneurons. Neuron 67, 294–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbs, L.K. , Kaplan, A.R. , Lemos, J.C. , Matsui, A. , Rubinstein, M. & Alvarez, V.A. (2016) Dopamine regulation of lateral inhibition between striatal neurons gates the stimulant actions of cocaine. Neuron 90, 1100–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards, S. , Whisler, K.N. , Fuller, D.C. , Orsulak, P.J. & Self, D.W. (2007) Addiction‐related alterations in D1 and D2 dopamine receptor behavioral responses following chronic cocaine self‐administration. Neuropsychopharmacology 32, 354–366. [DOI] [PubMed] [Google Scholar]
- Epstein, D.H. , Preston, K.L. , Stewart, J. & Shaham, Y. (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche, K.D. , Jones, P.S. , Williams, G.B. , Turton, A.J. , Robbins, T.W. & Bullmore, E.T. (2012) Abnormal brain structure implicated in stimulant drug addiction. Science 335, 601–604. [DOI] [PubMed] [Google Scholar]
- Ferguson, S.M. , Eskenazi, D. , Ishikawa, M. , Wanat, M.J. , Phillips, P.E. , Dong, Y. , Roth, B.L. & Neumaier, J.F. (2011) Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14, 22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, C.P. , Mark, G.P. & Williams, J.T. (2006) Properties and opioid inhibition of mesolimbic dopamine neurons vary according to target location. J Neurosci 26, 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford, C.P. , Phillips, P.E. & Williams, J.T. (2009) The time course of dopamine transmission in the ventral tegmental area. J Neurosci 29, 13344–13352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Free, R.B. , Chun, L.S. , Moritz, A.E. et al (2014) Discovery and characterization of a G protein‐biased agonist that inhibits beta‐arrestin recruitment to the D2 dopamine receptor. Mol Pharmacol 86, 96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, R.A. , Tran‐Nguyen, L.T. , Weber, S.M. , Khroyan, T.V. & Neisewander, J.L. (2002) Effects of 7‐OH‐DPAT on cocaine‐seeking behavior and on re‐establishment of cocaine self‐administration. Pharmacol Biochem Behav 72, 623–632. [DOI] [PubMed] [Google Scholar]
- Gallo, E.F. , Salling, M.C. , Feng, B. , Moron, J.A. , Harrison, N.L. , Javitch, J.A. & Kellendonk, C. (2015) Upregulation of dopamine D2 receptors in the nucleus accumbens indirect pathway increases locomotion but does not reduce alcohol consumption. Neuropsychopharmacology 40, 1609–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz, S.C. , Bunzow, J.R. & Williams, J.T. (2015a) Spontaneous inhibitory synaptic currents mediated by a G protein‐coupled receptor. Neuron 78, 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz, S.C. , Robinson, B.G. , Buck, D.C. , Bunzow, J.R. , Neve, R.L. , Williams, J.T. & Neve, K.A. (2015b) Distinct regulation of dopamine D2S and D2L autoreceptor signaling by calcium. Elife 4, e09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen, C.R. , Engber, T.M. , Mahan, L.C. , Susel, Z. , Chase, T.N. , Monsma, F.J. Jr. & Sibley, D.R. (1990) D1 and D2 dopamine receptor‐regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432. [DOI] [PubMed] [Google Scholar]
- Gittis, A.H. , Hang, G.B. , LaDow, E.S. , Shoenfeld, L.R. , Atallah, B.V. , Finkbeiner, S. & Kreitzer, A.C. (2011) Rapid target‐specific remodeling of fast‐spiking inhibitory circuits after loss of dopamine. Neuron 71, 858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glajch, K.E. , Kelver, D.A. , Hegeman, D.J. , Cui, Q. , Xenias, H.S. , Augustine, E.C. , Hernandez, V.M. , Verma, N. , Huang, T.Y. , Luo, M. , Justice, N.J. & Chan, C.S. (2016) Npas1+ pallidal neurons target striatal projection neurons. J Neurosci 36, 5472–5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves, P.M. & Wilson, C.J. (1980) Fine structure of rat locus coeruleus. J Comp Neurol 193, 841–852. [DOI] [PubMed] [Google Scholar]
- Hernandez‐Lopez, S. , Tkatch, T. , Perez‐Garci, E. , Galarraga, E. , Bargas, J. , Hamm, H. & Surmeier, D.J. (2000) D2 dopamine receptors in striatal medium spiny neurons reduce L‐type Ca2+ currents and excitability via a novel PLC[beta]1‐IP3‐calcineurin‐signaling cascade. J Neurosci 20, 8987–8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley, M.J. & Sabatini, B.L. (2010) Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander, E. , Nunes, E. , DeCaria, C.M. , Quitkin, F.M. , Cooper, T. , Wager, S. & Klein, D.F. (1990) Dopaminergic sensitivity and cocaine abuse: response to apomorphine. Psychiatry Res 33, 161–169. [DOI] [PubMed] [Google Scholar]
- Holroyd, K.B. , Adrover, M.F. , Fuino, R.L. , Bock, R. , Kaplan, A.R. , Gremel, C.M. , Rubinstein, M. & Alvarez, V.A. (2015) Loss of feedback inhibition via D2 autoreceptors enhances acquisition of cocaine taking and reactivity to drug‐paired cues. Neuropsychopharmacology 40, 1495–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubner, C.B. & Moreton, J.E. (1991) Effects of selective D1 and D2 dopamine antagonists on cocaine self‐administration in the rat. Psychopharmacology (Berl) 105, 151–156. [DOI] [PubMed] [Google Scholar]
- Ito, K. , Haga, T. , Lameh, J. & Sadee, W. (1999) Sequestration of dopamine D2 receptors depends on coexpression of G‐protein‐coupled receptor kinases 2 or 5. Eur J Biochem/FEBS 260, 112–119. [DOI] [PubMed] [Google Scholar]
- Itokawa, M. , Toru, M. , Ito, K. , Tsuga, H. , Kameyama, K. , Haga, T. , Arinami, T. & Hamaguchi, H. (1996) Sequestration of the short and long isoforms of dopamine D2 receptors expressed in Chinese hamster ovary cells. Mol Pharmacol 49, 560–566. [PubMed] [Google Scholar]
- Jentsch, J.D. & Taylor, J.R. (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward‐related stimuli. Psychopharmacology (Berl) 146, 373–390. [DOI] [PubMed] [Google Scholar]
- Jomphe, C. , Tiberi, M. & Trudeau, L.E. (2006) Expression of D2 receptor isoforms in cultured neurons reveals equipotent autoreceptor function. Neuropharmacology 50, 595–605. [DOI] [PubMed] [Google Scholar]
- Jones, S.R. , Gainetdinov, R.R. , Hu, X.T. , Cooper, D.C. , Wightman, R.M. , White, F.J. & Caron, M.G. (1999) Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci 2, 649–655. [DOI] [PubMed] [Google Scholar]
- de Jong, J.W. , Roelofs, T.J. , Mol, F.M. , Hillen, A.E. , Meijboom, K.E. , Luijendijk, M.C. , van der Eerden, H.A. , Garner, K.M. , Vanderschuren, L.J. & Adan, R.A. (2015) Reducing ventral tegmental dopamine D2 receptor expression selectively boosts incentive motivation. Neuropsychopharmacology 40, 2085–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebabian, J.W. & Calne, D.B. (1979) Multiple receptors for dopamine. Nature 277, 93–96. [DOI] [PubMed] [Google Scholar]
- Kebabian, J.W. & Greengard, P. (1971) Dopamine‐sensitive adenyl cyclase: possible role in synaptic transmission. Science 174, 1346–1349. [DOI] [PubMed] [Google Scholar]
- Kennedy, R.T. , Jones, S.R. & Wightman, R.M. (1992) Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem 59, 449–455. [DOI] [PubMed] [Google Scholar]
- Khan, Z.U. , Mrzljak, L. , Gutierrez, A. , de la Calle, A. & Goldman‐Rakic, P.S. (1998) Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci USA 95, 7731–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkwal, G. , Brami‐Cherrier, K. , Lizardi‐Ortiz, J.E. , Nelson, A.B. , Ramos, M. , Del Barrio, D. , Sulzer, D. , Kreitzer, A.C. & Borrelli, E. (2016a) Parkinsonism driven by antipsychotics originates from dopaminergic control of striatal cholinergic interneurons. Neuron 91, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharkwal, G. , Radl, D. , Lewis, R. & Borrelli, E. (2016b) Dopamine D2 receptors in striatal output neurons enable the psychomotor effects of cocaine. Proc Natl Acad Sci USA 113, 11609–11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khroyan, T.V. , Barrett‐Larimore, R.L. , Rowlett, J.K. & Spealman, R.D. (2000) Dopamine D1‐ and D2‐like receptor mechanisms in relapse to cocaine‐seeking behavior: effects of selective antagonists and agonists. J Pharmacol Exp Ther 294, 680–687. [PubMed] [Google Scholar]
- Kohnomi, S. , Koshikawa, N. & Kobayashi, M. (2012) D(2)‐like dopamine receptors differentially regulate unitary IPSCs depending on presynaptic GABAergic neuron subtypes in rat nucleus accumbens shell. J Neurophysiol 107, 692–703. [DOI] [PubMed] [Google Scholar]
- Kourrich, S. & Thomas, M.J. (2009) Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci 29, 12275–12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzhikandathil, E.V. , Yu, W. & Oxford, G.S. (1998) Human dopamine D3 and D2L receptors couple to inward rectifier potassium channels in mammalian cell lines. Mol Cell Neurosci 12, 390–402. [DOI] [PubMed] [Google Scholar]
- Lacey, M.G. , Mercuri, N.B. & North, R.A. (1987) Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol 392, 397–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalchandani, R.R. , van der Goes, M.S. , Partridge, J.G. & Vicini, S. (2013) Dopamine D2 receptors regulate collateral inhibition between striatal medium spiny neurons. J Neurosci 33, 14075–14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, H. , Liu, Y. , Bell, M.I. , Gurevich, V.V. & Neve, K.A. (2009) A dopamine D2 receptor mutant capable of G protein‐mediated signaling but deficient in arrestin binding. Mol Pharmacol 75, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavine, N. , Ethier, N. , Oak, J.N. , Pei, L. , Liu, F. , Trieu, P. , Rebois, R.V. , Bouvier, M. , Hebert, T.E. & Van Tol, H.H. (2002) G protein‐coupled receptors form stable complexes with inwardly rectifying potassium channels and adenylyl cyclase. J Biol Chem 277, 46010–46019. [DOI] [PubMed] [Google Scholar]
- Lemos, J.C. , Friend, D.M. , Kaplan, A.R. , Shin, J.H. , Rubinstein, M. , Kravitz, A.V. & Alvarez, V.A. (2016) Enhanced GABA transmission drives bradykinesia following loss of dopamine D2 receptor signaling. Neuron 90, 824–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Qi, J. , Yamaguchi, T. , Wang, H.L. & Morales, M. (2012) Heterogeneous composition of dopamine neurons of the rat A10 region: molecular evidence for diverse signaling properties. Brain Struct Funct 218, 1159–1176. [DOI] [PubMed] [Google Scholar]
- Lindgren, N. , Usiello, A. , Goiny, M. , Haycock, J. , Erbs, E. , Greengard, P. , Hokfelt, T. , Borrelli, E. & Fisone, G. (2003) Distinct roles of dopamine D2L and D2S receptor isoforms in the regulation of protein phosphorylation at presynaptic and postsynaptic sites. Proc Natl Acad Sci USA 100, 4305–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y.F. , Civelli, O. , Grandy, D.K. & Albert, P.R. (1992) Differential sensitivity of the short and long human dopamine D2 receptor subtypes to protein kinase C. J Neurochem 59, 2311–2317. [DOI] [PubMed] [Google Scholar]
- Lobo, M.K. , Covington, H.E. , Chaudhury, D. , Friedman, A.K. , Sun, H. , Damez‐Werno, D. , Dietz, D.M. , Zaman, S. , Koo, J.W. , Kennedy, P.J. , Mouzon, E. , Mogri, M. , Neve, R.L. , Deisseroth, K. , Han, M.H. & Nestler, E.J. (2010) Cell type‐specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330, 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis, D.E. , Kurachi, Y. , Galper, J. , Neer, E.J. & Clapham, D.E. (1987) The beta gamma subunits of GTP‐binding proteins activate the muscarinic K+ channel in heart. Nature 325, 321–326. [DOI] [PubMed] [Google Scholar]
- Luscher, C. & Slesinger, P.A. (2010) Emerging roles for G protein‐gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci 11, 301–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice, N. , Mercer, J. , Chan, C.S. , Hernandez‐Lopez, S. , Held, J. , Tkatch, T. & Surmeier, D.J. (2004) D2 dopamine receptor‐mediated modulation of voltage‐dependent Na+ channels reduces autonomous activity in striatal cholinergic interneurons. J Neurosci 24, 10289–10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercuri, N.B. , Saiardi, A. , Bonci, A. , Picetti, R. , Calabresi, P. , Bernardi, G. & Borrelli, E. (1997) Loss of autoreceptor function in dopaminergic neurons from dopamine D2 receptor deficient mice. Neuroscience 79, 323–327. [DOI] [PubMed] [Google Scholar]
- Missale, C. , Nash, S.R. , Robinson, S.W. , Jaber, M. & Caron, M.G. (1998) Dopamine receptors: from structure to function. Physiol Rev 78, 189–225. [DOI] [PubMed] [Google Scholar]
- Mizuno, T. , Schmauss, C. & Rayport, S. (2007) Distinct roles of presynaptic dopamine receptors in the differential modulation of the intrinsic synapses of medium‐spiny neurons in the nucleus accumbens. BMC Neurosci 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore, R.J. , Vinsant, S.L. , Nader, M.A. , Porrino, L.J. & Friedman, D.P. (1998) Effect of cocaine self‐administration on dopamine D2 receptors in rhesus monkeys. Synapse 30, 88–96. [DOI] [PubMed] [Google Scholar]
- Morgan, D. , Grant, K.A. , Gage, H.D. , Mach, R.H. , Kaplan, J.R. , Prioleau, O. , Nader, S.H. , Buchheimer, N. , Ehrenkaufer, R.L. & Nader, M.A. (2002) Social dominance in monkeys: dopamine D2 receptors and cocaine self‐administration. Nat Neurosci 5, 169–174. [DOI] [PubMed] [Google Scholar]
- Mucha, R.F. , van der Kooy, D. , O'Shaughnessy, M. & Bucenieks, P. (1982) Drug reinforcement studied by the use of place conditioning in rat. Brain Res 243, 91–105. [DOI] [PubMed] [Google Scholar]
- Nader, M.A. , Daunais, J.B. , Moore, T. , Nader, S.H. , Moore, R.J. , Smith, H.R. , Friedman, D.P. & Porrino, L.J. (2002) Effects of cocaine self‐administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology 27, 35–46. [DOI] [PubMed] [Google Scholar]
- Nader, M.A. , Morgan, D. , Gage, H.D. , Nader, S.H. , Calhoun, T.L. , Buchheimer, N. , Ehrenkaufer, R. & Mach, R.H. (2006) PET imaging of dopamine D2 receptors during chronic cocaine self‐administration in monkeys. Nat Neurosci 9, 1050–1056. [DOI] [PubMed] [Google Scholar]
- Neisewander, J.L. , O'Dell, L.E. & Redmond, J.C. (1995) Localization of dopamine receptor subtypes occupied by intra‐accumbens antagonists that reverse cocaine‐induced locomotion. Brain Res 671, 201–212. [DOI] [PubMed] [Google Scholar]
- Neve, K.A. , Ford, C.P. , Buck, D.C. , Grandy, D.K. , Neve, R.L. & Phillips, T.J. (2013) Normalizing dopamine D2 receptor‐mediated responses in D2 null mutant mice by virus‐mediated receptor restoration: comparing D2L and D2S. Neuroscience 248, 479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi, A. , Snyder, G.L. & Greengard, P. (1997) Bidirectional regulation of DARPP‐32 phosphorylation by dopamine. J Neurosci 17, 8147–8155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orefice, L.L. , Waterhouse, E.G. , Partridge, J.G. , Lalchandani, R.R. , Vicini, S. & Xu, B. (2013) Distinct roles for somatically and dendritically synthesized brain‐derived neurotrophic factor in morphogenesis of dendritic spines. J Neurosci 33, 11618–11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini, C.A. , Robinson, S. , Morikawa, H. , Williams, J.T. & Palmiter, R.D. (2003) Dopamine controls the firing pattern of dopamine neurons via a network feedback mechanism. Proc Natl Acad Sci USA 100, 2866–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, S.M. , Chen, M. , Schmerberg, C.M. , Dulman, R.S. , Rodriguiz, R.M. , Caron, M.G. , Jin, J. & Wetsel, W.C. (2016) Effects of beta‐arrestin‐biased dopamine D2 receptor ligands on schizophrenia‐like behavior in hypoglutamatergic mice. Neuropsychopharmacology 41, 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, S.M. , Pack, T.F. & Caron, M.G. (2015a) Receptor, ligand and transducer contributions to dopamine D2 receptor functional selectivity. PLoS One 10, e0141637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson, S.M. , Pack, T.F. , Wilkins, A.D. , Urs, N.M. , Urban, D.J. , Bass, C.E. , Lichtarge, O. & Caron, M.G. (2015b) Elucidation of G‐protein and beta‐arrestin functional selectivity at the dopamine D2 receptor. Proc Natl Acad Sci USA 112, 7097–7102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, P.E. , Johns, J.M. , Lubin, D.A. , Budygin, E.A. , Gainetdinov, R.R. , Lieberman, J.A. & Wightman, R.M. (2003) Presynaptic dopaminergic function is largely unaltered in mesolimbic and mesostriatal terminals of adult rats that were prenatally exposed to cocaine. Brain Res 961, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picetti, R. , Saiardi, A. , Abdel Samad, T. , Bozzi, Y. , Baik, J.H. & Borrelli, E. (1997) Dopamine D2 receptors in signal transduction and behavior. Crit Rev Neurobiol 11, 121–142. [DOI] [PubMed] [Google Scholar]
- Romano, G.J. , Shivers, B.D. , Harlan, R.E. , Howells, R.D. & Pfaff, D.W. (1987) Haloperidol increases proenkephalin mRNA levels in the caudate‐putamen of the rat: a quantitative study at the cellular level using in situ hybridization. Brain Res 388, 33–41. [DOI] [PubMed] [Google Scholar]
- Salgado, H. , Tecuapetla, F. , Perez‐Rosello, T. , Perez‐Burgos, A. , Perez‐Garci, E. , Galarraga, E. & Bargas, J. (2005) A reconfiguration of CaV2 Ca2+ channel current and its dopaminergic D2 modulation in developing neostriatal neurons. J Neurophysiol 94, 3771–3787. [DOI] [PubMed] [Google Scholar]
- Self, D.W. , Barnhart, W.J. , Lehman, D.A. & Nestler, E.J. (1996) Opposite modulation of cocaine‐seeking behavior by D1‐ and D2‐like dopamine receptor agonists. Science 271, 1586–1589. [DOI] [PubMed] [Google Scholar]
- Sesack, S.R. , Aoki, C. & Pickel, V.M. (1994) Ultrastructural localization of D2 receptor‐like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci 14, 88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy, S.K. & Lefkowitz, R.J. (2011) beta‐Arrestin‐mediated receptor trafficking and signal transduction. Trends Pharmacol Sci 32, 521–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim, H.R. , Choi, T.Y. , Lee, H.J. , Kang, E.Y. , Yoon, S. , Han, P.L. , Choi, S.Y. & Baik, J.H. (2013) Role of dopamine D2 receptors in plasticity of stress‐induced addictive behaviours. Nat Commun 4, 1579. [DOI] [PubMed] [Google Scholar]
- Smith, Y. , Bevan, M.D. , Shink, E. & Bolam, J.P. (1998) Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience 86, 353–387. [DOI] [PubMed] [Google Scholar]
- Spealman, R.D. , Barrett‐Larimore, R.L. , Rowlett, J.K. , Platt, D.M. & Khroyan, T.V. (1999) Pharmacological and environmental determinants of relapse to cocaine‐seeking behavior. Pharmacol Biochem Behav 64, 327–336. [DOI] [PubMed] [Google Scholar]
- Surmeier, D.J. & Kitai, S.T. (1997) State‐dependent regulation of neuronal excitability by dopamine. Jpn J Psychopharmacol 17, 105–110. [PubMed] [Google Scholar]
- Surmeier, D.J. , Carrillo‐Reid, L. & Bargas, J. (2011) Dopaminergic modulation of striatal neurons, circuits, and assemblies. Neuroscience 198, 3–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla, F. , Koos, T. , Tepper, J.M. , Kabbani, N. & Yeckel, M.F. (2009) Differential dopaminergic modulation of neostriatal synaptic connections of striatopallidal axon collaterals. J Neurosci 29, 8977–8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper, J.M. , Koos, T. & Wilson, C.J. (2004) GABAergic microcircuits in the neostriatum. Trends Neurosci 27, 662–669. [DOI] [PubMed] [Google Scholar]
- Thanos, P.K. , Michaelides, M. , Umegaki, H. & Volkow, N.D. (2008) D2R DNA transfer into the nucleus accumbens attenuates cocaine self‐administration in rats. Synapse 62, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault, D. , Albert, P.R. , Pineyro, G. & Trudeau, L.E. (2011) Neurotensin triggers dopamine D2 receptor desensitization through a protein kinase C and beta‐arrestin1‐dependent mechanism. J Biol Chem 286, 9174–9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, G.M. & Huganir, R.L. (2004) MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci 5, 173–183. [DOI] [PubMed] [Google Scholar]
- Threlfell, S. , Lalic, T. , Platt, N.J. , Jennings, K.A. , Deisseroth, K. & Cragg, S.J. (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64. [DOI] [PubMed] [Google Scholar]
- Tritsch, N.X. & Sabatini, B.L. (2012) Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 76, 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch, N.X. , Ding, J.B. & Sabatini, B.L. (2012) Dopaminergic neurons inhibit striatal output through non‐canonical release of GABA. Nature 490, 262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello, A. , Baik, J.H. , Rouge‐Pont, F. , Picetti, R. , Dierich, A. , LeMeur, M. , Piazza, P.V. & Borrelli, E. (2000) Distinct functions of the two isoforms of dopamine D2 receptors. Nature 408, 199–203. [DOI] [PubMed] [Google Scholar]
- Verdejo‐Garcia, A. , Lawrence, A.J. & Clark, L. (2008) Impulsivity as a vulnerability marker for substance‐use disorders: review of findings from high‐risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32, 777–810. [DOI] [PubMed] [Google Scholar]
- Volkow, N.D. , Fowler, J.S. , Wolf, A.P. , Schlyer, D. , Shiue, C.Y. , Alpert, R. , Dewey, S.L. , Logan, J. , Bendriem, B. , Christman, D. et al (1990) Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 147, 719–724. [DOI] [PubMed] [Google Scholar]
- Volkow, N.D. , Fowler, J.S. , Wang, G.J. , Hitzemann, R. , Logan, J. , Schlyer, D.J. , Dewey, S.L. & Wolf, A.P. (1993) Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14, 169–177. [DOI] [PubMed] [Google Scholar]
- Volkow, N.D. , Wang, G.J. , Fowler, J.S. , Logan, J. , Hitzemann, R. , Ding, Y.S. , Pappas, N. , Shea, C. & Piscani, K. (1996) Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 20, 1594–1598. [DOI] [PubMed] [Google Scholar]
- Volkow, N.D. , Chang, L. , Wang, G.J. , Fowler, J.S. , Ding, Y.S. , Sedler, M. , Logan, J. , Franceschi, D. , Gatley, J. , Hitzemann, R. , Gifford, A. , Wong, C. & Pappas, N. (2001) Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158, 2015–2021. [DOI] [PubMed] [Google Scholar]
- Volkow, N.D. , Wang, G.J. , Telang, F. , Fowler, J.S. , Thanos, P.K. , Logan, J. , Alexoff, D. , Ding, Y.S. , Wong, C. , Ma, Y. & Pradhan, K. (2008) Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42, 1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. , Kai, L. , Day, M. , Ronesi, J. , Yin, H.H. , Ding, J. , Tkatch, T. , Lovinger, D.M. & Surmeier, D.J. (2006) Dopaminergic control of corticostriatal long‐term synaptic depression in medium spiny neurons is mediated by cholinergic interneurons. Neuron 50, 443–452. [DOI] [PubMed] [Google Scholar]
- Welter, M. , Vallone, D. , Samad, T.A. , Meziane, H. , Usiello, A. & Borrelli, E. (2007) Absence of dopamine D2 receptors unmasks an inhibitory control over the brain circuitries activated by cocaine. Proc Natl Acad Sci USA 104, 6840–6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickman, K.D. , Iniguez‐Lluhl, J.A. , Davenport, P.A. , Taussig, R. , Krapivinsky, G.B. , Linder, M.E. , Gilman, A.G. & Clapham, D.E. (1994) Recombinant G‐protein beta gamma‐subunits activate the muscarinic‐gated atrial potassium channel. Nature 368, 255–257. [DOI] [PubMed] [Google Scholar]
- Wilson, C.J. & Groves, P.M. (1980) Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol 194, 599–615. [DOI] [PubMed] [Google Scholar]
- Witten, I.B. , Lin, S.C. , Brodsky, M. , Prakash, R. , Diester, I. , Anikeeva, P. , Gradinaru, V. , Ramakrishnan, C. & Deisseroth, K. (2010) Cholinergic interneurons control local circuit activity and cocaine conditioning. Science 330, 1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, D.F. , Wagner, H.N. Jr. , Tune, L.E. , Dannals, R.F. , Pearlson, G.D. , Links, J.M. , Tamminga, C.A. , Broussolle, E.P. , Ravert, H.T. , Wilson, A.A. , Toung, J.K. , Malat, J. , Williams, J.A. , O'Tuama, L.A. , Snyder, S.H. , Kuhar, M.J. & Gjedde, A. (1986) Positron emission tomography reveals elevated D2 dopamine receptors in drug‐naive schizophrenics. Science 234, 1558–1563. [DOI] [PubMed] [Google Scholar]
- Woolverton, W.L. (1986) Effects of a D1 and a D2 dopamine antagonist on the self‐administration of cocaine and piribedil by rhesus monkeys. Pharmacol Biochem Behav 24, 531–535. [DOI] [PubMed] [Google Scholar]
- Yan, Z. , Song, W.J. & Surmeier, J. (1997) D2 dopamine receptors reduce N‐type Ca2+ currents in rat neostriatal cholinergic interneurons through a membrane‐delimited, protein‐kinase‐C‐insensitive pathway. J Neurophysiol 77, 1003–1015. [DOI] [PubMed] [Google Scholar]
- Zald, D.H. , Cowan, R.L. , Riccardi, P. , Baldwin, R.M. , Ansari, M.S. , Li, R. , Shelby, E.S. , Smith, C.E. , McHugo, M. & Kessler, R.M. (2008) Midbrain dopamine receptor availability is inversely associated with novelty‐seeking traits in humans. J Neurosci 28, 14372–14378. [DOI] [PMC free article] [PubMed] [Google Scholar]


