Abstract
Background
Postpartum anemia is associated with maternal and perinatal morbidity. Population-level data may inform guideline development for postpartum anemia screening. Our objectives were to evaluate the associations between potential predictors (predelivery anemia and postpartum hemorrhage (PPH)) with severe postpartum anemia after cesarean section.
Study Design and Methods
Data were collected from 70,939 hospitalizations for cesarean section performed at Kaiser Permanente Northern California facilities between 2005 and 2013. Severe postpartum anemia was defined as a hemoglobin < 8 g/dl before hospital discharge. Using multivariable logistic regression, we assessed the associations between predelivery anemia and PPH with severe postpartum anemia. Distributions of these characteristics among women with severe postpartum anemia were evaluated.
Results
The overall rate of severe postpartum anemia was 7.3%; 95% confidence interval (CI) = 7.1 – 7.4. Severe postpartum anemia was strongly associated with a predelivery hemoglobin between 10 and 10.9 g/dl (adjusted odds ratio (aOR) 5.4; 95% CI = 4.89– 5.91), predelivery hemoglobin <10 g/dl (aOR 30.6; 95% CI = 27.21– 34.6, and PPH (aOR 8.45; 95% CI = 7.8–9.16). The proportions of women with severe postpartum anemia were highest for those experiencing PPH but no predelivery anemia (12.2%; 95% CI = 11.0 – 13.6), and those who did not incur PPH nor predelivery anemia (10.7%; 95% CI = 9.6 – 12.0).
Conclusions
Our findings suggest that PPH and predelivery anemia are strong independent risk factors for severe postpartum anemia. Optimization of patients’ hemoglobin prior to delivery may reduce the incidence of severe anemia after cesarean section.
Keywords: Cesarean Section, Anemia, Hemorrhage
INTRODUCTION
In the United States, the prevalence of anemia during pregnancy has been reported to be as high as 241 per 1000 pregnant women.1 To address this issue, The American College of Obstetricians and Gynecologists (ACOG) have published recommendations for managing anemia during pregnancy.2 These include: screening all pregnant women for anemia during pregnancy, and iron supplementation for women with iron deficiency anemia. By comparison, postpartum anemia has received less attention and is an underappreciated women’s health issue. Morbidities linked to postpartum anemia include: depression,3,4 fatigue,5 and impaired cognition6. These patient-centric outcomes can have important negative impacts on maternal-child bonding and the mother’s ability to provide newborn care.7
Because symptoms of maternal anemia are non-specific,8 anemia can only be detected by measuring the hemoglobin level. If postpartum anemia goes unrecognized, mothers may face a greater degree of physical or emotional hardship than non-anemic mothers. Therefore, to limit the likelihood of anemia-related morbidity, population-level data are needed to inform guideline development for postpartum anemia screening.
Predelivery anemia and PPH are presumed to confer the greatest risks to women for postpartum anemia.8 Women who undergo cesarean section may be particularly susceptible to postpartum anemia because their risk of PPH is higher than women undergoing vaginal delivery.9,10 However, there is a dearth of studies examining the frequency of anemia after cesarean section and the relations between predelivery anemia and PPH with postpartum anemia. These studies will be important in advancing clinical knowledge and providing key epidemiologic data for guiding discussions about optimizing Patient Blood Management in obstetrics.
The main objectives of this analysis were to describe the incidence of severe postpartum anemia, assess the relationships between severe postpartum anemia with blood loss at delivery and predelivery anemia, and to estimate the frequencies of these characteristics among women with severe postpartum anemia. In our secondary analyses, we calculated rates of postpartum anemia testing after cesarean section and examined characteristics of women who did not undergo postpartum hemoglobin testing. For these analyses, we employed data from Kaiser Permanente Northern California (KPNC), a pre-paid integrated healthcare delivery system in the United States.
MATERIALS AND METHODS
This study was approved by the KPNC and Stanford University institutional review boards, and the Committee for the Protection of Human Subjects of the California Health and Human Services Agency.
Data Source and Study Cohorts
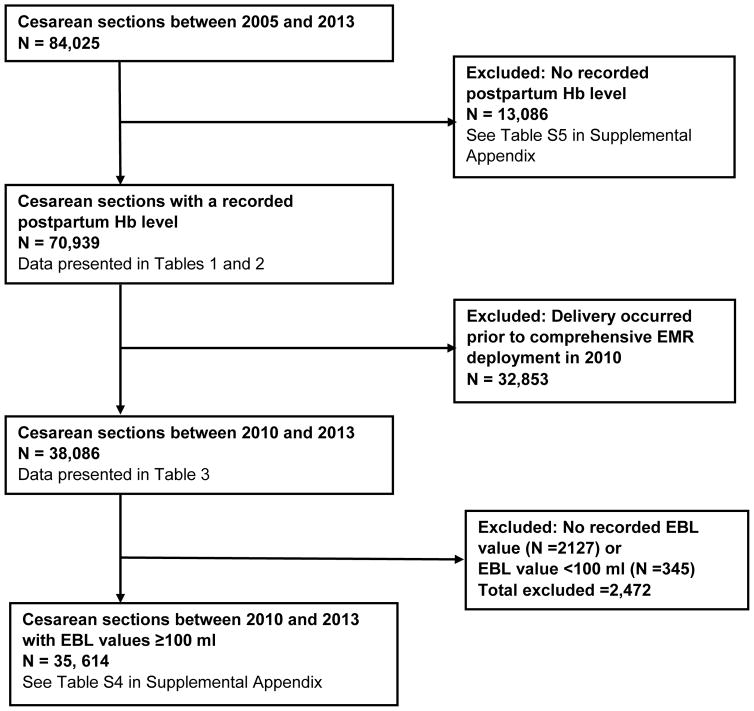
We used the existing research infrastructure of an integrated health care delivery system (KPNC) in California, United States. All KPNC hospitals and clinics use information systems that are linked by a common medical record number. For this study, data were obtained from linked electronic databases within KPNC, cleaned and processed using methods previously described.11–13 A flow diagram depicts the cohorts used for our primary, subgroup, and secondary analyses (Figure 1). For our primary analysis, the study cohort comprised of 70,939 women with a measured postpartum hemoglobin value who underwent cesarean section at a KPNC obstetric center between January 1, 2005 and December 31, 2013. We excluded women who were aged <15 years at the time of delivery. Because EBL data were available from the KPNC electronic medical records (EMRs) from 2010 onwards, we performed a subgroup analysis using a cohort comprised of 38,086 women who underwent cesarean section between January 1, 2010 and December 31, 2013. For our secondary analyses, we assessed characteristics of 13,086 women who delivered between 2005 and 2013 with no recorded postpartum hemoglobin value.
Figure 1.
Flow Chart.
EBL = estimated blood loss; EMR = electronic medical record; Hb = hemoglobin; PPH = postpartum hemorrhage
Variables
For our primary analysis, the outcome of interest was severe postpartum anemia, defined by World Health Organization (WHO) criteria 14 as a postpartum hemoglobin <8 g/dl, measured closest to the day of hospital discharge. To further substantiate this cutpoint, PPH guidelines published by several national societies (The Royal College of Obstetricians and Gynecologists and an interdisciplinary consensus group from Germany, Austria, and Switzerland [DACH]) indicate that a hemoglobin of 8 g/dl or more should be a therapeutic goal.15,16
The main independent variables of interest were: predelivery anemia and PPH. Predelivery anemia was determined using the antenatal hemoglobin level closest to the day of cesarean section. During pregnancy, the WHO define mild, moderate, and severe anemia as a hemoglobin between 10–10.9 g/dl, 7–9.9 g/dl, and <7 g/dl, respectively.14 As only 11 women in our study cohort had a predelivery hemoglobin <7 g/dl, we reclassified predelivery anemia as follows: ‘mild’ for hemoglobin values between 10–10.9 g/dl, and ‘moderate-severe’ for hemoglobin values <10 g/dl. We identified PPH using International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) diagnosis codes for PPH (see Table 1 in the Supplementary Appendix).
For our subgroup (n=38,086) who delivered between 2010 and 2013 (Figure 1: flow diagram), instead of the ICD-9 code for PPH, we used EBL data from the EMR to classify grades of blood loss severity at delivery. Descriptions of the methods of EBL measurement or estimation were not available in KPNC databases. Based on literature review,9,17–19 we classified normal blood loss, non-severe PPH, and severe PPH based on the following EBL criteria: <1000 ml, 1000 – 1499 ml and ≥1500 ml respectively. Due to uncertainties about the accuracy of very low EBL values, we classified any EBL<100 ml as missing data (n=345).
Other covariates in our analyses included patient and clinical characteristics, specified a priori, as potential confounders between the main independent variables and severe postpartum anemia. These were determined according to clinical relevance and prior literature review.8,20 Demographic characteristics included: age, race or ethnic group, and year of delivery. Medical characteristics included: hereditary or acquired coagulation disorders and thrombocytopenia. Obstetric and peripartum characteristics included: gestational age at delivery, grand multiparity, number of prior cesarean deliveries, obesity, labour, maternal hypertensive disease, placenta previa, antepartum hemorrhage, and transfusion. Maternal age, number of prior cesarean deliveries, gestational age at delivery, and year of delivery were extracted from linked KPNC databases. Data on mothers’ self-reported race and ethnicities were obtained from the State of California Birth Certificate Database. We used ICD-9 codes to identify diagnoses and procedures associated with each delivery hospitalization (Table S1 in the Supplemental Appendix).
STATISTICAL ANALYSIS
We calculated the number of days between predelivery hemoglobin measurement and cesarean section, discharge hemoglobin measurement and cesarean section, and the postpartum length of stay. Overall rates of anemia were calculated, and Wilson’s method was used to derive 95% confidence intervals.21 To evaluate temporal trends, we calculated annual rates of severe postpartum anemia at discharge. We also calculated annual rates of severe postpartum anemia for deliveries that were accompanied with ICD-9 codes for PPH or transfusion. Temporal trends were assessed using the χ2 test for linear trend in proportions.
We performed bivariate analyses to compare the distribution of patient and obstetric characteristics between women with vs. without severe postpartum anemia. We used multivariable logistic regression to assess the associations between the predelivery hemoglobin categories and PPH with severe postpartum anemia. We created separate logistic models to account for the influence of patient and obstetric characteristics, and year of delivery. Sequential models were constructed with increasing levels of adjustment for the following sets of covariates: model 1 = only predelivery hemoglobin categories and PPH; model 2 = covariates in model 1 + year of birth; model 3 = covariates in model 2 + patient demographics (including: maternal age, race/ethnicity); model 4 = covariates in model 3 + medical/obstetric characteristics (including: obesity, grand multiparity, hereditary or acquired coagulation disorders, thrombocytopenia, gestational age, number of prior cesarean deliveries, hypertensive disorders of pregnancy, labor or induction of labor, placenta previa, and antepartum hemorrhage). Individual hospitals were accounted for as random effects in each model. For each model, we calculated odds ratios for severe postpartum anemia at discharge as a function of the key variables of interest. To estimate the proportion of women with severe postpartum anemia that would have been eliminated if one of the main exposures (predelivery anemia or PPH) had been eliminated, we calculated population attributable fractions (based on adjusted odds ratios from non-hierarchical multivariable logistic models). Using full models, we also added interaction terms to investigate whether effect modification occurred between transfusion with PPH and predelivery anemia, respectively.
For our subgroup analyses, we examined data from 38,086 cesarean sections that occurred between 2010 and 2013 (see Figure 1: flow diagram). We calculated absolute rates of severe postpartum anemia according to the presence or absence of PPH (based on available EBL data) and predelivery anemia. We also analyzed data from 35,614 cesarean sections where EBL data were not missing (see Figure 1: flow diagram) to examine the relations between pre-delivery anemia and PPH (using EBL categories) with severe postpartum anemia.
Lastly, in our secondary analyses, we performed multivariable logistic regression to identify characteristics that may explain why 13,086 women who underwent cesarean section between 2005 and 2013 did not undergo testing for postpartum anemia (see Figure 1: flow diagram).
All analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC) and STATA version 12 (Stata Corp., College Station, TX).
RESULTS
Between 2005 and 2013, we identified 84,025 hospitalizations for cesarean delivery. The median postpartum length of stay was 3 days (interquartile range (IQR) = 2– 3); data were missing for 187 hospitalizations. Pre-delivery and post-delivery hemoglobin values were available for 48,439 (57.6%) and 70,939 (84.4%) hospitalizations respectively (Figure 1: flow diagram). The median time period between the predelivery hemoglobin level and cesarean section was 0 days (IQR = 0 – 1), and the median time period between cesarean section and the discharge hemoglobin level was 1 day (IQR = 1 – 2).
Among women with a recorded postpartum hemoglobin value, the rate of severe postpartum anemia was 7.3% (95% confidence interval [CI] = 7.1 – 7.4). Between 2005 and 2013, a modest increase occurred in the rate of severe postpartum anemia (P for trend=0.04; Figure S1 in Supplemental Appendix).
Characteristics of Women with Severe Postpartum Anemia
Maternal and obstetric characteristics of women with and without severe postpartum anemia at discharge are presented in Table 1. (The proportions of women with severe postpartum anemia for each maternal/obstetric characteristic (row percentages) are presented in Table S2 in Supplementary Appendix). On univariate analyses, women with mild or moderate-severe predelivery anemia and those with PPH were more likely to have severe postpartum anemia. Table 2 shows the crude and adjusted logistic regression models between predelivery anemia and PPH with postpartum anemia. For each predictor, the odds of severe postpartum anemia was relatively unchanged after adjustment for birth year, medical, and obstetric characteristics; in model 4, the adjusted odds ratio was 5.4 among women with mild predelivery anemia (95% CI=4.89– 5.91); 30.6 among those with moderate-severe predelivery anemia (95% CI=27.21– 34.6), and 8.45 among those with PPH (95% CI=7.8– 9.16). (Point estimates for all covariates in model 4 are presented in Table S2 in Supplementary Appendix). The proportions of severe postpartum anemia that could be attributed to PPH was 13.7% (95% CI=12.6–14.8%) and predelivery anemia (Hb<11 g/dl) was 30.6% (95% CI=28.7–32.4%).
Table 1.
Patient Characteristics of Women with and without Severe Postpartum Anemia who underwent Cesarean Section between 2005 and 2013.
| Patient Characteristics | No Severe Anemia (n=65,796) | Severe Anemia (n=5,143) | P value |
|---|---|---|---|
| Maternal age (y): | <0.001 | ||
| <20 | 1475 (2.2%) | 232 (4.5%) | |
| 20–34 | 44,439 (67.6%) | 3492 (67.9%) | |
| ≥35 | 19,882 (30.2%) | 1419 (27.6%) | |
| Race/ethnicity: | <0.001 | ||
| White | 26,606 (39.8%) | 1564 (30.4%) | |
| Black | 5618 (8.5%) | 512 (10%) | |
| Hispanic | 16,064 (24.4%) | 1453 (28.2%) | |
| Asian | 15,399 (23.4%) | 1442 (28.1%) | |
| Other | 1866 (2.9%) | 134 (2.6%) | |
| Unknown | 643 (1.0%) | 38 (0.7%) | |
| Prepartum Hb level (g/dl): | <0.001 | ||
| ≥11 g/dl | 40,597 (61.7%) | 2264 (44%) | |
| 10 – 10.9 g/dl | 3,267 (5%) | 857 (16.7%) | |
| <10 g/dl | 661 (1%) | 793 (15.4%) | |
| Hb missing | 21,271 (32.3%) | 1229 (23.9%) | |
| Hereditary or acquired coagulation disorder† | 91 (0.1%) | 93 (1.8%) | <0.001 |
| Gestational age at delivery: | <0.001 | ||
| <37 weeks | 1378 (2.1%) | 119 (2.3%) | |
| 37–41 weeks | 60,736 (92.3%) | 4615 (89.7%) | |
| >41 weeks | 3682 (5.6%) | 409 (8%) | |
| Grand multiparity† | 163 (0.3%) | 32 (0.6%) | <0.001 |
| Number of prior CSs: | <0.001 | ||
| 0 | 54,711 (83.2%) | 4460 (86.7%) | |
| 1 | 10,267 (15.6%) | 620 (12.1%) | |
| ≥2 | 818 (1.2%) | 63 (1.2%) | |
| Obesity† | 10,323 (15.7%) | 709 (13.8%) | <0.001 |
| Thrombocytopenia† | 1258 (1.9%) | 163 (3.2%) | <0.001 |
| Preeclampsia† | 3445 (5.2%) | 466 (9.1%) | <0.001 |
| Labor† | 30,716 (46.7%) | 3145 (61.2%) | <0.001 |
| Placenta previa† | 1131 (1.7%) | 173 (3.4%) | <0.001 |
| Antepartum hemorrhage † | 1191 (1.8%) | 236 (4.6%) | <0.001 |
| Postpartum hemorrhage † | 3144 (4.8%) | 1466 (28.5%) | <0.001 |
| RBC transfusion† | 364 (0.6%) | 1183 (23%) | <0.001 |
Data presented as n (%). Column percentages are presented in each cell.
Variables identified according to diagnosis or procedure codes in the International Classification of Diseases, 9th Revision, Clinical Modification.
CS = Cesarean section; Hb = hemoglobin; RBC = red blood cell
Table 2.
Associations between Severe Postpartum Anemia with Predelivery Hemoglobin and Postpartum Hemorrhage*
| Model 1a | Model 2b | Model 3c | Model 4d | |
|---|---|---|---|---|
| OR (95% CI) | aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| PPH | 9.57 (8.85 – 10.34) | 9.82 (9.08 – 10.62) | 9.57 (8.85 – 10.36) | 8.45 (7.8 – 9.16) |
| Prepartum Hb level (g/dl): | ||||
| > 11 | Reference | Reference | Reference | Reference |
| 10 –10.9 | 5.05 (4.61 – 5.54) | 5.06 (4.61 – 5.54) | 5.10 (4.65 – 5.60) | 5.38 (4.89 – 5.91) |
| < 10 | 26.7 (23.75 – 30.0) | 26.9 (23.92 – 30.23) | 27.06 (24.03 – 30.46) | 30.6 (27.1 – 34.6) |
| Missing Hb | 1.16 (1.08 – 1.25) | 1.16 (1.07 – 1.25) | 1.19 (1.10 – 1.28) | 1.54 (1.42 – 1.67) |
Denominator consists of 70,939 patients (Figure 1); dependent variable is severe postpartum anemia (N = 5,143)
unadjusted model
adjusted for year of birth as a fixed effect, and individual hospital as a random effect.
adjusted for year of birth, maternal age, and race/ethnicity as fixed effects, and individual hospital as a random effect.
adjusted for year of birth, maternal age, race/ethnicity, grand multiparity, hereditary or acquired coagulation disorders, gestational age at delivery, number of prior cesarean deliveries, obesity, thrombocytopenia, pre-eclampsia, labor, placenta previa, antepartum hemorrhage as fixed effects, and individual hospital as a random effect.
aOR = adjusted odds ratio; CI = confidence interval; Hb = hemoglobin; PPH = postpartum hemorrhage
Interactions according to transfusion were significant, therefore we performed stratified analyses to account for the presence/absence of transfusion (Table S3 in the Supplementary Appendix). In the non-transfused cohort, PPH, mild predelivery anemia, and moderate-severe anemia remained independently associated with severe postpartum anemia. In contrast, in the transfused cohort, the adjusted odds ratio was 2.12 (95% CI=1.37– 3.26) among those with mild predelivery anemia; 1.77 (95% CI=1.11– 2.81) among those with moderate-severe predelivery anemia; and 0.64 (95% CI=0.49 – 0.84) among those with PPH.
Subgroup Analyses
In our subgroup analyses, we examined data from 38,086 women who underwent cesarean section between 2010 and 2013 (Figure 1: flow diagram). Where available, EBL data were extracted from the EMR, and PPH was classified according to EBL severity. We calculated proportions for severe postpartum anemia according to the presence or absence of the following key factors: predelivery anemia ± PPH ± transfusion (Table 3). Of those with severe postpartum anemia, 12.2% (95% CI=11.0 – 13.6) women had PPH but did not incur predelivery anemia nor transfusion, and 10.7% (95% CI=11.0 – 13.6) women had a normal EBL, no predelivery anemia and did not receive transfusion.
Table 3.
Characteristics of 2,376 Women with Severe Postpartum Anemia who underwent Cesarean Section between 2010 and 2013.
| Patients with Severe Postpartum Anemiaa | ||
|---|---|---|
| N | % | |
| No Predelivery Anemia | ||
| No PPH: | ||
| Not Transfused | 255 | 10.73 |
| Transfused | 68 | 2.86 |
| PPH: | ||
| Not Transfused | 290 | 12.21 |
| Transfused | 62 | 2.61 |
| Severe PPH: | ||
| Not Transfused | 141 | 5.93 |
| Transfused | 136 | 5.72 |
| No Documented EBL: | ||
| Not Transfused | 50 | 2.1 |
| Transfused | 21 | 0.88 |
| Mild Predelivery Anemia | ||
| No PPH: | ||
| Not Transfused | 171 | 7.2 |
| Transfused | 19 | 0.8 |
| PPH: | ||
| Not Transfused | 101 | 4.25 |
| Transfused | 24 | 1.01 |
| Severe PPH: | ||
| Not Transfused | 25 | 1.05 |
| Transfused | 35 | 1.47 |
| No Documented EBL: | ||
| Not Transfused | 17 | 0.72 |
| Transfused | 5 | 0.21 |
| Severe Predelivery Anemia | ||
| No PPH: | ||
| Not Transfused | 215 | 9.05 |
| Transfused | 20 | 0.84 |
| PPH: | ||
| Not Transfused | 62 | 2.61 |
| Transfused | 21 | 0.88 |
| Severe PPH: | ||
| Not Transfused | 13 | 0.55 |
| Transfused | 17 | 0.72 |
| No Documented EBL: | ||
| Not Transfused | 16 | 0.67 |
| Transfused | 5 | 0.21 |
| No Predelivery Hb | ||
| No PPH: | ||
| Not Transfused | 234 | 9.85 |
| Transfused | 43 | 1.81 |
| PPH: | ||
| Not Transfused | 135 | 5.68 |
| Transfused | 33 | 1.39 |
| Severe PPH: | ||
| Not Transfused | 43 | 1.81 |
| Transfused | 53 | 2.23 |
| No Documented EBL: | ||
| Not Transfused | 37 | 1.56 |
| Transfused | 9 | 0.38 |
This cohort consists of 2,376 women among the 38,086 women who underwent cesarean section between 2010 and 2013 (see Figure 1); this subset developed severe postpartum anemia. Column percentages are presented.
EBL = estimated blood loss; Hb = hemoglobin; PPH = postpartum hemorrhage
For 35,614 women with a recorded EBL≥100 ml,(see Figure 1: flow diagram), we performed multivariate logistic regression to assess the relationships between predelivery hemoglobin and PPH (using EBL categories) with severe postpartum anemia. The results are presented in Table S4 in the Supplementary Appendix. Compared to women with an EBL<1000 ml, women who experienced moderate PPH (EBL=1000 – 1499 ml) or severe PPH (EBL≥1500 ml) had a 3.9 and 13.4 fold increased odds of severe postpartum anemia respectively.
Secondary Analyses
For our secondary analyses, we examined 13,086 women who underwent cesarean section who did not have a recorded postpartum hemoglobin (see Figure 1; flow diagram). Characteristics of women with no recorded postpartum hemoglobin value are presented in Table S5 in the Supplemental Appendix. Women who delivered <37 weeks’ gestational age had the highest odds of not undergoing postpartum hemoglobin testing (aOR=156.9). Women of unknown ethnicity and those without a predelivery hemoglobin value had a 3-fold increased odds of not getting tested. In contrast, the odds of not getting tested were lowest for women who underwent one or at least two prior cesareans compared to women who underwent primary cesarean section.
Within our full subgroup (n=38,086), we calculated proportions for women with missing hemoglobin values at discharge (Table S6 in Supplementary Appendix); those with missing predelivery hemoglobin values who experienced normal blood loss and no transfusion accounted for the highest proportion (56.7%; 95% CI=55.4 – 58.0).
DISCUSSION
With anemia management and blood loss minimization approaches being central tenets of good Patient Blood Management practice for surgical and medical patients,22–24 our findings that predelivery anemia and PPH are strong risk factors for severe postpartum anemia are timely and topical. Because predelivery anemia is a modifiable risk factor, our findings should prompt key stakeholders, including transfusion medicine specialists, obstetricians, and anesthesiologists, to examine ways to better detect and treat anemia prior to cesarean section. Until Patient Blood Management guidelines that are applicable to obstetrics are published, we recommend that anemia screening after cesarean section be considered for patients with predelivery anemia, no recorded predelivery hemoglobin level, or those who experience PPH.
In our analyses, predelivery anemia and PPH were identified as strong risk factors for severe postpartum anemia after cesarean section. After accounting for potential maternal and obstetric covariates in our sequential models, the magnitude of these associations remained relatively unchanged. These findings have important clinical relevance for several reasons. Firstly, predelivery anemia and PPH are not infrequent; predelivery anemia affects 25% of pregnant women 1 and the rate of PPH during cesarean section is approximately 9%25. Secondly, there is underappreciation of the negative health burden associated with postpartum anemia. Postpartum anemia may precipitate or worsen depressive symptoms, cognitive function, fatigue, reduced work performance, and maternal-infant bonding.3–8 Thirdly, few recommendations exist for detecting severe anemia after cesarean section. The Centers for Disease Control recommend targeted screening for anemia between four to six weeks postpartum.26 However, the value of this guidance is uncertain as acute or subacute postpartum anemia can develop much earlier (at 24–48 hr postpartum).8,20 Therefore, if severe anemia goes unrecognized after cesarean section, these women may face greater emotional or physical hardship than non-anemic mothers after hospital discharge. Fourthly, because predelivery anemia is a modifiable antepartum risk factor, the early identification and treatment of predelivery anemia may reduce the incidence of severe postpartum anemia after cesarean section. In the non-obstetric surgical setting, similar approaches have been recommended for the management of preoperative anemia by experts in Patient Blood Management.22,27
Although predelivery anemia and PPH were important risk factors, a high proportion of women with severe postpartum anemia (10.7%) had a ‘normal’ EBL and no predelivery anemia. Even with quantitative data for blood loss, clinicians are prone to underestimating blood loss as the volume of actual blood loss increases.28,29 As a consequence, screening for postpartum anemia during the early postpartum period may be helpful for early diagnosis and initiation of therapy,8 especially for those with predelivery anemia or who experience PPH.30 Of note, specific recommendations for detecting and treating women with iron deficiency anemia among pregnant and non-pregnant women have been published by an expert multidisciplinary group, sponsored by the Society for the Advancement of Blood Management.31 These recommendations may serve as an important foundation for formally establishing Patient Blood Management guidelines in obstetrics.
We acknowledge that others have also observed an association between PPH and early postpartum anemia.20,32–34 However, the majority of these studies focused on women who underwent vaginal delivery, and data on the prevalence of severe postpartum anemia after cesarean delivery is more limited. Horowitz et al. reported that the incidence of severe anemia (hemoglobin<8 g/dl) after elective cesarean delivery is 0.5%.35 However, this small cohort was sourced from a single obstetric center and did not include intrapartum cesarean sections, thus study generalizability is limited.
In our study, transfusion modified the associations between PPH and predelivery anemia with severe postpartum anemia. Because we relied upon ICD-9 codes to identify women who received transfusions, we cannot determine the context, triggers, timing or appropriateness of transfusion. This has clinical relevance because transfusion is recognized to be an important indicator of maternal morbidity.36 Less is known about the use of transfusion therapy for treating postpartum anemia in non-bleeding patients who are intravascularly replete. Medical and transfusion societies, such as the AABB and the American College of Critical Care Medicine, recommend that transfusion is not beneficial if the hemoglobin level is greater than 10 g/dl, but may have benefit when the hemoglobin level is less than 6–8 g/dl.37,38 In the setting of PPH, a hemoglobin level >8 g/dl is recommended as a therapeutic goal by several obstetric societies.15,16 However, maternal outcomes associated with moderate or severe anemia among women managed with a restrictive approach are not well understood. Among women with acute postpartum anemia (hemoglobin levels between 4.8 – 7.9 g/dl), Prick et al. observed that maternal fatigue, upto 6 weeks postpartum, is only marginally improved with transfusion compared to a non-transfusion based approach.39 In a separate study, Van der Woude et al. observed that maternal health status and fatigue were not different, upto 5 weeks postpartum, between women with vs. without postpartum anemia (hemoglobin <10.5 g/dl vs. hemoglobin ≥10.5 g/dl).40 Further research is needed to investigate long-term cognitive, functional, and psychological outcomes of women with moderate or severe postpartum anemia in the absence of transfusion.
Based on our analysis, the proportion of women with severe postpartum anemia who did not undergo predelivery hemoglobin testing and who did not experience PPH or transfusion was relatively high (9.9%). Therefore, it is unclear whether these women had unrecognized predelivery anemia and/or underestimated blood loss. Furthermore, 16% women in our cohort did not undergo postpartum hemoglobin testing. Women undergoing preterm cesarean delivery had the highest odds of not getting tested (aOR=261), whereas the odds were lowest for women with at least one prior cesarean. These findings emphasize the need for formal guidelines or protocols to standardize anemia screening approaches after cesarean section.
The main strengths of our study are that we obtained postpartum hemoglobin levels for 70,939 cesarean sections, with recorded clinical data on blood loss available for over 50% of our cohort. However, our study has several limitations. For our main analysis, we identified PPH using ICD-9 codes, therefore misclassification is a potential concern. Non-differential misclassification can typically bias results towards the null. Using EBL data to classify PPH, we still observed a strong associations between PPH and severe postpartum anemia in our follow-up analysis. Nonetheless, we acknowledge that the true exposure effect of blood loss severity on our primary outcome may have been underestimated. We could not account for all potential confounders in our logistic models, such as labor augmentation with oxytocin, type and volume of iv fluids administered prior to delivery, and body mass index. However, the point estimates for PPH and chronic anemia remained relatively unchanged in our sequential models, thus these associations are unlikely to be markedly affected by residual confounders. We did not assess whether women received medication, such as oral or intravenous iron, to prevent or treat postpartum anemia. Stabilization of maternal hemoglobin values occurs between 5–7 days postpartum.8 As the median length of postpartum stay was 3 days, postpartum hemoglobin levels may not have completely stabilized by the time of hospital discharge. Due to the observational nature of our study design, we could not examine factors that influence physicians’ decision to transfuse or screen for anemia before or after cesarean section. Although obstetric agencies are focusing attention on initiatives to reduce major maternal morbidity,41,42 future studies are needed to determine whether improvements in anemia screening and the use of non-RBC based therapies can improve patient-centric outcomes, including debilitating maternal quality of life disorders, such as fatigue and depression.
Using a contemporary obstetric cohort, our results provide insight into the epidemiology of severe postpartum anemia after cesarean section. With anemia detection, diagnosis and treatment recognized as key facets of Patient Blood Management, we encourage transfusion medicine and other health specialists to consider strategies to potentially reduce the incidence of severe postpartum anemia. Until national guidelines are updated, hospitals and providers may need to consider anemia screening after cesarean section for women with at least one of the following: predelivery anemia, no predelivery hemoglobin level, and PPH.
Supplementary Material
Supplemental Figure 1. The rate of severe postpartum anemia prior to hospital discharge by year, between 2005 and 2013. Error bars denote 95% CI.
Table S1. Diagnoses, Procedures and the Associated ICD-9 CM Codes
Table S2. Proportions with Severe Postpartum Anemia According to Each Characteristic and Adjusted Odds Ratios in the Final Multivariable Model.
Table S3. Associations between Severe Postpartum Anemia with Postpartum Hemorrhage and Predelivery Hemoglobin Level Stratified by Receipt and Non-Receipt of Transfusion.
Table S4. Sensitivity Analyses Examining the Associations between Severe Postpartum Anemia with Predelivery Hemoglobin Levels and Estimated Blood Loss for Women undergoing Cesarean Section between 2010 and 2013.
Table S5. Patient Characteristics of 13,086 Women who did not undergo Postpartum Hemoglobin Testing after Cesarean Section between 2005 and 2013
Table S6. Characteristics of 5,843 Women with Missing Postpartum Hemoglobin Values who underwent Cesarean Section between 2010 and 2013.
Acknowledgments
Source of work: Secondary analyses of data sourced from databases based at the Division of Research at Kaiser Permanente Northern California, Oakland, California.
Financial Support: This study was supported and funded internally by the Department of Anesthesiology, Perioperative and Pain Medicine, Stanford University School of Medicine and the Division of Research at Kaiser Permanente Northern California, Oakland, California. This work received funding support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant number: K23HD070972). Dr. Butwick is supported by an award from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (K23HD070972).
Footnotes
Conflicts of Interest:
No authors have any conflicts of interest to declare.
References
- 1.World Health Organization. Worldwide prevalance of anemia 1993–2005: WHO Global Database on Anemia. WHO; Geneva: 2008. [Google Scholar]
- 2.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No 95: anemia in pregnancy. Obstet Gynecol. 2008;112:201–7. doi: 10.1097/AOG.0b013e3181809c0d. [DOI] [PubMed] [Google Scholar]
- 3.Gaynes BN, Gavin N, Melzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Evidence Report/Technology Assessment No.119. Rockville, MD: Agency for Healthcare Research and Quality; 2005. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corwin EJ, Murray-Kolb LE, Beard JL. Low hemoglobin level is a risk factor for postpartum depression. J Nutr. 2003;133:4139–42. doi: 10.1093/jn/133.12.4139. [DOI] [PubMed] [Google Scholar]
- 5.Lee KA, Zaffke ME. Longitudinal changes in fatigue and energy during pregnancy and the postpartum period. J Obstet Gynecol Neonatal Nurs. 1999;28:183–91. doi: 10.1111/j.1552-6909.1999.tb01983.x. [DOI] [PubMed] [Google Scholar]
- 6.Beard JL, Hendricks MK, Perez EM, Murray-Kolb LE, Berg A, Vernon-Feagans L, Irlam J, Isaacs W, Sive A, Tomlinson M. Maternal iron deficiency anemia affects postpartum emotions and cognition. J Nutr. 2005;135:267–72. doi: 10.1093/jn/135.2.267. [DOI] [PubMed] [Google Scholar]
- 7.Murray-Kolb LE, Beard JL. Iron deficiency and child and maternal health. Am J Clin Nutr. 2009;89:946S–50S. doi: 10.3945/ajcn.2008.26692D. [DOI] [PubMed] [Google Scholar]
- 8.Milman N. Postpartum anemia I: definition, prevalence, causes, and consequences. Ann Hematol. 2011;90:1247–53. doi: 10.1007/s00277-011-1279-z. [DOI] [PubMed] [Google Scholar]
- 9.Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265–72. doi: 10.1111/j.1471-0528.2008.01859.x. [DOI] [PubMed] [Google Scholar]
- 10.Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anesth Analg. 2010;110:1368–73. doi: 10.1213/ANE.0b013e3181d74898. [DOI] [PubMed] [Google Scholar]
- 11.Escobar GJ, Greene JD, Hulac P, Kincannon E, Bischoff K, Gardner MN, Armstrong MA, France EK. Rehospitalisation after birth hospitalisation: patterns among infants of all gestations. Arch Dis Child. 2005;90:125–31. doi: 10.1136/adc.2003.039974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Med Care. 2008;46:232–9. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 13.Selby JV. Linking automated databases for research in managed care settings. Ann Intern Med. 1997;127:719–24. doi: 10.7326/0003-4819-127-8_part_2-199710151-00056. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Vitamin and Mineral Nutrition Information System. Department of Reproductive Health and Research, WHO; Geneva: 2011. Haemoglobin concentrations for the diagnosis of anemia and assessment of severity. [Google Scholar]
- 15.Schlembach D, Mortl MG, Girard T, Arzt W, Beinder E, Brezinka C, Chalubinski K, Fries D, Gogarten W, Hackeloer BJ, Helmer H, Henrich W, Hosli I, Husslein P, Kainer F, Lang U, Pfanner G, Rath W, Schleussner E, Steiner H, Surbek D, Zimmermann R. Management of postpartum hemorrhage (PPH): algorithm of the interdisciplinary D-ACH consensus group PPH (Germany - Austria - Switzerland) Anaesthesist. 2014;63:234–42. doi: 10.1007/s00101-014-2291-1. [DOI] [PubMed] [Google Scholar]
- 16.Royal College of Obstetricians and Gynaecologists. Prevention and management of postpartum haemorrhage (Green-top Guideline 52) RCOG; London: 2009. [Google Scholar]
- 17.Dahlke JD, Mendez-Figueroa H, Maggio L, Hauspurg AK, Sperling JD, Chauhan SP, Rouse DJ. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. 2015;213:76e1–e10. doi: 10.1016/j.ajog.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 18.Magann EF, Evans S, Hutchinson M, Collins R, Lanneau G, Morrison JC. Postpartum hemorrhage after cesarean delivery: an analysis of risk factors. South Med J. 2005;98:681–5. doi: 10.1097/01.SMJ.0000163309.53317.B8. [DOI] [PubMed] [Google Scholar]
- 19.Skjeldestad FE, Oian P. Blood loss after cesarean delivery: a registry-based study in Norway, 1999–2008. Am J Obstet Gynecol. 2012;206:76e1–7. doi: 10.1016/j.ajog.2011.07.036. [DOI] [PubMed] [Google Scholar]
- 20.Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW. Prevalence and risk factors for early postpartum anemia. Eur J Obstet Gynecol Reprod Biol. 2010;150:126–31. doi: 10.1016/j.ejogrb.2010.02.030. [DOI] [PubMed] [Google Scholar]
- 21.Brown LD, Cai TT, DasGupta A. Interval Estimation for a Binomial Proportion. Stat Sci. 2001;16:101–17. [Google Scholar]
- 22.Goodnough LT, Shander A. Patient blood management. Anesthesiology. 2012;116:1367–76. doi: 10.1097/ALN.0b013e318254d1a3. [DOI] [PubMed] [Google Scholar]
- 23.SABM and The Society for Hospital Medicine. Anemia Prevention and Management Program Implementation Guide. Philadelphia, PA: SHM; 2015. [Google Scholar]
- 24.Shander A, Isbister J, Gombotz H. Patient blood management: the global view. Transfusion. 2016;56(Suppl 1):S94–102. doi: 10.1111/trf.13529. [DOI] [PubMed] [Google Scholar]
- 25.Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Pract Res Clin Obstet Gynaecol. 2008;22:999–1012. doi: 10.1016/j.bpobgyn.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm Rep. 1998;47:1–29. [PubMed] [Google Scholar]
- 27.Munoz M, Gomez-Ramirez S, Kozek-Langeneker S, Shander A, Richards T, Pavia J, Kehlet H, Acheson AG, Evans C, Raobaikady R, Javidroozi M, Auerbach M. 'Fit to fly': overcoming barriers to preoperative haemoglobin optimization in surgical patients. Br J Anaesth. 2015;115:15–24. doi: 10.1093/bja/aev165. [DOI] [PubMed] [Google Scholar]
- 28.Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol. 2008;199:519e1–7. doi: 10.1016/j.ajog.2008.04.049. [DOI] [PubMed] [Google Scholar]
- 29.Toledo P, McCarthy RJ, Hewlett BJ, Fitzgerald PC, Wong CA. The accuracy of blood loss estimation after simulated vaginal delivery. Anesth Analg. 2007;105:1736–40. doi: 10.1213/01.ane.0000286233.48111.d8. [DOI] [PubMed] [Google Scholar]
- 30.Milman N. Postpartum anemia II: prevention and treatment. Ann Hematol. 2012;91:143–54. doi: 10.1007/s00277-011-1381-2. [DOI] [PubMed] [Google Scholar]
- 31.Friedman AJ, Shander A, Martin SR, Calabrese RK, Ashton ME, Lew I, Seid MH, Goodnough LT. Iron deficiency anemia in women: a practical guide to detection, diagnosis, and treatment. Obstet Gynecol Surv. 2015;70:342–53. doi: 10.1097/OGX.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 32.Nicol B, Croughan-Minihane M, Kilpatrick SJ. Lack of value of routine postpartum hematocrit determination after vaginal delivery. Obstet Gynecol. 1997;90:514–8. doi: 10.1016/s0029-7844(97)00354-2. [DOI] [PubMed] [Google Scholar]
- 33.Swaim LS, Perriatt S, Andres RL, Paradissis J, Watson MN. Clinical utility of routine postpartum hemoglobin determinations. Am J Perinatol. 1999;16:333–7. doi: 10.1055/s-2007-993881. [DOI] [PubMed] [Google Scholar]
- 34.Petersen LA, Lindner DS, Kleiber CM, Zimmerman MB, Hinton AT, Yankowitz J. Factors that predict low hematocrit levels in the postpartum patient after vaginal delivery. Am J Obstet Gynecol. 2002;186:737–44. doi: 10.1067/mob.2002.121255. [DOI] [PubMed] [Google Scholar]
- 35.Horowitz E, Yogev Y, Ben-Haroush A, Rabinerson D, Feldberg D, Kaplan B. Routine hemoglobin testing following an elective Cesarean section: is it necessary? J Matern Fetal Neonatal Med. 2003;14:223–5. doi: 10.1080/jmf.14.4.223.225. [DOI] [PubMed] [Google Scholar]
- 36.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120:1029–36. doi: 10.1097/aog.0b013e31826d60c5. [DOI] [PubMed] [Google Scholar]
- 37.Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013;381:1845–54. doi: 10.1016/S0140-6736(13)60650-9. [DOI] [PubMed] [Google Scholar]
- 38.Goodnough LT, Shah N. Is there a "magic" hemoglobin number? Clinical decision support promoting restrictive blood transfusion practices. Am J Hematol. 2015;90:927–33. doi: 10.1002/ajh.24101. [DOI] [PubMed] [Google Scholar]
- 39.Prick BW, Jansen AJ, Steegers EA, Hop WC, Essink-Bot ML, Uyl-de Groot CA, Akerboom BM, van Alphen M, Bloemenkamp KW, Boers KE, Bremer HA, Kwee A, van Loon AJ, Metz GC, Papatsonis DN, van der Post JA, Porath MM, Rijnders RJ, Roumen FJ, Scheepers HC, Schippers DH, Schuitemaker NW, Stigter RH, Woiski MD, Mol BW, van Rhenen DJ, Duvekot JJ. Transfusion policy after severe postpartum haemorrhage: a randomised non-inferiority trial. BJOG. 2014;121:1005–14. doi: 10.1111/1471-0528.12531. [DOI] [PubMed] [Google Scholar]
- 40.Van Der Woude D, Pijnenborg JM, Verzijl JM, Van Wijk EM, De Vries J. Health status and fatigue of postpartum anemic women: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2014;181:119–23. doi: 10.1016/j.ejogrb.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 41.D'Alton ME, Main EK, Menard MK, Levy BS. The National Partnership for Maternal Safety. Obstet Gynecol. 2014;123:973–7. doi: 10.1097/AOG.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 42.Main EK, Goffman D, Scavone BM, Low LK, Bingham D, Fontaine PL, Gorlin JB, Lagrew DC, Levy BS. National Partnership for Maternal Safety: Consensus Bundle on Obstetric Hemorrhage. Obstet Gynecol. 2015;126:155–62. doi: 10.1097/AOG.0000000000000869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The rate of severe postpartum anemia prior to hospital discharge by year, between 2005 and 2013. Error bars denote 95% CI.
Table S1. Diagnoses, Procedures and the Associated ICD-9 CM Codes
Table S2. Proportions with Severe Postpartum Anemia According to Each Characteristic and Adjusted Odds Ratios in the Final Multivariable Model.
Table S3. Associations between Severe Postpartum Anemia with Postpartum Hemorrhage and Predelivery Hemoglobin Level Stratified by Receipt and Non-Receipt of Transfusion.
Table S4. Sensitivity Analyses Examining the Associations between Severe Postpartum Anemia with Predelivery Hemoglobin Levels and Estimated Blood Loss for Women undergoing Cesarean Section between 2010 and 2013.
Table S5. Patient Characteristics of 13,086 Women who did not undergo Postpartum Hemoglobin Testing after Cesarean Section between 2005 and 2013
Table S6. Characteristics of 5,843 Women with Missing Postpartum Hemoglobin Values who underwent Cesarean Section between 2010 and 2013.