Abstract
Background
Sepsis remains a serious clinical problem despite intensive research efforts and numerous attempts to improve outcome by modifying the inflammatory response. Substance P, the principal ligand for the neurokinin-1 receptor (NK-1R), is a potent pro-inflammatory mediator that exacerbates inflammatory responses and cardiovascular parameters in sepsis.
Location
University basic science research laboratory.
Methods
The current study examined whether inhibition of the NK-1R with a specific antagonist (CJ-12,255) would improve survival in the cecal ligation and puncture (CLP) model of sepsis in adult female outbred mice. NK-1R treatment at the initiation of sepsis improved survival in CLP sepsis (NK-1RA survival = 79% vs. vehicle = 54%). Delaying therapy for as little as 8 hours post CLP failed to provide a survival benefit. NK-1RA treatment did not prevent the sepsis induced decrease in circulating white blood cells, augment the early (6 h post CLP) recruitment of inflammatory cells to the peritoneum or improve phagocytic cell killing of pathogens. However, the NK-1R antagonist significantly reduced both circulating and peritoneal cytokine concentrations. Additionally, the cardiovascular parameter pulse distension (a surrogate for stroke volume) was improved in the NK-1RA group during the first six hours of sepsis and there was a significant reduction in loss of fluid into the intestine.
Conclusion
These data show that early activation of the NK-1R by substance P decreases sepsis survival through multiple mechanisms including depressing stroke volume, increasing fluid loss into the intestine and increasing inflammatory cytokine production.
Keywords: cytokines, leukocytes, neuropeptides, inflammation, heart rate, bacteria
Introduction
Despite the significant research investments made to understand the pathophysiology of sepsis, in hopes of discovering targeted pharmacologic therapies, no single therapy has proved to be effective [1, 2]. Therapeutic approaches such as attenuating acute inflammation by cytokine inhibition (i.e. IL-1, TNF-alpha, etc.), globally suppressing inflammation with steroid administration, or altering the innate immune system with anti-toll like receptor-4 agents have been futile [1, 3, 4]. Managing the 750,000 cases of sepsis each year remains a significant burden to our healthcare system [5]. With a mortality rate of 30%, sepsis accounts for a significant numbers of deaths in non-coronary intensive care units (ICUs) [6], although recent data has shown that sepsis mortality has stabilized [7]. The consensus definitions for sepsis were revised in 2016 to emphasize that sepsis represents a dysregulated host response to infection [8].
Substance P (SP), a pro-inflammatory neuropeptide belonging to the tachykinin family, plays an integral role in the inflammatory cascades affecting the vascular response in acute inflammation. This vascular response has been referred to as neurogenic inflammation. Following a noxious stimulus, such as a septic insult, SP is released by sensory neurons and inflammatory cells and binds to the neurokinin 1 receptor (NK-1R), which can be found throughout the CNS, peripheral tissues, endothelial cells and other inflammatory cells. This leads to ERK1/2 activation with subsequent NF-KB activation and clinical sequelae as exhibited by the systemic inflammatory response with multi-organ dysfunction [9].
It was recently shown that SP release exerts a significant inflammatory response in a polymicrobial model of animal sepsis. Using cecal ligation and puncture (CLP) in mice, a well described model of sepsis in animals [10], investigators showed that plasma and lung SP concentrations peak within one hour following an infectious challenge [11]. Moreover, the inflammatory response within the lungs, defined by pro-inflammatory cytokine release and increased myeloperoxidase levels, was attenuated when the NK-1R was blocked [12]. Similar findings were reproduced in a CLP model using genetically modified mice that lack the ability to produce SP [11].
Previous literature suggests that the NK-1R may be a potential target for therapeutic modulation given its key role in the pathogenesis of sepsis. However, no clear mechanism has been elucidated to explain its therapeutic benefit. Blocking SP binding to the NK-1R may improve sepsis survival by decreasing cytokine production, increasing inflammatory cell recruitment to the site of infection or improving cardiac output. In this study, we characterized the mechanisms by which inhibiting the SPNK-1R axis improves survival using the CLP-induced sepsis model. We tested the hypothesis that NK-1R blockade would modulate the local and systemic inflammatory responses and attenuate the cardiovascular dysfunction frequently observed in sepsis.
Methods
Animals
Female, Institute of Cancer Research (ICR) mice aged 8-10 weeks were acclimated to the lab for 2 weeks in a humidity and temperature controlled housing with a 12 hour light:dark diurnal cycle prior to any intervention. Food and water was provided ad libitum for the entire duration of the study period. All studies were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine and performed following NIH guidelines.
CLP Model
Polymicrobial peritonitis was induced by subjecting mice to CLP as first published [13] with minor modifications [14, 15]. Briefly, general anesthesia was induced with 5% isoflurane with 3% for maintenance. The abdominal wall was prepped with Chloroprep antibacterial solution (BiMeda, Le Sueur, MN) followed by a 1 cm incision through the abdominal wall. Ligation of 75% of the cecal length and double puncture with a 16-gauge needle was performed. Prior to abdominal wall closure, a neurokinin-1 receptor antagonist (NK-1RA), CJ-12,255, 25 mg/kg (Pfizer, Groton, CT) prepared in 0.5 mL of 0.9% saline was administered intraperitoneally (IP); control mice received the vehicle. The dose was selected based on the literature [16]. Prior publications in experimental animals show that NK-1R antagonists in normal animals do not alter heart rate or other baseline measurements (data not shown and [17, 18]), so this control group was not studied. CJ-12,255 was administered IP for a total of 6 doses every 12 hours. Mice were resuscitated with 1 mL of 37°C normal saline with buprenorphine (0.5 mg/kg) injected subcutaneously (SC). Postoperative analgesia was maintained with buprenorphine 0.5 mg/kg administered every 12 hours SC for 48 hours. Antibiotic treatment with imipenem 25 mg/kg (Merck, West Point, PA) was started two hours after surgery and administered SC every 12 hours for 6 doses.
To assess the therapeutic efficacy of the NK-1RA, a delayed treatment protocol was performed. A group of ICR mice underwent CLP after which they were risk stratified based on their 6 hour post-CLP plasma IL-6 levels to predict which mice would succumb to death (Die-P) in the first five days post CLP and which would survive (Live-P). Using receiver operator characteristic curves, an IL-6 cutoff of 16 ng/mL was used to predict survival, as described previously [19-21]. Administration of CJ-12,255 was delayed by 8 hours following CLP and administered to risk stratified mice as described below [22].
Blood and Peritoneal Fluid Collection
Blood was collected by facial vein puncture in 20μL quantities at various time points as described previously [23, 24]. Blood was collected prior to CLP and at 6, 24, 48, 72 and 120 hours. The blood was immediately diluted in 180 μL of PBS:EDTA (3.3mM) and used for evaluation of cytokines and complete blood count with differential with a Hemavet instrument (CDC Technologies, Oxford, CT). In a separate experiment, groups of ICR mice were sacrificed at either 6 or 24 hours post CLP using a cocktail of ketamine 87 μg/gm and xylazine 13 μg/gm mixed in normal saline. Using sterile technique, the peritoneal cavity was lavaged with 1 mL of warm Hanks balanced salt solution (HBSS) containing 50 mM EDTA which was used for microbiology cultures and peritoneal cytokine analysis. Another 24 mL of fluid was used to lavage the peritoneal cavity. After collection, the fluid was combined with the first ml and filtered through a 70 m nylon filter (BD Biosciences, San Jose, CA) to remove peritoneal debris. The peritoneal fluid was centrifuged at 450 × g for 5 minutes to isolate the cell pellet used to count total cells using a Beckman-Coulter particle counter model ZF (Coulter Electronics, Hialeah, FL). Cytospin slides were prepared from the harvested cells and stained with Diff-quick (Dade Behring, Newark, DE).
Peritoneal Bacterial Cultures after CLP
The peritoneal lavage fluid collected 6 and 24 hours after CLP was cultured on 5% sheep blood agar plates (Remel, Lenexa, KS), the plates were incubated at 37°C in aerobic and anaerobic conditions for 16 hours. The number of colonies was then counted and number of CFUs calculated.
Enzyme-Linked Immunosorbent Assay (ELISA)
ELISAs were performed on plasma and peritoneal lavage samples using matched antibody pairs for capture and detection (R&D Systems Inc., Minneapolis, MN). An aliquot of plasma or peritoneal fluid was used to determine IL-6, MIP-2 and IL-1RA by ELISA as previously described [25].
Opsonization/Phagocytosis Assay
Phagocytosis by peritoneal cells following sepsis was measured as previously published [26]. Briefly, peritoneal cells (4 million/mL) were incubated with opsonized pHrodo E. coli bioparticles (Life Technologies) at a ratio of 20 bioparticles:cell. Incubations were performed at 37°C or on ice (negative control). Cells were then stained with GR-1 (neutrophils) and CD11b (macrophages, BD biosciences). Flow cytometric (FACS) analysis was used to determine phagocytic activity of neutrophils and macrophages.
Evaluation of Cardiovascular Parameters
Cardiovascular parameters were measured using the Mouse Ox collar sensor and software (Starr Life Sciences Corp, Oakmont, PA) as described [27]. To minimize the external stress, the noninvasive cervical collar was applied for 5 minutes before obtaining heart rate and pulse distension values. The measured parameters included heart rate and pulse distension, a surrogate for stroke volume.
Leakage of plasma proteins into intestines
Leakage of plasma proteins into the intestines has been previously described following CLP [28]. Evans's blue will bind to albumin and may be used to measure leakage of albumin into the lumen of the intestine and measured by near-infrared spectroscopy. For these experiments, animals were injected with 0.2% bodyweight of a 1% (w/v) Evans’ Blue solution in PBS by tail vein injection 2h prior to sacrifice. Mice were sacrificed as above to collect blood from the retroorbial venous plexus into 100uL of heparin. A 3mm section of intestine in the most edematous area was removed, opened, and placed lumen side down for scanning on an Odyssey near-infrared scanner (LiCor, Lincoln, NE). Image settings were Intensity 2.0, Focus offset 1.5mm, quality high, resolution 42um. Background was subtracted and integrated intensity of the section was obtained. 100uL of plasma was analyzed in a 96-well plate using settings: intensity 5.0, focus offset 3.0mm, quality high, resolution 84um with background subtraction. Integrated intensity of the well was obtained and used to calculate the ratio of blood:intestine so that a higher value indicates less extravasation of albumin.
Statistical Analysis
Statistical analysis was performed using Prism 5 (GraphPad Software, San Diego, CA). For survival analysis, a Kaplan-Meier curve and log rank survival was performed. All values were expressed in mean ± SE. Student's t-test was used to compare data between two groups. For repeated measures data, such as the cardiovascular parameters, the data were analyzed using repeated measures ANOVA with Bonferroni correction. The area under the curve for the temporal cytokine response was calculated for each mouse individually and then summed. Since cytokines are not detectable prior to CLP, time 0 values were assigned a value equal to one half the lower limit of detection of the assay.
Results
Early blockade of NK-1R improves sepsis survival in outbred mice
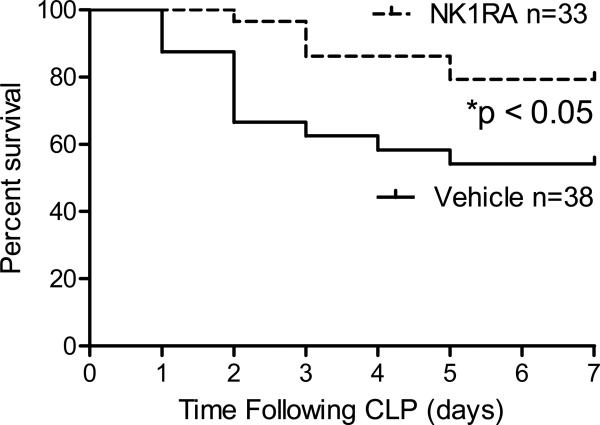
The first experiment evaluated whether an NK-1R antagonist would improve survival in the murine CLP model of sepsis. For these studies, the NK-1R antagonist was given immediately after CLP since prior work demonstrated that substance P is released within the first hour post-CLP and returns to baseline levels within 16 hours [11]. Survival was significantly higher in mice treated with NK-1RA. Mice who received the vehicle had a 50% survival which improved to nearly 80% when mice were treated with NK-1RA (Fig 1).
Figure 1. NK-1R antagonism results in improved survival in the acute phase of sepsis following CLP.
Mice treated with NK-1RA (n = 33) at the time of CLP had a 79% survival rate compared to those that received the vehicle with 54% survival (n = 38). *p < 0.05 by log rank survival analysis.
Our hypothesis is that SP released during the first hours of the septic response modulates immune and cardiovascular function to improve survival. This hypothesis was tested by delaying treatment. Previously, we and others published that plasma levels of IL-6 obtained 6 hours post CLP accurately predict mortality [19, 21, 22]. For the current study, all mice underwent CLP and plasma was collected 6 hours post-CLP. The IL-6 concentrations were then used to stratify mice into those predicted to live (Live-P) or predicted to die (Die-P). NK-1RA treatment was started 8 hours post-CLP, creating 4 groups of mice, listed in Table I. Delaying administration of NK-1RA failed to improve pulse distension, reduce IL-6, or improve survival in any of the groups. (Table I).
Table I.
Mice were stratified into predicted to live (Live-P) or predicted to die (Die-P) on the basis of plasma levels of IL-6. Mice were treated with the NK-1RA or vehicle by intraperitoneal injection starting 8 hours after the onset of sepsis.
| Group (n) | Treatment started 8 hours post CLP | Pulse distension | IL-6 (ng/ml) | 5 day survival |
|---|---|---|---|---|
| Live-P (11) | NK-1RA | 370 ± 36 | 4 ± 2 | 88% |
| Live-P (9) | Vehicle | 375 ± 37 | 1 ± 0.5 | 85% |
| Die-P (12) | NK-1RA | 258 ± 36 | 35 ± 13 | 38% |
| Die-P (12) | Vehicle | 247 ± 27 | 52 ± 16 | 23% |
Pharmacological blockade of NK-1R reduces plasma cytokines in sepsis
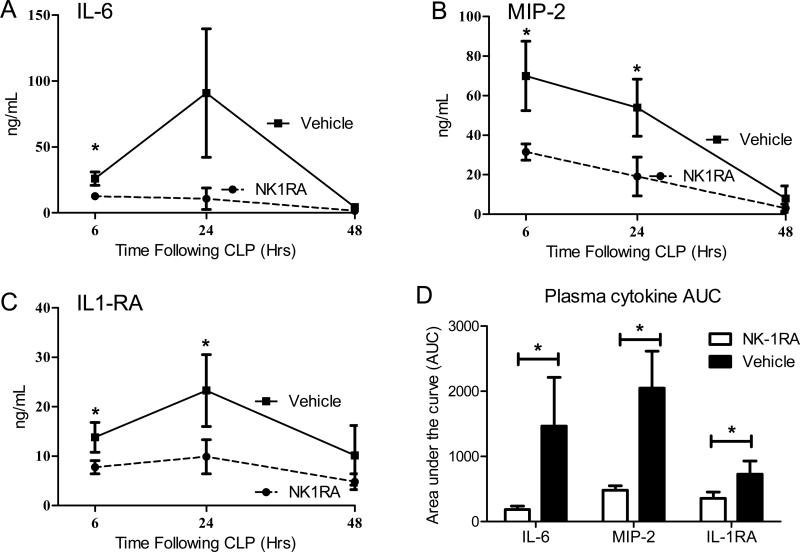
The septic response in CLP mice includes significant production of pro-inflammatory cytokines as well as cytokine inhibitors [22, 29]. These outbred ICR mice were subjected to our standard CLP model and treated with the NK-1RA immediately after CLP. Intraperitoneal administration of NK-1RA every 12 hours was continued during the first 3 days of sepsis. Serial collection of plasma from the facial vein allowed for evaluation of the temporal pattern of systemic inflammatory response assessed by the plasma levels of IL-6 (Fig 2A), MIP-2 (Fig 2B), and IL-1RA (Fig 2C). Mice treated with the NK-1RA at the time of CLP had significantly lower concentrations following CLP (Fig. 2). The total cytokine response through the first 48 hours was determined by calculating the area under the curve (AUC) for individual mice to allow a better analysis of the cytokine and cytokine inhibitor response during this early phase. For IL-6, MIP-2, and IL-1RA the AUC was significantly less for the NK-1RA treated mice compared to normal saline mice (Fig 2D). These data demonstrate that treatment with NK-1RA decreased both pro-inflammatory cytokines (IL-6 and MIP-2) as well as suppressing the cytokine inhibitor IL-1RA.
Figure 2. NK-1R antagonism attenuates the plasma cytokine levels.
Outbred female mice treated with NK-1RA (n = 14) following 16 gauge double puncture CLP had lower plasma cytokines including (A) pro-inflammatory IL-6, (B) chemokine MIP-2, and (C) anti-inflammatory IL-1RA early following CLP induced sepsis compared to vehicle mice (n = 11). The area under the curve (AUC) was significantly reduced by treatment with NK-1RA. Each value is the mean ± SEM. *p < 0.05 at the indicated time points comparing vehicle to NK1RA.
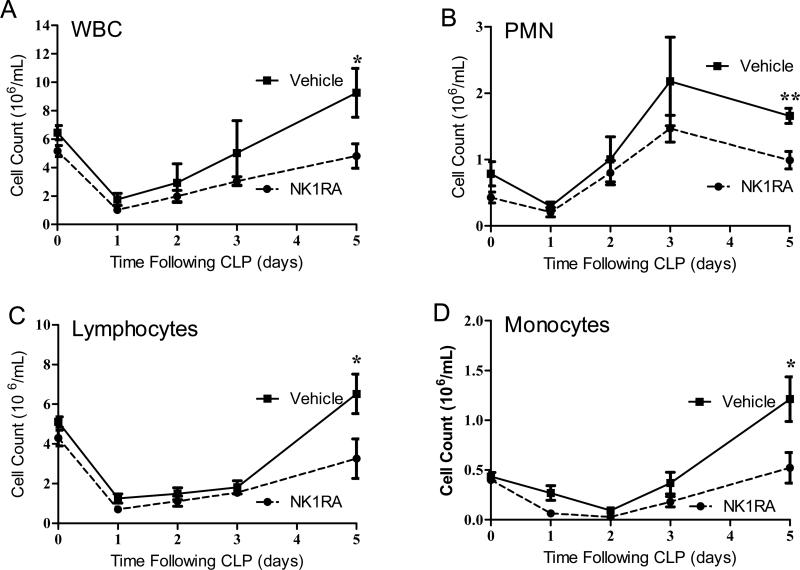
NK-1RA does not prevent early decreases in WBC
Peritoneal sepsis induces a transient reduction in the white blood cell (WBC) count which rebounds after 3-5 days [15]. During the first three days of the septic response, the decrease in WBC still occurred despite the blockade of the NK-1R. Figure 3 demonstrates that the baseline WBC was 5 × 106 cells in the blood. As described by Hotchkiss et al., the WBC drops dramatically shortly after septic challenge as a result of lymphocyte apoptosis [30], a trend observed in both the NK-1RA treated mice and mice that received the vehicle. By the last day of the acute phase of sepsis (day 5 [15]), it became evident that mice treated with NK-1RA had significantly lower WBC counts compared to the vehicle controls (Fig. 3A). This holds true among all peripheral blood cell populations including neutrophils (Fig 3B), lymphocytes (Fig. 3C), and monocytes (Fig 3D). As the cellular response in acute inflammation recovered, the WBC rebounded above baseline in vehicle treated mice while in the NK-1RA treated mice the WBC count levels only recovered to baseline.
Figure 3. Circulating white blood cells are only reduced at day 5 post CLP with NK-1RA.
(A) White blood cells including (B) PMN, (C) lymphocytes and (D) monocytes were reduced only at day 5 post CLP with NK-1RA (NK-1RA n = 8; vehicle n = 9). Additionally, by post CLP day 5, mice treated with NK-1RA recovered to baseline levels while the vehicle mice rebounded above baseline. *p < 0.05, **p<0.01 comparing the two groups.
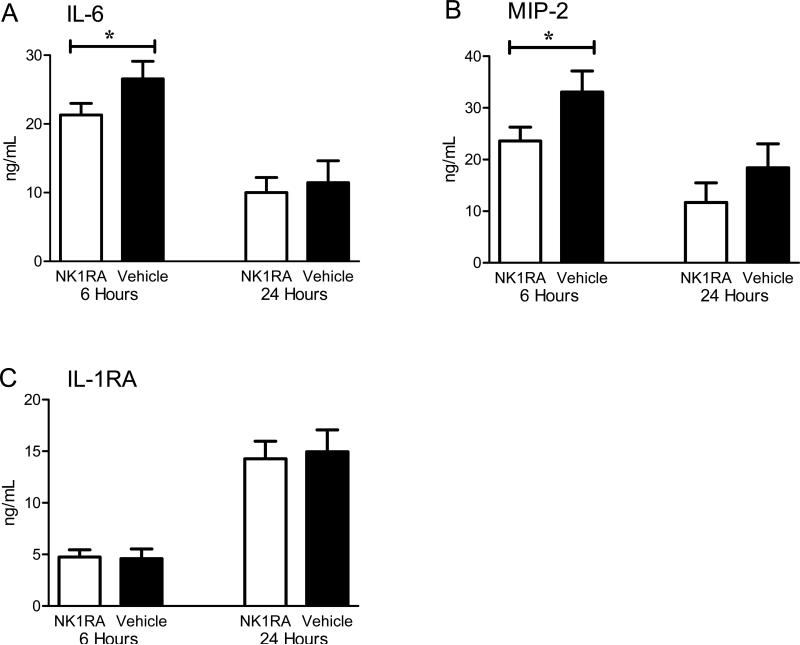
NK-1RA decreases peritoneal IL-6 and MIP-2
Since administration of NK-1RA decreased plasma cytokine concentrations, the local inflammatory effects compartmentalized within the peritoneum were examined. Peritoneal lavage was performed in mice sacrificed either 6 or 24 hours following CLP. Those sacrificed at 6 hours received just one dose of NK-1RA while those sacrificed at 24 hours received 3 doses. Paralleling the systemic inflammatory response, the concentrations of IL-6 and MIP-2 in the peritoneal fluid at 6 hours were significantly lower in mice that received the NK-1RA as compared with the saline controls (Fig. 4A-B). Mice treated with the NK-1RA had equivalent concentrations of IL-6 and MIP-2 at 24 hours. No difference was apparent in the anti-inflammatory IL-1RA concentration within the peritoneum at either of the early time points (Fig. 4C).
Figure 4. NK-1R antagonism partially attenuates the local cytokine production.
Mice were sacrificed at 6 and 24 hours post CLP and peritoneal lavage performed. Mice treated with NK-1RA had lower levels of (A) IL-6 and (B) MIP-2 early after CLP (n = 23) compared to mice given the vehicle (n = 16). These inflammatory markers were not significantly different 24 hours post CLP with treatment (n = 16) vs control (n = 16). There was no difference in (C) IL-1RA levels at either time point. *p < 0.05 vehicle compared to NK-1RA.
NK-1RA does not decrease 6 hour peritoneal cell recruitment
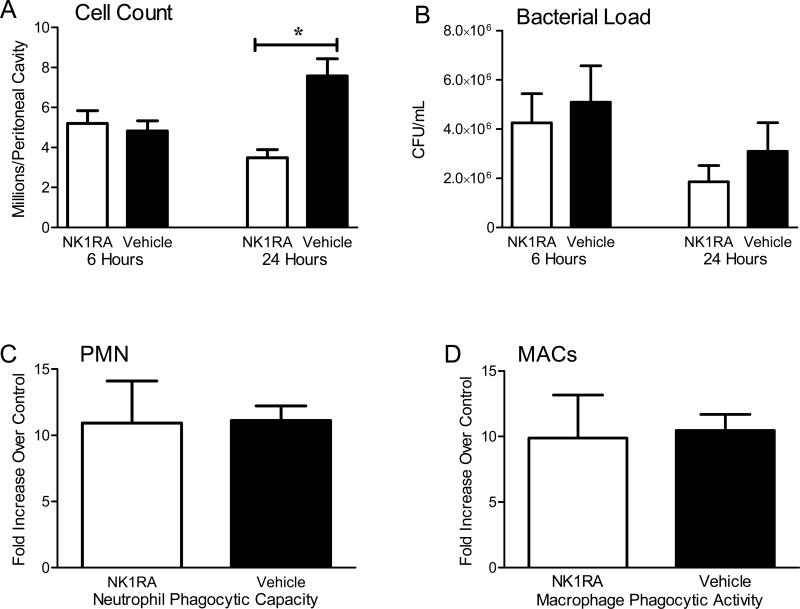
The local cytokine release within the peritoneum has been shown to play a pivotal role in the recruitment of inflammatory cells that play an important role in the eradication of the enteric pathogens associated with cecal injury [24, 31]. Since the NK-1RA reduced MIP-2 production within the peritoneal cavity (Fig 4B) we studied whether polymorphonuclear leukocyte (PMN) recruitment would be impaired. While there were essentially no neutrophils found in the sterile abdomen, millions of these inflammatory cells are rapidly recruited to the infected peritoneum within hours following CLP. NK-1RA treatment did not reduce the number of inflammatory cells recruited to the peritoneum at 6 hours. However, a substantial difference in the inflammatory cell count was apparent by 24 hours following CLP; mice treated with NK-1RA had significantly fewer peritoneal cells compared to mice treated with the vehicle (Fig. 5A). Moreover, the number of peritoneal inflammatory cells decreased between 6 and 24 hours in NK-1RA treated mice while the mice given the vehicle had an increase in the number of peritoneal cells. The recruitment of inflammatory cells within 6 hours of CLP has been shown to be important in the control of sepsis [24], and NK-1R treatment did not alter this early response.
Figure 5. NK-1R antagonism attenuates 24 hour peritoneal inflammatory cell recruitment but not improve phagocytic cell function.
(A) There was no difference in the cell counts within the peritoneum at 6 hours (NK-1RA n = 23, vehicle n = 16). At 24 hours the mice treated with NK-1RA not only had decreased cell counts compared to the vehicle (n = 16 for both groups), but also lower values than treated mice at 6 hours. (B) NK-1RA treatment did not affect bacterial burden at either time point suggesting that infectious burden was similar between the two groups (n = 16 for both groups). Hypothesizing that these findings were attributed to improved cell efficiency at killing bacterial burden, an ex vivo phagocytosis assay of isolated (C) PMN (n = 5 per group) and (D) macrophages (n = 5 per group) was performed. There was no difference in phagocytic cell function with NK-1RA treatment. *p < 0.05 vehicle compared to NK-1RA.
NK-1RA does not decrease bacterial growth or improve phagocytic cell function
To determine whether there would be any difference in the growth of peritoneal bacteria, serial dilutions of the peritoneal lavage were cultured on blood agar plates in both aerobic and anaerobic conditions. There were no significant differences in the number of bacteria within the peritoneal cavity at 6 or 24 hours post CLP (Fig 5B). Since there were similar numbers of peritoneal bacteria, it also indicates that the administration of CJ-12,255 into the peritoneal cavity did not alter bacterial replication. Since there were fewer inflammatory cells at 24 hours post CLP, but equivalent numbers of bacteria, we evaluated whether cells in NK-1R treated mice functioned more efficiently within the peritoneum. In other words, fewer peritoneal neutrophils at 24 hours post CLP (Fig 5A) were still able to control the local infection (Fig 5B). To evaluate phagocytic function more closely, peritoneal neutrophils and macrophages were harvested from both treated and control mice 24 hours following CLP. These cells were then incubated with exogenous E. coli labeled with a pH sensitive probe in an assay that determines if the bacteria have been incorporated into phagolysosomes [26, 32]. Specific cell surface markers were used to distinguish phagocytic activity of neutrophils versus macrophages. Antagonism of the NK-1R did not alter the phagocytic capacity of either neutrophils (Fig 5C) or macrophages (Fig 5D).
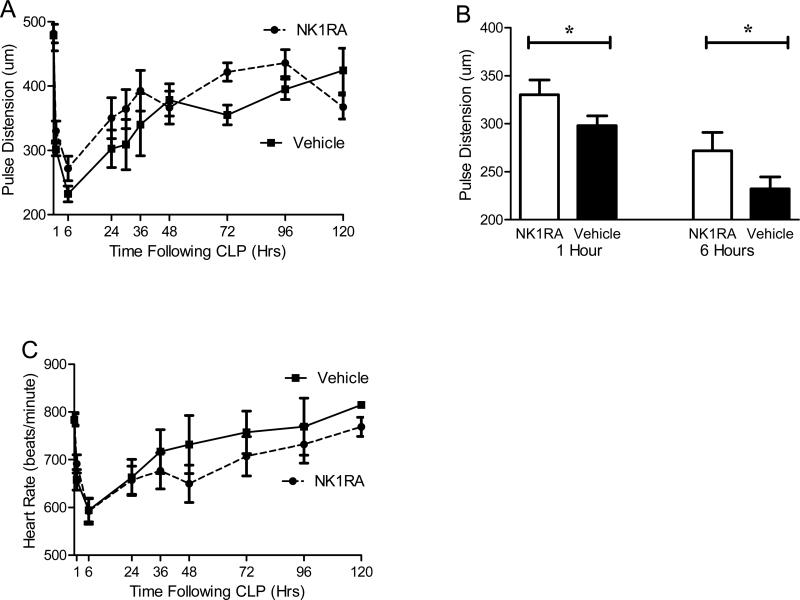
NK-1R blockade improves early cardiac output
Monitoring physiologic parameters and vital signs may provide insight into the cardiovascular responses during the septic challenge, critical components in sepsis management. In order to better characterize the hemodynamic changes, vital signs were measured serially using a non-invasive cervical collar probe. The probe senses local blood flow at the level of the carotid artery to measure pulse distension (a stroke volume surrogate) and heart rate. Treatment with the NK-1RA decreased the early decline in pulse distension following CLP (Fig 6A). To highlight the early improvement in pulse distension the values at one and six hours post CLP were graphed separately (Fig 6B). At these early time points, antagonism of NK-1R reduced the decline in pulse distension compared to vehicle.
Figure 6. Cardiovascular parameters improved with NK-1R Antagonism.
Using a noninvasive infrared cervical probe, cardiovascular parameters including pulse distension (a surrogate for stroke volume) and heart rate were obtained following CLP. (A) In the NK-1R antagonist treated mice, the sepsis induced decrease in pulse distension was less than the decrease observed in vehicle treated mice (NK-1RA, n=15, vehicle n=12). (B) To highlight the early changes, the pulse distension at 1 hour and 6 hours post CLP is displayed, which shows that the NK-1RA mice maintained their pulse distension better than the mice only given vehicle. (C) NK-1RA mice had essentially the same changes in heart rates throughout the first 48 hours of sepsis (NK-1RA n=12, Vehicle n=15).
Other cardiovascular parameters were also studied since the central nervous system helps regulate heart rate. Neurons within the paraventricular nucleus express NK-1R which modulate stress induced changes in heart rate [33]. In our studies, treatment with the NK-1RA did not alter the heart rate (Fig 6C). This finding is likely due to the fact that the NK-1RA used in these studies does not cross the blood brain barrier and thus does not affect the paraventricular nucleus responsible for regulating heart rate.
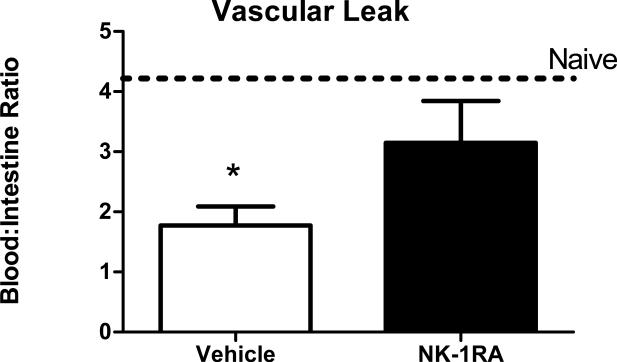
Vascular leak into the intestines has been demonstrated following CLP [28]. Decreased early cardiac output at 1 and 6 hours (Fig 6B) should exacerbate the damage to the intestine and increase vascular leak. This was measured by injecting Evans’ blue dye intravenously 22 hours after CLP and sacrificing mice 2 hours later. Figure 7 shows that mice subjected to CLP had significant leakage into the intestine compared to naïve mice. Treatment with the NK-1RA significantly reduced the vascular leak to levels similar to naïve animals.
Figure 7. NK-1R antagonist treatment reduces vascular leak into the intestines.
Vascular leak was measured by albumin extravasation into the intestine 24 hours after CLP. Mice subjected to CLP had increased vascular leak compared to naïve mice (dotted horizontal line). Treatment with NK-1R significantly prevented this vascular leak. *=p<.05 vehicle vs NK-1RA.
Discussion
The current study takes advantage of recent evidence demonstrating that SP peaks early in acute inflammation following CLP [9, 11]. We have demonstrated that modulating the inflammatory effects of SP with a receptor antagonist in the initial septic response improves survival in the murine CLP sepsis model. Blocking the NK-1R exerts an anti-inflammatory effect coupled with improved cardiovascular function during the first 6 hours of murine peritonitis. However, the cytokine reduction, especially within the peritoneum, was relatively mild. Additionally, there was no significant change in circulating white blood cells, 6 hour peritoneal cell recruitment or phagocytic cell function with NK-1R blockade. While there was a significant reduction in both the systemic and local cytokine response following the inhibition of NK-1R, this reduced cytokine response does not explain why mice exhibited improved survival in severe sepsis. Better survival with NK-1R antagonism probably occurs through multiple mechanisms.
Cardiovascular responses during sepsis are important determinants of survival. Cardiovascular depression—defined by reduced mean arterial pressure, stroke volume, and cardiac output—has been demonstrated in experimental pre-clinical models of sepsis including pneumonia and CLP. Interestingly, the stroke volume curves obtained by Zanotti-Cavazzoni assessing fluid resuscitation [34] are nearly identical to the pulse distension curves obtained in our study. While the end result in both studies demonstrated improved survival secondary to improved cardiovascular performance, the mechanisms are different. Whereas the former study adopted the goal directed resuscitation philosophy to improve survival in severe sepsis in the murine model of CLP, we pharmacologically attenuated the NK-1R interaction. A 2016 study defined that physiological deterioration after CLP occurred within 8 hours [35], results nearly identical to our data. This early deterioration also reinforces why delayed treatment with the NK-1R antagonist at 8 hours was not effective.
Prior publications using SP knockout (SP-KO) mice to evaluate the role of SP in the mortality of CLP-induced sepsis [11] showed that the SP-KO mice had better survival after CLP, consistent with our current findings. Despite these similarities, there are important differences between the studies. In the Puneet studies [11] there was 100% mortality within 24 hours in the wild type mice, and the SP-KO mice still had 100% mortality by 5 days. In contrast, our study had 50% mortality by 7 days in the vehicle treated mice which was improved to 80% with the NK-1R blockade. The role of NK-1R in the clearance of bacteria was studied using a model of staphylococcal sepsis. Verdrengh and Tarkowski showed that NK-1R knockout mice had increased bacteria in the kidneys and decreased survival [36] which differ from our results. These differences may relate to different pathogens and complete loss of SP signaling throughout the host rather than the NK-1R antagonist treatment used in the current study. The concept that NK-1R antagonism given at the onset of sepsis reduces the signaling pathways, i.e. NF-κB, that results in cytokine production has also been reported [37].
Using a different model of infection we have previously published that early blockade of the SP receptor decreases survival [38], results different than the current findings. There are several explanations for these discordant results. In the prior publication, mice were subjected to mild traumatic brain injury (mTBI) and 2 days later gram negative pneumonia was induced with Pseudomonas aeruginosa, so there are substantial differences in the models. While both pneumonia and peritonitis cause sepsis, the disease processes are very different. In the mTBI model NK-1R was blocked for 2 days prior to the start of the infection, while in the current manuscript the blockade began when the infection began. Finally, in the mTBI model there was nearly 100% lethality among the control mice while the current study had approximately 50% lethality. Prior work has demonstrated that the effectiveness of therapy depends on the mortality, i.e. therapies effective in high mortality models are not effective in lower mortality models [22].
Neuropeptides have not been widely studied in clinical sepsis despite their potent immunoregulatory and vasoactive properties. Moreover, the little data that has been published is controversial. While one study suggests that SP is not elevated in sepsis [39], another one concludes SP levels are elevated and, more importantly, SP levels correlate with mortality [40]. This controversy has probably been resolved by the recent publication of a larger clinical study showing that serum levels of substance P correlate with survival [41]. Our current study demonstrates the SP blockade is probably not an appropriate therapeutic target, since it made so quickly in the septic response. However, the neuropeptide SP does play a significant role in the mortality of sepsis.
Acknowledgements
The authors wish to thank Terry Hsieh for performing the vascular permeability studies in Figure 6.
Dr. Remick received support for article research from the National Institutes of Health (NIH). His institution received funding (This work was supported in part by NIH grant R01 GM97320, R21 AI112887, and T32 GM86308) and received funding from the NIH. Dr. Mella received support for article research from the NIH. Dr. Chiswick received support for article research from the NIH. Dr. Stepien received support for article research from the NIH. Dr. Moitra received support for article research from the NIH. Dr. Duffy received support for article research from the NIH.
Footnotes
Copyright form disclosures:
Dr. Stucchi disclosed that he does not have any potential conflicts of interest.
References
- 1.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA. 2011;306(23):2614–2615. doi: 10.1001/jama.2011.1853. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Liu KD. New Strategies for Effective Therapeutics in Critically Ill Patients. JAMA. 2016;315(8):747–748. doi: 10.1001/jama.2016.0661. [DOI] [PubMed] [Google Scholar]
- 3.Fisher CJ, Jr., Dhainaut JF, Opal SM, Pribble JP, Balk RA, Slotman GJ, Iberti TJ, Rackow EC, Shapiro MJ, Greenman RL, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome. Results from a randomized, double-blind, placebo-controlled trial. Phase III rhIL-1ra Sepsis Syndrome Study Group. JAMA. 1994;271(23):1836–1843. [PubMed] [Google Scholar]
- 4.Fisher CJ, Jr., Agosti JM, Opal SM, Lowry SF, Balk RA, Sadoff JC, Abraham E, Schein RM, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. The Soluble TNF Receptor Sepsis Study Group. N Engl J Med. 1996;334(26):1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 5.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Parrillo JE, Parker MM, Natanson C, Suffredini AF, Danner RL, Cunnion RE, Ognibene FP. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med. 1990;113(3):227–242. doi: 10.7326/0003-4819-113-3-227. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis*. Crit Care Med. 2014;42(3):625–631. doi: 10.1097/CCM.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ang SF, Moochhala SM, MacAry PA, Bhatia M. Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: involvement of substance P and ERK-NFkappaB signaling. PLoS One. 2011;6(9):e24535. doi: 10.1371/journal.pone.0024535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remick DG, Newcomb DE, Bolgos GL, Call DR. Comparison of the mortality and inflammatory response of two models of sepsis: lipopolysaccharide vs. cecal ligation and puncture. Shock. 2000;13(2):110–116. doi: 10.1097/00024382-200013020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Puneet P, Hegde A, Ng SW, Lau HY, Lu J, Moochhala SM, Bhatia M. Preprotachykinin-A gene products are key mediators of lung injury in polymicrobial sepsis. J Immunol. 2006;176(6):3813–3820. doi: 10.4049/jimmunol.176.6.3813. [DOI] [PubMed] [Google Scholar]
- 12.Hegde A, Zhang H, Moochhala SM, Bhatia M. Neurokinin-1 receptor antagonist treatment protects mice against lung injury in polymicrobial sepsis. J Leukoc Biol. 2007;82(3):678–685. doi: 10.1189/jlb.0407217. [DOI] [PubMed] [Google Scholar]
- 13.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 14.Ebong SJ, Call DR, Bolgos G, Newcomb DE, Granger JI, O'Reilly M, Remick DG. Immunopathologic responses to non-lethal sepsis. Shock. 1999;12(2):118–126. doi: 10.1097/00024382-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74(9):5227–5235. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassidy MR, Sheldon HK, Gainsbury ML, Gillespie E, Kosaka H, Heydrick S, Stucchi AF. The neurokinin 1 receptor regulates peritoneal fibrinolytic activity and postoperative adhesion formation. J Surg Res. 2014;191(1):12–18. doi: 10.1016/j.jss.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 17.Boscan P, Kasparov S, Paton JF. Somatic nociception activates NK1 receptors in the nucleus tractus solitarii to attenuate the baroreceptor cardiac reflex. Eur J Neurosci. 2002;16(5):907–920. doi: 10.1046/j.1460-9568.2002.02131.x. [DOI] [PubMed] [Google Scholar]
- 18.Boscan P, Dutschmann M, Herbert H, Paton JF. Neurokininergic mechanism within the lateral crescent nucleus of the parabrachial complex participates in the heart-rate response to nociception. J Neurosci. 2005;25(6):1412–1420. doi: 10.1523/JNEUROSCI.4075-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17(6):463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Iskander KN, Craciun FL, Stepien DM, Duffy ER, Kim J, Moitra R, Vaickus LJ, Osuchowski MF, Remick DG. Cecal ligation and puncture-induced murine sepsis does not cause lung injury. Crit Care Med. 41(1):159–170. doi: 10.1097/CCM.0b013e3182676322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vyas D, Javadi P, Dipasco PJ, Buchman TG, Hotchkiss RS, Coopersmith CM. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R1048–1053. doi: 10.1152/ajpregu.00312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osuchowski MF, Connett J, Welch K, Granger J, Remick DG. Stratification is the key: inflammatory biomarkers accurately direct immunomodulatory therapy in experimental sepsis. Crit Care Med. 2009;37(5):1567–1573. doi: 10.1097/CCM.0b013e31819df06b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weixelbaumer KM, Raeven P, Redl H, van Griensven M, Bahrami S, Osuchowski MF. Repetitive low-volume blood sampling method as a feasible monitoring tool in a mouse model of sepsis. Shock. 2010;34(4):420–426. doi: 10.1097/SHK.0b013e3181dc0918. [DOI] [PubMed] [Google Scholar]
- 24.Craciun FL, Schuller ER, Remick DG. Early enhanced local neutrophil recruitment in peritonitis-induced sepsis improves bacterial clearance and survival. J Immunol. 2010;185(11):6930–6938. doi: 10.4049/jimmunol.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemzek JA, Siddiqui J, Remick DG. Development and optimization of cytokine ELISAs using commercial antibody pairs. J Immunol Methods. 2001;255(1-2):149–157. doi: 10.1016/s0022-1759(01)00419-7. [DOI] [PubMed] [Google Scholar]
- 26.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol. 2011;186(4):2444–2453. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, Gallagher-Colombo SM, Solomides CC, Cengel KA, Christofidou-Solomidou M. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer. 13179 doi: 10.1186/1471-2407-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez JA, Samocha AJ, Liang Z, Burd EM, Farris AB, Coopersmith CM. Inhibition of IKKbeta in enterocytes exacerbates sepsis-induced intestinal injury and worsens mortality. Crit Care Med. 2013;41(10):e275–285. doi: 10.1097/CCM.0b013e31828a44ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osuchowski MF, Craciun F, Weixelbaumer KM, Duffy ER, Remick DG. Sepsis chronically in MARS: systemic cytokine responses are always mixed regardless of the outcome, magnitude, or phase of sepsis. J Immunol. 2012;189(9):4648–4656. doi: 10.4049/jimmunol.1201806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotchkiss RS, Swanson PE, Cobb JP, Jacobson A, Buchman TG, Karl IE. Apoptosis in lymphoid and parenchymal cells during sepsis: findings in normal and T- and B-celldeficient mice. Crit Care Med. 1997;25(8):1298–1307. doi: 10.1097/00003246-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 31.Seeley EJ, Barry SS, Narala S, Matthay MA, Wolters PJ. Noradrenergic neurons regulate monocyte trafficking and mortality during gram-negative peritonitis in mice. J Immunol. 2013;190(9):4717–4724. doi: 10.4049/jimmunol.1300027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, et al. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci U S A. 2009;106(15):6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Feetham CH, Barrett-Jolley R. NK1-receptor-expressing paraventricular nucleus neurones modulate daily variation in heart rate and stress-induced changes in heart rate variability. Physiol Rep. 2014;2(12) doi: 10.14814/phy2.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanotti-Cavazzoni SL, Guglielmi M, Parrillo JE, Walker T, Dellinger RP, Hollenberg SM. Fluid resuscitation influences cardiovascular performance and mortality in a murine model of sepsis. Intensive Care Med. 2009;35(4):748–754. doi: 10.1007/s00134-008-1360-9. [DOI] [PubMed] [Google Scholar]
- 35.Lewis AJ, Yuan D, Zhang X, Angus DC, Rosengart MR, Seymour CW. Use of Biotelemetry to Define Physiology-Based Deterioration Thresholds in a Murine Cecal Ligation and Puncture Model of Sepsis. Crit Care Med. 2016 doi: 10.1097/CCM.0000000000001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verdrengh M, Tarkowski A. The impact of substance P signalling on the development of experimental staphylococcal sepsis and arthritis. Scand J Immunol. 2008;67(3):253–259. doi: 10.1111/j.1365-3083.2007.02065.x. [DOI] [PubMed] [Google Scholar]
- 37.Hegde A, Koh YH, Moochhala SM, Bhatia M. Neurokinin-1 receptor antagonist treatment in polymicrobial sepsis: molecular insights. Int J Inflam. 2010;2010:601098. doi: 10.4061/2010/601098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang S, Stepien D, Hanseman D, Robinson B, Goodman MD, Pritts TA, Caldwell CC, Remick DG, Lentsch AB. Substance P mediates reduced pneumonia rates after traumatic brain injury. Crit Care Med. 2014;42(9):2092–2100. doi: 10.1097/CCM.0000000000000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnalich F, Sanchez JF, Martinez M, Jimenez M, Lopez J, Vazquez JJ, Hernanz A. Changes in plasma concentrations of vasoactive neuropeptides in patients with sepsis and septic shock. Life Sci. 1995;56(2):75–81. doi: 10.1016/0024-3205(94)00416-p. [DOI] [PubMed] [Google Scholar]
- 40.Beer S, Weighardt H, Emmanuilidis K, Harzenetter MD, Matevossian E, Heidecke CD, Bartels H, Siewert JR, Holzmann B. Systemic neuropeptide levels as predictive indicators for lethal outcome in patients with postoperative sepsis. Crit Care Med. 2002;30(8):1794–1798. doi: 10.1097/00003246-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 41.Lorente L, Martin MM, Almeida T, Hernandez M, Ferreres J, Sole-Violan J, Labarta L, Diaz C, Jimenez A. Association between serum substance P levels and mortality in patients with severe sepsis. J Crit Care. 2015 doi: 10.1016/j.jcrc.2015.05.012. [DOI] [PubMed] [Google Scholar]