Abstract
Meningiomas are the most common primary intracranial tumors. Since the adhesions in the plane of dissection are of interest in surgical planning, we suggest that digital image subtraction of FLAIR data from the T2 sequence of MRI may represent better the CSF spaces in the brain–tumor interface and may be a predictor of the intraoperative cleavage plane. From 2006 to 2016, 83 convexity meningiomas were resected in the Department of Neurosurgery of the University Hospital Complex of Vigo, an analysis of preoperative MRI was performed to assess peritumoral edema (PTE), tumor volume, among others; a digital subtraction of T2-FLAIR sequences was performed and analyzed in relationship to the cleavage plane described in the intraoperative report and postoperative neurological deficits. Simpson grade 1 resection was achieved in 85.54%, the overall 5-year PFS was 93.75%. Our rate of permanent new neurological deficit was 4.82% and the overall complication rate of 14.46%. The grade of PTE was proportional to tumor volume, 20 ± 2.8, 30 ± 5.3, and 34 ± 4.3 cm3 for grades 1, 2, and 3, respectively, positive cleft sign on image subtraction was predictive of good intraoperative cleavage plane and low grade cleavage plane (P = 0.04), and was a protective factor for postoperative neurological deficit (P = 0.02). Positive cleft sign in T2-FLAIR digital subtraction image is an independent predictor of good intraoperative cleavage plane, PTE is an independent predictor of the bad cleavage plane. Negative cleft sign in the image subtraction and a bad intraoperative cleavage plane are predictors of postoperative neurological deficit.
Keywords: meningioma, brain-tumor interface, peritumoral edema, cleavage plane, image subtraction
Introduction
Meningiomas are the most common primary intracranial tumors, representing between 35 and 40% of symptomatic brain tumors in adults.1) According to the 2016 WHO classification of tumors of the central nervous system, grade I tumors are of slow growth and benign histological features, on the other hand, Grades II and III have more aggressive behavior and tumor recurrences.2)
Magnetic resonance imaging (MRI) is the study of choice in the evaluation of intracranial neoplasms, both for diagnosis, surgical planning and follow-up. The extent of resection and histological grade are the most important factors related to progression-free survival (PFS) after meningioma surgery.3) The brain MRI features suggested as a predictor of histologic grade are the heterogeneity of the lesion and its margins4–6), the degree of enhancement, peritumoral edema (PTE) among others.6–8)
Moreover, in surgical planning, brain MRI provide information not only regarding the location and morphology of space-occupying lesions, but also on indirect data suggesting pial invasion of essentially extra-axial tumors. It has been described in classical literature the cleft sign on T2 and the absence of PTE as favorable factors for the resection of meningiomas.9–12)
Since the magnitude of adhesions in the plane of dissection is a point of interest for surgical planning, especially when this involves proximity to eloquent areas of the cortex, we consider important to evaluate the correlation of intraoperative findings with qualitative and quantitative data of standard MRI sequences.
It is thought that the cleft sign on T2 represents cerebrospinal fluid (CSF) spaces in the brain–tumor interface (BTI), although this sign does not only seem to always represent fluid, but also the tumor capsule given in the absence of suppression in FLAIR sequences.13)
Based on the concept of digital image subtraction technique, which consists of an arithmetic subtraction process of matching pixels in two images generating a third image, we suggest that the removal of the FLAIR sequence data from the T2 sequence, both highly sensitive for edema but inversely sensitive for CSF signal, may represent better the CSF spaces in the BTI.
This study uses the technique of digital image subtraction from the CSF sensitive sequences T2 and FLAIR for generating subtraction images and relate them with the intraoperative cleavage plane subjectively perceived by the surgeon.
Materials and Methods
Patient selection and data collection
A cross-sectional retrospective study was designed considering all patients admitted to the Department of Neurosurgery of the University Complex Hospital of Vigo between 2006 and 2016 with the diagnosis of pure convexity meningioma, with tumor resection and histology confirmed as meningioma indifferently of histological grade. In this study, we excluded other tumors despite its proximity that could introduce surgical bias. Among these, we do not consider parasagittal meningiomas because of its proximity to the venous sinuses and sphenoid ridge meningiomas as its implantation site has different characteristics of resectability. We considered as exclusion criteria for the patients who lack any of the usual brain MRI sequences or those were considered of sub-optimal quality, who had previous surgery on the same site or near the tumor site, or who had received prior brain radiotherapy regardless of the indication.
Brain MRI analysis
Digital image subtraction was generated from images stored in the local PACS DICOM, using all axial slices corresponding to the location of the tumor except slices of the top and bottom of the tumor, as they are not directly perpendicular to the plane of the slice, we used axial slices to achieve uniformity in our sample and because most of our cases larger diameters were never significantly predominant in any specific plane.
We proceeded to perform an image normalization to balance pixel intensities to RGB scale (minimum value of 0 and a maximum of 255) of both sequences, subsequently performing a digital subtraction using the Image Calculator function in the software (Fiji Is Just) ImageJ, 1.50 a, (Rasband WS, ImageJ, NIH, Bethesda, Maryland, USA) (Fig. 1).
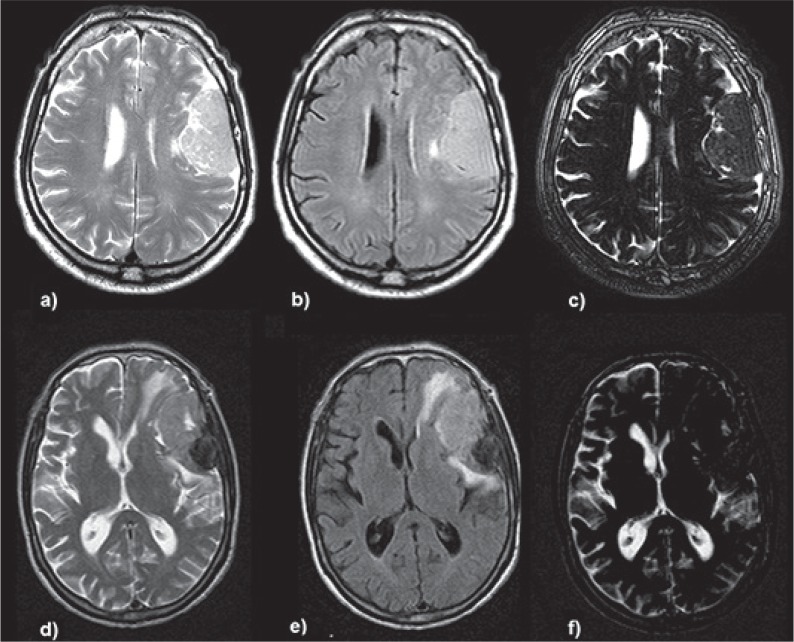
Fig. 1.
CSF sensitive digital image subtraction from T2-FLAIR sequences, two representative cases. Upper row: left parietal convexity meningioma. a) T2 sequence b) FLAIR sequence, c) digital T2-FLAIR subtraction image showing a positive CSF cleft sign with edema suppression, the surgical report describes Simpson 1 resection and good dissection plane on the entire surface of the interface, the patient had no postoperative neurological deficits. Lower row: left fronto-parietal convexity meningioma. d) T2 sequence e) FLAIR sequence f) digital subtraction image showing the lesion with no or minimal CSF cleft sign compared to T2 sequence, also note the peritumoral edema subtraction, the surgical report describes a Simpson 1 resection and bad cleavage plane mainly in its frontal aspect where the tumor was very attached to the cortical surface, the patient presented transient motor aphasia in the immediate postoperative period that fully recovered after 7 days of treatment with steroids and specific speech therapy.
An analysis of the BTI was made from the subtraction image considering a region of interest (ROI) generated from a line manually drawn on the tumor surface of the interface including automatically a range of 1 cm of thickness equally distributed on both sides of the line.
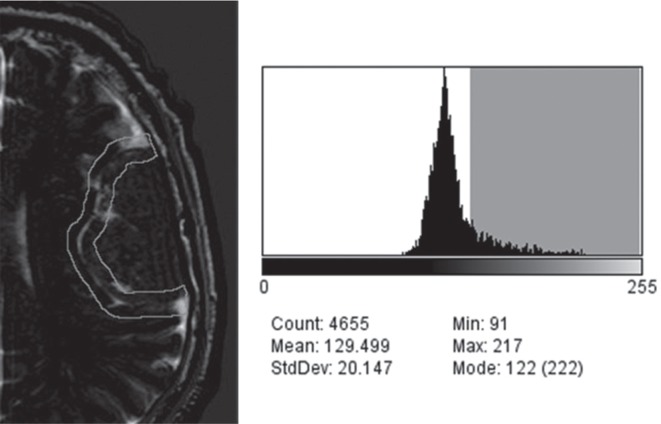
The designated ROI was automatically analyzed considering empirically an intensity greater of 140/255 as pure CSF signal and obtaining the ratio of that space over the total volume of the ROI, obtaining a percentage of theoretical occupation of CSF on the BTI. (Fig. 2).
Fig. 2.
Histogram of intensities in the region of interest. On the left, a region of interest manually drawn on the surface of a left parietal convexity meningioma, on the right a histogram of intensities generated from the ROI, highlighted in gray the intensities greater than 140 representing CSF spaces, the sum of these spaces was represented as a percentage over the total region of interest for quantitative analysis.
We also considered other radiological variables. The tumor volume was measured according to the frustum model between two slices and performing the sum of all segments. They are V = ∑ (h/3 × (A1 + A2 + (A1 × A2) 1/2)14), heterogeneity of contrast enhancement, the degree of brain PTE with the Enokizono scale, being grade 1 when there is no peritumoral brain edema, grade 2 when the brain PTE is less than 2 cm of maximum thickness measured in all cuts in FLAIR and edema grade 3 when PTE is greater than 2 cm of thickness at any of the FLAIR sequence slices15), the regularity of the brain interface surface, and the cleft sign in T2 also called arachnoid ring. All authors participated in the evaluation and consensus of measurements.
Analysis of pathology
Data regarding the result of the pathological examination of the surgical specimen were considered, considering variables regarding tumor grade and subtype according to the 2016 WHO classification of tumors of the central nervous system.2)
Variables related to surgical intervention and patient follow up
Data regarding the grade of tumor resection was collected using Simpson grading16), the arachnoid cleavage plane perceived by the surgeon grouped in good plane, bad plane, and indifferent according to the intraoperative sheet signed in all cases by the first surgeon (using the keywords: cleavage, plane, adhesion, difficult resection, pial). In addition, for some analyzes, we classify the cleavage plane by 4 grades. Grade 1: When the surgical description uses adjectives (very, fairly, pretty, too ...) for describing good cleavage plane. Grade 2: when the surgical report just mentions a good cleavage plane (without adjectivation). Grade 3: When the report just mentions a bad cleavage plane (without adjectivation), and Grade 4: When the surgical report uses adjectives (very fairly, pretty, too . . .) for describing bad cleavage plane, using the same criteria described in the first paragraph.
We also collected the complications and new neurological deficits associated with the surgery, postoperative adjuvant therapy, and the PFS.
Statistical analysis
Statistical analysis was performed using SPSS version 20.0 for MS Windows (Chicago, IL), considering general variables (age, gender) and specific variables described previously in methods. The quantitative data were obtained in the software (Fiji Is Just) ImageJ.
Nonparametric test of comparison of means, chi square test, and other statistical measures according to the main variables was performed and presented. The significance was defined as P < 0.05.
Patients who had an indifferent plane of dissection were excluded in the analysis of variables involving the intraoperative cleavage plane. For this analysis, the sample size is 38 patients.
Results
Demographic variables
From January 2006 to January 2016, 83 patients were admitted to the Neurosurgery Department of the University Hospital of Vigo with pathological diagnosis of pure convexity meningioma. The mean age of the population was 65.49 years (range 16–88 years), 46 patients (55.42%) were female and 37 male (44.58%). See Table 1.
Table 1.
Baseline characteristics of patient population
| Global | Good cleavage plane | Bad cleavage plane | P-value | OR | CI95 | |
|---|---|---|---|---|---|---|
| n = 83 | n = 24 | n = 14 | ||||
| Gender (male/female) | 37/46 | 9/16 | 10/4 | 0.04 | 4.16 | 1–17.3 |
| Age (mean ± SD) | 65.49 ± 13.1 | 64.44 ± 15.39 | 66.21 ± 11.97 | 0.71 | ||
| WHO grade (I/II) | 63/20 | 21/3 | 7/7 | 0.01 | 1.41 | 1–34.68 |
| Tumor volume (mean ± SD) | 26.99 ± 21.95 | 22.75 ± 23.25 | 32.72 ± 22.81 | 0.20 | ||
| Simpson grade (mean ± SD) | 1.17 ± 0.43 | 1.08 ± 0.28 | 1.57 ± 0.65 | 0.03 |
Extent of resection, pathology, and recurrence
Each observation unit corresponds to one patient and one surgery, 83 surgical resection of convexity meningioma were performed, regarding the extent of resection according to Simpson grade.16), grade 1 resection was possible in 85.54% of patients, grade 2 and grade 3 in 12.05% and 2.41% of patients, respectively; bone discarding because of infiltration was done in 15 cases (18.07%) using titanium mesh in all the cases cited.
Histologic grade was grade I in 75.9% of patients and grade II in 24.10% of them, the mean age of presentation of grade I was 66.4 years and 62.6 years for grade II. The histological subtype distribution was meningothelial (42.16%), atypical (24.10%), fibrous (14.46%), psammomatous (7.23%), angiomatous (4.82%), microcystic (3.61%), transitional (2.41%), and secretory (1.20%).
The average time of clinical and radiological follow-up was 43.52 months (range 2–113), 96.38% of patients showed no signs of radiological recurrence at follow-up MRI to date, 3.61% (three patients) had evidence of recurrence, two cases corresponding to a histology grade II and one case to grade I, of these, two cases corresponds to Simpson 1 and one case to Simpson 3; six cases (7.23%) for histology grade II received radiotherapy treatment. Assessing tumor recurrence by follow-up time, we have a PFS at 1 year of 97.06%, at 3 years of 93.75% at 5 years of 95.45% and at 7 years of 66.67%. The median time to recurrence was 28.25 months (range of 5–72 months).
Surgical morbidity and mortality
In our series, surgical resection of convexity meningioma presents a 30-day mortality after surgery essentially null (0%), and 85.54% of patients showed no immediate or late complication of surgery. The complications encountered were: five cases (6.02%) of extra-axial hematoma requiring surgical evacuation, four cases (4.82%) of hematoma in the surgical bed requiring surgical evacuation, one case of venous sinus injury requiring intraoperative repair, one case of CSF fistula, and one case of subdural empyema (1.20%).
Regarding functional status, 77.11% of patients showed no added neurological deficit, nine patients (10.84%) and six patients (7.23%) presented postoperative transitory limb motor paresis or transitory language deficit, respectively, overall 95.18% of patients showed no permanent deficit; being one case of permanent language deficit and three cases of permanent motor deficit, representing a total of 4.82% of new permanent neurological deficits associated with the intervention.
Preoperative brain MRI
Regarding tumor location, 51.81% of the lesions were located on the left side and 48.19% on the right side; of these the most common site was the right frontal convexity (36.14%), the left frontal convexity (26.51%), the left temporal convexity (10.84%), followed by the left parietal convexity and left cerebellar convexity (7.23%), right temporal convexity (4.82%), right parietal convexity (6.02%) and right occipital convexity (1.2%). The mean tumor volume was 26.99 cm3 (range 1–115 cm3). The gadolinium contrast enhancement in the T1C sequence was classified as homogeneous in 66.27% of cases and heterogeneous in 33.73% of cases; 10.84% of cases showed calcification on brain MRI confirmed by brain CT. BTI was considered regular in 69.88%, and irregular in 30.12% of cases.
Peritumoral edema
The degree of PTE was classified according to Enokizono scale, 40.96% of patients had no PTE edema in preoperative MRI, 31.33% of patients showed mild edema (less than 2 cm of maximum thickness measured in the FLAIR sequence), and 27.71% showed significant edema (more than 2 cm of maximum thickness measured in the FLAIR sequence). We found that the degree of PTE is directly related to tumor volume, with a mean of 20 ± 2.8, 30 ± 5.3 and 34 ± 4.3 (P = 0.03, ANOVA test) cm3 of volume in PTE grades 1, 2, and 3, respectively. When tumor edema is grouped into lesser and greater than 2 cm of maximum thickness, there is a significant difference in tumor volume (mean of 24 vs. 34 cm3, respectively) of both groups (P = 0.01, Mann–Whitney U test).
Using chi-square test, we found a statistically significant difference in the degree of PTE edema grade 3 in patients with tumor volume equal to or greater than 20 cm3 (OR 2.79, CI95 = 1.004–7.775, P = 0.045, chi-square test).
Both tumor volume and the grade of edema are independent of WHO histologic grade in the univariate analysis. The grade 3 PTE is associated with irregular tumors margins (OR 3.944, CI95 1.41–10.97, P = 0.007, chi-square test) and bad cleavage plane (OR 9.78, CI95 = 1.96–48.66, P = 0.0054, chi-square test).
Intraoperative cleavage plane
According to the surgical report, 54.22% of the narratives were inconclusive or indifferent in their interpretation, 28.92% of patients had good cleavage plane (reported mainly as without pial invasion, good cleavage plane, without arachnoid adhesions), and 16.87% of patients had poor cleavage plane (reported as pial invasion, subpial plane, bad arachnoid plane, tumor adhesion). In the classification by four groups (see methodology) considering only patients with surgical reports, 35.9% had grade 1, 28.21% had grade 2, 28.21% had grade 3 and 7.69% had grade 4 of cleavage plane.
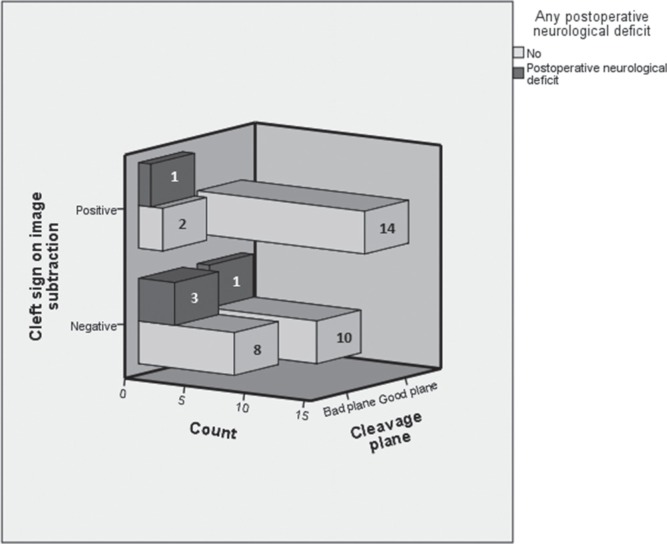
The presence of the cleft sign in T2 sequence is not associated with a good cleavage plane, (OR 2.93, CI95 = 0.55–15.63, P = 0.20, chi-square test), however, the cleft sign in the subtraction image is significantly associated with a good intraoperative cleavage plane (OR 4.667, CI95 = 1.04–20.95, P = 0.04, chi-square test) (see Fig. 3). The incidence of positive cleft sign in the subtraction sequence by cleavage grading in ascending order from 1 to 4 was 64.29%, 45.45%, 27.27%, and 0%, respectively, (P = 0.12, Kruskal Wallis test) (see Fig. 4).
Fig. 3.
Qualitatively assessed CSF spaces in the brain-tumor interface presented as a cleft sign in the subtraction image and its relationship with cleavage plane and postoperative neurological deficit.
Fig. 4.
Intraoperative cleavage plane grade and its relationship with the cleft sign on subtraction image.
The analysis of digital image subtraction showed a decrease of positivity in the cleft sign from 76% to 40%, the mean percentage of theoretical cleavage CSF space on the image subtraction was 21.6% (range 1–58%).
Considering the cleft sign only in T2 for predicting intraoperative cleavage plane provides a sensitivity of 88% but a specificity of 28.57% (PPV 69%, NPV 57%), using the cleft sign on the subtraction image, prognostic specificity increases to 78.57% at the expense of a reduction in sensitivity to 56% (PPV 82%, NPV 50%).
Regarding the quantitative approach of the study, we found a median of 19.6% and 17.06% in the groups of good and bad cleavage plane respectively (mean of 23.71% and 16.48%), using the mean comparison test found no significant association. (P = 0.22, Mann–Whitney U test).
The incidence of complications was statistically similar in both groups (0 and 8% in the groups of bad and good cleavage plane, respectively), regarding postoperative neurological deficits, 28.57% and 4% in groups of bad and good cleavage plane, respectively, presented postoperative neurological deficit, being this transitory in all cases (OR = 9.6, CI95 = 1–97, P = 0.028, chi-square test).
Similarly, the incidence of neurological deficit in groups of positive and negative cleft sign in the subtraction image was 12.1 and 30%, respectively, a statistically significant association was found as a protective factor (OR = 0.32, CI95 = 0.09–1.07, P = 0.04, Fisher’s test). See Fig. 3. The incidence of postoperative neurological deficit is 7.14%, 0%, 18.18%, and 66.67%, respectively, in grades 1 to 4 of cleavage plane in our ordinal grading system (P = 0.02, Kruskal Wallis test).
Complications in patients with cleft sign in the sequence of removal was 18 and 9% in groups of negative and positive cleft sign respectively although this association is not statistically significant (P = 0.259, OR = 0.456, chi-square test).
Discussion
It is widely described in the literature that the extent of resection in Simpson grade and histological grade according to WHO classification are the two most important factors related to PFS in the neurosurgical management of meningiomas, including the pure convexity meningiomas.17)
Extent of resection, pathology and recurrence
We reviewed three large series of patients undergoing surgery for convexity meningiomas in the literature, Morokoff et al.18), in 163 patients reviewed in 2008, found a Simpson grade 1 in 95% of surgical reports, Sanai et al.19), in 2010 presented a series of 141 convexity Meningiomas resected, founding a Simpson grade 0 and 1 in 87% of patients; a more recent report of Hasseleid et al.20) shows a grade 1 resection in 81% of the patients. In our series, considering surgical reports and outpatient reports, we found 85.54% of Simpson grade 1 resections, this finding is within the range reported by previous studies, being convexity meningiomas probably the most affordable to achieve a complete resection by location and surgical feasibility. Ten of our patients were considered Simpson 2 in the surgical report, in seven of these (70%) and in two cases (100%) of Simpson 3 resection the surgeon described dural adhesions to major cortical venous structures; despite being a small sample, the average size of tumors with Simpson 2 and 3 is 31.9 cm3 and 67.7 cm3, respectively, compared with 18.2 cm3 in tumors with Simpson 1 resection, this suggest that larger tumors present greater contact surface with the cerebral cortex and cortical venous structures so that the feasibility of resection is reduced.
The most common site in our series is frontal, data previously known from the above cited studies. The most common histologic grade found in the series of convexity meningiomas is the WHO grade I, with reports ranging from 88 to 95%.18–20) In our series, 76% of the convexity meningiomas resected corresponds to WHO grade I, which shows a relative greater incidence of atypical meningiomas in our series that should be considered regarding the results presented hereafter.
Although there are proportionately more cases of grade II tumor in our series, the 5-year PFS of 95.45% is comparable to that previously found by Morokoff et al. of 90%18) and Hasseleid et al. of 94%20), the low number of tumor recurrences in our series makes impossible further analysis relating other variables.
Surgical morbidity and mortality
Regarding neurological deficits and complications associated with convexity meningiomas surgery, Sanai et al.19) presented in 2010 a series of 141 patients being the largest to date to assess the safety profile of convexity meningioma surgery; the absence of mortality at 30-days after surgery is comparable with our findings, 1.4% of patients in this series required evacuation of extraxial hematoma post-surgical, in our series there is a higher incidence (6.02%), all extra-axial hematomas in our series occurred before 2012, being the peak 2007, since none of our study variables, nor the cleavage plane is related to bleeding complications in our series, we believe that improving surgical and postsurgical safety policies in our center may have played a major role in reducing the incidence of this complication that is reflected in their overall impact, and whose low incidence in the last 4 years is comparable to that reported in the international literature. CSF fistula and surgical site infection are rare complications and represent between 1 and 2% in all series including ours.18,20,21)
Few series report added neurological deficits, we believe any study regarding surgery involving brain tumor interface with manipulation of the surrounding brain tissue should consider this complication; Morokoff et al.18) in their series presented in 2008, found an incidence of 1.7% of new neurological deficit, which this author describes independently of other complications, in our series a case of permanent language deficit was the result of an evacuated subdural hematoma and one case of permanent motor deficit was related to surgical site infection in the form of subdural empyema, therefore, in our series, there are two cases of permanent motor deficit (2.4%) directly related to surgery without considering intermediate complications.
Preoperative brain MRI
From the radiological point of view, digital subtraction technique is widely used in the medical field to increase the sensitivity of images that otherwise its interpretation would be difficult and doubtful, in this regard previous studies22,23) have analyzed the role of subtraction images of brain MRI in the diagnosis of hemorrhage and enhancing brain lesions, more recently Ellington et al.24) have been widely used this technique in the study of contrast enhancement of glioblastoma multiforme; after searching the literature we found no studies that refer to subtractions in order to increase the sensitivity of CSF.
The FLAIR sequence is a predominantly T2-weighted acquisition, in which the signal CSF is suppressed by a pulse of initial inversion, this allows completely suppress CSF spaces from the image of the sequence, on the other hand the T2 sequence is a highly liquid sensitive sequence, so CSF signal is typically high as well as the signal derived from cerebral edema.25)
Based on these principles we believe that the removal of both sequences would delineate with greater sensitivity peritumoral arachnoid spaces described classically in the T2 sequence, with the added benefit of suppressing the brain PTE common for both sequences, being useful for the study of these spaces independently of other factors related to the pial invasion.
Peritumoral brain edema
Peritumoral brain edema in patients with grade I meningiomas and its relationship with tumor volume has been previously described by several authors9,26,27), they also found a higher incidence of irregular tumor margins suggesting pial invasion as a mechanism, our findings are consistent with previous. Vignes et al.28) suggest that the degree of PTE may be involved in preoperative morbidity and surgical resection difficulty. In our study, we found a significant relationship between PTE grade 3 and an intraoperative bad cleavage plane.
In our study, intraoperative bad cleavage plane was not related to tumor volume measured as a continuous or categorical variable so we can assume that the previously described associations are not a confounding variable.
Intraoperative cleavage plane
Our incidence of cleft sign on T2 is 76%, comparable with the incidence found by Alsaad et al. of 76%,29) Takeguchi et al. of 70%13) and Spagnoli et al. of 80%30), unlike the second study cited, in our series we didn’t qualitatively assessed the presence of clefts in the FLAIR sequence. In this regard, given the presence cleft sign in 52% of our suppression images, we assume the presence of signal compatible with CSF in nearly half of the T2 sequences with positive cleft sign, data that go against the first study previously cited, moreover our increased specificity of predicting the intraoperative cleavage plane using image subtraction compared to T2 sequence is a finding in favor for our theory.
The T2-FLAIR subtraction image allows us to perform two types of analysis, a qualitative regarding the meaning of the presence of cleft sign of arachnoid ring in T2 and its sensitivity in predicting a cleavage plane; in this regard considering the cleft sign only in T2 is not enough specific to be considered as a predictor, we found that using the subtraction image we can obtain good test measures (specificity: 78.57%, sensitivity: 56%, PPV: 82% and NPV: 50%), Ildan et al.9) previously described in 176 meningiomas from different locations, that cleft sign of CSF on T2 showed no relationship to the intraoperative cleavage plane, thus our study is consistent with these findings. We have also found that the incidence of positive cleft sign in the subtraction sequence is inversely proportional to the cleavage plane grade (see methodology), and this grading scale is proportional to the incidence of postoperative neurological deficits, findings that reinforces the results previously described.
The other aspect is the quantitative study. There is a mean difference of 7.23% of relative arachnoid space between good and bad cleavage plane group in our study, after using the corresponding statistical tests we cannot conclude that quantitative analysis measuring the relative occupation of CSF in the BTI is prognostic of bad cleavage plane, as an explanation we suggest that the relative amount of CSF in that space would not be as a continuous variable related to the incidence of good cleavage plane. The presence of a minimum of CSF detected by MRI on the BTI would be sufficient to obtain an adequate intraoperative plane without requiring a given thickness, that in our study it is dependent of an arbitrary measure of a one centimeter of interface.
Three of our patients in the negative cleft sign group and one in the positive cleft sign group presented permanent neurological deficit in the postoperative period, although it is a very low incidence, difficult to be used to draw conclusions about it, we consider important association found between bad cleavage plane and the presence of postoperative neurological deficit, which also we corroborated having found a higher incidence of postoperative neurological deficits in patients with a negative cleft sign in the subtraction image. This finding suggests that one might consider this sign as a predictive factor of surgical risk and can be considered in the comprehensive preoperative approach of the surgical treatment, this latter finding was corroborated in the total sample (not excluding patients with indifferent dissection plane), however, we suggest that more studies should be performed to corroborate our findings and extend the area of research to meningiomas in other locations if feasible.
Study limitations
Our study has some limitations: a low sample to characterize with adequate statistical power of association of CSF sensitive subtraction images with other prognostic variables such as histological grade, extent of resection and PFS, the relatively high frequency of neutral of indifferent intraoperative reports concerning the intraoperative cleavage plane.
While it is true that the grade of adhesion in the BTI can be different in different sites of the cortical surface, we think that the overall result of dissection plane determines the conception of the surgeon about the cleavage plane, as most of our positive reports were not regionally but generally descriptive. We consider this fact a limitation that exceeds the capacity of our design methodology. We consider that many of our limitations can be solved conducting a prospective study.
We also believe that the characterization of arachnoid spaces and CSF through the subtraction image technique presented in our study could be considered useful in the methodology of studies regarding relevant pathologies.
With the advent and development of new technologies in neuroimaging, we believe that software post-processing techniques based on clinical and pathophysiological reasoning of the clinical entities should always be taken into account both in clinical practice and in research.
Conclusions
The peritumoral subarachnoid space carachterized by CSF sensitive T2-FLAIR digital subtraction image qualitatively measured as the cleft sign is an independent predictor of intraoperative cleavage plane. Both the negative cleft sign in the image subtraction and bad intraoperative cleavage plane are predictors of postoperative neurological deficit.
Peritumoral brain edema is an independent predictor of intraoperatively bad cleavage plane in the resection of convexity meningiomas. As previous studies suggest, we emphasize the importance of complete resection of convexity meningiomas, which is associated with increased PFS.
Acknowledgement
The authors would like to thank all the nurses, physicians, radiologists and technicians of the University Hospital Complex of Vigo, who participated in the comprehensive care of our patients.
Footnotes
Conflicts of Interest Disclosure
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1). Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS: CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol 17 Suppl 4: iv1– iv62, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2). Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW: The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131: 803– 820, 2016. [DOI] [PubMed] [Google Scholar]
- 3). Yang SY, Park CK, Park SH, Kim DG, Chung YS, Jung HW: Atypical and anaplastic meningiomas: prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry 79: 574– 580, 2008. [DOI] [PubMed] [Google Scholar]
- 4). Kawahara Y, Nakada M, Hayashi Y, Kai Y, Hayashi Y, Uchiyama N, Nakamura H, Kuratsu J, Hamada J: Prediction of high-grade meningioma by preoperative MRI assessment. J Neurooncol 108: 147– 152, 2012. [DOI] [PubMed] [Google Scholar]
- 5). Lin BJ, Chou KN, Kao HW, Lin C, Tsai WC, Feng SW, Lee MS, Hueng DY: Correlation between magnetic resonance imaging grading and pathological grading in meningioma. J Neurosurg 121: 1201– 1208, 2014. [DOI] [PubMed] [Google Scholar]
- 6). Liu Y, Chotai S, Chen M, Jin S, Qi ST, Pan J: Preoperative radiologic classification of convexity meningioma to predict the survival and aggressive meningioma behavior. PLoS One 10: e0118908, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Liang RF, Xiu YJ, Wang X, Li M, Yang Y, Mao Q, Liu YH: The potential risk factors for atypical and anaplastic meningiomas: clinical series of 1,239 cases. Int J Clin Exp Med 7: 5696– 5700, 2014. [PMC free article] [PubMed] [Google Scholar]
- 8). Drape JL, Krause D, Tongio J: MRI of aggressive meningiomas. J Neuroradiol 19: 49– 62, 1992. [PubMed] [Google Scholar]
- 9). Ildan F, Tuna M, Gocer AP, Boyar B, Bagdatoglu H, Sen O, Haciyakupoglu S, Burgut HR: Correlation of the relationships of brain-tumor interfaces, magnetic resonance imaging, and angiographic findings to predict cleavage of meningiomas. J Neurosurg 91: 384– 390, 1999. [DOI] [PubMed] [Google Scholar]
- 10). Qi ST, Liu Y, Pan J, Chotai S, Fang LX: A radiopathological classification of dural tail sign of meningiomas. J Neurosurg 117: 645– 653, 2012. [DOI] [PubMed] [Google Scholar]
- 11). Taoka T, Yamada S, Sakamoto M, Akashi T, Miyasaka T, Ochi T, Wada T, Uchikoshi M, Nakase H, Kichikawa K: Accuracy for predicting adhesion between meningioma and the brain by using brain surface motion imaging: comparison between single and double acquisition methods. Neuroradiology 54: 1313– 1320, 2012. [DOI] [PubMed] [Google Scholar]
- 12). Lobato RD, Alday R, Gomez PA, Rivas JJ, Dominguez J, Cabrera A, Madero S, Ayerbe J: Brain oedema in patients with intracranial meningioma. Correlation between clinical, radiological, and histological factors and the presence and intensity of oedema. Acta Neurochir (Wien) 138: 485– 493; discussion 493–494, 1996. [DOI] [PubMed] [Google Scholar]
- 13). Takeguchi T, Miki H, Shimizu T, Kikuchi K, Mochizuki T, Ohue S, Ohnishi T: Evaluation of the tumor-brain interface of intracranial meningiomas on MR imaging including FLAIR images. Magn Reson Med Sci 2: 165– 169, 2003. [DOI] [PubMed] [Google Scholar]
- 14). Shally H, Chitharanjan K: Tumor volume calculation of brain from MRI slices. International Journal of Computer Science and Engineering Technology 4: 1126– 1132, 2013. [Google Scholar]
- 15). Enokizono M, Morikawa M, Matsuo T, Hayashi T, Horie N, Honda S, Ideguchi R, Nagata I, Uetani M: The rim pattern of meningioma on 3D FLAIR imaging: correlation with tumor-brain adhesion and histological grading. Magn Reson Med Sci 13: 251– 260, 2014. [DOI] [PubMed] [Google Scholar]
- 16). Simpson D: The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20: 22– 39, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Aizer AA, Bi WL, Kandola MS, Lee EQ, Nayak L, Rinne ML, Norden AD, Beroukhim R, Reardon DA, Wen PY, Al-Mefty O, Arvold ND, Dunn IF, Alexander BM: Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer 121: 4376– 4381, 2015. [DOI] [PubMed] [Google Scholar]
- 18). Morokoff AP, Zauberman J, Black PM: Surgery for convexity meningiomas. Neurosurgery 63: 427– 433; discussion 433–434, 2008. [DOI] [PubMed] [Google Scholar]
- 19). Sanai N, Sughrue ME, Shangari G, Chung K, Berger MS, McDermott MW: Risk profile associated with convexity meningioma resection in the modern neurosurgical era. J Neurosurg 112: 913– 919, 2010. [DOI] [PubMed] [Google Scholar]
- 20). Hasseleid BF, Meling TR, Ronning P, Scheie D, Helseth E: Surgery for convexity meningioma: Simpson Grade I resection as the goal: clinical article. J Neurosurg 117: 999– 1006, 2012. [DOI] [PubMed] [Google Scholar]
- 21). Black PM: How to Perform Surgery for Intracranial: (Convexity) Meningiomas. In: Sindou M, ed. Practical Handbook of Neurosurgery: From Leading Neurosurgeons. Vienna: Springer Vienna, 2009: 63– 70 [Google Scholar]
- 22). Lee VS, Flyer MA, Weinreb JC, Krinsky GA, Rofsky NM: Image subtraction in gadolinium-enhanced MR imaging. Am. J Roentgenol 167: 1427– 1432, 1996. [DOI] [PubMed] [Google Scholar]
- 23). Melhem ER, Mehta NR: Dynamic T1-weighted spin-echo MR imaging: The role of digital subtraction in the demonstration of enhancing brain lesions. J Magn Reson Imaging 9: 503– 508, 1999. [DOI] [PubMed] [Google Scholar]
- 24). Ellingson BM, Kim HJ, Woodworth DC, Pope WB, Cloughesy JN, Harris RJ, Lai A, Nghiemphu PL, Cloughesy TF: Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology 271: 200– 210, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). De Coene B, Hajnal JV, Gatehouse P, Longmore DB, White SJ, Oatridge A, Pennock J, Young I, Bydder G: MR of the brain using fluid-attenuated inversion recovery (FLAIR) pulse sequences. A J Neuroradiol 13: 1555– 1564, 1992. [PMC free article] [PubMed] [Google Scholar]
- 26). Simis A, Pires de Aguiar PH, Leite CC, Santana PA, Jr, Rosemberg S, Teixeira MJ: Peritumoral brain edema in benign meningiomas: correlation with clinical, radiologic, and surgical factors and possible role on recurrence. Surg Neurol 70: 471– 477; discussion 477, 2008. [DOI] [PubMed] [Google Scholar]
- 27). Tamiya T, Ono Y, Matsumoto K, Ohmoto T: Peritumoral brain edema in intracranial meningiomas: effects of radiological and histological factors. Neurosurgery 49: 1046– 1051; discussion 1051–1052, 2001. [DOI] [PubMed] [Google Scholar]
- 28). Vignes JR, Sesay M, Rezajooi K, Gimbert E, Liguoro D: Peritumoral edema and prognosis in intracranial meningioma surgery. J Clin Neurosci 15: 764– 768, 2008. [DOI] [PubMed] [Google Scholar]
- 29). Alsaad RH: Magnetic resonance imaging (MRI) paterrns of intracranial meningiomas. Basrah J Surg, 5: 113– 122, 2007. [Google Scholar]
- 30). Spagnoli MV, Goldberg HI, Grossman RI, Bilaniuk LT, Gomori JM, Hackney DB, Zimmerman RA: Intracranial meningiomas: high-field MR imaging. Radiology 161: 369– 375, 1986. [DOI] [PubMed] [Google Scholar]