Abstract
Binding of ouabain to cardiac Na+/K+-ATPase initiates cell signaling and causes contractility in cardiomyocytes. It is widely accepted that caveolins, structural proteins of caveolae, have been implicated in signal transduction. It is known that caveolae play a role in Na+/K+-ATPase functions. Regulation of caveolin-1 in ouabain-mediated cardiac signaling and contractility has never been reported. The aim of this study is to compare ouabain-induced cardiac signaling and contractility in wild-type (WT) and caveolin-1 knockout (cav-1 KO) mice. In contrast with WT cardiomyocytes, ouabain-induced signaling e.g., activation of phosphoinositide 3-kinase-α/Akt and extracellular signal-regulated kinases (ERK)1/2, and hypertrophic growth were significantly reduced in cav-1 KO cardiomyocytes. Interactions of the Na+/K+-ATPase α1-subunit with caveolin-3 and the Na+/K+-ATPase α1-subunit with PI3K-α were also decreased in cav-1 KO cardiomyocytes. The results from cav-1 KO mouse embryonic fibroblasts also proved that cav-1 significantly attenuated ouabain-induced ERK1/2 activation without alteration in protein and cholesterol distribution in caveolae/lipid rafts. Intriguingly, the effect of ouabain induced positive inotropy in vivo (via transient infusion of ouabain, 0.48 nmol/g body wt) was not attenuated in cav-1 KO mice. Furthermore, ouabain (1–100 μM) induced dose-dependent contractility in isolated working hearts from WT and cav-1 KO mice. The effects of ouabain on contractility between WT and cav-1 KO mice were not significantly different. These results demonstrated differential roles of cav-1 in the regulation of ouabain signaling and contractility. Signaling by ouabain, in contrast to contractility, may be a redundant property of Na+/K+-ATPase.
Keywords: caveolin-1, Na+/K+-ATPase, signaling, contractility, ouabain
Na+/K+-ATPase, the sodium pump, is a key membrane protein that regulates intracellular ion homeostasis by controlling active transport of sodium and potassium (23, 55). In the heart, cardiac glycosides such as ouabain bind to the pump and inhibit its activity and thereby increase myocardial intracellular calcium and contractility through influencing Na+/Ca2+ exchanger activity(30, 50). They are two noncovalently linked α- and β-subunits of the pump. The α-subunit is the catalytic subunit, and β is the regulatory subunit (5, 27, 56). The FXYD1 protein, i.e., phospholemman, is the third subunit in the heart that regulates the function of the enzyme (19). In rodents, adult cardiomyocytes express mainly two isoforms of Na+/K+-ATPase α-subunit, α1 (∼75%) and α2 (≤25%), which have low and high affinities for ouabain, respectively (4, 57, 60).
Concentrations of ouabain lower than α1 IC50 (∼0.1 mM) may not alter the global intracellular ion homeostasis but cause the pump to act as a signal transducer through interaction with neighboring proteins. There are multiple cell signaling pathways involved (28, 31, 46, 54, 69), including Src/EGFR/Ras (21), extracellular signal-regulated kinases 1/2 (ERK1/2) (29), phospholipase C (39), protein kinase C (42), phosphoinositide-3 kinase α (PI3Kα), and Akt (1, 38, 66). Additionally, in cultured cardiomyocytes, ouabain stimulates protein synthesis (1, 24, 25, 46, 66), an indicator of cardiac hypertrophy. These signaling functions of the Na+/K+-ATPase are initiated by interaction of ouabain with the α1-subunit within the caveolae (34, 35, 37). In rodent cardiomyocytes, the majority (∼75%) isoform is the ouabain-resistant isoform α1. The two isoforms of cardiomyocytes have ouabain sensitivities of about 2–3 orders of magnitude apart. The α2 ouabain IC50 is ∼1 μM. The signaling function through the α2-subunit is under the research and is not very clear.
Caveolae are 50–100 nm vesicular invaginations in the plasma membranes consisting of a structural protein called caveolin (49). There are three isoforms of caveolin in mammalian cells. Caveolin-1 (cav-1) is widely expressed but is found at particularly high levels in adipocytes, endothelial cells, and fibroblasts. Caveolin-2 (cav-2) interacts with caveolin-1, whereas caveolin-3 (cav-3) is expressed exclusively in myocytes. It has been well accepted that caveolae play an important role in regulation of signaling transduction and protein trafficking in hearts (12, 63). Caveolins contribute to the pathogenesis of cardiac hypertrophy, atherosclerosis, ischemic-reperfusion, and heart failure (12, 20, 45) by regulating ion transporters and membrane receptors (10, 47). Cav-3 is the major isoform expressed in cardiomyocytes. However, cav-1 is also present, but the role of cav-1 in cardiomyocytes is nearly unknown.
It has been demonstrated that the signaling function of the sodium pumps resides within the caveolae in the heart, vascular smooth muscle, and kidney (33, 34, 37, 62). Forty percent of the Na+/K+-ATPase α1-subunit and >90% of the fully glycosylated β1-subunit are localized in cardiac caveolae (35). Ouabain enhances interaction of cav-1 with Na+/K+-ATPase α1 and recruits downstream signaling proteins to caveolae (34, 36, 37) (43). Na+/K+-ATPase also stabilizes membrane distribution of cav-1 and regulates its trafficking (6). Previously, we have shown that ouabain binding to cardiac Na+/K+-ATPase causes activation of PI3K/Akt, ERK, and cardiac hypertrophy in adult cardiomyocytes in caveolae, which serve as a platform for these molecular events. In the current study, we hypothesized that caveolin-1 knockout (cav-1 KO) interrupts the interaction between Na+/K+-ATPase and signaling proteins and thereby impedes Na+/K+-ATPase signaling. Our findings demonstrate that caveolin-1 is pivotal for Na+/K+-ATPase-regulated cardiomyocyte signaling and cell growth but not for myocyte contractility.
MATERIALS AND METHODS
Animals.
cav-1 KO and genetically matched wild-type (WT) mice (stock no. 004585 and 101045, respectively) were purchased from Jackson Laboratories (Bar Harbor, ME). Offspring were bred at the college of Medicine and Life Sciences, University of Toledo. The genotypes were confirmed by PCR. Animals were housed in an animal facility in a 12 h light and 12 h dark cycle. Animal care and experiments were done following the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Toledo. All studies were carried out in mice 8–12 wk of age, at which time the body weights are indistinguishable between WT and cav-1 KO mice and the hearts of cav-1 KO mice do not exhibit reduced cardiac functions, changes in cardiac size, or other histological abnormalities that occur in older cav-1 KO mice (63).
Culture of adult mouse cardiac myocytes.
Isolation and culture of adult cardiomyocytes (ACM) were performed as previously described (1, 32). Mice were heparinized (5,000 U/kg) and anesthetized with Nembutal (100–140 mg/kg) via intraperitoneal injection. Hearts were excised and cannulated via the aorta and connected to a modified Langendorff perfusion apparatus. Isolated hearts were perfused for 5 min with calcium-free perfusion solution and followed with a solution containing 1 mg/ml type II collagenase (Worthington Biochemical, Lakewood, NJ) for 5–20 min. This procedure yielded ∼1.0 million viable rod-shaped cardiomyocytes from one heart. Cardiomyocytes were seeded to the culture dishes (precoated with 10 μg/ml laminin) at a density of 25,000 cells/ml in modified Eagle's medium, supplemented with 2 mM ATP, 2 mM glutamine, 10% fetal bovine Serum, 10 mM 2,3-butanedione monoxime (BDM), 100 U/ml penicillin, and 100 μg/ml streptomycin for 2 h in a 2% CO2 humidified incubator and then cultured overnight in a medium in which FBS was replaced by 0.1% bovine serum albumin. This medium was then changed to a fresh one that excluded BDM 30 min before the start of the indicated experiments.
Immortalized mouse embryonic fibroblast cell lines.
cav-1(+/+) and cav-1 (−/−) mouse embryonic fibroblast (MEF) cell lines were a generous gift from Dr. Julius C. Allen (Baylor College of Medicine, Houston, TX). Cells were grown in Dulbecco's modified Eagle's medium supplemented with antibiotics (penicillin and streptomycin) and 10% fetal bovine serum. The signaling and proliferation experiments were conducted after overnight serum starvation.
Cell proliferation assay.
Cell proliferation was measured by CyQUANT cell proliferation assay kit (Thermo Fisher Scientific, Carlsbad, CA) according to the manufacturer's instructions. In brief, cells were incubated different concentrations of ouabain for 96 h. Before the measurement, the cells were rinsed with PBS twice to remove the floating cells and then incubated with CyQUANT GR dye/cell-lysis buffer.
Analysis of mRNA expression by real-time PCR.
Total mRNA was extracted from isolated ACM with TRIZOL reagent according to the manufacturer's protocol. cDNA was synthesized from 3 μg RNA with the SuperScript III first-strand cDNA synthesis kit (Invitrogen, Carlsbad, CA). Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad, Berkeley, CA). Each sample was prepared in triplicate and loaded onto a 96-well PCR optical plate (Bio-Rad). The iCycler thermal cycler and the MyiQ software (Bio-Rad) were used for the real-time PCR experiment and for data analysis, respectively. Threshold cycles (Ct) values of >30 cycles were considered too little for significant amounts of cDNA copy numbers for data analysis and have been designated “low abundance” expression levels. In all cases, glyceraldehyde-3-phosphate dehydrogenase gene was used for data standardization and normalization. Gene expression levels and fold change comparisons were assessed using the ΔCt (cycle threshold) and ΔΔCt, respectively.
SDS-PAGE and Western blot analysis.
Cell lysates, homogenization of cardiac left ventricular tissues, and Western blotting were performed as we previously described (37, 38, 62). Primary antibodies were from BD Transduction Laboratories (US) [mouse anti-cav-1 (cat. #610058), anti-cav-2, anti-cav-3, and anti-PI3K p110α, anti-PI3K p85], Cell Signaling Technology (Danvers, MA) [rabbit anti-phospho473-Akt, anti-Akt, anti-phospho-ERK (for mouse heart) and anti-ERK], ABR (Na+/K+-ATPase α2), Millipore (Billerica, MA) (Na+/K+-ATPase β1), and Santa Cruz Biotechnology (Santa Cruz, CA) [anti-cav-1, anti-phospho-ERK (for myocytes), and secondary antibodies, goat anti-rabbit IgG-horseradish peroxidase (HRP), and goat anti-mouse IgG-HRP].
PI3Kα kinase activity assay.
This was conducted as previously described (1, 38, 65, 66). Briefly, cells were lysed in RIPA buffer with inhibitors. Equal amount of protein in each sample were immunoprecipitated with anti-PI3K p85α antibody (06-195; EMD Millipore, Billerica, MA). PI3K activity in the immunoprecipitates was assayed directly on the beads by a standard procedure with PI 0.6 mg/ml (Avanti Polar Lipids, Alabaster, AL) and [γ-32P] ATP (250 μM) as substrates. The product [γ-32P] PI(3)P was scanned by Typhoon Trio phosphorimager (GE Healthcare) and quantified by Image J software.
Immunoprecipitation.
Immunoprecipitation was conducted as previous described (1, 38, 65). Immunoprecipitation of cav-3 was modified slightly by using a lysate buffer containing 60 mM N-octylglucose, 20 mM Tris·Cl, pH 8.0, 150 mM NaCl, followed by washing buffer containing 60 mM N-octylglucose, 20 mM Tris·Cl, pH 8.0, 1% Triton X-100, 150 mM NaCl.
Protein synthesis assay.
Protein synthesis was measured using [3H]-leucine incorporation assay as previously described (1, 65, 66). Protein synthesis assay was used as indication of myocyte hypertrophy (1).
Immunofluorescence confocal microscopy.
Cells were prepared as previously described before (17, 34, 35, 39). In brief, cardiomyocytes were fixed in 2% paraformaldehyde, permeabilized in 0.02% Triton X-100, immunostained with anti-cav-1 (cat. #610058) or anti-cav-3 (cat. 610420) antibodies, and visualized with Alexa Fluor 488 (Molecular Probes) conjugated secondary antibodies. The coverslips were mounted with ProLong Gold antifade reagent (Molecular Probes). Confocal images were captured by a Leica TCS SP2 spectral confocal scanner and a Leica DMIRE2 microscope (Leica, Mannheim, Germany).
Echocardiography.
Left ventricular (LV) function and geometry of WT and KO mice were assessed with a 15 MHz probe and Philips Sonos 5500 Ultrasound system (Phillis Medical Systems, Bothell, WA) utilizing previously described techniques (18).
Transient ouabain infusion and measurement of cardiac function in intact animals.
LV hemodynamics were measured as previously described (16). Adult male mice were anesthetized with Nembutal (40–60 mg/kg ip). A high-fidelity, 1.4-French Millar Mikro-Tip transducer (model SPR-838; Millar Instruments, Houston, TX) was inserted into the right carotid artery and advanced into the LV to monitor cardiac performance. After a stabilization period of 10–15 min, signals were recorded continuously on an MPVS 400 pressure-volume conductance system (Millar Instruments). LV functions were recorded and analyzed by PVAN3.6 (Millar Instruments). The right femoral vein was cannulated for infusion of a bolus of ouabain (0.48 nmol/g body wt) or an equal volume of saline. LV function was recorded throughout drug or saline administration.
Working heart perfusion and analysis of LV function.
Mice were heparinized and anaesthetized. The hearts were excised and arrested in cold Krebs-Henseleit buffer (KHB) containing (in mmol/l) 118 NaCl, 4.7 KCl, 1.2 KH2PO4, 25 NaHCO3, 0.8 MgSO4, 1.85 CaCl2, 11.1 glucose, and 0.3 EGTA. The surplus tissues (lung, fat, and trachea) were removed rapidly, and the aorta was cannulated with a blunted 18-gauge needle in a working-heart perfusion apparatus (IH-SR; Hugo Sachs Elektronik/Harvard apparatus, Holliston, MA). The hearts were initially perfused in Langendorff mode with KHB equilibrated with 95% O2-5% CO2 at 37°C. The aortic pressure was set at 50 mmHg. The left atrium was cannulated with an 18-gauge steel cannula. The heart was then switched to the working heart mode. LV functions were recorded via a high-fidelity 1.0 F microconductance catheter (PVR-1045; Millar Instrument, Houston, TX) inserted into the LV chamber through the aorta needle. The catheter was linked to a Millar MPVS Ultra transducer. Atrial perfusion pressure was set at 12 mmHg, and afterload was set at 60 mmHg. After the heart was equilibrated for 10–15 min, LV function was analyzed with a Power Lab Chart 7 through a data acquisition system (PowerLab 8/30 and Bridge AMP, AD Instruments). LV contractility (dP/dt max and dP/dt min) was determined at baseline and steady-state conditions after the perfusion with different concentrations of ouabain.
Caveolar and noncaveolar membrane fractions.
MEF lysates were fractioned as previously described (34, 36, 37). Cells were lysed at 4°C in a solution containing 0.5 M Na2CO3 (pH 11), 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 1 mM NaF, 10 nM okadaic acid, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. The samples were then homogenized, sonicated, and centrifuged at 175,000 g in a SW41 rotor for 16 h by flotation through sucrose gradients at 4°C. After collection of 12 1-ml fractions, each fraction was diluted in 10 vol of MBS [25 mM 2-(N-morpholino) ethanesulfonic acid, 150 mM NaCl, pH 6.5] and centrifuged at 100,000 g for 1 h at 4°C. The membrane pellet from each fraction was resuspended in MBS and used immediately or frozen at −80°C.
Cholesterol assay.
Cholesterol content of membrane fraction was measured by the fluorescent Amplex red cholesterol assay kit (Molecular Probes, Eugene, OR) according to the manufacturer's instructions.
Statistical analysis.
Data are presented as means ± SE. Statistical analysis was performed by using SPSS software (IBM, version 21). Student's t-test was used to compare the means of two groups. One-way ANOVA, followed by the Bonferroni's post hoc test was used to compare the means of multiple groups. Two-way ANOVA was used to analyze the main effect of doses of ouabain and the main effect of mouse strains (WT and KO) and their interaction effect. Difference was considered statistically significant at P < 0.05.
RESULTS
Caveolin expression in isolated ACM from WT and cav-1 KO mice.
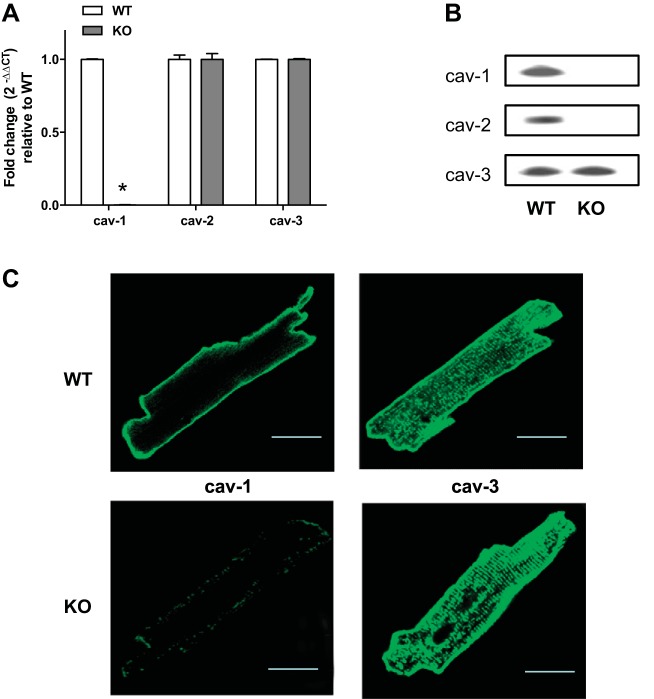
The expression of mRNA and protein for cav-1, cav-2, and cav-3 in isolated ACM from WT and KO mice was measured by real-time RT-PCR and Western blot, respectively. There was no change in expression of cav-2 and cav-3 mRNA, but expression of cav-1 mRNA was abolished in KO ACM (Fig. 1A). Proteins of three isoforms were expressed in WT ACM; only cav-3 was detectable in KO ACM (Fig. 1B). This is consistent with a previous report that shows the protein level of cav-2 is severely reduced in all cav-1 null tissues, but the expression of cav-2 mRNA remains unchanged (49). The distributions of cav-1 and 3 in ACM by fluorescence immunostaining are shown in Fig. 1C. Cav-3 is shown evenly in the peripheral sarcolemma and T-tubule membranes in ACM. Cav-1 was distributed mainly in the peripheral sarcolemma in WT but was greatly decreased in KO ACM.
Fig. 1.
Expression of caveolins in isolated adult cardiomyocytes (ACM) in wild-type (WT) and caveolin-1 knockout (cav-1 KO) mice. A: mRNA expression was expressed by fold changes assessed with specific primers by real-time PCR (n = 4). GAPDH is used an internal control. Means of Δcycle threshold (CT) from KO were compared with means of WT by Student's t-test, *P < 0.05 vs. WT. B: representative Western blots for caveolin proteins from 3 independent experiments. C: fluorescence confocal images show distributions of caveolins 1 and 3 in cultured mouse cardiomyocytes. Bar: 25 μm.
Effect of cav-1 KO on mouse heart in vivo and ouabain-induced Na/K-ATPase signaling and hypertrophy in ACM.
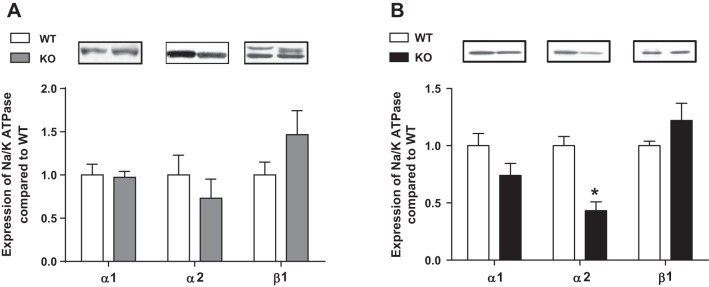
Echocardiographic evaluation showed no significant differences in LV size, geometry, or systolic function between WT and cav-1 KO mice at 10–12 wk of age (Table 1). Cav-1 KO did not alter expression of signaling proteins (Src, PI3Kα, Akt, and ERK1/2) in ACM but increased basal p-ERK1/2 level in LV (data not shown), which is consistent with the earlier report (10). Neither protein levels of Na+/K+-ATPase α1- nor β1-subunits were altered in KO ACM. However, protein expression of Na+/K+-ATPase α2-subunit was significantly reduced in KO ACM (Fig. 2).
Table 1.
Echocardiographic analysis of cardiac size and function in WT and cav-1 KO mice (10–12 wk)
| HR, bpm | LVEDD, mm | LVESD, mm | PWT, mm | SWT, mm | RWT, mm | M-Mode FS, % | B-Mode FS, % | |
|---|---|---|---|---|---|---|---|---|
| WT (n = 13) | 376 ± 21 | 3.3 ± 0.2 | 1.8 ± 0.2 | 1.1 ± 0.1 | 1.0 ± 0.1 | 6.8 ± 0.8 | 47 ± 4 | 52 ± 5 |
| KO (n = 9) | 404 ± 40 | 3.1 ± 0.1 | 1.7 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 7.3 ± 0.6 | 46 ± 3 | 51 ± 4 |
Values are averages ± SE.
WT, wild type; cav-1 KO, caveolin-1 knockout; bpm, beats per min; HR, heart rate; LVEDD, left ventricular end-diastolic internal diameter; LVESD, left ventricular end-systolic internal diameter; PWT, posterior wall thickness; SWT, septal wall thickness; RWT, relative wall thickness; FS, fractional shortening.
Fig. 2.
Comparison of Na+/K+-ATPase subunits in left ventricles (LV) and isolated ACM from WT and cav-1 KO mice. LV or ACM lysates from 12 wk old age-matched mice were subjected to Western blots for the α- and β-subunits of Na+/K+-ATPase subunits. A: LV (n = 4); B: ACM (n = 6). *P < 0.05 vs. WT.
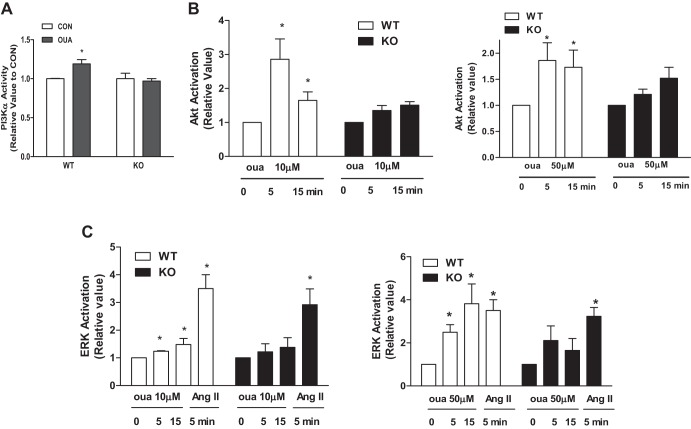
Knockout of cav-1 reduced ouabain (50 μM, 5–15 min)-mediated activation of PI3Kα, Akt, and ERK1/2 (Fig. 3). Furthermore, ouabain (10–50 μM, 12 h) increased protein synthesis in WT but not KO ACM (Fig. 4). Angiotensin II (ANG II)-induced ERK activation and -increased protein synthesis was not significantly altered (Figs. 3 and 4). These results suggest that cav-1 coordinates ouabain-induced Na+/K+-ATPase signaling and hypertrophy.
Fig. 3.
Effects of ouabain on signaling in cultured cardiomyocytes from WT and cav-1 KO mice. A: phosphoinositide-3 kinase (PI3K)α activity. Myocytes were incubated with or without 50 μM ouabain for 5 min. Cell lysates were immunoprecipitated (IP) with PI3K p85 subunit and assayed for PI3Kα activity as described in materials and methods (n = 4). *P < 0.05 vs. control (CON). B: Akt activation. Myocytes were incubated with 10, 50 μM ouabain for the indicated time (n = 4). Cell lysates were subjected to Western blots. Akt activation was assessed by the ratio of phosphorylated (p)-Akt/total Akt. C: ERK activation. Myocytes were incubated with 10, 50 μM ouabain or 100 nM ANG II for indicated time (n = 4). Cell lysates were subjected to Western blots. ERK activation was assessed by the ratio of p-ERK/ total ERK (n = 4). *P < 0.05 vs. 0 min. oua, Ouabain.
Fig. 4.
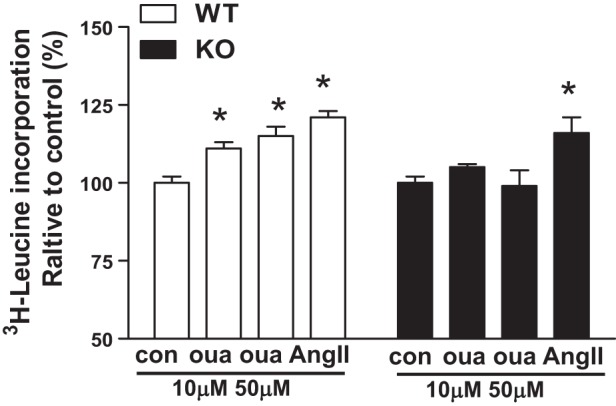
Effects of ouabain on hypertrophy in cultured cardiomyocytes from WT and cav-1 KO mice. Adult cardiac myocytes (ACM) were serum-deprived (4 h) and incubated with 1 μCi/ml radioactive labeled [3H]leucine in the presence or absence of ouabain or 100 nM ANG II for 12 h (n = 8). One-way ANOVA was used for analysis of ouabain dose effects. *P < 0.05 vs. con (vehicle).
Cav-1 KO and caveolar protein complexes.
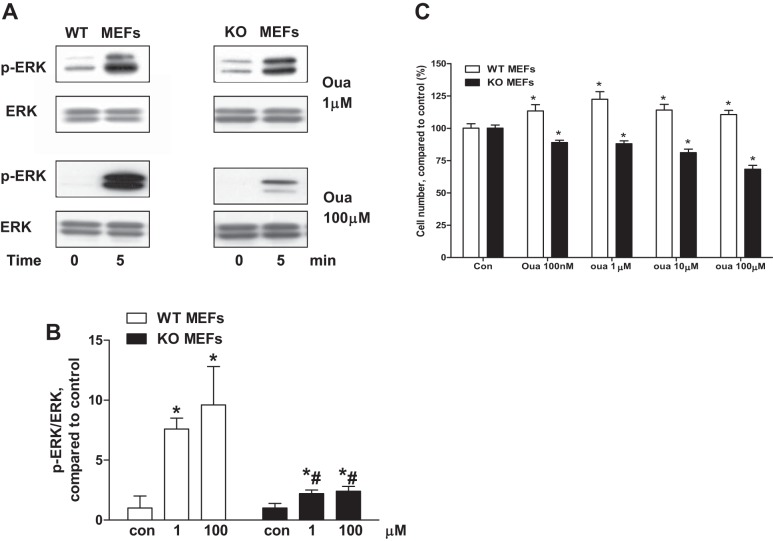
Immobilized MEFs from WT (cav-1+/+) and cav-1 KO (cav-1−/−) cell lines were used to compare caveolar protein and lipid distribution. Caveolar membrane fractions were isolated with a procedure that has been used successfully in our lab as described previously (34, 36, 37). Protein distribution and cholesterol content of the caveolar preparations were similar in the WT and cav-1 KO cells (data not shown). There is only one isoform α1-subunit of Na+/K+-ATPase expressed in MEFs. There were no significant alterations in Na+/K+-ATPase and its related signaling molecules of the caveolar fractions between WT and cav-1 KO MEFs. However, ouabain-induced activation of ERK was significantly reduced in cav-1 KO MEFs (Fig. 5, A and B). Ouabain-induced cell proliferation in WT MEFs was not observed in cav-1 KO MEFs (Fig. 5C). Conversely, ouabain-treated cav-1 KO cells demonstrated a consistent deterioration of cell numbers (Fig. 5C).
Fig. 5.
Ouabain-induced signaling in WT mouse embryonic fibroblasts (MEFs) and cav-1 KO MEFs. A: representative Western blots from 4 independent experiments. B: quantitation data on ERK activation. C: cells were treated by different concentrations of ouabain for 96 h. Cell proliferation was measured by CyQUANT assay (n = 8). One-way ANOVA and post hoc analysis were used to compare the ouabain concentration effects. *P < 0.05 vs. Con. Student's t-test was used to compare ouabain effects between WT and cav-1 KO MEFs, #P < 0.05 vs. WT MEFs at the same ouabain concentration.
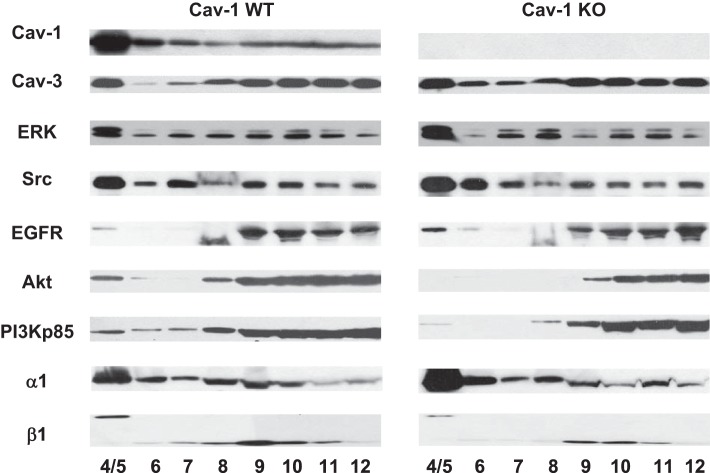
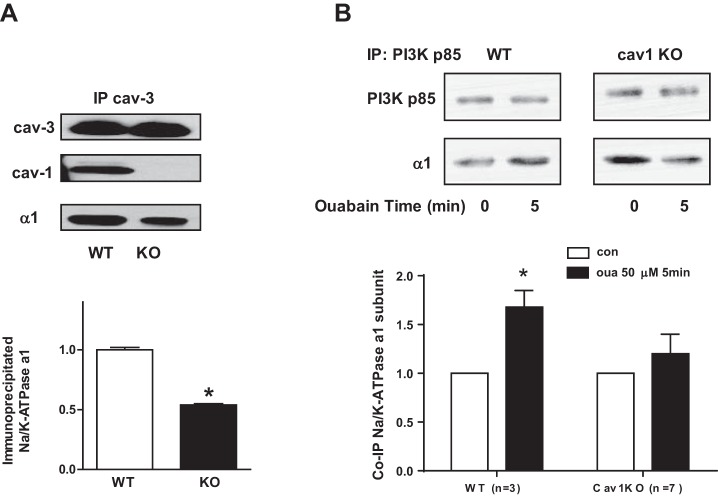
We also isolated caveolar membrane fractions from WT and cav-1 KO cardiomyocytes. Representative blots of the distribution are shown in Fig. 6. The distribution of total protein, α1 of Na+/K+-ATPase, and cav-3 was similar in WT and cav-1 KO cardiomyocytes caveolar preparation. However, β1 of Na+/K+-ATPase, PI3K, and Akt slightly reduced in caveolar fractions (Fig. 6). Moreover, immunoprecipitation assay showed that association of cav-3 and α1 of Na+/K+-ATPase dramatically reduced in isolated cav-1 KO cardiomyocytes (Fig. 7A). It suggests the sodium pump interacts with caveolin-3 partly through cav-1. Association of α1 of Na+/K+-ATPase and PI3K p85 regulated by ouabain also decreased in cav-1 KO cardiomyocytes (Fig. 7B). These data suggest that knockout of cav-1 disrupts the association of cav-3 and PI3Kα with the Na/K ATPase, which in turn could suppress ouabain-induced signaling as seen in Figs. 3 and 5.
Fig. 6.
Distribution of caveolar preparations and caveolins/Na+/K+-ATPase complex in WT and cav-1 KO cardiomyocytes. Representative blots from 2 experiments for caveolar membrane fractionation. Fractions 4 and 5 are considered as caveolar fractions. The same percentage of each fraction was used for loading.
Fig. 7.
Association of Na+/K+-ATPase with signaling proteins in cardiomyocytes from WT and cav-1 KO mice. A: cardiomyocyte lysates were IP with cav-3 antibody and co-IP with cav-1 and Na+/K+-ATPase α1-subunit. Top: representative blots. Bottom: the quantitative data (n = 5). *P < 0.05 vs WT. B: cardiomyocytes were exposed to 50 μM ouabain for 5 min. Cell lysates were subjected to IP with PI3K p85 antibody. Top: representative blots. Bottom: the quantitative data. *P < 0.05 vs. con.
Hearts of cav-1 KO mice retain ouabain-induced contractility.
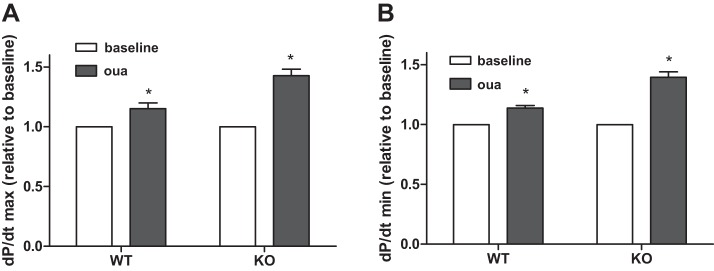
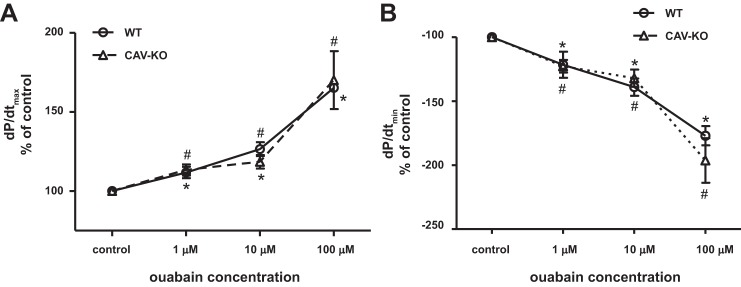
Ouabain (0.48 nmol/g body wt iv) was administered to anesthetized mice to determine the inotropic effect in vivo, resulting in an estimated blood concentration of <1 μM according to a previous report (15). After 2–5 min of infusion, the maximum rates of LV pressure rise and fall (LV dP/dt max and dP/dt min) were similarly increased in both WT and KO mice (Fig. 8). This observation of the in vivo ouabain inotropic effect might result from direct myocardial stimulation and extracardiac Na+/K+-ATPase. To further assess the direct inotropic effect of ouabain in cardiomyocytes, we examined ex vivo working hearts. After stabilization, a series of concentrations of ouabain (1, 10, 100 μM) were perfused through the hearts via the left atrium. Contractility was determined by an LV pressure-volume catheter directly inserted into the LV via the aorta. As shown in Fig. 9, ouabain induced contractility (LV dP/dt max and dP/dt min) in a dose-dependent manner in both WT and cav-1 KO hearts. After administration of ouabain (100 μM), LV dP/dt max (systolic contractility) was increased by 165 ± 2% in WT and 170 ± 18% in KO, and dP/dt min (diastolic contractility) was changed by −177 ± 7% in WT and −196 ± 17% in KO. Cardiac contractilities induced by ouabain were not significantly different between WT and cav-1 KO working hearts.
Fig. 8.
Ouabain induced cardiac positive inotropy in vivo. A high-fidelity, 1.4-French Millar Mikro-Tip transducer was advanced into the LV to monitor cardiac performance. The right femoral vein was cannulated for infusion of a bolus of ouabain (0.48 nmol/g body wt) or an equal volume of saline. LV function was recorded throughout drug or saline administration and analyzed off-line. A: dP/dt max; B: dP/dt min (n = 5). Paired t-test was used to compare with baseline and ouabain infusion. *P < 0.05 vs baseline.
Fig. 9.
Ouabain-induced contractility in perfused working hearts from WT and cav-1 KO mouse hearts. Measurements of steady-state cardiac function were made at 10 mmHg preload and 60 mmHg afterload. x-Axis is the log10 scale. A: dP/dt max; B: dP/dt min. Each data point represents mean ± SE. n = 6. Two-way ANOVA was used to analyze the effects of ouabain doses, mouse strains, and the interaction of both. There is no statistically different in contractility between the strains (WT and KO). One-way ANOVA and Bonferroni's post hoc test were used to compare ouabain dose effects. #P < 0.05 vs control in WT, *P < 0.05 vs. control in cav-KO.
DISCUSSION
Cav-1 KO causes a variety of pathophysiological consequences (26, 48, 52, 70). Cav-1 KO mice spontaneously develop cardiac hypertrophy 4–5 mo after birth concomitant with increased ERK1/2 activation in cardiac fibroblasts; however, cav-1 KO hearts still retain normal metabolic processes (13, 63). A previous report reveals normal cardiac function and structure (normal expression of troponin I or α-actinin) in young KO mice (9). These young KO mice have normal responses to physiological challenge or β-adrenergic stimulation (9). The young male KO mice used in the current study also show similar or normal cardiac function (Table 1).
There was controversy as to whether cav-1 is present in cardiomyocytes. This study and previous reports demonstrate that cav-1 exists in mouse, rat, monkey, and human cardiomyocytes (22, 44, 51, 61, 67). In cardiac myocytes, both cav-1 and cav-3 are involved in forming caveolae (53, 61). Few studies have reported the possible roles of cav-1 on Na+/K+-ATPase cardiac signaling and contractility.
Previous studies demonstrated that binding of ouabain to caveolar Na+/K+-ATPase activates cell signal pathways (34, 37, 62) and that caveolae contain the protein components necessary to complete ouabain-initiated cardiac signaling. Ouabain-induced PI3Kα/Akt and ERK1/2 signaling and hypertrophy were reduced in cav-1 KO cardiomyocytes. Addition of ouabain to the cav-1 KO MEFs also results in a significantly attenuated but not totally absent ERK1/2 response. Intriguingly, cav-1 KO significantly reduced downstream signaling (ERK1/2) by ouabain without affecting upstream protein content (EGFR, c-Src, and Na/K-ATPase α1-subunit) in caveolar fractions. Cav-1 KO MEFs have no visible caveolae but do have all ouabain signaling protein components lodged within lipid rafts, which by themselves are capable of signaling (e.g., ERK1/2 by ouabain). Hence, in cav-1 KO MEFs, the protein and lipid content in caveolae/lipid rafts may not alter in the absence of cav-1. However, cav-1 enhances the interaction of proteins that are compartmentalized in the lipid raft, which forms structurally intact caveolae in the presence of cav-1. The preliminary data in caveolar preparations from mouse cav-1 KO cardiomyocytes showed consistent results with cav-1 KO MEFs (data not shown). There was no alteration in protein distribution in the caveolar preparations in the absence of cav-1. Loss of cav-1 may reduce caveolar formation (7, 64). Furthermore, loss of cav-1 reduces the association of cav-3 with Na+/K+-ATPase and interaction of PI3Kα and Na+/K+-ATPase, suggesting cav-1 facilitates the interaction between the Na+/K+-ATPase and signaling proteins. The earlier study also reported that cav-1 colocalizes with cav-3 in ventricular myocytes (22).
Our data indicate that cav-1 is an unlikely regulator in ouabain-induced contractility. The distribution of cav-1 in cardiomyocytes also suggests that cav-1 may not have a role in cardiac contractility by ouabain. We postulate from the distribution of caveolins in cardiomyocytes that hetero-oligomer caveolae (cav-1/cav-3) are located in the peripheral membranes of the myocytes, providing the platform for the ouabain-Na+/K+-ATPase signaling. Conversely, ouabain's inotropic effect originates in caveolae in T-tubules (3, 14, 35, 58), where caveolin-3 and Na+/Ca2+ exchanger are present. Another supportive piece of evidence is that the cholesterol of membrane distribution was not significantly altered in the absence of cav-1. Previous reports reveal that varying in membrane cholesterol has measureable effect on Na+/K+-ATPase activity (40, 41). Ouabain-induced cardiac positive inotropy is enhanced in cav-1 KO mice (Fig. 8). It was not certain whether the hearts in cav-1 KO mice underwent hypertrophy, because it has been known for a long time that hypertrophic hearts exhibit an elevated inotropic effect of ouabain (2, 8, 59). Nevertheless, the mechanism of this alteration is not known.
A potential limitation of this study lies in the fact that the α2-subunit of Na+/K+-ATPase in cav-1 KO cardiomyocytes was reduced. Little work has been done on cardiac signaling by different α-isoforms of Na+/K+-ATPase. We could not neglect the possibility that the α2-subunit of Na+/K+-ATPase is involved in cardiac signaling. Overexpression of the α2-subunit of Na+/K+-ATPase prevents pathological cardiac hypertrophy by pressure overload in mice (11). However, a recent study reports that α2-subunit of Na+/K+-ATPase does not play a role in signaling transduction as the α1-subunit does when rat α2-subunit is overexpressed in α1-deficient porcine renal epithelial cells (68). The potential role of α2-subunit of Na+/K+-ATPase in cardiac signaling needs further clarification.
In conclusion, while much work has already been done on various signaling responses in cav-1 KO mice, the current report is the first to demonstrate that cav-1 is among the major regulators of Na+/K+-ATPase signaling in cardiomyocytes. Reduced α2-subunit may be directly due to cav-1 KO and also contribute to the regulation of Na+/K+-ATPase signaling.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-36573 (L. Liu) and Chinese National Natural Science Foundation NSFC 81300098 (Y. Bai).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Y.B., J.W., and L.L. conception and design of research; Y.B., J.W., D.L., E.E.M., X.Z., A.W., J.S., and L.L. performed experiments; Y.B., J.W., D.L., E.E.M., X.Z., and L.L. analyzed data; Y.B., J.W., E.E.M., J.L., B.J., R.G., and L.L. interpreted results of experiments; Y.B., J.W., D.L., X.Z., and L.L. prepared figures; Y.B., J.W., D.L., E.E.M., J.L., A.W., J.S., J.T., B.J., R.G., and L.L. approved final version of manuscript; D.L., E.E.M., J.L., J.S., J.T., B.J., R.G., and L.L. edited and revised manuscript; L.L. drafted manuscript.
ACKNOWLEDGMENTS
We acknowledge the long-term support of the late Dr. Julius C. Allen, who was an internationally recognized investigator in cardiovascular science and an early contributor to the biochemistry of the Na/K-ATPase at Baylor College of Medicine. We acknowledge the expertise of Dr. Alan Goodridge on scholarly writing. We thank Dr. Jiang Tian for valuable discussions. We also thank Marjorie Gable and Manoranjani Tillekeratne for technical assistance.
REFERENCES
- 1.Bai Y, Morgan EE, Giovannucci DR, Pierre SV, Philipson KD, Askari A, Liu L. Different roles of the cardiac Na+/Ca2+-exchanger in ouabain-induced inotropy, cell signaling, and hypertrophy. Am J Physiol Heart Circ Physiol 304: H427–H435, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrebi-Bertrand I, Lelievre LG, Mouas C, Swynghedauw B. Inotropic effect of ouabain in hypertrophied rat heart. Pflügers Arch 417: 247–254, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Berry RG, Despa S, Fuller W, Bers DM, Shattock MJ. Differential distribution and regulation of mouse cardiac Na+/K+-ATPase alpha1 and alpha2 subunits in T-tubule and surface sarcolemmal membranes. Cardiovasc Res 73: 92–100, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res 57: 897–912, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998. [DOI] [PubMed] [Google Scholar]
- 6.Cai T, Wang H, Chen Y, Liu L, Gunning WT, Quintas LE, Xie ZJ. Regulation of caveolin-1 membrane trafficking by the Na/K-ATPase. J Cell Biol 182: 1153–1169, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capozza F, Cohen AW, Cheung MW, Sotgia F, Schubert W, Battista M, Lee H, Frank PG, Lisanti MP. Muscle-specific interaction of caveolin isoforms: differential complex formation between caveolins in fibroblastic vs. muscle cells. Am J Physiol Cell Physiol 288: C677–C691, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Charlemagne D, Mansier P, Preteseille M, Swynghedauw B, Lelievre LG. Hypertrophied rat heart. New Na+, K+-ATPase-ouabain interactions in sarcolemma vesicles. Eur Heart J 5, Suppl F: 315–321, 1984. [DOI] [PubMed] [Google Scholar]
- 9.Chow AK, Daniel EE, Schulz R. Cardiac function is not significantly diminished in hearts isolated from young caveolin-1 knockout mice. Am J Physiol Heart Circ Physiol 299: H1183–H1189, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Cohen AW, Park DS, Woodman SE, Williams TM, Chandra M, Shirani J, Pereira de Souza A, Kitsis RN, Russell RG, Weiss LM, Tang B, Jelicks LA, Factor SM, Shtutin V, Tanowitz HB, Lisanti MP. Caveolin-1 null mice develop cardiac hypertrophy with hyperactivation of p42/44 MAP kinase in cardiac fibroblasts. Am J Physiol Cell Physiol 284: C457–C474, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Correll RN, Eder P, Burr AR, Despa S, Davis J, Bers DM, Molkentin JD. Overexpression of the Na+/K+ ATPase alpha2 but not alpha1 isoform attenuates pathological cardiac hypertrophy and remodeling. Circ Res 114: 249–256, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Das M, Das DK. Caveolae, caveolin, and cavins: potential targets for the treatment of cardiac disease. Ann Med 44: 530–541, 2012. [DOI] [PubMed] [Google Scholar]
- 13.Del Galdo F, Lisanti MP, Jimenez SA. Caveolin-1, transforming growth factor-beta receptor internalization, and the pathogenesis of systemic sclerosis. Curr Opin Rheumatol 20: 713–719, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Despa S, Brette F, Orchard CH, Bers DM. Na/Ca exchange and Na/K-ATPase function are equally concentrated in transverse tubules of rat ventricular myocytes. Biophys J 85: 3388–3396, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dostanic I, Paul RJ, Lorenz JN, Theriault S, Van Huysse JW, Lingrel JB. The alpha2-isoform of Na-K-ATPase mediates ouabain-induced hypertension in mice and increased vascular contractility in vitro. Am J Physiol Heart Circ Physiol 288: H477–H485, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem 279: 54053–54061, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Duda M, Dlouhy M, Minarik L, Skoumal P. [The gastroesophageal junction. Present views of its function and our experimental studies]. Acta Univ Palacki Olomuc Fac Med 120: 301–318, 1988. [PubMed] [Google Scholar]
- 18.Gao S, Ho D, Vatner DE, Vatner SF. Echocardiography in mice. Curr Protoc Mouse Biol 1: 71–83, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geering K. Function of FXYD proteins, regulators of Na, K-ATPase. J Bioenerg Biomembr 37: 387–392, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Gvaramia D, Blaauboer ME, Hanemaaijer R, Everts V. Role of caveolin-1 in fibrotic diseases. Matrix Biol 32: 307–315, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem 275: 27832–27837, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Hagiwara Y, Nishina Y, Yorifuji H, Kikuchi T. Immunolocalization of caveolin-1 and caveolin-3 in monkey skeletal, cardiac and uterine smooth muscles. Cell Struct Funct 27: 375–382, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Holmgren M, Wagg J, Bezanilla F, Rakowski RF, De Weer P, Gadsby DC. Three distinct and sequential steps in the release of sodium ions by the Na+/K+-ATPase. Nature 403: 898–901, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Huang L, Kometiani P, Xie Z. Differential regulation of Na/K-ATPase alpha-subunit isoform gene expressions in cardiac myocytes by ouabain and other hypertrophic stimuli. J Mol Cell Cardiol 29: 3157–3167, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Huang L, Li H, Xie Z. Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J Mol Cell Cardiol 29: 429–437, 1997. [DOI] [PubMed] [Google Scholar]
- 26.Jasmin JF, Rengo G, Lymperopoulos A, Gupta R, Eaton GJ, Quann K, Gonzales DM, Mercier I, Koch WJ, Lisanti MP. Abstract 14567: Caveolin-1 deficiency exacerbates cardiac dysfunction and reduces survival in mice with myocardial infarction. Circulation 122: A14567, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaplan JH. Biochemistry of Na,K-ATPase. Ann Rev Biochem 71: 511–535, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kaplan JH. A moving new role for the sodium pump in epithelial cells and carcinomas. Sci STKE 2005: pe31, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem 273: 15249–15256, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Larbig R, Torres N, Bridge JH, Goldhaber JI, Philipson KD. Activation of reverse Na+-Ca2+ exchange by the Na+ current augments the cardiac Ca2+ transient: evidence from NCX knockout mice. J Physiol 588: 3267–3276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefranc F, Kiss R. The sodium pump alpha1 subunit as a potential target to combat apoptosis-resistant glioblastomas. Neoplasia 10: 198–206, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D, Wu J, Bai Y, Zhao X, Liu L. Isolation and culture of adult mouse cardiomyocytes for cell signaling and in vitro cardiac hypertrophy. J Vis Exp: 51357, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Liang M, Liu L, Malhotra D, Xie Z, Shapiro JI. Ouabain-induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int 67: 1844–1854, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Abramowitz J, Askari A, Allen JC. Role of caveolae in ouabain-induced proliferation of cultured vascular smooth muscle cells of the synthetic phenotype. Am J Physiol Heart Circ Physiol 287: H2173–H2182, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Askari A. Beta-subunit of cardiac Na+-K+-ATPase dictates the concentration of the functional enzyme in caveolae. Am J Physiol Cell Physiol 291: C569–C578, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Liu L, Askari A. On the importance and mechanism of amplification of digitalis signal through Na+/K+-ATPase. Cell Mol Biol (Noisy-le-grand) 52: 28–30, 2006. [PubMed] [Google Scholar]
- 37.Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie Z, Askari A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol 284: C1550–C1560, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K-Akt signaling pathway with digitalis-induced hypertrophy of cardiac myocytes. Am J Physiol Cell Physiol 293: C1489–C1497, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Liu LJ, Mohammadi K, Kometiani P, Xie ZJ, Askari A. Sodium pump as a signal transducer: Ouabain-induced activation of PLC-g in cardiac myocytes. Biophys J 84: 267a–268a, 2003. [Google Scholar]
- 40.Lucio FJ, Hendry BM, Ellory JC. The effects of cholesterol depletion on the sodium pump in human red cells. Exp Physiol 76: 437–443, 1991. [DOI] [PubMed] [Google Scholar]
- 41.Makarov VL, Kuznetsov SR. Increased Na+,K+-pump activity in erythrocytes of rabbits fed cholesterol. Int J Exp Pathol 76: 93–96, 1995. [PMC free article] [PubMed] [Google Scholar]
- 42.Mohammadi K, Kometiani P, Xie Z, Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem 276: 42050–42056, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Morrill GA, Kostellow AB, Askari A. Caveolin-Na/K-ATPase interactions: Role of transmembrane topology in non-genomic steroid signal transduction. Steroids 77: 1160–1168, 2012. [DOI] [PubMed] [Google Scholar]
- 44.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J 21: 1565–1574, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Patel HM, Mast JL, Sinoway LI, Muller MD. Effect of healthy aging on renal vascular responses to local cooling and apnea. J Appl Physiol (1985) 115: 90–96, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng M, Huang L, Xie Z, Huang WH, Askari A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J Biol Chem 271: 10372–10378, 1996. [DOI] [PubMed] [Google Scholar]
- 47.Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-l-arginine methyl ester and angiotensin II. Endocrinology 151: 1236–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pojoga LH, Romero JR, Yao TM, Loutraris P, Ricchiuti V, Coutinho P, Guo C, Lapointe N, Stone JR, Adler GK, Williams GH. Caveolin-1 ablation reduces the adverse cardiovascular effects of N-omega-nitro-l-arginine methyl ester and angiotensin II. Endocrinology 151: 1236–1246, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Razani B, Lisanti MP. Caveolin-deficient mice: insights into caveolar function human disease. J Clin Invest 108: 1553–1561, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reuter H, Henderson SA, Han T, Ross RS, Goldhaber JI, Philipson KD. The Na+-Ca2+ exchanger is essential for the action of cardiac glycosides. Circ Res 90: 305–308, 2002. [DOI] [PubMed] [Google Scholar]
- 51.Robenek H, Weissen-Plenz G, Severs NJ. Freeze-fracture replica immunolabelling reveals caveolin-1 in the human cardiomyocyte plasma membrane. J Cell Mol Med 12: 2519–2521, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubin J, Schwartz Z, Boyan BD, Fan X, Case N, Sen B, Drab M, Smith D, Aleman M, Wong KL, Yao H, Jo H, Gross TS. Caveolin-1 knockout mice have increased bone size and stiffness. J Bone Miner Res 22: 1408–1418, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Rybin VO, Grabham PW, Elouardighi H, Steinberg SF. Caveolae-associated proteins in cardiomyocytes: caveolin-2 expression and interactions with caveolin-3. Am J Physiol Heart Circ Physiol 285: H325–H332, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Schoner W, Scheiner-Bobis G. Endogenous cardiac glycosides: hormones using the sodium pump as signal transducer. Semin Nephrol 25: 343–351, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Skou JC. The Na,K-pump. Methods Enzymol 156: 1–25, 1988. [DOI] [PubMed] [Google Scholar]
- 56.Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta 988: 185–220, 1989. [DOI] [PubMed] [Google Scholar]
- 57.Sweadner KJ, Herrera VL, Amato S, Moellmann A, Gibbons DK, Repke KR. Immunologic identification of Na+,K+-ATPase isoforms in myocardium. Isoform change in deoxycorticosterone acetate-salt hypertension. Circ Res 74: 669–678, 1994. [DOI] [PubMed] [Google Scholar]
- 58.Swift F, Tovsrud N, Enger UH, Sjaastad I, Sejersted OM. The Na+/K+-ATPase alpha2-isoform regulates cardiac contractility in rat cardiomyocytes. Cardiovasc Res 75: 109–117, 2007. [DOI] [PubMed] [Google Scholar]
- 59.Szabo J, Nosztray K, Szegi J. Changes in sarcolemmal adenosine triphosphatase activity and in ouabain sensitivity of rat myocardium in isoproterenol-induced cardiac hypertrophy. Pol J Pharmacol Pharm 41: 553–559, 1989. [PubMed] [Google Scholar]
- 60.Verdonck F, Volders PG, Vos MA, Sipido KR. Intracellular Na+ and altered Na+ transport mechanisms in cardiac hypertrophy and failure. J Mol Cell Cardiol 35: 5–25, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Volonte D, McTiernan CF, Drab M, Kasper M, Galbiati F. Caveolin-1 and caveolin-3 form heterooligomeric complexes in atrial cardiac myocytes that are required for doxorubicin-induced apoptosis. Am J Physiol Heart Circ Physiol 294: H392–H401, 2008. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem 279: 17250–17259, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Williams TM, Lisanti MP. The Caveolin genes: from cell biology to medicine. Ann Med 36: 584–595, 2004. [DOI] [PubMed] [Google Scholar]
- 64.Woodman SE, Cheung MW, Tarr M, North AC, Schubert W, Lagaud G, Marks CB, Russell RG, Hassan GS, Factor SM, Christ GJ, Lisanti MP. Urogenital alterations in aged male caveolin-1 knockout mice. J Urol 171: 950–957, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Wu J, Akkuratov EE, Bai Y, Gaskill CM, Askari A, Liu L. Cell signaling associated with Na+/K+-ATPase: activation of phosphatidylinositide 3-kinase IA/Akt by ouabain is independent of Src. Biochemistry 52: 9059–9067, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu J, Li D, Du L, Baldawi M, Gable ME, Askari A, Liu L. Ouabain prevents pathological cardiac hypertrophy and heart failure through activation of phosphoinositide 3-kinase alpha in mouse. Cell Biosci 5: 64, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wunderlich C, Schmeisser A, Heerwagen C, Ebner B, Schober K, Braun-Dullaeus RC, Schwencke C, Kasper M, Morawietz H, Strasser RH. Chronic NOS inhibition prevents adverse lung remodeling and pulmonary arterial hypertension in caveolin-1 knockout mice. Pulm Pharmacol Ther 21: 507–515, 2008. [DOI] [PubMed] [Google Scholar]
- 68.Xie J, Ye Q, Cui X, Madan N, Yi Q, Pierre SV, Xie Z. Expression of rat Na-K-ATPase alpha2 enables ion pumping but not ouabain-induced signaling in alpha1-deficient porcine renal epithelial cells. Am J Physiol Cell Physiol 309: C373–C382, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie Z, Askari A. Na+/K+-ATPase as a signal transducer. Eur J Biochem 269: 2434–2439, 2002. [DOI] [PubMed] [Google Scholar]
- 70.Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J Jr, Chien KR. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA 99: 11375–11380, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]