Abstract
PI 3-kinase (PI3K) occurs in the nuclei of a broad range of cell types, and various stimuli elicit PI3K nuclear translocation. However, little is known about the biological function of nuclear PI3K. Here we show that nuclear PI3K and its upstream regulator PIKE mediate the antiapoptotic activity of nerve growth factor (NGF) in the isolated nuclei. The nuclei from NGF-treated PC12 cells, EGF-treated HEK293 cells and HeLa cells are resistant to DNA fragmentation initiated by activated cell-free apoptosome. Nuclei from constitutively active PI3K adenovirus-infected cells display the same resistance as those treated by NGF, whereas PI3K inhibitors, dominant-negative PI3K or PIKE abolishes it. Knockdown of either PI3K or PIKE diminishes the antiapoptotic activity of NGF. PI (3,4,5)P3 alone mimics the antiapoptotic activity of NGF, for which nuclear Akt is required. These results demonstrate that PIKE/nuclear PI3K signaling through nuclear PI (3,4,5)P3 and Akt plays an essential role in promoting cell survival.
Keywords: apoptosis, DNA fragmentation, NGF, nuclear PI 3-kinase, PIKE
Introduction
PIKE-S is a brain-specific nuclear GTPase that enhances PI 3-kinase (PI3K) activity and is regulated by protein 4.1N (Ye et al, 2000). Nerve growth factor (NGF) treatment leads to PIKE-S activation by triggering the nuclear translocation of PLC-γ1, which acts as a physiological guanine-nucleotide-exchange factor (GEF) for PIKE-S through its SH3 domain. This action is independent of its lipase catalytic activity (Ye et al, 2002). Recently, we have identified a novel form of PIKE, designated PIKE-L, which, unlike the nuclear PIKE-S, localizes in both the cytoplasm and the nucleus. PIKE-L binds to Homer, an adaptor protein known to link metabotropic glutamate receptors (mGluR I) to multiple intracellular targets, including the inositol 1,4,5 trisphosphate receptor. The activation of mGluR I enhances the formation of an mGluR I–Homer–PIKE-L complex, leading to activation of PI 3-kinase activity and prevention of neuronal apoptosis (Rong et al, 2003).
NGF activates a variety of signaling cascades, but the PI3K/Akt pathway is particularly important for mediating neuronal survival under a wide variety of circumstances (Brunet et al, 2001). NGF, by binding to the TrkA receptor, elicits the recruitment of PI3k to the vicinity of the plasma membrane, where the catalytic subunit of PI3k generates the D3-phosphoinositols PIP2 and PIP3, which in turn lead to the membrane translocation of Akt through binding to its pleckstrin homology (PH) domain. At the plasma membrane, Akt is phosphorylated by the protein kinases PDK1 and PDK2 (Brunet et al, 2001). The active Akt inhibits apoptosis by impinging on the cytoplasmic and nuclear machinery through phosphorylation. For instance, Akt phosphorylates the proapoptotic Bcl-2 family member BAD, thereby inhibiting BAD proapoptotic functions (Datta et al, 1997; del Peso et al, 1997). In addition, Akt also regulates apoptosis by modulating the expression of apoptotic genes in the nucleus. For example, Akt controls a major class of transcription factors—the Forkhead box transcription factor—by phosphorylation and inhibiting their ability to induce the expression of death genes (Biggs et al, 1999; Brunet et al, 1999). PI3k and Akt predominantly locate in the cytoplasm, but they also occur in the nucleus, or translocate to there upon stimulation (Andjelkovic et al, 1997; Marchisio et al, 1998; Bavelloni et al, 1999; Neri et al, 1999; Tanaka et al, 1999; Borgatti et al, 2003). While cytoplasmic PI3k/Akt signaling has been well characterized, little is known about the nuclear counterpart.
In this report, we show that PIKE/nuclear PI3K signaling mediates the antiapoptotic actions of NGF through nuclear PI (3,4,5)P3 and nuclear Akt, which synergistically inhibit DFF40/CAD DNA fragmentation activity.
Results
The nuclei from NGF-treated PC12 cells resist DNA fragmentation
The PI3K/Akt pathway is sufficient and, in some cases, necessary for trophic-factor-induced cell survival of several neuronal cell types (Dudek et al, 1997; Miller et al, 1997; Philpott et al, 1997). NGF elicits translocation of PI3K and its downstream effectors into the nucleus; however, the biological function of nuclear PI3K remains elusive. We hypothesized that nuclear PI3K protects DNA fragmentation during programmed cell death. The experimental procedures are depicted as shown (Figure 1A). To test this hypothesis, we isolated nuclei from PC12 cells, treated with 50 ng/ml NGF for 0, 10, 30 min, and 1 h, then added the nuclei to the activated in vitro cell-free apoptotic solution, consisting of HEK293 cell cytosol supplemented with purified active caspase 3 (Liu et al, 1997). DNA fragmentation is evident in 0 and 10 but not 30 min or 1 h NGF-treated samples, indicating that 30 min–1 h NGF treatment activates pathways inhibiting caspase-3-activated DFF40/CAD (DNA Fragmentation Factor/Caspase-Activated DNase) (Figure 1B, top panel). Immunoblotting reveals a similar temporal pattern for cleavage of PARP (Figure 1B, bottom panel). DFF45 from control nuclei is cleaved in the cell-free apoptotic solution, indicating that caspase-3 is active in the cell-free apoptotic solution (Figure 1C). Nuclear integrity examined by DAPI staining demonstrates the nuclei from control PC12 cells display enormous condensed chromatin and apoptotic bodies; in contrast, the 30 min NGF-treated PC12 cell nuclei are intact (Figure 1D). Almost no intact nucleus is found in the control liver nuclei sample. Preincubation of PC12 cells with transcription and translation inhibitors does not alter the effects of NGF (data not shown). These observations suggest that NGF triggers a rapid protective action against both active caspase-3 and CAD, independent of transcription and translation. To examine which fraction contains the activity, we preincubated various amounts of cytosolic and nuclear extracts with the cell-free apoptotic solution and determined DNA cleavage with the control nuclei. DNA fragmentation assay reveals antiapoptotic activity specific for nuclear but not cytosolic fractions from NGF-treated PC12 cells (Figure 1E). Growth factors trigger PI3K nuclear translocation and nuclear PI (3,4,5)P3 synthesis in a variety of cell lines (Tanaka et al, 1999). To investigate whether this effect could be expanded to other cell types, we pretreated HEK293 and HeLa cells with EGF, isolated the nuclei and examined DNA fragmentation. A robustly inhibitory effect occurs in the samples pretreated with EGF; by contrast, evident DNA fragmentation is observed in the absence of EGF (Figure 1F). These findings demonstrate that growth factor treatment could rapidly accumulate antiapoptotic activity in the nucleus.
Figure 1.
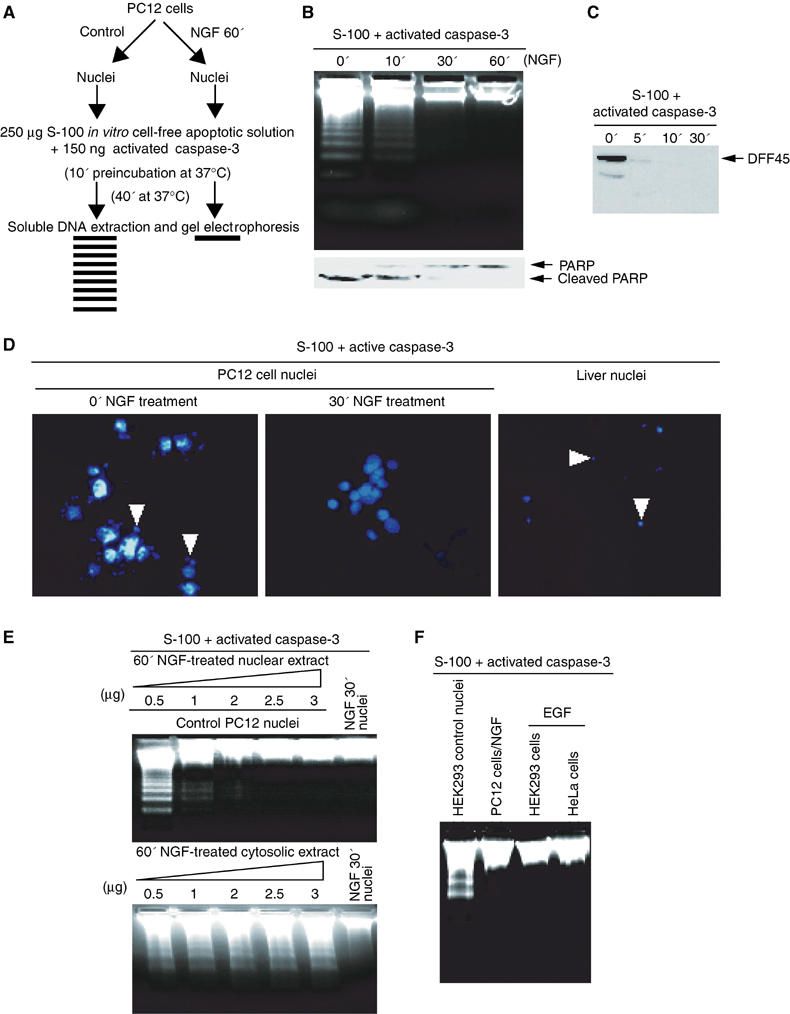
The nuclei from NGF-treated PC12 cells resist DNA fragmentation in cell-free apoptotic solution. (A) Experimental procedures employed in the DNA fragmentation assay. (B) DNA fragmentation assay. The nuclei were isolated from PC12 cells, which were treated with 50 ng/ml NGF (0, 10, 30 min and 1 h) and incubated in the activated cell-free apoptotic solution for 40 min. The fragmented DNA was extracted and resolved on 2% agarose. (C) DFF45 from the control nucleus was cleaved by active caspase-3 in HEK293 cytosolic fraction S-100. (D) Nuclear morphology assay with DAPI staining. The nuclei were isolated from PC12 cells treated with NGF and incubated in apoptotic solution for 40 min and stained with DAPI. The rat liver nuclei were employed as a positive control. (E) PC12 cells were treated with NGF in regular medium for 30 min, and the nuclear and cytosolic extracts of PC12 cells were prepared. Different amounts of the extracts were preincubated with the apoptotic solution for 10 min, then the control nuclei were introduced for DNA fragmentation assay. Nuclear, but not cytosolic, fraction inhibits active apoptosome-triggered DNA fragmentation. (F) The nuclei from HEK293 and HeLa cells, treated with 100 μg/ml EGF for 15 min, are resistant to DNA fragmentation in cell-free apoptotic solution. By contrast, evident DNA fragmentation is observed in control HEK293 cell nuclei without EGF treatment. The nuclei from PC12 cells treated with NGF were employed as positive control.
Nuclear PI3K mediates antiapoptotic actions of NGF
To determine whether nuclear PI3K is involved in NGF-regulated cell survival, we treated PC12 cells with NGF. After 30 min treatment, we isolated nuclei and pre-incubated the nuclei with the specific PI3K inhibitors Wortmannin (20 nM) and LY294002 (10 μM), or vehicle solution for 30 min, and examined the DNA fragmentation. We observe DNA fragmentation in PI3K inhibitor-treated samples, but not in the control group (Figure 2A and B), indicating that nuclear PI3K is necessary for NGF to protect DNA from cleaving by CAD or other endonucleases. Cytosolic PI3K might associate with the isolated nuclei and contribute to the protective effect. Accordingly, we examined PI3K activity in both cytosolic and nuclear fractions. Consistent with previous findings (Neri et al, 1994; Ye et al, 2000), cytosolic PI3K peaks at about 5 min upon NGF treatment and declines to baseline at 60 min, whereas nuclear PI3K lags with maximal activity at 30 min and decays slowly (Figure 2C, upper panel). Combined with the observation in Figure 1E, these results indicate that nuclear, but not cytosolic, PI3K accounts for this protective effect. The purity of both fractions was verified by immunoblotting with α-tubulin and PARP as cytosolic and nuclear markers (Figure 2C, lower panel). To explore further the role of nuclear PI3K in NGF-mediated cell survival, we infected PC12 cells with adenovirus expressing constitutively active or dominant-negative PI3K, and treated cells with or without NGF for 30 min. Regardless of NGF treatment, the nuclei infected by constitutively active PI3K are resistant to internucleosomal cleavage. In contrast, marked DNA fragmentation is observed in dominant-negative PI3K-infected nuclei in spite of NGF treatment (Figure 2D, upper panel). Dominant-negative Δp85 (deletion of amino acids 478–513) robustly inhibits both cytosolic and nuclear PI3K activity, whereas constitutively active p110* (K227E) markedly enhances it (Figure 2D, lower panel). To assess the subcellular localization of infected PI3K, we performed immunofluorescent staining and subcellular fractionation assay. Myc-p110* distributes in both the cytoplasm and the nucleus, and NGF elicits further nuclear translocation. However, in the absence of NGF, Δp85 predominantly occurs in the cytoplasm. NGF treatment triggers robust nuclear translocation of Δp85 (Figure 2E). To explore whether nuclear PI3K is required for the antiapoptotic effect, we immunodepleted PI3K from nuclear extract with anti-p110 antibody. Immunodepletion of PI3K abolishes the protective effect of nuclear extract, whereas rabbit IgG control fails. The lost activity could be reconstituted by a supplement of 1 μg recombinant PI3K protein or 10 μM PI (3,4,5)P3, but not by other phosphoinositols (Figure 2F, left panel). Compared with rabbit IgG control, p110 is substantially depleted from nuclear extract by the anti-p110 antibody (Figure 2F, right panels). To further evaluate the biological role of nuclear PI3K, we employed antennapedia peptide (Penetratin 1)-mediated intracellular delivery, a technique that has been widely and successfully used to knock down target genes (Troy et al, 2001; Rong et al, 2003). PC12 cells were pretreated with Penetratin 1-conjugated antisense or sense oligonucleotides of p110 α, stimulated with or without NGF for 30 min, and the isolated nuclei assayed in the activated cell-free apoptotic solution. Pronounced DNA fragmentation occurs in antisense but not sense p110 α-treated sample, despite NGF treatment (Figure 2G, left panel). Consistent with this observation, p110 α protein levels are substantially decreased in antisense but not sense oligonucleotide-treated cells. By contrast, PARP is unaffected. Akt phosphorylation is substantially decreased in p110-knockdown cells compared with sense control (Figure 2G, middle panels). The cytosolic and nuclear PI3K activity from oligonucleotide-treated cells correlates with Akt activation (Figure 2G, right panels). Thus, nuclear PI3K is sufficient and necessary for protecting DNA from fragmentation by NGF.
Figure 2.
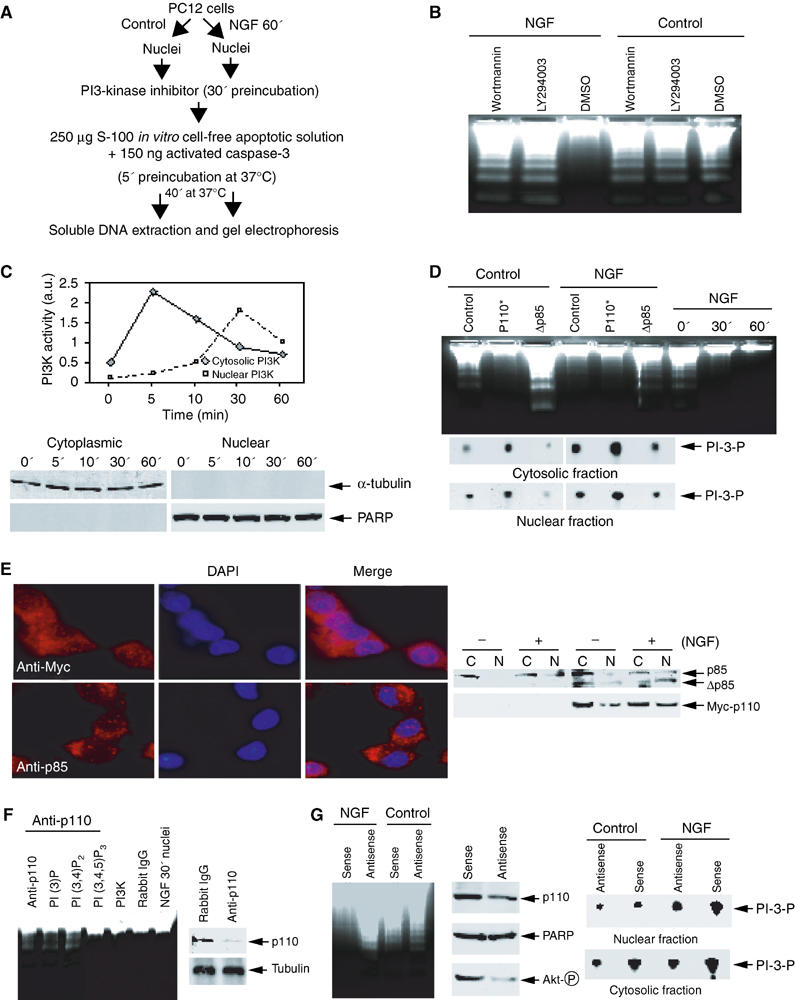
Nuclear PI3K is implicated in NGF-regulated cell survival. (A) Experimental procedures employed in the DNA fragmentation assay. (B) PI3K inhibitors abolish the antiapoptotic effect of NGF in the nucleus. PC12 cells were treated with NGF for 60 min. The isolated nuclei were pre-incubated with two specific PI3K inhibitors Wortmannin (20 nM), Ly294002 (10 μM), and vehicle solution, respectively, for 30 min. After removal of the inhibitors, the nuclei were incubated in apoptotic solution at 37°C for 40 min. The soluble DNA were extracted and analyzed. (C) Cytosolic and nuclear PI3K activity in NGF-treated PC12 cells (upper panel). The identity of cytosolic and nuclear fractions was verified by immunoblotting with anti-α-tubulin and anti-PARP (lower panels). (D) Nuclear PI3K is sufficient and necessary for the antiapoptotic effect of NGF in the nucleus. PC12 cells were infected with control adenovirus and adenovirus expressing constitutively active or dominant-negative PI3K. After 24 h, the infection efficiency was verified by GFP expression under fluorescent microscope. The isolated nuclei from NGF-treated or nontreated PC12 cells were analyzed in apoptotic solution (upper panel). In vitro PI3K activity assay of cytosolic and nuclear fractions from adenovirus-infected PC12 cells (lower panels). (E) PI3K nuclear translocation in PC12 cells infected with adenovirus expressing Myc-p110* and dominant-negative p85. The infected cells were treated with NGF for 30 min, followed by fixation and staining with anti-Myc and anti-p85 antibodies, respectively. Evident nuclear translocation occurred for p110 and p85 proteins (left panels). Similar effects were observed in biochemical fractionations (right panels). (F) Immunodepletion of PI3K from the nuclear extract abolishes its antiapoptotic effect. NGF-treated nuclear extract (10 μg) was preincubated with 2 μl anti-p110 antibody/20 μl protein-A/G conjugated beads at 4°C for 3 h, and the supernatant was supplemented with various phosphoinositol lipids or recombinant PI3K. Evident DNA fragmentation occurs when PI3K was immunodepleted (lane 1), whereas it is potently inhibited when recombinant PI3K was added back (lane 5). By contrast, rabbit IgG control failed to impair the activity (lane 6). Moreover, introduction of 10 μM PI (3,4,5)P3 but not PI (3)P or PI (3,4)P2 reconstitutes the inhibitory effect (left panel). P110 is specifically removed from nuclear extract by anti-p110 antibody (right panels). (G) Nuclear PI3K is required for the antiapoptotic effect of NGF in the nucleus. Serum-starved PC12 cells were treated with Penetratin 1-conjugated sense or antisense oligonucleotides of p110 α for 6 h, followed by 30 min NGF treatment. The isolated nuclei were analyzed in activated apoptotic solution (left panel). The protein level of p110 α and Akt phosphorylation status were verified by Western blotting. Compared to sense oligonucleotide, antisense markedly diminishes p110 α expression. By contrast, PARP protein level is not affected. Akt phosphorylation is substantially decreased in p110-knocked down cells (middle panels). Cytosolic and nuclear PI3K activity is decreased in p110-knocked down PC12 cells (right panels).
PIKE regulates the antiapoptotic activity of NGF
We have previously demonstrated that NGF activates PIKE, a brain-specific nuclear GTPase, which subsequently mediates the activation of nuclear PI3K (Ye et al, 2000). To explore the role of PIKE in NGF-mediated inhibition of DNA fragmentation, we infected PC12 cells with adenovirus expressing wild-type or dominant-negative PIKE (K413AS414N), and treated with or without NGF for 30 min. Negligible DNA fragmentation occurs in control and wild-type PIKE-infected PC12 cells. In contrast, dominant-negative PIKE-infected cells display pronounced DNA fragmentation. Without NGF treatment, we observe DNA fragmentation in all cells (Figure 3A), indicating that PIKE is required for regulating NGF-mediated cell survival. To further elucidate the role of PIKE, we prepared stably transfected PC12 cells with an inducible form of wild-type or dominant-negative PIKE. After NGF stimulation, nuclei from cells transfected with empty vector or wild-type PIKE display negligible DNA fragmentation, whereas marked DNA fragmentation is observed in the nuclei of dominant-negative PIKE-transfected cells. In the absence of NGF treatment, DNA cleavage is demonstrable in the nuclei from cells transfected with all the three constructs (Figure 3B, left panels). PIKE-L isoform employed in these experiments distributes in both the cytoplasm and the nucleus (Rong et al, 2003). Thus, wild-type PIKE strongly enhances both cytosolic and nuclear PI3K activity, whereas dominant-negative PIKE substantially inhibits it in response to NGF treatment (Figure 3B, right panels). To further assess the requirement of PIKE for NGF inhibition of DNA cleavage, we treated PC12 cells with Penetratin 1-conjugated antisense or sense oligonucleotides of PIKE, stimulated with or without NGF for 30 min, and assayed the isolated nuclei in the activated cell-free apoptotic solution. NGF stimulation of sense oligonucleotide-treated cells diminishes DNA cleavage in the nucleus. By contrast, nuclei from antisense oligonucleotide-treated cells display DNA fragmentation regardless of NGF treatment (Figure 3C, left panel). Expression of both PIKE-L and -S proteins is substantially decreased in antisense but not sense oligonucleotide-treated cells. As a control, the expression level of PARP is not affected (Figure 3C, middle panels). PIKE knockdown substantially decreases PI3K activity in both cytosolic and nuclear fractions (Figure 3C, right panels).
Figure 3.
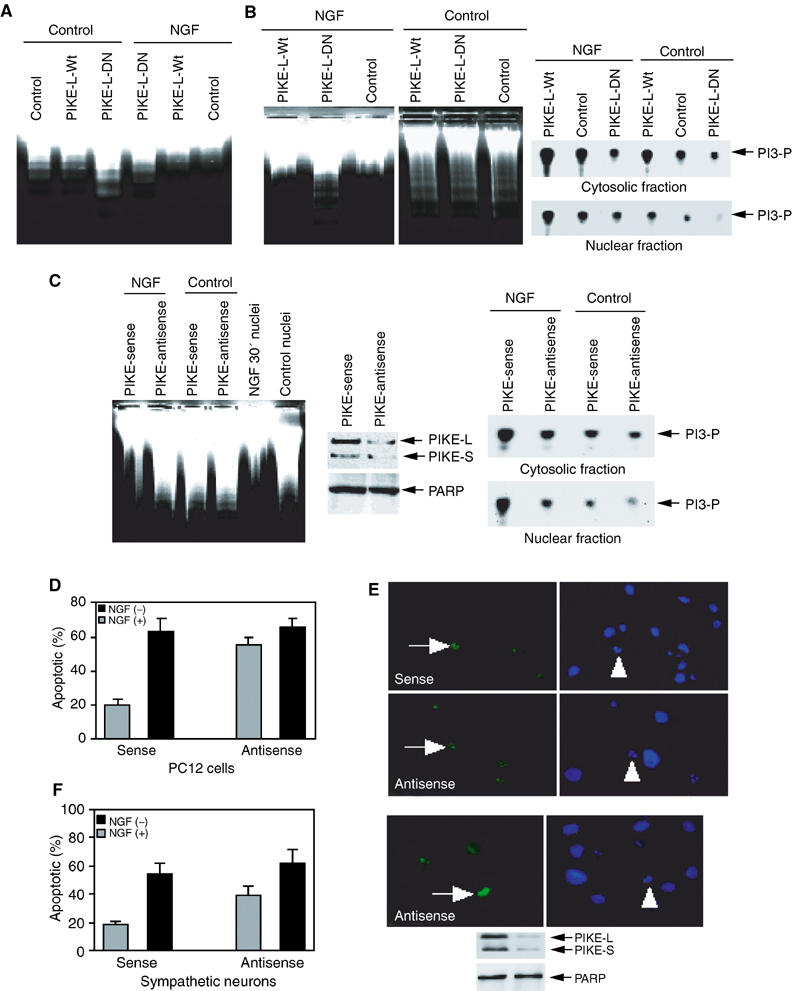
PIKE regulates the antiapoptotic effect of NGF in the nucleus. (A) Dominant-negative PIKE abrogates the antiapoptotic effect of NGF. PC12 cells were infected with control adenovirus and adenovirus expressing wild-type or dominant-negative PIKE. After 24 h, the infection efficiency was verified by GFP expression under a fluorescent microscope. The isolated nuclei from NGF-treated or nontreated PC12 cells were analyzed in apoptotic solution. (B) The stably transfected PC12 cells were induced to express wild-type or dominant-negative PIKE for 24 h, and GFP expression was verified under fluorescent microscope, then followed by NGF or vehicle solution stimulation for 1 h. The isolated nuclei were analyzed in activated apoptotic solution (left panel). Dominant-negative PIKE inhibits cytosolic and nuclear PI3K from induced PIKE stable cell lines (right panels). (C) Serum-starved PC12 cells were treated with Penetratin 1-conjugated sense or antisense oligonucleotides of PIKE for 6 h, followed by 1 h NGF treatment. The isolated nuclei were analyzed in activated cell-free apoptotic solution (left panel). Compared to sense oligonucleotide, antisense markedly diminishes both PIKE-L and -S protein expression. By contrast, PARP protein level is not affected (middle panels). In vitro PI3K activity assay of cytosolic and nuclear PI3K from oligonucleotide-treated cells. PIKE knockdown diminishes NGF-provoked PI3K activity in both the cytoplasm and the nucleus (right panels). (D) PIKE mediates the antiapoptotic effect of NGF in PC12 cells. PC12 cells were treated with Penetratin 1-conjugated sense or antisense oligonucleotides of PIKE for 6 h and induced apoptosis by 250 nM staurosporine for 24 h. (E) TUNEL assay and DAPI staining of staurosporine-treated cells. (F) PIKE mediates the antiapoptotic effect of NGF in sympathetic neurons. Sympathetic neurons were treated with Penetratin 1-conjugated sense or antisense oligonucleotides of PIKE for 6 h and treated with 250 nM staurosporine for 24 h in the presence or absence of NGF. TUNEL assay and DAPI staining of staurosporine-treated apoptotic sympathetic neurons (500 cells were counted under different fields) (right upper panels). Both PIKE-L and -S were markedly knocked down, whereas Tubulin was not changed (right lower panels). Numbers of treated cells in apoptosis were calculated as means (±s.d.) of five determinations and are representative of three experiments.
To investigate whether PIKE mediates NGF antiapoptotic actions in intact cells, we knocked down PIKE in PC12 cells employing Penetratin 1-conjugated antisense or sense oligonucleotides. The apoptotic activity was analyzed with TUNEL assay and DAPI staining for chromatin condensation and fragmentation. Staurosporine elicits 2.5-fold increase in apoptosis in antisense oligonucleotide-treated cells compared to sense control. However, in the absence of NGF, similar apoptotic activity occurs in both antisense and sense oligonucleotide-treated cells (Figure 3D). The TUNEL assay and DAPI staining on PC12 cells are depicted in Figure 3E. We extended these studies to sympathetic neurons. Consistent with our results in PC12 cells, apoptosis is robustly increased in antisense but not sense PIKE oligonucleotide-pretreated neurons (Figure 3F, left panel). The TUNEL assay and DAPI staining on sympathetic neurons are depicted (right upper panels). Expression of both PIKE-L and -S proteins is substantially decreased in antisense but not sense oligonucleotide-treated cells. As a control, PARP is not affected (right lower panels). Combined with our in vitro DNA fragmentation results, these observations establish that PIKE, acting through nuclear PI3K, plays a critical role in protecting DNA from internucleosomal cleavage.
Nuclear PI (3,4,5)P3 mediates the antiapoptotic effects of NGF
Phosphatidylinositols occur in the nucleus of various types of cells, and mediate a broad range of nuclear processes including chromatin structure, pre-mRNA splicing, cell cycle progression and nuclear response of DNA damage (Boronenkov et al, 1998; Yokogawa et al, 2000; Gozani et al, 2003). PI (3,4,5)P3 and PI (3,4)P2 promote cell survival by activating Akt/PKB, which phosphorylates components of the apoptotic machinery. High concentrations of PI (4,5)P2 directly inhibit caspases 8 and 9. Moreover, PI (4,5)P2 and PI (3,4,5)P3 but not PI (3,4)P2 bind and inhibit caspase 3 (Mejillano et al, 2001). To examine whether the resistance to the activated apoptosome in the nuclei from NGF-treated PC12 cells involves nuclear phosphatidylinositol lipids, we pre-incubated a variety of phosphatidylinositol (10 μM) lipids with the activated cell-free apoptotic solution for 10 min at 37°C (Figure 4A, upper panel). Western blotting analysis reveals apoptotic cleavage of DFF45 (Figure 4A, lower panel) and Lamin A/C (Figure 4C, upper panel). DNA fragmentation assay of control nuclei reveals internucleosomal cleaved DNA, indicating that none of the phosphatidylinositol lipids directly inhibits the activated endonucleases (Figure 4C, lower panel). Our observation that nuclear PI3K is critical for mediating NGF antiapoptotic action (Figure 2), combined with the failure of PI (3,4)P2 and PI (3,4,5)P3 to inhibit CAD-initiated DNA fragmentation (Figure 4C), indicates that downstream signals from nuclear receptors of PI (3,4,5)P3 but not lipids themselves exert the antiapoptotic activity. Accordingly, we examined whether PI (3,4)P2 or PI (3,4,5)P3 can mimic NGF's effect by pre-incubating a variety of phosphoinositol lipids (10 μM) with control nuclei for 30 min at 37°C, and performed DNA fragmentation assay after removal of the lipids (Figure 4B, top panel). Strikingly, PI (3,4,5)P3 pretreatment prevents DFF45 (Figure 4B, bottom panel) and lamin A/C cleavage in the control nuclei (Figure 4C, upper panel). We have observed the same effect on PARP cleavage (data not shown). Consistent with these observations, PI (3,4,5)P3 pretreatment protects control nuclear DNA from degradation, whereas PI (3,4)P2, PI (4,5)P2 and PI-3-P fail (Figure 4C, lower panel), suggesting that PI (3,4,5)P3 accounts for the antiapoptotic actions of NGF in the nucleus.
Figure 4.
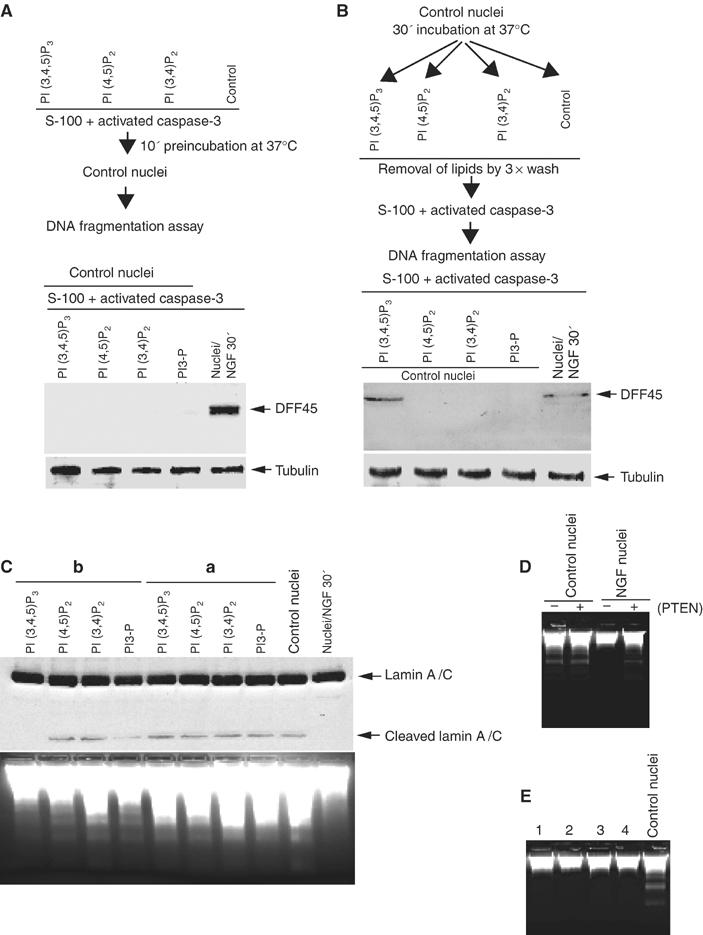
Phosphoinositol lipid PI (3,4,5)P3 mediates the antiapoptotic effect of NGF in the nucleus. (A) Phosphoinositol lipids do not directly inhibit active caspase-3. Experimental procedure scheme (upper panel). Preincubation of the lipids with the activated apoptotosome failed to prevent DFF45 cleavage in the control nuclei (lower panel). (B) PI (3,4,5)P3 pretreatment protects DFF45 in the control nuclei from apoptotic degradation. Experimental procedure scheme (upper panel). Various phosphoinositol lipids (10 μM) were preincubated with the nuclei from PC12 cells for 45 min, and removed by three times washing with buffer B, then the pretreated nuclei were analyzed in apoptotic solution. DFF45 in control nucleus pretreated with PI (3,4,5)P3 but not other lipids is intact. The nuclei from NGF-stimulated PC12 cells were employed as a positive control (lower panel). α-Tubulin was employed as loading control (bottom panel). (C) PI (3,4,5)P3 pretreatment protects the control nucleus from apoptotic degradation. PI (3,4,5)P3, but not other phosphoinositol lipids, protects lamin A/C cleavage in the pretreated control nuclei (upper panel). Consistent with DFF45 cleavage in A, none of the phosphoinositol lipids directly inhibits caspase-3-triggered lamin A/C cleavage. DNA fragmentation analysis correlates with protein cleavage assay (lower panel). (D) PTEN phosphotase abolishes the antiapoptotic effect in the nuclei from NGF-treated PC12 cells. PTEN (150 ng) preincubated with the nuclei for 15 min at 37°C before DNA fragmentation assay. (E) Cycloheximide pretreatment does not inhibit the antiapoptotic actions of NGF or PIP3. Cycloheximide (100 μg/ml) was preincubated with PC12 cells for 30 min and then treated with NGF (lane 1), or at the same time as NGF added (lane 2), cycloheximide preincubated with the nuclei from NGF-treated cells (lane 3), cycloheximide preincubated with the control nuclei from PC12 cells, followed by PIP3 treatment (lane 4), and the control nuclei (last lane) was employed as control.
To further test the antiapoptotic role of nuclear PI (3,4,5)P3, we pretreated the nuclei with or without 150 ng PTEN for 15 min at 37°C, and analyzed DNA fragmentation. PTEN pretreatment abolishes the protective effect of NGF, whereas marked DNA degradation occurs in the control nuclei, regardless of PTEN treatment (Figure 4D). PI (3,4,5)P3 pretreatment with the control nuclei renders them the inhibitory activity. To explore whether this process involves any new macromolecule transcription/translation, we preincubated PC12 cells with cycloheximide, followed by NGF treatment, or isolated the control nuclei from PC12 cells, then pretreated with cycloheximide, followed by PI (3,4,5)P3 incubation. None of these treatments abrogates the antiapoptotic activity of NGF or PI (3,4,5)P3 (Figure 4E), indicating that no new protein synthesis is required for PI (3,4,5)P3 to mediate this protective effect.
Nuclear Akt is required for antiapoptotic activity of NGF
One of the well-characterized downstream targets of PI (3,4,5)P3 is Akt, which plays a critical role in inhibiting apoptosis in the cytoplasm. NGF stimulates phosphorylated Akt to translocate to the nucleus of PC12 cell (Borgatti et al, 2003). To determine the role of nuclear translocated Akt in the antiapoptotic action of NGF, we infected PC12 cells with adenovirus expressing wild-type Akt, constitutively active Akt (T308DS473D) (Foran et al, 1999) or dominant-negative Akt, a kinase-inactive, phosphorylation-deficient Akt construct (K179AT308AS473A) (Wang et al, 1999), and treated cells with or without NGF for 30 min. Upon NGF treatment, the nuclei infected by control, wild-type or constitutively active Akt are resistant to internucleosomal cleavage. In contrast, evident DNA fragmentation is observed in dominant-negative Akt-infected nuclei in spite of NGF treatment, indicating that nuclear Akt is required for NGF-mediated antiapoptotic signaling. However, without NGF treatment, all the nuclei display demonstrable DNA degradation even infected with constitutively active Akt, suggesting that Akt activation alone is not sufficient to inhibit DNA cleavage (Figure 5A). The expression of infected Akt and kinase activity are verified (data not shown). To investigate the role of nuclear Akt, we generated stably transfected PC12 cells with an inducible form of nuclear localization signal (NLS)-tagged Akt constructs. Immunofluorescent staining with anti-Myc antibody reveals that constitutively active Akt predominantly localizes in the nucleus, whereas dominant-negative Akt distributes in both the cytoplasm and the nucleus (Figure 5B, left panels). Biochemical fractionation confirms the immunofluorescent staining findings. These observations are consistent with previous discovery that activation of Akt is required for its nuclear translocation (Borgatti et al, 2003). The identity and purity of both the cytosolic and nuclear fractions were, respectively, verified with anti-PARP and anti-tubulin antibodies (Figure 5B, right upper panels). Dominant-negative Akt robustly inhibits Akt activity in both fractions, while constitutively active Akt markedly increases it (Figure 5B, right lower panels). Upon induction and NGF stimulation, the nuclei from cells transfected with empty vector or Myc-NLS-Akt-CA display negligible DNA fragmentation; whereas robust DNA fragmentation is observed in the nuclei of dominant-negative Myc-NLS-Akt-transfected cells (DN1 and DN2 clones). In the absence of NGF treatment, DNA cleavage is evident in nuclei from cells transfected with all constructs, though DNA fragmentation is slightly less in constitutively active Akt cells (Figure 5C). To investigate whether nuclear Akt mediates the antiapoptotic effect of NGF through sequestrating PI (3,4,5)P3 from other nuclear targets, we express GST-tagged Akt PH domain and PIKE PH domain in 293 cells, respectively, and treated transfected cells with or without EGF. DNA fragmentation assay demonstrates that the nuclei from EGF-treated cells but not control cells resist DNA degradation, regardless of PH domain expression. The expression of both constructs was verified (Figure 5D). Immunofluorescent staining shows that Akt-PH predominantly occurs in the cytoplasm, while PIKE-PH domain exclusively distributes in the nucleus (data not shown). Collectively, these observations indicate that nuclear Akt is necessary but not sufficient to mediate the antiapoptotic action of NGF. Presumably, other PI (3,4,5)P3 nuclear receptor downstream effectors cooperatively antagonize apoptosis with nuclear Akt.
Figure 5.
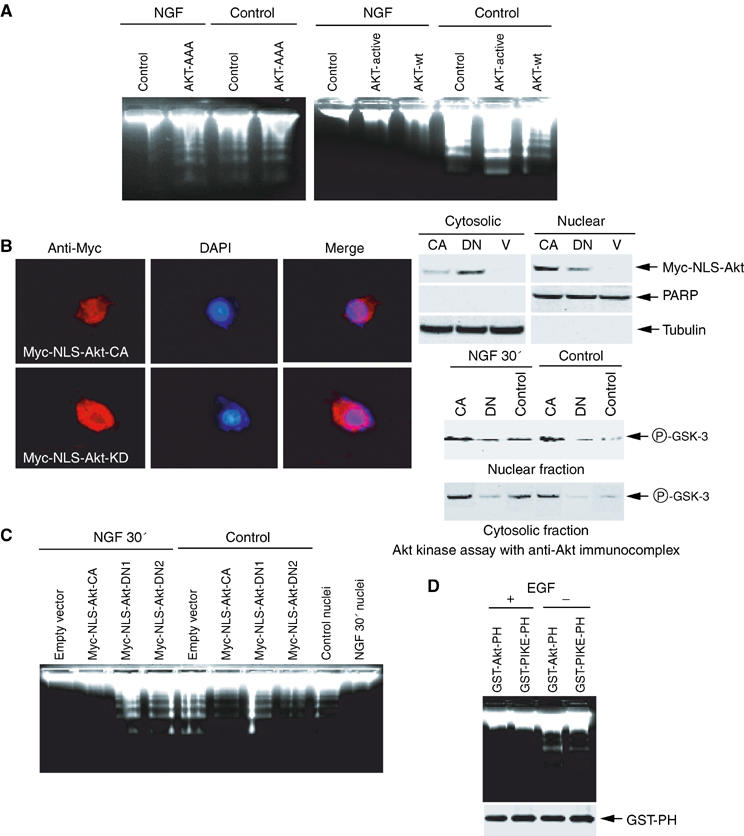
Nuclear Akt is required for the antiapoptotic action of NGF. (A) PC12 cells were infected with control adenovirus and adenovirus expressing dominant-negative Akt (left panel) or wild-type, constitutively active Akt (right panel). After 24 h, the infection efficiency was verified by GFP expression under a fluorescent microscope. The isolated nuclei from PC12 cells were analyzed in an activated cell-free apoptotic solution. (B) Characterization of stably transfected Akt in Myc-NLS-Akt cells. Stably transfected PC12 cells were cultured in tetracycline-free medium overnight, followed by fixation and staining with anti-Myc antibody. Constitutively active (CA)-NLS-Akt predominantly exists in the nucleus, whereas dominant-negative (DN)-NLS-Akt occurs in both the cytoplasm and the nucleus (left panels). Biochemical fractionation and immunoblotting analysis verify the subcellular localization of transfected Akt (right, upper panels). In vitro Akt kinase assay with anti-Akt immunocomplex from the cytosolic and nuclear fractions of PC12 cells, treated with or without NGF (right, lower panels). (C) Stably transfected PC12 cells were induced to express constitutively active or dominant-negative Akt (DN1 and DN2) for 24 h, and GFP expression was verified under a fluorescent microscope, then followed by NGF or vehicle solution stimulation for 30 min. The isolated nuclei were analyzed in the activated apoptotic solution. (D) Expression of PH domains does not interfere with the antiapoptotic actions of EGF. HEK293 cells were transfected with mammalian expression GST-Akt-PH or GST-PIKE-PH, and treated with or without EGF for 15 min. The isolated nuclei were analyzed with DNA fragmentation assay (upper panel). The expression of transfected constructs was verified (lower panel).
Discussion
Our discovery that PIKE/nuclear PI3K signaling mediates the antiapoptotic actions of NGF demonstrates that nuclear PI3K protects DNA from internucleosomal cleavage during apoptosis. This conclusion is underscored by the compelling evidence from manipulating PI3K upstream nuclear effector PIKE. Our findings suggest a continuity of survival-promoting effect from cytoplasmic to nuclear PI3K. Nuclear PI3K is also implicated in RNA processing and transport (Boronenkov et al, 1998; Bunney et al, 2000). Thus, the functions of nuclear PI3K in the nucleus are as diverse and extensive as those of cytoplasmic PI3K.
NGF and other agents promote survival of serum-deprived PC12 cells and sympathetic neurons even when macromolecular synthesis is blocked (Rukenstein et al, 1991), supporting post-translational influences of NGF on DNA fragmentation and cell survival, consistent with a proposed protein/phosphorylation-driven pathway (Batistatou and Greene, 1991). The early- and the late-phase effects of NGF in promoting cell survival might be due to transcription-dependent and independent mechanisms. Conceivably, the rapid antiapoptotic activity might involve a protein phosphorylation pathway suppressing the action of a constitutively expressed endonuclease activity. The late transcription-dependent mechanism might involve survival-promoting genes expression. The transcription/translation-independent resistance to the activated apoptosome displayed by NGF-treated PC12 cells implicates this pathway as a molecular mechanism for the antiapoptotic action of NGF (Figure 1). This observation is directly supported by cycloheximide pretreatment experiment. Regardless of cycloheximide introduction procedures, the nuclei from NGF-treated PC12 or PI (3,4,5)P3-treated control nuclei are resistant to DNA fragmentation in cell-free apoptotic solution (Figure 4E).
The protective activity of NGF may involve at least three possible mechanisms: (1) cytoplasmic proteins translocate to the nucleus in response to NGF to inhibit the endonuclease activity; (2) nuclear proteins are activated by NGF to inhibit the endonuclease activity; (3) NGF regulates DNA modification or insulation from endonucleases. These possibilities are supported by the ability of the endonuclease inhibitor ATA to promote long-term survival of NGF-deprived PC12 cells and sympathetic neurons (Batistatou and Greene, 1993). In current paradigm of apoptosis cascade: caspase activation>DFF45/ICAD degradation>activation of DFF40/CAD endonuclease>DNA fragmentation, PI3K/PI (3,4,5)P3 functions upstream of caspase activation through Akt, which phosphorylates procaspase-9 and Bad to inhibit their proapoptotic activities. However, our results demonstrate that PI (3,4,5)P3 pretreatment with control nuclei prevents DFF45 cleavage elicited by active caspase-3 in S-100 apoptotic solution, and this inhibitory effect does not arise from direct caspase-3 inhibition by PI (3,4,5)P3 (Figure 4). These observations indicate that PI (3,4,5)P3 acts not only upstream of caspase-3 activation in the cytoplasm, but also functions downstream of it in the nucleus to serve as a final checkpoint before internucleosomal DNA fragmentation occurs, presumably through putative nuclear receptors of PI (3,4,5)P3. We have depicted this model in Figure 6. Growth factors trigger PI3K nuclear translocation in various cell lines (Tanaka et al, 1999). Our results demonstrate that the nuclei from HEK293 and HeLa cells, pretreated with EGF, reveal comparable antiapoptotic effect, even though neither of the cell lines expresses the upstream PIKE-S or L isoform (Figure 1F). In agreement with the notion that PI (3,4,5)P3 is a ubiquitous second messenger, our findings suggest that the antiapoptotic function of nuclear PI3K/PI (3,4,5)P3 signaling does not restrict to PC12 cells or neurons, it could also be generalized to other cell types.
Figure 6.
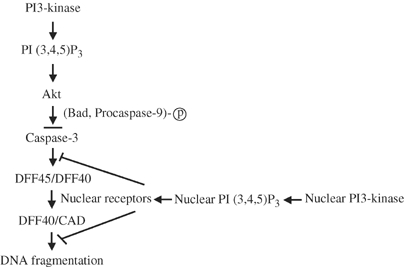
PI3K/PI (3,4,5)P3 signaling inhibits apoptosis both upstream and downstream of caspase-3 activation.
Pretreatment with isolated nuclei by PI3K inhibitors abolishes the antiapoptotic effect of NGF, suggesting that ongoing nuclear PI3K activity, the production of nuclear PI (3,4,5)P3, is required for this effect (Figure 2A). ATM, ATR and DNA double-strand break repair enzyme DNA-PK proteins are intimately involved in sensing DNA damage. They share the PI-3-like kinase domain, but they do not function as lipid kinases, but rather as serine–threonine protein kinases (Plumb et al, 1999; Rotman and Shiloh, 1999; Bao et al, 2001). It is possible that PI3K inhibitors could also affect these DNA damage sensors' kinase activity and interfere with their DNA integrity maintenance effect, contributing to trigger DNA fragmentation.
Both PI (3,4)P2 and PI (3,4,5)P3 bind to Akt and activate it. Surprisingly, pretreatment with control nuclei by PI (3,4)P2 has no effect on preventing apoptosis, while PI (3,4,5)P3 elicits potent activity (Figure 4). These observations argue that nuclear PI (3,4,5)P3 plays an essential role in promoting cell survival in the nucleus. On the contrary, PI (3,4)P2 is dispensable for this effect. Conceivably, PI (3,4,5)P3 triggers nuclear signaling cascades antagonizing active caspase-3 and CAD through the nuclear effectors, which PI (3,4)P2 does not activate. Nuclear Akt is required for the antiapoptotic actions of NGF (Figure 5). How does nuclear Akt regulate this effect? Clearly, it is not due to sequestration of PI (3,4,5)P3 from other nuclear targets, because overexpression of GST-Akt-PH domain (localized in the cytoplasm, data not shown) or GST-PIKE-PH domain (localized in the nucleus, data not shown) in HEK293 cells does not diminish the antiapoptotic effect of the nuclei from EGF-treated cells (Figure 5D). Presumably, unknown downstream nuclear targets of Akt are implicated in this process. However, constitutively active nuclear Akt alone is not sufficient to protect DNA from degradation (Figure 5), arguing that both nuclear PI (3,4,5)P3 receptors and nuclear Akt downstream effectors are necessary for the antiapoptotic effect of NGF in the nucleus.
Our data demonstrate that nuclear antiapoptotic machinery can be initiated after 30 min NGF stimulation. PIKE/nuclear PI3K signaling is probably mediated through nuclear PI (3,4,5)P3. The downstream effectors of nuclear PI (3,4,5)P3, including Akt, synergistically suppress apoptosis. This idea is indirectly supported by the recent finding that nuclear PI-5-P modulates p53 activity and cell death via interaction with its nuclear receptor ING2 (Gozani et al, 2003). Characterization of the PIKE/nuclear PI3K signaling downstream targets and nuclear receptors of PI (3,4,5)P3, which inhibit the activated caspases and endonucleases upon NGF stimulation, will provide insight into the mechanisms of NGF-promoted neuronal cell survival.
Materials and methods
Cells and reagents
PC12 cells were maintained in medium A (DMEM with 10% fetal bovine serum (FBS), 5% horse serum and 100 U penicillin–streptomycin) at 37°C with 5% CO2 atmosphere in a humidified incubator. All PC12 cells employed in the experiments are in naive but not differentiated form. The PIKE and Myc-NLS-Akt stably transfected PC12 cells (Tet-off cell line) were cultured in medium B (85% DMEM, 10% horse serum, 5% FBS, 100 μg/ml G418, 100 μg/ml hygromycin B, 2 μg/ml tetracycline and 100 U penicillin–streptomycin). The transfected genes were induced by culturing in medium C (85% DMEM, 10% horse serum, 5% FBS, 100 μg/ml G418, 100 μg/ml hygromycin B and 100 U penicillin–streptomycin) for 24 h. NGF was from Roche. Penetratin 1 was from Oncor, Inc. Sense and antisense oligonucleotides were from MWG Biotech. Anti-p85, p110, PARP antibodies were from Santa Cruz Biotechnology, Inc. Phospho-Akt-473 or 308 and Akt antibodies were from Cell Signaling. Anti-CAD and anti-DFF45 antibodies were from Upstate Biotechnology, Inc. The constitutively active p110*K227E mutant and dominant-negative Δp85 have been described before (Rodriguez-Viciana et al, 1996). The adenovirus expressing active p110*K227E mutant and Δp85 were gifts from Dr Harold A Franch at the Department of Medicine, Emory University. All phosphatidylinositol lipids were from Echelon Bioscience Inc. All the chemicals not included above and p110-γ protein were from Sigma.
Preparation of PC12 cell nuclei
PC12 cells were cultured in medium A. At various time points after NGF treatment, PC12 cells were harvested by centrifugation at 1800 g for 10 min at 4°C. After washed once with ice-cold PBS, the cell pellet was suspended in 5 v of ice-cold buffer A (20 mM Hepes (pH 7.5), 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT and 0.1 mM PMSF) supplemented with protease inhibitors. After sitting on ice for 15 min, cells were disrupted by passing 15 times in a douncer. The nuclei were centrifuged at 1000 g for 10 min at 4°C and resuspended in 2 v buffer A, and centrifuged at 25 000 g for another 30 min at 4°C. The purified nuclei were resuspended in buffer B (20 mM Hepes (pH 7.9), 20% glycerol (v/v), 0.1 M KCl, 0.2 mM EDTA, 0.5 mM DTT, and 0.5 mM PMSF) at 8.5 × 107 nuclei/ml and stored in multiple aliquots at −80°C.
Cell-free apoptotic solution preparation and DNA fragmentation assay
The procedures are exactly as described (Liu et al, 1997). Briefly, the pellets of 293 cells were washed once with ice-cold PBS and resuspended in 5 v of buffer A, supplemented with protease inhibitors. After sitting on ice for 15 min, the cells were broken by passing 15 times through a G22 needle. After centrifugation in a microcentrifuge for 5 min at 4°C, the supernatants were further centrifuged at 10 000 g for 30 min in an ultracentrifuge (Beckman). The resulting supernatants were used for in vitro apoptosis assay. The purified activated caspase 3 was added into S-100 extract to initiate the caspase cascade. After 1 h incubation at 37°C, the nuclei from PC12 cells, which were grown in medium A treated with or without NGF, were introduced and incubated for various time points. The DNA fragmentation assay was performed exactly as described (Liu et al, 1997).
PI (3,4,5)P3 triggers antiapoptotic actions in the control PC12 nucleus
Water-soluble PI (3,4,5)P3, PI (4,5)P2, PI (3,4)P2 and PI (3)P lipids (10 μM each) (Echlon, Inc.) were incubated with 1 million control PC12 nuclei for 30 min at 37°C in buffer A. After three times wash with 500 μl buffer A, the treated nuclei were introduced into 200 μg active S-100 and incubated at 37°C for another 45 min. The DNA fragmentation assay was performed as described above.
Subcellular fractionation and in vitro PI 3-kinase assay
The cytosolic and nuclear fractions from transfected or infected cells were prepared according to the manufacturer's protocol (Pierce, Nuclear and Cytoplasmic Extraction Reagent). Transfected PI3K was immunoprecipitated by anti-p110 antibody from cell lysate as described above, and washed with the following buffers: three times with Buffer A (PBS, 1% NP-40, 1 mM DTT); two times with Buffer B (PBS, 0.5 M LiCl, 1 mM DTT); two times with Buffer C (10 mM Tris-HCl pH 7.4, 0.1 M NaCl, 1 mM DTT). The following procedures were exactly as described (Ye et al, 2002).
Infection with adenovirus expressing wild-type and dominant-negative PIKE
Adenovirus expressing wild-type and dominant-negative (K413AS414N) PIKE-L were prepared. The virus was purified by CsCl banding with 1011–1012 plaque-forming units, and introduced into PC12 cells and cultured overnight. The GFP was monitored with immunofluorescent microscope.
TUNEL assay for apoptotic PC12 cells and sympathetic neurons
The cells were treated with Penetratin 1-conjugated oligonucleotide, and induced apoptosis with 250 nM staurosporine in the presence or absence of NGF. DNA strand breaks which occur in PC12 cells and sympathetic neurons were labeled in situ, in individual fixed and permeabilized cells, with biotinylated dUTP, using the terminal deoxynucleotidyl transferase or nick translation assays (Gorczyca et al, 1993). The procedures are exactly as described in manufacturer's brochure (Roche, In-situ cell death detection kit).
Penetratin 1-conjugated oligonucleotide knockdown assay
Sense and antisense oligonucleotides with 5′ SH group modification were resuspended in deionized water, an equimolar ratio of Penetratin1 (Oncor) was added, and the mixture was incubated at 37°C for 1 h. The yield of the reaction, estimated by SDS–PAGE followed by Coomassie blue staining, was >50%. The conjugated Penetranin1 was added into serum-starved PC12 cells 6 h before NGF treatment.
Acknowledgments
This work is supported by grants from the National Institute of Health (RO1, NS045627) to K Ye. We thank Dr Solomon H Snyder for critical reading of the manuscript. We are also grateful to Dr Paul A Wade for inspiring discussion and suggestions.
References
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA (1997) Role of translocation in the activation and function of protein kinase B. J Biol Chem 272: 31515–31524 [DOI] [PubMed] [Google Scholar]
- Bao S, Tibbetts RS, Brumbaugh KM, Fang Y, Richardson DA, Ali A, Chen SM, Abraham RT, Wang XF (2001) ATR/ATM-mediated phosphorylation of human Rad17 is required for genotoxic stress responses. Nature 411: 969–974 [DOI] [PubMed] [Google Scholar]
- Batistatou A, Greene LA (1991) Aurintricarboxylic acid rescues PC12 cells and sympathetic neurons from cell death caused by nerve growth factor deprivation: correlation with suppression of endonuclease activity. J Cell Biol 115: 461–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistatou A, Greene LA (1993) Internucleosomal DNA cleavage and neuronal cell survival/death. J Cell Biol 122: 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelloni A, Santi S, Sirri A, Riccio M, Faenza I, Zini N, Cecchi S, Ferri A, Auron P, Maraldi NM, Marmiroli S (1999) Phosphatidylinositol 3-kinase translocation to the nucleus is induced by interleukin 1 and prevented by mutation of interleukin 1 receptor in human osteosarcoma Saos-2 cells. J Cell Sci 112: 631–640 [DOI] [PubMed] [Google Scholar]
- Biggs WH III, Meisenhelder J, Hunter T, Cavenee WK, Arden KC (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96: 7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgatti P, Martelli AM, Tabellini G, Bellacosa A, Capitani S, Neri LM (2003) Threonine 308 phosphorylated form of Akt translocates to the nucleus of PC12 cells under nerve growth factor stimulation and associates with the nuclear matrix protein nucleolin. J Cell Physiol 196: 79–88 [DOI] [PubMed] [Google Scholar]
- Boronenkov IV, Loijens JC, Umeda M, Anderson RA (1998) Phosphoinositide signaling pathways in nuclei are associated with nuclear speckles containing pre-mRNA processing factors. Mol Biol Cell 9: 3547–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868 [DOI] [PubMed] [Google Scholar]
- Brunet A, Datta SR, Greenberg ME (2001) Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol 11: 297–305 [DOI] [PubMed] [Google Scholar]
- Bunney TD, Watkins PA, Beven AF, Shaw PJ, Hernandez LE, Lomonossoff GP, Shanks M, Peart J, Drobak BK (2000) Association of phosphatidylinositol 3-kinase with nuclear transcription sites in higher plants. Plant Cell 12: 1679–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91: 231–241 [DOI] [PubMed] [Google Scholar]
- del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G (1997) Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278: 687–689 [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME (1997) Regulation of neuronal survival by the serine–threonine protein kinase Akt. Science 275: 661–665 [DOI] [PubMed] [Google Scholar]
- Foran PG, Fletcher LM, Oatey PB, Mohammed N, Dolly JO, Tavare JM (1999) Protein kinase B stimulates the translocation of GLUT4 but not GLUT1 or transferrin receptors in 3T3-L1 adipocytes by a pathway involving SNAP-23, synaptobrevin-2, and/or cellubrevin. J Biol Chem 274: 28087–28095 [DOI] [PubMed] [Google Scholar]
- Gorczyca W, Gong J, Darzynkiewicz Z (1993) Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Cancer Res 53: 1945–1951 [PubMed] [Google Scholar]
- Gozani O, Karuman P, Jones DR, Ivanov D, Cha J, Lugovskoy AA, Baird CL, Zhu H, Field SJ, Lessnick SL, Villasenor J, Mehrotra B, Chen J, Rao VR, Brugge JS, Ferguson CG, Payrastre B, Myszka DG, Cantley LC, Wagner G, Divecha N, Prestwich GD, Yuan J (2003) The PHD finger of the chromatin-associated protein ING2 functions as a nuclear phosphoinositide receptor. Cell 114: 99–111 [DOI] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X (1997) DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89: 175–184 [DOI] [PubMed] [Google Scholar]
- Marchisio M, Bertagnolo V, Colamussi ML, Capitani S, Neri LM (1998) Phosphatidylinositol 3-kinase in HL-60 nuclei is bound to the nuclear matrix and increases during granulocytic differentiation. Biochem Biophys Res Commun 253: 346–351 [DOI] [PubMed] [Google Scholar]
- Mejillano M, Yamamoto M, Rozelle AL, Sun HQ, Wang X, Yin HL (2001) Regulation of apoptosis by phosphatidylinositol 4,5-bisphosphate inhibition of caspases, and caspase inactivation of phosphatidylinositol phosphate 5-kinases. J Biol Chem 276: 1865–1872 [DOI] [PubMed] [Google Scholar]
- Miller TM, Tansey MG, Johnson EM Jr, Creedon DJ (1997) Inhibition of phosphatidylinositol 3-kinase activity blocks depolarization- and insulin-like growth factor I-mediated survival of cerebellar granule cells. J Biol Chem 272: 9847–9853 [DOI] [PubMed] [Google Scholar]
- Neri LM, Martelli AM, Borgatti P, Colamussi ML, Marchisio M, Capitani S (1999) Increase in nuclear phosphatidylinositol 3-kinase activity and phosphatidylinositol (3,4,5) trisphosphate synthesis precede PKC-zeta translocation to the nucleus of NGF-treated PC12 cells. FASEB J 13: 2299–2310 [PubMed] [Google Scholar]
- Neri LM, Milani D, Bertolaso L, Stroscio M, Bertagnolo V, Capitani S (1994) Nuclear translocation of phosphatidylinositol 3-kinase in rat pheochromocytoma PC 12 cells after treatment with nerve growth factor. Cell Mol Biol (Noisy-le-grand) 40: 619–626 [PubMed] [Google Scholar]
- Philpott KL, McCarthy MJ, Klippel A, Rubin LL (1997) Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol 139: 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumb MA, Smith GC, Cunniffe SM, Jackson SP, O'Neill P (1999) DNA-PK activation by ionizing radiation-induced DNA single-strand breaks. Int J Radiat Biol 75: 553–561 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J (1996) Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J 15: 2442–2451 [PMC free article] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K (2003) PI3 kinase enhancer–Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci 6: 1153–1161 [DOI] [PubMed] [Google Scholar]
- Rotman G, Shiloh Y (1999) ATM: a mediator of multiple responses to genotoxic stress. Oncogene 18: 6135–6144 [DOI] [PubMed] [Google Scholar]
- Rukenstein A, Rydel RE, Greene LA (1991) Multiple agents rescue PC12 cells from serum-free cell death by translation- and transcription-independent mechanisms. J Neurosci 11: 2552–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Horiguchi K, Yoshida T, Takeda M, Fujisawa H, Takeuchi K, Umeda M, Kato S, Ihara S, Nagata S, Fukui Y (1999) Evidence that a phosphatidylinositol 3,4,5-trisphosphate-binding protein can function in nucleus. J Biol Chem 274: 3919–3922 [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Hohl JB, Angelastro JM, Greene LA, Shelanski ML (2001) Death in the balance: alternative participation of the caspase-2 and -9 pathways in neuronal death induced by nerve growth factor deprivation. J Neurosci 21: 5007–5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Somwar R, Bilan PJ, Liu Z, Jin J, Woodgett JR, Klip A (1999) Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol 19: 4008–4018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K, Aghdasi B, Luo HR, Moriarity JL, Wu FY, Hong JJ, Hurt KJ, Bae SS, Suh PG, Snyder SH (2002) Phospholipase C gamma 1 is a physiological guanine nucleotide exchange factor for the nuclear GTPase PIKE. Nature 415: 541–544 [DOI] [PubMed] [Google Scholar]
- Ye K, Hurt KJ, Wu FY, Fang M, Luo HR, Hong JJ, Blackshaw S, Ferris CD, Snyder SH (2000) Pike. A nuclear gtpase that enhances PI3kinase activity and is regulated by protein 4.1N. Cell 103: 919–930 [DOI] [PubMed] [Google Scholar]
- Yokogawa T, Nagata S, Nishio Y, Tsutsumi T, Ihara S, Shirai R, Morita K, Umeda M, Shirai Y, Saitoh N, Fukui Y (2000) Evidence that 3′-phosphorylated polyphosphoinositides are generated at the nuclear surface: use of immunostaining technique with monoclonal antibodies specific for PI 3,4-P(2). FEBS Lett 473: 222–226 [DOI] [PubMed] [Google Scholar]


