Abstract
Convection-enhanced delivery (CED) circumvents the blood–brain barrier by delivering agents directly into the tumor and surrounding parenchyma. CED can achieve large volumes of distribution by continuous positive-pressure infusion. Although promising as an effective drug delivery method in concept, the administration of therapeutic agents via CED is not without challenges. Limitations of distribution remain a problem in large brains, such as those of humans. Accurate and consistent delivery of an agent is another challenge associated with CED. Similar to the difficulties caused by immunosuppressive environments associated with gliomas, there are several mechanisms that make effective local drug distribution difficult in malignant gliomas. In this review, methods for local drug application targeting gliomas are discussed with special emphasis on CED. Although early clinical trials have failed to demonstrate the efficacy of CED against gliomas, CED potentially can be a platform for translating the molecular understanding of glioblastomas achieved in the laboratory into effective clinical treatments. Several clinical studies using CED of chemotherapeutic agents are ongoing. Successful delivery of effective agents should prove the efficacy of CED in the near future.
Keywords: drug delivery , convection-enhanced delivery , blood–brain barrier , malignant glioma
Introduction
The success of molecular-targeted therapies for several cancers, including non small-cell lung cancer,1) melanoma,2) and chronic myelogenous leukemia,3) has opened a new era in cancer treatment. This therapeutic strategy holds significant promise for the treatment of malignant gliomas. Numerous molecular-targeted agents have been tested over the past decade, mostly with unsuccessful results. Only one agent, bevacizumab, a monoclonal antibody against vascular endothelial growth factor (VEGF), has been granted approval (2009) by the US Food and Drug Administration (FDA) for treating recurrent glioblastoma.4–6) Other agents, although successful against other cancers, have failed to demonstrate efficacy against malignant gliomas. Drug delivery has been proposed as a possible explanation for this failure. Although the blood–brain barrier (BBB) at the core of brain tumors may be disrupted, heterogeneous incomplete disruption still prevents effective drug delivery. Local drug delivery can overcome this obstacle; however, effective local drug delivery remains a challenge.
Convection-enhanced delivery (CED) circumvents the BBB by delivering agents directly into the tumor and surrounding parenchyma by continuous positive-pressure infusion.7) CED can achieve large volumes of distribution because the diffusive spread is not limited by concentration gradients.8) Importantly, CED provides direct access to the tumor bed, thus resulting in high local concentrations of the drug with minimal systemic absorption.9) Currently, CED has been clinically tested in the treatment of neurodegenerative diseases, such as Parkinson’s disease10,11) and neuro-oncology.12,13) The administration of therapeutic agents via CED is not without its challenges, such as the accurate and consistent delivery of the agent. In this review, methods for local drug application against gliomas are discussed, with special emphasis on CED.
Local Delivery of Therapeutics against Gliomas
The only FDA-approved strategy to deliver chemotherapeutic agents locally to a brain tumor site is Gliadel®, a carmustine wafer that releases bis-chloroethylnitrosourea (BCNU) gradually after its placement in the resection cavity. Brem et al.14) published a report on 222 patients in a phase III randomized, multicenter, placebo-controlled trial evaluating the safety and efficacy of Gliadel® in patients with recurrent glioblastoma. In the report, they demonstrated the efficacy of Gliadel® wafers against recurrent high-grade gliomas. Two-phase III, randomized, double-blind, placebo-controlled clinical trials in patients with newly diagnosed high-grade gliomas have been conducted subsequently. Both trials showed greater survival for patients treated with Gliadel® implants.15) The second trial, which included 240 patients,16,17) showed an increase in the median survival rate from 11.6 months for the placebo group to 13.9 months for the Gliadel® group. The results of these studies have provided strong evidence on the efficacy of local chemotherapy against this devastating disease. However, the use of Gliadel® has been compromised for several reasons. First, the efficacy proven by the previous studies was only against radiation therapy. No chemotherapy was provided for patients in the control group. The efficacy of Gliadel® used in combination with the Stupp regimen18) has yet to be proved. Another limitation involves tissue penetration. The penetration of BCNU after placement of carmustine wafers has been shown to be limited to a maximum of 6 mm from the site of the wafers.19)
Intrathecal infusion is another often used method that bypasses the BBB. Intrathecal infusion of methotrexate is often used against lymphomas or meningeal carcinomatosis or some pediatric malignancies. Although this treatment sometimes is effective against these diseases, efficacy against gliomas has not been proven so far.
Recently, the results of a randomized, open-label, phase III trial for adenovirus-mediated gene therapy using sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT) were reported. These results showed that intraoperative perilesional injection of sitimagene ceradenovec (1 × 1012 viral particles) followed by intravenous injection of ganciclovir after resection increased time to death or re-intervention in patients with newly diagnosed supratentorial glioblastoma although the intervention did not improve overall survival.20)
Promise of CED against Gliomas
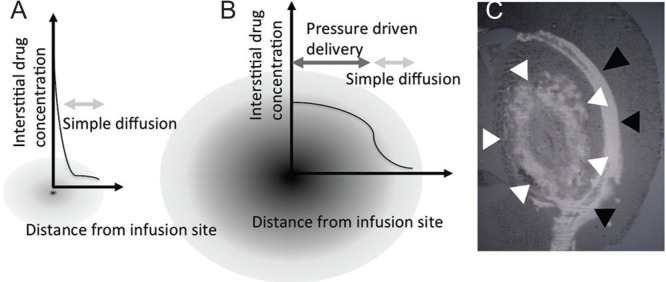
Patients with malignant gliomas often experience local recurrences. Therefore, strategies that enable effective local drug delivery are an important field of research. In the previous report, we reported on the patient whose brain stem recurrent glioblastoma once disappeared with CED of nimustine hydrochloride. In this patient, the treatment successfully eliminated the enhanced tumor without damaging brainstem.21) Principally, CED is an approach to deliver macromolecules by imposing a convective flow field in the extra-cellular space.7) Pressure gradients maintained at the infusion catheter tip generate this convective flow. In the homogenous tissue, the distribution by CED will be controlled by this pressure gradient. After the positive pressure infusion, infused molecules then spread diffusively with the simple diffusion.22) Therefore, it is demonstrated that CED can produce a plateau of drug concentration at the vicinity of infusion site without making a peak concentration at the infusion site (Fig. 1). For this reason, local toxicity of drugs effectively delivered by CED is usually defined by the concentration of the drugs rather than the dose.23) These mechanisms together may work for successful elimination of the tumor without severely damaging the local brain tissue. One more characteristics that make CED promising is that the pressure driven distribution usually extends into the perivascular or peri-fiber areas which is the same path as glioma invasion.24) Therefore, it may be effective even against invading tumor cells.
Fig. 1.
Schematic drawings of interstitial drug concentration after simple injection or drug disposition (A) and after convection-enhanced delivery (B). Image of brain slice of rodent after convection-enhanced delivery of liposomal doxorubicin (C). Distribution of doxorubicin within stratum (white arrowhead) is observed along leakage along the nerve fiber tract (black arrowhead).
Obstacles for Effective Local Drug Delivery
Local drug delivery to normal brain tissue is itself a challenge. Pathological conditions make this even more challenging (Table 1). Although the efficacy of CED has been demonstrated in a rodent brain tumor model,25) there are many more obstacles in human settings. The heterogeneity of the tumor tissue makes consistent drug distribution difficult. The most common and malignant glioma is glioblastoma, which was once called glioblastoma multiforme because of its heterogeneity. Human glioblastomas are characterized by pseudopalisading necrosis in a garland-like arrangement of hypercellular tumor nuclei lining up around tumor necrosis containing pyknotic nuclei. Further features include hemorrhage and multinuclear giant cells. Malignant tumors can erode the surrounding normal tissue, and the more erosive types of cancer have more destructive actions.26) If these cancer clusters erode adjacent normal or tumor vessels, microscopic hemorrhage may occur at any place or time within or adjacent to cancer tissues, and fibrin clots immediately form in situ to stop the bleeding. The fibrin clots are subsequently replaced by collagenous stroma in a process similar to that in normal wound healing and other nonmalignant diseases. The fibrin clot formation in cancer lasts for as long as the cancer cells survive in the body and occurs silently. Therefore, this is called a “malignant cycle of blood coagulation.”27) All these processes contribute to heterogeneous tissue rearrangement.
Table 1.
Summary of obstacles against effective local drug delivery in malignant gliomas
|
Increased tumor vascular permeability is another important obstacle that prevents consistent distribution of drugs after local delivery. In the neoplastic tissue, increased production of kinin, the generation of which is triggered by activated Hageman factor (also known as intrinsic coagulation factor XII), and VEGF improves tumor vascular permeability.27,28) Recently, an extrinsic coagulation factor known as tissue factor has been shown to apparently activate VEGF production.29) Hence, both intrinsic and extrinsic coagulation factors may be involved in tumor vascular permeability and tumor-induced blood coagulation. It is known that this increased vascular permeability affects both the interstitial distribution of drugs delivered systemically and the clearance of drugs disposed of into tumor parenchyma.
The biophysical microenvironment of the tumor can also be an obstacle.30) Biophysical forces, including stromal stiffening,31) interstitial pressure,32) and fluid flow,33) have a number of effects on the drug distribution and ability of therapy to induce a desired response in cancer cells. It is known that the tumor microenvironment becomes mechanically stiff because of accumulation and reorganization of extracellular matrix proteins and activation of stromal fibroblasts.31) In addition, because the tissue in which a cancer develops is a confined space with limited inlets and outlets for cells, fluids, and wastes, the cancer growth and by-products push against the surrounding environment until the cancer eventually invades the tissue.30) This process results in a buildup of pressure from the inside out and can lead to a vast pressure differential between the tumor and the tissue in which it resides. This increased interstitial pressure, together with a heterogeneous pressure gradient in cancer, leads to inconsistent drug distribution in the setting of local intraparenchymal drug delivery. In addition, as required by the principle, it is necessary to keep positive pressure at the catheter tip for effective delivery. Reflux through the catheter insertion tract compromises the distribution because it results in loss of pressure at the catheter tip. Increased interstitial pressure may affect the reflux. It is known that a catheter with a smaller outer diameter rather than a larger diameter is more resistant to reflux. Step-design catheters are used to halt the reflux.34)
Effective Local Drug Delivery
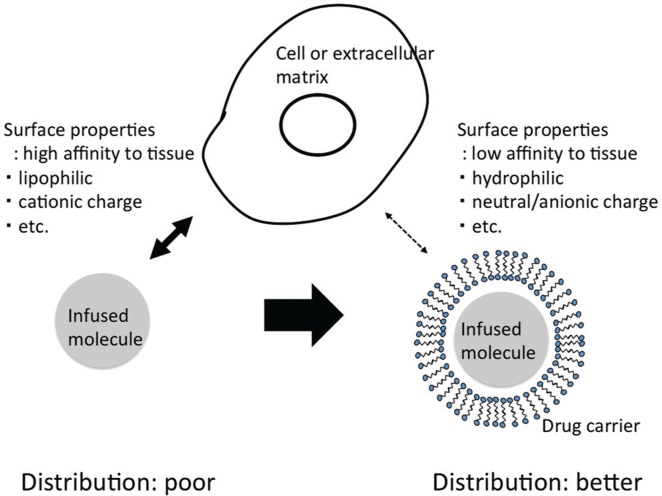
Distribution volume is a key property that affects the antitumor efficacy of therapeutic agents delivered by CED. However, locally applied agents with different physical and chemical properties have demonstrated different distribution volumes during CED into rodent brains.35) The infusates will not distribute if the molecules have high affinity to the tissues. This often happens in case that the molecules have a cationic charge or the molecules are lipophilic. Therefore, chemotherapeutic agents that are tested with CED should be formulated specifically to achieve the required distribution. The distribution characteristics of individual agents delivered by CED are difficult to evaluate, but we previously demonstrated the effectiveness of some drug carriers, such as liposomes and micelles.36,37) Doxorubicin delivered in a liposome or micelle formulation was shown to be effective in a rodent brain tumor model. Use of drug carriers reduced the possibility of inconsistent drug distribution caused by the physical properties of the infusates. Here, the drug carriers can compensate the surface properties of delivered molecules and contribute to more robust and consistent distribution (Fig. 2). Obviously, in addition, it is necessary to overcome obstacles created inside the tumor microenvironment. One possible approach is to add a therapeutic that reduces the interstitial pressure. We have demonstrated that the use of steroids resulted in better distribution after CED.38) A recently introduced potent anti-edema agent, bevacizumab, may also contribute from this point of view.39) Another possibility that we and others have considered is the use of ultrasound (US). Application of US has been shown to facilitate drug distribution in various drug distribution strategies.40) This property has also been demonstrated in local brain delivery.41) We have reported on the development of a US-facilitated drug delivery system that demonstrated consistent and more robust distribution of infusate when US was applied at the site of drug infusion.42)
Fig. 2.
The infusates will not distribute if the molecules have high affinity to tissues. Use of the drug carriers can compensate the surface properties of delivered molecules and contribute to more robust and consistent distribution.
Together with the above-mentioned strategies for overcoming obstacles to local drug delivery, visualization of the drug distribution is another important consideration. It is obvious that visualization of an infusion can improve distribution and safety. Strategies for real-time monitoring of drug distribution have been developed.43–45) Use of magnetic resonance imaging contrast agents may be a problem in clinical settings because no contrast-enhancement agents are currently approved for intraparenchymal delivery although safety has been demonstrated in several clinical studies.21) T2-weighted imaging46) or diffusion-weighted imaging47) methods for monitoring drug delivery via CED have also been reported and should be considered when applying CED to clinics.
Effective Delivery and Effective Tumor Killing
Assuming that we have achieved sufficiently robust distribution and covered the entire targeted area, is it possible to cure gliomas? This is another important question that must be answered. There are few drugs that are effective against gliomas. The only chemotherapeutic agents that have demonstrated efficacy in randomized phase III studies are certain alkylating agents, such as BCNU, 1-(2-chloroethyl)-3-cyclohexyl-1-nitrosourea (CCNU), Gliadel®, and temozolomide. Even the efficacy of bevacizumab, which is often used in clinics, has yet to be proven in a randomized phase III trial. Many molecular-targeted agents, which are quite effective against other cancers, have failed to show their efficacy against gliomas. Of course, the unsatisfactory results of molecular-targeted agents may be a consequence of poor drug distribution, but cellular-resistant mechanisms of gliomas against several agents have been elucidated. It is known that even if pathways of apoptosis are triggered in glioma cells, they may survive.48) From this point of view, trials using chemotherapeutic agents remain promising although they sometimes induce nonspecific toxicity.
Clinical Development
CED clinical trials have been conducted with various agents. As summarized in the review by Healy AT,49) these agents include conventional chemotherapies, cytotoxin-ligand conjugates targeting cell surface receptors, monoclonal antibodies with or without radioactive isotope conjugates, antisense oligonucleotides, and liposomal vectors engineered to deliver gene therapy (Table 2).50–66) To date, two-phase III trials have been initiated in patients with brain tumors. One trial used TransMID (Tf-CRM107), which is a fusion toxin conjugating transferrin with mutant diphtheria toxin, and the others used cintredekin besudotox, which is also a fusion toxin that conjugates recombinant human IL-13 with mutant pseudomonas exotoxin. Both failed to show statistically significant survival benefits, but the latter demonstrated a significant improvement in progression-free survival although this was not a prespecified analysis. Progression-free survival of recurrent glioblastoma patients who received CED of cintredekin besudotox has been shown to be significantly longer than that of patients who received Gliadel® intracavitary chemotherapy wafers (17.7 vs. 11.4 weeks; p = 0.0008).50,51) The overall survival periods for the cintredekin besudotox group and Gliadel® wafers group were 45.3 vs. 39.8 weeks (p = 0.476), respectively. Other drugs are in Phase I/II studies awaiting further development.
Table 2.
CED clinical trials against gliomas
| Molecules delivered with CED | Description | Current status | Reference |
|---|---|---|---|
| Cintredekin besudotox | conjugate of human IL-13 and truncated Pseudomonas exotoxin | Phase III (PRECISE) failed to show survival benefit against first recurrent GBM / Phase I well tolerated with RT+TMZ against newly diagnosed MG | 12, 50, 51, 52 |
| Tf-CRM107 (TransMID) | conjugate of transferrin and a point mutant of diphtheria toxin | Promising data with Phase II, but low efficacy in Phase III | 53, 54 |
| TP-38 | conjugate of transforming growth factor-alpha and Pseudomonas exotoxin | Phase II demonstrated survival benefit against recurrent or progressive MG or metastasis | 55 |
| NBI-3001 (PRX-321) | conjugate of IL-4 and pseudomonas exotoxin | Phase II demonstrated survival benefit against recurrent MG | 56, 57 |
| Cotara | 131I-labeled chimeric monoclonal antibody | Safety and feasibility demonstrated against 51 MG cases (Phase I/II) | 58 |
| Murine mAB 425 | antagonistic mAb against EGFR | Planned schedule could not be completed owing to inflammatory reactions (Phase I) against recurrent or inoperable MG | 59 |
| Paclitaxel | chemotherapeutic agent | Phase I/II study against recurrent MG reported high response rate but associated with a significant incidence of treatment associated complications | 13, 60 |
| Topotecan | chemotherapeutic agent | Phase Ib study against recurrent malignant glioma demonstrated safety and favorable PFS and OS of 23 and 60 weeks. Study against pediatric DIPG was tested in two cases. | 61, 62 |
| Nimustine hydrochloride | chemotherapeutic agent | Tumor regression was reported in a case with pediatric pontine GBM. Phase I, on going. | 21 |
| Carboplatin | chemotherapeutic agent | Phase I on going against recurrent or progressive GBM | 63 |
| Trabedersen | antisense oligonucleotide for transforming growth factor-beta | Phase IIb against recurrent or refractory MG demonstrated efficacy and safety. | 64 |
| CpG-28 | Immunostimulating oligodeoxynucleotides containing CpG motifs (CpG-ODN) | Phase II against recurrent GBM demonstrated modest activity on the 6-month PFS | 65 |
| LIPO-HSV-1-tk | HSV-1-tk in cationic liposomes | Phase I/II against recurrent GBM demonstrated safety and efficacy | 66 |
DIPG: diffuse intrinsic pontine glioma, EGFR: epidermal growth factor receptor, GBM: Glioblastoma, HSV: herpes simplex virus, IL: Interleukin, mAB: monoclonal antibody, MG: malignant glioma, OS: overall survival, PFS: progression free survival, RT: radiation therapy, TMZ: Temozolomide.
Promise for Local Drug Delivery in the Immunotherapy Era
Recently, tremendous progress has been achieved in the field of immunotherapy against cancers. Anti-(PD1) and (PDL1) treatments have demonstrated surprising antitumor efficacy in several types of cancers. Additionally, in the settings of malignant gliomas, immunosuppressive checkpoints mediated by indoleamine-pyrrole 2,3-dioxygenase (IDO), cytotoxic T lymphocyte antigen 4 (CTLA4), and PD1/PDL1 are known to have critical roles in glioma progression and in the efficacy of immunotherapies.67) Combined blockade of these immunosuppressive checkpoints in a glioma model has elicited long-term survival, and clinical studies are planned.67,68) Use of these treatment strategies may begin a new era in the treatment of gliomas. Local drug delivery in this context may become a promising approach to combination therapy because local modifications of cancer microenvironments may yield tumor antigens locally and disrupt the immunosuppressive environment maintained by tumors.69)
Conclusions
CED can potentially be a platform for translating molecular understanding of glioblastoma achieved in the laboratory into effective clinical treatments. Although the strategy has not yet been very successful in clinics, continuous efforts on loop-style development, translation from bedside to clinics, and feedback from clinics to bedside should eventually create success in this field and may provide a cure against this formidable disease.
Acknowledgement
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan (#26293319 to R.S.) and Translational Research Network Program from Japan Agency for Medical Research and Development (AMED).
Footnotes
Conflicts of Interest Disclosure
The authors declare that they have no competing interests.
References
- 1). Smith J. Erlotinib: small-molecule targeted therapy in the treatment of non-small-cell lung cancer. Clin Ther 27: 1513– 1534, 2005. [DOI] [PubMed] [Google Scholar]
- 2). Ribas A, Hersey P, Middleton MR, Gogas H, Flaherty KT, Sondak VK, Kirkwood JM: New challenges in endpoints for drug development in advanced melanoma. Clin Cancer Res 18: 336– 341, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Habeck M: FDA licences imatinib mesylate for CML. Lancet Oncol 3: 6, 2002. [DOI] [PubMed] [Google Scholar]
- 4). Cohen MH, Shen YL, Keegan P, Pazdur R: FDA drug approval summary: bevacizumab (Avastin) as treatment of recurrent glioblastoma multiforme. Oncologist 14: 1131– 1138, 2009. [DOI] [PubMed] [Google Scholar]
- 5). Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA: Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 27: 740– 745, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T: Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27: 4733– 4740, 2009. [DOI] [PubMed] [Google Scholar]
- 7). Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH: Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A 91: 2076– 2080, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH: Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg 90: 315– 320, 1999. [DOI] [PubMed] [Google Scholar]
- 9). Yun J, Rothrock RJ, Canoll P, Bruce JN: Convection-enhanced delivery for targeted delivery of antiglioma agents: the translational experience. J Drug Deliv 2013: 107573, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Eberling JL, Jagust WJ, Christine CW, Starr P, Larson P, Bankiewicz KS, Aminoff MJ: Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology 70: 1980– 1983, 2008. [DOI] [PubMed] [Google Scholar]
- 11). Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P: Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9: 589– 595, 2003. [DOI] [PubMed] [Google Scholar]
- 12). Kunwar S: Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl 88: 105– 111, 2003. [DOI] [PubMed] [Google Scholar]
- 13). Mardor Y, Roth Y, Lidar Z, Jonas T, Pfeffer R, Maier SE, Faibel M, Nass D, Hadani M, Orenstein A, Cohen JS, Ram Z: Monitoring response to convection-enhanced taxol delivery in brain tumor patients using diffusion-weighted magnetic resonance imaging. Cancer Res 61: 4971– 4973, 2001. [PubMed] [Google Scholar]
- 14). Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, Morawetz R, Schold SC: Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet 345: 1008– 1012, 1995. [DOI] [PubMed] [Google Scholar]
- 15). Valtonen S, Timonen U, Toivanen P, Kalimo H, Kivipelto L, Heiskanen O, Unsgaard G, Kuurne T: Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery 41: 44– 49, 1997. [DOI] [PubMed] [Google Scholar]
- 16). Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, Whittle IR, Jääskeläinen J, Ram Z: A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro-oncology 5: 79– 88, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Westphal M, Ram Z, Riddle V, Hilt D, Bortey E, Executive Committee of the Gliadel Study Group : Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 148: 269– 275, 2006. [DOI] [PubMed] [Google Scholar]
- 18). Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. National Cancer Institute of Canada Clinical Trials Group : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352: 987– 96, 2005. [DOI] [PubMed] [Google Scholar]
- 19). Fung LK, Ewend MG, Sills A, Sipos EP, Thompson R, Watts M, Colvin OM, Brem H, Saltzman WM: Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res 58: 672– 684, 1998. [PubMed] [Google Scholar]
- 20). Westphal M, Ylä-Herttuala S, Martin J, Warnke P, Menei P, Eckland D, Kinley J, Kay R, Ram Z, ASPECT Study Group : Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol 14: 823– 833, 2013. [DOI] [PubMed] [Google Scholar]
- 21). Saito R, Sonoda Y, Kumabe T, Nagamatsu K, Watanabe M, Tominaga T: Regression of recurrent glioblastoma infiltrating the brainstem after convection-enhanced delivery of nimustine hydrochloride. J Neurosurg Pediatr 7: 522– 526, 2011. [DOI] [PubMed] [Google Scholar]
- 22). Raghavan R, Brady ML, Rodríguez-Ponce MI, Hartlep A, Pedain C, Sampson JH. Convection-enhanced delivery of therapeutics for brain disease, and its optimization. Neurosurg Focus 20: E12, 2006. [DOI] [PubMed] [Google Scholar]
- 23). Zhang R, Saito R, Mano Y, Kanamori M, Sonoda Y, Kumabe T, Tominaga T. Concentration rather than dose defines the local brain toxicity of agents that are effectively distributed by convection-enhanced delivery. J Neurosci Methods 222: 131– 137, 2014. [DOI] [PubMed] [Google Scholar]
- 24). Krauze MT, Saito R, Noble C, Bringas J, Forsayeth J, McKnight TR, Park J, Bankiewicz KS: Effects of the perivascular space on convection-enhanced delivery of liposomes in primate putamen. Exp Neurol 196: 104– 111, 2005. [DOI] [PubMed] [Google Scholar]
- 25). Saito R, Bringas JR, Panner A, Tamas M, Pieper RO, Berger MS, Bankiewicz KS: Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res 64: 6858– 6862, 2004. [DOI] [PubMed] [Google Scholar]
- 26). Matsumura Y: The drug discovery by nanomedicine and its clinical experience. Jpn J Clin Oncol 44: 515– 525, 2014. [DOI] [PubMed] [Google Scholar]
- 27). Matsumura Y, Kimura M, Yamamoto T, Maeda H: Involvement of the kinin-generating cascade in enhanced vascular permeability in tumor tissue. Jpn J Cancer Res 79: 1327– 1334, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Senger DR, Galli SJ, Dvorak AM, Peruzzi CA, Harvey VS, Dvorak HF: Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 21: 983– 985, 1983. [DOI] [PubMed] [Google Scholar]
- 29). Dvorak HF, Rickles FR: Malignancy and hemostasis. in Colman RW, Marder VJ, Clowes AW, George JN, Goldharber SZ. (eds): Hemostasis and Thrombosis: Basic principles and Clinical Practice. 5th edn Philadelphia: LippinCottWilliams & Wilkins, 2006, pp. 851– 873 [Google Scholar]
- 30). Munson JM, Shieh AC: Interstital fluid flow in cancer: implications for disease progression and treatment. Cancer Manag Res 6: 317– 328, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). Butcher DT, Alliston T, Weaver VM: A tense situation: forcing tumour progression. Nat Rev Cancer 9: 108– 122, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32). Heldin CH, Rubin K, Pietras K, Ostman A: High interstitial fluid pressure – an obstacle in cancer therapy. Nat Rev Cancer 4: 806– 813, 2004. [DOI] [PubMed] [Google Scholar]
- 33). Teo CS, Hor Keong Tan W, Lee T, Wang CH: Transient interstitial fluid flow in brain tumors: effect on drug delivery. Chem Eng Sci 60: 4803– 4821, 2005. [Google Scholar]
- 34). Krauze MT, Saito R, Noble C, Tamas M, Bringas J, Park JW, Berger MS, Bankiewicz K: Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg 103: 923– 929, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Saito R, Krauze MT, Noble CO, Tamas M, Drummond DC, Kirpotin DB, Berger MS, Park JW, Bankiewicz KS: Tissue affinity of the infusate affects the distribution volume during convection-enhanced delivery into rodent brains: implications for local drug delivery. J Neurosci Methods 154: 225– 232, 2006. [DOI] [PubMed] [Google Scholar]
- 36). Kikuchi T, Saito R, Sugiyama S, Yamashita Y, Kumabe T, Krauze M, Bankiewicz K, Tominaga T: Convection-enhanced delivery of polyethylene glycol-coated liposomal doxorubicin: characterization and efficacy in rat intracranial glioma models. J Neurosurg 109: 867– 873, 2008. [DOI] [PubMed] [Google Scholar]
- 37). Inoue T, Yamashita Y, Nishihara M, Sugiyama S, Sonoda Y, Kumabe T, Yokoyama M, Tominaga M: Therapeutic efficacy of a polymeric micellar doxorubicin infused by convection-enhanced delivery against intracranial 9L brain tumor models. Neuro-oncology 11: 151– 157, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Yang X, Saito R, Nakamura T, Zhang R, Sonoda Y, Kumabe T, Forsayeth J, Bankiewicz K, Tominaga T: Peri-tumoral leakage during intra-tumoral convection-enhanced delivery has implications for efficacy of peri-tumoral infusion before removal of tumor. Drug Deliv 23: 781– 786, 2016. [DOI] [PubMed] [Google Scholar]
- 39). Ferrara N, Hillan KJ, Gerber HP, Novotny W: Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov 3: 391– 400, 2004. [DOI] [PubMed] [Google Scholar]
- 40). Mitragotri S: Healing sound: the use of ultrasound in drug delivery and other therapeutic applications. Nat Rev Drug Discov 4: 255– 260, 2005. [DOI] [PubMed] [Google Scholar]
- 41). Liu Y, Paliwal S, Bankiewicz KS, Bringas JR, Heart G, Mitragotri S, Prausnitz MR: Ultrasound-enhanced drug transport and distribution in the brain. AAPS PharmSciTech 11: 1005– 1017, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Mano Y, Saito R, Haga Y, Matsunaga T, Zhang R, Chonan M, Haryu S, Shoji T, Sato A, Sonoda Y, Tsuruoka N, Nishiyachi K, Sumiyoshi A, Nonaka H, Kawashima R, Tominaga T: Intraparenchymal ultrasound application and improved distribution of infusate with convection-enhanced delivery in rodent and nonhuman primate brain. J Neurosurg 124: 1490– 500 [DOI] [PubMed] [Google Scholar]
- 43). Lonser RR, Walbridge S, Garmestani K, Butman JA, Walters HA, Vortmeyer AO, Morrison PF, Brechbiel MW, Oldfield EH: Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg 97: 905– 913, 2002. [DOI] [PubMed] [Google Scholar]
- 44). Saito R, Bringas JR, McKnight TR, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Park JW, Berger MS, Bankiewicz KS: Distribution of liposomes into brain and rat brain tumor models by convection-enhanced delivery monitored with magnetic resonance imaging. Cancer Res 64: 2572– 2579, 2004. [DOI] [PubMed] [Google Scholar]
- 45). Saito R, Krauze MT, Bringas JR, Noble C, McKnight TR, Jackson P, Wendland MF, Mamot C, Drummond DC, Kirpotin DB, Hong K, Berger MS, Park JW, Bankiewicz KS: Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol 196: 381– 389, 2005. [DOI] [PubMed] [Google Scholar]
- 46). Richardson RM, Gimenez F, Salegio EA, Su X, Bringas J, Berger MS, Bankiewicz KS: T2 imaging in monitoring of intraparenchymal real-time convection-enhanced delivery. Neurosurgery 69: 154– 163, 2011. [DOI] [PubMed] [Google Scholar]
- 47). Mardor Y, Rahav O, Zauberman Y, Lidar Z, Ocherashvilli A, Daniels D, Roth Y, Maier SE, Orenstein A, Ram Z: Convection-enhanced drug delivery: increased efficacy and magnetic resonance image monitoring. Cancer Res 65: 6858– 6863, 2005. [DOI] [PubMed] [Google Scholar]
- 48). Saito R, Mizuno M, Hatano M, Kumabe T, Yoshimoto T, Yoshida J: Two different mechanisms of apoptosis resistance observed in interferon-beta induced apoptosis of human glioma cells. J Neurooncol 67: 273– 280, 2004. [DOI] [PubMed] [Google Scholar]
- 49). Healy AT, Vogelbaum MA: Convection-enhanced drug delivery for gliomas. Surg Neurol Int 6: S59– S67, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50). Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, Shaffrey M, Ram Z, Piepmeier J, Prados M, Croteau D, Pedain C, Leland P, Husain SR, Joshi BH, Puri RK, PRECISE Study Group : Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro-oncology 12: 871– 881, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51). Mueller S, Polley MY, Lee B, Kunwar S, Pedain C, Wembacher-Schröder E, Mittermeyer S, Westphal M, Sampson JH, Vogelbaum MA, Croteau D, Chang SM: Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J Neurooncol 101: 267– 277, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52). Kunwar S, Prados MD, Chang SM, Berger MS, Lang FF, Piepmeier JM, Sampson JH, Ram Z, Gutin PH, Gibbons RD, Aldape KD, Croteau DJ, Sherman JW, Puri RK, Cintredekin Besudotox Intraparenchymal Study Group. : Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 25: 837– 844, 2007. [DOI] [PubMed] [Google Scholar]
- 53). Laske DW, Youle RJ, Oldfield EH: Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med 3: 1362– 1368, 1997. [DOI] [PubMed] [Google Scholar]
- 54). Weaver M, Laske DW: Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol 65: 3– 13, 2003. [DOI] [PubMed] [Google Scholar]
- 55). Sampson JH, Akabani G, Archer GE, Berger MS, Coleman RE, Friedman AH, Friedman HS, Greer K, Herndon JE, Kunwar S, McLendon RE, Paolino A, Petry NA, Provenzale JM, Reardon DA, Wong TZ, Zalutsky MR, Pastan I, Bigner DD: Intracerebral infusion of an EGFR-targeted toxin in recurrent malignant brain tumors. Neuro-oncology 10: 320– 329, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56). Weber F, Asher A, Bucholz R, Berger M, Prados M, Chang S, Bruce J, Hall W, Rainov NG, Westphal M, Warnick RE, Rand RW, Floeth F, Rommel F, Pan H, Hingorani VN, Puri RK: Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol 64: 125– 137, 2003. [DOI] [PubMed] [Google Scholar]
- 57). Weber FW, Floeth F, Asher A, Bucholz R, Berger M, Prados M, Chang S, Bruce J, Hall W, Rainov NG, Westphal M, Warnick RE, Rand RW, Rommell F, Pan H, Hingorani VN, Puri RK: Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir Suppl 88: S93– 103, 2003. [DOI] [PubMed] [Google Scholar]
- 58). Patel SJ, Shapiro WR, Laske DW, Jensen RL, Asher AL, Wessels BW, Carpenter SP, Shan JS: Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery 56: 1243– 1252, 2005. [DOI] [PubMed] [Google Scholar]
- 59). Wersäll P, Ohlsson I, Biberfeld P, Collins VP, von Krusenstjerna S, Larsson S, Mellstedt H, Boethius J: Intratumoral infusion of the monoclonal antibody, mAb 425, against the epidermal-growth-factor receptor in patients with advanced malignant glioma. Cancer Immunol Immunther 44: 157– 164, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60). Lidar Z, Mardor Y, Jonas T, Pfeffer R, Faibel M, Nass D, Hadani M, Ram Z: Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg 100: 472– 479, 2004. [DOI] [PubMed] [Google Scholar]
- 61). Anderson RC, Kennedy B, Yanes CL, Garvin J, Needle M, Canoll P, Feldstein NA, Bruce JN: Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J Neurosurg Pediatr 11: 289– 295, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62). Bruce JN, Fine RL, Canoll P, Yun J, Kennedy BC, Rosenfeld SS, Sands SA, Surapaneni K, Lai R, Yanes CL, Bagiella E, DeLaPaz RL: Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery 69: 1272– 1279, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63). White E, Bienemann A, Taylor H, Hopkins K, Cameron A, Gill S: A phase I trial of carboplatin administered by convection-enhanced delivery to patients with recurrent/progressive glioblastoma multiforme. Contemp Clin Trials 33: 320– 331, 2012. [DOI] [PubMed] [Google Scholar]
- 64). Bogdahn U, Hau P, Stockhammer G, Venkataramana NK, Mahanatra AK, Suri A, Balasubramaniam A, Nair S, Oliushine V, Parfenov V, Poverennova I, Zaaroor M, Jachimczak P, Ludwig S, Schmaus S, Heinrichs H, Schlingensiepen KH, Trabedersen Glioma Study Group : Targeted therapy for high-grade glioma with the TGF-β2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro-oncology 13: 132– 142, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65). Carpentier A, Metellus P, Ursu R, Zohar S, Lafitte F, Barrié M, Meng Y, Richard M, Parizot C, Laigle-Donadey F, Gorochov G, Psimaras D, Sanson M, Tibi A, Chinot O, Carpentier AF: Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro-oncology 12: 401– 408, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66). Voges J, Reszka R, Gossmann A, Dittmar C, Richter R, Garlip G, Kracht L, Coenen HH, Sturm V, Wienhard K, Heiss WD, Jacobs AH: Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol 54: 479– 487, 2003. [DOI] [PubMed] [Google Scholar]
- 67). Castro MG, Baker GJ, Lowenstein PR. Blocking immunosuppressive checkpoints for glioma therapy: the more the Merrier! Clin Cancer Res 20: 5147– 5149, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68). Calinescu AA, Kamran N, Baker G, Mineharu Y, Lowenstein PR, Castro MG. Overview of current immunotherapeutic strategies for glioma. Immunotherapy 7: 1073– 1104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69). Candolfi M, Kroeger KM, Muhammad AK, Yagiz K, Farrokhi C, Pechnick RN, Lowenstein PR, Castro MG: Gene therapy for brain cancer: combination therapies provide enhanced efficacy and safety. Curr Gene Ther 9: 409– 421, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]