Abstract
The view that only the production and deposition of Aβ plays a decisive role in Alzheimer's disease has been challenged by recent evidence from different model systems, which attribute numerous functions to the amyloid precursor protein (APP). To investigate the potential cellular functions of APP and its paralogs, we use transgenic Drosophila as a model. Upon overexpression of the APP-family members, transformations of cell fates during the development of the peripheral nervous system were observed. Genetic analysis showed that APP, APLP1 and APLP2 induce Notch gain-of-function phenotypes, identified Numb as a potential target and provided evidence for a direct involvement of Disabled and Neurotactin in the induction of the phenotypes. The severity of the induced phenotypes not only depended on the dosage and the particular APP-family member but also on particular domains of the molecules. Studies with Drosophila APPL confirmed the results obtained with human proteins and the analysis of flies mutant for the appl gene further supports an involvement of APP-family members in neuronal development and a crosstalk between the APP family and Notch.
Keywords: Alzheimer, APP, Drosophila, Notch, Numb
Introduction
The APP, whose proteolytic fragment Aβ accumulates in the brain of Alzheimer's disease (AD) patients, is the founding member of the APP protein family. In mice as well as in humans, a single APP and two APP-like genes (APLP1, APLP2), encoding type I transmembrane proteins, have been identified. APP-family members have been implicated in many processes regulating neuronal activity (see Turner et al, 2003). Inactivation of the APP gene in mice does not result in lethality, but rather in changes in locomotion and memory impairment. These functional studies have been complicated by a partial redundancy among the three APP-family members. Whereas double knockouts of APP–APLP1 are viable, APP–APLP2 and APLP1–APLP2 double knockouts are lethal with no obvious phenotype (Heber et al, 2000).
In this light, it is intriguing that mutations in the Drosophila APP homolog appl have been correlated to a neurodegenerative phenotype. Although in Drosophila the appl gene seems not to be required for viability (Luo et al, 1992), loss- and gain-of-function studies have revealed a role for APPL in synapse differentiation and in axonal transport (Torroja et al, 1999a, 1999b; Gunawardena and Goldstein, 2001). Recently, Tschape et al (2002) have shown that the neurodegenerative phenotype in the mutant loechrig is strongly enhanced by appl mutants.
The identification of proteins that bind to the highly conserved intracellular domain (ICD) provided another source of insight into the functions of the APP family. Proteins containing phosphotyrosine-binding domains (PTB), like mouse and human homologs of Disabled (Dab-1, Dab-2), X11α and Fe65, can bind to the NPTY motif of the APP family and regulate trafficking, processing and transcriptional modulation (see Turner et al, 2003). Roncarati et al (2002) show a binding of APP to Numb and Numb-like in mouse brain lysates and an interaction with Notch signaling in cell culture. This is an interesting result since the processing of APP shows many hallmarks of Notch receptor-related signal transduction mechanisms (see Selkoe and Kopan, 2003). Notch signaling itself is important for the development of many organs and tissues by determining cell fates.
An important pathological feature of AD is the formation of senile plaques by the deposition of Aβ peptides and the formation of neurofibrillary tangles in the brain. However, the fact that in earlier APP-transgenic mouse models amyloid plaques are never accompanied by tangles and that in a new triple-transgenic model synaptic dysfunctions manifest prior to plaque and tangle formation makes it likely that these events might only play a role in later stages of AD (see Price et al, 1998; Oddo et al, 2003). Therefore, potential neurotoxic effects of Aβ peptides, accumulating C-terminal fragments (CTFs) and/or by impaired APP functions, have been suggested. Influences on APP processing could also result in the accumulation of full-length molecules or unusual CTFs from all APP-family members, since the processing of APP, APLP1 and APLP2 is very similar (Scheinfeld et al, 2002). In consequence, gain- or loss-of-function phenotypes could be induced of all APP-family members, and it will be of crucial interest for the estimation of the possible side effects of therapies and for the understanding of the primary events inducing late-onset AD, to uncover and understand all possible functions of the APP family and fragments thereof.
To address the question of APP function with respect to the development of a whole organism, we have chosen Drosophila melanogaster as a model system and gain-of-function genetics as a tool (Fossgreen et al, 1998). In this report, we demonstrate by loss- and gain-of-function experiments that the human APP-family members APP, APLP1, APLP2 and Drosophila APPL can interfere with the development of the Drosophila PNS by inducing Notch gain- and loss-of-function phenotypes in the mechano-sensory organs (MSOs). Our results also suggest that the phenotypes observed are the consequence of a putative crosstalk between the APP family and the Notch pathway, with Numb and Dab playing central roles as mediators.
Results
APP affects MSO development
We have previously reported that the expression of human APP in Drosophila induces a blistered wing phenotype (Fossgreen et al, 1998). In order to identify additional developmental processes affected by the expression of APP-family members, we used a broad range of GAL4 driver lines allowing the expression in different tissues, and observed an influence of the APP family on the development and differentiation of adult MSOs. These phenotypes were first observed using the apterous-GAL4 driver (ap-GAL4), an enhancer trap line expressing GAL4 in the dorsal compartment of the wing imaginal disc. For subsequent studies, exclusive expression in the sensory organ cell lineage was achieved by the use of scabrous-GAL4 (sca-GAL4; Kramer et al, 1995).
In Drosophila, each MSO of the adult thorax is derived from a sensory organ precursor cell (SOP), which itself is singled out by lateral inhibition from a group of ectodermal cells (Figure 1A; reviewed by Lai and Orgogozo, 2004). During this process, the presumptive SOP activates the Notch receptor of the neighboring cells, forcing them into epidermal fate. Within the progeny of the SOP, cellular diversity is ensured by differential activation of Notch signaling through the asymmetric segregation of Numb and Pon, Partner of Numb (see Schweisguth, 2004). Consequently, interference with Notch signaling during MSO development results in a variety of phenotypes depending on the penetrance and the time point when a gain or a loss in Notch function is introduced. During lateral inhibition, a Notch gain-of-function results in the loss of MSOs by inducing epidermal fate in all cells. A gain in Notch function during the first division of the SOP transforms the pIIb lineage into pIIa fate, thereby duplicating the external cells if normal Notch signaling is restored. This is accompanied by a loss of the internal cells (Figure 1B). However, if the gain in Notch function persists, all cells are transformed into socket cells (Figure 1C).
Figure 1.
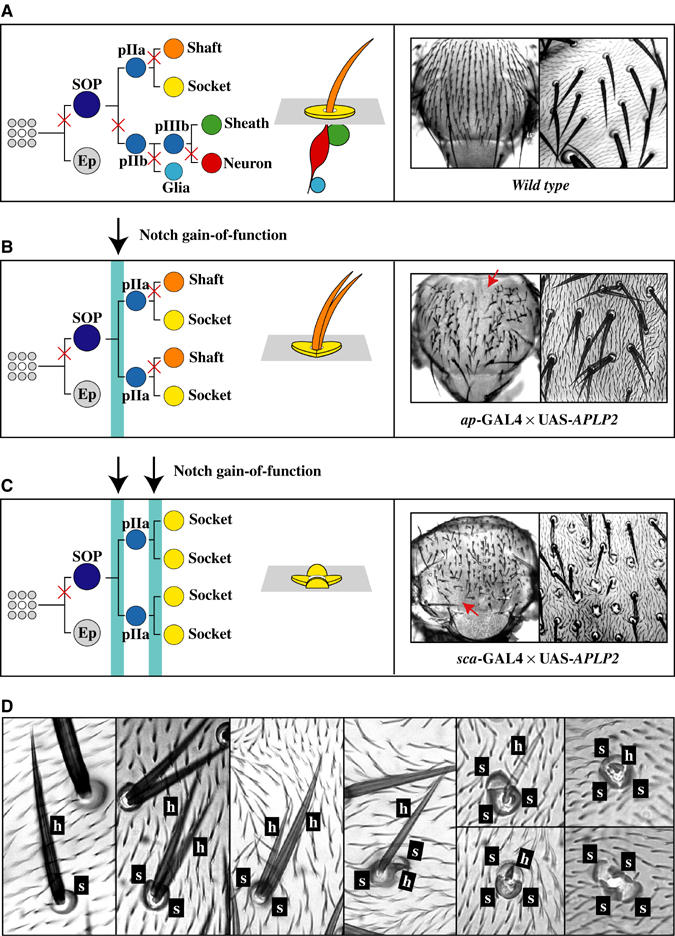
MSO formation in wt Drosophila and in flies expressing APP, APLP1 and APLP2. (A) Outline of the cell lineage which gives rise to a MSO. At each division, the asymmetric segregation of Numb protects one of the progeny from the activation of Notch (indicated by a cross). The MSOs on the thorax of a wt fly are displayed. (B) Outline of the phenotype induced by a gain in Notch function during the first division of the SOP. The MSOs on the thorax of a fly expressing APLP2 are shown. (C) Outline of the phenotype when a gain in Notch function is induced during all the divisions of a SOP. The MSOs on the thorax of a fly expressing APLP2 are displayed. (D) Overview of the different possible structures of the external cells of MSOs on the thorax of flies expressing members of the human APP family displayed in high magnification. Ep, epidermal cell; pIIa/b, primary precursor cell IIa/b; h, hair; s, socket.
Expression of human APP, APLP1, APLP2 and APP/APLP2 (a chimera between APP and APLP2; APLP2 sequences replace the APP.ICD and the Aβ domain) during MSO development causes transformations of cell lineages (Figures 1B–D and 2C). We observed duplicated shaft and socket cells as well as transformations from shafts to sockets, resembling known Notch gain-of-function phenotypes. The most extreme phenotype induced was the formation of patches of naked cuticle (Figure 1B and C, arrows). In addition, we noticed a broad range of Notch gain-of-function phenotypes resulting in SOPs with different-sized shafts and sockets exhibiting the possible stages of transformations from a shaft to a socket cell (Figure 1D). As shown in Figure 2A–D, the observed cell fate transformations depend on the dosage of the expressed APP-family transgenes, and different APP-family members show diverse strengths of activity. Whereas only high expression levels of APP resulted in MSO phenotypes, such phenotypes were obtained with lower levels of APLP1, and at even lower levels of APLP2. In agreement with the appearance of the external phenotypes, visualization of the internal neuronal and sheath cells of the MSOs by antibody staining of pupal nota revealed a reduced number of internal cells in these flies (Figure 2C).
Figure 2.
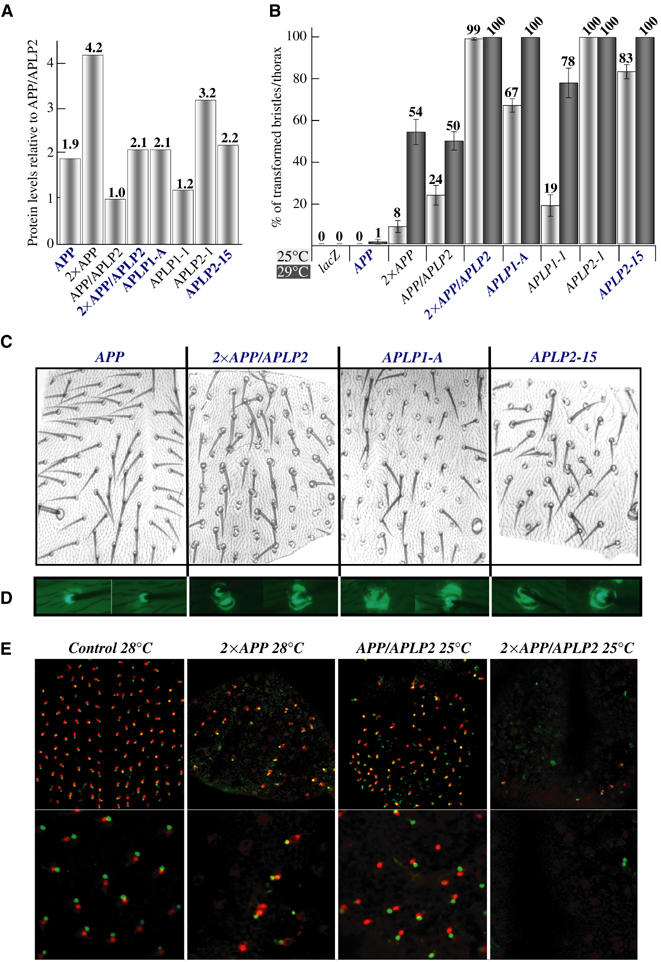
The severity of visible phenotypes induced by APP-family members during MSO development depends on the amount and nature of the expressed proteins. (A) Quantification of the protein amounts expressed by different transgenic lines. Marked with blue are all the lines which have comparable expression levels. For details see Supplementary data. (B) Quantification of the amount of MSOs with an increased number of external cells in flies expressing different members of the APP family. In this and in the following figures, MSOs on the thorax have been studied and sca-GAL4 has been used if not otherwise stated. Raising the flies at 28–29°C, the temperature at which GAL4 induction is most efficient, increases expression of the constructs and the severity of the phenotypes. (C) Examples from (B) are displayed. (D) Expression of EGFP under the ASE5 enhancer visualizes socket cell identities (Barolo et al, 2000) in the transformed MSOs from C. (E) Immunostaining of pupal nota for the neuronal marker Elav (red) and the sheath cell-specific marker Prospero (green) visualizes the number of internal MSO cells in APP or APP/APLP2-expressing flies. In this and in the following figures, 2 × indicates the usage of two copies of an UAS transgene.
Genetic interaction between APP and Notch
To generate flies with an intermediate bristle phenotype, which could be used for genetic interaction assays, an APP/APLP2 transgene was recombined onto the sca-GAL4 chromosome (named sca-APP/APLP2). This line displayed bristle phenotypes which could be enhanced or suppressed. Gain- or loss-of-function mutations in genes encoding known members of the Notch signaling cascade were crossed to these sca-APP/APLP2 flies, and the effect on the bristle phenotype was scored. A reduction in Notch signaling (N55e11; Suppressor of Hairless, Su(H)SF8) suppressed the APP/APLP2 induced phenotypes (Figure 3A and B). In contrast, a rise in Notch signaling (Nspl1; numb15; Hairless, HP8) enhanced the phenotypes. To confirm that the genetic interactions are not restricted to the APP/APLP2-induced phenotypes, sca-GAL4 was recombined onto Su(H) and numb mutant chromosomes, or introduced into Notch and H mutant lines. These lines were then crossed with APP and APLP1, revealing identical genetic interactions (Figure 3A and B).
Figure 3.
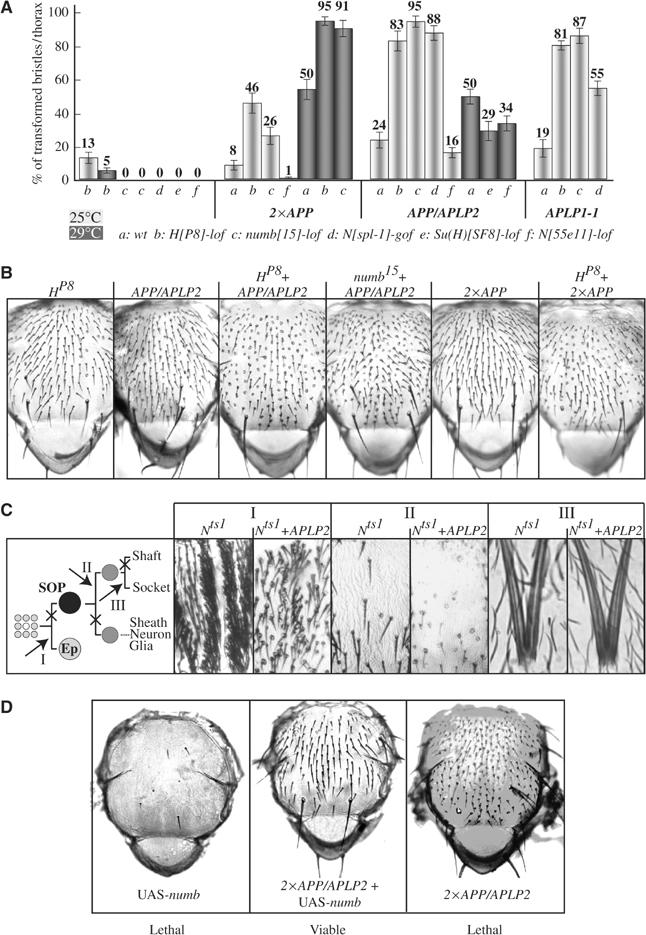
APP-family members interact genetically with components of the Notch signaling pathway. (A) Quantification of the sensitivity of the APP-family induced phenotypes to an increase or loss in Notch signaling. (B) Examples from (A) are visualized. (C) Notch loss-of-function phenotypes induced at three different time points (I–III) during MSO formation in a wt and APLP2 overexpression background. (D) Phenotypes induced by the overexpression of Numb, co-expression of Numb and APP/APLP2, and expression of APP/APLP2 alone.
To determine whether APP-family members directly target the Notch pathway, sca-GAL4 was combined with a Notch loss-of-function temperature-sensitive allele (Nts1). Incubation of these flies at the restrictive temperature of 29°C during different time points of MSO formation resulted in typical Notch loss-of-function phenotypes, which could not be rescued by overexpression of APLP2 (Figure 3C). Nevertheless, APLP2 was able to induce transformations in any wild-type (wt) MSO which developed in this genetic background due to temporal asynchrony (Figure 3C-II). The genetic interaction between sca-APP/APLP2 and numb, a negative regulator of Notch, was further investigated using UAS-numb transgenes (Figure 3B). Overexpression of Numb in the SOP inhibits bristle formation, thus displaying bold patches of cuticle, and the flies die shortly after hatching. In contrast to the Notch-ts phenotypes, this phenotype was suppressed by the expression of high levels of APP/APLP2. At the same time, the APP/APLP2-induced bristle phenotypes were also inhibited, as indicated by the presence of wt bristles in the now viable Numb-APP/APLP2 co-expressing flies.
In summary, our genetic studies indicate that APP-family members directly target the Notch signaling pathway during MSO development. The fact that functional Notch receptors are required for the induction of the phenotypes and that APP/APLP2 can overcome ectopically expressed Numb in its inhibition of Notch provides evidence that APP-family members may act at the level of Numb.
The NPTY motif of APP is essential for the induction of Notch gain-of-function phenotypes
To identify protein domains and motifs in the amino-acid sequence of APP required for the induction of bristle phenotypes, mutant constructs were generated (Figure 4A). The functional properties of these constructs were then tested in an enhancement assay (Figure 4B and C). The intermediate bristle phenotype of sca-APP/APLP2 flies was enhanced by an additional copy of wt APP or otherwise functional APP constructs. In contrast, APP molecules with mutations in essential domains had no effect (nonfunctional). From the results obtained with this assay and with our set of APP constructs, we conclude that the extracellular domain (ECD) and the ICD are important for the interference of APP with MSO development. Deletion of one of these domains rendered the protein into a nonfunctional form. Interestingly, within the ICD, only the NPTY motif proved to be essential for the particular function tested. Point mutations as well as a deletion of this motif failed to enhance the APP/APLP2-induced phenotype (Figure 4B). Most strikingly, the deletion of the NPTY motif or the ICD behaved as dominant-negative forms of APP, and were able to suppress the phenotype induced by wt APP/APLP2 in a dosage-sensitive and statistically significant manner (Figure 4C and D). Furthermore, whereas the expression of functional constructs in a wt background resulted in the already described Notch gain-of-function phenotypes, the expression of the APP.ΔNPTY and the APP-ΔICD-GFP constructs resulted in a loss of macrochaete (Figure 4E; data not shown).
Figure 4.
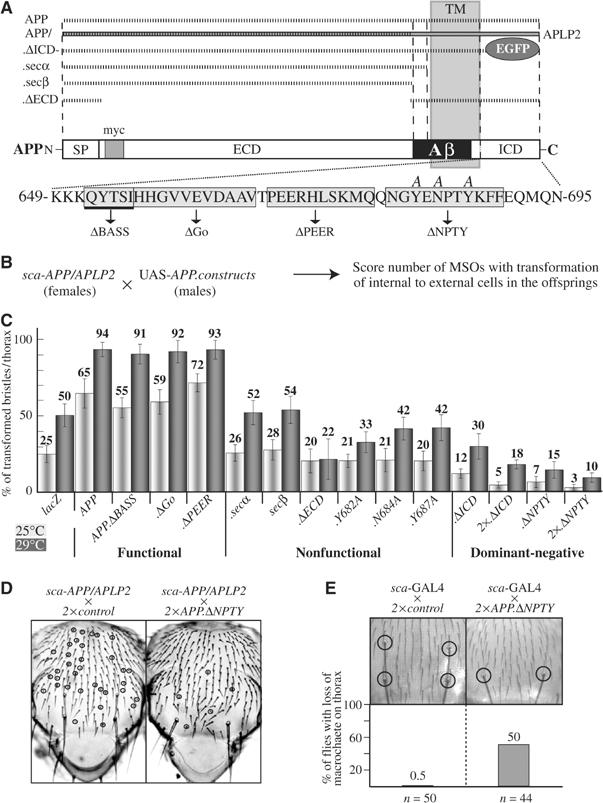
Full-length APP molecules with an intact NPTY motif are required for the induction of Notch gain-of-function phenotypes. (A) Schematic overview of mutated APP molecules used. (B) Outline of the enhancement assay. (C) Quantification and classification of the results of the enhancement assay. (D) Example for the dominant-negative effect of the APP.ΔNPTY construct. (E) Quantification of the phenotypes induced by the expression of APP.ΔNPTY in a wt background. SP, signal peptide; TM, transmembrane; myc, myc-tag.
Taken together, our studies with mutated APP constructs suggest a receptor-like function for APP and underline the importance of the NPTY motif for this function.
Numb and Disabled are binding partners of APP
The NPTY motif has been shown to be important for the binding of PTB-domain-containing proteins to APP. As Numb also contains a PTB domain, which is important for its function, binding to Notch and asymmetric localization (Knoblich et al, 1997), we tested whether the ICD of human APP can bind in vitro to Drosophila Numb and the N-terminal domain of Dab. As displayed in Figure 5A, both Dab and Numb bound the bacterially expressed GST-APP.ICD. In the case of Dab, its binding to APP.ICD was fully abolished upon deletion of the NPTY motif or introduction of point mutations. This motif is also essential for the binding of APP.ICD to Numb. However, the fact that the binding of Numb can only be reduced and not completely abolished by the introduction of NPTY mutations might indicate differences in the binding capabilities of Dab and Numb to APP.ICD. This result could be confirmed with human Dab2 and Numb (see Supplementary data).
Figure 5.
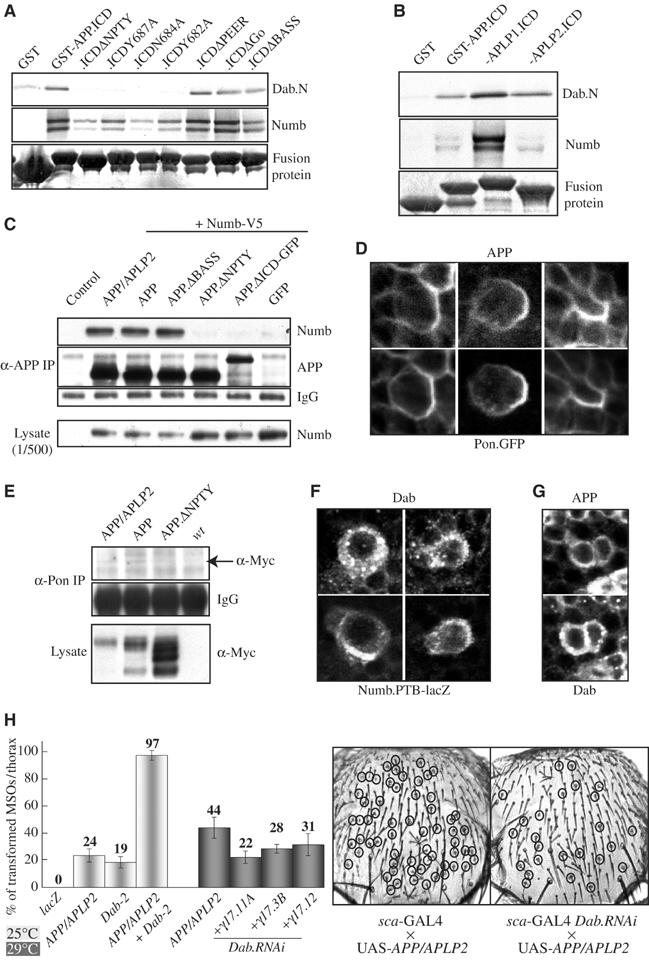
Interaction between APP, Numb and Dab in vitro and in vivo. (A) Binding studies performed with the bacterially expressed GST-tagged ICD of APP, the different mutated forms, GST and the in vitro expressed N-terminus of Dab (Dab.N) or full-length Numb. (B) Comparison of the in vitro binding affinity of Dab and Numb to the different human APP-family members. (C) Co-immunoprecipitation of APP, APP/APLP2 and Numb from S2-cells. (D) Immunostaining of pupal nota co-expressing APP and Pon.GFP. (E) Co-immunoprecipitation of APP, APP/APLP2 and Pon from pupae. (F, G) Immunostaining of pupal nota co-expressing Dab and Numb.PTB-lacZ (F), and APP and Dab (G) in the MSO cell lineage. (H) Quantification of the sensitivity of the APP/APLP2-induced MSO phenotypes to an increase or reduction in Dab protein amount.
The direct interaction between APP and Numb in vitro could imply that the observed Notch gain-of-function phenotypes result from an unregulated titration of Numb away from Notch by the ectopic expression of APP-family members. To exclude this possibility, several experiments were carried out. Considering that the APP-family members show differences in their competence to interfere with Notch signaling in Drosophila, we compared the in vitro binding affinity of APP, APLP1 and APLP2 to Dab and Numb. Whereas the binding affinity of APP and APLP2 proved to be identical, APLP1 showed a much higher affinity to Dab and especially to Numb (Figure 5C). This result stands in contrast to the in vivo situation where APLP2 showed the strongest, APLP1 moderate and APP only very weak phenotypes. Western blot analysis with specific antibodies did not reveal any significant differences in expression levels in vivo, which could account for the differences in phenotype induction (Figure 2A). To address this question further, we analyzed the binding affinity of Numb to APP and the APP/APLP2 chimera in S2 cells. Both proteins have identical Myc-tagged N-termini, but show differences in the induction of Notch gain-of-function phenotypes during MSO development due to their different C-terminal domains. Nevertheless, the binding affinity of APP and APP/APLP2 to Numb in transfected S2 cells proved to be weak but identical (Figure 5C). Also, Western blot analysis from dissected pupal tissue did not reveal any significant differences in the expression, stability or processing of APP and APP/APLP2 during MSO development (see Supplementary data; data not shown). Furthermore, visualization of the distribution of APP during MSO development by antibody staining of pupal nota revealed an asymmetric segregation and co-localization of APP with Pon (Figure 5D). A similar result was obtained for APP/APLP2 (data not shown). This notion goes hand in hand with the observation that both APP and APP/APLP2, but not APP.ΔNPTY, can be co-immunoprecipitated with Pon from extracts of pupae (Figure 5E), indicating that APP and APP/APLP2 are included with the same affinity into Numb-Pon-containing complexes. These co-immunoprecipitation experiments have been complicated by unspecific cross-reactions between the IgG contained in the rabbit anti-Pon serum and the anti-APP or anti-Myc antibodies used for detection. For the very same reason, similar experiments with a rabbit anti-Numb serum failed. Nevertheless, the results obtained so far suggest an in vivo interaction between Numb, Pon and APP/APLP2 as well as APP.
In contrast to APP, Pon and Numb, the distribution of Dab during SOP development is highly dynamic, which makes the interpretation of the data in these small cells difficult. Big vesicle-like structures can be observed, which only sometimes overlap with APP or Numb immunoreactivity (Figure 5F and G). Further studies with a GFP-tagged form of Dab, together with Golgi and ER markers, will have to be performed in future to analyze the localization of Dab during PNS development in more detail.
Taken together, these studies show that Drosophila Numb and Dab can bind to the NPTY motif of APP, but we observed no correlation between the binding affinities of different APP-family members to Numb and Dab in vitro, and their effectiveness to induce Notch gain-of-function phenotypes in vivo. The fact that APP becomes asymmetrically segregated during MSO development like APP/APLP2, probably by a direct interaction with Numb, although it does not induce phenotypes to the same extent like APP/APLP2, clearly indicates that the recognition of APP and APP/APLP2 by the machinery responsible for the asymmetric segregation of molecules during SOP division is not sufficient for phenotype induction.
Dab and Nrt but not Abl play a role in the APP-induced phenotypes
We have shown that the NPTY motif that is highly conserved in all identified APP homologs is the only essential motif within the ICD of APP required for the interference with MSO development. To further analyze the possible contribution of Dab to the phenotypes induced by the APP family, we used gain- and loss-of-function genetics (Figure 5H, and see Supplementary data, Table I). Whereas the overexpression of Dab enhanced the sca-APP/APLP2-induced phenotypes, a reduction in the amount of Dab by RNA interference (RNAi) leads to suppression. Induction of Dab.RNAi in a wt background had no effect. In Drosophila, Dab and Enabled (Ena) have been identified through genetic interaction with the Abelson (Abl) tyrosine kinase (Gertler et al, 1990), and in mice and in humans two homologs of Dab have been isolated. Again, we performed gain- and loss-of-function genetics to further analyze these factors (see Supplementary data). In summary, our genetic studies led to the identification of two molecules, Nrt and Dab, which have so far not been implicated in MSO development, as important and essential factors for the induction of Notch gain-of-function phenotypes by APP-family members. Strikingly, both proteins can induce Notch gain-of-function phenotypes also in the absence of APP if overexpressed during MSO formation. At the same time, these studies excluded an involvement of Abl and Ena in the interference of APP-family members with MSO development and revealed a conservation in function between Drosophila Dab and human Dab-2, but not between Dab and mouse Dab-1.
Drosophila APPL and MSO development
To confirm the results obtained with the human APP-family members, the Drosophila homolog of APP, APPL, was analyzed. In the course of their studies, White and co-workers have generated several transgenic Drosophila lines allowing the GAL4-driven expression of different appl constructs (Figure 6A; Torroja et al, 1999a, 1999b). Especially interesting for this study is a construct with a small deletion within the ECD (APPL secretion defective (APPL.sd)). Western blot analysis indicated that, in contrast to wt APPL, this mutant protein does not undergo proteolytic processing, which under normal conditions would result in the release of the ECD from APPL. The different appl constructs were overexpressed during MSO development by the use of sca-GAL4 and the resulting phenotypes scored. Strikingly, while APPL.sd induces very strong Notch gain-of-function phenotypes, wt APPL does not induce any phenotype even though the expression levels of the constructs were similar (Figure 6A and B). Furthermore, the results obtained with the human APP-family members regarding the importance of the NPTY motif and the genetic interactions could be confirmed (Figure 6B and C). The deletion of parts of the conserved second ECD, E2, decreased phenotype induction. This cannot be explained by lower levels of protein expressed, as suggested by the Western blot in Figure 6A, since for this Western blot an antibody against the ECD of APP has been used which recognizes parts of this domain. When we used our antibody against the ICD, which is identical between ΔE1 and ΔE2, protein levels were similar (data not shown).
Figure 6.
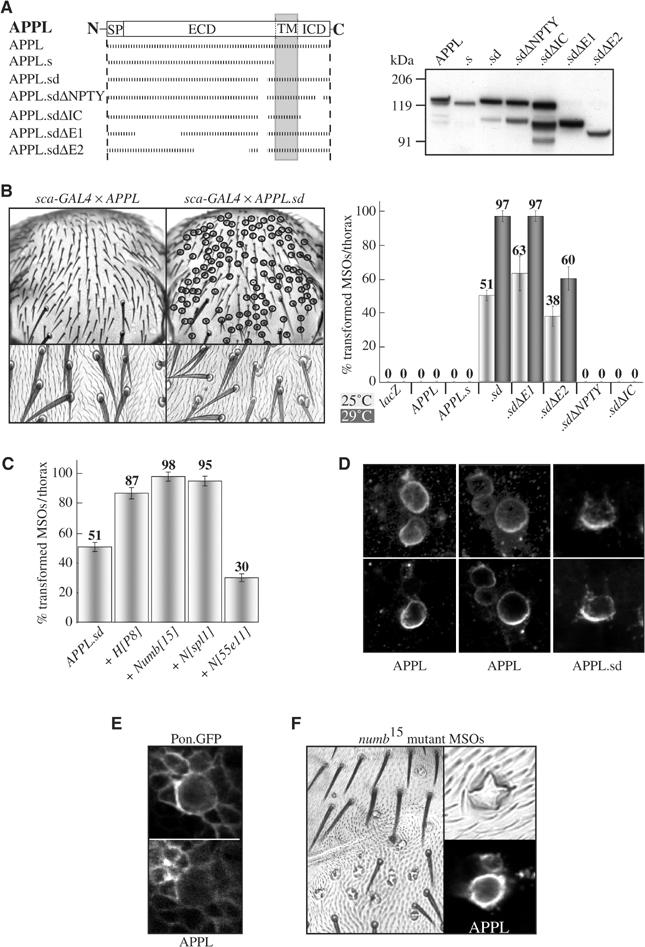
Influence of Drosophila APPL on MSO development. (A) Overview of the mutated APPL molecules used and their respective expression level revealed by an α-APPL-ECD antibody. (B) Quantification of the number of transformed bristles on the thorax of flies expressing different APPL transgenes. (C) Quantification of the sensitivity of the APPL.sd-induced phenotypes to an increase or loss in Notch signaling. (D) Immunostaining of pupal nota for APPL and APPL.sd during MSO development. Upper panel: average projections of stacks of confocal images. Lower panel: single sections. (E) Immunostaining of pupal nota co-expressing APPL and Pon.GFP during MSO development. (F) Immunostaining of pupal nota expressing APPL during MSO development in numb mutant cell clones.
The difference in phenotype induction between APPL and APPL.sd provided us with another opportunity to address the question of whether APP-family members achieve their interference with Notch signaling by titrating Numb away from the Notch receptor solely by direct, unregulated binding. Taking into account that (a) APPL.sd differs from APPL only by a 33 aa deletion within the ECD, (b) the expression level is identical and (c) an increase in stability of APPL.sd has never been observed (Figure 3 in Torroja et al, 1996; Figure 6A; data not shown), we decided to analyze their localization in differentiating SOPs. Although in wt flies APPL could only be detected at higher levels in the determined MSO neurons (data not shown), ectopically expressed APPL and APPL.sd were readily detectable in all cells. During MSO development, APPL as well as APPL.sd displayed an asymmetric segregation and co-localization with Pon.GFP (Figure 6D and E). In Figure 6D, average projections of stacks of confocal images along the z-axis are arranged together with single sections to show that the asymmetric signal is not caused by the position of the cells to the focal plane. This result again clearly indicates that the segregation event is uncoupled from the phenotype induction since APPL does not induce phenotypes, whereas APPL.sd induces very strong phenotypes, and that APPL and APPL.sd have the same affinity to Numb in vivo. To confirm that the asymmetric localization of APPL depends on Numb, numb mutant SOP cells were generated by the use of the FRT/FLP technique in an APPL overexpression background. The loss of numb during MSO development resulted in a four-socket phenotype due to constant activation of Notch signaling in all MSO cells and also the asymmetric segregation of APPL could not be observed anymore (Figure 6F).
The suggestion that APPL.sd is secretion defective was based on Western blot analysis, but these studies were not accompanied by studies with an antibody specific for the ICD of Drosophila APPL. In the course of another project, we have raised an antibody against the ICD of APPL (A Loewer, R Paro, G Merdes, in preparation). This enabled us to analyze the processing of APPL in more detail and to directly test for the formation of CTFs from APPL.sd. Surprisingly, our results suggest that there is no difference between the processing of APPL and APPL.sd, which could account for the different phenotypic effects of the two molecules (see Supplementary data).
To test whether endogenous APPL plays a role during the development of the PNS of Drosophila, we examined flies carrying a deletion of the appl gene (appld). An isogenized line was analyzed with respect to the number and structure of adult MSOs and, as documented in Figure 7A, we found a reduction in scutellar and sternopleural MSO number in each individual. This observation is concordant with the recent identification of a P-element insertion within the appl gene as a quantitative trait locus causing a variation in MSO number (Norga et al, 2003). Furthermore, if we try to restore APPL expression in appld mutant flies, the MSO defects are suppressed by 30%. A more penetrant rescue could not be obtained as the general overexpression of APPL leads to lethality. However, since the genetic background of the original appld mutant is obscured by the fact that (a) it has been generated by recombining two chromosomal deficiencies, and that (b) appl is located directly adjacent to the achaete–scute complex which can influence the number of MSOs as well, an appl-specific RNAi construct was generated. Induction of appl.RNAi during MSO development resulted in a loss of scutellar MSOs similar to the appld mutation (Figure 7A). Furthermore, the viability of the flies on our standard medium was greatly reduced, an effect which we already had observed for appld and which has been reported by Tschape et al (2002) as well. The RNAi effect of the construct in transgenic animals was confirmed by Western blot (see Supplementary data). Immunostainings from pharate appld mutant flies revealed the absence not only of the external cells of the macrochaete but also the absence of neurons (Figure 7B) and sheath cells (not shown), and therefore of the complete MSO, whereas the inner and external cells of microchaete are readily detectable on the same thorax.
Figure 7.
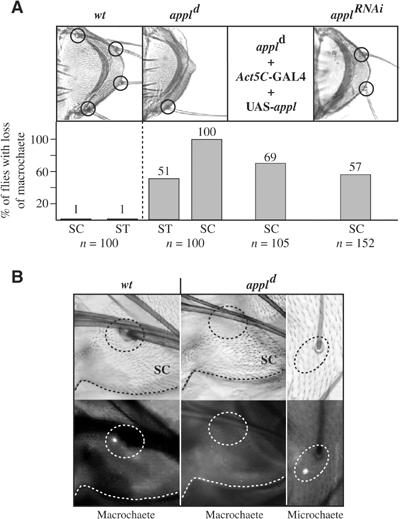
Requirement of APPL for PNS development. (A) Quantification of the phenotypes in the external MSO cells in different appl mutant genetic backgrounds. The scutellum of a wt Drosophila, the appld mutant and a fly expressing an appl.RNAi construct under the control of sca-GAL4 are shown. (B) wt and appld pharate adults (upper panel) were immunostained for the neuronal marker Elav (lower panel). SC, scutellum; ST, sternopleura.
Taken together, our studies with APPL confirm the results obtained with the human APP family regarding the induced MSO phenotypes and the importance of the NPTY motif. In addition, we demonstrate that a 33 aa deletion within the EC domain converts APPL into a far more potent molecule for interference with PNS development without changing its metabolism, processing or localization. Finally, we provide evidence that endogenous APPL plays a role during the development of the PNS of Drosophila.
Discussion
Our studies show that the ectopic expression of human APP-family members induced Notch gain-of-function phenotypes during the development of the adult PNS. The severity of the induced phenotypes not only depended on the dosage and the particular APP-family member, but also on particular domains of the molecules. This led us to identify the NPTY motif as the only critical motif within the ICD for the interference with PNS development and for the interaction of APP with Numb/Pon and Dab in vitro and in vivo. Genetic interactions suggest a direct influence of APP-family members on the Notch signaling cascade, identify Numb as a potential target and provide evidence for an involvement of Dab and Nrt in the induction of the phenotypes. Studies with Drosophila APPL confirmed the results obtained with the human proteins and demonstrate a previously undescribed involvement of endogenous appl in PNS development. Consequently, these results lead us to propose that a crosstalk between the APP family and Notch exists.
An interaction between APP and Numb has recently been demonstrated (Roncarati et al, 2002). In mouse brain lysates as well as in cell culture, APP or APP.ICD bound to all four isoforms of Numb and to Numb-like. Surprisingly, in this study, the processing of APP and the release of the ICD of APP resulted in an inhibition of Notch signaling. Numb is a negative regulator of Notch signaling and binds directly via its PTB domain to Notch (Knoblich et al, 1997). Therefore, a direct interaction between APP and the PTB domain of Numb should result in an increase rather than in a decrease of Notch activation. From the known crystal structure of PTB–NPTY interactions, a trimeric complex between Notch, APP and Numb seems unlikely (Yun et al, 2003). In our study, the induced Notch gain-of-function phenotypes, the strong genetic interaction, the dependence of the asymmetric localization of APPL on Numb and the direct binding between APP and Numb support a crosstalk between Notch signaling and APP-family members. One explanation for the APP induced Notch gain-of-function phenotypes during MSO development would indeed be the sequestration and inactivation of Numb by APP-family members. However, we provide several lines of evidence that, if APP competes with Notch for the binding to Numb, this binding and competition must be highly regulated and requires factors which have not been known to be involved in MSO development previously.
First, expression of the human APP-family proteins induced cell fate transformations during MSO development in a dosage- and construct-dependent manner, but the potency in phenotype induction of the different proteins did not correlate with their in vitro and in vivo binding affinity to Numb. Nevertheless, the NPTY motif proved to be essential both for binding to Numb and phenotype induction, suggesting that the binding to Numb might be necessary but not sufficient for phenotype induction. This implies that there is at least one additional factor which plays an important role and which must have different affinities to the APP-family members than Numb, for example, strong binding to APLP2 but weak binding to APP. Second, deletion of the ECD of APP results in an inactive molecule, which can no longer induce any phenotypes. This stands in contrast to all in vitro binding studies that have been performed between the NPTY motif of APP and PTB-containing proteins in cell culture. In these assays, the affinity of such a molecule to Fe65, Dab-1/2, X11L, Numb and Numb-like did not change significantly. Third, APP molecules with a deletion of the NPTY motif could suppress the phenotypes induced by wt APP and induce the loss of macrochaete in wt flies. Such a dominant-negative effect can only be explained if APP-family members have a receptor-like function. In this scenario, APP.ΔNPTY would compete with wt APP or APPL for ligand binding, but could not relay the ‘signal', for example, crosstalk to Notch and/or inactivating Numb. Another possibility would be the necessity of homodimer formation. Such a dimer formation has been postulated (Scheuermann et al, 2001), but so far no in vivo data are available. Furthermore, structural data do not provide any evidence for a dimerization of APP molecules prior to the binding of PTB-containing proteins. Fourth, overexpression of Drosophila APPL induced only very weak phenotypes, whereas the overexpression of APPL.sd induced very strong phenotypes. The difference in phenotype induction could not be correlated with significant differences in expression levels, metabolism or processing. This was surprising, since APPL.sd had been generated to impair secretion and therefore processing. As a consequence, we postulate that the 33 aa deletion in APPL.sd changes the conformation of the ECD, confirming again the important role the ECD plays in determining the potency of the APP-family members for interference with PNS development. Fifth, overexpression of APLP2 resulted in bold patches, suggesting that presumptive SOPs are transformed into epidermal cells by the induction of a Notch gain-of-function phenotype very early during MSO development. This step during PNS development is known to be independent of Numb and functions via the lateral inhibition mechanism, indicating that APP-family members can also interact with Numb-independent Notch signaling processes. During these processes, so far unknown factors might take over the role of Numb as negative regulator of Notch to add an additional level of control to the system. From the literature, it seems to be clear that endocytosis is important for inhibition and for the promotion of Notch signaling, but almost nothing is known about the factors directly involved in these events (summarized by Schweisguth, 2004). Finally, ectopically expressed APPL and APPL.sd as well as APP and APP/APLP2 were asymmetrically localized during MSO development and co-localization and co-immunoprecipitation with Pon could be demonstrated in vivo. This is an interesting result since APPL and APP induced only weak phenotypes, but APPL.sd and APP/APLP2 induced very strong phenotypes. Nevertheless, both types of proteins were recognized with the same efficiency by the Numb-dependent machinery responsible for the asymmetric distribution of factors during MSO development, thus completely uncoupling this event from phenotype induction. This implicates that the phenotype induction occurs after completion of the separation of the SOP siblings and that APP, even if it binds to Numb, does not compete with other binding partners of Numb for asymmetric segregation.
During MSO development, the asymmetric distribution of Numb ensures that the siblings arising from one mother cell show a difference in response to the activation of the Notch receptor. Numb is responsible for the asymmetric segregation of α-adaptin and binds both the ICD of Notch and α-adaptin (Berdnik et al, 2002), suggesting that Numb may regulate Notch by controlled endocytosis. The difference in response to Notch signaling is further amplified by the asymmetric localization of the E3 ubiquitin ligase Neuralized, which upregulates the endocytosis of the Notch ligand Delta (Le Borgne and Schweisguth, 2003). However, one has to take into account that it has also been reported that Numb can bind the ICD of Notch after release, inhibit the ability of this ICD to cause nuclear translocation of Su(H) and can inhibit Notch signaling during wing development by ectopic misexpression (Frise et al, 1996). Therefore, even if it is very tempting to suggest that Numb solely regulates Notch by endocytotic mechanisms, there might still be other Numb functions.
Nevertheless, more and more evidence is emerging that regulated endocytosis is an important general feature for the modulation of developmental signals (summarized by Piddini and Vincent, 2003). In this respect, it is especially intriguing that we have identified Drosophila Dab as an essential factor for the interaction of APP with Notch signaling. Whereas the overexpression of Dab enhanced the phenotype induced by APP, a reduction of the endogenous protein level by RNAi suppressed the phenotype. Notch gain-of-function phenotypes during MSO development can be induced by expression of high levels of Dab alone. This is remarkable since it has been proposed that the mammalian Dab-2 homologs belong to a family of cargo-specific adaptor proteins, like Numb and β-Arrestin, which regulate cargo selection and pit formation (Morris and Cooper, 2001; Mishra et al, 2002). Accordingly, APP molecules could induce the observed phenotypes during PNS development, influencing endocytosis and processing of Notch with the help of Dab. A function for APP as endocytotic receptor is supported by the finding that full-length APP is internalized via clathrin-coated vesicles (Marquez-Sterling et al, 1997). Furthermore, a direct interaction between Drosophila Dab and Notch has been published previously (Giniger, 1998). We have been able to reproduce these binding studies, but the observed binding of Dab to Notch in vitro is very weak in comparison to the binding affinity of Su(H) or Numb (data not shown). However, additional studies suggest not only the presence of a second Notch-binding motif within the C-terminal domain of Dab, but also reveal the presence in vivo of a complex which contains Notch and Dab in Drosophila embryos (LeGall and Giniger, personal communication, 2003). The second binding motif could allow a direct interaction between the Notch receptor and APP mediated by Dab, and it will be of great interest to elucidate the role of Dab with respect to Notch and APP signaling in the future.
That a crosstalk between the APP amily and Notch receptors also takes place in the mammalian system is not only implied by the data of Roncarati et al (2002) but also by the results of Abraham and colleagues. In a search for APP-binding proteins, they identified Notch 2 by employing a proteomic approach. An impermeable crosslinker to only crosslink plasma membrane proteins on HEK293 cells stably overexpressing APP751 was used, and an APP-containing complex was seen at 250 kDa. A similar complex was also observed when rat E18 primary neurons were virally infected with APP751. The neuron-derived complex was immunopurified and sent for MALDI-TOF identifying Notch 2 as the major protein (Abraham, CR, personal communication, 2004).
Originally, mutations in the dab gene were isolated by genetic interactions with the Drosophila Abl homolog (Gertler et al, 1990). It has recently been reported that these mutations have been erroneously attributed and that all mutations isolated as dab alleles in fact affect the nrt locus (Liebl et al, 2003). Nrt is a single-pass type-II transmembrane protein and belongs to the family of neuronal cell adhesion molecules (N-CAMs; Speicher et al, 1998). Nrt mutants are viable and fertile, but its function in growth cone guidance can be revealed in combination with other N-CAM mutants. Since we used the originally described dab alleles for our first genetic studies, we identified mutations in nrt as dominant suppressors of the APP-induced phenotype and also the overexpression of Nrt itself induces very strong and very specific Notch gain-of-function phenotypes. However, our genetic studies ruled out an involvement of Abl in the APP-induced phenotype. Preliminary genetic data suggest a genetic interaction between appl and nrt mutations resulting in lethality of the otherwise viable alleles. Additional experiments will be necessary both in Drosophila and vertebrates to further explore this interaction. Especially, the isolation of new mutants for Drosophila dab and appl generated in a clearly defined genetic background, and their use for genetic interactions with Notch, numb and nrt, should provide insights into the mechanisms underlying the potential functions of APP-family members in endocytosis, Notch signaling and PNS development. However, the identification of appl as a quantitative trait locus already provides evidence for a function of appl during PNS development (this paper; Norga et al, 2003).
Although it has not been established that the binding interactions between APP, Numb and Dab are functionally important in AD, signaling pathways emanating from aberrant APP function, as it occurs in AD, may influence Dab/Numb and thus Notch activity. Also, the use of drugs to lower APP processing and Aβ production could result in altered APP functions and an interference with Notch signaling in the adult brain. As already mentioned, an interaction between APP and Numb and Numb-like in the mouse brain has been demonstrated and there is accumulating evidence for a role of the Notch signaling pathway not only in early events during cell fate specifications but also in stem cells, in already differentiated neuronal cells and in neurodegeneration in the adult vertebrate nervous system (summarized by Gaiano and Fishell, 2002; Selkoe and Kopan, 2003). Furthermore, the view that only the production and deposition of Aβ plays a decisive role in AD has been challenged by recent evidence from different model systems which attribute numerous functions to APP and derivatives thereof (reviewed by Turner et al, 2003). These findings together with our data make it likely that alterations in the processing of APP either during the onset and progression of AD or by the use of therapeutics may result in loss- as well as in gain-of-function phenotypes contributing to the disease or side effects.
Materials and methods
Unless otherwise mentioned, standard molecular techniques were used.
Genetic crosses and fly stocks
Expression of APP transgenes was generally tested using homogenized adult heads derived from crosses of UAS constructs with Glass-GAL4 for Western blotting (see Supplementary data). For each UAS construct, different transgenic lines were tested and two lines from each construct expressing comparable amounts of proteins were used. For the quantification of changes in the number of external cells in MSOs, ⩾100 MSOs/thorax of ⩾10 different flies were analyzed. Fly stocks were obtained from the Bloomington Drosophila Stock Center (Indiana University), the Szeged Drosophila Stock Center (Hungary), or from published and available material. See Supplementary data for details about the genotype of the flies and temperature used.
Cloning of UAS constructs
The new pUAST-APP constructs contain an N-terminal myc-tag. The plasmid pUAST-APP.secα encodes 1–613 aa, pUAST-APP.secβ encodes 1–596 aa of APP695. Mutations within the ICD of APP695 were generated by the PCR-based megaprimer method for site-directed mutagenesis, and RNAi constructs were cloned into pWIZ (see Supplementary data for details).
Binding studies
PCR-amplified fragments of wt or mutant human APP695, APLP1 and APLP2 cDNAs were cloned into pGEX-4T-2 and expressed in Escherichia coli BL21(DE3) as recommended by Pharmacia. The coding sequences of numb and of dab (1–362 aa) were amplified by PCR, cloned into the pCR-II-TOPO vector and expressed as S35-labeled proteins (TNT® Quick System, Promega). In all, 10 μg of the GST-fusion proteins or 20 μg of GST bound to glutathione–Sepharose were incubated with the 35S-labeled proteins for 14 h at 4°C in 20 mM Tris (pH 8.0), 150 mM NaCl, 0.2% Triton X-100, washed twice, boiled in 2 × SDS sample buffer and subjected to SDS–PAGE. For the transfection of S2-cells, numb and GAL4 cDNAs were cloned into the pMT-V5-His vector and cells were transfected with the help of Effectene reagent according to the suppliers (Invitrogen/Qiagen). Lysis and IPs were performed in PBS, 1% NP-40, CompleteEDTAfree (Roche). Before homogenization, pupae were frozen and ground in liquid nitrogen.
Immunohistochemistry
Immunohistochemistry of pupal nota was performed essentially as described by Nagel et al (2000) and Berdnik et al (2002). Antibodies were obtained from the Developmental Studies Hybridoma Bank (NICHD, University of Iowa) or from published material (see Supplementary data). The α-APPL-ICD antibody will be published elsewhere.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Table I
Supplementary Table II
Acknowledgments
We thank R Carthew, S Cohen, F Fogerty, M Fortini, L Garcia-Alonso, E Giniger, Y Jan, J Knoblich, D Kretzschmar, G Multhaup, M Piovant, A Preiss, S Scheuermann and F Schweisguth for fly stocks, cDNAs and antibodies, A Preiss for teaching how to dissect pupal nota, E Giniger and C Abraham for sharing unpublished results, and M Müller for excellent technical assistance. Our apologies go to the authors of many important contributions that could not be discussed/cited due to space limitation. This work was supported by a grant from the DFG (SFB 488) to KB and RP, and by a grant to GM (SPP ‘Cellular Mechanisms of AD').
References
- Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, Posakony JW (2000) A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell 103: 957–969 [DOI] [PubMed] [Google Scholar]
- Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich JA (2002) The endocytic protein alpha-Adaptin is required for numb-mediated asymmetric cell division in Drosophila. Dev Cell 3: 221–231 [DOI] [PubMed] [Google Scholar]
- Fossgreen A, Bruckner B, Czech C, Masters CL, Beyreuther K, Paro R (1998) Transgenic Drosophila expressing human amyloid precursor protein show gamma-secretase activity and a blistered-wing phenotype. Proc Natl Acad Sci USA 95: 13703–13708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frise E, Knoblich JA, Younger-Shepherd S, Jan LY, Jan YN (1996) The Drosophila Numb protein inhibits signaling of the Notch receptor during cell–cell interaction in sensory organ lineage. Proc Natl Acad Sci USA 93: 11925–11932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiano N, Fishell G (2002) The role of notch in promoting glial and neural stem cell fates. Annu Rev Neurosci 25: 471–490 [DOI] [PubMed] [Google Scholar]
- Gertler FB, Doctor JS, Hoffmann FM (1990) Genetic suppression of mutations in the Drosophila abl proto-oncogene homolog. Science 248: 857–860 [DOI] [PubMed] [Google Scholar]
- Giniger E (1998) A role for Abl in Notch signaling. Neuron 20: 667–681 [DOI] [PubMed] [Google Scholar]
- Gunawardena S, Goldstein LS (2001) Disruption of axonal transport and neuronal viability by amyloid precursor protein mutations in Drosophila. Neuron 32: 389–401 [DOI] [PubMed] [Google Scholar]
- Heber S, Herms J, Gajic V, Hainfellner J, Aguzzi A, Rulicke T, von Kretzschmar H, von Koch C, Sisodia S, Tremml P, Lipp HP, Wolfer DP, Müller U (2000) Mice with combined gene knock-outs reveal essential and partially redundant functions of amyloid precursor protein family members. J Neurosci 20: 7951–7963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN (1997) The N terminus of the Drosophila Numb protein directs membrane association and actin-dependent asymmetric localization. Proc Natl Acad Sci USA 94: 13005–13010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, West SR, Hiromi Y (1995) Cell fate control in the Drosophila retina by the orphan receptor seven-up: its role in the decisions mediated by the ras signaling pathway. Development 121: 1361–1372 [DOI] [PubMed] [Google Scholar]
- Lai EC, Orgogozo V (2004) A hidden program in Drosophila peripheral neurogenesis revealed: fundamental principles underlying sensory organ diversity. Dev Biol 269: 1–17 [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Schweisguth F (2003) Unequal segregation of neuralized biases Notch activation during asymmetric cell division. Dev Cell 5: 139–148 [DOI] [PubMed] [Google Scholar]
- Liebl EC, Rowe RG, Forsthoefel DJ, Stammler AL, Schmidt ER, Turski M, Seeger MA (2003) Interactions between the secreted protein amalgam, its transmembrane receptor neurotactin and the Abelson tyrosine kinase affect axon pathfinding. Development 130: 3217–3226 [DOI] [PubMed] [Google Scholar]
- Luo L, Tully T, White K (1992) Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron 9: 595–605 [DOI] [PubMed] [Google Scholar]
- Marquez-Sterling NR, Lo AC, Sisodia SS, Koo EH (1997) Trafficking of cell-surface beta-amyloid precursor protein: evidence that a sorting intermediate participates in synaptic vesicle recycling. J Neurosci 17: 140–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM (2002) Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J 21: 4915–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM, Cooper JA (2001) Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2: 111–123 [DOI] [PubMed] [Google Scholar]
- Nagel AC, Maier D, Preiss A (2000) Su(H)-independent activity of hairless during mechano-sensory organ formation in Drosophila. Mech Dev 94: 3–12 [DOI] [PubMed] [Google Scholar]
- Norga KK, Gurganus MC, Dilda CL, Yamamoto A, Lyman RF, Patel PH, Rubin GM, Hoskins RA, Mackay TF, Bellen HJ (2003) Quantitative analysis of bristle number in Drosophila mutants identifies genes involved in neural development. Curr Biol 13: 1388–1396 [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39: 409–421 [DOI] [PubMed] [Google Scholar]
- Piddini E, Vincent JP (2003) Modulation of developmental signals by endocytosis: different means and many ends. Curr Opin Cell Biol 15: 474–481 [DOI] [PubMed] [Google Scholar]
- Price DL, Tanzi RE, Borchelt DR, Sisodia SS (1998) Alzheimer's disease: genetic studies and transgenic models. Annu Rev Genet 32: 461–493 [DOI] [PubMed] [Google Scholar]
- Roncarati R, Sestan N, Scheinfeld MH, Berechid BE, Lopez PA, Meucci O, McGlade JC, Rakic P, D'Adamio L (2002) The gamma-secretase-generated intracellular domain of beta-amyloid precursor protein binds Numb and inhibits Notch signaling. Proc Natl Acad Sci USA 99: 7102–7107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinfeld MH, Ghersi E, Laky K, Fowlkes BJ, D'Adamio L (2002) Processing of beta-amyloid precursor-like protein-1 and -2 by gamma-secretase regulates transcription. J Biol Chem 277: 44195–44201 [DOI] [PubMed] [Google Scholar]
- Scheuermann S, Hambsch B, Hesse L, Stumm J, Schmidt C, Beher D, Bayer TA, Beyreuther K, Multhaup G (2001) Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer's disease. J Biol Chem 276: 33923–33929 [DOI] [PubMed] [Google Scholar]
- Schweisguth F (2004) Notch signaling activity. Curr Biol 14: R129–R138 [PubMed] [Google Scholar]
- Selkoe D, Kopan R (2003) Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci 26: 565–597 [DOI] [PubMed] [Google Scholar]
- Speicher S, Garcia-Alonso L, Carmena A, Martin-Bermudo MD, de la Escalera S, Jimenez F (1998) Neurotactin functions in concert with other identified CAMs in growth cone guidance in Drosophila. Neuron 20: 221–233 [DOI] [PubMed] [Google Scholar]
- Torroja L, Chu H, Kotovsky I, White K (1999a) Neuronal overexpression of APPL, the Drosophila homologue of the amyloid precursor protein (APP), disrupts axonal transport. Curr Biol 9: 489–492 [DOI] [PubMed] [Google Scholar]
- Torroja L, Luo L, White K (1996) APPL, the Drosophila member of the APP-family, exhibits differential trafficking and processing in CNS neurons. J Neurosci 16: 4638–4650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroja L, Packard M, Gorczyca M, White K, Budnik V (1999b) The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci 19: 7793–7803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschape JA, Hammerschmied C, Muhlig-Versen M, Athenstaedt K, Daum G, Kretzschmar D (2002) The neurodegeneration mutant lochrig interferes with cholesterol homeostasis and Appl processing. EMBO J 21: 6367–6376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner PR, O'Connor K, Tate WP, Abraham WC (2003) Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol 70: 1–32 [DOI] [PubMed] [Google Scholar]
- Yun M, Keshvara L, Park CG, Zhang YM, Dickerson JB, Zheng J, Rock CO, Curran T, Park HW (2003) Crystal structures of the Dab homology domains of mouse disabled 1 and 2. J Biol Chem 278: 36572–36581 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure 6
Supplementary Table I
Supplementary Table II


