Abstract
We describe the efficacy and technical aspects of infiltrated preoperative embolization of meningioma by penetration of very dilute glue. In this method, a 13% n-butyl-cyanoacrylate (NBCA)-lipiodol mixture is injected extremely slowly from the middle meningeal artery (MMA) in a similar manner to plug and push injection of ethylene vinyl alcohol copolymer mixed with tantalum and dimethyl sulfoxide (Onyx®) after the tortuous side feeders are proximally embolized. The glue is infiltrated into small tumor arteries and extends to inaccessible feeders from deep meningeal arteries. Since 2011, we have used this technique in the embolization of 32 cases preoperatively diagnosed with meningioma. Intratumoral embolization was possible in 30 cases (94%), and a greater than 50% reduction in contrast area of contrast-enhanced T1-weighted MR imaging (T1-WI) was achieved in 18 cases (56%). Two cases achieved complete devascularization, showing a remarkable shrinkage in tumor size after embolization. If excessive reflux of embolization and the resulting migration of glue into normal arteries is achieved, this method provides extremely effective devascularization on surgical extirpation. It might also be applicable to surgically untreatable meningiomas as a semi-radical treatment option.
Keywords: meningioma/TH, therapeutic embolization, N-butyl-cyanoacrylate
Introduction
Transarterial embolization for hypervascular meningiomas has been used as a standard preoperative measure for reducing intraoperative blood loss and facilitating removal.1,2) Although conventional embolization methods using particle injection or proximal feeder occlusion with coils facilitate surgery, hemostasis is often hampered by residual skull-base feeders or pial arteries recruited due to hemodynamic imbalance following embolization of dural feeders.3–5)
Penetrating embolization with a slow and extensive injection of liquid material has been proposed for the treatment of arteriovenous malformations.6) An example of this method is the “plug and push” method with ethylene vinyl alcohol copolymer mixed with tantalum and dimethyl sulfoxide (Onyx®; Medtronic, Minneapolis MN, USA).7) This technique perfuses the embolic material into all branches from a single feeder only. However, although Onyx may be useful as a material for preoperative embolization of AVMs,8) it is not approved for use with intracranial lesions, except for arteriovenous shunts.
Here, we describe our use of the “plug and push” technique using a 13% N-butyl-cyanoacrylate (NBCA) glue solution in iodized oil (Lipiodol®, Laboratoire Guerbet, Roissy-Charles-de-Gaulle Cedex, France) for embolization in a series of surgically challenging meningiomas. Using this method, we were able to successfully embolize these tumors along with multiple axial feeders.
Materials and Methods
Subjects were 32 consecutive patients who underwent preoperative transarterial embolization for meningioma between January 2011 and December 2014. Approval for all interventional procedures was received from our Institutional Review Board. Off-label use of NBCA for meningiomas was explained and informed consent was obtained from each patient and his or her family. Under general anesthesia, conventional angiography (Artis zee BA Twin, Siemens, Berlin, Germany) via a transfemoral approach was performed through a 6-Fr-long sheath. Bilateral external and internal carotid and vertebral angiography is mandatory even in unilateral lesions to clarify the angiographic architecture of dural and pial feeders. Understanding the contribution of pial supply and the course of drainage routes is important in formulating an embolization strategy. The appropriate vessels for embolization were selected based on the preceding angiography, which usually included the middle meningeal artery (MMA), and occasionally other dural arteries.
Preliminary coil/particle embolization
First, the proximal segments of tortuous feeders that are difficult to approach are embolized with particles or coils. Particularly large feeders from the superficial temporal artery are exceptionally embolized at the proximal portion. If the catheter can be safely positioned, the anterior falcine artery from the ophthalmic artery and the posterior meningeal artery from the vertebral artery are occasionally embolized to avoid reflux into the parent arteries.
NBCA glue embolization
The MMA is always selected last. A distal access catheter (Cerulean® 4 Fr; Medikit, Tokyo, Japan) is placed in all cases to afford distal access. A microcatheter (Marathon®; Medtronic, Minneapolis, MN, USA) is advanced as distally as possible into the main feeder. Concentration of NBCA was chosen 13% in all cases. That is because 13% NBCA has low adhesion, and there is no difficulty of pulling out the microcatheter. The 13% NBCA is prepared and warmed to decrease its viscosity9) and very slowly advanced into the catheter. When the solution is visualized at the microcatheter tip, injection is temporarily halted. If very cautious injection advances the NBCA glue, injection is continued very slowly. If not, the injection is suspended again and repeated after several seconds to start the penetration of glue into the tumor. When the extension of glue into the drainers is noted into the anastomosed feeders from pial or dural arteries connecting with important functional arteries, the injection is stopped and restarted a few seconds later. This pattern of intermittent injection allows the glue to penetrate throughout the tumor. Although the glue cast usually reaches the feeders from the contralateral side, the direction of the tip of the glue cast should be carefully confirmed. When the forward movement of the glue stops and all subsequent glue is refluxed to the catheter-positioned feeder, or if there is further reflux into the normal pial feeders, the injection is stopped and the microcatheter is removed. If the contralateral angiogram shows remaining feeders, the method is repeated until the dural supply vanishes from the external carotid system.
Due to the dilute concentration of the glue, the microcatheter can be pulled out without difficulty, even if the length of reflux extends more than 1 cm. Postoperatively, the patient undergoes frequent checks of vital signs and neurological status. Since a large amount of glue may often cause a headache, analgesic agents or sedatives are given as needed, and methylprednisolone is administered at 250 mg/day for 2 days for its anti-inflammatory effect. Postprocedural magnetic resonance imaging (MRI; Signa HDxt 1.5T, GE, Connecticut, USA) is usually performed within two days to assess the extent of ischemic necrosis inside the tumor. Surgical resection is usually performed within one week before any remaining pial feeders are recruited.
Results
We performed preoperative transarterial embolization for meningioma in 32 patients between January 2011 and December 2014. Tumor locations are shown in Table 1. The tumor was supplied by the MMA in 29 cases, an accessory meningeal artery in seven, the superficial temporal artery (STA) in 12, the ethmoidal artery from the ophthalmic artery in three, meningeal branches from the internal carotid artery in four, the occipital artery in one, and the posterior meningeal artery in one. Embolization and surgical outcomes are shown in Table 2. The microcatheter was easily pulled out after embolization in all cases. A diffusion-weighted MRI (DWI) positive sign in the tumor represents tumor infarction and subsequent necrosis. The interval between embolization and surgical operation was 1–90 days (average: 11.2 days). Two cases achieved remarkable tumor shrinkage during the waiting period.
Table 1.
Patient characteristics
| Total patients (n) | 32 |
| Female, n (%) | 17 (53.1) |
| Mean age, years (range) | 62.5 (38–83) |
| Presentation (n) | |
| Seizure | 9 |
| Hemiparesis | 5 |
| Visual disturbance | 3 |
| Asymptomatic | 15 |
| Tumor location (n) | |
| Convexity | 18 |
| Parasagittal | 4 |
| Sphenoid ridge | 3 |
| Petroclival | 3 |
| Falx | 2 |
| Tuberculum sellae | 1 |
| Anterior skull base | 1 |
| Maximum tumor diameter, mm (range) | 50.9 (21–83) |
| Pial feeders, n (%) | 13 (40.6) |
Table 2.
Clinical findings
| Mean number of embolized feeders, n (range) | 2.3 (1–4) |
| Complete devascularization from dural artery, n (%) | 32 (100) |
| NBCA distribution, n (%) | |
| Intratumoral | 30 (93.8) |
| Feeder (%) | 2 (6.2) |
| Injection time of NBCA, sec (range) | 364 (157–989) |
| Injection volume of NBCA, ml (range) | 1.21 (0.38–2.76) |
| Microcatheter gluing (%) | 0 (0) |
| >50% reduction of gadolinium (%) | 18 (56.3) |
| DWI positive in the tumor (%) | 19 (59.4) |
| Complications, n | |
| Permanent | 0 |
| Transient | 2 |
| Intratumoral hemorrhage | 0 |
| Surgical Results (Simpson Grade), n (%) | |
| 1 | 19 (59.3) |
| 2 | 9 (28.1) |
| 3 | 2 (6.3) |
| 4 | 2 (6.3) |
Representative Cases
Case 1
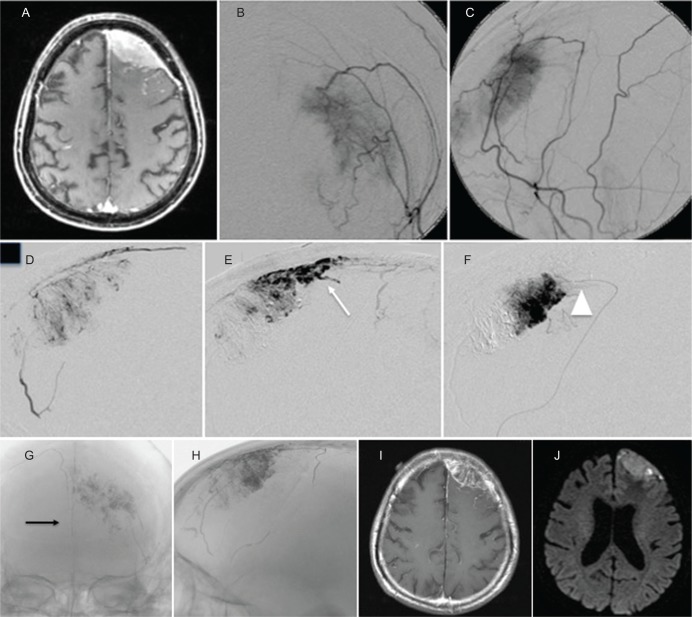
A 75-year-old man presented with general convulsion. Contrast-enhanced T1-weighted MRI (T1WI) showed a left convexity meningioma with en plaque dural enhancement, and angiography showed a tumor stain from the MMA and STA. NBCA embolization was performed via the MMA and STA over a 15 min injection. A cast of NBCA distributed into the tumor, including the en plaque area, as well as into a branch of the contralateral falcine artery, resulting in complete feeder occlusion. After embolization, the tumor stain disappeared, and the enhanced area was remarkably diminished on MRI. Surgical resection was performed five days after embolization with markedly little intraoperative blood loss (Simpson Grade 1) (Fig. 1).
Fig. 1.
(Case 1) Contrast-enhanced MRI T1-weighted image (T1WI) revealed a left convexity meningioma with en plaque lesion (A). Left external carotid angiogram (B: antero-posterior view, C: lateral view) showed tumor stain from MMA and STA. Embolization was performed via the posterior branch of the left MMA over a 15 min injection (D, E, F, white arrowhead: Microcatheter tip). X-ray after embolization showed NBCA cast distributed to the tumor including the en plaque area (white arrow) and simultaneously into contralateral feeders (black arrow) from the falcine artery (G, H). After embolization, most of the enhanced area on contrast-enhanced T1WI disappeared (I) and changed to a necrotic area on DWI (J).
Case 2
A 55-year-old woman presented with left hemiparesis. An enhanced MRI revealed a right convexity meningioma 71 mm in diameter, and angiography revealed a tumor stain with MMA distribution; the tumor was also supplied via pial feeders. The NBCA mixture was injected from the MMA with care taken not to extend the injection into the ophthalmic artery. After this, the tumor was no longer visualized on angiography. Contrast-enhanced T1WI and DWI after embolization showed marked devascularization and necrosis of most of the tumor, which was totally resected five days after embolization without any complications (Simpson Grade 1) (Fig. 2).
Fig. 2.
(Case 2) Contrast-enhanced T1WI revealed a large right convexity meningioma (A). Right external carotid angiogram (B: antero-posterior view, C: lateral view) showed tumor stain from the MMA anterior branch. NBCA glue was injected from the anterior branch of the MMA without migration to the ophthalmic artery (D, E, F). X-ray image after embolization showed NBCA cast distributed to the tumor (G, H). Contrast-enhanced T1WI and DWI after embolization revealed that part of the tumor had undergone necrosis (I, J).
Case 3
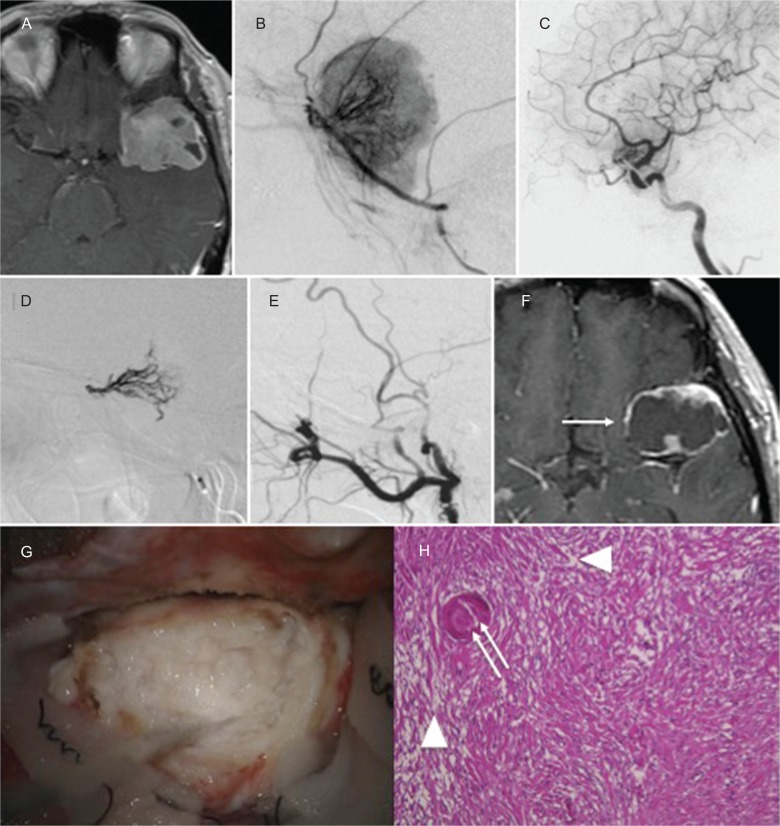
A 60-year-old woman presented without symptoms. Contrast-enhanced T1WI showed a left sphenoid ridge meningioma. The tumor was supplied by the left MMA and recurrent meningeal artery from the ophthalmic artery. NBCA was injected into the MMA and recurrent artery from the ophthalmic artery, and complete obliteration of feeders was achieved. The enhanced MRI taken 3 weeks later demonstrated remarkable shrinkage of the tumor. Intraoperatively, the tumor seemed to be degenerated and necrotic, and it was easily removed without bleeding. Histopathologic findings showed necrotic changes in the tumor and NBCA cast in the tumor vessels (Fig. 3).
Fig. 3.
(Case 3) Contrast-enhanced T1WI revealed a left sphenoid ridge meningioma (A). Left external carotid angiogram (B) and internal carotid angiogram (C) showed that the tumor was supplied by the left MMA and recurrent meningeal artery from the ophthalmic artery. NBCA was injected into the MMA and recurrent artery from the ophthalmic artery (D). After embolization, the tumor was devascularized (E). MRI taken 3 weeks after embolization demonstrated remarkable shrinkage (F: white arrow) of the tumor. Intraoperative photographs revealed a whitish and degenerated tumor (G). Histopathologic findings (H) showed necrotic changes in the tumor (white arrowhead) and NBCA cast in the tumor vessels (white double arrow).
Discussion
Slow injection of dilute glue is known to be useful for achieving occlusion of an isolated sinus of a dural arteriovenous fistula (DAVF).10) In sinus-type DAVF, the sinus functions as a lake of inflow feeders, and shunt flow drains directly into the veins. In contrast, a hypervascular tumor is fed primarily by terminal arteries, along with some feeders, including small intratumoral AV shunts. Since the purpose of embolization in DAVF is to block the shunt and drainage route, glue should be directed into the drainage route to fill the isolated sinus itself.11)
The goal of meningioma embolization is ischemic necrosis of the tumor. Occlusion of the feeders is sufficient to achieve devascularization.5) The method most commonly used to prepare a tumor for resection to date is proximal feeder occlusion.9) However, recruited pial or unembolized feeders sometimes cause troublesome bleeding when the tumor is cut.4,5) NBCA is generally used for the embolization of various organs in the filed of interventional radiology under the institutional approval. Microsphere, approved embolic material, is easily injected and can achieve proper feeder occlusion.
However, it never reaches the level of arteriole and also has a high risk of early recanalization. Even without the demerit of non-approved material, NBCA is the indispensable liquid embolic material with much difference of the behavioral characteristics. In our method, highly diluted glue is advanced into the small tumor arteries, allowing it to reflux into and occlude the other feeders through the intratumoral network. In this way, we can embolize inaccessible feeders from important vessels in one session. However, overzealous injection must be avoided, particularly when the glue enters the anterior falcine artery or dural arteries from the internal carotid artery or unexpectedly flows out into a draining vein. In these cases, the injection should be immediately stopped, and catheter withdrawal should be considered if reflux is observed. In tumors in a paramedian location with bilateral MMA supply, injected glue will easily advance into the contralateral MMA. In this case, glue tends to enter only one of the feeders crossing the midline, and further injection often fails to reach all feeders from the contralateral MMA, occasionally requiring embolization of the remaining contralateral feeders.
Microscopic observation of the tumor shows the effect of glue embolization into the smaller vessels, as the proportion of necrotic tissue in the center of the tumor is larger than that in cases with conventional proximal feeder occlusion. On intraoperative fluorescent microscopy, a sufficiently embolized tumor is less reactive than non-embolized areas. Although no significant differences in intraoperative blood loss between conventional and glue embolization methods have been noted, surgeons report easier hemostasis with piecemeal resection, particularly in deeper areas or behind the tumor. Further, no side effects have been associated with this method.
Several reports recently described an embolization method for meningioma using Onyx®.3) The slower precipitation of Onyx® over other liquid agents is advantageous in some respects, as the glue can then penetrate deeper into tumor vessels. Like NBCA, administration may be intermittently stopped in cases of early detection of intracranial anastomoses or evidence of non-targeted embolization.3) However, NBCA is more cost-effective and results in fewer imaging artifacts in MRI.
Periprocedural complications related to transarterial embolization of meningiomas include cerebral infarction, intra- or peritumoral hemorrhage, cranial nerve palsy, vessel perforation, scalp necrosis, and groin hematoma.3) Overall complication rates range from 0% to 21%.3,4) In our series, periprocedural complications occurred in only two cases (6.3%), and these cases were accompanied by focal cerebral infarction with transient symptoms. Raper et al. noted that hemorrhage may be more common after embolization with glue than with particles, because the liquid may migrate distally and embolize physiologically important draining veins.12) In our series, however, neither intratumoral hemorrhage nor aggravation of brain edema after embolization were observed on computed tomography or MRI. Aihara et al. reported that the radiopacity of NBCA is useful for controlling maneuvers compared with other embolic materials.13) Similarly, our very slow, meticulous injection of very diluted NBCA glue was adequately visualized under two-dimensional continuous digital subtraction angiography.
Our method may allow for safer and easier surgical excision of hypervascular meningiomas, as embolization with NBCA glue appears to be associated with spontaneous shrinkage due to intratumoral necrosis. This method may represent a semi-radical treatment option for difficult-to-resect meningiomas or for patients who are not candidates for surgery.
Footnotes
Conflicts of Interest Disclosure
There were no conflicts of interest. All authors who are members of The Japan Neurosurgical Society (JNS) have registered online self-COI Disclosure Statement Forms through the website for JNS members.
References
- 1). Gruber A, Killer M, Mazal P, Bavinzski G, Richling B: Preoperative embolization of intracranial meningiomas: a 17-years single center experience. Minim Invasive Neurosurg 43: 18– 29, 2000. [DOI] [PubMed] [Google Scholar]
- 2). Shah AH, Patel N, Raper DM, Bregy A, Ashour R, Elhammady MS, Aziz-Sultan MA, Morcos JJ, Heros RC, Komotar RJ: The role of preoperative embolization for intracranial meningiomas. J Neurosurg 119: 364– 372, 2013. [DOI] [PubMed] [Google Scholar]
- 3). Shah A, Choudhri O, Jung H, Li G: Preoperative endovascular embolization of meningiomas: update on therapeutic options. Neurosurg Focus 38: E7, 2015. [DOI] [PubMed] [Google Scholar]
- 4). Sluzewski M, van Rooij WJ, Lohle PN, Beute GN, Peluso JP: Embolization of meningiomas: comparison of safety between calibrated microspheres and polyvinyl-alcohol particles as embolic agents. Am J Neuroradiol 34: 727– 729, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Wakhloo AK, Juengling FD, Van Velthoven V, Schumacher M, Hennig J, Schwechheimer K: Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: assessment of two embolization techniques. Am J Neuroradiol 14: 571– 582, 1993. [PMC free article] [PubMed] [Google Scholar]
- 6). Debrun GM, Aletich V, Ausman JI, Charbel F, Dujovny M: Embolization of the nidus of brain arteriovenous malformations with n-butyl cyanoacrylate. Neurosurgery 40: 112– 121, 1997. [PubMed] [Google Scholar]
- 7). Weber W, Kis B, Siekmann R, Kuehne D: Endovascular treatment of intracranial arteriovenous malformations with Onyx: technical aspects. Am J Neuroradiol 28: 371– 377, 2007. [PMC free article] [PubMed] [Google Scholar]
- 8). Weber W, Kis B, Siekmann R, Jans P, Laumer R, Kuehne D: Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery 61: 244– 254, 2007. [DOI] [PubMed] [Google Scholar]
- 9). Kominami S, Watanabe A, Suzuki M, Mizunari T, Kobayashi S, Teramoto A: Preoperative embolization of meningiomas with N-butyl cyanoacrylate. Interv Neuroradiol 18: 133– 139, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Nelson PK, Russell SM, Woo HH, Alastra AJ, Vidovich DV: Use of a wedged microcatheter for curative trans-arterial embolisation of complex intracranial dural arterio-venous fistulae: indications, endovascular technique, and outcome in 21 patients. J Neurosurg 98: 498– 506, 2003. [DOI] [PubMed] [Google Scholar]
- 11). Toulgoat F, Mounayer C, Túlio Salles Rezende M, Piotin M, Spelle L, Lazzarotti G, Desal H, Moret J: Trans-arterial embolisation of intracranial dural arterio-venous malformations with ethylene vinyl alcohol copolymer (Onyx18). J Neuroradiol 33: 105– 114, 2006. [DOI] [PubMed] [Google Scholar]
- 12). Raper DM, Starke RM, Henderson F, Jr, Ding D, Simon S, Evans AJ, Jane JA, Sr, Liu KC: Preoperative embolization of intracranial meningiomas: efficacy, technical considerations, and complications. Am J Neuroradiol 35: 1798– 1804, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Aihara M, Naito I, Shimizu T, Fujimaki H, Asakura K, Miyamoto N, Yoshimoto Y: Preoperative embolization of intracranial meningiomas using n-butyl cyanoacrylate. Neuroradiol 57: 713– 719, 2015. [DOI] [PubMed] [Google Scholar]