Abstract
Reinforcement occurs when hybridization between closely related lineages produces low‐fitness offspring, prompting selection for elevated reproductive isolation specifically in areas of sympatry. Both premating and postmating prezygotic behaviors have been shown to be the target of reinforcing selection, but it remains unclear whether remating behaviors experience reinforcement, although they can also influence offspring identity and limit formation of hybrids. Here, we evaluated evidence for reinforcing selection on remating behaviors in Drosophila pseudoobscura, by comparing remating traits in females from populations historically allopatric and sympatric with Drosophila persimilis. We found that the propensity to remate was not higher in sympatric females, compared to allopatric females, regardless of whether the first mated male was heterospecific or conspecific. Moreover, remating behavior did not contribute to interspecific reproductive isolation among any population; that is, females showed no higher propensity to remate following a heterospecific first mating than following a conspecific first mating. Instead, we found that females are less likely to remate after initial matings with unfamiliar males, regardless of species identity. This is consistent with one scenario of postmating sexual conflict in which females are poorly defended against postcopulatory manipulation by males with whom they have not coevolved. Our results are generally inconsistent with reinforcement on remating traits and suggest that this behavior might be more strongly shaped by the consequences of local antagonistic male–female interactions than interactions with heterospecifics.
Keywords: allopatry, coevolution, reproductive isolation, sexual conflict, speciation, sympatry
1. Introduction
Because hybridization between incompletely isolated species can be costly—in terms of reduced fecundity or offspring survival or fertility—selection is expected to favor traits that reduce the frequency or consequences of these matings in nature (Dobzhansky, 1940). This “reinforcement” of incomplete reproductive isolation is thought to play a key role in speciation, especially where there is secondary contact between close relatives (Ortiz‐Barrientos, Grealy, & Nosil, 2009). Reinforcement has frequently been examined in the context of selection on premating traits, such as courtship displays or behaviors, which can act to prevent heterospecific matings (e.g., Rundle & Schluter, 1998; Saetre et al., 1997). However, postmating traits could also be subject to reinforcing selection (Servedio & Noor, 2003) as could traits that integrate pre‐ and postmating responses, such as postmating control of paternity via variable remating rate (Kisdi, 2003; Marshall, Arnold, & Howard, 2002). Control over mate and paternity choice has been shown to evolve rapidly in response to antagonistic coevolution between the sexes (e.g., Manier, Belote et al., 2013; Miller & Pitnick, 2002; Rice, 1996). Such rapidly evolving reproductive traits can potentially drive divergence between populations and might contribute strongly to reproductive isolation (Gavrilets, 2000; Howard, 1999; Howard, Palumbi, Birge, & Manier, 2009; Manier, Lupold et al., 2013; Martin & Hosken, 2003; Panhuis, Butlin, Zuk, & Tregenza, 2001; Parker & Partridge, 1998; Rice, 1998; Ritchie, 2007). Therefore, both mating and remating behaviors are potentially interesting candidates for examining the evolution of isolating mechanisms between species, including in the context of reinforcement.
One key expectation under reinforcement is that populations that are historically sympatric with closely related heterospecifics will show stronger isolation than populations that are historically allopatric (Butlin, 1987; Servedio & Noor, 2003). This is because only sympatric populations will have experienced selection to avoid producing lower fitness hybrid offspring. Mate choice during the first mating has been observed to show patterns consistent with reinforcement (e.g., Higgie, Chenoweth, & Blows, 2000; Noor, 1995; Rundle & Schluter, 1998; Saetre et al., 1997), whereby sympatric females are more discriminating against heterospecifics than are allopatric females. In comparison with initial mate choice, whether remating rates respond to reinforcement is largely unknown (Marshall et al., 2002; but see Matute, 2010; and Section “4”). Decreasing the time to remating (latency) or increasing the propensity to remate allows females to manipulate paternity, including after mating with a suboptimal male (variously called a “rescue effect” (Fricke, Arnqvist, & Amaro, 2006) or the “trading up” hypothesis (Byrne & Rice, 2005)). Because mating with heterospecifics is generally suboptimal, remating rate could respond to reinforcing selection such that sympatric females increase their propensity to remate with conspecifics following a heterospecific mating (Marshall et al., 2002). It is also possible that exposure to heterospecifics could generally increase remating rates of females in such populations, regardless of first male identity. In comparison, females from populations that are geographically allopatric are not expected to elevate remating responses.
Nonetheless, making predictions about remating rate is complex because remating behaviors are the product of both female choice and male manipulation. For example, in Drosophila, females are known to exhibit cryptic female choice by controlling number of mates and/or by preferentially using sperm from some male partners (Manier et al., 2010; Lupold et al., 2013; Manier, Lupold, Pitnick, & Starmer, 2013). In turn, male Drosophila seminal fluid proteins transferred during copulation are known to suppress female remating rate, increase oviposition rate, and reduce lifespan, potentially resulting in net fitness reductions for females (Parker & Partridge, 1998; Sirot et al., 2009; and references therein). The resulting antagonistic male–female coevolution acting on these traits can lead females to be poorly defended against males with whom they have not coevolved (Parker & Partridge, 1998; Rice, 1998). Under this scenario, for example, allopatric females that are less equipped to defend against heterospecific encounters might exhibit reduced remating rates, even when remating would be individually beneficial. It can, however, be difficult to make general predictions about the direction of female responses to unfamiliar mates, because this is expected to depend on which sex is “ahead” in the coevolutionary arms race, which can vary depending upon the precise details of these male–female interactions (Long, Montgomerie, & Chippindale, 2006; reviewed Tregenza, Wedell, & Chapman, 2006, and see Section “4”).
We sought to examine whether remating rates might respond to reinforcing selection in a Drosophila species pair that is a canonical example of reinforcement of premating isolation. Drosophila persimilis and Drosophila pseudoobscura are recently diverged (500 kya) sister species with distinct but significantly overlapping ranges (Schaeffer & Miller, 1991; Wang, Wakeley, & Hey, 1997; Machado, Kliman, Markert, & Hey, 2002). D. pseudoobscura has a wide geographic range in North America, stretching west from the Pacific to close to the Mississippi River and far south into Central America; D. persimilis has a far narrower range completely sympatric with D. pseudoobscura and not extending farther east than the Sierra Nevada and Cascade Mountain ranges (Figure 1). These species exhibit incomplete reproductive isolation and hybridize in the laboratory; natural hybrids, while rare, have been found in the wild (Dobzhansky, 1973; Kulathinal, Stevison, & Noor, 2009). In addition, mate choice patterns consistent with reinforcement have been directly demonstrated in this species pair, whereby allopatric D. pseudoobscura females mate at a higher rate with D. persimilis males than do D. pseudoobscura females from sympatric populations (Noor, 1995; Noor & Ortiz‐Barrientos, 2006), although see Anderson and Kim (2005, 2006) for more complex patterns of isolation between sympatric and allopatric populations. Additionally, a recent study evaluating other components of reproductive isolation in this species pair (Castillo & Moyle, 2016) found no difference in first mating rates between allopatric and sympatric D. pseudoobscura paired with D. persimilis males. The well‐established ranges and prior focus on evaluating reinforcement in this species pair make it particularly suited for examining whether remating rate might also respond to reinforcing selection.
Figure 1.
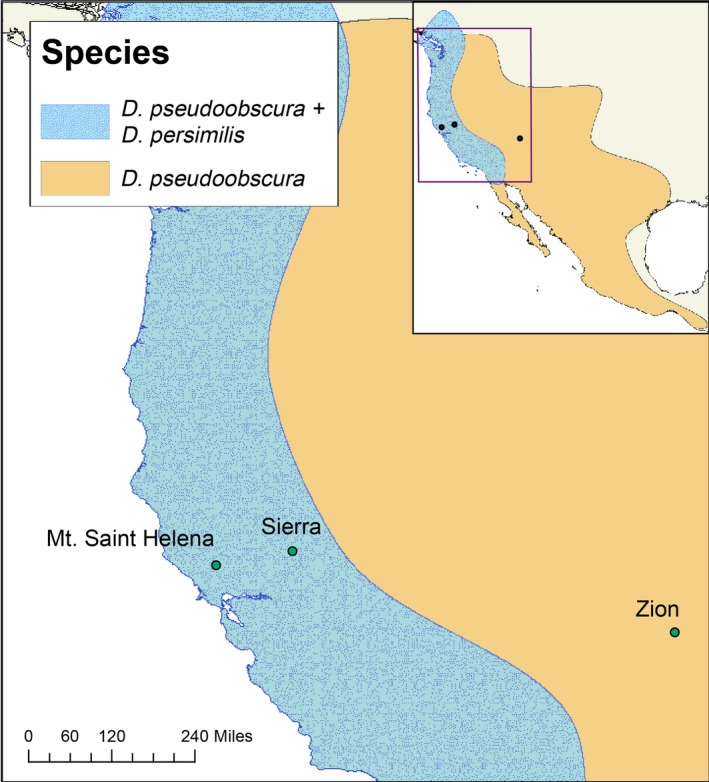
Collection locations for Drosophila pseudoobscura and Drosophila persimilis study populations. Mt. Saint Helena and Sierra are sympatric locations (both species); Zion is an allopatric site (D. pseudoobscura only). Inset: North American range maps for the two species; the range of D. persimilis is entirely contained within the broader D. pseudoobscura range
Our primary goal in this study was to evaluate evidence for reinforcing selection on remating behaviors of D. pseudoobscura females, using populations historically allopatric and sympatric with D. persimilis. To do so, we evaluated mating traits in females from three target D. pseudoobscura populations: two populations sympatric with D. persimilis, and one allopatric population (Figure 1). These populations were a subset of those examined in a larger parallel study of patterns of first mating and conspecific sperm precedence between these species (Castillo & Moyle, 2016). Following a first mating with either a heterospecific or a conspecific male, females were given the opportunity to remate with a male from their own population. We assessed whether female remating response depends on identity of the first mated male and, specifically, whether the propensity to remate depends upon female population identity (allopatric/sympatric). If remating behaviors have evolved in response to the presence of heterospecifics, we expected that D. pseudoobscura females from sympatric sites would more readily remate if their first mating was with a heterospecific male, consistent with an evolved response to limit the number of hybrid offspring sired from this first mating. An alternative expectation is that females from sympatric populations remate at a higher rate irrespective of first male identity, as a simpler response to potentially suboptimal first matings.
2. Methods
2.1. D. pseudoobscura and D. persimilis collection and maintenance
All stocks were reared on standard media prepared by the Bloomington Drosophila Stock Center and were kept at room temperature (~22°C). We used a subset of isofemale lines from a larger panel that were collected in the summers of 2013 and 2014 at three sites (Figure 1). Allopatric D. pseudoobscura were collected at Zion National Park, UT (kindly provided by N. Phadnis). Sympatric D. pseudoobscura and D. persimilis were collected at two sites: Mt. St. Helena, CA (D. pseudoobscura collected by A. Hish/M. Noor, and D. persimilis collected by D. Castillo), and, near Meadow Vista and Forest Hill, CA (called here “Sierra”; Figure 1) (D. pseudoobscura and D. persimilis collected by D. Castillo). For both sympatric populations, both species were present in field collections and can be considered truly co‐occurring/sympatric. Our three focal populations are a subset of four populations used in a parallel study that evaluated evidence for reinforcement on first matings, and on conspecific sperm precedence (Castillo & Moyle, 2016). (The current study excludes analysis of an additional allopatric population from Lamoille Canyon, NV). All but one of the six isofemale lines from our three populations are shared in common with the other study (MSH3 is not used in Castillo & Moyle, 2016), enabling us to compare remating data from both experiments here (see below), as well as reassess the prior first mating result with data obtained from our first mating observations.
2.2. Mating and remating assay
To examine remating behaviors in females from our three target D. pseudoobscura populations, we used a design in which each female was initially paired with one of five different types of male (males from each of the three D. pseudoobscura populations and two D. persimilis populations). Five‐day‐old virgin females were transferred individually without anesthesia to vials with individual 5‐day‐old virgin males and allowed to mate for 24 hr before the male was removed. Females were then allowed to lay for 9 days, a refractory period that pilot trials indicated gives ample time for females to become receptive to males again. Those that produced larvae (and therefore were guaranteed to have mated with the first male) were then given the opportunity to remate with a second, 5‐day‐old virgin male. The second male was always from the same population as the target female, to ensure females would mate most readily during the second mating. This procedure was performed for each combination of our three female populations and five first‐male types (15 total cross‐combinations). Two complete experimental blocks were performed for each cross‐combination, using two unique isofemale lines from each population. Within each experimental block, a minimum of eight biological replicates were carried out for each combination of first and second male matings.
For each first male pairing, mating behavior was directly observed for 3 hr, and copulation latency (time to start of copulation) and duration (time from start to end of copulation) were recorded. Following the 3‐hr observation period, pairs were maintained together for an additional 21 hr, and vials were checked 7 days later for larvae to determine whether mating occurred within first 24 hr but outside the initial 3‐hr observation window. This allowed us to assess whether female population origin influences mating behavior in the first male mating, and whether this varied according to male population identity. For each second male pairing, female mating behavior was assessed in terms of copulation latency and mating duration within the first 3 hr of pairing. This allowed us to evaluate whether females vary their remating behavior in response to the population and/or species identity of their first mate, in addition to whether these responses differed in females from allopatric versus sympatric sites. Finally, differences among isofemale lines in overall propensity to remate following conspecific first matings were used to confirm that there was heritable genetic variation for this trait within D. pseudoobscura (See Section “3”). Detailed mating procedures are provided in Supporting Information.
After completing at least eight replicates, we found that copulation duration during the first mating was indistinguishable among all crosses, and copulation latency was either similarly rapid (<10 min) in all conspecific pairings, or inconsistently and rarely observed within the first 3 hr in heterospecific pairings (See Section “3”). Based on these findings, for the remaining 14 replicates (which primarily focused on heterospecific second pairings), first matings were no longer directly observed for the first 3‐hr period, but were instead simply scored for presence/absence of larvae 7 days after co‐housing each male–female pair for 24 hr. Regardless of this change for first matings, remating behavior was always assessed as observed copulation, and copulation latency and duration, within the first 3 hr of co‐housing.
To assess whether detected remating differences could be explained by differences in sperm usage and depletion between different cross‐types, we tracked progeny production of two isofemale lines, one allopatric and one sympatric D. pseudoobscura strain, across 7 days. As with all first matings, individual virgin females were mated overnight with either a male from their own population, a D. pseudoobscura male from a different population, or a D. persimilis male. Males were removed after 24 hr. We found no significant differences in number of progeny produced from own population males, conspecific males from a different population, or heterospecific males, for either allopatric or sympatric isofemale lines, consistent with a previous study that found no evidence for noncompetitive gamete isolation contributing to reproductive isolation in this species pair (Lorch & Servedio, 2005). Allopatric females produced an average of 86 progeny, which did not differ based on whether they mated with males from their own population versus different population conspecifics (β = 9.400; p = .603) or versus heterospecifics (β = 7.487; p = .609). Similarly, there was no difference in the number of progeny produced for sympatric females (77 progeny) when mated with males from their own population versus different population conspecifics (β = 2.667; p = .913) or versus heterospecifics (β = 7.667; p = .702).
Although Castillo and Moyle (2016) did not observe remating directly, data from that experiment can be used to glean some additional information about remating rates in sympatric versus allopatric females. Similar to the design here, in that study, virgin D. pseudoobscura females were housed with D. persimilis males for 24 hr and then, following a period of 7 days, were given the opportunity to remate with D. pseuodoobscura males. Progeny after this second mating were scored (D. persimilis male was marked with a visible marker, and hybrid males are sterile), providing information on whether females remated or not. Females are inferred to have failed to remate if all progeny after second mating were hybrid; that is, if all males were sterile and all females carried the visible mutation. These data were used as an additional test of whether allopatric and sympatric females differed in their propensity to remate (see Section “3”).
2.3. Statistics
A chi‐square test of independence was used to compare overall D. pseudoobscura female mating rates in first pairings with conspecific versus heterospecific males. To make more specific comparisons among groups, we used logistic regression on presence/absence of larvae after mating (mating was considered a binary variable). Logistic regressions were used to assess differences in the mating probabilities of all females during their first matings, and during remating trials, depending upon whether they were initially paired with first males of three classes: males from their own population, males from a different conspecific population, or heterospecific males. Probabilities of mating and of remating were also specifically compared between D. pseudoobscura females historically allopatric and sympatric with D. persimilis. For all logistic regressions, differences between mating types were inferred by examining significance of the regression coefficients. Negative coefficients signified categories where matings were less likely to occur, and positive coefficients signified that mating was more likely to occur.
To analyze quantitative copulation latency, we primarily used Cox proportional hazard models in the survival package (Therneau, 2013) in R, which let us take into account the mating and remating rates as well as probability of mating within our 3‐hr observation. For one comparison (allopatric vs. sympatric remating latency), we used parametric survival regression (see Supporting Information for details). We included female genotype in the proportional hazard models to account for correlated observations within a given isofemale line (see Supporting information for details). Survival curves for a specific mating category were considered different when the coefficient from the model was significantly different than the zero. Negative coefficients signified categories where matings occurred more slowly than baseline, and positive coefficients signified that mating occurred more quickly than baseline. Baseline was always mating involving males from the females own population. Finally, a chi‐square test of independence was used to compare overall D. pseudoobscura remating rates between allopatric and sympatric females, using the remating data integrated from Castillo and Moyle (2016).
3. Results
3.1. Initial mate choice contributes to reproductive isolation between species but is not stronger in sympatry
We confirmed that D. pseudoobsura females discriminate against D. persimilis males; while almost all conspecific matings were successful (164/168), only 25% of heterospecific pairings resulted in mating (93/369), a significant difference in mating propensity (χ2 = 239.70; p < 2.2 × 10−16). Logistic regressions similarly indicated that the proportion of heterospecific matings was significantly lower than the proportion of first matings either with males from a different conspecific population (β = 3.5927; p = 8.31 × 10−10) or with males from the females own population (β = 4.0073; p = 7.13 × 10−05) (Figure 2). There was no difference in the propensity to mate of females paired with males from their own population versus males from different conspecific populations (β = 0.4146; p = .722) (Figure 2), indicating that female choice in conspecific first matings was not sensitive to the population origin of the conspecific male.
Figure 2.
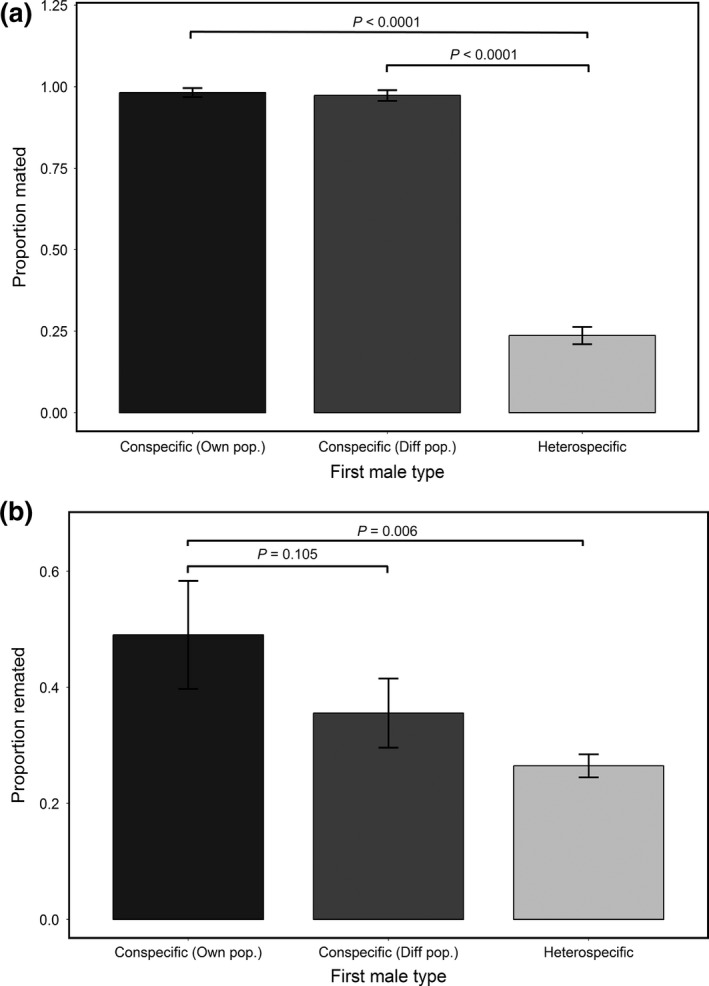
Mating (panel a) and remating (panel b) probabilities of D. pseudoobscura females following first matings with males from their own population (Own pop), a different conspecific population (Diff pop), and heterospecific males. p‐Values are from logistic regressions (see Section “3”)
To test for patterns consistent with reinforcement on first mating, we fit a logistic regression to first mating success according to whether female D. pseudoobscura were from a population that was allopatric or sympatric with D. persimilis. We found no significant difference between allopatric and sympatric females in probability of mating with a heterospecific first male (β = −0.1079; p = .623), consistent with prior observations of mating patterns that used more isofemale lines and one additional allopatric population comparison (Castillo & Moyle, 2016). We did not analyze differences in copulation latency between allopatric versus sympatric females in heterospecific first matings because too few of these mating events occurred within the directly observed first 3 hr of cohousing. Only 16 of 133 directly observed heterospecific pairings resulted in copulations within the first 3 hr, corresponding to 14% and 15% of sympatric and allopatric female pairings. An additional 20 of the 133 directly observed pairings resulted in progeny, but these matings occurred within the subsequent (unobserved) 21‐hr period of co‐housing.
3.2. Sympatric females are slower to mate with conspecific males in first matings
If exposure to heterospecifics has resulted in general changes in intrinsic mating behavior, rather than specific responses directed at heterospecific genotypes, allopatric and sympatric females should differ in mating responses to conspecific males. Allopatric and sympatric females did not differ in probability of mating when paired with conspecific males (regardless of their population of origin) (β = −17.22; p = .994). However, females from sympatric populations took significantly longer to initiate copulation with conspecific males than did allopatric females, despite persistent courtship by males (β = −0.2693; p = .0096). When we simultaneously tested for an effect of sympatry and for the population of origin of the conspecific male, mating latency did not differ according to the specific population of the conspecific male (β = 0.0584; p = .6717) but the difference between sympatry and allopatry remained (β = −0.2720; p = .0076). In other words, sympatric females are slower to initiate copulation, regardless of the population identity of the first conspecific male (i.e., own vs. other conspecific population) with which they are paired. It is possible that this is a subtle behavioral response to past selection imposed by heterospecifics: If female D. pseudoobscura in sympatry have adapted to encountering heterospecific males, they might be more circumspect in their initial mating decisions in general. This longer latency might contribute to fewer accidental heterospecific matings, especially under less restrictive conditions than those imposed by our laboratory co‐housing experiment.
3.3. Remating varies depending on the identity of the first mating male, but does not contribute to interspecific reproductive isolation or to enhanced isolation in sympatry
To evaluate whether D. pseudoobscura females differed in their readiness to remate depending on the identity of the first male they mated with, we compared the frequency of remating and the copulation latency in remating trials following three classes of first mating: with conspecific males from their own population, with conspecific males from a different population, or with heterospecific males. We found that analyses of both mating probability and latency to copulation indicate that remating happens more readily when females first mate with familiar (own population) males, than when initially mated with unfamiliar conspecifics or with heterospecifics. In terms of remating probability, females initially mated to their own population males were significantly more likely to remate compared with females initially mated to a D. persimilis male (β = −0.98291; p = .00555), although the probability of remating did not differ significantly between females previously mated with conspecific males from their own population versus from a different conspecific population (β = −0.55603; p = .10471). In terms of latency to copulation, females first mated with their own male remated more quickly (had shorter latency) than females initially mated with either conspecifics from different populations (β = −0.5213; p = .02195) or heterospecifics (β = −0.8035; p = .000526) (Figure 3). (Although trending in this direction, copulation latency was not significantly shorter in females initially mated with conspecifics from a different population versus with heterospecific males [i.e., the confidence intervals on β coefficients overlap]). These observations also indicate there is no generalized female D. pseudoobscura response to increase remating following heterospecific first matings.
Figure 3.
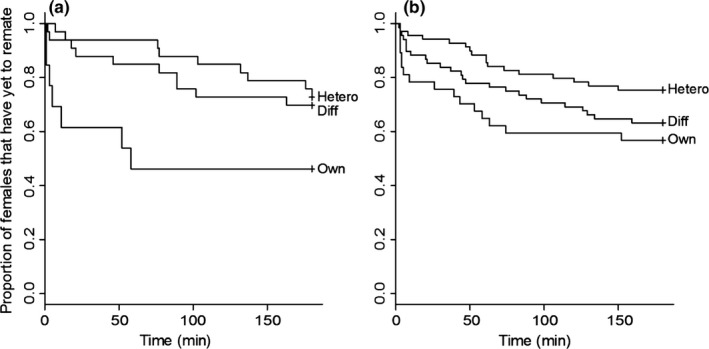
Survival curves showing remating latencies of allopatric (a) and sympatric female (b) D. pseudoobscura when mated to different classes of first males: Own (conspecific male from the same population as the female); Diff (conspecific male from a different population); Hetero (heterospecific male). Figure S1 shows survival curves of remating latency separately for each isofemale line
To test for patterns consistent with reinforcement on remating, we assessed whether allopatric versus sympatric females differ in their remating behaviors following first matings with D. persimilis males. We found that they did not differ in their probability of remating (β = −0.2851; p = .5447), or in how rapidly they remated (parametric survival regression; β = 0.252; p = .5280), following a heterospecific first mating. Finally, using a second set of mating data from Castillo and Moyle (2016), we examined the number of females that failed to remate compared to the total number of remating trials scored, and found there was no significant difference in remating rate between females from allopatric versus sympatric populations (χ2 = 0.1445; df = 1, p = .7029). Note that we detected significant differences among D. pseudoobscura isofemale lines in their overall propensity to remate following a conspecific first mating (Wald's χ2; df = 5; p = .0352), indicating there is genetic variance for remating behavior available to selection in this species.
3.4. Allopatric and sympatric females do not differ in remating behavior with conspecifics
To investigate whether allopatric versus sympatric females differ in their intrinsic propensity to remate, we compared remating probability and latency between allopatric and sympatric females that had first mated with conspecifics; we found that they did not differ in their probability of remating (β = 0.1586, p = .6569) or in their latency to copulate in remating trials (β = 0.1616, p = .5840). When we simultaneously tested for an effect of sympatry and for the population of origin of the first mated conspecific male, allopatric and sympatric females still did not differ in remating latency (β = 0.0961, p = .7437); however, we did detect a first male population effect, such that remating occurred more rapidly when females had mated first with a conspecific from their own population (β = −0.4975; p = .0239). This is consistent with our findings that females overall mate quickest following own‐male first matings. Sympatric and allopatric females did not differ in remating latency following own‐male matings (β = −0.3492, p = .1570).
4. Discussion
In this study, our primary goal was to evaluate whether sympatric D. pseudoobscura females remate more quickly or at a higher rate when previously mated to a heterospecific D. persimilis, as expected if remating behavior has responded to reinforcing selection in sympatry. We found no evidence for reinforcement effects on remating, in either probability of remating or in latency to copulation, when females had previously mated to heterospecifics. Sympatric females were also no more likely or faster to remate after conspecific first matings. Therefore, our results indicate little evidence that remating behavior in our sympatric populations has responded specifically to reinforcing selection. In addition, our results also imply that our sympatric D. pseudoobscura females do not show a generalized change in remating behavior (either an increased general propensity to remate or to remate more quickly) in order to minimize the consequences of suboptimal (especially heterospecific) matings. Our results differ from the only other study (of which we are aware) to compare remating rates between females allopatric and sympatric with a closely related conspecific species. In it, Matute (2010) found that D. yakuba females sympatric with D. santomea exhibit greater remating rates after heterospecific matings, compared to D. yakuba females that are allopatric, a pattern that is consistent with the expectations of reinforcement on remating, but that could also be explained by less direct effects.
Given that there is genetic variation for D. pseudoobscura female remating behavior (see Section “3”), one potential explanation for our findings is that selection on remating behavior is insufficiently strong or consistent to elicit a substantial evolutionary response. That is, if females are only infrequently exposed to the consequences of completed heterospecific matings, then selection on traits that mitigate these consequences could be relatively weak. In our study, only ~14% of D. pseudoobscura females mated with D. persimilis males within 3 hr of enforced co‐housing, and D.pseudoobscura females do not produce fewer progeny when mating with heterospecific males (see Section “2”, and Lorch & Servedio, 2005). In comparison, in Matute's (2010) study that detected enhanced remating in sympatric D. yakuba females, ~30% of D. yakuba females mated with a D. santomea male within a 1‐hr observation period (Matute, 2010; Table S4) and females produce fewer progeny in heterospecific crosses, potentially contributing to the different outcomes of that study and our data here. This relatively high first mating rate between D. yakuba females and D. santomea males should impose stronger selection on sympatric D. yakuba to evolve remating habits that reduce the negative effects of heterospecific matings. Alternatively, because female receptivity is also known to be influenced by the number of sperm in storage (the “sperm effect”; Manning, 1962, 1967), D. yakuba sympatric females might remate more rapidly because they experience more acute sperm depletion following heterospecific matings (as inferred in Matute, 2010), rather than the because of past reinforcing selection for higher remating in response to suboptimal (interspecies) matings. It is difficult to disentangle these two hypotheses without information on remating rates with conspecific males (remating in D. yakuba was examined only after heterospecific matings).
Alternatively, other forces might be more critical in shaping D. pseudoobscura remating behavior than exposure to heterospecifics. In particular, remating behaviors are determined by complex interactions between males and females, some of which might act in ways counter to reinforcing selection imposed by exposure to heterospecifics. There is substantial evidence for sexually antagonistic coevolution acting on remating traits (Arnqvist & Rowe, 2005; Crudgington, Beckerman, Brustle, Green, & Snook, 2005; Parker & Partridge, 1998); in these cases, individuals are expected to be well equipped to respond to antagonistic measures employed by others from their own population, but potentially poorly defended against individuals with whom they have not coevolved.
Intriguingly, our observations of remating behavior are consistent with these outcomes of local coevolution due to sexual antagonism. We found that females mated previously to male conspecifics of their own population remated significantly more quickly and/or more frequently than females previously mated with conspecific males from a different population or with heterospecifics; remating was least frequent after mating with heterospecific males. These observations suggest that increased sexual familiarity results in females better able to combat male postcopulatory manipulation (“molecular coercion”; Parker & Partridge, 1998) via the seminal fluid in ejaculate. A similar pattern has been previously observed in Bean Weevils, in which matings involving increasingly more distantly related first males resulted in increasingly reduced rates of female remating; first matings with heterospecific males elicited the greatest postcopulatory egg production and the lowest remating rate (Fricke et al., 2006). In both cases, females appear to be more able to resist suppression of remating by local males in comparison with unfamiliar males.
Nonetheless, it should be noted that while our data are consistent with one scenario of sexual conflict, patterns of remating that result from sexual conflict dynamics can be complex, and are not necessarily consistently predictable from population or species crosses (Tregenza et al., 2006). In particular, previous work indicates that the outcome of interactions with less familiar males is dependent on which sex is “ahead” in any particular instance (Long et al., 2006; Tregenza et al., 2006). Our findings are consistent with females being “behind” relative to foreign males, but “ahead” of local males; however, alternative patterns can be detected in these kinds of comparisons. For example, Long et al. (2006) performed crosses between six sister laboratory populations that had been isolated for 600+ generations and found within‐population variation among females in whether they performed better or worse following mating with foreign males compared to local males; they concluded this was due to segregating variation for whether the female was “ahead” or “behind” the specific male genotype to which she was mated. These and other studies indicate that potentially more complex patterns can equally be consistent with conflict scenarios, compared to the one we infer from our observations. Other interpretations of our finding are also possible. For example, the patterns we observed could be due to cryptic female choice for foreign or rare male sperm. This would be especially curious in heterospecific matings, as hybrid inviability makes it strongly disadvantageous for females to preferentially choose sperm from heterospecific males, and for this reason we favor the sexual conflict interpretation. Regardless, our data are clearly inconsistent with reinforcement shaping responses in this trait.
In addition to examining remating traits, our experimental design allowed us to reassess evidence of reinforcement in first matings involving these populations. As with a parallel larger study with many of the same isofemale lines (Castillo & Moyle, 2016), we found no evidence for reinforcement in first mating between our populations. Discrimination against heterospecific males was not stronger in historically sympatric females, the most straightforward expectation of a response to reinforcing selection. This is curious, as previous studies have detected significantly stronger sexual isolation in sympatric D. pseudoobscura females (Noor, 1995; Noor & Ortiz‐Barrientos, 2006). At least two factors could potentially contribute to our observed differences. First, sympatric populations might be polymorphic for high discrimination alleles (as suggested in Barnwell & Noor, 2008), and we happened to use lines that discriminate differently compared to previous studies. Second, Anderson and Kim (2005, 2006) have argued that gene flow among D. pseudoobscura populations has contributed to homogenizing mating discrimination traits between allopatric and sympatric sites. Interestingly, the range of mean heterospecific mating rates for sympatric isofemale lines is broad in both our analysis and in Noor's (1995) study (range = 0.22–0.52, 0.16–0.37, respectively). In addition, the sympatric lines in our study have somewhat higher heterospecific mating rates (mean = 0.346) compared with Noor's (1995) sympatric lines (mean = 0.252), whereas our allopatric lines mated with heterospecifics at considerably lower rates than Noor's (mean = 0.319 vs. 0.45 in Noor, 1995). These differences suggest indirect evidence that genetic polymorphism within sympatric populations and gene flow/homogenization between D. pseudoobscura populations might both contribute to differences between our findings and those in Noor (1995).
Regardless of these observations for first matings, our primary analysis of remating suggests that factors such as local sexual coevolution could act counter to reinforcing selection. Even when advantageous for females to manipulate the genetic identity of offspring via remating—such as following matings with heterospecific males—our results suggest that behavioral manipulation of females by male seminal proteins could supersede this response. It has been broadly recognized that sexually antagonistic coevolution and reproductive character displacement can interfere with each other, producing suboptimal outcomes for one or both processes. Interestingly, this potential tension between intraspecific and interspecific sexual interactions is more often described in terms of reproductive character displacement hampering optimal outcomes of intraspecific sexual selection, rather than the reverse (Ortiz‐Barrientos et al., 2009; Pfennig & Pfennig, 2012). Here, we infer that intraspecific sexual dynamics might instead overwhelm the action of reinforcing selection, producing complex outcomes for remating behaviors within and between species.
Conflict of Interest
None declared.
Supporting information
Acknowledgments
We would like to thank M. Noor, A. Hish, and N. Phadnis for providing strains used in this experiment, and Donn Castillo for help with collecting strains. Collections were completed with assistance from IU Biology Department travel awards to DMC. Research was supported by Indiana University Department of Biology funding to LCM, DMC, and JSD.
Davis, J. S. , Castillo, D. M. and Moyle, L. C. (2017), Remating responses are consistent with male postcopulatory manipulation but not reinforcement in D. pseudoobscura . Ecology and Evolution, 7: 507–515. doi: 10.1002/ece3.2628
References
- Anderson, W. W. , & Kim, Y. K. (2005). Sexual isolation between sympatric and allopatric populations of Drosophila pseudoobscura and D. persimilis . Behavior Genetics, 35, 305–312. [DOI] [PubMed] [Google Scholar]
- Anderson, W. W. , & Kim, Y. K. (2006). A further analysis of sexual isolation between sympatric and allopatric populations of Drosophila pseudoobscura and D. persimilis—Rejoinder to Noor and Ortiz‐Barrientos. Behavior Genetics, 36, 328–330. [DOI] [PubMed] [Google Scholar]
- Arnqvist G., & Rowe L. (Eds.) (2005). Sexual conflict. Princeton, NJ, USA: Princeton University Press. [Google Scholar]
- Barnwell, C. V. , & Noor, M. A. F. (2008). Failure to replicate two mate preference QTLs across multiple strains of Drosophila pseudoobscura . Journal of Heredity, 99, 653–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin, R. (1987). Speciation by reinforcement. Trends in Ecology & Evolution, 2, 8–13. [DOI] [PubMed] [Google Scholar]
- Byrne, P. G. , & Rice, W. R. (2005). Remating in Drosophila melanogaster: An examination of the trading‐up and intrinsic male‐quality hypotheses. Journal of Evolutionary Biology, 18, 1324–1331. [DOI] [PubMed] [Google Scholar]
- Castillo, D. , & Moyle, L. C. (2016). Reinforcement of conspecific sperm precedence weakens sexual selection in sympatric populations of Drosophila . bioRxiv, 071886; doi:10.1101/071886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crudgington, H. S. , Beckerman, A. P. , Brustle, L. , Green, K. , & Snook, R. R. (2005). Experimental removal and elevation of sexual selection: Does sexual selection generate manipulative males and resistant females? The American Naturalist, 165, S72–S87. [DOI] [PubMed] [Google Scholar]
- Dobzhansky, T. (1940). Speciation as a stage in evolutionary divergence. The American Naturalist, 74, 312–321. [Google Scholar]
- Dobzhansky, T. (1973). Is there gene exchange between Drosophila pseudoobscura and Drosophila persimilis in their natural habitats. The American Naturalist, 107, 312–314. [Google Scholar]
- Fricke, C. , Arnqvist, G. , & Amaro, N. (2006). Female modulation of reproductive rate and its role in postmating prezygotic isolation in Callosobruchus maculatus . Functional Ecology, 20, 360–368. [Google Scholar]
- Gavrilets, S. (2000). Rapid evolution of reproductive barriers driven by sexual conflict. Nature, 403, 886–889. [DOI] [PubMed] [Google Scholar]
- Higgie, M. , Chenoweth, S. , & Blows, M. W. (2000). Natural selection and the reinforcement of mate recognition. Science, 290, 519–521. [DOI] [PubMed] [Google Scholar]
- Howard, D. J. (1999). Conspecific sperm and pollen precedence and speciation. Annual Review of Ecology and Systematics, 30, 109–132. [Google Scholar]
- Howard, D. J. , Palumbi, S. R. , Birge, L. M. , & Manier, M. K. (2009). Sperm and speciation In Birkhead T. R., Hosken D. J., & Pitnick S. (Eds.), Sperm biology: An evolutionary perspective (pp. 367–403). Oxford, UK: Elsevier. [Google Scholar]
- Kisdi, E. (2003). Reinforcement with multiple mating. Trends in Ecology & Evolution, 18, 165–166. [Google Scholar]
- Kulathinal, R. J. , Stevison, L. S. , & Noor, M. A. F. (2009). The genomics of speciation in Drosophila: Diversity, divergence, and introgression estimated using low‐coverage genome sequencing. PLoS Genetics, 5(7): e1000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, T. , Montgomerie, R. , & Chippindale, A. K. (2006). Quantifying the gender load: Can population crosses reveal interlocus sexual conflict?. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 361(1466), 363–374. doi:10.1098/rstb.2005.1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch, P. D. , & Servedio, M. R. (2005). Postmating‐prezygotic isolation is not an important source of selection for reinforcement within and between species in Drosophila pseudoobscura and D. persimilis . Evolution, 59, 1039–1045. [PubMed] [Google Scholar]
- Lupold, S. , Pitnick, S. , Berben, K. S. , Blengini, C. S. , Belote, J. M. , & Manier M. K. (2013). Female mediation of competitive fertilization success in Drosophila melanogaster . Proceedings of the National Academy of Sciences of the United States of America, 110, 10693–10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado, C. A. , Kliman, R. M. , Markert, J. A. , & Hey, J. (2002). Inferring the history of speciation from multilocus DNA sequence data: The case of Drosophila pseudoobscura and close relatives. Molecular Biology and Evolution, 19, 472–488. [DOI] [PubMed] [Google Scholar]
- Manier, M. K. , Belote, J. M. , Berben, K. S. , Lupold, S. , Ala‐Honkola, O. , Collins, W. F. , & Pitnick, S. (2013). Rapid diversification of sperm precedence traits and processes among three sibling Drosophila species. Evolution, 67, 2348–2362. [DOI] [PubMed] [Google Scholar]
- Manier, M. K. , Belote, J. M. , Berben, K. S. , Novikov, D. , Stuart, W. T. , & Pitnick, S. (2010). Resolving mechanisms of competitive fertilization success in Drosophila melanogaster . Science, 328, 354–357. [DOI] [PubMed] [Google Scholar]
- Manier, M. K. , Lupold, S. , Belote, J. M. , Starmer, W. T. , Berben, K. S. , Ala‐Honkola, … Pitnick, S. (2013). Postcopulatory sexual selection generates speciation phenotypes in Drosophila . Current Biology, 23, 1853–1862. [DOI] [PubMed] [Google Scholar]
- Manier, M. K. , Lupold, S. , Pitnick, S. , & Starmer, W. T. (2013). An analytical framework for estimating fertilization bias and the fertilization set from multiple sperm‐storage organs. The American Naturalist, 182, 552–561. [DOI] [PubMed] [Google Scholar]
- Manning, A. (1962). Sperm factor affecting receptivity of Drosophila melanogaster females. Nature, 194, 252–253. [Google Scholar]
- Manning, A. (1967). Control of sexual receptivity in female Drosophila . Animal Behaviour, 15, 239–250. [DOI] [PubMed] [Google Scholar]
- Marshall, J. L. , Arnold, M. L. , & Howard, D. J. (2002). Reinforcement: The road not taken. Trends in Ecology & Evolution, 17, 558–563. [Google Scholar]
- Martin, O. Y. , & Hosken, D. J. (2003). The evolution of reproductive isolation through sexual conflict. Nature, 423, 979–982. [DOI] [PubMed] [Google Scholar]
- Matute, D. R. (2010). Reinforcement of gametic isolation in Drosophila . PLoS Biology, 8(3): e1000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, G. T. , & Pitnick, S. (2002). Sperm‐female coevolution in Drosophila . Science, 298, 1230–1233. [DOI] [PubMed] [Google Scholar]
- Noor, M. A. (1995). Speciation driven by natural selection in Drosophila . Nature, 375, 674–675. [DOI] [PubMed] [Google Scholar]
- Noor, M. A. F. , & Ortiz‐Barrientos, D. (2006). Simulating natural conditions in the laboratory: A re‐examination of sexual isolation between sympatric and allopatric populations of Drosophila pseudoobscura and D. persimilis . Behavior Genetics, 36, 322–327. [DOI] [PubMed] [Google Scholar]
- Ortiz‐Barrientos, D. , Grealy, A. , & Nosil, P. (2009). The genetics and ecology of reinforcement implications for the evolution of prezygotic isolation in sympatry and beyond In Schlichting C. D. & Mousseau T. A. (Eds.), Year in evolutionary biology 2009 (pp. 156–182). New York, USA: New York Academy of Sciences. [DOI] [PubMed] [Google Scholar]
- Panhuis, T. M. , Butlin, R. , Zuk, M. , & Tregenza, T. (2001). Sexual selection and speciation. Trends in Ecology & Evolution, 16, 364–371. [DOI] [PubMed] [Google Scholar]
- Parker, G. A. , & Partridge, L. (1998). Sexual conflict and speciation. Philosophical Transactions of the Royal Society B: Biological Sciences, 353, 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig, D. W. , & Pfennig, K. S. (2012). Evolution's Wedge: Competition and the origins of diversity. San Francisco, CA, USA: University of California Press. [Google Scholar]
- Rice, W. R. (1996). Sexually antagonistic male adaptation triggered by experimental arrest of female evolution. Nature, 381, 232–234. [DOI] [PubMed] [Google Scholar]
- Rice, W. R. (1998). Intergenomic conflict, interlocus antagonistic coevolution, and the evolution of reproductive isolation In Howard D. J. & Berlocher S. H. (Eds.), Endless forms: Species and Speciation (pp. 261–270). New York: Oxford University Press. [Google Scholar]
- Ritchie, M. G. (2007). Sexual selection and speciation Annual review of ecology evolution and systematics, 38, 79‐102. [Google Scholar]
- Rundle, H. D. , & Schluter, D. (1998). Reinforcement of stickleback mate preferences: Sympatry breeds contempt. Evolution, 52, 200–208. [DOI] [PubMed] [Google Scholar]
- Saetre, G. P. , Moum, T. , Bures, S. , Kral, M. , Adamjan, M. , et al. (1997). A sexually selected character displacement in flycatchers reinforces premating isolation. Nature, 387, 589–592. [Google Scholar]
- Schaeffer, S. W. , & Miller, E. L. (1991). Nucleotide sequence analysis of ADH genes estimates the time of geographic isolation of the Bogota population of Drosophila pseudoobscura . Proceedings of the National Academy of Sciences of the United States of America, 88, 6097–6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servedio, M. R. , & Noor, M. A. F. (2003). The role of reinforcement in speciation: Theory and data. Annual Review of Ecology Evolution and Systematics, 34, 339–364. [Google Scholar]
- Sirot, L. K. , LaFlamme, B. A. , Sitnik, J. L. , Rubinstein, C. D. , Avila, F. W. , et al. (2009). Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Advances in Genetics, 68, 23–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau, T. M. (2013) A package for survival analysis in S. R package version 2.37‐4. [Google Scholar]
- Tregenza, T. , Wedell, N. , & Chapman, T. (2006). Introduction. Sexual conflict: A new paradigm? Philosophical Transactions of the Royal Society B: Biological Sciences, 361(1466), 229–234. doi:10.1098/rstb.2005.1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R. L. , Wakeley, J. , & Hey, J. (1997). Gene flow and natural selection in the origin of Drosophila pseudoobscura and close relatives. Genetics, 147, 1091–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


