Abstract
Pancreatic Ductal Adenocarcinoma (PDAC) is one of the deadliest malignancies lacking effective therapeutic strategies. Here we show that the non-canonical IκB-related kinase, IKBKE, is a critical oncogenic effector during KRAS-induced pancreatic transformation. Loss of IKBKE inhibits the initiation and progression of pancreatic tumors in mice carrying pancreatic specific KRAS activation. Mechanistically, we demonstrate that this pro-tumoral effect of IKBKE involves the activation of GLI1 and AKT signaling, and independent of the levels of activity of the NFκB pathway. Further analysis reveals that IKBKE regulates GLI1 nuclear translocation, and promotes the reactivation of AKT post-inhibition of mTOR in PDAC cells. Interestingly, combined inhibition of IKBKE and mTOR synergistically blocks pancreatic tumor growth. Together, our findings highlight the functional importance of IKBKE in pancreatic cancer, support the evaluation of IKBKE as a therapeutic target in PDAC, and suggest IKBKE inhibition as a strategy to improve efficacy of mTOR inhibitors in the clinic.
Keywords: IKBKE, KRAS, AKT, mTOR, pancreatic cancer
INTRODUCTION
Pancreatic Ductal Adenocarcinoma (PDAC) is the most common and aggressive type of pancreatic cancer (1, 2). KRAS mutation, detected in >90% of PDAC cases, is a critical oncogenic event during PDAC initiation and progression (3). Mutant KRAS is known to activate multiple signaling pathways to promote its oncogenic activity, including MAPK, PI3K, RAL-A/B, and NF-κB pathways (4–10); however, the stimulation of these cascades does not explain the pleotropic effects of mutant KRAS. The identification of additional downstream pathways is critical to define the mechanism underlying KRAS-induced pancreatic tumorigenesis, and targeting these pathways may enable a more effective therapeutic strategy for PDAC treatment.
We previously demonstrated that Hedgehog ligand independent GLI activity is critical for KRAS-induced pancreatic transformation in vivo(11), and identified IKBKE as a downstream target of GLI1 in PDAC cells (11). IKBKE and its closely related kinase, TBK1, were originally identified as the non-canonical IκB kinases involved in regulation of NF-kB signaling (12, 13). The oncogenic activity of IKBKE/TBK1 has been linked to NF-κB activation in several tumorigenic contexts (14–18). TBK1 has also been reported to be activated by KRAS via a RALB-dependent mechanism to promote tumor cell survival (19). Despite their potential importance, the genetic requirement of these kinases in tumorigenesis, including KRAS-induced PDAC formation, has not been demonstrated, and it is not clear which pathways play major roles downstream of IKBKE in vivo.
Here, we show that IKBKE function is critical for KRASG12D dependent pancreatic transformation in vivo. Surprisingly we find that IKBKE is not essential for cytokine/NF-κB activation during pancreatic tumorigenesis; however, it engages in reciprocal regulation of GLI, regulates mTOR-independent AKT activity, and promotes AKT re-activation upon mTOR inhibition in PDAC cells in vitro and in vivo.
MATERIALS AND METHODS
Mouse strains
P48Cre, LSL-KrasG12D, and Ikbke−/− mice were obtained from Jackson Laboratories. P48Cre;LSL-KrasG12D;Ikbke−/− mice were obtained via interbreeding P48Cre mice with LSL-KrasG12D;Ikbke−/− mice. All mouse experiments were performed according to the guidelines of IACUC at University of Massachusetts Medical School.
Tissue collection and histology
Upon euthanasia, pancreatic tissue was fixed in 4% paraformaldehyde for 24 hours. For paraffin sections, tissue was dehydrated and embedded in paraffin blocks. Paraffin sections were stained with hematoxylin and eosin (H&E) using standard reagents and protocols. Human PDAC tissue microarray was obtained from UMass Tissue Bank and Genvelop.
Immunohistochemistry and immunoblotting
For immunohistochemistry, antigen retrieval was conducted in Sodium Citrate solution (pH 6.0) for 30 minutes. Sections were blocked in a buffer containing 5% BSA and 0.1% Triton X-100 in PBS and incubated overnight at 4 °C in primary antibodies diluted in blocking buffer. Primary antibodies used were: Ki67 (1:500, Abcam), phospho-AKT (1:50, Cell Signaling), IKBKE (1:50, Santa Cruz) for mouse sections, IKBKE (1:100, Sigma) for human sections, TBK1 (1:100, Cell Signaling), p65 (1:50, Cell Signaling), Amylase (1:800, Sigma), and Insulin (1:100, Abcam). Signal detection was accomplished with biotinylated secondary antibodies in the Vectastain ABC kit (Vector Labs).
For immunoblotting, the primary antibodies used were Flag-HRP (1:1,000 Sigma); β-Actin (1:1,000, Sigma); phospho-AKT S473 (1:1,000, Cell Signaling), phospho-Akt T308 (1:1000, Cell Signaling), phospho-ERK (1:1,000, Cell Signaling); total AKT (1:1,000, Cell Signaling); total ERK (1:1,000, Cell Signaling); IKBKE (1:1,000, Sigma), TBK1 (1:1000, Cell Signaling), phospho-S6K (1:1000, Cell Signaling), phospho-4EBP1 (1:1000, Cell Signaling), p65 (1:1000, Cell Signaling), PCNA (1:1000, Abcam), β-Tubulin (1:1000, Cell Signaling), Cleaved PARP Asp 214 (1:1000, Cell Signaling), and GLI1 (1:1000, Cell Signaling). HRP-conjugated secondary antibodies used for detection were obtained from Jackson Laboratories.
Cell lines
293T (CRL-3216), Panc-1 (CRL-1469), and MiaPaca-2 (CRL-1420) cell lines were obtained from the ATCC repository. Cell line characterization by ATCC is conducted by STR analysis. Cell lines were expanded, and cryogenically frozen upon acquisition to establish stocks, and stored in liquid nitrogen until use. Cell lines were cultured for a maximum of 3 months before experimentation. Mycoplasma testing was conducted using the Universal Mycoplasma Detection kit (ATCC), and cell lines were also analyzed for morphology, and proliferation before experimentation.
Cell proliferation, apoptosis and soft-agar assays
Cell proliferation, apoptosis, and soft-gar assays were conducted as previously described(11).
Lentiviral shRNA knockdown experiments
Cells were infected with pLKO-based lentiviruses encoding shRNAs targeting human Gli1 (#1: CATCCATCACAGATCGCATTT; #2: GCTCAGCTTGTGTGTAATTAT), KRAS (#1: GAGGGCTTTCTTTGTGTATTT; #2: TGAAGATATTCACCATTATAG), TBK1 (#1: GCAGAACGTAGATTAGCTTAT; #2:GCGGCAGAGTTAGGTGAAATT) and IKBKE (#1: TGGGCAGGAGCTAATGTTTCG; #2: GAGCATTGGAGTGACCTTGTA). Infected cells were selected in 5 μg/mL puromycin for 4 days prior to conducting assays.
Luciferase reporter analysis
NF-κB luciferase (p65-Luc) was a gift from Dr. Francis Chan (University of Massachusetts Medical School, Worcester, MA). Reporters were co-transfected with the expression vectors for Gli3T, IKBKE, IKBKE K38A, Gli1-AHA using lipofectamine 2000. IKBKE promoter luciferase was generated by cloning a 300bp region upstream of the human IKBKE transcription start site into a PGL3 luciferase vector. Luciferase assays were conducted 48 hours after transfection using the dual-luciferase reporter kit (Promega). Assays were conducted in triplicate.
Quantitative RT-PCR
cDNA synthesis was conducted using Invitrogen SuperScript II kit. Primers used for qRT-PCR were human IKBKE (forward: 5′-TGCGTGCAGAAGTATCAAGC-3′; reverse: 5′-TACAGGCAGCCACAGAACAG-3′); mouse Ikbke (forward: 5′-GCGGAGGCTGAATCACCAG-3′; human GAPDH (forward: 5′-ATGGGGAAGGTGAAGGTCG-3′; reverse: 5′-GGGGTCATTGATGGCAACAATA-3′); mouse Gapdh (forward: 5′-AGGCCGGTGCTGAGTATGTC-3′; reverse: 5′-TGCCTGCTTCACCACCTTCT-3′); human GLI1 (forward: 5′-CCAGCGCCCAGACAGAG-3′; reverse:5′-GGCTCGCCATAGCTACTGAT-3′); mouse Gli1 (forward: 5′-GTCGGAAGTCCTATTCACGC-3′; reverse: 5′-CAGTCTGCTCTCTTCCCTGC-3′); human PTCH1 (forward: 5′-CCACAGAAGCGCTCCTACA-3′; reverse 5′-CTGTAATTTCGCCCCTTCC-3′); mouse Ptc1 (forward: 5′-AACAAAAATTCAACCAAACCTC-3 ′reverse: 5′-TGTCTTCATTCCAGTTGATGTG-3′); human IL1A (forward: ATCATGTAAGCTATGGCCCACT; reverse: CCTTCCCGTTGGTTGCTACTA), mouse Il1a (forward: 5′-TCTATGATGCAAGCTATGGCTCA-3′; reverse: 5′-CGGCTCTCCTTGAAGGTGA-3′); human TNFA (forward: CCTCTCTCTAATCAGCCCTCTG; reverse: GAGGACCTGGGAGTAGATGAG), mouse Tnf (forward: 5′-CAGGCGGTGCCTATGTCTC-3′; reverse: 5′-CGATCACCCCGAAGTTCAGTAG-3′); human BCL2L1 (forward: CTGCTGCATTGTTCCCATAG-3′; reverse: 5′-TTCAGTGACCTGACATCCCA-3′), mouse Bcl2l1 (forward: 5′-ACATCCCAGCTTCACATAACCC-3′; reverse: 5′-CCATCCCGAAAGAGTTCATTCAC-3′); human BCL2 (forward: 5′-ATGTGTGTGGAGAGCGTCAA-3′; reverse: 5′-CGTACAGTTCCACAAAGGCA-3′); and mouse Bcl2 (forward: 5′-GCTACCGTCGTGACTTCGC-3′; reverse: 5′-CCCCACCGAACTCAAAGAAGG-3′). All qPCR assays were conducted in triplicate.
Nuclear and cytoplasmic fractionation
For nuclear and cytoplasmic fractionation, Panc-1 cells were infected either with shIKBKE#1, or with shRNA targeting GFP, and selected with puromycin for 4 days. Nuclear and cytoplasmic fractions were separated using a kit from G Biosciences according to manufacturer’s protocol.
Xenograft mouse models
MiaPaca-2 PDAC cells were stably infected either with the doxycycline inducible lentiviral vectors pTRIPZ and ptet-pLKO (Addgene) expressing shRNAs targeting either IKBKE or mTOR. These stable cell lines were then injected mice at a concentration of 1 × 107 cells in a volume of 100 ul in 1:1 ratio with matrigel in subcutaneously in the flanks of NOD-SCID mice (Jackson Laboratory). Tumors were allowed to grow to a volume of ~100 mm3 after which Doxycycline was administered in drinking water at a concentration of 100 μg/ml. Tumors were allowed to grow for 30 days and measured every 3 days using calipers. Tumor volume was calculated using the formula w2 × l. Tumors were dissected and whole tumors were imaged after 30 days of Doxycycline treatment.
RESULTS
IKBKE acts downstream of KRAS to promote PDAC initiation
Immunohistochemistry (IHC) analysis of IKBKE expression in a Tissue Microarray of human pancreatic cancer samples (N=105) showed IKBKE expression is high in the majority of human PDAC samples, but minimal in normal human pancreas (Figure 1A–B, S1A–D, I). The closely related non-canonical IkB-related kinase, TBK1, was also expressed in human PDAC, although in a less degree (Figure S1E–I). To further explore their connection to KRAS in PDAC, we conducted shRNA mediated knockdown of KRAS in human PDAC cells carrying oncogenic mutant KRAS. Knockdown of this GTPase led to a significant decrease in expression of IKBKE, but not TBK1 in Panc-1 and MiaPaca2 PDAC cells (Figures S2A–D) as measured by the immunoblotting and quantitative RT-PCR analyses. In addition, we showed that knockdown of IKBKE in human PDAC cells resulted in significant increase in apoptosis, marked decrease in cell proliferation, as well as transformation as measured by the soft agar colony formation assay (Figure S2E–J). Together, our data suggests that IKBKE is a downstream target of KRAS that may mediate its oncogenic effect in PDAC.
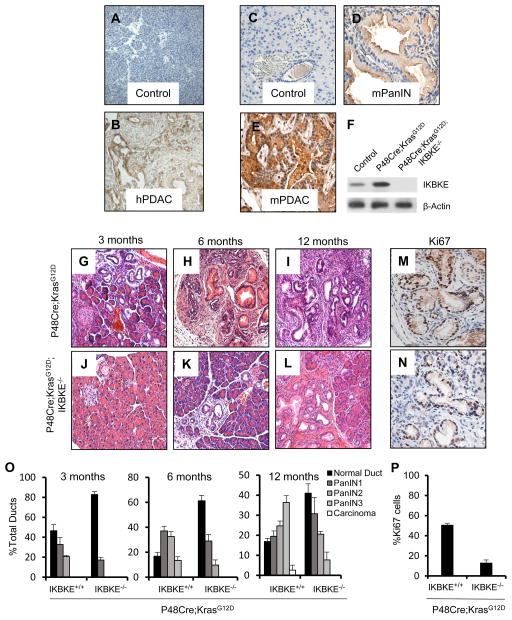
Figure 1. IKBKE requirement in Kras-induced pancreatic tumorigenesis.
Immunohistochemistry staining of IKBKE in human (A–B), and mouse pancreas (C–E). IKBKE protein levels are significantly higher in human PDAC samples (B) compared to wild type pancreas (A). PanIN lesions (D), and PDAC tissue (E) derived from P48Cre;KRASG12D mice have significantly higher IKBKE compared to wild type pancreas (C). (F) Western blot showing IKBKE levels in pancreas of 12 month old P48Cre;KrasG12D and P48Cre;KrasG12D;IKBKE−/− mice. IKBKE is not expressed in pancreas from P48Cre;KrasG12D;IKBKE−/− mice but is expressed in P48Cre;KrasG12D mice. (G–I) Representative H&E staining images of pancreas of 3 month old (G), 6 month old (H), and 12 month old (I) P48Cre;KrasG12D mice. (J–L) Representative H&E staining images of pancreas of 3 month old (J), 6 month old (K), and 12 month old (L) P48Cre;KrasG12D;IKBKE−/− mice. (M) Quantification of grade of PanIN lesions in age matched P48Cre;KrasG12D and P48Cre;KrasG12D;IKBKE−/− mouse pancreas at ages 3 months, 6 months, and 12 months. Comparison of age matched pancreas indicates significantly delayed initiation and progression of pancreatic neoplasms in P48Cre;KrasG12D;IKBKE−/− mice compared to P48Cre;KrasG12D mice. (N–O) Immunohistochemistry for Ki67 in 6 month old P48Cre;KrasG12D (N), and P48Cre;KrasG12D;IKBKE−/− (O) mice indicates decreased number of proliferative cells (P). Error bars represent Standard Deviation.
IKBKE is required for pancreatic neoplastic transformation in vivo
To further test the biological role of IKBKE in vivo, we utilized a mouse model carrying whole body knockout of Ikbke (21). The Ikbke−/− mice had histologically normal pancreatic architecture at 12 months of age (N=6) as compared to wild type mice (Figure S3A–B). IHC staining of the acinar cell marker amylase, and islet cell marker insulin also showed normal pancreatic differentiation (Figure S3C–F). These results suggest that IKBKE is not required for development of pancreas.
To examine whether IKBKE function is specifically required during KRAS-induced pancreatic transformation, we utilized the mouse model in which an oncogenic allele of Kras (KRASG12D) is targeted to the endogenous locus and expressed specifically in the pancreatic epithelium using Cre-recombinase expressed under the P48 (Ptf1a) promoter (22). As expected, the P48Cre;LSL-KrasG12D mice developed pancreatic intraepithelial neoplasia (PanIN) lesions of histological grades ranging from PanIN1-3, depending on the age of the mice, with some 12 month old mice displaying full blown PDAC (Figure 1G–I). Consistent with our IHC analysis in human PDAC, we found that IKBKE expression was also highly upregulated in mouse PanIN lesions as well as in PDAC compared to wild type mouse pancreas (Figure 1C–E).
To achieve simultaneous KRAS activation and IKBKE loss in the pancreas (Figure 1F), we generated P48Cre;KrasG12D;Ikbke−/− mice, and analyzed pancreas at ages 3 months, 6 months, and 12 months. We found that the P48Cre;LSL-KrasG12D (N=15) mice developed PanIN grade 1 and instances of grade 2 lesions at the age of 3 months, with increased number of PanIN 2 and 3 grade lesions at 6 months (Figure 1G, H). The normal pancreatic architecture was lost by 12 month age, with advanced grade lesions covering majority of the pancreas (Figure 1I), and instances of adenocarcinoma in some mice. In contrast, pancreas of P48Cre;LSL-KrasG12D;Ikbke−/− mice (N=24) were relatively normal at these 3 time points, with some low grade PanIN lesions at 6 and 12 month time points (Figure 2J–L). Quantification of PanIN lesions from H&E stained sections obtained from the mouse pancreas samples also showed significantly inhibition of initiation and delayed onset of pancreatic neoplastic transformation in P48Cre;LSL-KrasG12D;Ikbke−/− mice (Figure 2O). We also conducted Ki67 staining on stage matched lesions from P48Cre;LSL-KrasG12D and P48Cre;LSL-KrasG12D/;Ikbke−/− mice. PanIN lesions from P48Cre;LSL-KrasG12D;Ikbke−/− mice had less Ki67 positive cells compared to similar staged lesions from P48Cre;LSL-KrasG12D mice (Figure 2M, N, P, and Figure S4). This data indicates that IKBKE loss may impair cell proliferation, and therefore delay progression of the PanIN lesions. Together, our findings suggest a critical requirement of IKBKE in both KRAS-induced initiation and progression of pancreatic transformation.
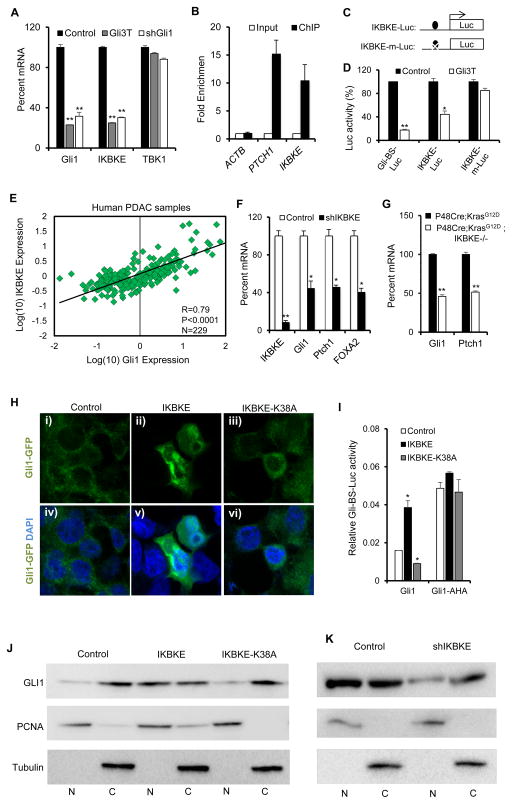
Figure 2. Reciprocal IKBKE-Gli1 interaction in PDAC cells.
(A) qPCR analysis in Panc-1 cells showing GLI1, IKBKE, and TBK1 mRNA expression after inhibition of GLI activity using a dominant negative GLI (Gli3T), or shRNA targeting GLI1. (B) Quantitative PCR of DNA enriched using ChIP against Flag-tag in Panc-1 cells infected with Gli3T-Flag indicates significant enrichment of IKBKE promoter region as well as the promoter of a known GLI target gene (PTCH1), but not control ACTB promoter region. (C) Schematic showing location of GLI binding site and mutated GLI binding site upstream of luciferase reporters. (D) Relative luciferase activity in Panc-1 cells expressing Gli-BS luciferase reporter (Gli-BS-Luc), IKBKE luciferase containing the wild type GLI binding site (IKBKE-Luc), and IKBKE luciferase carrying a mutated GLI binding site (IKBKE-m-Luc), with or without GLi3T ectopic expression. (E) Correlation of GLI1 and IKBKE mRNA expression in human PDAC tissue samples (n=229). Pearson coefficient of R = 0.79 indicates high degree of correlation between GLI1, and IKBKE expression in the tumors. (F) Quantitative RT-PCR analysis of transcription of IKBKE and the known GLI target genes, GLI1, PTCH1, and FOXA2, in Panc-1 cells with IKBKE knockdown. (G) Quantitative RT-PCR in tissue samples indicates significantly reduced expression of GLI target genes, Gli1 and Ptch1, in 6 month old P48Cre;KrasG12D;Ikbke−/− mouse pancreas compared to age-matched P48Cre;Kras;G12D pancreas. (H) Subcellular localization of Gli1-GFP fusion protein in transfected 293T cells with (i–iii) and without (iv–vi) overlay with DAPI. While GLI1 is localized mainly in the cytoplasm without IKBKE expression (i, iv), IKBKE expression drives nuclear localization of GLI1 (ii, v). Expression of a kinase-dead version of IKBKE (K38A) causes cytoplasmic retention of GLI1 (iii, vi). (I) Co-expression of IKBKE, but not the kinases-dead version of IKBKE, with GLI1 synergistically increases GLI transcriptional activity as measured by Gli-BS Luciferase activity in 293T cells. IKBKE expression has no effect on the activity of Gli1-AHA, a mutant form of Gli1 that is constitutively localized to the nucleus. Error bars represent Standard Deviation. (J) Western blot analysis of nuclear and cytoplasmic fractions indicates that ectopic coexpression with wild type but not catalytically inactive (K38A) IKBKE leads to significant nuclear localization of GLI1. PCNA, and Tubulin are used as controls for nuclear (N) and cytoplasmic fractions (C) respectively. (K) Western blot analysis of nuclear and cytoplasmic fractions in Panc-1 cells indicates decrease in endogenous GLI1 nuclear localization after shRNA mediated knockdown of IKBKE. Statistical significance was determined using a Student’s two-tailed t-test. * P<0.05; ** P<0.01.
NF-κB activity in PDAC cells is independent of IKBKE
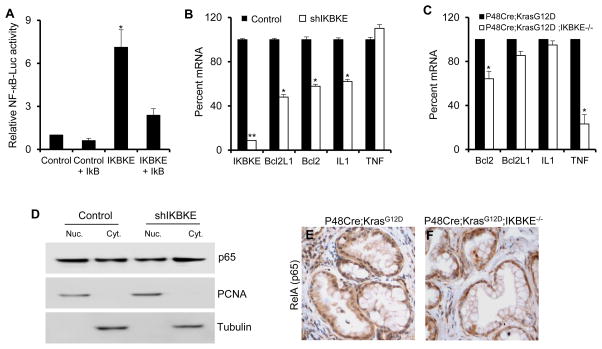
IKBKE was initially identified as an IκB kinase involved in regulation of NF-κB signaling (12). The cytokine-NF-κB axis has been shown to play an important role in pancreatic tumorigenesis (9, 10, 29). Thus, we examined the possible role of IKBKE in NF-κB activation in PDAC. We found that, although IKBKE overexpression could activate the NF-κB luciferase reporter activity in an IκB dependent manner (Figure 3A), IKBKE knockdown in PDAC cells did not significantly affect the expression of several known NF-κB target genes and cytokines (Figure 3B). In addition, we did not detect significant downregulation of NF-κB target gene expression in the pancreata of P48Cre;KrasG12D;Ikbke−/− mice compared to P48Cre;KrasG12D mice (Figure 3C).
Figure 3. IKBKE does not play a significant role in regulating NF-kB activity in PDAC.
(A) NF-κB luciferase activity in 293T cells is significantly increased in response to IKBKE ectopic expression. IKBKE activation of NF-κB activity can be inhibited by a dominant negative version of IκB suppressor. (B) qPCR analysis of mRNA levels of NF-κB target genes, BCL2L1, BCL2, IL1 and TNFA, in Panc-1 cells with and without IKBKE knockdown. (C) Quantitative RT-PCR analysis of mRNA levels of NF-κB target genes, Bcl2L1, Bcl2, Il1 and Tnf, in pancreas of 6 month old P48Cre;KrasG12D, and P48Cre;KrasG12D;Ikbke−/− mice. (D) Western blot showing RELA (p65) in nuclear and cytoplasmic fractions of Panc-1 cells with and without shRNA knockdown of IKBKE. PCNA and Tubulin are used as controls for nuclear and cytoplasmic fractions respectively. (E–F) Immunohistochemistry for p65 in stage matched PanIN lesions in P48Cre; KrasG12D (E), and P48Cre;KrasG12D;Ikbke−/− (F) mouse pancreas. Error bars represent Standard Deviation. Statistical significance was determined using a Student’s two-tailed t-test. * P<0.05; ** P<0.01
Nuclear localization of the NF-κB subunit p65, a hallmark for NF-κB pathway activation, was not affected by IKBKE knockdown in PANC1 cells (Figure 3D). Furthermore, we compared subcellular localization of p65 in stage-matched PanIN lesions of P48Cre;KrasG12D;Ikbke−/− and P48Cre;KrasG12D mice using immunohistochemistry. We found that nuclear p65 was present in the PanIN lesions and there was no significant difference in nuclear localization of p65 between lesions of P48Cre; KrasG12D;Ikbke−/− and P48Cre;KrasG12D mice (Figure 3E-F). Our findings indicate that IKBKE does not appear to play a major role in NF-κB activation in KRAS-induced pancreatic tumorigenesis, and that IKBKE oncogenic activity in the context of PDAC is mediated by NF-κB independent mechanisms.
IKBKE is a direct target of GLI1 in PDAC cells
Our expression profiling analysis in human PDAC cells identified IKBKE as a candidate GLI1 downstream target gene (11). Consistent with this idea, we found that inhibition of GLI transcriptional activity by either the dominant-negative Gli3T repressor (11), or shRNA mediated knockdown of GLI1 in Panc-1 cells decreased IKBKE but not TBK1 mRNA levels (Figure 2A), suggesting that TBK1 does not act downstream of GLI1. In addition, chromatin immunoprecipitation in Gli3T-expressing Panc-1 cells showed significant enrichment of GLI protein in the IKBKE promoter region 130 bp upstream of the transcriptional start site, as well as the promoter region of the known GLI target gene, PTCH1 (Figure 2B). Sequence analysis of the IKBKE promoter region revealed the existence of a candidate GLI binding site (GACTTCCCA) (Figure 2C). We generated IKBKE promoter-driven luciferase reporter constructs with a wild-type (IKBKE-Luc) or mutated GLI binding site (IKBKE-m-Luc) (Figure 2C). We showed that Gli3T was able to inhibit the luciferase activity of IKBKE-Luc, as well as Gli-BS-Luc, a luciferase reporter carrying 8 consecutive GLI canonical binding sites (24). However, it failed to block IKBKE-m-Luc activity in Panc1 cells (Figure 2D). Together, these findings suggest that IKBKE is a direct transcriptional target of GLI in PDAC cells.
We next compared mRNA expression levels of IKBKE vs GLI1 in human PDAC patient samples, and found a strong correlation (R = 0.79, P<0.0001) between the expression of the two genes in human PDAC samples (Figure 2E). This tight correlation led us to further explore the IKBKE-GLI1 interaction. Despite the importance of the Hh ligand-independent GLI1 activity in PDAC (11, 25), the upstream mechanism regulating this non-canonical GLI1 activation is not well understood. Interestingly, we found that IKBKE knockdown in Panc-1 cells led to a significant decrease in mRNA levels of the GLI target genes GLI1, FOXA2, and PTCH1 (Figure 2F). Pancreas tissue from P48Cre;KrasG12D;IKBKE−/− mice also exhibited significantly lower levels of mRNA of the GLI1 target genes compared to P48Cre; KrasG12D mice (Figure 2G), suggesting that IKBKE may be involved in regulating GLI activity in pancreatic tumor cells.
We and others have shown that regulation of the intracellular localization of GLI1, a nuclear-cytoplasmic shuttling protein, is important for its transcriptional activity (26–28). We found that, when IKBKE and GFP-fused version of GLI1 were co-expressed, IKBKE promotes the nuclear translocalization of GLI1 (Figure 2H, J), measured by immunofluorescence staining and western blot analysis. This IKBKE effect was dependent on its kinase activity, as a kinase-dead IKBKE mutant (K38A), was not able to promote GLI1 translocation (Figure 2H, J). To further test IKBKE and GLI interaction, we utilized a mutant version of GLI1 (Gli1-AHA) that is constitutively localized to the nucleus (26). We found that co-expression of IKBKE, but not IKBKE-K38A, led to a synergistic increase in the activation of wild type GLI1 (Figure 2I). However, co-expression of IKBKE or IKBKE-K38A did not significantly affect the activity of Gli1-AHA (Figure 2I). More importantly, when endogenous IKBKE was knocked down in MiaPaCa2 PDAC cells by shRNA, it markedly increased the cytoplasmic fraction of Gli1 protein (Figure 2K). Taken together, these data uncover a previously unknown IKBKE-mediated Gli regulation in PDAC cells, and suggest that IKBKE modulates GLI activity by controlling its nuclear localization.
IKBKE promotes AKT activation in PDAC
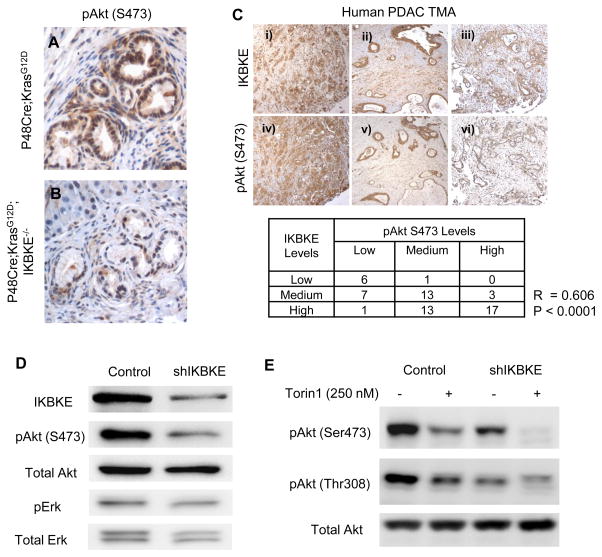
Further analysis of the mechanism found that levels of phosphorylated AKT, a substrate of IKBKE at both Serine-473 and Threonine-308 sites (insert ref) were markedly decreased in the stage-matched PanIN lesions of P48Cre; KrasG12D;Ikbke−/− mouse pancreas compared to P48Cre;KrasG12D mice (Figure 4A–B). Furthermore, the phospho-AKT levels in a tissue microarray of human PDAC samples (N=62) significantly correlated between AKT phosphorylation and expression levels of IKBKE in the samples as indicated by a Pearson coefficient of R = 0.606 (P<0.0001) (Figure 4C). In addition, we showed that IKBKE knockdown in Panc-1 cells led to a decrease in the phosphorylation of AKT but not ERK (Figure 4D), thus suggesting IKBKE-dependent phosphorylation of AKT in PDAC.
Figure 4. IKBKE regulates AKT activation in PDAC cells in vivo and in vitro.
(A–B) Immunohistochemistry for AKT phosphorylation at Serine-473 in stage matched PanIN lesions of P48Cre;KrasG12D (A), and P48Cre;KrasG12D;IKBKE−/− (B) mouse pancreas. P48Cre;KrasG12D;IKBKE−/− PanIN lesions have significantly reduced AKT phosphorylation compared to P48Cre;KrasG12D lesions. (C) Correlation of IKBKE protein levels and AKT Serine-473 phosphorylation levels in human PDAC tissue microarray. Representative images of immunohistochemistry for IKBKE (i–iii) and phospho-AKT Serine-473 (iv–vi) in matched human PDAC tissue samples. (D) Western blot showing phosphorylation of AKT and ERK in serum starved Panc-1 cells in response to shRNA mediated IKBKE knockdown. IKBKE knockdown leads to decrease in phosphorylation of AKT at Serine-473, but not ERK phosphorylation. (E) Western blot of Panc-1 cells with or without inhibition of mTOR using Torin-1, and shRNA mediated knockdown of IKBKE. Inhibition of IKBKE and mTOR leads to decrease in phosphorylation of AKT at Serine-473 and Threonine-308.
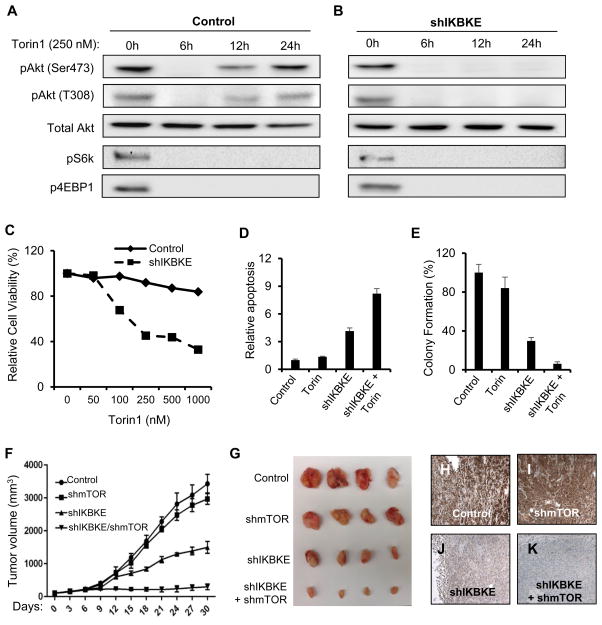
The mTORC2 complex is known to phosphorylate AKT at Serine-473 and thereby mediate AKT activation (33). In Panc-1 cells treated with the mTOR kinase inhibitor, Torin-1, AKT phosphorylation was down-regulated (Figure 4E). However, AKT phosphorylation was further reduced in cells with both mTOR inhibition and IKBKE knockdown (Figure 4E), suggesting that IKBKE mediated AKT phosphorylation is likely mTOR independent, and that both pathways are involved in AKT activation in PDAC cells. mTOR inhibitors have been approved for treatment of certain malignancies (34, 35). However, they appear to be ineffective in treating PDAC (36). One of the problems concerning the clinical utility of mTOR inhibitors is the reactivation of AKT post-inhibition of mTOR (37, 38). It has been reported that in breast cancer cells, mTOR kinase inhibition resulted in sustained inhibition of AKT phosphorylation at Serine-473; however, the compensatory activation of upstream receptor tyrosine kinase (RTK) and subsequent re-phosphorylation of AKT at Threonine-308 leads to AKT re-activation and resistance to mTOR inhibition (38). Interestingly, we found that, in Panc-1 cells treated with Torin-1, AKT phosphorylation at Serine-473 and Threonine-308 was initially inhibited 6 hours post-treatment; however, phosphorylation at both sites was restored as early as 12 hours after Torin-1 treatment (Figure 5A). Phosphorylation of other mTOR targets S6K and 4EBP1 continued to be inhibited even 24 hours after treatment (Figure 5A), suggesting that reactivation of AKT is mTOR-independent and may be mediated by an RTK-independent mechanism.
Figure 5. IKBKE mediates AKT reactivation post-mTOR inhibition.
(A–B) Western blot showing phosphorylation of Akt and other mTOR substrates, S6K and 4EBP1, in control Panc-1 cells (A) and Panc-1 cells with IKBKE knockdown (B) in response to treatment with Torin-1 up to 24 hours. (C) Relative cell viability of Panc-1 cells measured by MTT assay 5 days after treatment with increasing dosage of the mTOR inhibitor Torin-1 in the presence and absence of IKBKE knockdown. (D) Cleaved-Caspase 3 staining in Panc-1 cells indicates that while mTOR inhibition alone using Torin-1 does not lead to a significant increase in apoptosis, combined inhibition of IKBKE and mTOR leads to a significant increase in apoptosis. (E) Soft agar colony formation assay in Panc-1 cells indicates that while mTOR inhibition alone does not affect tumorigenicity of the cells, combined inhibition of mTOR and IKBKE leads to significant decrease in tumorigenicity. (F) Tumor volume measurement indicates significant decrease in growth of MiaPaca-2 cell line derived xenograft tumors (n = 6) in response to inducible IKBKE knockdown, and synergistic effect with combined IKBKE and mTOR knockdown while mTOR knockdown has no effect. (G) Representative images of xenograft tumors 4 weeks after induction of shRNA indicate significant reduction in tumor volume in response to IKBKE knockdown and synergistic reduction with combined IKBKE/mTOR knockdown. (H–K) Representative IHC images showing phosphorylation of AKT at Serine-473 in xenograft tumors derived from Control (H), shmTOR (I), shIKBKE (J), and shIKBKE/shmTOR (K) MiaPaca-2 cell lines. Error bars represent Standard Deviation. Statistical significance was determined using a Student’s two-tailed t-test. * P<0.05; ** P<0.01
Because of the IKBKE-AKT connection, we decided to test whether IKBKE is involved in AKT reactivation in PDAC cells. We found that in Panc-1 cells with shRNA mediated IKBKE knockdown, the reactivation of AKT post-inhibition of mTOR was ablated (Figure 5B). Furthermore, although mTOR inhibition alone did not significantly affect the survival of Panc1 and MiaPaCa2 cells (Figure 5C, S5A–D), knockdown of IKBKE sensitized these cells to the mTOR inhibitor. IKBKE knockdown combined with mTOR inhibition led to a synergistic decrease in cell viability, significant increase in apoptosis (Figure 5D), and inhibition of transformation of PDAC cells (Figure 5C–E, S5B–D). To test the combined IKBKE and mTOR inhibition as a potential PDAC therapeutic strategy in vivo, we generated human MiaPaCa2 cell lines stably expressing doxycycline inducible shRNAs targeting IKBKE, mTOR, or both (Figure S5). We subcutaneously injected 1 × 107 cells from each of the stable cell lines into the flanks of NOD-SCID mice. The tumors (N=6 per cell line) were allowed to grow to a size of ~100 mm3 before doxycycline treatment. Tumor volume was measured every 3 days for the next 30 days before the animals were sacrificed. We found that IKBKE knockdown decreased tumor growth in the mice, and mTOR knockdown did not significantly affect tumor formation. However, combined knockdown of mTOR and IKBKE resulted in a synergistic inhibition of xenograft tumor growth in vivo (Figure 5F, G). To evaluate the molecular characteristics of tumor growth inhibition by mTOR and IKBKE knockdown, we conducted immunohistochemical staining for phospho-AKT and phospho-S6K. S6K phosphorylation was blocked in tumors derived from shmTOR and shIKBKE/shmTOR cell lines (Figure S6D, J) but maintained in the tumors derived from control and shIKBKE cell lines (Figure S6A, G). We found that in spite of mTOR knockdown, AKT phosphorylation at Serine-473 in the tumors was maintained at high levels (Figure 5H, I), while combined knockdown of IKBKE and mTOR led to obliteration of AKT phosphorylation (Figure 5J, K, S6K). Together, our findings implicate a critical role for IKBKE in AKT activation in PDAC cells both in vitro and in vivo.
DISCUSSION
The nearly universal presence of activating mutations in the KRAS oncoprotein in PDAC suggest that inhibiting key downstream signaling nodes may be an effective therapeutic strategy in this disease. However, the identities of the key downstream signaling nodes remain poorly understood. Indeed, targeting of well-characterized signaling molecules such as MEK, PI3K and mTOR have all been ineffective thus far in PDAC. Here, we demonstrate that inhibition of the atypical IkB kinase IKBKE may be an important component of combinatorial therapeutic strategies in PDAC.
The IkB kinase-related kinases, IKBKE and TBK1, were originally identified by their ability to act as IkB kinases to regulate NF-kB signaling (12, 13). Later on it was reported that these versatile kinases engage multiple different substrates, including CYLD, TRAF2, FOXO3, and AKT (16, 30–32, 39, 40). However, the context-dependent downstream regulation by IKBKE/TBK1 in diseases and cancers is not well understood. Our study highlights the in vivo importance of IKBKE in Kras-induced pancreatic tumorigenesis, and suggests that IKBKE, but not TBK1, is likely the major IkB kinase-related kinase involved in PDAC. Interestingly, previously published studies indicated that TBK1 inhibition represented a synthetic lethal interaction with mutationally activated KRAS in colorectal, lung and breast cancer cells (19). The differential requirement for IKBKE and TBK1 may reflect their different expression levels in PDAC specimens. In addition, TBK1 is known to be activated downstream of the monomeric GTPase RALB, itself a downstream effector of KRAS (41). Yet, Lim et al demonstrated that RALA facilitates, while RALB impedes, KRAS-induced transformation in PDAC cells (42). Thus, it is intriguing that the selective dependence of IKBKE in PDAC may be the consequence of differential functional requirement of RALA/B in this tumorigenic context.
Critically, our results also suggest that IKBKE is largely dispensable for NF-kB activation in PDAC, but functions as one of the key regulators for AKT activity. Moreover, our studies also reveal a novel mechanism underlying AKT reactivation upon mTOR inhibition. In contrast to RTK-mediated AKT reactivation at T308 in breast cancer cells (38), IKBKE dependent AKT reactivation in PDAC cells involves both S473 and T308 phosphorylation, highlighting the complex signaling mechanisms contributing to AKT reactivation and resistance to mTOR inhibition in different tumors. Of note, concurrent inhibition of mTOR and IKBKE kinases profoundly impaired the growth of pancreatic cancer xenografts. While additional in vivo studies are required to validate the efficacy of this therapeutic approach, our observations provide a rationale for testing combination of IKBKE and mTOR inhibitors that are currently in clinical development for treating Kras-driven PDAC.
Our study also identified GLI1 nuclear translocation and transcriptional activity signaling as a novel downstream pathway regulated by IKBKE. It remains to be determined whether IKBKE mediated promotion of Gli1 nuclear localization is via direct phosphorylation or indirect regulation. Nonetheless, given our finding that non-canonical activation of GLI transcription factors stimulates IKBKE gene expression (11), these results indicate the presence of an oncogenic feed forward mechanism downstream of mutant KRAS with potential therapeutic implications. Additional studies directed toward the identification and characterization of other critical pathways downstream of IKBKE and GLI may therefore shed further light on the mechanisms underlying PDAC development and therapeutic resistance.
Supplementary Material
Acknowledgments
Funding Sources: J. Mao is supported by NIH grant R01DK099510 and American Cancer Society grant RSG-11-040-01-DDC, and M. E. Fernandez-Zapico is supported by NIH grant R01CA136526
National Institutes of Health grant R01DK099510 (to JM) and American Cancer Society grant RSG-11-040-01-DDC (to JM), and R01CA136526 (to MEFZ) were the major support for this work. We also thank members of Mao, Lewis and Fernandez-Zapico labs for helpful discussion.
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi AK. Familial pancreatic cancer: genetic advances. Genes Dev. 2014;28:1–7. doi: 10.1101/gad.228452.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 4.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 5.Hanrahan AJ, Solit DB. RAF/MEK dependence of KRAS-mutant pancreatic ductal adenocarcinomas. Cancer Discov. 2012;2:666–669. doi: 10.1158/2159-8290.CD-12-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamad NM, Elconin JH, Karnoub AE, Bai W, Rich JN, Abraham RT, Der CJ, Counter CM. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002;16:2045–2057. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, Baldwin AS., Jr Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- 8.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, Jacks T. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–107. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, Cruz-Monserrate Z, Wang H, Ji B, Logsdon CD. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–1528. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rajurkar M, De Jesus-Monge WE, Driscoll DR, Appleman VA, Huang H, Cotton JL, Klimstra DS, Zhu LJ, Simin K, Xu L, et al. The activity of Gli transcription factors is essential for Kras-induced pancreatic tumorigenesis. Proc Natl Acad Sci U S A. 2012;109:E1038–1047. doi: 10.1073/pnas.1114168109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimada T, Kawai T, Takeda K, Matsumoto M, Inoue J, Tatsumi Y, Kanamaru A, Akira S. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 13.Chariot A, Leonardi A, Muller J, Bonif M, Brown K, Siebenlist U. Association of the adaptor TANK with the I kappa B kinase (IKK) regulator NEMO connects IKK complexes with IKK epsilon and TBK1 kinases. J Biol Chem. 2002;277:37029–37036. doi: 10.1074/jbc.M205069200. [DOI] [PubMed] [Google Scholar]
- 14.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, Sjostrom SK, Garraway LA, Weremowicz S, Richardson AL, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 15.Guan H, Zhang H, Cai J, Wu J, Yuan J, Li J, Huang Z, Li M. IKBKE is over-expressed in glioma and contributes to resistance of glioma cells to apoptosis via activating NF-kappaB. J Pathol. 2011;223:436–445. doi: 10.1002/path.2815. [DOI] [PubMed] [Google Scholar]
- 16.Shen RR, Zhou AY, Kim E, Lim E, Habelhah H, Hahn WC. IkappaB kinase epsilon phosphorylates TRAF2 to promote mammary epithelial cell transformation. Mol Cell Biol. 2012;32:4756–4768. doi: 10.1128/MCB.00468-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbie TU, Alexe G, Aref AR, Li S, Zhu Z, Zhang X, Imamura Y, Thai TC, Huang Y, Bowden M, et al. Targeting an IKBKE cytokine network impairs triple-negative breast cancer growth. J Clin Invest. 2014;124:5411–5423. doi: 10.1172/JCI75661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Z, Aref AR, Cohoon TJ, Barbie TU, Imamura Y, Yang S, Moody SE, Shen RR, Schinzel AC, Thai TC, et al. Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discov. 2014;4:452–465. doi: 10.1158/2159-8290.CD-13-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462:108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, et al. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19:313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tenoever BR, Ng SL, Chua MA, McWhirter SM, Garcia-Sastre A, Maniatis T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science. 2007;315:1274–1278. doi: 10.1126/science.1136567. [DOI] [PubMed] [Google Scholar]
- 22.Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 23.Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CV. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki H, Hui C, Nakafuku M, Kondoh H. A binding site for Gli proteins is essential for HNF-3beta floor plate enhancer activity in transgenics and can respond to Shh in vitro. Development. 1997;124:1313–1322. doi: 10.1242/dev.124.7.1313. [DOI] [PubMed] [Google Scholar]
- 25.Nolan-Stevaux O, Lau J, Truitt ML, Chu GC, Hebrok M, Fernandez-Zapico ME, Hanahan D. GLI1 is regulated through Smoothened-independent mechanisms in neoplastic pancreatic ducts and mediates PDAC cell survival and transformation. Genes Dev. 2009;23:24–36. doi: 10.1101/gad.1753809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mao J, Maye P, Kogerman P, Tejedor FJ, Toftgard R, Xie W, Wu G, Wu D. Regulation of Gli1 transcriptional activity in the nucleus by Dyrk1. J Biol Chem. 2002;277:35156–35161. doi: 10.1074/jbc.M206743200. [DOI] [PubMed] [Google Scholar]
- 27.Kogerman P, Grimm T, Kogerman L, Krause D, Unden AB, Sandstedt B, Toftgard R, Zaphiropoulos PG. Mammalian suppressor-of-fused modulates nuclear-cytoplasmic shuttling of Gli-1. Nat Cell Biol. 1999;1:312–319. doi: 10.1038/13031. [DOI] [PubMed] [Google Scholar]
- 28.Sheng T, Chi S, Zhang X, Xie J. Regulation of Gli1 localization by the cAMP/protein kinase A signaling axis through a site near the nuclear localization signal. J Biol Chem. 2006;281:9–12. doi: 10.1074/jbc.C500300200. [DOI] [PubMed] [Google Scholar]
- 29.Ying H, Elpek KG, Vinjamoori A, Zimmerman SM, Chu GC, Yan H, Fletcher-Sananikone E, Zhang H, Liu Y, Wang W, et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-kappaB-cytokine network. Cancer Discov. 2011;1:158–169. doi: 10.1158/2159-8290.CD-11-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo JP, Coppola D, Cheng JQ. IKBKE protein activates Akt independent of phosphatidylinositol 3-kinase/PDK1/mTORC2 and the pleckstrin homology domain to sustain malignant transformation. J Biol Chem. 2011;286:37389–37398. doi: 10.1074/jbc.M111.287433. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Xie X, Zhang D, Zhao B, Lu MK, You M, Condorelli G, Wang CY, Guan KL. IkappaB kinase epsilon and TANK-binding kinase 1 activate AKT by direct phosphorylation. Proc Natl Acad Sci U S A. 2011;108:6474–6479. doi: 10.1073/pnas.1016132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ou YH, Torres M, Ram R, Formstecher E, Roland C, Cheng T, Brekken R, Wurz R, Tasker A, Polverino T, et al. TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol Cell. 2011;41:458–470. doi: 10.1016/j.molcel.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 34.Voss MH, Molina AM, Motzer RJ. mTOR inhibitors in advanced renal cell carcinoma. Hematol Oncol Clin North Am. 2011;25:835–852. doi: 10.1016/j.hoc.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP, et al. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193–198. doi: 10.1200/JCO.2008.18.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J, Guichard S, Rosen N. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 2011;1:248–259. doi: 10.1158/2159-8290.CD-11-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hutti JE, Shen RR, Abbott DW, Zhou AY, Sprott KM, Asara JM, Hahn WC, Cantley LC. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKepsilon promotes cell transformation. Mol Cell. 2009;34:461–472. doi: 10.1016/j.molcel.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo JP, Tian W, Shu S, Xin Y, Shou C, Cheng JQ. IKBKE phosphorylation and inhibition of FOXO3a: a mechanism of IKBKE oncogenic function. PLoS One. 2013;8:e63636. doi: 10.1371/journal.pone.0063636. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, Balakireva MG, Romeo Y, Kopelovich L, Gale M, Jr, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–170. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- 42.Lim KH, Baines AT, Fiordalisi JJ, Shipitsin M, Feig LA, Cox AD, Der CJ, Counter CM. Activation of RalA is critical for Ras-induced tumorigenesis of human cells. Cancer Cell. 2005;7:533–545. doi: 10.1016/j.ccr.2005.04.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.