Abstract
Sonic hedgehog (Shh) is found within gastric parietal cells and processed from a 45-kDa to a 19-kDa bioactive protein by an acid- and protease-dependent mechanism. To investigate whether Shh is associated with the parietal cell membrane compartment that becomes exposed to both acid and proteolytic enzymes during acid secretion, the cellular location of Shh within resting and stimulated gastric parietal cells was examined. Immunofluorescence microscopy of rabbit stomach sections showed that Shh colocalized predominantly with parietal and pit, not chief/zymogen or neck, cell markers. In resting and histamine-stimulated rabbit gastric glands Shh was expressed only in parietal cells close to H+-K+-ATPase-containing tubulovesicular and secretory membranes with some colocalizing with γ-actin at the basolateral membrane. Gastric gland microsomal membranes were prepared by differential and sucrose gradient centrifugation and immunoisolation with an anti-H+-K+-ATPase-α subunit antibody. The 45- and 19-kDa Shh proteins were detected by immunoblot in immunopurified H+-K+-ATPase-containing membranes from resting and stimulated gastric glands, respectively. Incubating glands with a high KCl concentration removed Shh from the membranes. Histamine stimulated 19-kDa Shh secretion from gastric glands into the medium. In human gastric cancer 23132/87 cells cultured on permeable membranes, histamine increased 19-kDa Shh secretion into both apical and basolateral media. These findings show that Shh is a peripheral protein associated with resting and stimulated H+-K+-ATPase-expressing membranes. In addition, Shh appears to be expressed at or close to the basolateral membrane of parietal cells.
Keywords: rabbit gastric glands, tubulovesicles, 23132/87 cells, transepithelial electrical resistance, pepsinogen A, processing
the stomach, in particular parietal cells, is not only the site of acid secretion but also is known to secrete numerous factors that modulate the growth and differentiation of the gastric epithelium. Such factors include TGF-α, Wnt, FGFs, amphiregulin, heparin-binding EGF, and sonic hedgehog (Shh) (1, 7, 22, 29). One favored explanation linking gastric atrophy and progression to cancer is that it is the loss of these factors that subsequently leads to disruption of normal cell proliferation and differentiation.
The Shh signaling pathway, fundamental to cell differentiation during early gastric development (19, 25), is also expressed in the adult stomach, where it is believed to regulate epithelial cell differentiation (30, 31). Shh is present in the adult oxyntic mucosa, in particular within parietal cells (29, 31). Unlike Shh from several nonmammalian species that is processed via autocatalytic cleavage of the 45-kDa nascent protein to smaller biologically active molecules of 19 and 25 kDa (5, 21, 24), in gastric parietal cells Shh is processed and secreted by a hormonally regulated (24, 29), acid- and protease-dependent [pepsinogen A (PgA)] mechanism (35). Consistent with this recently discovered processing mechanism are reports showing that Shh expression is also regulated by changes in acidity (8, 9). However, the cellular location of Shh within the parietal cell and the mechanism by which it is secreted when the cell is stimulated are unknown. The present study further characterizes the processing and secretion mechanisms by determining the precise location of the Shh protein in resting and stimulated gastric parietal cells using immunomicroscopy and immunodetection of Shh in subcellular membrane fractions from rabbit gastric glands.
A unique feature of the parietal cell is the secretory canaliculus, a membrane network that branches into the cytoplasm with one or more openings to the gastric lumen (15). In resting parietal cells, the H+-K+-ATPase resides in tubulovesicles within the cytoplasm adjacent to a small collapsed canaliculus. Following stimulation the tubulovesicles fuse with the canaliculus and apical membrane, leading to a large expanded secretory canaliculus containing the H+-K+-ATPase or H+ pump and Cl− (ClC-2) and K+ (various) channels. This morphological transformation together with activation of the channels result in increased acid secretion (10, 15, 16, 27). Using confocal microscopy the present study shows that Shh is localized in parietal cells in rabbit gastric glands. In particular, Shh appeared to colocalize with intracellular H+-K+-ATPase-containing tubulovesicles in resting parietal cells and with the expanded H+-K+-ATPase-containing secretory canaliculus when stimulated with the acid secretagogue histamine. Cellular fractionation indicated that 45-kDa and 19-kDa Shh proteins were present in resting and stimulated H+-K+-ATPase-containing membranes, respectively, but could be removed by high salt incubations. Expression of Shh at the basolateral membrane of parietal cells was also evident, colocalizing with γ-actin. Histamine stimulated Shh secretion as the 19-kDa peptide from gastric glands. Moreover, in a polarized Shh- and H+-K+-ATPase-expressing human gastric cancer cell line (23132/87) (8) cultured on permeable membranes, histamine stimulated secretion of the 19-kDa processed protein into the apical and basolateral media.
MATERIALS AND METHODS
Rabbit gastric gland isolation and treatment.
Gastric glands were isolated from anesthetized New Zealand White rabbits as previously described (2). Briefly, rabbits (1–1.5 kg) were anesthetized with ketamine (50 mg/kg) and xylazine (5 mg/kg). The aorta was then exposed and clamped, and a catheter was inserted for perfusion with PBS at high pressure. After the stomach was cleared of blood, it was collected and washed in PBS. The oxyntic mucosa was stripped off and cut into small pieces, then digested in MEM (Fisher Scientific) supplemented with 2 mg/ml glucose, 20 mM HEPES, 0.125 mg/ml collagenase (Sigma) and 2 mg/ml BSA (Sigma). Intact glands were allowed to settle for 15 min and were washed three times with MEM medium. Isolated gastric glands were then treated with cimetidine (10−4 M, resting) or histamine (10−4 M, stimulated) for 30 min and analyzed by confocal microscopy or Western blot. Media from resting and stimulated gastric glands were collected and subjected to gel filtration chromatography for analysis of secreted Shh. All rabbit studies were approved by the University of Cincinnati Institutional Animal Care and Use Committee, which maintains an American Association of Assessment and Accreditation of Laboratory Animal Care facility.
Immunofluorescence staining for confocal microscopy.
Longitudinal sections of rabbit fundic gastric tissue were fixed in 4% paraformaldehyde/PBS and paraffin embedded, and 5-μm sections were prepared. Antigen retrieval was performed after deparaffinization by heating the slides for 10 min at 100°C in 0.01 M sodium citrate. Nonspecific antigenic sites were blocked with 5% BSA in Tris buffered saline/0.1% Tween 80 (TBS-T) for 30 min before incubating with a 1:200 dilution of monoclonal 5E1 anti-Shh antibody (Developmental Studies Hybridoma Bank, University of Iowa) at 4°C for 16 h followed by a 1-h incubation with a 1:100 dilution of anti-mouse Alexa Fluor 555 secondary antibody (Invitrogen/Molecular Probes). Tissue was then counterstained with 1 μg H+-K+-ATPase-α subunit (Affinity BioReagents) antibody that was prelabeled with Alexa Fluor 488 IgG1 mouse Zenon primary antibody kit (Invitrogen/Molecular Probes), 20 μg/ml Alexa Fluor 488 Griffonia simplicifolia (GSII) (Invitrogen/Molecular Probes), or Ulex europaeus (UEAI) FITC conjugate (Sigma). To detect chief cells, tissue immunostained for Shh and parietal cells was then counterstained with 1:500 sheep anti-PgA (Acris) followed by a 1-h incubation with a 1:100 dilution of anti-sheep Alexa Fluor 633 antibody (Invitrogen/Molecular Probes) at room temperature.
Gastric glands settled on poly-d-lysine-coated coverslips and 23132/87 cells grown on Transwells were fixed with 4% paraformaldehyde, and nonspecific antigen sites were blocked with 5% BSA in TBS-T for 1 h before incubation for 16 h with a 1:200 dilution of monoclonal 5E1 anti-Shh antibody at 4°C followed by a 1-h incubation with a 1:100 dilution of anti-mouse Alexa Fluor 488 secondary antibody (Invitrogen/Molecular Probes). Gastric glands were then counterstained with 1 μg of H+-K+-ATPase-α subunit antibody that was prelabeled with Alexa Fluor 555 IgG1 mouse Zenon primary antibody kit for 1 h at room temperature, or 5 μg/μl Alexa Fluor 633 Phalloidin (Invitrogen, Molecular Probes) according to the manufacturer's protocol. 23132/87 cells were also immunostained with 1:200 dilution of mouse anti-Shh 5E1 or mouse anti-zonula occludens (ZO)-1 (FITC) antibody (Zymed) at a dilution of 1:50 for 1 h at room temperature. All coverslips were mounted onto slides with Prolong Gold Antifade Reagent Mounting Medium (Molecular Probes) and analyzed with a Zeiss LSM510 META confocal microscope. The average fluorescence intensity was obtained by use of Metamorph software (Universal Imaging, West Chester, PA). The cytoplasm of each individual cell was outlined and the average fluorescence intensity was measured in the Shh, H+-K+-ATPase, PgA, GSII, or UEAI marker channel. Background was subtracted using the average fluorescence intensity in each channel for a negative region.
Flow cytometry.
Rabbit gastric cells were isolated from anesthetized New Zealand White rabbits as previously described (18). After collagenase digestion of the rabbit gastric mucosa described above, cellular debris was removed by straining the gland suspension through a 40-μm cell strainer (BD Biosciences). Dissociated gastric cells that were left in the suspended medium (after glands had settled) were collected, washed and permeabilized using the Fix & Perm Cell Permeabilization Kit (CALTAG Laboratories) according to the manufacturer's protocol. Cells were then labeled using 1:200 dilution of 5E1 Shh antibody for 30 min at room temperature, washed (PBS/0.1% BSA), and stained using 1:200 dilution of phycoerythrin (PE)/Cy5.5-conjugated anti-mouse secondary antibody (CALTAG Laboratories). Cells were then counterstained with 1 μg of H+-K+-ATPase-α subunit antibody that was prelabeled with R-PE IgG1 mouse Zenon primary antibody kit for flow cytometry (Invitrogen/Molecular Probes), 1:500 sheep PgA antibody followed by 1:200 dilution of anti-sheep FITC secondary antibody (CALTAG Laboratories) or 5 μg/μl Alexa Fluor 488 phalloidin. Cells labeled with the isotype controls (sheep IgG FITC, mouse IgG1 PE-Cy5.5, mouse IgG1 R-PE) were included in all experiments. Cells were labeled with the appropriate primary, secondary, or conjugated antibodies for 30 min at room temperature. Labeled cells were then analyzed by cytometry using a Coulter Elite ESP Cell Sorter (Beckman-Coulter Electronics, Hialeah, FL). A total of 10,000 cells per gastric preparation were analyzed. Positive cells were gated from the total gastric cell preparation and reported as a percent of the 10,000 cells counted.
Isolation and immunopurification of H+-K+-ATPase-containing membranes.
Protein (∼32 mg total) was extracted from 3 ml of resting and stimulated rabbit gastric glands by homogenizing the cells in lysis buffer containing 300 mM NaCl, 30 mM Tris, 2 mM MgCl2, 2 mM CaCl2, 1% Triton X-100, pH 7.4 and supplemented with protease inhibitor tablets (Roche). Microsomal membranes were prepared as described by Karvar et al. (18). The entire homogenate was centrifuged sequentially at 3,200 g for 10 min (P1: nuclei, plasma membranes), 11,000 g for 10 min (P2: mitochondria) and 100,000 g for 1 h (P3: H+K+-ATPase tubulovesicles, endoplasmic reticulum). The supernatant from the P3 centrifugation was also analyzed as the cytosolic fraction (cyt) (18). P1 (stimulated) and P3 (resting) fractions that contained the majority of the H+-K+-ATPase were then further purified on a discontinuous sucrose gradient [20, 27, and 33% (wt/vol) sucrose] and centrifuged at 100,000 g for 2 h. Membrane fractions from the 10–20% (S20), 20–27% (S27), and 27–33% (S33) gradient interface and sucrose pellet were collected, diluted 1:5 with 10% sucrose, and centrifuged at 100,000 g for 1 h. The S20 pellets from resting and stimulated glands were resuspended in 500 μl of PBS containing protease inhibitor tablets (Roche) and used for the immunoisolation.
For immunoisolation as described by Calhoun and Goldenring (6), 750 μg of magnetic Dynabeads were washed three times in PBS/0.1% BSA (wt/vol), blocked with PBS/1% BSA at 4°C for 30 min, washed 2 times with PBS/0.1% BSA and then coated with either nonimmune IgG2b or a monoclonal anti-H+-K+-ATPase-α subunit (Affinity BioReagents) antibody overnight at 4°C. The magnetic beads were then washed three times at room temperature with PBS/0.1% BSA and incubated with the entire 500 μl resting or stimulated gastric membranes collected from the 10–20% sucrose gradient interface at 4°C for 16 h. Beads were then washed three times with PBS and resuspended in 40 μl SDS loading buffer, boiled and analyzed for expression of H+-K+-ATPase and Shh by Western blot.
Gel filtration chromatography.
Shh was detected in apical and basolateral media using previously published methods (29, 36). Media were concentrated to 0.5 ml on Centricon YM-10 spin columns (Millipore, Bedford, MA) and loaded onto a Superose-12 gel filtration column that was equilibrated with 0.01% NP-40 in PBS. The molecular mass standards used to calibrate the column were albumin (67 kDa), ovalbumin (43 kDa), chymotrypsinogen A (25 kDa), and ribonuclease A (13.7 kDa). Fractions 32–44 (500 μl fractions) were collected after the void volume, and every two fractions were pooled and immunoprecipitated as previously described (36) by using the monoclonal 5E1 anti-Shh antibody (Developmental Studies Hybridoma Bank, University of Iowa). Protein A/G agarose beads were then resuspended in 20 μl of SDS-loading buffer, boiled, and analyzed by Western blot using a rabbit anti-Shh antibody (Santa Cruz Biotechnology, H-160).
Identification of integral and peripheral membrane proteins.
To characterize Shh as an integral or peripheral membrane protein salt sensitivity was determined. Resting and stimulated gastric glands were washed in buffered isotonic sucrose (300 mM sucrose, 5 mM Tris, 0.2 mM EDTA, pH 7.5) and then suspended in this medium containing 0, 50, 100, or 150 mM KCl. Each tube containing the glands in various KCl concentrations were then permeabilized with digitonin (20 μg/ml) by gently shaking at 37°C for 0, 5, and 10 min. After digitonin permeabilization glands were centrifuged and equal amounts (10 μg) of each pellet and supernatant sample were analyzed for H+-K+-ATPase and Shh by Western blot.
Cell culture conditions and TEER measurements.
Human gastric adenocarcinoma 23132/87 cells purchased from DSMZ (German Collection of Microorganisms and Cell Cultures) were grown in DMEM-medium (Fisher Scientific) supplemented with 10% fetal calf serum and 5% penicillin-streptomycin. Cells were seeded at a density of 3 X 105 cells/tissue culture insert (Transwell permeable membrane supports, 0.4 μM polyester membrane, 12-well plate, Costar Corning). Medium was changed every 24 h and cells were cultured for 7 days until they reached maximum stable resistance. Transepithelial electrical resistance (TEER) was measured via an epithelial-volt-ohm-meter (EVOM, World Precision Instruments). Histamine (100 μM) was added to either the apical or basolateral side of the cells for 6 h. Medium was collected from both the apical and basolateral compartments of the Transwell, concentrated, and subjected to gel filtration chromatography. Shh immunoreactivity was then examined by Western blot.
Western blot analysis.
Samples were loaded on a 4–20% SDS-polyacrylamide gradient gel. Protein was transferred to membranes (Hybond-C Extra Nitrocellulose, Fisher Scientific) which were then blocked with Detector Block (KPL, Gaithersburg, MD) for 1 h at room temperature followed by an overnight incubation with a 1:200 dilution of the goat polyclonal anti-Shh (Santa Cruz, sc-1194) or 1:4,000 anti-mouse H+-K+-ATPase-α subunit (Affinity BioReagents). The membranes were washed three times for 5 min and incubated for an additional 1 h at room temperature with a 1:2,000 dilution of Alexa Fluor 680 secondary anti-goat, or mouse antibodies (Invitrogen, Molecular Probes). The same membrane was analyzed with antibodies specific for first H+-K+-ATPase-α subunit and then Shh. Proteins were visualized and quantified with the Odyssey Infrared Imaging System software.
Statistical analysis.
Significance of the results was tested by an unpaired t-test with commercially available software (GraphPad Prism, GraphPad Software, San Diego, CA). A P value <0.05 was considered significant.
RESULTS
Expression of Shh within gastric parietal and pit cells.
The expression pattern of Shh in rabbit fundic gastric mucosal cells was investigated by using 5-μm sections immunostained for Shh and a combination of parietal (H+-K+-ATPase), neck (GSII), pit (UEAI), and chief (PgA) cell markers as shown in Fig. 1. Shh clearly stained parietal cells (Fig. 1A, red; Supplemental Fig. S1), which are readily identified by their distinctive morphology, abundance within the neck compartment of the gastric unit, and staining with anti-H+-K+-ATPase (Fig. 1B, green). PgA-positive cells (Fig. 1C, blue) did not stain with Shh. Figure 1D shows the same section with the three immunostains merged and clearly shows that Shh colocalizes (yellow) with H+-K+-ATPase in the parietal cells and does not localize in the chief cells where PgA is found (see inset in Fig. 1D). GSII lectin-positive cells (green) localized to the neck region of the gastric unit and in close proximity to the parietal cells (Fig. 1, E–H) that were virtually devoid of Shh (red) and PgA (blue). Identification of gastric pit cells using UEAI lectin immunostaining (green) revealed that there was some expression of Shh in the pit cell region of the gastric gland (Fig. 1, I–L; Supplemental Fig. S1). To semiquantify these findings the average fluorescence intensity per cell for each marker was measured. Figure 2 shows scatterplots of Shh vs. H+-K+-ATPase (parietal cells), PgA (chief cells), UEAI (pit cells), or GSII (neck cells) (Fig. 2). H+-K+-ATPase positive parietal cells were positive for Shh (Fig. 2A), as were UEAI lectin-positive pit region cells (Fig. 2B). In contrast, PgA-positive chief cells (Fig. 2C) and GSII-positive neck cells (Fig. 2D) were less often positive for or virtually devoid of significant Shh. These findings were also supported by coefficients of variance for normalized ratios of markers to Shh: 34% for parietal cells, 45% for pit cells, 69% for chief cells, and 60% for neck cells.
Fig. 1.
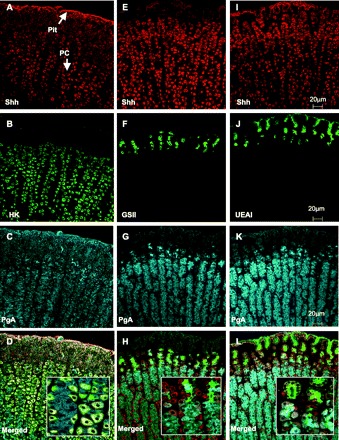
Expression of sonic hedgehog (Shh) and cell lineage markers in the rabbit gastric mucosa. Rabbit stomach was sectioned and immunostained for Shh (A, E, I, red) together with cell markers: H+-K+-ATPase (HK) for parietal cells (B, green), Griffonia simplicifolia (GSII) for neck cells (F, green), Ulex europaeus (UEAI) for pit cells (J, green), and pepsinogen A (PgA) for chief cells (C, G, K, blue). Merged images are shown in D for Shh/H+-K+-ATPase/PgA, L for Shh/UEAI/PgA, and H for Shh/GSII/PgA, and high-power magnification is shown in the insets. Arrows indicate expression of Shh in parietal cells (PC) and pit cells. Colocalization is indicated by yellow color. Scale bar indicated is the same for A–L.
Fig. 2.
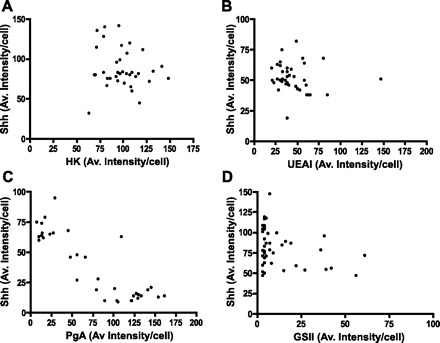
Distribution of Shh within the rabbit gastric mucosa. Scatterplots of average fluorescence intensity/cell quantified from 3 individual rabbit stomach sections as shown in Fig. 1 showing Shh vs. H+-K+-ATPase (HK) (parietal cells; A), UEAI (pit cells; B), PgA (chief cells; C), and GSII (neck cells; D). For each section 10–15 cells were quantified for a total of 3 sections (30–45 cells total). Av., average.
Rabbit gastric cells prepared by enzymatic digestion were also immunostained for Shh, H+-K+-ATPase, PgA, and F-actin (phalloidin) and analyzed by flow cytometry. Figure 3A shows the shift in the peak representing cells positive for Shh compared with the isotype control (negative control or background). Light scatter analysis of these Shh positive gated cells shows that 99.51% of cells were also positive for H+-K+-ATPase and F-actin (Fig. 3B). In Fig. 3C light scatter analysis of Shh-positive cells then gated for H+-K+-ATPase and PgA shows that only 1.18% of the cells were positive for these three markers, whereas 98.80% of the cells were positive for Shh and H+-K+-ATPase, and virtually none (0.02%) of the cells were positive for Shh and PgA. Summarized data from three gastric cell preparations are shown in Fig. 3D. Of the Shh-positive cells, 95.0 ± 2.1% also expressed H+-K+-ATPase and F-actin. Significantly fewer (P < 0.0005) Shh-positive cells, only 4.7 ± 1.8%, also expressed H+-K+-ATPase and PgA, which may be a measure of background staining. These data are consistent with the confocal images shown in Figs. 1 and 2 and demonstrate that Shh is predominantly expressed within the parietal and pit cells, not in chief or neck cells.
Fig. 3.
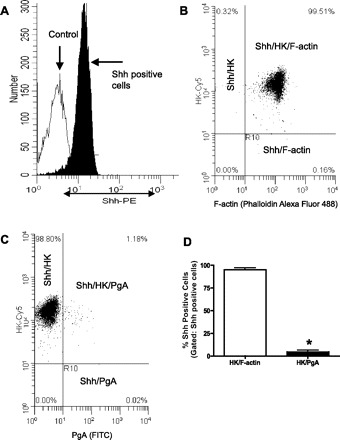
Analysis of Shh-expressing cells in a crude gastric cell preparation by flow cytometry. A: distribution of Shh-positive cells in a crude gastric cell preparation. The gated positive Shh cells shift away from the isotype (negative) control. PE, phycoerythrin. Light scatter analysis of Shh gated cells additionally gated for H+-K+-ATPase and F-actin (B) and for H+-K+-ATPase and PgA (C). D: summary of data expressed as percentage of Shh-positive cells that also express H+-K+-ATPase/F-actin and H+-K+-ATPase/PgA from 3 individual rabbit gastric cell preparations. *P < 0.0005 compared with Shh/H+-K+-ATPase/F-actin-positive cells.
Distribution of Shh in rabbit gastric glands.
Shh has been shown to be processed and secreted from parietal cells, a process that is dependent on stimulation of acid secretion and activation of PgA to the active enzyme pepsin A (35). Such findings suggest that Shh protein may be processed and secreted at the apical membrane of parietal cells. To further investigate the acid- and protease-dependent processing mechanism, the present study focused on the apical expression of Shh within parietal cells. The cellular distribution of Shh within parietal cells was studied in resting and stimulated rabbit gastric glands immunostained for Shh (red), H+-K+-ATPase (green), and F-actin (blue) and analyzed by confocal microscopy. The results are shown in Fig. 4. H+-K+-ATPase and Shh were clearly evident in parietal cells of both resting (Fig. 4, A and B) and stimulated (Fig. 4, D and E) rabbit gastric glands. H+-K+-ATPase and Shh appeared to colocalize to the tubulovesicular and collapsed intracellular canalicular region of resting parietal cells (Fig. 4C). In stimulated parietal cells H+-K+-ATPase and Shh were both evident at or close to the expanded apical canalicular membrane with also some diffuse localization of Shh in the parietal cells (Fig. 4F). F-actin staining of gastric glands showed clearly the morphological differences between resting (Fig. 4G) and stimulated (Fig. 4I) parietal cells that was consistent with images of rabbit gastric glands in previously published data (13, 14). F-actin and Shh also appeared to colocalize to the intracellular canalicular region of resting parietal cells (Fig. 4H) and at the expanded apical canalicular membrane in stimulated parietal cells (Fig. 4J). Colocalization of Shh with H+-K+-ATPase suggests association of Shh with the canalicular membrane. Some Shh was also evident at or close to the basolateral membrane (Fig. 4C).
Fig. 4.
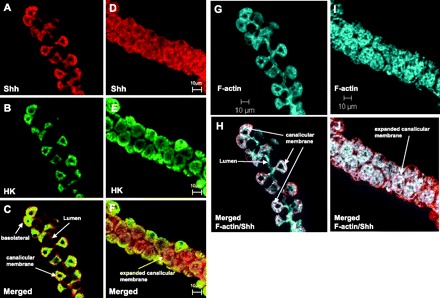
Cellular localization of Shh in parietal cells of resting and stimulated rabbit gastric glands. Confocal images of resting (A, B, C, G, H) and stimulated (D, E, F, I, J) rabbit gastric glands immunostained for Shh (Alexa Fluor 488, red) and H+-K+-ATPase (Alexa Fluor 555, green). F-actin staining (Alexa Fluor 633, blue) is shown for resting (G) and stimulated (I) glands. Merged images for Shh and H+-K+-ATPase are shown for resting (C) and stimulated (F) glands (yellow). Merged images for Shh and F-actin are shown for resting (H) and stimulated (J) glands (white). Gastric glands were treated with 10−4 M cimetidine (resting) or histamine (stimulated) for 30 min before fixation. Scale bar indicated is the same for A–F.
Shh in gastric membrane fractions.
It is not possible from the immunoconfocal microscopy data shown in Figs. 1–4 to identify which Shh form (nascent 45 kDa or processed 19 kDa) is present in parietal cells. To investigate the association of Shh with the canalicular membrane and the protein form present in both resting and activated parietal cells, Shh was first assayed in crude subcellular fractions from rabbit gastric glands by Western blot using antibodies specific for Shh and H+-K+-ATPase (Fig. 5). In crude cell fractions of resting glands a 45-kDa Shh immunoreactive band corresponding to the precursor protein was found to be concentrated in the microsomal membrane pellet (P3) together with immunoreactive bands for H+-K+-ATPase (Fig. 5A, lanes 1–4). When this P3 fraction was further purified over a discontinuous sucrose gradient, the greatest recovery of the 45-kDa Shh immunoreactive band was observed in the 10–20% (S20) interface also together with H+-K+-ATPase (Fig. 5A, lanes 5–8). Stimulation of acid secretion in parietal cells is characterized by a change in the distribution of the H+-K+-ATPase from the P3 microsomal membrane fraction to the P1 plasma membrane fraction (5). To determine whether Shh remains in the same fraction as H+-K+-ATPase after histamine stimulation and whether any Shh processing has been induced, a similar subcellular fractionation protocol was performed using stimulated rabbit gastric glands. Figure 5B shows that in histamine-stimulated gastric glands the nascent 45-kDa Shh precursor disappears from the P3 microsomal membrane fraction and a 19-kDa Shh immunoreactive band (ShhNp) consistent with the processed Shh protein appears predominantly in the P1 plasma membrane fraction (Fig. 5B, lanes 1–4). Concomitantly the well-established shift in H+-K+-ATPase expression from the P3 to the P1 fraction (34) is evident upon stimulation (Fig. 5B, lanes 1–4), which is consistent with recruitment of H+-K+-ATPase to the canalicular membrane (15). With further purification of P1 over a discontinuous sucrose gradient, the greatest recovery of the processed 19-kDa Shh immunoreactive band was observed in the 10–20% (S20) interface, together with H+-K+-ATPase (Fig. 5B, lanes 5–8), and the 45-kDa protein was absent. Stimulation of acid secretion with histamine was also confirmed by measuring the 14C-aminopyrine uptake ratio. The aminopyrine ratio increased significantly (P < 0.05) from 22 ± 5 to 116 ± 24 (n = 3 gastric gland preparations).
Fig. 5.
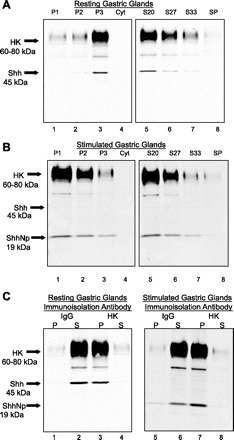
Localization of Shh in gastric H+-K+-ATPase-containing membranes. Membrane fractions were prepared from resting (A) and stimulated (B) rabbit gastric glands and analyzed by Western blot using antibodies specific for Shh or H+-K+-ATPase-α subunit. The entire gland homogenate (32 mg total) was centrifuged sequentially at 3,200 g for 10 min (P1: nuclei, plasma membranes), 11,000 g for 10 min (P2: mitochondria) and 100,000 g for 1 h (P3: tubulovesicles, endoplasmic reticulum). H+-K+-ATPase-containing membrane fraction P3 (resting glands) and P1 (stimulated glands) were further purified through a discontinuous sucrose gradient (S20%, S27%, S33%, sucrose gradient pellet, SP). A total of 10 μg of protein for each sample was loaded per well. Cyt, cytosolic fraction. C: immunoisolated H+-K+-ATPase-containing apical membranes prepared from resting and stimulated rabbit gastric glands were analyzed by Western blot using antibodies specific for Shh or H+-K+-ATPase-α subunit. Membranes from the 10–20% (S20) sucrose gradient were immunopurified with magnetic beads coated with either nonimmune IgG2b or anti-H+-K+-ATPase antibody. P, immunoadsorbed material (bead pellet); S, nonadsorbed material (supernatant); Shh, 45-kDa precursor protein; ShhNp, 19-kDa processed protein.
To confirm association of Shh with resting and stimulated H+-K+-ATPase-containing membranes, membranes from the 10–20% (S20) sucrose interface (Fig. 5, A and B) were further immunopurified by use of magnetic beads coated with anti-H+-K+-ATPase antibody. H+-K+-ATPase and Shh immunoreactivity of membrane fractions, immunoadsorbed pellet, and nonadsorbed supernatant were then analyzed by Western blot. Control experiments using IgG were also carried out. The results from resting and stimulated rabbit gastric glands are shown in Fig. 5C. Shh immunoreactivity was detected on immunoblots of enriched H+-K+-ATPase-containing membranes (pellets) and in the nonabsorbed (IgG treated) supernatants (Fig. 5C). Resting gastric glands showed only the 45-kDa precursor Shh protein and stimulated glands showed only the 19-kDa processed Shh protein. Immunoblots from three individual immunoisolations were quantified using the Odyssey Infrared Imaging System software and expressed as percentages of total recovery (means ± SE). In immunopurified membranes 93.0 ± 3.4 and 90.8 + 1.5% of Shh 45-kDa and 19-kDa immunoreactivity and 85.0 ± 1.2 and 87.0 ± 2.9% of the H+-K+-ATPase were recovered in the bead pellet of resting and stimulated glands, respectively. These results indicate that a major portion of Shh (whether precursor or processed protein) is associated (immunopurified) with the H+-K+-ATPase-containing membranes of parietal cells in both resting and stimulated gastric glands.
Nature of association of Shh with H+-K+-ATPase-containing membranes.
To characterize the association of Shh with the H+-K+-ATPase-containing membranes, resting and stimulated rabbit gastric glands were suspended in isotonic sucrose media with 0, 50, 100, or 150 mM KCl and permeabilized with digitonin. Glands were collected before the addition of digitonin (time 0) and after 5-, 10-, and 20-min incubations at 37°C. The glands were immediately separated into a supernatant (cytoplasmic contents) and pellet (membrane-bound proteins). Equal protein concentrations of pellet and supernatant were loaded onto a 4–20% gradient gel and analyzed for expression of H+-K+-ATPase and Shh. At time 0, H+-K+-ATPase and 45-kDa Shh precursor in resting glands (Fig. 6A) and H+-K+-ATPase and 19-kDa processed Shh in stimulated glands (Fig. 7A) were entirely in the pellet sample. Within 5 min of incubation with KCl (50, 100, and 150 mM) both the Shh precursor (Fig. 6B) and processed (Fig. 7B) proteins were observed in the supernatant of resting and stimulated glands, respectively. H+-K+-ATPase remained in the pellet sample regardless of salt concentration (Figs. 6 and 7) in all cases. Dissociation of Shh (both precursor and processed forms) with high salt indicates that it is a peripheral (not integral) membrane protein associated with H+-K+-ATPase-containing membranes in both resting and stimulated parietal cells. These findings suggest that histamine induces processing of the nascent 45-kDa precursor to the 19-kDa Shh secreted protein.
Fig. 6.
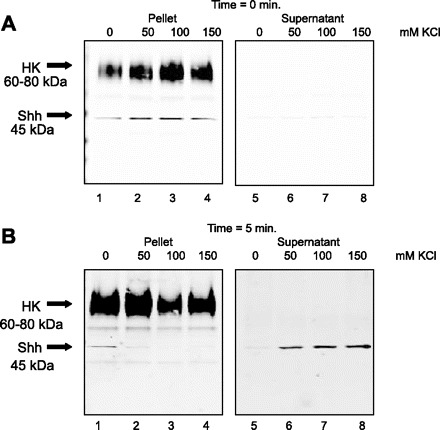
Release of Shh from permeabilized resting rabbit gastric glands in response to increased salt concentration. Resting rabbit gastric glands were permeabilized with 20 μg/ml digitonin in isotonic sucrose, incubated with 0, 50, 100, or 150 mM KCl for 5 min. Pellet (glands) and supernatant proteins (10 μg) were separated on a 4–20% SDS gradient gel and immunoblotted for Shh and H+-K+-ATPase. Immunoblots for time 0 (control, A) and 5 min after digitonin incubation (B) are shown.
Fig. 7.
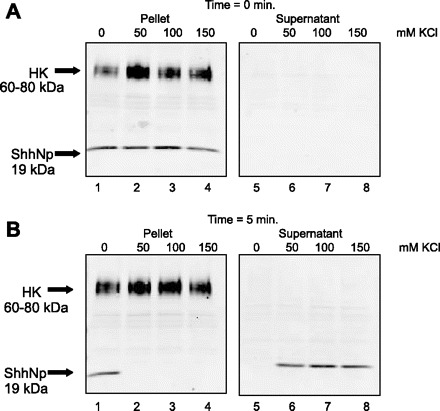
Release of Shh from permeabilized stimulated rabbit gastric glands in response to increased salt concentration. Stimulated rabbit gastric glands were permeabilized with 20 μg/ml digitonin in isotonic sucrose, incubated with 0, 50, 100, or 150 mM KCl for 5 min. Pellet (glands) and supernatant proteins (10 μg) were separated on a 4–20% SDS gradient gel and immunoblotted for Shh and H+-K+-ATPase. Immunoblots for time 0 (control, A) and 5 min after digitonin incubation (B) are shown.
Histamine-stimulated secretion of Shh in gastric glands and in polarized 23132/87 gastric cancer cells.
To investigate whether histamine stimulated secretion of the 19-kDa Shh processed protein, the medium was collected from resting and histamine-stimulated gastric glands, concentrated, purified by gel filtration chromatography, immunoprecipitated with the 5E1 Shh specific antibody, and analyzed by Western blot. The results are shown in Fig. 8.
Fig. 8.
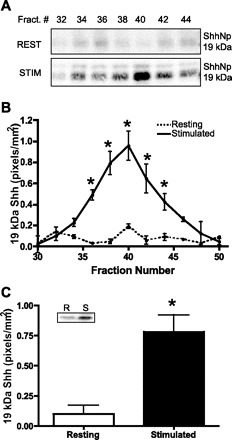
Secretion of 19-kDa Shh from resting and stimulated rabbit gastric glands. A: gastric glands were treated with either cimetidine [resting (rest)] or histamine [stimulated (stim)] for 30 min. Glands were allowed to settle and media were collected, concentrated, and subjected to gel filtration chromatography. Column fractions were collected and immunoprecipitated by use of the monoclonal 5E1 Shh antibody and analyzed by Western blot. The 19-kDa protein is shown. B: 19-kDa Shh protein in fractions 30–50 was quantified via the Odyssey Infrared Imaging System software. The data are shown as means ± SE for 3 individual rabbit gastric gland isolates expressed as Shh (pixels/mm2). *Significantly different from resting with P < 0.005, 0.02, or 0.05. C: fractions 32–44 were collected, pooled, and immunoprecipitated by use of the monoclonal 5E1 anti-Shh antibody and then analyzed by Western blot with a rabbit anti-Shh antibody. 19-kDa Shh protein was quantified by the Odyssey Infrared Imaging System software. Data are shown as means ± SE for 3 individual rabbit gastric gland isolates expressed as Shh (pixels/mm2). *P < 0.02 vs. resting. Inset: representative immunoblot. R, resting; S, stimulated.
Western blots of the gel filtration fractions from the media collected from equivalent amounts of resting and stimulated gastric glands showed that the 19-kDa Shh protein was the predominant form detected as shown in Fig. 8A. Low 19-kDa Shh immunoreactivity was seen in all fractions of medium from resting glands. In medium from stimulated glands, greater 19-kDa Shh immunoreactivity was evident. These results were quantified from fractions 30–50 with the Odyssey Infrared Imaging System software. The data are shown as means ± SE for three individual rabbit gastric gland isolates expressed as 19-kDa Shh in pixels per square millimeter (Fig. 8B). Low 19-kDa Shh immunoreactivity was detected throughout fractions 30–50 collected from the media of resting gastric glands. In media collected from stimulated gastric glands, the major immunoreactive band in fraction 40 (0.96 ± 0.14 pixels/mm2) was significantly greater than that in media from resting glands (0.19 ± 0.02 pixels/mm2). For further quantitation of resting vs. stimulated secreted 19-kDa Shh, fractions 32–44 were pooled, immunoprecipitated with the monoclonal 5E1 anti-Shh antibody, analyzed by Western blot using a rabbit anti-Shh antibody, and quantified with the Odyssey Infrared Imaging System software. Figure 8C shows a representative immunoblot (inset) and means ± SE of 19-kDa Shh (pixels/mm2) from three separate experiments. Treatment of gastric glands with histamine resulted in a significant (P < 0.02) increase of secreted 19-kDa Shh from 0.10 ± 0.07 to 0.78 ± 0.14 pixels/mm2. The 45-kDa precursor protein was not detected in media collected from either resting or stimulated gastric glands, suggesting that the precursor form is not secreted. Therefore, in rabbit gastric glands, as shown with other models (mouse and canine parietal cells), histamine stimulated secretion of the processed 19-kDa Shh protein.
It was evident from the confocal images presented in Fig. 4 that Shh also localized to the basolateral membrane of parietal cells. Thus, to study the basolateral distribution of Shh within parietal cells, rabbit gastric glands were immunostained for Shh together with the basolateral marker γ-actin (Fig. 9, A–C) and examined by confocal microscopy. In addition to the x-y image, a series of optical sections were then taken from identical x-z and y-z planes. As noted previously, Shh appeared to localize at the canalicular (apical/secretory) membrane of parietal cells (Fig. 9A) and also at or close to the basolateral membrane, with γ-actin localizing as expected specifically only to the basolateral membrane of the gastric epithelial cells (Fig. 9B). Upon merging of the two images in the x-y plane, some Shh colocalized with γ-actin at the basolateral membrane (yellow) and some Shh was present at the canalicular (apical/secretory) membrane (red) (Fig. 9C). This was particularly evident in the merged images of the x-z and y-z optical slices. A series of 0.5-μm optical sections taken in the z-axis of the gland are shown in Fig. 9D. Images in Fig. 9, A–C, were taken at 6.50 μm (z-axis) (Fig. 9D). When the section was focused at 2.50 and 12.00 μm, Shh was equally evident at the canalicular membrane (Fig. 9D). These data suggest that Shh may be expressed and perhaps secreted at both apical and basolateral membranes of parietal cells.
Fig. 9.
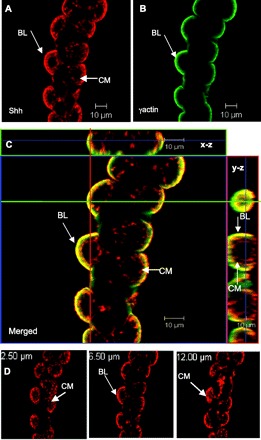
Expression of Shh at the basolateral membrane of gastric parietal cells. Rabbit gastric glands were immunostained for Shh (red, A) and the basolateral membrane marker, γ−actin (green, B). The merged image is shown in C, together with optical sections spaced 0.5 μm in the z-axis of glands (x-z and y-z). This image was taken at 6.50 μm. The green line in the x-y image is the location of the x-z slice. The red line in the x-y image is the location of the y-z slice. Colocalization is indicated by the yellow color. D: Shh immunofluorescence in the gastric gland from A at 3 different focal planes (2.50, 6.50, 12.00 μm). A series of 0.5-μm optical sections in the z-axis of the gland are shown. BL, basolateral membrane; CM, canalicular membrane.
It is not possible to differentiate in rabbit gastric glands between apical and basolateral secretion of Shh. Therefore an in vitro polarized cell culture model using the human gastric cancer cell line 23132/87, known to retain characteristics of parietal cells, contain the H+-K+-ATPase, and secrete Shh (8), was examined as a possible approach to this question. Gastric 23132/87 cells were grown on permeable membranes, with medium on and therefore accessibility to both apical and basolateral sides. First it was necessary to examine whether these cells formed a tight barrier between apical and basolateral compartments. TEER, which reflects tight junction formation, was measured over 8 days. As shown in Fig. 10A, TEER of gastric 23132/87 cells increased significantly (P < 0.0005) from 528 ± 27 (n = 6) Ω/cm2 to a maximum of 983 ± 67 (n = 6) Ω/cm2 after 6 days and remained stable until day 8. In addition, immunofluorescent confocal microscopy with an antibody specific for ZO-1, a tight junction marker, was carried out and showed that tight junctions were formed in monolayers of 23132/87 cells (Fig. 10B). Therefore localization of Shh was investigated in both x-y and x-z planes by immunofluorescent confocal microscopy of polarized gastric 23132/87 cells. Figure 10C shows that Shh is present predominantly at the apical surface of the gastric 23132/87 cell monolayer with a small amount at the basolateral surface. This model was then used to investigate whether histamine could stimulate secretion of 19-kDa Shh into the apical and/or basolateral media bathing the cells. Apical and basolateral media of untreated and histamine-treated 23132/87 cells cultured on permeable membranes were collected, concentrated, purified by gel filtration chromatography, immunoprecipitated with anti-Shh antibody, and then identified on Western blots. Figure 10D shows that 19-kDa Shh was found in apical but not basolateral medium from untreated cells and there was no change in either media when histamine was added apically. In contrast, as shown in Fig. 10E, when cells were treated with histamine on the basolateral side where histamine H2 receptors are present (8), there was not only a significant increase in 19-kDa Shh secreted into the apical medium compared with untreated cells, but moreover 19-kDa Shh was now also detected in the basolateral media of histamine-treated cells. These data demonstrate that histamine induces secretion of 19-kDa Shh into both apical and basolateral media of polarized 23132/87 cells and present a novel in vitro cell culture system that will be useful in further analysis of Shh processing and secretion in gastric cancer.
Fig. 10.
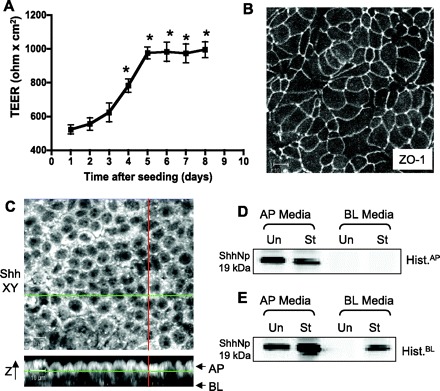
Apical and basolateral secretion of 19-kDa Shh from human gastric adenocarcinoma cell line 231232/87. A: transepithelial electrical resistance (TEER) of 23132/87 cells grown on permeable membranes. *P < 0.0005 compared with TEER at day 1 (n = 6). B: immunofluorescence of 23132/87 cell monolayer immunostained with tight junction marker zonula occludens (ZO-1). Size bar = 10 μm. C: confocal microscopy image of x-y and x-z sections of 23132/87 immunostained with Shh. Size bar = 10 μm. D and E: cells were cultured on permeable membranes and 100 μM histamine (Hist) was added either to the apical medium (D, control, St) or left untreated (Un); or added to the basolateral medium (E, St) or left untreated (Un). After 6 h apical (AP) and basolateral (BL) media were collected, concentrated, and subjected to gel filtration chromatography. Fractions 32–44 were collected, pooled, and immunoprecipitated with the monoclonal 5E1 anti-Shh antibody and analyzed by Western blot using a rabbit anti-Shh antibody. Shown are representative blots from 6 separate experiments.
DISCUSSION
Shh is among numerous factors secreted by parietal cells that are believed to regulate the growth and differentiation of the gastric epithelium (1, 7, 22, 29). The present study extends previous knowledge of gastric Shh by presenting new evidence for the cellular location of Shh within the acid-secreting parietal cells. Specifically, the data presented in gastric glands show that Shh resides close to the intracellular H+-K+-ATPase-containing membranes (tubulovesicles) of resting parietal cells. When a secretagogue such as histamine stimulates acid secretion, tubulovesicles fuse with the apical canalicular membrane and H+-K+-ATPase and Shh move with these structural changes of stimulated parietal cells. Although the present findings demonstrate that Shh is expressed at the H+-K+-ATPase-containing membranes where the protein would be accessible to acid and pepsin A for processing, basolateral Shh expression, which was also observed, suggests an active autocatalytic mechanism for basolateral secretion of bioactive Shh.
Immunofluorescent labeling of rabbit stomach using markers specific for Shh and different cell lineages revealed that Shh protein is predominantly expressed within parietal and pit cells. Quantitation of this data using average fluorescent intensities of individual cells confirmed that Shh was expressed with markers for parietal (H+-K+-ATPase) and pit (UEAI) cells. Although these data are consistent with other studies including analysis of stomachs collected from mouse, Mongolian gerbils, and human (9, 28, 30) there are reports of the absence of Shh expression in pit cells (31). These discrepancies have been attributed to species differences, antibody, and method specificity. However, despite this controversy, it is clear that Shh is highly expressed in the acid-secreting parietal cells, the primary focus of the present study. In contrast to its expression in parietal and pit cells, Shh was not expressed in chief (PgA) or neck (GSII) cells. These findings suggest that the previously reported protease-dependent Shh processing mechanism (35) must occur such that Shh would be accessible to the active enzyme pepsin A that is secreted from nonparietal chief cells, which are, however, in close proximity to parietal cells.
Confocal microscopy of rabbit gastric glands demonstrated that Shh was localized at apical H+-K+-ATPase-containing membranes and also at basolateral membranes of parietal cells. Rabbit gastric glands are tubular structures in which the epithelial cells (mainly parietal and chief cells) are organized side by side along the central lumen. Since parietal cells maintain their polarized structure in isolated gastric glands, this model is excellent for the study of Shh cellular and intracellular distribution. In resting glands Shh colocalized with both H+-K+-ATPase and F-actin within the intracellular membranes, presumably tubulovesicles and perhaps collapsed canalicular membrane. In stimulated glands Shh coexpressed together with H+-K+-ATPase and F-actin close to or within the expanded canalicular membrane. Some diffuse Shh expression along the gland was also evident. This is consistent with a protease-dependent processing mechanism of Shh (35). Further characterization of the location of Shh by fractionation and immunoisolation of gastric gland membranes showed that 45-kDa Shh was found with resting and 19-kDa Shh was found with stimulated H+-K+-ATPase-containing membranes. Thus stimulation of acid secretion by histamine resulted in the 45-kDa precursor Shh protein being processed to the 19-kDa Shh protein. Analysis of media collected from resting and stimulated glands showed that increased secretion of the 19-kDa Shh protein occurred from stimulated, but not resting glands.
Previous reports using purified canine parietal cells or mouse gastric mucosal cells show that when parietal cells are stimulated to secrete acid, Shh is processed from the 45-kDa precursor form such that the COOH-terminus domain is removed and the biologically active 19-kDa NH2-terminal domain remains and could bind to the hedgehog receptor patched (Ptch), which was localized to parietal cells (29, 35). The NH2-terminal 19-kDa protein has been shown to be cholesterol modified at its COOH terminus and palmitoylated at its NH2 terminus (17). In support of apical expression of Shh, polarized 292T cells transfected with green fluorescent protein fused with the COOH terminus of the human Shh protein show that expression of Shh protein was concentrated at the lateral and apical surfaces of the plasma membrane (33). Interestingly, apical areas are often enriched in cholesterol and the likely site of posttranslational modification (33). For example, the apical secretory membrane of the parietal cell also contains a high lipid content that is critical for selective permeability of water and ions, but not urea or ammonia (20). The presence of Shh in H+-K+-ATPase-containing membranes suggests that the protein may be either processed or posttranslationally modified at the secretory apical membrane of parietal cells after stimulation. The data in the present study suggest that the Shh 45-kDa precursor is translocated to the secretory canalicular membrane during tubulovesicular fusion and recruitment of H+-K+-ATPase, where it is processed and secreted.
The present study also showed that both 45- and 19-kDa Shh proteins were dissociated from H+-K+-ATPase-containing membranes with increasing salt concentration, consistent with a peripheral membrane protein that is palmitoylated (4). However, further biochemical analysis is required to characterize Shh secreted from parietal cells. Collectively, the confocal and immunoisolation data suggest that Shh is processed via a protease-dependent mechanism at the apical membrane of parietal cells. This is consistent with secretion of the 19-kDa protein that is stable under high concentrations of salt, detergent (11), and acid (29, 35).
In addition to apical expression, confocal images using an antibody specific for γ-actin revealed colocalization of Shh at the basolateral membrane. In support of the confocal data, histamine treatment of a polarized Shh- and H+-K+-ATPase-expressing human gastric cancer cell line (23132/87) (8) resulted in basolateral as well as apical Shh secretion. Basolateral expression of Shh is consistent with the autocatalytic processing mechanism (24), since there would be no access to pepsin A as could occur in the luminal compartment. Some Shh is associated with the basolateral membrane. The possibility that some Shh may also be associated with secretory organelles such as Golgi and endoplasmic reticulum, that are commonly found close to the basolateral membrane, remains to be determined. It should be noted, that the data collected from the 23132/87 cells must be interpreted with caution given that these cells originate from a human gastric adenocarcinoma. Constitutively activated hedgehog processing may contribute to the generation of overexpressed Shh protein that has been observed in gastric cancer cell lines (3). Given the strong correlation between overexpressed Shh signaling and gastric cancer (3), the 23132/87 cell line culture would be an appropriate model to study Shh processing and secretion in gastric cancer.
The findings reported in the present study extend the understanding of the expression and secretion of Shh in the stomach. In addition, it is possible to hypothesize on the precise role of Shh as a morphogen within the stomach. Secretion of Shh at the apical membrane of parietal cells suggests that (if externalized) Shh could be cleaved by pepsin A and then be able to bind to its receptor Ptch at the luminal side of the gastric epithelium, if present. Certainly there are a number of examples of luminal growth factors. For example, EGF and TGF-α regulate the maintenance of epithelial renewal (12), and in the intestine and stomach trefoil peptides promote repair following apical secretion (23). Evidence of apical expression of the Ptch receptor has emerged from studies using primary cilia projecting from vertebrate cell surfaces that extend into the extracellular environment and function as sensors for changes in Shh levels (26). However, Shh was also localized at the basolateral membrane, which suggests that the protein could play a role as a regulator of gastric function such as acid secretion that is consistent with expression of Ptch along the oxyntic gland and particularly on the parietal cell lineage (3, 32). This underscores the necessity for further detailed investigation into the function of Shh within the gastric mucosa using both in vitro models and hedgehog signaling mutant mice. In addition, further work is needed to understand at the molecular level the precise steps involved in the processing and secretion of Shh in the gastric mucosa.
GRANTS
This work was supported by start-up funds from the Department of Molecular and Cellular Physiology, University of Cincinnati (Y. Zavros).
Supplementary Material
Acknowledgments
The expert assistance from Dr. M. Montrose and C. Closson (Live Microscopy Core, University of Cincinnati) with confocal microscopy is acknowledged. Dr. D. J. Robbins (Dartmouth Medical School) and Dr. J. Cuppoletti (Molecular and Cellular Physiology, University of Cincinnati) are thanked for helpful discussions during the course of this work. Dr. R. S. Franco and M. Palascak (Internal Medicine-Hematology/Oncology) are thanked for assistance with flow cytometry analysis.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.BeauchampRDBeauchamp RD, Barnard JA, McCutchen CM, Cherner JA, Coffey RJ Jr. Localization of transforming growth factor alpha and its receptor in gastric mucosal cells. J Clin Invest 84: 1017–1023, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.BerglindhTBerglindh T, Obrink KJ. A method for preparing isolated glands from the rabbit gastric mucosa. Acta Physiol Scand 96: 150–159, 1976. [DOI] [PubMed] [Google Scholar]
- 3.BermanDMBerman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, Beachy PA. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature 425: 846–851, 2003. [DOI] [PubMed] [Google Scholar]
- 4.BijlmakersMJBijlmakers MJ, Marsh M. The on-off story of protein palmitoylation. Trends Cell Biol 13: 32–42, 2003. [DOI] [PubMed] [Google Scholar]
- 5.BumcrotDABumcrot DA, Takada R, McMahon AP. Proteolytic processing yields two secreted forms of sonic hedgehog. Mol Cell Biol 15: 2294–2303, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CalhounBCCalhoun BC, Goldenring JR. Two Rab proteins, vesicle-associated membrane protein 2 (VAMP-2) and secretory carrier membrane proteins (SCAMPs), are present on immunoisolated parietal cell tubulovesicles. Biochem J 325: 559–564, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ChenMCChen MC, Lee AT, Karnes WE, Avedian D, Martin M, Sorvillo JM, Soll AH. Paracrine control of gastric epithelial cell growth in culture by transforming growth factor-α. Am J Physiol Gastrointest Liver Physiol 264: G390–G396, 1993. [DOI] [PubMed] [Google Scholar]
- 8.DimmlerADimmler A, Brabletz T, Hlubek F, Hafner M, Rau T, Kirchner T, Faller G. Transcription of sonic hedgehog, a potential factor for gastric morphogenesis and gastric mucosa maintenance, is up-regulated in acidic conditions. Lab Invest 83: 1829–1837, 2003. [DOI] [PubMed] [Google Scholar]
- 9.El-ZaatariMGAEl-Zaatari MGA, McKenzie AJ, Powe DG, Scotting PJ, Watson SA. Cyclopamine inhibition of the sonic hedgehog pathway in the stomach requires concomitant acid inhibition. Regul Pept 146: 131–139, 2008. [DOI] [PubMed] [Google Scholar]
- 10.ForteJGForte JG, Forte TM, Black JA, Okamoto C, Wolosin JM. Correlation of parietal cell structure and function. J Clin Gastroenterol 1: 17–27, 1983. [DOI] [PubMed] [Google Scholar]
- 11.GoetzJAGoetz JA, Singh S, Suber LM, Kull FJ, Robbins DJ. A highly conserved amino-terminal region of sonic hedgehog is required for the formation of its freely diffusible multimeric form. J Biol Chem 281: 4087–4093, 2006. [DOI] [PubMed] [Google Scholar]
- 12.GokeMGoke M, Podolsky DK. Regulation of the mucosal epithelial barrier. Baillieres Clin Gastroenterol 10: 393–405, 1996. [DOI] [PubMed] [Google Scholar]
- 13.HanzelDKHanzel DK, Urushidani T, Usinger WR, Smolka A, Forte JG. Immunological localization of an 80-kDa phosphoprotein to the apical membrane of gastric parietal cells. Am J Physiol Gastrointest Liver Physiol 256: G1082–G1089, 1989. [DOI] [PubMed] [Google Scholar]
- 14.HanzelDKHanzel DK, Reggio H, Bretscher A, Forte JG, Mangeat P. The secretion-stimulated 80K phosphoprotein of parietal cells is ezrin, and has properties of a membrane cytoskeletal linker in the induced apical microvilli. EMBO J 10: 2363–2373, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.HirstBHHirst BH, Forte JG. Redistribution and characterization of (H+ + K+)-ATPase membranes from resting and stimulated gastric parietal cells. Biochem J 231: 641–649, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ForteJGForte JG. K+ channels in the secretory membrane of the parietal cell focus on “Gastric parietal cell secretory membrane contains PKA- and acid-activated Kir21 K+ channels.” Am J Physiol Cell Physiol 286: C478–C479, 2004. [DOI] [PubMed] [Google Scholar]
- 17.JohnsonRLJohnson RL, Tabin C. The long and short of hedgehog signaling. Cell 81: 313–316, 1995. [DOI] [PubMed] [Google Scholar]
- 18.KarvarSKarvar S, Zhu L, Crothers J Jr, Wong W, Turkoz M, Forte JG. Cellular localization and stimulation-associated distribution dynamics of syntaxin-1 and syntaxin-3 in gastric parietal cells. Traffic 6: 654–666, 2005. [DOI] [PubMed] [Google Scholar]
- 19.KimJHKim JH, Huang Z, Mo R. Gli3 null mice display glandular overgrowth of the developing stomach. Dev Dyn 234: 984–991, 2005. [DOI] [PubMed] [Google Scholar]
- 20.LandeMBLande MB, Priver NA, Zeidel ML. Determinants of apical membrane permeabilities of barrier epithelia. Am J Physiol Cell Physiol 267: C367–C374, 1994. [DOI] [PubMed] [Google Scholar]
- 21.LeeJJLee JJ, Ekker SC, von Kessler DP, Porter JA, Sun BI, Beachy PA. Autoproteolysis in hedgehog protein biogenesis. Science 266: 1528–1537, 1994. [DOI] [PubMed] [Google Scholar]
- 22.NamKTNam KT, Varro A, Coffey RJ, Goldenring JR. Potentiation of oxyntic atrophy-induced gastric metaplasia in amphiregulin-deficient mice. Gastroenterology 132: 1804–1819, 2007. [DOI] [PubMed] [Google Scholar]
- 23.PodolskyDKPodolsky DK, Lynch-Devaney K, Stow JL, Oates P, Murgue B, DeBeaumont M, Sands BE, Mahida YR. Identification of human intestinal trefoil factor. Goblet cell-specific expression of a peptide targeted for apical secretion. J Biol Chem 268: 6694–6702, 1993. [PubMed] [Google Scholar]
- 24.PorterJAPorter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, Beachy PA. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature 374: 363–366, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Ramalho-SantosMRamalho-Santos M, Melton DA, McMahon AP. Hedgehog signals regulate multiple aspects of gastrointestinal development. Development 127: 2763–2772, 2000. [DOI] [PubMed] [Google Scholar]
- 26.RohatgiRRohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science 317: 372–376, 2007. [DOI] [PubMed] [Google Scholar]
- 27.SherryAMSherry AM, Malinowska DH, Morris RE, Ciraolo GM, Cuppoletti J. Localization of ClC-2 Cl− channels in rabbit gastric mucosa. Am J Physiol Cell Physiol 280: C1599–C1606, 2001. [DOI] [PubMed] [Google Scholar]
- 28.ShiotaniAShiotani A, Iishi H, Uedo N, Ishiguro S, Tatsuta M, Nakae Y, Kumamoto M, Merchant JL. Evidence that loss of sonic hedgehog is an indicator of Helicobacter pylori-induced atrophic gastritis progressing to gastric cancer. Am J Gastroenterol 100: 581–587, 2005. [DOI] [PubMed] [Google Scholar]
- 29.StepanVStepan V, Ramamoorthy S, Nitsche H, Zavros Y, Merchant JL, Todisco A. Regulation and function of the sonic hedgehog signal transduction pathway in isolated gastric parietal cells. J Biol Chem 280: 15700–15708, 2005. [DOI] [PubMed] [Google Scholar]
- 30.SuzukiHSuzuki H, Minegishi Y, Nomoto Y, Ota T, Masaoka T, van den Brink GR, Hibi T. Down-regulation of a morphogen (sonic hedgehog) gradient in the gastric epithelium of Helicobacter pylori-infected Mongolian gerbils. J Pathol 206: 186–197, 2005. [DOI] [PubMed] [Google Scholar]
- 31.van den BrinkGRvan den Brink GR, Hardwick JC, Nielsen C, Xu C, ten Kate FJ, Glickman J, van Deventer SJ, Roberts DJ, Peppelenbosch MP. Sonic hedgehog expression correlates with fundic gland differentiation in the adult gastrointestinal tract. Gut 51: 628–633, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Den BrinkGRVan Den Brink GR, Hardwick JC, Tytgat GN, Brink MA, Ten Kate FJ, Van Deventer SJ, Peppelenbosch MP. Sonic hedgehog regulates gastric gland morphogenesis in man and mouse. Gastroenterology 121: 317–328, 2001. [DOI] [PubMed] [Google Scholar]
- 33.VincentSVincent S, Thomas A, Brasher B, Benson JD. Targeting of proteins to membranes through hedgehog auto-processing. Nat Biotechnol 21: 936–940, 2003. [DOI] [PubMed] [Google Scholar]
- 34.WolosinJMWolosin JM, Forte JG. Changes in the membrane environment of the (K+ + H+)-ATPase following stimulation of the gastric oxyntic cell. J Biol Chem 256: 3149–3152, 1981. [PubMed] [Google Scholar]
- 35.ZavrosYZavros Y, Waghray M, Tessier A, Todisco A, Gumucio DL, Samuelson LC, Dlugosz A, Merchant JL. Reduced pepsin A processing of sonic hedgehog in parietal cells precedes gastric atrophy and transformation. J Biol Chem 282: 33265–33274, 2007. [DOI] [PubMed] [Google Scholar]
- 36.ZengXZeng X, Goetz JA, Suber LM, Scott WJ Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature 411: 716–720, 2001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


