Abstract
A rapid, sensitive and validated method for the determination of fusaric acid (FA) in several Fusarium strains and different commercial food and feed products is reported based on ultra-performance liquid chromatography. This method requires only crude sample by a simple extraction with methanol, and requires a very short time of 8 min for completion. Separation of FA was performed at injection volume of 1 μl with a 20:80 (v/v) water/acetonitrile mobile phase containing 0.1 % formic acid at a flow rate of 0.05 ml/min and detected with UV at 220 nm. Nice linearity and good correlation coefficient (R2 > 0.99) were obtained in the concentration range of 1–200 μg/ml. Validation was demonstrated using blank samples spiked at three different concentrations with standard solution, and the method yielded more than 98.2 % recovery efficiencies and below 2.56 % R.S.D. when applied in the analysis of FA produced by Fusarium verticillioides and a set of transgenic strains of this fungus. Satisfactory recoveries in the range of 79.1–105.8 % and R.S.D lower than 10 % were also obtained for the tested commercial food and feed products. The concentration FA detection in the transgenic strains ranged from 9.65 to 135 μg/kg (0.29–4.05 μg per gram of biomass). However, FA was not detected in most of the commercial products with the exception of niblet, oatmeal, red kidney bean and soybean, for which the concentrations of FA ranged from 2.5 to 18 μg/kg (below the permitted maximum). These results show that the proposed method has a great potential application to analyze FA from different sources rapidly.
Keywords: Mycotoxin, Fusaric acid (FA), UPLC, Food and feed products, Fusarium
Introduction
Fusaric acid (5-butylpicolinic acid, FA) is a broad-spectrum mycotoxin produced by various Fusarium species, including F. heterosporum, F. verticillioides and F. oxysporum [1, 2]. This compound is thought to be directly related to the pathogenesis of vascular wilt, damping off, and root rot diseases of numerous vegetable crops [3–8]. In addition to the suggested role in plant pathogenesis, FA is also a potential health hazard as it easily contaminates agricultural commodities [9–11]. Consumption of FA-contaminated feedstuffs and food may cause severe disorders in animals and humans, including impairment in nerve [12–14], cardiovascular [15] and immune systems [16, 17], as well as some mammalian tumor cell lines [16]. Even though FA exhibits low acute toxicity in animals, synergism with certain mycotoxins combined with FA can enhance toxicity [13, 18].
Several methods are in use for the detection of FA in agricultural commodities and cell culture, involving high-performance liquid chromatography (HPLC) coupled with UV or ESI–MS/MS [19–21]. In addition, GC–MS methods and several qualitative methods such as the use of luminescent bacterium assay have been developed [22]. However, these methods involving HPLC in FA analysis require at least 20 min for completion [21], and it is also less sensitive than UPLC (requires about 20 μl injection volume while UPLC only needs 1 μl). The GC–MS method has almost never been used since it was reported in 1995, and the major disadvantage is its complex extraction and analysis process [14]. What’s more, FA at a low level usually cannot be detected, because of poor recovery during sample treatment or a relatively low instrumental sensitivity. Therefore, it is necessary to develop an efficient, simple and reproducible method for detection of FA produced by the pathogen. More recently, the introduction of UPLC has attracted interest in the determination of mycotoxins in foodstuffs as it exhibits advantages of fast analysis, greater resolution, higher peak capacity, and sensitivity compared to HPLC. Using a proper solid stationary phase, UPLC can analyze multiple samples in a shorter time period compared to HPLC and has employed in determination of a variety of mycotoxins, including aflatoxins [23], ochratoxin [24] and deoxynivalenol [25]. We devised and optimised an UPLC protocol for the determination of FA in cell cultures and commercial food and feed stocks.
Materials and Methods
Reagents, Strains and Culture Conditions
FA standard substance (purity 99.4 %) was purchased from Sigma-Aldrich (St. Louis, MO, USA) in 1-mg package. Analytic-grade methanol (MeoH) for FA extraction was supplied by Sangon Biotech Co., Ltd (Shanghai, China). UPLC-grade acetonitrile (MeCN) and trifluoroacetic acid (TFA) for mobile phase were bought from Sigma-Aldrich (St. Louis, MO, USA). UPLC-grade water was obtained from a Milli-Q Gradient water system (Bedford, MA, USA). FA standard stock solution (1 mg/ml) was prepared by dissolving accurately weighed portion of the standard in MeoH. Then, the standard stock solution was further diluted by MeoH to obtain the standard working solution (50 μg/ml). For calibration curve, the standard stock solution was sequentially diluted with MeoH to prepare a series of standard solutions with concentrations of 1, 10, 20, 50, 100, 150 and 200 μg/ml. All of the prepared standard solutions were stored in brown vials at 4 °C. Commercial food and feed including peanut, two rice samples (sticky and black rice), three maize samples (maize, corn flakes and niblet), two wheat samples (oatmeal and millfeed), and seven bean samples (red kidney bean, red bean, pinto bean, black soybean, white bean, mung bean and soybean) were purchased from a local supermarket in Fuzhou (China).
Fusarium verticillioides A0149 (FGSC number 7600), the wild-type strain, was kindly donated by Shandong University. Seven transgenic strains of F. verticillioides A0149 were obtained in our laboratory by random insertions of plasmid PII99 into the wide type. The wild-type strain was maintained on potato dextrose agar (PDA) plates containing 50 % (v/v) sea water, and the transgenic strains were maintained on the same plates adding with 50 μg/ml geneticin.
Fusaric Acid Extraction
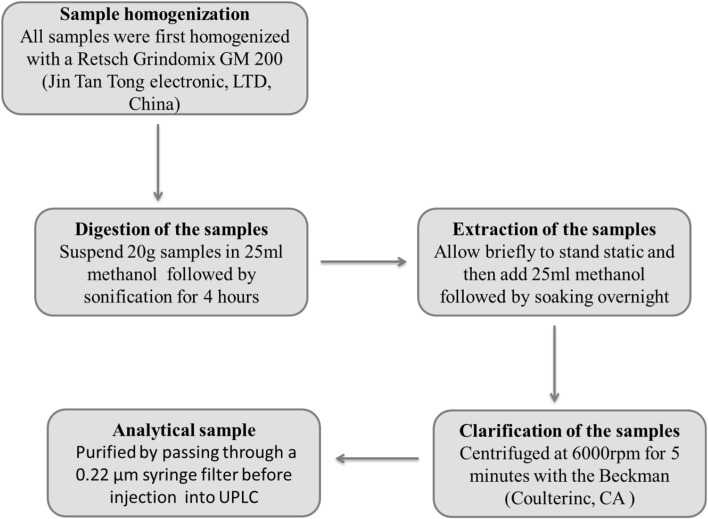
To extract FA from F. verticillioides, the solid cultures were chopped into small pieces and then soaked in MeoH three times under continuous ultrasonication for 4 h. The supernatants were centrifuged at 6000 rpm for 5 min and stored at 4 °C for UPLC analysis. The extraction of FA from fifteen agricultural products was conducted based on the method described by Noser [26] with some modifications (Fig. 1). In brief, all samples were first homogenised with a Retsch Grindomix GM 200 (Jin Tan Tong electronic, LTD, China). Then an aliquot of 20 g sample was weighed and transferred to centrifuge tubes (100 ml). Samples were extracted by soaking with 25 ml MeoH under continuous ultrasonication. After 4 h, another 25 ml MeoH was added and the samples were kept to be extracted overnight. Afterwards, the mixture was centrifuged at 6000 rpm for 5 min, and the supernatant was transferred into an intubation tube for UPLC–MS/MS analysis. In all cases, the extracts were filtered through a 0.22 µm syringe filter before using for analysis.
Fig. 1.
The protocol for FA extraction in commercial food and feed products
UPLC Analyses
A waters acquity UPLC system (Waters, Millford, MA, USA) consisting of a vacuum degasser, an auto-sampler and a binary pump, a BEH reverse-phase C18 column (10 cm × 2.1 mm, 1.7 μm) were used for the analysis of FA. The column temperature was maintained at 25 °C with the detection wavelength set at 220 nm [27]. The mobile phase “A” was water/TFA (1000:1, v/v) and the phase “B” was acetonitrile containing TFA (1000:1, v/v). To optimize the UPLC condition to obtain the best chromatogram with the lowest noise over signal ratio and shorter running time, the following UPLC conditions were experimented: (1) Mobile phase composition (A to B) varied at 10:90, 20:80, and 30:70 were tested with injection volumes and flow rate kept constant at 1 μl and 0.05 ml/min, respectively. (2) Flow rate was varied (0.02, 0.05 and 0.07 ml/min) with injection volumes and mobile phase composition maintained at 1 μl and 20:80, respectively.(3) different injection volumes (1, 2 and 3 μl)was performed keeping the composition of mobile phase 20:80 and flow rate of 0.05 ml/min fixed. The identification and quantification of FA were performed by comparing the retention times and peak areas of tested samples with the calibration curve prepared with authentic standard.
Determination of FA in Different Strains of Fusarium verticillioides and in Fifteen Food and Feed Products
The optimised protocol was applied to FA analysis in cell cultures of F. verticillioides and in fifteen food and feed products purchased from the local market in order to investigate its practical applicability. The wild type strain of F. verticillioides and seven transgenic strains (named M1–M7) were cultured on PDA plates containing 50 % sea water (containing both 50 μg/ml geneticin for transgenic strains) at 28 °C for 9 days. Afterward, the strains were scraped out from the surface of the medium and the solid medium was extracted with MeoH for UPLC analysis. Determination of FA in food and feed products was conducted based on the modified protocol as mentioned above.
Accuracy and Precision
Intra- and inter-day variations were chosen to determine the accuracy and precision of the developed assay according to recovery experiments by spiking blank samples at three different concentrations as follows. Solutions with low (10 μg/ml), middle (100 μg/ml) and high (200 μg/ml) concentrations of the calibration curve were prepared by spiking cell cultures of wild type strain of F. verticillioides or extracts of niblet, oatmeal, red kidney bean and soybean with appropriately diluted standard solution of FA (1 mg/ml). The relative standard deviation (R.S.D.) was taken as a measure of precision and the accuracy was calculated based on the ratio of FA amount measured actually. For intra-day precision, each low, middle, and high quality control sample was analyzed thrice within 1 day while for inter-day test, this experiment was performed once each day, continuously 3 days.
Results
Optimization of UPLC Conditions
Effect of Mobile Phase Composition
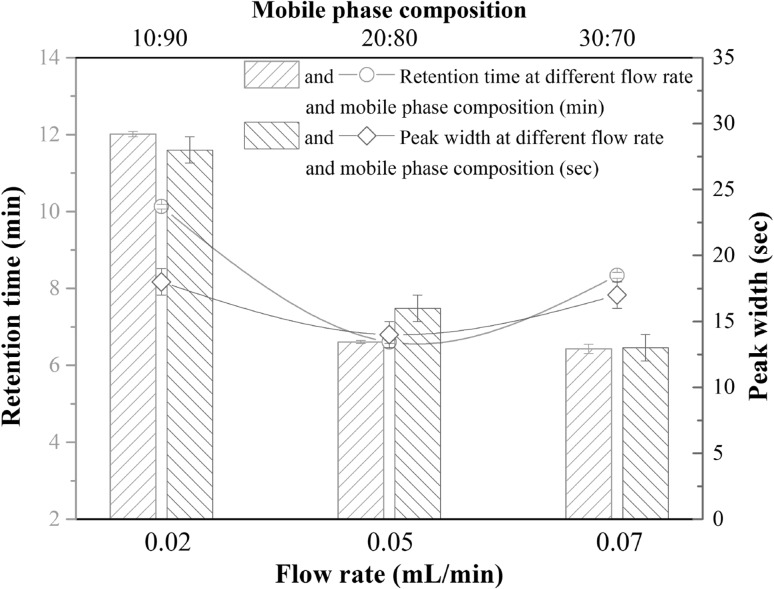
To optimize UPLC condition for FA determination, a set of gradient elution were designed. The effect of differing mobile phase compositions (mobile phase A to B at 10:90, 20:80, and 30:70 v/v levels) with an injection volumes of 1 μl and flow rate of 0.05 ml/min (with column pressure of 4400 psi) was shown in Fig. 2. Based on the results, we can see that the 20:80 of mobile phase A to B has the minimum retention time about 6.61 min and the narrowest peak width of 14.8 s at half height in comparison to the other two ratios of mobile phase A and B tested.
Fig. 2.
Effects of mobile phase composition (water: acetonitrile, line) and flow rate (column) on retention time and peak width at half height
Effect of Mobile Phase Flow Rate
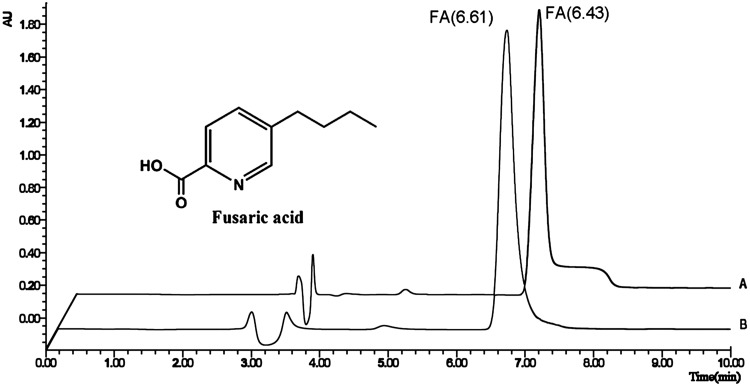
Different flow rates were evaluated in terms of retention time, peak shape and peak width at half height (Fig. 2). There was no significant difference in retention time, which was at 6.61 min and 6.43 min for 0.05 ml/min flow (column pressure of 4400 psi) and 0.07 ml/min flow (column pressure of 4842 psi), respectively. Good peak symmetry with narrow peaks (16.8 s and 12.3 s respectively) was also achieved. However, an increase in the retention time to 12.21 min with 0.02 ml/min flow (column pressure of 3986 psi), and a broad peak width of 28.8 s were observed under this condition. Figure 3 shows that the flow rate at 0.05 ml/min was better than that at 0.07 ml/min because it had a satisfactory peak shape without tailing.
Fig. 3.
Chemical structure of FA and different UPLC chromatograms at 0.07 ml/min (A) and 0.05 ml/min flow (B)
Effect of Injection Volume
Tests with different injection volumes (1, 2 and 3 μl) indicated that the 1 μl injection yielded the lowest noise signal in the chromatograms and allowed the entire running time to be reduced to 8.0 min (data not shown).
Calibration Curve (Linearity)
The method was validated for linearity, and the calibration curve (Y = 44681x-99453) with R2 value of 0.9992 was obtained. The high correlation coefficient value indicated good linearity between investigated compound concentrations and their peak areas in the studied range of 1–200 μg/ml.
Fast Quantitative Determination of FA in Fusarium Strains
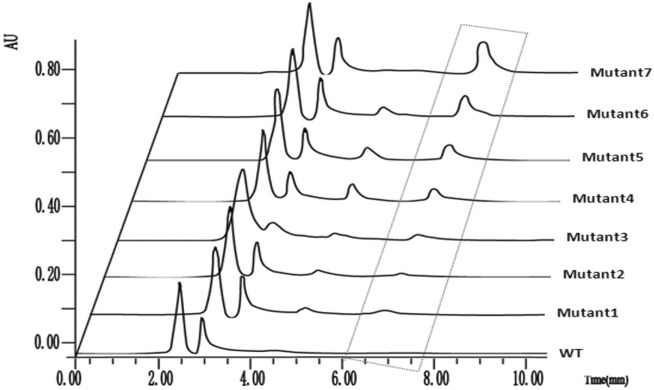
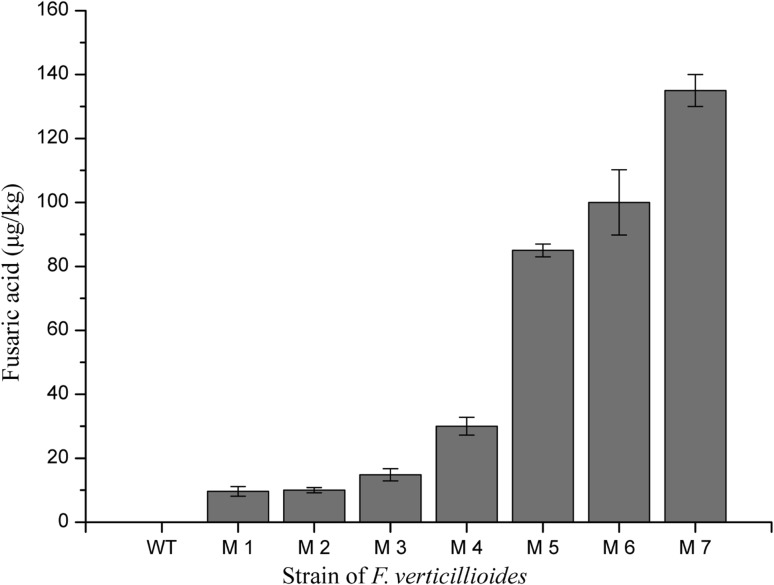
This protocol was applied for the analysis of FA in some transgenic strains of F. verticillioides. Eight samples were routinely injected and analyzed. The column pressure during the elution was around 4400 psi without big fluctuations. The UPLC chromatographic resolutions of 8 samples are all in 8 min (Fig. 4). The concentration of FA in each sample was calculated by converting the peak area to its molar concentration using the calibration curve, and for per kg of agar culture, the yield of FA ranged from 9.65 to 135 μg. The data could be converted to 0.29–4.05 μg per gram of biomass since an average of 1.5 g mycelium (1/30 time of agar culture, wet weight) was produced on each plate after 9-days cultivation (Fig. 5).
Fig. 4.
Chromatograms of the wild type strain of F. verticillioides (WT) and its transgenic strains. Transgenic strains of F. verticillioides 711 were obtained by random insertions of plasmid PII99 to the wild type (WT) strain
Fig. 5.
Concentrations of FA detected in the wild type strain (WT) and transgenic strains (M1–M7) of F. verticillioides
Survey of FA Contaminated Commercial Food and Feed Products
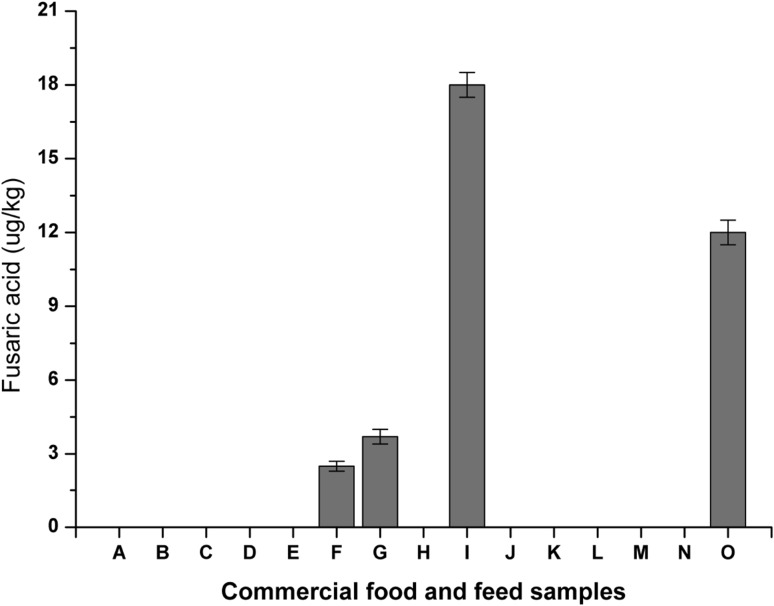
The established method was also used to examine 15 commercial food and feed products collected from the local market. The analytical results of FA in these samples are summarized in Fig. 6. The concentration of FA in each sample was calculated by converting the peak area to its molar concentration according to the calibration curve. FA was detected in 4 out of 15 samples. The highest content of FA was determined in a red kidney bean sample (18 μg/kg) and the lowest content of FA was observed in a niblet sample (2.5 μg/kg). The column pressure during the elution was around 4400 psi without big fluctuations.
Fig. 6.
FA levels in the analyzed samples purchased from the China market. A–O were sticky rice, maize, black rice, peanut, corn flakes, niblet, oatmeal, millfeed, red kidney bean, red bean, pinto beans, black soybean, white bean, mung bean and soybean, respectively
Precision and Accuracy
The wild type strain of F. verticillioides and four commercial products based on analytical results (Fig. 6) were used as blank sample, and recoveries and precision were determined by spiking these blank samples with standard solution in duplicate at the spiking levels indicated above. For the cell culture of F. verticillioides strains, the results obtained were satisfactory both in inter-day and intra-day tests. Recovery values varied from 98.2 to 99.6 % demonstrating that there was no interference from endogenous components of cell culture in the procedure used. The RSD ratios of intra- and inter-day studies ranged from 1.21 to 2.48 % indicating that the proposed method was highly precise (Table 1). Satisfactory results were also observed in niblet, oatmeal, red kidney bean and soybean. The average recovery rates were between 70 and 120 % and precisions (R.S.D., %) were less than 10 % (Table 2), which meet the regulations of European Food Safety Authority (EFSA) [28].
Table 1.
The results of inter-day (n = 3) and intra-day (n = 3) precision and accuracy in the wild type strain of F. verticillioides
| Nominal concentration (μg/ml) | Mean concentration founda (μg/ml) | Mean accuracyb (%) | Precision (R.S.D., %) | Confidence interval (CI) |
|---|---|---|---|---|
| Inter-day (n = 3) | ||||
| 10 | 9.96 | 99.6 | 1.21 | 10 ± 0.14 |
| 100 | 98.42 | 98.4 | 2.56 | 100 ± 3.34 |
| 200 | 198.23 | 99.1 | 1.17 | 200 ± 4.12 |
| Intra-day (n = 3) | ||||
| 10 | 9.95 | 99.5 | 1.84 | 10 ± 0.18 |
| 100 | 98.17 | 98.2 | 2.48 | 100 ± 2.13 |
| 200 | 198.01 | 99.0 | 1.26 | 200 ± 3.42 |
aMean value of three determinations at three concentration levels for inter-day and intra-day respectively
bAll the mean accuracies were calculated against their nominal concentrations
Table 2.
The results of inter-day (n = 3) and intra-day (n = 3) precision and accuracy in commercial food and feed products
| Samples | Mean accuracy (%) | Precision (R.S.D., %, Intra-day) (n = 3) | Precision (R.S.D., %, Inter-day) (n = 3) |
|---|---|---|---|
| Niblet | 81.2 | 8.3 | 8.7 |
| Oatmeal | 79.1 | 6.4 | 6.9 |
| Red kidney bean | 90.3 | 7.5 | 8.6 |
| Soybean | 105.8 | 4.2 | 6.1 |
Discussion
This study has established a useful method using UPLC to simultaneously analyze FA in fungal culture and some food and feed commodities. For the optimization of UPLC, the best chromatograms for FA with the lowest noise were obtained using a mobile phase consist of water and acetonitrile (both containing 0.1 % TFA) of 20–80 volume ratio at a flow rate of 0.05 ml/min with injection volume of 1 μl. Under the designated conditions, FA got eluted at 6.61 min, and all analytes were completely resolved within 8 min, which was much shorter than currently used methods. The method was found to be linear over an analytical range of 1–200 μg/ml with high correlation coefficients (Y = 44681X-99453, R2 = 0.9992). It was first achieved in application in analysis of cell cultures of Fusarium strains, and perfect results were also observed when it was applied to analyse FA in food and feed products. The method was validated and reasonable recoveries in Fusarium strains (ranged from 98.2 to 99.6 %) and in food and feed products (ranged from 81.2 to 105.8 %) were achieved. Satisfactory precisions (R.S.D., %) in Fusarium strains and food and feed products less than 3.0 % and 10 % respectively were also obtained. Although there has not been a commission recommendation on the maximum level of FA from authoritative organizations, we find that the FA contents in all the commercial food and feed samples are below to that of the permitted maximum of T-2 toxin, another fusarium-toxin showing stronger toxicity than FA, established by the European Food Safety Authority (EFSA) in the commission recommendation 2013/165/EU [29]. It means that all the tested food and feed products from market in Fuzhou are safe. In conclusion, the method may serve as a reference for developing analytical standards on FA by regulatory agencies conveniently in a short time following simple pretreatment without any kind of pre-concentration.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 31070053). The authors gratefully acknowledge the staff of microbiology laboratory of Fujian Normal University.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Brown DW, Butchko RA, Busman M, Proctor RH. Identification of gene clusters associated with fusaric acid, fusarin, and perithecial pigment production in Fusarium verticillioides. Fungal Genet Biol. 2012;49:521–532. doi: 10.1016/j.fgb.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Bacon CW, Porter JK, Norred WP, Leslie JF (1996) Production of fusaric acid by Fusarium species. Appl Environ Microb 62:4039–4043. http://www.ncbi.nlm.nih.gov/pubmed/8899996 [DOI] [PMC free article] [PubMed]
- 3.Sundararajan KS, Subbaraj R, Chandrashekaran MK, Shunmugasundaram S. Influence of fusaric acid on circadian leaf movements of the cotton plant, Gossypium hirsutum. Planta. 1978;144:111–112. doi: 10.1007/BF00385016. [DOI] [PubMed] [Google Scholar]
- 4.Jiao J, Zhou B, Zhu X, Gao Z, Liang Y. Fusaric acid induction of programmed cell death modulated through nitric oxide signalling in tobacco suspension cells. Planta. 2013;238:727–737. doi: 10.1007/s00425-013-1928-7. [DOI] [PubMed] [Google Scholar]
- 5.Dong X, Ling N, Wang M, Shen Q, Guo S. Fusaric acid is a crucial factor in the disturbance of leaf water imbalance in Fusarium-infected banana plants. Plant Physiol Biochem. 2012;60:171–179. doi: 10.1016/j.plaphy.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Ghag SB, Shekhawat UK, Ganapathi TR. Native cell-death genes as candidates for developing wilt resistance in transgenic banana plants. AoB Plants. 2014;6:1037–1049. doi: 10.1093/aobpla/plu037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang M, Sun Y, Sun G, Liu X, Zhai L, Shen Q, Guo S. Water balance altered in cucumber plants infected with Fusarium oxysporum f. sp. cucumerinum. Sci Rep. 2015;5:7722–7729. doi: 10.1038/srep07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Zuo C, Deng G, Kuang R, Yang Q, Hu C, Sheng O, Zhang S, Ma L, Wei Y, Yang J, Liu S, Biswas MK, Viljoen A, Yi G. Contamination of bananas with beauvericin and fusaric acid produced by Fusarium oxysporum f. sp. cubense. PLoS One. 2013;8:70226–70237. doi: 10.1371/journal.pone.0070226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimshoni JA, Cuneah O, Sulyok M, Krska R, Galon N, Sharir B, Shlosberg A. Mycotoxins in corn and wheat silage in Israel. Food Addit Contam Part A. 2013;30:1614–1625. doi: 10.1080/19440049.2013.802840. [DOI] [PubMed] [Google Scholar]
- 10.Saremi H, Okhovvat SM (2006) Mycotoxin producing Fusarium species associated with plant disease on potato, wheat, corn and animal diseases in northwest Iran. Commun Agric Appl Biol Sci 71:1175–1185. http://www.ncbi.nlm.nih.gov/pubmed/17390876 [PubMed]
- 11.Smith TK, McMillan EG, Castillo JB (1997) Effect of feeding blends of Fusarium mycotoxin-contaminated grains containing deoxynivalenol and fusaric acid on growth and feed consumption of immature swine. J Anim Sci 75:2184–2191. http://www.ncbi.nlm.nih.gov/pubmed/9263067 [DOI] [PubMed]
- 12.Lindner G, Grosse G (1986) The effect of fusaric acid on nerve tissue cultured in vitro. Mikrosk Anat Forsch 100:262–272. http://www.ncbi.nlm.nih.gov/pubmed/3487895 [PubMed]
- 13.Smith TK, MacDonald EJ (1991) Effect of fusaric acid on brain regional neurochemistry and vomiting behavior in swine. J Anim Sci 69:2044–2049. http://www.ncbi.nlm.nih.gov/pubmed/1712354 [DOI] [PubMed]
- 14.Porter JK, Bacon CW, Wray EM, Hagler WM., Jr Fusaric acid in Fusarium moniliforme cultures, corn, and feeds toxic to livestock and the neurochemical effects in the brain and pineal gland of rats. Nat Toxins. 1995;3:91–100. doi: 10.1002/nt.2620030206. [DOI] [PubMed] [Google Scholar]
- 15.Velasco M, Gilbert CA, Rutledge CO, McNay JL (1975) Antihypertensive effect of a dopamine beta hydroxylase inhibitor, bupicomide: a comparison with hydralazine. Clin Pharmacol Ther 18:145–153. http://www.ncbi.nlm.nih.gov/pubmed/1097150 [DOI] [PubMed]
- 16.Wang H, Ng TB (1999) Pharmacological activities of fusaric acid (5-butylpicolinic acid). Life Sci 65:849–856. http://www.ncbi.nlm.nih.gov/pubmed/10465344 [DOI] [PubMed]
- 17.Swamy HV, Smith TK, Karrow NA, Boermans HJ (2004) Effects of feeding blends of grains naturally contaminated with fusarium mycotoxins on growth and immunological parameters of broiler chickens. Poult Sci 83:533–543. http://www.ncbi.nlm.nih.gov/pubmed/15109051 [DOI] [PubMed]
- 18.Voss KA, Porter JK, Bacon CW, Meredith FI, Norred WP (1999) Fusaric acid and modification of the subchronic toxicity to rats of fumonisins in F. moniliforme culture material. Food Chem Toxicol 37:853–861. http://www.ncbi.nlm.nih.gov/pubmed/10506009 [DOI] [PubMed]
- 19.Appell M, Jackson MA, Wang LC, Ho CH, Mueller A. Determination of fusaric acid in maize using molecularly imprinted SPE clean-up. J Sep Sci. 2014;37:281–286. doi: 10.1002/jssc.201301065. [DOI] [PubMed] [Google Scholar]
- 20.Amalfitano C, Pengue R, Andolfi A, Vurro M, Zonno MC, Evidente A. HPLC analysis of fusaric acid, 9,10-dehydrofusaric acid and their methyl esters, toxic metabolites from weed pathogenic Fusarium species. Phytochem Anal. 2002;13:277–282. doi: 10.1002/pca.648. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita K, Yamazaki K, Komatsu S, Numazawa M. Fusaric acid as a novel proton-affinitive derivatizing reagent for highly sensitive quantification of hydroxysteroids by LC-ESI-MS/MS. J Am Soc Mass Spectrom. 2010;21:249–253. doi: 10.1016/j.jasms.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Jiang G, Yang B, Dong X, Feng L, Lin S, Chen F, Ashraf M, Jiang Y. A luminescent bacterium assay of fusaric acid produced by Fusarium proliferatum from banana. Anal Bioanal Chem. 2012;402:1347–1354. doi: 10.1007/s00216-011-5546-6. [DOI] [PubMed] [Google Scholar]
- 23.Ventura M, Guillen D, Anaya I, Broto-Puig F, Lliberia JL, Agut M, Comellas L. Ultra-performance liquid chromatography/tandem mass spectrometry for the simultaneous analysis of aflatoxins B1, G1, B2, G2 and ochratoxin A in beer. Rapid Commun Mass Spectrom. 2006;20:3199–3204. doi: 10.1002/rcm.2723. [DOI] [PubMed] [Google Scholar]
- 24.Waskiewicz A, Beszterda M, Bocianowski J, Golinski P. Natural occurrence of fumonisins and ochratoxin A in some herbs and spices commercialized in Poland analyzed by UPLC-MS/MS method. Food Microbiol. 2013;36:426–431. doi: 10.1016/j.fm.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Kostelanska M, Zachariasova M, Lacina O, Fenclova M, Kollos AL, Hajslova J. The study of deoxynivalenol and its masked metabolites fate during the brewing process realised by UPLC-TOFMS method. Food Chem. 2011;126:1870–1876. doi: 10.1016/j.foodchem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 26.Noser J, Schneider P, Schmutz H, Rother M. Determination of 6 Alternaria toxins with UPLC-MS/MS and their occurrence in tomatoes and tomato products from the Swiss market. Mycotoxin Res. 2011;27:265–271. doi: 10.1007/s12550-011-0103-x. [DOI] [PubMed] [Google Scholar]
- 27.Lattanzio VMT, Solfrizzo M, Powers S, Visconti A. Simultaneous determination of aflatoxins, ochratoxin A and fusarium toxins in maize by liquid chromatography/tandem mass spectrometry after multitoxin immunoaffinity cleanup. Rapid Commun Mass Spectrom. 2007;21:3253–3261. doi: 10.1002/rcm.3210. [DOI] [PubMed] [Google Scholar]
- 28.Commission EU (2006) Commission Regulation (EC) No.1881/2006 of 19th December 2006. Setting maximum levels for certain contaminants in foodstuffs. Off J Eur Union L364:15–24. http://ec.europa.eu/food/safety/animal-feed/index_en.htm
- 29.Commission regulation on the presence of T-2 and HT-2 toxin in cereals and cereal products (2013). Commission Regulation (EC) 2013/165/EU, Off J Eur Union L91:12–15. http://ec.europa.eu/food/safety/animal-feed/index_en.htm