Abstract
Acoustic information propagates from the ear to the brain via spiral ganglion neurons that innervate hair cells in the cochlea. These afferents include unmyelinated type II fibers that constitute 5 % of the total, the majority being myelinated type I neurons. Lack of specific genetic markers of type II afferents in the cochlea has been a roadblock in studying their functional role. Unexpectedly, type II afferents were visualized by reporter proteins induced by tyrosine hydroxylase (TH)-driven Cre recombinase. The present study was designed to determine whether TH-driven Cre recombinase (TH-2A-CreER) provides a selective and reliable tool for identification and genetic manipulation of type II rather than type I cochlear afferents. The “TH-2A-CreER neurons” radiated from the spiral lamina, crossed the tunnel of Corti, turned towards the base of the cochlea, and traveled beneath the rows of outer hair cells. Neither the processes nor the somata of TH-2A-CreER neurons were labeled by antibodies that specifically labeled type I afferents and medial efferents. TH-2A-CreER-positive processes partially co-labeled with antibodies to peripherin, a known marker of type II afferents. Individual TH-2A-CreER neurons gave off short branches contacting 7–25 outer hair cells (OHCs). Only a fraction of TH-2A-CreER boutons were associated with CtBP2-immunopositive ribbons. These results show that TH-2A-CreER provides a selective marker for type II versus type I afferents and can be used to describe the morphology and arborization pattern of type II cochlear afferents in the mouse cochlea.
Keywords: hair cells, cochlea, hearing, afferent, type II fiber, dopamine
INTRODUCTION
Numerous specializations distinguish the mammalian cochlea from the auditory organ of other vertebrates. Among these are two distinct classes of afferent neurons that differ by number, size, and myelination and innervation pattern. Acoustic information is transmitted centrally by the predominant (95 %) myelinated type I afferents, each of which contacts a single inner hair cell. In contrast, unmyelinated type II afferents extend hundreds of microns along the cochlear coil to receive input from many outer hair cells (OHCs). Despite this extended dendritic arbor, type II afferents are only weakly activated by hair cell glutamate release (Weisz et al. 2009; Weisz et al. 2012) and are insensitive to sound (Brown 1994; Robertson 1984; Robertson et al. 1999), although they project centrally in parallel with neighboring type I afferents (Berglund and Brown 1994; Brown et al. 1988; Brown and Ledwith 1990; Morgan et al. 1994). Emerging evidence suggests that type II afferents instead respond to tissue damage (Flores et al. 2015; Liu et al. 2015) perhaps to serve as cochlear nociceptors. Further insight into type II function will require knowledge of their central connectivity, as well as animal models in which type II function can be altered selectively. Such studies will be advanced by identification of biomarkers, genes whose activity can be used to alter type II function specifically in behaving animal models. One potential biomarker is peripherin, a type III intermediate filament protein expressed mainly in peripheral neurons or central neurons projecting to the periphery. Peripherin antibodies label type II but not type I afferents in young rodent cochleas (Hafidi 1998; Hafidi et al. 1993; Huang et al. 2007b; Reid et al. 2004). A complication is that peripherin is expressed by all cochlear afferents at perinatal ages (Hafidi et al. 1993; Huang et al. 2007b). Other candidates include voltage-gated sodium and potassium channels. For example, firing properties differ significantly between type II and type I SGNs in cochlear explants from young rats (Jagger and Housley 2003; Reid et al. 2004), but whether this reflects qualitative differences in ion channel expression remains to be determined. It is possible that relative protein levels, rather than unique expression profiles, determine activity patterns, as appears to be the case for tonotopic differences among type I SGNs (Adamson et al. 2002; Reid et al. 2004), comprehensively reviewed in (Rusznak and Szucs 2009).
The present study establishes an alternative gene product for the selective manipulation of type II versus type I cochlear afferents. Tyrosine hydroxylase (TH) is the enzyme that converts the amino acid l-tyrosine to l-3,4-dihydroxyphenylalanine (l-DOPA), leading on to synthesis of dopamine, norepinephrine, and epinephrine, and as such is usually considered to be a marker of catecholaminergic neurons. A Th 2a-CreER mouse line was acquired to study dopaminergic lateral olivocochlear (LOC) efferents. As expected, a population of LOCs as well as sympathetic neurons were labeled in these cochleas after P15, as will be described in a separate study (Wu and Glowatzki, in progress). Unexpectedly however, Th 2a-CreER cochleas also showed a pattern of reporter protein expression reminiscent of type II afferent morphology. Previous immunohistochemical studies in mice (Darrow et al. 2006), rats (Trigueiros-Cunha et al. 2003), and guinea pig (Eybalin et al. 1993) reported that tyrosine hydroxylase is expressed by the lateral efferents originating near the lateral superior olive (LSO) and in type I cochlear afferents, rather than the type II-like pattern observed here. Thus, we compared TH-2A-CreER-positive neurons with immunolabeling of type I afferent and efferent neurons of the cochlea. The present studies were designed to determine whether TH-2A-CreER expression provides a selective tool for the genetic manipulation of type II cochlear afferents and to add to description of type II afferent morphology in the mouse cochlea.
MATERIALS AND METHODS
Animals
All animal procedures were carried out in accordance with the protocols approved by the Johns Hopkins Animal Care and Use committee. The generation of Th 2A-CreER mice will be described in detail in a subsequent publication. In brief, a T2A- peptide CreER cassette was inserted just before the 3′ UTR of the tyrosine hydroxylase gene using a recombineering protocol, to allow for efficient transcription of both tyrosine hydroxylase and Cre recombinase. Reporter line Ai3 +/− [B6.Cg-Gt(ROSA)26Sortm3(CAG-EYFP)Hze/J, #007903] mice were obtained from The Jackson Laboratory, and Ai9 +/− [B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, #007905] mice were obtained from Dr. Michael Deans, University of Utah. Prph1-cre [C57BL/6-Tg(Prph1-cre)35Dmd/Mmucd, #000120] sperm was obtained from the Mutant Mouse Resource and Research Centers (NIH). Mice carrying this transgene were recovered by the Transgenic Core Laboratory (Johns Hopkins University). Mice of either sex were used in the study.
Animals for Transmission Electron Microscopy (TEM)
Serial section electron microscopy was carried out on mid or basal turn cochlear segments of one 21-day-old mouse from each of four different strains: FVB/NJ—basal turn, C57BL/6—basal turn, 129S6 × CBA/CaJ—medial turn, and FVB129.J2—medial turn.
Tamoxifen Injection
Tamoxifen (Sigma #T5648) was dissolved in corn oil (Sigma #C8267) at a concentration of 10 mg/ml and subjected to sonication for up to 2 h at room temperature. Tamoxifen was stored in the dark, and stocks were made fresh for every use. For studying the phenotype of postnatal day (P) 8 animals, P1, P3, and P5 pups were injected with tamoxifen in their milk spot using an insulin syringe with an ultrafine needle (BD, 22G). For analysis at 3–8 weeks, tamoxifen was administered by gavage (Table 1).
TABLE 1.
Tamoxifen injection regimen at different ages
| Tamoxifen injection regimen | |||
|---|---|---|---|
| Tamoxifen dose | Administration age | Administration mode | Analysis age |
| 30–60 μg per animal | P1, P3, P5 | Intragastric injection | P8 |
| 1.0 mg/10 g body weight | P15, P20 | Gavage | P25–P56 |
Immunohistochemistry
The primary antibodies used in this study were anti-GFP (goat polyclonal, Sicgen #AB0020, 1:5000), anti-mCherry (goat polyclonal, Sicgen #AB0040, 1:200-1:1000), anti-TuJ1 (rabbit monoclonal, Covance #MRB-435P, 1:300), anti-α3 Na+/K+ ATPase (mouse monoclonal, Thermo Fisher #MA3-915, 1:500), anti-CtBP2 (mouse monoclonal, BD Biosciences #612044 1:200), anti-tyrosine hydroxylase (rabbit polyclonal, Millipore #657012, 1:500-1:1000), anti-myosin VI (rabbit polyclonal, Sigma #M5187, 1:500), anti-myosin VIIa (mouse monoclonal, DSHB #MYO7A 138-1, 1:300-1:500; data not shown; used for identification of hair cells in Fig. 4D), and anti-peripherin (mouse monoclonal, Chemicon #MAB1527; rabbit polyclonal, Chemicon #AB1530; rabbit polyclonal, Abcam #ab4666). They produced similar labeling pattern in our experience. Figure 3, in particular, was performed with Chemicon MAB1527. Mice were subjected to deep anesthesia by isoflurane inhalation and decapitated; the cochleas were harvested from the temporal bones and perfused with 4 % paraformaldehyde (PFA) through the round and oval windows. The tissue was post-fixed for 1 h at 4 °C, washed with phosphate buffer solution (1× PBS), and dissected into apical, medial, and basal turns. The cochlear turns were incubated in 30 % sucrose for 10 min, subjected to a quick freeze (−80 °C) and thaw (37 °C) and then washed with 1× PBS. The tissues were then incubated in a blocking and permeabilizing solution containing 10 % normal donkey serum, 1 % BSA, and 0.5 % Triton X-100 in 1× PBS for 1 h at room temperature. Primary antibodies were applied in the same solution that had been diluted 1:1 with 1× PBS. The tissues were incubated in the primary antibody solution for 48 h at room temperature. After three washes in 1× PBS, the tissues were incubated with Alexa Fluor-conjugated secondary antibodies (Molecular Probes) used at 1:1000 dilution for 1–2 h at room temperature. Tissues were mounted in FluorSave antifade mounting medium (CalBiochem). Images were acquired via a LSM700 confocal microscope (Zeiss Axio Imager Z2) using 10× and 25× water immersion, and 40× oil N.A. 1.30 immersion objectives. Images were processed using ImageJ, Photoshop CS6 (Adobe) and illustrator CS6 (Adobe). Image analysis was carried out using Zen software (Zeiss) and Imaris (Bitplane).
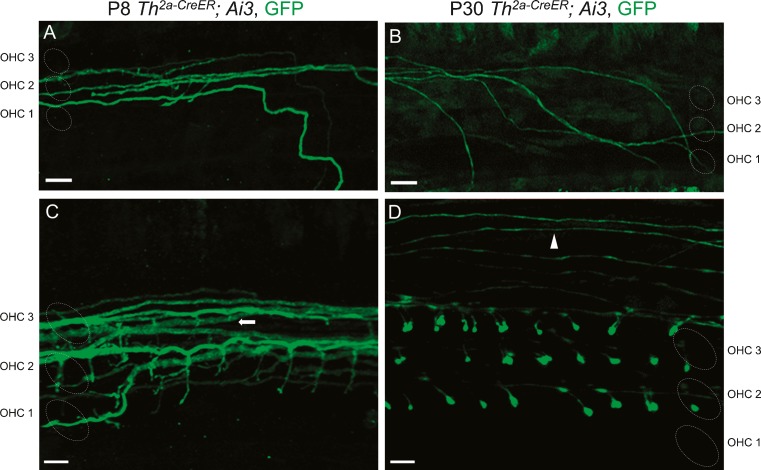
FIG. 4.
Type II afferent morphology changes during development. Apical cochlear turns of Th 2a-CreER; Ai3 mice were analyzed at P8 (A, C) and P30 (B, D). A Type II afferents (green, anti-GFP) execute right-angle basal-ward turns after crossing the tunnel of Corti at P8. B Type II trajectory (green) is curvilinear at P30. C Type II terminal projections (green) were filamentous at P8. D Enlarged terminal boutons (green) were found on OHCs at P30. While type II fibers ran underneath the three rows of OHCs at younger ages (C, arrow), at older ages, they additionally appeared on the periphery beyond the rows of OHCs (D, arrowhead). Scale bar represents 10 μm in A, B and 5 μm in C, D.
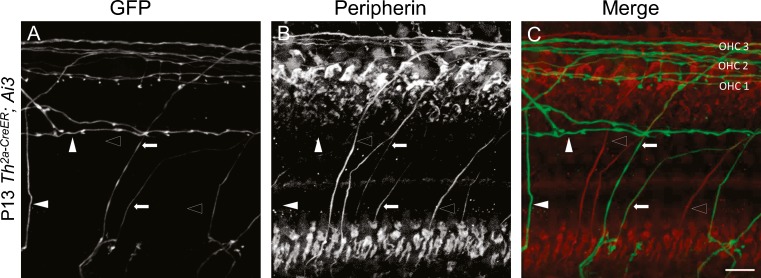
FIG. 3.
Some TH-2A-CreER-labeled neurons are peripherin-positive. Organ of Corti (cochlear apex) from P13 Th 2a-CreER; Ai3 mice immunolabeled with antibodies against GFP (A), and peripherin (B). Arrows indicate type II fibers immunopositive for both GFP and peripherin. Open arrow heads indicate type II fibers expressing peripherin, but not EYFP. Solid arrow heads indicate type II fibers expressing EYFP that appear to be peripherin-negative. C Overlay of GFP (green) and peripherin (red) channels. Images are presented as maximum intensity projections of a stack of confocal micrographs from the apical turn. Panel B is oversaturated posthoc to illustrate relatively weak peripherin signal for illustration. TH-2A-Cre ER labeling is intentionally sparse due to low-dose tamoxifen, so not every type II fiber is EYFP-positive. Peripherin immunolabel was relatively weak; nonetheless, it appears that some TH-2A-Cre ER neurons had very low, or no, peripherin expression (solid arrowheads). Scale bar represents 20 μm.
TEM
Tissue was obtained and processed according to published methods (Fuchs et al. 2014). In brief, euthanasia and tissue extraction was carried out according to approved animal protocols (Johns Hopkins Institutional Animal Care and Use Committee). Tissue was fixed with 1 % osmium (OsO4) and 1 % potassium ferricyanide [FeK3(CN)6] in 0.1 M sym-collidine-HCl buffer (pH 7.4) rinsed with 0.1 M maleate buffer (pH 7.4) in 5 % EDTA in 0.1 M phosphate buffer (pH 7.4–7.8). Tissues were embedded in Araldite and then sectioned at 40 μm parallel to the modiolus. These thick sections were re-embedded in Epon between Aclar sheets (EMS) and desired cochlear segments cut out for thin sectioning. Chosen sections were re-embedded in Epon blocks for ultra-thin sectioning (Leica Ultracut S) parallel to the modiolar axis (perpendicular to the organ of Corti). Serial 65-nm sections were collected onto Formvar-coated slot grids, for image acquisition on a Hitachi H7600 TEM at 80 kV at 30,000 magnification. Digital images (2120 × 2120 pixels) were collected at 8- or 16-bit depth and analyzed as 8-bit files. Images were imported into Reconstruct software (Fiala 2005) for assembly, calibration, and alignment. The outline of the hair cell, efferent and afferent neuronal contacts, and the postsynaptic cistern were traced. Synaptic ribbons and associated vesicles were traced when present. Partial reconstructions were made of the synaptic pole of 26 OHCs that included 35 afferent boutons.
RESULTS
Tyrosine Hydroxylase Is Expressed in a Subset of Neurons in the Cochlea
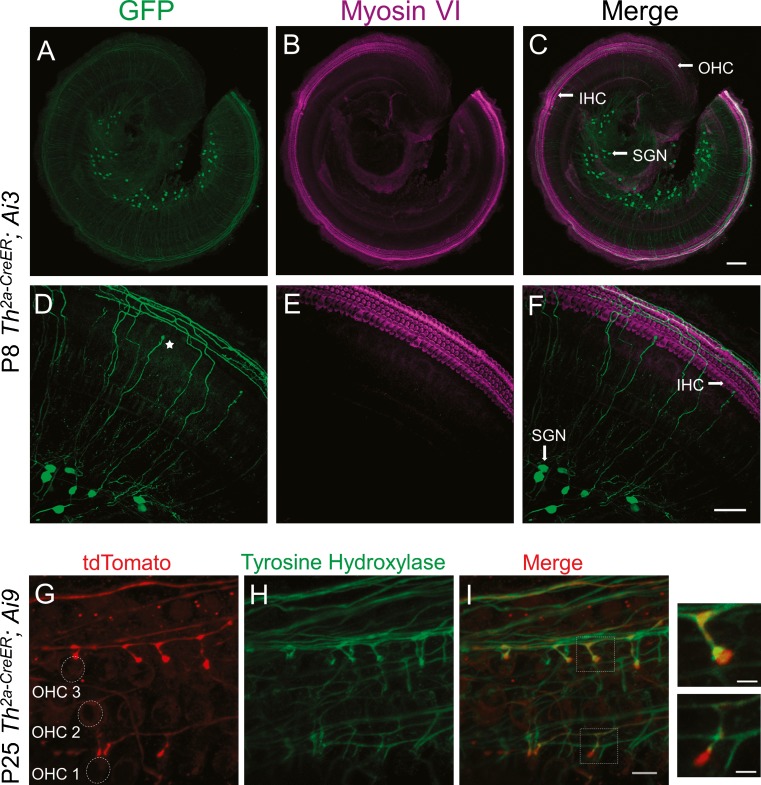
To investigate the expression of tyrosine hydroxylase (TH) in the mouse cochlea, Th 2a-CreER; Ai3 or Th 2a-CreER; Ai9 mice were treated with tamoxifen to induce Cre recombinase-mediated expression of reporter proteins, and examined at various ages (see Table 1). For most experiments, a low concentration of tamoxifen was used, to allow for sparse labeling, so that the anatomy of individual labeled fibers could be investigated. The cochlear epithelium from Th 2a-CreER; Ai3 mice at postnatal day (P) 8 was labeled with antibodies against GFP (which recognize as well the EYFP molecule) (Fig. 1A) and against myosin VI, a cytoskeletal protein that can be used to identify IHCs and OHCs in the cochlea (Fig. 1B, E). GFP antibodies labeled a subset of spiral ganglion neurons (Fig. 1A) giving rise to thin fibers with a trajectory like that of type II afferents (Fig. 1D): radiating from the spiral lamina and crossing the tunnel of Corti to turn towards the base of the cochlea, traveling beneath the rows of OHCs. Hereafter, these will be referred to as “TH-2A-CreER” neurons. While TH-2A-CreER neurons could be observed in basal cochlea (Table 2), reporter expression was more reliable and robust in the cochlear apex. Thus, examples from apical cochlea are shown for sparse labeling experiments. In 3–4-week-old cochleas, additionally to type II-like fibers, putative dopaminergic lateral efferent terminals and sympathetic fibers were labeled (data not shown, Wu and Glowatzki, in preparation). When tamoxifen was administered early (during the first postnatal week) and reporter expression observed before postnatal day 13, some labeled fibers had enlarged terminals near inner hair cells (Fig. 1D, star). These few fibers (∼10 % of labeled fibers) bear a passing resemblance to putative type I processes seen in the first postnatal week (Druckenbrod and Goodrich 2015). These “IHC-ending” neurons were not found when tamoxifen was administered at P15 or P30.
FIG. 1.
Cochlear type II-like neurons express tyrosine hydroxylase (TH). A Apical cochlear turn (P8) of Th 2a-CreER; Ai3 mice labeled with antibodies to GFP (green). A subset of neuronal somata in the spiral ganglion and projecting fibers are seen. B Antibodies to myosin VI label IHCs and OHCs in same tissue (magenta). C Merge of GFP-myosin VI labels. D, E, F Maximum intensity projections of higher magnification confocal image stacks show GFP-immunopositive fibers crossing the tunnel of Corti to make a basal-ward turn into the spiral bundles beneath OHCs, characteristic for type II fibers. A star (D) marks one of a small fraction of labeled fibers that end near the inner hair cells only in young cochleas. G tdTomato (red) expressed in Th 2a-CreER; Ai9 cochlea labels type II-like fibers and boutons among OHCs (P25). H Antibody labeling of TH (green) on same tissue. I Merge of tdTomato and TH labels shows overlap of both labels except for the terminal boutons, where TH immunolabel was lower (insets). Scale bar represents 100 μm (A–C), 50 μm (D–F), 10 μm (G–I), and 3 μm for inset.
TABLE 2.
1–2-month-old Th2a-CreER; Ai3 or Th2a-CreER; Ai9 mice were given low dose of tamoxifen and analyzed for the number of OHCs contacted by a single fiber
| Synaptic bouton counts | ||||
|---|---|---|---|---|
| Fiber number | Total synaptic branches | Branches in major synaptic area | Age | Location |
| F1 | 12 | 12 | 4 weeks | Apex |
| F2 | 17 | 17 | 4 weeks | Apex |
| F3 | 15 | 15 | 4 weeks | Apex |
| F4 | 10 | 10 | 4 weeks | Apex |
| F5 | 13 | 13 | 4 weeks | Apex |
| F6 | 10 | 10 | 4 weeks | Base |
| F7 | 12 | 12 | 4 weeks | Base |
| F8 | 25 | 14 | 4 weeks | Apex |
| F9 | 11 | 11 | 8 weeks | Apex |
| F10 | 8 | 8 | 6 weeks | Apex |
| F11 | 8 | 5 | 6 weeks | Apex |
| F12 | 9 | 9 | 6 weeks | Apex |
| F13 | 9 | 9 | 7 weeks | Apex |
| F14 | 7 | 7 | 7 weeks | Apex |
| F15 | 8 | 5 | 7 weeks | Apex |
| Avg ± SD | 11.6 ± 4.6 | 10.5 ± 3.5 | ||
To determine if TH protein was present in the labeled type II-like fibers, cochlear explants from Th 2a-CreER; Ai9 mice were double-labeled with antibodies against TH and tdTomato, here shown at P25 (Fig. 1G–I). TH immunolabeling co-localized with the tdTomato fluorescence in type II-like fibers traveling beneath the rows of OHCs. In the terminal-most boutons, tdTomato was significantly brighter (Fig. 1I, insets).
TH-2A-CreER Neurons Are Neither Type I Afferents nor Medial Efferents
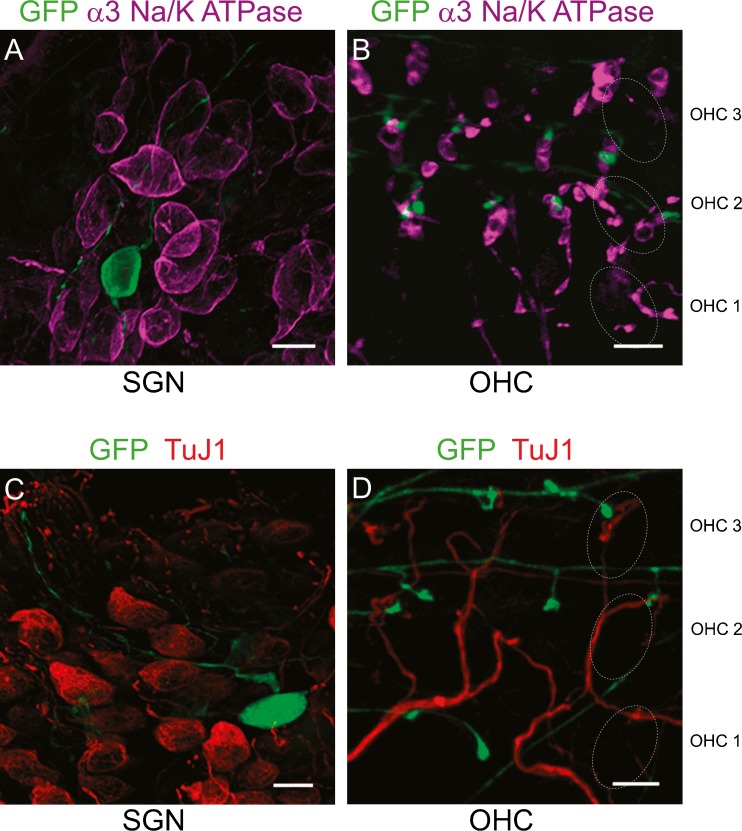
To confirm the identity of labeled neurons in Th 2a-CreER; Ai3 cochleas as type II fibers, molecular markers against other neuronal populations were used. It has been shown that α3 Na+/K+ ATPase is expressed specifically in type I afferents and medial efferents and is absent from type II afferents (McLean et al. 2009). Cochlear whole mounts of P30 Th 2a-CreER; Ai3 mice were co-immunolabeled with antibodies against α3 Na+/K+ ATPase to identify type I afferents and medial efferents. TH-2A-CreER fibers were clearly distinct from both type I afferents and medial efferents (Fig. 2). Neuronal somata in the spiral ganglion were immunopositive for either α3 Na+/K+ ATPase, or GFP, but not both (Fig. 2A). Synaptic endings among the OHCs were either larger α3 Na+/K+ ATPase-positive medial efferent terminals, or smaller GFP-immunopositive type II-like afferent terminals (Fig. 2B). The distinct identity of TH-2A-CreER neurons was further demonstrated by double labeling with antibodies against the neuron-specific class III beta-tubulin (TuJ1). TuJ1 immunolabeling has been previously reported as selective for type I neurons (Xing et al. 2012). At P30, neither GFP-immunopositive neuronal somata nor GFP-immunopositive neuronal processes among OHCs co-localized with neurons strongly labeled for TuJ1 (Fig. 2C, D), type I afferents and medial olivocochlear efferents, respectively.
FIG. 2.
TH-2A-CreER does not label type I afferents or efferents. A Spiral ganglion neurons (P30 cochlear apex) in Th 2a-CreER; Ai3 mice labeled with antibodies to GFP (green) or α3 Na+/K+ ATPase (magenta-type I afferents) show no overlap between these two types. B Outer hair cell region shows TH-2A-CreER boutons (green) on OHCs, distinct from medial efferent terminals (magenta-α3 Na+/K+ ATPase). C Spiral ganglion neurons (P30 cochlear apex) in Th 2a-CreER; Ai3 mice labeled with antibodies to GFP (green) or TuJ1 (red) show no overlap between these two types. D Outer hair cell region shows TH-2A-CreER boutons (green) on OHCs, distinct from TuJ1 labeled medial efferent terminals (red). Dotted ovals indicate position of OHC rows. Scale bar represents 10 μm.
Some TH-2A-CreER Neurons Were Peripherin-Positive
Further evidence for the identification of TH-2A-CreER neurons was obtained by co-immunolabeling with an antibody against peripherin. Peripherin is a type III intermediate filament expressed in the neurons of the peripheral nervous system and a subset of neurons in the central nervous system, which extend their processes into peripheral organs (Escurat et al. 1990; Gorham et al. 1990) (Hafidi et al. 1993) (Ryugo et al. 1991). It has been used as a specific marker for type II cochlear afferents (Huang et al. 2007b). Some TH-2A-CreER EYFP-positive neurons were immunolabeled for peripherin, supporting their identification as type II afferents (Arrows, Fig. 3A–C). It was expected that not all peripherin-positive neurons were labeled with GFP antibodies (Open arrow heads, Fig. 3A–C), as a low concentration of tamoxifen was used to induce only sparse Cre-mediated recombination. Unexpectedly, however, some TH-2A-CreER EYFP-positive neurons with type II morphology appeared to be peripherin-negative (solid arrowheads, Fig. 3A–C).
Developmental Maturation of Type II Afferent Trajectories and Terminations
To examine morphological maturation, the dendritic arbors of TH-2A-CreER type II afferents were detailed in the cochleas of mice before and after the onset of hearing. The trajectory and shape of terminal branches of TH-2A-CreER fibers changed between P8 and P30. At P8, type II fibers had a sharply rectilinear trajectory along the rows of OHCs, as though confined to narrow channels. However, at P30, their trajectory was more curvilinear, without distinctive right-angle turns (Fig. 4A, B). This change in morphology was consistent throughout apical, medial, and basal turns of the cochlea. Also, spiral processes of type II fibers appeared to be larger diameter at P8 than those at P30 (Fig. 4A, B). At P8, the average fiber diameter was 1.26 ± 0.20 μm (SD) over total length of 1618 μm (n = 14 from six cochlear preparations), which is statistically different (t(21) = 6.98, p = 6.78×10−7) from the average fiber diameter at P30, 0.70 ± 0.12 μm (SD) over total length of 1149 μm (n = 9 from four cochlear preparations). P8 vs P30 fiber diameter ratio was 1.8.
While type II fibers ran underneath the three rows of OHCs at younger ages (Fig. 4C, arrow), at older ages, they additionally appeared on the periphery beyond the rows of OHCs (Fig. 4D, arrowhead). The terminal branches of type II afferents were filamentous at P8, with small terminal swellings. At P30, stouter branches appeared with enlarged terminal boutons (Fig. 4D).
Single TH-2A-CreER Fibers Contact Many OHCs Within Concentrated Synaptic Zones
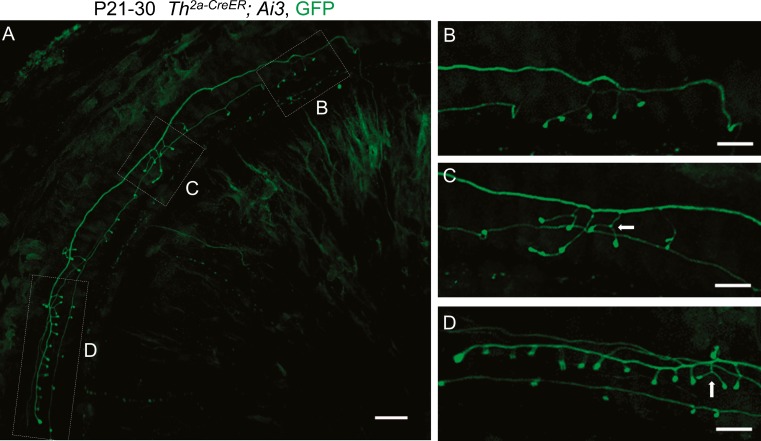
After crossing the tunnel of Corti, a single type II afferent is thought to contact as many as 30–60 OHCs, depending on species and cochlear location (Berglund and Ryugo 1987; Brown 1987; Ginzberg and Morest 1983; Jagger and Housley 2003; Perkins and Morest 1975; Simmons and Liberman 1988; Weisz et al. 2009). Previous studies in postnatal rats have shown that an individual type II fiber contacted ∼23 OHCs on average (Martinez-Monedero et al. 2016) and was functionally coupled to at least 10 OHCs (Weisz et al. 2012). Given the low average probability of release, computational modeling suggested that glutamate release from 24 OHCs would be required to cause type II afferent spiking in rat (Weisz et al. 2014). The trajectory and putative synaptic contacts of single TH-2A-CreER type II fibers were examined after low-dose tamoxifen to produce sparse labeling (few neurons) in 1–2-month-old mice. Single TH-2A-CreER neurons crossed the tunnel of Corti to travel hundreds of microns towards the cochlear base. Most of the TH-2A-CreER type II fibers contained a single spiral process, from which the majority of terminal branches projected out to end in enlarged boutons on OHCs. Less commonly seen were fibers with bifurcated spiral processes that could innervate adjacent rows of OHCs. Each fiber usually contained a major arborization zone (“synaptic area”) in which the largest number of terminal branches occurred (Fig. 5A, area D). Subsidiary clusters with fewer terminal branches could also be found in some fibers (Fig. 5B, C) (Table 2). The average number of terminal branches in the major synaptic area from a single TH-2A-CreER type II fiber was 10.5 ± 3.5 (n = 15), and the average total number of branches per fiber was 11.6 ± 4.6 (n = 15) (Table 2). Some terminal branches had multiple bouton-like swellings in series (Fig. 5C) or formed “wishbones” with two boutons (Fig. 5C, D; arrows). Less certain from this experiment is whether two different TH-2A-CreER fibers could contact the same outer hair cell (but see below). We next sought to establish functionality by comparing TH-2A-CreER projections with the locus of presynaptic ribbons labeled with antibodies against CtBP2.
FIG. 5.
Branching pattern of an individual type II afferent fiber. Maximum intensity projections of confocal image stacks taken in the cochlear apex of P30 Th 2a-CreER; Ai3 mice (A). After crossing the tunnel of Corti, type II fibers turn towards the base, sporadically extending a few branches (B, C) before a terminal arborization with 14 terminations (D). B–D Enlargements of minor and major arborizations. Some terminal branches formed “wishbones” with two boutons (arrows). Scale bar represents 10 μm.
Synaptic Structures of Type II Afferents
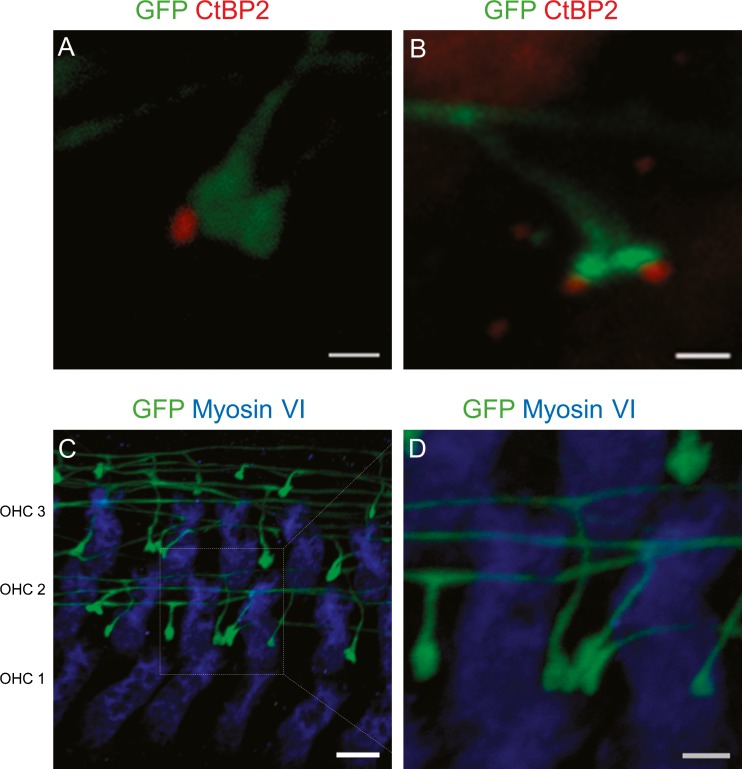
For these experiments, a high dose of tamoxifen (Table 1) was used to maximize visualization of TH-2A-CreER neurons. With large numbers of labeled TH-2A-CreER fibers, it was clear that not all bouton endings were associated with CtBP2-labeled puncta (Fig. 6A, B). Neighboring terminals could be ribbon-associated or ribbon-free. With high-dose tamoxifen, it proved possible to observe cases in which more than one type II afferent contacted a single OHC. Featured here are three contacts arising from three different type II afferent fibers (Fig. 6C, D).
FIG. 6.
Synaptic structure of type II afferents. Organ of Corti from 3–4-week-old Th 2a-CreER; Ai3 mice apical cochlear turns were immunolabeled for the presynaptic ribbon protein CtBP2/RIBEYE (red), myosin VI, and GFP. A Type II afferent boutons make synaptic contacts with outer hair cells with or without an associated synaptic ribbon. B Two type II afferent boutons from a single fiber associated with two ribbons. C Three afferent boutons from three different type II afferents making synapses on one OHC. D Magnified image that shows boutons from three fibers. Scale bar represents 1 μm in A, B, D and E and 5 μm in C.
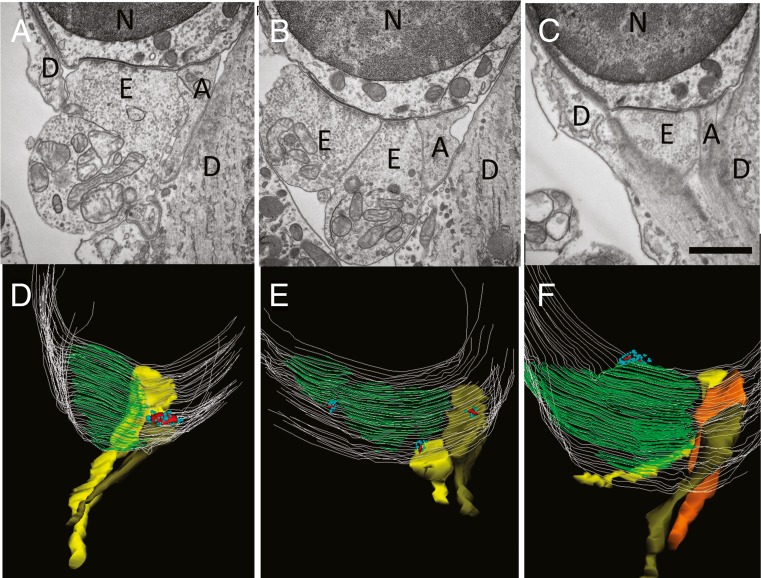
The association of type II fiber terminals with ribbons was examined further using transmission electron microscopy and serial reconstruction of the synaptic pole of OHCs in the mouse cochlea. Synaptic arrangements were quantified in OHCs from the medial or basal cochlear turns of four individual wild-type mice (P21) of different strains (see “Materials and Methods” section). Afferent contacts were readily distinguished from efferent contacts by their vesicle-free, finely granular cytoplasm, typically smaller hair cell contact area, and the absence of a postsynaptic cistern (Fig. 7). Synaptic ribbons of OHCs had a central electron dense core and a cluster of nearby small clear vesicles that were usually fewer in number and more heterogeneous in size and disposition than those found at inner hair cell ribbons (Fuchs and Glowatzki 2015; Weisz et al. 2012). Thirty-five afferent boutons on 26 OHCs were reconstructed. Among these, 18 were associated with presynaptic ribbons. In some cases, neighboring boutons were ribbon-less (Fig. 7). In two cases, “orphan” ribbons also were found that were not allied with afferent boutons. Afferent contacts were always adjacent to much larger efferent synapses (shown here by the synaptic cistern co-extensive with efferent contacts), and the entire synaptic pole was partially or completely bracketed by Deiters’ cell processes.
FIG. 7.
Transmission electron micrographs and reconstructions of OHC synapses. A–C Single thin (65 nm) sections from three different OHCs (basal turn, FVB/NJ mouse, P21). Scale bar = 1 μm. Large vesiculated efferent terminals cover most of the synaptic pole and lie opposite to a postsynaptic cistern seen at this magnification as a thickening of the hair cell plasma membrane. Smaller non-vesiculated, granular afferents are opposite hair cell ribbons in approximately half the cases. D–F Z-axis projections of 3D reconstructions. A–C provide example single sections from D–F reconstructions. Hair cell membrane in light gray lines, postsynaptic cisterns colored green. Afferent terminals in yellow, olive, or orange. Synaptic ribbons colored red, nearby vesicles turquoise. Sometimes ribbons appear “misplaced,” e.g., lying over the postsynaptic efferent cistern. Abbreviations N nucleus of outer hair cell, E efferent, A afferent, D Deiters’ cell.
DISCUSSION
This work was designed to validate a Th 2a-CreER mouse line for the study of type II cochlear afferents. Cochlear whole mounts from Th 2a-CreER; Ai3 or Th 2a-CreER; Ai9 mice treated with tamoxifen displayed a small subset of labeled spiral ganglion neurons. They projected from the spiral ganglion into the outer spiral bundle, forming terminal branches onto numerous OHCs hundreds of microns basal to the tunnel of Corti crossing, as typically found for type II afferents. TH-2A-CreER neurons were not labeled by antibodies that specifically labeled type I afferents or medial efferents, but could co-label with tyrosine hydroxylase (TH) antibodies. These results demonstrated the specific expression of Th in type II spiral ganglion neurons and validated the Th 2a-CreER mouse line for selective labeling of type II fibers. TH-2A-CreER type II neurons were mostly found in the cochlear apex, suggesting a tonotopic variation of Th expression levels in type II neurons. Molecular heterogeneity among type II afferents is the subject of ongoing work exploring TH and other candidate biomarkers.
This somewhat surprising outcome merits further discussion, particularly given the observation that some TH-2A-CreER neurons appeared to be peripherin-negative. Many laboratories have described preferential labeling of type II afferents by peripherin antibodies, e.g., (Flores-Otero and Davis 2011; Hafidi 1998; Huang et al. 2007a; McLean et al. 2009; Wang and Green 2011). However, various factors can limit the use of peripherin as a type II biomarker. Peripherin immunolabeling can vary with species and tonotopic position (Mou et al. 1998). Further, peripherin antibodies label type I as well as type II afferents in the perinatal cochlea (Hafidi et al. 1993). In any event, it is not known whether all type II afferents express peripherin equally, so in that sense the, present differential peripherin immunolabeling of TH-2A-CreER type II afferents may not be atypical.
We sought also to characterize type II afferent innervation by crossing a transgenic Prph1-cre mouse (Zhou et al. 2002) with Ai9 reporter mice. Although small somata in dorsal root ganglia were reliably labeled in these mice as previously reported, only a few (2–3) type II afferents expressed tdTomato after Cre recombination (data not shown), discouraging use of this Prph1-cre mouse model for studying type II afferents. Genetic knockout of the peripherin gene was reported to ablate type II afferents (Froud et al. 2015), and a peripherin-GFP fusion mouse has been used to label spiral ganglion neuronal somata (Sundaresan et al. 2016). While peripherin-GFP labeled neuronal somata and many fibers within the spiral ganglion, it was not reported whether that expression pattern was specific to type II processes within the organ of Corti. Given the utility of the TH-2A-CreER mouse line for labeling type II afferents, peripherin was not pursued further.
By adjusting the level of tamoxifen, it was possible to vary from sparse to dense labeling. Higher amounts of tamoxifen produced multiple labeled terminals on each OHC, and these could arise from different type II fibers. When compared to the distribution of presynaptic ribbons labeled with CtBP2 antibodies, only some TH-2A-CreER boutons were associated with ribbons. Ribbon-associated and ribbon-lacking boutons could be adjacent on one OHC, as could two ribbon-associated boutons. The presence of ribbon-associated and ribbon-free afferent synapses was also seen in 3D reconstructions from serial section TEM of mouse OHCs. These showed that half of all afferent contacts were ribbon-free, reminiscent of earlier reports of ribbon-less contacts in various species (Dunn and Morest 1975; Francis and Nadol 1993; Liberman et al. 1990; Nadol 1983; Sobkowicz et al. 1986). Pre- and postsynaptic immunolabeling in rat showed that only half of the postsynaptic densities in type II boutons were associated with ribbons in OHCs (Martinez-Monedero 2016). Interestingly, only those postsynaptic densities immunopositive for the AMPAR subunit GluA2 were associated with ribbons. While the present study does not address this last point directly, the quantitative similarity in arrangement of type II afferent boutons with OHC ribbons in both rats (54 %) and mice (51 %) suggests similar underlying molecular organization.
With sparse labeling, it was possible to determine the arborization patterns of single type II afferents. Several regions of branching were observed in TH-2A-CreER type II afferents, one of which included the majority of branches. Using biocytin to fill individual fibers in postnatal rats, an average of 16 terminal branches were found (Martinez-Monedero et al. 2016), which is similar to the result from this study in 1–2-month-old mice, where an average of ∼12 endings per fiber were counted. Intracellular recording suggested that at least 10 OHCs are presynaptic to each type II afferent (Weisz et al. 2012). Although derived from quite different methods, species, ages, and cochlear regions, type II structure and function correspond reasonably well.
TH expression in lateral efferent fibers also was observed here as in guinea pig (Eybalin et al. 1993) and mouse (Darrow et al. 2006). Previously, TH expression also was reported to occur transiently during postnatal maturation of type I cochlear afferents in rats, but specifically not in type II (peripherin-positive) afferents (Trigueiros-Cunha et al. 2003). However, Th 2A-CreER-driven reporter expression was found in type II afferents, and not type I afferents in the present study. Immunolabeling with TH antibody also showed the presence of TH protein in type II afferents out to 5 weeks of age. In any event, neither class of afferents is known to engage in catecholaminergic transmission, although transmission to central targets of type II afferents has not been studied. It remains to be determined whether type II afferents express other components required for dopaminergic or other catecholaminergic signaling (aromatic l-amino acid decarboxylase, the vesicular dopamine transporter VMAT2, etc.) since TH expression alone is not a guarantor of catecholaminergic synaptic transmission. For example, TH-positive neurons in locus ceruleus generate glutamatergic postsynaptic currents (Holloway et al. 2013) while TH-positive neurons in striatum are GABA-ergic and do not release dopamine (Xenias et al. 2015).
This leaves unanswered what role TH does play in such cells. Among those considered elsewhere is that l-DOPA may serve as an activity-dependent intracellular signaling molecule for cells prone to synaptic rearrangement (Ugrumov 2009). This is of some interest with respect to type II afferents, many of whose terminal branches appear to be lacking presynaptic ribbons, suggesting weaker or no function. Perhaps, type II afferents remain plastic throughout life, altering connectivity as the cochlea ages, or is subject to insult. For example, OHCs of SK2-null mice with diminished efferent innervation have increased numbers of ribbon-associated type II boutons compared to wild-type (Fuchs et al. 2014). Whatever its actual function proves to be, the selective expression of TH-dependent Cre recombinase enables genetic manipulation of type II versus type I cochlear afferents. Such an experimental model will be useful in the context of development, damage, and disease, but even more fundamentally, to establish the function of TH-expressing type II cochlear afferents.
Acknowledgments
Supported by NIDCD R01DC006476 and R01DC012957 to EG, NIDCD R01DC011741 to PAF, NIDCD P30 DC005211 to the Center for Hearing and Balance, NS050274 to the Department of Neuroscience Multiphoton Imaging Core, and by support from by the John Mitchell, Jr. Trust and the David M. Rubenstein Fund for Hearing Research. We thank H. Hiel and M. Lehar for the help with electron microscopy. AZ was supported by the Howard Hughes Medical Institute.
Author Contributions
PV, JSW, and EG designed research; PV and JSW performed research and analyzed data; AZ designed and produced the Th 2a-CreER mouse; and PV, JSW, PF, and EG discussed results and wrote the paper.
Compliance with Ethical Standards
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
Pankhuri Vyas and Jingjing Sherry Wu contributed equally to this work.
Paul Fuchs and Elisabeth Glowatzki contributed equally to this work.
Contributor Information
Pankhuri Vyas, Email: pvyas1@jhmi.edu.
Jingjing Sherry Wu, Email: jwu57@jhmi.edu.
Amanda Zimmerman, Email: amanda_zimmerman@hms.harvard.edu.
Paul Fuchs, Email: pfuchs1@jhmi.edu.
Elisabeth Glowatzki, Phone: 410-502-7008, Email: eglowat1@jhmi.edu.
References
- Adamson CL, Reid MA, Mo ZL, Bowne-English J, Davis RL. Firing features and potassium channel content of murine spiral ganglion neurons vary with cochlear location. J Comp Neurol. 2002;447:331–350. doi: 10.1002/cne.10244. [DOI] [PubMed] [Google Scholar]
- Berglund AM, Brown MC. Central trajectories of type II spiral ganglion cells from various cochlear regions in mice. Hear Res. 1994;75:121–130. doi: 10.1016/0378-5955(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Berglund AM, Ryugo DK. Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol. 1987;255:560–570. doi: 10.1002/cne.902550408. [DOI] [PubMed] [Google Scholar]
- Brown MC. Morphology of labeled efferent fibers in the guinea pig cochlea. J Comp Neurol. 1987;260:605–618. doi: 10.1002/cne.902600412. [DOI] [PubMed] [Google Scholar]
- Brown MC. Antidromic responses of single units from the spiral ganglion. J Neurophysiol. 1994;71:1835–1847. doi: 10.1152/jn.1994.71.5.1835. [DOI] [PubMed] [Google Scholar]
- Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;278:581–590. doi: 10.1002/cne.902780409. [DOI] [PubMed] [Google Scholar]
- Brown MC, Ledwith JV., 3rd Projections of thin (type-II) and thick (type-I) auditory-nerve fibers into the cochlear nucleus of the mouse. Hear Res. 1990;49:105–118. doi: 10.1016/0378-5955(90)90098-A. [DOI] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, Liberman MC. Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol. 2006;498:403–414. doi: 10.1002/cne.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckenbrod NR, Goodrich LV. Sequential retraction segregates SGN processes during target selection in the cochlea. J Neurosci. 2015;35:16221–16235. doi: 10.1523/JNEUROSCI.2236-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RA, Morest DK. Receptor synapses without synaptic ribbons in the cochlea of the cat. Proc Natl Acad Sci U S A. 1975;72:3599–3603. doi: 10.1073/pnas.72.9.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escurat M, Djabali K, Gumpel M, Gros F, Portier MM. Differential expression of two neuronal intermediate-filament proteins, peripherin and the low-molecular-mass neurofilament protein (NF-L), during the development of the rat. J Neurosci. 1990;10:764–784. doi: 10.1523/JNEUROSCI.10-03-00764.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eybalin M, Charachon G, Renard N. Dopaminergic lateral efferent innervation of the guinea-pig cochlea: immunoelectron microscopy of catecholamine-synthesizing enzymes and effect of 6-hydroxydopamine. Neuroscience. 1993;54:133–142. doi: 10.1016/0306-4522(93)90389-W. [DOI] [PubMed] [Google Scholar]
- Fiala JC. Reconstruct: a free editor for serial section microscopy. J Microsc. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Flores-Otero J, Davis RL. Synaptic proteins are tonotopically graded in postnatal and adult type I and type II spiral ganglion neurons. J Comp Neurol. 2011;519:1455–1475. doi: 10.1002/cne.22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores EN, Duggan A, Madathany T, Hogan AK, Marquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, Garcia-Anoveros J. A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol. 2015;25:606–612. doi: 10.1016/j.cub.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis HW, Nadol JB., Jr Patterns of innervation of outer hair cells in a chimpanzee: I. Afferent and reciprocal synapses. Hear Res. 1993;64:184–190. doi: 10.1016/0378-5955(93)90004-K. [DOI] [PubMed] [Google Scholar]
- Froud KE, Wong AC, Cederholm JM, Klugmann M, Sandow SL, Julien JP, Ryan AF, Housley GD. Type II spiral ganglion afferent neurons drive medial olivocochlear reflex suppression of the cochlear amplifier. Nat Commun. 2015;6:7115. doi: 10.1038/ncomms8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Glowatzki E. Synaptic studies inform the functional diversity of cochlear afferents. Hear Res. 2015;330:18–25. doi: 10.1016/j.heares.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Lehar M, Hiel H. Ultrastructure of cisternal synapses on outer hair cells of the mouse cochlea. J Comp Neurol. 2014;522:717–729. doi: 10.1002/cne.23478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzberg RD, Morest DK. A study of cochlear innervation in the young cat with the Golgi method. Hear Res. 1983;10:227–246. doi: 10.1016/0378-5955(83)90056-4. [DOI] [PubMed] [Google Scholar]
- Gorham JD, Baker H, Kegler D, Ziff EB. The expression of the neuronal intermediate filament protein peripherin in the rat embryo. Brain Res Dev Brain Res. 1990;57:235–248. doi: 10.1016/0165-3806(90)90049-5. [DOI] [PubMed] [Google Scholar]
- Hafidi A. Peripherin-like immunoreactivity in type II spiral ganglion cell body and projections. Brain Res. 1998;805:181–190. doi: 10.1016/S0006-8993(98)00448-X. [DOI] [PubMed] [Google Scholar]
- Hafidi A, Despres G, Romand R. Ontogenesis of type II spiral ganglion neurons during development: peripherin immunohistochemistry. Int J Dev Neurosci. 1993;11:507–512. doi: 10.1016/0736-5748(93)90024-8. [DOI] [PubMed] [Google Scholar]
- Holloway BB, Stornetta RL, Bochorishvili G, Erisir A, Viar KE, Guyenet PG. Monosynaptic glutamatergic activation of locus coeruleus and other lower brainstem noradrenergic neurons by the C1 cells in mice. J Neurosci. 2013;33:18792–18805. doi: 10.1523/JNEUROSCI.2916-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LC, Thorne PR, Housley GD, Montgomery JM (2007a) Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development [DOI] [PubMed]
- Huang LC, Thorne PR, Housley GD, Montgomery JM. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development. 2007;134:2925–2933. doi: 10.1242/dev.001925. [DOI] [PubMed] [Google Scholar]
- Jagger DJ, Housley GD. Membrane properties of type II spiral ganglion neurones identified in a neonatal rat cochlear slice. J Physiol. 2003;552:525–533. doi: 10.1111/j.1469-7793.2003.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW, Pierce S. Afferent and efferent innervation of the cat cochlea: quantitative analysis with light and electron microscopy. J Comp Neurol. 1990;301:443–460. doi: 10.1002/cne.903010309. [DOI] [PubMed] [Google Scholar]
- Liu C, Glowatzki E, Fuchs PA. Unmyelinated type II afferent neurons report cochlear damage. Proc Natl Acad Sci U S A. 2015;112:14723–14727. doi: 10.1073/pnas.1515228112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Monedero, R. 2016. GluA2-containing AMPA receptors distinguish ribbon-associated from ribbonless afferent contacts on rat cochlear hair cells. 3. [DOI] [PMC free article] [PubMed]
- Martinez-Monedero R, Liu C, Weisz C, Vyas P, Fuchs PA, Glowatzki E (2016) GluA2-containing AMPA receptors distinguish ribbon-associated from ribbonless afferent contacts on rat cochlear hair cells. eNeuro 3 [DOI] [PMC free article] [PubMed]
- McLean WJ, Smith KA, Glowatzki E, Pyott SJ. Distribution of the Na, K-ATPase alpha subunit in the rat spiral ganglion and organ of corti. J Assoc Res Otolaryngol. 2009;10:37–49. doi: 10.1007/s10162-008-0152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan YV, Ryugo DK, Brown MC. Central trajectories of type II (thin) fibers of the auditory nerve in cats. Hear Res. 1994;79:74–82. doi: 10.1016/0378-5955(94)90128-7. [DOI] [PubMed] [Google Scholar]
- Mou K, Adamson CL, Davis RL. Time-dependence and cell-type specificity of synergistic neurotrophin actions on spiral ganglion neurons. J Comp Neurol. 1998;402:129–139. doi: 10.1002/(SICI)1096-9861(19981207)402:1<129::AID-CNE9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Nadol JB., Jr Serial section reconstruction of the neural poles of hair cells in the human organ of Corti. II. outer hair cells. Laryngoscope. 1983;93:780–791. doi: 10.1288/00005537-198306000-00015. [DOI] [PubMed] [Google Scholar]
- Perkins RE, Morest DK. A study of cochlear innervation patterns in cats and rats with the Golgi method and Nomarkski Optics. J Comp Neurol. 1975;163:129–158. doi: 10.1002/cne.901630202. [DOI] [PubMed] [Google Scholar]
- Reid MA, Flores-Otero J, Davis RL. Firing patterns of type II spiral ganglion neurons in vitro. J Neurosci. 2004;24:733–742. doi: 10.1523/JNEUROSCI.3923-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res. 1984;15:113–121. doi: 10.1016/0378-5955(84)90042-X. [DOI] [PubMed] [Google Scholar]
- Robertson D, Sellick PM, Patuzzi R. The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hear Res. 1999;136:151–158. doi: 10.1016/S0378-5955(99)00120-3. [DOI] [PubMed] [Google Scholar]
- Rusznak Z, Szucs G. Spiral ganglion neurones: an overview of morphology, firing behaviour, ionic channels and function. Pflugers Arch. 2009;457:1303–1325. doi: 10.1007/s00424-008-0586-2. [DOI] [PubMed] [Google Scholar]
- Ryugo DK, Dodds LW, Benson TE, Kiang NY. Unmyelinated axons of the auditory nerve in cats. J Comp Neurol. 1991;308:209–223. doi: 10.1002/cne.903080208. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Liberman MC. Afferent innervation of outer hair cells in adult cats: II. Electron microscopic analysis of fibers labeled with horseradish peroxidase. J Comp Neurol. 1988;270:145–154. doi: 10.1002/cne.902700112. [DOI] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GL, Levenick CV. Distribution of synaptic ribbons in the developing organ of Corti. J Neurocytol. 1986;15:693–714. doi: 10.1007/BF01625188. [DOI] [PubMed] [Google Scholar]
- Sundaresan S, Kong JH, Fang Q, Salles FT, Wangsawihardja F, Ricci AJ, Mustapha M. Thyroid hormone is required for pruning, functioning and long-term maintenance of afferent inner hair cell synapses. Eur J Neurosci. 2016;43:148–161. doi: 10.1111/ejn.13081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigueiros-Cunha N, Renard N, Humbert G, Tavares MA, Eybalin M. Catecholamine-independent transient expression of tyrosine hydroxylase in primary auditory neurons is coincident with the onset of hearing in the rat cochlea. Eur J Neurosci. 2003;18:2653–2662. doi: 10.1046/j.1460-9568.2003.02989.x. [DOI] [PubMed] [Google Scholar]
- Ugrumov MV. Non-dopaminergic neurons partly expressing dopaminergic phenotype: distribution in the brain, development and functional significance. J Chem Neuroanat. 2009;38:241–256. doi: 10.1016/j.jchemneu.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011;31:7938–7949. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–1129. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz CJ, Glowatzki E, Fuchs PA. Excitability of type II cochlear afferents. J Neurosci. 2014;34:2365–2373. doi: 10.1523/JNEUROSCI.3428-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz CJ, Lehar M, Hiel H, Glowatzki E, Fuchs PA. Synaptic transfer from outer hair cells to type II afferent fibers in the rat cochlea. J Neurosci. 2012;32:9528–9536. doi: 10.1523/JNEUROSCI.6194-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenias HS, Ibanez-Sandoval O, Koos T, Tepper JM. Are striatal tyrosine hydroxylase interneurons dopaminergic? J Neurosci. 2015;35:6584–6599. doi: 10.1523/JNEUROSCI.0195-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Samuvel DJ, Stevens SM, Dubno JR, Schulte BA, Lang H. Age-related changes of myelin basic protein in mouse and human auditory nerve. PLoS One. 2012;7 doi: 10.1371/journal.pone.0034500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Nepote V, Rowley DL, Levacher B, Zvara A, Santha M, Mi QS, Simonneau M, Donovan DM. Murine peripherin gene sequences direct Cre recombinase expression to peripheral neurons in transgenic mice. FEBS Lett. 2002;523:68–72. doi: 10.1016/S0014-5793(02)02936-8. [DOI] [PubMed] [Google Scholar]