Abstract
Bioluminescence imaging is an effective tool for in vivo investigation of molecular processes. We have demonstrated the applicability of bioluminescence imaging to spatiotemporally monitor gene expression in cardioregulatory brain nuclei during the development of cardiovascular disease, via incorporation of firefly luciferase into living animals, combined with exogenous d-luciferin substrate administration. Nevertheless, d-luciferin uptake into the brain tissue is low, which decreases the sensitivity of bioluminescence detection, particularly when considering small changes in gene expression in tiny central areas. Here, we tested the hypothesis that a synthetic luciferin, cyclic alkylaminoluciferin (CycLuc1), would be superior to d-luciferin for in vivo bioluminescence imaging in cardiovascular brain regions. Male C57B1/6 mice underwent targeted delivery of an adenovirus encoding the luciferase gene downstream of the CMV promoter to the subfornical organ (SFO) or paraventricular nucleus of hypothalamus (PVN), two crucial cardioregulatory neural regions. While bioluminescent signals could be obtained following d-luciferin injection (150 mg/kg), CycLuc1 administration resulted in a three- to fourfold greater bioluminescent emission from the SFO and PVN, at 10- to 20-fold lower substrate concentrations (7.5–15 mg/kg). This CycLuc1-mediated enhancement in bioluminescent emission was evident early following substrate administration (i.e., 6–10 min) and persisted for up to 1 h. When the exposure time was reduced from 60 s to 1,500 ms, minimal signal in the PVN was detectable with d-luciferin, whereas bioluminescent images could be reliably captured with CycLuc1. These findings demonstrate that bioluminescent imaging with the synthetic luciferin CycLuc1 provides an improved physiological genomics tool to investigate molecular events in discrete cardioregulatory brain nuclei.
Keywords: subfornical organ, hypothalamus, d-luciferin, CycLuc1, cardiovascular disease
long-lasting changes in central nervous system (CNS) function, in both physiological and pathophysiological settings, require changes in gene expression. These processes, driven in part by transcription factors, ultimately lead to changes in protein expression that influence everything from synaptic plasticity to neuromodulatory/neurotrophic factor production, to synaptic structures (16, 21, 25). It is important to consider that traditional experimental approaches to examine gene expression in the CNS represent a “snapshot” of the underlying complex dynamics involved in activation and deactivation, as they are typically performed at a single time point due to the time involved and amount of tissue required. Indeed, genes can be upregulated (and downregulated) in a variety of patterns from sustained, to biphasic, to sinusoidal, to a transient surge (3, 13, 17, 37). In this context, in vivo bioluminescence imaging has emerged as a novel approach to quantify and longitudinally map gene expression in the CNS of living animals (3, 6, 9, 37).
In vivo bioluminescence imaging utilizes luciferase-dependent luminescence that is due to a chemical reaction with its corresponding substrate (15, 33). Using luciferase-expressing transgenic animals or luciferase viral constructs restricted to discrete CNS nuclei, photon emission with tissue-penetrating wavelengths can be detected with a highly sensitive charge-coupled device camera (6, 7). This approach allows for noninvasive spatiotemporal observation of molecular processes in vivo in both healthy and disease conditions (3, 5, 31, 37). The most common choices for luciferase and substrate are firefly luciferase and the naturally occurring d-luciferin, respectively (6, 7). d-Luciferin is advantageous as it is nontoxic and stable in live animals, permitting repeated administration for longitudinal imaging (3, 5, 26, 37). Moreover, in vivo bioluminescence imaging data can be obtained within a few minutes following systemic administration (3, 5, 26, 37). We have recently shown that in vivo bioluminescence imaging with firefly luciferase reporter constructs and d-luciferin can be used to monitor transcription factor events in discrete cardiovascular nuclei during the development of hypertension (3, 37). In particular, we have demonstrated the utility of this approach to quantify molecular events in the circumventricular subfornical organ (SFO) and paraventricular nucleus of the hypothalamus (PVN) in murine models of hypertension (3, 37). These CNS nuclei are intricately connected, and alterations in these neural networks are well known to contribute to the development of cardiovascular diseases (10, 12, 36).
While in vivo bioluminescence imaging using the aforementioned approach is beneficial to examine underlying molecular events in individual CNS nuclei, several shortcomings of d-luciferin as a substrate have been raised. These include: 1) a high concentration is required for standard in vivo imaging, which could become toxic in the long-term (11), 2) low cell permeability (14, 29), and 3) a heterogeneous tissue distribution including low uptake into the brain (2). Importantly, recent findings have proposed an alternative luciferase substrate, cyclic alkylaminoluciferin (CycLuc1) (11). CycLuc1 has been shown to elicit greater photon emission than d-luciferin at much lower concentrations (11). Relative to d-luciferin, CycLuc1 may be particularly useful for CNS imaging because of an improved accessibility to luciferase (higher lipophilicity and lower Km value) and a red shifted wavelength that facilitates tissue penetration (11). However, previous findings using CycLuc1 are based upon broad expression of luciferase including whole body luciferase expressing animals and large peripheral tumors, as well as in mice with extensive luciferase expression throughout the brain (11). Whether CycLuc1 is superior to d-luciferin when firefly luciferase is expressed at lower levels and restricted to individual cardiovascular nuclei, such as the SFO or PVN, remains unknown.
The current investigation was performed to determine the utility of CycLuc1 for in vivo monitoring of gene expression in individual cardiovascular brain regions. We hypothesized that relative to d-luciferin, CycLuc1 would enhance the sensitivity of in vivo bioluminescence imaging of gene expression, thus providing an improved physiological genomics tool to investigate molecular events in discrete CNS nuclei under healthy and disease (i.e., cardiovascular disease) conditions.
METHODS
Animals.
Male C57BL/6 mice (8–10 wk of age) were obtained from Jackson Laboratories or in-house colonies. Mice were provided access to food and water ad libitum and were housed in animal facilities with a 12 h light-dark cycle. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the George Washington University and met the standard guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Adenoviral targeting of the SFO and PVN.
A recombinant adenoviral vector encoding firefly luciferase driven off the human cytomegalovirus promoter (AdCMV-luc, 1.0 × 1011 plaque-forming units/ml) was obtained from the Iowa Gene Transfer Vector Core (26). Previous work has shown that the use of these adenoviral vectors using a CMV promoter results in expression of the transgene in both neuronal and glial cell types (30). Mice were anesthetized intraperitoneally (ip) [Ketamine (100 mg/kg) mixed with Xylazine (10 mg/kg)] and placed in a stereotaxic frame. The surface of the skull was visualized with a dissecting microscope and leveled between bregma and lambda. AdCMV-luc was targeted to the SFO or PVN as we have previously described (3, 35, 37–39) via a custom pressure injection system and pulled glass pipettes using the following coordinates: SFO (500 μl): 3.2 mm ventral from the dorsal surface of the skull at 0.3 mm rostral and 1.0 lateral to bregma; PVN (200 μl bilaterally): 4.8 mm from the dorsal surface of the skull at 0.6 mm rostral and 0.3 mm bilateral to bregma. Following surgery, mice were provided ∼3 wk of recovery before undergoing in vivo bioluminescence imaging.
Immunohistochemistry.
Mice that had received AdCMV-luc targeted to the SFO or PVN were transcardially perfused with ice-cold 4% paraformaldehyde (PFA) in PBS. Brains were removed and postfixed overnight with 4% PFA in PBS, which was followed by incubation in 30% sucrose for 48 h. The brains were embedded in optimal cutting temperature fixation medium and were then cryosectioned (30 μm). Sections were blocked with 5% BSA/10% normal goat serum in PBS for 1 h, followed by overnight incubation with a primary antibody to firefly luciferase (Abcam, ab185924, 1:500 dilution) at 4°C. Sections were washed three times with PBS, followed by incubation with a fluorophore-conjugated secondary antibody (Abcam, ab150077, 1:1,000 dilution) for 2 h at room temperature. Fluorescent images were acquired using a confocal microscope (LSM510, Zeiss).
In vivo bioluminescence imaging.
All bioluminescent images were obtained using an IVIS Lumina K machine (Perkin Elmer, Waltham, MA). Animals were anesthetized with isoflurane, and the surface of the head was shaved and cleaned with hair removal cream. Subsequently, the substrate was administered ip, and the mice were transferred to the light-sealed imaging cabinet of the IVIS machine and positioned in a nose cone to maintain anesthesia. Bioluminescent images were acquired with a charge-coupled device camera cooled to −80°C to achieve maximal sensitivity. Images were acquired every 2 min for 20 min (a total of 10 images) with an exposure time of 60 s, medium binning, F/Stop = 1, and EM gain off. Images were collected in a similar fashion at 60 and 120 min postsubstrate administration.
Experimental protocol.
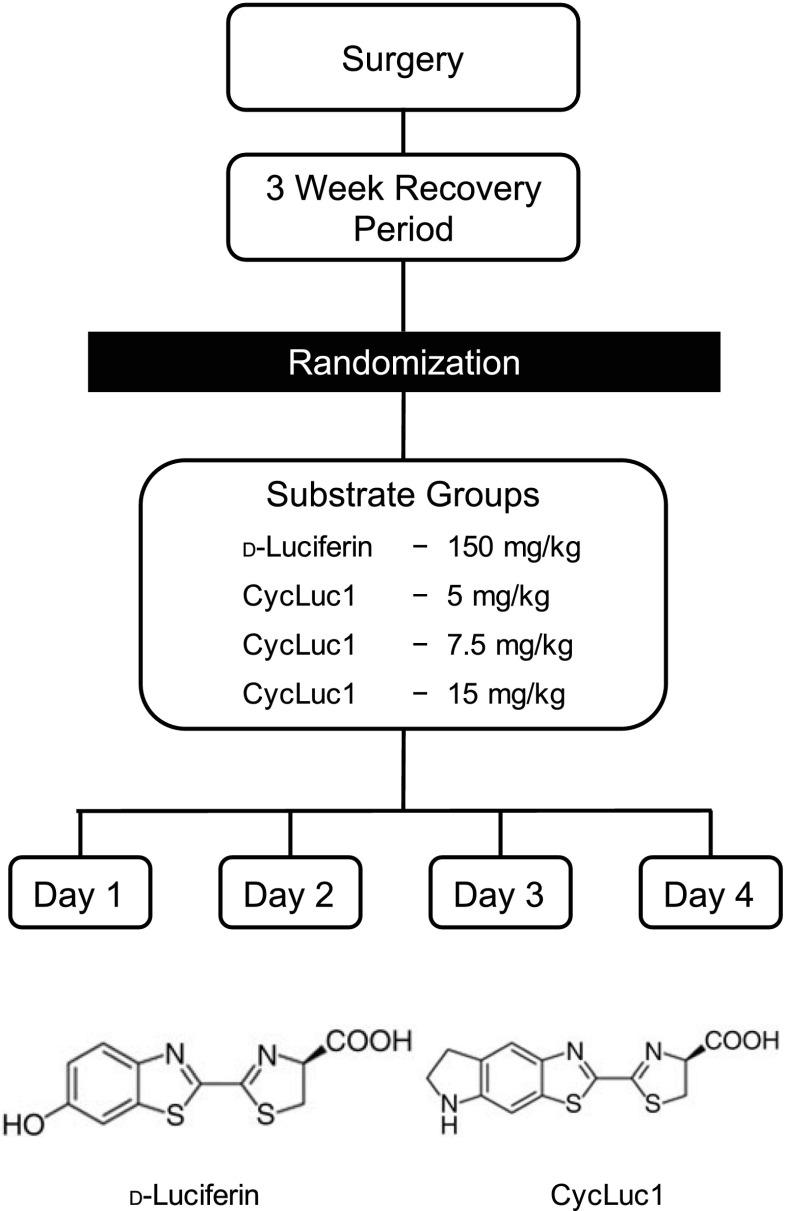
The experimental protocol consisted of 4 consecutive days (Fig. 1). On day 1, mice were anesthetized with isoflurane and randomly assigned to one of four groups that received the following substrates: d-luciferin (150 mg/kg ip) (Perkin Elmer, Boston, MA) or three different doses of the synthetic aminoluciferin CycLuc1 (5, 7.5, and 15 mg/kg ip) (EMD Millipore, Billerica, MA). On subsequent days, each mouse was assigned in a randomized fashion to the other groups, such that each animal was tested in all four substrate groups over 4 consecutive days (i.e., randomized crossover design).
Fig. 1.
Diagram of the experimental protocol. Following subfornical organ (SFO) or paraventricular nucleus of hypothalamus (PVN)-targeted delivery of AdCMV-luc and 3 wk of recovery, mice were randomly assigned to receive the substrates d-luciferin (150 mg/kg ip) or 3 different doses of the synthetic alkylaminoluciferin CycLuc1 (5, 7.5, and 15 mg/kg ip), and bioluminescent imaging was performed. On subsequent days, each mouse was assigned in a randomized fashion to the other groups, such that each mouse was tested in all 4 substrate groups over 4 consecutive days (i.e., randomized crossover design). Molecular structures for d-luciferin and CycLuc1 are also presented.
Real-time “kinetic” imaging.
In addition to acquisition of bioluminescence data using standard prolonged exposure times (i.e., 60 s) (3, 26, 37), we also evaluated the utility of CycLuc1 and d-luciferin to monitor gene expression on a rapid timescale. This approach, termed “kinetic” by the IVIS Lumina K system manufacturer, involves acquisition of images on a timescale of milliseconds, thus permitting “real-time” collection of data. Mice were anesthetized with isoflurane and randomly assigned to either d-luciferin (150 mg/kg ip) or CycLuc1 (15 mg/kg ip) before imaging. Individual bioluminescent images were acquired 10 min postsubstrate administration at an exposure of 60 s. Subsequently, continuous images were acquired at an exposure of 1,500 ms.
Image analysis.
Image data were analyzed with Xenogen Living Image v4.5.1 software as described (3, 26, 37). Briefly, a region of interest was drawn over the head using the same settings for all animals, followed by quantification of the average radiance (photons/s/cm2/sr) and photon flux (photons/s). It is important to note that the bioluminescent images appear as widespread emission across the entire head due to scattering of light during exit from the skull (i.e., Figs. 2, 4, and 6). However, luciferase expression is restricted to the SFO or PVN (Figs. 2B and 4B), thus allowing for the determination of gene expression in individual CNS nuclei (3, 26, 37). For presentation purposes, a color scale adjustment and 7×7 smoothing were performed equally across images to facilitate optimal comparisons between images. For kinetic images, the average background radiance was obtained from the dorsolumbar region of the mouse, which was then subtracted from the head region of interest for quantification (28).
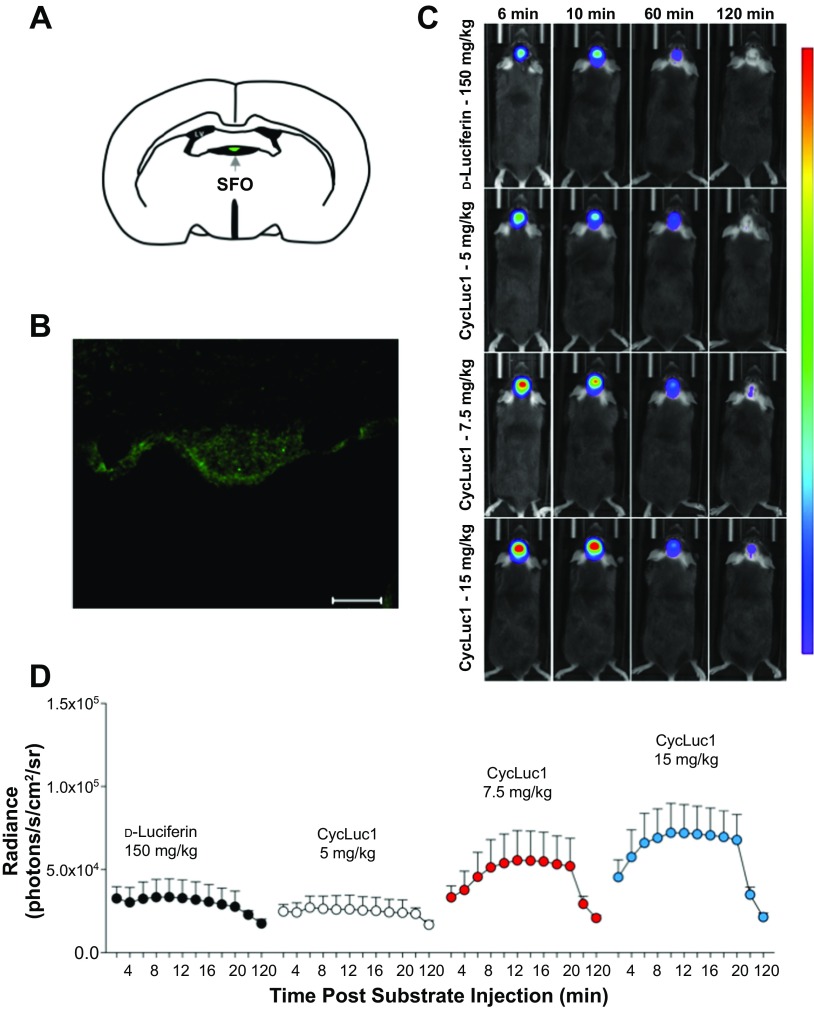
Fig. 2.
CycLuc1 enhances in vivo bioluminescence imaging of gene expression in the SFO. A: schematic of the SFO. LV, lateral ventricle. B: representative immunohistochemistry demonstrating robust firefly luciferase expression in the SFO following targeted AdCMV-luc administration. Scale bar = 50 μm. C: original bioluminescent images of photon emission from the SFO at 6, 10, 60, and 120 min after substrate injection of d-luciferin (150 mg/kg) or CycLuc1 (5, 7.5, and 15 mg/kg). All images are from the same animal and plotted on the same scale. The areas of high photon emission are displayed in red, and the areas of low photon emission are displayed in blue. D: temporal measurements of SFO bioluminescent emission from 2 to 120 min following substrate administration; n = 8.
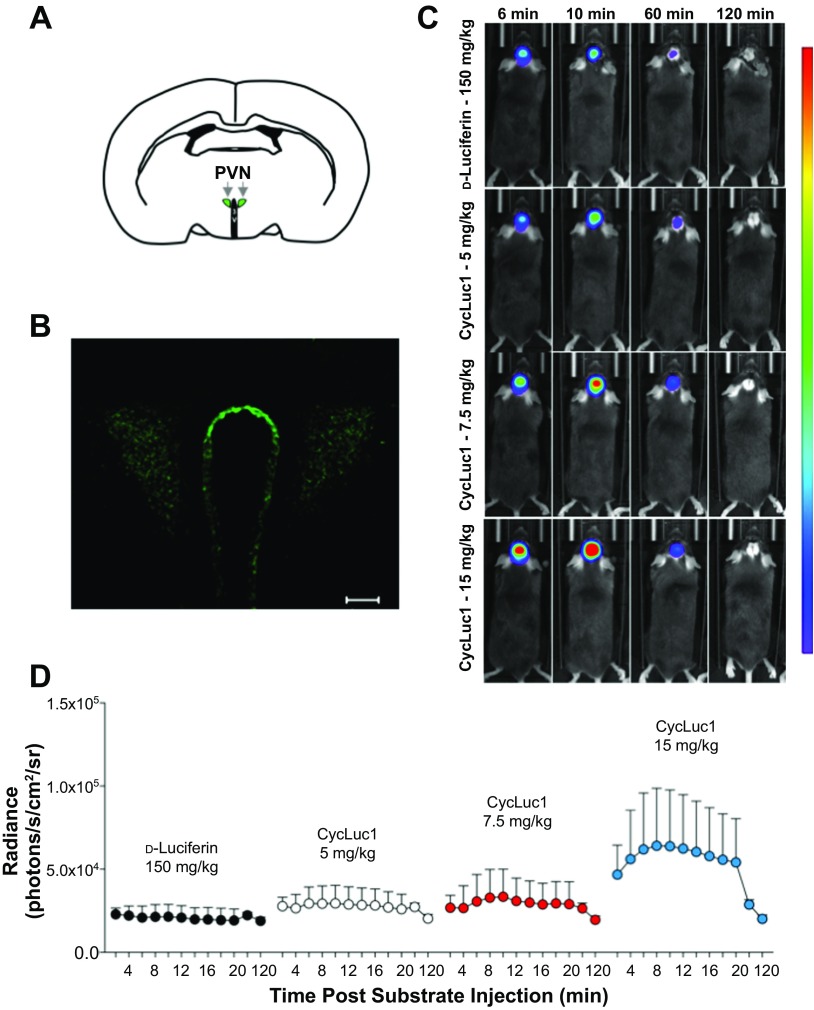
Fig. 4.
CycLuc1 enhances in vivo bioluminescence imaging of gene expression in the PVN. A: schematic of the PVN. 3V, third ventricle. B: representative immunohistochemistry demonstrating robust firefly luciferase expression in the PVN following targeted AdCMV-luc administration. Scale bar = 50 μm. C: original bioluminescent images of photon emission from the PVN at 6, 10, 60, and 120 min after substrate injection of d-luciferin (150 mg/kg) or CycLuc1 (5, 7.5, and 15 mg/kg). All images are from the same animal and plotted on the same scale. The areas of high photon emission are displayed in red, and the areas of low photon emission are displayed in blue. D: temporal measurements of PVN bioluminescent emission from 2 to 120 min following substrate administration; n = 8.
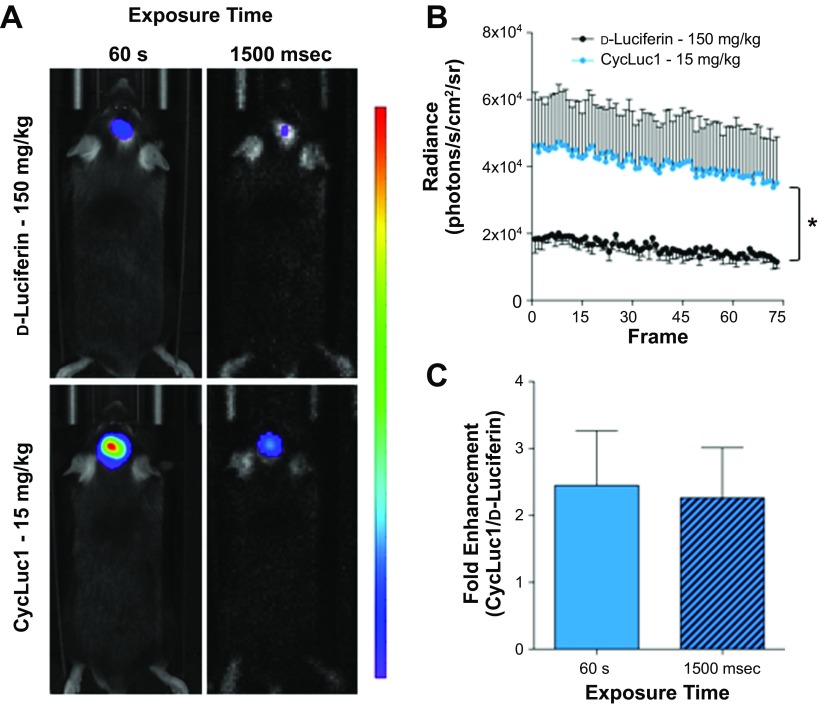
Fig. 6.
CycLuc1 allows for millisecond acquisition of in vivo bioluminescence images from the PVN. A: original bioluminescent images, using a standard exposure of 60 s or a rapid exposure of 1,500 ms, of photon emission from the PVN after d-luciferin or CycLuc1 substrate administration. All images are plotted on the same scale. B: the average radiance during continuous capture of 1,500 millisecond images (75 frames) following d-luciferin or CycLuc1 administration (n = 3/group). C: fold enhancement of CycLuc1 (CycLuc1/ d-luciferin) bioluminescent emission from the PVN with a 60 s or 1,500 millisecond exposure (n = 3/group). *P < 0.05 when comparing the area under the curve (see text for additional details).
Statistical analysis.
Data are expressed as means ± SE. A repeated one-way measures ANOVA and Student's unpaired t-test were used to compare between groups, with Tukey's post hoc comparisons when appropriate. The alpha level was set at P < 0.05.
RESULTS
CycLuc1 enhances in vivo bioluminescence imaging of gene expression in the SFO.
Given that dysregulation in the SFO is well recognized to contribute to impairments in autonomic, neurohumoral, and cardiovascular regulation (4, 10, 12, 35, 41), we first tested the effectiveness of CycLuc1 for in vivo imaging of gene expression in this circumventricular region. Following SFO-targeted delivery of a luciferase reporter construct (AdCMV-luc), mice were administered the substrates d-luciferin or CycLuc1 (intraperitoneally) in a randomized fashion on different days (Fig. 1). We used the most common d-luciferin dose (150 mg/kg) (3, 11, 26, 37), and 10–30 fold lower CycLuc1 doses (5–15 mg/kg) were administered based on previous work demonstrating enhanced sensitivity with synthetic luciferin substrates (11). As shown in Fig. 2B, selective targeting of AdCMV-luc to the SFO results in robust expression of luciferase in this region.
We initially evaluated the time course of luciferase-dependent light emission from the SFO following substrate administration. Figure 2, C and D, illustrates original bioluminescent images and the average radiance (i.e., photons/s/cm2/sr) from 2 to 120 min after substrate injection, respectively. In line with previous work (8, 26), the bioluminescent signal increased following d-luciferin injection within 10 min and returned back toward baseline levels by 120 min. Interestingly, CycLuc1 administration resulted in a much greater SFO bioluminescence emission, relative to d-luciferin (Fig. 2, C and D), which was evident in a dose-dependent manner early following substrate administration and persisted for up to 60 min. This enhanced photon emission was most prominent at CycLuc1 concentrations of 15 and 7.5 mg/kg that reflect 10- to 20-fold lower doses than d-luciferin, respectively and when quantified as an area under the curve (AUC) to reflect the total bioluminescent emission over the imaging time course (2.78 × 106 vs. 4.03 × 106 vs. 4.95 × 106 AUC; d-luciferin vs. CycLuc1 7.5 mg/kg vs. CycLuc1 15 mg/kg, P < 0.05). Furthermore, a CycLuc1 dose of 5 mg/kg produced a comparable bioluminescent signal to a 30-fold higher d-luciferin dose of 150 mg/kg (Fig. 2, C and D; 2.78 × 106 vs. 2.61 × 106 AUC; d-luciferin vs. CycLuc1 5 mg/kg, P > 0.05). Similar findings were obtained when evaluating the data as total photon flux (photons/s; data not shown).
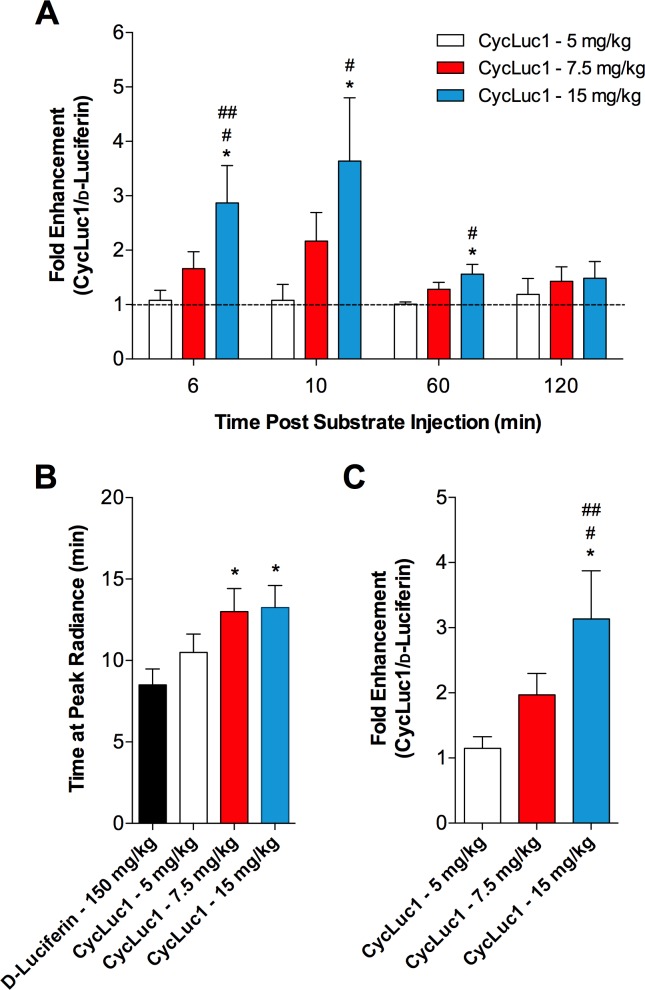
We also examined several individual time points to further understand the temporal patterns of luciferase-dependent photon emission following d-luciferin and CycLuc1 administration. Previous findings indicate that peak bioluminescence in the CNS occurs at ∼10 min after d-luciferin injection (8, 26). In line with this, dose-dependent enhancements in the bioluminescent signal were found with CycLuc1, relative to d-luciferin, at 10 min postsubstrate injection, with the highest CycLuc1 dose of 15 mg/kg yielding an almost fourfold higher bioluminescence signal than d-luciferin (Fig. 3A). Similar CycLuc1 associated improvements in photon emission were found early postsubstrate administration (i.e., 6 min; Fig. 3A). At 1 h after substrate injection, the average radiance had decayed substantially in all groups; however, the signal following administration of CycLuc1 at 15 mg/kg was still higher than that following d-luciferin, and this difference became indistinguishable at 2 h postinjection (Fig. 3A). Importantly, the findings presented in Fig. 3A assume a similar temporal profile between different substrates (i.e., d-luciferin and CycLuc1 both peak at the same time). In this context, the maximal signal occurred slightly, albeit significantly, later following CycLuc1 injection at 7.5 and 15 mg/kg when compared with d-luciferin (Fig. 3B). Therefore, to account for slight differences in the time to peak bioluminescence, we also evaluated the fold enhancement of CycLuc1 from the maximal bioluminescent signal in each animal. Importantly, this did not influence the findings with dose-dependent enhancements in light emission from the SFO evident following CycLuc1 administration, relative to d-luciferin (Fig. 3C).
Fig. 3.
Relative enhancement of CycLuc1 for in vivo bioluminescence imaging of gene expression in the SFO. A: fold enhancement of CycLuc1 (CycLuc1/d-luciferin) bioluminescent emission from the SFO at 6, 10, 60, and 120 min after substrate injection. Values are normalized to the d-luciferin condition, which is represented at a value of 1 by the dashed line. B: the time at which the peak radiance occurred after substrate injection. C: fold enhancement of CycLuc1 at the peak radiance. n = 8. *P < 0.05 vs. d-luciferin; #P < 0.05 vs. CycLuc1 5 mg/kg; ##P < 0.05 vs. CycLuc1 7.5 mg/kg.
CycLuc1 enhances in vivo bioluminescence imaging of gene expression in the PVN.
The findings in Figs. 2 and 3 indicate that CycLuc1 enhances bioluminescent evaluation of gene expression in the SFO. Importantly, the SFO is situated outside of the blood-brain barrier, presumably allowing for easy access of luciferase substrates. Therefore, we also performed similar experiments focused on the PVN, which is protected by the blood-brain barrier and situated deep below the surface of the skull, thus necessitating strong light emission for in vivo imaging. Previous findings have determined that small lipophilic compounds such as d-luciferin and CycLuc1 can penetrate the blood-brain barrier by simple diffusion (24) or are taken up by transporters expressed in epithelial, neuronal, and glial cells in the brain (18, 27, 34).
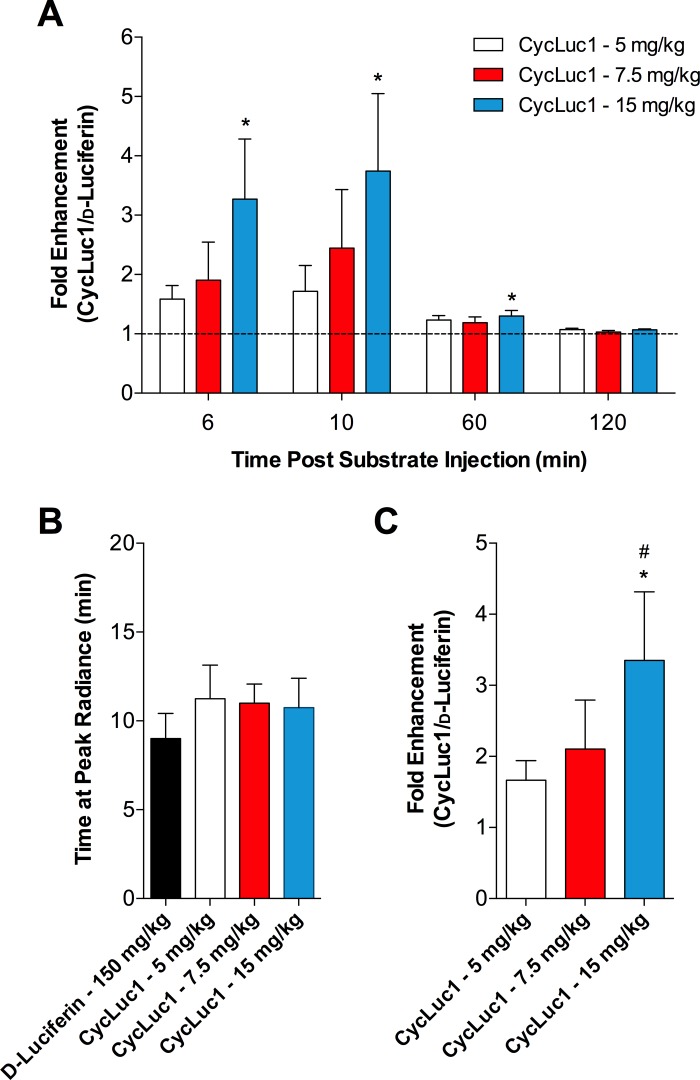
We first confirmed that AdCMV-luc targeted to the PVN results in bilateral luciferase expression in this region (Fig. 4B). In vivo, similar to the SFO, the bioluminescent signal from the PVN was greater following CycLuc1 relative to d-luciferin (Fig. 4, C and D; 2.44 × 106 vs. 3.03 × 106 vs. 4.19 × 106 AUC; d-luciferin vs. CycLuc1 7.5 mg/kg vs. CycLuc1 15 mg/kg, P = 0.07). At 6 and 10 min postsubstrate injection, 15 mg/kg of CycLuc1 yielded an approximate threefold greater signal compared with d-luciferin (Fig. 5A). However, lower doses of CycLuc1 were not statistically different from d-luciferin. The average radiance decayed substantially by 60 min after injection, yet CycLuc1 at 15 mg/kg still produced a greater bioluminescence relative to d-luciferin (Fig. 5A). The difference in signals between groups was not evident by 2 h postsubstrate injection. Figure 5B indicates that the maximal signal occurred at ∼9–10 min postsubstrate injection and was not different between groups. Thus, the fold enhancement of CycLuc1, relative to d-luciferin, at the peak signal (in addition to discrete time points; Fig. 5A), also indicated a significantly improved bioluminescent emission, particularly with CycLuc1 at a dose of 15 mg/kg (Fig. 5C).
Fig. 5.
Relative enhancement of CycLuc1 for in vivo bioluminescence imaging of gene expression in the PVN. A: fold enhancement of CycLuc1 (CycLuc1/d-luciferin) bioluminescent emission from the PVN at 6, 10, 60, and 120 min after substrate injection. Values are normalized to the d-luciferin condition, which is represented at a value of 1 by the dashed line. B: the time at which the peak radiance occurred after substrate injection. C: fold enhancement of CycLuc1 at the peak radiance; n = 8. *P < 0.05 vs. d-luciferin; #P < 0.05 vs. CycLuc1 5 mg/kg.
CycLuc1 allows for millisecond acquisition of in vivo bioluminescence images from deep brain nuclei.
Standard bioluminescent imaging with firefly luciferase and d-luciferin is performed with image acquisition times from seconds to minutes to allow for sufficient capture of emitted photons (6). However, the ability to acquire data in a continuous and quick manner may allow for imaging of conscious animals and/or rapid cellular events (28). We therefore determined the applicability of CycLuc1 for bioluminescent imaging on a timescale of milliseconds. Mice that had undergone PVN-targeted delivery of AdCMV-luc were administered d-luciferin (150 mg/kg) or CycLuc1 (15 mg/kg), and a standard bioluminescence image with an exposure time of 60 s was acquired at 10 min after substrate injection. Subsequently, continuous images at an exposure of 1,500 ms were then captured. Bioluminescent images with a 60 s and 1,500 ms exposure are shown in Fig. 6A. Consistent with the findings in Fig. 4, acquisition of PVN photon emission with a 60 s exposure time was greater following CycLuc1 administration, compared with d-luciferin (Fig. 6A). When the exposure time was reduced to 1,500 ms, minimal signal was detectable with d-luciferin, whereas bioluminescent images could be reliably and continuously captured with CycLuc1 (Fig. 6, A and B; 1.15 × 106 vs. 2.94 × 106 AUC; d-luciferin 150 mg/kg vs. CycLuc1 15 mg/kg, P < 0.05). Interestingly, the fold difference in emission output between CycLuc1 and d-luciferin with a 60 s exposure time was comparable to that when using a much shorter exposure time of 1,500 ms (Fig. 6C).
DISCUSSION
The major finding of this study is that replacing standard d-luciferin with the synthetic CycLuc1 enhances the sensitivity of in vivo bioluminescence gene detection from individual brain cardiovascular control areas including the SFO and PVN. Furthermore, our findings also indicate that CycLuc1 may be useful for bioluminescent detection of rapid cellular events in individual brain nuclei. Collectively, these findings significantly strengthen in vivo bioluminescent imaging as a physiological genomics tool to evaluate gene expression in the brain in healthy and disease settings.
Even though d-luciferin combined with firefly luciferase can be used to evaluate molecular mechanisms in vivo, bioluminescence imaging in the brain is particularly challenging (6). The skull, which affects the amount of light emission, coupled with the blood-brain barrier, which limits access of d-luciferin to the brain parenchyma, both influence the sensitivity of CNS in vivo bioluminescence imaging (6). Organ uptake kinetics and biodistribution data using radiolabeled d-luciferin have suggested very low d-luciferin uptake in the brain relative to the liver, intestine, and kidney (2). In line with this, recent findings suggest that bioluminescent signals due to d-luciferin are robust at the site of administration (i.e., abdominal cavity), but weaker in more distant tissues (i.e., brain) (11). In an effort to overcome these shortcomings, CycLuc1 has been recently developed for in vivo bioluminescence imaging (11, 14). Importantly, previous work demonstrated an improved sensitivity for imaging broad luciferase expression in the brain when using CycLuc1, compared with d-luciferin (11). The current findings support and extend this work by illustrating that CycLuc1 enhances in vivo bioluminescence imaging of low-level luciferase expression in discrete CNS cardioregulatory nuclei.
We first examined the potential of CycLuc1 to improve imaging of gene expression in the SFO. This circumventricular structure lacks a blood-brain barrier and is thus anatomically exposed to circulating hormones, electrolytes, and metabolites. As such, the SFO plays a crucial role in linking peripheral circulating factors with downstream CNS circuitry, and molecular alterations within the SFO are well known to contribute to cardiovascular diseases (10, 12, 32, 35, 38, 41). Given the location of the SFO outside of the blood-brain barrier, in vivo bioluminescence imaging should not be limited by d-luciferin having to cross into the CNS. Indeed, we have recently employed this technology with d-luciferin and found complex temporal activation of the transcription factor nuclear factor-κB in the SFO during the development of essential hypertension in mice (37).
Although yet to be determined, the current work using ubiquitous expression of luciferase demonstrates that improved imaging sensitivity with CycLuc1 will aid in future investigations such as these due to superior detection of small changes in gene expression. This is supported by an enhanced photon emission early after CycLuc1 substrate administration (i.e., 6 min; Fig. 3A), a continued increase and later peak signal than d-luciferin (i.e., continued distribution and accumulation in SFO; Fig. 3B), and a sustained bioluminescent emission for up to 1 h postsubstrate administration (Fig. 3A). While it is unclear exactly how 10- to 20-fold lower concentrations of CycLuc1, relative to d-luciferin, led to a much greater bioluminescence from the SFO, a lower Km, prolonged half-life, and a red-shifted emission wavelength that allows for greater tissue penetration likely contribute to CycLuc1-mediated improvements in bioluminescence imaging of the SFO (1, 11, 14, 23, 24).
In addition to the SFO, the PVN is also a key neural region involved in cardiovascular, autonomic, and neurohumoral regulation. Along with a pituitary gland related neurosecretory role, PVN neurons project to other cardiovascular regulatory regions, as well as directly to sympathetic preganglionic neurons in the intermediolateral cell column of the spinal cord (12). It is well established that dysregulation in the PVN, including factors such as reactive oxygen species and inflammatory cytokines, contributes to cardiovascular pathogenesis (3, 20, 36, 40). The use of bioluminescence imaging represents a unique opportunity to image such disease-related processes. However, the PVN presents a challenge for in vivo imaging, as its deep location requires a strong light emission to penetrate significant amounts of tissue and the skull, along with the presence of a blood-brain barrier, necessitating efficient delivery of substrate into the PVN. In this regard, relative to the conventional d-luciferin substrate, CycLuc1 may more readily cross the blood-brain barrier by simple diffusion due to its increased lipophilicity (24), thus allowing access to luciferase expression in a deep brain region such as the PVN. As mentioned above, the lower Km value and red wavelength shift that allows for better tissue penetration (1, 11) would also likely contribute to more sensitive bioluminescence detection in the PVN. While in vivo bioluminescence imaging has been used to track molecular mechanisms in the PVN during cardiovascular disease development, it is important to note that in this single investigation we had to use a high number of animals in each group (∼20) because of significant variability in bioluminescence in the PVN when using d-Luciferin (3). In this context, the current finding that CycLuc1 elicited near triple the amount of light emission from the PVN, compared with d-Luciferin, represents a significant advancement in our ability to image molecular processes in deep cardiovascular nuclei.
The majority of in vivo bioluminescence investigations to date have required long exposure times, on the order of seconds to minutes, to capture sufficient signal that is greater than the background noise (28). While beneficial, long exposure times limit study design to investigation of sustained molecular events in anesthetized animals. Given the significant enhancements provided by CycLuc1 when using a 1 min exposure time, we reasoned that the use of this synthetic luciferin might also be beneficial for imaging rapid and transient processes in the CNS. Our findings indicate that CycLuc1 allows for in vivo bioluminescent imaging deep within individual CNS nuclei on a millisecond timescale. Although speculative, these findings suggest that CycLuc1 could be used in freely moving animals without the use of anesthesia. While the isoflurane anesthesia that is used for in vivo imaging is fairly brief, the use of anesthesia may confound chronic longitudinal investigations because of the chronic stress imposed on the animal along with potential influence of anesthetics on CNS signaling processes (19, 22). Furthermore, rapid exposure times may permit investigation of dynamic cellular events, such as signal transduction pathways or calcium signaling, in discrete brain nuclei (28). While the current findings support the possible use of CycLuc1 in such studies, future work is needed to further confirm the sensitivity and applicability of our approach when using rapid exposure times.
In conclusion, we have established the effectiveness of CycLuc1, coupled with adenovirus-mediated gene transfer of luciferase reporters, for in vivo bioluminescence imaging of gene expression in discrete cardiovascular brain regions including the SFO and PVN. This approach provides a significant advancement in our ability to observe and quantify low-level biological events in deep brain tissue under pathophysiological or healthy conditions. Furthermore, our findings also suggest the possibility that real-time bioluminescence recordings with a millisecond acquisition time scale, when coupled with CycLuc1, could be used to evaluate transient and rapid cellular events, while also potentially negating the need for anesthesia. Collectively, bioluminescent imaging with the synthetic luciferin CycLuc1 provides an improved physiological genomics tool to longitudinally and spatiotemporally investigate molecular events in discrete cardioregulatory brain nuclei in vivo.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant HL-116776.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
H.S., C.H., and C.N.Y. conception and design of research; H.S., C.H., and C.N.Y. performed experiments; H.S., C.H., and C.N.Y. analyzed data; H.S., C.H., and C.N.Y. interpreted results of experiments; H.S., C.H., and C.N.Y. prepared figures; H.S., C.H., and C.N.Y. drafted manuscript; H.S., C.H., and C.N.Y. edited and revised manuscript; H.S., C.H., and C.N.Y. approved final version of manuscript.
REFERENCES
- 1.Adams ST Jr, Miller SC. Beyond d-luciferin: expanding the scope of bioluminescence imaging in vivo. Curr Opin Chem Biol 21: 112–120, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger F, Paulmurugan R, Bhaumik S, Gambhir SS. Uptake kinetics and biodistribution of 14C-d-luciferin–a radiolabeled substrate for the firefly luciferase catalyzed bioluminescence reaction: impact on bioluminescence based reporter gene imaging. Eur J Nucl Med Mol Imaging 35: 2275–2285, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burmeister MA, Young CN, Braga VA, Butler SD, Sharma RV, Davisson RL. In vivo bioluminescence imaging reveals redox-regulated activator protein-1 activation in paraventricular nucleus of mice with renovascular hypertension. Hypertension 57: 289–297, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao X, Peterson JR, Wang G, Anrather J, Young CN, Guruju MR, Burmeister MA, Iadecola C, Davisson RL. Angiotensin II-dependent hypertension requires cyclooxygenase 1-derived prostaglandin E2 and EP1 receptor signaling in the subfornical organ of the brain. Hypertension 59: 869–876, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, Contag CH. Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA 101: 221–226, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contag CH. Molecular imaging using visible light to reveal biological changes in the brain. Neuroimaging Clin N Am 16: 633–654, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Contag CH, Bachmann MH. Advances in in vivo bioluminescence imaging of gene expression. Annu Rev Biomed Eng 4: 235–260, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Contag CH, Spilman SD, Contag PR, Oshiro M, Eames B, Dennery P, Stevenson DK, Benaron DA. Visualizing gene expression in living mammals using a bioluminescent reporter. Photochem Photobiol 66: 523–531, 1997. [DOI] [PubMed] [Google Scholar]
- 9.Contag PR, Olomu IN, Stevenson DK, Contag CH. Bioluminescent indicators in living mammals. Nat Med 4: 245–247, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Cottrell GT, Ferguson AV. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul Pept 117: 11–23, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Evans MS, Chaurette JP, Adams ST Jr, Reddy GR, Paley MA, Aronin N, Prescher JA, Miller SC. A synthetic luciferin improves bioluminescence imaging in live mice. Nat Methods 11: 393–395, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson AV. Angiotensinergic regulation of autonomic and neuroendocrine outputs: critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 89: 370–376, 2009. [DOI] [PubMed] [Google Scholar]
- 13.Hager GL, McNally JG, Misteli T. Transcription dynamics. Mol Cell 35: 741–753, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harwood KR, Mofford DM, Reddy GR, Miller SC. Identification of mutant firefly luciferases that efficiently utilize aminoluciferins. Chem Biol 18: 1649–1657, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hastings JW. Chemistries and colors of bioluminescent reactions: a review. Gene 173: 5–11, 1996. [DOI] [PubMed] [Google Scholar]
- 16.Herdegen T, Zimmermann M. Immediate early genes (IEGs) encoding for inducible transcription factors (ITFs) and neuropeptides in the nervous system: functional network for long-term plasticity and pain. Prog Brain Res 104: 299–321, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298: 1241–1245, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res 24: 1227–1251, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Kohtala S, Theilmann W, Suomi T, Wigren HK, Stenberg T, Elo-Uhlgen L, Rokka A, Rantamäki T. Brief isoflurane anesthesia produces prominent phosphoproteomic changes in the adult mouse hippocampus. ACS Chem Neurosci 7: 749–756, 2016. [DOI] [PubMed] [Google Scholar]
- 20.Li HB, Qin DN, Ma L, Miao YW, Zhang DM, Lu Y, Song XA, Zhu GQ, Kang YM. Chronic infusion of lisinopril into hypothalamic paraventricular nucleus modulates cytokines and attenuates oxidative stress in rostral ventrolateral medulla in hypertension. Toxicol Appl Pharmacol 279: 141–149, 2014. [DOI] [PubMed] [Google Scholar]
- 21.Loebrich S, Nedivi E. The function of activity-regulated genes in the nervous system. Physiol Rev 89: 1079–1103, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandal PK, Saharan S, Penna O, Fodale V. Anesthesia issues in central nervous system disorders. Curr Aging Sci 9: 116–143, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Mofford DM, Adams ST Jr, Reddy GS, Reddy GR, Miller SC. Luciferin amides enable in vivo bioluminescence detection of endogenous fatty acid amide hydrolase activity. J Am Chem Soc 137: 8684–8687, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mofford DM, Miller SC. Luciferins behave like drugs. ACS Chem Neurosci 6: 1273–1275, 2015. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Cadahia B, Drobic B, Davie JR. Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol 89: 61–73, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JR, Infanger DW, Braga VA, Zhang Y, Sharma RV, Engelhardt JF, Davisson RL. Longitudinal noninvasive monitoring of transcription factor activation in cardiovascular regulatory nuclei using bioluminescence imaging. Physiol Genomics 33: 292–299, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Ronaldson PT, Davis TP. Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol Rev 65: 291–314, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roncali E, Savinaud M, Levrey O, Rogers KL, Maitrejean S, Tavitian B. New device for real-time bioluminescence imaging in moving rodents. J Biomed Opt 13: 054035, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Shinde R, Perkins J, Contag CH. Luciferin derivatives for enhanced in vitro and in vivo bioluminescence assays. Biochemistry 45: 11103–11112, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Sinnayah P, Lindley TE, Staber PD, Cassell MD, Davidson BL, Davisson RL. Selective gene transfer to key cardiovascular regions of the brain: comparison of two viral vector systems. Hypertension 39: 603–608, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney TJ, Mailander V, Tucker AA, Olomu AB, Zhang W, Cao Y, Negrin RS, Contag CH. Visualizing the kinetics of tumor-cell clearance in living animals. Proc Natl Acad Sci USA 96: 12044–12049, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension 62: 118–125, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson T, Hastings JW. Bioluminescence. Annu Rev Cell Dev Biol 14: 197–230, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Huang W, Prasad PD, Seth P, Rajan DP, Leibach FH, Chen J, Conway SJ, Ganapathy V. Functional characteristics and tissue distribution pattern of organic cation transporter 2 (OCTN2), an organic cation/carnitine transporter. J Pharmacol Exp Ther 290: 1482–1492, 1999. [PubMed] [Google Scholar]
- 35.Young CN, Cao X, Guruju MR, Pierce JP, Morgan DA, Wang G, Iadecola C, Mark AL, Davisson RL. ER stress in the brain subfornical organ mediates angiotensin-dependent hypertension. J Clin Invest 122: 3960–3964, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young CN, Davisson RL. Angiotensin-II, the brain, hypertension: an update. Hypertension 66: 920–926, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young CN, Li A, Dong FN, Horwath JA, Clark CG, Davisson RL. Endoplasmic reticulum and oxidant stress mediate nuclear factor-kappaB activation in the subfornical organ during angiotensin II hypertension. Am J Physiol Cell Physiol 308: C803–C812, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young CN, Morgan DA, Butler SD, Mark AL, Davisson RL. The brain subfornical organ mediates leptin-induced increases in renal sympathetic activity but not its metabolic effects. Hypertension 61: 737–744, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Young CN, Morgan DA, Butler SD, Rahmouni K, Gurley SB, Coffman TM, Mark AL, Davisson RL. Angiotensin type 1a receptors in the forebrain subfornical organ facilitate leptin-induced weight loss through brown adipose tissue thermogenesis. Mol Metab 4: 337–343, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang ZH, Wei SG, Francis J, Felder RB. Cardiovascular and renal sympathetic activation by blood-borne TNF-alpha in rat: the role of central prostaglandins. Am J Physiol Regul Integr Comp Physiol 284: R916–R927, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res 95: 210–216, 2004. [DOI] [PubMed] [Google Scholar]