Abstract
During urinary bladder filling the bladder urothelium releases chemical mediators that in turn transmit information to the nervous and muscular systems to regulate sensory sensation and detrusor muscle activity. Defects in release of urothelial mediators may cause bladder dysfunctions that are characterized with aberrant bladder sensation during bladder filling. Previous studies have demonstrated release of ATP from the bladder urothelium during bladder filling, and ATP remains the most studied purine mediator that is released from the urothelium. However, the micturition cycle is likely regulated by multiple purine mediators, since various purine receptors are found present in many cell types in the bladder wall, including urothelial cells, afferent nerves, interstitial cells in lamina propria, and detrusor smooth muscle cells. Information about the release of other biologically active purines during bladder filling is still lacking. Decentralized bladders from C57BL/6 mice and Cynomolgus monkeys (Macaca fascicularis) were filled with physiological solution at different rates. Intraluminal fluid was analyzed by high-performance liquid chromatography with fluorescence detection for simultaneous evaluation of ATP, ADP, AMP, adenosine, nicotinamide adenine dinucleotide (NAD+), ADP-ribose, and cADP-ribose content. We also measured ex vivo bladder filling pressures and performed cystometry in conscious unrestrained mice at different filling rates. ATP, ADP, AMP, NAD+, ADPR, cADPR, and adenosine were detected released intravesically at different ratios during bladder filling. Purine release increased with increased volumes and rates of filling. Our results support the concept that multiple urothelium-derived purines likely contribute to the complex regulation of bladder sensation during bladder filling.
Keywords: urothelium, adenosine 5′-triphosphate release, nicotinamide adenine dinucleotide release
sensory transduction in the urinary bladder is likely attributed to the interplay between the urothelium and the release of urothelially derived chemical mediators (2, 4, 12, 37). A key substance that the urothelium releases during bladder distention, ATP, stimulates afferent neurons in the lamina propria and triggers the micturition cycle (7, 19, 33, 39, 54). Multiple pathways have been suggested to mediate urothelial ATP release, including vesicle exocytosis and membrane channels and transporters (3, 36, 38, 41, 49). Mechanically induced urothelial release of ATP is reported to be regulated by extracellular Ca2+ and intracellular cAMP (34, 35) and to depend on mechanical stretch and not on hydrostatic pressure (58). Yet, it is recognized that the regulation of ATP release is still poorly understood (15). Purinergic receptors have been found located on various cell types, including urothelial cells (10, 52), smooth muscle cells (24), suburothelial myofibroblasts (56), platelet-derived growth factor receptor α-positive (PDGFRα+) cells (30, 31), and nerve terminals (1). Purinergic signaling in these cell types has been demonstrated to increase sensory neuron excitability (8) and to modulate smooth muscle tone (49). Therefore, urothelially derived ATP may bind to any of the purine receptors to activate autocrine and paracrine pathways and facilitate bladder sensory signaling. Indisputably ATP is the most studied extracellular nucleotide in the bladder (7, 39), yet a clear understanding about the dependence of urothelial ATP release on bladder filling is still lacking.
Another purine substance, adenosine, has been shown to be present in human urine (25, 53) and to be released in rabbit isolated uroepithelium upon changes of hydrostatic pressure (60). Adenosine receptors are expressed on urothelial cells, afferent neurons, and detrusor smooth muscle (55, 60). The dominant effect of adenosine appears to be inhibition of bladder contractility via various adenosine receptor activations. It is a widespread notion that high extracellular concentrations of adenosine are the result of extracellular hydrolysis of ATP by ectonucleotidases (61). More recently, it has been emphasized that other extracellular adenine nucleotides such as nicotinamide adenine dinucleotide (NAD+) and its metabolite ADP-ribose (ADPR) can also produce extracellular adenosine (16, 40, 61). In fact, NAD+ and ADPR have been demonstrated to be significant sources of adenosine (16); however, such mechanisms have not been investigated in the bladder. Release of adenosine during bladder filling has not been investigated in detail.
We have demonstrated that NAD+ and ADPR are released spontaneously and upon neural stimulation in isolated blood vessels, urinary bladder, and large intestine of different species (6, 16, 18, 27, 40, 47). Interestingly, the neurogenic release of NAD+ and ADPR has been found to exceed significantly the neural release of ATP in the human bladder (6). NAD+ and ADPR stimulate purine receptors (e.g., P2Y1 purine receptor) and membrane ion channels [e.g., small-conductance Ca2+-activated potassium (SK3) channels] in smooth muscles (26, 40). Interestingly, cells that express highly P2Y1 receptors and SK3 channels have been found in the murine bladder (30, 31). NAD+ inhibits contractility of human bladder detrusor muscle (6). It remains to be determined whether NAD+ and ADPR are released from the bladder urothelium during bladder filling and how these mediators contribute to regulation of bladder functions.
Aberrant purinergic signaling in the urothelium has been suggested to contribute to bladder dysfunction in injury or disease or during aging, since ATP release is increased in many functional disorders of the urinary bladder (13, 44, 50, 51, 57). It is currently unknown whether purines other than ATP also participate in lower urinary tract disorders. A better understanding of purinergic regulation of sensory transduction by urothelium-derived purines will hopefully lead to more effective treatments of bladder disorders.
The goal of the present study was to investigate the type of purines that are released from the bladder mucosa during bladder filling at different flow rates using ultrasensitive high-performance liquid chromatography with fluorescence detection (HPLC-FLD) methodologies and to determine the relationship of purine release during bladder filling with in vivo cystometry parameters and ex vivo bladder filling pressures.
METHODS
Animals
Male C57BL/6 mice (2–3 mo old) were purchased from Jackson Laboratory (Bar Harbor, MN). For studies using isolated bladders (see below), mice were killed by sedation with isoflurane followed by cervical dislocation and exsanguination. The bladder was removed and placed in oxygenated cold Krebs-bicarbonate solution (KBS) with the following composition (in mM): 118.5 NaCl, 4.2 KCl, 1.2 MgCl2, 23.8 NaHCO3, 1.2 KH2PO4, 11.0 dextrose, and 1.8 CaCl2 (pH 7.4). Urinary bladders of Cynomolgus monkeys (Macaca fascicularis) were obtained from Charles River Preclinical Services (Reno, NV). Monkeys, sedated with ketamine (10 mg/kg) and 0.7 ml beuthanasia-D (Schering-Plough AH, Kenilworth, NJ), were exsanguinated (Charles River Laboratories) for reasons unrelated to this project. All experimental procedures were approved by the University of Nevada Institutional Animal Use and Care Committee.
Cystometry in Conscious Unrestrained Mice
Surgical procedure.
Mice were anesthetized with isoflurane, and the urinary bladder was exposed via a midline abdominal incision. A saline-filled polyethylene tubing (PE-10) with the end flared by heat was inserted in the bladder lumen through a small incision in the bladder dome and secured with 6-0 nylon suture. The other end of the tubing was sealed, tunneled subcutaneously, and externalized at the back of the neck. The tubing was then placed in the subcutaneous space, and abdominal and neck incisions were closed (45). Buprenorphine SR-LAB (1 mg/kg) was administered for analgesia postoperatively, and mice were allowed to recover for 72 h before cystometry experiments were performed.
Cystometry experiments.
Mice were placed unrestrained in recording cages with a balance placed below the cage to measure voided volumes. The tubing was exteriorized at the neck and connected to a syringe pump with an in-line pressure transducer, and saline was infused at previously reported rates of 15 (9, 41), 25 (21, 22), or 100 (11, 29) μl/min to elicit repetitive urinary bladder contractions. Changes in intravesical pressure were recorded using a Small Animal Cystometry System (Med Associates, St. Albans, VT). Baseline pressure, maximum micturition pressure, infused volume, voided volume, and intervoiding intervals were recorded for each micturition cycle. After the experiment, mice were killed by isoflurane sedation followed by cervical dislocation.
Ex Vivo Bladder Pressure During Filling
Isolated C57BL/6 mouse bladders were catheterized via the urethra with PE-20 tubing flared at one end by heat and secured with 6-0 silk suture. Bladders were placed in an organ bath and perfused continuously with oxygenated KBS (37°C). The other end of the tubing was connected via a three-way stopcock to a syringe pump (Kent Scientific, Torrington, CT) and to a pressure transducer (AD Instruments, Colorado Springs, CO). KBS was infused in the bladder at a rate of 15 or 100 μl/min, and bladder pressure was recorded using a PowerLab data acquisition system (AD Instruments). The infusion was stopped when pressure reached 30 mmHg (maximum voiding pressure in mice; see Ref. 46), and the bladder was emptied by disconnecting the tubing from the syringe pump. At least four reproducible pressure-volume curves were recorded at each infusion rate per mouse.
Release of Purines from the Mucosa of Decentralized (Ex Vivo) Bladders
Isolated C57BL/6 mouse bladders were catheterized via the urethra with PE-20 tubing heat flared at one end and secured with 6-0 silk suture. Bladders were placed in 1-ml organ baths and perfused continuously with oxygenated KBS (37°C). After equilibration, bladders were distended via an infusion pump (15–100 μl/min) with 50 (low distension), 100 (low distension), and 200 (high distension) μl KBS (60–90 min between distensions). These volumes were chosen based on data from cystometry experiments and experiments monitoring ex vivo bladder filling pressures (see results). Bladder filling was performed at either 15 or 100 μl/min to match the low and high flow rates used in cystometry experiments. The intraluminal fluid was drained from the bladders and collected in ice-cold Eppendorf tubes. Isolated monkey bladders were catheterized via the urethra with larger-diameter (1.6 mm) tubing, placed in 50-ml organ baths and perfused with oxygenated KBS at 37°C. Bladders were distended with 10 and 50 ml KBS via an infusion pump (10 ml/min), and the intraluminal fluid was collected in ice-cold tubes. Samples from mouse and monkey bladders were acidified to pH 4.0 with citrate phosphate buffer and subjected to 1,N6-etheno derivatization at 80°C for 40 min for HPLC analysis (5, 28, 32, 40). Detection sensitivity of 1,N6-etheno-derivatized purines exceeds significantly the detection sensitivity of nonderivatized purines (5). A schematic diagram of the isolated whole bladder model used to collect intraluminal samples for analysis of urothelially released purines is shown in Fig. 3A.
Fig. 3.
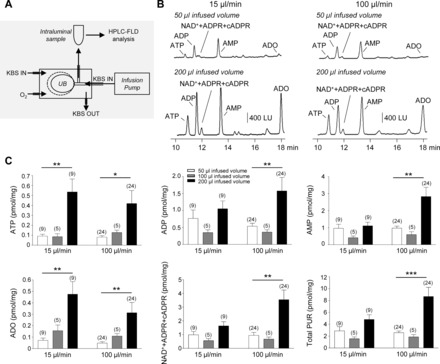
Increased urothelial release of purines occurs during filling of mouse bladders. A: schematic diagram of the isolated whole bladder model used to collect intraluminal samples for analysis of urothelially released purines. Bladders, placed in 1-ml organ baths and perfused with oxygenated Krebs solution (KBS), were filled with KBS via an infusion pump. The intraluminal solution was collected and processed for detection of purines by high-performance liquid chromatography with fluorescence detection (HPLC-FLD) (described in methods). UB, urinary bladder. B: chromatograms of intraluminal fluid collected during low (50 μl) and high (200 μl) infused volumes of isolated C57BL/6 mouse bladders at 15 (left) and 100 (right) μl/min infusion rates. The release of purines from the urothelium is increased during bladder filling. Of note, the samples in B were diluted 10× to obtain chromatography signals in scale. LU, luminescence units. C: averaged data (means ± SE) summarizing the bladder filling-evoked release of purines in pmol/mg tissue from mouse bladder urothelium at 15 and 100 μl/min infusion rates. Total purines (Total PUR) is the sum of ATP, ADP, AMP, adenosine (ADO), and NAD++ADPR+cADPR. Significant difference between groups indicated with horizontal bars (*P < 0.05, **P < 0.01, and ***P < 0.001, data analyzed with 1-way ANOVA or paired t-test). No. of experiments is in parentheses. White bars, 50 μl infused volume; gray bars, 100 μl infused volume; black bars, 200 μl infused volume.
HPLC Assay of 1,N6-Etheno-Derivatized Nucleotides/Nucleosides in Bladder Intraluminal Fluid
A reverse-phase gradient Agilent Technologies 1200 liquid chromatography system equipped with a fluorescence detector (Agilent Technologies, Wilmington, DE) was used to detect the 1,N6-etheno-derivatized nucleotides as described previously (28, 40). The mobile phase consisted of 0.1 M KH2PO4 (pH 6.0) as eluent A. Eluent B consisted of 65% eluent A and 35% methanol. Gradient elution was employed according to the following linear program: time 0, 0% eluent B; 18 min, 100% eluent B. The flow rate was 1 ml/min, and run time was 20 min. Column and autosampler temperatures were maintained at 25 and 4°C, respectively. The fluorescence detector was set to record 1,N6-etheno-derivatized nucleotide and nucleoside signals at an excitation wavelength of 230 nm and emission wavelength of 420 nm (5). The amount of nucleotide/nucleoside in each sample was calculated from calibration curves of nucleotide standards run simultaneously with each set of unknown samples. Results, normalized for sample volume and tissue weight, were expressed in picomoles per milligram of tissue.
HPLC Fraction Analysis
As we published previously, the endogenous NAD+, ADPR, and cyclic ADPR convert to 1,N6-etheno-ADPR (eADPR) during etheno derivatization at high temperature and elute at 11.9 min (40, 47). Nonderivatized cADPR, ADPR, and NAD+ elute at 7.9, 9.4, and 11.3 min, respectively, but remain under detection limit. To identify the composition of the eADPR (standing for NAD++ADPR+cADPR), we performed an HPLC fraction analysis (40, 47). Nonderivatized intraluminal fluid samples collected during bladder filling were processed through an HPLC-FLD system. An Agilent Technologies 1200 Analytical Fraction Collector was employed to collect 400-μl fractions corresponding to retention times of nonderivatized cADPR (7.9-min fraction), ADPR (9.4-min fraction), and NAD (11.3-min fraction). Fractions were then subjected to etheno derivatization and reanalyzed by HPLC for 1,N6-etheno-ADPR content. Contribution of NAD+, ADPR, and cADPR to the eADPR peak was calculated as percentage of the NAD++ADPR+cADPR mixture.
Statistics
Data presented are means ± SE. Means are compared by a two-tailed paired or unpaired t-test or by one-way ANOVA for comparison of more than two groups followed by a post hoc Bonferroni multiple-comparison test (GraphPadPrism, version 3; GraphPad Software, San Diego, CA). A probability value <0.05 was considered significant.
RESULTS
Cystometry Recordings in Conscious C57BL/6 Mice
Continuous cystometry experiments in unrestrained C57BL/6 mice demonstrated repetitive urinary bladder contractions in response to intravesical infusion of saline (15, 25, and 100 μl/min; Fig. 1). There was no difference in bladder capacity or voided volumes at 15, 25, or 100 μl/min infusion rates (P > 0.05), whereas intervoiding intervals were significantly reduced at higher infusion rates (15 vs. 25 μl/min, P < 0.001; 15 vs. 100 μl/min, P < 0.001; 25 vs. 100 μl/min, P < 0.001; Table 1). Baseline bladder pressures and maximum micturition pressures were higher with increasing infusion rates; however, the change in bladder pressure during each micturition cycle (maximum micturition pressure − baseline pressure) was similar at all infusion rates (P > 0.05, Table 2).
Fig. 1.
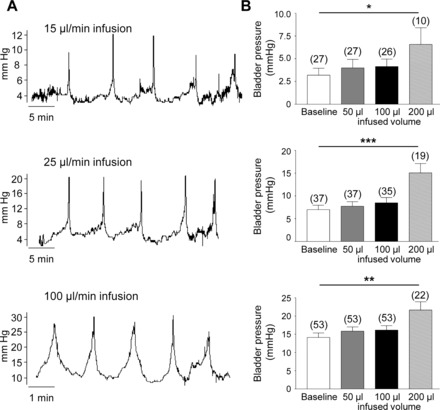
Cystometry recordings in C57BL/6 mice. A: representative cystometrogram recordings using continuous intravesical infusion of saline in conscious C57BL/6 mice. Top, middle, and bottom traces are at 15, 25, and 100 μl/min infusion rates, respectively. B: bar graphs (means ± SE) summarizing bladder pressures at baseline and at 50, 100, and 200 μl infused volumes of saline. Top, middle, and bottom graphs show data from 15, 25, and 100 μl/min infusion rates, respectively. *P < 0.05, **P < 0.01, and ***P < 0.001, significant difference from baseline pressure. Note that there is no change in pressure from baseline with 50 or 100 μl infused volumes of saline, whereas pressure is significantly increased with 200 μl infusion of saline. No. of observations from 3–5 mice are in parentheses.
Table 1.
Bladder capacity, voided volumes, and IVI of conscious C57BL/6 mice during intravesical infusion of saline at 15, 25, and 100 μl/min
| 15 μl/min | n | 25 μl/min | n | 100 μl/min | n | |
|---|---|---|---|---|---|---|
| Bladder capacity, μl | 185.59 ± 12.96 | 27 | 213.81 ± 14.10 | 37 | 195.23 ± 7.78 | 53 |
| Voided volume, μl | 113.67 ± 11.04 | 26 | 128.78 ± 7.39 | 30 | 140.18 ± 5.80 | 34 |
| IVI, min | 12.37 ± 0.86 | 27 | 8.55 ± 0.56*** | 37 | 1.95 ± 0.08***○○○ | 53 |
Values are means ± SE; n, no. of observations from 3–5 mice.
IVI, intervoiding intervals.
P < 0.001, significant difference from 15 μl/min (
) and from 25 μl/min (
).
Table 2.
Micturition pressures of C57BL/6 mice during intravesical infusion of saline at 15, 25, and 100 μl/min
| 15 μl/min | n | 25 μl/min | n | 100 μl/min | n | |
|---|---|---|---|---|---|---|
| Baseline pressure, mmHg | 3.19 ± 0.76 | 27 | 7.00 ± 0.96** | 37 | 14.13 ± 1.22***○○○ | 53 |
| Maximum micturition pressure, mmHg | 12.33 ± 1.23 | 27 | 19.34 ± 2.01** | 37 | 29.91 ± 1.77***○○○ | 53 |
| Pressure change during micturition cycle, mmHg | 12.17 ± 0.74 | 27 | 12.34 ± 1.32 | 37 | 15.77 ± 1.26 | 53 |
Values are means ± SE; n, no. of observations from 3–5 mice.
P < 0.01 and
P < 0.001, significant difference from 15 μl/min;
P < 0.001, significant difference from 25 μl/min.
There was no change in bladder pressure from baseline pressure when 50 or 100 μl of saline were infused in the bladders (P > 0.05 at all infusion rates). However, 200 μl infusion of saline in the bladder resulted in a significant increase in pressure from baseline pressure (P < 0.05, 0.001, and 0.01 at 15, 25, and 100 μl/min infusion rates, respectively; Fig. 1). These results indicate that 50- or 100-μl infusions of saline in the bladder correspond to low bladder filling pressure, whereas 200-μl infusions corresponds to high bladder pressure; therefore, the release of purines during filling of isolated mouse bladders (see below) was analyzed in intraluminal fluid collected from the bladder upon filling with 50, 100, and 200 μl of KBS.
Ex Vivo Murine Bladder Filling Pressure Recordings
Pressure traces from ex vivo bladder filling experiments in mice demonstrated that, similar to the results from in vivo cystometry experiments, infusion of 50 and 100 μl of KBS in bladders at 15 and 100 μl/min produced insignificant changes in pressure from baseline pressure (P > 0.05), whereas infusion of 200 μl KBS significantly enhanced bladder pressures above baseline pressure (P < 0.001, Fig. 2). Increasing bladder filling rates resulted in less time to reach the voiding pressure range.
Fig. 2.
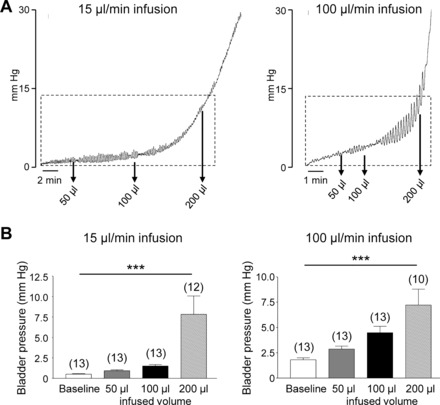
Ex vivo murine bladder filling pressures. A: pressure traces from ex vivo bladder filling experiments in mice at 15 (left) and 100 (right) μl/min infusion rates. Pressure scale (mmHg) applied to all traces. Regions indicated by boxes represent the bladder filling phase before typically micturition occurs (i.e., 0–12 mmHg). Arrows indicate bladder pressures at infused volumes of 50, 100, and 200 μl. B: bar graphs (means ± SE) summarizing bladder pressures at baseline and at 50, 100, and 200 μl infusion of KBS. ***P < 0.001, significant difference from baseline pressure. Note that at 15 and 100 μl/min there is no change in pressure from baseline with 50 or 100 μl infusion of KBS, whereas pressure is significantly increased with 200 μl infusion of KBS. No. of observations in replicates from 3 mice are in parentheses.
Release of Purines from the Mucosa During Ex Vivo Bladder Filling
In C57/Bl6 mouse bladders (Fig. 3) filled with KBS at two different rates, HPLC-FLD analysis demonstrated the presence of ATP, ADP, AMP, NAD++ADPR+cADPR, and adenosine in intraluminal fluid collected during filling of bladders with 50, 100, or 200 μl KBS. As discussed in HPLC Fraction Analysis, NAD+, ADPR, and cADPR elute as one peak at 11.9 min in samples that undergo 1,N6-etheno derivatization and, therefore, the peak at 11.9 min is designated as NAD++ADPR+cADPR. Results from a detailed HPLC fraction analysis demonstrate that NAD+ is the dominant compound in this mixture as discussed in HPLC Fraction Analysis of NAD++ADPR+cADPR. Also note that the fluorescent properties of etheno-derivatized NAD+ are significantly lower than the properties of etheno-derivatized ATP (17) and, therefore, chromatography signals that are generated by NAD+ may appear smaller than the signals generated by ATP. In fact, the amounts of released NAD+ exceeded the amounts of ATP at all infused volumes and infusion rates (Fig. 3C); thus, at 15 μl/min infusion rate (n = 9), P was 0.004 and 0.001 at 50 and 200 μl infused volume, respectively. At 100 μl/min (n = 24), P was 0.0006 and 0.0002 at 50 and 200 μl infused volume, respectively.
The release of purines was generally similar at 50 and 100 μl fluid volumes but was enhanced when the bladders were filled with 200 μl fluid, at both 15 and 100 μl/min flow rates (Fig. 3C). This pattern was clearly demonstrated for all purines at 100 μl/min and for ATP and adenosine at either 15 or 100 μl/min. The trend of increased release at 200 μl bladder filling was also observed at 15 μl/min infusion rate for ADP, AMP, NAD++ADPR+cADPR, and total purines (e.g., ATP+ADP+AMP+adenosine +NAD++ADPR+cADPR), but did not reach significance.
In Cynomolgus monkey bladders (Fig. 4), HPLC-FLD analysis demonstrated the presence of ATP, ADP, NAD++ADPR+cADPR, AMP, and adenosine in intraluminal fluid collected during 10 ml infused volume (low stretch) of bladders at 10 ml/min filling rate. Higher distention (50 ml infused volume) of monkey bladders resulted in increased amounts in the intraluminal fluid of all detected purines, but reached statistical significance for adenosine (Fig. 4). These results demonstrate remarkable similarities between patterns of intraluminal purine release during filling of murine and monkey bladders.
Fig. 4.
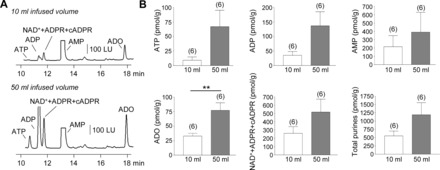
Increased urothelial release of purines occurs during filling of monkey bladders. A: chromatograms of intraluminal fluid collected during low (10 ml) and high (50 ml) distention of isolated Cynomolgus monkey bladders at 10 ml/min infusion rate. The release of purines from the urothelium is increased during bladder filling. Of note, the samples in A were diluted 5× to obtain chromatography signals in scale. B: averaged data (means ± SE) summarizing the bladder filling-evoked release of purines in pmol/g tissue from monkey bladder urothelium. **P < 0.01, significant difference from 10 ml infusion. No. of experiments is in parentheses.
HPLC Fraction Analysis of NAD++ADPR+cADPR
An HPLC fraction analysis was carried out to determine the composition of the 11.9-min eADPR (NAD++ADPR+cADPR) peak at both 15 and 100 μl/min infusion rate of mouse bladders. At 15 μl/min, at low bladder distention (50 μl infused KBS) the 11.9-min peak was comprised of 91.5% NAD+, 6.7% ADPR, and 1.8% cADPR, whereas at higher distention (200 μl infused KBS) the peak was comprised of 97.3% NAD+, 1.5% ADPR, and 1.2% cADPR. At 100 μl/min and 50 μl infused KBS, the 11.9-min peak was comprised of 81% NAD+, 11.8% ADPR, and 7.2% cADPR, whereas at 200 μl infused volume the peak was comprised of 90% NAD+, 7.5% ADPR, and 3.5% cADPR. Therefore, NAD+ was the dominant substance behind the 11.9-min peak at both low and high degrees of bladder filling.
DISCUSSION
This study shows the first direct evidence for urothelial release of multiple purines during filling of decentralized (ex vivo) bladders of mice and nonhuman primates. Specifically, we demonstrated here that bladder filling results in intravesical release of remarkable amounts of ATP, ADP, AMP, NAD+, ADPR, cADPR and adenosine, suggesting that multiple purines, in addition to ATP, are likely present in the bladder mucosa and thus could affect multiple cell types in the bladder wall. Therefore, these studies raise the possibility that the regulation of bladder sensation and the micturition cycle by urothelium-derived extracellular purines is more complex than typically considered.
ATP is the most investigated purine mediator that is released from the bladder mucosa (1, 2, 20). It is released during bladder distention (3, 19, 33, 54) and activates P2X2/P2X3 receptors on afferent nerve fibers to convey information to the central nervous system where voiding can be initiated (1, 54). When administered intravesically, ATP induces bladder overactivity (42), suggesting a prominent role of ATP in the afferent control of bladder function. ATP can also affect bladder functions by activating receptors on smooth muscle cells (7, 39) or by activating interstitial cells in the detrusor muscle that are positive for PDGFRα (31). Therefore, understanding the mechanisms of ATP release during bladder distention will be important for understanding broad mechanisms of bladder regulation during bladder filling. Adenosine that is produced in the urothelium (60) has been suggested to stimulate or inhibit afferent nerve activity depending on the adenosine receptor expressed (43, 60).
Many studies make assumptions about mechanisms of purinergic regulation by examining effects of purines on P1 adenosine receptors or P2 purine receptors on effector cells. Such studies commonly assume that endogenous purine receptor agonists are released in the extracellular space without providing direct evidence for this. The majority of studies that do analyze ATP release in the bladder commonly use the luciferin luciferase chemiluminescence assay (48). This assay is sensitive but is restricted to ATP. Therefore, possible release of other purines generally remains undetected, although functional studies using receptor agonists and antagonists suggest possible roles for purines in addition to ATP. Clearly, there is important new information that could be obtained by measuring simultaneously the full endogenous complement of extracellular purines that are released from the urothelium during bladder filling. In this study we used highly sensitive HPLC techniques to evaluate simultaneously the release of ATP, ADP, AMP, adenosine and NAD+, ADPR, and cADPR in the bladder lumen at different degrees and rates of bladder filling.
We preformed conscious cystometry in mice and recorded ex vivo bladder pressure during filling to determine the bladder fill volumes that corresponded to “low” bladder pressure/distention during the filling phase and “high” bladder pressure/distention when the pressure was approaching the typical range in which voiding is initiated. Our cystometry data demonstrated that, while baseline pressures and maximum micturition pressures were increased with higher filling rates, the overall change in bladder pressure during each micturition cycle was the same at all infusion rates. Moreover, bladder capacity (i.e., volume of fluid infused before micturition occurred) was the same at all infusion rates, suggesting similar voiding patterns at low and high rates of fluid infusion, although micturition occurred more frequently at the higher infusion rates. Basal pressures appeared to be higher in in vivo cystometry than in ex vivo pressure recordings likely due to the influence of the central nervous system. Closer examination of the volume-pressure relationships in in vivo cystometry as well as in ex vivo pressure recordings demonstrated that 50 and 100 μl filling volumes can be considered low distention volumes, whereas 200 μl filling volume could be considered as high filling volume. Thus, the pressure generated by 50 or 100 μl infused volumes in conscious cystometry experiments were close to the basal pressure at all flow rates, whereas the pressure generated by 200 μl infused volume was higher and close to the pressure range when voiding occurs (Fig. 1). Also, 200 μl infused volume roughly corresponded to the bladder “capacity” determined in cystometry experiments (Table 1). Generally similar data were obtained in measuring ex vivo bladder pressures: at either 15 or 100 μl/min, 50 and 100 μl infused volumes generated similar filling pressures that were not significantly different from the baseline pressures. Two hundred microliter infused volume generated pressure close to the typical pressure range when voiding occurs. As anticipated, increase in flow rates was accompanied with a decreased time for reaching the high-limit pressure.
Based on these two sets of experiments, we measured urothelial purine release at low (50 and 100 μl) and high (200 μl) filling volumes at two flow rates, namely 15 μl/min, which is considered to be a physiological rate for mice, and 100 μl/min, which could be considered relevant to circumstances in which higher rate of bladder filling occurs (e.g., stimulated diuresis by diuretics). Therefore, the purine release studies were performed within the physiological volume range of bladder function and at physiologically relevant filling rates. Finally, to eliminate complicating influences of the central nervous system, we carried out the release experiments in decentralized bladders from mice and nonhuman primates. Evaluation of intraluminal content of ATP in the bladder is an established approach in studies of urothelial release of ATP (e.g., Refs. 3, 11, and 13). This approach has advantages to other approaches that are commonly used to examine ATP release from primary cultured cells (that generally grow nonpolarized and may not represent the urothelium in vivo) or surgically removed bladder urothelium sheets that are placed in Ussing chambers and may also release ATP from cells damaged during dissection or from dead cells that accumulate during the experiment (3). Importantly, we measured the content of purines in the entire intraluminal volume that was collected at different infused volumes, which also brings advantages to studies that evaluate purine content in aliquots of intraluminal samples and thus may underestimate the amounts of ATP in higher-volume samples that are collected during bladder distention.
In confirmation of previous work, we found that intraluminal fluid collected during bladder filling contained ATP and adenosine. The intravesical ATP and adenosine amounts were increased at higher bladder distention in both mice and nonhuman primates. It is commonly believed that extracellular ATP is degraded quickly by extracellular ATPases (eNTPDases) (20, 61) to AMP, which then is degraded to adenosine by 5′-nucleotidase (CD73) (61). Presence of eNTPDase3 and alkaline phosphatase was demonstrated on urothelial cells (58), suggesting that ATP would be degraded in the urothelium. However, immunohistochemistry analysis suggests that CD73 is absent in the mouse urothelium (60). Therefore, it is presently unclear whether adenosine is released on its own from the urothelium during distention of the bladder (60) or, alternatively, enzymes that are different from CD73 might participate in adenosine production from purine precursors such as ATP, NAD+, or ADPR (61). ADP, another product of ATP that was suggested previously to regulate detrusor smooth muscle contractility (59), was also found present in the intraluminal fluid during bladder filling.
Another interesting finding in the present study was that large amounts of NAD+ and relatively smaller amounts of metabolites ADPR and cADPR were found intraluminally during bladder filling. In fact, NAD+ represented ∼80–98% of the composite NAD++ADPR+cADPR peak as determined by HPLC fraction analysis, whereas ADPR and cADPR represented ∼1.5–12 and 3–7% of the composite peak, respectively. The extracellular metabolism of purines, including NAD+, is particularly complex (14), and more studies should be carried out to determine whether the degradation of NAD+ depends on the degree and rate of bladder distention. In fact, ATP and adenosine metabolism during bladder filling should also be investigated because extracellular bioavailability of purines is a result of released minus removed (metabolized) substances. NAD+ and ADPR have been demonstrated to be novel enteric neurotransmitters in the large intestine (16, 18, 40). In the bladder, we demonstrated previously that large amounts of NAD+ are released upon action potential firings in bladder preparations from different species, including human beings (6, 47). We also demonstrated that exogenous NAD+ inhibits the contractility of isolated detrusor muscle strips (6). Moreover, NAD+ and ADPR are reported to activate P2Y1 receptor-mediated mechanisms (23, 40), but no information is available about possible effects of extracellular NAD+ and ADPR on sensory transduction in the bladder. The present study demonstrated for the first time that NAD+ and ADPR are released from the bladder mucosa during bladder filling. Further studies are warranted to determine the role of extracellular NAD+ and ADPR in afferent nerve activity and in regulation of the micturition cycle in vivo.
The similarities between urothelial purine release during filling of mouse and monkey bladders suggest that the mechanisms we observed likely have broad implications across species.
It is not currently possible to define the specific cell type(s) that is the primary source of mucosa-derived purines in the bladder during bladder filling while keeping the cell syncytium intact. Nevertheless, the evidence for urothelial release of multiple purines during bladder filling is compelling and merits future studies to understand the mechanisms that underlie the release and formation of bioactive purines that we determined to be released from the urothelium during bladder filling. It will be important to understand whether individual purines (e.g., ATP, NAD+, and adenosine) have preferential mechanisms of release, whether these mechanisms are selectively activated at different stages of bladder filling, or whether the rate of extracellular purine metabolism changes during bladder filling. Finally, an important question to be addressed would be to determine how defects in urothelial purine release contribute to bladder pathologies that are characterized with bladder overactivity or underactivity.
GRANTS
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants DK-41315 and DK-098388.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.D. and V.N.M.-Y. conception and design of research; L.D., S.H., R.C., A.Y., and V.N.M.-Y. performed experiments; L.D., S.H., R.C., A.Y., and V.N.M.-Y. analyzed data; L.D., S.H., S.D.K., and V.N.M.-Y. interpreted results of experiments; L.D., S.H., and V.N.M.-Y. prepared figures; L.D. and V.N.M.-Y. drafted manuscript; L.D., S.D.K., and V.N.M.-Y. edited and revised manuscript; L.D., S.H., R.C., A.Y., S.D.K., and V.N.M.-Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Charles River Preclinical Services in Reno, NV, for providing primate bladders, Core B and Nancy Horowitz for maintaining mouse colonies, and Sheerien Manzoor for technical assistance. We also thank Dr. Margaret A. Vizzard and Susan E. Malley (University of Vermont) for providing excellent training in cystometry of unrestrained mice.
REFERENCES
- 1.Andersson KE. Purinergic signalling in the urinary bladder. Auton Neurosci 191: 78–81, 2015. [DOI] [PubMed] [Google Scholar]
- 2.Apodaca G, Balestreire E, Birder LA. The uroepithelial-associated sensory web. Kidney Int 72: 1057–1064, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Beckel JM, Daugherty SL, Tyagi P, Wolf-Johnston AS, Birder LA, Mitchell CH, de Groat WC. Pannexin 1 channels mediate the release of ATP into the lumen of the rat urinary bladder. J Physiol 593: 1857–1871, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birder L, Andersson KE. Urothelial signaling. Physiol Rev 93: 653–680, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobalova J, Bobal P, Mutafova-Yambolieva VN. High-performance liquid chromatographic technique for detection of a fluorescent analogue of ADP-ribose in isolated blood vessel preparations. Analytical Biochemistry 305: 269–276, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Breen LT, Smyth LM, Yamboliev IA, Mutafova-Yambolieva VN. β-NAD is a novel nucleotide released on stimulation of nerve terminals in human urinary bladder detrusor muscle. Am J Physiol Renal Physiol 290: F486–F495, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Burnstock G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal 10: 103–155, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature 377: 428–431, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Takahashi S, Zhong S, Hosoda C, Zheng HY, Ogushi T, Fujimura T, Ohta N, Tanoue A, Tsujimoto G, Kitamura T. Function of the lower urinary tract in mice lacking alpha1d-adrenoceptor. J Urol 174: 370–374, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Chopra B, Gever J, Barrick SR, Hanna-Mitchell AT, Beckel JM, Ford AP, Birder LA. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol 294: F821–F829, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins VM, Daly DM, Liaskos M, McKay NG, Sellers D, Chapple C, Grundy D. OnabotulinumtoxinA significantly attenuates bladder afferent nerve firing and inhibits ATP release from the urothelium. BJU Int 112: 1018–1026, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Daly DM, Collins VM, Chapple CR, Grundy D. The afferent system and its role in lower urinary tract dysfunction. Curr Opin Urol 21: 268–274, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Daly DM, Nocchi L, Liaskos M, McKay NG, Chapple C, Grundy D. Age-related changes in afferent pathways and urothelial function in the male mouse bladder. J Physiol 592: 537–549, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Figueiredo LF, Gossmann TI, Ziegler M, Schuster S. Pathway analysis of NAD+ metabolism. Biochem J 439: 341–348, 2011. [DOI] [PubMed] [Google Scholar]
- 15.Dunning-Davies BM, Fry CH, Mansour D, Ferguson DR. The regulation of ATP release from the urothelium by adenosine and transepithelial potential. BJU Int 111: 505–513, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Durnin L, Hwang SJ, Ward SM, Sanders KM, Mutafova-Yambolieva VN. Adenosine 5′-diphosphate-ribose (ADPR) is a neural regulator in primate and murine large intestine along with beta-NAD+. J Physiol 590: 1921–1941, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durnin L, Moreland N, Lees A, Mutafova-Yambolieva VN. A commonly used ecto-ATPase inhibitor, ARL-67156, blocks degradation of ADP more than the degradation of ATP in murine colon. Neurogastroenterol Motil 28: 1370–1381, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Durnin L, Sanders KM, Mutafova-Yambolieva VN. Differential release of beta-NAD(+) and ATP upon activation of enteric motor neurons in primate and murine colons. Neurogastroenterol Motil 25: e194–e204, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes–a possible sensory mechanism? J Physiol 505: 503–511, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry CH, Vahabi B. The role of the mucosa in normal and abnormal bladder function. Basic Clin Pharmacol Toxicol doi:10.1111/bcpt.12626, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, Owsianik G, Liedtke W, Daelemans D, Dewachter I, Van LF, Voets T, De RD, Nilius B. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest 117: 3453–3462, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Girard BM, Malley SE, Mathews MM, May V, Vizzard MA. Intravesical PAC1 receptor antagonist, PACAP(6–38), reduces urinary bladder frequency and pelvic sensitivity in NGF-OE mice. J Mol Neurosci 59: 290–299, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafsson AJ, Muraro L, Dahlberg C, Migaud M, Chevallier O, Khanh HN, Krishnan K, Li N, Islam MS. ADP ribose is an endogenous ligand for the purinergic P2Y1 receptor. Mol Cell Endocrinol 333: 8–19, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Heppner TJ, Bonev AD, Nelson MT. Elementary purinergic Ca2+ transients evoked by nerve stimulation in rat urinary bladder smooth muscle. J Physiol 564: 201–212, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heyne N, Benohr P, Muhlbauer B, Delabar U, Risler T, Osswald H. Regulation of renal adenosine excretion in humans–role of sodium and fluid homeostasis. Nephrol Dial Transplant 19: 2737–2741, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol 590: 1957–1972, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. beta-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 140: 608–617, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-Nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 140: 608–617, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jusuf AA, Kojima S, Matsuo M, Tokuhisa T, Hatano M. Vesicourethral sphincter dysfunction in ncx deficient mice with an increased neuronal cell number in vesical ganglia. J Urol 165: 993–998, 2001. [PubMed] [Google Scholar]
- 30.Lee H, Koh BH, Peri LE, Sanders KM, Koh SD. Functional expression of SK channels in murine detrusor PDGFR+ cells. J Physiol 591: 503–513, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H, Koh BH, Peri LE, Sanders KM, Koh SD. Purinergic inhibitory regulation of murine detrusor muscles mediated by PDGFRalpha+ interstitial cells. J Physiol 592: 1283–1293, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levitt B, Head RJ, Westfall DP. High-pressure liquid chromatographic-fluorometric detection of adenosine and adenine nucleotides: application to endogenous content and electrically induced release of adenyl purines in guinea pig vas deferens. Anal Biochem 137: 93–100, 1984. [DOI] [PubMed] [Google Scholar]
- 33.Lewis SA, Lewis JR. Kinetics of urothelial ATP release. Am J Physiol Renal Physiol 291: F332–F340, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto-Miyai K, Kagase A, Yamada E, Yoshizumi M, Murakami M, Ohba T, Kawatani M. Store-operated Ca2+ entry suppresses distention-induced ATP release from the urothelium. Am J Physiol Renal Physiol 300: F716–F720, 2011. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto-Miyai K, Yamada E, Yoshizumi M, Kawatani M. The regulation of distention-induced ATP release from urothelium by the adenylyl cyclase-cyclic AMP pathway. Biomed Res 33: 153–157, 2012. [DOI] [PubMed] [Google Scholar]
- 36.McLatchie LM, Fry CH. ATP release from freshly isolated guinea-pig bladder urothelial cells: a quantification and study of the mechanisms involved. BJU Int 115: 987–993, 2015. [DOI] [PubMed] [Google Scholar]
- 37.Merrill L, Gonzalez EJ, Girard BM, Vizzard MA. Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol 13: 193–204, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyamoto T, Mochizuki T, Nakagomi H, Kira S, Watanabe M, Takayama Y, Suzuki Y, Koizumi S, Takeda M, Tominaga M. Functional role for Piezo1 in stretch-evoked Ca(2)(+) influx and ATP release in urothelial cell cultures. J Biol Chem 289: 16565–16575, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mutafova-Yambolieva VN, Durnin L. The purinergic neurotransmitter revisited: a single substance or multiple players? Pharmacol Ther 144: 162–191, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mutafova-Yambolieva VN, Hwang SJ, Hao X, Chen H, Zhu MX, Wood JD, Ward SM, Sanders KM. beta-Nicotinamide adenine dinucleotide is an inhibitory neurotransmitter in visceral smooth muscle. Proc Natl Acad Sci USA 104: 16359–16364, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Negoro H, Urban-Maldonado M, Liou LS, Spray DC, Thi MM, Suadicani SO. Pannexin 1 channels play essential roles in urothelial mechanotransduction and intercellular signaling. PLoS One 9: e106269, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandita RK, Andersson KE. Intravesical adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol 168: 1230–1234, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Prakasam HS, Herrington H, Roppolo JR, Jackson EK, Apodaca G. Modulation of bladder function by luminal adenosine turnover and A1 receptor activation. Am J Physiol Renal Physiol 303: F279–F292, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruggieri MR., Sr Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction. Nat Clin Pract Urol 3: 206–215, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol 298: R534–R547, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith PP, Kuchel GA. Continuous uroflow cystometry in the urethane-anesthetized mouse. Neurourol Urodyn 29: 1344–1349, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smyth LM, Bobalova J, Mendoza MG, Lew C, Mutafova-Yambolieva VN. Release of β-nicotinamide adenine dinucleotide upon stimulation of postganglionic nerve terminals in blood vessels and urinary bladder. J Biol Chem 279: 48893–48903, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Stanley PE, Williams SG. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem 29: 381–392, 1969. [DOI] [PubMed] [Google Scholar]
- 49.Sui G, Fry CH, Montgomery B, Roberts M, Wu R, Wu C. Purinergic and muscarinic modulation of ATP release from the urothelium and its paracrine actions. Am J Physiol Renal Physiol 306: F286–F298, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol 290: C27–C34, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol 166: 1951–1956, 2001. [PubMed] [Google Scholar]
- 52.Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int 93: 1344–1348, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Vidotto C, Fousert D, Akkermann M, Griesmacher A, Muller MM. Purine and pyrimidine metabolites in children's urine. Clin Chim Acta 335: 27–32, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci 21: 5670–5677, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winder M, Tobin G, Zupancic D, Romih R. Signalling molecules in the urothelium. Biomed Res Int 2014: 297295, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559: 231–243, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology 63: 17–23, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Yu W. Polarized ATP distribution in urothelial mucosal and serosal space is differentially regulated by stretch and ectonucleotidases. Am J Physiol Renal Physiol 309: F864–F872, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu W, Sun X, Robson SC, Hill WG. ADP-induced bladder contractility is mediated by P2Y12 receptor and temporally regulated by ectonucleotidases and adenosine signaling. FASEB J 28: 5288–5298, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu W, Zacharia LC, Jackson EK, Apodaca G. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol 291: C254–C265, 2006. [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann H, Zebisch M, Strater N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8: 437–502, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


