Abstract
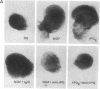
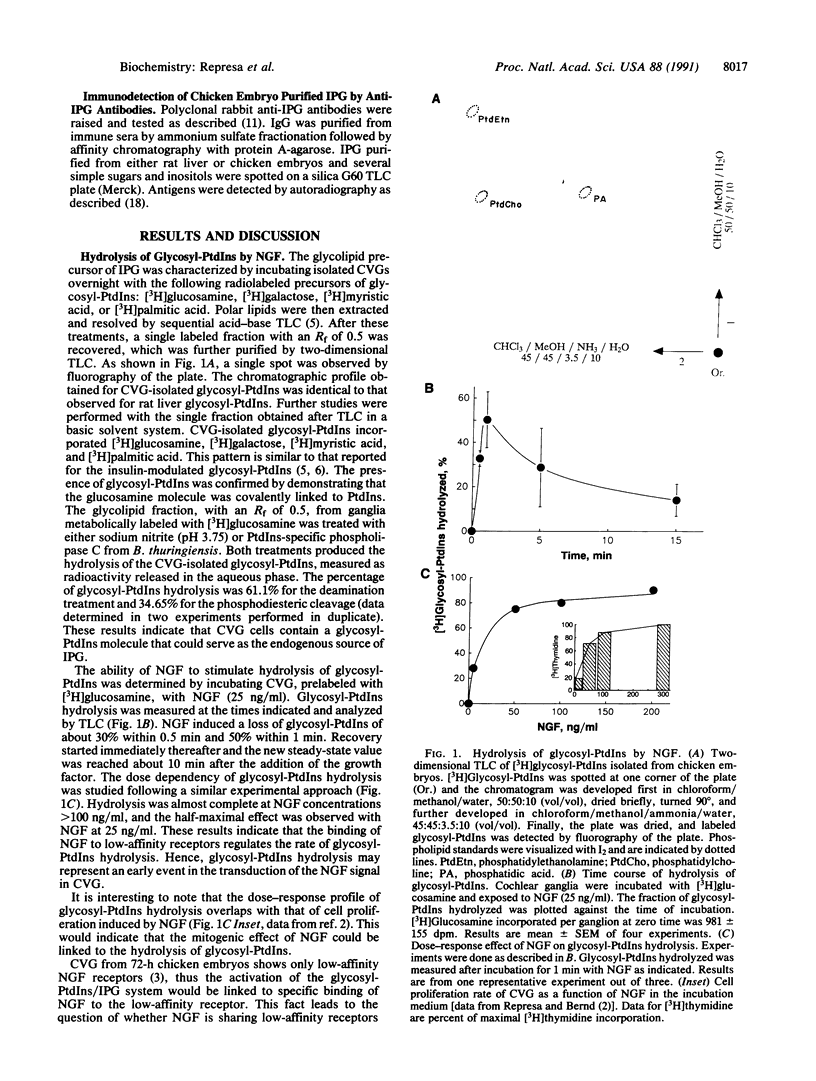
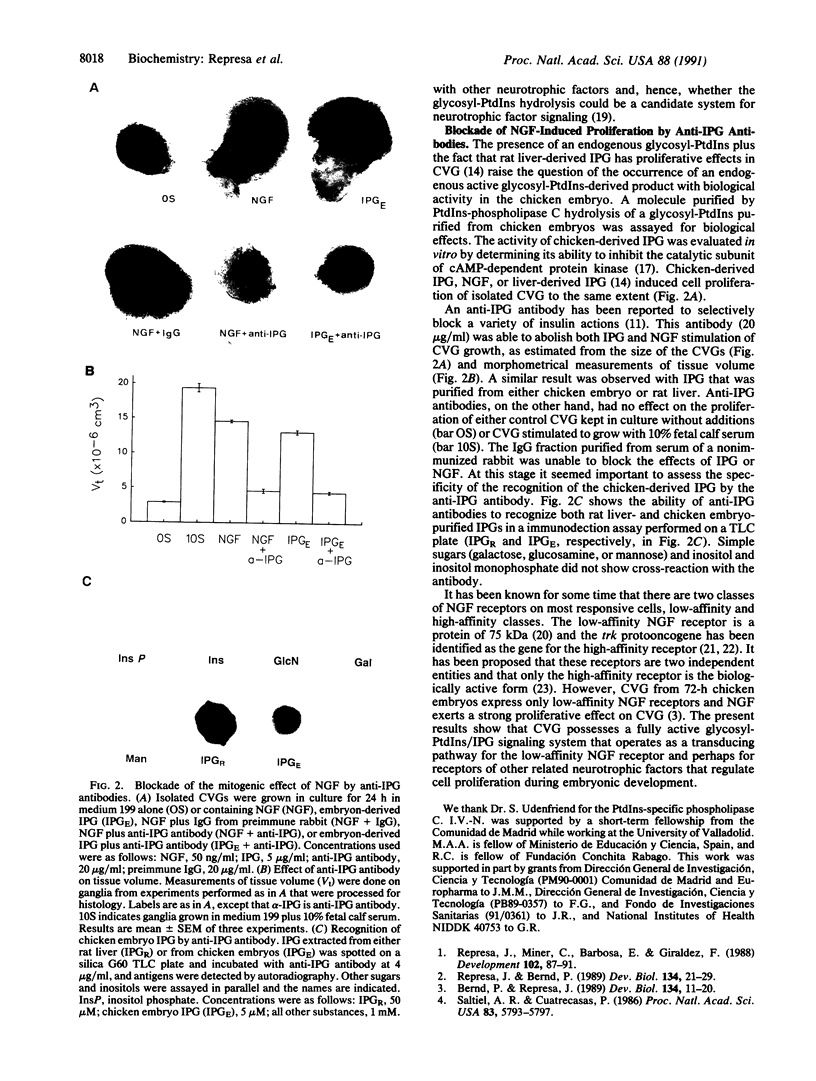
Nerve growth factor (NGF) exerts a variety of actions during embryonic development. At the early stages of inner ear development, NGF stimulates cell proliferation, an effect mediated through low-affinity receptors. We have studied the possibility that the glycosyl-phosphatidylinositol/inositol phosphoglycan (glycosyl-PtdIns/IPG) system is involved in transmitting this NGF signal. Endogenous glycosyl-PtdIns was characterized in extracts of cochleovestibular ganglia (CVGs) that incorporated [3H]glucosamine, [3H]galactose, [3H]myristic acid, and [3H]palmitic acid. Incubation of CVG with NGF produced a rapid and transient hydrolysis of glycosyl-PtdIns. Hydrolysis was complete at 100 ng/ml, and the half-maximal effect occurred at 25 ng/ml, overlapping with the concentration dependence of the mitogenic effect of NGF. An IPG was isolated from embryonic extracts. It had biological effects similar to those reported for the insulin-induced IPG in other tissues. It exerted a powerful mitogenic effect on CVG, comparable to that of NGF. Both the IPG- and NGF-induced cell proliferation were blocked by anti-IPG antibodies that recognized the endogenous IPG on a silica plate immunoassay. These results show that CVG possesses a fully active glycosyl-PtdIns/IPG signal transduction system and that the proliferative effects associated with NGF binding to low-affinity receptors require IPG generation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernd P., Represa J. Characterization and localization of nerve growth factor receptors in the embryonic otic vesicle and cochleovestibular ganglion. Dev Biol. 1989 Jul;134(1):11–20. doi: 10.1016/0012-1606(89)90073-0. [DOI] [PubMed] [Google Scholar]
- Chan B. L., Chao M. V., Saltiel A. R. Nerve growth factor stimulates the hydrolysis of glycosylphosphatidylinositol in PC-12 cells: a mechanism of protein kinase C regulation. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1756–1760. doi: 10.1073/pnas.86.6.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Giraldez F., Represa J. J., Borondo L., Barbosa E. Polarization and density of Na-pumps in the inner ear of the chick embryo during early stages of development. Development. 1987 Jun;100(2):271–278. doi: 10.1242/dev.100.2.271. [DOI] [PubMed] [Google Scholar]
- Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature. 1991 Apr 25;350(6320):678–683. doi: 10.1038/350678a0. [DOI] [PubMed] [Google Scholar]
- Johnson D., Lanahan A., Buck C. R., Sehgal A., Morgan C., Mercer E., Bothwell M., Chao M. Expression and structure of the human NGF receptor. Cell. 1986 Nov 21;47(4):545–554. doi: 10.1016/0092-8674(86)90619-7. [DOI] [PubMed] [Google Scholar]
- Kelly K. L., Mato J. M., Merida I., Jarett L. Glucose transport and antilipolysis are differentially regulated by the polar head group of an insulin-sensitive glycophospholipid. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6404–6407. doi: 10.1073/pnas.84.18.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Jing S. Q., Nanduri V., O'Rourke E., Barbacid M. The trk proto-oncogene encodes a receptor for nerve growth factor. Cell. 1991 Apr 5;65(1):189–197. doi: 10.1016/0092-8674(91)90419-y. [DOI] [PubMed] [Google Scholar]
- Larner J., Huang L. C., Suzuki S., Tang G., Zhang C., Schwartz C. F., Romero G., Luttrell L., Kennington A. S. Insulin mediators and the control of pyruvate dehydrogenase complex. Ann N Y Acad Sci. 1989;573:297–305. doi: 10.1111/j.1749-6632.1989.tb15006.x. [DOI] [PubMed] [Google Scholar]
- Machicao F., Mushack J., Seffer E., Ermel B., Häring H. U. Mannose, glucosamine and inositol monophosphate inhibit the effects of insulin on lipogenesis. Further evidence for a role for inositol phosphate-oligosaccharides in insulin action. Biochem J. 1990 Mar 15;266(3):909–916. [PMC free article] [PubMed] [Google Scholar]
- Magnani J. L., Nilsson B., Brockhaus M., Zopf D., Steplewski Z., Koprowski H., Ginsburg V. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J Biol Chem. 1982 Dec 10;257(23):14365–14369. [PubMed] [Google Scholar]
- Mato J. M., Kelly K. L., Abler A., Jarett L. Identification of a novel insulin-sensitive glycophospholipid from H35 hepatoma cells. J Biol Chem. 1987 Feb 15;262(5):2131–2137. [PubMed] [Google Scholar]
- Ragsdale C., Woodgett J. Neurobiology. trking neurotrophic receptors. Nature. 1991 Apr 25;350(6320):660–661. doi: 10.1038/350660a0. [DOI] [PubMed] [Google Scholar]
- Represa J. J., Miner C., Barbosa E., Giraldez F. Bombesin and other growth factors activate cell proliferation in chick embryo otic vesicles in culture. Development. 1988 May;103(1):87–96. doi: 10.1242/dev.103.1.87. [DOI] [PubMed] [Google Scholar]
- Represa J., Bernd P. Nerve growth factor and serum differentially regulate development of the embryonic otic vesicle and cochleovestibular ganglion in vitro. Dev Biol. 1989 Jul;134(1):21–29. doi: 10.1016/0012-1606(89)90074-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Tébar A., Dechant G., Barde Y. A. Binding of brain-derived neurotrophic factor to the nerve growth factor receptor. Neuron. 1990 Apr;4(4):487–492. doi: 10.1016/0896-6273(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Romero G., Gámez G., Huang L. C., Lilley K., Luttrell L. Anti-inositolglycan antibodies selectively block some of the actions of insulin in intact BC3H1 cells. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1476–1480. doi: 10.1073/pnas.87.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Cuatrecasas P. Insulin stimulates the generation from hepatic plasma membranes of modulators derived from an inositol glycolipid. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5793–5797. doi: 10.1073/pnas.83.16.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel A. R., Sorbara-Cazan L. R. Inositol glycan mimics the action of insulin on glucose utilization in rat adipocytes. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1084–1092. doi: 10.1016/0006-291x(87)90519-5. [DOI] [PubMed] [Google Scholar]
- Varela-Nieto I., Represa J., Avila M. A., Miner C., Mato J. M., Giraldez F. Inositol phospho-oligosaccharide stimulates cell proliferation in the early developing inner ear. Dev Biol. 1991 Feb;143(2):432–435. doi: 10.1016/0012-1606(91)90095-k. [DOI] [PubMed] [Google Scholar]
- Varela I., Alvarez J. F., Clemente R., Ruiz-Albusac J. M., Mato J. M. Asymmetric distribution of the phosphatidylinositol-linked phospho-oligosaccharide that mimics insulin action in the plasma membrane. Eur J Biochem. 1990 Mar 10;188(2):213–218. doi: 10.1111/j.1432-1033.1990.tb15392.x. [DOI] [PubMed] [Google Scholar]
- Villalba M., Kelly K. L., Mato J. M. Inhibition of cyclic AMP-dependent protein kinase by the polar head group of an insulin-sensitive glycophospholipid. Biochim Biophys Acta. 1988 Jan 18;968(1):69–76. doi: 10.1016/0167-4889(88)90045-6. [DOI] [PubMed] [Google Scholar]