Abstract
Neuronal Kv7 channels underlie a voltage-gated non-inactivating potassium current known as the M-current. Due to its particular characteristics, Kv7 channels show pronounced control over the excitability of neurons. We will discuss various factors that have been shown to drastically alter the activity of this channel such as protein and phospholipid interactions, phosphorylation, calcium, and numerous neurotransmitters. Kv7 channels locate to key areas for the control of action potential initiation and propagation. Moreover, we will explore the dynamic surface expression of the channel modulated by neurotransmitters and neural activity. We will also focus on known principle functions of neural Kv7 channels: control of resting membrane potential and spiking threshold, setting the firing frequency, afterhyperpolarization after burst firing, theta resonance, and transient hyperexcitability from neurotransmitter-induced suppression of the M-current. Finally, we will discuss the contribution of altered Kv7 activity to pathologies such as epilepsy and cognitive deficits.
Keywords: M-current, Kv7, KCNQ, Excitability, Channel trafficking, Epilepsy
Introduction
Potassium channels, being the most diverse of all ion channels, underlie a robust number of functions controlling the excitability of neurons. They are responsible for such aspects as setting the resting membrane potential, and reducing excitability as well as controlling the duration, shape and firing frequency of action potentials. Potassium channels are localized to all subcellular compartments critical for electrical conduction of excitatory inputs. Therefore, it is not surprising that many of these channels are gated by specific activators (such as membrane potential or neurotransmitters) and regulated by complex molecular pathways.
Kv7 channels produce an outward potassium current with characteristics that make them distinct from other voltage-gated potassium channels [1, 2]; lacking inactivation, slow gating kinetics (an order of magnitude slower than other voltage-gated potassium channels at room temperature as well as physiological temperatures [3, 4]) and activation near resting membrane potential [5, 6]. Neuronal Kv7 subunits are widely expressed in the central as well as peripheral nervous system [7, 8], where its steady outwardly rectifying current functions as “brakes” for neurons receiving persistent excitatory input. A somewhat unique characteristic of the M-current is derived from the circumstances by which it was discovered. Namely, upon activation of muscarinic acetylcholine receptors (M1 and M3), both of which being Gq-coupled receptors, there is subsequent robust suppression of the M-current, leading to a transient increase in excitability. Suppression of the M-current has been shown to lower the action potential threshold, increase afterdepolarization and depolarize axonal resting potential [2, 9]. Over the past several years, it has been revealed that activation of numerous Gq/11-coupled receptors suppress the channel [10–12].
Since the initial discovery of the M-current in bullfrog sympathetic ganglion neurons, there have been numerous breakthroughs identifying neuronal types expressing the M-current as well as mediators of the channel [9, 13]. However, it took nearly two decades to determine the molecular identity [7]. The lapse in time that was required to elucidate Kv7 as the underling subunits of the M-current was largely due to the lack of available tools able to link cloned channels to native M-current [14]. On the heels of this discovery, numerous facets involved in the modulation of the Kv7 channel complex have also been uncovered. However, though the M-current has consistently been discussed as playing a pivotal role in neuroplasticity, due to its prominent expression throughout the brain and its pronounced suppression via neurotransmitters, only recently have we begun to understand how dynamic Kv7 channel function contributes to higher brain functions.
Components of the Kv7 channel complex
The M-current is conducted through a voltage-gated potassium channel comprised of tetrameric assemblies by members of Kv7 α subunit homomers or heteromers derived from KCNQ1-5 genes. Kv7.1 is the predominant subunit of the Kv7 family that is found in cardiomyocytes [15, 16] (Fig. 1a). Kv7.1 channel generates the slowly activating delayed rectifier current (I Ks) that contributes to the repolarization phase after action potential initiation. Kv7.1 channel is also expressed in the cochlea of the inner ear [17], and vascular smooth muscles [18].
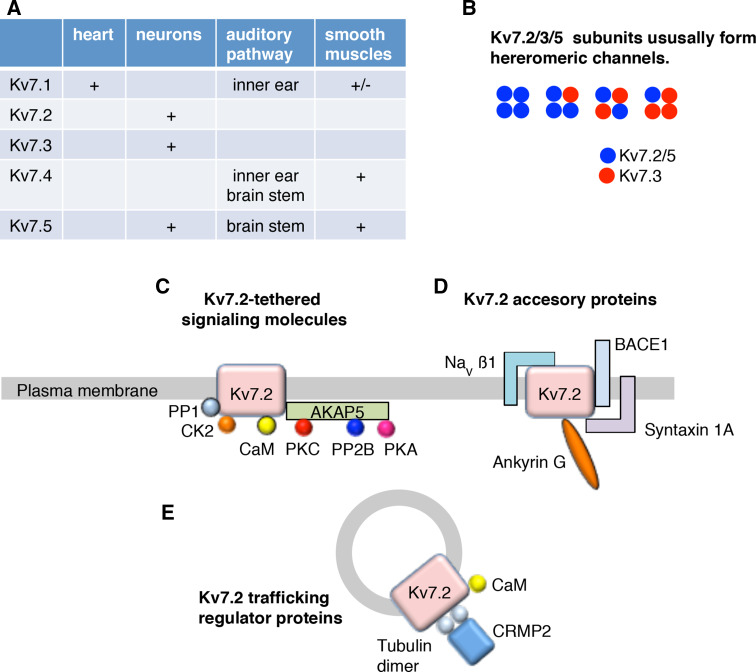
Fig. 1.
Summary of Kv7 channel family and their binding proteins. a Subtypes of Kv7 channel family and their expression pattern. b Summary of subunit composition with Kv7.2. 7.3 and 7.5 subunits, which is widely observed in neurons. c Schematic summary of signaling proteins that are tethered to Kv7.2 subunit. PP1 protein phosphatase 1, CK2 CK2 protein kinse, CaM calmodulin, PKC protein kinse C, PP2B protein phosphatase 2B, PKA protein kinse A. d Schematic summary of accessory proteins for Kv7.2 subunit. Navβ1 sodium channel β subunit 1, BACE1 β-site amyloid precursor protein cleaving enzyme 1. e Schematic summary of identified regulator proteins for Kv7.2 channel trafficking. CRMP2 collapsin response mediator protein 2
A wide variety of neurons in the CNS as well as peripheral neurons express Kv7.2, 7.3, and 7.5 subunits, which are considered to generate the neuronal M-current [1, 2]. It is widely believed that heteromeric Kv7.2/3 or Kv7.3/5 channels are the dominant subunit composition (Fig. 1b) [1, 2] since Kv7.3 has been shown to facilitate surface expression of other Kv7 subunits especially in Xenopus oocytes [19] and immunohistochemical studies show co-localization of these subunits in many areas [8, 20]. However, a recent study using conditional knock-out mice of KCNQ2 or 3 genes showed abolished M-current in Kv7.2 deficient neurons, but normal M-current in Kv7.3-deficient neurons, which suggests that Kv7.2 homomeric channels can be fully functional without Kv7.3 subunit in the CNS neurons [21].
Kv7.4 is a subtype selectively expressed in the auditory pathway including hair cells of the inner ear (Fig. 1a) [22–24]. In addition to neurons, Kv7.4 and Kv7.5 are also expressed in various smooth muscle cells including vascular [18, 25] as well as gastrointestinal tract (Fig. 1a) [26, 27].
With the advent of discovering the molecular identity of the M-current, many aspects of the Kv7 channel complex have been brought to light (Fig. 1c–e): protein–protein and protein–lipid interactions, as well as channel phosphorylation [7, 8, 28]. Understanding the components of the Kv7 channel complex has provided the necessary insight for understanding the requirements for basal channel function, its modulation, as well as the pathways involved in neurotransmitter-induced M-current suppression.
Phosphatidylinositol-4,5-bisphosphate (PIP2) is an anionic phospholipid found on the intracellular leaflet of the plasma membrane. PIP2 is a co-factor for numerous ion channels and transporters [29], including Kv7, that alters channel function and often is obligatory for activation [30–33]. While it has been known that PIP2 interaction with Kv7 is mandatory for channel function [34, 35], more recently it was shown that PIP2 has multiple sites of interaction within the channel, with varying effects [36]. One such PIP2 interaction modulates coupling the voltage-sensing to the pore-gating domain [37–40]. Functionally, PIP2 interaction within the voltage-sensing domain of the S4–S5 linker has been attributed to increasing open probability, thus voltage conductance [40]. Another key interaction site lies within the proximal C-terminus of the Kv7.2 subunit that overlaps with the calmodulin (CaM) binding site, a key target for M-current suppression [39, 41, 42]. Notably, while depletion of PIP2 in Kv7.2 and Kv7.5 homomeric channels leads to profound current suppression, heteromeric assemblies incorporating the Kv7.3 subunit, with its higher affinity to PIP2, offers resistance to PIP2 turnover [40].
Both N and C termini of Kv7 subunits locate within the intracellular side and are rich with sites of protein interaction. Within the amino terminus lies a consensus site for phosphorylation by protein kinase A, which in Xenopus oocytes has been shown to increase Kv7 currents [19]. Protein kinase CK2 and protein phosphatase 1 (PP1) also tether to Kv7 subunits (Fig. 1c), and modulate the channel through the phosphorylation state of an essential auxiliary unit CaM [43]. The carboxyl terminus is relatively long compared to other potassium channels, containing numerous sites of interaction [42]. Among these are the aforementioned sites for PIP2, CaM, A-kinase anchoring protein 5, AKAP5 (also known as AKAP79/150), as well as ankyrin G (Fig. 1d). Interaction of the channel with many of these factors is highly modulated by neurotransmitters activating Gq/11-coupled receptors [44, 45].
AKAPs are facilitators of second messenger signaling events that tether signaling enzymes to target substrates at the plasma membrane, such as Kv7 channels (Fig. 1c) [28, 46, 47]. This anchoring protein is located in the plasma membrane through PIP2 binding and a dynamic post-translational fatty acid modification known as palmitoylation [48]. AKAP5-bound Protein kinase C (PKC) is kept in proximity to neuronal Kv7 channels, which has been shown to be important for the specificity of PKC-induced dissociation of CaM from Kv7.2 subunits [8, 28, 46].
CaM plays roles in the trafficking of Kv7 channels to the plasma membrane [49, 50], functions as the Ca2+ sensor, and promotes channel interaction with PIP2 (Fig. 1c) [44, 51]. CaM interacts with Kv7 subunits through two α helical regions (A and B) running antiparallel in the C-terminal tail. There has been controversy regarding whether calcium facilitates or reduces CaM binding to Kv7 [52, 53]. A recent crystal structure study using Kv7.1 suggests that the story is not that simple [54]. The study shows that the calcium-free C-lobe of CaM interacts with helix A of Kv7 and the calcium-containing N-lobe of CaM interacts with helix B of Kv7 [54]. However, Kv7.2 has been shown to have higher affinity for CaM at helix B, regardless of [Ca2+] [55], whereas the C-lobe of CaM has higher affinity to [Ca2+] and is dynamically bound to helix A in a calcium-dependent manner [56].
Studies during the last several years identified an increasing number of accessory proteins for the Kv7.2 subunit (Fig. 1d). One of the earliest examples is ankyrin G, which is an underlying molecule for anchoring Kv7.2 at the axon initial segment as well as the Node of Ranvier [57, 58]. The SNARE protein syntaxin 1A is a plasma membrane protein, which serves as a docking site for synaptic vesicles. Syntaxin 1A has been demonstrated to interact with both the cytoplasmic carboxyl and amino termini of Kv7.2 subunit, which slows channel activation and decreases current amplitude (Fig. 1d) [59, 60]. Navβ1 was originally considered as a beta subunit unique to voltage-gated sodium channels (Nav) [61]. More recently, the promiscuity of this auxiliary protein was uncovered, first in its modulation of Kv4 [62], and more recently Kv1 and Kv7 subfamilies [63] (Fig. 1d). Navβ1 slows Kv7.2 channel activation at depolarized potentials [63]. An interesting addition to accessory proteins of the Kv7.2 subunit is β-site amyloid precursor protein cleaving enzyme 1 (BACE1), which was originally identified to produce neurotoxic β-amyloid [64] and known to cleave Navβs and increase sodium currents [65]. Physical interaction of BACE1, even with enzymatic inactive BACE1, can change the gating kinetics and functional expression of Kv7 channels with the exception of Kv7.3 homomeric channels [66]. However, effects through cleavage of Kv7.2-bound Navβ have not been characterized to date.
Channel trafficking of Kv7 is another mechanism for regulating neuronal excitability (Fig. 1e). It has been noted that the majority of Kv7 channel subunits stay in cytoplasmic vesicles rather than being transported to the plasma membrane [49]. Detailed analyses of CaM-dependent regulation of Kv7 channel trafficking revealed that CaM regulates exit of Kv7 channels from ER [49, 50]. In addition, our recent proteomic study identified tubulin dimer together with Collapsin response mediator protein-2 (CRMP-2) play a role in channel trafficking of Kv7.2 channels at post-Golgi vesicles (Fig. 1e) [67].
Integrated channel suppression
Various neurotransmitters activating Gq-protein coupled receptors induce profound transient suppression of the M-current [68–70]. It has been demonstrated that stimulation by luteinizing hormone releasing hormone [9], purinergic P2Y [13], substance P [71], 5-HT2 serotonin [72], as well as activation of M1/M3 muscarinic receptors [5, 73, 74], metabotropic glutamate receptors [75], κ and δ opioid receptors [76], and AT1 angiotensin receptors [77, 78] all suppress the M-current.
Gq/11 mediated pathway
Perhaps most well characterized is M-current suppression subsequent to activation of Gq/11-coupled muscarinic acetylcholine receptors [79, 80]. It is well documented that reduction in PIP2 leads to marked suppression of the M-current (Fig. 2a) [81–83]. Also, PKC was one of the first modulators proposed for neurotransmitter-induced M-current suppression [84]. This was for a time debated due to confounding and at times contradicting pharmacological data such as PKC inhibitors at times showing no interference with muscarinic suppression of the M-current [12, 85]. These reports were reconciled once it was determined that AKAP-tethered PKC is protected from certain PKC inhibitors [86, 87]. Indeed, a key PKC phosphorylation site on the Kv7.2 subunit has been shown to induce inhibition equivalent to muscarinic suppression of the channel [28, 44, 88]. Therefore, both depletion of PIP2 and activation of PKC are targeted downstream of Gq-coupled receptor activation.
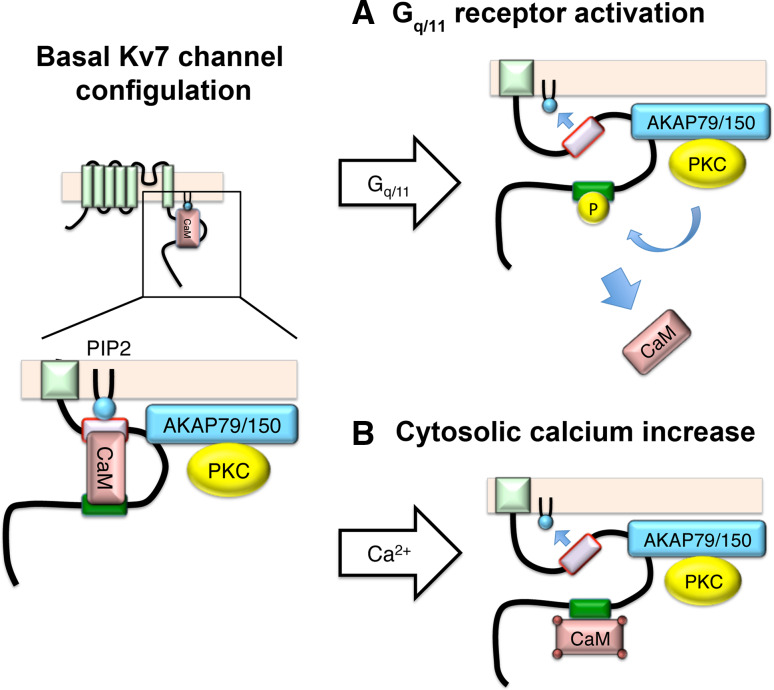
Fig. 2.
Schematic summary of molecular configuration of Kv7 channel and its modulation modified from Ref [29]. a Activation of Gq/11 coupled receptor induces depletion of PIP2 as well as activation of PKC, which phosphorylates Kv7 channel leading to dissociation of CaM and unstabilized PIP2 interaction. b Increase in cytosolic calcium induces change in CaM conformation, and configuration between CaM and Kv7 channel, which leads to unstabilized PIP2 interaction
The Gq-protein mediated pathway occurs as follows: subsequent to activation of Gq/11-coupled receptors, such as the M1 muscarinic receptor, comes activation of phospholipase C (PLC) causing hydrolysis of PIP2 into diacylglycerol (DAG) and inositol triphosphate (IP3), leading to the activation of PKC. Activated PKC, tethered to the M-channel complex through AKAP5, phosphorylates the C-terminus of the Kv7.2 subunit, which overlaps with the channel’s CaM binding site. Phosphorylation of the Kv7.2 subunit dissociates CaM from the channel, which destabilizes its interaction with PIP2 along with the concomitant reduction in PIP2 due to its hydrolysis. Together, these signaling pathways downstream of Gq/11 activation lead to an amplified response that synergistically suppresses the M-channel [44].
Calcium-CaM pathway
Another well-established pathway of M-current suppression is induced by increases in intracellular calcium sensed by channel-bound CaM (Fig. 2b) [44, 89, 90]. Bradykinin receptors have been demonstrated to use this pathway [90].
The physical interaction of calcium-free CaM bound to helix A of Kv7 subunits is integral to maintaining channel affinity to PIP2 [44]. Consequently, calcium-bound CaM decreases Kv7.2 channel efficacy for PIP2, thereby inhibiting the M-current [44, 90, 91]. On the other hand, a recent study showed that splicing variants of Kv7.4 are differentially modulated by CaM [92]. This mechanism may explain why bradykinin-induced suppression of the M-current is usually smaller than that by muscarinic agonists.
Kv7 channel physiology
The voltage-dependent characteristics of the M-current lend it to various roles associated with controlling excitability in the brain. Kv7 channels activate within subthreshold potentials, approximately at −60 mV. Additionally, unlike many other voltage-gated potassium channels, Kv7 does not inactivate, therefore, as long as the membrane remains depolarized Kv7 current will persist [7]. The outcome is the effective stabilization of the membrane potential throughout the duration that neurons receive subthreshold excitatory inputs [93]. Relatively slow activation kinetics indicate that Kv7 channels do not appreciably alter single action potential amplitude or duration [94]; rather, the functional consequence of M-current is to clamp the membrane at more negative potentials, preventing repetitive action potential firing. Moreover, the persistent outward current functions to control numerous aspects of neuronal excitability as follows.
Setting membrane potential
Due to its activation at subthreshold potentials, Kv7 channels have been implicated in controlling the resting membrane potential (RMP) at the axon initial segment (AIS) and unmyelinated portions of the axons, the nodes of Ranvier, as well as axon terminals. Examples have been shown in sympathetic, cortical and hippocampal neurons such as visceral sensory neurons and within the calyx of Held [95–97]. M-current control of RMP is also a major determinant for increasing the recovery of inactivated channels (i.e., axonal Na+ and A-type K+ channels) at the nodes of Ranvier, allowing for consecutive action potential spiking [4, 97, 98]. Therefore, axonal Kv7 channels at nodes of Ranvier exert two contradicting functions: increasing the action potential threshold but also increasing excitability by promoting recovery of sodium channels from inactivation [4, 20]. In addition, Kv7 channels are known to reach the axon terminal and regulate synaptic release through regulating RMP [97, 99]. What is confusing is that inhibition of Kv7 channel sometimes facilitates synaptic transmission, such as in calyx of Held [97] and hippocampal synaptosomes [99], while suppressing synaptic transmission in Schaffer neurons [100]. These contradicting effects are assumed to be the results of difference in inactivation of calcium channels and sodium channels [99, 100]. On the other hand, post-synaptic responses can be also affected by perisomatic Kv7 channels by changing integration of excitatory postsynaptic potentials at the AIS, dampening synaptic transmission of prolonged subthreshold stimulus [101, 102].
Afterhyperpolarization
As a consequence of continuous spiking, numerous types of neurons produce three types of an afterhyperpolarization (AHP) that contribute to refractory periods: fast AHP lasting 2–5 ms, medium AHP with durations ranging from 50 to 100 ms (mAHP), and a slow AHP lasting 0.1–2 s (sAHP).
Medium AHP is produced through slowly activating and long-lasting outward K+ current that is composed in part by a component that requires Ca2+ influx [103, 104] and has been shown to control the time where neurons are refractory to further excitatory input. Channels contributing to the generation of mAHP are Kv7 channels as well as SK2 calcium-activated potassium channels and HCN channels [80, 103, 105]. However, it has been reported that distinct channels are responsible for mAHP depending on membrane potentials and neuronal types [104, 106].
Slow AHP is involved in neuronal plasticity and is implicated as a major component during learning and establishing memory within the hippocampus [34, 38]. The identity of the potassium channel responsible for the slow AHP had proven elusive until recently. While Kv7 has been suggested as a contributor of sAHP, as afferent cholinergic stimulation reduces effective sAHP amplitude [107], one confounding factor discredited its involvement: the sAHP is active at membrane potentials more negative than M-current activity is typically observed. Activation of the sAHP is known to have a Ca2+ dependent component, and neuronal calcium sensor proteins such as hippocalcin have been implicated in the activation of potassium channels that constitute the sAHP [108, 109]. A physiological consequence of hippocalcin activation is the downstream production of PIP2. In a recent report evidence was given for shifting Kv7 open probability to more negative potentials through increased interaction with PIP2 [40, 110]. Further support comes from the observation that BACE1 knockout mice have reduced sAHP. BACE1 has been shown to cause a leftward shift in Kv7 voltage activation, within the voltage range where sAHP is active [66].
Interspike interval
High-frequency bursts of action potentials leads to Kv7 channel activation. Consequently, this gradually increases spike interval, which is also known as spike frequency adaptation [80, 111]. Recently M-current was also shown to regulate the firing frequency of tonically firing neurons in rat entorhinal cortex layer II stellate cells [112], as well as neurons of the retrotrapezoid nucleus [113], which, during continuous current input, initially fire with short interspike intervals that quickly lead to refractory periods with minimal firing [112]. Furthermore, Kv7 current has also been shown to control the rate of firing in dopaminergic neurons of the ventral tegmental area [94] and hippocampal neurons [114]. Thus, the M-current robustly controls the frequency at which neurons are able to fire while receiving continuous excitatory input [115].
Theta resonance
Another physiological function of Kv7 channels, in conjunction with HCN channels, is facilitating the responsiveness to oscillating subthreshold membrane potential within theta frequencies (2–7 Hz), which functions as a band pass filter [116]. This function has predominantly been characterized in pyramidal neurons in the hippocampus [116, 117]. Interestingly, two distinct channel types with different ion species, gating kinetics, and subcellular localization (somatic Kv7 channels and dendritic HCN channels) produce a synergized function [116, 117]. Theta resonance has been considered integral to inducing synchronous activity within the local circuit and shown to be a necessary component of neuroplasticity as well as learning and memory [118–120]. Network oscillations at the theta frequency have been shown to be important for hippocampal function, such as exploration [121] and working memory such as navigation of mazes [122, 123]. As such, disruption of M-channel activity, such as conditional knockout of Kv7.2 subunits, reduces hippocampal theta resonance and consequently impairs animal performance in spatial memory tasks [124].
Transient neuronal hyperexcitability
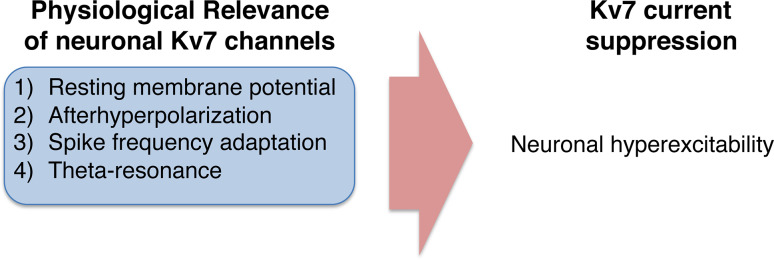
Suppression of Kv7 current leads to the transient removal of above functions, leading to neural hyperexcitability (Fig. 3). Consequently, channel suppression allows for the accumulation of excitatory inputs that lead to burst firing or “complex spikes” [125]. In addition, suppression of Kv7 channel leads to concomitant reduction in action potential threshold such as what is seen in dentate granule cells after stimulation by cholinergic fibers [126]. Thus, reduction in action potential threshold by Kv7 channel suppression makes action potential propagation more permissive, where weaker stimuli can invoke neuronal spiking with higher probability, and increases the likelihood of spontaneous firing, which underlies burst firing in some neurons [4]. Alternatively, ablation of Kv7 current by conditional knock-out produces a similar effect [21]. Furthermore, since the M-current is activated during high-frequency firing, M-current suppression allows neurons to respond to high-frequency inputs. Moreover, this bursting has been shown to be one component of memory coding after learning in subcortical regions [14, 127]. Suppression of axonal M-current also increases the likelihood of the back-propagation of action potential spikes into the apical dendrites, which has been demonstrated to promote long-term potentiation [101, 128].
Fig. 3.
Summary of physiological relevance of neuronal Kv7 channel and its modulation
Kv7 channel augmentation
Just as there are multiple mechanisms for M-current suppression, there are multiple mechanisms for augmenting the M-current. Early examples include neurotransmitters such as somatostatin [129], corticostatin [130] and dynorphin [131]. Since phosphorylation of certain residues of Kv7 channels are known to cause robust suppression, it follows that some protein phosphatases preserve neuronal Kv7 channel activity. One example is Protein Phosphatase 2A, (PP2A), which was demonstrated to counteract channel inhibition via phosphorylation by Glycogen Synthase Kinase 3β, (GSK3β) [132, 133]. While phosphorylation of Kv7 subunits is typically associated with channel suppression, phosphorylation of Kv7-bound CaM by protein kinase CK2 has been shown to facilitate CaM interaction with the channel, increasing PIP2 efficacy and increasing channel amplitude while remaining sensitive to increases in intracellular Ca2+ [43, 134]. Another signaling pathway known to augment the M-current (specifically, channels containing Kv7.2/4/5 subunits) is through the increase in an intracellular reactive oxygen species, ROS [135, 136]. It has been shown that the oxidation of three cysteine residues lying within the S2-S3 linker increases the Po of Kv7 channels [135]. Another recently identified pathway for ROS induced augmentation is methylation of arginine residues of Kv7.2 subunit by arginine methyltransferase 1 (Prmt1), which promotes Kv7.2 interaction with PIP2 [136]. This study also suggests that partial methylation of arginine residues in Kv7 channels is essential for maintaining basal M-current [136].
Auxiliary units of potassium channels such as members of the KCNE family are common co-factors that associate with pore-forming subunits and alter channel activity, such as voltage dependence of activation. Recently β-secretase BACE1 was revealed to associate with members of the Kv7 family in neurons (Fig. 1d). Interestingly, it is the physical interaction and not the enzymatic activity of BACE1 protein that is responsible for the leftward shift in the voltage conductance of the M-current. This leftward shift was accompanied by a change in Kv7 channel kinetics, accelerating activation as well as slowing channel deactivation [66]. While PIP2 is necessary for M-current function, it has also been revealed that increasing PIP2 levels caused Kv7 channels to open at deeper potentials, similar to the effect seen with BACE1 [40, 110]. Interestingly, the combination of BACE1 interaction with the channel along with elevating PIP2 levels causes even greater leftward shift in channel activation [40]. These revelations bring credence to the growing consensus that Kv7 channels are a major part of the burst firing-activated outward current underlying sAHP, which is known to be active at membrane potentials lower than where the M-current has been thought to be active.
Another association with Kv7.4 subunits has recently been uncovered. Kv7 channels in vascular smooth muscle are positively regulated by G-protein βγ subunits, enhancing the rate of Kv7 channel activation and shifting the voltage dependence of activation so that the channel is open at deeper potentials [137]. While it is important to note that this phenomenon has yet to be confirmed in CNS neurons, G-protein βγ subunits may prove to be an underlying factor responsible for enhancement of Kv7 activity by as seen with G-protein coupled receptor activation by somatostatin [129] and dynorphins [131].
Subcellular localization
A key characteristic determining the roles of neuronal Kv7 channels lies in their subcellular localization. Kv7 channels are highly concentrated at the distal end of the AIS and nodes of Ranvier by the protein ankyrin G. The AIS functions as the gatekeeper of action potential initiation by controlling the threshold at which excitatory presynaptic potentials transmit action potentials down the axon. Ankyrin G also functions to localize Kv7.2 and to a lesser extent Kv7.3 channels at unmyelinated regions of the axon, known as Nodes of Ranvier [57, 58, 138]. At these nodes, Kv7 channels (Kv7.2/7.3) co-cluster with voltage-gated sodium channels and are thought to underlie the slow outward potassium current, stabilizing RMP [4, 98]. In agreement with its axonal localization, selective suppression of axonal Kv7 channel increases spike afterdepolarization, facilitating action potential firing [125]. Interestingly, in a study where myelination of the axon was removed, Kv7 channels dispersed along the axon, which had the net effect of increasing neural excitability [139]. This was postulated to be through stabilizing the RMP throughout the axon, thereby increasing the availability of Nav channels as previously described [4, 20].
Kv7 channels have also been reported to be expressed in the perisomatic region outside the AIS [57, 140, 141]. Somatic function of the M-current is implicated in excitatory synaptic potential integration as well as counteracting afterdepolarization [57, 142, 143]. Perisomatic Kv7 channel has been shown to change the amplitude and shape of somatic EPSPs [102]. There is also accumulating evidence that Kv7 channels may be expressed at apical dendrites [140, 142, 144]. Due to their relative scarcity at this location, the function of Kv7 channels within dendrites is still controversial, though it has been proposed to contribute to conditions where there is dendritic hyperexcitability, as in the case of persistent excitatory presynaptic input [142, 144], however, further clarification of Kv7 channel function outside the axon is still needed.
Finally, Kv7.2 has been shown to localize to presynaptic terminals [99]. Augmentation of Kv7 channels at this location attenuates neurotransmitter release [99] whereas suppression of Kv7 current by Gq activation increases activation of voltage-gated calcium channels leading to increased transmission [145].
Surface expression
Under basal conditions, Kv7 channels have very low turnover rate throughout the axon [146]. However, surface expression of Kv7 channels is upregulated after neuronal stimulation [147]. Paradoxically, persistent excitation of neurons leads to pronounced internalization of Kv7 channel at the AIS, which is largely irreversible [148]. Li and colleagues demonstrated that persistent activation through glutamate induced a Ca2+ and PKC-dependent internalization of Kv7 channel. Similar irreversible suppression of Kv7 channel was observed with cholinergic stimulation onto dentate granule cells, causing an increase in their intrinsic excitability [126]. It was revealed that cholinergic stimulation onto the AIS of dentate granule neurons leads to persistent activation of T-type Ca2+ channels, causing robust M-current suppression that does not recover after washout. Conversely, deprivation of afferent axonal stimulus, as shown in neurons of the avian cochlear nucleus, leads to pronounced increase in surface expression of Kv7.2 channels and concomitant reduction of Kv1 channels at the AIS [149]. This reduces the fast activating Kv1 current, thereby increasing excitability, while preserving axonal RMP by Kv7 channels.
CaM tethered to Kv7 subunits is important for of Kv7 channel exit from the endoplasmic reticulum and is necessary for basal surface expression of the channel at the plasma membrane [49, 55]. Alternatively, a recent study uncovered a form of induced surface translocation subsequent to muscarinic suppression of the channel tested in a heterologous system in which Kv7 channels in post-Golgi vesicles were determined to be the predominant source of this form of over-recovery of the M-current, mediated through a CRMP-2 pathway [67] (Fig. 1e).
Pathology
The genes encoding Kv7.2 and Kv7.3 have been shown to have numerous mutations that induce epilepsies and encephalopathies. Most common of these pathologies is known as benign familial neonatal seizures (BFNS), a form of autosomal dominant idiopathic epileptic syndrome. The generalized seizures induced by these mutations occur within the first days after birth and have a high likelihood of spontaneously receding within the first 2 months [150–153]. Mutations leading to epileptic activity are predominantly autosomal dominant, inducing loss of channel function such as from missense, truncation, and non-native splice variants that reduce functional Kv7 channel at the plasma membrane [154, 155]. Many of these mutations lead to reduction in basal M-current and subsequent hyperexcitability [156]. This is also true in the cases of early onset epileptic encephalopathy, though usually with more severe channel suppression [152]. As of yet, encephalopathies have only been detected from missense mutations in KCNQ genes and tend to occur de novo [152, 157, 158]. One example is through an alternative substitution of a previously described select amino acid mutation, which is known to cause BFNS, instead leading to a severe form of encephalopathy. It was determined that the functional difference in this severe form of single amino acid mutation is derived from not only a reduction of functional expression of Kv7 channels but also loss of localization of Kv7 channels to the AIS [152]. As a consequence of the growing literature on pathologies associated with Kv7 mutations, there has been an effort to develop genetically engineered animals to recapitulate and understand the pathologies of the numerous implicated mutations and better understand underlying causes [159, 160] as well as the effectiveness of potential therapies [161].
M-current activity is an important obstacle for seizure onset [162, 163]. However, gain of function mutations in Kv7 channels have also been uncovered in patients suffering from epileptic encephalopathies [164–166]. One such mutation was found within the voltage-sensing domain of Kv7.2 and Kv7.3 subunits, stabilizing the channel in the open conformation, reducing the voltage dependence of activation [165]. Interestingly, while these mutations decrease the intrinsic excitability in neurons where they are expressed, there is a marked increase in network excitability within the CA1 of the hippocampus. This suggests the grave importance in Kv7 channel control of action potential propagation, particularly at its axonal localization.
Conclusion
The specific characteristics of Kv7 channels lend it to function as powerful brakes against continuous neural activity. As an extreme example, retigabine-induced augmentation of the M-current has seen great success as an antiepileptic therapy [167], and more recently it was determined that one of the predominant antiepileptic drugs, valproic acid, owes part of its protective activity to the preservation of the M-current during seizures [168]. In contrast, removal of Kv7.2 increases susceptibility to seizures and leads to deficits in hippocampal-dependent spatial memory [124] as well as fear memory in mice [169]. However, continuous ablation of hyperactivity in neurons expressing Kv7 channels may disrupt cognitive functions such as memory encoding, indicating the importance of transient channel suppression. Neurotransmitters are intricately involved in higher brain functions and acetylcholine-induced suppression of the M-current was among the first examples of modulating neuronal excitability [79]. It would follow, therefore, that suppressing the activity of Kv7 channels such as what is seen by afferent cholinergic stimulation [126] would provide a means to allow plasticity in neural excitability with great spatial and temporal resolution. Even so, work is ongoing to determine the intricacies of M-current suppression in higher brain functions.
Abbreviations
- PKC
Protein kinase C
- PP1
Protein phosphatase 1
- PP2A
Protein phosphatase 2A
- AKAP
A-kinase anchoring protein
- PLC
Phospholipace C
- DAG
Diacylglycerol
- IP3
Inositol triphosphate
- PIP2
Phosphatidylinositol 4,5-bis phosphate
- CaM
Calmodulin
- RMP
Resting membrane potential
- AIS
Axon initial segment
- GSK3β
Glycogen synthase kinase 3β
- CRMP-2
Collapsin response mediator protein 2
- Nav
Voltage-gated sodium chanel
- AHP
Afterhyperpolarization
- SNARE
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- EPSPs
Excitatory post-synaptic potentials
- ROS
Reactive oxygen species
- BFNC
Benign familial neonatal convulsions
References
- 1.Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1(1):21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- 2.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6(11):850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- 3.Miceli F, Cilio MR, Taglialatela M, Bezanilla F. Gating currents from neuronal K(V)7.4 channels: general features and correlation with the ionic conductance. Channels (Austin) 2009;3(4):274–283. doi: 10.4161/chan.3.4.9477. [DOI] [PubMed] [Google Scholar]
- 4.Battefeld A, Tran BT, Gavrilis J, Cooper EC, Kole MH. Heteromeric Kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J Neurosci. 2014;34(10):3719–3732. doi: 10.1523/JNEUROSCI.4206-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halliwell JV, Adams PR. Voltage-clamp analysis of muscarinic excitation in hippocampal neurons. Brain Res. 1982;250(1):71–92. doi: 10.1016/0006-8993(82)90954-4. [DOI] [PubMed] [Google Scholar]
- 6.Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90(1):1–19. doi: 10.1016/S0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- 7.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282(5395):1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- 8.Cooper EC, Aldape KD, Abosch A, Barbaro NM, Berger MS, Peacock WS, Jan YN, Jan LY. Colocalization and coassembly of two human brain M-type potassium channel subunits that are mutated in epilepsy. Proc Natl Acad Sci USA. 2000;97(9):4914–4919. doi: 10.1073/pnas.090092797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams PR, Brown DA. Luteinizing hormone-releasing factor and muscarinic agonists act on the same voltage-sensitive K+-current in bullfrog sympathetic neurones. Br J Pharmacol. 1980;68(3):353–355. doi: 10.1111/j.1476-5381.1980.tb14547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21(15):5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adelman JP, Maylie J, Sah P. Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol. 2012;74:245–269. doi: 10.1146/annurev-physiol-020911-153336. [DOI] [PubMed] [Google Scholar]
- 12.Shapiro MS, Roche JP, Kaftan EJ, Cruzblanca H, Mackie K, Hille B. Reconstitution of muscarinic modulation of the KCNQ2/KCNQ3K(+) channels that underlie the neuronal M current. J Neurosci. 2000;20(5):1710–1721. doi: 10.1523/JNEUROSCI.20-05-01710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams PR, Brown DA, Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook L, Nickolson VJ, Steinfels GF, Rohrbach KW, Denoble VJ. Cognition enhancement by the acetylcholine releaser DuP 996. Drug Dev Res. 1990;19(3):301–314. doi: 10.1002/ddr.430190308. [DOI] [Google Scholar]
- 15.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384(6604):80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 16.Nakajo K, Ulbrich MH, Kubo Y, Isacoff EY. Stoichiometry of the KCNQ1–KCNE1 ion channel complex. Proc Natl Acad Sci USA. 2010;107(44):18862–18867. doi: 10.1073/pnas.1010354107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neyroud N, Tesson F, Denjoy I, Leibovici M, Donger C, Barhanin J, Faure S, Gary F, Coumel P, Petit C, Schwartz K, Guicheney P. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome. Nat Genet. 1997;15(2):186–189. doi: 10.1038/ng0297-186. [DOI] [PubMed] [Google Scholar]
- 18.Ng FL, Davis AJ, Jepps TA, Harhun MI, Yeung SY, Wan A, Reddy M, Melville D, Nardi A, Khong TK, Greenwood IA. Expression and function of the K(+) channel KCNQ genes in human arteries. Br J Pharmacol. 2011;162(1):42–53. doi: 10.1111/j.1476-5381.2010.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396(6712):687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- 20.Kole MH, Cooper EC. Axonal Kv7.2/7.3 channels: caught in the act. Channels (Austin) 2014;8(4):288–289. doi: 10.4161/chan.29965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soh H, Pant R, LoTurco JJ, Tzingounis AV. Conditional deletions of epilepsy-associated KCNQ2 and KCNQ3 channels from cerebral cortex cause differential effects on neuronal excitability. J Neurosci. 2014;34(15):5311–5321. doi: 10.1523/JNEUROSCI.3919-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcotti W, Johnson SL, Holley MC, Kros CJ. Developmental changes in the expression of potassium currents of embryonic, neonatal and mature mouse inner hair cells. J Physiol. 2003;548(Pt 2):383–400. doi: 10.1113/jphysiol.2002.034801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci USA. 2000;97(8):4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006;25(3):642–652. doi: 10.1038/sj.emboj.7600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brueggemann LI, Mackie AR, Cribbs LL, Freda J, Tripathi A, Majetschak M, Byron KL. Differential protein kinase C-dependent modulation of Kv7.4 and Kv7.5 subunits of vascular Kv7 channels. J Biol Chem. 2014;289(4):2099–2111. doi: 10.1074/jbc.M113.527820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohya S, Asakura K, Muraki K, Watanabe M, Imaizumi Y. Molecular and functional characterization of ERG, KCNQ, and KCNE subtypes in rat stomach smooth muscle. Am J Physiol Gastrointest Liver Physiol. 2002;282(2):G277–G287. doi: 10.1152/ajpgi.00200.2001. [DOI] [PubMed] [Google Scholar]
- 27.Greenwood IA, Ohya S. New tricks for old dogs: KCNQ expression and role in smooth muscle. Br J Pharmacol. 2009;156(8):1196–1203. doi: 10.1111/j.1476-5381.2009.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, Higashida H. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6(6):564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314(5804):1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLaughlin S, Wang J, Gambhir A, Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu Rev Biophys Biomol Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 32.Rusten TE, Stenmark H. Analyzing phosphoinositides and their interacting proteins. Nat Methods. 2006;3(4):251–258. doi: 10.1038/nmeth867. [DOI] [PubMed] [Google Scholar]
- 33.Li Y, Zaydman MA, Wu D, Shi J, Guan M, Virgin-Downey B, Cui J. KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity. Proc Natl Acad Sci USA. 2011;108(22):9095–9100. doi: 10.1073/pnas.1100872108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci. 2003;4(11):885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Gamper N, Hilgemann DW, Shapiro MS. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25(43):9825–9835. doi: 10.1523/JNEUROSCI.2597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eckey K, Wrobel E, Strutz-Seebohm N, Pott L, Schmitt N, Seebohm G. Novel Kv7.1-phosphatidylinositol 4,5-bisphosphate interaction sites uncovered by charge neutralization scanning. J Biol Chem. 2014;289(33):22749–22758. doi: 10.1074/jbc.M114.589796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zaydman MA, Silva JR, Delaloye K, Li Y, Liang H, Larsson HP, Shi J, Cui J. Kv7.1 ion channels require a lipid to couple voltage sensing to pore opening. Proc Natl Acad Sci USA. 2013;110(32):13180–13185. doi: 10.1073/pnas.1305167110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Ouyang M, Ganellin CR, Thomas SA. The slow afterhyperpolarization: a target of beta1-adrenergic signaling in hippocampus-dependent memory retrieval. J Neurosci. 2013;33(11):5006–5016. doi: 10.1523/JNEUROSCI.3834-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zaydman MA, Cui J. PIP2 regulation of KCNQ channels: biophysical and molecular mechanisms for lipid modulation of voltage-dependent gating. Front Physiol. 2014;5:195. doi: 10.3389/fphys.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KS, Duignan KM, Hawryluk JM, Soh H, Tzingounis AV. The voltage activation of cortical KCNQ channels depends on global PIP2 levels. Biophys J. 2016;110(5):1089–1098. doi: 10.1016/j.bpj.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suh BC, Horowitz LF, Hirdes W, Mackie K, Hille B. Regulation of KCNQ2/KCNQ3 current by G protein cycling: the kinetics of receptor-mediated signaling by Gq. J Gen Physiol. 2004;123(6):663–683. doi: 10.1085/jgp.200409029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haitin Y, Attali B. The C-terminus of Kv7 channels: a multifunctional module. J Physiol. 2008;586(Pt 7):1803–1810. doi: 10.1113/jphysiol.2007.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kang S, Xu M, Cooper EC, Hoshi N. Channel-anchored protein kinase CK2 and protein phosphatase 1 reciprocally regulate KCNQ2-containing M-channels via phosphorylation of calmodulin. J Biol Chem. 2014;289(16):11536–11544. doi: 10.1074/jbc.M113.528497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosenko A, Kang S, Smith IM, Greene DL, Langeberg LK, Scott JD, Hoshi N. Coordinated signal integration at the M-type potassium channel upon muscarinic stimulation. EMBO J. 2012;31(14):3147–3156. doi: 10.1038/emboj.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hughes S, Marsh SJ, Tinker A, Brown DA. PIP2-dependent inhibition of M-type (Kv7. 2/7.3) potassium channels: direct on-line assessment of PIP2 depletion by Gq-coupled receptors in single living neurons. Pflügers Arch Eur J Physiol. 2007;455(1):115–124. doi: 10.1007/s00424-007-0259-6. [DOI] [PubMed] [Google Scholar]
- 46.Hoshi N, Langeberg LK, Scott JD. Distinct enzyme combinations in AKAP signalling complexes permit functional diversity. Nat Cell Biol. 2005;7(11):1066–1073. doi: 10.1038/ncb1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5(12):959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 48.Delint-Ramirez I, Willoughby D, Hammond GR, Ayling LJ, Cooper DM. Palmitoylation targets AKAP79 protein to lipid rafts and promotes its regulation of calcium-sensitive adenylyl cyclase type 8. J Biol Chem. 2011;286(38):32962–32975. doi: 10.1074/jbc.M111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Etxeberria A, Aivar P, Rodriguez-Alfaro JA, Alaimo A, Villace P, Gomez-Posada JC, Areso P, Villarroel A. Calmodulin regulates the trafficking of KCNQ2 potassium channels. FASEB J. 2008;22(4):1135–1143. doi: 10.1096/fj.07-9712com. [DOI] [PubMed] [Google Scholar]
- 50.Alaimo A, Gomez-Posada JC, Aivar P, Etxeberria A, Rodriguez-Alfaro JA, Areso P, Villarroel A. Calmodulin activation limits the rate of KCNQ2 K+ channel exit from the endoplasmic reticulum. J Biol Chem. 2009;284(31):20668–20675. doi: 10.1074/jbc.M109.019539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alberdi A, Gomis-Perez C, Bernardo-Seisdedos G, Alaimo A, Malo C, Aldaregia J, Lopez-Robles C, Areso P, Butz E, Wahl-Schott C, Villarroel A. Uncoupling PIP2-calmodulin regulation of Kv7.2 channels by an assembly destabilizing epileptogenic mutation. J Cell Sci. 2015;128(21):4014–4023. doi: 10.1242/jcs.176420. [DOI] [PubMed] [Google Scholar]
- 52.Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci. 2006;9(3):305–310. doi: 10.1038/nn1647. [DOI] [PubMed] [Google Scholar]
- 53.Kosenko A, Hoshi N. A change in configuration of the calmodulin-KCNQ channel complex underlies Ca2+-dependent modulation of KCNQ channel activity. PLoS One. 2013;8(12):e82290. doi: 10.1371/journal.pone.0082290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sachyani D, Dvir M, Strulovich R, Tria G, Tobelaim W, Peretz A, Pongs O, Svergun D, Attali B, Hirsch JA. Structural basis of a Kv7.1 potassium channel gating module: studies of the intracellular c-terminal domain in complex with calmodulin. Structure. 2014;22(11):1582–1594. doi: 10.1016/j.str.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Alaimo A, Alberdi A, Gomis-Perez C, Fernandez-Orth J, Gomez-Posada JC, Areso P, Villarroel A. Cooperativity between calmodulin-binding sites in Kv7.2 channels. J Cell Sci. 2013;126(Pt 1):244–253. doi: 10.1242/jcs.114082. [DOI] [PubMed] [Google Scholar]
- 56.Alaimo A, Alberdi A, Gomis-Perez C, Fernandez-Orth J, Bernardo-Seisdedos G, Malo C, Millet O, Areso P, Villarroel A. Pivoting between calmodulin lobes triggered by calcium in the Kv7.2/calmodulin complex. PLoS One. 2014;9(1):e86711. doi: 10.1371/journal.pone.0086711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24(5):1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26(10):2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Regev N, Degani-Katzav N, Korngreen A, Etzioni A, Siloni S, Alaimo A, Chikvashvili D, Villarroel A, Attali B, Lotan I. Selective interaction of syntaxin 1A with KCNQ2: possible implications for specific modulation of presynaptic activity. PLoS One. 2009;4(8):e6586. doi: 10.1371/journal.pone.0006586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Etzioni A, Siloni S, Chikvashvilli D, Strulovich R, Sachyani D, Regev N, Greitzer-Antes D, Hirsch JA, Lotan I. Regulation of neuronal M-channel gating in an isoform-specific manner: functional interplay between calmodulin and syntaxin 1A. J Neurosci. 2011;31(40):14158–14171. doi: 10.1523/JNEUROSCI.2666-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brackenbury WJ, Isom LL. Na channel beta subunits: overachievers of the ion channel family. Front Pharmacol. 2011;2:53. doi: 10.3389/fphar.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marionneau C, Carrasquillo Y, Norris AJ, Townsend RR, Isom LL, Link AJ, Nerbonne JM. The sodium channel accessory subunit Navbeta1 regulates neuronal excitability through modulation of repolarizing voltage-gated K(+) channels. J Neurosci. 2012;32(17):5716–5727. doi: 10.1523/JNEUROSCI.6450-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen HM, Miyazaki H, Hoshi N, Smith BJ, Nukina N, Goldin AL, Chandy KG. Modulation of voltage-gated K+ channels by the sodium channel beta1 subunit. Proc Natl Acad Sci USA. 2012;109(45):18577–18582. doi: 10.1073/pnas.1209142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo Y, Bolon B, Kahn S, Bennett BD, Babu-Khan S, Denis P, Fan W, Kha H, Zhang J, Gong Y, Martin L, Louis JC, Yan Q, Richards WG, Citron M, Vassar R. Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat Neurosci. 2001;4(3):231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 65.Wong HK, Sakurai T, Oyama F, Kaneko K, Wada K, Miyazaki H, Kurosawa M, De Strooper B, Saftig P, Nukina N. beta Subunits of voltage-gated sodium channels are novel substrates of beta-site amyloid precursor protein-cleaving enzyme (BACE1) and gamma-secretase. J Biol Chem. 2005;280(24):23009–23017. doi: 10.1074/jbc.M414648200. [DOI] [PubMed] [Google Scholar]
- 66.Hessler S, Zheng F, Hartmann S, Rittger A, Lehnert S, Volkel M, Nissen M, Edelmann E, Saftig P, Schwake M, Huth T, Alzheimer C. beta-Secretase BACE1 regulates hippocampal and reconstituted M-currents in a beta-subunit-like fashion. J Neurosci. 2015;35(8):3298–3311. doi: 10.1523/JNEUROSCI.3127-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang L, Kosenko A, Yu C, Huang L, Li X, Hoshi N. Activation of m1 muscarinic acetylcholine receptor induces surface transport of KCNQ channels through a CRMP-2-mediated pathway. J Cell Sci. 2015;128(22):4235–4245. doi: 10.1242/jcs.175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown D. M-currents: an update. Trends Neurosci. 1988;11(7):294–299. doi: 10.1016/0166-2236(88)90089-6. [DOI] [PubMed] [Google Scholar]
- 69.Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- 70.Zaika O, Lara LS, Gamper N, Hilgemann DW, Jaffe DB, Shapiro MS. Angiotensin II regulates neuronal excitability via phosphatidylinositol 4,5-bisphosphate-dependent modulation of Kv7 (M-type) K+ channels. J Physiol. 2006;575(Pt 1):49–67. doi: 10.1113/jphysiol.2006.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jones SW. Muscarinic and peptidergic excitation of bull-frog sympathetic neurones. J Physiol. 1985;366:63–87. doi: 10.1113/jphysiol.1985.sp015785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Colino A, Halliwell JV. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987;328(6125):73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- 73.Marrion NV, Smart TG, Marsh SJ, Brown DA. Muscarinic suppression of the M-current in the rat sympathetic ganglion is mediated by receptors of the M1-subtype. Br J Pharmacol. 1989;98(2):557–573. doi: 10.1111/j.1476-5381.1989.tb12630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bernheim L, Mathie A, Hille B. Characterization of muscarinic receptor subtypes inhibiting Ca2+ current and M current in rat sympathetic neurons. Proc Natl Acad Sci USA. 1992;89(20):9544–9548. doi: 10.1073/pnas.89.20.9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charpak S, Gahwiler BH, Do KQ, Knopfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- 76.Moore SD, Madamba SG, Schweitzer P, Siggins GR. Voltage-dependent effects of opioid peptides on hippocampal CA3 pyramidal neurons in vitro. J Neurosci. 1994;14(2):809–820. doi: 10.1523/JNEUROSCI.14-02-00809.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Constanti A, Brown DA. M-currents in voltage-clamped mammalian sympathetic neurones. Neurosci Lett. 1981;24(3):289–294. doi: 10.1016/0304-3940(81)90173-7. [DOI] [PubMed] [Google Scholar]
- 78.Shapiro MS, Wollmuth LP, Hille B. Angiotensin II inhibits calcium and M current channels in rat sympathetic neurons via G proteins. Neuron. 1994;12(6):1319–1329. doi: 10.1016/0896-6273(94)90447-2. [DOI] [PubMed] [Google Scholar]
- 79.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- 80.Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/S0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- 81.Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35(3):507–520. doi: 10.1016/S0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- 82.Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP(2) activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37(6):963–975. doi: 10.1016/S0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- 83.Winks JS, Hughes S, Filippov AK, Tatulian L, Abogadie FC, Brown DA, Marsh SJ. Relationship between membrane phosphatidylinositol-4,5-bisphosphate and receptor-mediated inhibition of native neuronal M channels. J Neurosci. 2005;25(13):3400–3413. doi: 10.1523/JNEUROSCI.3231-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Higashida H, Brown DA. Two polyphosphatidylinositide metabolites control two K+ currents in a neuronal cell. Nature. 1986;323(6086):333–335. doi: 10.1038/323333a0. [DOI] [PubMed] [Google Scholar]
- 85.Bosma MM, Hille B. Protein kinase C is not necessary for peptide-induced suppression of M current or for desensitization of the peptide receptors. Proc Natl Acad Sci USA. 1989;86(8):2943–2947. doi: 10.1073/pnas.86.8.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoshi N, Langeberg LK, Gould CM, Newton AC, Scott JD. Interaction with AKAP79 modifies the cellular pharmacology of PKC. Mol Cell. 2010;37(4):541–550. doi: 10.1016/j.molcel.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Smith IM, Hoshi N. ATP competitive protein kinase C inhibitors demonstrate distinct state-dependent inhibition. PLoS One. 2011;6(10):e26338. doi: 10.1371/journal.pone.0026338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tunquist BJ, Hoshi N, Guire ES, Zhang F, Mullendorff K, Langeberg LK, Raber J, Scott JD. Loss of AKAP150 perturbs distinct neuronal processes in mice. Proc Natl Acad Sci USA. 2008;105(34):12557–12562. doi: 10.1073/pnas.0805922105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kirkwood A, Lisman JE. Action potentials produce a long-term enhancement of M-current in frog sympathetic ganglion. Brain Res. 1992;580(1–2):281–287. doi: 10.1016/0006-8993(92)90955-9. [DOI] [PubMed] [Google Scholar]
- 90.Gamper N, Shapiro MS. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J Gen Physiol. 2003;122(1):17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bal M, Zhang J, Hernandez CC, Zaika O, Shapiro MS. Ca2+/calmodulin disrupts AKAP79/150 interactions with KCNQ (M-Type) K+ channels. J Neurosci. 2010;30(6):2311–2323. doi: 10.1523/JNEUROSCI.5175-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sihn CR, Kim HJ, Woltz RL, Yarov-Yarovoy V, Yang PC, Xu J, Clancy CE, Zhang XD, Chiamvimonvat N, Yamoah EN. Mechanisms of Calmodulin Regulation of Different Isoforms of Kv7.4 K+ Channels. J Biol Chem. 2016;291(5):2499–2509. doi: 10.1074/jbc.M115.668236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156(8):1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Koyama S, Appel SB. Characterization of M-current in ventral tegmental area dopamine neurons. J Neurophysiol. 2006;96(2):535–543. doi: 10.1152/jn.00574.2005. [DOI] [PubMed] [Google Scholar]
- 95.Wladyka CL, Kunze DL. KCNQ/M-currents contribute to the resting membrane potential in rat visceral sensory neurons. J Physiol. 2006;575(Pt 1):175–189. doi: 10.1113/jphysiol.2006.113308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guan D, Higgs MH, Horton LR, Spain WJ, Foehring RC. Contributions of Kv7-mediated potassium current to sub- and suprathreshold responses of rat layer II/III neocortical pyramidal neurons. J Neurophysiol. 2011;106(4):1722–1733. doi: 10.1152/jn.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Huang H, Trussell LO. KCNQ5 channels control resting properties and release probability of a synapse. Nat Neurosci. 2011;14(7):840–847. doi: 10.1038/nn.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schwarz JR, Glassmeier G, Cooper EC, Kao TC, Nodera H, Tabuena D, Kaji R, Bostock H. KCNQ channels mediate IKs, a slow K+ current regulating excitability in the rat node of Ranvier. J Physiol. 2006;573(Pt 1):17–34. doi: 10.1113/jphysiol.2006.106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martire M, Castaldo P, D’Amico M, Preziosi P, Annunziato L, Taglialatela M. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci. 2004;24(3):592–597. doi: 10.1523/JNEUROSCI.3143-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vervaeke K, Gu N, Agdestein C, Hu H, Storm JF. Kv7/KCNQ/M-channels in rat glutamatergic hippocampal axons and their role in regulation of excitability and transmitter release. J Physiol. 2006;576(Pt 1):235–256. doi: 10.1113/jphysiol.2006.111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah MM, Migliore M, Valencia I, Cooper EC, Brown DA. Functional significance of axonal Kv7 channels in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 2008;105(22):7869–7874. doi: 10.1073/pnas.0802805105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shah MM, Migliore M, Brown DA. Differential effects of Kv7 (M-) channels on synaptic integration in distinct subcellular compartments of rat hippocampal pyramidal neurons. J Physiol. 2011;589(Pt 24):6029–6038. doi: 10.1113/jphysiol.2011.220913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Storm JF. An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol. 1989;409:171–190. doi: 10.1113/jphysiol.1989.sp017491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gu N, Vervaeke K, Hu H, Storm JF. Kv7/KCNQ/M and HCN/h, but not KCa2/SK channels, contribute to the somatic medium after-hyperpolarization and excitability control in CA1 hippocampal pyramidal cells. J Physiol. 2005;566(Pt 3):689–715. doi: 10.1113/jphysiol.2005.086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA. 1999;96(8):4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mateos-Aparicio P, Murphy R, Storm JF. Complementary functions of SK and Kv7/M potassium channels in excitability control and synaptic integration in rat hippocampal dentate granule cells. J Physiol. 2014;592(4):669–693. doi: 10.1113/jphysiol.2013.267872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sanchez G, Rodriguez MJ, Pomata P, Rela L, Murer MG. Reduction of an afterhyperpolarization current increases excitability in striatal cholinergic interneurons in rat parkinsonism. J Neurosci. 2011;31(17):6553–6564. doi: 10.1523/JNEUROSCI.6345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow afterhyperpolarization in hippocampal pyramidal cells. Neuron. 2007;53(4):487–493. doi: 10.1016/j.neuron.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andrade R, Foehring RC, Tzingounis AV. The calcium-activated slow AHP: cutting through the Gordian knot. Front Cell Neurosci. 2012;6:47. doi: 10.3389/fncel.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Q, Zhou P, Chen Z, Li M, Jiang H, Gao Z, Yang H. Dynamic PIP2 interactions with voltage sensor elements contribute to KCNQ2 channel gating. Proc Natl Acad Sci USA. 2013;110(50):20093–20098. doi: 10.1073/pnas.1312483110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Aiken SP, Lampe BJ, Murphy PA, Brown BS. Reduction of spike frequency adaptation and blockade of M-current in rat CA1 pyramidal neurones by linopirdine (DuP 996), a neurotransmitter release enhancer. Br J Pharmacol. 1995;115(7):1163–1168. doi: 10.1111/j.1476-5381.1995.tb15019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nigro MJ, Mateos-Aparicio P, Storm JF. Expression and functional roles of Kv7/KCNQ/M-channels in rat medial entorhinal cortex layer II stellate cells. J Neurosci. 2014;34(20):6807–6812. doi: 10.1523/JNEUROSCI.4153-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hawryluk JM, Moreira TS, Takakura AC, Wenker IC, Tzingounis AV, Mulkey DK. KCNQ channels determine serotonergic modulation of ventral surface chemoreceptors and respiratory drive. J Neurosci. 2012;32(47):16943–16952. doi: 10.1523/JNEUROSCI.3043-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lawrence JJ, Saraga F, Churchill JF, Statland JM, Travis KE, Skinner FK, McBain CJ. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci. 2006;26(47):12325–12338. doi: 10.1523/JNEUROSCI.3521-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurones in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hu H, Vervaeke K, Storm JF. Two forms of electrical resonance at theta frequencies, generated by M-current, h-current and persistent Na + current in rat hippocampal pyramidal cells. J Physiol. 2002;545(Pt 3):783–805. doi: 10.1113/jphysiol.2002.029249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ge L, Liu XD. Electrical resonance with voltage-gated ion channels: perspectives from biophysical mechanisms and neural electrophysiology. Acta Pharmacol Sin. 2016;37(1):67–74. doi: 10.1038/aps.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201(4351):160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- 119.Huerta PT, Lisman JE. Heightened synaptic plasticity of hippocampal CA1 neurons during a cholinergically induced rhythmic state. Nature. 1993;364(6439):723–725. doi: 10.1038/364723a0. [DOI] [PubMed] [Google Scholar]
- 120.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33(3):325–340. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- 121.Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26(1):1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 122.Sarnthein J, Petsche H, Rappelsberger P, Shaw GL, von Stein A. Synchronization between prefrontal and posterior association cortex during human working memory. Proc Natl Acad Sci USA. 1998;95(12):7092–7096. doi: 10.1073/pnas.95.12.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Olvera-Cortes E, Guevara MA, Gonzalez-Burgos I. Increase of the hippocampal theta activity in the Morris water maze reflects learning rather than motor activity. Brain Res Bull. 2004;62(5):379–384. doi: 10.1016/j.brainresbull.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 124.Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8(1):51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- 125.Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24(19):4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Martinello K, Huang Z, Lujan R, Tran B, Watanabe M, Cooper EC, Brown DA, Shah MM. Cholinergic afferent stimulation induces axonal function plasticity in adult hippocampal granule cells. Neuron. 2015;85(2):346–363. doi: 10.1016/j.neuron.2014.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Young MB, Thomas SA. M1-muscarinic receptors promote fear memory consolidation via phospholipase C and the M-current. J Neurosci. 2014;34(5):1570–1578. doi: 10.1523/JNEUROSCI.1040-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frick A, Magee J, Johnston D. LTP is accompanied by an enhanced local excitability of pyramidal neuron dendrites. Nat Neurosci. 2004;7(2):126–135. doi: 10.1038/nn1178. [DOI] [PubMed] [Google Scholar]
- 129.Moore SD, Madamba SG, Joels M, Siggins GR. Somatostatin augments the M-current in hippocampal neurons. Science. 1988;239(4837):278–280. doi: 10.1126/science.2892268. [DOI] [PubMed] [Google Scholar]
- 130.de Lecea L, Criado JR, Prospero-Garcia O, Gautvik KM, Schweitzer P, Danielson PE, Dunlop CL, Siggins GR, Henriksen SJ, Sutcliffe JG. A cortical neuropeptide with neuronal depressant and sleep-modulating properties. Nature. 1996;381(6579):242–245. doi: 10.1038/381242a0. [DOI] [PubMed] [Google Scholar]
- 131.Madamba SG, Schweitzer P, Siggins GR. Dynorphin selectively augments the M-current in hippocampal CA1 neurons by an opiate receptor mechanism. J Neurophysiol. 1999;82(4):1768–1775. doi: 10.1152/jn.1999.82.4.1768. [DOI] [PubMed] [Google Scholar]
- 132.Borsotto M, Cavarec L, Bouillot M, Romey G, Macciardi F, Delaye A, Nasroune M, Bastucci M, Sambucy JL, Luan JJ, Charpagne A, Jouet V, Leger R, Lazdunski M, Cohen D, Chumakov I. PP2A-Bgamma subunit and KCNQ2 K+channels in bipolar disorder. Pharmacogenomics J. 2007;7(2):123–132. doi: 10.1038/sj.tpj.6500400. [DOI] [PubMed] [Google Scholar]
- 133.Wildburger NC, Laezza F. Control of neuronal ion channel function by glycogen synthase kinase-3: new prospective for an old kinase. Front Mol Neurosci. 2012;5:80. doi: 10.3389/fnmol.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhang M, Meng XY, Cui M, Pascal JM, Logothetis DE, Zhang JF. Selective phosphorylation modulates the PIP2 sensitivity of the CaM-SK channel complex. Nat Chem Biol. 2014;10(9):753–759. doi: 10.1038/nchembio.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gamper N, Zaika O, Li Y, Martin P, Hernandez CC, Perez MR, Wang AY, Jaffe DB, Shapiro MS. Oxidative modification of M-type K(+) channels as a mechanism of cytoprotective neuronal silencing. EMBO J. 2006;25(20):4996–5004. doi: 10.1038/sj.emboj.7601374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kim HJ, Jeong MH, Kim KR, Jung CY, Lee SY, Kim H, Koh J, Vuong TA, Jung S, Yang H, Park SK, Choi D, Kim SH, Kang K, Sohn JW, Park JM, Jeon D, Koo SH, Ho WK, Kang JS, Kim ST, Cho H. Protein arginine methylation facilitates KCNQ channel-PIP2 interaction leading to seizure suppression. Elife. 2016 doi: 10.7554/eLife.17159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stott JB, Povstyan OV, Carr G, Barrese V, Greenwood IA. G-protein betagamma subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc Natl Acad Sci USA. 2015;112(20):6497–6502. doi: 10.1073/pnas.1418605112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roper J, Schwarz JR. Heterogeneous distribution of fast and slow potassium channels in myelinated rat nerve fibres. J Physiol. 1989;416:93–110. doi: 10.1113/jphysiol.1989.sp017751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hamada MS, Kole MH. Myelin loss and axonal ion channel adaptations associated with gray matter neuronal hyperexcitability. J Neurosci. 2015;35(18):7272–7286. doi: 10.1523/JNEUROSCI.4747-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shah M, Mistry M, Marsh SJ, Brown DA, Delmas P. Molecular correlates of the M-current in cultured rat hippocampal neurons. J Physiol. 2002;544(Pt 1):29–37. doi: 10.1113/jphysiol.2002.028571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kapfhamer D, Berger KH, Hopf FW, Seif T, Kharazia V, Bonci A, Heberlein U. Protein Phosphatase 2a and glycogen synthase kinase 3 signaling modulate prepulse inhibition of the acoustic startle response by altering cortical M-Type potassium channel activity. J Neurosci. 2010;30(26):8830–8840. doi: 10.1523/JNEUROSCI.1292-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yue C, Yaari Y. Axo-somatic and apical dendritic Kv7/M channels differentially regulate the intrinsic excitability of adult rat CA1 pyramidal cells. J Neurophysiol. 2006;95(6):3480–3495. doi: 10.1152/jn.01333.2005. [DOI] [PubMed] [Google Scholar]
- 143.Hu H, Vervaeke K, Storm JF. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci. 2007;27(8):1853–1867. doi: 10.1523/JNEUROSCI.4463-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen X, Johnston D. Properties of single voltage-dependent K(+) channels in dendrites of CA1 pyramidal neurones of rat hippocampus. J Physiol. 2004;559(Pt 1):187–203. doi: 10.1113/jphysiol.2004.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sun J, Kapur J. M-type potassium channels modulate Schaffer collateral-CA1 glutamatergic synaptic transmission. J Physiol. 2012;590(16):3953–3964. doi: 10.1113/jphysiol.2012.235820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Benned-Jensen T, Christensen RK, Denti F, Perrier JF, Rasmussen HB, Olesen SP. Live imaging of Kv7.2/7.3 cell surface dynamics at the axon initial segment: high steady-state stability and calpain-dependent excitotoxic downregulation revealed. J Neurosci. 2016;36(7):2261–2266. doi: 10.1523/JNEUROSCI.2631-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Zhang J, Shapiro MS. Activity-dependent transcriptional regulation of M-Type (Kv7) K(+) channels by AKAP79/150-mediated NFAT actions. Neuron. 2012;76(6):1133–1146. doi: 10.1016/j.neuron.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li C, Lu Q, Huang P, Fu T, Li C, Guo L, Xu X. Activity-dependent downregulation of M-Type (Kv7) K(+) channels surface expression requires the activation of iGluRs/Ca(2)(+)/PKC signaling pathway in hippocampal neuron. Neuropharmacology. 2015;95:154–167. doi: 10.1016/j.neuropharm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 149.Kuba H, Yamada R, Ishiguro G, Adachi R. Redistribution of Kv1 and Kv7 enhances neuronal excitability during structural axon initial segment plasticity. Nat Commun. 2015;6:8815. doi: 10.1038/ncomms9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279(5349):403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- 151.Castaldo P, del Giudice EM, Coppola G, Pascotto A, Annunziato L, Taglialatela M. Benign familial neonatal convulsions caused by altered gating of KCNQ2/KCNQ3 potassium channels. J Neurosci. 2002;22(2):RC199. doi: 10.1523/JNEUROSCI.22-02-j0003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abidi A, Devaux JJ, Molinari F, Alcaraz G, Michon FX, Sutera-Sardo J, Becq H, Lacoste C, Altuzarra C, Afenjar A, Mignot C, Doummar D, Isidor B, Guyen SN, Colin E, De La Vaissiere S, Haye D, Trauffler A, Badens C, Prieur F, Lesca G, Villard L, Milh M, Aniksztejn L. A recurrent KCNQ2 pore mutation causing early onset epileptic encephalopathy has a moderate effect on M current but alters subcellular localization of Kv7 channels. Neurobiol Dis. 2015;80:80–92. doi: 10.1016/j.nbd.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 153.Borgatti R, Zucca C, Cavallini A, Ferrario M, Panzeri C, Castaldo P, Soldovieri MV, Baschirotto C, Bresolin N, Dalla Bernardina B, Taglialatela M, Bassi MT. A novel mutation in KCNQ2 associated with BFNC, drug resistant epilepsy, and mental retardation. Neurology. 2004;63(1):57–65. doi: 10.1212/01.WNL.0000132979.08394.6D. [DOI] [PubMed] [Google Scholar]
- 154.Schwake M, Pusch M, Kharkovets T, Jentsch TJ. Surface expression and single channel properties of KCNQ2/KCNQ3, M-type K+ channels involved in epilepsy. J Biol Chem. 2000;275(18):13343–13348. doi: 10.1074/jbc.275.18.13343. [DOI] [PubMed] [Google Scholar]
- 155.Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci USA. 2006;103(23):8870–8875. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Soldovieri MV, Miceli F, Taglialatela M. Driving with no brakes: molecular pathophysiology of Kv7 potassium channels. Physiology (Bethesda) 2011;26(5):365–376. doi: 10.1152/physiol.00009.2011. [DOI] [PubMed] [Google Scholar]
- 157.Dedek K, Fusco L, Teloy N, Steinlein OK. Neonatal convulsions and epileptic encephalopathy in an Italian family with a missense mutation in the fifth transmembrane region of KCNQ2. Epilepsy Res. 2003;54(1):21–27. doi: 10.1016/S0920-1211(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 158.Weckhuysen S, Ivanovic V, Hendrickx R, Van Coster R, Hjalgrim H, Moller RS, Gronborg S, Schoonjans AS, Ceulemans B, Heavin SB, Eltze C, Horvath R, Casara G, Pisano T, Giordano L, Rostasy K, Haberlandt E, Albrecht B, Bevot A, Benkel I, Syrbe S, Sheidley B, Guerrini R, Poduri A, Lemke JR, Mandelstam S, Scheffer I, Angriman M, Striano P, Marini C, Suls A, De Jonghe P. Extending the KCNQ2 encephalopathy spectrum: clinical and neuroimaging findings in 17 patients. Neurology. 2013;81(19):1697–1703. doi: 10.1212/01.wnl.0000435296.72400.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Singh NA, Otto JF, Dahle EJ, Pappas C, Leslie JD, Vilaythong A, Noebels JL, White HS, Wilcox KS, Leppert MF. Mouse models of human KCNQ2 and KCNQ3 mutations for benign familial neonatal convulsions show seizures and neuronal plasticity without synaptic reorganization. J Physiol. 2008;586(14):3405–3423. doi: 10.1113/jphysiol.2008.154971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tomonoh Y, Deshimaru M, Araki K, Miyazaki Y, Arasaki T, Tanaka Y, Kitamura H, Mori F, Wakabayashi K, Yamashita S, Saito R, Itoh M, Uchida T, Yamada J, Migita K, Ueno S, Kitaura H, Kakita A, Lossin C, Takano Y, Hirose S. The kick-in system: a novel rapid knock-in strategy. PLoS One. 2014;9(2):e88549. doi: 10.1371/journal.pone.0088549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ihara Y, Tomonoh Y, Deshimaru M, Zhang B, Uchida T, Ishii A, Hirose S. Retigabine, a Kv7.2/Kv7.3-channel opener, attenuates drug-induced seizures in knock-in mice harboring Kcnq2 mutations. PLoS One. 2016;11(2):e0150095. doi: 10.1371/journal.pone.0150095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lothman EW, Bertram EH, 3rd, Stringer JL. Functional anatomy of hippocampal seizures. Prog Neurobiol. 1991;37(1):1–82. doi: 10.1016/0301-0082(91)90011-O. [DOI] [PubMed] [Google Scholar]
- 163.Meriaux C, Franck J, Park DB, Quanico J, Kim YH, Chung CK, Park YM, Steinbusch H, Salzet M, Fournier I. Human temporal lobe epilepsy analyses by tissue proteomics. Hippocampus. 2014;24(6):628–642. doi: 10.1002/hipo.22246. [DOI] [PubMed] [Google Scholar]
- 164.Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, Deprez L, Smets K, Hristova D, Yordanova I, Jordanova A, Ceulemans B, Jansen A, Hasaerts D, Roelens F, Lagae L, Yendle S, Stanley T, Heron SE, Mulley JC, Berkovic SF, Scheffer IE, de Jonghe P. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71(1):15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- 165.Miceli F, Soldovieri MV, Ambrosino P, De Maria M, Migliore M, Migliore R, Taglialatela M. Early-onset epileptic encephalopathy caused by gain-of-function mutations in the voltage sensor of Kv7.2 and Kv7.3 potassium channel subunits. J Neurosci. 2015;35(9):3782–3793. doi: 10.1523/JNEUROSCI.4423-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Devaux J, Abidi A, Roubertie A, Molinari F, Becq H, Lacoste C, Villard L, Milh M, Aniksztejn L. A Kv7.2 mutation associated with early onset epileptic encephalopathy with suppression-burst enhances Kv7/M channel activity. Epilepsia. 2016 doi: 10.1111/epi.13366. [DOI] [PubMed] [Google Scholar]
- 167.Wickenden AD, Yu W, Zou A, Jegla T, Wagoner PK. Retigabine, a novel anti-convulsant, enhances activation of KCNQ2/Q3 potassium channels. Mol Pharmacol. 2000;58(3):591–600. doi: 10.1124/mol.58.3.591. [DOI] [PubMed] [Google Scholar]
- 168.Kay HY, Greene DL, Kang S, Kosenko A, Hoshi N. M-current preservation contributes to anticonvulsant effects of valproic acid. J Clin Invest. 2015;125(10):3904–3914. doi: 10.1172/JCI79727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Santini E, Porter JT. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J Neurosci. 2010;30(37):12379–12386. doi: 10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]