Abstract
Sleep influences the cardiovascular, endocrine, and thermoregulatory systems. Each of these systems may be affected by the activity of hypocretin (orexin)-producing neurons, which are involved in the etiology of narcolepsy. We examined sleep in male rats, either hypocretin neuron-ablated orexin/ataxin-3 transgenic (narcoleptic) rats or their wild-type littermates. We simultaneously monitored electroencephalographic and electromyographic activity, core body temperature, tail temperature, blood pressure, electrocardiographic activity, and locomotion. We analyzed the daily patterns of these variables, parsing sleep and circadian components and changes between states of sleep. We also analyzed the baroreceptor reflex. Our results show that while core temperature and heart rate are affected by both sleep and time of day, blood pressure is mostly affected by sleep. As expected, we found that both blood pressure and heart rate were acutely affected by sleep state transitions in both genotypes. Interestingly, hypocretin neuron-ablated rats have significantly lower systolic and diastolic blood pressure during all sleep stages (non-rapid eye movement, rapid eye movement) and while awake (quiet, active). Thus, while hypocretins are critical for the normal temporal structure of sleep and wakefulness, they also appear to be important in regulating baseline blood pressure and possibly in modulating the effects of sleep on blood pressure.
Keywords: circadian, blood pressure, orexins, body temperature
sleep is involved in the regulation of peripheral organ function, such as endocrine and cardiovascular function, as specific changes in peripheral organ systems occur during both rapid eye movement (REM) and non-REM (NREM) sleep. One well described sleep-associated cardiovascular change is the decrease in blood pressure that occurs during sleep in a variety of species, a phenomenon generally referred to as “dipping” (2, 9, 50, 53). The sleep-related decrease in blood pressure occurs in addition to the blood pressure changes induced by the circadian clock and changes in body posture that typically accompany sleeping behaviors (10, 53). A loss of the normal reduction in blood pressure during sleep (“non-dipping” status, defined as a <10% drop in blood pressure during sleep) is a predictive measure for increased cardiovascular morbidity (40). Dipping is thought to be influenced by a change in the activity of the autonomic nervous system as nondippers have an increase in sympathetic tone at sleep onset as opposed to the normal reduction in sympathetic and increase in parasympathetic tone that typically occurs during sleep (24, 60).
Sleep state transitions are also accompanied by changes in the cardiovascular system. For example, when going from NREM sleep to REM sleep, blood pressure and heart rate increase and become less stable (12, 21, 22, 28, 29, 37, 45–48). These cardiovascular changes that occur during NREM to REM transitions may, in part, underlie the observation of increased prevalence of heart attacks in the early morning hours (36), a time at which transitions to REM sleep are more frequent.
Another physiological function under the control of the autonomic nervous system that is affected by sleep stages is thermoregulation. The relationship between thermoregulation and sleep is reciprocal. Core temperature is lower during sleep, or when sleep propensity is high. This is accompanied by a decrease in metabolic heat production (4, 5) and an increase in peripheral vasodilation (42, 54). The increase in heat loss that precedes sleep onset likely contributes to the induction of sleep (27) as this increase in peripheral temperature likely causes a drop in core and brain temperature that activates sleep-promoting temperature-sensitive hypothalamic neurons (34). These changes are likely induced by both circadian- and sleep-related mechanisms (13, 18).
Hypocretins are a pair of excitatory neuropeptides (hypocretin-1 and hypocretin-2, also referred to as orexin A and orexin B, respectively), exclusively expressed by a group of neurons in the lateral and perifornical hypothalamus (11). The loss or dysfunction of hypocretin neurons results in the sleep disorder narcolepsy (30), a significant sleep disorder characterized by cataplexy, excessive daytime sleepiness, and sleep fragmentation. Hypocretin neurons project profusely throughout the hypothalamus and into various brain areas, e.g., the median preoptic area, the neocortex, the lateral hypothalamus, the tuberomammillary nucleus, the periaqueductal gray, the ventral tegmental area, and the locus ceruleus, which are implicated in the control of sleep and wakefulness (57), cardiovascular function (43, 49), energy expenditure (32, 59), and thermoregulation (3). The hypocretin system connects these diverse systems that have limited direct anatomic connections with one another.
Given the connections between the cardiovascular and the thermoregulatory systems with sleep behavior, and the possible role of hypocretins in integrating these various systems, the aim of our study was to better characterize the interrelationship between sleep, the cardiovascular system, and thermoregulation. We also hypothesized that the dysfunction of hypocretin neurons and the low hypocretin secreted levels would affect and change these interrelations. Role of hypocretin in such would be revealed.
METHODS
Sprague-Dawley orexin/ataxin-3 transgenic rats, expressing a truncated Machado-Joseph disease gene product (ataxin-3) with an expanded polyglutamine stretch specifically in hypocretin neurons (17), were cross-bred with wild-type rats to create littermates that were either hemizygous (one copy of orexin/ataxin-3) or wild type. PCR with specific primers confirmed the presence of the ataxin transgene as appropriate. In orexin/ataxin-3 rats, there is a gradual and selective loss of hypocretin neurons as a result of a hypocretin promoter-driven accumulation of the cytotoxic gene product poly-Q-ataxin, such that at 5 wk, cerebrospinal fluid (CSF) hypocretin-1 concentrations are 70% of wild type and at 8 wk, concentrations are 20% of wild type as was reported in the same line of animals from our laboratory (58). This is phenotypically similar to human narcolepsy, which is characterized by a near total loss of hypocretin-expressing neurons (39, 52).
Male wild-type and transgenic littermates were housed at 23 ± 1°C under a 12:12-h light:dark cycle. Water and food were given ad libitum. At the age of 6–8 wk (220–300 g), a transmitter (C50-PXT, Data Sciences, St. Paul, MN) capable of measuring blood pressure, electrocardiogram (ECG), core temperature, and locomotor activity was implanted in the abdominal cavity of each rat. During the surgical implantation, rats were anaesthetized with isoflurane (2%, in medical-grade oxygen). The descending aorta was cannulated for monitoring of blood pressure. Two biopotential leads were connected to the thorax for ECG recordings. Four stainless steel screw electrodes were positioned in the skull to sit on the surface of the cortex and were used to record the electroencephalogram (EEG). Two miniature screw electrodes were inserted 2 mm on either side of the sagittal sinus and 3 mm anterior to bregma (frontal cortex). The other two screw electrodes were located 3 mm on either side of the sagittal sinus and 6 mm behind bregma (occipital cortex). To record muscle activity (electromyogram, EMG), two flexible multistranded wires were inserted in the nuchal muscles. The six electrodes (4 EEG, 2 EMG) were inserted into a plastic plug that was secured onto the skull using dental cement. After at least 1 wk of recovery, the rats were placed in the experimental cage. The electrodes were connected to a cable attached to a commutator allowing free movement. Another transmitter, capable of measuring skin temperature and locomotor activity (e-mitter, Minimitter, Bend, OR) and housed in a Plexiglas device, was placed outside the rat's tail, 2 cm from the tail base. The device was custom made, put on the rat tail, ∼1 cm from tail base, according to previous publication (16). It enabled the measurement of tail skin temperature, noninvasively, and isolated from the environment (16). Rats were allowed to become accustomed with the environment and the equipment for at least 1 wk before the beginning of the recordings.
Signals from EEG and EMG were amplified (Grass, West Warwick, RI) and simultaneously recorded onto a computer (SleepSign, Kissei, Japan). Signals of core temperature, blood pressure, and ECG were transferred via receivers (RPC-1, Data Sciences) on the side of the cage to a computer using Dataquest A.R.T 4.1 software (Data Sciences). Data from tail temperature and locomotor activity were collected from separate receivers (ER, Minimitter) placed underneath the cage and transferred to a computer using VitalView software (Minimitter).
The data from EEG recordings were filtered at 50 Hz (low pass filter) and 0.5 Hz (high pass filter) using a Grass electroencephalograph and continuously sampled at 512 Hz. EEG and EMG recordings were manually scored on a computer (SleepSign, Kissei, Japan) in 5-s epochs for wake, NREM sleep, and REM sleep. This time epoch was chosen to increase the accuracy of the sleep scoring. Wake (W) was identified by the presence of desynchronized EEG and high EMG activity. NREM sleep consisted of high-amplitude slow waves together with an EMG tone low relative to W. REM sleep was identified by the presence of regular theta activity coupled with low EMG activity relative to NREM sleep.
The entire study was approved and conducted in accordance with the guidelines of Stanford's Administrative Panel on Laboratory Animal Care.
Analyses.
Two-way ANOVA were used to compare the effect of different genotype and time on both composite (e.g., number of wake periods) and continuous (e.g., 24-h core temperature) variables, followed by post hoc Student's t-tests with Bonferroni correction as appropriate. Significance was set when the P value was less than α = 0.05. Other statistical tests are specified in the text.
To examine the changes that occurred at times of transitions between states of consciousness (e.g., between W and NREM), each transition (± at most 60 s of a continuous state) that occurred in each of six variables (core temperature, tail temperature, systolic blood pressure, diastolic blood pressure, pulse pressure, heart rate) was parsed. All available data were included in this analysis. A one-way ANOVA was used to determine if there was a significant effect of time on a given variable in a given transition (individual test α = 0.01, overall test α = 0.05, as adjusted for 5 comparisons per variable). In variables in which there was a significant effect of time at the transition, curve fitting was used to determine amplitude parameters. To account for time of day changes in baseline, data were transformed before fitting by determining change scores from baseline; baseline was calculated as the average (≤60 s) of the leading state. As appropriate, data were fit either with a Gaussian
or a Boltzmann
equation. In the Gaussian, y0 is the baseline, xc is the x-coordinate of the midpoint of the peak, A is the amplitude of the peak, and w is the width of the peak at half-amplitude. In the Boltzmann, A1 is the starting asymptote, A2 is the final asymptote, x0 is the x-coordinate of the midpoint of the linear portion of the curve, and dx is a power term for the steepness of the linear portion of the curve. The amplitude of change in the Boltzmann was calculated as A2 − A1. For visualization, data from a single physiological variable were z-score transformed within transitions and then averaged within animal and then between rats of a single genotype. Curve-fitting procedures used the Levenberg-Marquardt algorithm to minimize error (OriginPro8, OriginLab, Northampton MA).
Analyses of the baroreceptor-heart rate reflex sensitivity were done using the sequence technique (6, 51). (Spontaneous beat-to-beat blood pressure fluctuations and their corresponding heart rate changes enable calculation of a reliable index of baroreceptor-heart rate reflex sensitivity in rat). Briefly, the HemoLab Software (ver. 9.0, available at http://www.haraldstauss.com/HemoLab/HemoLab.html) was used to identify sequences of three or more heart beats where systolic blood pressure increased and interbeat interval lengthened (so-called “up sequences”) or where systolic blood pressure decreased and interbeat interval shortened (so-called “down sequences”). Baroreceptor-heart rate reflex sensitivity was defined as the slope of the relationship between changes in systolic blood pressure and changes in interbeat interval. Baroreflex engagement was determined by the number of sequences relative to the number of heart beats. All bouts of specific W or sleep states that lasted at least 120 s were selected for baroreflex analysis.
RESULTS
General sleep pattern.
Manually scored sleep variables are presented in Table 1 for the entire 24 h and separated into day and night. As expected, there were significant day/night differences in the duration, percent time, and bout length of wakefulness in both the wild type and transgenic rats. There was also a corresponding day/night difference in the amount and percent of NREM sleep in both genotypes. Additionally, the transgenic rats displayed fewer and shorter NREM bouts at night compared with the daytime. REM stages were significantly longer at night in the transgenic rats compared with their wild-type littermates. Another difference between genotypes was that the wild-type rats only entered REM sleep from NREM sleep, while the transgenic rats also experienced 1.92 ± 0.6 direct transitions from W to REM sleep per 24 h, so called DREMs (14, 55). These occurred equally during the day (0.92 ± 0.3) and night (1.08 ± 0.2) (P values > 0.1, Mann-Whitney).
Table 1.
Sleep pattern of wild-type and transgenic rats (n = 8) in 24 h, and separately during the day and the night
| 24 h |
Day |
Night |
||||
|---|---|---|---|---|---|---|
| WT | TG | WT | TG | WT | TG | |
| Wake | ||||||
| No. of periods | 201.33 ± 18.66 | 192.08 ± 12.21 | 96.00 ± 11.96 | 100.42 ± 15.87 | 106.33 ± 7.86 | 102.83 ± 9.82 |
| Total time, min | 558.18 ± 36.95 | 590.58 ± 37.18 | 191.79 ± 16.41 | 200.16 ± 12.04 | 366.29 ± 31.26† | 388.84 ± 31.65† |
| % | 38.87 ± 2.72 | 40.12 ± 2.60 | 26.66 ± 2.3 | 27.38 ± 1.400 | 50.91 ± 4.35† | 52.86 ± 4.45† |
| Mean time per stage, s | 182.44 ± 30.53 | 190.71 ± 23.93 | 133.66 ± 25.59 | 132.21 ± 20.03 | 228.24 ± 36.02† | 245.5 ± 44.69† |
| NREM | ||||||
| No. of periods | 371.11 ± 24.58 | 358.83 ± 24.40 | 211.72 ± 15.24 | 199.17 ± 13.10 | 159.61 ± 10.99 | 160.00 ± 13.64† |
| Total time, min | 782.21 ± 42.61 | 777.08 ± 34.22 | 471.33 ± 20.11 | 479.59 ± 11.54 | 310.87 ± 26.65† | 293.43 ± 27.24† |
| % | 54.31 ± 2.75 | 53.76 ± 2.28 | 65.50 ± 2.50 | 66.05 ± 1.68 | 43.20 ± 3.70† | 41.48 ± 3.71† |
| Mean time per stage, s | 128.22 ± 5.71 | 132.76 ± 5.84 | 136.84 ± 7.32 | 146.51 ± 8.04 | 117.83 ± 6.07 | 113.47 ± 4.78† |
| REM | ||||||
| No. of periods | 98.00 ± 15.18 | 83.50 ± 20.04 | 56.72 ± 7.10 | 51.5 ± 14.14 | 41.83 ± 8.84 | 31.92 ± 7.21 |
| Total time, min | 98.53 ± 13.45 | 82.96 ± 13.10 | 56.28 ± 4.78 | 45.68 ± 6.53 | 42.37 ± 10.73 | 36.93 ± 7.86 |
| % | 6.82 ± 0.88 | 6.12 ± 0.95 | 7.84 ± 0.7 | 6.57 ± 0.86 | 5.89 ± 1.49 | 5.66 ± 1.18 |
| Mean time per stage, s | 61.64 ± 3.03 | 70.31 ± 8.71 | 62.06 ± 4.17 | 63.36 ± 9.44 | 59.85 ± 4.87 | 79.36 ± 6.32* |
Data are presented as means ± SE. WT, wild type; TG, transgenic; NREM, non-rapid eye movement sleep; REM, rapid eye movement sleep. %, Time in a given sleep state (wake, NREM, REM) expressed as a percentage of total period under consideration (24 h; or 12 h for Day and for Night).
P < 0.05 vs. WT.
P < 0.05 vs. day of the same genotype.
Twenty-four hour pattern of physiological parameters.
Variation over 24 h of core and skin temperatures, systolic and diastolic blood pressure, pulse pressure, heart rate, and locomotor activity are presented in Fig. 1. In both control and transgenic rats, there was a significant effect of time of day on core body temperature, systolic and diastolic blood pressure, heart rate, and locomotor activity. Tail temperature and pulse pressure did not change significantly over the day. Although systolic and diastolic blood pressure, pulse pressure, core body and tail temperatures and locomotor activity tended to be lower in transgenic rats, compared with the wild-type rats, none of these effects were significant (0.1 > P values > 0.05).
Fig. 1.
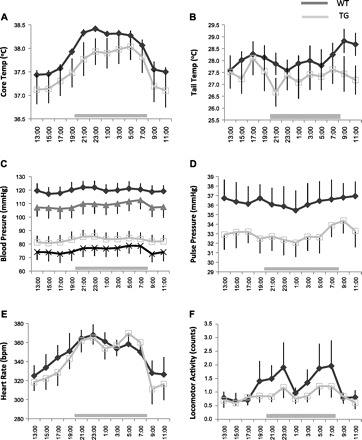
Measurements for 24 h of core body temperature (A), tail temperature (B), systolic and diastolic blood pressure (C), pulse pressure (D), heart rate (E), and locomotor activity (F). Each graph represents data collected from 8 rats in each group: wild type (WT; black) and transgenic (TG; gray). In C, systolic and diastolic blood pressure, respectively, are represented by closed diamonds and open rectangles (WT) or triangles and × (TG). Data were collected every 10 s and averaged for every 2 h within, then between rats, and are presented as means ± SE. Horizontal gray bar represents the time of darkness. Lights were on from 8 AM until 8 PM. bpm, beats/min.
Effects of sleep states on physiological parameters.
We also analyzed the temperature and cardiovascular data for specific sleep (NREM, REM) and W [quiet wake (QW), active wake (AW)] states, irrespective of time of day (Fig. 2). In the wild-type animals, core body temperature, systolic and diastolic blood pressure, and heart rate were significantly affected by the specific sleep/wake state, with core temperature, systolic and diastolic blood pressure, and heart rate all being low during both NREM and REM sleep, compared with quiet wake. All physiological parameters were similar in quiet wake and active wake. By further parsing the data according to day/night (Fig. 3), we found that core temperature was significantly (P < 0.001) higher during each state (active wake, quiet wake, NREM, REM) when that state occurred at night then when that state occurred during the daytime. Heart rate was also significantly higher (P = 0.03) during quiet wake, when that state occurred at night than when that state occurred during the daytime, but not active wake, NREM or REM, (Fig. 3). This additive affect of the time of day likely represents the combination of the circadian (daily) rhythm and the immediate effects of sleep/activity and light/dark on core body temperature and heart rate. Although systolic and diastolic blood pressure showed state-specific variation (Fig. 2), there was no additional contribution noted when the data were divided into epochs occurring during the day and night (Fig. 3). In addition to lack of a time of day variation, tail temperature and pulse pressure were also not significantly different among the different sleep and wake states.
Fig. 2.
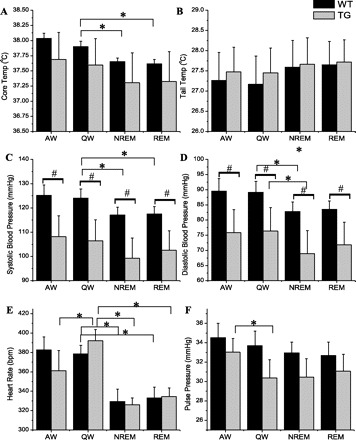
Core body temperature (A), tail temperature (B), systolic (C) and diastolic (D) blood pressure, heart rate (E), and pulse pressure (F), in each wake/sleep state, in WT (black) and TG (gray) rats. Each bar represents an average of 6–8 rats in specific wake/sleep stage: active wake (AW), quiet wake (QW), non-rapid eye movement sleep (NREM), and rapid eye movement sleep (REM). Data were collected over 48 h, averaged within, then between rats, and are presented as means ± SE. *P < 0.05 vs. QW, #P < 0.05 vs. WT in the same sleep/wake state.
Fig. 3.
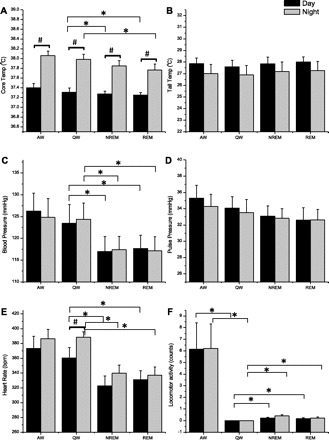
Core body temperature (A), tail temperature (B), systolic blood pressure (C), pulse pressure (D), heart rate (E), and locomotor activity (F) in each wake/sleep state, during the day (black) and during the night (gray) in WT rats. Each bar represents an average of 6–8 rats in specific wake/sleep stage: AW, QW, NREM sleep, and REM sleep. Data were collected over 48 h, averaged within, then between rats, and are presented as means ± SE. *P < 0.05 vs. QW, #P < 0.05 vs. day in the same sleep/wake state.
In the transgenic animals, core body temperature, diastolic blood pressure, heart rate, and pulse pressure were significantly affected by the specific sleep/wake state. Core body temperature, diastolic blood pressure, and heart rate were all lower in NREM compared with quiet wake. Heart rate was also lower in REM and active wake compared with quiet wake. Pulse pressure was higher in active wake compared with quiet wake. As with the wild-type rats, core temperature and heart rate were significantly (P < 0.05) higher during each state (active wake, quiet wake, NREM, REM) when that state occurred at night compared with when that state occurred during the daytime. In addition, diastolic blood pressure was significantly (P < 0.05) higher during the night time occurrences of active wake, quiet wake, and NREM, but significantly (P < 0.05) lower during the night time occurrence of REM. Systolic blood pressure during NREM and REM at night was significantly (P < 0.05) higher in NREM and lower in REM compared with that which occurred during NREM and REM during the day. Pulse pressure during active wake at night was significantly (P < 0.05) lower than during active wake during the day. Tail temperature was not significantly different among the different sleep and wake states.
In comparing the transgenic rats to their wild-type littermates, the transgenic rats had significantly lower systolic and diastolic blood pressure in all subdivided states (active wake, quiet wake, NREM, REM) (Fig. 2). There were no differences between the genotypes within the subdivided states for core body temperature, tail temperature, heart rate, or pulse pressure.
Transitions.
We next examined changes in blood pressure, heart rate, and temperature during the transitions between different wake/sleep states, starting from 60 s before until 60 s after the transition, according to EEG and EMG scoring. There were no consistent changes in tail temperature, pulse pressure, or core temperature during any of the transitions in either of the genotypes (NREM to REM, NREM to W, REM to NREM, REM to W, or W to NREM; consistency defined as no more than one animal exhibiting a significant effect of time during the transition as determined by ANOVA). There were no consistent changes in any of the three aforementioned variables, as well as systolic blood pressure, diastolic blood pressure, and heart rate, in the direct transitions from W to REM or in the transitions from REM to NREM. In the transition from NREM to W, REM to W, and W to NREM, there were significant changes in systolic blood pressure, diastolic blood pressure, and heart rate (Fig. 4). In the transition from NREM to REM, there were significant changes in systolic blood pressure and diastolic blood pressure, but not heart rate, although a clear trend in the same direction was seen (Fig. 4). Given the temporal discrimination of our data, we are unable to determine if the changes in systolic blood pressure, diastolic blood pressure, and heart rate preceded, followed, or occurred during the transition between EEG/EMG-defined states of consciousness. To determine the amplitude shift between states, we fit with either a sigmoid curve (Boltzmann) or a spike curve (Gaussian), as appropriate (Fig. 4) (see methods). Between the two genotypes, we found no difference in amplitude shifts during the transitions (Ws > 10, Ps > 0.5, Wilcoxon U-test). As such, for the following statistics, the two genotypes are combined. The transition from NREM to W was characterized by a sigmoid pattern that has a baseline plateau (NREM), a linear change, and a final plateau (W). In this transition, there was a 2.82 ± 0.683 mmHg increase in systolic blood pressure, 2.27 ± 0.536 mmHg increase in diastolic blood pressure, and 14.7 ± 3.49 beats/min increase in heart rate. The transition back from W to NREM was also characterized by a sigmoid pattern. In this transition, there was a 1.06 ± 0.649 mmHg decrease in systolic blood pressure, 1.88 ± 0.743 mmHg decrease in diastolic blood pressure, and 16.0 ± 5.38 beats/min decrease in heart rate. In the transition from NREM to REM, which was also sigmoidal, there was a 2.07 ± 1.26 mmHg increase in systolic blood pressure and 2.16 ± 1.23 mmHg increase in diastolic blood pressure. The change between REM and W was characterized by a spike of activity around the time of transition: a stable baseline followed by a sharp increase and an immediate sharp decrease back to the same stable baseline (Fig. 4). Systolic blood pressure decreased 3.85 ± 1.12 mmHg and diastolic blood pressure decreased 3.02 ± 1.08 mmHg during the spike, but heart rate increased 16.9 ± 4.98 beats/min. Thus, while the changes in blood pressure and heart rate both increased or both decreased during shifts between wake and NREM, the opposite occurred during shifts between REM and W, with blood pressure decreasing and heart rate increasing.
Fig. 4.
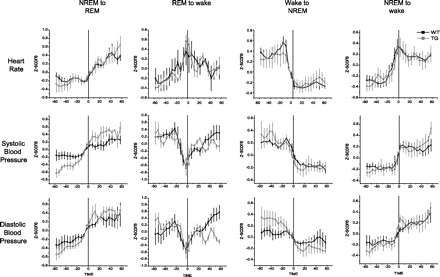
Patterns of systolic and diastolic blood pressure and heart rate occurring during transitions between sleep/wake states are presented. Data were z-score transformed within rats and then averaged between rats (6–8 rats/group) and are shown as means ± SE for the WT (black) and TG (gray) rats. Data from 60 s before until 60 s after transition point are shown.
Baroreceptor-heart rate reflex.
Baroreceptor-heart rate reflex (BRR) function was assessed by the sensitivity (or gain) and the engagement (relative number of sequences) of the reflex in wild-type and transgenic rats during each specific sleep/wake state. There was no difference in the gain or number of BRR sequences between the genotypes or among quiet wake, active wake, or NREM (Table 2). There was also no difference in the number of sequences in REM sleep compared with the other states or between genotypes (Table 2). In wild-type rats, there was, however, a significant increase in the BRR gain in REM sleep compared with all other states (P < 0.05) or to the REM in the transgenic rats (P < 0.05). We then subdivided BRR into up (increase in blood pressure leads to a decrease in heart rate) and down (decrease in blood pressure leads to an increase in heart rate) sequences. In the down sequences, a change in the gain of BRR was again detected only in REM sleep (REM 2.8 ± 0.2, P < 0.05; active wake 1.9 ± 0.1, quiet wake 1.5 ± 0.1, NREM 1.7 ± 0.1, Ps > 0.05). In the up sequences, there were no differences in gain between the different states of sleep and W (REM 2.0 ± 0.2, P < 0.05; active wake 1.7 ± 0.1, quiet wake 1.9 ± 0.5, NREM 1.9 ± 0.1, Ps > 0.05). The transgenic rats have the same gain value in up and down sequences in REM sleep, as well as in other states.
Table 2.
Baroreceptor-heart rate reflex gain and number of sequences by sleep state (active wake, quite wake, NREM sleep, and REM sleep) and genotype (wild type and transgenic)
| BRR Gain, ms/mmHg |
No. of BRR Sequences |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT |
TG |
WT |
TG |
|||||||||
| Total | Up seq | Down seq | Total | Up seq | Down seq | Total | Up seq | Down seq | Total | Up seq | Down seq | |
| AW | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 1.9 ± 0.1 | 1.7 ± 0.1 | 2.0 ± 0.2 | 0.9 ± 0.3 | 0.4 ± 0.1 | 0.5 ± 0.1 | 0.8 ± 0.3 | 0.3 ± 0.1 | 0.5 ± 0.2 |
| QW | 1.8 ± 0.1 | 1.9 ± 0.5 | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.6 ± 0.2 | 0.5 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.9 ± 0.2 | 0.4 ± 0.1 | 0.5 ± 0.2 |
| NREM | 1.7 ± 0.2 | 1.9 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.2 | 1.8 ± 0.1 | 1.6 ± 0.1 | 1.3 ± 0.4 | 0.8 ± 0.3 | 0.5 ± 0.1 | 0.9 ± 0.3 | 0.4 ± 0.1 | 0.5 ± 0.1 |
| REM | 2.5 ± 0.1# | 2.0 ± 0.2 | 2.8 ± 0.2# | 2.0 ± 0.1* | 1.9 ± 0.2 | 2.0 ± 0.1* | 1.1 ± 0.4 | 0.4 ± 0.2 | 0.7 ± 0.3 | 1.7 ± 0.3 | 0.7 ± 0.1† | 1.0 ± 0.3† |
Data are presented as means ± SE. AW, active wake; QW, quiet wake; BRR, baroreceptor-heart rate reflex. Up seq and Down seq refer, respectively, to sequences of 3 or more heart beats where systolic blood pressure increased and interbeat interval lengthened (so-called “up sequences”) or where systolic blood pressure decreased and interbeat interval shortened (so-called “down sequences”).
P < 0.05 vs. WT.
P < 0.05 vs. all other states of consciousness.
DISCUSSION
By simultaneously monitoring multiple physiological parameters in conscious rats, we attempted to tease apart the direct and indirect effects of sleep, as well as find the manner in which the hypocretin peptides might influence sleep-associated changes in cardiovascular regulation.
We hypothesized that hypocretin is involved in the connections between sleep, the cardiovascular system, and the thermoregulatory system. Indeed we found that the loss of hypocretin led to modifications in blood pressure in different sleep/wake states.
As expected, we observed a diurnal variation in core temperature, systolic and diastolic blood pressure, heart rate, and locomotor activity in both genotypes. Neither tail temperature nor pulse pressure covaried with the 24-h cycle. Surprisingly, there were relatively few differences in the sleep parameters of the two genotypes, despite the partial loss of hypocretin neurons in the transgenic rats (58). Previous studies of these rats have used either 20-s (7) or 12-s (59) intervals in which sleep and wake were scored; the present study used 5-s intervals, since we thought that the shorter the bout is, the more accurate the scoring is. Beuckmann et al. (7) report significant changes in several parameters of REM sleep and an increased fragmentation of sleep and W; Zhang et al. (59) reported fewer alterations of REM and no change in fragmentation. In our study, besides the presence of direct transitions from wake to REM, a pathognomonic hallmark of hypocretin neuron loss that was also observed in the two previous studies, we identified only a single difference between the genotypes (mean time per REM sleep episode during the night). It is likely that the dichotomy in the data reported in our manuscript and the previous two publications is due to the variation in the time window used to score sleep. A longer scoring window would subsume short episodes of REM into other episodes and, thereby, give the impression of less REM sleep. Previous studies (59) show that there is a significant shift to shorter sleep episodes in the orexin/ataxin-3 transgenic rats compared with their wild-type littermates; we observe a similar shift to an increase in the number of short REM sleep episodes in the transgenic rats (data not shown). We believe that the fact that we scored shorter bouts made our results more accurate. These observations are also consistent with the idea that the loss of hypocretin neurons leads to a change in the length of discrete sleep episodes, not a change in the amount of sleep or W. It, furthermore, agrees with the hypothesis that blocking hypocretin signaling only increases REM during the active (night) portion of the diurnal cycle, as was found using a microinjection of short interfering RNAs (siRNA) targeting preproorexin mRNA into the rat perifornical hypothalamus (10). In this study, as in ours, wakefulness and NREM sleep were unaffected.
Our findings over the 24 h confirm two independent regulatory systems of the cardiovascular and thermoregulatory systems: a diurnal effect and a direct sleep/wake effect. We find additive effects between sleep/wake and a diurnal pattern in core temperature and heart rate data. In contrast, our data have showed only a direct effect of sleep/wake, without any independent diurnal contribution, in systolic and diastolic blood pressure. This effect should be further studied using other direct methods to confirm the implication that blood pressure is controlled by sleep/wake state and not the circadian clock. The additive effects of time of day and sleep/wake on core temperature is consistent with results derived from human forced desynchrony studies that show independent sleep/wake and circadian influences on this parameter (8). The effects of sleep on heart rate have been extensively described in the human literature (38), although a circadian contribution has been less well studied (19). The influence of behavior, including sleep/wake, on blood pressure regulation is well established but the contribution of a circadian component is less so (41). It has been suggested that the loss of rhythmic variation of blood pressure regulation in suprachiasmatic nucleus (SCN, the location of the circadian pacemaker in mammals)-lesioned rats is evidence for an endogenous component to blood pressure regulation. However, the ablation of the SCN is also accompanied by a loss of rhythmic behavior so if the behavior is causing the blood pressure variation, it is not unexpected that there is a loss of daily variation in blood pressure (20, 55). Our data, however, would argue against a significant circadian component to blood pressure modulation. Using a forced desynchrony technique in humans would be one way to disentangle the contribution of behavior and endogenous circadian regulation of blood pressure and this remains to be done in humans.
The loss of hypocretin neurons did not appear to affect the diurnal or direct sleep/wake regulation of core body or tail temperature, heart rate, or pulse pressure. It did, however, have a significant impact on blood pressure regulation such that the loss of hypocretin neurons in the transgenic rats was associated with a lower systolic and diastolic blood pressure in all four of our subdivided states (active and quiet wake, NREM and REM sleep). In addition to the effect of sleep, the division to active and quiet wake was done to abolish the effect of locomotor activity on the examined parameter. As locomotor activity is generally lower in transgenic rats, this could have affect blood pressure, as well as other parameters. The lower blood pressure found here is in agreement with the observation that central application of hypocretin-1 or -2 can augment sympathetic outflow and systemic arterial pressure in anesthetized rats (33, 49). Furthermore, preprohypocretin knockout mice have a lower baseline arterial pressure (23). It appears, therefore, that hypocretin neurons are necessary in rodents for the normal expression of blood pressure across both sleep and W and that the lack of such input results in lower systemic blood pressure. The effect of hypocretin on the cardiovascular system can derive from several possible pathways. It may be related to its effect on the sympathetic nerve system, as was previously reported (49). It may also evolve from hypocretin effect on the hypothalamic-pituitary-adrenal axis that was also previously reported, either direct effect on the adrenal to produce catecholamines, or central effect in the hypothalamus. In accordance, the change in hypocretin-1 release in narcoleptic patients was hypothesized to affect their sympathetic activity and blood pressure (44). A comprehensive study on this subject was not published so far, presumably since most narcolepsy patients are treated with sympathomimetic drugs.
Transitions between specific sleep/wake states are important regulatory phases. They may influence on the occurrence probability of the upcoming state or to be an external expression of underlying mechanisms or modifications that leads to the change of sleep state. If changes are detected in specific transition between sleep/wake states, it may be related to changes in this state prevalence during the day and in total sleep behavior. Transitions between sleep/wake states were accompanied by reproducible changes in blood pressure and heart rate in both genotypes. In transitions from NREM sleep to W, heart rate increased as did systolic and diastolic blood pressure (Fig. 4). In transitions from W to NREM sleep, there was a decrease in both heart rate and systolic and diastolic blood pressure (Fig. 4). These changes were likely secondary to the decreased sympathetic and increased parasympathetic tones present in NREM sleep, compared with W. This would also explain the increase in heart rate and systolic and diastolic blood pressure in the transition from NREM to REM, as there is decreased sympathetic and increased parasympathetic tone in NREM sleep compared with REM sleep. During the transition from REM sleep to W, however, there is an upward spike in heart rate and a downward spike in both systolic and diastolic blood pressure (Fig. 4). The drop in blood pressure accompanied by an increase in heart rate is likely the consequence of the baroreceptor reflex. The gain of the down sequences (the amount heart rate increases in response to a drop in blood pressure) is elevated during REM sleep compared with both wake and NREM, while the number of times the reflex is invoked is similar between quiet wake, active wake, NREM sleep, and REM sleep (Table 2).
In humans, the diurnal rhythm of distal skin temperature precedes that of core body temperature by about 2 h. Kräuchi et al. (25) have hypothesized that, in humans, increases in peripheral heat loss lead to changes in core and brain temperature that are causal or permissive for sleep onset mechanisms. Previous results on thermoregulation in rats suggested that rats are able to actively regulate core temperature by changing vascular resistance in the tail artery (increased resistance leading to less heat loss, and vice versa) (15, 56). Previous studies also showed the effect of sleep on tail temperature, as depending on environmental temperature (1). Our results, however, were different from what has previously been reported. Tail temperature in the rats did not demonstrate a significant diurnal rhythmic pattern, although the pattern looks similar to what was previously reported in humans by Kräuchi et al. (26) and using a greater number of animals it might have been significant. Our results, however, indicate that heat transfer through the tail is not a major factor in the diurnal regulation of basal (i.e., not challenged by exercise or changes in environmental temperature) core body temperature in rats. Our results also indicate that heat transfer through the tail is not a consequential factor in the sleep state-related changed in core temperature. Zhang et al. (59) showed in mice that energy expenditure and respiratory quotient are significantly changed with time of day and correlate with the diurnal pattern of core body temperature. Given the lack of diurnal variation in tail temperature and the presence of a diurnal variation in core temperature, the most parsimonious explanation would be that, in rats, changes in heat production have a dominant role in the regulation of the observed circadian and diurnal rhythms of core body temperature.
As was reported from our lab, using the same line of rats, the orexin/ataxin-3 transgenic rats lose their hypocretin-producing neurons with age such that at 8 wk, CSF hypocretin-1 concentrations are 20% of wild type (58). While reduced, these hypocretin neurons can still be activated in response to various stimuli, e.g., sleep deprivation (58). While this model displays an incomplete loss of hypocretin-containing neurons, it may still be a valid model of human narcolepsy. Human narcolepsy is characterized by lumbar CSF hypocretin-1 concentrations <110 pg/ml (35) or <30% of normal levels (31). Many (∼25%) individuals with narcolepsy in fact have low, but detectable, amounts of lumbar CSF hypocretin-1 (35). Thus, while not modeling a total absence of hypocretin neurons, we believe that the orexin/ataxin-3 rats are an adequate model of human narcolepsy.
In conclusion, by simultaneously recording multiple physiological signals, we were able to examine the interrelationship of different systems that are all affected by or affect sleep. We demonstrate that hypocretins are likely involved in the enhancement of blood pressure and regulated autonomic control. We also show that there is a strong sleep/wake modulation of blood pressure and a relative absence of a circadian component (i.e., the diurnal rhythm in blood pressure in rats is likely caused by the diurnal distribution of sleep and wake and not an underlying, direct circadian modulation of blood pressure). Our data also implicate the modulation of metabolic activity, rather than heat loss through tail anastamoses, as the mechanism responsible for the daily variation in core body temperature in rats.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
REFERENCES
- 1. Alföldi P, Rubicsek G, Cserni G, Obál F., Jr Brain and core temperatures and peripheral vasomotion during sleep and wakefulness at various ambient temperatures in the rat. Pflügers Arch 417: 336–341, 1990. [DOI] [PubMed] [Google Scholar]
- 2. Antic V, Van Vliet BN, Montani JP. Loss of nocturnal dipping of blood pressure and heart rate in obesity-induced hypertension in rabbits. Auton Neurosci 90: 152–157, 2001. [DOI] [PubMed] [Google Scholar]
- 3. Balasko M, Szelenyi Z, Szekely M. Central thermoregulatory effects of neuropeptide Y and orexin A in rats. Acta Physiol Hung 86: 219–222, 1999. [PubMed] [Google Scholar]
- 4. Berger RJ, Palca JW, Walker JM, Phillips NH. Correlations between body temperatures, metabolic rate and slow wave sleep in humans. Neurosci Lett 86: 230–234, 1988. [DOI] [PubMed] [Google Scholar]
- 5. Berger RJ, Phillips NH. Comparative aspects of energy metabolism, body temperature and sleep. Acta Physiol Scand Suppl 574: 21–27, 1988. [PubMed] [Google Scholar]
- 6. Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens 3, Suppl 3: S79–S81, 1985. [PubMed] [Google Scholar]
- 7. Beuckmann CT, Sinton CM, Williams SC, Richardson JA, Hammer RE, Sakurai T, Yanagisawa M. Expression of a poly-glutamine-ataxin-3 transgene in orexin neurons induces narcolepsy-cataplexy in the rat. J Neurosci 24: 4469–4477, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown EN, Choe Y, Luithardt H, Czeisler CA. A statistical model of the human core-temperature circadian rhythm. Am J Physiol Endocrinol Metab 279: E669–E683, 2000. [DOI] [PubMed] [Google Scholar]
- 9. Charloux A, Piquard F, Geny B, Ehrhart J, Brandenberger G. Circulating endothelin parallels arterial blood pressure during sleep in healthy subjects. Regul Pept 119: 133–138, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Thakkar MM, Winston S, Bolortuya Y, Basheer R, McCarley RW. REM sleep changes in rats induced by siRNA-mediated orexin knockdown. Eur J Neurosci 24: 2039–2048, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Lecea L, Sutcliffe JG. The hypocretins/orexins: novel hypothalamic neuropeptides involved in different physiological systems. Cell Mol Life Sci 56: 473–480, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. del Bo A, Ledoux JE, Tucker LW, Harshkfield GA, Reis DJ. Arterial pressure and heart rate changes during natural sleep in rat. Physiol Behav 28: 425–429, 1982. [DOI] [PubMed] [Google Scholar]
- 13. Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett 166: 63–68, 1994. [DOI] [PubMed] [Google Scholar]
- 14. Fujiki N, Cheng T, Yoshino F, Nishino S. Specificity of direct transition from wake to REM sleep in orexin/ataxin-3 transgenic narcoleptic mice. Exp Neurol 217: 46–54, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon CJ. Thermal biology of the laboratory rat. Physiol Behav 47: 963–991, 1990. [DOI] [PubMed] [Google Scholar]
- 16. Gordon CJ, Puckett E, Padnos B. Rat tail skin temperature monitored noninvasively by radiotelemetry: characterization by examination of vasomotor responses to thermomodulatory agents. J Pharmacol Toxicol Methods 47: 107–114, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30: 345–354, 2001. [DOI] [PubMed] [Google Scholar]
- 18. Hiddinga AE, Beersma DG, Van den Hoofdakker RH. Endogenous and exogenous components in the circadian variation of core body temperature in humans. J Sleep Res 6: 156–163, 1997. [DOI] [PubMed] [Google Scholar]
- 19. Hilton MF, Umali MU, Czeisler CA, Wyatt JK, Shea SA. Endogenous circadian control of the human autonomic nervous system. Comput Cardiol 27: 197–200, 2000. [PubMed] [Google Scholar]
- 20. Janssen BJ, Tyssen CM, Duindam H, Rietveld WJ. Suprachiasmatic lesions eliminate 24-h blood pressure variability in rats. Physiol Behav 55: 307–311, 1994. [DOI] [PubMed] [Google Scholar]
- 21. Kanamori N, Sakai K, Sei H, Bouvard A, Salvert D, Vanni-Mercier G, Jouvet M. Effect of decerebration on blood pressure during paradoxical sleep in cats. Brain Res Bull 37: 545–549, 1995. [DOI] [PubMed] [Google Scholar]
- 22. Kanamori N, Sakai K, Sei H, Salvert D, Vanni-Mercier G, Yamamoto M, Jouvet M. Power spectral analysis of blood pressure fluctuations during sleep in normal and decerebrate cats. Arch Ital Biol 132: 105–115, 1994. [PubMed] [Google Scholar]
- 23. Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol 285: R581–R593, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Kodama Y, Iwase S, Mano T, Cui J, Kitazawa H, Okada H, Takeuchi S, Sobue G. Attenuation of regional differentiation of sympathetic nerve activity during sleep in humans. J Auton Nerv Syst 74: 126–133, 1998. [DOI] [PubMed] [Google Scholar]
- 25. Kräuchi K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol Behav 90: 236–245, 2007. [DOI] [PubMed] [Google Scholar]
- 26. Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol Regul Integr Comp Physiol 267: R819–R829, 1994. [DOI] [PubMed] [Google Scholar]
- 27. Kräuchi K, Cajochen C, Werth E, Wirz-Justice A. Warm feet promote the rapid onset of sleep. Nature 401: 36–37, 1999. [DOI] [PubMed] [Google Scholar]
- 28. Lacombe J, Nosjean A, Meunier JM, Laguzzi R. Computer analysis of cardiovascular changes during sleep-wake cycle in Sprague-Dawley rats. Am J Physiol Heart Circ Physiol 254: H217–H222, 1988. [DOI] [PubMed] [Google Scholar]
- 29. Lenzi P, Cianci T, Guidalotti PL, Leonardi GS, Franzini C. Brain circulation during sleep and its relation to extracerebral hemodynamics. Brain Res 415: 14–20, 1987. [DOI] [PubMed] [Google Scholar]
- 30. Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98: 365–376, 1999. [DOI] [PubMed] [Google Scholar]
- 31. Lin L, Nishino S, Zeitzer JM, Bassetti C, Billiard M, Broughton R, Dauvilliers Y, Guilleminault C, Honda Y, Lammers GJ, Overeem S, Pollmächer T, Mayer G, Montplaisir J, Silber M, Thorpy M, Mignot E. Guidelines for the appropriate use of CSF measurements to diagnose narcolepsy. In: Narcolepsy and Hypersomnia, edited by Bassetti C, Billiard M, Mignot E. New York: Informa Healthcare, 2006, p. 663–670. [Google Scholar]
- 32. Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochem Biophys Res Commun 253: 241–245, 1998. [DOI] [PubMed] [Google Scholar]
- 33. Matsumura K, Tsuchihashi T, Abe I. Central orexin-A augments sympathoadrenal outflow in conscious rabbits. Hypertension 37: 1382–1387, 2001. [DOI] [PubMed] [Google Scholar]
- 34. McGinty D, Gong H, Suntsova N, Alam MN, Methippara M, Guzman-Marin R, Szymusiak R. Sleep-promoting functions of the hypothalamic median preoptic nucleus: inhibition of arousal systems. Arch Ital Biol 142: 501–509, 2004. [PubMed] [Google Scholar]
- 35. Mignot E, Lammers GJ, Ripley B, Okun M, Nevsimalova S, Overeem S, Vankova J, Black J, Harsh J, Bassetti C, Schrader H, Nishino S. The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 59: 1553–1562, 2002. [DOI] [PubMed] [Google Scholar]
- 36. Mitler MM, Carskadon MA, Czeisler CA, Dement WC, Dinges DF, Graeber RC. Catastrophes, sleep, and public policy: consensus report. Sleep 11: 100–109, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Obal F, Jr, Beranek L, Brandenberger G. Sleep-associated variations in plasma renin activity and blood pressure in the rat. Neurosci Lett 179: 83–86, 1994. [DOI] [PubMed] [Google Scholar]
- 38. Penzel T, Kantelhardt JW, Lo CC, Voigt K, Vogelmeier C. Dynamics of heart rate and sleep stages in normals and patients with sleep apnea. Neuropsychopharmacology 28, Suppl 1: S48–S53, 2003. [DOI] [PubMed] [Google Scholar]
- 39. Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 6: 991–997, 2000. [DOI] [PubMed] [Google Scholar]
- 40. Pickering TG, Kario K. Nocturnal non-dipping: what does it augur? Curr Opin Nephrol Hypertens 10: 611–616, 2001. [DOI] [PubMed] [Google Scholar]
- 41. Portaluppi F, Waterhouse J, Minors D. The rhythms of blood pressure in humans. Exogenous and endogenous components and implications for diagnosis and treatment. Ann NY Acad Sci 783: 1–9, 1996. [DOI] [PubMed] [Google Scholar]
- 42. Raymann RJ, Swaab DF, Van Someren EJ. Skin temperature and sleep-onset latency: changes with age and insomnia. Physiol Behav 90: 257–266, 2007. [DOI] [PubMed] [Google Scholar]
- 43. Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res 831: 248–253, 1999. [DOI] [PubMed] [Google Scholar]
- 44. Scrima L, Garlick I, Victor Y, Miller BR, Wang L. Narcolepsy patients' blood pressure. Sleep Res 26: 502, 1997. [Google Scholar]
- 45. Sei H, Morita Y. Acceleration of EEG theta wave precedes the phasic surge of arterial pressure during REM sleep in the rat. Neuroreport 7: 3059–3062, 1996. [DOI] [PubMed] [Google Scholar]
- 46. Sei H, Morita Y. Effect of ambient temperature on arterial pressure variability during sleep in the rat. J Sleep Res 5: 37–41, 1996. [DOI] [PubMed] [Google Scholar]
- 47. Sei H, Morita Y, Morita H, Hosomi H. Long-term profiles of sleep-related hemodynamic changes in the postoperative chronic cat. Physiol Behav 46: 499–502, 1989. [DOI] [PubMed] [Google Scholar]
- 48. Sei H, Sakai K, Kanamori N, Salvert D, Vanni-Mercier G, Jouvet M. Long-term variations of arterial blood pressure during sleep in freely moving cats. Physiol Behav 55: 673–679, 1994. [DOI] [PubMed] [Google Scholar]
- 49. Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol Regul Integr Comp Physiol 277: R1780–R1785, 1999. [DOI] [PubMed] [Google Scholar]
- 50. Somers VK, Dyken ME, Mark AL, Abboud FM. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 328: 303–307, 1993. [DOI] [PubMed] [Google Scholar]
- 51. Stauss HM, Moffitt JA, Chapleau MW, Abboud FM, Johnson AK. Baroreceptor reflex sensitivity estimated by the sequence technique is reliable in rats. Am J Physiol Heart Circ Physiol 291: H482–H483, 2006. [DOI] [PubMed] [Google Scholar]
- 52. Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron 27: 469–474, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Van de Borne P, Nguyen H, Biston P, Linkowski P, Degaute JP. Effects of wake and sleep stages on the 24-h autonomic control of blood pressure and heart rate in recumbent men. Am J Physiol Heart Circ Physiol 266: H548–H554, 1994. [DOI] [PubMed] [Google Scholar]
- 54. Van Someren EJ. Mechanisms and functions of coupling between sleep and temperature rhythms. Prog Brain Res 153: 309–324, 2006. [DOI] [PubMed] [Google Scholar]
- 55. Witte K, Schnecko A, Buijs RM, van der Vliet J, Scalbert E, Delagrange P, Guardiola-Lemaitre B, Lemmer B. Effects of SCN lesions on circadian blood pressure rhythm in normotensive and transgenic hypertensive rats. Chronobiol Int 15: 135–145, 1998. [DOI] [PubMed] [Google Scholar]
- 56. Young AA, Dawson NJ. Evidence for on-off control of heat dissipation from the tail of the rat. Can J Physiol Pharmacol 60: 392–398, 1982. [DOI] [PubMed] [Google Scholar]
- 57. Zeitzer JM, Nishino S, Mignot E. The neurobiology of hypocretins (orexins), narcolepsy and related therapeutic interventions. Trends Pharmacol Sci 27: 368–374, 2006. [DOI] [PubMed] [Google Scholar]
- 58. Zhang S, Lin L, Kaur S, Thankachan S, Blanco-Centurion C, Yanagisawa M, Mignot E, Shiromani PJ. The development of hypocretin (orexin) deficiency in hypocretin/ataxin-3 transgenic rats. Neuroscience 148: 34–43, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang S, Zeitzer JM, Sakurai T, Nishino S, Mignot E. Sleep/wake fragmentation disrupts metabolism in a mouse model of narcolepsy. J Physiol 581: 649–663, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ziegler MG. Sleep disorders and the failure to lower nocturnal blood pressure. Curr Opin Nephrol Hypertens 12: 97–102, 2003. [DOI] [PubMed] [Google Scholar]


