Abstract
Comparative auditory studies make it possible both to understand the origins of modern ears and the factors underlying the similarities and differences in their performance. After all lineages of land vertebrates had independently evolved tympanic middle ears in the early Mesozoic era, the subsequent tens of millions of years led to the hearing organ of lizards, birds, and mammals becoming larger and their upper frequency limits higher. In extant species, lizard papillae remained relatively small (<2 mm), but avian papillae attained a maximum length of 11 mm, with the highest frequencies in both groups near 12 kHz. Hearing-organ sizes in modern mammals vary more than tenfold, up to >70 mm (made possible by coiling), as do their upper frequency limits (from 12 to >200 kHz). The auditory organs of the three amniote groups differ characteristically in their cellular structure, but their hearing sensitivity and frequency selectivity within their respective hearing ranges hardly differ. In the immediate primate ancestors of humans, the cochlea became larger and lowered its upper frequency limit. Modern humans show an unusual trend in frequency selectivity as a function of frequency. It is conceivable that the frequency selectivity patterns in humans were influenced in their evolution by the development of speech.
Keywords: evolution, hearing, amniote, lizard, bird, human, cochlea
Introduction
It was clear to the earliest researchers in hearing, for example, the anatomist Retzius (1884) and the physiologist von Békésy (1960), that a solid understanding of hearing organs needs to be rooted in a comparative approach. These very early authors described the anatomy and the physiology of a great variety of ears, from fish to humans. They were led to this broad approach by the conviction that the human ear, like all other vertebrate ears, is the product of a long evolutionary process and is best understood in that context.
Unfortunately, there are frequent and profound misunderstandings of what this conclusion means. Before the Darwin-Wallace revolution of the mid nineteenth century, humans were viewed as the top rung of a ladder of progress that began with the lowest worms, without, however, explaining how the various rungs of the ladder were related. In fact, this ladder, or scale, of life goes back to ancient Greece and Aristotle’s teachings. It pleased the human mind to view itself as the pinnacle of existence and, in different religions, as the pinnacle of some kind of creation. Darwin and Wallace’s (1858) ideas on natural selection as the mechanism of evolution rendered such ideas not only untenable but misleading. The concept of evolution by natural selection has since been established and hugely strengthened by developments in paleontology, stratigraphy, age dating, anatomy, physiology, and genetics, building a consistent foundation for all of modern biology and medicine (Futuyma 2008). The title of a famous article by the evolutionary biologist Theodosius Dobzhansky (1973) is still true: Nothing in biology makes sense except in the light of evolution. Each living species is best viewed as the tip of a tiny twig at the outside of a very large and complexly-branched tree. All the other little twigs represent other species that, when traced back down the larger twigs and then branches of the tree turn out to be more or less closely related, depending on how far back in tree growth (time) it was since they split from a common ancestor. Instead of humans being the top rung of some mythical ladder, we find ourselves to be the very talented but otherwise unremarkable representatives of a small side branch of the primate subgroup of mammals.
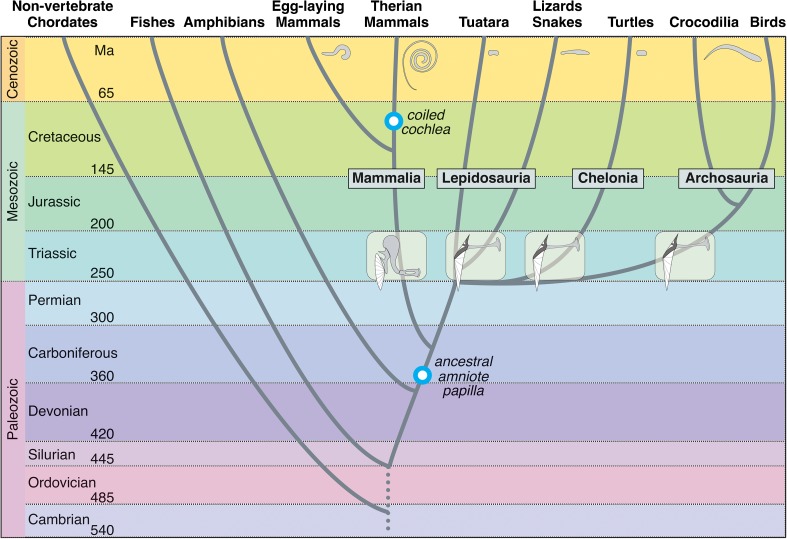
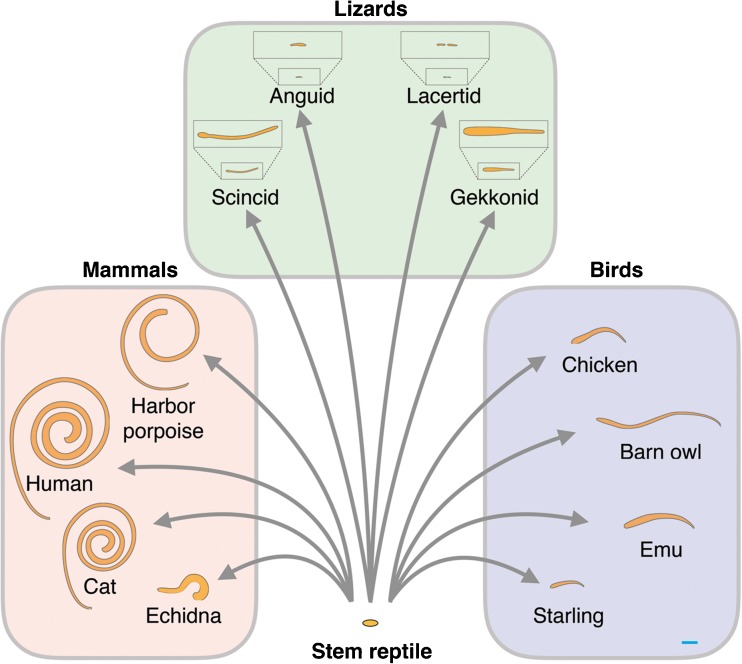
Remarkably, however, Aristotle’s ladder continues to lurk in the background of modern thinking and even biologists sometimes succumb to the use of terms that derive from it (Omland et al., 2008). Thus, the idea still lingers that mammals evolved from lizards and birds, which is impossible, since the ancestors of mammals arose long before lizards and birds graced the landscape (Fig. 1). Also, the idea that there are “higher” and “lower” vertebrates stems from the concept of the ladder, as does the term “primitive,” it would be better if these words were eliminated from modern biological literature. Instead of “lower,” the group can simply be given its name (fishes, amphibians, lizards, birds, etc.) or, in the rarer cases where all are meant, simply “non-mammalian” vertebrates. When terms are needed to describe earlier and later representatives of a group, it has proven to be unambiguous to refer to ancestral forms (or ancestral characteristics) (whose origins lie near the roots of the group) and recent forms to refer to more modern such forms or characteristics. Paleontologists often refer to “stem” or “crown” mammals as those critically important groups that lie in time close to the origin of mammals or enclose all relevant groups under one umbrella term.
FIG. 1.
Highly schematic representation of the amniote phylogenetic tree over 500 million years to illustrate the approximate time of origin of particular features of auditory systems. Amniotes arose from the earliest amphibian tetrapods early in the paleozoic and presumably inherited from them a simple hearing organ (the lower blue ring marks the latest time possible for the origin of the ancestral amniote papilla). Apart from the lineages to the turtles and the Tuatara, that remained ancestral in a number of respects, three main lineages to modern amniotes are distinguished: Mammalian ancestors, that arose first; The archosaur line that led to the dominant land organisms of the Mesozoic (only the crocodile-alligator and bird groups survived to modern times); and Lepidosaurs (mostly lizards and snakes). The tympanic middle ear (inserts) originated independently in all groups during the Triassic, initiating the evolution of elongation and unique cellular configurations of the different inner-ear papillae. Amphibians also independently evolved a single-ossicle middle ear, but it is not yet known exactly when this occurred. Monotreme mammals do not have a coiled cochlea, coiling originated at the root of the marsupial-placental lineages (original diagram provided by Ulrike Sienknecht).
A whole new science has arisen around the need to establish relationships between groups of organisms and the times of their divergences, using cladistics, molecular biology, and special statistical techniques. The results bear no resemblance whatever to a ladder or even dozens of ladders. Through the new methodologies, we now have a much better understanding of where humans fit in the great pattern of vertebrate life and understand that the human ear, like all ears of recent vertebrates, is the product of a long evolutionary process. It has also become clear that this process in mammals ran parallel to the evolution of ears of other groups of land vertebrates, with the result that the ears of lizards and their relatives, of crocodilians and birds, and of mammals evolved largely independently and show characteristic differences. Since all arose from a distant common ancestor, however, they have many ancestral properties in common and use similar physiological solutions to the problems of the analysis of sound (Manley and Köppl 1998). Thus, the ears of all land vertebrates offer fascinating insights into the ways that ears can be built and into mechanisms that are common to the ears of mammals and other groups.
What Is the Worth of Comparative Auditory Research?
Since most research is funded by society in general, i.e., the taxpayers, systems were established for judging the “usefulness” and “relevance” of projects prior to decisions about funding, with the criterion “scientific value” usually also playing a rôle. The question is, of course: Useful and relevant to whom and for what purpose? There are two broad categories that can form the basis for such allocations:
Research that can at least be broadly categorized as “applied.” In hearing research, these are projects whose goals are—at least in the short and medium term—directly related to medical problems in Otolaryngology. In the meantime, this category includes the huge and of course highly important work on understanding aging and genetic diseases involving ears, etc. However, even brief reflection will make it obvious that something can only be “applied” if there is also something already known, which leads to the second category.
Basic research is an enterprise unique to advanced human civilizations. One of the ways in which humans differ from even their closest animal relatives is in their unbounded curiosity and their need to communicate. Since the beginning of time, humans have been creative in their approach to their environment in that through curiosity, they gleaned information that made it easier, for example, to react to emergencies and ensure survival and possible reproduction. It was thus established early as an essential feature of human society to have a culture of simply wanting to know and understand things about our world and ourselves. This is the idea underlying basic research in all fields of knowledge, including hearing research. Indeed, it is the basis of all human cultural enterprise. Without basic research, applied research must dry up and reach an impasse.
In recent times, justifying research expenditures for basic research has been under attack, with some fundamental opposition on the basis of its lack of “usefulness” (but see above). Especially, the insidious tendency for neoliberal ideology to progressively dominate even cultural and political structures (Brown 2015) has led to decisions that massively weaken and pauperize cultural and research endeavors. Educated and culture-based human thought, however, is essential. All such enterprises can only flourish with unfettered creativity and the liberty to develop free of the restraints of commerce, continuing to enable society to enrich itself and to reap new rewards of knowledge. That is why, within every research field, basic science needs adequate space to breathe. This should not be confused with undirected or poorly motivated research: Basic research is as stringently argued and hypothesis-driven as applied research, perhaps even more so because the goals are inherently less obvious.
The History of Land Vertebrates as Seen from the Ear
In this review, it will be necessary to restrict discussion to the major groups of land vertebrates, ignoring some of the larger and most of the smaller ones. For the present purposes, land vertebrates are restricted to the amniotes, i.e., those groups derived from an ancestral “stem reptile” whose lineage evolved a shell around the egg and thus leapt the final hurdle to emancipation from a life in water. With the evolution of this amniotic egg, the embryo was provided with the basic needs for its sheltered development. These needs were mediated by the evolution of several membranes that developed outside of the embryo’s body itself, connecting, for example, the embryo to the inner wall of the eggshell, enabling the uptake of oxygen. An additional membrane surrounded the embryo, enclosing it in a fluid buffer: This is the amnion, and provided the name for the group of amniotes. The third membrane stored waste products until they could be discarded at hatching. These three membranes survive even in amniotes that no longer lay eggs (most mammals, a number of live-bearing lizards) and there serve other purposes (such as building part of the placenta).
Fossil evidence indicates that, following the origin of tetrapods about 400 million years ago (Ma), it took close to 100 Ma until the stem reptile group began to break up into several lineages (Fig. 1; Carroll 1987). About 320 Ma ago, a lineage known as the mammal-like reptiles arose and subsequently evolved separately from all other groups, later giving rise to the true mammals. These were the Synapsida; the name derives from the presence of a single hole in the temporal region of the skull. Following their origin, two other major lineages evolved, the Lepidosauria and the Archosauria, both Diapsida (two holes in the temporal region), and were the last successful lineages arising from the stem reptiles. The Lepidosauria today includes the squamates (lizards and snakes) and their close relatives and a tiny, ancestral sister group known as the rhynchocephalians. The latter group is included here, since the ears of its remaining living representatives, the Tuatara “lizard” (it is not a true lizard), known only from islands off New Zealand, provide very useful comparative data.
The second major diapsid lineage, the Archosauria, today includes all the birds and the Crocodilia (crocodiles, alligators and gavials). In the past, this lineage also included groups such as the dinosaurs that, for space reasons, will not be further discussed here, but for whom interesting information is available on their ears and hearing (Gleich et al. 2005; Walsh et al. 2009). Related to the archosaurs is an early offshoot, the turtles (Fig. 1), that is also included here since their ears provide useful comparative insight into the evolution of ears. As will be described below, the three major groups all inherited the same, simple type of inner ear from their common ancestor, but subsequent to their split evolved in full independence, developing unique and characteristic types of inner-ear structure in each lineage.
What Kind of Inner Ear Did All Amniotes Inherit?
Hair cells, the sensory cells forming the basis of all vertebrate hearing organs, arose very early in the history of animals. They are prominent in modern representatives of non-vertebrate chordates, such as sea squirts, and perhaps even traceable back to the earliest animals that possessed true tissues, the cnidarians (sea anemonies and their relatives; for reviews, see Coffin et al. 2004; Manley and Ladher 2007). All vertebrate hair cells conform to a clear generalized pattern and are homologous (in evolutionary terms, homology is the shared ancestry of a structure in different taxa).
Where the basilar papilla of non-mammalian amniotes (and its later relative, the mammalian organ of Corti) came from and when it originated is still an open question. One of the reasons the present discussion does not deal with the (fascinating!) group of amphibians is that neither of their one or two hearing organs is clearly homologous to the amniote basilar papilla (Smothermann and Narins 2004). Thus, the modern amphibians, that are placed in their own group (Lissamphibia) and differ in many respects from the ancestral amphibians from which the amniotes arose, are no help in deciding on the origin of the basilar papilla. The amniote basilar papilla is defined as a patch of sensory hair cells that is supported on a free basilar membrane and lies (at least originally) between the saccular and lagenar maculae.
Only one other enigmatic clue to the ancestry of the basilar papilla exists. In the embryos of the coelacanth fish Latimeria (coelacanths are a small group of bony fishes and are among the fishes most closely related to land vertebrates), Fritzsch (1987) found a structure in the inner ear that had some resemblances to the amniote basilar papilla. Unlike all other (vestibular) sensory areas within the inner ear it consisted of hair cells suspended over a free membrane and its position within the complex structure of the inner ear was also similar to that of amniote basilar papillae. However, modern systematic studies indicate that the (also sarcopterygian) lung fishes are even more closely related to land vertebrates than is the coelacanth (Liang et al. 2013) and lung fish inner ears show no evidence of a basilar papilla. At present, we do not know whether lung fishes lost this structure (and it is therefore ancestral) or whether the coelacanths independently developed their own version of it. In the much larger groups of the cartilaginous fishes (sharks and rays) or the ray-finned fishes (most other modern fish groups), there is no evidence for a basilar papilla and hearing is mediated by one or more of the vestibular macular receptors, generally the sacculus (Ladich 2013).
Questions related to defining the ancestral condition are the competence of the science of systematics. Using diverse approaches, including statistical analyses of very large data bases on anatomical characteristics, it is usually possible to identify the most likely ancestral form. In the present case, two amniote groups, the Tuatara and the turtles, stand out as showing the most ancestral characteristics (even though they have in other respects, such as the Plastron of the turtles, clear but unique specializations). Both the turtles and the Tuatara were studied early in auditory research (Miller 1978; Wever 1978; Sneary 1988). Basilar papillae are found in both groups; these are small (~1 mm) strips of hair cells that are supported by a freely-suspended basilar membrane and that arise during individual development between the more ancestral hair-cell organs of the saccular and the lagenar maculae. The ancestral character of turtle (Archosauria) and Tuatara (Lepidosauria) basilar papillae does not conclusively mean that the papilla was present or even arose in stem amniotes; it is simply the most parsimonious explanation.
Thus, we are left uncertain with regard to the origin of the amniote hearing organ, except that comparative studies do provide a good idea of what the putative ancestral amniote basilar papilla looked like. It should, however, be pointed out at this stage that the earliest representatives of all three major amniote groups did not have anything resembling a middle ear. Thus, if there were a common origin of the basilar papilla before the separation of the major amniote lines, the sound input to this ancestral amniote hearing organ was almost certainly restricted to loud, low-frequency sounds that reached the inner ear through diverse tissue paths. And thus the situation remained in all groups for the subsequent 70 Ma, first changing dramatically during the geological period known as the Triassic. During this first era of the Mesozoic, all lineages independently evolved middle ears (Clack 2002) and thus kick-started an unparalleled period of expansion and specialization of the inner-ear auditory organs (Fig. 1).
The Origin of Middle Ears
The origin of the mammalian middle ear from other skull elements has been understood since the early decades of the twentieth century, and its elucidation was one of the earliest triumphs of comparative anatomy and paleontology. What was not then clear, however, is that middle ears in all amniote groups did not arise until relatively late in their evolution. Previously, it had been assumed that, since major changes were necessary in the body of vertebrates when they left aquatic environments to live—at least as adults—on land, the ears would also have benefitted from change and therefore have evolved a middle ear. This idea was strongly supported by the presence of a deep notch caudo-laterally on both sides of the skulls of transitional (fish-amphibian) fossils and that was interpreted as the position of an eardrum and called appropriately (as was then surmised) the “otic notch.” It took decades of painstaking work, including re-examination of the earliest transitional fossils, to show that this was false (Clack 2002). Whatever the notch was for (it could, for example, have provided space for muscles to expand during closure of the mouth, as is conceived for the skull openings used to name the different skull types in amniotes), it was not the location of an eardrum. Amniote middle ears arose much, much later.
Interestingly, the re-examination of fossils did provide interesting information in some ancient fish on middle-ear-like structures (Clack et al. 2003) that operate in a similar way to some (independently evolved) structures known from modern lungfishes (Christensen-Dalsgaard et al. 2011). In these, a bubble of gas is maintained in a position inside the head and lateral to the inner ear. The surface of this bubble provides a density interface that would vibrate when sound waves pass—otherwise without major effect—through the fishes body and thus enhance sensitivity to underwater sound. The emphasis here is on underwater sound; these were not middle ears in the classical sense.
Two kinds of middle ear, single- and three-ossicle types, arose in amniotes (and, incidentally, in the ancestors of Lissamphibians), and these are sufficiently different in origin and structure as to necessitate separate discussion.
The “Single Ossicle” Tympanic Middle Ear
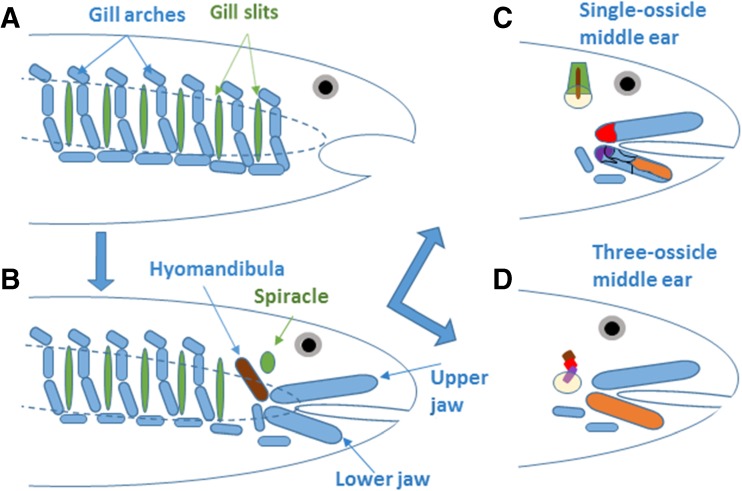
Although structurally consisting of more than one element, functionally, the middle ears of lepidosaurs and archosaurs (that evolved independently in the Triassic, Clack 2002) use a single ossicle or bone. In many cases, substantial parts of this structure are in fact cartilaginous and not bony, which is in part related to how it works. The main ossicular component in these middle ears is the columella, which was given this name before its evolutionary history had been deciphered. In fact, the columella is the hyomandibula, an element known from the gill-support structures (branchial arches) of fish ancestors. Fish have, of course, gills and these are supported in their structure by cartilaginous or bony rods arranged in groups from dorsal to ventral and lying between each of the pairs of gill slits (Fig. 2A, B). In the early history of fishes, the first set of such structures at the front of the head moved beneath the neurocranium and formed jaws—the jawed fishes arose (Carroll 1987). As a result of this, the frontal gill slits and branchial arches were distorted and moved and one dorsal arch component that originally lay behind the jaws was moved forward and used to support the rear end of the upper jaw by connecting it to the neurocranium (Fig. 2B). This is the hyomandibula, a structure with one of the most checkered histories of all vertebrate body components. Beginning its evolution as a gill support, it evolved into a jaw support (it still is in many fishes), later changing yet again to enter the middle ear in land vertebrates (Carroll 1987). Next to the hyomandibula, one of the gill slits was constricted upwards, becoming a narrow tube above the rear of the jaw joint and known as the spiracle. In cartilaginous fishes such as sharks and rays, this spiracle still exists and, in rays that spend long periods lying hidden on the ocean floor, sting rays for example, plays a critical role as an inhalent opening for breathing and thus prevents sand entering the mouth. In non-mammalian amniotes, it reappears and surrounds the middle ear (Fig. 2C).
FIG. 2.
Highly schematic representation of the endochondral skeletal elements of various vertebrate skulls. A The 6 gill slits (green) and their supporting skeletal elements (light blue) that line the outer edge of the pharynx (dashed blue line) in an ancestral jawless fish that was a plankton—or detritus—feeder with no ability to bite prey or larger food items. B Over time, new species evolved that had turned the first gill-support arch into an upper and lower jaw, forcing the first gill slit upwards. That gill slit is then called the spiracle in such species and forms the basis for the middle-ear cavities in later non-mammals. The jaws were supported at the rear by an enlarged element of the second arch, known as the hyomandibula (brown). C In much later non-mammalian land vertebrates, the hyomandibula (brown) becomes the columella of the middle ear and lies inside the spiracle (green), making contact to a newly-formed eardrum (yellow). In the upper jaw, the quadrate is shown in red. In the lower jaw, two of the seven bones are colored; the articular is purple; the dentary is orange. D In mammals, the lower jaw only consists of the dentary (orange). The articular = malleus (purple) and the quadrate = incus (red) have joined the columella = stapes (brown) in the middle ear. The malleus contacts a newly formed eardrum (yellow). The neurocranium is not shown.
The hyomandibula thus had a long history as a jaw support in fishes and early land vertebrates, a history that ended in all amniote groups in the Triassic period. By this time, the skull consisted of two major skeletal complexes. One, the older, lay deep in the head and consisted of the neurocranium and the gill arches, including the jaws, whose bone was formed within and thus replaced the older cartilaginous elements (endochondral ossification, Fig. 2). Outside this, newer elements were formed within the skin (dermal ossification) and formed a very large area of plates that covered the inner skull and were connected to it at many locations. Due to changes in skull structure, in all groups except mammals, the hyomandibula became unnecessary as a support for the upper jaw region and, instead, moved into the revived spiracle, which had formed a middle-ear space that was—and still is in modern representatives—widely open to the mouth cavity (Carroll 1987). At its inner end, the hyomandibula (Fig. 2C), which is then termed the columella, expanded to form a footplate that inserted into the oval window of the inner ear and lay very close to the basilar papilla. At its lateral, outside end, the columella was extended by a—mostly cartilaginous—“extracolumella” that formed finger-like projections in several directions both supporting the columella in the center of the spiracle and, with one extension, connecting along the inside of the newly-formed eardrum with a tip near the center. The eardrum is formed by tissues from the outer skin and from the mouth cavity and becomes very thin (typically less than 15–20 μm). Thus, the elements of a single-ossicle middle ear evolved, an event that occurred independently at least twice in the origin of lepidosaurs and archosaurs.
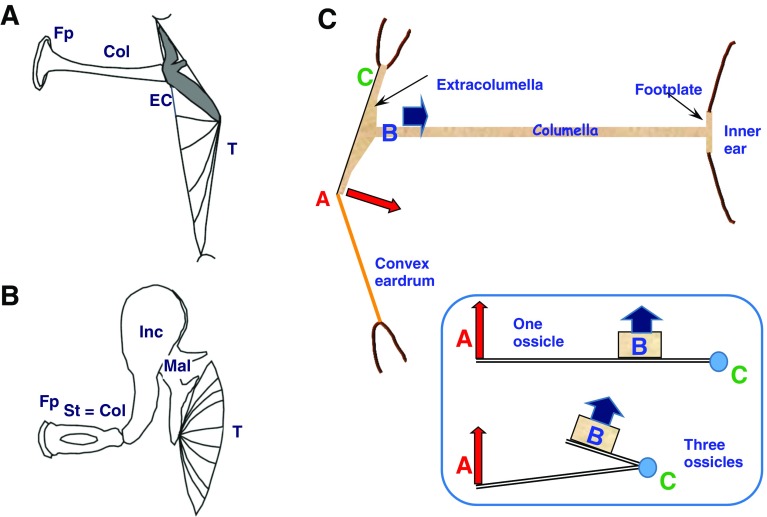
Functionally, this kind of middle ear, operative in present-day, non-mammalian amniotes, forms a secondary lever system (Fig. 3; e.g., Manley 1972a). This leverage forms part of what is usually termed an impedance-matching device that is thought to better match the changes in air pressure associated with sound in the low-impedance air environment to the much higher impedance of the fluids in the inner ear that enclose the basilar papilla. The tip of the extracolumella, located in the center of the eardrum, is moved through a larger distance than the location of the connection to the columella itself (Fig. 3). Thus, the columella is moved through a smaller distance but with greater force. This “lever ratio” is, in most middle-ear systems, about 2:1. In addition, other characteristics act to increase the force at the columella footplate, including the much greater area of the eardrum collecting the sound input from the air as compared to the area of the columellar footplate. Physiological studies of such systems (Manley 1972a; Werner and Wever 1972; Wever 1978; Saunders 1985) have shown that without it, hearing sensitivity is diminished by up to 60 dB. At frequencies that are beyond the processing capacity of the basilar papilla, less of the input energy from the middle ear is accepted by the inner ear, leading to a rise in impedance that causes the lever system to bend (it is mainly cartilage) and to be reduced in its efficiency (Manley 1972b).
FIG. 3.
Middle ear configurations and function. A Non-mammalian, single-ossicle type of middle ear. B Mammalian, three-ossicle type of middle ear. Both A and B are as seen in a transverse section of the head. Dorsal is upwards; lateral is to the right. T tympanic membrane or eardrum, EC extracolumella, Col Columella=stapes, FP footplate, Mal malleus, Inc Incus, St stapes. C Schematic of the function of the non-mammalian middle ear. The extracolumellar tip is at A (red) and an increase in sound (air) pressure moves this location inwards. The extracolumella rotates around a fulcrum at C (green). The columella inserts into the extracolumella at B (blue) and the columella with its footplate moves inwards with less amplitude but greater force than at A. On the lower right, the lever arrangements of the two middle-ear types are illustrated. A and B after Christensen-Dalsgaard and Manley, 2014.
The “Three-Ossicle” Tympanic Middle Ear
Mammalian ancestors evolved a different kind of middle ear by adding two elements from the old jaw joint to the columella, resulting in the three-ossicle middle ear that is one of the defining characteristics of mammals (review in Manley 2010). Thus, any amniote that has this kind of middle ear (and, as will be discussed, a correspondingly modified jaw joint) is a mammal, a definition based on bony structures that can be recognized in fossil material. Other features of mammals, such as milk glands to feed the young, and hair, are less useful with regard to interpreting fossils.
It is one of the remarkable coincidences of amniote evolution that just at the time when pre-mammals were evolving a middle ear, they were simultaneously re-organizing the almost contiguous jaw-joint region. All non-mammalian land vertebrates use a so-called primary jaw joint, it being the “original version” in these organisms (Carroll 1987). This joint is between the quadrate bone of the upper skull and the articular bone of the lower jaw. The lower jaw of non-mammals consists of up to seven bones (Fig. 2C). During the origin of true mammals, this was reduced to one single bone, the dentary. In mammals, this bone articulates in the—now secondary—jaw joint with the squamosal bone of the upper skull (Fig. 2D). During the transition, mammal ancestors had a double jaw joint (seen in fossils such as the appropriately-named Diarthrognathus, Carroll 1987).
Over time, the deeper-lying, primary joint between the articular and the quadrate bones was eliminated from the double jaw articulation and its component bones moved into the future middle-ear region (Fig. 2D). During the re-organization of the jaw joint, the upper skull was also reorganized, such that the hyomandibula (the same bone as the columella and in mammals called the stapes), which previous to that time was a massive bone that had prevented relative movement between the inner and outer bony regions of the upper skull during feeding, also became redundant. This bone retained its close approximation to the inner-ear region via its footplate but evolved articulations to the articular (now called the incus) that in turn retained its (old jaw joint) articulation with the quadrate (now called the malleus).
These events involved a region of the skull that lay just inside the jaw joint and thus lower in the head than the middle-ear region of non-mammals. It thus did not involve the ancestral spiracular space (Manley 2012) that makes up the middle-ear spaces of non-mammals. A new space around the new middle ear evolved independently, as did a connection to the mouth cavity (the Eustachian tubes, that are not homologous to the equivalent spiracular connections in non-mammals; Takechi and Kuratani 2010). This Eustachian tube connection is essential for maintaining normal air pressures in the middle-ear spaces, since the middle-ear epithelium is continuously absorbing gasses from the air. A new eardrum also evolved that lay at a more ventral position than that of the non-mammals, as demonstrated, e.g., by differences in middle-ear and tympanic membrane development in mammals and non-mammals (Kitazawa et al., 2014). The eardrum formed a tight connection to the malleus that evolved an “umbo,” an extension along the eardrum to—roughly—its center.
The newly-evolved and definitive mammalian tympanic middle ear in fact evolved at least twice, in the ancestors of therian mammals (pouched marsupials and placentals) and in the egg-laying monotremes, such as the echidnas and platypus. The monotremes had initiated a separate lineage at a time when all mammal ancestors already had the elements of middle ears, but these were not yet in their final configuration (Luo 2011). Interestingly, there was a long transition period between the actual origin of the mammalian three-ossicle middle ear connections and its final reduction in size and full emancipation from the other skull elements. Specifically, the malleus maintained a connection to the lower jaw over a long period of time, especially in (egg-laying) monotremes. This “transitional” middle ear has been the subject of intensive study (e.g., Luo 2011). In fact, in the embryos of modern monotreme mammals, the malleus only separates from the lower jaw just before birth.
Functionally, the three-ossicle middle ear is equivalent to its single-ossicle cousin in most respects. The lever system in mammals is a primary one, but the effect is the same, the area ratios are similar to those of non-mammals and the main effect is a very large improvement in auditory threshold. In mammals, just as in non-mammals, the middle ear works with little frictional loss (4–6 dB) up to the frequency at which the inner ear “runs out of frequencies” (Fig. 4). That is, at frequencies beyond the limit of hearing of the inner ear of the particular species, the middle ear system ceases to function well in sound transmission (Manley et al., 1972; Manley and Johnstone 1974; Ruggero and Temchin 2002).
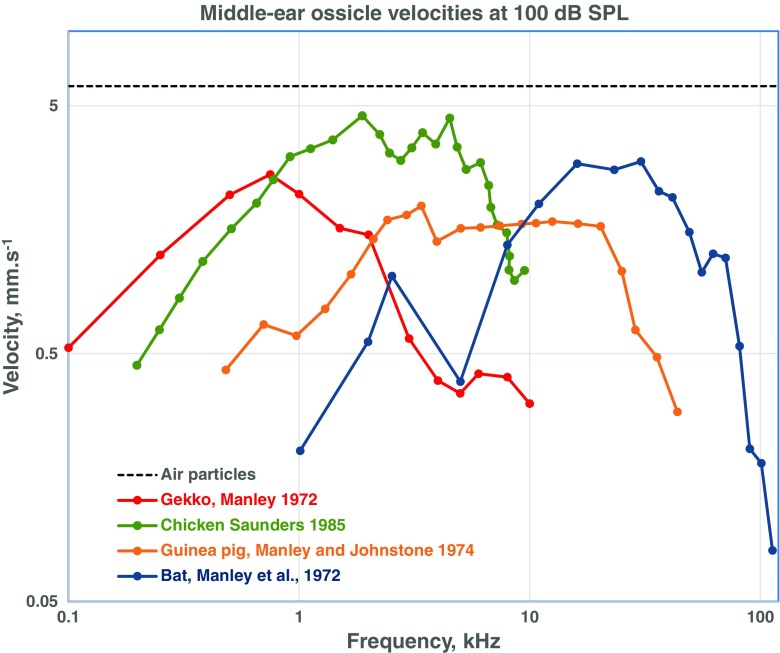
FIG. 4.
Middle-ear transmission characteristics of four species of land vertebrates, a lizard (Tokay gecko), the chicken, the guinea pig, and a bat (Eptesicus). Shown are (horizontal, dashed black line) the velocity of air particles as a function of frequency at 100 dB SPL, the stimulus driving the middle ears. In each species, the velocity of the center of the eardrum (tip of the extracolumella or tip of the malleus umbo) rises with frequency and falls again at higher frequencies. The upper frequency limit of good transmission is governed by the inner ear (see text).
Later, mammals evolved additional features not seen in non-mammals. First, at some point, in therian mammals, flaps of skin behind the external ear canal expanded and formed ear lobes, or pinnae that, in modern species, can funnel so much sound to the eardrum as to produce an additional 20 dB of hearing sensitivity. In non-mammals, pinnae did not evolve, although one group of birds, the owls, evolved feathery facial disks that can amplify sound input equivalently. Together with asymmetrical ears in some owls, the facial disk endows these birds with a unique sound-localization ability, enabling them to catch rodents in complete darkness (Konishi 1993).
Second, mammals evolved middle-ear spaces that, since they arose de novo and well after the origin of the different mammalian lineages, can differ quite strongly between groups. What are termed bullae are in fact not necessarily of the same origin and their support structures can be bony, cartilaginous, or even membranous (Novacek 1977). These bullae can sometimes be very large indeed and be responsible for improvements in sensitivity to sounds in particular hearing ranges (Heffner et al. 2001).
These quite remarkable evolutionary events resulted in a huge improvement in the transmission of sound from the outside world into the inner ear. This initiated changes in inner ears of all groups that greatly improved their ability to process the input. Those changes were in the hearing organ itself, which evolved in each group along unique trajectories and in almost all cases grew in size and processing capacity but also in the brain centers processing the input information (the latter is not part of this review).
Middle-Ear Function
Middle ears are machines that transmit sound from air to fluid. Of interest to the inner ear are the sound pressures, sound frequencies, and durations. Sound pressure is a reflection of the velocity of the air particles and, at any frequency, the velocity of air particles at a given sound pressure will, by definition, be constant. At 100 dB SPL, the velocity of air particles is 6 mm s−1. In Figure 4, the velocity of the equivalent structure of the middle ear (here, the tip of the extracolumella or the tip of the umbo of the malleus in mammals, both near the center of the eardrum) is shown at a sound pressure of 100 dB SPL across a range of frequencies. The dashed line at 6 mm.s−1 is the velocity of the air particles driving the eardrums. For each species, whether gecko, chicken, guinea pig, or bat, the colored functions show the absolute velocity of the eardrum tip as a function of frequency. The relatively flat portion of each function (prominent in the mammals) indicates the “loss” incurred mainly by frictional forces (i.e., how much below the dashed line do the functions lie), which is about 3 to 10 dB compared to the air particles. The differences between the functions of mammals and non-mammals in this respect are quite small. In other words, within their respective best frequency ranges, the middle-ear systems are equivalently “good.”
What is obviously different is the frequency range when compared between species. Whereas the non-mammals gecko and chicken show strong responses at a few hundred Hz, the mammals do not reach equivalent responses until about 1 kHz. At the other extreme, whereas gecko response levels are well down at 5 kHz and those of the chicken at 7 kHz, the guinea pig system is not reduced to the same levels until 40 kHz and the bat until 70 kHz. These large differences reflect the frequencies that are “accepted” by the inner ear. As noted above, frequencies outside this specific range in each species are not well transmitted through the middle ear. This reflects the fact that the hearing range of any given species is primarily determined not by the middle ear but by the inner ear (Manley 1972b; Ruggero and Temchin 2002). It is thus to the inner ear that we now must turn to elucidate the evolution of these differences in hearing range and in other features of the basilar papillae of amniote groups.
The Origins of Characteristic Differences in Inner-Ear Structure
The typical structural features of the inner-ear auditory papillae of different amniote groups that will be described below can be traced in their origin to relatively early time periods following the evolution of tympanic middle ears. First, in both birds and crocodilians, the structure of the hearing organ with regard, for example, to the presence and distributions of cell types is very similar. It is thus reasonable to assume that this kind of structural configuration had been reached or at least initiated at the latest near the time of separation of their ancestral lineages (160 Ma., Fig. 1). Similar reasoning can be applied to the lepidosaurs, in which we find that in the Tuatara, which separated early from the other lepidosaur lineages (Fig. 1), the typical cellular configuration of the papilla is simpler than that of lizard papillae. In the Tuatara, as in turtles, there is only one type of hair cell and all hair cells have the same orientation. The most parsimonious conclusion is that the more complex lizard-typical structure arose after the Tuatara lineage split from the squamates (lizards and snakes). It is, however, not known whether lepidosaurs (i.e., including Tuataras) evolved a tympanic middle ear only once. Lastly, in mammals, the typical structure of the hearing organ that we know as the organ of Corti is basically seen in all recent groups of mammals, with, however, some clear differences between the monotremes and the therian mammals. It can thus be assumed that the organ of Corti arose before these lineages split and therefore between 220 and 150 Ma.
The above structural comparison indicates that in each lineage, the evolution of a tympanic middle ear had profound consequences for the further evolution of inner-ear hearing organs. The reduction in the sizes of bones and the new configurations creating tympanic middle ears would have not only improved auditory transmission in general but especially that of higher frequencies. This additional input no doubt added selection pressures on the hearing organ to optimally transduce the information and transmit it to the brain. One might, of course, ask the question: If hearing is important and there are laws of physics governing how hearing systems can function efficiently, why does each group have its own, unique structural pattern? As we will see, answers to this question lie in the fact that, although because of their history these ears look different, in particular elements of the quality of their function, they are essentially the same. Of course, tiny hearing organs cannot achieve what a huge inner-ear papilla can achieve, but the qualitative differences seen in diverse modern representatives of many groups are in fact smaller than anticipated, as will be demonstrated below. The typical structural patterns of the auditory papillae of lepidosaurs, archosaurs, and mammals are so different (Figs. 5 and 6) that each needs to be discussed in turn.
FIG. 5.
Highly schematic illustration of the phylogenetic radiation of the form of the auditory papilla in amniote vertebrates (the scale bar on the top right is 1 mm). In general, the papillar size increased during the evolution of the three major lineages (lepidosaurs are here represented by the lizards, archosaurs by the birds), but the extent of the elongation differed greatly between groups. On the right are shown four examples of papillae from modern birds, the barn-owl papilla being the longest known (~12 mm). In therian mammals, the cochlea is coiled (left panel) to a different extent and length in different groups. In the egg-laying monotreme mammals, here represented by the spiny anteater (Echidna), however, the cochlea remained uncoiled and relatively short. In lizards (top panel), all cochleae are less than 2 mm in length and the length and shape are family-specific. Some papillae are even divided into sub-papillae (e.g., Lacertidae). Because the papillae are so small, each papilla outline drawing is also shown at ×2.5 magnification. Original diagram provided by Ulrike Sienknecht.
FIG. 6.
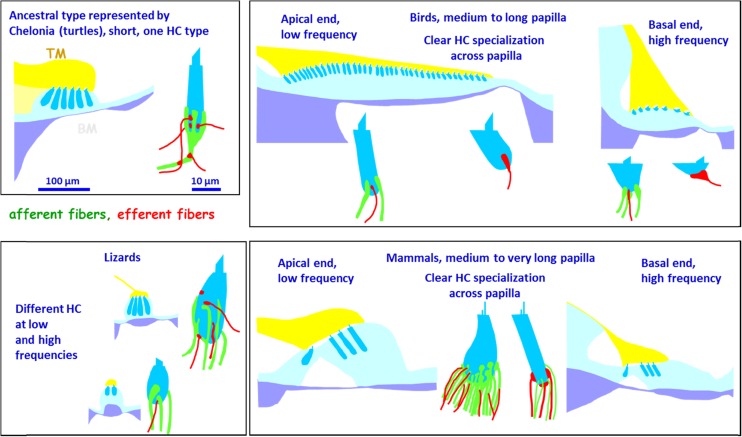
Schematic representations of transverse sections through the papillae of four groups of amniotes (BM basilar membrane—lilac, supporting cells—light blue, hair cells—dark blue, tectorial membrane—yellow), together with sketches of the hair-cell types encountered with afferent (green) and efferent (red) innervating nerve fibers. The top left panel shows the papilla of a turtle and its single type of hair cell. The lower left panel shows cross sections through the basal (top) and apical (bottom) papillae areas with low-frequency hair-cell type (below) and high-frequency hair-cell type (above). The bird papilla is illustrated in the top right panel, apical to the left and basal on the right. In each case, one neurally lying tall hair cell and one abneurally lying short hair cell is drawn. The mammalian organ of Corti is illustrated in the lower right panel, with an apical cross-section on the left and a basal one on the right. One inner hair cell, with many afferent fibers and tiny efferent endings and one outer hair cell with few afferent and large efferent endings, is shown. Modified after Manley and Köppl 1998 and used with permission.
The Hearing Organ of Lepidosaurs
The hearing papillae of lepidosaurs have remained relatively the smallest among modern amniotes (Manley and Köppl 1998). The sister subgroup to the lizards and snakes is formed by the Genus Sphenodon, the Tuatara, which is anatomically the most ancestral of the lepidosaurs. Its hearing papilla consists of a 1-mm-long patch of 225 hair cells whose bundles are all oriented in the same direction and are covered by a tectorial membrane (Wever 1978). This is known as a unidirectional hair-cell orientation pattern. The additional bidirectional areas known from lizards, and which have been lost again by snakes, apparently evolved following the split of their lineage from Sphenodon.
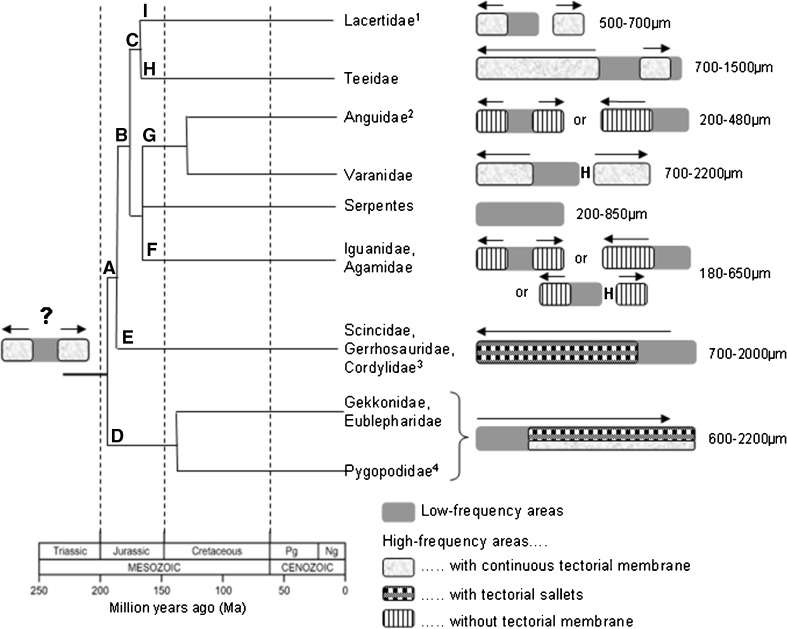
The morphology of lizard papillae has been very extensively documented, mainly by Wever (1978) using light microscopy, and Miller (1980, 1992) using scanning and transmission electron microscopy (SEM and TEM). Both authors reported family-typical configurations of the distributions of the two kinds of hair-cell areas, but only Miller recognized that not only was the bundle orientation different but also the morphology of the hair-cell bodies and even their innervation patterns differed systematically. Thus, even though as an exception the low-frequency hair-cell area of skink lizards is bidirectionally oriented in some groups, the hair cell structure is otherwise of the “unidirectional type” (Fig. 6; Miller 1992). One result of these studies was the ability to compare anatomies from many families with their times of divergence as derived from genetic data, as shown in Figure. 7. This led to the conclusion that by the time of their origin, the ancestral lizards had already evolved two new bidirectionally-oriented hair-cell areas, each of which flanked the central, ancestral, unidirectional area at the apical and basal ends. From physiological studies (see below), it was clear that the unidirectionally oriented hair-cell area responded only to low frequencies up to about 1 kHz. The new hair-cell areas responded to higher frequencies, leading to an extension of the hearing range of lizards beyond that known from the Tuatara (upper limit about 1 kHz; Wever 1978). The “new” frequency ranges were, however, initially represented twice as mirror-images at both ends of the papillae and were thus redundant. In subsequent evolutionary steps that led to a diverse group of lizard families, in many cases, one of these areas was modified or lost, resulting in a reduction or loss of redundancy and, in the latter case, a continuous tonotopic organization (Fig. 7). In snakes, only the ancestral hair-cell patch was retained—presumably a result of the secondary loss of the tympanic middle ear. Other interesting variations are found among the papillae of diverse lizard families, particularly involving the anatomical specialization of or even complete loss of the tectorial membrane. These changes had very interesting physiological consequences. One of the most important is that in lizards that lack a tectorial membrane and have so-called “free-standing” hair cells, the sensitivity and the selectivity of auditory-nerve afferents is much poorer (see below and Manley 2002; Manley and Köppl 2008 for reviews).
FIG. 7.
Schematic diagram of the relationships of some squamate families. The geological time frame and eras are shown on the bottom left. On this scale, squamates originated about 200 Ma, with most families coming into existence before the end of the Mesozoic, 65 Ma. Using the terminology of Vidal and Hedges (2009), the major nodes showing origins of major groups are as follows: (a) Unidentata; (b) Episquamata; (c) Laterata; (d) Gekkota; (e) Scinciformata; (f) Iguania; (g) Anguimorpha; (h) Teiformata; and (i) Lacertibaenia. Not shown on this diagram are the Dibamidae, which are a sister group to all other squamates and 20 additional lizard families that have as yet not played a role in lizard bioacoustic studies. Notes: (1) Amphisbaenia are included here, (2) Helodematidae belong here, (3) Xantusiidae belong here, (4) The closest relatives of pygopods are the diplodactyline geckos. The groups (f), (g) and the snakes (Serpentes) are all ancestrally equipped with poison glands and are now classified together under the group name Toxicofera. To the right of each lizard family’s name is a schematic sketch of the typical structure of the papilla in terms of the groups of hair cells (low and high-frequency) and their typical tectorial covering together with a range of lengths (mm) for the papillae in that family or those families. The diagram patterns and the types of tectorial membrane are shown below. “H” indicates that there is a hiatus along the papilla, creating two sub-papilla separated by a stretch of limbus; these patches lack hair cells. The sizes are estimated from Wever (1978) and are for fixed, embedded material (add 15 % for sizes in life). In each papilla, the basal end is to the left. The arrows above each papilla show the direction of tonotopicity in the high-frequency area (above 1 kHz). Pg paleogene, Ng neogene. After Manley (2011).
Wever (1978) also studied the hearing abilities of hundreds of lizard species using the technique for which he was at that time already well known, as the co-discoverer of the cochlear microphonic (Wever and Bray 1930). Since the cochlear microphonic is a summed potential representing the receptor potentials of many hair cells, it has no true threshold. To study hearing abilities, the lack of a true threshold is obviously a major disadvantage, but at least comparisons can be made between species and different frequencies, provided that in each papilla the number of hair cells representing any particular frequency range is the same and can thus be expected to produce equivalent signal amplitudes. Unfortunately, it is not but can vary tenfold between papillae. Generally, Wever chose the fixed level of 1 μV peak-to-peak for his lizard studies (Wever 1978). There is, however, an even more fundamental flaw in the reasoning behind using this technique on lizards, a singular disadvantage that Wever apparently did not recognize. The microphonic potentials from hair-cell areas that are bidirectionally oriented (i.e., all high-frequency responses) consist of two sets of signals that are 180° out of phase. If the two sets of hair cells having the opposite bundle orientation are precisely the same in size, there will be no signal at all, since complete cancelation can occur (Manley et al. 2001).
Thus, it comes as no surprise that Wever generally found the best “sensitivities” at frequencies below 1 kHz, where in almost all species the hair cells have the same orientation. Of course, as the sound level rises, hair cells whose best threshold is at 1 kHz will also respond to higher frequencies. Higher-frequency hair-cell areas are bidirectionally oriented, but often the arrangement of cells is not so regular (Köppl, 1988; Köppl and Authier 1995) and thus full cancelation of the out-of-phase components produced by hair cells of opposite orientations would not be expected. Nevertheless, Wever’s microphonic data tend to greatly overestimate the high-frequency thresholds. As an example, in the Tokay gecko, primary neural data at 2–3 kHz demonstrate thresholds that are 40 dB more sensitive than Wever’s CM criterion would suggest, although matching quite well at 500 Hz (Eatock et al. 1981). In sum, it is an unfortunate fact that the largest set of physiological data on lizard hearing is essentially impossible to interpret. The only clearly usable data are cases in which microphonic responses were measured before and after an intervention (such as the complete removal of the middle ear except for the columellar footplate)—in such cases, the difference in microphonic sensitivity is reliable (e.g., Werner and Wever 1972).
Subsequently, several authors have studied the responses of auditory-nerve fibers in different species of lizards (reviews in Manley 1990; Manley and Köppl 2008) and more recently, data on sensitivity and frequency selectivity have also been derived from the suppression of spontaneous otoacoustic emissions (SOAE; review in Manley and van Dijk 2008). Neural data showed that most lizard species hear from roughly 50 Hz to 5 kHz, but the responses of individual nerve fibers are temperature sensitive (Eatock and Manley 1981) and in general, daytime temperatures of many lizard species would be higher than those used in laboratory studies. In one subfamily of geckos, exceptionally high frequency responses up to 14 kHz were found (Manley and Kraus 2010). As noted below, when sensitivity and frequency selectivity of neural units of lizards are compared to those of other amniotes, the differences are small. The main exception to this is in those families in which the papillae are tiny (~100 μm) and the tectorial membrane has been completely lost (Fig. 8). The loss permits the retention of differentiated frequency responses despite the extremely small dimensions, but at the cost of a partial loss of selectivity and sensitivity (Authier and Manley 1995; Manley 1997).
FIG. 8.
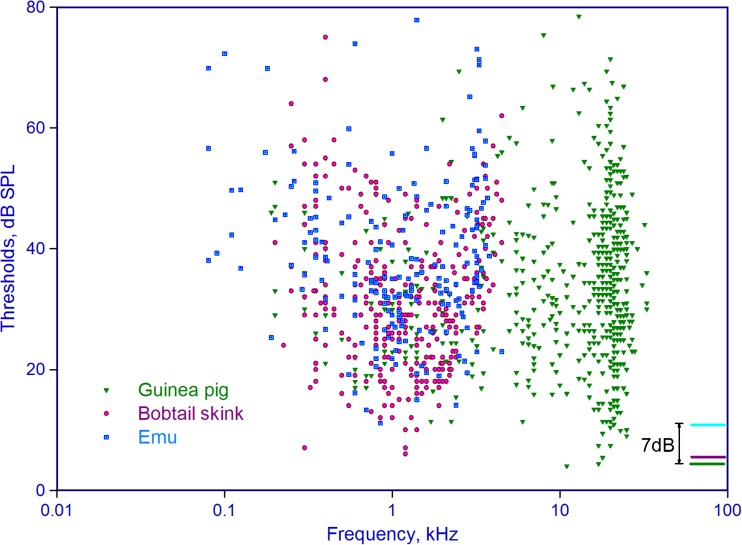
A comparison of the threshold response sound levels of many auditory-nerve fibers from a lizard, the Bobtail skink (red circles), the Emu (blue squares), and the Guinea pig (green triangles). On the lower right is a comparison of the sensitivity of the most sensitive fiber measured in each species.
In most lepidosaurs, the evolution of a middle ear was thus likely followed by the development of high-frequency responding hair-cell areas, in some cases in papillae up to 2 mm in length and with 2000 hair cells. Each lizard family lineage shows typical anatomical patterns, presumably the result of variations in the specific selective pressures influencing papillar evolution (Manley 2002). Some of the anatomical specializations permitted even the tiniest of papillae to retain frequency-selective responses up to and beyond 5 kHz. The most specialized papillae are found in the only nocturnal family of lizards, the geckos (Manley and Köppl 2008).
One of the most remarkable features of lizard ears is that they almost always robustly produce SOAE and the rates of occurrence hugely exceed those known from archosaurs and mammals (Manley 1997; Manley and Köppl 2008). SOAE are observed only over the range of the high-frequency hair-cell area(s). Their suppression tuning curves are as sensitive and as frequency selective as (indeed in their shape identical to) neural tuning curves of primary afferent fibers (Köppl and Manley 1994). The SOAE show behaviors (e.g., their calcium sensitivity, Manley et al. 2004) that were predicted by in-vitro studies of active processes in isolated hair cells or small groups of hair cells (reviewed in Hudspeth et al. 2000), providing an additional, strong indicator that SOAE are generated by hair cells. Furthermore, the unique bidirectional orientation patterns of the hair-cell areas made it possible to demonstrate that the SOAE are produced by the hair-cell bundles (Manley et al. 2001) and not as in therian mammals by prestin molecules changing the length of hair cell somata (Zheng et al. 2000; see below).
The Hearing Organ of Archosaurs
The evolution of a tympanic middle ear in the ancestral archosaurs also resulted in an elongation of the auditory papilla and in modern species, lengths between 2 and 4 mm in small birds and crocodilians and up to 12 mm in the barn owl are known (Gleich et al. 2004). Studies of related fossil species such as dinosaurs show cochlear dimensions that fall near the lower end of this range and such data has been used to estimate the hearing ranges of fossil species (Gleich et al. 2005; Walsh et al. 2009). In archosaurs, unlike in lepidosaurs, no new kind of hair cell evolved. Instead, a gradation of hair-cell dimensions across the width of the—widening—papilla was established. The hair cells are not arranged in systematic rows but, with supporting cells between, in a complex cell mosaic. The papillae of modern archosaurs are in general the widest of all amniote papillae, especially at the apical (low frequency) end, where there can be up to 50 hair cells in a transverse section (in the emu, Fischer 1998). Because of the papillar width and the fact that hair cells of all groups are roughly the same size, the total number of hair cells in archosaurs can be very high (e.g., 16,300 in the barn owl, Fischer et al. 1988 innervated by 31,000 afferents, Köppl 1997a) in spite of the papillae not being very long. The hair cells near the neural edge of the papilla are taller—sometimes much taller—than the hair cells at the abneural edge, with a steady gradation between the extremes in birds and a less steady gradation in crocodilians (Gleich et al. 2004). The hair-cell height also falls towards the base of the organ, with the net result that the tallest of the hair cells lie apical-neural, the shortest (that may be only 3 μm high) lie basal-abneural (Fig. 6).
The above pattern of hair-cell size led early to the use of the terms “tall” and “short” hair cells (review in Smith 1985), but this was a continuum and nothing was known that could clearly separate them. Thus, the arbitrary decision was made to define “tall” as being a cell that is taller than it is wide. Nonetheless, it was suspected that there must be some functional correlate of the size difference. Much later, Fischer (1992) discovered using TEM that in the chicken, the most abneural hair cells completely lacked connections to afferent auditory nerve fibers. Instead, the efferent fibers, that make only small bouton synapses on the taller hair cells, make very large synapses on these short hair cells. Fischer thus suggested ignoring the height gradient but keeping the term “short hair cell” for cells lacking afferent connections. This lack of an ability to communicate sensory responses to the brain is extremely unusual for any sensory receptor and seems to be quite illogical. Across the tall hair-cell area (roughly the neural half of the papilla), there was an early indication that the sensitivities of connecting afferents changed systematically, being the most sensitive near the neural edge of the papilla (the range of sensitivities between auditory nerve fibers is large; Gleich 1989). This suggested that abneural areas of the avian papilla were insensitive to sound.
At that time, information concerning the ability of vestibular hair-cell bundles to amplify stimuli (Hudspeth et al. 2000) suggested that avian hair cells lacking afferent connections may represent a special, micromechanically-active population acting as sound amplifiers (Manley et al. 1988). Modeling the function of the avian papilla on the assumption of mechanically active short hair cells suggested that they would be able to actively move the tectorial membrane in a radial direction. Due to its particular anatomy, that would lead to the largest amplitudes of motion (and thus the highest cell sensitivities) near the neural edge of the papilla (Steele 1997), as found by Gleich (1989).
The discovery of SOAE in a bird, the barn owl, indicated that indeed, at least some of the hair cells were spontaneously micromechanically active. Suppression of the SOAE produced tuning curves (suppression tuning curves, STC) that were, as in lizards, as sensitive and as frequency selective as afferent neural tuning curves (Taschenberger and Manley 1997). Much more recently, studies of avian hair cells suggest the involvement of the avian variety of the molecule prestin in this activity (Beurg et al. 2013). In general, the avian papilla is clearly a highly specialized hearing organ that provides a great deal of information on the acoustic environment to the brain (review in Köppl 2015). The case of the barn owl indicates that in some species, profound re-arrangement of the organ’s anatomy has occurred, producing an acoustic fovea that provides much space for analyzing the frequencies used in the localization of prey (Köppl et al. 1993). A more recent study suggests that Kiwi also may possess an acoustic fovea (Corfield et al. 2011).
The accumulated evidence indicates that archosaurs have evolved a papilla that has hair-cell patterns indicating a division of labor between those cells responding to sound and transmitting the data to the brain and a second set that perhaps uses both prestin and also an active bundle mechanism to amplify the input stimulus and alter the mechanics of the papilla. This is a remarkable case of parallel evolution to the phenomena known from therian mammals (see below). Although it is clear that in birds the two hair-cell populations have distinct innervation patterns, we do not yet know whether with regard to their contributions to active processes these are two fully distinct sets of cells.
Earlier data from pigeon physiology (Schermuly and Klinke 1990), behavior (Kreithen and Quine 1979) and more recently from chicken behavior (Hill et al. 2014) indicate that, in contrast to both lizards and most mammals, at least some birds are sensitive to extremely low frequency sounds (2 Hz). Assuming that all of the sensitivity is due to the auditory papilla, this brings their hearing abilities into a frequency range that may be the result of self-induced pressure (i.e., altitude) changes during flight. An alternative has been suggested that at least pigeons may use their sensitivity to infrasonic frequencies that are transmitted over huge distances for orientation in space (Hagstrum and Manley 2015).
One final interesting feature of avian papillae deserves mentioning; avian hair cells can be regenerated from supporting cells if they are damaged and die. This ability is perhaps the result of the lower degree of supporting-cell specialization as compared to the mammalian cochlea (Cotanche 1999). Even without damage, this regenerative capacity may compensate for degenerative aging effects in the cochlea of birds (see Köppl 2015 for a review).
Thus, although restricted in its response frequency range at the high-frequency end to between 4 kHz (in, e.g., crocodilians, Manley 1970; Smolders and Klinke 1986) and 12 kHz (in barn owls, Köppl 1997b), which is fully comparable to lizards, the archosaur papilla represents a highly evolved hearing organ of unique structure and, probably, unique functional details.
The Hearing Organ of Mammals
So much has been written about the cochlea of mammals that this review concentrates on evolutionary and unique aspects. As noted previously (Manley 2012), it is the structure of the organ of Corti that clearly characterizes the mammalian cochlea. Since the uncoiled cochlea of egg-laying (monotreme) mammals also has an organ of Corti, the organ’s typical structure almost certainly long preceded cochlear coiling in the therians: Note that the monotreme lineage diverged from the therian lineage well before cochlear coiling evolved (Fig. 1). The monotreme cochlea has inner hair cells, pillar cells forming a tunnel of Corti and outer hair cells (for a review, see Vater et al. 2004). These cell types, are, however, each represented in more numerous rows than in therian mammals. There are several rows of inner hair cells, at least two sets of inner and outer pillar cells, etc. Because of this, and despite the relative shortness of the monotreme cochlea (<7 mm), the total number of inner hair cells (2700 in the spiny anteater, Ladhams and Pickles, 1996) rivals the numbers seen in cochleae of cats and humans. In fact, the therian cochlea may represent a monotreme-like cochlea that elongated after coiling by re-arranging the cell groups into the smaller numbers of rows typical for therians.
The therian coiled cochlea first formed after the innovation of the three-ossicle middle ear that defines mammals (review in Manley 2012) and just before the two therian groups, marsupials and placentals, split into separate lineages (Fig. 1). At that time, the cochlea was only about 4 mm long. Since the cochlear structure of therians is highly uniform, it is likely that their typical cellular arrangement, with few hair-cell rows, arose before this split. As is well known, the mammalian organ of Corti has a prominent division of labor between the sound-receptor inner and the amplifying outer hair cells, with a concomitant differentiation of the afferent and efferent innervations. As noted above, an independently-evolved but perhaps similarly-acting division of labor is seen in archosaurs. Mammals also show other cochlear specializations that are also seen in birds, such as differences in length (from about 6 to 70 mm) and the evolution of acoustic foveae in certain bats (Vater et al. 2004).
It has been speculated that some therian ancestors of ~150 Ma that possessed ~4 mm uncoiled cochleae (that perhaps still contained a lagena, restricting the length of the basilar membrane to <3 mm) already processed ultrasonic frequencies (e.g., Luo et al. 2010; Ruf et al. 2009). However, this is unlikely. This speculation, based solely on the presence of bony secondary laminae associated with the apomorphy of the bony “invasion” of the hearing organ (Manley 2012), does not take into account that other major evolutionary steps were necessary before the modifications enabling high-frequency hearing were completed. These were, for example, changes in the membrane protein prestin permitting high-frequency responses, changes in the biochemistry and biophysics of the stria vascularis, and changes to the tectorial membrane and the transduction apparatus that would have followed the later loss of the lagenar macula and the resulting fall in calcium concentrations (Manley 2012). Monotreme hearing is limited to frequencies below 15 kHz, even though the cochlea is 2–3 times longer than cochleae of mid-Mesozoic species.
The earliest fully coiled cochlea is attributed to marsupials of the late Cretaceous (about 65 Ma., Meng and Fox, 1995). Coiled cochleae are characterized by the integration of the organ of Corti and of the peripheral neural elements into a bony complex, which provided laminae as stiff supports for the basilar membrane and surrounded in bone the nerve fascicles that then enter the cochlea through the cribriform plate. The coiling of the cochlea thus made it possible to supply neural innervation from a central modiolus and have afferents of similar lengths to all frequencies. Although the latter has been emphasized as of advantage (Fox and Meng, 1997), it is unlikely to have been an important selection pressure for coiling, since in modern mammals, fiber diameters (that influence conduction velocities) are not identical, varying in the rat from 1 to 3 μm (Perge et al., 2012) and in the guinea pig from 1.5 to 4 μm (Gleich and Wilson 1993). Conduction velocities in myelinated axons increase disproportionately with the fiber diameter, suggesting that variations in fiber diameter can, if advantageous, easily compensate for differences in axon length.
The further evolution of the cochlea was specific to the different sub-groups of mammals. Mammals diverged hugely during several subsequent geological periods (Bininda-Emonds et al. 2007) and despite the relatively constant basic structure of their cochleae, differences in cochlear length and various specializations did evolve. Not only did cochleae attain very large differences in lengths but the two groups pursuing the localization of prey using ultrasonic frequencies in air (bats) and high ultrasonics in water (toothed whales) independently evolved the same specializations of the molecule prestin that correlate with this ability (Li et al. 2010; Liu et al. 2010). Some bat species also evolved acoustic foveae, cochlear regions in which the length of the organ of Corti devoted to specific, narrow frequency ranges is greatly enlarged (Vater et al. 2004).
Comparing the “Performance” of Different Types of Amniote Inner Ear
As was noted above, the two kinds of tympanic middle ear are, within their particular frequency ranges, fully comparable in their functionality. Thus, differences in hearing abilities must largely be due to the performance of the inner ears. Such a comparison is to some degree difficult, since it can usually only be made using studies of single elements such as auditory-nerve fibers. Thus, such comparisons fail to provide information on perhaps profound differences that may only be obvious at the behavioral level, especially signal processing that requires large numbers of neurons that are not found, for example, in the small brain centers of lizards. Nonetheless, some comparisons are possible at a superficial level. Comparing the sensitivity of auditory-nerve fibers between three unspecialized members of the lizards (the Bobtail skink, Tiliqua), birds (the emu, Dromaius) and mammals (the guinea pig, Cavia) show that the sensitivity of the most sensitive fibers of each species are within maximally 7 dB of each other (Fig. 8). Given that small calibration differences in different experimental set-ups and different ear canals may fully account for these minor differences, the remarkable conclusion is reached that the sensitivity of amniote inner ears is—despite all the complex anatomical differences noted above—the same (Manley 2000). This of course ignores any advantages gained later in evolution from having external sound collectors such as mammalian pinnae (see above).
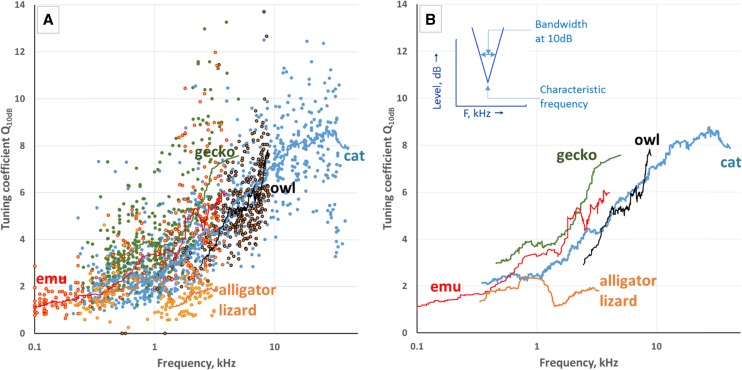
A comparison of frequency selectivity at the level of the auditory nerve is also instructive. All vertebrate hearing organs carry out frequency analysis, mostly by spreading the frequency sensitivities of the individual cellular elements along a monotonic tonotopic gradient (Manley and Köppl 1998). Figure 9 compares frequency selectivity of single auditory afferent fibers in different species by calculating the Q10 value. This is a common measure of selectivity calculated by dividing the best frequency of a single-neuron tuning curve (say, 1 kHz) by the bandwidth between the tuning curve flanks at the level of 10 dB above the best threshold (Fig. 9 insert in the right panel). If that bandwidth is 400 Hz, the Q10 at 1 kHz is 2.5. A narrower bandwidth would of course produce a higher Q10 value (sharper tuning) and vice versa. Figure 9 compares the frequency selectivities of a large number of single fibers recorded from the auditory nerve in the Tokay gecko (Gekko), a lizard with a well-developed papilla, the emu (Dromaius) and the barn owl (Tyto), unspecialized and specialized birds, respectively, and the cat (Felis), a moderately specialized mammal. All of these species have a tectorial membrane over the hair cells. For comparison, the equivalent nerve data from the Alligator lizard (Gerrhonotus, now known as Elgaria) in which the hair-cell bundles are free of tectorial material (extracted from Weiss et al., 1976) are shown. The right-hand panel shows only locally-weighted fits to each of the species’ data clouds; the individual data points have been removed. In keeping with the fact that the audible frequency ranges of these species differ, the regression curves appear displaced along the frequency axis. The range of Q10 values that each species displays is, however, with the possible exception of low-frequency data from the emu and the clear exception of data from the alligator lizard, essentially the same. The range in no way correlates with the huge anatomical differences described above in the different amniote groups. Since a number of morphological features are involved in tuning selectivity (such as the space devoted to a single octave, the pattern of afferent innervation, the form of the tectorial membrane), Figure 9 is not intended as a general analysis of tuning in the three groups of amniotes. Its purpose is more to show that equivalent sharp frequency tuning has been achieved by representatives of all groups. The fact that the gecko shows the sharpest tuning between about 3 and 5 kHz is likely to be due to the fact that its tectorial membrane is divided into small, semi-independent sections called sallets and this is likely to reduce coupling to neighboring frequency regions. Mammals and birds, in comparison, have only continuous, often thick, tectorial structures. The poorest tuning is shown by the Alligator lizard, illustrating one of the effects of the lack of a tectorial membrane (Authier and Manley 1995; review in Manley and Köppl 2008).
FIG. 9.
A comparison of the tuning coefficients (Q10dB; the method of calculation is shown at the top of B) of many auditory nerve fibers in representative species of different amniote groups; a lizard (Tokay gecko, own original data in green), two birds, the Emu (own original data in red) and the Barn owl (original data courtesy of C. Köppl in brown), and one mammal, the cat (original data courtesy of C. Liberman in light blue). Additionally, data are shown for a lizard species that lacks a tectorial membrane, the Alligator lizard, in yellow (extracted from Weiss et al. 1976). In A, each data point represents the Q10dB value for a single auditory-nerve fiber and the appropriately colored lines are locally-weighted regressions. In B, only the locally-weighted regressions are shown.
The comparison of these two quite basic features of all hearing organs suggests that these functions are based on common elements of papillae—such as hair cells—that, despite other morphological differences, retain their basic physiology unchanged. Seen on this level, the 300 million years of separate evolution of lepidosaurs, archosaurs, and mammals apparently led to only small differences. Thus, questions as to the importance of any observed differences in morphology need to be posed in other ways. The most obvious, and major, difference between the groups is the generally higher upper frequency limit of mammals, a probable result of selection pressures that arose through the integration of bone into the organ of Corti, itself possibly a response to the newly-evolved, stiff mammalian middle ear. This difference should not, however, be over-emphasized. The birds and lizards with the highest upper frequency limits (~12 and ~14 kHz, respectively) are equivalent to the upper limits of some mammals (e.g., the Elephant, at 12 kHz, Heffner and Heffner 1982; Mole rat at 12.6 kHz, Müller et al., 1992). This is, of course, not just a question of huge animals hearing low frequencies. Some of the lowest hearing is shown by small rodents (chipmunks, groundhogs, and hamsters hear below 100 Hz; Heffner et al. 2001), and the highest upper frequency limits in mammals (well over 100 kHz) are also shown by some very large marine mammals (Ketten 2000).
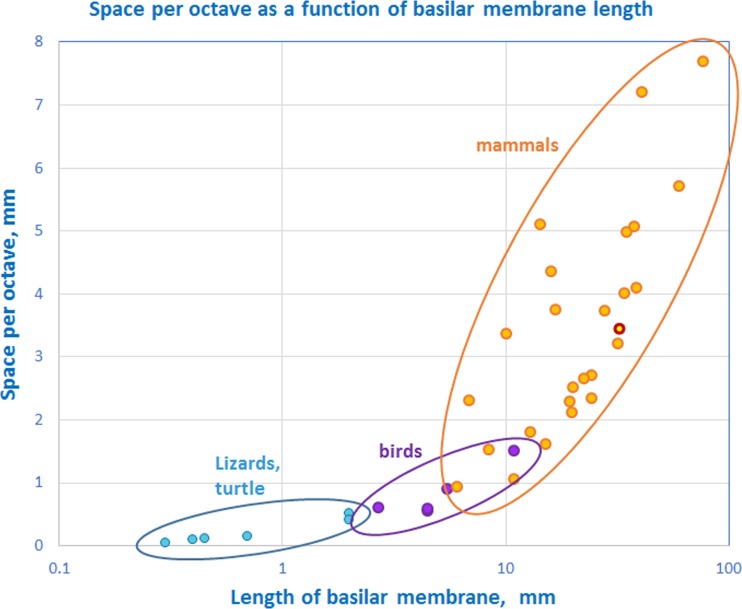
In general, there seems to be a dichotomy between those mammals with poor low-frequency hearing (mostly small mammals such as rodents and bats) and those with good low-frequency hearing (a wide variety of mammals of all sizes, including some rodents; Heffner and Heffner 1982). Thus, many small mammals have evolved cochleae in which the frequency map at the low end begins first at between 0.5 and 1 kHz (Heffner et al. 2001). The loss of the lower-frequency octaves (that, due to the octave-based tonotopic distribution, require much space) in these lineages was presumably essential to provide enough space for the high-frequency octaves. Thus, the mammalian cochlea is a flexible instrument in which the frequency limits and the total length of the cochlea strongly influence the amount of space devoted to particular frequency ranges. These features have been molded by evolutionary forces within each group and produced specific cochlear configurations that—apart from being a very useful, general-purpose source of information on potentially important survival cues—also transduces the sound information of particular use to that group, such as for communication signals. Between mammal species, the space available per octave varies greatly (Fig. 10).
FIG. 10.
The space per octave in millimeters as a function of the length of the hearing organ for different species of lizards and a turtle (blue dots), birds (purple dots), and mammals (orange dots). The data point for humans has a red surround. Modified after Manley 1971, updated and supplemented.
What About Humans?
Of particular interest to humans is of course what is known about the evolution of hearing in the group known as primates and, within that group, in our closest relatives, the hominids. Fortunately, in recent years, much new information has been gleaned from comparative studies of primate cochleae, although anatomical knowledge exceeds physiological studies. In 1969, on the basis of comparisons of audiograms of different species of living primates, and based on the assumption that the audiograms of living representatives of more ancient groups have remained unchanged over long periods of time, Masterton et al. 1969 wrote: “Low-frequency hearing improved markedly in mankind’s line of descent, but the kind and degree of improvement are not unique among mammalian lineages. High sensitivity developed in the earliest stages of man’s lineage and has remained relatively unchanged since the simian level. The frequency of the lowest threshold has declined in Man’s lineage.”
More recent studies provide extensive information on cochlear dimensions among primate groups and lead to similarly interesting conclusions. An analysis of 10 primate taxa revealed that the volume of the cochlea negatively correlates with the high frequency limit of hearing, i.e., large cochleae have low upper-frequency limits. Remarkably, this is independent of body mass and phylogeny, suggesting that cochlear size is functionally related to the range of audible frequencies in primates (Kirk and Gosselin-Ildari 2009). The most ancestral primates and their relatives (such as tree shrews, Tupaia) had good high-frequency but relatively poor low-frequency hearing. In the lineage of monkeys and apes, cochlear size increased, and low-frequency hearing improved (Coleman and Boyer 2012). Between the more ancestral gibbons and their relatives and the hominid lineage (that includes Gorilla, Chimpanzees, extinct representatives of the human line and humans themselves), there was an additional, significant jump in cochlear size that correlates with further improvements in low-frequency hearing (Braga et al. 2015). To quote Braga et al. (2015): “Premodern (Homo erectus) and modern human cochleae ... show cochlear relative lengths and oval window areas larger than expected for their body mass, two features corresponding to increased low-frequency sensitivity more recent than 2 million years ago..... The cochlea in the genus Homo is uniquely hypertrophied.” It is thus not surprising that humans have one of the lowest upper-frequency limits of all mammals. Thus, even after human ancestors separated from chimpanzee lineages about 7 Ma ago (Diamond 2002), it is possible that significant changes in cochlear morphology occurred. Do these evolutionary trends indicate something special about modern hominid cochleae?
There are claims that the frequency selectivity of the human cochlea is unexpectedly high (Shera et al. 2002), about twice as high as measured directly in the auditory nerve of, for example, the cat. This is surprising, since so far the cat has rated as a moderately specialized mammal and since physical principles dictate that any sharpening of tuning selectivity comes at the price of a loss in the ability to distinguish signals closely-spaced in time. It is not possible to place sharp microelectrodes in the auditory nerve of living humans, so other techniques need necessarily be used to assess frequency selectivity. Since selectivity is also dependent on the sound-pressure level used to measure it, any technique should ideally use as low sound pressures as technically possible. Shera et al. (2002) used a complex technique that involves first inducing a small otoacoustic emission at many different frequencies sequentially (stimulus-frequency otoacoustic emissions, SFOAE) and then suppressing these using a close-by added tone while measuring the amplitude and phase of the SFOAE. The authors argue that this is a valid measure of cochlear tuning selectivity. Interestingly, the values the authors measured correlate with the frequency spacing that is observed between peak amplitude frequencies of human SOAE; these correlated data are thus clearly indicating something detailed about cochlear function (Manley et al., 2015). But are they usable as indicators of frequency selectivity at the level of the auditory nerve, the input to the brain?
Other techniques have also been used to measure selectivity, but they depend on the conscious responses of experimental subjects, judging whether a tone they are presented with has or has not been masked by another, added tone, and thus involve the additional activity of brain centers. Such psychoacoustical data indicate a fairly constant and moderate, certainly not very high, selectivity (e.g., Glasberg and Moore 1990; Eustaquio and Lopez-Poveda 2010). A technique that is potentially of great import is one that has been used widely in non-mammals, namely the suppression of SOAE. As noted above, in many lizard species and in the only bird species that is known to show SOAE, the barn owl, the shape, and the tuning selectivity of suppression tuning curves (STC) of SOAE match very well the same data as measured directly for single auditory-nerve afferent fibers. Figure 11 shows samples of the two data sets for the Bobtail skink Tiliqua (Köppl and Manley 1994).
FIG. 11.
A comparison of the shape and selectivity of the frequency tuning curves of auditory nerve fiber thresholds (left panel) and the 3 dB suppression tuning of single spontaneous otoacoustic emissions (right panel) for the Bobtail skink Tiliqua rugosa. After Manley and Köppl 2008.
Since primates seem to be the only mammalian group whose representatives produce many SOAE peaks in spectra measured in the external ear canal, and humans show a very high incidence of SOAE (Lonsbury-Martin and Martin 2008), these can be used as an indirect measure of cochlear tuning selectivity. This is not a new idea, but curiously, it has never been systematically applied. In the 1980s and 1990, four groups examined STC for—in each study—just a few human SOAE and the frequency range covered in all studies was similar and narrow (Schloth and Zwicker 1983; Rabinowitz and Widin 1984; Frick and Matthies 1988; Zwicker and Peisl 1990). In a recent study, we examined STC of 63 SOAE at frequencies across a wide range of frequencies up to above 10 kHz in young humans (Manley and van Dijk, 2016). The STC had best sensitivities close to the individual person’s auditory threshold at that frequency; in other words, the tips of the STC were measured very close to behavioral thresholds. Overall, the frequency selectivity was, as in the psychoacoustical studies, moderate and not especially high. In the same frequency range, these data match those of the earlier studies of human SOAE-STC. Interestingly, a recent study of the suppression tuning selectivity of SFOAE showed, at least in two frequency ranges, that the STC selectivity was also only moderate (Charaziak et al. 2013). Since in all non-mammalian species, SOAE-STC selectivity and neural selectivity match quite well, there is no obvious reason as to why the human STC data are not applicable as an estimate of cochlear neural selectivity.
Interestingly, when the human SOAE-STC data are compared to neural data from the Macaque monkey (a reasonably closely-related primate), the human selectivity is actually higher at frequencies below about 2 kHz but progressively lower at increasingly high frequencies (Fig. 12). Since human speech mainly contains lower frequencies (Fig. 12), it can be speculated that in the human lineage, the processing of speech signals has acted as a selective pressure on cochlear evolution. If so, the time available was relatively short, since languages certainly did not evolve until well after the human lineage split from the closest hominid relatives, the chimpanzees, about 7 Ma ago (Diamond 2002) and perhaps evolved in much less than the last 1 Ma. Is this realistic? There is no doubt that the efficient processing of language signals was not only critically important to human survival but certainly one of and perhaps the most important selective pressure ensuring the fantastic success of humans after what Diamond (2002) calls the “Great leap forward” that occurred 40,000 years ago.
FIG. 12.
The frequency tuning selectivity of the Q3dB selectivity of suppression tuning of single spontaneous otoacoustic emissions in humans (red dots and red fit line, after Manley and van Dijk, 2016 and used with permission) and a fit to the Qerb of single auditory-nerve fibers in the Macaque monkey (blue line, after Joris et al., 2011). The green curve represents the speech spectrum for the German language averaged over a period of 1 min (spectrum from http://www.proav.de/index.html?http&&&www.proav.de/audio/speech-level.html).
Although in the past evolution has been regarded as a slow, methodical process, more recent evidence indicates that this impression is a distortion due to the fact that most evolutionary selective pressures are in fact stabilizing ones that tend to maintain the status quo. Species such as the well-known Horseshoe crab, known from today and from the Palaeozoic, have remained unchanged over vast periods of time. On the other hand, some modern studies paint a totally different picture. For example, the cichlid fishes of some lakes in Africa, where there are hundreds of species unique to each lake, have all arisen during the last 250,000 years (Meyer et al. 1990). In a similar vein, a recent study of a population of Darwin’s finches demonstrated that as the result of a prolonged drought, easily measurable changes in the size of body parts occurred within 2 years—at most a few generations (Grant and Grant 2000). In humans, although the generation times would be ten times longer than in the fishes, the issue at stake is not the derivation of hundreds of new species, but the modification of a sense organ to best process communication signals vital to survival and thus to reproduction. Are several thousand generations enough? Whether the human cochlea has evolved parallel to the development of speech is a fascinating question that may be open to test by future research.
Acknowledgments
The themes of this review were the basis of the Award of Merit address at the 2016 Midwinter Meeting of the Association for Research in Otolaryngology. I thank the ARO for the honor of the award, the many colleagues, and students whose cooperation over 45 years helped in the development of the ideas expressed in this review and Christine Köppl and Pim an Dijk for their introductions to my talk. Special thanks also to Ulrike Sienknecht for creating two of the figures and the Deutsche Forschungsgemeinschaft (DFG) that funded most of the comparative work of my research group.
Compliance with Ethical Standards
Conflict of Interest
The author declares that he has no conflict of interest.
Footnotes
This paper was given as the Award of Merit lecture at the 2016 ARO Midwinter Meeting in San Diego.
References
- Authier S, Manley GA. A model of frequency tuning in the basilar papilla of the Tokay gecko, Gekko gecko. Hear Res. 1995;82:1–13. doi: 10.1016/0378-5955(94)00138-G. [DOI] [PubMed] [Google Scholar]
- Békésy G (1960) Experiments in Hearing. In: Wever EG (ed) McGraw-Hill, New York
- Beurg M, Tan X, Fettiplace R. A prestin motor in chicken auditory hair cells: active force generation in a nonmammalian species. Neuron. 2013;79:69–81. doi: 10.1016/j.neuron.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bininda-Emons ORP, Cardillo M, Jones KE, MacPhee RDE, Beck RMD, et al. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- Braga J, Loubes J-M, Descouens D, Dumoncel J, Thackeray JF, et al. Disproportionate cochlear length in genus Homo shows a high phylogenetic signal during apes’ hearing evolution. PLoS One. 2015 doi: 10.1371/journal.pone.0127780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown W. Undoing the demos. New York: Zone Books; 2015. [Google Scholar]
- Carroll RL. Vertebrate palaeontology and evolution. New York: Freeman; 1987. [Google Scholar]
- Charaziak KK, Souza P, Siegel JH. Stimulus-frequency otoacoustic emission suppression tuning in humans: comparison to behavioral tuning. JARO. 2013;14:843–862. doi: 10.1007/s10162-013-0412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Manley GA. The malleable middle ear: an underappreciated player in the evolution of hearing in vertebrates. In: Köppl C, Manley GA, Popper AN, Fay RR, editors. Insights from comparative hearing research. New York: Springer; 2014. pp. 157–191. [Google Scholar]
- Christensen-Dalsgaard J, Brandt C, Wilson M, Wahlberg M, Madsen PT. Hearing in the African lungfish (Protopterus annectens): pre-adaptation to pressure hearing in tetrapods? Biol Lett. 2011;7:139–141. doi: 10.1098/rsbl.2010.0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack JA. Patterns and processes in the early evolution of the tetrapod ear. J Neurobiol. 2002;53:251–264. doi: 10.1002/neu.10129. [DOI] [PubMed] [Google Scholar]
- Clack JA, Ahlberg PE, Finney SM, et al. A uniquely specialized ear in a very early tetrapod. Nature. 2003;425:65–69. doi: 10.1038/nature01904. [DOI] [PubMed] [Google Scholar]
- Coffin A, Kelley M, Manley GA, Popper AN. Evolution of sensory hair cells. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer; 2004. pp. 55–94. [Google Scholar]
- Coleman MN, Boyer DM. Inner ear evolution in primates through the Cenozoic: implications for the evolution of hearing. Anat Rec. 2012;295:615–631. doi: 10.1002/ar.22422. [DOI] [PubMed] [Google Scholar]
- Corfield J, Kubke MF, Parsons S, Wild JM, Köppl C. Evidence for an auditory fovea in the New Zealand kiwi (Apteryx mantellii) PLoS One. 2011;6:e23771. doi: 10.1371/journal.pone.0023771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotanche DA. Structural recovery from sound and aminoglycoside damage in the avian cochlea. Audiol Neurootol. 1999;4:271–285. doi: 10.1159/000013852. [DOI] [PubMed] [Google Scholar]
- Darwin CR, Wallace AR. On the tendency of species to form varieties; and on the perpetuation of varieties and species by natural means of selection. J Proc Linn Soc Lond Zoology. 1858;3:45–50. doi: 10.1111/j.1096-3642.1858.tb02500.x. [DOI] [Google Scholar]
- Diamond J. The rise and fall of the third chimpanzee. London: Vintage books; 2002. [Google Scholar]
- Dobzhansky T. Nothing in biology makes sense except in the light of evolution. Am Biol Teach. 1973;35:125–129. doi: 10.2307/4444260. [DOI] [Google Scholar]
- Eatock RA, Manley GA. Auditory-nerve fibre activity in the tokay gecko: II. Temperature effect on tuning. J Comp Physiol A. 1981;142:219–226. doi: 10.1007/BF00605740. [DOI] [Google Scholar]
- Eustaquio-Martin A, Lopez-Poveda EA. Isoresponse versus isoinput estimates of cochlear filter tuning. J Assoc Res Otolaryngol. 2010;12:281–299. doi: 10.1007/s10162-010-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer FP. Quantitative analysis of the innervation of the chicken basilar papilla. Hear Res. 1992;61:167–178. doi: 10.1016/0378-5955(92)90048-R. [DOI] [PubMed] [Google Scholar]
- Fischer FP. Hair-cell morphology and innervation in the basilar papilla of the emu (Dromaius novaehollandiae) Hear Res. 1998;34:87–101. doi: 10.1016/0378-5955(88)90053-6. [DOI] [PubMed] [Google Scholar]
- Fischer FP, Köppl C, Manley GA. The basilar papilla of the barn owl: a quantitative morphological SEM analysis. Hear Res. 1988;34:87–102. doi: 10.1016/0378-5955(88)90053-6. [DOI] [PubMed] [Google Scholar]
- Fox RC, Meng J. An X-radiographic and SEM study of the osseous inner ear of multituberculates and monotremes (Mammalia): implications of mammalian phylogeny and evolution of hearing. Zool J Linnean Soc. 1997;121:249–291. doi: 10.1111/j.1096-3642.1997.tb00339.x. [DOI] [Google Scholar]
- Frick LR, Matthies ML. Effects of external stimuli on spontaneous otoacoustic emissions. Ear Hear. 1988;9:190–197. doi: 10.1097/00003446-198808000-00004. [DOI] [PubMed] [Google Scholar]
- Fritzsch B. Inner ear of the coelacanth fish Latimeria has tetrapod affinities. Nature. 1987;327:153–154. doi: 10.1038/327153a0. [DOI] [PubMed] [Google Scholar]
- Futuyma DJ. Evolution. 2. Sunderland, MA: Sinauer Associates; 2008. [Google Scholar]
- Glasberg BR, Moore BCJ. Derivation of auditory filter shapes from notched-noise data. Hear Res. 1990;47:103–138. doi: 10.1016/0378-5955(90)90170-T. [DOI] [PubMed] [Google Scholar]
- Gleich O. Auditory primary afferents in the starling: correlation of function and morphology. Hear Res. 1989;37:255–268. doi: 10.1016/0378-5955(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Gleich O, Wilson S. The diameters of guinea pig auditory nerve fibres: distribution and correlation with spontaneous rate. Hear Res. 1993;71:69–79. doi: 10.1016/0378-5955(93)90022-S. [DOI] [PubMed] [Google Scholar]
- Gleich O, Fischer FP, Köppl C, Manley GA. Hearing organ evolution and specialization: archosaurs. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer; 2004. pp. 224–255. [Google Scholar]
- Gleich O, Dooling RJ, Manley GA. Audiogram, body mass, and basilar papilla length: correlations in birds and predictions for extinct archosaurs. Naturwiss. 2005;92:595–598. doi: 10.1007/s00114-005-0050-5. [DOI] [PubMed] [Google Scholar]
- Grant PR, Grant BR. Unpredictable evolution in a 30-year study of Darwin’s finches. Science. 2000;296:707–711. doi: 10.1126/science.1070315. [DOI] [PubMed] [Google Scholar]
- Hagstrum JT, Manley GA. Releases of surgically deafened homing pigeons indicate that aural cues play a significant role in their navigational system. J Comp Physiol A. 2015;201:983–1001. doi: 10.1007/s00359-015-1026-3. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Heffner HE. Hearing in the elephant (Elephas maximus): absolute sensitivity, frequency discrimination and sound localization. J Comp Physiol Psychol. 1982;96:926–944. doi: 10.1037/0735-7036.96.6.926. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Koay G, Heffner HE. Audiograms of five species of rodents: implications for the evolution of hearing and the perception of pitch. Hear Res. 2001;157:139–152. doi: 10.1016/S0378-5955(01)00298-2. [DOI] [PubMed] [Google Scholar]
- Hill EM, Koay G, Heffner RS, Heffner HE. Audiogram of the chicken (Gallus gallus domesticus) from 2 Hz to 9 kHz. J Comp Physiol A. 2014;200:863–870. doi: 10.1007/s00359-014-0929-8. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, Choe Y, Mehta AD, Martin P. Putting ion channels to work: mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc Natl Acad Sci U S A. 2000;97:11765–11772. doi: 10.1073/pnas.97.22.11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketten D. Cetacean ears. In: Whitlow WL, Popper AN, Fay RR, editors. Hearing by whales and dolphins; Springer Handbook of Auditory Research vol.12. New York: Springer; 2000. pp. 43–108. [Google Scholar]
- Joris PX, Bergevin C, Kalluri R, McLaughlin M, Michelet P, van der Heijden M, Shera C. Frequency selectivity in Old-World monkeys corroborates sharp cochlear tuning in huamns. Proc Natl Acad Sci. 2011;108:17516–17520. doi: 10.1073/pnas.1105867108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EC, Gosselin-Ildari AD. Cochlear labyrinth volume and hearing abilities in primates. Anat Rec. 2009;292:765–776. doi: 10.1002/ar.20907. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, Takechi M, Hirasawa T, Adachi N, Narboux-Nême N, et al. Developmental genetic bases behind the independent origin of the tympanic membrane in mammals and diapsids. Nat Commun. 2014;6:6853. doi: 10.1038/ncomms7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C. Morphology of the basilar papilla of the bobtail lizard Tiliqua rugosa. Hear Res. 1988;35:209–228. doi: 10.1016/0378-5955(88)90119-0. [DOI] [PubMed] [Google Scholar]
- Köppl C. Number and axon calibres of cochlear afferents in the barn owl. Audit Neurosci. 1997;3:313–334. [Google Scholar]
- Köppl C. Frequency tuning and spontaneous activity in the auditory nerve and cochlear nucleus magnocellularis of the Barn Owl Tyto alba. J Neurophysiol. 1997;77:364–377. doi: 10.1152/jn.1997.77.1.364. [DOI] [PubMed] [Google Scholar]
- Köppl C. Avian hearing. In: Scanes CG, editor. Avian physiology. 6th. Amsterdam: Elsevier; 2015. pp. 71–87. [Google Scholar]
- Köppl C, Gleich O, Manley GA (1993) An auditory fovea in the barn owl cochlea. J Comp Physiol A 171:695–704
- Köppl C, Manley GA. Spontaneous otoacoustic emissions in the bobtail lizard. II: interactions with external tones. Hear Res. 1994;72:159–170. doi: 10.1016/0378-5955(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Köppl C, Authier S. Quantitative anatomical basis for a model of micromechanical frequency tuning in the Tokay gecko, Gekko gecko. Hear Res. 1995;82:14–25. doi: 10.1016/0378-5955(94)00139-H. [DOI] [PubMed] [Google Scholar]
- Konishi M. Listening with two ears. Sci Am. 1993;268:66–73. doi: 10.1038/scientificamerican0493-66. [DOI] [PubMed] [Google Scholar]
- Kreithen ML, Quine DB. Infrasound detection by the homing pigeon: a behavioural audiogram. J Comp Physiol A. 1979;129:1–4. doi: 10.1007/BF00679906. [DOI] [Google Scholar]
- Ladhams A, Pickles JO. Morphology of the monotreme organ of Corti and macula lagena. J Comp Neurol. 1996;366:335–347. doi: 10.1002/(SICI)1096-9861(19960304)366:2<335::AID-CNE11>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Ladich F. Diversity in hearing in fishes: ecoacoustical, communicative, and developmental constraints. In: Köppl C, Manley GA, Popper AN, Fay RR, editors. Insights from comparative hearing research; Springer Handbook of Auditory Research Vol.49. New York: Springer; 2013. pp. 289–321. [Google Scholar]
- Li Y, Liu Z, Shi P, Zhang J. The hearing gene prestin unites echolocating bats and whales. Curr Biol. 2010;20:R55–R56. doi: 10.1016/j.cub.2009.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Shen XX, Zhang P. One thousand two hundred ninety nuclear genes from a genome-wide survey support lungfishes as the sister group of tetrapods. Mol Biol Evol. 2013;30:1803–1807. doi: 10.1093/molbev/mst072. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cotton JA, Shen B, Han X, Rossiter SJ, Zhang S. Convergent sequence evolution between echolocating bats and dolphins. Curr Biol. 2010;20:R53–R54. doi: 10.1016/j.cub.2009.11.058. [DOI] [PubMed] [Google Scholar]
- Lonsbury-Martin BL, Martin GK. Otoacoustic emissions: basic studies in mammalian models. In: Manley GA, Fay RR, Popper AN, editors. Active processes and otoacoustic emissions; springer handbook of auditory research vol.30. New York: Springer; 2008. pp. 261–303. [Google Scholar]
- Luo Z-X. Developmental patterns in Mesozoic evolution of mammal ears. Annu Rev Ecol Evol Syst. 2011;42:355–380. doi: 10.1146/annurev-ecolsys-032511-142302. [DOI] [Google Scholar]
- Luo Z-X, Ruf I, Schultz JA, Martin T. Fossil evidence on evolution of inner ear cochlea in Jurassic mammals. Proc R Soc B. 2010;278:28–34. doi: 10.1098/rspb.2010.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. Frequency sensitivity of auditory neurones in the caiman cochlear nucleus. Z vergl Physiol. 1970;66:251–256. doi: 10.1007/BF00297828. [DOI] [Google Scholar]
- Manley GA (1971) Some aspects of the evolution of hearing in vertebrates. Nature 230:506–509 [DOI] [PubMed]
- Manley GA. Frequency response of the ear of the tokay gecko. J Exp Zool. 1972;181:159–168. doi: 10.1002/jez.1401810203. [DOI] [PubMed] [Google Scholar]
- Manley GA. Frequency response of the middle ear of geckos. J Comp Physiol. 1972;81:251–258. doi: 10.1007/BF00693630. [DOI] [Google Scholar]
- Manley GA. Diversity in hearing-organ structure and the characteristics of spontaneous otoacoustic emissions in lizards. In: Lewis ER, Long GR, Lyon RF, Narins PM, Steele CR, editors. Diversity in auditory mechanics. Singapore: World Scientific Publishing; 1997. pp. 32–38. [Google Scholar]
- Manley GA (1990) Peripheral hearing mechanisms in reptiles and birds. Heidelberg, Germany: Springer-Verlag
- Manley GA. Cochlear mechanisms from a phylogenetic viewpoint. Proc Natl Acad Sci. 2000;97:11736–11743. doi: 10.1073/pnas.97.22.11736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA. Evolution of structure and function of the hearing organ of lizards. J Neurobiol. 2002;53:202–211. doi: 10.1002/neu.10115. [DOI] [PubMed] [Google Scholar]
- Manley GA. An evolutionary perspective on middle ears. Hear Res. 2010;263:3–8. doi: 10.1016/j.heares.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Manley GA (2011) Lizard auditory papillae: an evolutionary kaleidoscope. Hear Res 273:59–64 [DOI] [PubMed]
- Manley GA. Evolutionary paths to mammalian cochleae. JARO. 2012;13:733–743. doi: 10.1007/s10162-012-0349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Johnstone BM. Middle-ear function in the guinea pig. J Acoust Soc Am. 1974;56:571–576. doi: 10.1121/1.1903292. [DOI] [PubMed] [Google Scholar]
- Manley GA, Köppl C. Phylogenetic development of the cochlea and its innervation. Curr Opin Neurobiol. 1998;8:468–474. doi: 10.1016/S0959-4388(98)80033-0. [DOI] [PubMed] [Google Scholar]
- Manley GA, Köppl C. What have lizard ears taught us about auditory physiology? Hear Res. 2008;238:3–11. doi: 10.1016/j.heares.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Manley GA, Kraus JEM. Exceptional high-frequency hearing and matched vocalizations in Australian pygopod geckos. J Exp Biol. 2010;213:1876–1885. doi: 10.1242/jeb.040196. [DOI] [PubMed] [Google Scholar]
- Manley GA, Ladher R. Phylogeny and evolution of ciliated mechano-receptor cells. In: Hoy RR, Shepherd GM, Basbaum AI, Kaneko A, Westheimer G, editors. The senses: a comprehensive reference. Amsterdam: Elsevier; 2007. pp. 1–34. [Google Scholar]
- Manley GA, Van Dijk P. Otoacoustic emissions in amphibians, lepidosaurs and archosaurs. In: Manley GA, Fay RR, Popper AN, editors. Active processes and otoacoustic emissions in hearing; Springer Handbook of Auditory Research Vol. 30. New York: Springer; 2008. pp. 211–260. [Google Scholar]
- Manley GA, Van Dijk P (2016) Frequency selectivity of the human cochlea: suppression tuning of spontaneous otoacoustic emissions. Hear Res in press [DOI] [PubMed]
- Manley GA, Irvine DRF, Johnstone BM. Frequency response of the bat tympanic membrane. Nature. 1972;237:112–113. doi: 10.1038/237112a0. [DOI] [Google Scholar]
- Manley GA, Gleich O, Brix J, Kaiser A. Functional parallels between hair-cell populations of birds and mammals. In: Duifhuis H, Horst JW, Wit HP, editors. Basic issues in hearing. London: Academic Press; 1988. pp. 64–71. [Google Scholar]
- Manley GA, Kirk D, Köppl C, Yates GK. In-vivo evidence for a cochlear amplifier in the hair-cell bundle of lizards. Proc Natl Acad Sci U S A. 2001;98:2826–2831. doi: 10.1073/pnas.041604998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley GA, Sienknecht U, Köppl C. Calcium modulates the frequency and amplitude of spontaneous otoacoustic emissions in the bobtail skink. J Neurophysiol. 2004;92:2685–2693. doi: 10.1152/jn.00267.2004. [DOI] [PubMed] [Google Scholar]
- Manley GA, Köppl C, Bergevin C. Common substructure in otoacoustic emission spectra of land vertebrates. In: Karavitaki KD, Corey DP, editors. Mechanics of hearing: protein to perception, AIP conference proceedings 1703. Melville, NY: American Institute of Physics; 2015. pp. 090012/1–090012/5. [Google Scholar]
- Masterton B, Heffner H, Ravizza R. The evolution of human hearing. J Acoust Soc Am. 1969;45:966–985. doi: 10.1121/1.1911574. [DOI] [PubMed] [Google Scholar]
- Meng J, Fox RC. Therian petrosals from the Oldman and milk river formations (Late Cretaceous), Alberta, Canada. J Vertebr Paleontol. 1995;15:122–130. doi: 10.1080/02724634.1995.10011212. [DOI] [Google Scholar]
- Meyer A, Kocher TD, Basasibwaki P, Wilson AC. Monophyletic origin of Lake Victoria cichlid fishes suggested by mitochondrial DNA sequences. Nature. 1990;347:550–553. doi: 10.1038/347550a0. [DOI] [PubMed] [Google Scholar]
- Miller MR. Scanning electron microscope studies of the papilla basilaris of some turtles and snakes. Am J Anat. 1978;151:409–435. doi: 10.1002/aja.1001510306. [DOI] [PubMed] [Google Scholar]
- Miller MR. The reptilian cochlear duct. In: Popper AN, Fay RR, editors. Comparative studies of hearing in vertebrates. NewYork, Heidelberg, Berlin: Springer-Verlag; 1980. pp. 169–204. [Google Scholar]
- Miller MR. The evolutionary implications of the structural variations in the auditory papilla of lizards. In: Webster DB, Fay RR, Popper AN, editors. The evolutionary biology of hearing. New York: Springer; 1992. pp. 463–487. [Google Scholar]
- Müller M, Laube B, Burda H, Bruns V. Structure and function of the cochlea in the African mole rat (Cryptomys hottentotus): evidence for a low frequency acoustic fovea. J Comp Physiol. 1992;171:469–476. doi: 10.1007/BF00194579. [DOI] [PubMed] [Google Scholar]
- Novacek MJ. Aspects of the problem of variation, origin and evolution of the eutherian auditory bulla. Mammal Rev. 1977;7:131–149. doi: 10.1111/j.1365-2907.1977.tb00366.x. [DOI] [Google Scholar]
- Omland KE, Cook LG, Crisp MD. Tree thinking for all biology: the problem with reading phylogenies as ladders of progress. BioEssays. 2008;30:854–867. doi: 10.1002/bies.20794. [DOI] [PubMed] [Google Scholar]
- Perge JA, Niven JE, Mugnaini E, Balasubramanian V, Sterling P. Why do axons differ in caliber? J Neurosci. 2012;32:626–638. doi: 10.1523/JNEUROSCI.4254-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz WM, Widin GP. Interaction of spontaneous oto-acoustic emissions and external sounds. J Acoust Soc Amer. 1984;76:1713–1720. doi: 10.1121/1.391618. [DOI] [PubMed] [Google Scholar]
- Retzius G (1884) Das Gehörorgan der Wirbelthiere. Morphologisch-histologische Studien. Band II. Das Gehörorgan der Reptilien, der Vögel und der Säugethiere
- Ruf I, Luo Z-X, Wible JR, Martin T. Petrosal anatomy and inner ear structures of the late Jurassic Henkelotherium (Mammalia, Cladotheria, Dryolestoidea): insight into the early evolution of the ear region in cladotherian mammals. J Anat. 2009;214:679–693. doi: 10.1111/j.1469-7580.2009.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Temchin AN. The roles of the external, middle, and inner ears in determining the bandwidth of hearing. PNAS. 2002;99:13206–13210. doi: 10.1073/pnas.202492699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JC. Auditory structure and function in the bird middle ear: an evaluation by SEM and capacitative probe. Hear Res. 1985;18:253–268. doi: 10.1016/0378-5955(85)90042-5. [DOI] [PubMed] [Google Scholar]
- Schermuly L, Klinke R. Infrasound sensitive neurones in the pigeon’s cochlear ganglion. J Comp Physiol A. 1990;166:355–363. doi: 10.1007/BF00204808. [DOI] [PubMed] [Google Scholar]
- Shera C, Guinan JJ, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci U S A. 2002;99:3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloth E, Zwicker E. Mechanical and acoustical influences on spontaneous oto-acoustic emissions. Hear Res. 1983;11:285–293. doi: 10.1016/0378-5955(83)90063-1. [DOI] [PubMed] [Google Scholar]
- Smith CA. Inner ear. In: AS K, McLeland J, editors. Form and function in birds. London: Academic Press; 1985. pp. 273–310. [Google Scholar]
- Smolders JWT, Klinke R. Synchronized responses of primary auditory fibre populations in Caiman crocodilus (L.) to single tones and clicks. Hear Res. 1986;24:89–103. doi: 10.1016/0378-5955(86)90052-3. [DOI] [PubMed] [Google Scholar]
- Smothermann M, Narins P. Evolution of the amphibian ear. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer; 2004. pp. 164–199. [Google Scholar]
- Sneary MG. Auditory receptor of the red-eared turtle: I General ultrastructure. J Comp Neurol. 1988;276:573–587. doi: 10.1002/cne.902760410. [DOI] [PubMed] [Google Scholar]
- Steele C. Three-dimensional mechanical modeling of the cochlea. In: Lewis ER, Long GR, Lyon RF, Narins PM, Steele CR, editors. Diversity in auditory mechanics. Singapore: World Scientific Publishing; 1997. pp. 455–461. [Google Scholar]
- Takechi M, Kuratani S. History of studies on mammalian middle ear evolution: a comparative morphological and developmental biology perspective. J Exp Zool (Mol Dev Evol) 2010;314B:417–433. doi: 10.1002/jez.b.21347. [DOI] [PubMed] [Google Scholar]
- Taschenberger G, Manley GA. Spontaneous otoacoustic emissions in the barn owl. Hear Res. 1997;110:61–76. doi: 10.1016/S0378-5955(97)00070-1. [DOI] [PubMed] [Google Scholar]
- Vater M, Meng J, Fox R. Hearing organ evolution and specialization: early and later mammals. In: Manley GA, Popper AN, Fay RR, editors. Evolution of the vertebrate auditory system. New York: Springer; 2004. pp. 256–288. [Google Scholar]
- Vidal N, Hedges, SB (2009) The molecular evolutionary tree of lizards, snakes, and amphisbaenians. Comptes Rendus Biol. 332:129–139 [DOI] [PubMed]
- Weiss TF, Mulroy MJ, Turner RG, Pike CL. Uning of single fibers in the cochlear nerve of the alligator lizard: relation to receptor morphology. Brain Res. 1976;115:71–90. doi: 10.1016/0006-8993(76)90823-4. [DOI] [PubMed] [Google Scholar]
- Walsh SA, Barrett PM, Milner AC, Manley GA, Witmer LM. Inner ear anatomy is a proxy for deducing auditory capability and behaviour in reptiles and birds. Proc R Soc B. 2009;276:1355–1360. doi: 10.1098/rspb.2008.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner YL, Wever EG. The function of the middle ear in lizards: Gekko gecko and Eublepharis macularius (Gekkonoidea) J Exp Zool. 1972;179:1–16. doi: 10.1002/jez.1401790102. [DOI] [Google Scholar]
- Wever EG. The reptile ear. Princeton N.J: Princeton Univ Press; 1978. [Google Scholar]
- Wever EG, Bray CW. Auditory nerve impulses. Science. 1930;71:215. doi: 10.1126/science.71.1834.215. [DOI] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- Zwicker E, Peisl W. Cochlear preprocessing in analog models, in digital models and in human inner ear. Hear Res. 1990;44:209–216. doi: 10.1016/0378-5955(90)90081-Y. [DOI] [PubMed] [Google Scholar]