Abstract
Background
Triple negative breast cancer (TNBC) occurs at higher frequency in African Americans compared with Caucasians. It is unclear if the biology of TNBC is different in African American versus Caucasians. In this study, we sought to evaluate racial differences in the molecular pathology of TNBC.
Methods
Using data from The Cancer Genome Atlas, we identified TNBC patients with information on race. We analyzed differences in clinical characteristics, tumor somatic mutations, and gene expression patterns by race from whole exome and microarray data.
Results
1104 patients were identified, of which 178 had TNBC. TNBC was more frequent in African American than Caucasians (33.3% vs 14.9%). Although, more African American than Caucasians overall were classified as basal-like from PAM50 gene expression (34.8% vs 16.1%), no differences in the TNBC cohort were observed. Median tumor somatic mutation counts were higher in African American versus Caucasians (39.5 vs 34), but no racial differences in the mutation counts in TNBC were observed. Somatic mutation analysis revealed racial differences in specific high prevalence genes in all patients- [TP53: 46% in African American vs 27% in Caucasians; PIK3CA: 23% in African American vs 34% in Caucasians; and MLL3: 12% in African American vs 6% in Caucasians]. TNBC patients did not have any specific high prevalence genes associated with racial differences. There were no racial differences in gene expression patterns in selected genes involved in breast cancer biology. Overall, African Americans had shorter TTP and worse DFS. Racial differences in clinical outcomes were not observed in TNBC.
Conclusion
The mutational landscape of TNBC is similar between African Americans and Caucasians. The higher frequency of TNBC in African Americans is therefore not associated with a different genomic profile of common established tumor regulatory pathway genes. Other modifiable factors may exist that contribute to the racial disparity in TNBC.
Keywords: triple negative breast cancer, genomics, survival
Introduction
Although breast cancer mortality rates have been dropping since 1990 due to earlier detection and improved treatment modalities, substantial racial disparities in outcome persist. Breast cancer occurs at a lower frequency in black women, but is associated with a poorer overall and breast cancer specific survival.[31, 34] Outcome disparities persist even after controlling for socioeconomic factors such as access to care.[7, 23] Blacks with breast cancer are also more likely to present at earlier ages than whites, and to have tumors with more aggressive characteristics, such as high histologic grade and triple negative breast cancers (TNBC).[1, 6] In the Carolina Breast Cancer Study, the prevalence of TNBC was highest (39%) among black premenopausal women compared to other groups of patients (14–16%).[4] It could be suggested that the preponderance of TNBC among black women could be due to their younger age at diagnosis, but even in older age groups, blacks are more likely than whites to present with TNBC.[11] This subtype has a less favorable prognosis than the others, particularly Luminal A subtype, which has the highest survival rates.[4]
It is possible that racial disparities in outcome are being driven by those with hormone receptor (HR) positive cancers versus TNBC. Black women with HR positive breast cancer have a much higher mortality rate in the first two years after diagnosis than whites,[34] but among those with TNBC, there is conflicting data on survival outcomes according to race.[16, 19, 21, 26, 28, 29, 33, 34] Although studies in breast cancer patients in general have identified racial differences in genomic profiles,[10, 13] it is unclear if these differences are being driven by those with HR positive cancers or TNBC. To address this lack of data, we sought to investigate racial differences in the genetic landscape of TNBC.
Methods
Patient population
From the publicly available Cancer Genome Atlas (TCGA) database, we identified 1104 patients with primary breast cancer, who had undergone whole exome next generation sequencing with a somatic mutation report. Patients were diagnosed between 1988 and 2013, and had clinical information was submitted to the TCGA between March 2010 and January 2015. Patients without information on race were excluded.
Statistical analysis
Chi squared or fisher’s exact test and analysis of variance were used, to compare baseline categorical and continuous variables across race groups. Tumor-specific somatic mutations from whole exome next generation sequencing were analyzed. Sequencing was done at minimum coverage of 70% and at a depth of 20X as previously described.[3] We included genes with a prevalence of at least 5% in the TCGA breast cancer database. Gene expressions were assessed with TCGA microarray data. Two sample t tests were used to identify differentially expressed genes (DEGs) between African Americans and Caucasians. P values per gene level from hypothesis testing were adjusted to address multiple testing issues by the false discovery rate adjustment method. Differences in gene expression between race groups were calculated to sort gene list, then top DEGs as well as other genes relevant in breast cancer biology were selected. Java Treeview was used to generate a heatmap to graphically represent gene expression values by race. To determine whether race was differentially associated with disease free survival (DFS), the Cox proportional hazards model was applied and non-breast cancer deaths were treated as competing risk events. Kaplan Meier method and log rank test were used to show differences in DFS by race. All statistical analyses were performed by SAS (version 9.4, SAS Institute, Cary, NC). Statistical significance was assessed as two sided P <0.05.
Results
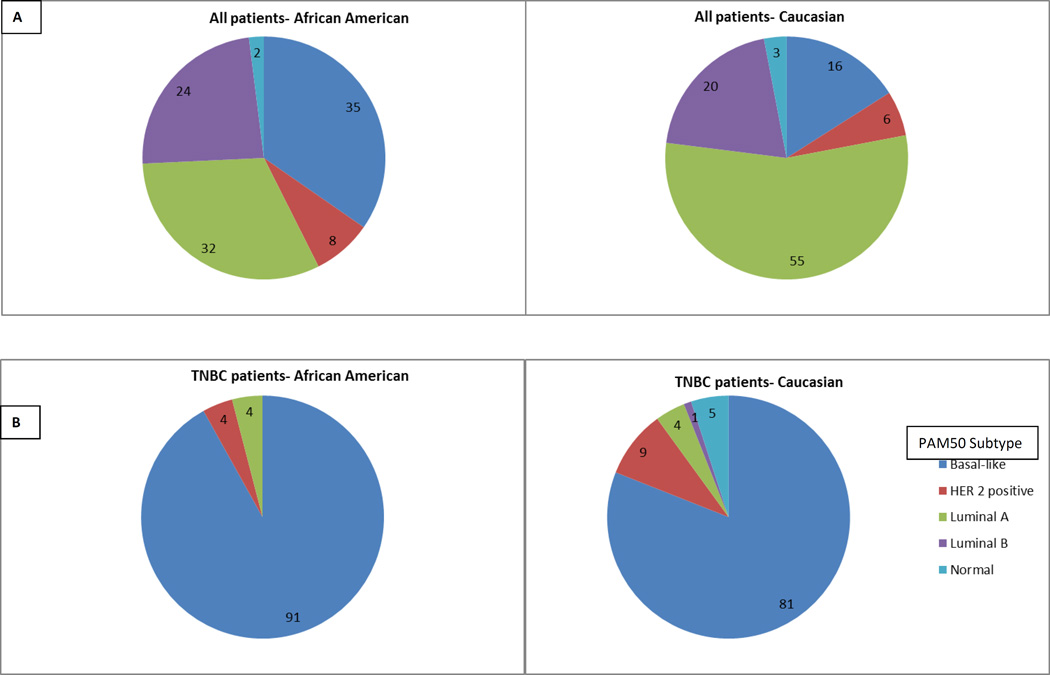
Table 1 shows the patient characteristics in all breast cancer and TNBC patients only, according to race. A total of 1,104 patients with primary breast cancer were identified, of which 178 (16.1%) had TNBC. Of the 947 breast cancer patients with data on race, 183 (19.3%) were African American, while 764 (80.7%) were Caucasians. African Americans were more likely to have TNBC than Caucasian patients (33.3% versus 14.9%, p <.001). In all breast cancer patients, there were significantly more basal-like tumors by PAM50 categorization in African Americans versus Caucasians (34.9% versus 16.1%; p = .001) (Figure 1A). In the TNBC cohort, there were no racial differences in PAM50 categorization (91% versus 80.6%; p = .842) (Figure 1B).
Table 1.
Patient and tumor characteristics for entire cohort and TNBC cohort
| All patients (N=1104) | |||
|---|---|---|---|
| Variable | African American (%) |
White (%) | p |
| Diagnosis age in years | 54.5 | 59.0 | .057 |
| Interquartile range | 47–67 | 49–67 | |
| TNBC | 33.3 | 14.9 | <.001 |
| HER2 positive | 15.9 | 13.5 | .430 |
| Stage | |||
| 1 | 19.3 | 18.9 | .424 |
| 2 | 59.4 | 55.9 | |
| 3 | 18.7 | 23.7 | |
| 4 | 2.4 | 1.5 | |
| PAM50 Subtype | |||
| Basal-like | 34.9 | 16.1 | .001 |
| HER2 positive | 7.6 | 5.79 | |
| Luminal A | 31.8 | 54.5 | |
| Luminal B | 24.2 | 20.3 | |
| Normal | 1.5 | 3.3 | |
| TNBC patients only (N=178) | |||
| Diagnosis age in years | 53.5 | 54.0 | .196 |
| Interquartile range | 48–67 | 46–62 | |
| Stage | |||
| 1 | 20.8 | 21.0 | .859 |
| 2 | 63.0 | 64.1 | |
| 3 | 16.7 | 13.6 | |
| 4 | 0 | 1.9 | |
| PAM50 Subtype | |||
| Basal-like | 91.3 | 80.6 | .842 |
| HER2 positive | 4.3 | 9.2 | |
| Luminal A | 4.3 | 4.1 | |
| Luminal B | 0 | 1.0 | |
| Normal | 0 | 5.1 | |
Figure 1. PAM50 classification by gene expression analysis.
Pie chart A- Shows all patients subdivided by race, Pie chart B- TNBC patients only subdivided by race. Dark blue indicates basal-like, red is HER2 positive, green is Luminal A, purple is Luminal B, light blue is Normal.
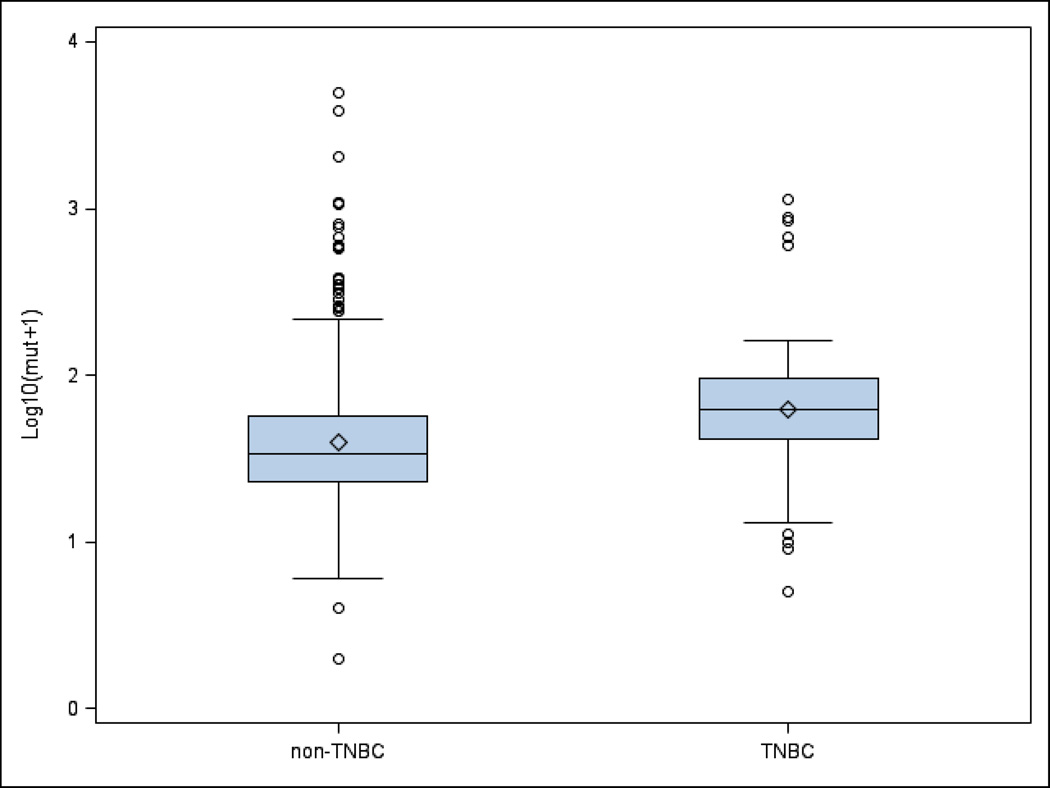
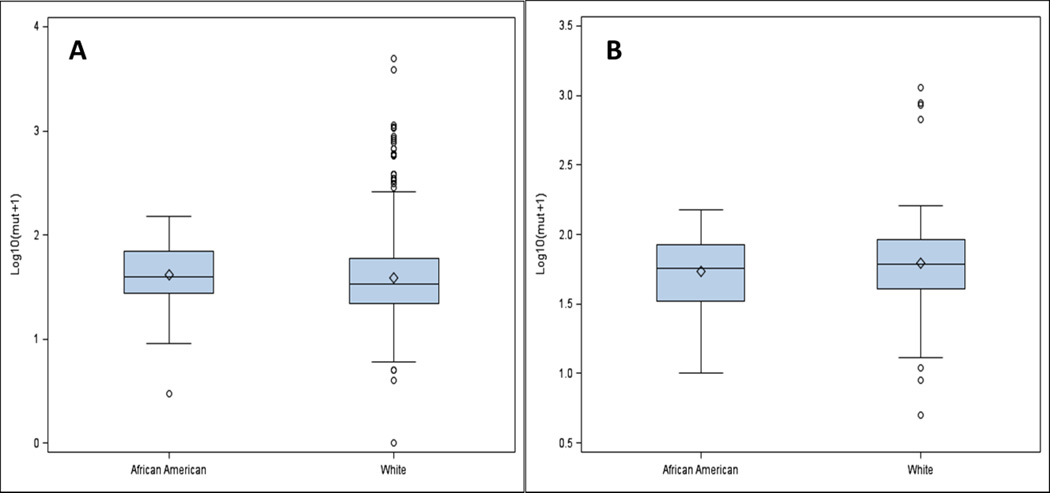
The median somatic mutation number per tumor was higher in all African American versus Caucasian patients with breast cancer overall (39.5 versus 34; p = .022). Although there were more somatic mutation numbers per tumor in the cohort of TNBC patients compared with all breast cancers (Figure 2), there were no racial differences in the TNBC cohort (56 versus 60; p = .399). Figure 3 shows the distribution of mutation numbers per tumor distribution in all patients and in TNBC patients according to race.
Figure 2. Distribution of somatic mutation number per tumor for TNBC versus non-TNBC.
Patients. Box and whisker plot shows the distribution of somatic mutation number per tumor for TNBC versus non-TNBC patients with interquartile range, median, and upper and lower quartiles.
Figure 3.
Distribution of somatic mutation number per tumor for A- all patients and B- TNBC patients only. Box and whisker plots shows the distribution of somatic mutation number per tumor for A- all patients subdivided by race, B-TNBC patients only subdivided by race with interquartile range, median, and upper and lower quartiles.
Somatic mutation analysis revealed racial differences in high prevalence (>5% in the TCGA dataset) genes (TP53, PIK3CA, MLL3) in all breast cancer patients, irrespective of clinical subtype (Table 2). TP53 alterations were observed in 46% of all African Americans versus 27% of all Caucasians; p value <0.001, PIK3CA alterations: 23% in all African Americans versus 34% in all Caucasians; p value 0.021, and MLL3 alterations: 12% in all African Americans versus 6% in all Caucasians; p value 0.034. The TNBC patients did not have any high prevalence genes associated with racial differences between African Americans and Caucasians. Table 2 also shows the differences in high prevalence genes between the TNBC versus non-TNBC patients (CDH1, GATA3, MAP3K1, PIK3CA, TP53, TTN).
Table 2.
Gene mutation frequencies by race and TNBC status
| Frequencies by race | |||
|---|---|---|---|
| Gene mutation | African American (%) |
White (%) | p |
| P53 | 46.3 | 27.3 | <.001 |
| PIK3CA | 23.1 | 33.8 | .021 |
| MLL3 | 11.6 | 6.1 | .033 |
| Frequencies by TNBC status | |||
| TNBC (%) | Non-TNBC (%) | ||
| CDH1 | 1.36 | 13.5 | <.001 |
| GATA3 | 0.68 | 11.0 | <.001 |
| MAP3K1 | 0.68 | 8.24 | <.001 |
| PIK3CA | 10.9 | 37.0 | <.001 |
| TP53 | 71.4 | 20.8 | <.001 |
| TTN | 23.3 | 15.0 | .014 |
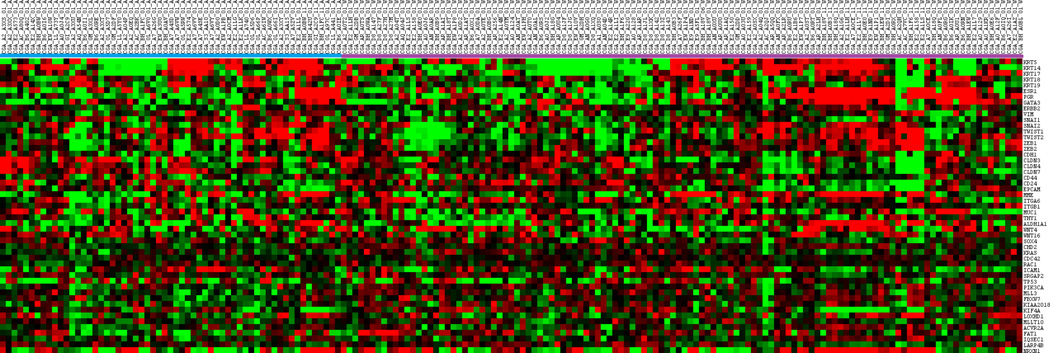
Microarray data was analyzed in order to delineate racial differences in gene expression patterns. The top 20 up- or down-regulated genes with the highest standardized mean racial differences in expression are listed in Table 3. Other important genes in selected pathways in breast cancer biology were also evaluated. Genes selected included basal markers (KRT5, KRT14, KRT 17), luminal (KRT18, KRT19, ERS1, PgR, GATA3, ERBB2), epithelial to mesenchymal transition-EMT (VIM, SNAI1, SNAI2, TWIST1, TWST2, ZEB1, ZEB2, CDH1, CLDN3, CLDN4, CLDN7), stem cell/epithelial differentiation (CD44, CD24, EPCAM, MME, ITGA6, ITGB1, MUC1, THY1, ALDH1A1, WNT4, WNT16, SOX4), and cell migration/adhesion/metastasis (CDH2, KRAS, CDC42, RAC1, ICAM1, SRGAP2). We also included our previously described somatically mutated high prevalence genes with racial differences in all patients (TP53, PIK3CA, MLL3, FBXW7, KIAA2018, KIF4A, LOXHD1, MLLT10, ACVR2A, FAT1, IQSEC1, LARP4B, NRXN1). The cluster heat map for TNBC patients showed no apparent clustering by race (Figure 4). Evaluated of expression differences in other specific genes with potential therapeutic implications in TNBC (AR, PTEN, RB) also did not reveal racial differences-data not shown.
Table 3.
Top 20 up and down-regulated DEGs
| Gene Symbol | Standardized mean difference1 |
False Discovery Rate p value |
|---|---|---|
| All patients | ||
| CRYBB2 | 1.365 | 9.96E-33 |
| FAM3A | 1.216 | 3.17E-29 |
| CROCCL1 | 1.107 | 8.86E-36 |
| SCXB | 1.102 | 2.28E-24 |
| PIF1 | 1.093 | 3.54E-35 |
| TRABD | 1.087 | 5.72E-35 |
| TSPO | 1.087 | 5.04E-24 |
| C6orf108 | 1.086 | 3.08E-26 |
| MIIP | 1.084 | 2.92E-23 |
| C21orf70 | 1.082 | 4.07E-23 |
| TOP1MT | 1.069 | 1.57E-25 |
| NACA2 | 1.065 | 3.46E-22 |
| PWP2 | 1.063 | 3.51E-21 |
| BAX | 1.063 | 1.45E-33 |
| LOC90784 | −1.327 | 7.72E-21 |
| LRRC37A2 | −1.212 | 2.48E-25 |
| C14orf167 | −1.170 | 1.16E-22 |
| ARHGEF12 | −1.119 | 1.33E-22 |
| MFAP3 | −1.076 | 8.28E-24 |
| PJA2 | −1.070 | 2.22E-23 |
| TNBC patients only | ||
| CROCCL1 | 1.328 | 3.38E-10 |
| NACA2 | 1.202 | 2.40E-06 |
| PRSS45 | 1.141 | 1.57E-05 |
| DDX51 | 1.127 | 1.93E-07 |
| LOC442459 | 1.066 | 1.11E-06 |
| OGFOD2 | 1.015 | 3.92E-06 |
| TREML4 | 1.014 | 0.000106 |
| TUBB8 | 0.993 | 7.15E-06 |
| CAPN10 | 0.976 | 1.10E-05 |
| GLI4 | 0.973 | 1.10E-05 |
| UCKL1 | 0.935 | 2.43E-05 |
| EXD 3 | 0.934 | 2.43E-05 |
| ZNF707 | 0.927 | 0.000141 |
| ZNF276 | 0.922 | 3.33E-05 |
| LOC90784 | −1.600 | 7.93E-08 |
| LRRC37A2 | −1.125 | 1.57E-05 |
| ZNF816A | −1.062 | 1.11E-06 |
| N FATC3 | −0.954 | 0.000112 |
| ADAL | −0.948 | 1.91E-05 |
| LPP | −0.944 | 2.05E-05 |
Positive numbers indicate African American with higher gene expression than Caucasians
Figure 4.
Heat map representation of selected genes in TNBC only patients. Each row represents a gene and each column represents a patient. African American (A) patients located on the right hand side, while Caucasian (C) patients located on the left. Red indicates upregulation, green indicated downregulation, black indicates no change.
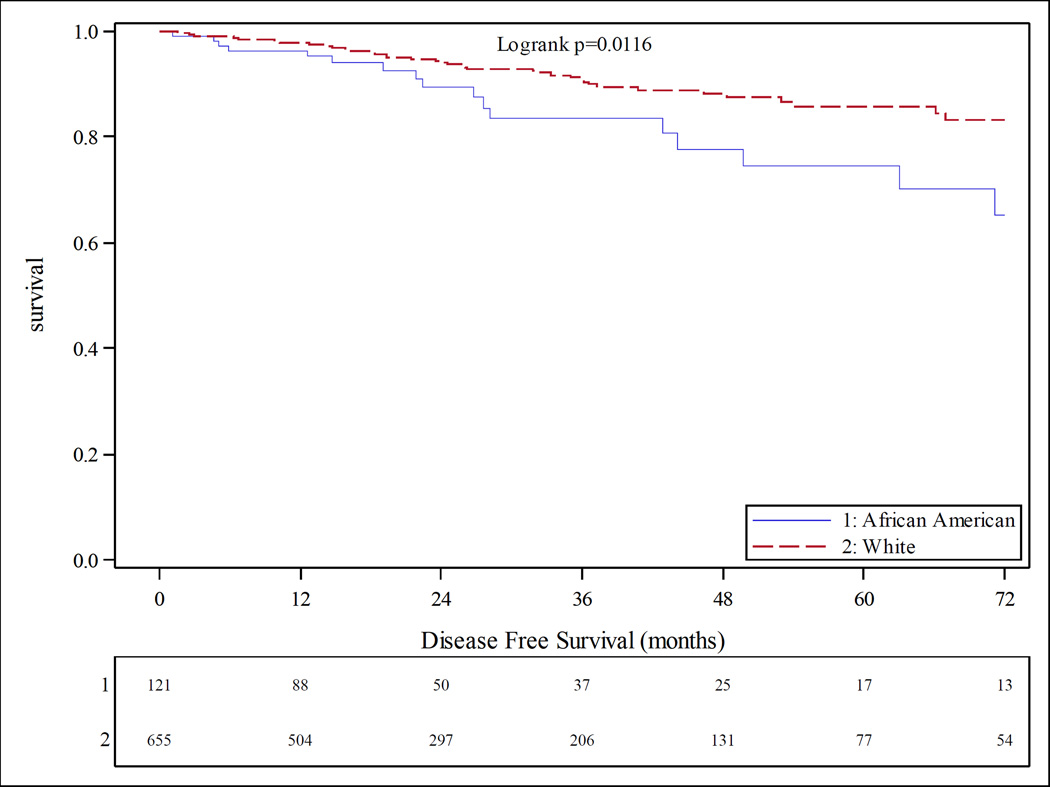
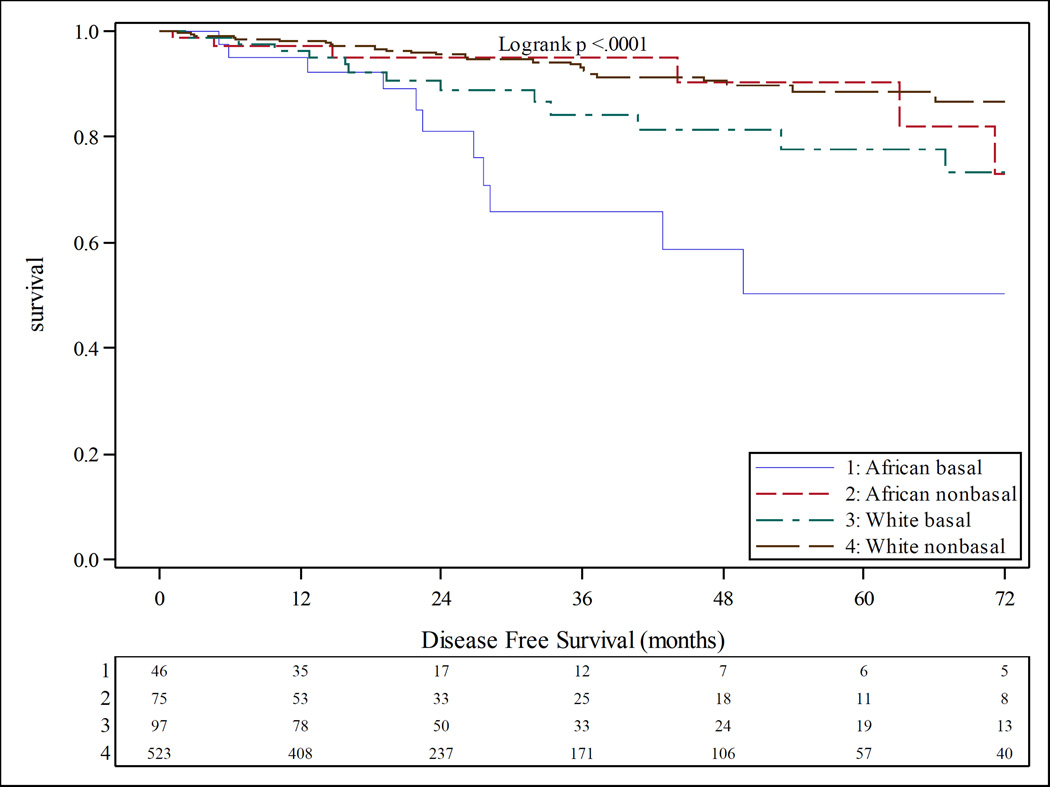
At six years of follow up, African American with breast cancer had a shorter time to progression (TTP) (hazard ratio 1.5, p value 0.012) as well as worse disease free survival (DFS) than Caucasians (Figure 5). African Americans with basal-like tumors by PAM50 subtyping had the worst DFS outcomes (Figure 6). No racial differences in TTP or DFS were observed in the TNBC patients.
Figure 5.
Kaplan-Meier product limit estimates of disease free survival stratified by race at 6 years follow-up. Blue survival curve indicates African American, red curve indicates Caucasian.
Figure 6.
Kaplan-Meier product limit estimates of disease free survival stratified by PAM50 status and race. Blue survival curve indicates African American basal-like, red curve indicates African American non basal-like, green curve indicates Caucasian basal-like, brown curve indicates Caucasian non basal-like.
Discussion
Our study is one of the few to systematically examine racial differences in molecular characteristics specifically in patients with TNBC. We evaluated racial differences in clinical characteristics and outcome, somatic mutations both quantitatively and qualitatively, as well as gene expression patterns. In the current analysis of 1,104 patients, we demonstrated racial differences in the frequency of TNBC, basal-like tumors, and number of somatic mutations per tumor in patients with unselected primary breast cancer. African Americans had a higher prevalence of TNBC, more basal-like tumors by PAM50 classification, higher somatic mutations per tumor, higher TP53 mutations, and lower PIK3CA mutations. In the TNBC cohort, there were no racial differences in clinical or molecular characteristics. These findings suggest that the mutational landscape and outcome of primary breast cancer is different between African Americans and Caucasians, but appears to be similar in both races in those with TNBC.
Our results are consistent with and build upon another large population-based study showing differences in somatic mutations in TP53, PIK3CA by race, more basal-like tumors by PAM50 classification in African Americans, and greater intratumor genetic heterogeneity in African Americans and those with HR positive disease.[15] This study also showed no racial differences in somatic mutation frequency in the TNBC cohort.
These findings have two implications. First, racial disparities in breast cancer are likely being driven by differences in those with HR positive disease. Although previous studies have shown inconsistent results regarding racial differences in outcome in patients with TNBC,[1, 8, 19, 25, 29, 32] we have previously shown that survival outcomes between African Americans and Caucasians with TNBC are similar.[26] Other large studies have shown that in those with HR positive breast cancer, survival outcomes among African Americans are significantly worse than Caucasians.[25, 32, 35] An analysis comparing the outcomes of African American with non-African Americans breast cancer patients treated on a large randomized phase III adjuvant trial, reported inferior disease free and overall survival outcomes for HR positive African Americans patients.[32] TNBC patients did not have any differences in outcome by race. Possible explanations for this phenomenon include variations in treatment due to access or compliance, comorbidities, reduced ER expression in African Americans, or higher frequency of obesity in African Americans and consequently higher levels of endogenous estrogens. It is important to note that HR positive breast cancer is also genomically diverse,[27] and it could be postulated that racial differences in this subtype may be due to underlying genomic or epigenetic instability,[24] or differences in genes involved in DNA repair[9] or hormone metabolism.[17]
Secondly, the higher frequency of TNBC in African Americans is unlikely due to differences in molecular underpinnings, but other modifiable factors. In the Carolina Breast Cancer Study (CBCS), Millikan et al. showed that TNBC was associated with different risk factors related to obesity and reproductive history compared with HR positive breast cancer. Patients with TNBC were more likely to have an advanced age at first full term pregnancy (AFFTP), higher parity combined with reduced breastfeeding duration or number of children breastfed, and early onset weight gain.[22] The authors also make reference to a proposed theory concerning the prognosis of breast cancer being predetermined by the spectrum of mutations the precursor cells obtain early in the carcinogenesis process,[2] suggesting that perhaps poor prognostic cancers, such as TNBC, have a different etiology than more favorable cancers. It is therefore feasible to speculate that African Americans exposed to certain endocrine or metabolic risk factors earlier on may be predisposed to aggressive breast cancers via early insults to precursor cells. Recent data from the Nurses’ Health Study II also showed that physical activity between menarche and AFFTP reduces the risk of breast cancer in women with a long interval between these two events,[18] also suggesting that early life exposures affect breast cancer risk. Public health interventions such as reducing obesity and promoting breastfeeding may have an impact in reducing the incidence of TNBC in African Americans.
The data from the current study must be interpreted in light of its limitations. We are not able to report the impact of sociodemographic variables on outcome in this study. While it is feasible that the worse outcome observed in African Americans with breast cancer may be due to sociodemographic factors, we would have expected to see a similar finding in the TNBC cohort if these factors explained majority of the disparities. The absence of systemic therapy information did not allow us to control for the impact of chemotherapy on clinical outcome, or even explore the association of neoadjuvant chemotherapy with mutation burden in those with residual disease. As African Americans tend to be more obese than Caucasians,[5, 14] it is possible they have worse outcomes due to reduced systemic exposure to chemotherapy from dose capping in patients with higher body surface areas.[12, 20, 30] However, poorer survival in African Americans should also have been apparent in the TNBC cohort if this was a contributory factor. We did not evaluate germline genomics, such as genes involved in immune mechanisms, inflammation, drug metabolism, etc., therefore, we cannot rule out racial differences in germline genomic that could potentially affect the tumor microenvironment or response to treatment. Lastly, the cancer registry nature of the TCGA database is not conducive to study mechanisms underlying the associations between molecular factors and clinical outcome.
In conclusion, this study did not indicate any racial differences in the molecular characteristics and clinical outcome in patients with TNBC. The known racial disparities in breast cancer outcome in HR positive patients suggest an underlying role of estrogens. These data may also help to further understand the influence of genomics in breast cancer disparities and possibly direct research to reducing the racial gap in patients with HR positive breast cancer.
Acknowledgments
Funding: This study was funded by Grant Number 1K12CA167540 through the National Cancer Institute (NCI) at the National Institutes for Health (NIH) and Grant Number UL1 TR000448 through the Clinical and Translational Science Award (CTSA) program of the National Center for Advancing Translational Sciences (NCATS) at the NIH. The authors also wish to acknowledge the support of the Siteman Cancer Center Biostatistics Core and the NCI Cancer Center Support Grant P30 CA091842. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCI, NCATS, or NIH.
Footnotes
Compliance with Ethical Standards
Ethical approval: This article does not directly contain any studies with human participants performed by any of the authors. The results published here are in whole or part based upon data generated by TCGA managed by the NCI and NHGRI. Information about TCGA can be found at http://cancergenome.nih.gov. Informed consent was obtained from all individual participants included in TCGA.
Conflict of Interest: Foluso Ademuyiwa, Yu Tao, Jingqin Luo, Cynthia Ma, and Katherine Weilbaecher declare no conflicts of interest.
References
- 1.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 2.Bernards R, Weinberg RA. A progression puzzle. Nature. 2002;418:823. doi: 10.1038/418823a. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. Jama. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease C, Prevention. Differences in prevalence of obesity among black, white, and Hispanic adults - United States, 2006–2008. MMWR. Morbidity and mortality weekly report. 2009;58:740–744. [PubMed] [Google Scholar]
- 6.Chen VW, Correa P, Kurman RJ, Wu XC, Eley JW, Austin D, Muss H, Hunter CP, Redmond C, Sobhan M, et al. Histological characteristics of breast carcinoma in blacks and whites. Cancer Epidemiol Biomarkers Prev. 1994;3:127–135. [PubMed] [Google Scholar]
- 7.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL, Prentice R. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. Journal of the National Cancer Institute. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 8.Dawood S, Broglio K, Kau SW, Green MC, Giordano SH, Meric-Bernstam F, Buchholz TA, Albarracin C, Yang WT, Hennessy BT, Hortobagyi GN, Gonzalez-Angulo AM. Triple receptor-negative breast cancer: the effect of race on response to primary systemic treatment and survival outcomes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:220–226. doi: 10.1200/JCO.2008.17.9952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duell EJ, Millikan RC, Pittman GS, Winkel S, Lunn RM, Tse CK, Eaton A, Mohrenweiser HW, Newman B, Bell DA. Polymorphisms in the DNA repair gene XRCC1 and breast cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001;10:217–222. [PubMed] [Google Scholar]
- 10.Field LA, Love B, Deyarmin B, Hooke JA, Shriver CD, Ellsworth RE. Identification of differentially expressed genes in breast tumors from African American compared with Caucasian women. Cancer. 2012;118:1334–1344. doi: 10.1002/cncr.26405. [DOI] [PubMed] [Google Scholar]
- 11.Gapstur SM, Dupuis J, Gann P, Collila S, Winchester DP. Hormone receptor status of breast tumors in black, Hispanic, and non-Hispanic white women. An analysis of 13,239 cases. Cancer. 1996;77:1465–1471. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1465::AID-CNCR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 12.Griggs JJ, Sorbero ME, Lyman GH. Undertreatment of obese women receiving breast cancer chemotherapy. Archives of internal medicine. 2005;165:1267–1273. doi: 10.1001/archinte.165.11.1267. [DOI] [PubMed] [Google Scholar]
- 13.Grunda JM, Steg AD, He Q, Steciuk MR, Byan-Parker S, Johnson MR, Grizzle WE. Differential expression of breast cancer-associated genes between stage- and age-matched tumor specimens from African- and Caucasian-American Women diagnosed with breast cancer. BMC research notes. 2012;5:248. doi: 10.1186/1756-0500-5-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson CL, Wang NY, Yeh HC, Szklo M, Dray-Spira R, Brancati FL. Body-mass index and mortality risk in U.S. blacks compared to whites. Obesity. 2014;22:842–851. doi: 10.1002/oby.20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keenan T, Moy B, Mroz EA, Ross K, Niemierko A, Rocco JW, Isakoff S, Ellisen LW, Bardia A. Comparison of the Genomic Landscape Between Primary Breast Cancer in African American Versus White Women and the Association of Racial Differences With Tumor Recurrence. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:3621–3627. doi: 10.1200/JCO.2015.62.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kroenke CH, Sweeney C, Kwan ML, Quesenberry CP, Weltzien EK, Habel LA, Castillo A, Bernard PS, Factor RE, Kushi LH, Caan BJ. Race and breast cancer survival by intrinsic subtype based on PAM50 gene expression. Breast cancer research and treatment. 2014;144:689–699. doi: 10.1007/s10549-014-2899-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature genetics. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Tobias DK, Sturgeon KM, Rosner B, Malik V, Cespedes E, Joshi AD, Eliassen AH, Colditz GA. Physical activity from menarche to first pregnancy and risk of breast cancer. International journal of cancer. 2016;139:1223–1230. doi: 10.1002/ijc.30167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund MJ, Trivers KF, Porter PL, Coates RJ, Leyland-Jones B, Brawley OW, Flagg EW, O’Regan RM, Gabram SG, Eley JW. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast cancer research and treatment. 2009;113:357–370. doi: 10.1007/s10549-008-9926-3. [DOI] [PubMed] [Google Scholar]
- 20.Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21:4524–4531. doi: 10.1200/JCO.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Ma H, Lu Y, Malone KE, Marchbanks PA, Deapen DM, Spirtas R, Burkman RT, Strom BL, McDonald JA, Folger SG, Simon MS, Sullivan-Halley J, Press MF, Bernstein L. Mortality risk of black women and white women with invasive breast cancer by hormone receptors, HER2, and p53 status. BMC cancer. 2013;13:225. doi: 10.1186/1471-2407-13-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast cancer research and treatment. 2008;109:123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24:1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 24.Ng CK, Pemberton HN, Reis-Filho JS. Breast cancer intratumor genetic heterogeneity: causes and implications. Expert review of anticancer therapy. 2012;12:1021–1032. doi: 10.1586/era.12.85. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien KM, Cole SR, Tse CK, Perou CM, Carey LA, Foulkes WD, Dressler LG, Geradts J, Millikan RC. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:6100–6110. doi: 10.1158/1078-0432.CCR-10-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacheco JM, Gao F, Bumb C, Ellis MJ, Ma CX. Racial differences in outcomes of triple-negative breast cancer. Breast cancer research and treatment. 2013;138:281–289. doi: 10.1007/s10549-012-2397-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. The New England journal of medicine. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 28.Perez CA, Zumsteg ZS, Gupta G, Morrow M, Arnold B, Patil SM, Traina TA, Robson ME, Wen YH, McCormick B, Powell SN, Ho AY. Black race as a prognostic factor in triple-negative breast cancer patients treated with breast-conserving therapy: a large, single-institution retrospective analysis. Breast cancer research and treatment. 2013;139:497–506. doi: 10.1007/s10549-013-2550-x. [DOI] [PubMed] [Google Scholar]
- 29.Sachdev JC, Ahmed S, Mirza MM, Farooq A, Kronish L, Jahanzeb M. Does race affect outcomes in triple negative breast cancer? Breast cancer : basic and clinical research. 2010;4:23–33. [PMC free article] [PubMed] [Google Scholar]
- 30.Shayne M, Crawford J, Dale DC, Culakova E, Lyman GH Group ANCS. Predictors of reduced dose intensity in patients with early-stage breast cancer receiving adjuvant chemotherapy. Breast cancer research and treatment. 2006;100:255–262. doi: 10.1007/s10549-006-9254-4. [DOI] [PubMed] [Google Scholar]
- 31.Smigal C, Jemal A, Ward E, Cokkinides V, Smith R, Howe HL, Thun M. Trends in breast cancer by race and ethnicity: update 2006. CA: a cancer journal for clinicians. 2006;56:168–183. doi: 10.3322/canjclin.56.3.168. [DOI] [PubMed] [Google Scholar]
- 32.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr, Wood WC, Davidson NE. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. Journal of the National Cancer Institute. 2012;104:406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tao L, Gomez SL, Keegan TH, Kurian AW, Clarke CA. Breast Cancer Mortality in African-American and Non-Hispanic White Women by Molecular Subtype and Stage at Diagnosis: A Population-Based Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2015;24:1039–1045. doi: 10.1158/1055-9965.EPI-15-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Partridge AH. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33:2254–2261. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright JL, Reis IM, Zhao W, Panoff JE, Takita C, Sujoy V, Gomez CR, Jorda M, Franceschi D, Hurley J. Racial disparity in estrogen receptor positive breast cancer patients receiving trimodality therapy. Breast. 2012;21:276–283. doi: 10.1016/j.breast.2011.11.003. [DOI] [PubMed] [Google Scholar]