Abstract
The characteristics of human otoacoustic emissions (OAEs) have not been thoroughly examined above the standard audiometric frequency range (>8 kHz). This is despite the fact that deterioration of cochlear function often starts at the basal, high-frequency end of the cochlea before progressing apically. Here, stimulus-frequency OAEs (SFOAEs) were obtained from 0.5 to 20 kHz in 23 young, audiometrically normal female adults and three individuals with abnormal audiograms, using a low-to-moderate probe level of 36 dB forward pressure level (FPL). In audiometrically normal ears, SFOAEs were measurable at frequencies approaching the start of the steeply sloping high-frequency portion of the audiogram (∼12–15 kHz), though their amplitudes often declined substantially above ∼7 kHz, rarely exceeding 0 dB SPL above 8 kHz. This amplitude decline was typically abrupt and occurred at a frequency that was variable across subjects and not strongly related to the audiogram. In contrast, certain ears with elevated mid-frequency thresholds but regions of normal high-frequency sensitivity could possess surprisingly large SFOAEs (>10 dB SPL) above 7 kHz. When also measured, distortion-product OAEs (DPOAEs) usually remained stronger at higher stimulus frequencies and mirrored the audiogram more closely than SFOAEs. However, the high-frequency extent of SFOAE and DPOAE responses was similar when compared as a function of the response frequency, suggesting that middle ear transmission may be a common limiting factor at high frequencies. Nevertheless, cochlear factors are more likely responsible for complexities observed in high-frequency SFOAE spectra, such as abrupt amplitude changes and narrowly defined response peaks above 10 kHz, as well as the large responses in abnormal ears. These factors may include altered cochlear reflectivity due to subtle damage or the reduced spatial extent of the SFOAE generation region at the cochlear base. The use of higher probe levels is necessary to further evaluate the characteristics and potential utility of high-frequency SFOAE measurements.
Keywords: stimulus-frequency otoacoustic emissions, distortion-product otoacoustic emissions, high frequencies, forward pressure level
INTRODUCTION
In response to a tone, sensitive ears emit sound at the frequency of stimulation, referred to as stimulus-frequency (SF) otoacoustic emission (OAE) or SFOAE. These emissions are thought to arise via reflections of the stimulus-driven traveling wave off of micromechanical irregularities distributed along the cochlear partition, with the dominant sources being located near the peak of the traveling wave (Zweig and Shera 1995) and/or somewhat more basal (e.g., Choi et al. 2008; Sisto et al. 2015; see also Siegel et al. 2005). As SFOAEs presumably provide a window onto the active outer hair cell (OHC)-mediated processes that amplify vibrations near the tonotopic region of the stimulus, they have been used as a noninvasive probe of both cochlear sensitivity (Ellison and Keefe 2005) and frequency tuning (Shera et al. 2002). However, despite the fact that sensitivity and tuning are highly vulnerable to insult at the basal, high-frequency end of the cochlea, the characteristics and potential utility of SFOAEs—or other OAEs, for that matter—have not been thoroughly examined above the standard audiometric range (>8 kHz).
High-frequency OAE measurements have primarily been limited by the technical challenge of delivering adequate and accurately calibrated stimulus levels to the eardrum, in part due to the presence of standing waves in the sealed ear canal (e.g., Siegel 1994). Thus far, techniques have been developed to permit extended high-frequency recordings of distortion-product OAEs (DPOAEs) evoked by two-tone stimuli (Dreisbach and Siegel 2001, 2005; Hecker et al. 2011; Poling et al. 2014), transient-evoked OAEs (TEOAEs; Goodman et al. 2009; Keefe et al. 2011; Keefe 2012), as well as OAEs evoked by broadband noise (termed “cochlear reflectance,” or CR, when expressed as a ratio of the response to the stimulus; Rasetshwane and Neely 2012). However, aside from preliminary reports (Dreisbach 1999), there have yet to be systematic high-frequency investigations of OAEs evoked by single tones (i.e., SFOAEs).
At least at low stimulus levels, SFOAEs, TEOAEs, and CR are all thought to arise via the same reflection mechanism. These responses may therefore provide similar information or even be spectrally equivalent (Kalluri and Shera 2007a; Sisto et al. 2013; Rasetshwane and Neely 2012). Nevertheless, SFOAEs likely afford the clearest window onto the underlying reflection mechanism—a mechanism proposed to be fundamentally distinct from the nonlinear distortion which produces DPOAEs (Shera and Guinan 1999). Though nonlinear methods are typically required to extract SFOAE responses from the evoking stimulus pressure (see Kalluri and Shera 2007b and also “METHODS”), the measured response to a low-level tone likely originates from a narrow region of the cochlea and is relatively uncontaminated by nonlinear interactions, such as suppression and intermodulation distortion, which may occur during broadband stimulation. Conversely, the use of tonal rather than transient stimuli also permits higher levels to be delivered at any given frequency. This allows measurement of responses over a wider range of stimulus levels, including those commonly used to elicit DPOAEs, and those at which nonlinear distortion may also contribute to or dominate the SFOAE response (Talmadge et al. 2000; Goodman et al. 2003; though see also Schairer et al. 2006).
The mechanisms of SFOAE generation presumably operate across all frequencies where hearing is sensitive (up to 12–16 kHz in humans), if not up to the highest audible frequencies (20 kHz). However, while TEOAE and CR responses have been measured at frequencies up to 16 kHz, their magnitudes decline at frequencies somewhat lower. Median TEOAE amplitudes reported by Goodman et al. (2009) for 73 dB peak sound pressure level (SPL) clicks are reduced by 15 dB from 8 to 16 kHz, while a similar decline in mean CR responses (for low-level stimuli) from Rasetswhane and Neely (2012) starts at ∼4–6 kHz. This amplitude decline is also consistent with the observation that spontaneous OAEs (SOAEs) primarily occur below 4 kHz and are rarely measurable above 8 kHz (e.g., Talmadge et al. 1993). As SOAEs are thought to arise from self-sustaining reflections of SFOAE energy between its site(s) of origin and the middle ear boundary, they should only be present when SFOAE generation is strong (Kemp 1979; Shera 2003). In contrast, DPOAE amplitudes are relatively flat with frequency up to stimulus frequencies of at least 10–12 kHz (Dreisbach and Siegel 2001; Dreisbach et al. 2006; Poling et al. 2014).
The above evidence suggests that the SFOAE generation mechanism may grow weak at frequencies somewhat below the upper range of sensitive hearing and/or that high-frequency responses are limited by the transmission characteristics of the middle ear (e.g., Puria 2003). However, the high-frequency decline in TEOAE and CR magnitudes could also be attributed to calibration-related variations in stimulus pressure or other methodological issues. For instance, the click stimulus used by Goodman et al. (2009) was reduced in amplitude above 12 kHz, thus limiting responses at higher frequencies. Additionally, CR measurement requires separating OAE energy from residual, short-latency artifacts in the time-frequency domain, effectively precluding observation of energy above 16 kHz and possibly attenuating responses at lower frequencies (Rasetshwane and Neely 2012). It is not clear if SFOAEs would exhibit a similar frequency dependence when evoked by equally calibrated tones and analyzed in the frequency domain.
To address this, the present report characterizes SFOAE responses from 0.5 to 20 kHz in young, normal-hearing individuals, as well as in several ears with abnormal audiometric profiles. Stimuli were calibrated in terms of forward pressure level (FPL), a method which involves decomposing the stimulus pressure measured in the ear canal into its forward- and reverse-going pressure components (e.g., Scheperle et al. 2008, 2011). Referencing stimuli to the forward pressure reduces the influence of standing waves on the stimulus delivered to the ear and has been shown to yield repeatable behavioral threshold measurements at frequencies up to 20 kHz with changes in probe insertion depth (Souza et al. 2014). So as to assess potential constraints on SFOAE generation and measurement, the frequency dependence of SFOAE responses was compared to the configuration of the behavioral threshold curve and, in some cases, DPOAE profiles over the same frequency range.
METHODS
Subjects
Behavioral threshold and SFOAE measurements were obtained for one ear each of 26 female adults aged 18–25 (mean ± standard deviation (SD) = 20.9 ± 1.9 years). Twenty-three subjects were audiometrically normal (defined below) and contributed to the primary data set, while three others with abnormal audiometric results served as case studies. All subjects had normal otoscopic and tympanometric findings and no history of chronic ear infections or other ear pathology or surgery. Subjects provided written, informed consent and were compensated monetarily. All procedures were approved by the Institutional Review Board at Northwestern University.
Audiometric status was assessed via standard clinical audiometry during an initial screening, with pure-tone thresholds obtained in 5 dB steps. Audiometrically normal subjects were defined as those with thresholds for air-conducted stimuli ≤15 dB hearing level (HL) at all octave frequencies 0.25–8 kHz, as well as 3 and 6 kHz, and no differences between thresholds for air- and bone-conducted stimuli exceeding 15 dB at a single frequency, or 10 dB at two frequencies (bone conduction thresholds were obtained only up to 4 kHz). Individuals with a threshold >20 dB HL at one or more frequencies, but no marked differences between thresholds for air- and bone-conducted stimuli (see criteria above), were considered for inclusion as case studies. One subject who initially provided a normal audiogram was found to have elevated thresholds when she returned for subsequent testing, possibly due to noise exposure at an event in the interim. This subject was included as one of the three case studies and is described in greater detail later on.
The study was limited to female subjects to improve the feasibility of obtaining full data sets from individual ears. In preliminary measurements, it was found that male subjects were typically slower and less reliable in completing the threshold measurements. Male subjects also produced higher levels of physiological noise and more artifactual responses during the OAE measurements. As the present study required ∼4 h of testing, and formed part of a set of related and more time-consuming experiments, the choice was therefore made to limit all studies to female subjects. Due to the relatively small number of subjects, the inclusion of only female subjects was also hoped to reduce sources of variability in the relationships between the threshold and SFOAE measures. While this precluded the observation of any sex-related differences, preliminary high-frequency SFOAE responses from three male subjects were not inconsistent with the findings shown here.
General Protocol
Following the initial screening measurements, high-resolution threshold and SFOAE measurements were obtained from ∼0.5 to 20 kHz using custom equipment and procedures, and with stimuli calibrated in terms of FPL (rather than HL or SPL), as described in the following subsections. Measurements were made over the course of two 2-h sessions separated by an average of 8 days (SD = 6.2 days; median = 7 days; range = 1–30 days). Behavioral pure-tone thresholds were measured in the first 20–30 min of each session. Subjects then watched a subtitled DVD for approximately 60–90 min during which SFOAE and SOAE recordings were obtained. DPOAEs were also measured in eight audiometrically normal subjects and all three of the subjects with abnormal audiograms.
Equipment
Measurements were made in a sound-attenuating audiometric booth with the subject seated in a comfortable recliner. In the initial screening session, standard clinical audiometry was performed with an Interacoustics Audio Traveller AA220 (Interacoustics, Assens, Denmark), and tympanometric readings were obtained with a GSI Tympstar Middle Ear Analyzer (Grason-Stadler Inc., Eden Prairie, MN). For all other measurements, signals were generated and recorded using custom software written in C++, MATLAB (The MathWorks Inc., Natick, MA), and MaxMSP (https://cycling74.com/) running on an Apple Macintosh computer. Digital-to-analog and analog-to-digital conversions were performed with a MOTU 828 mkII FireWire interface (Mark of the Unicorn, Cambridge, MA), using 24-bit resolution and a sampling rate of 96 kHz. Stimulus signals were passed through an Etymotic Research ER H4C amplifier (Etymotic Research Inc., Elk Grove Village, IL) and presented via one of two MB Quart 13.01 HX speakers (Maxxsonics, Chicago, IL). Flexible plastic tubing connected the speakers to the sound ports of an ER-10B+ OAE probe, which was coupled to the ear canal with an ER10-14 foam tip. All acoustic measurements were made with the ER-10B+ microphone and preamplifier (set to 20 dB gain) and were compensated for the magnitude and phase of the microphone transfer function (Siegel 2007; Rasetshwane and Neely 2011a).
To reduce the influence of any sudden or gradual changes in probe position over time, silicone earmold material (Insta-mold Products Inc., Oaks, PA) was injected around the probe following insertion into the ear canal. The silicone filled the concha and the upper, frontal portion of the triangular fossa. Comparison of wideband ear canal responses obtained during repeated in situ calibrations (typically at the beginning, middle, and end of each session) confirmed that the probe position was highly stable over the course of each test session.
Stimulus Calibration
Stimuli were calibrated in terms of FPL using MATLAB code provided by Dr. Shawn Goodman. FPL calibration requires use of the probe’s Thévenin-equivalent source pressure and impedance to decompose the pressure measured in situ by the probe into its forward- and reverse-going components (i.e., components propagating toward and away from the eardrum, respectively). Details regarding the theory and calculations involved in this calibration method are provided elsewhere (e.g., Scheperle et al. 2008, 2011; Souza et al. 2014) and described only briefly here.
First, the Thévenin-equivalent source pressure and impedance of the probe were calculated from pressure measurements made in known acoustic loads. This was achieved by inserting the probe into a brass tube (8 mm inner diameter) terminated at the other end by a copper rod of equivalent diameter, adjusting the insertion depth of the rod in the tube to produce six effective cavity lengths (typically 2.96, 3.67, 4.18, 5.38, 6.74, and 7.92 cm) and recording the response to a wideband chirp at each length. The source pressure and impedance were then derived by minimizing the error in the solution to the set of equations describing the theoretical acoustic impedances of the cavities. Due to ongoing refinement of the calibration procedure, slightly different cavity lengths were used in some of the earlier measurements. Source characteristics were re-calculated on an approximately weekly basis.
During each test session, the ear canal response to the wideband chirp stimulus was obtained after the probe was positioned and sealed in place. Equations relating the source pressure, source impedance, and measured ear canal pressure were used to determine the ear canal impedance, surge impedance (using the approach of Rasetshwane and Neely 2011b), and pressure reflectance, from which the forward and reverse components of the total measured pressure could also be calculated. Correction factors needed to achieve the appropriate forward pressure were then computed across frequency and applied to all subsequent stimuli.
Note that for stimuli presented at a constant FPL, the SPL measured by the probe still exhibits peaks and valleys across frequency due to standing waves in the ear canal. These patterns depend on the insertion depth of the probe and the ear canal pressure reflectance. Across subjects, the measured probe SPL was typically ∼4–6 dB above the nominal FPL value below 1 kHz, as well as at the half-wave ear canal resonances (near 6–8 and 12–16 kHz), but could fall as much as 15 dB below the nominal FPL value at the quarter-wave nulls in between. Similar patterns were obtained with the probe placed in an ear simulator (IEC 60318-4; Bruel and Kjaer 4157). However, the SPL measured by the ear simulator microphone (at its terminal end) varied much more smoothly with frequency and was ∼2.5–6 dB above the nominal FPL value from 0.5 to 20 kHz or about 4 dB higher on average. This is consistent with the terminal SPL being the sum of the forward and reflected pressures at the ear simulator microphone, thus varying with frequency according to the magnitude and phase of the simulator’s pressure reflectance. To the extent that characteristics of our subjects’ ears were similar to those of the ear simulator, the SPL near the eardrum was approximately 2.5–6 dB above a given FPL value.
Behavioral Threshold Measurement
Pure-tone thresholds were obtained at 40 frequencies from 0.125 to 20.159 kHz using a modified, fixed-frequency Békésy tracking procedure (Lee et al. 2012). Thresholds were obtained at 0.125, 0.25, 0.5, and 0.75 kHz and then in 1/3rd-octave steps from 1 to 3.175 kHz, 1/9th-octave steps from 3.703 to 11.758 kHz, and 1/18th-octave steps from 11.758 to 20.159 kHz, so as to better characterize thresholds at high frequencies.
At each frequency, pure-tone stimuli (250 ms duration with 25 ms rise/fall times) were pulsed twice per second with an interstimulus interval of 250 ms. Subjects pressed or released a button when the stimulus was deemed audible or inaudible, respectively, thus marking a “reversal.” The stimulus level was stepped by 6 dB per presentation prior to the second reversal and by 2 dB per presentation thereafter. After six ascending runs (i.e., with stimulus level crossing from below to above threshold), stimulus levels at the midpoints between reversals were calculated for each ascending run, excluding the first two. The average midpoint level was taken as the threshold if the standard error (SE) was less than 1 dB; otherwise, additional ascending runs were completed until this criterion was met.
Thresholds were obtained from 0.125 to 10.079 kHz in the first test session and from 6.35 to 20.159 kHz in the second session. Thresholds for the seven frequencies overlapping between sessions were averaged in the final threshold curve for each subject and were generally very similar. Across all overlapping frequencies and subjects, the mean absolute difference in threshold between the two sessions was 2.47 dB (SD = 1.88 dB).
SFOAE Measurement
SFOAE responses were obtained from 0.458 to 20.16 kHz with an ∼1/100th-octave resolution for a single, low-to-moderate probe level of 36 dB FPL. As mentioned in the “Stimulus Calibration” subsection, the SPL measured at the terminal end of an ear simulator varied between ∼2.5 and 6 dB above a nominal FPL value for the frequency range considered here, suggesting that the 36-dB FPL stimulus may have produced roughly 40 dB SPL near the eardrum in a given subject.
A step size of 1/100th octave was chosen to accurately characterize the fine structure of the responses. Additionally, this helped to avoid any ambiguities in unwrapping of the SFOAE phase, particularly at high frequencies, where phase accumulation between adjacent measurement points could approach 0.2 cycles. Due to the time-consuming nature of such measurements, SFOAE responses were unfortunately not obtained for multiple probe levels. A relatively low-level probe was chosen so as to facilitate comparisons with previous reports at lower frequencies, which have typically used probe levels ≤40 dB SPL. Additionally, higher probe levels may produce responses with contributions from a broader cochlear region and/or both reflection and nonlinear distortion sources (Shera and Guinan 1999). The low probe level used here therefore offered a window primarily onto the reflection mechanism. Nevertheless, use of a higher probe level would likely have produced more responses above the noise floor, particularly at high frequencies, where both cochlear and middle ear factors may limit the response.
At frequencies below ∼8 kHz, SFOAEs were evoked by continuously swept tones and extracted from the total ear canal pressure via the “compression” method (Kemp 1980). At higher frequencies, SFOAEs were evoked by discrete tones and extracted via the “suppression” method (Kemp and Chum 1980). The different measurement paradigms were used to efficiently obtain responses at low frequencies, while avoiding artifacts at higher frequencies, as described below. SFOAE amplitudes and phases for the different frequency spans were stitched together at the midpoints of their overlap to create the final SFOAE profile for each subject.
Swept-Tone Compression Method
In the first test session, SFOAEs evoked by continuously swept tones were obtained from 0.458 to 8.724 kHz in four 1.25-octave spans, with 0.25-octave overlap between adjacent spans. Stimuli were logarithmically swept at a rate of 1/6th octave/s or 7.5 s per 1.25 octave span. For each span, presentation of the swept-tone at 36 dB FPL (the probe stimulus) was followed by presentation of the same stimulus at 56 dB FPL (the reference stimulus). Note that only a single sound source was used in this paradigm, with both the probe and reference stimuli presented in sequence via the same sound source. The SFOAE evoked by each probe sweep was then estimated by scaling down the response to the reference sweep by 20 dB and subtracting it from the probe response. Due to the nonlinear, compressive growth of SFOAE amplitudes at moderate-to-high stimulus levels, this subtraction removed an estimate of the stimulus pressure, as well as any linearly growing SFOAE components, leaving a nonlinear SFOAE “residual.”
The average SFOAE residual was calculated from 32 repetitions of the interleaved 36 and 56 dB FPL stimuli. While no automatic artifact-rejection procedures were implemented, subjects were provided 10 s breaks after every 30 s of swept-tone measurements, as cued by a short, low-frequency tone. Subjects were instructed to restrict swallowing and movement to these 10-s breaks. In addition, the ear canal spectrum was continuously monitored for evidence of swallowing or other physiological noise, and recordings were manually paused and restarted if such events occurred. While these procedures were entirely subjective, examination of the resulting noise floors (typically <−10 dB SPL) suggested that they were adequate for our purposes. In a few cases, time constraints or excessive physiological noise (a noise floor >0 dB SPL in the averaged response) required that only 16 or 24 responses be included in the final average.
A least squares fit (LSF) analysis (Long and Talmadge 1997; Long et al. 2008) was applied to the average probe, reference, and SFOAE residual waveforms to compute the amplitude and phase as a function of probe frequency. The LSF analysis window was 200 ms wide and applied to the measured waveforms in 60 ms steps, such that there was 140 ms of overlap between adjacent windows. This achieved amplitude and phase estimates at frequencies separated by 1/100th octave. Data within each 200 ms segment were windowed by a Hann function prior to analysis. To compute the noise floor, the polarity of every other residual was inverted before averaging and applying the LSF analysis. SFOAE phases were compensated for the stimulus phase at each frequency by subtracting the reference sweep phase.
Preliminary attempts to use the swept-tone compression paradigm (with 7.5 s sweeps) at high frequencies resulted in excessively high noise floors and SFOAE residuals with phases that were often flat across frequency. Such responses were likely artifacts due to uncanceled stimulus pressure in the residual, possibly due to subtle shifts in probe position over time. Measurements in an ear simulator did not have high noise floors but did reveal some artifactual, low-level (<−10 dB SPL) SFOAE residuals above 10 kHz, presumably due to nonlinearity in the sound source. One approach to address these issues could have been to use shorter swept tones and the suppression, rather than compression, method for extracting the SFOAE. Shorter sweeps could have reduced acoustic variability between stimulus presentations, and the use of a second suppressor tone presented via a separate sound source (instead of a higher level reference sweep from the same sound source) could have eliminated artifacts due to system nonlinearity. However, with such a swept-tone suppression paradigm, the SFOAE residual could be contaminated by incomplete cancelation of not only the probe but also the suppressor and the SFOAE evoked by the suppressor. Though the LSF analysis could hypothetically be optimized to only capture components with delays consistent with those of the SFOAE at the probe frequency (e.g., Kalluri and Shera 2013), this may not be practical at very high frequencies. The probe, suppressor, and evoked SFOAEs would all be close in frequency and have similarly short delays and therefore would not be easily separable in time. Thus, for simplicity, a more traditional approach using discrete tones and the suppression method was employed at high frequencies.
Discrete-Tone Suppression Method
In the second test session, SFOAEs were evoked by discrete tones presented from 7.128 to 20.16 kHz in ∼1/100th-octave steps (151 frequencies total). Each 250 ms presentation of the probe tone alone was followed by a 250-ms presentation of the probe plus a 56-dB FPL suppressor tone with frequency 47 Hz below that of the probe. In practice, the probe tone was presented continuously (with 5 ms on/off-ramps applied to the beginning and end of the total waveform) and the suppressor was pulsed on and off at appropriate intervals (with 5 ms on/off-ramps applied to each presentation) via a separate sound source. Presentation intervals always contained an integer number of stimulus cycles at the probe frequency.
A suppressor presented 20 dB above the probe level presumably eliminated much of the SFOAE evoked at the probe frequency (e.g., Kalluri and Shera 2007b; Keefe et al. 2008), such that the probe-plus-suppressor interval yielded an estimate of the probe pressure alone. SFOAE residuals were therefore obtained by subtracting each response to the probe-plus-suppressor from the previous response to the probe alone. The average residual was computed from 32 repetitions of the probe and probe-plus-suppressor intervals for a total measurement time of 16 s at each frequency. SFOAE amplitudes, phases, and noise floors were all calculated as above for the swept-tone measurements, using the LSF analysis. To avoid contamination of the residual by the onset or offset of the probe and suppressor, the first and last 5 ms of the responses were removed prior to analysis, resulting in a 240-ms analysis window.
Ear simulator measurements confirmed that SFOAE residuals from a passive cavity were indistinguishable from the noise floor, which was roughly −20 to −25 dB SPL from 7 to 15 kHz and −5 to −15 dB SPL above 15 kHz. Variation in the noise floor amplitude was due to compensation for the microphone transfer function and is clear in the data presented in the “RESULTS.” As with the swept-tone compression method, no automatic artifact-rejection procedures were implemented, though recordings were manually paused and restarted online when swallowing or other physiological noise was noted in the real-time spectrum of the microphone signal. Subjects were instructed to restrict swallowing and movement to 10 s breaks provided after every 32 s of measurement time.
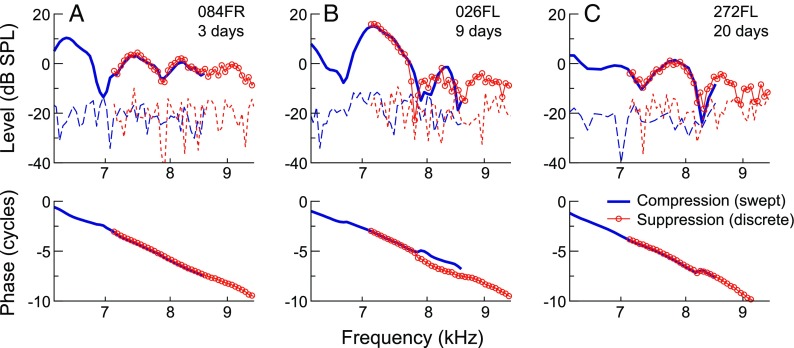
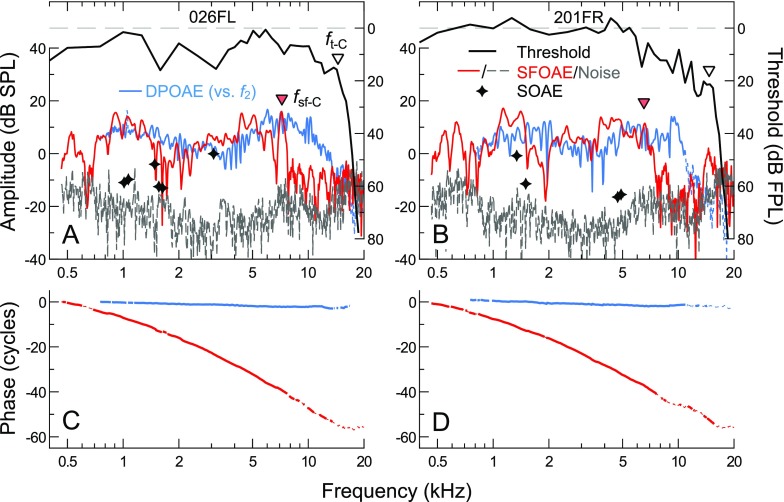
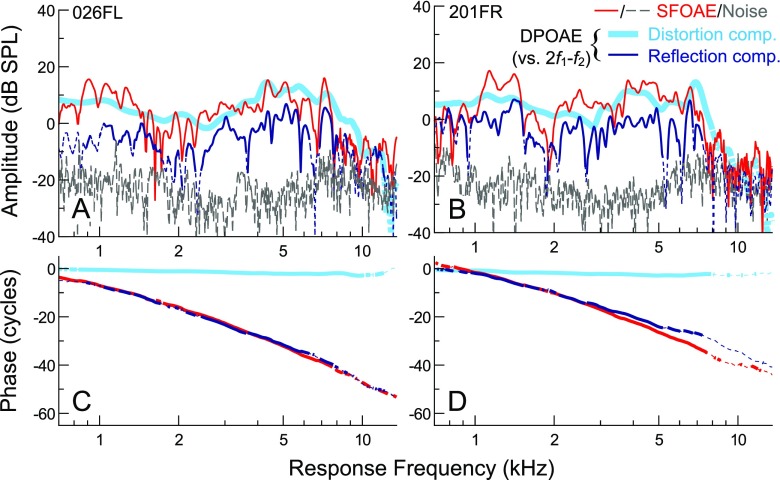
Combined use of the different SFOAE measurement methods was supported by previous demonstrations that SFOAEs evoked with discrete or swept tones and extracted via suppression or compression methods are largely equivalent (Kalluri and Shera 2007b, 2013). Consistent with this, Figure 1 illustrates that SFOAE amplitudes and phases obtained with the two methods were highly similar within the frequency range of overlap between the two test sessions. As the measurements were separated by up to 30 days (20 days for the data shown), this agreement also suggests that SFOAE responses could be relatively stable over time.
FIG. 1.
Comparison of SFOAE responses obtained with the two measurement paradigms used in this study, the combination of which allowed efficient measurements at low frequencies and reduced artifactual responses at high frequencies. SFOAE amplitudes and phases obtained via the swept-tone compression (blue lines) and discrete-tone suppression (red circles/lines) methods are shown for three subjects (A–C), with noise floors indicated by long or short dashed lines, respectively. Measurements were obtained in two different sessions, with the separation in days shown next to the subject identifiers. Despite up to 20 days between test sessions for these subjects, SFOAE amplitudes and phases obtained by the two methods were highly similar within the ∼0.25-octave span of overlap between measurements. While phase jumps in opposite directions were occasionally observed near amplitude valleys (B), these were also sometimes observed in repeated measures using the same method (not shown).
SOAE Measurement
Three-minute ear canal recordings were made in the absence of stimulation to determine the presence of any SOAEs, which appear as narrowband signals in the ear canal spectrum. A fast Fourier transform (FFT) was performed on each second of the recording, and the median amplitude (across all 180 spectra) for each 1-Hz-wide frequency bin was used to construct the final spectrum. SOAE recordings were typically made at the end of the test session. While the presence or absence of SOAEs did not factor into any of the final analyses, SOAE amplitudes/frequencies are indicated in figures showing data from individual subjects, providing a more complete picture of a given ear’s OAE profile.
DPOAE Measurement
When time allowed, DPOAEs were evoked by two logarithmically swept primary tones (Long et al. 2008) with instantaneous frequencies f 1 and f 2 (f 2 > f 1; f 2/f 1 = 1.16) and levels L 1 and L 2 (51 and 36 dB FPL, respectively). After averaging the responses to 16 stimulus repetitions, the amplitude and phase of the 2f 1-f 2 DPOAE were obtained as a function of frequency using the LSF analysis. Primaries were swept at a rate of 1/6th-octave/s over separate low- and high-frequency spans, with f 2 swept from 743.3 to 4204.5 kHz or 3535.5 to 20 kHz, respectively, and f 1 swept such that f 2/f 1 was held constant. A narrow primary ratio (compared to the more standard 1.2-1.22) was used so as to evoke higher DPOAE levels at the high frequencies and low stimulus levels of interest here (Dreisbach and Siegel 2001).
Case Study Protocol
Two subjects with abnormal clinical audiograms completed the test protocol and measurements as described above. Of these, one had a mild, bilateral mid-frequency loss (20–30 dB HL thresholds from 1 to 4 kHz), and the other had a mild, unilateral high-frequency loss (25 dB HL threshold at 8 kHz). These audiometric configurations were confirmed by subsequent threshold measurements obtained with the Békésy tracking procedure.
A modified test protocol was implemented for a third subject, who provided a normal audiogram during the initial screening but returned for testing 1 month later with a mild-to-moderate threshold elevation from 0.25 to 6 kHz. Though a second clinical audiogram was not obtained, thresholds assessed via Békésy tracking were 20–40 dB above those from the audiometrically normal subjects. The subject reported that her hearing may have been affected due to noise exposure at an event 5 days prior. To track any changes in thresholds, SFOAEs, and DPOAEs between the two scheduled test sessions, the protocol was abbreviated so that measurements could be obtained across the entire frequency range in a single 2-h session. This was achieved by reducing the frequency resolution of the threshold measurements and reducing the number of swept-tone stimulus repetitions used in the SFOAE measurements from 32 to 20. Discrete-tone SFOAEs were obtained at frequencies up to 12.3 kHz without reducing the number of stimulus repetitions. This same protocol was then repeated 5 days later.
SFOAE Phase-Gradient Delays
SFOAE phase-gradient delays were computed as the negative slope of the unwrapped SFOAE phase curve. Slopes were calculated for the three-point (1/50th octave) span centered on each probe frequency. As SFOAE delays fluctuate widely across frequency and subject, group data were characterized with a robust loess fit to data with SNR ≥9 dB (computed using the “smooth” function in MATLAB, with a smoothing span of 0.2). The 95 % confidence interval (CI) for the loess trend was determined via bootstrap resampling, with the group data at each frequency randomly resampled prior to calculating the trend. The CI was constructed from the results of repeating this procedure 1000 times.
DPOAE Component Separation
While the primary generation mechanism for the 2f 1-f 2 DPOAE is thought to be distinct from that of SFOAEs, the measured DPOAE may actually contain contributions from both the distortion and reflection mechanisms. The “distortion component” arises in the cochlear region where the excitation patterns elicited by f 1 and f 2 overlap, presumably near the peak of the f 2 response, and then reverse-propagates out to the ear canal. Additionally, an SFOAE-like “reflection component” results when distortion propagates apically to its own characteristic place, i.e., the place tuned to 2f 1-f 2, and is then subject to the same scattering mechanism which gives rise to SFOAEs. The distortion and reflection components are distinguished by their differing phase behaviors with frequency: flat vs. steeply sloping, respectively.
To more precisely compare the DPOAE and SFOAE responses based on the presumed mechanism of generation, DPOAEs were separated into the distortion and reflection components according to their different phase gradients. This was accomplished with an inverse FFT-based approach described previously (Poling et al. 2014). First, in the frequency domain, a Hann window was applied to the complex DPOAE pressure in 50 Hz steps (after appropriate interpolation of the responses), with the length of the window varied on a logarithmic scale according to the cochlear place-frequency map (Greenwood 1990). The window length ranged from 400 to 1874 Hz from the lowest to highest DPOAE frequency. For each window, an impulse response (IPR) function was determined and rectangular time-domain filters then applied to the IPR to extract components with different delays. A peak in the IPR with two minima falling between −2 and 10 ms was identified as the distortion component, while the reflection component was identified as a second peak falling between 2 and 15 ms. The amplitudes and phases of the distortion and reflection components were then taken from the FFT of the data within their respective time windows. Though DPOAEs were originally measured at frequencies between 0.54 and 14.48 kHz, component estimates were limited to frequencies between 0.7 and 13.5 kHz due to edge effects of the windowing used in the procedure. This range included the high-frequency extent of the measured responses and thus does not limit interpretation of the results.
RESULTS
Group Data
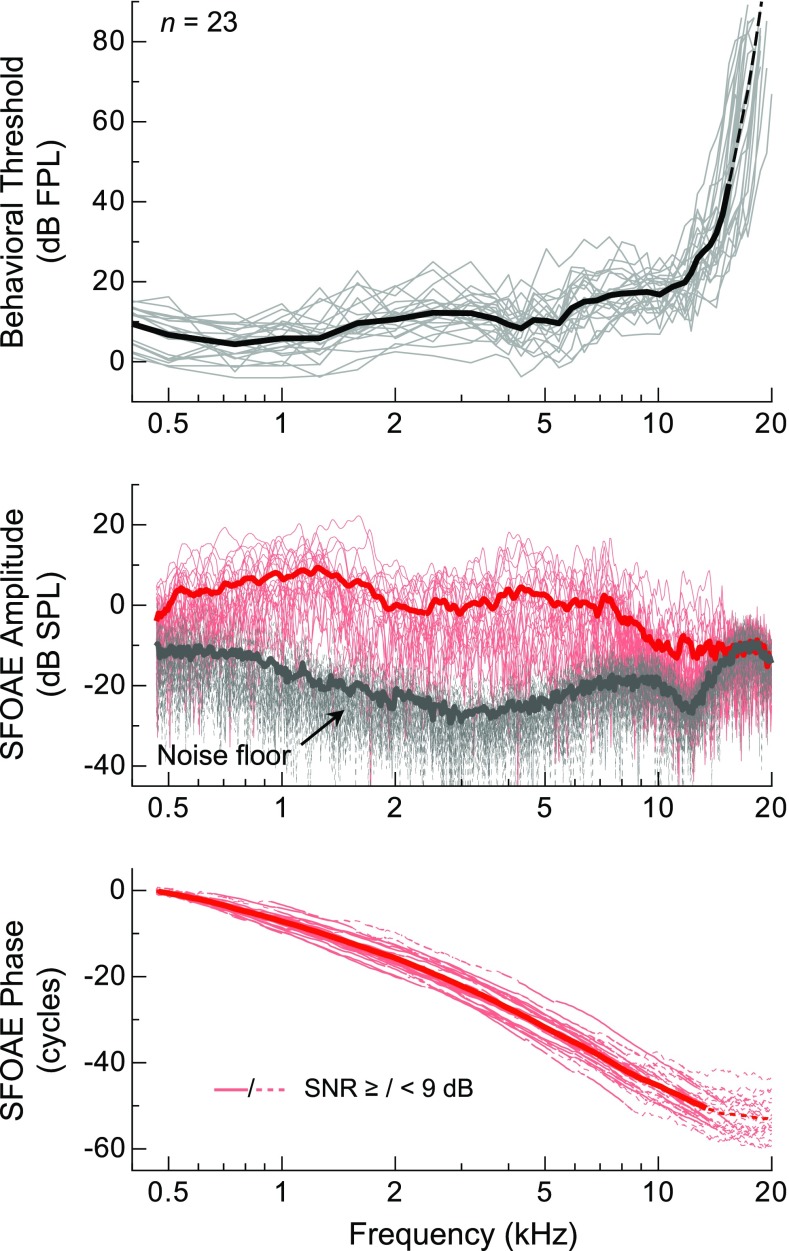
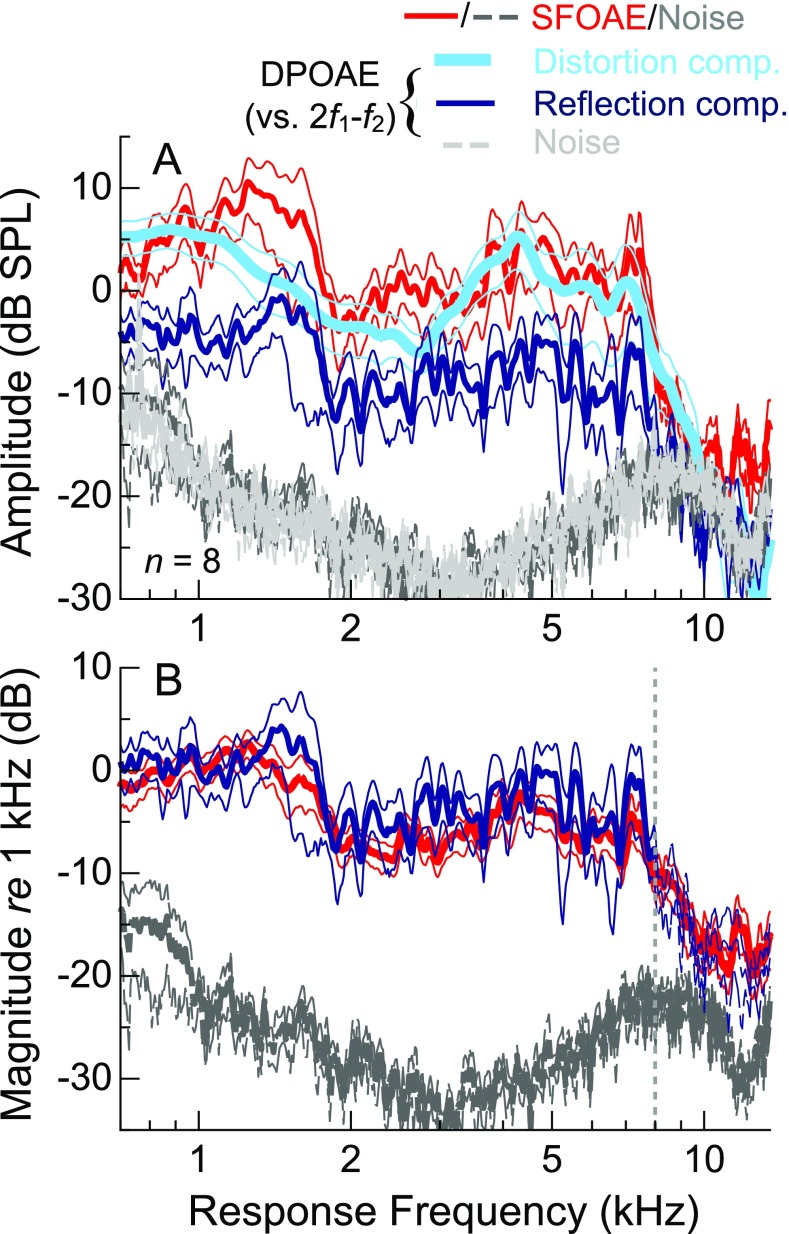
Average SFOAE amplitudes and behavioral thresholds from audiometrically normal subjects mirrored one another over much of the frequency range examined, as shown in Figure 2. Consistent with previous reports of thresholds from adults of a similar age range (e.g., Lee et al. 2012), thresholds were lowest near 1 kHz (mean = 4.3 dB FPL at 0.75 kHz) and relatively sensitive (<∼20 dB FPL) at frequencies up to 12–15 kHz, or the so-called corner frequency of the audiogram, where the steep high-frequency slope of the threshold curve starts. Likewise, SFOAE amplitudes were largest near 1 kHz, with the mean approaching 10 dB SPL at 1.25 kHz, and relatively flat from 2 to 7.5 kHz (∼0 dB SPL on average). However, while SFOAEs were often measurable above the noise floor up to ∼15 kHz, their amplitudes typically declined above 7 kHz. Amplitudes rarely exceeded 0 dB SPL beyond 8 kHz, and responses were often indistinguishable from the noise floor at higher frequencies. The observation of lower-level responses above 15–16 kHz was precluded by the reduced sensitivity of the probe microphone at these frequencies, which resulted in a rising noise floor following compensation for the microphone transfer function. At least above 2 kHz, the frequency dependence of the measurement noise floor was largely due to having compensated for the response of the ER-10B+ microphone.
FIG. 2.
Individual (thin) and average (thick lines) behavioral thresholds and SFOAE amplitudes, noise floors, and phases for a probe level of 36 dB FPL. Average threshold calculations above 15.4 kHz (thin dashed line) required linear extrapolation of some of the individual curves due to non-responses above this frequency. Dashed gray portions of the individual SFOAE phase curves indicate frequencies where the SNR was less than 9 dB. The dashed portion of the average phase curve indicates where fewer than half of the data points satisfied this criterion.
Despite the lower SFOAE amplitudes (and SNR) at high frequencies, steeply sloping phase curves were often observed out to 15–16 kHz, indicating the presence of low-level responses up to these frequencies. Delays calculated from the negative slope of the phase curves (phase-gradient delays) decreased from about 16 to 1.5 ms from 0.5 to 16 kHz, as shown in Figure 3A. Unfortunately, delay estimates became less reliable at higher frequencies due to the sparsity of data with sufficient SNR. The flattening of the individual and average phase curves at higher frequencies suggests that most of the responses were dominated by noise or uncanceled stimulus artifact.
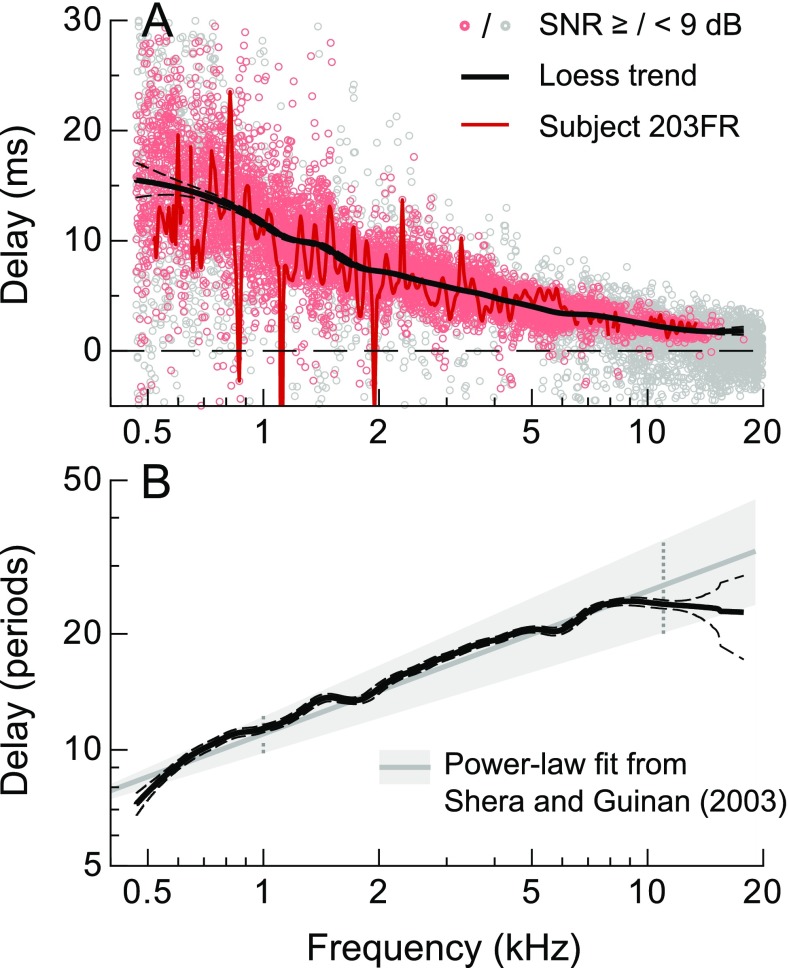
FIG. 3.
A SFOAE phase-gradient delays calculated from the data in Figure 2. The solid/dashed lines indicate the mean and 95 % CI for the robust loess fit (span = 0.2) to all data with SNR ≥ 9 dB (red symbols; gray symbols indicate SNR < 9 dB). Delays from a representative ear are shown with the red line (only data with SNR ≥9 shown for clarity), illustrating that the large variation in the group data was primarily due to quasiperiodic fluctuations with frequency observed in individual ears. B Mean and 95 % CI for the robust loess fit to the delays expressed in stimulus periods (solid and dashed lines). Also shown is the power-law fit provided by Shera and Guinan (2003), which was based on data extending to slightly lower frequencies (gray line, shaded area indicates the 95 % CI). The dashed vertical lines at 1 and 11 kHz indicate the frequency range of the data used to derive the power-law fit.
When expressed in stimulus periods, a quantity theoretically proportional to cochlear tuning (Shera et al. 2010), delays increased from ∼7.5 periods at 0.5 kHz to a plateau of ∼24 periods above 7 kHz, as shown in Figure 3B. The robust loess fit to all data with SNR ≥9 dB is shown by the thick solid line, with dashed lines indicating the 95 % CI for the fit (computed via a bootstrap resampling procedure described in the “METHODS”). The delay plateau coincided in frequency with the decline in average SFOAE amplitudes, consistent with there being a change in the properties of SFOAE generation, and perhaps cochlear tuning, at this frequency. At least at lower frequencies, the trend agreed well with the power-law fit provided by Shera and Guinan (2003) (gray line; shaded area indicates the 95 % CI), which was based on SFOAE delays obtained between 1 and 11 kHz.
Note that the wide variation in phase-gradient delays across subjects was primarily due to the quasiperiodic fluctuations in delay observed across frequency within individual ears (delays for a representative subject are shown with the red line in Fig. 3A). Such fluctuations in the phase-gradient delay correspond to fluctuations in actual time-domain estimates of SFOAE latency (Kalluri and Shera 2013) and may be attributed to variations in the dominant SFOAE generation regions and/or multiple intracochlear reflections between these sites and the middle ear (e.g., Konrad-Martin and Keefe 2003; Shera and Bergevin 2012).
Spectral Shapes of Average Threshold Sensitivity and SFOAE Amplitude Curves
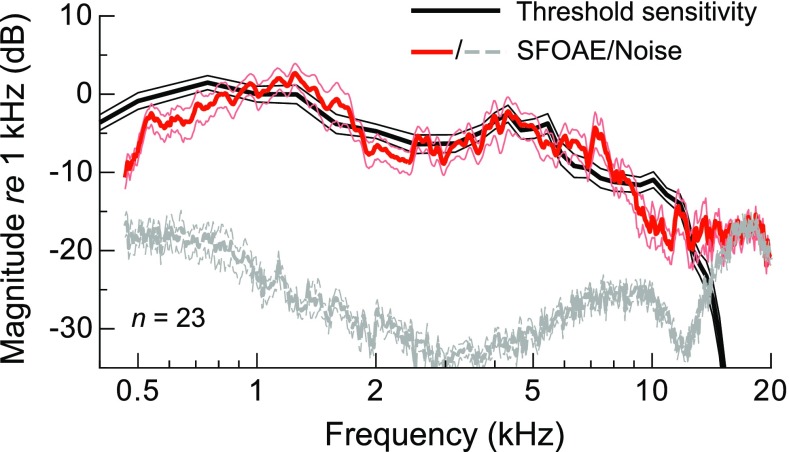
The average threshold and SFOAE amplitude curves are more explicitly compared in Figure 4, highlighting the similarity in their spectral shapes over much of the frequency range examined. To facilitate comparison, thresholds were converted to units of sensitivity by inversion, and both the average sensitivity and SFOAE amplitude curves were normalized to their respective magnitudes at 1 kHz. Thin lines indicate ±1 SE. While the curves had a similar frequency dependence between 1 and 5 kHz, SFOAE amplitudes declined more rapidly than sensitivity below 1 kHz and between 7 and 12 kHz. The steep high-frequency decline in SFOAE amplitude near 7–8 kHz was accompanied by only a gradual decline in sensitivity and preceded the corner frequency of the average threshold curve by approximately 0.5 octaves. The rapid decline in sensitivity above 12 kHz was accompanied by little change in mean SFOAE amplitude, though this was primarily due to averaging across subjects with different audiometric and SFOAE profiles. Low-level SFOAE responses were not observed above the audiometric corner frequency in individual ears, as shown in the following subsection.
FIG. 4.
Average threshold sensitivity (i.e., inverted thresholds) and SFOAE amplitudes normalized to their respective magnitudes at 1 kHz, with thin lines indicating ±1 SE. The average noise floor was normalized with reference to the average SFOAE amplitude at 1 kHz.
Individual Data
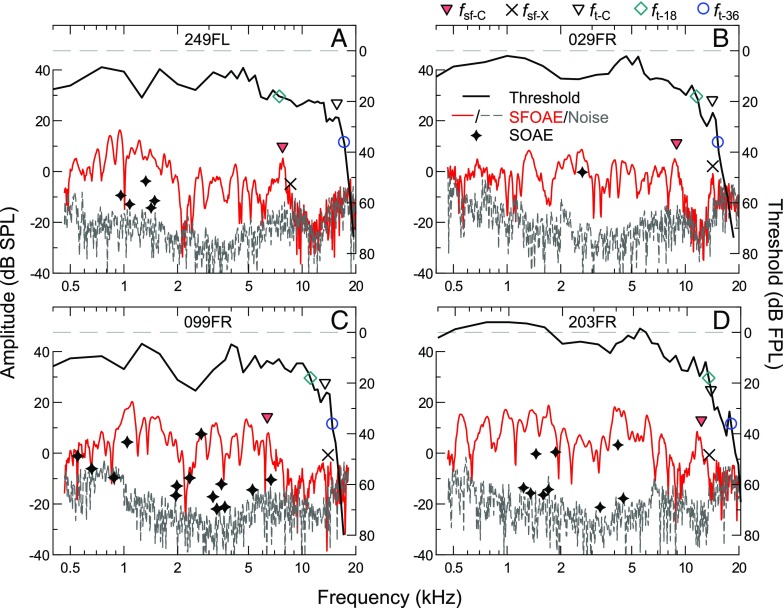
While discrepancies between the shapes of the average SFOAE amplitude and threshold sensitivity curves may appear subtle, SFOAE amplitudes often declined rather abruptly in individual ears, as shown in Figure 5. The frequency where this decline occurred was variable across subjects and typically not associated with any notable changes in thresholds. For comparison, threshold values in Figure 5 are plotted upside down (see right axes), but are not normalized, in contrast to the average sensitivity curve in Figure 4. Above the SFOAE amplitude decline, SFOAE responses were often indistinguishable from the noise floor, as for the subject shown in panel A. However, in some ears, either a lone SFOAE amplitude peak (panel B) or steady, low-level SFOAE responses (panel C) were observed at frequencies up to, but not above, the corner frequency of the audiogram. In the few cases where relatively large (>0 dB SPL) SFOAE responses were observed above 10 kHz, the high-frequency extent of these responses aligned well with the audiometric corner frequency (panel D). Nevertheless, an amplitude decline at lower frequencies was still evident in such cases (at ∼6 kHz for the subject in D). Also shown in Figure 5 are the frequencies and amplitudes of any SOAEs (indicated by stars), which were typically not observed above 8 kHz and never in the absence of measurable SFOAEs. The low incidence of SOAEs at frequencies higher than that of the SFOAE amplitude decline is consistent with presumed dependence of SOAEs on the mechanisms of SFOAE generation (e.g., Shera 2003).
FIG. 5.
Individual thresholds (black lines) and SFOAE amplitudes/noise floors (red/dashed gray lines) for four subjects (A–D), demonstrating the diversity of SFOAE responses at high frequencies. SOAE frequencies and amplitudes are indicated by stars. As described in the main text, threshold and SFOAE corner frequencies (f t-C and f sf-C) were determined and are marked with open and filled triangles, respectively. Also indicated is the last frequency where the SFOAE SNR was at least 9 dB within a 1/9th-octave span (f sf-X; x’s), as well as the 18- and 36-dB FPL threshold intercepts (f t-18 and f t-36; open diamonds and circles, respectively). These threshold and SFOAE “landmark” frequencies are compared in Figure 6.
Comparison of Audiometric and SFOAE “Landmark” Frequencies
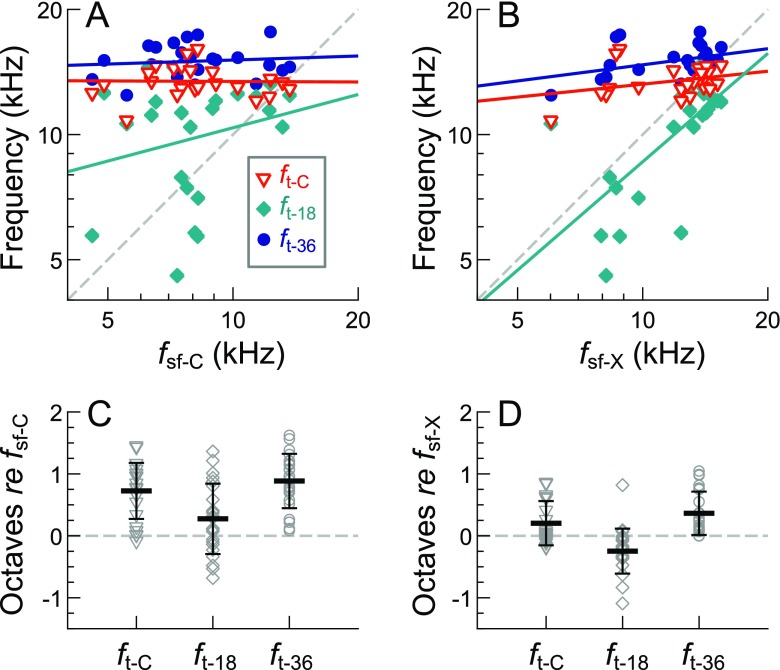
Despite the impression that the SFOAE amplitude decline was a notable feature in the individual SFOAE profiles, the frequency where this occurred was not strongly related to the audiometric configuration. To assess this, threshold and SFOAE corner frequencies (f t-C and f sf-C, indicated by open and filled triangles, respectively, in Fig. 5) were determined by first smoothing the response curves with a moving one-third-octave average and then calculating the slope of the linear fit to the surrounding smoothed, one-third-octave span at each frequency. Moving from high to low frequencies, f t-C was identified as the highest frequency where the slope of the smoothed threshold curve became less than 55 dB per octave. Likewise, f sf-C was defined as the highest frequency where the slope became less negative than −4 dB per octave and where the average SNR for a one-third-octave band extending below that frequency was at least 9 dB, so as to avoid f sf-C being influenced by isolated amplitude peaks (as in Fig. 5B). These criteria were designed such that the objectively determined corner frequencies generally matched subjective assessments.
As shown in Figure 6A, C, f sf-C varied widely across ears (mean ± 1 SD = 8.48 ± 2.57 kHz) but tended to fall far below f t-C (mean ± 1 SD = 0.73 ± 0.45 octaves). The two corner frequencies were not significantly correlated (p > 0.05 as assessed via Pearson’s product–moment correlation). To further assess the relationship between the SFOAE corner frequency and the audiogram, f sf-C was compared to the highest-frequency 18 and 36 dB FPL threshold intercepts, f t-18 and f t-36 (indicated by teal diamonds and blue circles in Fig. 5). These intercepts are potentially relevant to the overall spectral shape of the SFOAE responses, as the first (f t-18) marks the transition from relatively sensitive to less sensitive thresholds (based on an assessment of the range of typical mid-frequency thresholds), while the second (f t-36) marks where the stimulus level transitioned from audible to inaudible. Nevertheless, neither of these intercepts were significantly correlated with f sf-C, which fell an average (±1 SD) of 0.27 ± 0.56 octaves and 0.89 ± 0.44 octaves below f t-18 and f t-36, respectively. Upon re-plotting all threshold curves with the frequency axis normalized to f sf-C (not shown), thresholds tended to gently slope upward starting about two thirds of an octave below f sf-C, though there was no marked change in either the absolute value or slope of the threshold curve at f sf-C.
FIG. 6.
Relationships between audiometric and SFOAE landmark frequencies. A The SFOAE corner frequency (f sf-C) was not significantly correlated (p > 0.05) with the threshold corner frequency (f t-C; red triangles) or the frequencies of the 18- and 36-dB FPL threshold intercepts (f t-18 and f t-36; teal diamonds and blue circles). Linear regression fits (solid lines) and Pearson’s product–moment correlations were computed after converting all frequencies to an octave scale. B The high-frequency extent of measurable SFOAEs (f sf-X) was often close in frequency to f t-C and f t-36, though was only significantly correlated with f t-18 (R 2 (21) = 0.45, p = 0.00046). C, D Frequency differences (in octaves) between the audiometric and SFOAE landmark frequencies. Black horizontal lines and error bars indicate the average frequency difference ± 1 SD.
Since low-level responses were observed above the SFOAE corner frequency, the relationship between the high-frequency extent of measurable SFOAEs (f sf-X; x’s in Fig. 5) and the audiometric “landmark” frequencies (f t-C, f t-18, and f t-36) was also examined. For each subject, f sf-X was defined as the highest frequency where SFOAE amplitudes averaged over the surrounding 1/9th-octave bin had an SNR of at least 9 dB. While f sf-X also varied across ears (mean ± 1 SD = 12.01 ± 2.75 kHz), this frequency was more related to the audiometric landmarks than f sf-C (Fig. 6B, D), tending to occur just below f t-36 (mean ± 1 SD = 0.36 ± 0.35 octaves) and closer to f t-C (0.20 ± 0.36 octaves below). While not significantly correlated with either f t-C or f t-36, most of the data points clustered near or above unity (dashed line), and the median difference between f sf-X and f t-C was only 0.04 octaves. In addition, f sf-X was significantly correlated with f t-18 (R 2 (21) = 0.45, p = 0.00046), as is evident in Figure 6B, occurring an average of 0.25 (±0.36) octaves above this threshold intercept. Re-plotting threshold curves with the frequency axis normalized to f sf-X (not shown) confirmed this relationship and indicated that f sf-X tended to fall where thresholds exceeded ∼24 dB FPL.
Thus, while it was difficult to account for the SFOAE corner frequency based on the audiometric configuration, the high-frequency extent of measurable SFOAEs was limited to frequencies approaching the threshold corner frequency and was related to a departure from what might be considered sensitive hearing. However, absolute threshold sensitivity still provided little indication of SFOAE amplitude at high frequencies. When averaged in non-overlapping one-third-octave bins from 8 to 16 kHz (i.e., with bins centered at 8, 10.1, 12.7, and 16 kHz), thresholds and SFOAE amplitudes were very weakly correlated within each bin. The highest and only significant correlation was between thresholds and SFOAE amplitudes for the bin centered at 12.7 kHz (R 2 (21) = 0.25, p = 0.015). Nevertheless, all linear regression slopes were negative, such that higher thresholds tended to be associated with lower SFOAE amplitudes at the same frequency.
Comparison of SFOAE and DPOAE Responses in Normal Ears
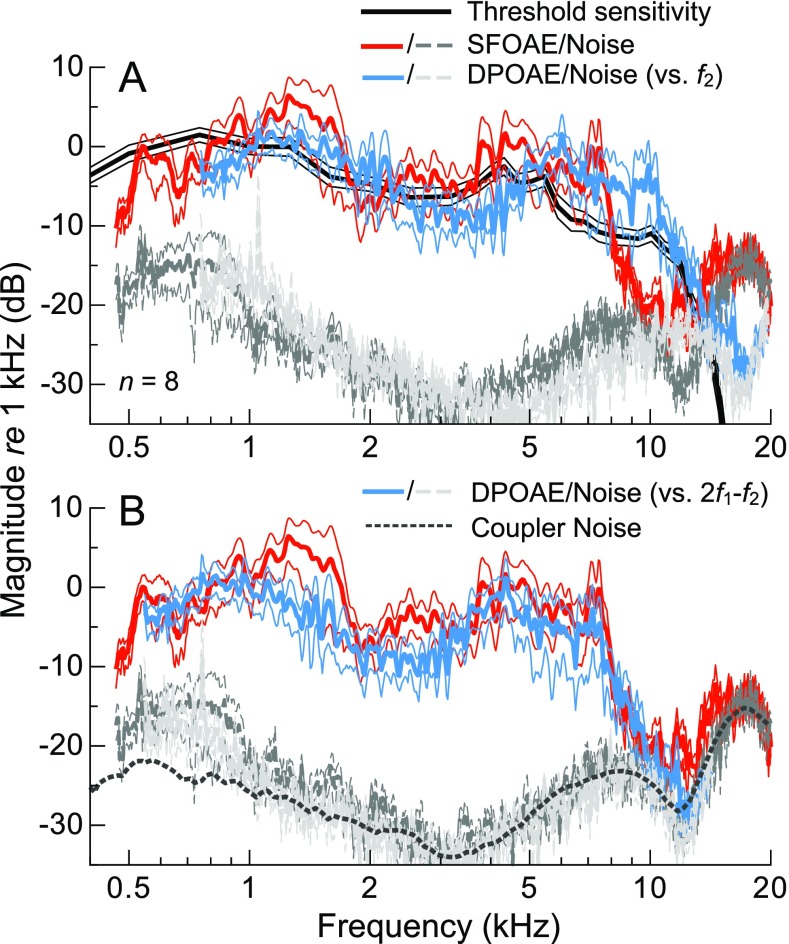
In a subset of eight subjects, DPOAEs were measured in response to low-level primary tones (L 1, L 2 = 51, 36 dB FPL) swept with a relatively narrow frequency ratio (f 2/f 1 = 1.16). The average 2f 1-f 2 DPOAE magnitude plotted vs. f 2 is compared with the average sensitivity and SFOAE magnitude curves from these subjects in Figure 7A. Plotting DPOAEs as a function of f 2 is conventional, as the response presumably originates near or basal to the tonotopic location of f 2, where the excitation patterns elicited by f 1 and f 2 overlap. Using this convention, DPOAE and SFOAE magnitudes had a similar shape in the mid-frequency region, but the high-frequency decline in DPOAE magnitudes occurred approximately 0.5 octaves above that of the SFOAE curve, aligning well with the decline in behavioral sensitivity. Thus, for low-level stimuli, the frequency dependence of behavioral sensitivity was better matched by DPOAE rather than SFOAE amplitudes, at least when the former was considered as a function of f 2.
FIG. 7.
Average threshold sensitivity, SFOAE amplitude, and 2f 1-f 2 DPOAE amplitude curves, all normalized to their respective values at 1 kHz, for a subset of eight subjects. Thin lines indicate ±1 SE. When DPOAEs are plotted vs. f 2, (A) the high-frequency decline in the average DPOAE spectrum occurs about ∼0.5 octaves above that for SFOAEs and better matches the decline in average sensitivity. Due to plotting vs. f 2 rather than 2f 1-f 2, the average DPOAE noise floor (light dashed gray lines; normalized re the DPOAE magnitude at 1 kHz) appears to be a shifted version of the SFOAE noise floor (darker gray lines). When DPOAEs are instead plotted vs. 2f 1-f 2, (B) the offset between the high-frequency extents of the mean DPOAE and SFOAE magnitude curves is largely eliminated, suggesting a possible common dependence of SFOAEs and DPOAEs on the response frequency. The similar shapes of the DPOAE and SFOAE noise floors are due to compensating responses for the transfer function of the probe microphone and are not of biological origin. To illustrate this, the noise floor from a measurement in an ear simulator is also shown (after one-third-octave smoothing, for clarity, and vertical shifting to align with the SFOAE and DPOAE noise floors).
However, as shown in Figure 7B, plotting DPOAE amplitudes vs. 2f 1-f 2 eliminated much of the offset between the average SFOAE and DPOAE profiles, as 2f 1-f 2 was 0.47 octaves below f 2. Accordingly, the linear correlation between mean DPOAE magnitudes (with an SNR of at least 6 dB) and mean SFOAE magnitudes was greatly improved when DPOAEs were considered as a function of 2f 1-f 2 rather than f 2 (R 2 (415) increased from 0.40 to 0.66, though both correlations were highly significant, with p < 0.0001). A common dependence of SFOAE and DPOAE magnitude on the response frequency could suggest that the high-frequency extent of both emission types was limited by the transmission properties of the middle ear. In other words, an abrupt decrease in the transmission of energy from the cochlea to ear canal near 7–8 kHz would explain a decrease in OAE amplitude at the same response frequency, regardless of the stimulus frequency. Alternatively, or in addition, the 0.5-octave offset between the mean response magnitudes when DPOAEs were plotted vs. f 2 could suggest that, for a given stimulus frequency, the dominant generation region for SFOAEs is located more basally than that of DPOAEs. These possibilities are considered further in the “DISCUSSION.” While the SFOAE and DPOAE noise floors also shared a common dependence on the response frequency, note that their spectral shapes were largely due to compensating for the transfer function of the probe microphone. Figure 7B also shows the noise floor measured in an ear simulator (after one-third-octave smoothing, for clarity), which had a similar shape above 1 kHz.
Individual data from two subjects in Figure 8 further illustrate that DPOAEs plotted vs. f 2 had similar magnitudes and spectral shapes as SFOAEs up to f sf-C but typically varied in a manner more consistent with the threshold curve at higher frequencies, though the agreement was by no means perfect (e.g., Fig. 8B). While DPOAEs generally remained stronger at higher frequencies, two of the eight subjects possessed SFOAE amplitude peaks just above the frequency where DPOAE amplitudes declined into the noise floor, roughly near f t-C (as in Fig. 8B). At lower frequencies, SFOAE amplitudes also sometimes peaked locally while both sensitivity and DPOAE amplitudes declined (not shown). The SFOAE and DPOAE amplitude profiles from individual ears were therefore not simply offset from one another in frequency, as may be suggested by the average data in Figure 7.
FIG. 8.
A, B Thresholds (black lines), SFOAE amplitudes/noise floors (red/dashed gray lines), and DPOAE amplitudes (blue lines) for two subjects. DPOAEs are plotted as a function of f 2. DPOAE noise floors are not shown for clarity, though thin dashed portions of the DPOAE amplitude curves indicated SNR <9 dB. For reference, threshold and SFOAE corner frequencies (f t-C and f sf-C) are also shown with open and filled triangles, respectively. SOAE frequencies and amplitudes are indicated by stars. C, D SFOAE and DPOAE phase as a function of frequency for the same two subjects, demonstrating that the two response types exhibit different phase behaviors (steeply sloping vs. flat with frequency) over a wide frequency range. Thin dashed portions of the curves indicate SNR <9 dB.
The primary mechanisms underlying SFOAE and DPOAE generation are theoretically distinguishable by the phase behavior of the resulting emissions, i.e., rapid vs. little phase rotation with frequency for reflection- and distortion-source OAEs, respectively (Shera and Guinan 1999). Consistent with this, SFOAE and DPOAE phase gradients were steep and flat, respectively, across all frequencies where the SNR was adequate (Fig. 8C, D; dashed portions of the curves indicate SNR <9 dB). While this indicates that the measured DPOAEs were dominated by the distortion mechanism, the responses may have included significant contributions from the reflection mechanism as well. This is because distortion not only propagates basally from its generation site (near the f 2 region) to the ear canal, but it may also propagate apically to the region tuned to 2f 1-f 2, thus eliciting an SFOAE-like component at the distortion frequency. The contribution of such reflection components to the DPOAE could partially explain the similarity of the average SFOAE and DPOAE magnitudes when plotted vs. response frequency.
To address this, an IFFT-based approach was used to separate the distortion and reflection components on the basis of their different phase gradients (see “METHODS” for details). Figure 9 compares the separated DPOAE components and SFOAE responses for the two subjects whose data were shown in Figure 8. Average component amplitudes are summarized for all eight subjects in Figure 10. DPOAE components are plotted vs. 2f 1-f 2 so as to appropriately compare the reflection component with the SFOAE, though note that both components should also have some spectral dependence on f 2, since forward- and backward-propagating distortion originates from near the place tuned to this frequency. As is evident from the individual and average data, the reflection component did indeed contribute to the measured DPOAE responses. Though the relative levels of the two components varied with frequency and could occasionally be equivalent, the reflection component was typically ∼10 dB below the level of the distortion component. Consistent with this, the gross amplitude and phase of the measured DPOAE largely resembled those of the distortion component.
FIG. 9.
Comparison of SFOAE amplitudes (A, B) and phases (C, D) with those of the DPOAE distortion and reflection components, plotted vs. 2f 1-f 2. Data are from the subjects whose raw DPOAE responses are shown in Figure 8. Responses are only compared from 0.7 to 13.5 kHz due to edge effects of the DPOAE component separation procedure. The amplitude of the DPOAE reflection component (dark blue line) was ∼10 dB below that of the distortion component (thick light blue line) and the SFOAE (red line). However, the overall spectral shape and fine structure as well as the phase of the reflection component were highly similar to those of the SFOAEs from the same ear. DPOAE noise floors are not shown for clarity. Dashed portions of the DPOAE component amplitude and phase curves indicate SNR <9 dB.
FIG. 10.
A Average (±1 SE) amplitudes of SFOAE responses and separated DPOAE components from the data previously compared in Figure 7. DPOAE component amplitudes are plotted vs. 2f 1-f 2, facilitating comparison of the reflection component and SFOAE, which are thought to arise via the same mechanisms. B Average (±1 SE) SFOAE and DPOAE reflection component magnitudes normalized to their respective levels at 1 kHz. The spectral shapes of the average responses are highly similar, consistent with the observations in the individual data shown in Figure 9. The average SFOAE noise floor is shown normalized to the average SFOAE response at 1 kHz. The dashed vertical line indicates where the average reflection component fell below the DPOAE noise floor, which is not shown for clarity.
As anticipated, the overall spectral shape, fine structure, and phase of the reflection component were highly similar to those of the SFOAE responses from the same ear when the DPOAE was plotted vs. 2f 1-f 2. The agreement between the overall spectral shape of the SFOAE and reflection component is highlighted in Figure 10B, which shows the average response magnitudes normalized to their values at 1 kHz. Reliable comparison is unfortunately limited to frequencies below 8 kHz (see dashed vertical line), as the average reflection component amplitude fell within or below the average DPOAE noise floor above this frequency (for clarity, only the SFOAE noise floor is shown). Note that since both SFOAE and DPOAE amplitudes declined when the frequency of the response exceeded ∼8 kHz, examination of the DPOAE reflection component does not provide an alternate test of the high-frequency extent of the SFOAE responses. In fact, when plotted vs. the response frequency, SFOAEs could be measured at higher frequencies than DPOAEs (Fig. 9B). However, this also does not contradict the primary finding that DPOAE responses typically remained stronger at higher stimulus frequencies.
SFOAE and DPOAE Responses in Ears with Abnormal Audiometric Profiles
The complex relationships among SFOAEs, DPOAEs, and behavioral thresholds are illustrated further by ears with mid-frequency and/or punctate cochlear lesions in Figures 11 and 12. Data from three such ears reveal that (1) large low-to-mid-frequency SFOAE responses can be measured in an ear with mild, high-frequency loss (25 dB HL at 8 kHz; Fig. 11A, C), (2) large high-frequency SFOAEs can be obtained in an ear with a mild, low-to-mid frequency loss (20–30 dB HL thresholds from 1 to 4 kHz; Fig. 11B, D), and that (3) following noise exposure, SFOAEs may be reduced at certain frequencies but enhanced at others (Fig. 12). Responses for each subject are shown along with the average threshold and SFOAE curves previously shown in Figure 2 (thick gray lines; average DPOAE amplitudes not shown for clarity) and are described in detail below. While separated DPOAE components are not shown, the distortion component always resembled a smoothed version of the total DPOAE. Due to the dominance of the distortion component, DPOAEs are plotted vs. f 2 (as in Figs. 7A and 8). Exceptions to this dominance are noted when relevant. While we presume that the audiometric abnormalities from these ears are cochlear in origin, any spectral variation in the efficiency of reverse middle ear transmission would shape the total DPOAE as a function of 2f 1-f 2. Plotting DPOAEs vs. f 2 therefore obscures any common effects of reverse transmission on SFOAEs and DPOAEs.
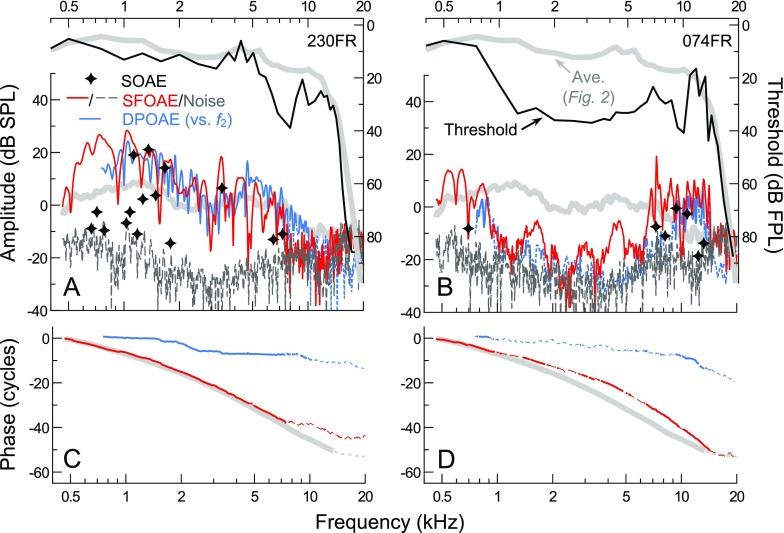
FIG. 11.
A, B Thresholds, SFOAEs, and DPOAE responses for two subjects with abnormal audiometric configurations. Average threshold and SFOAE amplitude curves from Figure 2 are shown for reference (thick gray lines). SFOAE and DPOAE phases are shown in C, D. DPOAEs are plotted vs. f 2, due to the dominance of the responses by the distortion component (separated components are not shown). Subject 230FR (A, C) had the largest low-mid frequency SFOAEs observed in this study despite a mild high-frequency threshold notch. Subject 074FR (B, D) had an extensive, mild mid-to-high frequency loss but abnormally large SFOAE amplitudes at high frequencies, even at those where thresholds exceeded the average by 15–20 dB. Multiple SOAEs (stars) were also observed between 7.3 and 13.1 kHz. In contrast, strong DPOAEs were only measurable near frequencies where thresholds approached the average curve. Dashed portions of the phase curves and DPOAE amplitude curves indicate SNR <9 dB (DPOAE noise floors not shown for clarity).
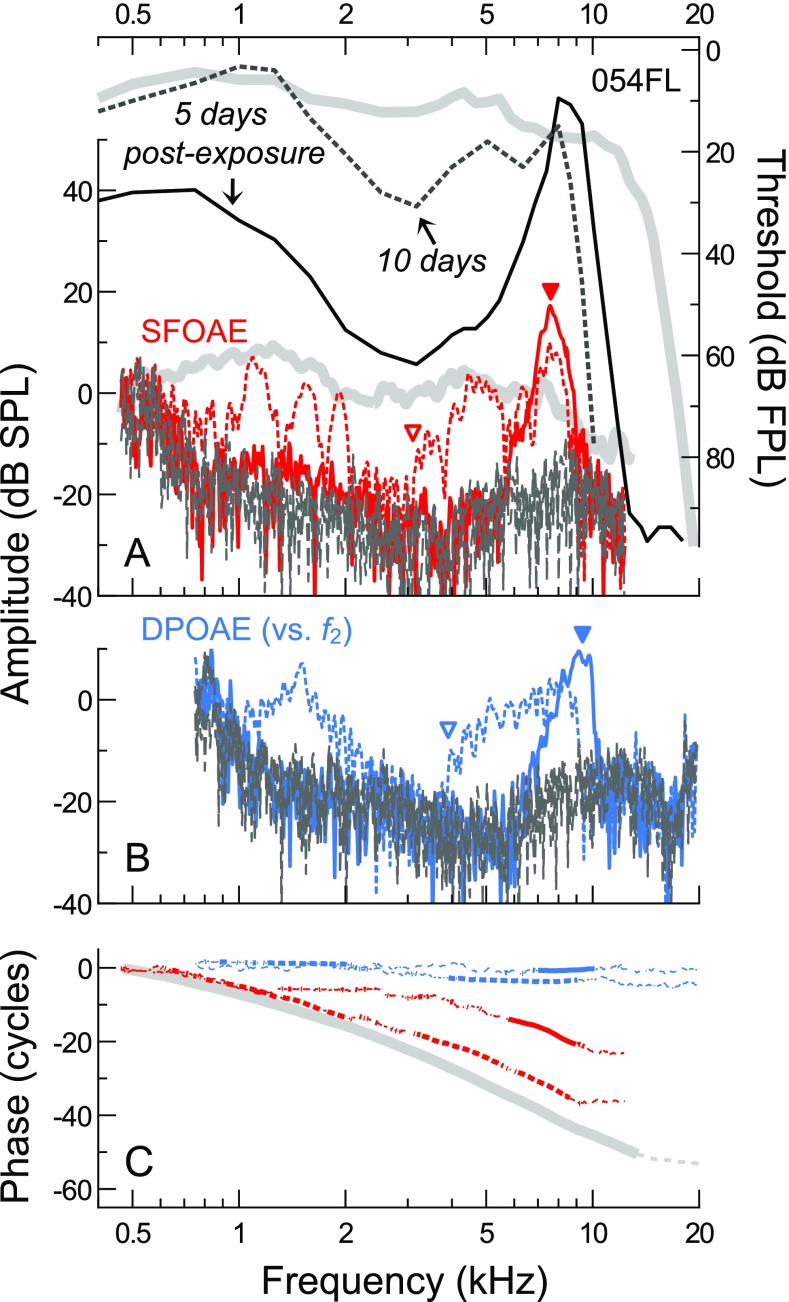
FIG. 12.
A, B Thresholds, SFOAEs, and DPOAE responses for subject 054FL obtained 5 and 10 days after noise exposure (solid and dashed lines, respectively). C SFOAE and DPOAE phases, with thin dashed portions of the curves indicating SNR <9 dB. DPOAE responses are plotted vs. f 2, as in Figure 11. Average thresholds and SFOAE amplitudes/phases from the normal-hearing subjects are shown with thick gray lines in A and C. Though a normal standard clinical audiogram was obtained pre-exposure, pre-exposure tracking thresholds were not available. To highlight the subtle frequency offset between the SFOAE and DPOAE responses, solid triangles indicate the positions of the high-frequency SFOAE/DPOAE amplitude peaks at 5 days post-exposure, and open triangles indicate where the responses became measurable above the noise floor for frequencies >3 kHz at 10 days post-exposure.
Subject 230FR (Fig. 11A, C) possessed abnormally large SFOAE amplitudes at low-to-mid frequencies (approaching 30 dB SPL at 1 kHz) and multiple high-level SOAEs, despite a mild, notch-like threshold elevation between 6 and 10 kHz. Modest SFOAE amplitudes, as well as several low-level SOAEs, were present out to 7.3 kHz, roughly one third of an octave above where thresholds started to rise. DPOAEs were measurable for f 2 frequencies even one third of an octave higher, though component analysis (not shown) indicated that these responses included a large reflection component. Thus, even when sensitivity near the f 2 place is reduced, any distortion generated at this location may propagate to more sensitive, apical locations tuned to 2f 1-f 2, resulting in a significant reflection component in the total DPOAE response. While thresholds improved above 8 kHz and approached average levels near 11 kHz, SFOAEs and DPOAEs did not increase in amplitude above the threshold notch.
In contrast, subject 074FR (Fig. 11B, D) possessed abnormally large SFOAE amplitudes and multiple SOAEs at high frequencies (>6 kHz), despite a mild threshold elevation from 1 to 10 kHz (∼20−25 dB re average). While the steep increase in thresholds from 0.75 to 1 kHz was accompanied by a sharp decline in SFOAE and DPOAE amplitudes, low-level SFOAE responses were observed throughout the mid-frequency region where thresholds exceeded 30 dB FPL and DPOAEs fell close to the noise floor. The SFOAE phase slope was shallower than average in this frequency region, corresponding to abnormally short delays between ∼1 and 4 kHz. SFOAE amplitudes dramatically increased above 6 kHz, approximately two thirds of an octave below the frequency where thresholds approached the average curve, with large SFOAEs observed at frequencies where thresholds were still 8–21 dB higher than average. The relatively narrow region of measurable DPOAEs above 8 kHz aligned better with the contour of the threshold curve at high frequencies. Though the reflection component contributed strongly to the DPOAE response (as indicated by the sloping DPOAE phase, as well as component analysis), this does not change the interpretation of the data. The distortion component had the same general magnitude and shape as the total DPOAE response.
While the etiology of subject 074FR’s hearing loss is unknown, data from another subject suggest that mid-frequency hearing loss and enhanced high-frequency SFOAEs could be associated with noise exposure. Figure 12 shows thresholds, SFOAEs, and DPOAE responses for subject 054FL obtained 5 and 10 days following recreational noise exposure. A normal clinical audiogram was obtained previously from this subject, with thresholds from 0.5 to 8 kHz of 10 dB HL or less, but pre-exposure threshold tracking data were unfortunately not available for comparison. At 5 days post-exposure, thresholds were elevated by 20–50 dB re average across the low-to-mid frequency range, and the high-frequency slope of the threshold curve was positioned about 0.5 octaves lower than average, resulting in very poor sensitivity above 10 kHz. SFOAE responses consisted of a single, narrowly defined peak, with a maximum amplitude of 17.5 dB SPL at 7.6 kHz, greatly exceeding the average at this frequency. The SFOAE peak roughly corresponded to the narrow region of good sensitivity between 8 and 9.3 kHz, indicating that the noise exposure did not affect, or may have even enhanced, cochlear responses within this frequency range (again, pre-exposure tracking thresholds were not available). SFOAEs were otherwise largely absent below 5.7 kHz and above 9.6 kHz, though there were some low-level responses near 1 kHz, where the probe level exceeded threshold.
SFOAE amplitudes obtained at 10 days post-exposure (dashed red line) improved in a manner that roughly mirrored the threshold recovery (see dashed gray line). Response amplitudes approached the average curve below 2 kHz and above 3 kHz but were close to the noise floor for the intermediate frequencies where thresholds remained elevated. Thresholds and SFOAEs unfortunately did not improve above 8 kHz; in fact, the high-frequency threshold slope shifted downward by an additional 1/5th octave. Interestingly, the high-frequency SFOAE peak shifted little in frequency between sessions, though was reduced in amplitude by an amount similar to the inter-session change in thresholds at 8 kHz.
Changes in DPOAE amplitudes between sessions were similar to those observed for SFOAEs at most frequencies. However, the shift in the high-frequency extent of the DPOAE curve better matched the downward shift of the threshold corner frequency. Additionally, at 10 days post-exposure, mid-frequency SFOAE responses emerged from the noise floor at ∼3 kHz, approximately 0.3 octaves below where the DPOAEs had adequate SNR (compare red/blue open triangles). This offset was similar to that observed between the high-frequency SFOAE and DPOAE amplitude peaks at 5 days post-exposure (filled triangles) and consistent with that observed previously for the high-frequency extents of the average SFOAE and DPOAE profiles when the latter were plotted vs. f 2 (Fig. 7A). Thus, while there does not appear to be a single rule that governs the relationship between SFOAE and DPOAE profiles in both audiometrically normal and abnormal ears, data from this subject reinforce the general finding that DPOAEs evoked by low-level tones are measurable up to higher stimulus frequencies than SFOAEs and may more accurately reflect features present in the behavioral audiogram.
DISCUSSION
Relationships Among SFOAEs, TEOAEs, and CR
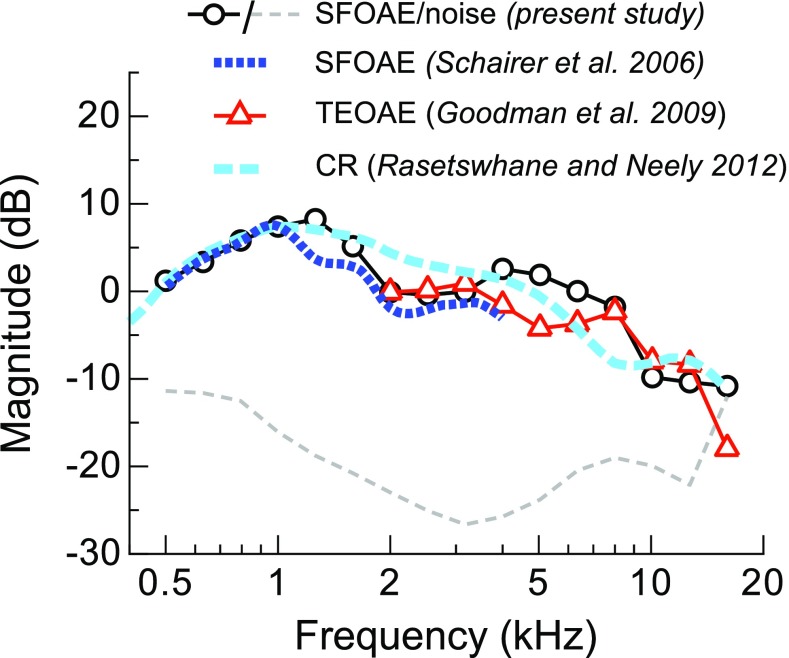
The mechanisms underlying SFOAE generation are also thought to give rise to OAEs evoked by transient stimuli (i.e., TEOAEs) and broadband noise (i.e., CR; Rasetshwane and Neely 2012). Consistent with this, the SFOAE amplitudes and delays presented here were highly similar to those of SFOAEs at lower frequencies (e.g., Schairer et al. 2006; Shera et al. 2010), as well as TEOAEs (Goodman et al. 2009; Keefe 2012), and CR (Rasetshwane and Neely 2012). The similarity in overall spectral shape among these response types is illustrated in Figure 13, which shows SFOAE amplitudes from the present study averaged in 1/3rd-octave bins along with previously reported means/medians (see legend). Compared to SFOAEs and TEOAEs, CR has a slightly more bandpass shape, with magnitudes declining around 4–5 kHz. This may be due to the wider age range of the subjects (15–65 years) included in Rasetshwane and Neely (2012) compared to in the present study (18–25 years) and in Goodman et al. (2009) (14–29 years), likely resulting in weaker mean responses at high frequencies. CR could also be more influenced by nonlinear interactions, such as suppression, due to the broadband stimulus. Regardless, the similar frequency dependence of these responses up to 16 kHz suggests that the high-frequency decline reported for TEOAEs and CR was not primarily due to issues related to calibration or methodology. Instead, the present data indicate that such high-frequency responses are more fundamentally limited by cochlear and/or middle ear factors.
FIG. 13.
Similarity of the spectral shapes of SFOAEs, TEOAEs, and CR. Shown are the SFOAE amplitudes and noise floors from the present study averaged in 1/3rd-octave bins from 0.5 to 16 kHz (circles and dashed line, respectively), along with the mean amplitudes of SFOAEs evoked by 40 dB SPL tones from 0.5 to 4 kHz (from Fig. 11 of Schairer et al. 2006), median 1/3rd-octave averaged amplitudes for TEOAEs evoked by 73 dB peak-equivalent SPL clicks from 2 to 16 kHz (from Fig. 4 of Goodman et al. 2009), and the mean CR amplitude for a 40-dB SPL stimulus (from Fig. 7 of Rasetshwane and Neely 2012). SFOAE amplitudes are shown in dB SPL, with CR and TEOAE magnitudes shifted to match the average SFOAE amplitude from this study at 1 and 2 kHz, respectively.
Comparison with DPOAEs
SFOAE and DPOAE responses had similar magnitudes and spectral shapes at low-to-mid frequencies but differed in their behavior at high frequencies when DPOAEs were considered as a function of f 2. In most audiometrically normal and abnormal ears, DPOAEs were measurable at higher stimulus frequencies, and their amplitudes (plotted vs. f 2) better mirrored the audiogram. Individual and average DPOAE spectra resembled those previously reported for larger subject groups and comparable stimulus levels, though different calibration methods (Dreisbach and Siegel 2001; Dreisbach et al. 2006; Poling et al. 2014), adding confidence to our findings. Since DPOAE responses were dominated by the distortion component, which must originate in the region of overlap between the responses to f 1 and f 2 (and thus, near or basal to the f 2 region), DPOAEs may be used to assess more basal cochlear locations than SFOAEs when responses are evoked by low-level stimuli.
This conclusion is not changed by the finding that DPOAEs also contained a significant SFOAE-like component over much of the measured frequency range, with a magnitude ∼10 dB below that of the distortion component (as in Poling et al. 2014). The spectral characteristics of the reflection component were highly similar to those of the SFOAE from the same ear, thus extending a previous demonstration of this relationship at frequencies below 3 kHz (Kalluri and Shera 2001). The lower absolute magnitude of the reflection component compared to the SFOAE indicates that the effective level of the distortion propagating to the 2f 1-f 2 region was less than that of a 36-dB FPL tone and/or that the presence of the primary tones had nonlinear effects on the generation of the reflection component (Kalluri and Shera 2001). Differences in the fine structure and phase of the SFOAEs and reflection components may therefore also be attributable to either of these scenarios.
Limiting Factors at High Frequencies
While SFOAEs were generally weak above 7 kHz, responses were often still measurable above the noise floor at frequencies approaching the audiometric corner frequency (f t-C). This suggests that SFOAE generation extends throughout the cochlear base, as the f t-C is thought to be the characteristic frequency of the basal-most cochlear location with functional afferent synapses (Ruggero and Temchin 2002). At least in young subjects with low thresholds at f t-C, this location is likely near the basal end of the cochlea. Though it is tempting to interpret f t-C as the upper frequency limit of SFOAE generation, observation of responses above f t-C was perhaps also limited by the reduced sensation level of the probe and the lower microphone sensitivity at these frequencies. Use of higher probe levels would aid in further characterizing the high-frequency extent of SFOAE generation and its relationship to f t-C.
The decline in SFOAE amplitudes at frequencies much lower than f t-C could be attributed to reductions in (1) high-frequency middle ear transmission, (2) the spatial extent of SFOAE generation near the cochlear base, and/or (3) basal OHC status. In particular, a reduction in reverse transmission of OAE energy via the middle ear (i.e., from the cochlea to the ear canal) at a frequency near ∼7–8 kHz could explain the alignment of the average SFOAE and DPOAE spectra when the latter were plotted vs. 2f 1-f 2 (the response frequency). A decline in forward transmission of the stimulus to the cochlea is also possible, though behavioral thresholds did not markedly change near the SFOAE corner frequency. To the extent that SFOAEs grow compressively at the probe level used here, a small decrease in the effective stimulus level alone would result in an even smaller decrease in emission amplitude. A reduction in reverse transmission would more effectively attenuate the responses and would better explain a decline in the SFOAE and DPOAE amplitudes at the same response frequency.
Nevertheless, the present data cannot distinguish between the influence of middle ear and cochlear factors, and the overall amplitude decline near 7 kHz may also be partly or primarily cochlear in origin. For instance, the decline in DPOAE amplitudes at response frequencies of 7 kHz occurred at f 2 frequencies of ∼10 kHz, near the corner frequency of the audiogram. Assuming a cochlear origin for the audiometric and DPOAE corner frequencies, then the alignment of the high-frequency extents of the SFOAE and DPOAE spectra when plotted vs. the response frequency may simply be coincidental. In addition, it is difficult to explain many of the complexities observed in high-frequency SFOAE spectra in terms of middle ear factors. These features included abrupt drops in SFOAE amplitude, patchy responses, and isolated amplitude peaks near the corner frequency of the audiogram. The transmission properties of the middle ear would presumably filter the responses more smoothly with frequency, though abrupt notches in reverse transmission may be possible. The presence of large high-frequency SFOAEs in an ear with elevated mid-frequency thresholds (Fig. 11B) is also most likely not due to a difference in middle ear properties.
Thus, the overall decline in SFOAE amplitudes near 7 kHz could potentially also be attributed to changes in the spatial extent and/or efficiency of the SFOAE generation region at high frequencies. For instance, SFOAEs may be generated over a relatively broad region extending basal to the peak of the traveling wave (e.g., Avan et al. 1991; Siegel et al. 2005), such that their amplitudes are reduced as this region grows increasingly narrow. A dependence of SFOAEs (and TEOAEs) on cochlear regions basal to the probe frequency place has been suggested by the findings that, in humans, thresholds at a given frequency may be most predictive of response amplitudes at lower frequencies (Avan et al. 1991; Ellison and Keefe 2005). Additionally, in animal models, responses at low-to-mid frequencies can be influenced by damage to basal locations (e.g., Avan et al. 1995; Withnell et al. 2000; Charaziak and Siegel 2015). However, similar evidence for basal OAE sources has also been reported for DPOAEs, though at higher stimulus levels (Arnold et al. 1999; Martin et al. 2010). Alternatively, SFOAEs may simply be more sensitive than thresholds and DPOAEs to cochlear status. Reduced high-frequency SFOAE amplitudes could therefore indicate very subtle changes in basal OHC function due to aging or exposure-related damage. If SFOAEs were generated over a significant basal extent and highly sensitive to subtle dysfunction within this region, then this could readily explain the decline in SFOAE amplitudes up to an octave or more below the audiometric corner frequency.
The relationship between SFOAE amplitudes and the extent and/or status of the cochlear region involved in their generation is likely complex. For instance, if SFOAEs were generated over a broad region, then SFOAE amplitudes might be expected to decline smoothly with frequency as this region gradually becomes more restricted. Instead, SFOAEs often dropped abruptly in amplitude, and the SFOAE corner frequency, at least as defined here, was not consistently related to any feature of the audiogram. Though thresholds tended to rise between ∼5 kHz and f t-C, consistent with a slight decline in basal OHC function, threshold levels at and near the SFOAE corner frequency were highly variable across subjects. The presence of narrowly defined response peaks at frequencies near f t-C is also difficult to explain, particularly when they occurred at frequencies where thresholds tended to increase. One possibility is that high-frequency SFOAE sources are distributed such that they tend to cancel in phase, with release from cancelation occurring due to acute irregularities (e.g., damaged or missing OHCs) or restriction of the generation region to the very basal end of the cochlea.
Spectral complexities could also be explained by interactions between distinct, spatially segregated sources. Time-domain analyses suggest that TEOAEs, CR, and SFOAEs are comprised of multiple components with different latencies (Goodman et al. 2009; Rasetshwane and Neely 2012; Sisto et al. 2013), with short-latency components arising from regions with characteristic frequencies between 0.2 and 0.6 octaves higher than those which give rise to long-latency components (Moleti et al. 2013; Moleti et al. 2014; Lewis and Goodman 2015). As the probe frequency is increased, the more basal, shorter-latency components would progressively disappear. Depending on the relative phases of the dominant components, this could produce abrupt drops in SFOAE amplitude, as well as local amplitude peaks. However, preliminary time-frequency analyses of the present data revealed no consistent patterns in the contributions of long- and short-latency components at high frequencies. As the response amplitudes were very low above 7 kHz, it is possible that analysis of data collected at higher probe levels would yield clearer results.
Enhancement of SFOAEs by Damage
The relationship between SFOAEs and cochlear sensitivity at high frequencies may be complicated if certain types of damage actually enhance SFOAE amplitudes. Here, the largest high-frequency SFOAEs were measured in an ear with a mid-to-high frequency hearing loss, even at frequencies where thresholds were elevated and where DPOAEs were not measurable. In another subject, large SFOAEs were also found for a narrowly defined high-frequency region following noise exposure. These data are consistent with previous demonstrations that transitions in threshold sensitivity and/or regions of cochlear damage may be associated with enhanced TEOAEs (Kakigi et al. 1998; Raveh et al. 1998) and SOAEs (Ruggero et al. 1983, 1984; Clark et al. 1984; Powers et al. 1995) at frequencies near the transition or near the characteristic frequency of the damaged region. Selective cochlear damage could enhance OAEs by releasing adjacent, functional OHCs from negative feedback (Ruggero et al. 1983), or more generally, by increasing the mechanical irregularities that produce reflection-source OAEs.
Of particular note is that the effects of noise exposure are most pronounced between 3–6 kHz and above 11 kHz (Fausti et al. 1981; Büchler et al. 2012). This pattern is consistent with the region of spared hearing sensitivity and SFOAEs found post-exposure in Figure 12. If noise exposure can either reduce or enhance OAEs between 6 and 11 kHz, this may partially explain why Ellison and Keefe (2005) found the weakest correlations between thresholds and SFOAEs at 8 kHz, where SFOAEs with high SNR (∼20 dB) could be obtained even when thresholds exceeded 50 dB HL. Goodman et al. (2009) also found the weakest correlation between high-frequency TEOAEs and thresholds at 8 kHz. Regardless of the source of these weaker associations, the possibility that cochlear damage may enhance OAEs while elevating behavioral thresholds complicates attempts to screen or monitor for such damage using OAEs alone. However, if enhancements are more commonly observed for reflection- rather than distortion-source emissions, combined SFOAE and DPOAE measurements may be useful for identifying particular types of lesions.
CONCLUSIONS
SFOAEs evoked using a low-to-moderate probe level are measurable up to ∼15 kHz, though substantially decline in amplitude above 7 kHz. This high-frequency amplitude decline is possibly due to reduced middle ear transmission, the reduced spatial extent of the SFOAE generation region, and/or subtle OHC dysfunction at the cochlear base. However, more acute damage to the mid-basal region may actually enhance high-frequency SFOAE amplitudes, thus complicating the interpretation of such measurements. Though DPOAE amplitudes better mirror the behavioral audiogram in both abnormal and normal ears, it is possible that combined high-frequency DPOAE and SFOAE measurements may be useful in discriminating the effects of aging and noise exposure (or other insults) on cochlear function. Future work could clarify and extend the present findings by comparing high-frequency SFOAE and DPOAE responses over a broader range of stimulus levels, in both male and female subjects.
ACKNOWLEDGMENTS
This work was supported by NIH/NIDCD grant F31 DC013710 and by the School of Communication at Northwestern University. The authors would like to thank the three anonymous reviewers whose helpful comments clarified the manuscript. Additionally, thanks are due to Shawn Goodman and Stephen Neely for providing the MATLAB code and support in implementing the FPL calibration procedure, Simon Henin for code to perform the LSF analysis, as well as Jonathan Siegel for many stimulating discussions. Preliminary analyses of a portion of the data shown here were presented at the 2014 Mechanics of Hearing Workshop in Cape Sounio, Greece and recently published in the conference proceedings (Dewey and Dhar 2015).
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest
The authors declare that they have no conflict of interest.
Contributor Information
James B. Dewey, Phone: +1- 650 498 6268, Email: jbdewey@stanford.edu
Sumitrajit Dhar, Email: s-dhar@northwestern.edu.
REFERENCES
- Arnold DJ, Lonsbury-Martin BL, Martin GK. High-frequency hearing influences lower-frequency distortion-product otoacoustic emissions. Arch Otolaryngol Head Neck Surg. 1999;125:215–222. doi: 10.1001/archotol.125.2.215. [DOI] [PubMed] [Google Scholar]
- Avan P, Bonfils P, Loth D, Narcy P, Trotoux J. Quantitative assessment of human cochlear function by evoked otoacoustic emissions. Hear Res. 1991;52:99–112. doi: 10.1016/0378-5955(91)90191-B. [DOI] [PubMed] [Google Scholar]
- Avan P, Bonfils P, Loth D, Elbez M, Erminy M. Transient-evoked otoacoustic emissions and high-frequency acoustic trauma in the guinea pig. J Acoust Soc Am. 1995;97:3012–3020. doi: 10.1121/1.411866. [DOI] [PubMed] [Google Scholar]
- Büchler M, Kompis M, Hotz MA. Extended frequency range hearing thresholds and otoacoustic emissions in acute acoustic trauma. Otol Neurotol. 2012;33:1315–1322. doi: 10.1097/MAO.0b013e318263d598. [DOI] [PubMed] [Google Scholar]
- Charaziak KK, Siegel JH. Tuning of SFOAEs evoked by low-frequency tones is not compatible with localized emission generation. J Assoc Res Otolaryngol. 2015;16:317–329. doi: 10.1007/s10162-015-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Lee SY, Parham K, Neely ST, Kim DO. Stimulus-frequency otoacoustic emission: measurements in humans and simulations with an active cochlear model. J Acoust Soc Am. 2008;123:2651–2669. doi: 10.1121/1.2902184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WW, Kim DO, Zurek PM, Bohne BA. Spontaneous otoacoustic emissions in chinchilla ear canals: correlation with histopathology and suppression by external tones. Hear Res. 1984;16:299–314. doi: 10.1016/0378-5955(84)90119-9. [DOI] [PubMed] [Google Scholar]
- Dewey JB and Dhar S (2015) Wideband profiles of stimulus-frequency otoacoustic emissions in humans. In: Karavitaki KD, Corey DP (eds) Mechanics of hearing: protein to perception. American Institute of Physics, Melville, NY, pp 090018-1–090018-5.
- Dreisbach LE (1999) Characterizing the 2f1-f2 distortion-product otoacoustic emission and its generators measured from 2 to 20 kHz in humans. Doctoral dissertation, Northwestern University, Evanston, Illinois
- Dreisbach LE, Siegel JH. Distortion-product otoacoustic emissions measured at high frequencies in humans. J Acoust Soc Am. 2001;110:2456–2469. doi: 10.1121/1.1406497. [DOI] [PubMed] [Google Scholar]
- Dreisbach LE, Siegel JH. Level dependence of distortion-product otoacoustic emissions measured at high frequencies in humans. J Acoust Soc Am. 2005;117:2980–2988. doi: 10.1121/1.1880792. [DOI] [PubMed] [Google Scholar]
- Dreisbach LE, Long KM, Lees SE. Repeatability of high-frequency distortion-product otoacoustic emissions in normal-hearing adults. Ear Hear. 2006;27:466–479. doi: 10.1097/01.aud.0000233892.37803.1a. [DOI] [PubMed] [Google Scholar]
- Ellison JC, Keefe DH. Audiometric predictions using stimulus-frequency otoacoustic emissions and middle ear measurements. Ear Hear. 2005;26:487–503. doi: 10.1097/01.aud.0000179692.81851.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausti SA, Erickson DA, Frey RH, Rappaport BZ, Schechter MA. The effects of noise upon human hearing sensitivity from 8000 to 20 000 Hz. J Acoust Soc Am. 1981;69:1343–1347. doi: 10.1121/1.385805. [DOI] [PubMed] [Google Scholar]
- Goodman SS, Fitzpatrick DF, Ellison JC, Jesteadt W, Keefe DH. High-frequency click-evoked otoacoustic emissions and behavioral thresholds in humans. J Acoust Soc Am. 2009;125:1014–1032. doi: 10.1121/1.3056566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SS, Withnell RH, Shera CA. The origin of SFOAE microstructure in the guinea pig. Hear Res. 2003;183:7–17. doi: 10.1016/S0378-5955(03)00193-X. [DOI] [PubMed] [Google Scholar]
- Greenwood DD. A cochlear frequency-position function for several species—29 years later. J Acoust Soc Am. 1990;87:2592–2605. doi: 10.1121/1.399052. [DOI] [PubMed] [Google Scholar]
- Hecker DJ, Lohscheller J, Bader CA, Delb W, Schick B, Dlugaiczyk J. A new method to analyze distortion product otoacoustic emissions (DPOAEs) in the high-frequency range up to 18 kHz using windowed periodograms. IEEE Trans Biomed Eng. 2011;58:2369–2377. doi: 10.1109/TBME.2011.2157154. [DOI] [PubMed] [Google Scholar]
- Kakigi A, Hirakawa H, Harel N, Mount RJ, Harrison RV. Basal cochlear lesions result in increased amplitude of otoacoustic emissions. J Otolaryngol. 1998;27:354–360. doi: 10.1159/000013806. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Distortion-product source unmixing: a test of the two-mechanism model for DPOAE generation. J Acoust Soc Am. 2001;109:622–637. doi: 10.1121/1.1334597. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Near equivalence of human click-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am. 2007;121:2097–2110. doi: 10.1121/1.2435981. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Comparing stimulus-frequency otoacoustic emissions measured by compression, suppression, and spectral smoothing. J Acoust Soc Am. 2007;122:3562–3575. doi: 10.1121/1.2793604. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Shera CA. Measuring stimulus-frequency otoacoustic emissions using swept tones. J Acoust Soc Am. 2013;134:356–368. doi: 10.1121/1.4807505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH. Moments of click-evoked otoacoustic emissions in human ears: group delay and spread, instantaneous frequency and bandwidth. J Acoust Soc Am. 2012;132:3319–3350. doi: 10.1121/1.4757734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Ellison JC, Fitzpatrick DF, Gorga MP. Two-tone suppression of stimulus frequency otoacoustic emissions. J Acoust Soc Am. 2008;123:1479–1494. doi: 10.1121/1.2828209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Goodman SS, Ellison JC, Fitzpatrick DF, Gorga MP. Detecting high-frequency hearing loss with click-evoked otoacoustic emissions. J Acoust Soc Am. 2011;129:245–261. doi: 10.1121/1.3514527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DT. The evoked cochlear mechanical response and the auditory microstructure—evidence for a new element in cochlear mechanics. Scand Audiol Supp. 1979;9:35–47. [PubMed] [Google Scholar]
- Kemp DT. Towards a model for the origin of cochlear echoes. Hear Res. 1980;2:533–548. doi: 10.1016/0378-5955(80)90091-X. [DOI] [PubMed] [Google Scholar]
- Kemp DT, Chum RA (1980) Observations on the generator mechanism of stimulus frequency acoustic emissions—two tone suppression. In: deBoer E, Viergever MA (eds) Psychophysical, physiological and behavioral studies in hearing. Delft University Press, Delft, pp 34–41.
- Konrad-Martin D, Keefe DH. Time-frequency analyses of transient-evoked stimulus-frequency and distortion-product otoacoustic emissions: testing cochlear model predictions. J Acoust Soc Am. 2003;114:2021–2043. doi: 10.1121/1.1596170. [DOI] [PubMed] [Google Scholar]
- Lee J, Dhar S, Abel R, Banakis R, Grolley E, Lee J, Zecker S, Siegel J. Behavioral hearing thresholds between 0.125 and 20 kHz using depth-compensated ear simulator calibration. Ear Hear. 2012;33:315–329. doi: 10.1097/AUD.0b013e31823d7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JD, Goodman SS. Basal contributions to short-latency transient-evoked otoacoustic emission components. J Assoc Res Otolaryngol. 2015;16:29–45. doi: 10.1007/s10162-014-0493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long GR, Talmadge CL. Spontaneous otoacoustic emission frequency is modulated by heartbeat. J Acoust Soc Am. 1997;102:2831–2848. doi: 10.1121/1.420339. [DOI] [PubMed] [Google Scholar]
- Long GR, Talmadge CL, Lee J. Measuring distortion product otoacoustic emissions using continuously sweeping primaries. J Acoust Soc Am. 2008;124:1613–1626. doi: 10.1121/1.2949505. [DOI] [PubMed] [Google Scholar]
- Martin GK, Stagner BB, Lonsbury-Martin BL. Evidence for basal distortion-product otoacoustic emission components. J Acoust Soc Am. 2010;127:2955–2972. doi: 10.1121/1.3353121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moleti A, Al-Maamury AM, Bertaccini D, Botti T, Sisto R. Generation place of the long- and short-latency components of transient-evoked otoacoustic emissions in a nonlinear cochlear model. J Acoust Soc Am. 2013;133:4098–4108. doi: 10.1121/1.4802940. [DOI] [PubMed] [Google Scholar]
- Moleti A, Sisto R, Lucertini M. Experimental evidence for the basal generation place of the short-latency transient-evoked otoacoustic emissions. J Acoust Soc Am. 2014;135:2862–2872. doi: 10.1121/1.4870699. [DOI] [PubMed] [Google Scholar]
- Poling GL, Siegel JH, Lee J, Lee J, Dhar S. Characteristics of the 2f1-f2 distortion product otoacoustic emission in a normal hearing population. J Acoust Soc Am. 2014;135:287–299. doi: 10.1121/1.4845415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers NL, Salvi RJ, Wang J, Spongr V, Qiu CX. Elevation of auditory thresholds by spontaneous cochlear oscillations. Nature. 1995;375:585–587. doi: 10.1038/375585a0. [DOI] [PubMed] [Google Scholar]
- Puria S. Measurements of human middle ear forward and reverse acoustics: implications for otoacoustic emissions. J Acoust Soc Am. 2003;113:2773–2789. doi: 10.1121/1.1564018. [DOI] [PubMed] [Google Scholar]
- Rasetshwane DM, Neely ST. Calibration of otoacoustic emission probe microphones. J Acoust Soc Am. 2011;130:EL238–243. doi: 10.1121/1.3632047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetshwane DM, Neely ST. Inverse solution of ear-canal area function from reflectance. J Acoust Soc Am. 2011;130:3873–3881. doi: 10.1121/1.3654019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasetshwane DM, Neely ST. Measurements of wide-band cochlear reflectance in humans. J Assoc Res Otolaryngol. 2012;13:591–607. doi: 10.1007/s10162-012-0336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raveh E, Mount RJ, Harrison RV. Increased otoacoustic-emission amplitude secondary to cochlear lesions. J Otolaryngol. 1998;27:354–360. [PubMed] [Google Scholar]
- Ruggero MA, Kramek B, Rich NC. Spontaneous otoacoustic emissions in a dog. Hear Res. 1984;13:293–296. doi: 10.1016/0378-5955(84)90083-2. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Rich NC, Freyman R. Spontaneous and impulsively evoked otoacoustic emissions: indicators of cochlear pathology? Hear Res. 1983;10:283–300. doi: 10.1016/0378-5955(83)90094-1. [DOI] [PubMed] [Google Scholar]
- Ruggero MA, Temchin AN. The roles of the external, middle, and inner ears in determining the bandwidth of hearing. Proc Natl Acad Sci. 2002;99:13206–13210. doi: 10.1073/pnas.202492699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schairer KS, Ellison JC, Fitzpatrick D, Keefe DH. Use of stimulus-frequency otoacoustic emission latency and level to investigate cochlear mechanics in human ears. J Acoust Soc Am. 2006;120:901–914. doi: 10.1121/1.2214147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperle RA, Goodman SS, Neely ST. Further assessment of forward pressure level for in situ calibration. J Acoust Soc Am. 2011;130:3882–3892. doi: 10.1121/1.3655878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperle RA, Neely ST, Kopun JG, Gorga MP. Influence of in situ, sound-level calibration on distortion-product otoacoustic emission variability. J Acoust Soc Am. 2008;124:288–300. doi: 10.1121/1.2931953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA. Mammalian spontaneous otoacoustic emissions are amplitude-stabilized cochlear standing waves. J Acoust Soc Am. 2003;114:244–262. doi: 10.1121/1.1575750. [DOI] [PubMed] [Google Scholar]
- Shera CA, Bergevin C. Obtaining reliable phase-gradient delays from otoacoustic emission data. J Acoust Soc Am. 2012;132:927–943. doi: 10.1121/1.4730916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ. Evoked otoacoustic emissions arise by two fundamentally different mechanisms: a taxonomy for mammalian OAEs. J Acoust Soc Am. 1999;105:782–798. doi: 10.1121/1.426948. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ. Stimulus-frequency-emission group delay: a test of coherent reflection filtering and a window on cochlear tuning. J Acoust Soc Am. 2003;113:2762–2772. doi: 10.1121/1.1557211. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci. 2002;99:3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Oxenham AJ. Otoacoustic estimation of cochlear tuning: validation in the chinchilla. J Assoc Res Otolaryngol. 2010;11:343–365. doi: 10.1007/s10162-010-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JH. Ear-canal standing waves and high-frequency sound calibration using otoacoustic emission probes. J Acoust Soc Am. 1994;95:2589–2597. doi: 10.1121/1.409829. [DOI] [Google Scholar]
- Siegel JH (2007) Calibration of otoacoustic emission probes. In: Robinette MS, Glattke TJ (eds) Otoacoustic emissions: clinical applications (3rd ed). New York, Thieme Medical, pp 403–427
- Siegel JH, Cerka AJ, Recio-Spinoso A, Temchin AN, van Dijk P, Ruggero MA. Delays of stimulus-frequency otoacoustic emissions and cochlear vibrations contradict the theory of coherent reflection filtering. J Acoust Soc Am. 2005;118:2434–2443. doi: 10.1121/1.2005867. [DOI] [PubMed] [Google Scholar]
- Sisto R, Moleti A, Shera CA. On the spatial distribution of the reflection sources of different latency components of otoacoustic emissions. J Acoust Soc Am. 2015;137:768–776. doi: 10.1121/1.4906583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisto R, Sanjust F, Moleti A. Input/output functions of different-latency components of transient-evoked and stimulus-frequency otoacoustic emissions. J Acoust Soc Am. 2013;133:2240–2253. doi: 10.1121/1.4794382. [DOI] [PubMed] [Google Scholar]
- Souza NN, Dhar S, Neely ST, Siegel JH. Comparison of nine methods to estimate ear-canal stimulus levels. J Acoust Soc Am. 2014;136:1768–1787. doi: 10.1121/1.4894787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge CL, Long GR, Murphy WJ, Tubis A. New off-line method for detecting spontaneous otoacoustic emissions in human subjects. Hear Res. 1993;71:170–182. doi: 10.1016/0378-5955(93)90032-V. [DOI] [PubMed] [Google Scholar]
- Talmadge CL, Tubis A, Long GR, Tong C. Modeling the combined effects of basilar membrane nonlinearity and roughness on stimulus frequency otoacoustic emission fine structure. J Acoust Soc Am. 2000;108:2911–2932. doi: 10.1121/1.1321012. [DOI] [PubMed] [Google Scholar]
- Withnell RH, Yates GK, Kirk DL. Changes to low-frequency components of the TEOAE following acoustic trauma to the base of the cochlea. Hear Res. 2000;139:1–12. doi: 10.1016/S0378-5955(99)00132-X. [DOI] [PubMed] [Google Scholar]
- Zweig G, Shera CA. The origin of periodicity in the spectrum of evoked otoacoustic emissions. J Acoust Soc Am. 1995;98:2018–2047. doi: 10.1121/1.413320. [DOI] [PubMed] [Google Scholar]