Abstract
Multiparametric magnetic resonance imaging (MRI) has become a critical clinical tool for diagnosing focal ischemic stroke severity, staging treatment, and predicting outcome. Imaging during the acute phase focuses on tissue viability in the stroke vicinity, while imaging during recovery requires the evaluation of distributed structural and functional connectivity. Preclinical MRI of experimental stroke models provides validation of non-invasive biomarkers in terms of cellular and molecular mechanisms, while also providing a translational platform for evaluation of prospective therapies. This brief review of translational stroke imaging discusses the acute to chronic imaging transition, the principles underlying common MRI methods employed in stroke research, and experimental results obtained by clinical and preclinical imaging to determine tissue viability, vascular remodeling, structural connectivity of major white matter tracts, and functional connectivity using task-based and resting-state fMRI during the stroke recovery process.
Introduction
Ischemic stroke is the third leading cause of death globally and the most common cause of complex chronic disability [1, 2]. Trends in demographics and health care have produced rising rates of cardiovascular disease but decreasing rates of stroke mortality [1], so that evermore people are living with the aftermath of ischemic stroke. Up to 75% of stroke survivors require some level of long-term assistance [3]. Despite the urgent need for better therapies at all stages from stroke onset to recovery, only recanalization therapy to restore perfusion is available to treat stroke acutely, and only about 2% of patients receive this intervention [4]. In the weeks to months after stroke, many pharmacotherapies and other novel strategies have demonstrated efficacy in animal studies, and in fact numerous drugs have shown promise for enhancing certain aspects of recovery in small clinical trials [5]. However, despite identification of promising pharmacotherapeutic adjuncts to tradition physical therapies, no drugs currently are approved in the United States to enhance stroke recovery.
One of the challenges for treating stroke and determining efficacy during recovery is “the astounding heterogeneity surrounding neuroplasticity and regeneration” [6], a heterogeneity that largely has been revealed in the clinical domain by neuroimaging. Imaging helps diagnose ischemic stroke, stage treatment, and monitor recovery by providing anatomical markers of lesion location and size, and functional markers of tissue status. For studying recovery processes and therapies, imaging can narrow inclusion criteria based on the original insult to reduce biological variance within groups. Non-invasive neuroimaging studies can help define the natural evolution of neurodegeneration and plasticity and the benefits of therapies through longitudinal studies. The translational nature of imaging can help elucidate cross-species variations in underlying processes and identify differences in responses to therapies, which so often fail during translation to the clinic.
The “neurovascular unit” provides a conceptual basis for framing the damage due to stroke and subsequent structural recovery mechanisms and pathways, which can be broadly categorized as angiogenesis, neurogenesis, and oligodendrogenesis [7, 8]. Neuroimaging markers rarely align precisely with these categories. Moreover, functional neuroimaging implicates disrupted connectivity that might represent nonlocal processes including diaschisis, vicariation, or disinhibition [9, 10]. One of the goals of pre-clinical imaging is to elucidate the relationship between imaging biomarkers and molecular or histological markers, and then to see how these markers evolve during acute stroke and recovery.
Although this special volume emphasizes preclinical stroke models and research, imaging provides a translational bridge across scales and species, and so this brief review describes both clinical and preclinical imaging methods and applications to studies of stroke recovery. Despite the wide variance in clinical studies compared to controlled experimental models, profound differences in neuroanatomy across species, and criticisms of some specific aspects of experimental stroke models, a recurring theme is the remarkable degree of concordance between preclinical and clinical imaging results. The main focus of this review is magnetic resonance imaging (MRI), which lacks the molecular specificity of positron emission tomography (PET) or the high resolution of semi-invasive optical imaging, but which provides excellent spatial resolution across whole brain and a multitude of methods that can be used to probe different aspects of tissue structure, function, and neuronal connectivity.
Acute Stroke: Core and Penumbra
Although it is widely agreed that complete recovery from complete brain ischemia can occur only for brief ischemic durations of less than about 10 minutes under normal physiological conditions, some recovery of tissue function can occur for severe ischemia up to an hour given prompt and complete reperfusion [11]. This time window is extended in regions with incomplete occlusion. Current clinical guidelines recommend endovascular clot retrieval (thrombectomy) for patients with image-proven occlusion of large cerebral artery in the anterior circulation [12], and reperfusion by intravenous thrombolysis using tissue plasminogen activator within 3 to 4.5 hours of stroke [13], after excluding hemorrhage by imaging using MRI or CT. At later time points, reperfusion offers less benefit and more risk of secondary injury. Nevertheless, imaging suggests that a subset of patients might benefit from treatment at times as long as 24 hours after stroke [14].
One of the roles of acute imaging is differentiation of regions that potentially can be saved from those that have suffered irreversible loss of function; this is particularly relevant in the clinic where stroke onset times may be unclear [15]. Conceptually, stroke-affected tissue is parcellated into a core region of severely damaged brain and a “penumbra” that is ischemic but still viable if adequate perfusion is efficiently restored [16]. In the acute stage shortly after stroke, clinical imaging assists initial treatment strategies and informs expectations. Preclinical imaging enables targeting for subsequent molecular or histological analysis, while also helping to define the evolution of tissue structure and function with and without intervention.
Positron emission tomography (PET) provided the first imaging indications of penumbrae by identifying tissue with compromised CBF but relatively preserved oxygen utilization due to an increased oxygen extraction fraction [17]. Another method employed by nuclear imaging defined the penumbra by comparing regional deficits in CBF with loss of GABAergic benzodiazepine receptors, which are widely abundant in healthy brain and indicate by absence an irreversible neuronal loss due ischemic injury [17]. Although PET provides gold-standard measurements of CBF, metabolism, and neuroreceptor densities, a disadvantage for acute clinical imaging is the lead-time required for synthesizing radioligands, as well as long acquisition times in sequential scans for each radioligand. High costs and limited spatial resolution restrict utility in small animal models.
Although the continued development of new radioligands, and the recent integration of simultaneous PET and MRI, might yet open new avenues for PET imaging during stroke recovery, PET has largely has been replaced in acute stroke imaging by MRI, which possesses better spatial and temporal resolution. Historically, MRI evaluated the penumbra at early time points in terms of a “perfusion-diffusion mismatch” between regions with diminished CBF and diminished water diffusion. Clinically, it is recognize that this mismatch does not optimally define the penumbra [16]. MRI measurements of perfusion typically overestimate the size of the core by underestimating collateral flow. Perfusion measurements overestimate the penumbra by including regions of benign oligemia that do not place the tissue at risk. Diffusion measurements overestimate the core region by including potentially salvageable regions of the penumbra. Nevertheless, the MRI perfusion-diffusion mismatch provides an approximate indication of the penumbra, and often this is the definition used to define tissue regions in subsequent investigations of stroke recovery.
More advanced methods have been developed for assessing potentially salvageable penumbral tissue that rely upon multi-parametric input. Bolus injection of paramagnetic contrast agent provides indices not only of CBF, but also of the mean transit time and time to peak response as reflections of delay and dispersion through the vascular bed. By incorporating this additional perfusion information with diffusion and potentially even other data like tissue T2, predictive acute-phase models can be created based upon outcomes defined at time points late after stroke [18, 19]. In animal models, image-based predictions of infarct volume and location are highly accurate in the absence of reperfusion treatments [20]. Additionally, several MRI research techniques under development may contribute to delineation of penumbrae and assessment of viability. pH-sensitive MRI offers the possibility to determine graded levels of ischemic acidosis [21], and diffusion kurtosis is a marker of local water restriction that may be more sensitive than mean diffusion to early structural damage [22].
The volume of penumbra demonstrating a perfusion-diffusion mismatch is transient, and the conversion of ischemic tissue to infarction in patients increases significantly with age and with pre-existing conditions of hypertension and diabetes [23], comorbidities that often are absent in preclinical models [24]. Within the highly heterogeneous human population, imaging may provide a useful patient selection tool for clinical trials in order to create comparable subject populations using predictive models [25]. Furthermore, the mismatch might provide predictions of final infarct volume that can be used as a treatment outcome to reduce inherent variability across patients [16]. Irrespective of whether the penumbra volume is estimated purely from imaging, or instead by combining imaging with neurological assessments, imaging is a critical clinical tool for staging treatment [14].
Preclinical stroke models generally conform to the critical post-stroke time windows identified in the clinical setting. Following permanent middle cerebral artery occlusion (MCAO) in rats, the penumbra slowly infarcts over a time window of about 3 hours [26, 27]. Incomplete occlusion models may extend the viability of a penumbra but exhibit more experimental variability [28]. Stroke outcome in preclinical models depends not only upon the time point of reperfusion, but also the nature of reperfusion: it has been argued that promptly reversed mechanical occlusion, as in the common rodent transient MCAO model, artificially extends the treatment window relative to clinical stroke by avoiding complicating factors like the no-reflow phenomenon that associate with slow reperfusion [27].
MRI of CBF
Regional cerebral blood flow is a parameter of critical interest in acute stroke and in evaluating reperfusion efficacy following thrombolysis. In the peri-acute phase following thrombolysis, the time window of impaired CBF may extend well beyond reperfusion due to incomplete recanalization, post-thrombolytic hyperemia, and the “no reflow” phenomena [29]. Some clinical and preclinical studies suggest that flow deficits persist weeks into the recovery stage. MRI provides several ways to measure CBF in the form of either dynamic susceptibility contrast imaging (DSC), which applies tracer-kinetic analysis to analyze the first passage of a bolus of an MRI contrast agent, or arterial spin labeling (ASL), which applies a magnetic label to proximal blood in alternating acquisitions and then observes the downstream effects on brain T1-weighted signals.
DSC is the most common clinical method for assessing perfusion in acute stroke due to high sensitivity and a short acquisition time. In this method, a paramagnetic contrast agent based upon gadolinium is rapidly injected as a bolus in a peripheral vein, and rapid imaging tracks the bolus passage through the brain. To correct for intravascular dispersion of bolus material, the tissue response is deconvolved with a local arterial input function [30]. Relative indices can be obtained for both CBF and CBV. The mean transit time (MTT), which is the ratio CBV/CBF by the central volume theorem [31], is particularly useful for visualizing altered perfusion as hyperintense regions with reduced CBF and potentially elevated CBV.
ASL provides an alternative to DSC that avoids the use of contrast agent. Due to an inherently weak signal, repeated signal averaging is required in order for ASL to build useful contrast. This requirement has restricted time-critical clinical applications, but modern implementations of pseudo-continuous ASL on clinical scanners [32] are driving a resurgence of interest in this method for a wide variety of clinical perfusion measurements, including stroke [33].
For pre-clinical stroke research in small animals, ASL offers a superior set of capabilities relative to DSC methods in terms of CBF measurements but sacrifices information about delay and dispersion that can be useful in acute-phase predictive models. DSC perfusion generally provides only relative measurements of CBF, and artifactual results can be obtained when the blood brain barrier is compromised due to leakage of contrast agent [30]. Due to the short MTT in rats and mice, very rapid imaging is required to adequately sample a bolus passage using DSC, and this limits volumetric coverage and image resolution. Conversely, ASL extends the time window for imaging, enabling whole brain coverage, higher spatial resolution, and repeated imaging. Long delays associated with collateral blood flow can reduce the accuracy of ASL measurements of CBF in principle, although these arterial transit artifacts have diagnostic quality in patients [34]. Arterial transit delays generally are less problematic in small rodent brains, due to shorter blood transit times, and also higher magnetic fields (typically used for rodent MRI), where the longitudinal relaxation time of water (T1) is lengthened. However, as a practical matter, manufacturers of preclinical small-bore scanners generally do not provide useful versions of continuous ASL, which requires dedicated RF labeling and imaging coils to work well. Pseudo-continuous ASL, which is the recommended clinical variant of ASL [32] and which does not require specialized hardware, also typically is not available to date on most small-bore systems used for preclinical MRI, although implementation of this method has been reported [35].
Although DSC and ASL are extensively used to define CBF deficits during acute stroke, these methods have been applied much less commonly to the chronic aftermath of stroke; nevertheless, there is some evidence that perfusion impairments extend well into the recovery period. In a heterogeneous clinical population with a mean post-stroke imaging time of 4 years, a study of ASL-based perfusion reported that CBF was decreased on average by 30% in peri-infarct tissue and 15% in the ipsilesional hemisphere; moreover, perfusion deficits correlated with lesion volumes defined by T2-weighted MRI [36]. In a preclinical comparison of sildenafil-treated and control rats, perilesional CBF was elevated in treated rats for up to 8 weeks [37]. These data suggest a need for further investigations of chronic perfusion abnormalities, together with correlations between CBF and functional markers of tissue status, as a potential pathway for intervention.
Lesion Evaluation by MRI
Stroke lesions are indicated by imaging methods that reveal aspects of tissue metabolism or microstructure. The main MRI markers of ischemic stroke include the apparent diffusion coefficient (ADC) and the tissue transverse relation time T2. Each marker evolves during the evolution of a lesion following an ischemic insult. ADC is a better early marker and T2 is a better late marker of lesion volume, although both measurements can be collected efficiently to evaluate lesion status.
ADC is sensitive to cytotoxic edema as an indicator of disrupted cellular metabolism. However, the precise temporal order and relative magnitude of the biological mechanisms contributing to ADC remain controversial. On a very rapid time scale of minutes following complete cessation of flow, initial changes in ADC reflect processes that are potentially reversible, including energy-dependent processes like cytoplasmic streaming [38] or cell swelling secondary to acidosis [39]. When ATP falls below a critical threshold of about 30% [40], membrane sodium and potassium pumps become impaired, and anoxic depolarization produces cellular swelling. MRI detects the shift in water between extracellular and intracellular compartments as a change in water diffusion; the reduced extracellular space further restricts diffusion, and a larger fraction of water enters the intracellular space where large proteins encumber diffusion. Most ADC lesions observed at early time points following stroke include terminal anoxic depolarization and irreversible brain damage in the core of the lesion.
Some prompt reversal of ADC generally is observed in penumbra regions following reperfusion, particularly in preclinical models of focal mechanical reversal using a vascular clip or intraluminal filament. In these cases, secondary delayed injury often occurs within hours, resulting in another reduction of ADC [41–43]. Ultimately, ADC in the lesion core will recover above baseline on a time scale of days as a result of vasogenic edema and loss of membrane structure. For this reason, ADC becomes a poor indicator of stroke lesion in the days following the ischemic insult.
Unlike ADC, tissue T2 is more strongly affected by vasogenic than cytotoxic edema. Lesions rarely are visible using endogenous T2 contrast until several hours after the ischemic insult [44], when vascular fluids penetrate the blood brain barrier due to breakdown of tight endothelial junctions. In contrast to ADC, tissue T2 is a monotonic index of dysfunction that continues to increase in the days and weeks following stroke. At time points longer than 48 hours post-stroke, lesion sizes determined by T2 correlate strongly with those determined by histology [45]. Because cerebrospinal fluid (CSF) also has a long T2, in practice it is necessary to either suppress (e.g., by FLAIR MRI) or identify CSF in order to use automated techniques to segment lesion volumes based upon T2.
Figure 1a shows typical results for T2 and ADC acquired at 48 hours after reversible 1-hour MCAO in a murine stroke model. At this time point, T2 is elevated in the stroke core region and also in cortical boundary areas, as demonstrated by T2w imaging at later time points (Fig. 1b) and by immunohistology at 8 weeks (Fig. 1c), demonstrated infiltrating vasogenic edema at the early time point. ADC is still depressed at 48 hours throughout the MCAO-supplied territory, but ADC distributions at 4 and 8 weeks (not shown) simply differentiate tissue from CSF; at this time point, an enlarged lateral ventricle accompanies the tissue loss.
Figure 1.
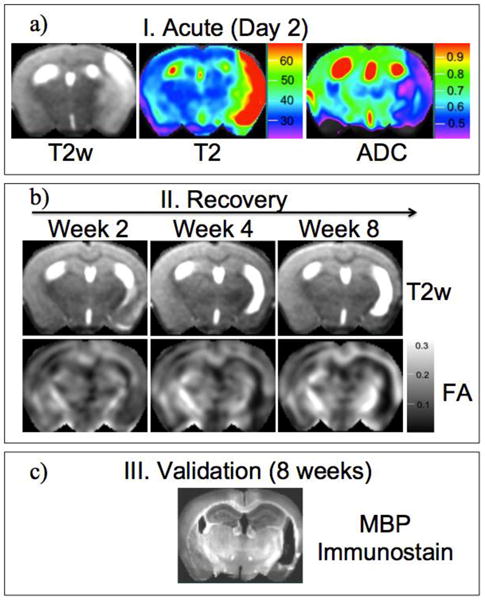
A typical preclinical design in a single mouse; a) the initial stroke infarct following 1-hour transient MCA occlusion characterized using T2-weighted imaging (T2w), quantitative T2 (ms), and the apparent diffusion coefficient (ADC in μm2/ms); b) longitudinal imaging of T2w signal and fractional anisotropy (FA) to assess renormalization of edema and remodeling of white matter as correlates for behavior (not shown); c) immunohistochemistry (MBP = myelin basic protein, converted from green florescence to gray-scale image for comparison with FA) to validate source of MRI signal.
Structural connectivity: imaging of white matter by diffusion MRI
MRI provides a powerful method for assessing indices of white matter integrity and remodeling following ischemic stroke through diffusion-based methods, which are sensitive to the random Brownian motion of water molecules and can probe tissue microstructure at spatial scales much smaller than the image resolution. In a heterogeneous environment like the brain, molecular mobility is locally anisotropic due to diffusional hindrances that arise from axonal membranes, myelin sheaths, or other geometrically ordered boundaries. By applying diffusion-weighting gradients along a series of different directions, preferential diffusion orientations can be identified. Using typical MRI spatial resolutions, voxels that exhibit clear biases in the direction of diffusion generally lie along major white matter pathways.
The simplest representation of diffusion anisotropy is obtained by diffusion tensor imaging (DTI), which depicts the net distribution of all diffusion within an image voxel as an ellipsoid; along major white matter bundles, the longest axis of the ellipsoid orients along the direction of the tract [46]. DTI requires the sampling of a minimum of six diffusion directions, but higher angular sampling densities provide more uniform sensitivity to all possible tract orientations [47]. Fractional anisotropy (FA) is a scalar representation of the local bias in diffusion orientation, with values confined between zero (isotropic diffusion with a spherical distribution) and one (a pencil-shaped tract along a single direction). The directionality defined by local diffusion tensors enables “tractography” along major white matter tracts [48], but achieving accurate pathways remains a topic of research due to complexities associated with crossing fibers and limited spatial resolution.
Measurements of FA by DTI are often considered as surrogates for white matter integrity in the context of stroke and other pathologies, but the underlying sources of ischemia-induced modulation of FA may be complex. In densely packed white matter bundles, axonal membranes and myelination both contribute to FA, with the former arguably producing a greater contribution [49]. Focal ischemia models have correlated FA reductions with both axonal loss and demyelination [50, 51]. More generally following tissue damage, and particularly in gray matter, several studies have cautioned against interpreting FA as a simple combination of axonal density and white matter myelination. FA correlated strongly with histological evidence for oriented reactive astrocytes following cervical cord damage in a rat model [52], and diffusion anisotropies arising in a rat model of traumatic brain injury were heavily influenced by gliosis, particularly in cortical areas [53]. The latter result was attributed both to microstructural anisotropies in fibrous astrocytes as well as to a tendency of gliosis to organize along lesion boundaries. As a marker of white matter integrity following stroke, DTI should be interpreted with caution, particularly in regions not clearly associated with major white matter tracts.
Most clinical DTI studies of motor pathways during stroke recovery have focused upon the pyramidal tract as a common outflow pathway that is subject to Wallerian anterograde degeneration downstream from motor stroke [54]. When stroke directly damages a portion of the corticospinal tract, as determined by DTI, the location of damage was found to be far more predictive of recovery outcome than the size of the lesion, and acute-stage DTI predicted motor outcome better than clinical scores [55].
Numerous preclinical studies of stroke recovery have incorporated DTI to assess the damage and recovery to white matter pathways or to measure changing DTI signals in penumbra regions. The majority of these studies have focused on the core and periphery/penumbra of the stroke lesion, rather than on major white matter bundles like the corpus collosum, using MCAO models of focal stroke in rats [37, 56–59]. Within hours of ischemic onset, FA drops in ischemic regions and reaches a nadir at several days post-stroke [59]. FA does not recover in ischemic core regions that exhibit non-reversible elevation of T2-weighted MRI signals, whereas regions exhibiting transient changes in T2 and ADC also show a subsequent recovery of FA over a period of many weeks [56].
Recovery of FA in penumbra regions occurs most rapidly during the first two weeks following stoke, with continued slow increases in FA for many weeks thereafter [37]. Penumbra FA values reportedly renormalize to pre-lesion or contralesional FA over several weeks [37, 56, 58, 59]. In some rats, the normal evolution of stroke recovery produces an “overshoot” of FA beyond contralesional values [58], and the overshoot has been reported to be consistent within penumbra border zones in cohorts treated by neural progenitor cells [56] or sildenafil [37]. In these studies, FA correlated with qualitative histological evidence for increased axonal density, suggestive of axonal remodeling along a lesion border zone. Consistent with clinical observations, experimental stroke in a rat model reduced FA in major white matter tracts (corpus callosum, cerebral peduncles, internal capsule) outside the core and penumbra areas due to anterograde or retrograde degeneration or widespread edema, and motor recovery correlated with preservation or restoration of corticospinal tract FA [60].
Figure 1b shows longitudinal FA results in a murine model to qualitatively illustrate recovery of FA at the level of the internal capsules, which form part of the corticospinal tract. In this animal, FA clearly increases for at least 8 weeks following stroke in the ipsilesional capsule and for 4 weeks in the contralesional capsule. As noted by by van Meer [60], gradually resolving edema produces time-changing cerebral swelling and midline shifts that complicate quantitative longitudinal analysis of FA along major white matter tracts.
Angiogenesis/Vascular imaging
There are no MRI methods that are specific for angiogenesis, a process that is thought to be beneficial during recovery in penumbral brain regions but one that is incompletely understood [61]. However, MRI provides methods for probing the integrity of the blood brain barrier (BBB) and for determining gross indices of vascular morphology, and these methods may prove useful for in vivo studies of angiogenesis.
Increased BBB permeability can be associated with both ischemic injury at early time points and vascular remodeling during later periods. Most clinical observations of hyper-intense MRI signal after administration of gadolinium occur during the acute phase when gadolinium-based contrast agents are used to assess perfusion deficits; these clinical observations presumably reflect ischemic injury. Due to the relative large size of these agents, leakage is slow and sometimes only apparent on follow-up scans, and spatial delineation of BBB dysfunction is non-specific due to gadolinium deposition in the cerebrospinal fluid [62]. Similarly in some rodent models of stroke, large changes are observed in the permeability of the blood-brain barrier to gadolinium complexes in the first few days [63] and presumably reflect persistent vascular damage. A few preclinical studies have demonstrated increased BBB permeability to gadolinium in penumbral regions up to 4 weeks [63, 64], and these results may represent vascular remodeling.
Another MRI method detects changes in vascular morphology by employing measurements of both total and microvascular blood volume after injection of contrast agent in order to compute an average vessel size or density [65]. Several alternative formulations of this MRI-based “vessel size index” or “microvascular density” attempt to be more quantitative or eliminate dependence on the contrast agent dose [66, 67]. Oncological applications have compared MRI indices with vessel immunostaining and demonstrated good correspondence [65, 66] with little differentiation between alternative formulations [68]. A rat stroke model also demonstrated good correspond between MRI and immunostaining except in the lesion core [69], where penetration of MRI intravascular contrast agent may be limited. Human post-mortem studies have reported increased microvessel density in penumbrae [70], whereas rat studies indicate a decrease in the MRI-derived quantity at several weeks post-stroke in penumbrae [63, 69].
To facilitate high-quality human studies, contrast agents used for studying vascular morphology ideally should have a long blood half-life to facilitate high-resolution imaging and a strong paramagnetic effect to provide robust measurements of CBV. Unfortunately, the class of small iron-based MRI contrast agents that are routinely used for preclinical imaging are not yet approved as MRI agents for clinical use, although initial clinical studies using ferumoxytol have been reported [71].
Functional status: task-based fMRI
Although a major focus of stroke treatment and associated imaging is tissue salvage and the assessment of peri-infarct status, remote dysfunction and plasticity often result from focal stroke [72]. Clinical and preclinical fMRI efforts to date have focused primarily on elucidating the nature of functional remapping of brain activity in relation to behavioral outcomes, but ultimately the goal of imaging is to provide predictive models of treatment benefits in order to guide approaches and stratify clinical trials, and to identify potentially reversible phenomena like diaschisis that might be treated by directed therapies.
There are numerous methods for non-invasively assessing changes in task-induced brain function based upon PET, SPECT, and MRI. Among these methods, fMRI using blood oxygen level dependent (BOLD) signal is the principle clinical method, and preclinical methods employ BOLD signal or CBV-weighted imaging after injection of a blood pool contrast agent. The preclinical CBV method significantly improves detection power relative to endogenous contrast mechanisms [73], but BOLD fMRI also has become an efficient preclinical tool due to the proliferation of very high-field small-bore scanners. While the CBV-weighted method offers a relatively simple index of vascular-mediated activation in the form of the percentage CBV change, BOLD signal depends upon a combination of competing changes in CBV, CBF, and oxygen utilization plus a strong dependence upon baseline physiology in the form of the local blood oxygenation [74].
Although there is a tremendous heterogeneity in clinical stroke foci and recovery, task-induced fMRI studies of upper limb recovery in patients have found early fMRI patterns of activation that include perilesional shifts of response foci and contralesional activation [75]. Greater injury generally associates with less lateralized fMRI responses [72], greater recovery associates with maintenance or reinstatement of normal brain activation patterns, and poor recovery associates with persistent contralesional fMRI signal [75]. Diaschisis has been implicated by transient post-stroke behavioral deficits and lack of fMRI signal in interconnected cortical regions outside the perfusion territory affected by the stroke [9].
In parallel with these clinical reports, preclinical fMRI investigations of rat stroke model functional recovery using forepaw stimulation have converged upon similar findings. Several days after unilateral permanent focal ischemia, fMRI signals were detected in the lesion periphery of affected cortex and also in ipsilesional cortex [76]. At several weeks post-stroke, similar patterns were observed [77] but with reduced contralesional involvement and greater laterization [76]. Larger lesions correlated with decreased laterality in fMRI responses [78], and reinstatement of fMRI signal in affected cortex correlated with reinstatement of electrical responsiveness [79] and improvement of neurological status [78]. Within affected cortex, fMRI responses generally have been reported to correlate with tissue structural integrity as assessed by ADC or T2 [79] or with perfusion status [80, 81]. However, behavioral dysfunction can persist longer than fMRI or structural deficits [80] and small changes in task-induced electrical activity can occur in the absence of a measurable BOLD response [79].
Several specific concerns have been raised for post-stroke function brain mapping. Although it may be possible in principle to use dynamic imaging of glucose metabolism [82] to study stroke clinically, or techniques like manganese uptake to study preclinical stroke recovery [83], studies to date have inferred function from vascular reactivity and thus depend upon the integrity of the local neurovascular unit, as well as the distributed brain circuitry. Perilesional BOLD responses to tactile stimuli following sensorimotor stroke were reported in a small subject cohort during subacute (1 week) and chronic phases (> 1 month) of recovery but not at an intermediate time point (2 weeks); secondary hyperperfusion of post-ischemic tissue might explain this phenomenon and represent a decoupling of BOLD and electrical signals [84]. This result emphasizes the need to supplement BOLD studies with measurements of tissue perfusion (e.g., ASL) to help disentangle competing effects from neurovascular reactivity and altered baseline perfusion; the latter can mask BOLD signal changes by increasing basal blood oxygenation. Preclinical results support the notion of BOLD-reactivity decoupling due to hyperperfusion; perilesional CBV responses were significantly larger than co-localized BOLD responses at two weeks after transient focal MCA occlusion [85]. Hemodynamic responses to sensorimotor stimulation also are somewhat delayed in aftermath of stroke in rat models [85, 86], and responses in stroke patients also are variable and generally delayed [87]. This can complicate detection of fMRI activation, although longer stimulation periods mitigate this problem.
Functional connectivity: resting state fMRI
Task-induced fMRI studies of stroke recovery require active participation of functionally impaired patients, and additionally rely upon specific task designs that might not activate all tissue of interest. These limitations have spurred interest in the use of resting-state fMRI (rfMRI), or “functional connectivity” [88], as a method to simultaneously interrogate all functional networks throughout the brain without requiring patient compliance. Paired with diffusion MRI as a complementary measurement of “structural connectivity” for the major white matter tracts, the ability to probe whole brain function in the absence of a task potentially could explain a greater degree of the wide behavioral variations following stroke [54].
The temporal structure of rfMRI signal (typically based upon BOLD signal, but also including methods sensitive to CBF or CBV) appears as apparently random fluctuations in any brain region. The signal contains significant contributions from uninteresting sources, including noise from detectors, subject movement, and cardiac and respiratory cycles. However, a portion of the signal correlates with fluctuations in electrical activity and can be used to reproducibly detect a consistent set of brain networks across healthy subjects [89]. The component of “noise” that is related to brain activity provides a window into the dynamic electrophysiology of brain activity viewed through a very slow filter imposed by the blood supply.
One of the notable features of rfMRI functional connectivity in healthy brain is the high degree of inter-hemispheric symmetry: right motor cortex correlates most strongly with left motor cortex, for instance. In the aftermath of stroke, a consistent finding in patients has been a reduction of inter-hemispheric connectivity across homologous cortical areas governing sensory perception, motor function, and visual attention [54, 90]. In the acute stage shortly after stroke, the degree of inter-hemispheric connectivity, but not intra-hemispheric connectivity, correlated with the severity of visual neglect or upper extremity impairment [91, 92]. Similarly, progressive restoration of inter-hemispheric connectivity correlated with progressive recovery of motor function [92]. Local lesions often lead to remote changes in functional connectivity that cannot be explained simply in terms of local structural damage [90, 91].
Preclinical rfMRI studies of stroke recovery in anesthetized rats using MRI or optical imaging also have emphasized the loss of inter-hemispheric connectivity between homotopic cortical regions as a key correlate of both stroke severity and functional recovery [60, 83, 93]. Early stages after experimental stroke exhibit decreased inter-hemispheric connectivity and increased intra-hemispheric connectivity, and both of these findings are consistent with changes in the transport rate of a locally injected tracer of neuronal tracts [83].
Interpretation of rfMRI data is subject to the same caveats attached to task-induced fMRI signal, plus some additional concerns. Changes in rfMRI connectivity in principle could reflect a loss of vascular reactivity that is not representative of local neuronal activity, or a loss of BOLD sensitivity due to increased blood oxygenation secondary to hyperperfusion or blunted metabolism. The current emphasis on inter-hemispheric connectivity as a marker for stroke severity and functional recovery might reflect a measurement bias: due to strong homotopic inter-hemispheric connectivity in healthy brain, loss of this connectivity following stroke is easiest to detect. Finally, rfMRI is based upon signal correlations that typically contain significant contributions from non-neuronal sources, and inferences can be corrupted by group-wise or time-wise differences in subject motion or physiology that can be pernicious [94].
Future
Although MRI often is viewed as a relatively mature modality, ongoing improvements in hardware, data acquisition methodologies, and analysis strategies continue to advance imaging capabilities. Some well-developed methods, like efficient ASL, only now are becoming routinely available in clinical practice and on preclinical systems; moreover, new developments like velocity-selective ASL may prove particular useful for quantitative measurements of perfusion following stroke [95]. Parallel imaging can significantly accelerate acquisition rates, which can improve data quality in numerous ways. MRI offers multiple ways to obtain imaging contrast that is relevant to stroke and recovery, yet multimodal methods (e.g., combined PET/MRI) will further expand the information that can be obtained in a single session.
Functional connectivity by rfMRI provides an example application that can be expected to see relatively significant improvements through a combination of acquisition and analysis method. Large arrays of radio frequency receiver coils are enabling accelerated data collection rates by simultaneous acquisition of multiple slices or reduction in the gradient encoding matrix. Such methods can be use to obtain whole-brain coverage with sampling rates that enable filtering of high-frequency cardiac and respiratory noise [94], thus improving the specificity of information provided by rfMRI. There still are unsolved questions about how much data to collect, how to spatially parcellate data into networks, and how to interpret data within and across subjects [96]. Given that relatively subtle effects like learning can influence rfMRI data [97, 98], there certainly must be more to learn about stroke recovery from rfMRI.
Gadolinium remains the only contrast agent now clinically approved for MRI. Preclinical studies clearly demonstrate the advantages of blood-pool iron oxide agents for functional imaging [73] and the possibility to obtain high-resolution images of CBV or indices of the microvasculature like the mean vessel size [63]. Human studies have employed similar agents in preliminary studies [71, 99], but regulatory approval will be required for routine clinical usage.
The advent of simultaneous PET and MRI offers the possibility to bring the molecular specificity of PET back to stroke research. PET is poorly suited to the time-sensitive realm of acute stroke, and the ability of PET systems to perform only a single measurement per session is a drawback for studies of stroke recovery using PET-only systems. However, concurrent PET complements anatomical and functional MRI with targeted molecular probes. Within this context of simultaneous PET/MRI, measurements of glucose metabolism can help interpret suspected diaschisis or luxury perfusion, or radioligands that target translocator proteins can assess neuro-inflammation as a possible sign of early lesion progression or late reactive astrocytes [100–102]. A multitude of combined approaches can be envisioned for clinical and basic studies, including such speculative possibilities as using MRI for tracking injected cells and PET for demonstrating biological activity, or combining evolving PET methods for imaging angiogenesis with complementary MRI markers [17]. New probes that target histone deacetylases [103] could facilitate imaging of oligodendrogenesis [104]. Such new research opportunities may spur radioligand development for novel preclinical and clinical imaging of stroke and other neuropathologies.
Acknowledgments
Funding: This work was funded in part by a grant from National Institutes of Health (P01NS055104).
Footnotes
Compliance with Ethical Standards
Conflict of Interest: The authors have no conflicts of interest to report.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Literature
- 1.Barquera S, Pedroza-Tobias A, Medina C, Hernandez-Barrera L, Bibbins-Domingo K, Lozano R, Moran AE. Global Overview of the Epidemiology of Atherosclerotic Cardiovascular Disease. Arch Med Res. 2015;46(5):328–38. doi: 10.1016/j.arcmed.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Flynn RW, MacWalter RS, Doney AS. The cost of cerebral ischaemia. Neuropharmacology. 2008;55(3):250–6. doi: 10.1016/j.neuropharm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 3.Miller EL, Murray L, Richards L, Zorowitz RD, Bakas T, Clark P, Billinger SA N American Heart Association Council on Cardiovascular, and C. the Stroke. Comprehensive overview of nursing and interdisciplinary rehabilitation care of the stroke patient: a scientific statement from the American Heart Association. Stroke. 2010;41(10):2402–48. doi: 10.1161/STR.0b013e3181e7512b. [DOI] [PubMed] [Google Scholar]
- 4.Kleindorfer D, Lindsell CJ, Brass L, Koroshetz W, Broderick JP. National US estimates of recombinant tissue plasminogen activator use: ICD-9 codes substantially underestimate. Stroke. 2008;39(3):924–8. doi: 10.1161/STROKEAHA.107.490375. [DOI] [PubMed] [Google Scholar]
- 5.Cramer SC. Drugs to Enhance Motor Recovery After Stroke. Stroke. 2015;46(10):2998–3005. doi: 10.1161/STROKEAHA.115.007433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krieger DW. Therapeutic drug approach to stimulate clinical recovery after brain injury. Front Neurol Neurosci. 2013;32:76–87. doi: 10.1159/000346419. [DOI] [PubMed] [Google Scholar]
- 7.Xing C, Hayakawa K, Lok J, Arai K, Lo EH. Injury and repair in the neurovascular unit. Neurol Res. 2012;34(4):325–30. doi: 10.1179/1743132812Y.0000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–98. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nhan H, Barquist K, Bell K, Esselman P, Odderson IR, Cramer SC. Brain function early after stroke in relation to subsequent recovery. J Cereb Blood Flow Metab. 2004;24(7):756–63. doi: 10.1097/01.WCB.0000122744.72175.9C. [DOI] [PubMed] [Google Scholar]
- 10.Jaillard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain. 2005;128(Pt 5):1122–38. doi: 10.1093/brain/awh456. [DOI] [PubMed] [Google Scholar]
- 11.Hossmann KA, Kleihues P. Reversibility of ischemic brain damage. Arch Neurol. 1973;29(6):375–84. doi: 10.1001/archneur.1973.00490300037004. [DOI] [PubMed] [Google Scholar]
- 12.Peisker T, Koznar B, Stetkarova I, Widimsky P. Acute stroke therapy: A review. Trends Cardiovasc Med. 2016 doi: 10.1016/j.tcm.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Del Zoppo GJ, Saver JL, Jauch EC, Adams HP, Jr C American Heart Association Stroke. Expansion of the time window for treatment of acute ischemic stroke with intravenous tissue plasminogen activator: a science advisory from the American Heart Association/American Stroke Association. Stroke. 2009;40(8):2945–8. doi: 10.1161/STROKEAHA.109.192535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez RG. Current state of acute stroke imaging. Stroke. 2013;44(11):3260–4. doi: 10.1161/STROKEAHA.113.003229. [DOI] [PubMed] [Google Scholar]
- 15.Wu O, Schwamm LH, Sorensen AG. Imaging stroke patients with unclear onset times. Neuroimaging Clin N Am. 2011;21(2):327–44. xi. doi: 10.1016/j.nic.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen F, Ni YC. Magnetic resonance diffusion-perfusion mismatch in acute ischemic stroke: An update. World J Radiol. 2012;4(3):63–74. doi: 10.4329/wjr.v4.i3.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heiss WD. Radionuclide imaging in ischemic stroke. J Nucl Med. 2014;55(11):1831–41. doi: 10.2967/jnumed.114.145003. [DOI] [PubMed] [Google Scholar]
- 18.Wu O, Koroshetz WJ, Ostergaard L, Buonanno FS, Copen WA, Gonzalez RG, Rordorf G, Rosen BR, Schwamm LH, Weisskoff RM, Sorensen AG. Predicting tissue outcome in acute human cerebral ischemia using combined diffusion- and perfusion-weighted MR imaging. Stroke. 2001;32(4):933–42. doi: 10.1161/01.str.32.4.933. [DOI] [PubMed] [Google Scholar]
- 19.Bouts MJ, I, Tiebosch A, van der Toorn A, Viergever MA, Wu O, Dijkhuizen RM. Early identification of potentially salvageable tissue with MRI-based predictive algorithms after experimental ischemic stroke. J Cereb Blood Flow Metab. 2013;33(7):1075–82. doi: 10.1038/jcbfm.2013.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu O, Sumii T, Asahi M, Sasamata M, Ostergaard L, Rosen BR, Lo EH, Dijkhuizen RM. Infarct prediction and treatment assessment with MRI-based algorithms in experimental stroke models. J Cereb Blood Flow Metab. 2007;27(1):196–204. doi: 10.1038/sj.jcbfm.9600328. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y, Zhou IY, Chan ST, Wang Y, Mandeville ET, Igarashi T, Lo EH, Ji X, Sun PZ. pH-sensitive MRI demarcates graded tissue acidification during acute stroke - pH specificity enhancement with magnetization transfer and relaxation-normalized amide proton transfer (APT) MRI. Neuroimage. 2016;141:242–249. doi: 10.1016/j.neuroimage.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheung JS, Wang E, Lo EH, Sun PZ. Stratification of heterogeneous diffusion MRI ischemic lesion with kurtosis imaging: evaluation of mean diffusion and kurtosis MRI mismatch in an animal model of transient focal ischemia. Stroke. 2012;43(8):2252–4. doi: 10.1161/STROKEAHA.112.661926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ay H, Koroshetz WJ, Vangel M, Benner T, Melinosky C, Zhu M, Menezes N, Lopez CJ, Sorensen AG. Conversion of ischemic brain tissue into infarction increases with age. Stroke. 2005;36(12):2632–6. doi: 10.1161/01.STR.0000189991.23918.01. [DOI] [PubMed] [Google Scholar]
- 24.Ergul A, Hafez S, Fouda A, Fagan SC. Impact of Comorbidities on Acute Injury and Recovery in Preclinical Stroke Research: Focus on Hypertension and Diabetes. Transl Stroke Res. 2016;7(4):248–60. doi: 10.1007/s12975-016-0464-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heiss WD, Kidwell CS. Imaging for prediction of functional outcome and assessment of recovery in ischemic stroke. Stroke. 2014;45(4):1195–201. doi: 10.1161/STROKEAHA.113.003611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duong TQ. Magnetic resonance imaging of perfusion-diffusion mismatch in rodent and non-human primate stroke models. Neurol Res. 2013;35(5):465–9. doi: 10.1179/1743132813Y.0000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hossmann KA. The two pathophysiologies of focal brain ischemia: implications for translational stroke research. J Cereb Blood Flow Metab. 2012;32(7):1310–6. doi: 10.1038/jcbfm.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hossmann KA. Cerebral ischemia: models, methods and outcomes. Neuropharmacology. 2008;55(3):257–70. doi: 10.1016/j.neuropharm.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Ames A, 3rd, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52(2):437–53. [PMC free article] [PubMed] [Google Scholar]
- 30.Wu O, Ostergaard L, Sorensen AG. Technical aspects of perfusion-weighted imaging. Neuroimaging Clin N Am. 2005;15(3):623–37. xi. doi: 10.1016/j.nic.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Meier P, Zierler KL. On the Theory of the Indicator-Dilution Method for Measurement of Blood Flow and Volume. J Appl Physiol. 1954;6:731–744. doi: 10.1152/jappl.1954.6.12.731. [DOI] [PubMed] [Google Scholar]
- 32.Alsop DC, Detre JA, Golay X, Gunther M, Hendrikse J, Hernandez-Garcia L, Lu H, Macintosh BJ, Parkes LM, Smits M, van Osch MJ, Wang DJ, Wong EC, Zaharchuk G. Recommended implementation of arterial spin-labeled perfusion MRI for clinical applications: A consensus of the ISMRM perfusion study group and the European consortium for ASL in dementia. Magn Reson Med. 2014 doi: 10.1002/mrm.25197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: clinical applications in the brain. J Magn Reson Imaging. 2015;41(5):1165–80. doi: 10.1002/jmri.24751. [DOI] [PubMed] [Google Scholar]
- 34.Zaharchuk G. Arterial spin labeling for acute stroke: practical considerations. Transl Stroke Res. 2012;3(2):228–35. doi: 10.1007/s12975-012-0159-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duhamel G, Callot V, Tachrount M, Alsop DC, Cozzone PJ. Pseudo-continuous arterial spin labeling at very high magnetic field (11.75 T) for high-resolution mouse brain perfusion imaging. Magn Reson Med. 2012;67(5):1225–36. doi: 10.1002/mrm.23096. [DOI] [PubMed] [Google Scholar]
- 36.Richardson JD, Baker JM, Morgan PS, Rorden C, Bonilha L, Fridriksson J. Cerebral perfusion in chronic stroke: implications for lesion-symptom mapping and functional MRI. Behav Neurol. 2011;24(2):117–22. doi: 10.3233/BEN-2011-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, Panda S, Davarani SP, Athiraman H, Li Q, Ewing JR, Chopp M. Magnetic resonance imaging investigation of axonal remodeling and angiogenesis after embolic stroke in sildenafil-treated rats. J Cereb Blood Flow Metab. 2008;28(8):1440–8. doi: 10.1038/jcbfm.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harkins KD, Galons JP, Divijak JL, Trouard TP. Changes in intracellular water diffusion and energetic metabolism in response to ischemia in perfused C6 rat glioma cells. Magn Reson Med. 2011;66(3):859–67. doi: 10.1002/mrm.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris NG, Zilkha E, Houseman J, Symms MR, Obrenovitch TP, Williams SR. The relationship between the apparent diffusion coefficient measured by magnetic resonance imaging, anoxic depolarization, and glutamate efflux during experimental cerebral ischemia. J Cereb Blood Flow Metab. 2000;20(1):28–36. doi: 10.1097/00004647-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 40.Obrenovitch TP, Scheller D, Matsumoto T, Tegtmeier F, Holler M, Symon L. A rapid redistribution of hydrogen ions is associated with depolarization and repolarization subsequent to cerebral ischemia reperfusion. J Neurophysiol. 1990;64(4):1125–33. doi: 10.1152/jn.1990.64.4.1125. [DOI] [PubMed] [Google Scholar]
- 41.Leithner C, Fuchtemeier M, Jorks D, Mueller S, Dirnagl U, Royl G. Infarct Volume Prediction by Early Magnetic Resonance Imaging in a Murine Stroke Model Depends on Ischemia Duration and Time of Imaging. Stroke. 2015;46(11):3249–59. doi: 10.1161/STROKEAHA.114.007832. [DOI] [PubMed] [Google Scholar]
- 42.Dijkhuizen RM, Knollema S, van der Worp HB, Ter Horst GJ, De Wildt DJ, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Dynamics of cerebral tissue injury and perfusion after temporary hypoxia-ischemia in the rat: evidence for region-specific sensitivity and delayed damage. Stroke. 1998;29(3):695–704. doi: 10.1161/01.str.29.3.695. [DOI] [PubMed] [Google Scholar]
- 43.van Lookeren Campagne M, Thomas GR, Thibodeaux H, Palmer JT, Williams SP, Lowe DG, van Bruggen N. Secondary reduction in the apparent diffusion coefficient of water, increase in cerebral blood volume, and delayed neuronal death after middle cerebral artery occlusion and early reperfusion in the rat. J Cereb Blood Flow Metab. 1999;19(12):1354–64. doi: 10.1097/00004647-199912000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Moseley ME, Cohen Y, Mintorovitch J, Chileuitt L, Shimizu H, Kucharczyk J, Wendland MF, Weinstein PR. Early detection of regional cerebral ischemia in cats: comparison of diffusion and T2 weighted MRI and spectroscopy. Magn Reson Med. 1990;14:330–346. doi: 10.1002/mrm.1910140218. [DOI] [PubMed] [Google Scholar]
- 45.Milidonis X, Marshall I, Macleod MR, Sena ES. Magnetic resonance imaging in experimental stroke and comparison with histology: systematic review and meta-analysis. Stroke. 2015;46(3):843–51. doi: 10.1161/STROKEAHA.114.007560. [DOI] [PubMed] [Google Scholar]
- 46.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13(4):534–46. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 47.Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: a Monte Carlo study. Magn Reson Med. 2004;51(4):807–15. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- 48.Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44(4):625–32. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 49.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 50.Fan SJ, Lee FY, Cheung MM, Ding AY, Yang J, Ma SJ, Khong PL, Wu EX. Bilateral substantia nigra and pyramidal tract changes following experimental intracerebral hemorrhage: an MR diffusion tensor imaging study. NMR Biomed. 2013;26(9):1089–95. doi: 10.1002/nbm.2922. [DOI] [PubMed] [Google Scholar]
- 51.Tuor UI, Morgunov M, Sule M, Qiao M, Clark D, Rushforth D, Foniok T, Kirton A. Cellular correlates of longitudinal diffusion tensor imaging of axonal degeneration following hypoxic-ischemic cerebral infarction in neonatal rats. Neuroimage Clin. 2014;6:32–42. doi: 10.1016/j.nicl.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwartz ED, Duda J, Shumsky JS, Cooper ET, Gee J. Spinal cord diffusion tensor imaging and fiber tracking can identify white matter tract disruption and glial scar orientation following lateral funiculotomy. J Neurotrauma. 2005;22(12):1388–98. doi: 10.1089/neu.2005.22.1388. [DOI] [PubMed] [Google Scholar]
- 53.Budde MD, Janes L, Gold E, Turtzo LC, Frank JA. The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain. 2011;134(Pt 8):2248–60. doi: 10.1093/brain/awr161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thiel A, Vahdat S. Structural and resting-state brain connectivity of motor networks after stroke. Stroke. 2015;46(1):296–301. doi: 10.1161/STROKEAHA.114.006307. [DOI] [PubMed] [Google Scholar]
- 55.Puig J, Pedraza S, Blasco G, Daunis IEJ, Prados F, Remollo S, Prats-Galino A, Soria G, Boada I, Castellanos M, Serena J. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol. 2011;32(5):857–63. doi: 10.3174/ajnr.A2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Q, Zhang ZG, Ding GL, Silver B, Zhang L, Meng H, Lu M, Pourabdillah-Nejed DS, Wang L, Savant-Bhonsale S, Li L, Bagher-Ebadian H, Hu J, Arbab AS, Vanguri P, Ewing JR, Ledbetter KA, Chopp M. MRI detects white matter reorganization after neural progenitor cell treatment of stroke. Neuroimage. 2006;32(3):1080–9. doi: 10.1016/j.neuroimage.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 57.Li L, Jiang Q, Ding G, Zhang L, Zhang ZG, Li Q, Panda S, Kapke A, Lu M, Ewing JR, Chopp M. MRI identification of white matter reorganization enhanced by erythropoietin treatment in a rat model of focal ischemia. Stroke. 2009;40(3):936–41. doi: 10.1161/STROKEAHA.108.527713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Zijden JP, van der Toorn A, van der Marel K, Dijkhuizen RM. Longitudinal in vivo MRI of alterations in perilesional tissue after transient ischemic stroke in rats. Exp Neurol. 2008;212(1):207–12. doi: 10.1016/j.expneurol.2008.03.027. [DOI] [PubMed] [Google Scholar]
- 59.Pitkonen M, Abo-Ramadan U, Marinkovic I, Pedrono E, Hasan KM, Strbian D, Durukan A, Tatlisumak T. Long-term evolution of diffusion tensor indices after temporary experimental ischemic stroke in rats. Brain Res. 2012;1445:103–10. doi: 10.1016/j.brainres.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 60.van Meer MP, Otte WM, van der Marel K, Nijboer CH, Kavelaars A, van der Sprenkel JW, Viergever MA, Dijkhuizen RM. Extent of bilateral neuronal network reorganization and functional recovery in relation to stroke severity. J Neurosci. 2012;32(13):4495–507. doi: 10.1523/JNEUROSCI.3662-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arai K, Jin G, Navaratna D, Lo EH. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J. 2009;276(17):4644–52. doi: 10.1111/j.1742-4658.2009.07176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ostwaldt AC, Rozanski M, Schaefer T, Ebinger M, Jungehulsing GJ, Villringer K, Fiebach JB. Hyperintense acute reperfusion marker is associated with higher contrast agent dosage in acute ischaemic stroke. Eur Radiol. 2015;25(11):3161–6. doi: 10.1007/s00330-015-3749-5. [DOI] [PubMed] [Google Scholar]
- 63.Moisan A, I, Favre M, Rome C, Grillon E, Naegele B, Barbieux M, De Fraipont F, Richard MJ, Barbier EL, Remy C, Detante O. Microvascular plasticity after experimental stroke: a molecular and MRI study. Cerebrovasc Dis. 2014;38(5):344–53. doi: 10.1159/000368597. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015;12:26. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dennie J, Mandeville JB, Boxerman JL, Packard SD, Rosen BR, Weisskoff RM. NMR imaging of changes in vascular mophology due to tumor angiogenesis. Magn Reson Med. 1998;40(6):793–799. doi: 10.1002/mrm.1910400602. [DOI] [PubMed] [Google Scholar]
- 66.Tropres I, Lamalle L, Peoc’h M, Farion R, Usson Y, Decorps M, Remy C. In vivo assessment of tumoral angiogenesis. Magn Reson Med. 2004;51(3):533–41. doi: 10.1002/mrm.20017. [DOI] [PubMed] [Google Scholar]
- 67.Jensen JH, Chandra R. MR imaging of microvasculature. Magn Reson Med. 2000;44(2):224–230. doi: 10.1002/1522-2594(200008)44:2<224::aid-mrm9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 68.Lemasson B, Valable S, Farion R, Krainik A, Remy C, Barbier EL. In vivo imaging of vessel diameter, size, and density: a comparative study between MRI and histology. Magn Reson Med. 2013;69(1):18–26. doi: 10.1002/mrm.24218. [DOI] [PubMed] [Google Scholar]
- 69.Bosomtwi A, Jiang Q, Ding GL, Zhang L, Zhang ZG, Lu M, Ewing JR, Chopp M. Quantitative evaluation of microvascular density after stroke in rats using MRI. J Cereb Blood Flow Metab. 2008;28(12):1978–87. doi: 10.1038/jcbfm.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Krupinski J, Kaluza J, Kumar P, Kumar S, Wang JM. Some remarks on the growth-rate and angiogenesis of microvessels in ischemic stroke. Morphometric and immunocytochemical studies. Patol Pol. 1993;44(4):203–9. [PubMed] [Google Scholar]
- 71.Christen T, Ni W, Qiu D, Schmiedeskamp H, Bammer R, Moseley M, Zaharchuk G. High-resolution cerebral blood volume imaging in humans using the blood pool contrast agent ferumoxytol. Magn Reson Med. 2012 doi: 10.1002/mrm.24500. [DOI] [PubMed] [Google Scholar]
- 72.Cramer SC. Functional imaging in stroke recovery. Stroke. 2004;35(11 Suppl 1):2695–8. doi: 10.1161/01.STR.0000143326.36847.b0. [DOI] [PubMed] [Google Scholar]
- 73.Mandeville JB. IRON fMRI measurements of CBV and implications for BOLD signal. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown GG, Perthen JE, Liu TT, Buxton RB. A primer on functional magnetic resonance imaging. Neuropsychol Rev. 2007;17(2):107–25. doi: 10.1007/s11065-007-9028-8. [DOI] [PubMed] [Google Scholar]
- 75.Buma FE, Lindeman E, Ramsey NF, Kwakkel G. Functional neuroimaging studies of early upper limb recovery after stroke: a systematic review of the literature. Neurorehabil Neural Repair. 2010;24(7):589–608. doi: 10.1177/1545968310364058. [DOI] [PubMed] [Google Scholar]
- 76.Dijkhuizen RM, Ren J, Mandeville JB, Wu O, Ozdag FM, Moskowitz MA, Rosen BR, Finklestein SP. Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc Natl Acad Sci U S A. 2001;98(22):12766–71. doi: 10.1073/pnas.231235598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abo M, Chen Z, Lai LJ, Reese T, Bjelke B. Functional recovery after brain lesion--contralateral neuromodulation: an fMRI study. Neuroreport. 2001;12(7):1543–7. doi: 10.1097/00001756-200105250-00048. [DOI] [PubMed] [Google Scholar]
- 78.Dijkhuizen RM, Singhal AB, Mandeville JB, Wu O, Halpern EF, Finklestein SP, Rosen BR, Lo EH. Correlation between Brain Reorganization, Ischemic Damage, and Neurologic Status after Transient Focal Cerebral Ischemia in Rats: A Functional Magnetic Resonance Imaging Study. J Neurosci. 2003;23(2):510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weber R, Ramos-Cabrer P, Justicia C, Wiedermann D, Strecker C, Sprenger C, Hoehn M. Early prediction of functional recovery after experimental stroke: functional magnetic resonance imaging, electrophysiology, and behavioral testing in rats. J Neurosci. 2008;28(5):1022–9. doi: 10.1523/JNEUROSCI.4147-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sicard KM, Henninger N, Fisher M, Duong TQ, Ferris CF. Long-term changes of functional MRI-based brain function, behavioral status, and histopathology after transient focal cerebral ischemia in rats. Stroke. 2006;37(10):2593–600. doi: 10.1161/01.STR.0000239667.15532.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sauter A, Reese T, Porszasz R, Baumann D, Rausch M, Rudin M. Recovery of function in cytoprotected cerebral cortex in rat stroke model assessed by functional MRI. Magn Reson Med. 2002;47(4):759–65. doi: 10.1002/mrm.10123. [DOI] [PubMed] [Google Scholar]
- 82.Villien M, Wey HY, Mandeville JB, Catana C, Polimeni JR, Sander CY, Zurcher NR, Chonde DB, Fowler JS, Rosen BR, Hooker JM. Dynamic functional imaging of brain glucose utilization using fPET-FDG. Neuroimage. 2014;100:192–9. doi: 10.1016/j.neuroimage.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van Meer MP, van der Marel K, Otte WM, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Correspondence between altered functional and structural connectivity in the contralesional sensorimotor cortex after unilateral stroke in rats: a combined resting-state functional MRI and manganese-enhanced MRI study. J Cereb Blood Flow Metab. 2010;30(10):1707–11. doi: 10.1038/jcbfm.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Binkofski F, Seitz RJ. Modulation of the BOLD-response in early recovery from sensorimotor stroke. Neurology. 2004;63(7):1223–9. doi: 10.1212/01.wnl.0000140468.92212.be. [DOI] [PubMed] [Google Scholar]
- 85.Kim YR, I, Huang J, Lee SR, Tejima E, Mandeville JB, van Meer MP, Dai G, Choi YW, Dijkhuizen RM, Lo EH, Rosen BR. Measurements of BOLD/CBV ratio show altered fMRI hemodynamics during stroke recovery in rats. J Cereb Blood Flow Metab. 2005;25(7):820–829. doi: 10.1038/sj.jcbfm.9600084. [DOI] [PubMed] [Google Scholar]
- 86.Dijkhuizen RM, van der Marel K, Otte WM, Hoff EI, van der Zijden JP, van der Toorn A, van Meer MP. Functional MRI and diffusion tensor imaging of brain reorganization after experimental stroke. Transl Stroke Res. 2012;3(1):36–43. doi: 10.1007/s12975-011-0143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Veldsman M, Cumming T, Brodtmann A. Beyond BOLD: optimizing functional imaging in stroke populations. Hum Brain Mapp. 2015;36(4):1620–36. doi: 10.1002/hbm.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34(4):537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 89.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–53. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carter AR, Shulman GL, Corbetta M. Why use a connectivity-based approach to study stroke and recovery of function? Neuroimage. 2012;62(4):2271–80. doi: 10.1016/j.neuroimage.2012.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67(3):365–75. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu H, Qin W, Chen H, Jiang L, Li K, Yu C. Contribution of the resting-state functional connectivity of the contralesional primary sensorimotor cortex to motor recovery after subcortical stroke. PLoS One. 2014;9(1):e84729. doi: 10.1371/journal.pone.0084729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bauer AQ, Kraft AW, Wright PW, Snyder AZ, Lee JM, Culver JP. Optical imaging of disrupted functional connectivity following ischemic stroke in mice. Neuroimage. 2014;99:388–401. doi: 10.1016/j.neuroimage.2014.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Murphy K, Birn RM, Bandettini PA. Resting-state fMRI confounds and cleanup. Neuroimage. 2013;80:349–59. doi: 10.1016/j.neuroimage.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zaharchuk G. Better late than never: the long journey for noncontrast arterial spin labeling perfusion imaging in acute stroke. Stroke. 2012;43(4):931–2. doi: 10.1161/STROKEAHA.111.644344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Smith SM, Vidaurre D, Beckmann CF, Glasser MF, Jenkinson M, Miller KL, Nichols TE, Robinson EC, Salimi-Khorshidi G, Woolrich MW, Barch DM, Ugurbil K, Van Essen DC. Functional connectomics from resting-state fMRI. Trends Cogn Sci. 2013;17(12):666–82. doi: 10.1016/j.tics.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lewis CM, Baldassarre A, Committeri G, Romani GL, Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106(41):17558–63. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Albert NB, Robertson EM, Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19(12):1023–7. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qiu D, Zaharchuk G, Christen T, Ni WW, Moseley ME. Contrast-enhanced functional blood volume imaging (CE-fBVI): Enhanced sensitivity for brain activation in humans using the ultrasmall superparamagnetic iron oxide agent ferumoxytol. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Catana C, Drzezga A, Heiss WD, Rosen BR. PET/MRI for neurologic applications. J Nucl Med. 2012;53(12):1916–25. doi: 10.2967/jnumed.112.105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Virdee K, Cumming P, Caprioli D, Jupp B, Rominger A, Aigbirhio FI, Fryer TD, Riss PJ, Dalley JW. Applications of positron emission tomography in animal models of neurological and neuropsychiatric disorders. Neurosci Biobehav Rev. 2012;36(4):1188–216. doi: 10.1016/j.neubiorev.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 102.Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res. 2015;6(1):4–8. doi: 10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]
- 103.Wang C, Schroeder FA, Wey HY, Borra R, Wagner FF, Reis S, Kim SW, Holson EB, Haggarty SJ, Hooker JM. In vivo imaging of histone deacetylases (HDACs) in the central nervous system and major peripheral organs. J Med Chem. 2014;57(19):7999–8009. doi: 10.1021/jm500872p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang ZG, Chopp M. Promoting brain remodeling to aid in stroke recovery. Trends Mol Med. 2015;21(9):543–8. doi: 10.1016/j.molmed.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]


