Abstract
Sec1/Munc18 (SM) proteins are central to intracellular transport and neurotransmitter release but their exact role is still elusive. Several SM proteins, like the neuronal N-Sec1 and the yeast Sly1 protein, bind their cognate t-SNAREs with high affinity. This has been thought to be critical for their function. Here, we show that various mutant forms of Sly1p and the Golgi-localized syntaxin Sed5p, which abolish their high-affinity interaction, are fully functional in vivo, indicating that the tight interaction of the two molecules per se is not relevant for proper function. Mutant Sly1p unable to bind Sed5p is excluded from core SNARE complexes, also demonstrating that Sly1p function is not directly coupled to assembled SNARE complexes thought to execute membrane fusion. We also find that wild-type Sly1p and mutant Sly1p unable to bind Sed5p directly interact with selected ER-to-Golgi and intra-Golgi nonsyntaxin SNAREs. The newly identified, direct interactions of the SM protein with nonsytaxin SNAREs might provide a molecular mechanism by which SNAREs can be activated to engage in pairing and assemble into fusogenic SNARE complexes.
Keywords: membrane fusion, Sec1/Munc18 family, Sly1p, SNARE assembly, vesicular transport
Introduction
Fusion of membranes in eukaryotic cells requires groups of related proteins, specific members of which fulfill their functions at particular steps in exo- and endocytotic trafficking. N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), Sec1/Munc18 family proteins, also termed SM proteins, and Ypt/Rab-GTPases are the most prominent proteins required for fusion to occur (Chen and Scheller, 2001; Gallwitz and Jahn, 2003; Jahn et al, 2003). Whereas SNAREs are type II membrane proteins that form helical bundles on opposing membranes to be fused (Sutton et al, 1998; Weber et al, 1998), Sec1/Munc18 family members are hydrophilic proteins that were first discovered in yeast. Those acting in exocytosis, Sec1p and Sly1p, are essential for cell viability (Novick and Schekman, 1979; Dascher et al, 1991; Ossig et al, 1991). Two other members of this family in yeast, Vps45p and Vps33p, participate in vesicular transport between Golgi and endosomes, and between endosomes and the vacuole and in homotypic vacuole fusion, respectively (Piper et al, 1994; Nichols et al, 1998; Sato et al, 2000; Seals et al, 2000). The mammalian homolog of yeast Sec1p, N-Sec1 (also termed Munc18), was shown to bind the SNARE syntaxin 1 with nanomolar affinity (Pevsner et al, 1994), indicating that SNAREs and SM proteins act in concert and at the heart of membrane fusion. Work in yeast showed that Sly1p and the syntaxin Sed5p, which act in ER-to-Golgi transport, interact with similarly high affinity (Grabowski and Gallwitz, 1997). However, N-Sec1 binds to a closed conformation of syntaxin 1 and prevents SNARE complex formation in vitro (Dulubova et al, 1999; Misura et al, 2000; Yang et al, 2000), whereas Sly1p in complex with Sed5p readily allows core SNARE complex generation (Peng and Gallwitz, 2002). Yeast Sec1p does not bind the isolated syntaxin Sso1p, but instead appears to associate with the preformed exocytic SNARE complex only (Carr et al, 1999). The different modes of SM protein–syntaxin interaction have also led to different views regarding their function, and negative and positive roles of these proteins for SNARE complex formation have been postulated (Gallwitz and Jahn, 2003).
It has been demonstrated recently that several Sec1 family members, Sly1p and Vps45p, bind to a short N-terminal peptide of their cognate SNAREs Sed5p and Tlg2p, respectively (Dulubova et al, 2002; Yamaguchi et al, 2002). This mode of interaction seems to prevail, whereas the N-Sec1–syntaxin1 interaction in neurotransmitter release is rather specialized. The recent elucidation of the crystal structure of yeast Sly1p complexed with the N-terminus of Sed5p revealed that the binding of the syntaxin involves a specially folded N-terminal domain of the SM protein, leaving untouched two other Sly1p domains that might play important regulatory roles (Bracher and Weissenhorn, 2002).
As the yeast Sec1 and Sly1 proteins are found to be associated with fully assembled SNARE complexes (Carr et al, 1999; Peng and Gallwitz, 2002), it is reasonable to assume that these proteins might influence the conformational cycle of SNAREs. Although it is unclear at present how SM proteins work in molecular terms, recent in vitro studies suggest that they play a role in the specificity of SNARE complex assembly (Peng and Gallwitz, 2002). The intriguingly tight binding of Sed5p to the outer surface of Sly1p domain I via a short N-terminal peptide of the syntaxin has several implications: it would allow the SM protein to fulfill a specific function by staying associated with the syntaxin during and after SNARE complex assembly, or Sly1p bound to the syntaxin could recruit to membranes to be fused other protein(s) engaged in special aspects of SNARE assembly or membrane fusion. The significance of other parts of Sly1p besides the Sed5p interacting region is, for example, shown by a single amino-acid substitution (E532K) in domain III that uncouples ER-to-Golgi transport from the need of Ypt1 GTPase function (Dascher et al, 1991; Ossig et al, 1991).
In an extensive mutational study, we have now made the surprising finding that the high-affinity binding of Sly1p to Sed5p is not important for the functioning of the two proteins in ER-to-Golgi transport. Neither the Golgi localization of the SM protein nor the secretion of proteins is significantly affected after abolishing Sed5p–Sly1p interaction. During this study, we also made the discovery that Sly1p and its mutant forms unable to interact with Sed5p can bind to specific nonsyntaxin SNAREs (Bet1p, Bos1p, Sft1p and Gos1p) that are involved in ER-to-Golgi and intra-Golgi transport. The results of the present investigation suggest that Sly1p, and possibly other SM proteins, may act in SNARE complex assembly through stepwise interactions with different SNARE proteins.
Results
High-affinity binding of the SM protein Sly1p to the Golgi syntaxin Sed5p is functionally dispensable
The yeast Sly1p and the neuronal N-Sec1/Munc18 protein bind their cognate syntaxins, Sed5p and syntaxin 1, with nanomolar affinity. However, this type of interaction appears not to be universal among all members of the two protein families. We therefore sought to investigate whether the function of the yeast SM protein Sly1p and the Golgi syntaxin Sed5p is linked to their tight interaction. Biochemical and crystallographic studies revealed that the N-terminal 20 amino-acid residues of Sed5p are responsible for binding to Sly1p (Yamaguchi et al, 2002). The crystal structure of Sly1p complexed with the N-terminal 45 amino acids of Sed5p also disclosed that five residues of Sly1p (L137, L140, A141, I153 and V156) form a hydrophobic pocket closely surrounding phenylalanine 10 of Sed5p, one of the critical residues involved in Sly1p binding (Bracher and Weissenhorn, 2002).
To test whether the hydrophobic pocket of Sly1p mediates the high-affinity binding to Sed5p, we expressed domain I (amino acids 1–167) of wild-type and mutant Sly1 proteins fused to the C-terminus of glutathione S-transferase (GST). Each mutant protein carried a single lysine or arginine substitution for one of the five hydrophobic amino acids, or a single substitution for either of two neighboring, charged and highly conserved residues (R134E or E138K) (Figure 1B). The fusion proteins were immobilized on glutathione–Sepharose beads and probed for their binding to His-tagged Sed5p. Sed5p binding to domain I of wild-type Sly1p and to two of the mutants, Sly1(R134E)p and Sly1(E138K)p, was similarly effective. In contrast, Sed5p binding to Sly1p mutants affecting the hydrophobic pocket was completely abolished. In addition, no interaction was seen with the C-terminal three quarters of Sly1p (Figure 1A). To test whether nontruncated Sly1 mutant protein was also unable to bind Sed5p, the full-length version of one of the mutants, Sly1(L140K)p, was prepared as a GST fusion, and the interaction with Sed5p was probed in GST pulldown assays. As shown in Figure 2A, wild-type Sly1p bound Sed5p efficiently (lane 2) but Sly1(L140K)p did not (lane 3). In pulldown experiments with GST-Sed5p, only traces of His-tagged Sly1(L140K) protein were found to bind to the syntaxin on beads (lane 6). These in vitro studies revealed that substitutions of hydrophobic residues of Sly1p, including L140K, previously shown to form crystal contacts to an N-terminal peptide of Sed5p (Bracher and Weissenhorn, 2002), disrupt the interaction with the syntaxin and that the hydrophobic pocket of Sly1p is necessary and sufficient for binding to Sed5p.
Figure 1.
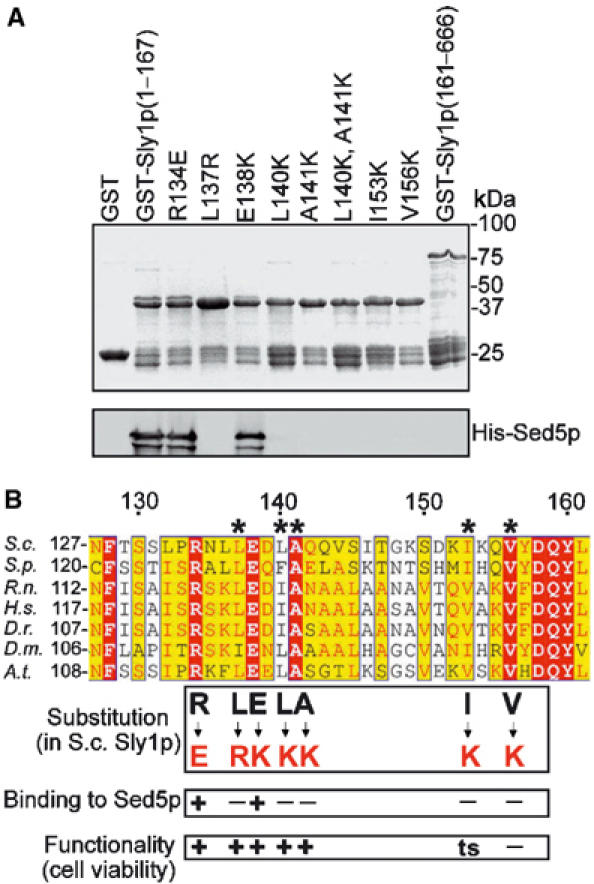
Sly1p mutants unable to bind to Sed5p are functional. (A) Fragments of wild-type and mutant Sly1 protein fused to GST were bound to glutathione–Sepharose and incubated with His-tagged Sed5p (0.2 μg/μl) purified from Escherichia coli. After extensive washing, the beads were mixed with SDS sample buffer, boiled and bound proteins were separated by SDS–PAGE and viewed by Coomassie blue staining (upper panel) or immunoblotting with affinity-purified anti-Sed5p antibodies (lower panel). (B) Sly1p sequences from different species were aligned by a Clustalw software. Conserved amino acids are highlighted by different colors. The five amino acids that form the hydrophobic Sed5-binding pocket are designated by asterisks. Amino-acid substitutions and their consequences are indicated.
Figure 2.
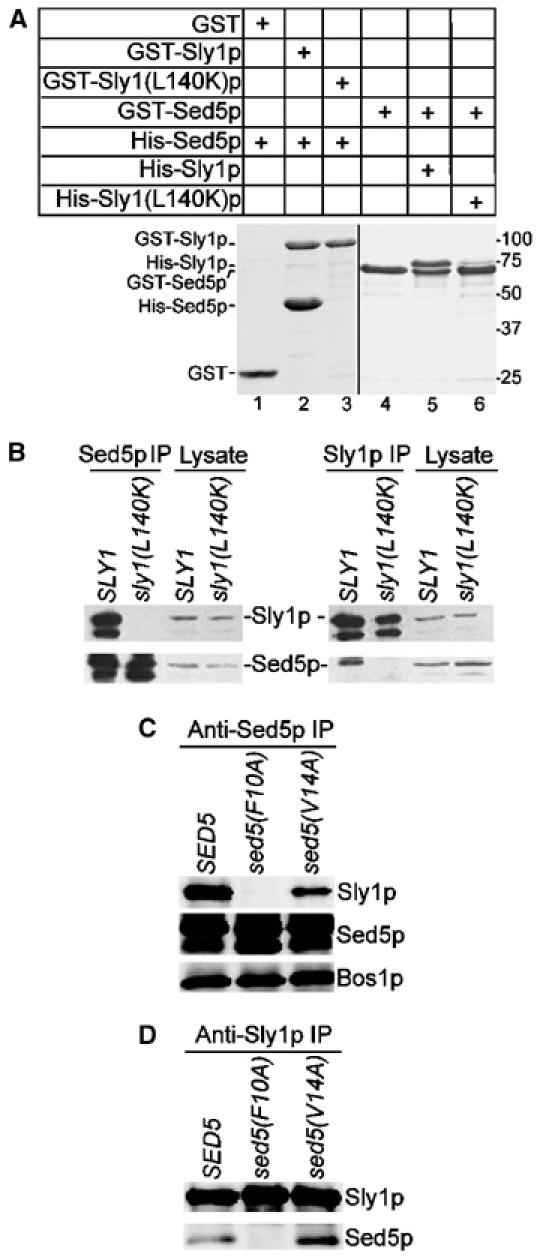
Sly1(L140K)p unable to bind Sed5p, or Sed5(F10A)p abolishing Sly1p binding is functional. (A) Full-length wild-type Sly1p, mutant Sly1(L140K)p and Sed5p fused to GST were immobilized on glutathione–Sepharose beads and incubated with His-tagged Sed5p (0.8 μg/μl), Sly1p (0.8 μg/μl) or Sly1(L140K)p (0.8 μg/μl), respectively. GST fusion proteins and protein complexes formed on beads were analyzed by SDS–PAGE and Coomassie blue staining. (B) Detergent lysates prepared from yeast strains expressing wild-type Sly1p (strain MSUC3D) and Sly1(L140K)p (strain RPY238) were subjected to immunoprecipitation with affinity-purified anti-Sed5p or anti-Sly1p antibodies. The immunoprecipitates along with a fraction of original lysates (2%) were analyzed on immunoblots. Note that both Sly1p and Sed5p were partially degraded. (C, D) Detergent lysates prepared from cells expressing wild-type Sed5p (strain RPY214), Sed5(F10A)p (strain RPY215) or Sed5(V14A)p (strain RPY216) were subjected to immunoprecipitation with anti-Sed5p and anti-Sly1p antibodies as in (B). Proteins co-precipitated with Sed5p or Sly1p were analyzed on immunoblots with antibodies indicated to the right.
As a complementary approach to the in vitro studies, we explored the interaction between Sly1(L140K)p and Sed5p in vivo. To our surprise, we found that Sly1(L140K)p expressed from a plasmid-contained gene was functionally active since it complemented both the temperature sensitivity of a sly1-1 mutant strain and the functional loss of Sly1p in a sly1 deletion strain. Therefore, we were able to construct a yeast strain in which Sly1(L140K)p was expressed from the genomic SLY1 locus as sole source of Sly1p. The mutant cells were perfectly viable and had no obvious growth defect. Detergent lysates prepared from cells of the mutant and its isogenic wild-type strain were subjected to immunoprecipitation with either anti-Sed5p or anti-Sly1p antibodies. In contrast to Sly1p, Sly1(L140K)p was not at all co-precipitated with Sed5p (Figure 2B). Likewise, Sed5p was completely absent from Sly1p immunoprecipitates obtained from Sly1(L140K)p-expressing cells (Figure 2B). We also introduced the other single substitutions shown in Figure 1A into full-length Sly1p and probed whether the mutant proteins were able to functionally replace the essential Sly1p. The results are summarized in Figure 1B. Of the five residues forming the hydrophobic pocket, every single substitution effectively abolished binding to Sed5p. Interestingly, three of these mutations (L137R, L140K and A141K) did not affect Sly1p function. The I153K substitution led to temperature sensitivity, whereas the substitution V156K caused lethality of mutant cells. The reason for the latter appears to be instability of Sly1(V156K)p (data not shown).
We further investigated whether Sed5p function was coupled to the tight interaction with Sly1p. Since phenylalanine in position 10 and valine in position 14 of Sed5p were previously shown to be critical for syntaxin's function and interaction with Sly1p (Yamaguchi et al, 2002), we introduced two single substitutions (F10A or V14A) into Sed5p and probed for functional activity of the mutant proteins. When expressed from a CEN plasmid, both Sed5(F10A)p and Sed5(V14A)p were able to support viability of cells with the genomic SED5 gene deleted. To test whether the mutant Sed5 proteins still bound to Sly1p in vivo, we constructed two strains in which either Sed5(F10A)p or Sed5(V14A)p was expressed as the sole source for Sed5p. Cells of these strains were perfectly viable. Detergent lysates from cells of both strains were prepared, and proteins immunoprecipitated with either anti-Sed5p or anti-Sly1p antibodies were subjected to immunoblot analysis (Figure 2C and D). No interaction between Sed5(F10A)p and Sly1p was observed. However, Sed5(V14A)p still bound to Sly1p, although at a reduced level. This is in accordance with in vitro GST pulldown assays, in which Sed5(F10A)p/Sly1p binding was completely abolished, whereas Sed5(V14A)p still showed an appreciable binding to the SM protein (Yamaguchi et al, 2002). Our results show that although the F10A substitution of Sed5p disrupted the binding to Sly1p, the function of the mutant protein was still maintained, which again demonstrates that the high-affinity binding of Sed5p to Sly1p is dispensable for the functioning of the two essential proteins.
At steady state, Sly1p and Sed5p are primarily associated with Golgi membranes. As the high-affinity interaction of the two essential proteins proved dispensable for cell viability, we investigated whether Sly1(L140K)p was still associated with Golgi membranes. For this purpose, yeast strains that expressed either GFP-Sly1p or GFP-Sly1(L140K)p from the fusion genes integrated into the SLY1 locus were constructed. Cells of both strains grew like wild type. Localization studies in living cells revealed that the mutant protein, like wild-type Sly1p, was primarily in dotted structures, which according to the significant overlap of Sly1 proteins and Emp47p, an established Golgi marker (Schröder et al, 1995), represented Golgi organelles (Figure 3). In living cells, the number of dots was smaller (on average 3–4 punctate structures in mutant and 5–6 in wild-type cells) and their fluorescence intensity somewhat weaker. The data show that a significant part of the mutant Sly1 protein was still associated with Golgi membranes.
Figure 3.
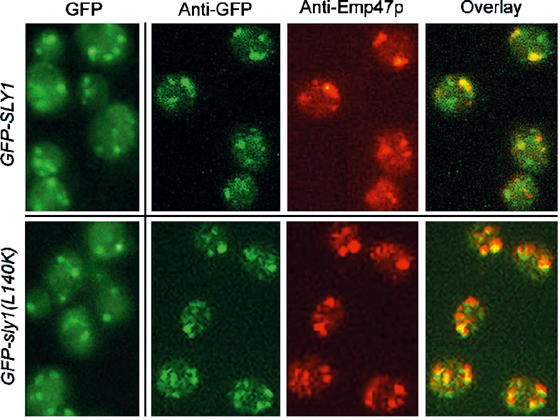
Wild-type Sly1p and mutant Sly1(L140K)p are localized to the Golgi membranes. Localization of GFP-Sly1p (strain RPY142) and GFP-Sly1(L140K)p (strain RPY220) was analyzed in living cells by fluorescence, or in fixed cells using anti-GFP and anti-Emp47p antibodies. Note the significant colocalization of GFP-Sly1p and GFP-Sly1(L140K)p with the Golgi protein Emp47p.
Vesicular protein transport was apparently unaffected in sly1(L140K) and sed5(F10A) mutant cells. Pulse-chase experiments (Figure 4A) showed normal maturation and transport kinetics of the vacuolar carboxypeptidase Y (CPY) whose ER and Golgi maturation intermediates (p1 and p2, respectively) and the mature vacuolar form (m) can be easily distinguished electrophoretically. Whereas at 35°C, the COPII mutant sec23-1 was fully blocked in ER-to-Golgi transport and accumulated the ER form only, even at 30 min of chase, the sly1(L140K) and the sed5(F10A) mutant cells had almost finished CPY maturation within 10 min, like wild-type cells. Likewise, invertase induced for 60 min was glycosylated and secreted completely normally at 25 and 35°C (Figure 4B).
Figure 4.
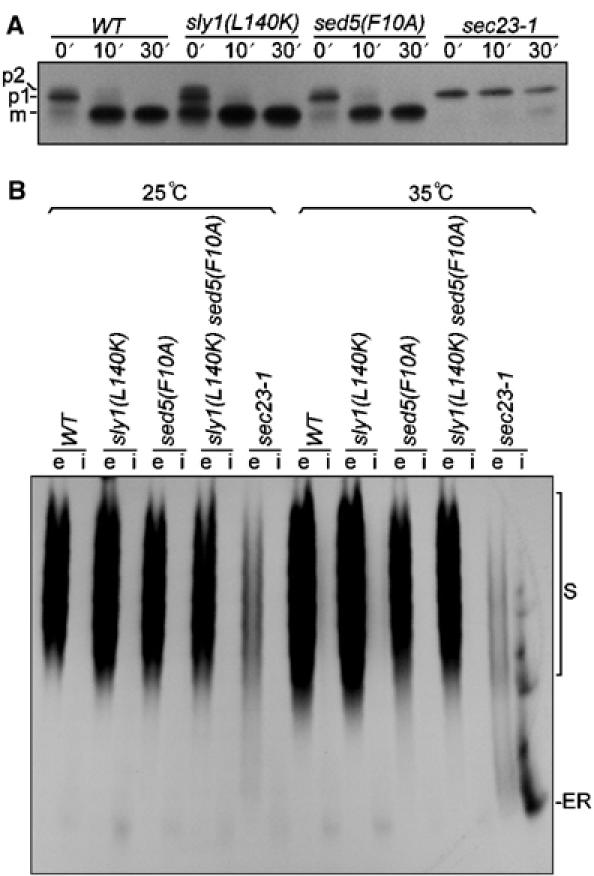
Analysis of CPY transport and invertase secretion in sly1(L140K) and sed5(F10A) mutants. (A) Cells of wild-type (MSUC3D), sly1(L140K) (RPY238), sed5(F10A) (RPY215) and sec23-1 (RH227-3A) strains were grown at 25°C before incubation at 35°C for 10 min. The cells were labeled with Tran35S-label at 35°C for 10 min and chased for 10 or 30 min. CPY was immunoprecipitated and analyzed by SDS–PAGE, followed by autoradiography. ER core-glycosylated (p1), Golgi-modified (p2) and mature form (m) of CPY are indicated. (B) Cells of wild-type, sly1(L140K), sed5(F10A), sly1(L140K)/sed5(F10A) double-mutant (RPY249) and sec23-1 strains grown at 25°C were transferred to 0.1% glucose medium to induce invertase synthesis for 60 min at either 25 or 35°C (nonpermissive temperatures). Spheroplasts were prepared and pelleted to obtain the periplasmic (e) and intracellular (i) fractions. Proteins of both fractions were separated on nondenaturing polyacrylamide gel, followed by activity staining of invertase. S, secreted invertase; ER, ER core-glycosylated invertase.
Genetic studies underlining irrelevance of Sly1p/Sed5p interaction for ER-to-Golgi transport
The findings described above indicated that the high-affinity interaction between Sly1p and Sed5p is irrelevant for proper functioning of the two proteins. However, another, admittedly less likely explanation had to be excluded. In analogy to our previous observation that mutant cells expressing Sly1(E532K)p, termed Sly1-20p, are able to grow in the absence of the otherwise essential GTPase Ytp1p (Dascher et al, 1991; Ossig et al, 1991), Sly1(L140K)p might allow cells to survive in the absence of Sed5p, and viability of mutant cells expressing Sed5p(F10A)p might no longer depend on Sly1p function. We therefore created sed5 and sly1 conditional mutants that relied on either Sly1(L140K)p or Sed5(F10A)p as the sole source of these proteins. Cells of both double-mutant strains were viable at permissive but inviable at nonpermissive temperatures, clearly indicating that both, the SM protein and the syntaxin, were still essential for protein transport and cell viability.
To assess whether the sly1(L140K) or the sly1-1 allele caused synthetic negative effects when combined with other mutations in ER-to-Golgi transport, we sought to create double mutants with the conditionally lethal ypt1-3 or uso1-1 alleles. The GTPase Ypt1p and the Golgi-associated Uso1 protein are, like Sly1p, essential for docking/fusion of ER-derived vesicles to Golgi membranes, Sly1p acting after vesicle tethering (Cao et al, 1998). We found that combining sly1-1 with either ypt1-3 or uso1-1 led to mutants with synthetic lethality, whereas sly1(L140K)/ypt1-3 and sly1(L140K)/uso1-1 mutant cells were viable at permissive temperatures, indicating again that high-affinity binding of Sly1p to Sed5p is functionally unimportant.
Our finding that the sly1(L140K) and sed5(F10A) alleles could also be combined without affecting cell viability and protein transport (Figure 4B) leads to the same conclusion.
Sly1(L140K)p is excluded from core SNARE complexes
We had previously shown that Sly1p binds to preassembled core SNARE complexes (Peng and Gallwitz, 2002). A further study concluded that Sec1p, an SM protein required for secretion, fulfills its function only when bound to assembled SNARE complexes in vivo (Carr et al, 1999). We therefore investigated whether Sly1(L140K)p, which is unable to bind to Sed5p, might still bind to SNARE complexes. The SNAREs involved in either ER-to-Golgi transport (Bos1p, Sec22p, Bet1p and Sed5p) or in intra-Golgi transport (Gos1p, Ykt6p, Sft1p and Sed5p) were assembled into core complexes, immobilized on glutathione–Sepharose beads and probed for the interaction with His-tagged wild-type Sly1 or Sly1(L140K)p. As shown in Figure 5A, the two core SNARE complexes formed in vitro bound wild-type Sly1p effectively but Sly1(L140K)p only in traces.
Figure 5.
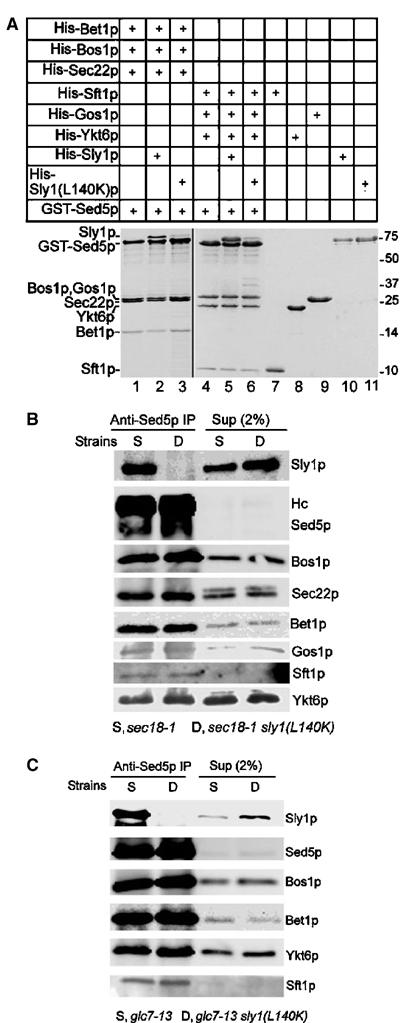
Sly1(L140K)p is excluded from core SNARE complexes. (A) SNARE complexes of ER-to-Golgi and intra-Golgi transport were assembled in solution from purified proteins. The complexes were incubated with His-tagged wild-type or mutant Sly1 protein. Core complexes and their associated Sly1p or Sly1(L140K)p were analyzed by SDS–PAGE and Coomassie blue staining. As determined by densitometry, the stoichiometry of Sed5p/Gos1p/Ykt6p/Sft1p in the complex (lane 4) was 1/1.3/1.3/1. Sly1p bound to the complex at a ratio of 1:1 (Sly1p to Sed5p, lane 5). (B) Cells of sec18-1 (strain YTX50) and sec18-1/sly1(L140K) (strain RPY237) were grown at 25°C to mid-log phase before incubation at 37°C for 30 min. Detergent lysates prepared from both strains were immunoprecipitated by anti-Sed5p antibodies. Proteins co-immunoprecipitated with Sed5p were analyzed on immunoblots. (C) Cells of glc7-13 (PAY702-4) and glc7-13/sly1(L140K) (strain RPY239) were grown and treated as in (B). Immunoprecipitation with anti-Sed5p antibodies and proteins co-immunoprecipitated with Sed5p were analyzed on immunoblots.
These results were complemented by in vivo experiments. As previously suggested, yeast cells defective in SNARE complex-disrupting ATPase (NSF) activity should have an advantage over wild-type cells when studying cis-SNARE complexes in vivo (Søgaard et al, 1994). Therefore, a strain carrying mutations in Sly1p and Sec18p (sec18-1/sly1(L140K)) was constructed, and along with its isogenic strain (sec18-1) was analyzed at permissive and nonpermissive temperatures. Detergent lysates from these strains were subjected to immunoprecipitation with anti-Sed5p antibodies so that the syntaxin was completely precipitated. As shown in Figure 5B, a fraction (about 6–8% of total) of the SNARE molecules involved in ER-to-Golgi transport and in intra-Golgi transport were co-precipitated with Sed5p from lysates of both the sec18-1 and the sec18-1/sly1(L140K) double-mutant strains, confirming that these SNAREs were in complex with Sed5p. Importantly, wild-type Sly1p but not Sly1(L140K)p was co-precipitated with Sed5p, indicating that Sly1(L140K)p was not associated with the Sed5p-containing SNARE complexes in vivo.
It was recently described that yeast protein phosphatase 1, the product of the GLC7 gene, is required at a final stage of membrane fusion (Peters et al, 1999), and that glc7 mutant cells at nonpermissive conditions might enrich trans-SNARE complexes (Bryant and James, 2003). In an attempt to take advantage of a glc7-13 strain for further exploring the possible interaction of Sly1(L140K)p with SNARE complexes in vivo, a glc7-13/sly1(L140K) double-mutant strain was constructed, and SNARE complexes were immunoprecipitated with anti-Sed5p antibodies from detergent lysates of mutant cells grown at nonpermissive temperatures. Although clear evidence for the existence of ER-to-Golgi and intra-Golgi SNARE complexes was obtained by the co-precipitation of Sed5p with cognate SNAREs, wild-type Sly1p but not Sly1(L140K)p was found in immunoprecipitates (Figure 5C), complementing the data in Figure 5B and demonstrating that the Sly1p function is not directly coupled to assembled SNARE complexes.
Sly1p directly and specifically interacts with nonsyntaxin v- and t-SNAREs
As shown above, a significant fraction of Sly1p unable to bind Sed5p was still on Golgi membranes. We therefore screened for a possible interaction of Sly1p with nonsyntaxin SNAREs. For this purpose, we systematically examined the binding of Sly1p to all SNAREs that have been implicated in Golgi membrane fusions. The cytosolic regions of the SNAREs fused to the C-terminus of GST were immobilized on glutathione–Sepharose beads and incubated with His-tagged Sly1p or Sly1(L140K)p (Figure 6A). After extensive washing, only four of the eight SNAREs tested exhibited significant binding to Sly1p: Bet1p and Bos1p, which are involved in ER-to-Golgi transport, as well as Sft1p and Gos1p, which act in intra-Golgi transport. Importantly, the four SNAREs also bound to Sly1(L140K)p. The syntaxin Tlg1p and the nonsyntaxin SNAREs Vti1p and Nyv1p, which act in transport from Golgi to endosomes and to vacuoles, did not interact with Sly1p. In addition, Ykt6p, an essential SNARE apparently involved in multiple transport steps (McNew et al, 1997; Dilcher et al, 2001; Kweon et al, 2003), exhibited significant interaction with Sly1(L140K)p but not with wild-type Sly1p (Figure 6A). This experiment led to three important conclusions. Firstly, the SM protein Sly1p not only binds to Sed5p but also interacts directly with specific nonsyntaxin SNAREs. Secondly, the L140K substitution in Sly1p abolishes the binding to the syntaxin Sed5p, but not to nonsyntaxin molecules. Thirdly, Sly1(L140K)p appears to acquire the ability to interact with Ykt6p, which itself has been shown to contribute to intra-Golgi but not to ER-to-Golgi core SNARE complex formation under physiological conditions.
Figure 6.
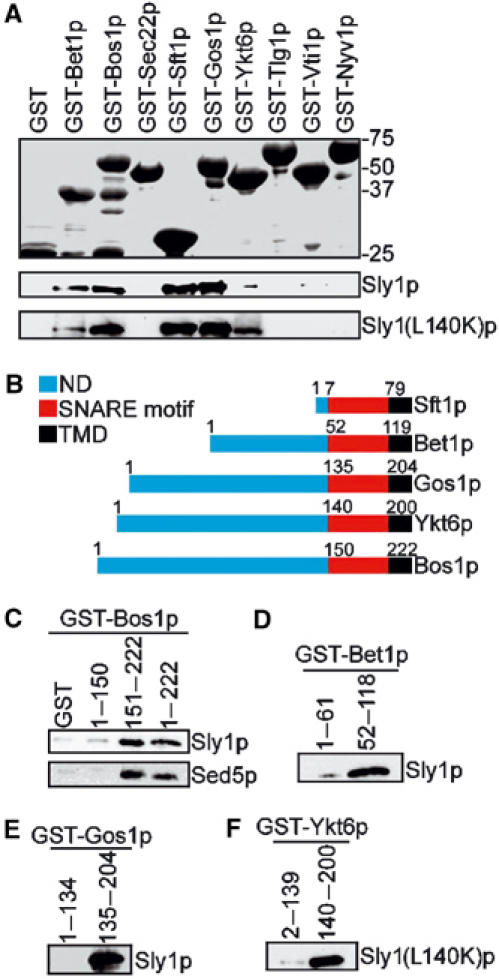
Sly1p directly binds to v-SNAREs and nonsyntaxin t-SNAREs. (A) Bacterially produced nonsyntaxin SNAREs of various protein transport steps fused to GST were immobilized on glutathione–Sepharose beads and incubated with purified Sly1p (0.2 μg/μl) or Sly1(L140K)p (0.2 μg/μl). Proteins bound on beads were analyzed by SDS–PAGE and Coomassie blue staining (upper panel) or by immunoblotting using anti-Sly1p antibodies (lower panel). (B) Domain structures of nonsyntaxin SNAREs Bet1p, Bos1p, Sft1p, Gos1p and Ykt6p. ND, N-terminal domain; TMD, transmembrane domain. (C–F) The N-terminal region or the SNARE motifs of different SNAREs fused to GST and bound to glutathione–Sepharose beads were incubated with Sly1p (0.2 μg/μl) or Sly1(L140K)p (0.2 μg/μl). GST fusions and bound proteins were analyzed by immunoblotting. In (B), the binding of Bos1p fragments to Sed5p (0.2 μg/μl) is shown.
Having shown that wild-type Sly1p and Sly1(L140K)p can interact with nonsyntaxin SNAREs in a nonpromiscous way, we sought to examine which part of the SNAREs bound to the SM protein. SNAREs are characterized by an evolutionarily conserved heptad repeat of 60–70 amino-acid residues, called SNARE motif, a preceding N-terminus and a transmembrane domain at the C-terminal end (Figure 6B). The SNARE motifs of three or four distinct SNAREs are engaged in the four-helix bundle of SNARE complexes (Sutton et al, 1998; Antonin et al, 2002). The N-terminus and the SNARE motif of individual SNAREs fused to GST were expressed in bacteria and their interaction with Sly1p or Sly1(L140K)p was probed in GST pulldown experiments. Sft1p has a very short N-terminal region (seven amino acids), but GST-Sft1(17–79)p efficiently bound Sly1p (Figure 6A), suggesting that the SNARE motif region of this protein largely accounted for the interaction. Likewise, the SNARE motifs of Bet1p, Bos1p and Gos1p strongly bound Sly1p, while the N-terminal regions did not (Figure 6C–E). We also examined the binding of Sly1(L140K)p to Ykt6p and found that it is its SNARE motif that interacts with the SM protein (Figure 6F). Therefore, this type of interaction differs from the binding of Sly1p to Sed5p in which case the N-terminal region of the syntaxin provides the short binding site for the SM protein.
As the SNARE motif regions are part of the four-helix bundle of trans-SNARE complexes, Sly1p is expected to be excluded from such complexes. To address this experimentally, we mixed GST-Sed5p immobilized on glutathione–Sepharose beads and His-tagged Bet1p and Bos1p, previously shown to form complexes in vitro (Tsui et al, 2001; Peng and Gallwitz, 2002), in the absence or presence of increasing amounts of Sly1(L140K)p, assuming that the binding of Sly1(L140K)p to Bet1p and Bos1p will interfere with binding of the nonsyntaxin SNAREs to Sed5p. This was in fact the case: as the concentration of Sly1(L140K)p increased, the amount of Bet1p and Bos1p bound to GST-Sed5p declined (Figure 7A). Likewise, during intra-Golgi core SNARE complex assembly in vitro, a decline of Sft1p, Ykt6p and Gos1p binding to Sed5p was observed with increasing concentrations of Sly1(L140K)p in the assembly reaction (Figure 7B), regardless of the duration of the reaction (Figure 7C).
Figure 7.
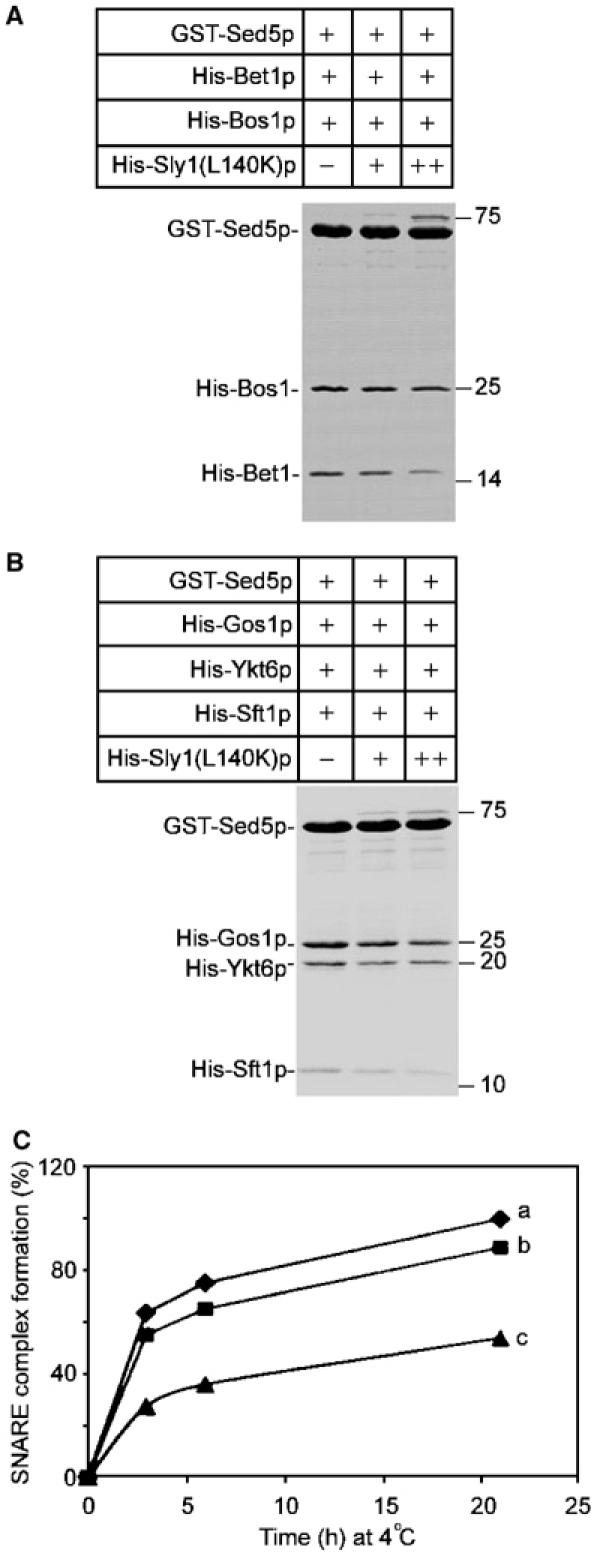
Excess of Sly1p inhibits nonsyntaxin SNARE incorporation into SNARE complexes in vitro. (A) GST-Sed5p (1 μM) bound to glutathione–Sepharose beads was incubated with Bet1p and Bos1p at equal molecular ratio in the absence or presence of increasing amounts of Sly1(L140K)p (0, 0.5 and 2 μM). Core SNARE complexes formed at 4°C overnight were analyzed by SDS–PAGE and Coomassie blue staining. Densitometric analysis of nonsyntaxin SNAREs at increasing Sly1(L140K)p concentrations gave the following ratios: Bos1p, 1/0.8/0.7; Bet1p, 1/0.7/0.4. (B) SNARE complexes with GST-Sed5p (1 μM), Gos1p, Ykt6p and Sft1p were formed and analyzed as in (A). Densitometric analysis of nonsyntaxin SNAREs at increasing Sly1(L140K)p concentrations gave the following ratios: Gos1p, 1/0.8/0.6; Ykt6p, 1/0.8/0.7; Sft1p, 1/0.7/0.4. (C) Kinetics of SNARE complex formation. GST-Sed5p (1 μM) was incubated at 4°C with equal amounts of Gos1p, Ykt6p and Sft1p in the absence (a), or presence of 0.5 μM (b) and 2 μM (c) Sly1(L140K)p. After 3, 6 and 21 h, assembled SNARE complexes were recovered on glutathione–Sepharose beads, separated by SDS–PAGE and stained with Coomassie blue. As a measure of SNARE complex formation, bands representing Sft1p were scanned densitometrically and the value obtained in the absence of Sly1(L140K)p at 21 h was taken as 100%.
In summary, our study demonstrates that in addition to its cognate syntaxin Sed5p, the SM protein Sly1p binds directly to distinct nonsyntaxin SNAREs involved in ER-to-Golgi and intra-Golgi transport. This newly discovered protein interaction is selective and involves the SNARE motif region of SNAREs. It is expected to have important functional implications.
Discussion
From studies of the past few years, it has been appreciated that different SM proteins and their corresponding syntaxins can interact in different ways. Set aside possible indirect interactions of some members of the two protein families in larger protein complexes (Gallwitz and Jahn, 2003), two prominent and so far best-studied examples are the bimolecular interactions of N-Sec1/Munc18 and syntaxin 1, relevant for neuronal secretion, and of Sly1p and the Golgi syntaxin Sed5p, critical for ER-to-Golgi and intra-Golgi transport in yeast. In both cases, binding of the SM protein to the corresponding syntaxin is characterized by its high affinity (Pevsner et al, 1994; Grabowski and Gallwitz, 1997). One would therefore have expected that this binding is of utmost biological significance. Indeed, transfection of mammalian cells with either the Sly1p-binding fragment of syntaxin 5, the homolog of the yeast Sed5p, or with the N-terminal Sed5p-interacting domain of Sly1p has been reported to cause Golgi fragmentation, presumably, as argued by the investigators, through interference of endogenous syntaxin 5 binding to Sly1p on Golgi membranes (Yamaguchi et al, 2002; Dulubova et al, 2003). Although effects on cell viability or protein transport were not analyzed in these studies, a recent investigation treating permeabilized cells with the Sly1p-binding N-terminal fragment of syntaxin 5 revealed that at high concentrations (mM) of the syntaxin 5 peptide, ER-to-Golgi transport could be inhibited by roughly 50% compared to controls (Williams et al, 2004). All three studies concluded that an intimate binding of Sly1p to syntaxin 5 is biologically important and required for transport in the early secretory pathway, although direct evidence for this conclusion could not be furnished.
The investigation with the yeast system that we have performed took advantage of the possibility to create cells that relied solely on either mutant Sly1p or Sed5p unable to bind its specific partner. Two of such disabled proteins, Sly1(L140K)p having lost its binding capacity for Sed5p, and Sed5(F10A)p lacking the ability to interact with Sly1p, were studied in detail and found to allow cells to grow and transport proteins completely undisturbed. Although in binding studies with purified bacterially produced proteins, traces of the mutant Sly1(L140K)p were often seen to be associated with matrix-bound Sed5p (see Figure 2A), binding of either Sly1(L140K)p to Sed5p or of Sed5(F10A)p to wild-type Sly1p was completely abolished in vivo as analyzed by co-precipitations from mutant cell lysates and protein identification on sensitive immunoblots. Even sly1(L140K)/sed5(F10A) double-mutant cells grew and secreted invertase normally. This is compelling evidence for the notion that the tight binding of Sly1p to the syntaxin Sed5p is unimportant with regard to the functioning of the two essential proteins. It is noteworthy in this context that neither of the two mutant proteins made its binding partner dispensable.
It is pertinent to ask what purpose the tight interaction of Sly1p and Sed5p serves in normally growing cells. Previous cell-free transport studies (Cao et al, 1998; Cao and Barlowe, 2000) indicated that Sly1p acts after transport vesicle tethering to Golgi membranes and in trans-SNARE complex formation and membrane fusion. Furthermore, in vitro studies showed that Sly1p bound to Sed5p can prevent the assembly of nonphysiological SNARE complexes (Peng and Gallwitz, 2002), which also supports the assumption that the SM protein's essential function is connected to the conformational cycle of SNAREs. Therefore, tight binding of Sly1p to the membrane-anchored Sed5p, which at steady state is primarily localized to Golgi membranes (Hardwick and Pelham, 1992; Banfield et al, 1994), could provide an efficient way to recruit the Sly1 protein to, or to keep it at, potential sites for trans-SNARE complex formation and membrane fusion. Surprisingly, interference with the tight interaction of Sly1p and Sed5p had only a moderate effect on Golgi localization of Sly1p, suggesting that other mechanism(s) exist(s) to recruit the SM protein to the Golgi target membrane. One such mechanism could be the binding of Sly1 wild-type and mutant proteins to specific nonsyntaxin SNAREs that we have discovered in the present study. Bet1p and Bos1p of the ER-to-Golgi SNARE complex, and Sft1p and Gos1p of the intra-Golgi complex bound Sly1p with their SNARE motif region, and it therefore appears that Sly1p–nonsyntaxin SNARE interactions are transient and should be interrupted after four-helix bundles in trans-SNARE complexes have been fully assembled. We had previously shown that the kinetics of core SNARE complex formation in vitro is not influenced by Sly1p bound to Sed5p (Peng and Gallwitz, 2002). However, SNARE assembly reactions performed in the present study were inhibited when increasing amounts of Sly1(L140K)p were mixed simultaneously with the SNAREs (Figure 7). This inhibition was presumably caused by making unavailable for complex formation a fraction of nonsyntaxin SNAREs bound to the SM protein. In living cells, association with Golgi membranes of mutant Sly1p unable to bind Sed5p could therefore be mediated by their binding to nonsyntaxin SNAREs residing on target membranes or on tethered transport vesicles. This does not exclude the possibility that the association of Sly1 mutant proteins to Golgi membranes might also involve other specific protein–protein interactions.
The apparent affinity of Sly1p for nonsyntaxin SNAREs as compared to that for Sed5p is certainly lower as revealed by in vitro binding analyses. Under the conditions used, only substoichiometric amounts of the nonsyntaxin SNAREs were recovered from the GST-Sly1p affinity beads and had to be identified on immunoblots. It is possible therefore that the dissociation rates of the Sly1p–nonsyntaxin SNARE interactions are higher than that of the Sly1p–Sed5p interactions. According to published (Yamaguchi et al, 2002) and our unpublished results, the same seems to hold for the interaction between Sly1p and the ER-localized syntaxin Ufe1p, which is of lower apparent affinity than Sly1p–Sed5p binding.
SNARE assembly is a complex process, which in vitro proceeds very slowly (Nicholson et al, 1998; Peng and Gallwitz, 2002; Fasshauer and Margittai, 2004). The slow assembly of exocytic complexes from neurons or yeast cells occurs stepwise whereby the plasma membrane-localized syntaxins, syntaxin 1 and Sso1p, respectively, and the nonsyntaxin SNAREs, SNAP-25 and Sec9p, respectively (each providing two SNARE motif regions for the final four-helix bundle), first associate to apparently form an only partly structured three-helix bundle (Fiebig et al, 1999). This structure then serves as an efficient binding site for the v-SNAREs synaptobrevin and Snc1p, respectively. The same is expected to hold for the assembly of ER-to-Golgi and intra-Golgi SNARE complexes where according to in vitro analyses, Sed5p/Bos1p/Sec22p and Sed5p/Gos1p/Ykt6p are the respective complexes on the target membrane, which bind their cognate vesicular SNAREs Bet1p and Sft1p, respectively (Parlati et al, 2000, 2002). Clearly, because of the very slow in vitro assembly of SNARE complexes, it must be assumed that, in vivo, acceleration and specificity of the assembly reaction is governed by distinct factors. Sly1p and/or associated proteins are likely candidates for such a role.
The newly discovered, remarkably specific interaction of Sly1p with nonsyntaxin SNAREs, which is not affected by mutating the Sed5p-binding site, must involve other regions of the SM protein than the hydrophobic pocket in domain I. This provides an attractive molecular mechanism for specific recognition of SNAREs assembling into fusogenic complexes whereby, in an initial step, Sly1p and/or associated proteins would bridge the t-SNARE complexes with their cognate v-SNAREs Bet1p or Sft1p, respectively. Under conditions where the SM protein lacks the ability to interact with the syntaxin, mutant Sly1p (and possibly associated proteins) bound to nonsyntaxin SNAREs Bos1p or Gos1p in partially structured t-SNARE complexes on the one side and to the corresponding v-SNAREs Bet1p or Sft1p on the other, would, after vesicle tethering, allow (I) the recognition of specific SNAREs to be paired, and (II) to catalyze their rapid assembly. It seems well possible that the v-SNAREs Bet1p and Sft1p and their corresponding nonsyntaxin t-SNAREs (Bos1p and Gos1p) have different binding sites on Sly1p, which would allow one SM protein molecule to bridge the two SNAREs in trans-SNARE assembly. An example for a protein that binds different SNAREs on different sites is the COPII component Sec24p (Miller et al, 2003; Mossessova et al, 2003).
From the experimental results described here, it follows that the SM protein Sly1 is not an obligatory component of trans-SNARE complexes, making unlikely a role in regulating membrane fusion after SNARE complex assembly (Carr et al, 1999). It rather appears possible that after vesicle tethering, the engagement of specific v- and t-SNAREs on opposite membranes might involve Sly1p regardless of whether it is bound to syntaxin or not. Since the SM protein is absolutely essential, this view leads to the prediction that abolishing Sly1p binding to both v- and t-SNAREs should significantly interfere with trans-SNARE complex assembly and hence with protein transport to and within the Golgi.
Materials and methods
Construction of plasmids and yeast strains
Domain I of Sly1p (residues 1–167), the entire cytoplasmic regions of Bet1p (1–118), Bos1p (1–222), Gos1p (1–204), Sft1p (1–78), Tlg1p (1–204), Vti1p (1–194), Ykt6p (1–200) and their truncations were amplified from genomic DNA by standard PCR. The PCR products were subcloned into pGEX-KG (Hakes and Dixon, 1992) for bacterial expression of GST fusion proteins, and into pET-19b (Novagen; for Gos1p and Sft1p), or pQE32 (Qiagen; for Ykt6p) for His-tagged proteins. Constructs expressing GST- and His-tagged Sly1p (full-length), GST- and His6-Sed5p (1–324), His6-Bet1p, His6-Bos1p, His6-Sec22p and GST-Sec22p without transmembrane domains have been described (Peng and Gallwitz, 2002). Plasmids expressing GST-Nyv1p (full-length) and GST-Sft1p (17–78) were provided by T Söllner (New York) and D Banfield (Hong Kong). The in vitro site-directed mutagenesis was performed by the QuickChange™ Site-Directed Mutagenesis Kit (Stratagene).
Yeast strains used in this study were as follows: MSUC3D (MATα ura3 leu2 his3 trp1 lys2), RPY238 (MATα sly1(L140K)∷LEU2 ura3 leu2 his3 trp1 lys2), RPY142 (MATα 2XGFP-SLY1 ura3, leu2, his3, trp1, lys2), RPY220 (MATa 2XGFP-sly1(L140K)∷LEU2 ura3 leu2 his3 trp1 lys2), RPY108 (MATα sly1-1 ura3 leu2 his3 trp1 lys2), YTX50 (MATa sec18-1 ura3 leu2 his3), RPY237 (MATa sec18-1 sly1(L140K)∷LEU2 ura3 leu2 his3 trp1), PAY702-4 (MATa trp1∷glc7-13∷TRP1 ade2-1 his3-11,15 leu2-3 trp1-1 ura3-1 can1-100 ssd1-d2 glc7∷LEU2 GAL+ FUS3 KSS1) and RPY239 (MAT a trp1∷glc7-13∷TRP1 sly1(L140K)∷LEU2 ura3 leu2 his3 trp1 lys2 glc7∷LEU2). Also, the sed5 deletion strain (Mata/a; his3D1/his3D1; leu2D0/leu2D0; lys2D0/LYS2; MET15/met15D0; ura3D0/ura3D0; sed5∷kanMX4/SED5), RPY214 (MATa his3D leu2D ura3D sed5∷kanMX4 pSED5, CEN, LEU2), RPY215 (MATa his3D leu2D ura3D sed5∷kanMX4 pSED5(F10A), CEN, LEU2), RPY216 (MATα his3D leu2D ura3D sed5∷kanMX4 pSED5(V14A), CEN, LEU2) and the double sed5/sly1 mutant strain RPY249 (MATa ura3 leu2 his3 lys2 sly1(L140K)∷LEU2 sed5∷kanMX4 pSED5(F10A), CEN, URA3) were used. Original ypt1-3, sec23-1 and uso1-1 mutants were obtained from H Riezman (Geneva) and G Waters (Princeton). Yeast strains were generated by standard genetic methods.
Protein expression, purification and pulldown assays
GST- and His-tagged proteins were purified as described (Peng and Gallwitz, 2002). GST pulldown experiments were performed as described (Peng and Gallwitz, 2002), except for Figure 6 where modifications were introduced. Briefly, 10 μl of 50% glutathione–Sepharose slurry (Pharmacia) was mixed with excessive amounts of GST fusion proteins at 4°C for 60 min to saturate the available binding sites on the beads. Beads were washed three times with buffer F (50 mM Tris–HCl, pH 7.5, 150 mM KCl, 10% glycerol, 0.5% Triton X-100, 2 mM β-mercaptoethanol) and mixed with purified His-tagged Sly1p or Sly1p(L140K)p in a total volume of 100 μl in buffer F. The reaction proceeded for 90 min at 4°C before five times washing with buffer F (500 μl each). Proteins bound on beads were eluted with SDS sample buffer, analyzed by SDS–PAGE and Coomassie blue staining or Western blotting.
Antibodies and immunoprecipitation
Antibodies against Sly1p, Sed5p, Bos1p, Bet1p, Sec22p and Emp47p have been described (Schröder et al, 1995; Peng and Gallwitz, 2002). Polyclonal antiserum against Ykt6p was made from rabbits. Anti-Gos1p and Sft1p antisera were provided by T Söllner (New York) and D Banfield (Hongkong). Monoclonal anti-GFP antibodies were purchased from Roche.
Immunoprecipitation experiments were performed as described (Peng and Gallwitz, 2002). For evaluation of temperature effects on the in vivo SNARE complex assembly, cells were first grown at 25°C to mid-log phase before incubation at nonpermissive temperatures for the time periods indicated in the figures.
Fluorescence microscopy
Cells expressing GFP fusions were grown in YPG medium. The fluorescence was viewed in living cells. Briefly, mid-log-phase cells were collected and washed once with sterile, distilled water, and three times with ice-cold PBS buffer. The washed cells were resuspended in ice-cold PBS buffer. Cell suspension (1.5 μl) was dropped on a 76 × 26 mm microscope slide, covered with a coverslip and subjected to immediate viewing. Fluorescence microscopy was performed with an Axioplan microscope equipped with a × 100 oil-immersion objective (Carl Zeiss Inc.) and with an FITC filter (excitation 485 nm, emission 535 nm). Images were recorded with a photometric CCD camera and processed by Photoshop software. Indirect immunofluorescence was performed as described (Schröder et al, 1995). Differently stained images were superimposed using analysis 3.0® software (Soft Imaging System GmbH, Münster, Germany).
Analysis of CPY maturation and invertase secretion
Pulse-chase experiments with carboxypeptidase Y (CPY) and native staining of invertase were performed as described (Peng et al, 2000).
In vitro SNARE complex assembly and interaction with wild-type and mutant Sly1 protein
To form complexes, GST-Sed5p (10 μg) was incubated with cognate SNAREs Bet1p, Bos1p, Sec22p or with Gos1p, Ykt6p and Sft1p in buffer F. Generally, the reaction proceeded at 4°C for 17–20 h before 10 μl of 50% glutathione–Sepharose slurry was added. The reaction continued for an additional 60 min. Beads were washed five times with buffer F. The protein complexes on beads were subjected to SDS–PAGE and Coomassie blue staining.
Binding of wild-type and mutant Sly1p to preformed SNARE complexes was carried out in 100 μl of buffer F by rotation at 4°C overnight. Beads were then washed, and bound proteins were analyzed by SDS–PAGE and Coomassie blue staining.
For the competition experiments in Figure 7, GST-Sed5p bound to glutathione–Sepharose beads was incubated with multiple SNARE proteins at an equal molar ratio. The binding reactions proceeded at 4°C for different periods of time in the absence or presence of increasing amounts of Sly1(L140K)p. After extensive washing, proteins bound to beads were eluted and analyzed by SDS–PAGE and Coomassie blue staining. The protein amount assembled into SNARE complexes was determined by densitometry (Peng and Gallwitz, 2002).
Acknowledgments
We thank Reinhard Jahn for comments on the manuscript, Peter Mienkus for technical assistance and Thomas Söllner, Andrea Mayer, Howard Riezman, David Banfield and Gerard Waters for antisera, yeast strains and plasmids. We thank Hans-Dieter Schmitt for discussions and Uwe Andag for purified GST-Sed5p. This work was supported by the Max Planck Society and by grants to DG from the Human Frontier Science Program and Fonds der Chemischen Industrie.
References
- Antonin W, Fasshauer D, Becker S, Jahn R, Schneider TR (2002) Crystal structure of the endosomal SNARE complex reveals common structural principles of all SNAREs. Nat Struct Biol 9: 107–111 [DOI] [PubMed] [Google Scholar]
- Banfield DK, Lewis MJ, Rabouille C, Warren G, Pelham HR (1994) Localization of Sed5, a putative vesicle targeting molecule, to the cis-Golgi network involves both its transmembrane and cytoplasmic domains. J Cell Biol 127: 357–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracher A, Weissenhorn W (2002) Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J 21: 6114–6124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant NJ, James DE (2003) The Sec1p/Munc18 (SM) protein, Vps45p, cycles on and off membranes during vesicle transport. J Cell Biol 161: 691–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Ballew N, Barlowe C (1998) Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J 17: 2156–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Barlowe C (2000) Asymmetric requirements for a Rab GTPase and SNARE proteins in fusion of COPII vesicles with acceptor membranes. J Cell Biol 149: 55–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr CM, Grote E, Munson M, Hughson FM, Novick P (1999) Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol 146: 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YA, Scheller RH (2001) SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98–106 [DOI] [PubMed] [Google Scholar]
- Dascher C, Ossig R, Gallwitz D, Schmitt HD (1991) Identification and structure of four yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the RAS superfamily. Mol Cell Biol 11: 872–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilcher M, Kohler B, von Mollard GF (2001) Genetic interactions with the yeast Q-SNARE VTI1 reveal novel functions for the R-SNARE YKT6. J Biol Chem 276: 34537–34544 [DOI] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J (1999) A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J 18: 4372–4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Arac D, Li H, Huryeva I, Min SW, Rizo J, Sudhof TC (2003) Convergence and divergence in the mechanism of SNARE binding by Sec1/Munc18-like proteins. Proc Natl Acad Sci USA 100: 32–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Yamaguchi T, Gao Y, Min SW, Huryeva I, Sudhof TC, Rizo J (2002) How Tlg2p/syntaxin 16 ‘snares' Vps45. EMBO J 21: 3620–3631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D, Margittai M (2004) A transient N-terminal interaction of SNAP-25 and syntaxin nucleates SNARE assembly. J Biol Chem 279: 7613–7621 [DOI] [PubMed] [Google Scholar]
- Fiebig KM, Rice LM, Pollock E, Brunger AT (1999) Folding intermediates of SNARE complex assembly. Nat Struct Biol 6: 117–123 [DOI] [PubMed] [Google Scholar]
- Gallwitz D, Jahn R (2003) The riddle of the Sec1/Munc-18 proteins—new twists added to their interactions with SNAREs. Trends Biochem Sci 28: 113–116 [DOI] [PubMed] [Google Scholar]
- Grabowski R, Gallwitz D (1997) High-affinity binding of the yeast cis-Golgi t-SNARE, Sed5p, to wild-type and mutant Sly1p, a modulator of transport vesicle docking. FEBS Lett 411: 169–172 [DOI] [PubMed] [Google Scholar]
- Hakes DJ, Dixon JE (1992) New vectors for high level expression of recombinant proteins in bacteria. Anal Biochem 202: 293–298 [DOI] [PubMed] [Google Scholar]
- Hardwick KG, Pelham HR (1992) SED5 encodes a 39-kD integral membrane protein required for vesicular transport between the ER and the Golgi complex. J Cell Biol 119: 513–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Lang T, Sudhof TC (2003) Membrane fusion. Cell 112: 519–533 [DOI] [PubMed] [Google Scholar]
- Kweon Y, Rothe A, Conibear E, Stevens TH (2003) Ykt6p is a multifunctional yeast R-SNARE that is required for multiple membrane transport pathways to the vacuole. Mol Biol Cell 14: 1668–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNew JA, Sogaard M, Lampen NM, Machida S, Ye RR, Lacomis L, Tempst P, Rothman JE, Sollner TH (1997) Ykt6p, a prenylated SNARE essential for endoplasmic reticulum–Golgi transport. J Biol Chem 272: 17776–17783 [DOI] [PubMed] [Google Scholar]
- Miller EA, Beilharz TH, Malkus PN, Lee MC, Hamamoto S, Orci L, Schekman R (2003) Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114: 497–509 [DOI] [PubMed] [Google Scholar]
- Misura KM, Scheller RH, Weis WI (2000) Three-dimensional structure of the neuronal-Sec1–syntaxin 1a complex. Nature 404: 355–362 [DOI] [PubMed] [Google Scholar]
- Mossessova E, Bickford LC, Goldberg J (2003) SNARE selectivity of the COPII coat. Cell 114: 483–495 [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Holthuis JC, Pelham HR (1998) The Sec1p homologue Vps45p binds to the syntaxin Tlg2p. Eur J Cell Biol 77: 263–268 [DOI] [PubMed] [Google Scholar]
- Nicholson KL, Munson M, Miller RB, Filip TJ, Fairman R, Hughson FM (1998) Regulation of SNARE complex assembly by an N-terminal domain of the t-SNARE Sso1p. Nat Struct Biol 5: 793–802 [DOI] [PubMed] [Google Scholar]
- Novick P, Schekman R (1979) Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci USA 76: 1858–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossig R, Dascher C, Trepte HH, Schmitt HD, Gallwitz D (1991) The yeast SLY gene products, suppressors of defects in the essential GTP-binding Ypt1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol Cell Biol 11: 2980–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F, McNew JA, Fukuda R, Miller R, Söllner TH, Rothman JE (2000) Topological restriction of SNARE-dependent membrane fusion. Nature 407: 194–198 [DOI] [PubMed] [Google Scholar]
- Parlati F, Varlamov O, Paz K, McNew JA, Hurtado D, Sollner TH, Rothman JE (2002) Distinct SNARE complexes mediating membrane fusion in Golgi transport based on combinatorial specificity. Proc Natl Acad Sci USA 99: 5424–5429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng R, De Antoni A, Gallwitz D (2000) Evidence for overlapping and distinct functions in protein transport of coat protein Sec24p family members. J Biol Chem 275: 11521–11528 [DOI] [PubMed] [Google Scholar]
- Peng R, Gallwitz D (2002) Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J Cell Biol 157: 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Andrews PD, Stark MJ, Cesaro-Tadic S, Glatz A, Podtelejnikov A, Mann M, Mayer A (1999) Control of the terminal step of intracellular membrane fusion by protein phosphatase 1. Science 285: 1084–1087 [DOI] [PubMed] [Google Scholar]
- Pevsner J, Hsu SC, Scheller RH (1994) n-Sec1: a neural-specific syntaxin-binding protein. Proc Natl Acad Sci USA 91: 1445–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Whitters EA, Stevens TH (1994) Yeast Vps45p is a Sec1p-like protein required for the consumption of vacuole-targeted, post-Golgi transport vesicles. Eur J Cell Biol 65: 305–318 [PubMed] [Google Scholar]
- Sato TK, Rehling P, Peterson MR, Emr SD (2000) Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell 6: 661–671 [DOI] [PubMed] [Google Scholar]
- Schröder S, Schimmöller F, Singer-Krüger B, Riezman H (1995) The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret-1 mutation in alpha-COP. J Cell Biol 131: 895–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price AA (2000) Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA 97: 9402–9407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Søgaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Sollner T (1994) A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell 78: 937–948 [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brünger AT (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature 395: 347–353 [DOI] [PubMed] [Google Scholar]
- Tsui MM, Tai WC, Banfield DK (2001) Selective formation of Sed5p-containing snare complexes is mediated by combinatorial binding interactions. Mol Biol Cell 12: 521–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE (1998) SNAREpins: minimal machinery for membrane fusion. Cell 92: 759–772 [DOI] [PubMed] [Google Scholar]
- Williams AL, Ehm S, Jacobson NC, Xu D, Hay JC (2004) rsly1 binding to syntaxin 5 is required for endoplasmic reticulum-to-Golgi transport but does not promote SNARE motif accessibility. Mol Biol Cell 15: 162–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Sudhof TC (2002) Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell 2: 295–305 [DOI] [PubMed] [Google Scholar]
- Yang B, Steegmaier M, Gonzalez L Jr, Scheller RH (2000) nSec1 binds a closed conformation of syntaxin1A. J Cell Biol 148: 247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]


