to the editor: Utilizing intravital multiphoton microscopy, Schießl and Castrop (19) examined glomerular albumin permeability and the effect of angiotensin II. Recent data relating to glomerular albumin permeability and proximal tubule cell albumin handling have renewed the debate regarding the importance of both facets in albuminuria. The utilization of multiphoton microscopy to study this phenomenon has divided the community as initial studies by Russo et al. (13) reported a glomerular sieving coefficient for albumin (GSCA) ∼50× greater than previously reported by techniques such as micropuncture (23). The model generated by these higher glomerular albumin permeability values relies on reabsorption and transcytosis of albumin and is gaining traction as numerous investigators have delineated proximal tubule (PT) proteins whose dysfunction results in albuminuria (5, 11, 18). Notably, three different investigators using diphtheria toxin receptor insertion into PT cells showed PT injury lead to a marked increase in albuminuria with normal glomerular light and electron microscopy morphology (5, 20, 25). In particular, Sekine et al. (20) (Fig. 3), Zhang et al. (25) (Fig. S4), and the review by Grgic et al. (5) (Fig. S4) show marked proteinuria and albuminuria, well into the nephrotic range, occurring temporally with PT dysfunction following one treatment with diphtheria toxin, and disappearing with PT recovery. There were no changes in glomerular morphology at either the light (25) or electron microscopic (5) levels.
Publications by Peti-Peterdi and Tanner in 2009 (10, 21), which were referenced by Scheiβl and Castrop (19), sowed doubt and confusion about the GSCA values reported by Russo et al. (12, 13). Briefly, Peti-Peterdi reported a low GSCA value that was not measured using albumin but by a broadly dispersed 70-kDa rhodamine dextran (10). On closer inspection, this dextran generated a time-dependent GSC as the smaller molecular weight (MW) fractions were cleared out early, leaving larger MW fractions to produce increasingly lower GSCs (16). In the second study conducted by Russo et al. (12), molecular calibration using a narrowly dispersed 69.7-kDa dextran produced a GSC values of 0.025 ± 0.0041, virtually identical to those obtained by Rippe's group using fractional clearance for the same sized dextran with similar dispersion characteristics (0.022) (1).
Schieβl and Castrop also cite Tanner's editorial (21) that attributed Russo's high-albumin GSCA to an out-of-focus fluorescence contributing signal into Bowman's space when using external (nondescanned) detectors and poor physiological preparations. However, Peti-Peterdi in all his studies (8, 10, 14) and Scheiβl and Castrop (19) used external (nondescanned) and generated very low GSCA numbers. This rules out implicating out-of-focus fluorescence emanating from the use of external detectors as the reason for our higher GSCA. Typically, out-of-focus fluorescence emission is not a problem with two-photon excitation due to the physics of the light generation at the point of excitation (2, 16). However, Theer and Denk (22) describe how out-of-focus fluorescence can become a problem at very deep imaging depths (+900 μm). Measurements made in the kidney for our studies are acquired at depths of 15–25 μm, so this is not a concern. Finally the use of internal detectors, compared with external nondescanned detectors, was shown (16) to decrease sensitivity, partially explaining the reported reduction in GSC. Numerous manuscripts from our laboratory (12, 13, 15–17) challenged Tanner's editorial, citing poor physiological preparations because our techniques are consistent, multiple parameters are measured and controlled, and values remain virtually identical between studies. In a paper by Comper and Russo (4), the GSCA for individual glomeruli remained time independent for the 90 min they were observed. Moreover, other studies done in collaboration with Tanner, using the same techniques, resulted in stable physiological parameters for several hours (3, 6, 7).
We believe the lack of agreement within published multiphoton data regarding GSCA is primarily due to factors affecting sensitivity in digital microscope systems that are not widely known or utilized (9). Our study on the effects of photodetector offset provides extensive characterization both in vitro and in vivo and was carried out using gradually increasing offset values (17). This lowers the system's ability to detect low-intensity values, thus minimizing signal recording. In our study, in vitro sensitivity calibration curves using a series of fluorescent albumin concentrations revealed a marked decrease in detector sensitivity with progressively increasing offsets in which more and more pixel values in the image are forced to zero. In in vivo studies looking at GSCA with the same series of offsets in identical glomeruli, the average GSC dropped from a peak 0.0152 ± 0.0047 for offset 38 (typically used in our studies), to 0.00017 ± 0.00003 for offset 52, where background values are essentially zero (see Fig. 1). In studies by Nakano et al. (8), they adjusted the detector offset for each individual glomerulus pre- and postinfusion of albumin to a lower background. For quantitative imaging, the offset is set once and should not be adjusted for the duration of the experiment (9, 24). Nakano et al. (8) may have done this in an effort to force tissue autofluorescence intensities to fall within those of electronic noise or even cell culture autofluorescence. However, intravital tissues are rich in naturally occurring autofluorescent structures, and adjusting the offset to force the fluorescence of these structures to zero results in a marked decrease in sensitivity, thus minimizing albumin detection as we have documented (17).
Fig. 1.
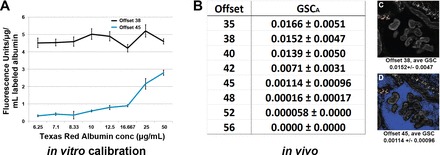
Photodetector offset influences glomerular albumin permeability values dramatically. The offset or “black level” settings determines the lower detection limits on the photomuliplier tube (PMT) detectors found in many laser scanning confocal and multi-photon microscopes. A: effect on detector sensitivity using 2 different offsets on a range of background subtracted Texas red albumin (TRA) solutions in vitro. The average intensity readings from the images were divided by the concentration of TRA to give a direct unit of sensitivity. This unit should be constant across the concentration range if sensitivity is unaffected, as shown when offset is set to 38. Using a higher numerical offset of 45, which forces background values closer to zero, markedly decreases the microscope's ability to correctly detect fluorescence in identical dishes. With detector sensitivity decreased particularly at lower TRA concentrations, the sensitivity value is no longer constant across the range for offset 45. B: list of background subtracted, glomerular sieving coefficients for albumin (GSCA) collected at different offsets for the same set of glomeruli. Note the rapidly decreasing GSCA seen starting at offset 42. There is a 262-fold decrease in GSCA in a comparison of offsets 38 and 52. C and D: identical images of a glomerulus after infusion of TRA, taken at offsets 38 and 45, respectively. The blue color seen in Bowman's space in D (offset 45) indicates the values located therein have a value of zero before background subtraction. Colors alerting the user to pixels in the image having intensity values above or below the detection limit of the detectors are standard in every microscope system (typically red for saturated values and blue or green for values at zero). The GSC for TRA in these experiments, where only the offset was changed for the image, averaged 0.015 ± 0.0047 using offset 38 and 0.00114 ± 0.00096 using offset 45. Values in this, and all of our studies, were background subtracted, with a reference image taken at the corresponding offsets.
The current work of Schieβl and Castrop (19) appears to have used settings causing this same lack of sensitivity produced when using higher offsets. Evaluation of a digital copy of Fig. 1B from their manuscript when it was downloaded, color decoded, blue channel selected, and thresholding was done of the corresponding blue region of the glomerulus revealed ∼87.3% of the pixels had a reading of zero. This would correspond to an offset of 42–45 in our study (17). This places the sensitivity well below the linear range for filtered albumin in an extrapolation to our study. (Note: the blue color warns the user the highlighted pixels have an intensity value of zero). To correctly adjust the offset, one should instead generate a “pre-” background image before infusion of a fluorescent compound and use those images to subtract the background values from identical image planes and regions taken postinfusion (15). The offsets should be set so that only a few pixels randomly display a value of zero (blue) and are not adjusted further. Indeed, factors critical in measuring GSCA were validated in our two previous studies (16, 17). Nuances in novel technologies such as the correct photodetector offset, critical in multiphoton microscopy, can lead to generation of erroneous data when inappropriately set. These parameters are not widely understood and must be identified and better established in future studies.
DISCLOSURES
No conflicts of interest, financial or otherwise, ad declared by the authors.
AUTHOR CONTRIBUTIONS
R.M.S. and B.A.M. drafted the manuscript; R.M.S. and B.A.M. prepared figures; R.M.S. and B.A.M. edited the manuscript; R.M.S. and B.A.M. revised the manuscript; R.M.S. and B.A.M. approved the final version of the manuscript.
REFERENCES
- 1.Asgeirsson D, Venturoli D, Fries E, Rippe B, Rippe C. Glomerular sieving of three neutral polysaccharides, polyethylene oxide and bikunin in rat. Effects of molecular size and conformation. Acta Physiol 191: 237–246, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Centonze VE, White JG. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys J 75: 2015–2024, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choong FX, Sandoval RM, Molitoris BA, Richter-Dahlfors A. Multiphoton microscopy applied for real-time intravital imaging of bacterial infections in vivo. Methods Enzymol 506: 35–61, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comper WD, Russo LM. The glomerular filter: an imperfect barrier is required for perfect renal function. Curr Opin Nephrol Hypertens 18: 336–342, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Grgic I, Campanholle G, Bijol V, Wang C, Sabbisetti VS, Ichimura T, Humphreys BD, Bonventre JV. Targeted proximal tubule injury triggers interstitial fibrosis and glomerulosclerosis. Kidney Int 82: 172–183, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melican K, Boekel J, Mansson LE, Sandoval RM, Tanner GA, Kallskog O, Palm F, Molitoris BA, Richter-Dahlfors A, Richter-Dahlfors A. Bacterial infection-mediated mucosal signalling induces local renal ischaemia as a defence against sepsis. Cell Microbiol 10: 1987–1998, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Melican K, Sandoval RM, Kader A, Josefsson L, Tanner GA, Molitoris BA, Richter-Dahlfors A. Uropathogenic Escherichia coli P and Type 1 fimbriae act in synergy in a living host to facilitate renal colonization leading to nephron obstruction. PLoS Pathog 7: e1001298, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, Nishiyama A, Peti-Peterdi J. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol 23: 1847–1856, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawley J. Editor. Handbook of Biological Confocal Microscopy. New York: Springer, 2006. [Google Scholar]
- 10.Peti-Peterdi J. Independent two-photon measurements of albumin GSC give low values. Am J Physiol Renal Physiol 296: F1255–F1257, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rangel-Filho A, Lazar J, Moreno C, Geurts A, Jacob HJ. Rab38 modulates proteinuria in model of hypertension-associated renal disease. J Am Soc Nephrol 24: 283–292, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russo LM, Sandoval RM, Campos SB, Molitoris BA, Comper WD, Brown D. Impaired tubular uptake explains albuminuria in early diabetic nephropathy. J Am Soc Nephrol 20: 489–494, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, Molitoris BA, Comper WD. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int 71: 504–513, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, Bates DO, Peti-Peterdi J. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol 23: 1339–1350, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval RM, Molitoris BA. Quantifying glomerular permeability of fluorescent macromolecules using 2-photon microscopy in Munich Wistar rats. J Visual Exp 17: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandoval RM, Wagner MC, Patel M, Campos-Bilderback SB, Rhodes GJ, Wang E, Wean SE, Clendenon SS, Molitoris BA. Multiple factors influence glomerular albumin permeability in rats. J Am Soc Nephrol 23: 447–457, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandoval RM, Wang E, Molitoris BA. Finding the bottom and using it. Intra Vital 2: 1–9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarav M, Wang Y, Hack BK, Chang A, Jensen M, Bao L, Quigg RJ. Renal FcRn reclaims albumin but facilitates elimination of IgG. J Am Soc Nephrol 20: 1941–1952, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schieβl IM, Castrop H. Angiotensin II AT2 receptor activation attenuates AT1 receptor-induced increases in the glomerular filtration of albumin: a multiphoton microscopy study. Am J Physiol Renal Physiol 305: F1189–F1200, 2013. [DOI] [PubMed] [Google Scholar]
- 20.Sekine M, Monkawa T, Morizane R, Matsuoka K, Taya C, Akita Y, Joh K, Itoh H, Hayashi M, Kikkawa Y, Kohno K, Suzuki A, Yonekawa H. Selective depletion of mouse kidney proximal straight tubule cells causes acute kidney injury. Transgenic Res 21: 51–62, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanner GA. Glomerular sieving coefficient of serum albumin in the rat: a two-photon microscopy study. Am J Physiol Renal Physiol 296: F1258–F1265, 2009. [DOI] [PubMed] [Google Scholar]
- 22.Theer P, Denk W. On the fundamental imaging-depth limit in two-photon microscopy. J Opt Soc Am A Opt Image Sci Vis 23: 3139–3149, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Tojo A, Endou H. Intrarenal handling of proteins in rats using fractional micropuncture technique. Am J Physiol Renal Fluid Electrolyte Physiol 263: F601–F606, 1992. [DOI] [PubMed] [Google Scholar]
- 24.Wilson LIMP, Sullivan KF, Kay SA. Green fluorescent proteins. Methods Cell Biol 58: 31–48, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Zhang MZ, Yao B, Yang S, Jiang L, Wang S, Fan X, Yin H, Wong K, Miyazawa T, Chen J, Chang I, Singh A, Harris RC. CSF-1 signaling mediates recovery from acute kidney injury. J Clin Invest 122: 4519–4532, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


