Abstract
The ability of the human immunodeficiency virus type 1 (HIV-1) to replicate in primary blood dendritic cells was investigated. Dendritic cells compose less than 1% of the circulating leukocytes and are nondividing cells. Highly purified preparations of dendritic cells were obtained using recent advances in cell fractionation. The results of these experiments show that dendritic cells, in contrast to monocytes and T cells, support the active replication of all strains of HIV-1 tested, including T-cell tropic and monocyte/macrophage tropic isolates. The dendritic cell cultures supported much more virus production than did cultures of primary unseparated T cells, CD4+ T cells, and adherent as well as nonadherent monocytes. Replication of HIV-1 in dendritic cells produces no noticeable cytopathic effect nor does it decrease total cell number. The ability of the nonreplicating dendritic cells to support high levels of replication of HIV-1 suggests that this antigen-presenting cell population, which is also capable of supporting clonal T-cell growth, may play a central role in HIV pathogenesis, serving as a source of continued infection of CD4+ T cells and as a reservoir of virus infection.
Full text
PDF
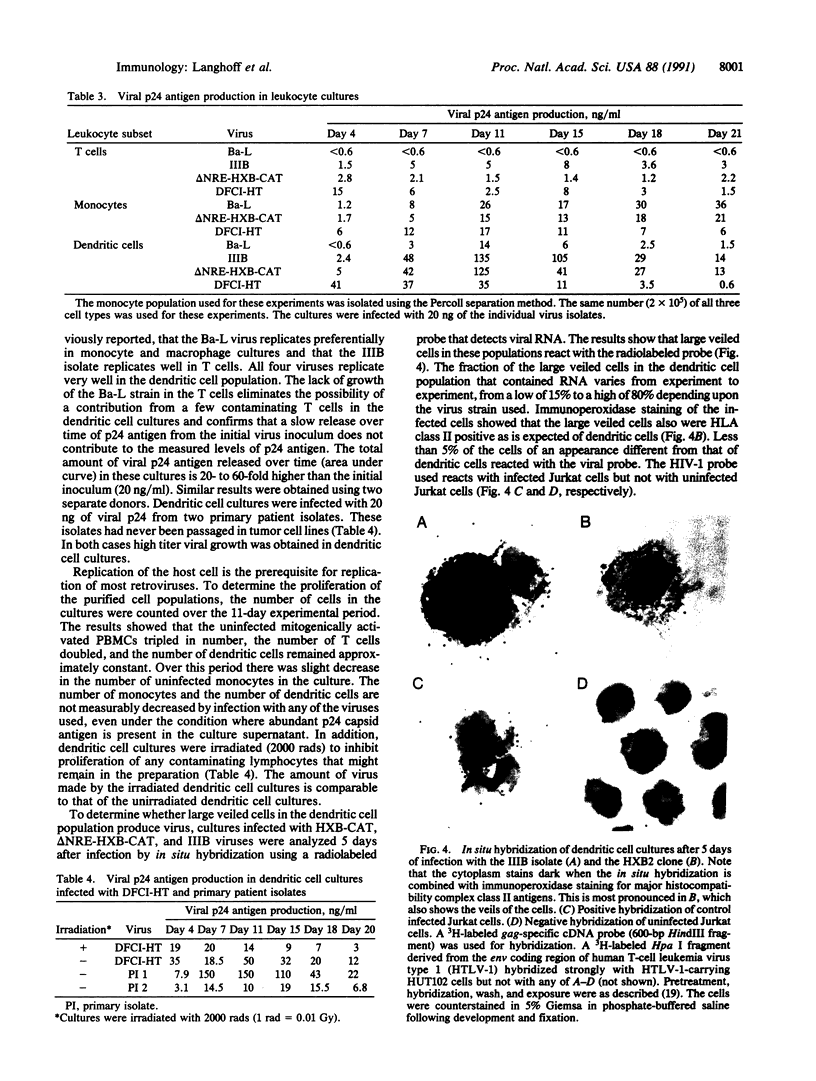

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Kupiec-Weglinski J. W., Hankins D. F., Morris P. J. Migration patterns of dendritic cells in the mouse. Homing to T cell-dependent areas of spleen, and binding within marginal zone. J Exp Med. 1988 Feb 1;167(2):646–651. doi: 10.1084/jem.167.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos H. J., Meyer F., de Veld J. C., Beelen R. H. Peritoneal dialysis fluid induces change of mononuclear phagocyte proportions. Kidney Int. 1989 Jul;36(1):20–26. doi: 10.1038/ki.1989.155. [DOI] [PubMed] [Google Scholar]
- Braathen L. R. Langerhans cells and HIV infection. Biomed Pharmacother. 1988;42(5):305–308. [PubMed] [Google Scholar]
- Cohen E. A., Terwilliger E. F., Jalinoos Y., Proulx J., Sodroski J. G., Haseltine W. A. Identification of HIV-1 vpr product and function. J Acquir Immune Defic Syndr. 1990;3(1):11–18. [PubMed] [Google Scholar]
- Faustman D. L., Steinman R. M., Gebel H. M., Hauptfeld V., Davie J. M., Lacy P. E. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3864–3868. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner S., Markovits P., Markovitz D. M., Kaplan M. H., Gallo R. C., Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986 Jul 11;233(4760):215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- Gartner S., Popovic M. Macrophage tropism of HIV-1. AIDS Res Hum Retroviruses. 1990 Aug;6(8):1017–1021. doi: 10.1089/aid.1990.6.1017. [DOI] [PubMed] [Google Scholar]
- Hart D. N., McKenzie J. L. Isolation and characterization of human tonsil dendritic cells. J Exp Med. 1988 Jul 1;168(1):157–170. doi: 10.1084/jem.168.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Krejci J., Malkovsky M., Colizzi V., Gautam A., Asherson G. L. The role of dendritic cells in the initiation of immune responses to contact sensitizers. I. In vivo exposure to antigen. Cell Immunol. 1985 Sep;94(2):427–434. doi: 10.1016/0008-8749(85)90266-7. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Macatonia S. E., Patterson S. HIV I infection of dendritic cells. Int Rev Immunol. 1990;6(2-3):163–175. doi: 10.3109/08830189009056627. [DOI] [PubMed] [Google Scholar]
- Knight S. C., Mertin J., Stackpoole A., Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6032–6035. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhoff E., McElrath J., Bos H. J., Pruett J., Granelli-Piperno A., Cohn Z. A., Steinman R. M. Most CD4+ T cells from human immunodeficiency virus-1 infected patients can undergo prolonged clonal expansion. J Clin Invest. 1989 Nov;84(5):1637–1643. doi: 10.1172/JCI114341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhoff E., Steinman R. M. Clonal expansion of human T lymphocytes initiated by dendritic cells. J Exp Med. 1989 Jan 1;169(1):315–320. doi: 10.1084/jem.169.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler R. I., Batchelor J. R. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982 Jan 1;155(1):31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard J., Khillan J. S., Gendelman H. E., Adachi A., Lorenzo S., Westphal H., Martin M. A., Meltzer M. S. The human immunodeficiency virus long terminal repeat is preferentially expressed in Langerhans cells in transgenic mice. AIDS Res Hum Retroviruses. 1989 Aug;5(4):421–430. doi: 10.1089/aid.1989.5.421. [DOI] [PubMed] [Google Scholar]
- Lu Y., Stenzel M., Sodroski J. G., Haseltine W. A. Effects of long terminal repeat mutations on human immunodeficiency virus type 1 replication. J Virol. 1989 Sep;63(9):4115–4119. doi: 10.1128/jvi.63.9.4115-4119.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Lau R., Patterson S., Pinching A. J., Knight S. C. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990 Sep;71(1):38–45. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Patterson S., Knight S. C. Suppression of immune responses by dendritic cells infected with HIV. Immunology. 1989 Jul;67(3):285–289. [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- Montagnier L., Gruest J., Chamaret S., Dauguet C., Axler C., Guétard D., Nugeyre M. T., Barré-Sinoussi F., Chermann J. C., Brunet J. B. Adaptation of lymphadenopathy associated virus (LAV) to replication in EBV-transformed B lymphoblastoid cell lines. Science. 1984 Jul 6;225(4657):63–66. doi: 10.1126/science.6328661. [DOI] [PubMed] [Google Scholar]
- Patterson S., Knight S. C. Susceptibility of human peripheral blood dendritic cells to infection by human immunodeficiency virus. J Gen Virol. 1987 Apr;68(Pt 4):1177–1181. doi: 10.1099/0022-1317-68-4-1177. [DOI] [PubMed] [Google Scholar]
- Schuler G., Steinman R. M. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985 Mar 1;161(3):526–546. doi: 10.1084/jem.161.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger E. F., Cohen E. A., Lu Y. C., Sodroski J. G., Haseltine W. A. Functional role of human immunodeficiency virus type 1 vpu. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5163–5167. doi: 10.1073/pnas.86.13.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger E. F., Godin B., Sodroski J. G., Haseltine W. A. Construction and use of a replication-competent human immunodeficiency virus (HIV-1) that expresses the chloramphenicol acetyltransferase enzyme. Proc Natl Acad Sci U S A. 1989 May;86(10):3857–3861. doi: 10.1073/pnas.86.10.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger E., Sodroski J. G., Rosen C. A., Haseltine W. A. Effects of mutations within the 3' orf open reading frame region of human T-cell lymphotropic virus type III (HTLV-III/LAV) on replication and cytopathogenicity. J Virol. 1986 Nov;60(2):754–760. doi: 10.1128/jvi.60.2.754-760.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschachler E., Groh V., Popovic M., Mann D. L., Konrad K., Safai B., Eron L., diMarzo Veronese F., Wolff K., Stingl G. Epidermal Langerhans cells--a target for HTLV-III/LAV infection. J Invest Dermatol. 1987 Feb;88(2):233–237. doi: 10.1111/1523-1747.ep12525402. [DOI] [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh L. J., Parry A., Scholes A., Seymour G. J. Modulation of CD4 antigen expression on human gingival Langerhans cells by gamma interferon. Clin Exp Immunol. 1987 Nov;70(2):379–385. [PMC free article] [PubMed] [Google Scholar]
- Witmer M. D., Steinman R. M. The anatomy of peripheral lymphoid organs with emphasis on accessory cells: light-microscopic immunocytochemical studies of mouse spleen, lymph node, and Peyer's patch. Am J Anat. 1984 Jul;170(3):465–481. doi: 10.1002/aja.1001700318. [DOI] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988 Jan;111(1):167–182. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]
- Zack J. A., Arrigo S. J., Weitsman S. R., Go A. S., Haislip A., Chen I. S. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990 Apr 20;61(2):213–222. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]