Abstract
This study analyzed effects of pressurization on wideband acoustic stapedial-muscle reflex (ASR) tests in infants cared for in normal newborn (NN) and neonatal intensive care units (NICU). Effects of hearing-screening outcomes on ASR threshold measurements were also evaluated, and a subsequent longitudinal study established normative threshold ranges over the first year after birth. An initial experiment compared thresholds in newborns measured at ambient pressure in the ear canal and at the tympanometric peak pressure. ASR thresholds for broadband noise were higher for ears that did not pass newborn hearing screening and ASR threshold was 14 dB higher for real-ear compared to coupler conditions. Effects of pressurization were significant for ears that passed screening; thus, ASR testing in infants should be conducted at tympanometric peak pressure. ASR threshold was significantly higher for ears that referred on transient evoked otoacoustic emissions and Auditory Brainstem Response (ABR) screening tests and also for ears with conductive and sensorineural hearing loss diagnosed by ABR. Developmental ASR changes were significant over the first year for both normal and NICU infants. Wideband pressurized ASR thresholds are a clinically relevant measure of newborn hearing screening and diagnostic outcomes.
Keywords: middle ear muscle reflexes, acoustic stapedial reflex, middle ear, development
INTRODUCTION
Early studies of the acoustic stapedial reflex (ASR) in newborns demonstrated that the ASR is present at birth, but is not reliably detected using low probe frequencies (below 0.66 kHz). This is because the impedances of the outer and middle ear differ in newborns. Higher frequency probe tones (0.8–1.8 kHz) detect the ASR reliably in newborns, but require stimulus levels above 100 dB SPL for tonal stimuli (McMillan et al. 1985; Sprague et al. 1985; Weatherby and Bennett 1980). ASR thresholds for broadband noise (BBN) stimuli are significantly lower than for pure tones, and ipsilateral presentation produces lower thresholds than contralateral presentation (Mazlan et al. 2009; Sprague et al. 1985). Test-retest reliability of the ASR for 1 and 2 kHz and BBN activators is “high” in healthy newborns (Mazlan et al. 2009).
Sound pressure level measured in ear canals of infants can be up to 20 dB higher than in adults due to smaller physical volumes (Bagatto et al. 2005), and there exists the risk of permanent threshold shift at overly high test SPLs in the ear canal (Hunter et al. 1999). At a maximum ASR presentation level typical of adult testing (110 dB SPL), the output in infant ears reaches 126 and 130 dB SPL at 1 and 2 kHz, respectively (McMillan et al. 1985) and could cause permanent damage to hearing. BBN is an attractive alternative stimulus to reduce the risk of iatrogenic hearing loss compared to pure tones since the stimulus energy is lower, distributed across the basilar membrane, ASR thresholds are lower and are less prone to ceiling effects.
An alternative wideband ASR method uses shifts in absorbed power measured with broadband clicks rather than the standard 0.226 kHz pure tone (Feeney and Keefe 1999, 2001). Wideband procedures use either BBN (Feeney and Keefe 2001) or tonal stimuli to evoke the ASR (Feeney et al. 2003). The resulting ASR threshold (ASRT) is 12–13 dB lower in adults than the standard clinical test (Feeney et al. 2003). The frequency region between about 1 and 2 kHz is optimal for detecting ASR shifts in infants (Keefe et al. 2010; Liu et al. 2008). This is because higher probe frequencies than the clinical standard using a 0.226-kHz probe tone are less affected by the flaccid ear-canal walls of newborns. An ipsilateral WB-ASRT, which is desirable for testing in infants, may be accomplished using a stimulus set with pulsed activators interleaved with clicks (Keefe et al. 2010).
Previous infant studies have reported WB-ASR thresholds only at ambient pressure in the ear canal (Feeney and Sanford 2005; Keefe et al. 2010). In contrast, clinical ASR tests are typically performed in older subjects at the tympanometric peak pressure (TPP). Reflex tests at TPP tend to maximize the magnitude of the ASR shift by equalizing pressure across the eardrum, which results in lower ASRTs than ambient-pressure tests. This is not necessarily the case for ASR testing in newborns because inserting the probe and pressurizing the ear canal may displace the ear-canal wall and change the cross-sectional area, resulting in a higher ASRT in the positive pressure condition (Holte et al. 1991). This maturational effect is most important in the first few months of life and disappears as the tympanic ring becomes fully ossified (Saunders et al. 1983). WB-ASRT testing has translational clinical potential in the initial screening exam and as a follow-up procedure to newborn hearing screening (NHS) and diagnosis in order to define middle-ear problems, cochlear hearing loss, and problems beyond the outer hair cells, such as auditory neuropathy spectrum disorder.
No published studies have evaluated whether WB ASRTs vary in infants tested in the newborn nursery relative to those tested in the NICU. In this study, WB-ASRTs were measured in pressurized and ambient-pressure conditions, in both the NN and NICU groups. For a given BBN activator used to elicit an ASR, the WB-ASRTs were measured in terms of the in-the-ear SPL of the activator as well as in terms of the SPL generated within a 2-cm3 coupler. The pressurized WB-ASRT was also measured longitudinally over the first year after birth on at least 3 and up to 5 visits to study development in infants with normal hearing. Finally, the ASRT was studied in relationship to newborn hearing screening tests (TEOAE and automated ABR) and normal or impaired hearing based on diagnostic ABR.
STUDYAIMS
Measure ASR shifts in newborns cared for in NN and NICU nurseries for activator SPLs in coupler and real-ear conditions.
Compare ASRTs measured at ambient pressure in the ear canal and at TPP.
Characterize normal development of pressurized wideband ASRT over the first year after birth in a longitudinal sample.
Evaluate whether ASRTs differed according to NHS and diagnostic ABR test outcomes.
MATERIALS AND METHODS
Subject Enrollment
This study was part of a multisite project on translational wideband tests developed for middle-ear, cochlear, and neural hearing loss in infants, children, and adults. Infants were enrolled in normal and NICU nurseries at a large urban hospital (Good Samaritan Hospital-GSH) and a Regional Neonatal Intensive Care Unit (Cincinnati Children’s Hospital-CCHMC) after they received NHS tests. Table 1 details the demographic characteristics of 748 infants enrolled in the study at birth, grouped according to their newborn hearing screening outcomes. An additional experiment on pressurized versus ambient ASRTs was conducted on the first 80 enrolled newborns, 40 from the Normal Newborn Nursery (NN), and 40 from the NICU.
TABLE 1.
Demographic characteristics of infants tested at screening, combined for the well-baby and NICU nurseries, grouped by results at the two-stage NHS exam. The mean and standard deviation (SD) of the ASRTs are summarized, in which a NR outcome was coded as an ASRT 5 dB higher than the maximum activator level
| Variable | Screen pass (n = 403) | TEOAE refer + automated ABR Pass (n = 237) | TEOAE refer + automated ABR Refer (n = 108) |
|---|---|---|---|
| Gender: | |||
| Male | 212 (52.6 %) | 136 (57.4 %) | 67 (62 %) |
| Female | 191 (47.4 %) | 101 (42.6 %) | 41 (38 %) |
| Race: | |||
| White | 252 (62.5 %) | 138 (58.23 %) | 53 (49.1 %) |
| Non-white | 151 (37.5 %) | 99 (41.27 %) | 55 (50.9 %) |
| Ethnicity: | |||
| Hispanic | 8 (2 %) | 5 (2.1 %) | 3 (2.8 %) |
| Non-Hispanic | 395 (98 %) | 232 (97.9 %) | 105 (96.3 %) |
| NICU >5 days: | |||
| No | 295 (73.2 %) | 215 (90.7 %) | 92 (85.2 %) |
| Yes | 108 (26.8 %) | 22 (9.3 %) | 16 (14.8 %) |
| Low birth weight: | |||
| No | 379 (94 %) | 226 (93.6 %) | 99 (91.7 %) |
| Yes | 24 (6 %) | 11 (4.6 %) | 9 (8.3 %) |
HL denotes hearing loss
The NHS protocol for the NN nursery consisted of two-stage screening using TEOAE. If the infant did not pass TEOAE in either ear, automated ABR was completed in the birthing hospital, usually on the next day. Both tests used Natus Medical, Inc. (Pleasanton, CA) hearing screening instrumentation. Following the two-stage screening, infants were classified as overall Pass or Refer. In the NICU, ABR testing combined with TEOAE or Distortion Product Otoacoustic Emission (DPOAE) testing was used due to higher risk for neurological problems, as recommended by the Joint Committee on Infant Hearing (JCIH 2007). WB-ASRT was measured after screening and over the first year after birth at follow-up study visits in which other tests were completed. These included a wideband acoustic test battery (ambient and tympanometric wideband absorbance, acoustic stapedial reflexes, transient evoked OAE using clicks and chirps), threshold tone-burst air, and bone-conduction ABR, DPOAE, and Visual Reinforced Audiometry (VRA). The protocol used in this study was approved by the Institutional Review Boards of Cincinnati Children’s Hospital Medical Center (CCHMC) and Good Samaritan Hospital (GSH), and informed consent was obtained from the parent(s) of all infants.
Test Sequence
For testing in the newborn nurseries, infants were fed, swaddled, and tested in their bassinet in a procedure room or the nursery. NICU infants were tested near discharge date when breathing room air in their isolette in the NICU. Screening TEOAEs were performed first, followed by the WB battery of tests in the following order: down-swept reflectance tympanogram, reflectance at ambient pressure, up-swept reflectance tympanogram, and WB ASRTs. In the experiment to investigate effects of middle-ear pressure on the ASRT, data were obtained under two ear-canal pressure conditions: ambient pressure and at TPP. The two pressure conditions were counterbalanced for test order for each ear and for the first ear tested. In the initial experiment, the reflectance at ambient pressure was the first test. Initial results showed that the probe insertion to achieve an adequate pressure seal was more easily accomplished in the down-swept tympanogram than in the ambient-pressure test or up-swept tympanogram. For this reason, the first test listed above for the main experiments was the down-swept reflectance tympanogram, followed by an ambient pressure reflectance measurement, then an up-swept reflectance tympanogram. For each measurement of a pressure reflectance of the ear at the probe tip, an absorbance was calculated as one minus the squared magnitude of this reflectance. Following the tympanogram sequence, WB-ASRT was then recorded at ambient and TPP in the pressure experiment, but only at TPP in the longitudinal study.
General Procedures
Wideband absorbance and ASRT data were acquired using a Titan ear-canal probe and modified AT-235 tympanometry hardware (both manufactured by Interacoustics: Wideband Tympanometry research system, Middelfart, DK). The Titan probe had two receiver ports to deliver sound stimuli and one microphone port to measure the acoustical pressure response. An additional port provided air pressure changes delivered by the tympanometry pump with modified firmware and controlled by custom software. Data were recorded using custom software running on a personal computer with a CardDeluxe sound card (22.05 kHz sample rate, 24 bit converters).
WB absorbance tympanograms were first obtained with pressure swept from +200 to −300 daPa, in both descending and ascending directions. Detailed methods used to calibrate and measure wideband absorbance tympanograms are described by Keefe et al. (2015). Briefly, the acoustic response to a train of clicks, with an inter-click interval of 46 ms, was measured during the pressure sweep. The acoustic absorbance was measured for each click response as a joint function of air pressure and frequencies from 0.2 to 8 kHz, which was analyzed as a 3-dimensional tympanogram. A frequency-averaged absorbance was calculated between 0.8 and 2 kHz as a function of air pressure in both a down-swept and up-swept pressure direction. The TPP was calculated for each sweep direction as the air pressure at which the frequency-averaged absorbance attained its maximum value. The final TPP used to acquire WB-ASRT data was the average of the pair of TPPs for both sweep directions. This averaging tended to remove any bias associated with the sweep polarity in adult ears (Liu et al. 2008).
ASR Test Procedures
Detailed methods used to measure the ipsilateral-ear WB-ASRT are fully described by Keefe et al. (2016) and are briefly summarized here. After inserting the probe into the infant ear canal, the five clicks in a pulsed-activator stimulus set were delivered by one receiver port and four-pulsed BBN activators were delivered by a second receiver port. Each activator was presented between a pair of clicks. This resulted in an ipsilateral reflex test as both the reference signals (i.e., the clicks) and the signals activating the ASR (i.e., the BBN pulses) were delivered to the same ear into which the probe was inserted. The initial, or “baseline,” click in each pulse-activator stimulus set measured the sound pressure response at the probe in the assumed absence of any ASR effect. The difference of each of the post-activator clicks with respect to this baseline click provided a set of four click difference waveforms. The presence of a sufficiently large shift in any of these click difference waveforms was associated with the presence of a measurable ASR; otherwise, the ASR was classified as absent. A silent duration of 1.58 s was placed between presentations of the pulsed-activator stimulus sets. This silence provided sufficient time for any ASR effect generated by the first stimulus set to decay to baseline values before the presentation of the next stimulus set.
All data were high-pass filtered below approximately 0.2 kHz to exclude low-frequency noise below the frequency range of interest and to improve the visual interpretation of the click difference waveforms that were displayed in real time to the test operator. In particular, the filter level was 3 dB down at 0.192 kHz and at least 30 dB down at all frequencies below 0.129 kHz. All data were analyzed and plotted after this high-pass filter was applied. The stimulus set was repeated once at each of 10 activator levels in 5 dB increments of activators ascending in level. Each activator level was specified by the SPL measured in a reference 2 cm3 coupler and also in reference to measures in the real ear, since infant ears are much smaller than the standard 2 cm3 coupler. These ranged in 5-dB steps for BBN activators from 35 to 80 dB SPL in the coupler. ASR responses were measured either at ambient pressure in the ear canal or at the TPP described above. The air pressure in the ear canal was continuously monitored during the ASR test at TPP using a pressure sensor within the probe assembly. Whenever the measured air pressure deviated by more than 10 daPa from the desired ambient pressure or TPP, the system paused the reflex test, adjusted the pump so as to produce the desired pressure, and then automatically re-started the reflex test at the current activator level. The ASR was detected in terms of the click differences in the sound power absorbed by the ear in response to the baseline click and to each of the four subsequent clicks. Illustrative examples of this ASR test are described by Keefe et al. (2016) for two infant ears tested at age 1 month.
Diagnostic ABR and VRA Procedures
Follow-up diagnostic threshold tone burst ABR was scheduled at age 1 month (corrected for gestational age if the infant was premature). Otoscopy (Welch Allyn pneumatic otoscope) and DPOAE testing using the Vivosonic Integrity system (Version 5.2) were performed prior to the diagnostic threshold ABR. For DPOAE, primary tone levels were set at SPLs of 65 dB (L1) and 55 dB (L2), and primary tone frequencies f1 and f2 were set with an f2/f1 ratio equal to 1.22. Pass criteria were SNR of 6 dB or greater at 3 of 5 test (f2) frequencies (2, 3, 4, 5.5, and 8 kHz). In addition, DPOAE levels were required to be at or above 0 dB SPL (Gorga et al. 2005). Diagnostic ABR examination was conducted by audiologists within a shielded double-walled sound-attenuated booth, using the Vivosonic Integrity system (Version 5.2). Stimuli for air-conduction tests were presented via insert earphones (Etymotic Research ER-3A) using pediatric ear foam tips, and stimuli for bone-conduction tests were presented via a B-71 bone oscillator. Recording methods and analysis were described by Elsayed et al. (2015). Tone-burst thresholds were collected for air and bone conduction at octave frequencies between 0.5 and 4 kHz. The minimum test protocol was to acquire air-conduction data for at least two frequencies between 0.5 and 4 kHz, and bone-conduction data for at least one of these corresponding frequencies. Responses were defined as normal for ABR if minimal responses for Wave V were present at or below 30 dB nHL at 1 and 2 kHz, and 20 dB nHL at 4 kHz, with no air-bone gaps exceeding 10 dB at any frequency.
VRA was completed using the Intelligent Hearing Systems Smart VRA device with insert earphones. Animated toys in smoked plexiglass boxes at 90° azimuth were used for reinforcement. Testing began with recorded speech stimuli at 35 dB HL using insert earphones to obtain minimal response levels, and a bracketing procedure was used, decreasing in 20 dB steps, then reversing to 10 dB steps to estimate threshold. If a participant responded to more than 1 of 3 control trials, re-training was done and stimulus intervals were lengthened to reduce false-positive responses. If these measures were not successful, the test was considered to be invalid and was repeated after a break on the same day or another day. If air conduction thresholds were greater than 20 dB, bone conduction was performed using the same technique in the worse ear with masking noise in the better ear. The minimal protocol to retain data included speech detection threshold for standard spondee words and pure tone air conduction results at 1 and 4 kHz. The criteria for a normal MRL was 25 dB HL or better for pure tones between 1 and 4 kHz and for speech awareness to recorded speech syllables, with no air bone gaps exceeding 10 dB.
Analysis and Statistics
Descriptive analyses were performed with calculation of means, medians, and standard deviations for continuous variables. Categorical variables were measured using frequency counts and proportions. Residual diagnostics were conducted to check model assumptions. Wilcoxon rank sum analysis of variance (ANOVA) due to non-normally distributed values with Dunn’s post-hoc pairwise comparison tests were used to compare the TPP values between 4 groups, NICU (pass), NICU (refer), well (pass), and well (refer) babies. Moreover, the non-parametric ANOVA was used to compare if the ASRT value was different for ambient versus TPP recording overall for the aforementioned four groups.
To study developmental changes in the normal longitudinal group, a linear mixed model with repeated measures was conducted to study the ASRT change with chronological age, controlling for gestational age, infant group (NICU or NN), race (Caucasian/White or African American, and others combined (non-white). The interaction of corrected age and race was tested in the model if race was significant. The corrected age was classified as: birth (1.5 days–0.25 month), 1 month (0.25–4), 6 months (4–8), 9 months (8–11), and 12 months (11–15). The least square (LS) mean was adjusted by any significant variables. Data were analyzed employing SAS statistical software, version 9.3 (SAS Institute, Cary, N.C.). A two-sided significance level was set at 0.05.
RESULTS
Example of Measured ASR Responses in an Infant Ear
Figure 1 (top) illustrates the ASRT results in the right ear of an infant that passed the newborn screening exam at 1 day, a tone burst ABR test at 9 weeks, and a VRA exam at 43 weeks. Figure 1 (bottom) shows the corresponding left-ear outputs at the same test dates for the same infant. This infant did not pass newborn screening in the left ear as a result of a transient middle-ear condition, which was possibly due to a positive middle ear pressure, a collapsing ear canal, or amniotic fluid in the middle ear. These test data were obtained from the ASR test performed at the TPP. The algorithm defined ASR status function of “present” or “absent” is indicated for the nine activator levels from 40 to 80 dB SPL as measured in the reference coupler. For the ASR tests on day 1, the WB-ASRT was 40 dB SPL for the right ear, and absent in the left ear. The left-ear measurement was classified as noisy, based on procedures described by Keefe et al. (2016). The ASRTs were 65 and 55 dB SPL in the normal right ear and were 70 and 55 dB SPL in the referred left ear at 9 and 43 weeks, respectively. In the pass ear in Figure 1, the TPP was large and positive at the youngest test age (102 daPa at 1 day) and tended toward more normal values at the older ages (e.g., 18 daPa at 9 weeks and 2 daPa at 43 weeks). In the refer ear in Figure 1, the TPP was initially very positive and slowly improved, with a TPP of 175 daPa at 1 day, 68 daPa at 9 weeks, and 12 daPa at 43 weeks.
FIG. 1.
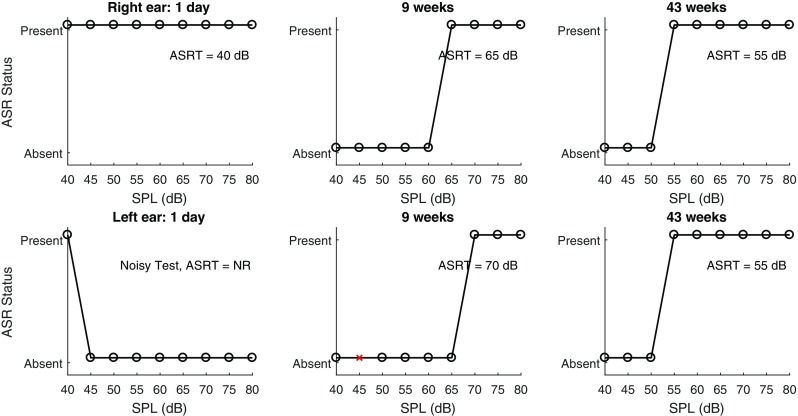
Right ear of an infant tested longitudinally that passed newborn screening at 1 day, ABR at 9 weeks, and visual reinforcement audiometry at 43 weeks. Left ear of the same infant that did not pass newborn screening at 1 day due to a middle ear condition. ABR was normal at 9 weeks and visual reinforcement audiometry was normal at 43 weeks. The normal right ear is shown in the top panels and the left ear that had a transient middle ear condition is shown in the bottom panels. Presence and absence of the ASRT is shown for each of the stimulus intensities at each age tested.
Although not plotted here, the relative amount of sound power absorbed by the ear was less at higher activator levels than at lower activator levels due to the ASR; similar infant-ear examples are described in Keefe et al. (2016). As explained in that report, the WB-ASR was detected based on analyses of the difference in absorbed sound power in the later clicks relative to the absorbed sound power from the baseline click. The procedure to determine the WB-ASRT included both a similarity test and a magnitude test. The magnitude test determined whether the shift in absorbed power at each activator level was sufficiently large to be considered an ASR shift. The similarity test determined whether the shifts in absorbed power across the range of activator levels were consistent with one another.
Tympanometric Peak Pressure in Newborns
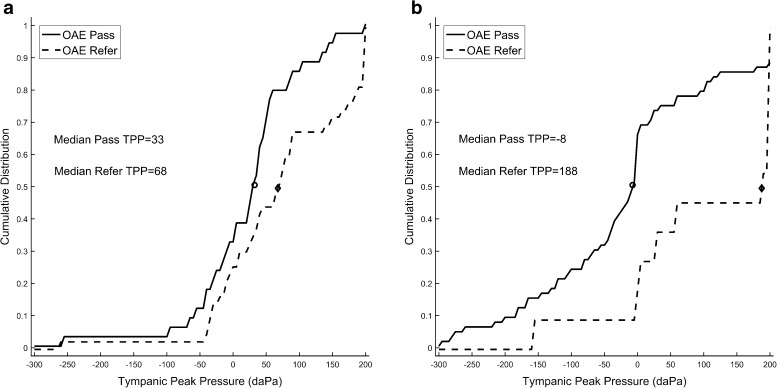
Tympanometric peak pressure was measured as an average of down- and up-swept tympanometry across low frequencies and is plotted in Figure 2. The cumulative distributions of TPP in NNs and in the NICU for initial NHS pass and refer sub-groups are shown in the a and b panels respectively. Due to non-normal distributions (Shapiro-Wilk normality test failed), Mann-Whitney U tests were performed. NN and NICU groups had different distributions of thresholds for TPP. The distributions were different for the NICU newborns, as the median TPP for NICU ears was 40 daPa, compared to 0 daPa for NN ears (U6874.5, p = 0.002). For both NN and NICU groups combined, ears that referred on NHS had a more positive TPP compared to ears that passed on NHS (72.5 daPa median difference, U = 5382, p < 0.001). NN ears that referred on screening had a significantly more positive TPP than ears that passed (U = 1107, p = 0.025). The pass and refer distributions of TPP were markedly different for the NICU newborns, as about 50 % of NICU refer ears had a TPP greater than 188 daPa. This amounted to a median difference of 190 daPa compared to NICU pass ears (U = 641.5, p = 0.003).
FIG. 2.
a, b Cumulative distribution of TPP in babies in the NN (a panel) and in the NICU group (b panel). Median TPP is shown by the open and filled circles.
ASRT in Newborns
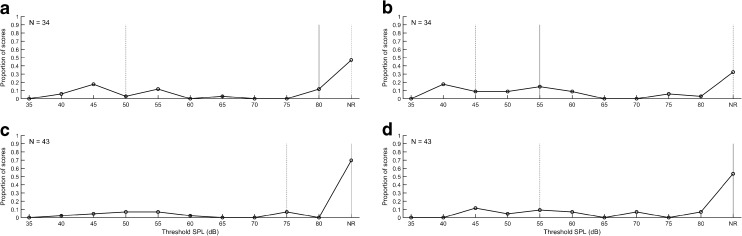
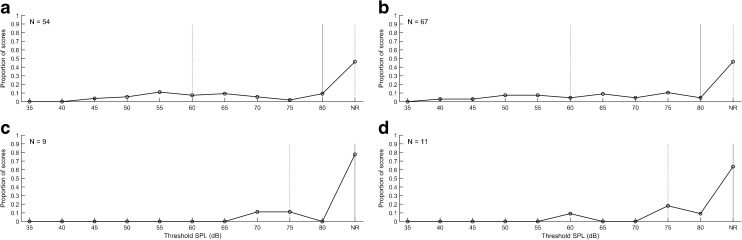
Because these differences in TPP could affect ASRT threshold and had not been previously studied relative to threshold effects, an experiment was conducted of ASRT at ambient pressure and at the average TPP value for up- and down-swept tympanometry. This experiment was completed for the first 80 ears in the NN and NICU subgroups. The median, interquartile range, and distribution of the WB-ASRTs are shown in Figure 3 for measurements at ambient pressure and at TPP for infants cared for in the NN nurseries who passed TEOAE screening in the initial NHS. Corresponding results for testing performed at ambient pressure and TPP, respectively, are depicted in the lower left and right panels of Figure 3 for newborns that referred on TEOAE screening. In Figure 4, the same distributions are shown for NICU newborn ears that passed or referred on the two-stage NHS exam. The distribution of ASRT values is shown in these figures for the pressure condition (TPP and ambient pressure) and the NN and NICU subgroups. The stimulus level was limited, as discussed previously, to 80 dB SPL.
FIG. 3.
Median (solid line), interquartile ranges (dotted lines), and distribution of ASRT at ambient pressure (a) and at TPP (b) for NN infants who passed the TEOAE test in the NHS exam. ASRT was also measured at ambient pressure (c) and TPP (d) for NN ears that referred on the TEOAE test.
FIG. 4.
Median, interquartile ranges and distribution of ASRT at ambient pressure (a) and at TPP (b) for infants cared for in NICUs who passed the two-stage NHS exam (n = 54–67 ears). c, d ASRT measured at ambient pressure and TPP for the NN infants that referred on the NHS exam (n = 9–11 ears).
If a measurable ASRT was not obtained at 80 dB relative to 2 cm3 coupler values, it was classified as a no response (NR). These NR results are shown in each panel on the extreme right side of the distribution. The proportion of NR values varied from 30 % of ears in the NN Pass group at TPP to 70 % of the NN Refer group at ambient pressure. In the NICU group, the proportion of NR values varied from 50 % of the Pass ears at TPP to 80 % of the Refer ears at ambient pressure. The proportion of NR values was compared for NN and NICU groups using chi square tests and were not significantly different for NR test results (p = 0.53). This is important because NR values may bias the threshold estimates since they are not included in the mean values.
Differences between the ASRT acquired with ambient and TPP conditions for NN and NICU infants were compared. This ASRT was quantified in terms of the SPL measured for the BBN activator in the 2 cm3 coupler. For purposes of comparing ASRT values calibrated to SPL in the 2 cm3 coupler, a NR on the ASR test was coded as ASRT of 85 dB SPL, which was 5 dB larger than the maximum activator level of 80 dB SPL. This coding preserved the rank order of ASRTs. A normality test (Shapiro-Wilk) failed (p < 0.05), so a Wilcoxon signed rank test was done to test screening subgroup differences. For all ears combined, the median ASRT was significantly lower with TPP (77.5 dB SPL) than with ambient pressure (85 dB SPL, W = −1104, p = 0.003). For ears that passed screening, the median ASRT was also significantly lower (72.5 dB in the TPP condition versus 80 dB for ambient pressure, W = −515, p = 0.017). For ears that referred on screening, median ASRT threshold was 85 dB SPL in both the TPP and ambient conditions. This 85 dB SPL value was the coding value for a NR. Thus, half the ears that referred had no measurable reflex at the output limit regardless of pressurization (see panels C and D of Figs. 5 and 6). Thus, pressurization improved ASRT for ears that passed screening, but not for ears that referred on screening.
FIG. 5.
Box and stem plots with median (center line), 95 % confidence limits (box) and range (stem) for ASRT, referenced to a 2-cm3 coupler (top panels) and in-the-ear SPL (bottom panels) for infants from birth (screening) to 12 months old in the NN group (left panels) and NICU (right panels). Age was corrected if an infant was born at less than 38 weeks gestation.
FIG. 6.
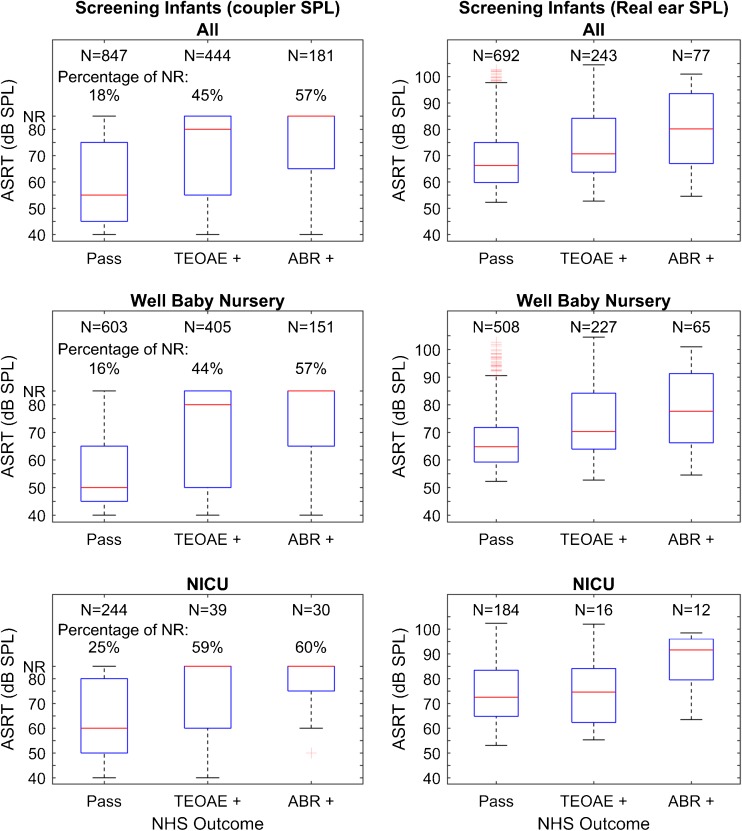
Screening results for ears that passed and referred on TEOAE and ABR. Results are shown for all ears combined, for well babies and for NICU. Box and whisker plots depicting median (centerline), IQRs (boxes), whiskers (the lesser of 1.5 IQRs above or below box or full data range) and individual outliers beyond the whiskers for BBN ipsilateral ASRT measured relative to 2 cm3 coupler values (left) and using real-ear SPL (right). Real-ear SPLs are plotted only for ears with measurable ASRT, i.e., excluding no response (NR).
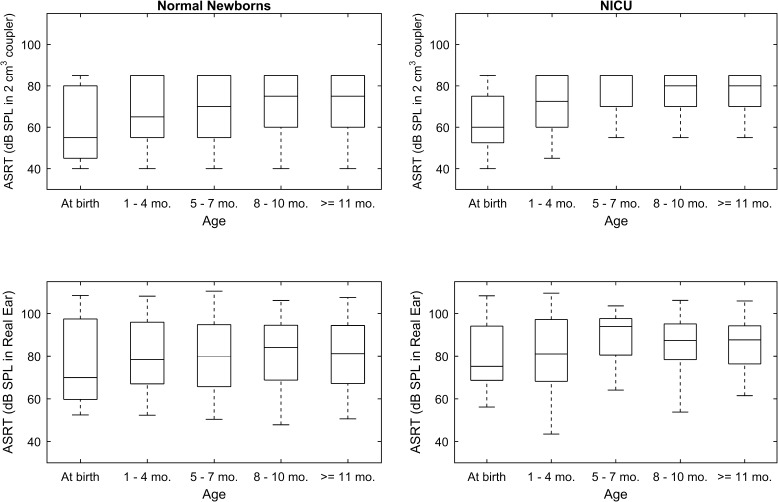
Development of ASRT in the First Year
In order to characterize development of the ASRT from birth (at screening) to 1 year old, results were obtained for infants who passed newborn screening, and who returned for follow-up testing at ages up to 15 months. To provide normative data across the first 15 months of life, results were included only for infants who also passed the diagnostic ABR and VRA exams. The ASRT results are depicted in Figure 5 as box and stem plots across the age range. ASRTs are referenced to the SPL in the 2 cm3 coupler (top panels) and to the in-the-ear SPL (bottom panels). The left panels are for the NN group, and the right panels are for infants cared for in the NICU. Age was corrected if the infant was born at less than 38 weeks gestation. For purposes of comparing ASRT values specified as in-the-ear SPL of the BBN activator, a NR on the ASR test was coded as an ASRT with an in-the-ear SPL that was 5 dB larger than the in-the-ear SPL at the maximum activator level. This coding preserved the rank order of ASRTs.
LS regression results are provided in Table 2 for the estimated mean and 95 % confidence interval (CI) at each age range. In this analysis, the study sample included 182 infants classified as normal (passed newborn hearing screen, normal ABR, normal VRA) with mean gestational age of 37.5 weeks at birth (range from 26 to 42 weeks). Girls comprised 45.6 % of this sample and 59.9 % were Caucasian, similar to the entire group as detailed in Table 1. In this analysis, only one ear was included from each subject that had the most complete data across the age range, since ASRT is highly correlated in the right and left ears. The ear effect was controlled by including data from the same proportion of right ears (50.1 %) and left ears (49.9 %).
TABLE 2.
Normative means and 95 % confidence intervals for WB-ASRT, with a BBN stimulus, controlled for gestational age at birth and race. Data include one ear with the most complete longitudinal results from 182 infants who passed newborn hearing screening, diagnostic ABR at 1 month and VRA at 9 months of age
| Outcome | Group | LS mean | 95 % CI | |
|---|---|---|---|---|
| Lower | Upper | |||
| ASRT referenced to 2 cm3 coupler (peSPL) | Corrected age | |||
| Birth | 60.6 | 57.5 | 63.7 | |
| 1 month | 66.1 | 63.1 | 69.1 | |
| 6 months | 70.5 | 67.0 | 73.9 | |
| 9 months | 71.7 | 68.6 | 74.7 | |
| 12 months | 70.3 | 66.8 | 73.8 | |
| Group | ||||
| NICU | 68.3 | 66.6 | 70.1 | |
| NN | 67.3 | 63.0 | 71.7 | |
| ASRT real-ear measures (peSPL) | Corrected Age | |||
| Birth | 76.0 | 72.6 | 79.3 | |
| 1 month | 78.8 | 75.5 | 82.0 | |
| 6 months | 81.7 | 77.9 | 85.5 | |
| 9 months | 81.0 | 77.7 | 84.4 | |
| 12 months | 79.4 | 75.6 | 83.2 | |
| Group | ||||
| NICU | 79.6 | 77.8 | 81.5 | |
| NN | 79.1 | 74.3 | 83.9 | |
LS mean least square mean
Gestational age at birth was significantly related to ASRT referenced to coupler values (p = 0.012) and to measurements made in the real ear (p = 0.016). ASRT referenced to the coupler was significantly related to age (p < 0.0001), but not to ASRT measured in the real ear. The regression model showed an overall increase in the threshold of the ASR from birth to 1 year of approximately 10 dB when referenced to a 2 cm3 coupler, likely due to the smaller infant ear canal volume at birth. Evidence for ear canal growth was that when referenced to the real ear measured values, ASRT increased only 3 dB from birth to 1 year. Mean and 95 % CI values are given in Table 2 for both groups combined across these ages.
ASRT in Pass Compared to Refer Newborn Screening Tests
Figure 6 illustrates box and whisker plots for ASRT, in ears that passed compared to those that referred for TEOAE and automated ABR newborn hearing screening. Median ASRT is shown as measured in the coupler and in the real ear. The overall one-way non-parametric ANOVA (Kruskal-Wallis) was significant (H = 251.855 with 5 degrees of freedom, p = <0.001). To isolate the group or groups that differed from the others, a multiple comparison procedure was performed that controlled familywise error (Dunn’s Method). Median ASRT was higher (Q = 11.5, p < 0.001) for NN ears that referred on TEOAE or ABR relative to those that passed. For NICU infants, median ASRT was higher for ears that referred on TEOAE (Q = 6.2, p < 0.02) or ABR (Q = 4.2, p < 0.001), relative to passed ears. NICU infants that passed on TEOAE or ABR screening had significantly higher ASRT thresholds compared to NNs that passed TEOAE or ABR (Q = 6.2, p < 0.001). Other pairwise comparisons were not significant.
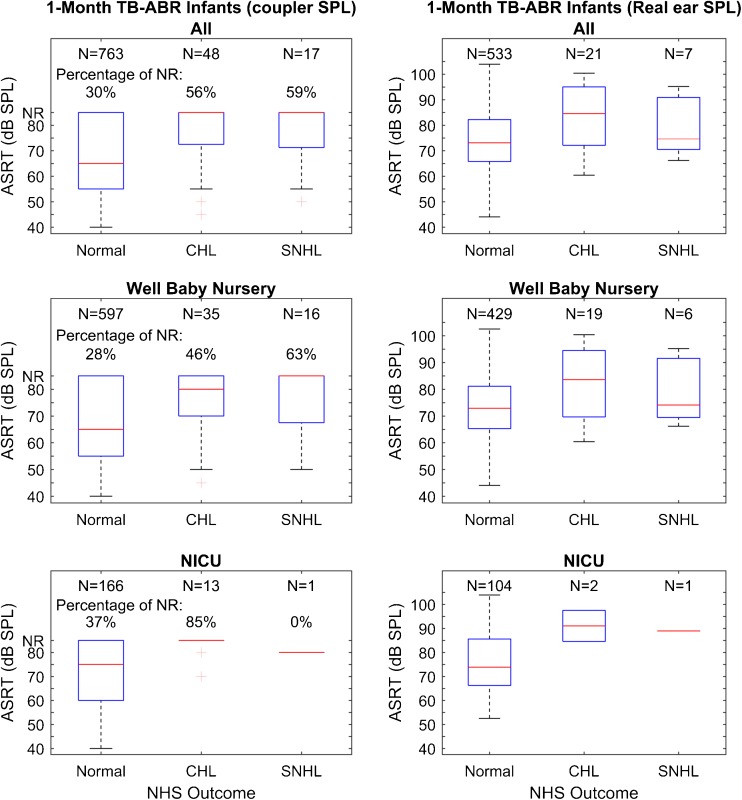
Figure 7 illustrates box and whisker plots for ASRT in ears that had normal hearing diagnosed by ABR compared to those that had CHL or SNHL. Median ASRT is shown, as measured in the coupler and in the real ear. An overall ANOVA (Kruskal-Wallis) was significant (H = 39.5 with 5 degrees of freedom, p = <0.001). To isolate the group or groups that differed from the others, a multiple comparison procedure was performed that controlled familywise error (Dunn’s Method). In this case, median ASRT was higher (Q = 3.4, p = 0.027) for NN ears with CHL on diagnostic ABR relative to normal hearing ears. ASRT was also higher in NICU ears with CHL on diagnostic ABR relative to NN normal hearing ears (Q = 3.4, p = 0.01). Other pairwise comparisons within groups (NN or NICU) were not significant.
FIG. 7.
Follow-up results for infants with normal hearing, conductive hearing loss (CHL) and sensorineural hearing loss (SNHL). Results are shown for all ears combined, for NN and for NICU groups. Box and whisker plots depicting median (centerline), IQRs (boxes), whiskers (the lesser of 1.5 IQRs above or below box or full data range) and individual outliers beyond the whiskers for BBN ipsilateral ASRT measured relative to 2 cm3 coupler values (left) and using real-ear SPL (right). Real-ear SPLs are plotted only for ears with measurable ASRT, i.e., excluding no response (NR).
DISCUSSION
Newborns present a special challenge to measure valid acoustic reflexes for several reasons. The small size and extreme flaccidity of the outer ear, coupled with frequent presence of amniotic fluid and vernix at birth, make the ASRT more difficult to record, especially for low-frequency stimuli. Internal noise is high in the newborn ear, and developmental changes in the first year after birth must be accounted for in establishing normal ranges for clinical application. In order to account for the stimulus level in smaller infant ears, one aim of this study was to measure ASR shifts for activator SPLs were in both coupler and real-ear conditions, since the stimulus level in the real ear of newborns varies between infants due to size differences and as the ear develops and the ear canal lengthens.
Related to this developmental issue, another aim of this study was to characterize normal development of pressurized wideband ASRT over the first year after birth. Results obtained in normal ears showed that the level required to elicit the ASRT increased from birth to 1 year by 10 dB when measured in the coupler, but by only 3 dB when measured in the real ear. Thus, real ear measures mostly accounted for the developmental difference due to ear canal size changes (7 dB), while approximately 3 dB increase in ASRT was due to factors other than maturation of ear-canal area and length. Factors such as increased stiffness of the outer ear could account for these developmental changes and has been demonstrated in longitudinal wideband reflectance measures in normal hearing infants from this well-baby and NICU cohort (Hunter et al. 2015). Specifically, absorbance decreased in the lower frequencies (<1 kHz), while absorbance increased with age from 1 to 4 kHz, and then decreased with age from 6 to 8 kHz. The largest changes in ASRT level for both coupler and real-ear measures occurred in the first 4 months, during the time that the outer ear canal becomes more ossified and rigid.
Although developmental data for clinical ASRT measured using clinical admittance methods are scarce, Mazlan et al. (2007) reported that mean ASRT for a 2 kHz pure tone increased from 73.1 to 79.6 dB SPL, while the ASRT for BBN increased from 59.4 to 65.8 from birth to 7 weeks. The corresponding ASRT for BBN in this study increased from 60.6 at birth to 66.1 dB SPL at 1 month measured in the coupler, which is in good agreement with data from Mazlan and colleagues. Another study in healthy neonates (2.5 days) reported a mean threshold for BBN of 57.1 dB HL (Kei 2012). It is unclear what the equivalent SPL was since it was not reported. The average ASRT found for other studies using BBN referenced to coupler values in infants were 64.3 dB SPL (.8 kHz probe tone) reported by Hirsch et al. (1992) and 73 dB SPL for a 1.2 kHz probe tone (Bennett and Weatherby 1982). Those values are higher than the present study with the BBN WB-ASRT found for screening Pass ears referenced to coupler values (56 dB SPL).
A further aim of this study was to compare ASRTs measured at ambient pressure in the ear canal and at TPP. Middle-ear pressure in newborns has not been systematically studied in relation to hearing screening and acoustic reflex recordings, perhaps because there is an assumption that Eustachian tube mechanisms that cause negative pressure in older children and adults (such as upper respiratory infections) are not an issue in newborns. However, our study revealed high rates of both positive and negative pressure that affected ASRT measurements at birth. NN and NICU groups had very different distributions of TPP that were related to hearing screen results. Ears that passed NHS had a more normal TPP (i.e., a TPP closer to 0 daPa) than ears that referred on NHS, especially in the NICU group. The distribution was skewed for the NICU newborns, as about half of NICU refer ears had a TPP greater than 188 daPa, a median difference of 196 daPa compared to pass ears. However, the proportion of “no response” ASRT values was not different in NN and NICU groups. Overall, TEOAE and ABR referrals at birth were associated with significantly more positive TPP for infants in the NICU, but not in the NNs. Infants in the NICU have more frequently been exposed to positive pressure ventilation. Although no infants were receiving ventilation at the time of the ASRT measurement, there could be residual positive middle ear pressure.
Sanford et al. (2009) reported TPP in normal newborns using wideband absorbance measures and clinical 1 kHz tympanometric measures. In that study, the TPP based on wideband tympanometry was centered on 0–25 daPa, with a mean of 21 daPa in Pass ears, while the distribution was skewed toward positive pressures for refer ears with a mean of 95 daPa. In the current study for the experimental group of 80 infants, the median TPP for wideband tympanometry using an average of upswept and downswept recordings was 33 daPa in the Pass NN group, 68 daPa in the NN Refer group, −8 daPa in the NICU Pass group, and 188 daPa in the NICU Refer group. Thus, results of this study agree well with those of Sanford et al. (2009) for NNs that passed or referred on hearing screening. Infants in the NICU had slightly less positive pressures in the pass screening sub-group, probably due to greater time since birth for middle-ear pressure equilibration. However, they had much higher positive pressure in the refer ears. As this is the first published report of TPP in relation to wideband ASRT in infants from the NICU population, comparative results are not available.
Sanford and Feeney (2008) studied maturation of tympanometric wideband acoustic transfer functions in human infants and reported broad notching at .5 kHz that suggested canal-wall resonance, and negative pressure asymmetries relative to positive pressures for 12 and 24-week-old infants. These low-frequency effects were attributed to flaccidity of the newborn ear canal and are consistent with the observation by Holte et al. (1991) that mean diameter changes in the newborn ear canal are much larger for negative pressures than for positive pressures. These differences between positive and negative pressure are likely due to collapse of the ear canal at high negative pressures. Thus, collapse of the ear canal at negative pressures may underlie at least some cases of positive pressure measurements observed in the present study and is a mechanism that could also cause OAE levels to be affected, particularly in lower frequencies. Positive pressure has a similar effect to negative pressure in terms of stiffness effects and the frequency range of effects on wideband reflectance measurements (Hunter et al. 2008; Margolis et al. 2001) and is well documented to affect OAE levels due to altered transmission properties (Hof et al. 2005; Trine et al. 1993). Thus, positive pressure in the middle ear, or collapsing ear canals due to flaccidity of the osseous portion of the canal, could be a mechanism for some OAE screening referrals.
The present study included an experiment to equilibrate middle ear pressure effects by recording the ASRT with and without adjustment based on the average TPP based on up-swept and down-swept wideband tympanometric recordings. Pressurization to equilibrate for TPP during ASRT recordings requires a more sophisticated apparatus during wideband recordings and is more difficult technically to administer due to the need to maintain hermetic seal in the tiny newborn ear. Some individual ears demonstrated better thresholds with pressurization and the median ASRT decreased by 25 dB for NNs that passed TEOAE screening. However, equilibration for TPP did not produce significantly better ASRT for ears that referred on hearing screening. This would be expected if the screening did not pass because of fluid in the middle ear rather than due to middle ear pressure. As shown in Figure 7, ears that were confirmed to have CHL at the time of the diagnostic ABR test did have significantly elevated ASRT after pressure equilibration, relative to ears with normal ABR results. This result could also occur in ears that have either a sensory hearing loss, or neural pathology, such as auditory neuropathy. A total of 18 ears had a confirmed sensorineural hearing loss at diagnostic ABR testing and also had elevated ASRT with pressure equilibration.
ASRT tests were equilibrated for TPP in the larger study, since improved ASRT in the Pass group should help to separate distributions between normal and impaired ears. It would have been time prohibitive to measure ASRTs under both ambient and TPP conditions in the larger study because of the goals to measure diagnostic ABR and VRA data at older ages in the same ears.
The fourth aim of this study was to evaluate whether ASRTs differed according to NHS and diagnostic ABR test outcomes. We found a significant, 11 dB elevation in BBN ASRT in ears that referred on TEOAE and a 13 dB elevation for ears that referred on automated ABR at birth, for the NICU group. For the well infants, we found a 26-dB elevation for ears that referred on TEOAE and a 33-dB elevation for ears that referred on ABR. In addition, the ASRT was elevated by 19 dB in ears that were found to have a CHL and by 15 dB for ears with a SNHL using diagnostic ABR. CHL changes the impedance characteristics of the middle ear, attenuating all stimuli reaching the cochlea and thus affecting absorbance of the middle ear, forward and reverse OAE transmission, ABR latency, and amplitude. The BBN ASRT was equally affected in our sample of newborns for CHL and SNHL; thus, a measure specific to middle ear transmission such as wideband absorbance tympanometry is needed to distinguish between these hearing loss types. A limitation of the maximum level we set for safety reasons is that the maximum level as measured in a 2-cm3 coupler was limited to 80 dB SPL. The upper limit translated into maximum real-ear levels of approximately 105 dB SPL, thus, was appropriate for the smallest infant ears. However, this upper limit may have made differences between CHL and SNHL ears less likely. The normal range for BBN ASRT reported in newborns using commercially available instruments and a 1-kHz probe tone is 50–85 dB HL (Mazlan et al. 2007). Keefe et al. (2010) reported WB-ASRT results using a BBN activator in 230 well newborns and found the median low-frequency WB-ASRT was elevated by 15 dB for ears that referred on DPOAE on the first day of age. Other tonal activators of the ASR at 1 and 2 kHz were tested in that study, but the resulting ASRTs showed smaller differences between pass and refer ears compared to ASRTs for the BBN activator. Infants cared for in the NICU are an important group for clinical application of WB-ASRT due to the higher risk for auditory neuropathy spectrum disorder, but they have not been previously studied using a wideband version of the ASRT. An acoustic reflex test that is brief, simple, and valid, as found in this study for both well and NICU infants, has translational potential for detection of neural disorders.
CONCLUDING COMMENTS
Tympanometric peak pressure was higher in ears of NNs that passed NHS than in NICU babies who passed screening, likely due to non-equalized middle ear pressure soon after birth, or effects of flaccid ear canals that cause an asymmetry in the tympanometric measures. Equilibration of TPP significantly improved ASRT in ears that passed hearing screening, but not in ears that referred on screening. Results of TEOAE and ABR screening showed that median ASRT was significantly higher in ears that referred on screening for both NN and NICU groups. NICU infants had higher ASRT than NN despite passing newborn hearing screening, which may be due to subtle differences in middle ear transmission or reflex activation by efferent pathways. The ASRT test is based on the assumption that a stapedius muscle contraction in an infant ear stiffens the annular ligament and thereby reduces vibration transmission from the middle ear into the cochlear fluid at the lowest test frequencies. While true for adult ears, this assumption has not specifically been assessed for infant ears. This assumption is built into the ASR test design that classifies an ASR shift as present or absent. Hunter et al. (2015) found that NICU infants had a somewhat more mature absorbance response than normal newborn infants, with lower absorbance at low and high frequencies, and higher absorbance in the mid-frequencies. Thus, the slight increase in ASRT found in NICU infants relative to NN despite passing newborn screening may be at least partly due to a more mature (stiffer) middle ear system at testing, since they were tested at older chronological ages. Test performance of WB-ASRT combined with wideband tympanometry is being studied in follow-up of these same infants, relative to a gold standard hearing test (diagnostic threshold ABR and audiometry), and will be the subject of future reports that are hoped to inform clinical application of these wideband acoustic test procedures.
Acknowledgments
The authors were assisted by John C. Ellison in preliminary analyses of these reflex data. Portions of these results were presented at the Association for Research in Otolaryngology in 2011 and the American Auditory Society in 2014. Research reported in this publication was supported by the National Institute of Deafness and other Communication Disorders of the National Institutes of Health under Award Number R01 DC010202 and an ARRA supplement (DC010202-01S1). Co-author Keefe is involved in commercializing devices to assess middle-ear function in infants. Lisa Hunter has received honoraria from Interacoustics, Inc. and Vivosonic, Inc. for educational lectures. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The content of this article does not represent the views of the Department of Veterans Affairs or of the US Government.
Abbreviations
- ABR
Auditory brainstem response
- ANOVA
Analysis of variance
- ASR
Acoustic stapedial reflex
- ASRT
ASR threshold
- BBN
Broad band noise
- CCHMC
Cincinnati Children’s Hospital Medical Center
- CHL
Conductive hearing loss
- DPOAE
Distortion Product Otoacoustic Emission
- GSH
Good Samaritan Hospital
- LS
Least Square
- nHL
Hearing Level referenced to normal hearing
- NHS
Newborn Hearing Screening
- NN
normal newborn
- NICU
Neonatal Intensive Care Unit
- TEOAE
Transient Evoked Otoacoustic Emission
- peSPL
Peak-to-peak equivalent sound pressure level
- SNHL
Sensorineural Hearing Loss
- SPL
Sound Pressure Level
- TPP
Tympanometric Peak Pressure
- VRA
Visual Reinforcement Audiometry
- WB-ASRT
Wideband ASRT
Compliance with Ethical Standards
Funding Source
Research reported in this publication was supported by the National Institute of Deafness and other Communication Disorders of the National Institutes of Health under Award Number R01 DC010202 and an ARRA supplement (DC010202-01S1). Co-author Keefe is involved in commercializing devices to assess middle-ear function in infants. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The content of this article does not represent the views of the Department of Veterans Affairs or of the United States Government.
Contributor Information
Lisa L. Hunter, Phone: 513-803-0532, Email: lisa.hunter@cchmc.org
Douglas H. Keefe, Email: Douglas.Keefe@boystown.org
M. Patrick Feeney, Email: patrick.feeney@va.gov.
Denis F. Fitzpatrick, Email: Denis.Fitzpatrick@boystown.org
References
- Bagatto MP, Moodie S, Scollie SD, Seewald RC, Moodie K, Pumford J, Liu KPR. Clinical protocols for hearing instrument fitting in the desired sensation level method. Trends Amplif. 2005;9:199–226. doi: 10.1177/108471380500900404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MJ, Weatherby LA. Newborn acoustic reflexes to noise and puretone signals. J Speech Hear Res. 1982;25:383–387. doi: 10.1044/jshr.2503.383. [DOI] [PubMed] [Google Scholar]
- Elsayed AM, Hunter LL, Keefe DH, Feeney MP, Brown DK, Meinzen-Derr JK, Baroch K, Sullivan-Mahoney M, Francis K, Schaid LG. Air and bone conduction click and tone-burst auditory brainstem thresholds using Kalman adaptive processing in Nonsedated normal-hearing infants. Ear Hear. 2015;36:471–481. doi: 10.1097/AUD.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH (1999)Acoustic reflex detection using wide-band acoustic reflectance, admittance, and power measurements. J Speech Lang Hear Res 42:1029–1041 [DOI] [PubMed]
- Feeney MP, Keefe DH. Estimating the acoustic reflex threshold from wideband measures of reflectance, admittance, and power. Ear Hear. 2001;22:316–332. doi: 10.1097/00003446-200108000-00006. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Sanford CA. Detection of the acoustic stapedius reflex in infants using wideband energy reflectance and admittance. J Am Acad Audiol. 2005;16:278–290. doi: 10.3766/jaaa.16.5.3. [DOI] [PubMed] [Google Scholar]
- Feeney MP, Keefe DH, Marryott LP. Contralateral acoustic reflex thresholds for tonal activators using wideband energy reflectance and admittance. J Speech Lang Hear Res. 2003;46:128–136. doi: 10.1044/1092-4388(2003/010). [DOI] [PubMed] [Google Scholar]
- Gorga MP, Dierking DM, Johnson TA, Beauchaine KL, Garner CA, Neely ST. A validation and potential clinical application of multivariate analysis of distortion product otoacoustic emission data. Ear Hear. 2005;26:593–607. doi: 10.1097/01.aud.0000188108.08713.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JE, Margolis RH, Rykken JR. A comparison of acoustic reflex and auditory brain stem response screening of high-risk infants. Ear Hear. 1992;13:181–186. doi: 10.1097/00003446-199206000-00007. [DOI] [PubMed] [Google Scholar]
- Hof JR, Anteunis LJ, Chenault MN, van Dijk P. Otoacoustic emissions at compensated middle ear pressure in children. Intl J Audiol. 2005;44:317–320. doi: 10.1080/14992020500057822. [DOI] [PubMed] [Google Scholar]
- Holte L, Margolis RH, Cavanaugh RM. Developmental changes in multifrequency tympanograms. Audiol. 1991;30:1–24. doi: 10.3109/00206099109072866. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Ries DT, Schlauch RS, Levine SC, Ward WD. Safety and clinical performance of acoustic reflex tests. Ear Hear. 1999;20:506–514. doi: 10.1097/00003446-199912000-00006. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Tubaugh L, Jackson JA, Propes S. Wideband middle ear power measurement in infants and children. J Am Acad Audiol. 2008;19:309–324. doi: 10.3766/jaaa.19.4.4. [DOI] [PubMed] [Google Scholar]
- Hunter LL, Keefe DH, Feeney MP, Fitzpatrick DF, Lin L. Longitudinal development of wideband reflectance tympanometry in normal and at-risk infants. Hear Res. 2015 doi: 10.1016/j.heares.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint Committee on Infant Hearing - JCIH (2007) Year 2007 Position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics 120(4):898-921 [DOI] [PubMed]
- Keefe DH, Fitzpatrick D, Liu YW, Sanford CA, Gorga MP. Wideband acoustic-reflex test in a test battery to predict middle-ear dysfunction. Hear Res. 2010;263:52–65. doi: 10.1016/j.heares.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Hunter LL, Feeney MP, Fitzpatrick D. Procedures for ambient-pressure and tympanometric tests of aural acoustic reflectance and admittance in human infants and adults. J Acoust Soc Am. 2015;138:3625–3653. doi: 10.1121/1.4936946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe DH, Feeney MP, Hunter LL, Fitzpatrick DH (2016) Aural acoustic stapedius-muscle reflex threshold procedures to test human infants and adults. J Assn Res Otolaryngol [DOI] [PMC free article] [PubMed]
- Kei J. Acoustic stapedial reflexes in healthy neonates: normative data and test-retest reliability. J Am Acad Audiol. 2012;23:46–56. doi: 10.3766/jaaa.23.1.5. [DOI] [PubMed] [Google Scholar]
- Liu YW, Sanford CA, Ellison JC, Fitzpatrick DF, Gorga MP, Keefe DH. Wideband absorbance tympanometry using pressure sweeps: system development and results on adults with normal hearing. J Acoust Soc Am. 2008;124:3708–3719. doi: 10.1121/1.3001712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RH, Paul S, Saly GL, Schachern PA, Keefe DH. Wideband reflectance tympanometry in chinchillas and human. J Acoust Soc Am. 2001;110:1453–1464. doi: 10.1121/1.1394219. [DOI] [PubMed] [Google Scholar]
- Mazlan R, Kei J, Hickson L, Stapleton C, Grant S, et al. High frequency immittance findings: newborn versus six-week-old infants. Int J Audiol. 2007;46:711–717. doi: 10.1080/14992020701525858. [DOI] [PubMed] [Google Scholar]
- Mazlan R, Kei J, Hickson L. Test-retest reliability of the acoustic stapedial reflex test in healthy neonates. Ear Hear. 2009;30:295–301. doi: 10.1097/AUD.0b013e31819c3ea0. [DOI] [PubMed] [Google Scholar]
- McMillan PM, Bennett MJ, Marchant CD, Shurin PA. Ipsilateral and contralateral acoustic reflexes in neonates. Ear Hear. 1985;6:320–324. doi: 10.1097/00003446-198511000-00008. [DOI] [PubMed] [Google Scholar]
- Sanford CA, Feeney MP. Effects of maturation on tympanometric wideband acoustic transfer functions in human infants. J Acoust Soc Am. 2008;124:2106–2122. doi: 10.1121/1.2967864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanford CA, Keefe DH, Liu YW, Fitzpatrick D, McCreery, et al. Sound-conduction effects on distortion-product otoacoustic emission screening outcomes in newborn infants: test performance of wideband acoustic transfer functions and 1-kHz tympanometry. Ear Hear. 2009;30:635–652. doi: 10.1097/AUD.0b013e3181b61cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JC, Kaltenback JA, Relkin EM. The structural and functional development of the outer and middle ear. In: Romand R, Romand MR, editors. Development of auditory and vestibular systems. New York: Academic Press; 1983. [Google Scholar]
- Sprague BH, Wiley TL, Goldstein R. Tympanometric and acoustic-reflex studies in neonates. J Speech Hear Res. 1985;28:265–272. doi: 10.1044/jshr.2802.265. [DOI] [PubMed] [Google Scholar]
- Trine MB, Hirsch JE, Margolis RH. The effect of middle ear pressure on transient evoked otoacoustic emissions. Ear Hear. 1993;14:401–407. doi: 10.1097/00003446-199312000-00005. [DOI] [PubMed] [Google Scholar]
- Weatherby LA, Bennett MJ. The neonatal acoustic reflex. Scand Audiol. 1980;9:103–110. doi: 10.3109/01050398009076343. [DOI] [PubMed] [Google Scholar]