Abstract
Nox4 and Nox2 are the most abundant NADPH oxidases (Nox) in the kidney and have been shown to contribute to hypertension, renal oxidative stress, and injury in Dahl salt-sensitive (SS) hypertensive rats. The present study focused on the role of Nox4 and p67phox/Nox2 in the generation of H2O2 and O2·− in the renal medullary thick ascending limb of Henle (mTAL) of SS rats in response to increasing luminal flow (from 5 to 20 nl/min). Nox4 and p67phox/Nox2 genes were found to be expressed in the mTAL of SS rats. Responses of SS rats were compared with those of SS rats with knockout of Nox4 (SSNox4−/−) or functional mutation of p67phox (SSp67phox−/−). Nox4 was the dominant source of increased intracellular H2O2 production in response to increased luminal flow as determined using the fluorescent dye peroxyfluor 6-AM (PF6-AM). The rate of mitochondrial H2O2 production [as determined by mitochondria peroxy yellow 1 (mitoPY1)] was also significantly reduced in SSNox4−/− compared with SS rats, but not in SSp67phox−/− rats. In contrast, intracellular superoxide (O2·−) production (the ratio of ethidium to dihydroethidium) in the mTAL of SSNox4−/− rats was nearly identical to that of SS rats in response to luminal flow, indicating that Nox4 made no measurable contribution. mTAL O2·− production was reduced in SSp67phox−/− compared with SS rats at the lower luminal flow of 5 nl/min and progressively increased when perfusion was changed to 20 nl/min. We conclude that increased mTAL luminal flow results in increases in intracellular and mitochondrial H2O2, which are dependent on the presence of Nox4, and that p67phox/Nox2 accounts solely for increases in O2·− production.
Keywords: kidney, Dahl salt-sensitive rat, medullary thick ascending limb, Nox4, p67phox, luminal flow, mitochondrial and cellular H2O2, O2·−
increased oxidative stress in the renal medulla induced by administration of H2O2 or the superoxide dismutase (SOD) inhibitor diethyldithiocarbamic acid (DETC) has been shown to result in salt-sensitive hypertension in Sprague-Dawley (SD) rats (27, 28, 44). Elevated levels of reactive oxygen species (ROS) have been observed in the renal medulla of Dahl salt-sensitive (SS) rats, and chronic medullary interstitial infusion of the antioxidants apocynin and/or catalase significantly reduced salt-sensitive hypertension in SS rats (45).
The sources and localization of ROS production within the kidney and the functional relevance of the various oxidative species have been of great interest but remain poorly understood. We have found that elevated ROS production within the renal outer medulla of SS rats can be largely attributed to the multicomponent NADPH oxidases (Nox) (45) that catalyze the reduction of molecular oxygen to superoxide (O2·−) and H2O2. Among the seven different members of the Nox family of enzymes, Nox2 (also named gp91phox) and Nox4 are expressed in the kidneys of rats (3, 5, 18, 21, 22, 30). Our data and findings of others indicate that Nox1 is expressed at very low levels in the medullary thick ascending limb of Henle (mTAL), and Nox5 has not been found in rodents (15, 21, 30). The p67phox cytosolic subunit [necessary for Nox2 enzyme activity (20)] and Nox4 have been found to be important determinants of salt sensitivity in SS rats based on observations that functional mutation of p67phox (Ncf2 gene) in SS (SSp67phox−/−) (14) rats and knockout of Nox4 in SS (SSNox4−/−) rats (9) reduced salt-induced hypertension and renal injury of SS rats by 40–50%.
We previously characterized blood pressure salt sensitivity and renal function in SSNox4−/− and SSp67phox−/− rats compared with SS rats (9, 14). SSNox4−/− and SSp67phox−/− rats exhibited significant reductions (∼50%) in salt-induced hypertension, albumin excretion, glomerular injury, and tubular necrosis. A dynamic analysis of sequential changes in glomerular filtration rate (GFR) and in medullary blood flow during development of hypertension in unanesthetized SSp67phox−/− rats compared with SS (wild-type) rats showed that SSp67phox−/− rats were protected from the pattern of early reductions of medullary blood flow and proteinuria during the 1st wk of a high-salt diet and from the reductions of GFR observed after 2 wk of a 4% NaCl diet in SS rats (13).
We have been particularly interested in the mTAL, a segment of the nephron that reabsorbs ∼25% of the filtered Na+ and, thus, is important in NaCl and water homeostasis. The mTAL of SS rats has been found to transport and reabsorb excess amounts of NaCl compared with the salt-resistant Dahl R rats (24, 39) and produces excess ROS compared with salt-resistant control strains of rats (33). We and others have found that increased tubular flow and luminal Na+ content stimulate ROS production in the mTAL of normotensive SD rats (8, 17). The present study was designed to determine the extent to which Nox4 and p67phox contribute to total intracellular O2·− and H2O2 production and to mitochondrial H2O2 production in the mTAL of SS rats in the face of increased luminal flow and Na+ delivery. Selective fluorescent dyes were used to determine changes within whole cell O2·− and H2O2 and mitochondrial H2O2 of microperfused mTAL, as we previously described (32, 36). Our findings indicate that Nox4 contributes to mTAL cellular and mitochondrial H2O2 production and is not involved in intracellular O2·− production in response to increased luminal flow in SS rats. The p67phox subunit contributes to a lesser extent to the increased cellular H2O2 and is primarily responsible for basal levels of O2·− production.
MATERIALS AND METHODS
Experimental animals.
Male Dahl SS rats [SS rat; Medical College of Wisconsin (MCW), Milwaukee, WI], SSNox4−/− rats, and SSp67phox−/− rats were obtained at weaning from colonies developed and maintained at the MCW under controlled environmental conditions. Parents and offspring were fed a purified AIN-76A rodent diet (Dyets, Bethlehem, PA) containing 0.4% NaCl until the experimental period, during which they were fed the 4.0% NaCl (high-salt) diet (Dyets); water was provided ad libitum. Rats were studied at 7–8 wk of age and 180–250 g body weight. All experimental protocols were approved by the MCW Institutional Animal Care and Use Committee. Generation of the knockout strains using zinc finger nuclease technology has been previously described for the SSp67phox−/− (14) and SSNox4−/− (9) rats. For the RNA studies, rats were maintained on the 0.4% NaCl diet from weaning or switched to the 4.0% NaCl diet for 7 days prior to collection of tissue. For the microscopy studies, rats were maintained on the 4.0% NaCl diet for 3 days prior to tubule isolation.
Modified enzymatic isolation of nephron segments (mTALs, glomeruli, proximal tubules, and outer medullary collecting ducts) for RNA analysis.
Rats were anesthetized with pentobarbital sodium (50 mg/kg), and the kidneys were cleared of blood by retrograde infusion with 10 ml of cold saline solution. An equal volume of a collagenase digestion solution [Hanks' balanced salt solution (HBSS) containing 20 mM HEPES (HBSS-H), pH 7.4, with collagenase type 2 (200 U/ml)] was infused at 4 ml/min, and the kidneys were removed, decapsulated, and maintained at 4°C. The outer medulla and cortex tissues were cut into small pieces and incubated in separate tubes with 1–2 ml of collagenase digestion solution at 37°C for 20 min in a hybridization oven, allowing for continuous rotation of the contents of the tubes to facilitate digestion. The digested tissue formed a gradient, with the heaviest and most undigested tissue settling on the bottom of the tube and the middle supernatant containing the nephron components already separated. The heavier, undigested tissue remaining in the original tube was further digested by addition of an equal volume of collagenase solution and incubation at 37°C. The first supernatant after the initial 20-min digestion of the cortex sample was used to isolate glomeruli. The second and third supernatants after 30 and 40 min of digestion of the cortical sample were used to isolate proximal tubules (PTs), which were mixed together. The first, second, and third supernatants (20, 30, and 40 min of digestion) of the outer medulla tissue were used to collect medullary thick ascending limb (mTAL) and mixed together. The fourth and fifth digestions of the outer medullary sample (60 and 70 min of incubation at 37°C) were used to collect the outer medullary collecting ducts (OMCDs) and mixed together. The supernatant from each digestion was removed and centrifuged at 100 g for 5 min at 4°C, and the pellet was washed twice with 1% BSA-HBSS-H by centrifugation. The final pellet was resuspended in 1% BSA-HBSS-H, and this suspension was placed in a small petri dish and visualized using a dissecting stereomicroscope (model M3Z, Leica) for manual separation and segregation of the nephron elements. OMCDs, mTALs, PTs, and glomeruli were isolated from the cortex and the outer medulla using this method and transferred to individual tubes that were then frozen in liquid nitrogen and stored at −80°C until RNA extraction. Photomicrographs of the four components of the nephron that were isolated using this digestion and isolation method are shown in Fig. 1A.
Fig. 1.
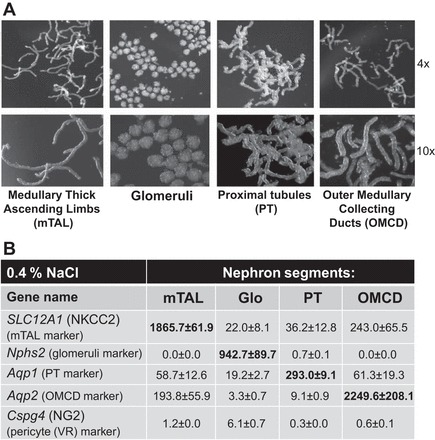
A: photomicrographs at ×4 and ×10 magnification show nephron components from kidneys of salt-sensitive (SS) rats following digestion and manual isolation prior to RNA extraction. mTAL, medullary thick ascending limb; PT, proximal tubule; OMCD, outer medullary collecting duct. B: summary of marker gene expression of the nephron components from RNA-seq analysis. Slc12a1 gene [encodes NKCC2 (Na+-K+-Cl− cotransporter)] was used as a marker of mTAL (16, 26, 47). Nphs2 (podocin), which is almost exclusively expressed in podocytes of fetal and mature kidney glomeruli, was used as a marker of glomeruli (6). Aqp1 (aquaporin-1 water channel protein) was used as a marker of PT (46). Aqp2 (aquaporin-2 collecting duct water channel protein) was used as a marker for the OMCD (10). Cspg4 [a chondroitin sulfate proteoglycan 4, also known as neuron-glial antigen 2 (NG2)] was used as a pericyte marker (vasa recta) (37). All values are expressed as fragments per kilobase of transcript per million mapped reads.
RNA sequencing.
TRIzol was used to extract RNA from each of the nephron segment samples, and the quality of the extracted RNA was determined by a bioanalyzer (model 2100, Agilent). Six rats with SS genetic background were used to obtain six RNA-seq libraries using ∼100 ng of total RNA pooled from two samples of each segment using an Ilumina TruSeq Stranded mRNA LT kit. Three libraries were prepared from rats fed the 0.4% NaCl diet and three from rats fed the 4.0% NaCl diet for 7 days. The libraries underwent cluster generation using the TruSeq PE Cluster Kit v3-cBot-HS and 100 cycles of paired-end sequencing using the TruSeq SBS Kit v3-HS. These libraries were multiplexed on one lane of a 300-Gb flow cell and sequenced using an Illumina HiSeq2000 sequencer, as previously described (19, 23). The same amount of RNA for RNA sequencing was used for all four segments (100 ng of total RNA) to enable a relative comparison of these segments. Purity of nephron segments is shown in Fig. 1B. The expression of segment markers was determined from RNA-seq data in the four isolated components of the nephron from rats with SS genomic background fed the 0.4% NaCl diet and showed that contamination of tubules with other segments was low (Fig. 1B).
Reagents and solutions.
HBSS (catalog no. 14025) was obtained from Gibco; HEPES (catalog no. H4034), BSA (catalog no. A9647), Tween 20 (catalog no. P7949), DETC (catalog no. 228680), and menadione (catalog no. M5750) from Sigma; collagenase type 2 (catalog no. LS004174) from Worthington Biochemical; Cell-Tak from BD Biosciences (Bedford, MA); and dihydroethidium (DHE; catalog no. D11347) from Molecular Probes. Mitochondria peroxy yellow 1 (mitoPY1) and peroxyfluor 6 (PF6)-AM were generously provided by Dr. Bryan Dickinson and Dr. Christopher Chang.
Nonenzymatic isolation of rat renal mTAL for fluorescence microscopy.
Rats were switched from the 0.4% to the 4.0% NaCl diet for 3 days before collection of the kidneys for the microscopy studies. On the day of study, the kidneys were flushed with 10 ml of chilled (4°C) HBSS-H (pH 7.4) and then hemisected to expose the inner strip of the outer medulla, which was removed, and the mTAL was isolated by microdissection at 4°C using the Leica M3Z stereomicroscope, as previously reported (36). The isolated mTAL tissue strip was placed on a glass coverslip coated with the tissue adhesive Cell-Tak in HBSS-H for fluorescence imaging. Several coverslips were prepared from each rat.
Microperfusion of mTAL.
Glass pipettes (Narishige) were pulled to an internal diameter of 10–15 μm, the tips were beveled and smoothed, and the pipettes were secured on a micromanipulator (World Precision Instruments, Sarasota, FL) mounted on the microscope stage. The micropipette tip was inserted into the open lumen of the mTAL, and the tubule was perfused with the HBSS-H solution with the flow rate controlled using a nano pump (model A1400, World Precision Instruments).
Fluorescence microscopy for determination of H2O2 responses to change in luminal flow.
As we described previously (36), intracellular and mitochondrial H2O2 levels in mTALs were determined by incubation of tubules with 5 μM PF6-AM (for intracellular H2O2) (11) or 5 μM mitoPY1 (for mitochondrial H2O2) (12) in HBSS-H for 30 min at 37°C. The mTALs were washed three times with HBSS-H to remove excess dye, and the coverslips were placed on a heated imaging chamber (Warner Instruments) maintained at 37°C. Several coverslips could be prepared from each kidney to permit different treatments of mTALs from the same rat, including nonperfused (no flow) time controls in each case. The number given for each experimental measurement corresponds to a separate rat in all cases. PF6-AM and mitoPY1 fluorescence images were obtained using a Nikon TE-2000U inverted microscope equipped with a 60/1.1 water immersion objective lens and a high-resolution digital camera (Photometrics Cascade 512B, Roper Scientific, Tucson, AZ) (1). Excitation was provided by a 175-W xenon arc lamp (model DG-4, Sutter Instrument, Novato, CA) at alternating wavelengths, and emission was controlled using an optical filter changer (Lambda 10-3, Sutter Instrument). PF6-AM and mitoPY1 were excited at 480/40 nm, and emission signals from 510/30 and 530/60 nm, respectively, were acquired every 1 min at the 5 nl/min flow rate and every 3 min at the 20 nl/min flow rate. PF6-AM and mitoPY1 signals were normalized by subtraction of the first time point value from each data point. A positive control stimulus was applied at the end of the experiment by administration of H2O2 (100 μM) to confirm that the tubule was viable and responsive and could be included in the analysis.
Fluorescence microscopy for determination of O2·− responses to changes in flow.
Tissue strips containing mTAL were loaded with DHE (50 mmol/l) in HBSS-H for 15 min at room temperature. The mTALs were washed three times for 5 min each with HBSS-H to remove excess dye, and the coverslips were placed on a heated imaging chamber maintained at 37°C. Fluorescence intensity of each image was quantified over an area of ∼10–15 mTAL cells using MetaFluor imaging software (Universal Imaging, Downingtown, PA). Fluorescence from regions of interest was measured every 10 s for 150 s at flow rates of 5 and 20 nl/min. The rate of O2·− production was determined as a change in ratio of the ethidium (Eth) fluorescent signal to the DHE fluorescent signal (Eth/DHE) in mTALs perfused at flow rates of 5–20 nl/min and nonperfused mTALs (no flow). We have found that using Eth/DHE to determine O2·− production in mTAL reduces measurement artifacts associated with cell volume changes and dye bleaching (31). A 445/40-nm and a 605/55-nm band-pass emission filter were used to collect DHE (380/40X–445/40E) and Eth (480/40X–605/55E), signals, respectively (35). Background Eth and DHE fluorescence signals were subtracted from the average intensity at all the regions of interest containing mTAL epithelial cells. We normalized the data by subtracting Eth/DHE value at the first time point from each data point. At the end of the experiment, the SOD inhibitor DETC (1 mM) and menadione (500 mM), a stimulant of O2·− production, were administered as a positive control for dye loading and cell viability. Regression analysis of the fluorescence ratios was performed, and differences in slopes (Pinteraction) were evaluated using two-way repeated-measures (RM) ANOVA (150 s of recording at 5 and 20 nl/min, total 15 measurements for each mTAL flow rate).
Detection of total O2·− and NADPH oxidase activity by 2-hydroxyethidium fluorescence.
Outer medullary homogenate protein (100 μg) isolated from SS and SSNox4−/− rats was incubated with DHE (20 mmol/l), with salmon testes DNA (0.5 mg/ml), and with or without 100 mmol/l diphenylene iodonium (Sigma), an inhibitor of NADPH oxidase. Oxyethidium fluorescence was measured at an excitation of 485 nm and an emission of 570 nm on a SpectraFluor microplate reader (Tecan) every 5 min for 35 min at 37°C (45). The maximal increase in fluorescence within 35 min was used as an index of total O2·− level. The portion of oxyethidium fluorescence after diphenylene iodonium inhibition was used as an index of NADPH oxidase activity.
Statistical analysis.
Values are means ± SE. Two-way RM ANOVA followed by a Holm-Sidak test (2-way RM ANOVA, SigmaPlot 12.0) was used to assess differences between the strains and between perfused and nonperfused mTALs within each strain. Pinteraction (obtained from 2-way RM ANOVA) was used to evaluate the differences in slopes. For NADPH activity measurements, significance was evaluated using a paired t-test. P < 0.05 was considered significant.
RESULTS
Nox4, p67phox, Nox1, Nox2, and Nox3 RNA expression in tubular segments and glomeruli of SS rats.
The average expression levels of Nox4, p67phox, Nox2, Nox1, and Nox3 of the isolated tubular segments and glomeruli of rats with SS genomic background fed the 0.4% NaCl diet or the 4.0% NaCl diet for 7 days are summarized in Table 1. It is evident from this RNA-seq analysis that Nox4 and Nox2 (Cybb) genes are expressed in the mTALs, OMCDs, and glomeruli of these rats and at quantitatively similar levels. Nox4 mRNA was expressed at ∼65-fold greater levels in the PTs than the mTALs of these rats, the relevance of which remains to be determined. The p67phox (Ncf2) mRNA was expressed in all tubular segments but at lower levels of expression than Nox2 (Cybb). Nox1 mRNA was expressed in the mTALs at levels only one-sixth of Nox2, but we cannot exclude Nox1 as a potential ROS generator. Nox3 was not detectable in any of the nephron segments or the glomeruli.
Table 1.
Gene expression of Nox1, Cybb (Nox2), Nox3, Nox4, and Ncf2 (p67phox) in isolated components of the nephron from rats with SS genomic background fed 0.4% and 4% NaCl for 7 days
| Nephron Segments |
||||
|---|---|---|---|---|
| Gene Name | mTAL | Glo | PT | OMCD |
| 0.4 % NaCl | ||||
| Nox4 | 3.6 ± 0.6 | 3.0 ± 1.7 | 210.4 ± 75.5 | 1.1 ± 0.3 |
| Ncf2 (p67phox) | 0.2 ± 0.0 | 0.8 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| Cybb (Nox2) | 3.5 ± 0.3 | 9.9 ± 0.9 | 2.6 ± 0.3 | 3.3 ± 0.4 |
| Nox1 | 0.7 ± 0.1 | 0.5 ± 0.0 | 0.6 ± 0.1 | 1.1 ± 0.1 |
| Nox3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 4 % NaCl for 7 days | ||||
| Nox4 | 3.0 ± 0.1 | 3.1 ± 1.2 | 155.9 ± 52.6 | 2.0 ± 0.4 |
| Ncf2 (p67phox) | 0.5 ± 0.1 | 0.8 ± 0.1 | 0.4 ± 0.1 | 0.3 ± 0.1 |
| Cybb (Nox2) | 5.6 ± 0.6 | 11.1 ± 0.2 | 6.1 ± 1.0 | 4.4 ± 0.8 |
| Nox1 | 0.6 ± 0.0 | 0.4 ± 0.1 | 0.7 ± 0.1 | 1.2 ± 0.1 |
| Nox3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
Values (means ± SE) are expressed as FPKM (fragments per kilobase of transcript per million mapped reads). Gene expression was determined by RNA-seq in isolated components of the nephron from rats with salt-sensitive (SS) genomic background. Glo, glomerulus; mTAL, medullary thick ascending limb of Henle; OMCD, outer medullary collecting duct; PT, proximal tubule.
Comparison of total intracellular H2O2 production in mTALs isolated from SS, SSNox4−/−, and SSp67phox−/− rats in response to increased tubular flow.
Intracellular mTAL H2O2 responses (PF6-AM fluorescence) to increased luminal flow (from 5 to 20 nl/min) were compared in SS, SSNox4−/−, and SSp67phox−/− rats fed the 4.0% NaCl diet for 3 days. Nonperfused mTALs of each strain were used as time controls. Figure 2A shows that mTALs of SS rats (n = 12) responded with significant elevation of intracellular H2O2 as luminal flow was increased compared with nonperfused mTALs (slopes were statistically different, Pinteraction < 0.01). In contrast, increases in intracellular H2O2 were absent in mTALs isolated from mutant SSNox4−/− rats (n = 8) and did not differ significantly from the nonperfused control mTALs (Fig. 2B). The mTALs of SSp67phox−/− rats (n = 8) exhibited reduced H2O2 responses compared with SS rats but not to the extent seen in SSNox4−/− mTALs and also were not significantly different from control nonperfused mTALs (Fig. 2C). These differences are more apparent in Fig. 2D, which summarizes the mTAL H2O2 responses to luminal flow in the three rat strains. Statistical comparisons of mean data at each time point at 5 and 20 nl/min between SS and SSp67phox−/− mTALs show that H2O2 production was reduced in the absence of p67phox (SSp67phox−/− rats) after 20 min of 20 nl/min flow, despite no significant differences in slopes between the two strains (Pinteraction = 0.2), indicating that Nox2 probably does contribute to the overall cellular increases in H2O2. The H2O2 production of mTALs from SSNox4−/− rats was significantly reduced compared with SS and SSp67phox−/− rats whether the mean data at each time point or slopes (Pinteraction < 0.01) were compared. This total elimination of the H2O2 response to flow in mTALs isolated from SSNox4−/− rats indicates that Nox4 was the major source of H2O2 production.
Fig. 2.
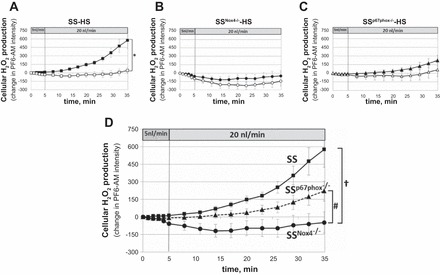
A–C: intracellular H2O2 responses [change in peroxyfluor 6 (PF6)-AM (PF6-AM) intensity] to an increase in mTAL luminal flow from 5 to 20 nl/min in SS (n = 12), SSNox4−/− (n = 8), and SSp67phox−/− (n = 8) rats fed 4.0% NaCl diet for 3 days prior to study (■, ●, and ▲). □, ○, and △, Fluorescence changes of nonperfused mTALs. *Significant differences between slopes of perfused and nonperfused mTALs within each strain [2-way repeated-measures (RM) ANOVA, Pinteraction < 0.05]. D: summary of responses to increased luminal flow in mTALs of SS (■), SSNox4−/− (●), and SSp67phox−/− (▲) rats. †Significant differences between slopes of SS and SSNox4−/− rats; #significant differences between slopes of SSNox4−/− and SSp67phox−/− rats (2-way RM ANOVA, Pinteraction < 0.05).
mTAL luminal flow induced mitochondrial H2O2 production in SS, SSNox4−/−, and SSp67phox−/− rats.
The mitochondria H2O2-specific fluorescent dye mitoPY1 was applied to measure responses to increased luminal flow in mTALs of rats fed the 4.0% NaCl diet for 3 days prior to the study. As summarized in Fig. 3, A and C, mitoPY1 fluorescence intensity significantly increased in the mTALs of SS and SSp67phox−/− rats in response to increased luminal perfusion compared with the nonperfused control mTALs (slope differences, Pinteraction < 0.05). However, intracellular H2O2 production did not differ significantly between perfused and nonperfused mTALs isolated from mutant SSNox4−/− rats (n = 8; Fig. 3B). Mitochondrial responses of mTALs isolated from SS, SSNox4−/−, and SSp67phox−/− rats relative to flow are summarized in Fig. 3D. At a luminal perfusion of 5 nl/min, the slopes were not statistically different between the rat strains. When luminal flow was increased to 20 nl/min, the rate of increase in mitochondrial H2O2 production was reduced in SSNox4−/− compared with SS rats (Pinteraction = 0.015), which was apparent only after 20 min. While the mitochondrial H2O2 production tended to be greater in SSp67phox−/− than SS rats, these differences were not statistically significant in terms of slopes or mean data at each time point (Fig. 3D).
Fig. 3.
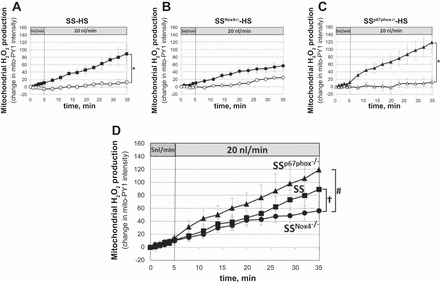
A–C: mitochondrial H2O2 responses [change in mitochondria peroxy yellow 1 (mitoPY1) intensity] to increased mTAL luminal flow from 5 to 20 nl/min in SS (n = 10), SSNox4−/− (n = 6), and SSp67phox−/− (n = 7) rats fed 4.0% NaCl diet for 3 days prior to study (■, ●, and ▲). □, ○, and △, Fluorescence changes of nonperfused mTALs. *Significant differences between slopes of perfused and nonperfused mTALs within each strain (2-way RM ANOVA, Pinteraction < 0.05). D: summary of responses to increased luminal flow in mTALs of SS (■), SSNox4−/− (●), and SSp67phox−/− (▲) rats. †Significant differences between slopes of SS and SSNox4−/− rats; #significant differences between slopes of SSNox4−/− and SSp67phox−/− rats (2-way RM ANOVA, Pinteraction < 0.05).
Intracellular O2·− production in mTALs isolated from SS, SSNox4−/−, and SSp67phox−/− rats in response to increased tubular flow.
Eth/DHE fluorescence responses were used to evaluate intracellular changes of O2·− in mTALs. The rate of O2·− production (slopes, Pinteraction) was significantly different between mTALs perfused at luminal flow of 5 and 20 nl/min and nonperfused mTALs of each strain (Fig. 4, A–C). Only mTALs of SSp67phox−/− rats did not show significant differences in slopes between mTALs perfused at 5 nl/min and nonperfused mTALs (Fig. 4C). Figure 4D shows that O2·− production (Eth/DHE) in mTALs of SS rats increased continuously throughout the 5 nl/min perfusion period and that although O2·− production continued to increase when luminal perfusion was changed to 20 nl/min, the rate of O2·− production (slope) was not further increased. Importantly, this response was nearly identical in mTALs of SSNox4−/− and SS rats (Fig. 4D), indicating that Nox4 did not contribute to the mTAL O2·− production. Consistent with these observations, NADPH oxidase activity determined from homogenates of outer medullary tissue of SSNox4−/− rats fed the 4.0% NaCl diet for 21 days showed no significant differences in total enzyme activity compared with SS rats (n = 5 for each strain). The rate of O2·− production was significantly lower in mTALs of SSp67phox−/− than SS and SSNox4−/− rats (slope differences, Pinteraction < 0.001; Fig. 4D) at the lower rates of luminal perfusion (5 nl/min). However, the response of SSp67phox−/− rats to 20 nl/min flow was similar to that of SS and SSNox4−/− rats (slopes were not significantly different; Fig. 4D). We conclude that Nox4 makes no measurable contribution to the tubular flow-induced increase in O2·− production, while p67phox/Nox2 contributes significantly to the basal production of O2·−, at low (5 nl/min), but not high (20 nl/min), rates of luminal flow.
Fig. 4.
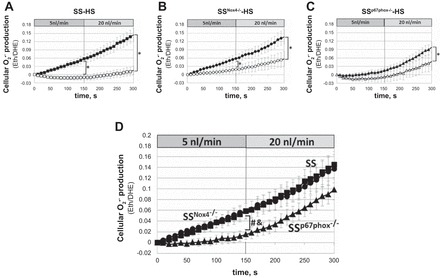
Intracellular superoxide (O2·−) production [ethidium-to-dihydroethidium (Eth/DHE) ratio] in response to increase in mTAL luminal flow from 5 to 20 nl/min in SS (n = 9), SSNox4−/− (n = 7), and SSp67phox−/− (n = 8) rats fed 4.0% NaCl diet for 3 days prior to study (■, ●, and ▲). □, ○, and △, nonperfused mTALs. *Significant differences between slopes of perfused and nonperfused mTALs within each strain (2-way RM ANOVA, Pinteraction < 0.05). D: summary of responses to increased luminal flow in mTALs of SS (■), SSNox4−/− (●), and SSp67phox−/− (▲) rats. &Significant differences between slopes of SS and SSp67phox−/− rats at 5 nl/min flow; #significant differences between slopes of SSNox4−/− and SSp67phox−/− rats at 5 nl/min flow (2-way RM ANOVA, Pinteraction < 0.05).
DISCUSSION
The present studies examined the contribution of Nox4 and the p67phox cytosolic subunit of Nox2 to ROS production in tubular epithelial cells of the mTAL in response to increased luminal flow and Na+ delivery in SS rats. Excess Na+ reabsorption and elevated ROS production in the mTAL and renal outer medulla are associated with blood pressure salt sensitivity of the SS rat (9, 14, 27, 28). In this study we confirmed that Nox4 and Nox2 are the most abundantly expressed Nox isoforms in the rat kidney (Table 1), as previously reported (4, 21, 30), and importantly we determined their functional contributions to ROS production in the mTAL. Development of the SSNox4−/− and SSp67phox−/− rat models has enabled us to overcome the limitations of nonselective inhibitors and the limited in vivo efficiency of mRNA antisense oligonucleotides and allowed us to begin exploring these relationships in the salt-sensitive model of hypertension.
Nox4 and p67phox/Nox2 contributions to mTAL cellular and mitochondrial H2O2 elevations in response to increased tubular flow.
Our results indicate that Nox4 is the dominant source of intracellular production of H2O2 in response to physiological elevations of luminal flow (from 5 to 20 nl/min). This was apparent given the complete elimination of the H2O2 responses to increased luminal flow in mTALs of SSNox4−/− rats (Fig. 2D). Although SSp67phox−/− rats tended to exhibit a reduced cellular H2O2 production compared with SS rats, the slope differences were not statistically significant. The contribution of Nox4 and p67phox/Nox2 to mitochondrial H2O2 production in the SS rat has not been studied. The rate of mitochondrial H2O2 production (slope) was significantly lower in mTALs of SSNox4−/− than SS rats, as became apparent after 20 min of luminal perfusion at 20 nl/min (Fig. 3D). This finding was in contrast to mTALs of SSp67phox−/− rats, in which the rate of mitochondrial H2O2 production tended to be even greater than in SS rats, although it was not significantly different.
Localization of Nox4 has been elusive given the limitations of immunohistochemical methods and lack of specificity of Nox4 antibodies, but studies have suggested that Nox4 may be present in mitochondria and contribute to ROS formation (2, 7, 25). The results of the present study could be consistent with the presence of Nox4 in the mitochondria, which was stimulated by increased mTAL luminal flow or Na+ delivery to produce H2O2. However, we were previously unable to detect the presence of Nox1, Nox2, or Nox4 in a proteomic analysis of mitochondria isolated from the mTAL nephron segment of SS rats (48). We therefore speculate that the reductions in the rate of mitochondrial H2O2 production in SSnox4−/− rats were in part due to 1) electron transport chain activity of complexes I and III perhaps due to reduced mTAL Na+ flux (36) and 2) reduced H2O2 production of membrane Nox4 and, thereby, less diffusion of H2O2 to the mitochondria. The kinetics of such responses could also explain why ∼20 min were required to detect the reduction in the rate of increase in mitochondrial H2O2 in SSnox4−/− rats.
We observed previously (33) that apocynin pretreatment of mTALs isolated from the SD rat had no effect on mitochondrial H2O2 responses (mitoPY1 fluorescence) to increased tubular flow (from 5 to 20 nl/min). Since apocynin inhibits the translocation of cytosolic subunits of Nox1 and Nox2 required for their activation (38, 42), which are not required for Nox4, these data indicate that H2O2 produced from Nox1 or/and Nox2 in the mTAL did not feed-forward to stimulate mitochondrial H2O2 production (36). However, since Nox4 is constitutively active and its activation does not require cytosolic proteins, it is possible that H2O2 produced from membrane Nox4 could diffuse into the mitochondria (feed-forward) and enhance the measured rate of rise of mitochondrial H2O2 production in SS rats.
Independent of mitochondrial responses, it is curious that the knockout of Nox4 (SSNox4−/−) completely eliminated any increases of cytosolic H2O2 in response to increased tubular flow. Nox2 remains present in the mTALs of SSNox4−/− rats, although at reduced expression levels, as we have reported (9), but one could expect continued O2·− and H2O2 production. An absence of any increase in cellular H2O2 with increased luminal flow was not expected and suggests some type of interaction between Nox4 and Nox2. It is possible that an additive response from the knockout of Nox4 together with reduction of p67phox/Nox2 expression in SSNox4−/− rats pushed cytoplasmic H2O2 below detection limits of the PF6-AM fluorescence probe.
We previously showed in isolated mTALs of SD rats an increase in both cytoplasmic H2O2 (PF6-AM fluorescence) and mitochondrial H2O2 (mitoPY1 fluorescence) when tubular flow was increased from 5 to 20 nl/min (36). Cytoplasmic H2O2 responses were significantly reduced by pretreatment with the electron transport chain inhibitors rotenone and antimycin A and by inhibition of mTAL Na+ transport with furosemide or ouabain. It was evident from these studies that mitochondrial H2O2 production was determined by mTAL Na+ transport and that mitochondrial H2O2 production made an important contribution to the overall increases in cellular H2O2 in response to luminal flow. It is likely that similar events contributed to the overall increases in intracellular H2O2 in the SS rats.
Nox4 and p67phox/Nox2 contributions to mTAL cellular O2·− responses to increased tubular flow.
In contrast to the observed H2O2 responses, mTAL production of O2·− was not measurably affected by the absence of Nox4, as seen by the nearly identical mTAL Eth/DHE responses of the SS and SSNox4−/− rats (Fig. 4D). This finding is consistent with evidence that Nox4 produces H2O2, rather than O2·−, which is a unique feature of Nox4 and is thought to be a consequence of a highly conserved histidine residue in the E-loop of Nox4 that promotes the rapid dismutation of O2·− within the protein itself (41, 43).
As might be anticipated, SSp67phox−/− rats exhibited significantly lower rates of mTAL O2·− production than SS and SSNox4−/− rats. Yet, this difference was observed only at the low luminal perfusion rate of 5 nl/min, and as luminal perfusion was increased to 20 nl/min, the rate of change in O2·− production (slope, Pinteraction) in SSp67phox−/− rats was similar to that in SS and SSNox4−/− rats. We believe that these observations are likely explained by the high concentrations of Na+ (149 mM) in the luminal perfusate used in our studies. We have found in SD rats that at lower mTAL luminal Na+ concentrations of 60 mM, increases in luminal flow from 5 to 20 nl/min resulted in a sharp rise of intracellular O2·− production, whereas tubules perfused at 149 mM Na+ exhibited a progressive rise of O2·− production, even at a luminal perfusion rate of 5 nl/min (1). Importantly, the rate of rise of O2·− production (Eth/DHE slopes) was not further influenced by increases in luminal perfusion to 20 nl/min, indicating that the Na+ transport stimulus was already driving the O2·− production to levels that could not be further enhanced by luminal flow. Since the present studies perfused the mTAL at luminal Na+ concentrations of 149 mM and the O2·− responses (Eth/DHE) were very similar to those observed in SD rats (1) and the O2·− responses of the SSp67phox−/− rats mimicked those of SD rats perfused at lower Na+ concentration (60 mM), these data suggest reduced rates of Na+ transport in the mTALs of SSp67phox−/− rats, which reduced the basal level of O2·− production. In support of this idea are observations of reduced blood pressure salt sensitivity and reduced levels of O2·− production in the outer medullary tissue and reduced outer medullary renal injury in SSp67phox−/− rats (14, 40). We recognize that the present observations are not consistent with observations by others who concluded that Nox2 was not involved in luminal flow-mediated O2·− production (Eth/DHE) in Nox2−/− mice and found that transduction of mTALs isolated from SD rats with a Nox4 siRNA adenovirus inhibited flow-stimulated O2·− production (21). While it is difficult to reconcile these differences, many laboratories have found that H2O2, and not O2·−, is the prominent product of Nox4 (5, 29, 34, 43), and we believe that the SSNox4−/− rat model system has meaningfully addressed the contributions of Nox4.
Conclusion.
We conclude that Nox4 is responsible for the increases in intracellular and, possibly, mitochondrial H2O2 observed with physiological increases in mTAL luminal flow but does not contribute to the increases in intracellular O2·−. In contrast, p67phox/Nox2 contributes little to the elevations of H2O2 and is responsible only for flow-mediated increases in intracellular O2·−.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-116264 (A. W. Cowley, Jr.), HL-82798 (A. W. Cowley, Jr.), and HL-122662 (A. W. Cowley, Jr.).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.Z. and C.Y. performed the experiments; N.Z. and C.Y. analyzed the data; N.Z., C.Y., and A.W.C. interpreted the results of the experiments; N.Z. and C.Y. prepared the figures; N.Z. and A.W.C. drafted the manuscript; N.Z., C.Y., and A.W.C. edited and revised the manuscript; N.Z., C.Y., and A.W.C. approved the final version of the manuscript; A.W.C. developed the concept and designed the research.
ACKNOWLEDGMENTS
The authors thank Meredith Skelton for careful review of the manuscript.
REFERENCES
- 1.Abe M, O'Connor P, Kaldunski M, Liang M, Roman RJ, Cowley AW Jr. Effect of sodium delivery on superoxide and nitric oxide in the medullary thick ascending limb. Am J Physiol Renal Physiol 291: F350–F357, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 106: 1253–1264, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araujo M, Wilcox CS. Oxidative stress in hypertension: role of the kidney. Antioxid Redox Signal 20: 74–101, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asaba K, Tojo A, Onozato ML, Goto A, Quinn MT, Fujita T, Wilcox CS. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 67: 1890–1898, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Boute N, Gribouval O, Roselli S, Benessy F, Lee H, Fuchshuber A, Dahan K, Gubler MC, Niaudet P, Antignac C. NPHS2, encoding the glomerular protein podocin, is mutated in autosomal recessive steroid-resistant nephrotic syndrome. Nat Genet 24: 349–354, 2000. [DOI] [PubMed] [Google Scholar]
- 7.Case AJ, Li S, Basu U, Tian J, Zimmerman MC. Mitochondrial-localized NADPH oxidase 4 is a source of superoxide in angiotensin II-stimulated neurons. Am J Physiol Heart Circ Physiol 305: H19–H28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cowley AW Jr, Abe M, Mori T, O'Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol 308: F179–F197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cowley AW Jr, Yang C, Zheleznova NN, Staruschenko A, Kurth T, Rein L, Kumar V, Sadovnikov K, Dayton A, Hoffman M, Ryan RP, Skelton MM, Salehpour F, Ranji M, Geurts A. Evidence of the importance of Nox4 in production of hypertension in Dahl salt-sensitive rats. Hypertension 67: 440–450, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deen PM, Weghuis DO, Sinke RJ, Geurts van Kessel A, Wieringa B, van Os CH. Assignment of the human gene for the water channel of renal collecting duct aquaporin 2 (AQP2) to chromosome 12 region q12→q13. Cytogenet Cell Genet 66: 260–262, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson BC, Peltier J, Stone D, Schaffer DV, Chang CJ. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol 7: 106–112, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickinson BC, Srikun D, Chang CJ. Mitochondrial-targeted fluorescent probes for reactive oxygen species. Curr Opin Chem Biol 14: 50–56, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans LC, Ryan RP, Broadway E, Skelton MM, Kurth T, Cowley AW Jr. Null mutation of the nicotinamide adenine dinucleotide phosphate-oxidase subunit p67phox protects the Dahl-S rat from salt-induced reductions in medullary blood flow and glomerular filtration rate. Hypertension 65: 561–568, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O'Connor PM, Cowley AW Jr. Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metab 15: 201–208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fulton DJ. Nox5 and the regulation of cellular function. Antioxid Redox Signal 11: 2443–2452, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gamba G, Friedman PA. Thick ascending limb: the Na+:K+:2Cl− co-transporter, NKCC2, and the calcium-sensing receptor, CaSR. Pflügers Arch 458: 61–76, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garvin JL, Hong NJ. Cellular stretch increases superoxide production in the thick ascending limb. Hypertension 51: 488–493, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci USA 97: 8010–8014, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, Cowley AW Jr. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension 65: 447–455, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han CH, Freeman JL, Lee T, Motalebi SA, Lambeth JD. Regulation of the neutrophil respiratory burst oxidase. Identification of an activation domain in p67(phox). J Biol Chem 273: 16663–16668, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Hong NJ, Garvin JL. NADPH oxidase 4 mediates flow-induced superoxide production in thick ascending limbs. Am J Physiol Renal Physiol 303: F1151–F1156, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huling JC, Pisitkun T, Song JH, Yu MJ, Hoffert JD, Knepper MA. Gene expression databases for kidney epithelial cells. Am J Physiol Renal Physiol 302: F401–F407, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaczorowski CC, Stodola TJ, Hoffmann BR, Prisco AR, Liu PY, Didier DN, Karcher JR, Liang M, Jacob HJ, Greene AS. Targeting the endothelial progenitor cell surface proteome to identify novel mechanisms that mediate angiogenic efficacy in a rodent model of vascular disease. Physiol Genomics 45: 999–1011, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirchner KA. Greater loop chloride uptake contributes to blunted pressure natriuresis in Dahl salt sensitive rats. J Am Soc Nephrol 1: 180–186, 1990. [DOI] [PubMed] [Google Scholar]
- 25.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107: 15565–15570, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lytle C, Xu JC, Biemesderfer D, Forbush B 3rd. Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol Cell Physiol 269: C1496–C1505, 1995. [DOI] [PubMed] [Google Scholar]
- 27.Makino A, Skelton MM, Zou AP, Cowley AW Jr. Increased renal medullary H2O2 leads to hypertension. Hypertension 42: 25–30, 2003. [DOI] [PubMed] [Google Scholar]
- 28.Makino A, Skelton MM, Zou AP, Roman RJ, Cowley AW Jr. Increased renal medullary oxidative stress produces hypertension. Hypertension 39: 667–672, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Massey KJ, Hong NJ, Garvin JL. Angiotensin II stimulates superoxide production in the thick ascending limb by activating NOX4. Am J Physiol Cell Physiol 303: C781–C789, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori T, Cowley AW Jr. Angiotensin II-NAD(P)H oxidase-stimulated superoxide modifies tubulovascular nitric oxide cross-talk in renal outer medulla. Hypertension 42: 588–593, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Mori T, Cowley AW Jr. Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 43: 341–346, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Mori T, O'Connor PM, Abe M, Cowley AW Jr. Enhanced superoxide production in renal outer medulla of Dahl salt-sensitive rats reduces nitric oxide tubular-vascular cross-talk. Hypertension 49: 1336–1341, 2007. [DOI] [PubMed] [Google Scholar]
- 34.Nisimoto Y, Diebold BA, Cosentino-Gomes D, Lambeth JD. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry (Mosc) 53: 5111–5120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Connor PM, Lu L, Liang M, Cowley AW Jr. A novel amiloride-sensitive H+ transport pathway mediates enhanced superoxide production in thick ascending limb of salt-sensitive rats, not Na+/H+ exchange. Hypertension 54: 248–254, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohsaki Y, O'Connor P, Mori T, Ryan RP, Dickinson BC, Chang CJ, Lu Y, Ito S, Cowley AW Jr. Increase of sodium delivery stimulates the mitochondrial respiratory chain H2O2 production in rat renal medullary thick ascending limb. Am J Physiol Renal Physiol 302: F95–F102, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan SY, Chang YT, Lin SL. Microvascular pericytes in healthy and diseased kidneys. Int J Nephrol Renovasc Dis 7: 39–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters EA, Hiltermann JT, Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med 31: 1442–1447, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension 17: 1018–1024, 1991. [DOI] [PubMed] [Google Scholar]
- 40.Salehpour F, Ghanian Z, Yang C, Zheleznova NN, Kurth T, Dash RK, Cowley AW Jr, Ranji M. Effects of p67phox on the mitochondrial oxidative state in the kidney of Dahl salt-sensitive rats: optical fluorescence 3-D cryoimaging. Am J Physiol Renal Physiol 309: F377–F382, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res 91: 406–413, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm 2008: 106507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takac I, Schroder K, Zhang L, Lardy B, Anilkumar N, Lambeth JD, Shah AM, Morel F, Brandes RP. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase Nox4. J Biol Chem 286: 13304–13313, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor NE, Cowley AW Jr. Effect of renal medullary H2O2 on salt-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol 289: R1573–R1579, 2005. [DOI] [PubMed] [Google Scholar]
- 45.Taylor NE, Glocka P, Liang M, Cowley AW Jr. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 47: 692–698, 2006. [DOI] [PubMed] [Google Scholar]
- 46.Verkman AS. Renal concentrating and diluting function in deficiency of specific aquaporin genes. Exp Nephrol 10: 235–240, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Stingo FC, Ahn KW, Liu P, Vannucci M, Laud PW, Skelton M, O'Connor P, Kurth T, Ryan RP, Moreno C, Tsaih SW, Patone G, Hummel O, Jacob HJ, Liang M, Cowley AW Jr. Increased proliferative cells in the medullary thick ascending limb of the loop of Henle in the Dahl salt-sensitive rat. Hypertension 61: 208–215, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheleznova NN, Yang C, Ryan RP, Halligan BD, Liang M, Greene AS, Cowley AW Jr. Mitochondrial proteomic analysis reveals deficiencies in oxygen utilization in medullary thick ascending limb of Henle in the Dahl salt-sensitive rat. Physiol Genomics 44: 829–842, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


