Abstract
Neural systems that index self-regulation have been associated with mental health outcomes, including risk for anxiety problems, from early in life. Yet, little is known about the environmental factors that may impact the development of neural systems of regulation. Behavioral work suggests that sensitive parenting, or parents’ ability to correctly interpret and respond to children’s signals, supports the development of regulation. Conversely, harsh parenting, or uninvolved or punitive parent behaviors, is thought to compromise developing regulatory systems. We recorded preschoolers’ baseline electroencephalography (EEG) and tested whether individual differences in delta-beta coupling were linked to sensitive or harsh parenting behaviors in mothers and fathers. Using Fisher’s r-to-z transform, we found that preschoolers whose fathers were low (vs. high) in harsh parenting showed greater coupling at parietal electrode sites (z = 2.66, p = 0.00); preschoolers whose fathers were high (vs. low) in harsh parenting showed greater coupling at frontal electrode sites (z = −2.14, p = 0.02). Heightened coupling at frontal electrodes was also visible for children who showed high (vs. low) levels of social fear (z = −2.11, p = 0.02), suggesting that enhanced frontal coupling may be associated with increased risk for anxiety problems. No differences in coupling were seen based on levels of sensitive parenting behaviors in mothers or fathers. Results provide initial evidence that harsh parenting behaviors in fathers are associated with differences in a general index of neural regulation in preschoolers, which may have implications for the development of social fear in early life.
Keywords: Delta-Beta Coupling, Parenting, Preschool, Self-Regulation
1. Introduction
Heightened fearfulness (Hirshfeld-Becker et al., 2007; Schwartz, Snidman, & Kagan, 1999; Spence, Rapee, McDonald, & Ingram, 2001), high, stable levels of social fear (Brooker et al., 2013), and out-of-context displays of fearfulness (Buss et al., 2013) predict increased risk for anxiety problems as early as infancy. These traits are associated with biological and behavioral systems of self-regulation (Degnan, Almas, & Fox, 2010; Phelps, Brooker, & Buss, 2016), disruptions in which may serve as mechanisms of early fear and risk for later disorder. Caregiver behaviors predict observed regulatory behaviors in preschoolers (Rubin, Cheah, & Fox, 2001). However, it is unclear whether caregiver behaviors similarly disrupt biological systems, impacting self-regulation at a more basic level. Understanding how self-regulation may be impacted at the level of neural activity may provide additional insight into the mechanisms by which the early environment may support or disrupt self-regulation in preschoolers. In the current study, we addressed this gap in the literature by using delta-beta coupling, a purported neural index of self-regulation (Knyazev, 2007; Knyazev & Slobodskaya, 2003), to examine differences in regulatory mechanisms associated with early parenting behaviors. We tested these associations at age 3 years, when neural systems are undergoing vast developmental change and when parents comprise the majority of the child’s environment (Kopp, 1982; Tsujimoto, 2008).
1.1 Delta-Beta Coupling
Noninvasive electroencephalograph (EEG) recordings provide temporally sensitive measures of neural activity. EEG can be used to identify associations between power in different frequency bands and relatively discrete forms of neural processing. For example, low-frequency oscillations in the delta band (0.5 Hz – 4 Hz) are positively correlated with motivational, reward and emotional processes in adults (Knyazev, 2007). Greater delta power has been similarly linked to enhanced activity in sub-cortical regions of the brain, including the limbic system, which is believed to underlie emotional and motivational functioning (Gray, 1982; Guyton, 1976). In children, delta (0.5 Hz – 2 Hz) is the predominant visible frequency in the EEG. However, delta power is also enhanced during periods of emotional processing (Knyazev et al., 2003). Similarly, excessive delta, which may indicate neural immaturity, is visible in children who were more physiologically reactive (Raine, Venables, Dalais, Mellingen, Reynolds, & Mednick, 2001) and children with behavioral disorders relative to typically-developing peers (Matsuura et al., 1993). Overall, previous findings suggest that delta is linked, from early in life, to subcortical processes reflecting emotional reactivity.
In contrast, high-frequency oscillations in the beta band are visible during awake states and periods of enhanced cognitive processing in both children (11 Hz– 18 Hz) and adults (12 Hz – 30 Hz; Bell, 1998; Ray & Cole, 1985; Stern, Ray, & Quigley, 2001). In adults, beta power is enhanced in contexts that call for attentional control and self-regulation, reflecting intra-cortical connections (Engel, Fries, & Singer, 2001; Ray & Cole 1985). In children ages 8 to 13, beta power is thought to play a similar role in self-regulation: low beta activity is linked to increased impulsivity and diagnosed Attention Deficit Hyperactivity Disorder (Barry, Clarke, & Johnstone, 2003; Callaway, Halliday, & Naylor, 1983). Thus, from a behavioral level, beta power appears to be linked to efforts to deploy regulatory behaviors, perhaps by exerting an inhibitory influence on arousal in subcortical systems (Robinson, 1999).
Recently, measures of delta-beta coupling have come of interest as putative indices of associations between reactive and self-regulatory systems. Delta-beta coupling refers to the correlation between neural activity in the delta and beta frequency bands, thus denoting complementary changes in delta and beta power. Although there is not currently a single, agreed-upon definition regarding the functional significance of delta-beta coupling, it is frequently purported to index crosstalk between the brain’s cortical and subcortical networks (Knyazev, 2007; Knyazev & Slobodskaya, 2003). From this perspective, delta-beta coupling reflects, in real time, efforts by cognitively-oriented cortical neural networks to downregulate emotion-based reactivity in subcortical networks. Indeed, greater delta-beta coupling, or greater positive correlations between delta and beta power, during resting baseline is associated with trait-level emotion propensities, including enhanced trait anxiety or anxiety symptoms (Knyazev, 2011; Miskovic et al., 2010) and greater trait-level inhibition (Putman, 2011) in adults. Increased baseline coupling is also visible in children of anxious parents (Miskovic et al., 2011a) and preschoolers who are prone to show high levels of fear in low-threat situations (Phelps et al., 2016).
In sum, although the research on delta-beta coupling in children is still in its early stages, evidence suggests that resting baseline delta-beta coupling may index trait-level propensities for cognitively-oriented neural systems to down-regulate neural systems of emotional reactivity. Thus, while we acknowledge that the functional implications of coupling are still being shaped by the expanding literature, we here refer to coupling as “neural regulation” based on the work cited above. Developmental and psychobiological theories suggest that the early environment plays an important role in shaping biological systems of self-regulation. For example, early sensitization theory posits that the early rearing environment programs biological templates for trait-level regulatory responses (Gunnar & Quevedo, 2007; Shirtcliff & Ruttle, 2010). However, few investigations have examined whether neural regulation as indexed by delta-beta coupling might be shaped by the early environment. If so, this neural system may serve as a mechanism by which the early environment affects regulatory capacities and putative risk for later disorder.
1.2 Parenting
Arguably the most prominent environmental mechanisms in early life for typically developing children are interactions with parents. Parent behaviors play an important role in the development of self-regulation (Kopp, 1982) which, in turn, has a significant impact on long-term mental health and well-being (John & Gross, 2004). Research suggests that sensitive and harsh parenting behaviors are particularly salient early influences on children’s developing regulatory capacities (Morris, Silk, Steinberg, Myers, & Robinson, 2007). Sensitive parenting, or a parent’s ability to correctly perceive, interpret, and respond promptly to children’s signals (Ainsworth, Bell, Stayton, & Richards, 1974), positively predicts cognitive abilities (NICHD Early Child Care Research Network, 2003) and emotional regulation (Eisenberg et al., 2003) in offspring. In contrast, greater harsh parenting, or parental uninvolvement, disengagement, or punitiveness (Knutson, DeGarmo, Koeppl, & Reid, 2005), is linked to diminished self-regulation, greater social wariness (Degnan, Henderson, Fox, & Rubin, 2008), and heightened risk for anxiety problems (Shanahan, Copeland, Costello, & Angold, 2008) during childhood and adolescence.
Studies using Event Related Potentials (ERPs), which measure electrical potentials to isolate quickly-occurring (i.e., on the order of milliseconds) neural processes in response to specific events (e.g., self-monitoring following a button-press response), and functional Magnetic Resonance Imaging (fMRI), a measure of the hemodynamic response that measures activation in specific neural structures, have shown that more harsh parenting is linked to hyperactivation of the amygdala (Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, 2006), and increased sensitivity to errors, (Brooker & Buss, 2014; Meyer et al., 2015), both of which indicate dysregulation and enhanced risk for anxiety problems during childhood. High rates of sensitive parenting, on the other hand, are linked to the healthy development of the corpus callosum, a brain region associated with high order self-regulation (Ghassabian et al., 2013; Kok et al., 2013). However, links between parenting practices and more global assessments of neural regulation remain unexamined, which limits our understanding of the extent to which early parenting behaviors may predict disruptions in general systems of self-regulation. Establishing such associations may be particularly important for children who are unlikely candidates for more specific measures of neural function such as those cited above. Therefore, this was one aim of the current study. We investigated whether the early parental environment – specifically harsh and sensitive parenting - was associated with neural regulation in preschool-aged children. We hypothesized that more parental harshness and less parental sensitivity would be associated with greater efforts at neural regulation, indexed as greater delta-beta coupling, during preschool.
It should also be noted that, to date, much of the work on the role of early parenting in shaping child outcomes has focused on maternal behaviors. However, paternal behaviors may be equally important for child development. For example, greater sensitive parenting in fathers has been associated with better cognitive regulation in children (Towe-Goodman et al., 2014). Greater sensitivity in fathers has also been linked to lower rates of behavioral problems and better social skills during periods of transition (Kazak, 2004), and lower autonomic reactivity in social contexts (Boyce et al., 2006). In contrast, harsher parenting in fathers predicts increased reactivity (Boyce et al., 2006), more aggression (Chang Schwartz, Dodge, & McBride-Chang, 2003), and a greater risk for mental health issues, including anxiety problems, between ages 7 and 9 (Boyce et al., 2006).
The effects of sensitive and harsh parenting may also rely on the sex of the parent. For example, although greater paternal harshness predicts increased aggression, maternal harshness predicts more problems with emotion regulation (Chang et al., 2003). Furthermore, paternal sensitivity predicts better problem solving skills and autonomy in toddlers and preschoolers even when high rates of mothers’ sensitive parenting behaviors are considered (Easterbrooks & Goldberg, 1984; Tamis-LeMonda, Shannon, Cabrera, & Lamb, 2004), suggesting that fathers make independent contributions to developing regulation in children. Thus, a second aim of the current study was to investigate associations between parenting behaviors and neural regulation separately for mothers and fathers. To do this, we tested associations between parenting and delta-beta coupling separately for mothers and fathers in order to understand whether patterns of effects might be similar across parents. We hypothesized that harshness in both mothers and fathers would be associated with enhanced delta-beta coupling.
Finally, we acknowledge that, without additional behavioral evidence, it may be hard to interpret whether links between parenting behaviors and neural regulation suggested adaptive or maladaptive tendencies. To aid with interpretation of findings, we also planned to test whether differences in neural regulation were linked to early social fear, an index of risk for anxiety problems in preschoolers. Similar patterns of parent-based differences in neural regulation and fearfulness may provide suggestive evidence as to whether such differences are adaptive or maladaptive. We predicted that children high in social fear would show increased delta-beta coupling relative to children low in social fear.
2. Method
2.1 Participants
Families with 3-year-old children were contacted through fliers, in-person recruitment at local events, and letters based on local birth records to participate in a longitudinal study of the development of cognition and emotion in preschoolers. Participants for this study included 107 children who visited the laboratory at the first wave of data collection (Mage=3.60, SD= 0.20; 58.3% female) and their mothers (Mage=35.81, SD= 5.06) and fathers (Mage=37.90, SD= 6.29). Children largely lived in two-parent households with heterosexual, biological parents. Participants were mostly Caucasian (95.8%, 3% Native American/Alaskan Native, 1% Asian) and Non-Hispanic/Latino (94.7%). Nearly half (48.7%) of families reported an annual household income of more than $61,000, though reported incomes ranged from less than $15,000 to more than $90,000 per year.
2.2 Procedure
Two weeks prior to the laboratory visit, primary and secondary caregivers (primarily biological mothers and fathers) were mailed a packet of questionnaires, which they were asked to complete independently. The primary caregiver, defined as the individual who was responsible for the majority of caretaking duties in the household, returned completed questionnaires when they accompanied their child to the lab. During the laboratory visit, children completed three tasks while EEG data were recorded: a resting baseline followed by age-appropriate flanker and go/no-go tasks. Following the EEG recordings, each child engaged in five different behavioral episodes designed to assess emotional reactivity and regulation in children. Parents were compensated $30.00 for their time in the laboratory and an additional $5.00 per completed questionnaire packet. Given the hypotheses of the current study regarding delta-beta coupling and early anxiety risk, measures of interest included baseline EEG, parent-reported parenting behaviors, and observed fear. Additional measures are not discussed further.
2.2.1 Delta-beta coupling
EEG data were acquired during a resting baseline using a 64-channel BioSemi Active Two recording system. Following the application of the electrode cap (Electro-Cap International, Inc.), the child was instructed to sit quietly and watch a 5-minute video clip of an interesting but emotionally neutral scene from the children’s show Magic School Bus™. EEG data were sampled at a rate of 2048 Hz. All electrodes were referenced to the Common Mode Sense and Driven Right Leg electrodes during recording.
Offline EEG data processing was performed in Brain Vision Analyzer (Brain Products: Gilching, Germany). Data were referenced to the average of the right and left mastoid channels, then high-pass (0.10 Hz) and low-pass (30 Hz) filtered in order to remove slow drift and high-frequency activity, respectively. Eye blink and eye movement artifacts were removed using the Gratton and Coles (1983) ocular correction algorithm. Similar to previous methods in research on delta-beta coupling in children (Miskovic et al., 2010; 2011a; 2011b; Phelps et al., 2016), data were then segmented into 1000 ms segments (50% overlap) and baseline corrected from 0–1000 ms. Segments that met one or more of the following criteria were marked as containing artifact: a voltage step of more than 75 µV/ms between data points, a voltage difference of 150µV within a single segment, a voltage difference of less than 0.5 µV within a 50 ms interval, or an absolute voltage of ±100 µV. Artifact free segments were subjected to a Fast-Fourier Transformation using a Hamming window with 50% segment width overlap. A mean of 237.29 segments (SD = 135.29) were retained for each participant.
Power (µV2) was derived in the delta (0.5–2.0 Hz) and beta (11.0–18.0 Hz) frequency bands (Marshall & Fox, 2006; Miskovic et al., 2011b). Consistent with previous work, delta and beta power were examined at the following channels: F3, F4, F7, F8, C3, C4, P3, P4 (Henderson, Marshall, Fox, & Rubin, 2004; Sutton et al., 2005). Power values were windsorized and natural log transformed to correct for nonnormal distributions. Similar to previous research, composites for delta and beta power were then formed for frontal (F3/4, F7/8), central (C3/4) and parietal (P3/4) electrode sites (Miskovic et al., 2011b; Phelps et al., 2016).
2.2.2 Parenting
To assess sensitive and harsh parenting behaviors, the Coping with Children’s Negative Emotions Scale (CCNES; Fabes, Eisenberg, & Bernzweig, 1990) was completed independently by mothers and fathers. This measure asked parents to report, using a 7-point scale (1 = very unlikely, 7 = very likely), the likelihood that they would display specific behaviors in response to negative or distressing situations that their child may experience. Reponses are believed to index general tendencies for parents to respond in certain ways to their child.
To distinguish between sensitive and harsh parenting, the five subscales of the CCNES were collapsed into two scales, which were validated using a Principal Components Analysis (PCA). PCAs were used to derive maternal and paternal composites separately. For both mothers and fathers, the sensitive parenting composite reflected high scores on the expressive encouragement (moms α = 0.95, dads α = 0.92), emotion-focused reactions (moms α = 0.86, dads α = 0.81), and problem focused reactions (moms α = 0.90, dads α = 0.88) subscales. Scores from each subscale were collapsed into a mean composite reflecting parental report of sensitive parenting (mean factor loading: moms = 0.77, dads = 0.86).
For mothers and fathers, the harsh parenting composite reflected high scores on the distress reaction (moms α = 0.80, dads α =0.87) and punitive/minimization reactions (moms α = 0.89, dads α = 0.87) subscales. Scores from each subscale were collapsed into a mean composite reflecting parental report of harsh parenting (mean factor loading: moms = 0.81, dads = 0.82).
2.2.3 Social fear
The Stranger Approach paradigm, a standardized laboratory episode, was used to elicit social fear in preschoolers (Goldsmith & Rothbart, 1999). During a three-minute episode, the child was instructed to play with age-appropriate toys while their primary caregiver sat in a chair across the room. Shortly thereafter, an unfamiliar male entered the room and approached the child in a non-threatening way while asking short, conversational questions (e.g., “are you having fun?”). The stranger remained in the room for 2 minutes. Parents were instructed to remain uninvolved throughout the episode.
Videos of the Stranger Approach were coded offline by trained graduate and undergraduate research assistants. Ratings of observed fear, including facial fear, decreased activity, avoidance, verbal hesitancy, gaze aversion, and fidgeting were assigned across nine coding epochs, each lasting approximately 10 seconds in length. Intensity of facial fear (κ = 0.73) was coded on a 4-point scale adapted from the AFFEX system, which differentiates emotion expressions based on three regions of the face (Izard, Dougherty, & Hembree, 1983). Codes ranged from no facial region showing codable fear (0) to the presence of fear across all three regions (brow, eye, and mouth) of the face (3). Decreased activity (κ = 0.78) was coded on a four point scale ranging from no sign of decreased activity (0) to high bodily fear such as freezing or tensing the body with little motion (3). Avoidance (κ = 0.84) was coded from no avoidance or standing in place (0) to high avoidance or going to the far corner of the room or to the parent (3). Verbal hesitancy (κ = 0.83) was coded on a three point scale based the child’s eager responses to the stranger’s questions (0) or no absence of response (2). Gaze aversion (κ = 0.79) was coded as the child showing no break in gaze from the stranger (0) to making no eye contact with the stranger (3). Fidgeting behaviors (κ = 0.88) were coded as either present (1) or absent (0).
All coders were trained by the second author and required to achieve a minimum reliability of 0.70 on all behaviors before coding independently. Approximately 40% of episodes were double-coded to calculate coding reliabilities and prevent drift. A factor analysis suggested that a single factor accounted for 41% of the variance in the original variables. Therefore, ratings of facial fear, activity decrease, avoidance, verbal hesitancy, gaze aversion and fidgeting were standardized and then mean composited into an index of social fear.
2.2.4 Missing Data
Five children were not included in analyses due to excessive EEG artifacts, seven children refused to be capped, and four children were excluded due to technical errors. In total, 91 children provided usable baseline EEG data. Fear behaviors could not be coded for seven children due to computer/technical errors, four fear episodes were not conducted due to the lack of a stranger and one child was excluded from the study procedure because he was too upset to continue participation. The CCNES was completed by 97 mothers and 71 fathers. Missing parent questionnaire data was due to parents not returning questionnaire packets to the laboratory, completing only some questionnaires from the packet, or leaving questionnaire items blank. A missing value analysis suggested that data were missing completely at random (Little’s MCAR χ2 = 72.58, p = 0.56).
2.2.5 Plan for Analysis
First, we conducted preliminary analyses using paired-samples t-tests to understand whether parenting behaviors differed between mothers and fathers. We also examined patterns of bivariate correlations among primary variables. Second, Pearson’s correlation coefficients were calculated across subjects and used to estimate delta-beta coupling at each electrode location. Third, we used Fisher’s r-to-z transformation to test for differences in delta-beta coupling between the following groups of children: children whose fathers reported high vs. low sensitivity, children whose fathers reported high vs. low harshness; children whose mothers reported high vs. low sensitivity, children whose mothers reported high vs. low harshness. All groupings were based on a mean split. To control for Type I error, Bonferroni adjusted alpha levels of 0.0063 were used for each test (α = 0.05/8). Finally, to aid in the interpretation of group differences in coupling, we used Fisher’s r-to-z transformation to test for differences in delta-beta coupling between children who displayed high vs. low levels of social fear.
3. Results
3.1 Preliminary analyses
Descriptive statistics are shown in Table 1. Mothers reported higher levels of sensitive parenting than fathers (t(68) = −5.71, p = 0.00). In contrast, mothers and fathers showed only a marginal difference in levels of harsh parenting (t(68) = 1.84, p = 0.07). Given some indication of differences between parents, fathers’ and mothers’ parenting behaviors were analyzed separately for all subsequent analyses.
Table 1.
Descriptive statistics for delta and beta power, parenting behaviors, and social fear
| N | Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|---|
| F3/4 Delta | 91 | 3.90 | 5.11 | 4.50 | 0.25 |
| F7/8 Delta | 91 | 3.88 | 5.14 | 4.47 | 0.25 |
| C3/4 Delta | 91 | 3.61 | 4.83 | 4.31 | 0.27 |
| P3/4 Delta | 91 | 2.77 | 4.87 | 4.15 | 0.33 |
| F3/4 Beta | 91 | −0.41 | 1.68 | 0.68 | 0.37 |
| F7/8 Beta | 91 | −0.59 | 1.68 | 0.64 | 0.37 |
| C3/4 Beta | 91 | −0.33 | 0.96 | 0.27 | 0.29 |
| P3/4 Beta | 91 | −0.97 | 0.99 | 0.33 | 0.33 |
| Father Sensitive | 71 | 1.00 | 6.94 | 5.02* | 0.92 |
| Father Harsh | 71 | 1.42 | 5.10 | 3.02+ | 0.74 |
| Mother Sensitive | 97 | 3.89 | 6.78 | 5.56* | 0.69 |
| Mother Harsh | 97 | 1.25 | 4.79 | 2.78+ | 0.78 |
| Social Fear | 93 | −1.46 | 1.61 | 0.02 | 0.63 |
Note:
denotes difference between maternal and paternal measures (p < 0.05) based on paired-samples t-test;
denotes difference between maternal and paternal measures (p < 0.10) based on paired-samples t-test
Bivariate correlations among variables are shown in Table 2. Less sensitive parenting in mothers was correlated with greater baseline delta power in children at C3/4 (r = −0.26, p = 0.02), and P3/4 (r = −0.36, p < 0.01). Less sensitive parenting in mothers was also correlated with greater baseline beta power in children at P3/4 (r = −0.24, p = 0.03). No other correlations between parenting and children’s delta or beta power were significant (rs < 0.24, ps > 0.05).
Table 2.
Bivariate correlations among delta power, beta power, parenting behaviors, and social fear.
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. F3/4 Delta | 0.64** | 0.71** | 0.52** | 0.09 | 0.05 | 0.26* | 0.07 | −0.15 | 0.00 | −0.16 | 0.11 | 0.10 |
| 2. F7/8 Delta | 0.56** | 0.34** | 0.17 | 0.29** | 0.29** | 0.07 | −0.13 | −0.05 | −0.10 | 0.02 | 0.08 | |
| 3. C3/4 Delta | 0.74** | 0.08 | 0.04 | 0.36** | 0.25* | −0.21 | −0.05 | −0.26* | 0.05 | 0.03 | ||
| 4. P3/4 Delta | 0.00 | 0.02 | 0.34** | 0.55** | −0.23 | −0.15 | −0.36** | 0.03 | −0.12 | |||
| 5. F3/4 Beta | 0.66** | 0.53** | 0.31** | −0.16 | −0.20 | 0.01 | 0.08 | −0.02 | ||||
| 6. F7/8 Beta | 0.53** | 0.39** | −0.18 | −0.13 | −0.06 | 0.16 | 0.09 | |||||
| 7. C3/4 Beta | 0.78** | −0.08 | −0.06 | −0.16 | 0.17 | −0.04 | ||||||
| 8. P3/4 Beta | −0.15 | −0.16 | −0.24* | 0.10 | −0.11 | |||||||
| 9. Father Sensitive | 0.11 | 0.47** | −0.18 | 0.05 | ||||||||
| 10. Father Harsh | 0.04 | 0.19 | −0.04 | |||||||||
| 11. Mother Sensitive | −0.18 | 0.06 | ||||||||||
| 12. Mother Harsh | 0.10 | |||||||||||
| 13 Social Fear |
p < 0.05
p < 0.01
Sensitive parenting was unrelated to harsh parenting for both fathers (r = 0.11, p = 0.35) and mothers (r = −0.18, p = 0.08). This lack of association suggested that harshness and sensitivity were independent constructs.
3.2 Delta-beta coupling and parent behaviors
Significant coupling was observed at F7/8 (r = 0.29, p = 0.01), C3/4 (r = 0.36, p < 0.001), and P3/4 site (r = 0.55, p < 0.001). Coupling was not significant at F3/4 (r = 0.09, p = 0.406; Table 2).
3.2.1 Paternal Behaviors
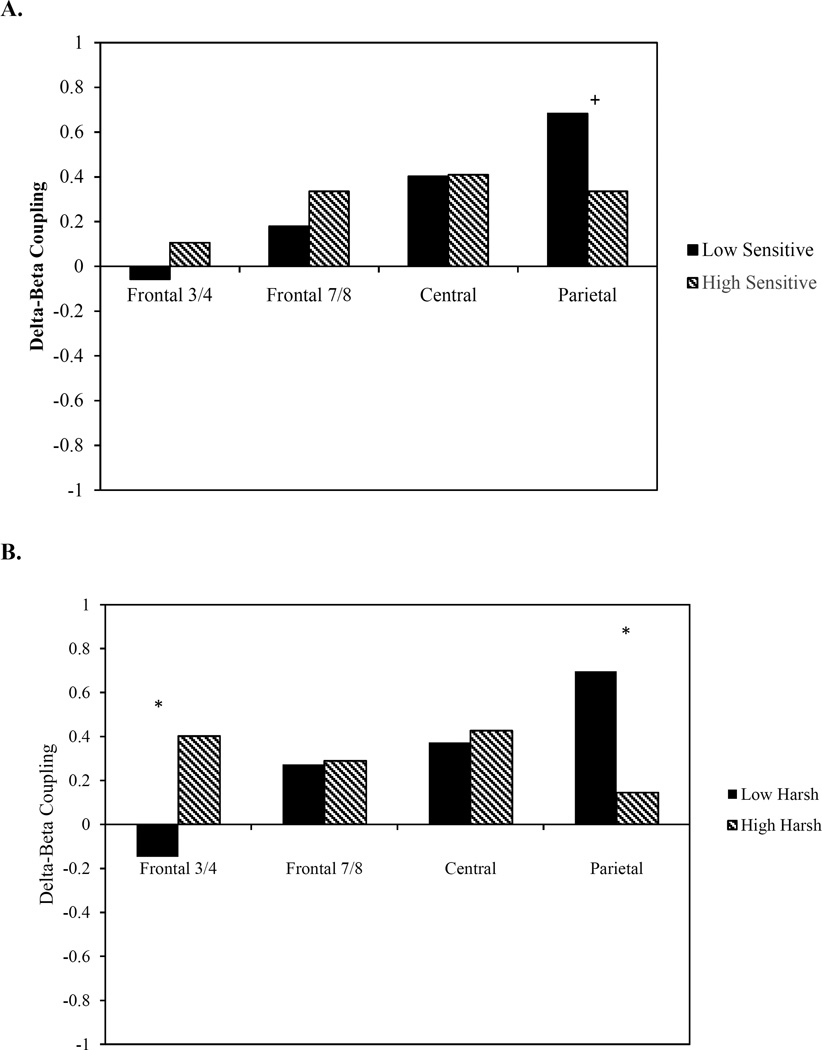
We then examined delta-beta coupling at each electrode location for children whose fathers were low (n = 27) and high in sensitive parenting (n = 35). A marginal group difference in coupling between low and high paternal sensitivity was observed at P3/4 (z = 1.81, p = 0.07). However, this difference was nonsignificant following Bonferroni correction. Coupling was not significantly different between children of low and high sensitive fathers at the other electrode sites (F3/4: z = 0.60, p = 0.27; F7/8: z = 0.62, p = 0.27; C3/4: z = 0.03, p = 0.45; Figure 1A).
Figure 1.
Group differences in delta-beta coupling based on levels of fathers’ (A) sensitive parenting and (B) harsh parenting
+ p < 0.10 *p < 0.05
We next examined delta-beta coupling for children whose fathers were low (n = 34) and high (n = 28) in harsh parenting. Group differences in coupling were observed at F3/4 (z = −2.14, p = 0.02) and P3/4 (z = 2.66, p < 0.01). Children whose fathers were high in harsh parenting showed significantly greater coupling at F3/4 compared to children with fathers low in harsh parenting. In contrast, at P3/4, children whose fathers were low in harsh parenting showed significantly greater coupling than those children with fathers high in harsh parenting. Only group differences at P3/4 remained significant following Bonferroni correction. There were no significant differences at F7/8 (z = −0.06, p = 0.48) or C3/4 (z = −0.23, p = 0.41) electrodes (Figure 1B).
3.2.2 Maternal behaviors
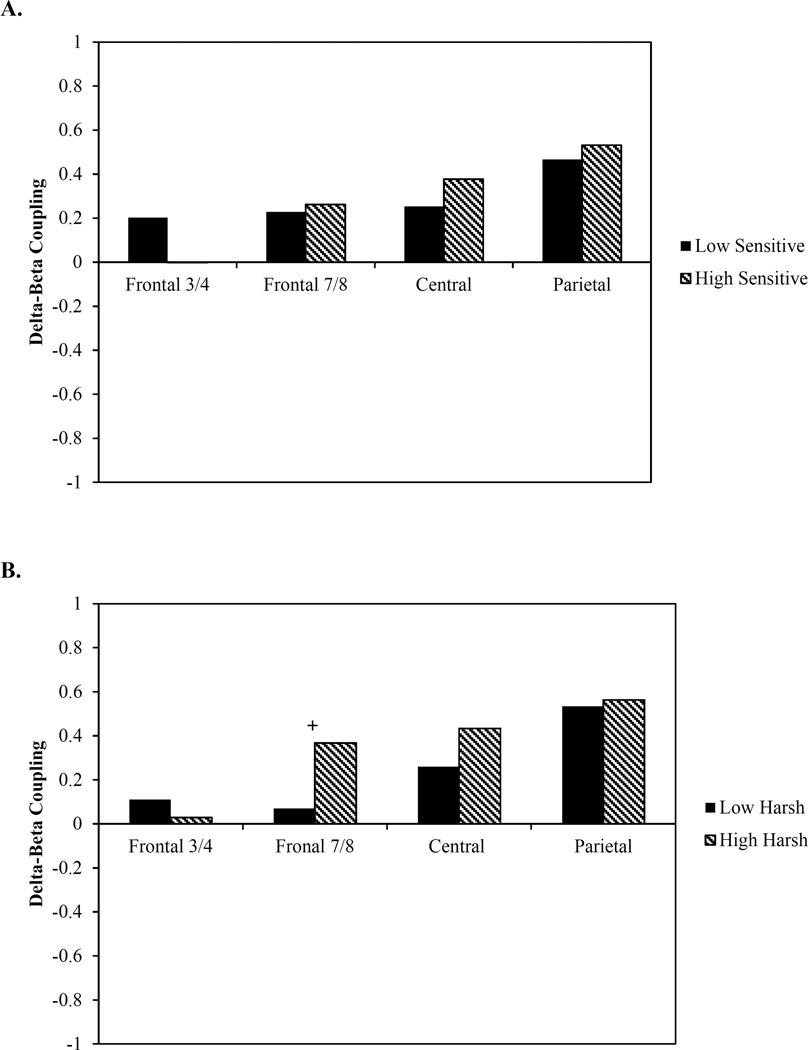
We then examined delta-beta coupling for children whose mothers were low (n = 41) and high (n = 40) in sensitive parenting. There were no group differences in coupling at any electrode sites (F3/4: z = 0.90, p = 0.18; F7/8: z = 0.15, p = 0.44; C3/4: z = 0.59, p =0.28; P3/4: z = 0.37, p = 0.36; Figure 2A).
Figure 2.
Group differences in delta-beta coupling based on levels of mothers’ (A) sensitive parenting and (B) harsh parenting
+ p < 0.10 *p < 0.05
Finally, we examined delta-beta coupling for children whose mothers were low (n = 41) and high (n = 40) in harsh parenting. We found a marginal difference in coupling between preschoolers with low and high harsh mothers in the F7/8 (z = −1.36, p = 0.09) site. Coupling was not different at any other electrode site (F3/4: z = 0.36, p = 0.36; C3/4: z = 0.86, p = 0.20; P3/4: z = 0.17, p = 0.43; Figure 2B).
3.3 Delta-beta coupling and observed social fear
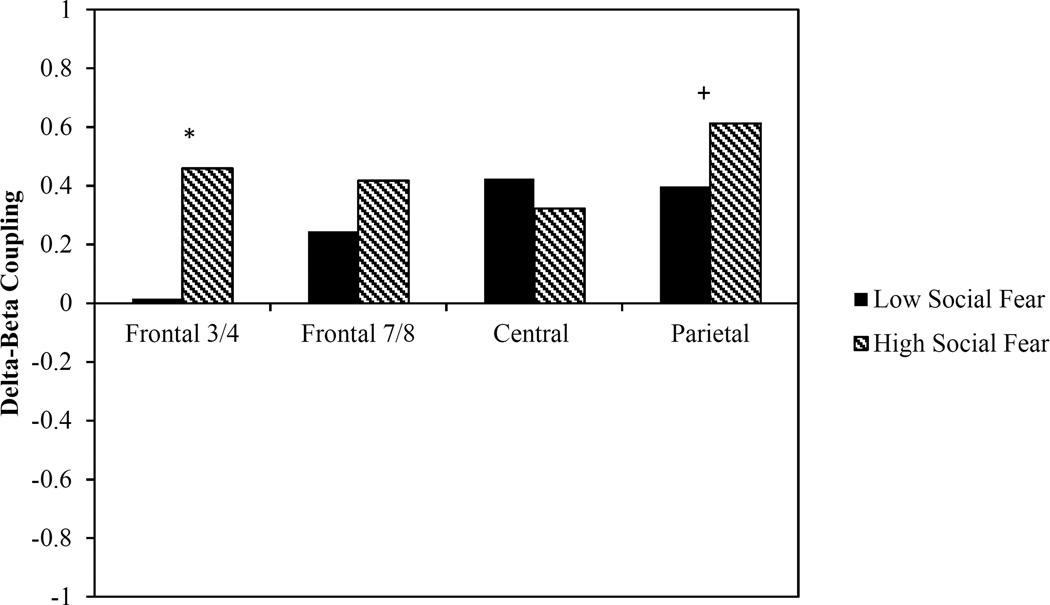
Finally, we tested for differences in coupling for children who were low (n = 44) and high (n = 39) in social fear. We found that children who were high in social fear had significantly greater coupling at F3/4 (z = −2.11, p = 0.02) and marginally greater coupling at P3/4 (z = −1.27, p = 0.10) relative to those children with low social fear. No group differences in coupling were found at F7/8 (z = −0.85, p = 0.20) or C3/4 (z = −0.51, p = 0.31; Figure 3).
Figure 3.
Group differences in delta-beta coupling based on levels of preschoolers’ social fear
+ p < 0.10 *p < 0.05
4. Discussion
Our results provide initial evidence that self-reported parenting behaviors in fathers, but not mothers, are associated with neural regulation indexed by delta-beta coupling in preschoolers. Findings appeared to be specific to fathers’ harsh behaviors, as sensitive parenting was not associated with any differences in coupling in preschoolers. Parallel analyses suggested that differences in coupling related to fathers’ levels of harsh parenting may have implications for the development of social fear in early life. Each set of findings is discussed in turn.
We found evidence for greater coupling at frontal electrode sites for children whose fathers showed high levels of harsh parenting relative to children whose fathers showed low levels of harsh parenting. Recall that delta-beta coupling is purported to provide a real-time index of efforts by cortical neural networks to down-regulate emotion-based reactivity in subcortical networks. Our results thus reflect the possibility that in contexts where children may need to employ high levels of regulation, such as when there is a greater threat of a harsh response from a parent, children may develop a propensity for greater neural regulation. This explanation is consistent with the idea of delta-beta coupling as a putative index of regulation at the neural level (Knyazev, 2007; Knyazev et al., 2006) as well as with previous work linking delta-beta coupling at frontal electrodes to levels of fear (Phelps et al., 2016), risk for anxiety problems, (Miskovic et al., 2011b), and anxious behaviors (Knyazev, 2011; Miskovic et al., 2010) in children and adults.
At parietal electrodes, in contrast, coupling was diminished for children of more harsh fathers relative to children of less harsh fathers. This pattern raises the possibility that harsh parenting not only alters propensities for regulation, but also the neural regions that support regulation in early development. Because our study uses EEG measures, conclusions about spatial boundaries of function are limited. Nonetheless, findings using more spatially precise measures have shown that parietal brain regions are important for regulatory processing in toddlers and children between ages 8 and 12, when frontal networks are still developing (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; Durston et al., 2002; Rothbart, Sheese, Rueda, & Posner, 2011). Specifically, as neutral networks mature and self-regulatory abilities improve, regulatory processing shifts from posterior to anterior locations. Thus, it is possible that the differences in coupling at frontal and parietal electrodes found here illustrate a normative pattern of development (i.e., reliance on parietal networks) for preschoolers with less harsh fathers, but also suggest that chronic exposure to harsh parenting may expedite the development of frontal regulatory networks in order to cope with harsh, punitive early environments. Similar patterns of effects have been observed for the development of neural markers of self-monitoring. Greater levels of harsh parenting during toddlerhood predict greater error-related negativity, a neural mechanism linked to performance and self-monitoring, during preschool and early childhood (Brooker & Buss, 2014; Meyer et al., 2015). Similarly, early maturing patterns of self-monitoring are associated with greater numbers of anxiety symptoms during late childhood and adolescence (Ladouceur, Dahl, Birmaher, Axelson, & Ryan, 2006). Further studies will be needed to directly test this possibility and the direction of effects. Nonetheless, this work provides preliminary evidence that paternal harshness may influence the development of neural systems of regulation in preschool aged children.
Although we found clear differences in patterns of coupling based on levels of paternal harshness, it is reasonable to question whether enhanced coupling in frontal regions – associated with high levels of paternal harshness - is truly maladaptive. At minimum, high baseline coupling reflects an inefficient use of neural resources to the extent that it reflects a mismatch of regulatory efforts with the eliciting context (Bonanno & Burton, 2013; Buss, 2011). However, we also provided evidence that increased frontal coupling was associated with greater social fear during preschool. As previously noted, high levels of social fear are linked to greater risk for anxiety problems in early life (Brooker et al., 2013; Brooker, Kiel, & Buss, in press; Buss et al., 2013), suggesting a possible biological mechanism of risk that is visible by age 3. Notably, paternal harshness was unrelated to levels of parietal coupling, again suggesting that these harsh behaviors impact the development of frontal regulatory networks in particular.
We found no evidence for differences in delta-beta coupling in preschoolers based on mothers’ levels of harsh parenting. At first glance, this suggests that the paternal environment plays a unique, potentially greater role in the development of anxiety risk relative to mothers. However, there is mixed evidence for the importance of maternal versus paternal behaviors in the development of children’s regulatory skills and internalizing problems. Numerous studies have suggested that fathers’ – but not mothers’ - harsh parenting during childhood is directly (Rinaldi & Howe, 2012) or indirectly associated on the development of internalizing symptoms in adulthood (South & Jarnecke, 2015). Reviews of the parenting literature have similarly suggested that fathers’ harsh parenting has a stronger effect on child outcomes than mothers’ harsh parenting (Loeber & Southamer-Loeber, 1986). However, other studies suggest that maternal harshness has a stronger effect on children’s outcomes relative to paternal harshness (Denham et al., 2000; Rothbaum & Weisz, 1994) or that the impact of maternal and paternal behaviors do not differ (Lytton & Romney, 1991). It is possible that these inconsistencies result from the presence of additional moderators on which the effects of parental behaviors are dependent. For example, the effect of harsh parenting on children’s aggressive outcomes has been suggested to depend on whether parents and children were of the same sex, such that fathers’ behaviors more strongly impact sons and mothers’ behaviors more strongly impact daughters (Deater-Deckard & Dodge, 1997; Fabes, 1994). Yet, other work showed that paternal harshness equally predicts behaviors in sons and daughters (McKee et al., 2007). Controlling for sex of child does not change the pattern of results we report here. It has also been suggested that differences in child-father attachments relations modulate the impact of paternal behaviors on child development, although such differences do not typically appear until later in childhood (Lieberman, Doyle, & Markiewicz, 1999). Thus, while this is not the first study to find evidence for different patterns of association between paternal and maternal behaviors on children’s developing biological systems (Brooker et al., 2015; Hastings et al., 2008), additional work is needed to clarify the mechanisms that underlie these effects.
Unexpectedly, no coupling differences were seen in preschoolers based on levels of parent sensitivity. Our sample shows high levels of sensitivity in general and the lack of any substantial differences could be due this narrow range. Sensitive parenting may also be associated with coupling in a nonlinear fashion, where by high and low levels would both be adverse. This association would not be evident from a correlation, which looks only at linear relationships. Another explanation could be that parental sensitivity is related to other regulatory systems. For example, sensitive parenting appears to buffer neuroendocrine responses in socially inhibited children (Kertes et al., 2009).
Our work suggests associations between a putative biological measure of self-regulation in children and the early parenting environment. Notably, all but one child in the current study was being raised by at least one parent to whom s/he was genetically related. Thus, there exists the possibility that genetic influences shared by the parent and child may be contributing both to parents’ behaviors and to children’s neural function (i.e., gene-environment correlation; Plomin, DeFries, & Loehlin, 1977). That is, the same genetic influences may simultaneously influence complex behaviors, such as parenting (Kendler & Baker, 2007), and biological systems that index self-regulation, including neural oscillations (Begleiter & Porjesz, 2006). As such, we cannot distinguish any single effect as purely environmental. This should be considered when interpreting the current results and should be further explored in a genetically-informative design.
This study is not without limitations. First, this work was done in a typically-developing sample that was unselected for anxiety risk. As a result, it is unknown which children will go on to develop problems. Additional research using a longitudinal design is currently underway that will allow us to test the implications of these early differences in coupling for long-term child outcomes. Second, our sample was composed of predominantly white families with two heterosexual parents, which may not generalize to the entire population. Additional work that investigates associations between parenting and neural regulation in nonwhite and/or single-parent families and in families with same-sex parents is needed. Third, although our findings are consistent with and expand on previous research regarding delta-beta coupling, the full function of delta-beta coupling at early developmental stages is still being explored. It will be important to continue to assess whether delta-beta coupling reflects similar regulatory processing in adults and children in order to fully explicate its early associations with risk for long-term disorder. Lastly, the coupling measure used here reflects a group-level assessment of coupling that does not fully capture individual differences. It will be beneficial to replicate this work using a within-person coupling measure, which will provide additional power as well as the opportunity to explore a dimensional approach to the effects of the early environment on neural systems of regulation.
It should also be noted that the parenting measure used here was based on parents’ self-report of the use of harsh and sensitive parenting behaviors with their child. Given that parent-report measures are associated, here, with observational measures and assessments of neural activity, we do not feel that our results can be explained as by reporting bias. However, self-report measures of parenting likely assess unique aspects of parent-child child interactions than do observational assessments; such differences between parent-report and observation are noted in other areas of developmental science (e.g., Zentner & Bates, 2008). Thus, interpretation our findings is limited to links between fathers’ perceptions of their harsh parenting behaviors and coupling in children. It will be important for future studies to replicate this work using other methods to assess parenting.
Overall, these findings reveal that paternal harshness in the early environment impacts at least one of the neural processes believed to underlie regulatory processing in children. Through converging evidence, our results appear to suggest that paternal harsh parenting influences the development of normative and atypical regulatory processing, with potential implications for children’s fear behaviors, as early as the preschool years.
Highlights.
Delta-beta coupling is associated with paternal but not maternal behaviors during preschool.
Greater paternal harshness is associated with greater frontal coupling.
Less paternal harshness is associated with greater parietal coupling.
Greater social fear is associated with greater frontal coupling.
Paternal harshness appears to affect the development of neural systems of regulation as early as age 3.
Acknowledgments
We thank the families who participated in this study and the staff members who helped with the recruitment of study participants and data collection. Data collection for this project was supported by K01 MH100240 from the National Institute of Mental Health (PI: Brooker) and P20 GM104417 (PI: Harmsen).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- Ainsworth MDS, Bell SM, Stayton DJ, Richards MPM. Infant-mother attachment and social development. In: Richards MP, editor. The Introduction of the Child into a Social World. London, UK: Cambridge University Press; 1974. pp. 99–135. [Google Scholar]
- Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clinical Neurophysiology. 2003;114(2):171–183. doi: 10.1016/s1388-2457(02)00362-0. http://dx.doi.org/10.1016/S1388-2457(02)00362-0. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Gentics of human brain oscillations. International Journal of Psychophysiology. 2006;60(2):162–171. doi: 10.1016/j.ijpsycho.2005.12.013. http://dx.doi.org/10.1016/j.ipsycho.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Bell MA. Neuroimaging in Child Neuropsychiatric Disorders. Berlin Heidelberg: Springer; 1998. The ontogeny of the EEG during infancy and childhood: Implications for cognitive development; pp. 97–111. [Google Scholar]
- Bonanno GA, Burton CL. Regulatory flexibility an individual differences perspective on coping and emotion regulation. Perspectives on Psychological Science. 2013;8(6):591–612. doi: 10.1177/1745691613504116. http://dx.doi.org/10.1177/1745691613504116. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Alkon A, Goldsmith HH, Kraemer HC, Kupfer DJ. Early father involvement moderates biobehavioral susceptibility to mental health problems in middle childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 2006;45(12):1510–1520. doi: 10.1097/01.chi.0000237706.50884.8b. http://dx.doi.org/10.1097/01.chi.0000237706.50884.8b. [DOI] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA. Harsh parenting and fearfulness in toddlerhood interact to predict amplitudes of preschool error-related negativity. Developmental Cognitive Neuroscience. 2014;9:148–159. doi: 10.1016/j.dcn.2014.03.001. http://dx.doi.org/10.1016/j.dcn.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Buss KA, Lemery-Chalfant K, Aksan N, Davidson RJ, Goldsmith HH. The development of stranger fear in infancy and toddlerhood: normative development, individual differences, antecedents, and outcomes. Developmental Science. 2013;16(6):864–878. doi: 10.1111/desc.12058. http://dx.doi.org/10.1111/desc.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Kiel EJ, Buss KA. Early social fear predicts kindergarteners’ socially anxious behaviors: Direct associations, moderation by inhibitory control, and differences from nonsocial fear. Emotion. doi: 10.1037/emo0000135. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker RJ, Neiderhiser JM, Leve LD, Shaw DS, Scaramella LV, Reiss D. Associations between infant negative affect and parent anxiety symptoms are bidirectional: Evidence from mothers and fathers. Frontiers in Psychology. 2015;6:1875. doi: 10.3389/fpsyg.2015.01875. http://dx.doi.org/10.3389/fpsyg.2015.01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. http://dx.doi.org/10.1016/S0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA. Which fearful toddlers should we worry about? Context, fear regulation, and anxiety risk. Developmental Psychology. 2011;47(3):804–819. doi: 10.1037/a0023227. http://dx.doi.org/10.1037/a0023227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss KA, Davis EL, Kiel EJ, Brooker RJ, Beekman C, Early MC. Dysregulated fear predicts social wariness and social anxiety symptoms during kindergarten. Journal of Clinical Child & Adolescent Psychology. 2013;42(5):603–616. doi: 10.1080/15374416.2013.769170. http://dx.doi.org/10.1080/15374416.2013.769170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway E, Halliday R, Naylor H. Hyperactive children’s event-related potentials fail to support underarousal and maturational-lag theories. Archives of General Psychiatry. 1983;40(11):1243–1248. doi: 10.1001/archpsyc.1983.01790100089012. http://dx.doi.org/10.1001/archpsyc.1983.01790100089012. [DOI] [PubMed] [Google Scholar]
- Chang L, Schwartz D, Dodge KA, McBride-Chang C. Harsh parenting in relation to child emotion regulation and aggression. Journal of Family Psychology. 2003;17(4):598–606. doi: 10.1037/0893-3200.17.4.598. http://dx.doi.org/10.1037/0893-3200.17.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, Dupuy FE, Heckel LD, McCarthy R, Selikowitz M, Johnstone SJ. Behavioural differences between EEG-defined subgroups of children with Attention-Deficit/Hyperactivity Disorder. Clinical Neurophysiology. 2011;122(7):1333–1341. doi: 10.1016/j.clinph.2010.12.038. http://dx.doi.org/10.1016/j.clinph.2010.12.038. [DOI] [PubMed] [Google Scholar]
- Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Excess beta activity in children with attention-deficit/hyperactivity disorder: an atypical electrophysiological group. Psychiatry research. 2001;103(2):205–218. doi: 10.1016/s0165-1781(01)00277-3. http://dx.doi.org/10.1016/S0165-1781(01)00277-3. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K, Dodge KA. Externalizing behavior problems and discipline revisited: Nonlinear effects and variation by culture, context, and gender. Psychological Inquiry. 1997;8(3):161–175. http://dx.doi.org/10.1207/s15327965pli0803_1. [Google Scholar]
- Degnan KA, Almas AN, Fox NA. Temperament and the environment in the etiology of childhood anxiety. Journal of Child Psychology and Psychiatry. 2010;51(4):497–517. doi: 10.1111/j.1469-7610.2010.02228.x. http://dx.doi.org/10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan KA, Henderson HA, Fox NA, Rubin KH. Predicting social wariness in middle childhood: The moderating roles of childcare history, maternal personality and maternal behavior. Social Development. 2008;17(3):471–487. doi: 10.1111/j.1467-9507.2007.00437.x. http://dx.doi.org/10.1111/j.1467-9507.2007.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham SA, Workman E, Cole PM, Weissbrod C, Kendziora KT, Zahn-Waxler C. Prediction of externalizing behavior problems from early to middle childhood: The role of parental socialization and emotion expression. Development and Psychopathology. 2000;12(1):23–45. doi: 10.1017/s0954579400001024. http://dx.doi.org/10.1017/S0954579400001024. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Uluğ AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5(4):F9–F16. http://dx.doi.org/10.1111/1467-7687.00235. [Google Scholar]
- Easterbrooks MA, Goldberg WA. Toddler development in the family: Impact of father involvement and parenting characteristics. Child Development. 1984;55(3):740–752. http://dx.doi.org/10.2307/1130126. [PubMed] [Google Scholar]
- Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nature Reviews Neuroscience. 2001;2(10):704–716. doi: 10.1038/35094565. http://dx.doi.org/10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Valiente C, Morris AS, Fabes RA, Cumberland A, Reiser M, Losoya S. Longitudinal relations among parental emotional expressivity, children’s regulation, and quality of socioemotional functioning. Developmental Psychology. 2003;39(1):3–19. doi: 10.1037//0012-1649.39.1.3. http://dx.doi.org/10.1037/0012-1649.39.1.3. [DOI] [PubMed] [Google Scholar]
- Fabes RA. Physiological, emotional, and behavioral correlates of gender segregation. New Directions for Child and Adolescent Development. 1994;1994(65):19–34. doi: 10.1002/cd.23219946504. http://dx.doi.org/10.1002/cd.23219946504. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N, Bernzweig J. Coping with children’s negative emotions scale (CCNES): Description and scoring. Tempe, AZ: Arizona State University; 1990. [Google Scholar]
- Ghassabian A, Herba CM, Roza SJ, Govaert P, Schenk JJ, Jaddoe VW, Tiemeier H. Infant brain structures, executive function, and attention deficit/hyperactivity problems at preschool age. A prospective study. Journal of Child Psychology and Psychiatry. 2013;54(1):96–104. doi: 10.1111/j.1469-7610.2012.02590.x. http://dx.doi.org/10.1111/j.1469-7610.2012.02590.x. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Rothbart MK. The laboratory temperament assessment battery. Locomotor Version. 1999;3 [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. http://dx.doi.org/10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Gray A. The neuropsychology of anxiety: An enquiry into the septo-hippocampal system. Oxford: Oxford University Press; 1982. [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. http://dx.doi.org/10.1146/annurev.psych.58.110405.08560. [DOI] [PubMed] [Google Scholar]
- Guyton AC. Organ physiology: Structure and fucntion of the nervous system. London: W.B. Saunders; 1976. [Google Scholar]
- Harmony T. The functional significance of delta oscillations in cognitive processing. Frontiers in Integrative Neuroscience. 2013;7:83. doi: 10.3389/fnint.2013.00083. http://dx.doi.org/10.3389/fnint.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings PD, Sullivan C, McShane KE, Coplan RJ, Utendale WT, Vyncke JD. Parental socialization, vagal regulation, and preschoolers’ anxious difficulties: Direct mothers and moderated fathers. Child Development. 2008;79(1):45–64. doi: 10.1111/j.1467-8624.2007.01110.x. http://dx.doi.org/10.1111/j.1467-8624.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Marshall PJ, Fox NA, Rubin KH. Psychophysiological and behavioral evidence for varying forms and functions of nonsocial behavior in preschoolers. Child Development. 2004;75(1):251–263. doi: 10.1111/j.1467-8624.2004.00667.x. http://dx.doi.org/10.1111/j.1467-8624.2004.00667.x. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Davis S, Harrington K, Rosenbaum JF. Behavioral inhibition in preschool children at risk is a specific predictor of middle childhood social anxiety: a five-year follow-up. Journal of Developmental & Behavioral Pediatrics. 2007;28(3):225–233. doi: 10.1097/01.DBP.0000268559.34463.d0. http://dx.doi.org/10.1097/01.DBP.0000268559.34463.d0. [DOI] [PubMed] [Google Scholar]
- Izard CE, Dougherty LM, Hembree EA. A system for identifying affect expressions by holistic judgments (AFFEX) Instructional Resources Center, University of Delaware; 1983. [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality. 2004;72(6):1301–1334. doi: 10.1111/j.1467-6494.2004.00298.x. http://dx.doi.org/10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Kazak AE. Fathers’ and mothers’ parenting behavior and beliefs as predictors of children’s social adjustment in the transition to school. Journal of Family Psychology. 2004;18(4):628–638. doi: 10.1037/0893-3200.18.4.628. http://dx.doi.org/10.1037/0893-3200.18.4.628. [DOI] [PubMed] [Google Scholar]
- Kendler K, Baker JH. Genetic influences on measures of the environment: A systematic review. Psychological Medicine. 2007;37(5):615–626. doi: 10.1017/S0033291706009524. http://dx.doi.org/10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Donzella B, Talge NM, Garvin MC, Van Ryzin MJ, Gunnar MR. Inhibited temperament and parent emotional availability differentially predict young children’s cortisol responses to novel social and nonsocial events. Developmental Psychobiology. 2009;51(7):521–532. doi: 10.1002/dev.20390. http://dx.doi.org/10.1002/dev.20390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson JF, DeGarmo D, Koeppl G, Reid JB. Care neglect, supervisory neglect, and harsh parenting in the development of children’s aggression: A replication and extension. Child Maltreatment. 2005;10(2):92–107. doi: 10.1177/1077559504273684. http://dx.doi.org/10.1177/1077559504273684. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience & Biobehavioral Reviews. 2007;31(3):377–395. doi: 10.1016/j.neubiorev.2006.10.004. http://dx.doi.org/10.1016/j.neubiorev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Cross-frequency coupling of brain oscillations: An impact of state anxiety. International Journal of Psychophysiology. 2011;80(3):236–245. doi: 10.1016/j.ijpsycho.2011.03.013. http://dx.doi.org/10.1016/j.ijpsycho.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Schutter DJ, van Honk J. Anxious apprehension increases coupling of delta and beta oscillations. International Journal of Psychophysiology. 2006;61(2):283–287. doi: 10.1016/j.ijpsycho.2005.12.003. http://dx.doi.org/10.1016/j.ijpsycho.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskaya HR. Personality trait of behavioral inhibition is associated with oscillatory systems reciprocal relationships. International Journal of Psychophysiology. 2003;48(3):247–261. doi: 10.1016/s0167-8760(03)00072-2. http://dx.doi.org/10.1016/S0167-8760(03)00072-2. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskaya HR, Safronova MV, Sorokin OV, Goodman R, Wilson GD. Personality, psychopathology and brain oscillations. Personality and Individual Differences. 2003;35(6):1331–1349. http://dx.doi.org/10.1016/S0191-8869(02)00353-7. [Google Scholar]
- Kok R, Lucassen N, Bakermans-Kranenburg MJ, van IJzendoorn MH, Ghassabian A, Roza SJ, Tiemeier H. Parenting, corpus callosum, and executive function in preschool children. Child Neuropsychology. 2014;20(5):583–606. doi: 10.1080/09297049.2013.832741. http://dx.doi.org/10.1080/09297049.2013.832741. [DOI] [PubMed] [Google Scholar]
- Kopp CB. Antecedents of self-regulation: A developmental perspective. Developmental psychology. 1982;18(2):199–214. http://dx.doi.org/10.1037/0012-1649.18.2.199. [Google Scholar]
- Ladouceur CD, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Increased error-related negativity (ERN) in childhood anxiety disorders: ERP and source localization. Journal of Child Psychology and Psychiatry. 2006;47(10):1073–1082. doi: 10.1111/j.1469-7610.2006.01654.x. http://dx.doi.org/10.1111/j.1469-7610.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Lieberman M, Doyle AB, Markiewicz D. Developmental patterns in security of attachment to mother and father in late childhood and early adolescence: Associations with peer relations. Child Development. 1999;70(1):202–213. doi: 10.1111/1467-8624.00015. http://dx.doi.org/10.1111/1467-8624.00015. [DOI] [PubMed] [Google Scholar]
- Loeber R, Stouthamer-Loeber M. Family factors as correlates and predictors of juvenile conduct problems and delinquency. In: Tonry M, Morris N, editors. Crim and Justice. Vol. 7. Chicago: University of Chicago Press; 1986. pp. 29–149. [Google Scholar]
- Lytton H, Romney DM. Parents’ differential socialization of boys and girls: A meta-analysis. Psychological Bulletin. 1991;109(2):267–296. http://dx.doi.org/10.1037/0033-2909.109.2.267. [Google Scholar]
- Marshall PJ, Fox NA. Infant EEG and ERP in relation to social and emotional development. In: DeHaan M, editor. Infant EEG and Event-Related Potentials. New York: Psychology Press; 2006. pp. 227–250. [Google Scholar]
- Matsuura M, Okubo Y, Toru M, Kojima T, He Y, Hou T, Shen Y, Lee CK. A cross-national EEG study of children with emotional and behavioral problems: A WHO collaboratoive study in the Western Pacific region. Biological Psychiatry. 1993;34:59–65. doi: 10.1016/0006-3223(93)90257-e. http://dx.doi.org/10.1016/0006-3223(93)90257-E. [DOI] [PubMed] [Google Scholar]
- McKee L, Roland E, Coffelt N, Olson AL, Forehand R, Massari C, Jones D, Gaffney CA, Zens MS. Harsh discipline and child problem behaviors: The roles of positive parenting and gender. Journal of Family Violence. 2007;22(4):187–196. http://dx.doi.org/10.1007/s10896-007-9070-6. [Google Scholar]
- Meyer A, Proudfit GH, Bufferd SJ, Kujawa AJ, Laptook RS, Torpey DC, Klein DN. Self-reported and observed punitive parenting prospectively predicts increased error-related brain activity in six-year-old children. Journal of Abnormal Child Psychology. 2015;43(5):821–829. doi: 10.1007/s10802-014-9918-1. http://dx.doi.org/10.1007/s10802-015-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR. The role of the family context in the development of emotion regulation. Social Development. 2007;16(2):361–388. doi: 10.1111/j.1467-9507.2007.00389.x. http://dx.doi.org/10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Ashbaugh AR, Santesso DL, McCabe RE, Antony MM, Schmidt LA. Frontal brain oscillations and social anxiety: A cross-frequency spectral analysis during baseline and speech anticipation. Biological Psychology. 2010;83(2):125–132. doi: 10.1016/j.biopsycho.2009.11.010. http://dx.doi.org/10.1016/j.biopsycho.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Campbell MJ, Santesso DL, Van Ameringen M, Mancini CL, Schmidt LA. Frontal brain oscillatory coupling in children of parents with social phobia: A pilot study. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011b;23(1):111–114. doi: 10.1176/jnp.23.1.jnp111. http://dx.doi.org/10.1176/appi.neuropsych.23.1.11. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Moscovitch DA, Santesso DL, McCabe RE, Antony MM, Schmidt LA. Changes in EEG cross-frequency coupling during cognitive behavioral therapy for social anxiety disorder. Psychological Science. 2011a;22(4):507–516. doi: 10.1177/0956797611400914. http://dx.doi.org/10.1177/0956797611400914. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Does quality of child care affect child outcomes at age 4 ½. Developmental Psychology. 2003;39(3):451–469. doi: 10.1037/0012-1649.39.3.451. http://dx.doi.org/10.1037/0012-1649.39.3.451. [DOI] [PubMed] [Google Scholar]
- Phelps RA, Brooker RJ, Buss KA. Toddlers’ dysregulated fear predicts delta–beta coupling during preschool. Developmental Cognitive Neuroscience. 2016;17:28–34. doi: 10.1016/j.dcn.2015.09.007. http://dx.doi.org/10.1016/j.dcn.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84(2):309–322. http://sx.doi.org/10.1037/0033-2909.84.2.309. [PubMed] [Google Scholar]
- Putman P. Resting state EEG delta-beta coherence in relation to anxiety, behavioral inhibition, and selective attentional processing of threatening stimuli. International Journal of Psychophysiology. 2011;80(1):63–68. doi: 10.1016/j.ijpsycho.2011.01.011. http://dx.doi.org/10.1016/j.ijpsycho.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Dalais C, Mellingen K, Reynolds C, Mednick SA. Early educational and health enrichment at age 3–5 years is associated with increased autonomic and central nervous system arousal and orienting at age 11 years: Evidence from the Mauritius Child Health Project. Psychophysiology. 2001;38:254–266. http://dx.doi.org/10.1111/1469-8986.3820254. [PubMed] [Google Scholar]
- Ray WJ, Cole HW. EEG Alpha Activity Reflects Attentional Demands, and Beta Activity Reflects Emotional and Cognitive Processes. Science. 1985;228(4700):750–752. doi: 10.1126/science.3992243. http://dx.doi.org/10.2307/1694556. [DOI] [PubMed] [Google Scholar]
- Rinaldi CM, Howe N. Mothers’ and fathers’ parenting styles and associations with toddlers’ externalizing, internalizing, and adaptive behaviors. Early Childhood Research Quarterly. 2012;27(2):266–273. http://dx.doi.org/10.1016/j.ecresq.2011.08.001. [Google Scholar]
- Robinson DL. The technical, neurological and psychological significance of “alpha”, “delta” and “theta” waves confounded in EEG evoked potentials: a study of peak latencies. Clinical Neurophysiology. 1999;110(8):1427–1434. doi: 10.1016/s1388-2457(99)00078-4. http://doi.org/10.1016/S1388-2457(99)00078-4. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Sheese BE, Rueda MR, Posner MI. Developing mechanisms of self-regulation in early life. Emotion Review. 2011;3(2):207–213. doi: 10.1177/1754073910387943. http://dx.doi.org/10.1177/1754073910387943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbaum F, Weisz JR. Parental caregiving and child externalizing behavior in nonclinical samples: a meta-analysis. Psychological Bulletin. 1994;116(1):55–74. doi: 10.1037/0033-2909.116.1.55. http://dx.doi.org/10.1037/0033-2909.116.1.55. [DOI] [PubMed] [Google Scholar]
- Rubin KH, Cheah CS, Fox N. Emotion regulation, parenting and display of social reticence in preschoolers. Early Education and Development. 2001;12(1):97–115. http://dx.doi.org/10.1207/s15566935eed1201_6. [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(8):1008–1015. doi: 10.1097/00004583-199908000-00017. http://dx.doi.org/10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Shanahan L, Copeland W, Costello EJ, Angold A. Specificity of putative psychosocial risk factors for psychiatric disorders in children and adolescents. Journal of Child Psychology and Psychiatry. 2008;49(1):34–42. doi: 10.1111/j.1469-7610.2007.01822.x. http://dx.doi.org/10.1111/j.1469-7610.2007.01822.x. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Ruttle P. Attachment and Early Disorders of Development. Munich: Klett-Cotta; 2010. Immunological and neuroendocrine dysregulation following early deprivation and stress. [Google Scholar]
- South SC, Jarnecke AM. Genetic and environmental influences on adult mental health: Evidence for gene-environment interplay as a function of maternal and paternal discipline and affection. Behavior Genetics. 2015;45(4):438–450. doi: 10.1007/s10519-015-9716-8. http://dx.doi.org/10.1007/s10519-015-9716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH, Rapee R, McDonald C, Ingram M. The structure of anxiety symptoms among preschoolers. Behaviour Research and Therapy. 2001;39(11):1293–1316. doi: 10.1016/s0005-7967(00)00098-x. http://dx.doi.org/10.1016/S0005-7967(00)00098-X. [DOI] [PubMed] [Google Scholar]
- Stern RM, Ray WJ, Quigley KS. Psychophysiological Recording. New York, New York: Oxford University Press; 2001. [Google Scholar]
- Sutton SK, Burnette CP, Mundy PC, Meyer J, Vaughan A, Sanders C, Yale M. Resting cortical brain activity and social behavior in higher functioning children with autism. Journal of Child Psychology and Psychiatry. 2005;46(2):211–222. doi: 10.1111/j.1469-7610.2004.00341.x. http://dx.doi.org/10.1111/j.1469-7610.2004.00341.x. [DOI] [PubMed] [Google Scholar]
- Tamis-LeMonda CS, Shannon JD, Cabrera NJ, Lamb ME. Fathers and mothers at play with their 2-and 3-year-olds: contributions to language and cognitive development. Child Development. 2004;75(6):1806–1820. doi: 10.1111/j.1467-8624.2004.00818.x. http://dx.doi.org/10.1111/j.1467-8624.2004.00818.x. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. http://dx.doi.org/10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Towe-Goodman NR, Willoughby M, Blair C, Gustafsson HC, Mills-Koonce WR, Cox MJ. Fathers’ sensitive parenting and the development of early executive functioning. Journal of Family Psychology. 2014;28(6):867. doi: 10.1037/a0038128. http://dx.doi.org/10.1037/a00381285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto S. The prefrontal cortex: Functional neural development during early childhood. The Neuroscientist. 2008;14(4):345–358. doi: 10.1177/1073858408316002. http://dx.doi.org/10.1177/1073858408316002. [DOI] [PubMed] [Google Scholar]
- Zentner M, Bates JE. Child temperament: An integrative review of concepts, research programs, and measures. European Journal of Developmental Science. 2008;2(1/2):7–37. http://dx.doi.org/10.3233/DEV-2008-21203. [Google Scholar]