Abstract
Objective
We tested the hypothesis that FMR1 expansions would result in global gene dysregulation as early as the second trimester of human fetal development.
Method
Using cell-free fetal RNA obtained from amniotic fluid supernatant and expression microarrays we compared RNA levels in samples from fetuses with premutation or full mutation allele expansions to control samples.
Results
We found clear signals of differential gene expression relating to a variety of cellular functions, including ubiquitination, mitochondrial function and neuronal/synaptic architecture, among others. Additionally, among the genes showing differential gene expression, we saw links to related diseases of intellectual disability and motor function. Finally, within the unique molecular phenotypes established for each mutation set, we saw clear signatures of mitochondrial dysfunction and disrupted neurological function. Patterns of differential gene expression were very different in male and female fetuses with premutation alleles.
Conclusion
These results support a model for which genetic misregulation during fetal development may set the stage for late clinical manifestations of FMR1-related disorders.
INTRODUCTION
FMR1 is an X-linked gene encoding the fragile-X mental retardation protein (FMRP). Mice demonstrate ubiquitous FMR1 expression by embryonic day ten1. In both mice and humans, expression is markedly higher in the brain and gonads than other tissues by adulthood1. FMRP accomplishes diverse cellular functions, including directing mRNA localization and translational regulation for hundreds of proteins, including many involved in synaptic plasticity and motor function2–5.
The 5’ untranslated region of FMR1 contains a triplet repeat CGG of variable length, with a normal range being <45 repeats. The full mutation allele (≥ 200 repeats, frequency 1:5,000 males /1:8,000 females6–7) results in gene hypermethylation and inhibition of FMR1 expression8–9. Fragile-X syndrome (FXS), characterized by seizures, anxiety, obsessive-compulsion, and language delay,10 is the most common genetic basis for intellectual disability (ID) and constitutes a leading monogenic cause of autism (1–2%)11. Many individuals with FXS are also mosaic for smaller, premutation length alleles; mosaicism among males with a FXS diagnosis is estimated at 19–41%12–13.
Expansions in the premutation range (55–200 repeats, frequency 1:209 females /1:430 males14) can result in increased FMR1 RNA expression. Twenty percent of female carriers of the premutation allele will develop fragile-X associated primary ovarian insufficiency (FXPOI), defined as reduced ovarian function, and in the extreme case, premature ovarian failure, a cessation of menses prior to age 4015–18. By the age of 60, many men (40%) and women (16%) with premutation alleles will develop fragile X-associated tremor/ataxia syndrome (FXTAS), a late-onset neurodegenerative disorder characterized by tremors, cognitive decline, gait ataxia and autonomic dysfunction, with neurological signatures of white matter disease and the loss of axons and myelin (reviewed19–21). FXTAS has also been demonstrated to co-occur with dementia and Parkinson’s disease in males and with Alzheimer’s disease in females19. Postmortem exams of human cortex and cerebellum tissues reveal that the premutation allele is associated with ubiquitin-positive intranuclear inclusions in neurons and astrocytes22–24. Although the likelihood of developing FXTAS decreases for lower range premutation alleles, an elevated risk for development of parkinsonian disorders remains, perhaps as a result of the mitochondrial dysfunction resulting from elevated FMR1 transcription25. Additional medical conditions associated with the premutation length allele include migraine, fibromyalgia, neuropathy, psychiatric disorders, hypothyroidism, hypertension, and immune-mediated disorders among others (reviewed26).
Historically, the premutation allele was thought to be related to only adult-onset disorders, but recent studies offer compelling evidence that clinical symptoms may manifest throughout the lifetime (reviewed20–21,26). Premutation mouse models have shown altered neuronal migration and differentiation in the embryonic neocortex27. Cultured neurons from neonatal premutation mice exhibit oxidative stress and mitochondrial dysfunction as well as elevated expression of stress proteins and shorter dendritic length with reduced branching, likely as a result of asynchronized calcium oscillations28–30. Human infants with the premutation allele have demonstrable deficits in visual motion processing31. Carriers of the premutation allele may present in childhood with anxiety, attention-deficit hyperactivity disorder and autism spectrum disorders32–34. In light of these observations, a recent review by Hagerman and Hagerman26 proposed that human carriers of the premutation allele are subject to lifelong disruptions in calcium regulation and mitochondrial function as a result of a background state of cellular dysfunction established early in development.
While the disorders associated with each allelic expansion have distinct clinical features and underlying molecular mechanisms, areas of overlap blur these distinctions. As such, the phrase “fragile X-associated disorder (FXD)” captures the continuity and breadth of clinical involvement resulting from all expansions.
To our knowledge, there are three studies showing that fetuses with the full mutation allele lose FMR1 expression in the first trimester35–37. Cell-free fetal RNA (cffRNA) in amniotic fluid supernatant (AFS) from fetuses with expanded FMR1 alleles offers the opportunity to examine transcription in a diverse array of fetal tissues. This approach has been used in prior studies of genetic conditions using AFS cffRNA38–42. Given that an FMR1 expansion allele is known to have molecular effects on somatic organs of the adult in addition to nervous system tissues43, we used AFS cffRNA to examine transcription in different tissue types from the developing fetus44.
METHODS
Ethical Approval/Samples
The procedures employed in this study were reviewed and approved by the Tufts Medical Center Institutional Review Board (Tufts Medical Center protocol #5582), and are in accordance with the guidelines set forth by this journal. Anonymized amniotic fluid supernatant (AFS) samples (n=40) were received from Integrated Genetics/LabCorp (Westborough, MA) and the New York State Institute for Basic Research in Developmental Disabilities (Staten Island, NY).
Genotyping
Fetal sex and FMR1 triplet repeat number were determined per routine clinical protocols for all samples, including control fetuses, by collaborating CLIA-certified diagnostic laboratories, using both Southern blot and PCR assays. Genotypes were subsequently grouped according to allele-size, corresponding to either normal (repeat size <45), premutation (55–200) or full mutation (>200) size. Samples with intermediate (repeat size 45–54) alleles were excluded from the analysis.
Expression Microarray Preparation and Data Analysis
Full details available in Supplementary Methods. Cell-free fetal RNA was extracted from residual amniotic fluid supernatant as described elsewhere45. Raw microarray CEL files, along with normalized values are publicly available at NCBI’s Gene Expression Omnibus46 using the GEO Series accession number GSE83556. Normalization of microarrays from all FMR1 allele groups was performed as a batch; methods for this and for identification of differential gene expression are described elsewhere38.
In Silico Functional Analyses
Full details available in Supplementary Methods. As is described elsewhere44, tissue-specific gene expression was assessed using BioGPS Gene Expression Atlas, http://biogps.org. Candidate genes of interest from male premutation cases were manually curated for disease-specific associations using NCBI’s Online Mendelian Inheritance in Man (OMIM, http://omim.org/) and PubMed (http://www.ncbi.nlm.nih.gov/pubmed, accessed 5/27/15). A Gene Set Enrichment Analysis47 with fetus-specific functional annotation, DFLAT48, was also undertaken (pre-ranked based on paired t-scores, FDR q ≤0.25). The advantage of GSEA with DFLAT over the previously used Ingenuity Pathway Analysis software is that DFLAT most accurately reflects the current understanding of fetal biology, including the rapid physiologic cellular proliferation in fetal tissues that may falsely present a signature of cancer when relying solely on adult annotations49.
RESULTS
FMR1 Expansions and Differential Gene Expression
The premutation male analysis data set contained 12 pairs of gestational age (GA)- and sex-matched fetuses with FMR1 CGG repeats in the normal or premutation length (Table 1, Table S1). Forty-five (45) probes representing 36 genes were significantly differentially expressed, with fold-change magnitude ≥2, a BH p-value ≤0.05, and direction of differential gene expression (DGE) consistent among at least 10 pairs (Table S2).
Table 1.
Amniotic Fluid Samples Used in Study
| Sample | GA | Number of Repeats | Diagnosis | Fetal Sex |
|---|---|---|---|---|
| Nl-1 | 15+5 | 29 | Normal | M |
| Nl-2 | 15+6 | 31 | Normal | M |
| Nl-3 | 16+2 | 24 | Normal | M |
| Nl-4 | 16+2 | 36 | Normal | M |
| Nl-5 | 16+3 | 24 | Normal | M |
| Nl-6 | 16+4 | 32 | Normal | M |
| Nl-7 | 16+6 | 29 | Normal | M |
| Nl-8 | 17+3 | 20 | Normal | M |
| Nl-9 | 17+5 | 25 | Normal | M |
| Nl-10 | 18+0 | 43 | Normal | M |
| Nl-11 | 20+2 | 30 | Normal | M |
| Nl-12 | 20+3 | 30 | Normal | M |
| Pm-1 | 15+0 | 67 | Premut | M |
| Pm-2 | 16+3 | 55 | Premut | M |
| Pm-3 | 16+4 | 56 | Premut | M |
| Pm-4 | 16+5 | 66 | Premut | M |
| Pm-5 | 16+5 | 66 | Premut | M |
| Pm-6 | 16+5 | 61 | Premut | M |
| Pm-7 | 16+6 | 99 | Premut | M |
| Pm-8 | 17+2 | 62 | Premut | M |
| Pm-9 | 17+5 | 57 | Premut | M |
| Pm-10 | 19+0 | 64 | Premut | M |
| Pm-11 | 19+4 | 60 | Premut | M |
| Pm-12 | 20+2 | 144, 165 | Premut | M |
| Nl-13 | 16+6 | 27, 29 | Normal | F |
| Nl-14 | 17+1 | 21, 30 | Normal | F |
| Nl-15 | 17+3 | 29, 30 | Normal | F |
| Nl-16 | 17+4 | 29, 30 | Normal | F |
| Nl-17 | 19+2 | 29, 30 | Normal | F |
| Nl-18 | 19+5 | 30, 43 | Normal | F |
| Pm-13 | 16+6 | 23, 55 | Premut | F |
| Pm-14 | 17+0 | 37, 56 | Premut | F |
| Pm-15 | 17+6 | 29, 64 | Premut | F |
| Pm-16 | 17+6 | 29, 64 | Premut | F |
| Pm-17 | 19+0 | 23, 83 | Premut | F |
| Pm-18 | 19+0 | 23, 83 | Premut | F |
| Fm-1 | 16+4 | 30, >200 | Full | F |
| Fm-2 | 17+5 | 30, >200 | Full | F |
| Fm-3 | 16+6 | 155, 171, >200 | Full | M |
| Fm-4 | 20+0 | >200 | Full | M |
GA, weeks+days gestational age; Nl, Normal; Pm, Premutation; Fm, Full mutation
The premutation female analysis data set contained 6 pairs of GA-and sex-matched fetuses with FMR1 CGG repeats in the normal or premutation length (Tables 1, 2 and S1). Due to the smaller sample size, a fold-change magnitude ≥2.5 was used to increase the specificity of results. With a BH p-value ≤ 0.05, and direction of differential gene expression (DGE) consistent among at least 5 pairs, 243 probes (174 unique genes) showed statistically significant differential expression (Table S3).
Table 2.
Analysis groups comprising this study.
| Characteristics | Criteria for Differential Gene Expression |
||||||
|---|---|---|---|---|---|---|---|
| Analysis Group |
Samples (Nl) |
Samples (Pm or Fm) |
GA Range |
Fold- Change Thres- hold |
Concor -dant Direct- ion |
BH-p Thres- hold |
Number of Different- ially Expressed Genes |
| Pm Male | 12 | 12 | 15–20 | 2 | 10/12 | 0.05 | 36 |
| Pm Female |
6 | 6 | 16–20 | 2.5 | 5/6 | 0.05 | 174 |
| Fm (2M, 2F) |
4 | 4 | 16–20 | 2.5 | 4/4 | 0.05 | 1, 257 |
GA, weeks gestational age; Nl, Normal; Pm, Premutation; Fm, Full mutation
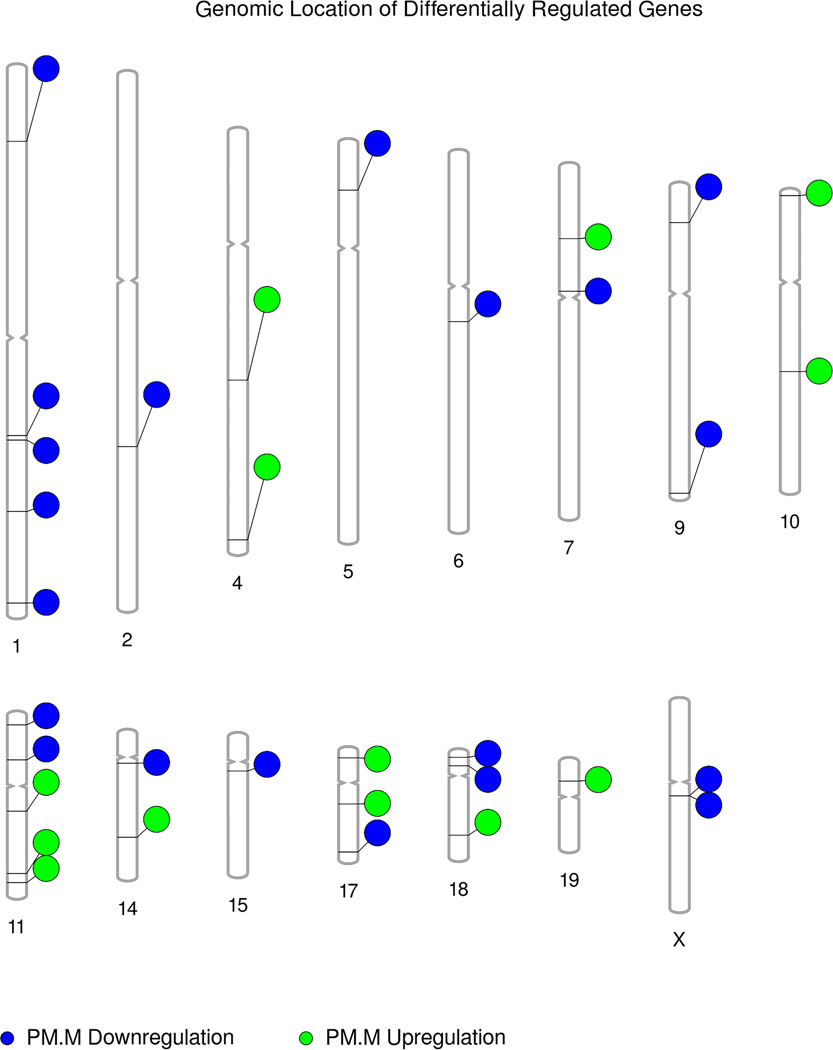
For both female and male premutation samples, differentially expressed genes were found throughout the genome (Figure 1, Figure S1). The increased numbers of samples in the male premutation set allowed greater stringency in the identification of candidate genes, resulting in fewer differentially expressed genes as compared to the female premutation data.
As proof of principle for our ability to report on multiple fetal tissues, Affymetrix probe sets showing statistically significant DGE in either male or female premutation samples were queried for tissue-specific expression using the publicly available BioGPS database. Probe sets corresponding to 13 genes were shown to be tissue-specific (Table 3). The tissues contributing cffRNA to the AFS are not limited to this list, but the presence of transcripts expressed in a tissue-specific manner illustrates that gene expression in these diverse sets of fetal tissues, including those of the central and peripheral nervous systems, was successfully detected by this experiment.
Table 3.
Probe sets known to have tissue-specific expression in humans. These probe sets showed significantly different gene expression in premutation males or females compared to their matched controls.
| Gene Symbol | Probe Set | Tissue | DGE | Magnitude Fold-Change |
|---|---|---|---|---|
| SNAP25 | 202508_s_at | Amygdala | Downregulation FP | 2.60 |
| TMEFF1 | 205122_at | Amygdala | Upregulation FP | 2.56 |
| HPCA | 205454_at | Caudate Nucleus | Downregulation MP | 2.96 |
| NCF1 | 204961_s_at | CD19+ B Cells (Neg. Sel.) | Downregulation FP | 3.43 |
| AL928768.3 | 215118_s_at | CD19+ B Cells (Neg. Sel.) | Upregulation FP | 2.85 |
| IRF5 | 205469_s_at | CD33+ Myeloid Cells | Upregulation FP | 3.14 |
| CD3D | 213539_at | CD4+ T Cells | Downregulation FP | 4.72 |
| L1CAM | 204584_at | Fetal Brain | Upregulation FP | 3.87 |
| UPB1 | 220507_s_at | Liver | Downregulation FP | 4.28 |
| SFTPD | 214199_at | Lung | Upregulation MP | 2.11 |
| ADAM12 | 213790_at | Placenta | Upregulation FP | 2.91 |
| AKR1B10 | 206561_s_at | Small Intestine | Downregulation FP | 3.26 |
| ARHGAP25 | 38149_at | Whole Blood | Upregulation FP | 2.53 |
DGE, Differential Gene Expression; MP, Male Premutation; FP, Female Premutation.
Due to the relative rarity of amniotic fluid supernatant samples from full mutation fetuses, only four pairs of GA- and sex-matched fetuses with either normal or full mutation length FMR1 alleles were available for this study (Table 1, Table S1). Although the sample size was too small to provide statistically significant conclusions, preliminary analyses can, in broad strokes, outline the altered terrain of fetal gene expression. There were 1,610 probes representing 1,257 genes that showed DGE, with fold-change magnitude ≥ 2.5, a BHp-value ≤ 0.05, and direction of differential regulation consistent among all four pairs (Table S4).
There were some commonalities in the gene families and general gene functions among the three sets of genes showing DGE, yet the majority of genes were unique to one sample set or another. Indeed, no single gene was found to be differentially regulated in both the male and female premutation data sets (Table S5). These unique signatures thus suggest specific molecular phenotypes associated with each FMR1 allele size group.
Trends in Gene Function and Human Disease
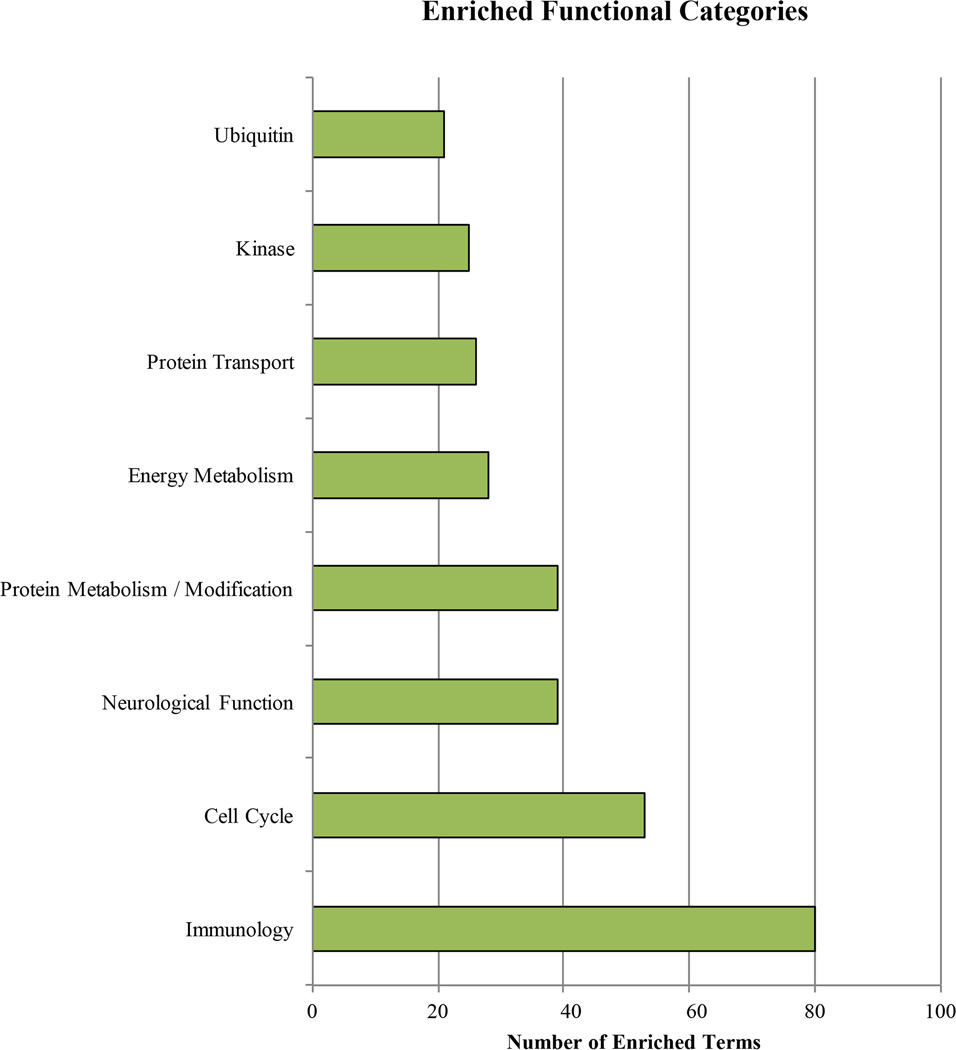
The GSEA analysis revealed an enrichment in gene ontologies related to mitochondrial function, the immune system, the cell cycle, regulation of mRNA and proteins, neurological development, hormone signaling, phosphorylation and ubiquitination, and cellular energy metabolism, among others. The female premutation dataset demonstrated four enriched terms, including RNA splicing, translation initiation and nuclear import of proteins bearing a nuclear localization signal. Analysis of the full mutation data set revealed enrichment for a diversity of terms, the most frequent of which relate to lipid biosynthesis and energy metabolism (Figure 2, Table S6).
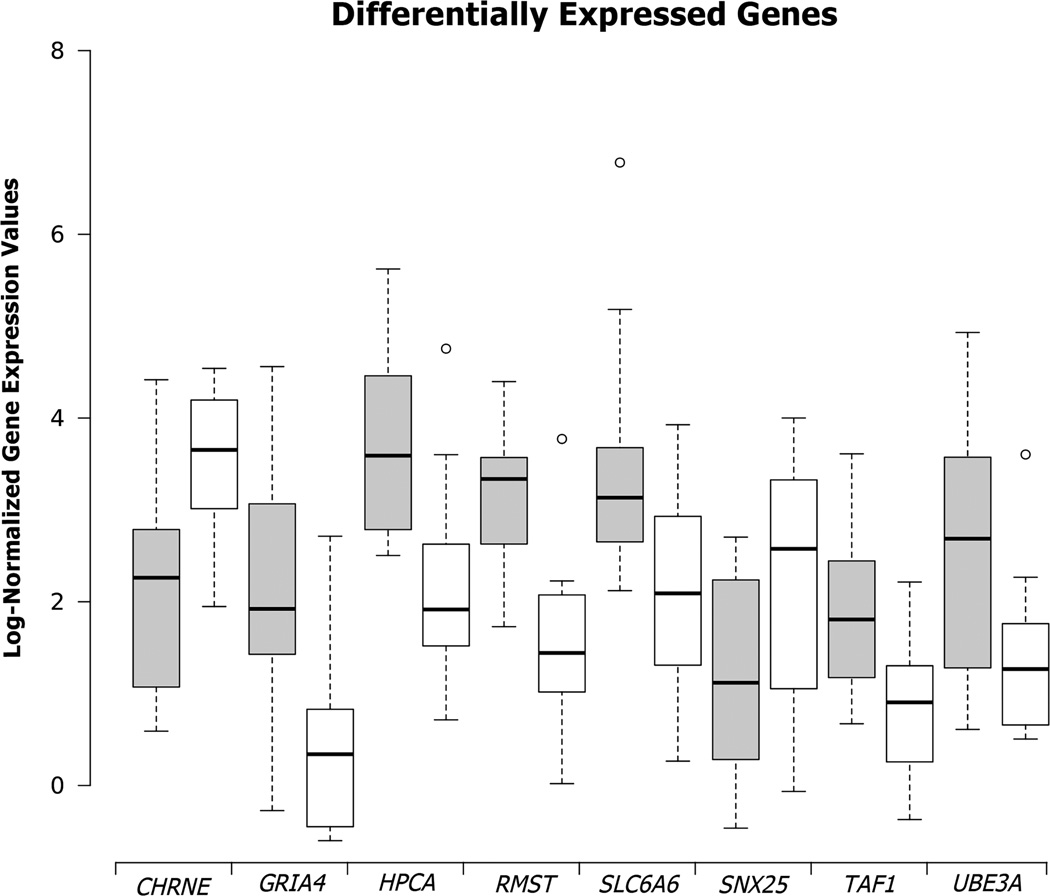
Cross-referencing the lists of both male and female premutation results with OMIM and NCBI PubMed databases revealed that many genes had previously known associations with other diseases. Genes of particular interest, given clinical implications in neurological diseases, include: CHRNE, GRIA4, HPCA, RMST, SLC6A6, TAF1, and UBE3A in the premutation male; and CP, FGFR2, GRIK2, GRIN2A, L1CAM, NRXN1, NTRK2, SIX3, SNAP25, and SNCA in the premutation female (Figure 3, Table S7).
DISCUSSION
To our knowledge, this is the first study of differential gene expression resulting from the expanded FMR1 allele in ongoing human pregnancies. This increases knowledge of the effects of FMR1 expansion alleles on human fetal development and suggests areas for further investigation of the pathophysiology of related adult-onset diseases. A major strength of this work is its rigorous design, made all the more notable in light of the technical difficulties associated with studying a living fetus. Prior studies of mammalian development and FMR1 gene expression relied on samples from animal studies, aborted human fetal tissues, induced pluripotent stem cells (iPSC), or cells cultured from amniocytes or chorionic villi, the latter three being limited to mitotically active cells and possibly affected by the culturing process27,35–37,50.
Biological variation among our samples was minimized through careful matching of sex and GA (± 7 days), both of which are known to affect fetal gene expression51. Rigorous statistical corrections were applied to limit the effects of multiple hypothesis testing in the identification of differential gene expression and altered developmental pathways. In order to minimize technical variation, all samples were processed by the same person using the same techniques. By reporting only genes for which DGE was observed in ≥10 of the 12 pairs for premutation males and ≥5 of the 6 pairs for premutation females, we are focusing only on the most consistent signals. This requirement unavoidably leads to possible under-reporting of some of the gene expression changes associated with various expansions in FMR1, particularly given the heterogeneity of clinical outcomes and the tissue-specific somaticisms that have been reported for some FMR1 expansions52–52.
While FMR1 is an X-linked gene, differentially expressed genes are found genome-wide, in line with our understanding of the complex and interrelated nature of expression regulation and the pleiotropic phenotypes associated with FMR1 mutations (Figure 1, Figure S1). The results for male and female premutation length FMR1 alleles are distinct from signatures for prior diseases we have studied, with only five probe sets reported in both42 (Table S8). Most of the genes reported here have not previously been studied in the context of FXD, with several notable exceptions (Table S9). Some other genes of interest from prior FXD studies do show suggested differential gene expression, defined as a relaxation of the fold-change magnitude threshold to ≥ 1.5 (Table S9). Further study and critical analyses are necessary to determine the validity of these latter suggested differences in gene expression.
There are multiple possible explanations for the fact that FMR1 does not show consistent differential gene expression in our studies. It may be that at this stage of development, changes in gene expression levels for FMR1 are negligible, but the physical presence of the premutation RNA molecule itself disrupts the regulation of gene expression of other genes. This possibility is in line with the toxic-gain-of function model, in which the expanded CGG repeat of the FMR1 RNA molecule itself binds directly to and sequesters one more cellular proteins, removing the proteins from normal cellular functioning (reviewed26). Another possibility is that at this stage of development, the effect on gene expression falls below detection level in our study, while still affecting transcriptional regulation of other genes. Finally, some of the cffRNA may have originated from undifferentiated fetal stem cells, in which FMR1 expression levels may not yet differ among allele types54.
DGE signals within each study set were enriched for certain molecular and physiological functions. Many of these functional terms were enriched regardless of whether the biological function was defined narrowly or broadly (Table S6), reflecting both the strength and the breadth of these findings. Selections chosen based on clinical significance are discussed below.
Neuro- and Neuromuscular Development and Calcium
Among the genes found misregulated in male premutation carriers are several with well-established links to ID (Table S7). UBE3A normally drives ubiquitination, marking proteins for degradation by the 26S proteasome – a process regulating synaptic development55. Loss of function of the maternally inherited UBE3A allele is the basis for Angelman syndrome, a genetic disease causing developmental delay, speech and cognitive impairments, tremors and seizures56. Changes in UBE3A expression have previously been linked to premutation length FMR1 alleles57. ABHD11, LAT2 and NCF1, which are DGE in the female premutation samples, and ADCY10, which is downregulated in the male premutation samples, are all located on 7q11, in a region deleted in Williams syndrome. Individuals with this genetic disorder exhibit abnormalities in cardiovascular, ophthalmologic, renal, connective and dental tissues, and variable neurological and cognitive abilities58–60. Given the overlapping clinical features between Williams syndrome and some juveniles or adults with FMR1 premutation mutations, it may be that affected cellular pathways are affected in both cases. Another example is UPB1, which was differentially expressed in female premutation samples. Mutations in UPB1 have been linked to Beta-ureidopropionase deficiency in a handful of patients, with effects that range from severe neurologic involvement with mental retardation and seizures to no discernable effects on neurologic development61.
In addition to genes implicated in ID, DGE was observed for several genes known to be important in neurodevelopment or neurodegeneration, including those modulating the concentration of intracellular Ca2+, which regulates dendritic growth and retraction. In the premutation male, DGE was seen for SLC6A6, which encodes a neurotransmitter transporter for the GABAA agonist taurine, modulating both neuronal excitability and calcium flux. These data provide the first evidence of Ca2+ signaling misregulation in the developing fetus of the premutation carrier, and lend support to the hypothesis that adult-onset consequences of the premutation allele could be traced to early fetal development. PCDH11X, which was differentially expressed in the female premutation set, belongs to a family of protocadherin genes that is especially prevalent in the central nervous system, and performs calcium-dependent cell adhesion and cell recognition functions. PCDH11X has been implicated in late-onset Alzheimer’s disease62. Premutation males also showed DGE of CHRNE, mutations of which have been shown to result in slow-channel congenital myasthenic syndrome, a postsynaptic neuromuscular junction disorder resulting in early-onset and progressive muscle weakness63. A similar phenotype is observed for mutations in SNAP25, which showed evidence of DGE in premutation females. SNAP25 encodes an acetyl choline receptor and has a role in calcium-triggered neuronal exocytosis. Similarly, psychomotor developmental delays have been seen in a family with a mutation in the gene CAD64. The DGE of CHRNE, SNAP25, and CAD suggests a possible link to the adult-onset neuromuscular clinical phenotype associated with the premutation allele.
Among the female premutation results were other differentially-expressed genes with important roles in synaptic functions, including mediation of synaptic transmission and synaptogenesis and dentritic spine morphology: GRIK2, GRIN2A, L1CAM and SRCIN1 (Table S7). Additional differentially regulated genes include: GRIA4, mutations in which result in absence epilepsy due to increased duration of synaptic responses in a mouse model65; NRXN1, mutations in which are known to alter synaptic function, resulting in epilepsy, ID and autism in human subjects66–68; SNCA proteins are thought to integrate presynaptic signaling, and aggregations of these proteins are hallmarks of neurodegenerative diseases, including Alzheimer’s disease69. Overexpression of SNCA is also linked to mitochondrial fragmentation in cell lines70.
Mitochondria
Mitochondria have several key cellular roles, including phospholipid biosynthesis and inter-organelle trafficking, glycosphingolipid anabolism, metabolism of ceramide and cholesterol, and intracellular Ca2+ homeostasis in mammalian cells71–73. The male premutation GSEA results show enrichment for pathways related to cell-death, cholesterol metabolism, phospholipid trafficking, carbohydrate metabolism, regulation of oxygen species and ion transport, organelle localization and transport, all of which suggest disrupted mitochondrial function (Table S6).
Mitochondrial disorders in the context of FMR1 expansions have previously been explored. Tasha et al.74 found FMRP to be physically associated with the heavy membrane of mitochondria in HeLa cells, suggesting a functional interaction. Ross-Inta et al.75 found evidence for disruption of ATP synthesis as well as evidence of low rates of NAD- and FAD-linked oxygen uptake in cultured fibroblasts from male premutation carriers. They propose that these mitochondrial deficits, found regardless of whether or not the premutation carriers had presented with FXTAS, predispose individuals to associated neurodegenerative disorders, such as Parkinson’s disease. A study of the Drosophila homolog of FMR1, dfmr1 suggests that another function of dfmr1 is to negatively regulate mitochondrial numbers and transport76. Cultured neurons from newborn premutation knock-in mice possess fewer mitochondria, and those mitochondria suffer from reduced mobility28. Hagerman21 posited that the neurodegeneration seen in FXS can be traced to the failure of mitochondria to meet the particularly high energy needs of neurons as compared to other cell types. Similarly, Hagerman and Hagerman26 suggest that this mitochondrial insufficiency reduces dendritic growth of premutation mouse neurons, resulting in long-term neurological dysfunction.
Differential expression of genes related to mitochondrial functioning was also found among the female premutation samples. These included: ACAD10, which has a role in mitochondrial fatty acid beta-oxidation; NDUFA10, which is involved in the transfer of electrons from NADH to the respiratory chain and is related to Leigh syndrome; and REXO2, which is necessary for mitochondrial protein synthesis and DNA repair.
We cannot know which fetuses among the male and female premutation carriers might go on to develop associated disorders such as FXTAS or FXPOI. However, the presence of these molecular signals suggests that a background state of cellular dysregulation far predates the clinical onset of symptoms. Indeed, Hagerman and Hagerman26 review several neuroimaging studies showing that CNS changes predate FXTAS, and propose that the transition from genetic predisposition to clinical manifestation may rely on a combination of additional mutations, or a variety of environmental insults encountered throughout life.
Preliminary Analyses from Full Expansion Alleles
With four full mutation samples and their matched controls, the limited size of the full mutation sample set makes the resulting data appropriate only for preliminary interpretation. The most interesting of these preliminary results relates to the consistent differential gene expression of genes previously studied in the context of neurological diseases, including FXD (Table S9). Due to the nature of genomic work, some of these findings may be spurious; however, they are worthy of preliminary consideration, particularly in light of the unique nature of these data. Among the results of interest is differential expression of NLGN4, mutations in which are recognized as a monogenic source of autism and X-linked mental retardation77–78. Genes previously studied in context of FXS full mutation include NRNX1 and UBE3A, discussed in detail above, as well as: DSCAM, MAPK3, NCS1, PRKCD and PRKCE (Table S9). Cvetkovska et al.79 found that Drosophila FMRP null mutants showed overexpression of DSCAM and synaptic targeting errors, suggesting a role for DSCAM in FXS-associated ID. Similarly, a Drosophila knockout model of dfmr1 showed decreased expression of the Drosophila homolog of NCS1 (frequenin) in the fourth day of life80, a finding mirrored in our full mutation samples. Gene expression downregulation was previously seen for MAPK3, PRKCD and PRKCE in fibroblasts from a mouse knockout mode81.
Molecular signatures for mitochondrial dysfunction are also observed in our full mutation samples, including consistent differential expression of NRF1, which encodes a transcription factor acting on nuclear genes encoding mitochondrial proteins. Similarly, the GO terms most enriched for the full mutation analysis included those related to lipid biosynthesis and transport, carbohydrate metabolism and nucleic acid metabolism, known functions of mitochondria.
CONCLUSIONS
Using a discovery-driven approach, we have revealed a rich landscape of altered gene expression throughout a variety of fetal tissues, with prominent signatures of mitochondrial and neurological dysfunction. Our work shows that the FMR1 expansions affect gene regulation as early as the second trimester, a developmental period previously unexplored in the context of FXD. Our results suggest that developmental aberrations beginning during this critical period of development may set the stage for later clinical manifestations. Future research might focus on prenatal use of preventative therapies, including antioxidants to combat oxidative stress or dietary therapies used for mitochondrial diseases so as to lower the lifetime risk of FXD.
Finally, further studies are needed to elucidate the relationship between transcription of the expanded allele and the molecular phenotypes we have reported. Indeed, the relationship between FMR1 transcription and FXTAS-associated mitochondrial disorders is by no means clear26. Future studies with larger sample sets and prospective tracking of life-time environmental risk and disease incidence would allow for a richer understanding of the complex relationship between the premutation allele and FXD.
Supplementary Material
WHAT’S ALREADY KNOWN ABOUT THIS TOPIC?
Expansions in the fragile X mental retardation 1 gene (FMR1) have been studied extensively in the context of their juvenile and adult-onset diseases.
To date, however, little is known about the effects of these genetic changes early in human development.
WHAT DOES THIS STUDY ADD?
This study presents for the first time an analysis of gene expression in developing fetuses with expanded FMR1 alleles (both premutation and full mutation)
In fetuses with premutation alleles, differential expression was observed in many genes, including those involved in ubiquitination, mitochondrial function and neuronal/synaptic architecture.
Male and female fetuses with premutations had very different transcription signatures.
Acknowledgments
We wish to thank Donna P. Slonim, PhD of Tufts University for assistance with GSEA and Fayçal Guedj, PhD of Tufts Medical Center for the critical review of the manuscript.
AUTHORS’ ROLES
SLN, and PMO, and ME procured the samples and edited the manuscript. SLN provided expertise in FMR1 analysis and medical genetics. DWB and LMZ designed the experiments and drafted the manuscript. HCW and LMZ performed the GSEA analysis. LMZ performed all other laboratory and computational experiments and data analyses.
Funding:
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD 042053-10 to Dr. Bianchi).
Footnotes
Disclosures:
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, et al. Tissue specific expression of FMR-1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 1993;3:36–43. doi: 10.1038/ng0193-36. [DOI] [PubMed] [Google Scholar]
- 2.Fridell RA, Benson RE, Hua J, et al. A nuclear role for the Fragile X mental retardation protein. EMBO J. 1996;15:5408–5414. [PMC free article] [PubMed] [Google Scholar]
- 3.Ling S-C, Fahrner PS, Greenough WT, Gelfand VI. Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc Nat Acad Sci USA. 2004;101:17428–17433. doi: 10.1073/pnas.0408114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dictenberg JB, Swanger SA, Antar LN, et al. A direct role for FMRP in activity-dependent dendritic mRNA transport links filopodial-spine morphogenesis to fragile x syndrome. Dev Cell. 2008;14:926–939. doi: 10.1016/j.devcel.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darnell JC, Van Driesche SJ, Zhang C, et al. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A. 2008;146A:1009–1016. doi: 10.1002/ajmg.a.32060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hill MK, Archibald AD, Cohen J, Metcalfe SA. A systematic review of population screening for fragile X syndrome. Genet Med. 2010;12:396–410. doi: 10.1097/GIM.0b013e3181e38fb6. [DOI] [PubMed] [Google Scholar]
- 8.Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67:1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 9.Pieretti M, Zhang FP, Fu YH, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66:817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 10.Boyle L, Kaufmann WE. The behavioral phenotype of FMR1 mutations. Am J Med Genet C Semin Med Genet. 2010;154C:469–476. doi: 10.1002/ajmg.c.30277. [DOI] [PubMed] [Google Scholar]
- 11.Reddy KS. Cytogenetic abnormalities and fragile-x syndrome in Autism Spectrum Disorder. BMC Med Genet. 2005;6:3. doi: 10.1186/1471-2350-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rousseau F, Heitz D, Biancalana V, et al. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991;325:1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- 13.Nolin SL, Glicksman A, Houck GE, et al. Mosaicism in fragile X affected males. Am J Med Genet. 1994;51:509–512. doi: 10.1002/ajmg.1320510444. [DOI] [PubMed] [Google Scholar]
- 14.Tassone F, Iong KP, Tong T-H, et al. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Med. 2012;4:100. doi: 10.1186/gm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz CE, Dean J, Howard-Peebles PN, et al. Obstetrical and gynecological complications in fragile X carriers: A multicenter study. Am J Med Genet. 1994;51:400–402. doi: 10.1002/ajmg.1320510419. [DOI] [PubMed] [Google Scholar]
- 16.Allingham-Hawkins DJ, Babul-Hirji R, Chitayat D, et al. Fragile X premutation is a significant risk factor for premature ovarian failure: the international collaborative POF in fragile X study—preliminary data. Am J Med Genet. 1999;83:322–325. [PMC free article] [PubMed] [Google Scholar]
- 17.Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. 2000;97:189–194. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 18.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 19.Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291:460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Arocena D, Hagerman PJ. Advances in understanding the molecular basis of FXTAS. Hum Mol Genet. 2010;19:R83–R89. doi: 10.1093/hmg/ddq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagerman PJ. Fragile X-associated tremor/ataxia syndrome (FXTAS): pathology and mechanisms. Acta Neuropathol. 2013;126:1–19. doi: 10.1007/s00401-013-1138-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greco CM, Hagerman RJ, Tassone F, et al. Neuronal intranuclear inclusions in a new cerebellar tremor/ataxia syndrome among fragile X carriers. Brain. 2002;125:1760–1771. doi: 10.1093/brain/awf184. [DOI] [PubMed] [Google Scholar]
- 23.Tassone F, Iwahashi C, Hagerman PJ. FMR1 RNA within the intranuclear inclusions of fragile X-associated tremor/ataxia syndrome (FXTAS) RNA Biol. 2004;1:103–105. doi: 10.4161/rna.1.2.1035. [DOI] [PubMed] [Google Scholar]
- 24.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 25.Loesch DZ, Godler DE, Evans A, et al. Evidence for the toxicity of bidirectional transcripts and mitochondrial dysfunction in blood associated with small CGG expansions in the FMR1 gene in patients with parkinsonism. Genet Med. 2011;13:392–399. doi: 10.1097/GIM.0b013e3182064362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagerman PJ, Hagerman RJ. Fragile X–associated tremor/ataxia syndrome. Ann NY Acad Sci. 2015;1338:58–70. doi: 10.1111/nyas.12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunningham CL, Martinez Cerdeño V, Navarro Porras E, et al. Premutation CGG-repeat expansion of the Fmr1 gene impairs mouse neocortical development. Hum Mol Genet. 2011;20:64–79. doi: 10.1093/hmg/ddq432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan, Eitan S, et al. Early mitochondrial abnormalities in hippocampal neurons cultured from Fmr1 premutation mouse model. J Neurochem. 2012;123:613–621. doi: 10.1111/j.1471-4159.2012.07936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao Z, Hulsizer S, Tassone F, et al. Clustered burst firing in FMR1 premutation hippocampal neurons: amelioration with allopregnanolone. Hum Mol Gen. 2012;21:2923–2935. doi: 10.1093/hmg/dds118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y, Tassone F, Berman RF, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet. 2010;19:196–208. doi: 10.1093/hmg/ddp479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gallego PK, Burris JL, Rivera SM. Visual motion processing deficits in infants with the fragile X premutation. J Neurodev Disord. 2014;6:29. doi: 10.1186/1866-1955-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorders in boys with fragile X premutation. J Dev Behav Pediatr. 2006;27:S137–S144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 33.Chonchaiya W, Tassone F, Ashwood P, et al. Autoimmune disease in mothers with the FMR1 premutation is associated with seizures in their children with fragile X syndrome. Hum Genet. 2010;128:539–548. doi: 10.1007/s00439-010-0882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chonchaiya W, Au J, Schneider A, et al. Increased prevalence of seizures in boys who were probands with the FMR1 premutation and co-morbid autism spectrum disorder. Hum Genet. 2012;131:581–589. doi: 10.1007/s00439-011-1106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutcliffe JS, Nelson DL, Zhang F, et al. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1:397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 36.Malter HE, Iber JC, Willemsen R, et al. Characterization of the full fragile X syndrome mutation in fetal gametes. Nat Genet. 1997;15:165–169. doi: 10.1038/ng0297-165. [DOI] [PubMed] [Google Scholar]
- 37.Willemsen R, Bontekoe CJ, Severijnen LA, Oostra BA. Timing of the absence of FMR1 expression in full mutation chorionic villi. Hum Genet. 2002;110:601–605. doi: 10.1007/s00439-002-0723-5. [DOI] [PubMed] [Google Scholar]
- 38.Slonim DK, Koide K, Johnson KL, et al. Functional genomic analysis of amniotic fluid cell-free mRNA suggests that oxidative stress is significant in Down syndrome fetuses. Proc Natl Acad Sci USA. 2009;106:9425–9429. doi: 10.1073/pnas.0903909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koide K, Slonim DK, Johnson KL, et al. Transcriptomic analysis of cell-free fetal RNA suggests a specific molecular phenotype in trisomy 18. Hum Genet. 2011;129:295–305. doi: 10.1007/s00439-010-0923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hui L, Slonim DK, Wick HC, et al. Novel neurodevelopmental information revealed in amniotic fluid supernatant transcripts from fetuses with trisomies 18 and 21. Hum Genet. 2012;131:1751–1759. doi: 10.1007/s00439-012-1195-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Massingham LJ, Johnson KL, Scholl TM, et al. Amniotic fluid RNA gene expression profiling provides insights into the phenotype of Turner syndrome. Hum Genet. 2014;133:1075–1082. doi: 10.1007/s00439-014-1448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zwemer LM, Bianchi DW. The amniotic fluid transcriptome as a guide to understanding fetal disease. Cold Spring Harb Perspect Med. 2015;5 doi: 10.1101/cshperspect.a023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunsaker MR, Greco CM, Spath MA, et al. Widespread non-central nervous system organ pathology in fragile X premutation carriers with fragile X-associated tremor/ataxia syndrome and CGG knock-in mice. Acta Neuropathol. 2011;122:467–479. doi: 10.1007/s00401-011-0860-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui L, Slonim DK, Wick HC, et al. The amniotic fluid transcriptome: a source of novel information about human fetal development. Obstet Gynecol. 2012;119:111–118. doi: 10.1097/AOG.0b013e31823d4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dietz JA, Johnson KL, Massingham LJ, et al. Comparison of extraction techniques for amniotic fluid supernatant demonstrates improved yield of cell-free fetal RNA. Prenatal Diagn. 2011;31:598–599. doi: 10.1002/pd.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edgar R, Domrachev M, Lash AE. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005:10215545–10215550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wick HC, Drabkin H, Ngu H, et al. DFLAT: functional annotation for human development. BMC Bioinformatics. 2014;15:45. doi: 10.1186/1471-2105-15-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edlow AG, Slonim DK, Wick HC, et al. The pathway not taken: understanding 'omics data in the perinatal context. Am J Obstet Gynecol. 2015;213:59.e1–59.e172. doi: 10.1016/j.ajog.2015.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6:407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larrabee PB, Johnson KL, Lai C, et al. Global gene expression analysis of the living human fetus using cell-free messenger RNA in amniotic fluid. JAMA. 2005;293:836–842. doi: 10.1001/jama.293.7.836. [DOI] [PubMed] [Google Scholar]
- 52.Pretto DI, Hunsaker MR, Cunningham CL, et al. Intranuclear inclusions in a fragile X mosaic male. Transl Neurodegener. 2013;2:10. doi: 10.1186/2047-9158-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lokanga RA, Entezam A, Kumari D, et al. Somatic expansion in mouse and human carriers of fragile X premutation alleles. Human Mutat. 2013;34:157–166. doi: 10.1002/humu.22177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eiges R, Urbach A, Malcov M, et al. Developmental study of fragile X syndrome using human embryonic stem cells derived from preimplantation genetically diagnosed embryos. Cell Stem Cell. 2007;1:568–577. doi: 10.1016/j.stem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 55.Greer PL, Hanayama R, Bloodgood BL, et al. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140:704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 57.Handa V, Goldwater D, Stiles D, et al. FEBS Lett Long CGG-repeat tracts are toxic to human cells: implications for carriers of Fragile X premutation alleles. 2005;579:2702–2708. doi: 10.1016/j.febslet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation. 1961;24:1311–1318. doi: 10.1161/01.cir.24.6.1311. [DOI] [PubMed] [Google Scholar]
- 59.Beuren AJ, Apitz J, Harmjanz D. Supravalvular aortic stenosis in association with mental retardation and certain facial appearance. Circulation. 1962;26:1235–1240. doi: 10.1161/01.cir.26.6.1235. [DOI] [PubMed] [Google Scholar]
- 60.Robinson WP, Waslynka J, Bernasconi F, et al. Delineation of 7q11.2 deletions associated with Williams-Beuren syndrome and mapping of a repetitive sequence to within and to either side of the common deletion. Genomics. 1996;34:17–23. doi: 10.1006/geno.1996.0237. [DOI] [PubMed] [Google Scholar]
- 61.Yaplito-Lee J, Pitt J, Meijer J, et al. Beta-ureidopropionase deficiency presenting with congenital anomalies of the urogenital and colorectal systems. Molec Genet Metab. 2008;93:190–194. doi: 10.1016/j.ymgme.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Carrasquillo MM, Zou F, Pankratz VS, et al. Genetic variation in PCDH11X is associated with susceptibility to late-onset Alzheimer disease. Nature Genet. 2009;41:192–198. doi: 10.1038/ng.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ohno K, Hutchinson DO, Milone M, et al. Congenital myasthenic syndrome caused by prolonged acetylcholine receptor channel openings due to a mutation in the M2 domain of the epsilon subunit. Proc Natl Acad Sci USA. 1995;92:758–762. doi: 10.1073/pnas.92.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng BG, Wolfe LA, Ichikawa M, et al. Biallelic mutations in CAD impair de novo pyrimidine biosynthesis and decrease glycosylation precursors. Hum Molec Genet. 2015;24:3050–3057. doi: 10.1093/hmg/ddv057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Beyer B, Deleuze C, Letts VA, et al. Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4. Hum Molec Genet. 2008;17:1738–1749. doi: 10.1093/hmg/ddn064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim HG, Kishikawa S, Higgins AW, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yan J, Noltner K, Feng J, et al. Neurexin 1alpha structural variants associated with autism. Neurosci Lett. 2008;438:368–370. doi: 10.1016/j.neulet.2008.04.074. [DOI] [PubMed] [Google Scholar]
- 68.Lintas C, Persico AM. Autistic phenotypes and genetic testing: state-of-the-art for the clinical geneticist. J Med Genet. 2009;46:1–8. doi: 10.1136/jmg.2008.060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ueda K, Fukushima H, Masliah E, et al. Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:11282–11286. doi: 10.1073/pnas.90.23.11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nakamura K, Nemani VM, Azarbal F, et al. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rintoul GL, Filiano AJ, Brocard JB, et al. Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J Neurosci. 2003;23:7881–7888. doi: 10.1523/JNEUROSCI.23-21-07881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yi M, Weaver D, Hajnóczky G. Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J Cell Biol. 2004;167:661–672. doi: 10.1083/jcb.200406038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lebiedzinska M, Szabadkai G, Jones AW, et al. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int J Biochem Cell Biol. 2009;41:1805–1816. doi: 10.1016/j.biocel.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 74.Tasha MS, Nouri K, Milroy LG, et al. Subcellular Fractionation and Localization Studies Reveal a Direct Interaction of the Fragile X Mental Retardation Protein (FMRP) with Nucleolin. 2014;9:e91465. doi: 10.1371/journal.pone.0091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ross-Inta C, Omanska-Klusek A, Wong S, et al. Evidence of mitochondrial dysfunction in fragile X-associated tremor/ataxia syndrome. Biochem J. 2010;429:545–552. doi: 10.1042/BJ20091960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yao A, Jin S, Li X, et al. Drosophila FMRP regulates microtubule network formation and axonal transport of mitochondria. Hum Mol Genet. 2011;20:41–63. doi: 10.1093/hmg/ddq431. [DOI] [PubMed] [Google Scholar]
- 77.Jamain S, Quach H, Betancur C, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Laumonnier F, Bonnet-Brilhault F, Gomot M, et al. X-linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cvetkovska V, Hibbert AD, Emran F, Chen BE. Overexpression of down syndrome cell adhesion molecule impairs precise synaptic targeting. Nat Neurosci. 2013;16:677–682. doi: 10.1038/nn.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tessier CR, Broadie K. The fragile x mental retardation protein developmentally regulates the strength and fidelity of calcium signaling in drosophila mushroom body neurons. Neurobiol Dis. 2011;41:147–159. doi: 10.1016/j.nbd.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Matic K, Eninger T, Bardoni B, et al. Quantitative phosphoproteomics of murine Fmr1-KO cell lines provides new insights into FMRP-dependent signal transduction mechanisms. J Proteome Res. 2014;13:4388–4397. doi: 10.1021/pr5006372. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.