Abstract
Serotonin (5-hydroxytryptamine, 5-HT) and its transporters and receptors are involved in a wide array of digestive functions. In particular, 5-HT4 receptors are known to mediate intestinal peristalsis and recent data in experimental animals have shown their role in neuronal maintenance and neurogenesis. This study has been designed to test whether prucalopride, a well-known full 5-HT4 agonist, exerts protective effects on neurons, including enteric neurons, exposed to oxidative stress challenge. Sulforhodamine B assay was used to determine the survival of SH-SY5Y cells, human enteric neurospheres, and ex vivo submucosal neurons following H2O2 exposure in the presence or absence of prucalopride (1 nM). Specificity of 5-HT4-mediated neuroprotection was established by experiments performed in the presence of GR113808, a 5-HT4 antagonist. Prucalopride exhibited a significant neuroprotective effect. SH-SY5Y cells pretreated with prucalopride were protected from the injury elicited by H2O2 as shown by increased survival (73.5 ± 0.1% of neuronal survival vs. 33.3 ± 0.1%, respectively; P < 0.0001) and a significant reduction of proapoptotic caspase-3 and caspase-9 activation in all neurons tested. The protective effect of prucalopride was reversed by the specific 5-HT4 antagonist GR113808. Prucalopride promotes a significant neuroprotection against oxidative-mediated proapoptotic mechanisms. Our data pave the way for novel therapeutic implications of full 5-HT4 agonists in gut dysmotility characterized by neuronal degeneration, which go beyond the well-known enterokinetic effect.
Keywords: 5-HT4 full agonist, enterokinetic drug, prucalopride, serotonin
the gastrointestinal tract serves as a major depot of the biogenic monoamine serotonin (5-hydroxytryptamine, 5-HT) with ∼95% of the body 5-HT localized in the gut (11). 5-HT is predominantly contained in enteroendocrine (i.e., enterochromaffin) cells (ECs) dispersed throughout the mucosal surface, but it is also synthesized and stored in a small proportion of neurons (2% of myenteric interneurons) of the enteric nervous system (ENS) (11, 12). The ENS expresses different types of 5-HT receptors (from 5-HT1 to 5-HT7) through which 5-HT modulates a remarkable number of physiological effects ranging from motility and secretion to mucosa growth and maintenance and neurogenesis (4, 19). For instance, 5-HT4, which is mainly localized to the ascending excitatory neuronal reflex pathways, plays a prominent role in modulating propulsive patterns of gut motility (i.e., peristalsis), by releasing acetylcholine and other excitatory mediators, thus resulting in a prokinetic activity (11, 12, 19, 21). Various 5-HT4 agonists have been developed to treat gastrointestinal disorders characterized by altered motility (25), including the 5-HT4 full agonist prucalopride, which significantly improves symptoms and quality of life of patients with severe chronic constipation with excellent safety and tolerance profile (22). Interestingly, 5-HT4 receptors have also been shown to modulate neuronal survival and neurogenesis (11). Indeed, in the central nervous system, 5-HT4 has been shown to be involved in synaptic plasticity crucial to cognitive processes and memory consolidation (25). Recent findings also provide evidence for a role of 5-HT4 in mediating neurogenesis and synaptic plasticity in the ENS (23). Furthermore, 5-HT4−/− mice showed loss of enteric neurons and reduced size of the surviving neurons at 1 mo in addition to delayed gastrointestinal transit (17). Finally, in vitro treatment with 5-HT4 agonists increased the number of enteric neurons developing from precursor cells and/or surviving in culture, supporting neurogenetic properties mediated by this receptor subtype (17).1
In this study, we tested the hypothesis that the 5-HT4 full agonist prucalopride exerted a protective effect against neuronal injury using an established model of oxidative stress and different types of human neuronal cell lines, primary human enteric neurospheres (2), and human submucosal neuron whole-mount preparations obtained from colonic biopsies (16). We demonstrated that 5-HT4 activation stimulated a specific protective response in the different neuronal cells analyzed, including human enteric neurons. Our findings open new avenues in the treatment of gastrointestinal disorders and homeostasis, especially in relation to neurodegeneration often underlying severe gut dysmotility.
METHODS
Neuronal cell lines and neurospheres culture.
Murine Neuro2A as well as human SH-SY5Y, SK-N-BE, S-K-NSH, LAN-1, and HEK293 (ATCC, Teddington, UK) cells were cultured in 95% air and 5% CO2 at 37°C in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% (vol/vol) fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Primary human enteric neurospheres were generated from human ENS stem cells containing neurosphere-like bodies according to a previously modified method (12). To produce oxidative stress, H2O2 was freshly prepared from 30% stock solution prior to each experiment. Cells were treated with 200 μM H2O2 in phosphate-buffered saline (PBS) for 30 min as previously indicated (3).
Western blotting.
Whole-cell lysates were obtained in immunoprecipitation (IP) buffer (Sigma-Aldrich, St. Louis, MO), protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN), and phosphatase inhibitors (Sigma-Aldrich) on ice for 15 min. Protein concentration was evaluated by using the DC protein concentration assay kit (Bio-Rad, Hercules, CA). Fresh biopsies were sonicated on Bioruptor Pico Sonication System (V 1.1) (Diagenode, Liege, Belgium) in 100 μl of IP buffer and inhibitor cocktail (Sigma-Aldrich). Bands were visualized by the enhancer chemiluminescence method (GE Healthcare, Little Chalfont, UK). Primary antibodies used were anti-5-HT4 (1:200; Abcam, Cambridge, UK), anti-HuC/D (1:200; Invitrogen, Carlsbad, CA), anti-p75 (1:200; Thermo Fisher Scientific, Waltham, MA), anti-γ-tubulin and anti-vinculin (1:10,000 and 1:50,000, respectively; Sigma-Aldrich), anti-caspase-3 and caspase-9 (both 1:200; Cell Signaling, Denver, CO), anti-neuronal nitric oxide synthase (nNOS) (1:50; Santa Cruz, Dallas, TX), and anti-peripheral choline acetyl transferase (pChAT) (1:100; Justus-Liebig University, Giessen, Germany). The experiments on neurospheres were performed on cells cultured for at least 14 days.
Cytotoxicity assay.
Sulforhodamine B colorimetric (SRB) assay was used for cell density determination. The method has been optimized for the toxicity screening of compounds to adherent cells in a 24-well plate (24). To determine the dose-response profiles of prucalopride, the SRB assay was performed by incubating cells for 0 (untreated), 30, and 60 min with prucalopride (Shire, Lexington, MA) at the following final concentrations: 10 mM, 10 μM, 10 nM, 1 nM, and 10 pM to establish a correct dose finding. Controls were exposed to 0.05% (vol/vol) dimethyl sulfoxide in culture medium while wells containing only culture medium served as blank. The selective antagonist GR113808 (10 nM) ([1-[2-[(methylsulfonyl)-amino]ethyl]-4-piperidinyl]methyl-1-methyl-1H-indole-3-carboxylate, Sigma-Aldrich) was applied 30 min before prucalopride administration (Fig. 1A). Absorbance was measured at λ = 540 nm by spectrophotometry.
Fig. 1.
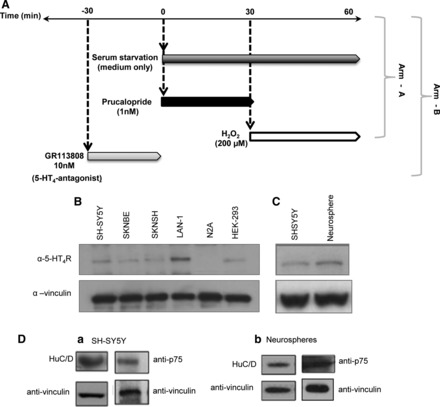
A: experimental design used in the present study. Arm-A: serum starvation (medium only) was applied at time 0 in cell cultures. Prucalopride (1 nM) was applied 30 min after challenge with H2O2 (200 μM). Arm-B: the 5-HT4 antagonist GR113808 (10 nM) was applied 30 min before either prucalopride or H2O2. B: Western blot of total cell lysates with antibody to 5-HT4 protein and with anti-vinculin in SH-SY5Y (lane 1), SK-N-BE (lane 2), SK-N-SH (lane 3), LAN-1 (lane 4), Neuro2A (N2A, lane 5), and HEK293 (lane 6). C: separate Western blot of total cell lysates with antibody to 5-HT4 protein and with anti-vinculin in SH-SY5Y (lane 1) and human enteric neurospheres (lane 2). D: total cell lysates prepared from SH-SY5Y (a) and human enteric neurospheres (b) immunoblotted with anti-HuC/D, anti-p75, and anti-vinculin antibodies.
Cell proliferation assay.
SH-SY5Y cells (3×105) were plated in duplicate and incubated with 10 μM bromodeoxyuridine (BrdU) (Millipore, Billerica, MA) for 1–48 h. BrdU-labeled cells adhered to the plate by centrifugation (300 g for 10 min) and then dried to the plate at room temperature for 1 h. Anti-mouse HRP-conjugated IgG antibody was used as secondary antibody. Absorbance measured at λ = 450 nm was used to calculate the BrdU labeling index.
Submucosal neuron whole-mount preparations from colonic biopsies.
A written, informed consent (protocol: PaBio-2011) was obtained from enrolled subjects (n = 3 female; age range: 30–55 yr) undergoing routine screening colonoscopy with biopsy sampling (n = 3 each subject from the descending colon). Subjects were recruited at the outpatient clinics of Internal Medicine and Gastroenterology Units of the Department of Medical and Surgical Sciences of University of Bologna. Biopsies were transferred to Sylgard-coated petri dish filled with ice-cold Hanks' solution and pinned flat, and the mucosa separated from the submucosa under a stereomicroscope (16). Fresh submucosal specimens were treated with prucalopride (1 nM) for 30 min in combination with H2O2 maintained in DMEM F12 medium. Biopsies were sonicated for eight cycles at 20–60 kHz.
Immunofluorescence.
Immunofluorescence was performed on neurospheres fixed in 4% paraformaldehyde, rinsed in PBS, and treated with blocking solution for 30 min. Previously described primary antibodies, i.e., rabbit anti-pChAT (1:100), mouse anti-nNOS (1:50), rabbit anti-5-HT4 (1:200), and HuC/D (1:200), were applied to neurospheres overnight at 4°C. Specificity experiments included primary antibody omission and preincubation with the respective homologous antigen. After washing with PBS, a donkey anti-rabbit Cy3, anti-rabbit Alexa488, anti-goat Alexa488, and anti-mouse Alexa488 (1:200, Thermo Fisher Scientific) secondary antibodies were applied for 30 min at room temperature. Fluoroshield DAPI solution (Sigma-Aldrich) was used as mounting solution and to counterstain nuclei. Image capturing was performed on a Nikon microscope using DS-5M digital camera (Nikon Instruments, Düsseldorf, Germany).
Statistical analysis.
Statistical analysis was performed with GraphPad Prism software v.5.0 (San Diego, CA). Statistical significance was defined as P < 0.05 as evaluated by one-way ANOVA and Tukey's multiple-comparison test. Statistical analysis of densitometry was performed using Image Lab Software (Bio-Rad).
RESULTS
Evaluation of the 5-HT4 expression in human enteric neurospheres and other neuronal cell lines.
The 5-HT4 protein expression was studied by Western blot analysis using human neuroblastoma cell lines SH-SY5Y, SK-N-BE, S-K-NSH, and LAN-1; the human embryonic kidney cell line HEK293; a mouse neural crest-derived cell line, Neuro2A (used as a negative control for antibody specificity); and human enteric neurospheres (Fig. 1, B and C). All the human cell lines analyzed showed 5-HT4 expression, whereas no signals were detected in the murine Neuro2A cells since the antibody used was specific for the human 5-HT4 isoform. Western blot analysis of human enteric neurospheres and SH-SY5Y showed that these cells also expressed the neuronal marker HuC/D and the progenitor/stem cell marker p75 (Fig. 1D), thus indicating that these cells were suitable for studies on neuroprotection.
The 5-HT4 agonist prucalopride protects SH-SY5Y cells from oxidative stress insult.
We tested different concentrations of the full 5-HT4 agonist prucalopride on SH-SY5Y cells exposed to the oxidative stress agent H2O2. The oxidative stress evoked by 200 μM of H2O2 resulted in a significant reduction of cell survival (33.3 ± 0.1%) compared with untreated cells (P < 0.001). Prucalopride at 1 nM concentration showed the best protective effect, with a significantly higher cell survival compared with cells exposed to H2O2 (73.5 ± 0.1% of neuronal survival vs. 33.3 ± 0.1%, respectively; P < 0.0001) (Fig. 2A). To verify the specificity of the 5-HT4-mediated protective effect, cells were exposed to the selective antagonist GR113808 (10 nM) applied 30 min before prucalopride administration (18). Pretreatment with GR113808 induced a significant reduction in cell survival, i.e., 23.3 ± 0.1 vs. the 73.5 ± 0.1% in the H2O2-treated cells exposed to prucalopride (P < 0.0001). These data are comparable to those obtained in presence of H2O2 alone, indicating that the observed protection was elicited via 5-HT4 activation (Fig. 2A). Untreated cells exposed to GR113808 at 10 nM concentration showed a survival rate of 81.3 ± 0.1%, which was comparable to that of vehicle-treated cells, indicating lack of toxicity of the 5-HT4 antagonist (Fig. 2A, P < 0.001). By contrast, prucalopride becomes toxic at high concentrations, with the highest toxic effect observed with 10 mM prucalopride as indicated by reduced survival (32 ± 1.2 and 29 ± 1.2% at 30 and 60 min, respectively) in cells unexposed to oxidative stress (cell survival being virtually 100%). Prucalopride at the lowest concentration tested (10 pM) failed to rescue neuronal cells from damage induced by oxidative stress (survival being 41 ± 0.8 vs. 32 ± 1.2%, respectively) (Fig. 2B).
Fig. 2.
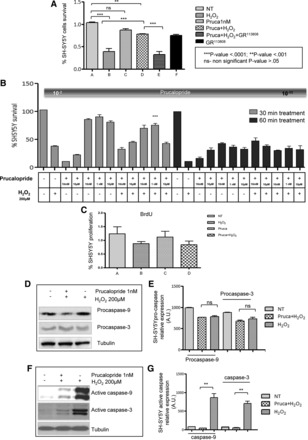
A: percentage of SH-SY5Y survived after treatment with 1 nM prucalopride alone (C bar) or in combination with 200 μM H2O2 (prucalopride+H2O2 vs. H2O2 **P < 0.0001) for 30 min (D bar) and GR113808 either alone [GR113808 vs. untreated (NT); ns] (F bar) or with 200 μM H2O2 (prucalopride+H2O2 vs. prucalopride+GR113808+H2O2 ***P < 0.0001) (E bar). A bar, NT are considered as 100% cell survival; B bar, H2O2 alone. B: dose finding was established by treating SH-SY5Y cells for 30 or 60 min with prucalopride at different concentrations (10 mM, 10 μM, 10 nM, 1 nM, and 10 pM). Compared with 30 min, 10 nM prucalopride for 60 min resulted in a reduced neuroprotective effect (cell survival 80 vs. 60%, respectively, P < 0.0001). C: BrdU analysis in the SH-SY5Y cell line. Percentage of SH-SY5Y proliferation after treatment with 1 nM prucalopride, alone (C bar) and in combination with 200 μM H2O2 for 30 min (D bar). A bar, NT; B bar, 200 μM H2O2 for 30 min. ANOVA and Tukey's multiple comparison tests did not reached statistical significance (P = 0.384). D and E: Western blotting of SH-SY5Y total lysates did not show differences in procaspase-3 and -9 expression; lane 1: NT; lane 2: 1 nM prucalopride (Pruca) and H2O2; lane 3: H2O2 alone. F and G: Western blotting of SH-SY5Y showing significant differences in active caspase-3 and -9 expression (**P < 0.001 vs. NT); lane 1: NT; lane 2: 1 nM prucalopride and H2O2; lane 3: H2O2 alone.
Evaluation of prucalopride in neurogenesis in vitro.
To determine whether prucalopride induces neurogenesis in vitro, we evaluated the BrdU incorporation 24 h after the treatment with different concentrations of the 5-HT4 agonist. Prucalopride did not evoke a significantly different incorporation of BrdU compared with untreated SH-SY5Y or H2O2-treated cells (P = 0.384) (Fig. 2C). This suggests that the effect of prucalopride treatment of SH-SY5Y cells exposed to oxidative stress is predominantly neuroprotective and not due to neuroproliferation.
Effect of prucalopride on caspase expression in neuronal cell lines and neurospheres.
To investigate the mechanisms underlying the neuroprotective effects mediated by prucalopride, we examined the expression of two apoptotic markers, i.e., caspase-3 and caspase-9, which are activated by oxidative stimuli. No changes in procaspase expression were found before and after treatments (Figs. 2, D and E, and 3, A and B), whereas a significant decrease of the active form of both oxidative stress-activated caspases-3 and caspase-9 was detected in SH-SY5Y cell lines (Fig. 2, F and G) and in human enteric neurospheres (Fig. 3, C and D) treated with 1 nM prucalopride.
Fig. 3.
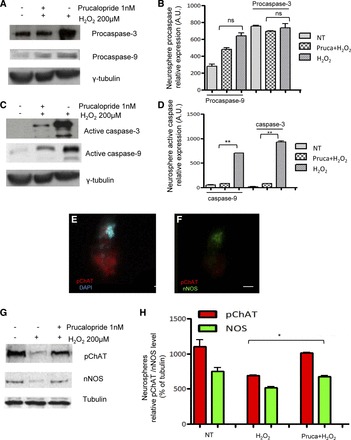
A and B: Western blotting of human enteric neurospheres did not show differences in procaspase-3 and -9 expression; lane 1: NT; lane 2: 1 nM prucalopride and H2O2; lane 3: H2O2 alone. C and D: Western blotting showed significant differences in active caspase-3 and -9 expression (**P < 0.001 vs. NT); lane 1: NT; lane 2: 1 nM prucalopride and H2O2; lane 3: H2O2 alone. E and F: note nitrergic (nNOS) (E) and cholinergic (pChAT) (F) immunoreactive neurons (scale bar 100 μm). G and H: Western blotting for nNOS and pChAT showed rescue of these 2 neuronal subsets after prucalopride treatment; lane 1: NT; lane 2: H2O2 alone; lane 3: H2O2+prucalopride (*P < 0.05 pChAT/nNOS prucalopride+H2O2 vs. H2O2).
Effect of prucalopride in different subgroups of neurons.
To determine whether the neuroprotective effect evoked by prucalopride occurred in selective subpopulations of enteric neurons, we investigated the effect of prucalopride following the oxidative stress injury on the expression of two major enteric neuron transmitters nNOS (neuronal nitric oxide synthase, the enzyme that produces nitric oxide) and pChAT (peripheral cholinacetyltransferase, the enzyme that catalyzes the synthesis of acetylcholine) in human enteric neurospheres. Expression of both nNOS and pChAT was identified by immunofluorescence and Western blot (Figs. 3, E–G). H2O2 treatment induced a quantitative reduction of both nNOS and pChAT proteins in neurospheres compared with the total protein. In contrast, prucalopride treatment prevented the oxidative-induced reduction of each subset of transmitters compared with H2O2 treatment without prucalopride (Fig. 3, H and I, P < 0.05).
Western blot analysis confirmed the expression of 5-HT4 in colonic biopsy-derived submucosae obtained during colonoscopy (Fig. 4A).
Fig. 4.
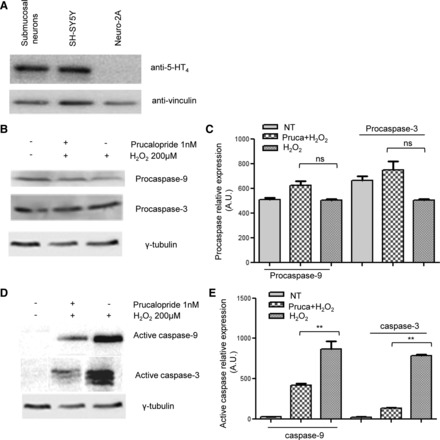
A: Western blot showing 5-HT4 expression in submucosal neuron whole mounts (lane 1) compared with human SH-SY5Y (lane 2, positive control) and murine Neuro2A (lane 3, negative control). B and C: Western blotting did not show differences in procaspase-3 and -9 expression; lane 1: NT; lane 2: 1 nM prucalopride+H2O2; lane 3: H2O2 alone. D and E: Western blotting showed significant differences in active caspase-3 and -9 expression (**P < 0.001 vs. NT); lane 1: NT; lane 2: 1 nM prucalopride+H2O2; lane 3: H2O2 alone.
After treatment with prucalopride, submucosal whole-mount preparations were exposed to H2O2 and the levels of activated caspases were measured. Procaspase expression did not vary with any treatment (Figs. 4, B and C). However, activated caspase-3 and caspase-9 were significantly reduced in prucalopride-treated biopsies compared with H2O2 only (Fig. 4, D and E, P < 0.001).
DISCUSSION
The results of this study provide strong evidence for a neuroprotective role of 5-HT4 subtype, which is expressed in human neuronal cell lines, neurospheres, and enteric neurons as confirmed by in vitro and ex vivo experiments. Stimulation of 5-HT4 with the specific, high-affinity full agonist prucalopride induced significant protection against the oxidative stress injury elicited by H2O2.
The protective effect of prucalopride on human neurons, including enteric neurons, was demonstrated by 1) the increased survival of human neuronal cell lines exposed to oxidative stress and 2) reduction of apoptosis in neuronal cell lines, human enteric neurospheres obtained from perinatal gut biopsies, and whole-mount preparation of submucosal neurons from adult colonic tissues, following oxidative stress challenge. The abolition of all of these effects by pretreatment with the 5-HT4 antagonist (GR113808) demonstrated that prucalopride-evoked protective effect was due to specific 5-HT4 activation. Taken together these results expand previous data showing that other 5-HT4 agonists (i.e., tegaserod and renzapride) protected primary cultures of mouse enteric neurons from apoptosis (17). Notably, our study indicated that the optimal neuroprotective effect was obtained with 1 nM prucalopride, which is at least 10 times lower compared with the doses used by Liu et al. (17) with either tegaserod or renzapride. The difference in terms of concentrations and related neuroprotective effect can be the result of a diverse efficiency in 5-HT4 receptor activation exhibited by the different agonists used in our vs. Liu et al. study (17).
The neural network supplying the digestive system, including the ENS, exerts a prominent regulatory role on several digestive functions (6). A tight association links gut dysfunction to ENS abnormalities characterized by neuronal damage and/or loss (15). In addition to the many effects elicited by serotoninergic pathways, 5-HT4 plays an important role in mediating 5-HT neuroprotective function as supported by the observation that mice lacking 5-HT4 displayed a markedly reduced number of submucosal and myenteric neurons in an age-dependent manner (11, 17). Oxidative stress is well known to trigger neuronal loss and represents a key target for neuroprotection. A neuroprotective effect of 5-HT against oxidative stress-induced cell death has been supported by the results of a study showing that 5-HT treatment increased the activity of antioxidant enzyme and counteracted lipid peroxidation in the brain in a rat model of Parkinson's disease (1).
Our study extends the data reported in the brain, by showing that prucalopride, a specific 5-HT4 receptor agonist, protects peripheral neurons against oxidative stress-mediated proapoptotic insults induced by exposure to H2O2, as indicated by the significant reduction in caspase-3 and caspase-9 activation, both key regulators of apoptosis in a variety of cells including the ENS (5, 7). The prucalopride-mediated neuroprotection in SH-SY5Y cell lines, human neurospheres, and ex vivo colonic submucosal neurons indicates that 5-HT4 counteracts proapoptotic pathways by influencing the activation of both initiator and effector caspases. Notably, the finding that prucalopride rescued the neuronal expression of nNOS as well as pChAT that was reduced by oxidative stress challenge suggests that damage of functionally distinct neuronal populations, i.e., nitrergic (inhibitory) and cholinergic (excitatory) neurons (6, 9), can be prevented by 5-HT4 stimulation. The 5-HT4 activity is mediated by PKA/CREB signaling (17), a system crucial for neurogenesis, and neuronal connectivity in the brain and pharmacological modulation of PKA have been proposed as possible therapeutic targets in neuronal degeneration, e.g., Alzheimer's and Parkinson's diseases (14, 20). Ideally, 5-HT4-mediated PKA activation might be exploited to restore neuronal circuitry also in enteric neuropathies.
Our results provide a pharmacological basis for prucalopride as a compound potentially useful in patients with enteric degeneration and loss of neurons, such as enteric neuropathies. These conditions are characterized by a severe abnormality of gastrointestinal motility and might represent the “tip of the iceberg” of functional digestive disorders (15). Enteric neuropathies can be either primary (i.e., of unknown etiology) or secondary to a variety of diseases including central neurodegenerative disorders. Patients with enteric neuropathies manifest a broad spectrum of digestive symptoms resulting from the severe abnormalities of gut motility. Typical examples of such conditions, which markedly impair the patients' quality of life and can be life threatening, include gastroparesis, chronic intestinal pseudo-obstruction, and severe slow-transit constipation/colonic inertia. The treatment of these conditions is mainly supportive and so far lacking a targeted, e.g., receptor-mediated, approach (9, 15).
Interestingly, a previous study reported that the 5-HT4 partial agonist tegaserod increased the number of neurons and length of neurites of in vitro cultured neural crest-derived enteric neurons, indicating that activation of enteric neural 5-HT4 receptors promotes neurogenesis (11, 17, 23). By contrast, our in vitro experiments did not show neurogenetic effects of prucalopride on human neuronal cell lines. This apparent discrepancy between our data and the previously reported results may be ascribed to a shorter period of analysis used in our investigation (24 h) compared with the Matsuyoshi et al. study (18) in which mosapride (another partial 5-HT4 agonist) was used for 15 days in animal models. Whether a longer period of treatment with prucalopride can also induce an increase in cell proliferation and/or differentiation (i.e., neurite outgrowth) remains to be elucidated.
In conclusion, the present data highlight for the first time that the specific 5-HT4 full agonist prucalopride evokes significant neuroprotective effects in neurons, including human enteric neurons, in vitro and ex vivo. Prucalopride is currently used for treatment of chronic constipation and has an excellent safety and tolerance profile. These findings widen the role of this compound beyond its well-defined enterokinetic property and support that 5-HT4 activation can be a target for pharmacological intervention in neurodegenerative diseases of the gut. Ad hoc-designed clinical trials are awaited to test the efficacy of 5-HT4 in stopping the progression of neuronal damage and loss in patients with severe gut dysmotility.
GRANTS
This work was supported by Ricerca Finalizzata RER2009 (Ita-MNGIE), Ministry of Health; the Italian Ministry of University and Research (PRIN/COFIN2009MFSXNZ_002); Telethon Grant GGP15171 (to R. De Giorgio); and NIH P30DK041301 grant “Imaging and Stem Cell Biology Core” (C. Sternini). R. De Giorgio is also the recipient of grants from “Fondazione del Monte di Bologna e Ravenna,” Bologna, Italy. N. Thapar is supported by Great Ormond Street Hospital Children's Charity (GOSHCC). D. Natarajan is funded through a GOSHCC grant (awarded to N. Thapar).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
F. Bianco, E. Bonora, N.T., U.V., C.S., and R.D.G. conception and design of research; F. Bianco, E. Bonora, D.N., M.V., F.T., F.G., E. Boschetti, and M.M. performed experiments; F. Bianco, E. Bonora, D.N., M.V., F.T., F.G., E. Boschetti, M.M., M.S., P.C., V.S., C.S., and R.D.G. analyzed data; F. Bianco, E. Bonora, D.N., M.V., N.T., F.T., F.G., E. Boschetti, U.V., F. Bazzoli, M.M., M.S., P.C., V.S., C.S., and R.D.G. interpreted results of experiments; F. Bianco, E. Bonora, and E. Boschetti prepared figures; F. Bianco, E. Bonora, U.V., and R.D.G. drafted manuscript; F. Bianco, E. Bonora, D.N., M.V., N.T., F.T., F.G., E. Boschetti, U.V., F. Bazzoli, M.M., M.S., P.C., V.S., C.S., and R.D.G. edited and revised manuscript; F. Bianco, E. Bonora, D.N., M.V., N.T., F.T., F.G., E. Boschetti, U.V., F. Bazzoli, M.M., M.S., P.C., V.S., C.S., and R.D.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the late Prof. Marcello Tonini, a renowned scientist and our friend, colleague, and (for many of us) mentor with a genuine enthusiasm for serotonin pharmacology and neurogastroenterology.
Footnotes
This article is the topic of an Editorial Focus by Michael D. Gershon (13a).
REFERENCES
- 1.Anderson G, Maes M. Neurodegeneration in Parkinson's disease: interactions of oxidative stress, tryptophan catabolites and depression with mitochondria and sirtuins. Mol Neurobiol 49: 771–783, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 130: 6387–6400, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Clement MV, Ponton A, Pervaiz S. Apoptosis induced by hydrogen peroxide is mediated by decreased superoxide anion concentration and reduction of intracellular milieu. FEBS Lett 440: 13–18, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Coupar IM, Desmond PV, Irving HR. Human 5-HT4 and 5-HT7 receptor splice variants: are they important? Curr Neuropharmacol 5: 224–231, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Danial N, Korsmeyer SJ. Cell death: critical control points. Cell 116: 205–219, 2004. [DOI] [PubMed] [Google Scholar]
- 6.De Giorgio R, Barbara G, Furness JB, Tonini M. Novel therapeutic targets for enteric nervous system disorders. Trends Pharmacol Sci 28: 473–481, 2007. [DOI] [PubMed] [Google Scholar]
- 7.De Giorgio R, Bovara M, Barbara G, Canossa M, Sarnelli G, De Ponti F, Stanghellini V, Tonini M, Cappello S, Pagnotta E, Nobile-Orazio E, Corinaldesi R. Anti-HuD-induced neuronal apoptosis underlying paraneoplastic gut dysmotility. Gastroenterology 125: 70–79, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Emmanuel A, Cools M, Vandeplassche L, Kerstens R. Prucalopride improves bowel function and colonic transit time in patients with chronic constipation: an integrated analysis. Am J Gastroenterol 109: 887–894, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Gale JD, Grossman CJ, Whitehead JW, Oxford AW, Bunce KT, Humphrey PP. GR113808: a novel, selective antagonist with high affinity at the 5-HT4 receptor. Br J Pharmacol 111: 332–338, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gershon MD, Liu MT. Serotonin and neuroprotection in functional bowel disorders. Neurogastroenterol Motil 19, Suppl 2: 19–24, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gershon MD, Tack J. The serotonin signaling system: from basic understanding to drug development for functional GI disorders. Gastroenterology 132: 397–414, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Gershon MD. 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes 20: 14–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Gershon MD. 5-HT4-mediated neuroprotection: a new therapeutic modality on the way? Am J Physiol Gastrointest Liver Physiol (March 24, 2016). doi: 10.1152/ajpgi.00120.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Irwin RW, Brinton RD. Allopregnanolone as regenerative therapeutic for Alzheimer's disease: translational development and clinical promise. Prog Neurobiol 113: 40–55, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowles CH, Lindberg G, Panza E, De Giorgio R. New perspectives in the diagnosis and management of enteric neuropathies. Nat Rev Gastroenterol Hepatol 10: 206–218, 2013. [DOI] [PubMed] [Google Scholar]
- 16.Lebouvier T, Coron E, Chaumette T, Paillusson S, Bruley des Varannes S, Neunlist M, Derkinderen P. Routine colonic biopsies as a new tool to study the enteric nervous system in living patients. Neurogastroenterol Motil 22: e11–e14, 2010. [DOI] [PubMed] [Google Scholar]
- 17.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci 29: 9683–9699, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuyoshi H, Kuniyasu H, Okumura M, Misawa H, Katsui R, Zhang GX, Obata K, Takaki M. A 5-HT4-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol Motil 22: 806–813, e226, 2010.20146727 [Google Scholar]
- 19.Mawe GM, Hoffman JM. Serotonin signaling in the gut — functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol 10: 473–486, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SY, Kim do Y, Kang JK, Park G, Choi YW. Involvement of activation of the Nrf2/ARE pathway in protection against 6-OHDA-induced SH-SY5Y cell death by α-iso-cubebenol. Neurotoxicology 44: 160–168, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Poole DP, Xu B, Koh SL, Hunne B, Coupar IM, Irving HR, Shinjo K, Furness JB. Identification of neurons that express 5-hydroxytryptamine4 receptors in intestine. Cell Tissue Res 325: 413–422, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Tack J, Camilleri M, Chang L, Chey WD, Galligan JJ, Lacy BE, Müller-Lissner S, Quigley EM, Schuurkes J, De Maeyer JH, Stanghellini V. Systematic review: cardiovascular safety profile of 5-HT(4) agonists developed for gastrointestinal disorders. Aliment Pharmacol Ther 35: 745–767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takaki M, Goto K, Kawahara I. The 5-hydroxytryptamine 4 receptor agonist-induced actions and enteric neurogenesis in the gut. J Neurogastroenterol Motil 20: 17–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc 1: 1112–1116, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Waeber C, Sebben M, Nieoullon A, Bockaert J, Dumuis A. Regional distribution and ontogeny of 5-HT4 binding sites in rodent brain. Neuropharmacology 33: 527–541, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Wong BS, Manabe N, Camilleri M. Role of prucalopride, a serotonin (5-HT-4) receptor agonist, for the treatment of chronic constipation. Clin Exp Gastroenterol 3: 49–56, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


