Abstract
TFIIS, an elongation factor encoded by DST1 in Saccharomyces cerevisiae, stimulates transcript cleavage in arrested RNA polymerase II. Two components of the RNA polymerase II machinery, Med13 (Srb9) and Spt8, were isolated as two-hybrid partners of the conserved TFIIS N-terminal domain. They belong to the Cdk8 module of the Mediator and to a subform of the SAGA co-activator, respectively. Co-immunoprecipitation experiments showed that TFIIS can bind the Cdk8 module and SAGA in cell-free extracts. spt8Δ and dst1Δ mutants were sensitive to nucleotide-depleting drugs and epistatic to null mutants of the RNA polymerase II subunit Rpb9, suggesting that their elongation defects are mediated by Rpb9. rpb9Δ, spt8Δ and dst1Δ were lethal in cells lacking the Rpb4 subunit. The TFIIS N-terminal domain is also strictly required for viability in rpb4Δ, although it is not needed for binding to RNA polymerase II or for transcript cleavage. It is proposed that TFIIS and the Spt8-containing form of SAGA co-operate to rescue RNA polymerase II from unproductive elongation complexes, and that the Cdk8 module temporarily blocks transcription during transcript cleavage.
Keywords: caffeine, mediator, mycophenolate, RNA polymerase, Saccharomyces cerevisiae
Introduction
All DNA-dependent RNA polymerases (Pol's) are endowed with an intrinsic ribonuclease activity that cleaves a few nucleotides from the 3′ end of the elongating transcript. This cleavage activity probably helps to backtrack elongation complexes that are out of register with the transcript 3′ end, allowing them to resume transcription (Fish and Kane (2002) and references therein). Transcript cleavage operates in bacterial and archaeal Pol's, and in the three eukaryotic enzymes (Pol I, II and III). In bacteria, it is activated by the GreA and GreB factors (Borukhov et al, 1993). In the eukaryotic Pol III enzyme, cleavage is catalysed by the enzyme alone and depends on its Rpc11 subunit (Chédin et al, 1998). In Pol II, it depends on the Rpb9 subunit (akin to Rpc11) and on a factor initially referred to as S-II in human cells (Natori et al, 1973; Izban and Luse, 1992; Reines, 1992) or P37 in yeast (Sawadogo et al, 1980), but now generally called TFIIS (Fish and Kane, 2002). Pol I also has a cleavage activity that does not depend on TFIIS (Tschochner, 1996) but may require Rpa12, a subunit paralogous to Rpb9 and Rpc11 (Nogi et al, 1993; Van Mullem et al, 2002a).
Yeasts (Saccharomyces cerevisiae and Schizosaccharomyces pombe) have only one form of TFIIS, in contrast to the multiple subforms present in vertebrates (Labhart and Morgan, 1998). The corresponding null mutants (dst1Δ) have little or no growth defects (Archambault et al, 1992; Exinger and Lacroute, 1992; Nakanishi et al, 1992; Williams and Kane, 1996), suggesting that TFIIS is only required under specific conditions or that it is functionally redundant with other transcription factors (Davie and Kane, 2000; Lindstrom and Hartzog, 2001; Ubukata et al, 2003). The structure of the yeast TFIIS–Pol II complex was recently determined at a resolution of 3.8 Å and reveals its probable mode of action (Kettenberger et al, 2003). In short, TFIIS binds the Rpb1/Rpb9 ‘jaw' of Pol II and inserts into the Pol II pore, contacting the catalytic site by its highly conserved C-end bearing an invariant RSADE motif. The two carboxylic amino acids of that motif are thought to contribute to metal coordination at the level of the enzyme active site. Remarkably, the RSADE motif is present in Rpa12 and Rpc11, but not in Rpb9. This suggests that these two subunits may themselves directly contact the catalytic sites in Pol I and Pol III, as their Pol's do not require TFIIS for cleavage.
The N-terminal domain of TFIIS (approximately corresponding to its first 132 amino acids) stays on the outer surface of Pol II, where it is available for interaction with other components of the transcription complex (Kettenberger et al, 2003). This N-terminal part is conserved in all eukaryotes sequenced so far. It also has significant homology to the N-end of MED26 (a component of the human Mediator formerly called CRSP70 or ARC70; Ryu et al, 1999; Bourbon et al, 2004) and of the three subforms of human Elongin A (Aso et al, 1995; Yamazaki et al, 2002). This domain was found here to engage in specific interactions with Spt8 and Srb9 (now called Med13; Bourbon et al, 2004). Spt8 defines a subform of the SAGA co-activator (Belotserkovskaya et al, 2000; Pray-Grant et al, 2002; Sterner et al, 2002; Wu and Winston, 2002). Med13 (Srb9) is associated with the evolutionarily conserved kinase Srb10 (Hengartner et al, 1998), now called Cdk8 (Bourbon et al, 2004). Spt8 and Med13 had not been connected so far to each other or to TFIIS, but we shall present evidence suggesting that both may be genuine partners of this factor.
Results
Spt8 and Med13 are two-hybrid partners of the N-terminal domain of TFIIS
Figure 1 presents the outcome of a two-hybrid screening using the entire TFIIS sequence fused to the Gal4BD(1–147) domain. The corresponding pVV70 plasmid was used as a bait against a random library of yeast genomic fragments fused to the Gal4AD(768–881) domain (Fromont-Racine et al, 1997; Flores et al, 1999). From a total of about 107 transformants obtained in strain Y190, 118 clones were selected by their ability to grow in the presence of 100 mM 3-amino-triazole, and were then shown to activate the lacZ reporter gene, as detected in a β-galactosidase assay (Figure 1C). Based on the DNA sequence of their inserts, they were allocated to 36 independent clones, defined, respectively, by 25 and 11 distinct but overlapping fragments of Spt8 and Med13. Thus, we consistently identified the same two partners and the same domains on these partners, which strongly argues for a saturating and specific two-hybrid screening.
Figure 1.
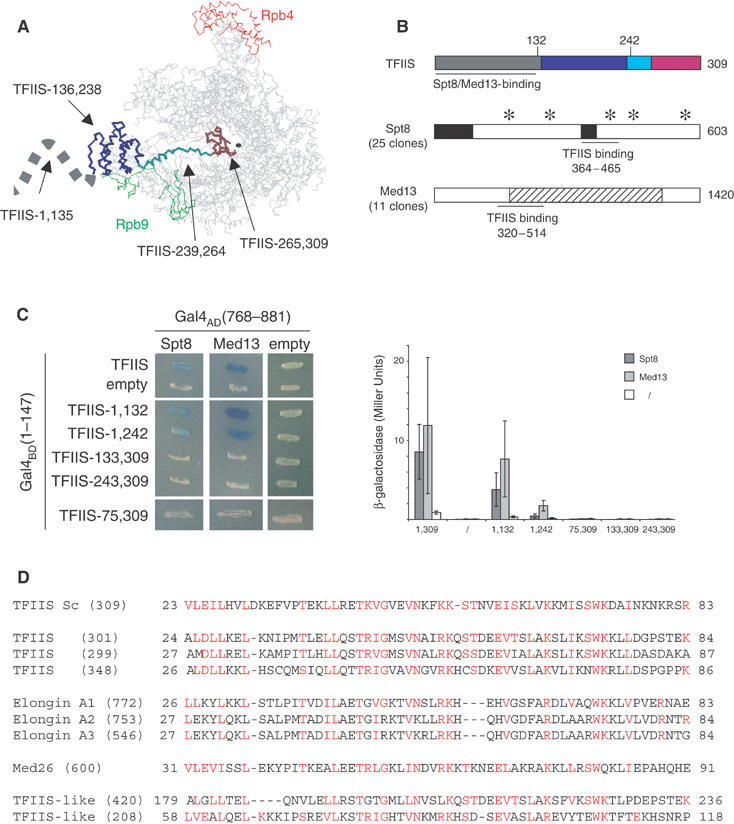
Spt8 and Med13 are TFIIS partners in a two-hybrid assay. (A) Spatial structure of the RNA polymerase II–TFIIS complex domain I, domain II, the inter-domain linker and domain III of TFIIS are shown in grey, blue, cyan and brown, respectively. The borders of each domain are taken from the crystal structure recently reported by Kettenberger et al (2003). The structure of the N-terminal domain I (positions 1–111, symbolised here by a dashed line) was solved in solution by nuclear magnetic resonance (Booth et al, 2000), but was not determined in association with Pol II. The Rpb4 and Rpb9 subunits of Pol II are indicated in red and green, respectively. The black sphere locates the catalytic Mg2+ A. This figure was prepared with the RASMOL software (www.umass.edu/microbio/rasmol/). (B) General organisation of Spt8, Med13 and TFIIS. TFIIS: The TFIIS domains are shown in the same code colour as in (A). A horizontal thick line denotes the minimal region supporting a two-hybrid interaction with Spt8 and Med13, based on the data shown in (C). Spt8: Stars and black boxes indicate WD40-like domains and acidic stretches, respectively. The horizontal thick line denotes the TFIIS-binding region (positions 364–465) as defined by the smallest domain common to the 25 pACT2-SPT8 clones identified by a two-hybrid screening using pVV70 as bait vector (Table II). An example of two-hybrid interaction is shown in (C). Med13: The stripped box corresponds to a region with strong homology between fungal forms of Med13. The horizontal thick line denotes the TFIIS-binding region (positions 320–514), as defined by the smallest domain common to the 11 pACT2-MED13 clones selected by two-hybrid screening. An example of two-hybrid interaction is shown in (C). (C) Two-hybrid interactions with TFIIS. Left panel: The complete coding sequence of TFIIS and various N-terminal or C-terminal fragments thereof were fused to the C-end of the GAL4BD(1–147) DNA-binding domain (plasmids pVV70–pVV75, Table II) and tested for their interaction with Spt8 (plasmid pSPT8-84) and Med13 (plasmid pMED13-111). Transformants were obtained in strain Y190 and tested at 30°C for β-galactosidase activity in an overlay assay (Flores et al, 1999). Right panel: β-Galactosidase activity was assayed as described by Miller (1972). The average values and their standard deviation were calculated from assays performed on three independent transformants, using the same plasmid combination as in the left panel. (D) Conservation of the TFIIS N-terminal domain. The S. cerevisiae (Sc) sequence of TFIIS was compared to the current human genome. Homology search was made using the Psi-blast algorithm and improved by manual inspection. The number in brackets indicates the length of each polypeptide. The two last sequences (accession number AAH35374 and XP294568) correspond to putative gene products that are related to TFIIS but lack the invariant RSADE motif.
Spt8 belongs to a subform of the yeast SAGA Pol II co-activator (Grant et al, 1997). This protein is essentially formed of WD40-like domains, with two acidic patches (Figure 1B). The 25 Spt8 clones isolated in the two-hybrid screening shared a 102 amino-acid segment between positions 364 and 465. This minimal TFIIS interacting domain includes one acidic patch and one of the WD40-like motifs. Med13 is a moderately conserved component of the Mediator (Borggrefe et al, 2002; Boube et al, 2002). It belongs to the Cdk8 module, where Cdk8 is a conserved kinase initially identified by its ability to phosphorylate the C-terminal domain (CTD) of the largest Pol II subunit (Hengartner et al, 1998). The 11 Med13 clones defined a 195 amino-acid segment (320–514) as the minimal TFIIS interacting domain. This domain is included in a region conserved in all fungal Med13 (Figure 1B). It bears no detectable similarity to the Spt8 target domain defined above.
Four domains can be recognised on the TFIIS structure and are collinear to its amino-acid sequence (Kettenberger et al, 2003; see also Figure 1A). The corresponding fragments were fused to the Gal4BD(1–147) domain and tested separately against Spt8 and Med13 in a two-hybrid assay. As shown in Figure 1C, the N-terminal part of TFIIS (amino acids 1–132) is necessary and sufficient to interact with both partners. NMR data have shown that this domain is made of four closely packed α helices (Booth et al, 2000). They are not included in the crystal structure (Kettenberger et al, 2003) but are evidently exposed on the outer surface of Pol II (Figure 1A), and are thus available for interactions with other components of the transcription machinery.
The N-half of this domain (amino acids 1–74) is important for the two-hybrid interaction since its deletion abolishes the two-hybrid response (Figure 1C). However, a fragment bearing amino acids 1–74 alone failed to interact with Spt8 or Med13 (data not shown). Interestingly, this region harbours a highly conserved motif corresponding to the α2, α3 and α4 helices (Figure 1D). A Psi-Blast survey of the human genome showed that this motif is present in nine distinct gene products, including three TFIIS isoforms (Labhart and Morgan, 1998), three forms of Elongin A (Aso et al, 1995; Booth et al, 2000; Yamazaki et al, 2002) and the MED26 component of the Mediator (Ryu et al, 1999; Bourbon et al, 2004).
TFIIS co-purifies with Med13 and its associated Cdk8 kinase in cell-free extracts
The two-hybrid data above suggested that Med13 may be a functional partner of TFIIS. We therefore examined if the immunopurification of Med13 from cell-free extracts leads to a co-purification of TFIIS and vice versa. Med13∷13Myc was barely detectable when introduced as a chromosomal allele, but could be readily detected when expressed from a replicative plasmid and then pulled down a significant amount of TFIIS∷3HA (Figure 2A). This occurred no matter whether cells expressed Spt8 (SPT8+) or not (spt8Δ). In a reciprocal experiment, TFIIS∷13Myc pulled down Med13∷3HA (Figure 2B).
Figure 2.
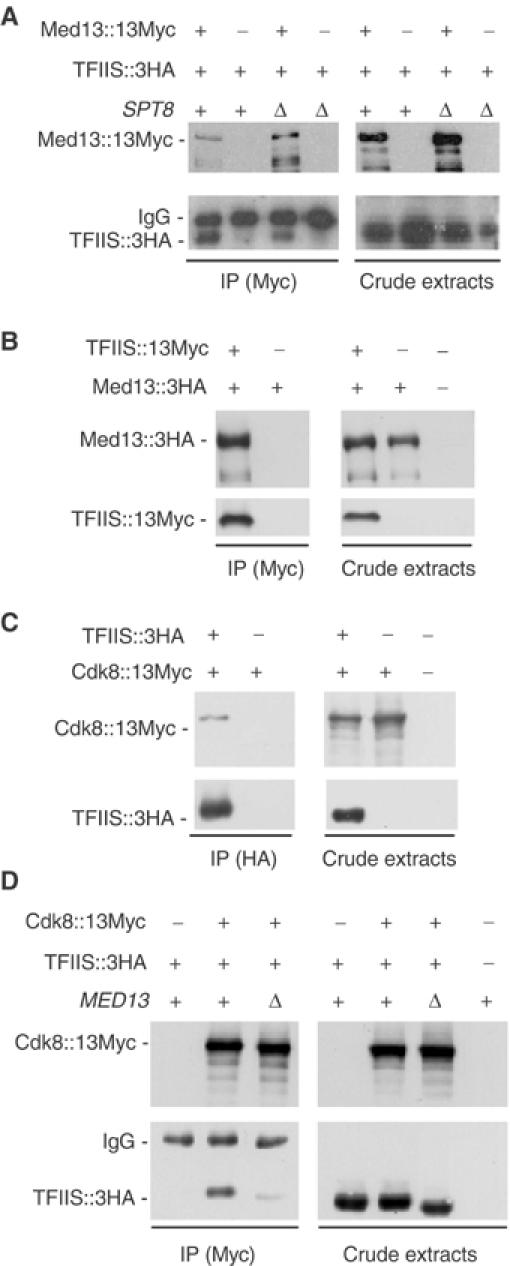
Co-purification of TFIIS with Med13 and Cdk8 in cell-free extracts. (A) Co-purification of TFIIS∷3HA with immunoprecipitated Med13∷13Myc. Strains YPH499 (SPT8+) and YBV61 (spt8Δ) were transformed with plasmids encoding the Med13∷13Myc fusion (pVV226) and/or the TFIIS∷3HA fusion (pVV227). Protein crude extracts were prepared as described (Van Mullem et al, 2002b). Med13∷13Myc was immunoprecipitated by mouse monoclonal anti-Myc antibodies. Proteins (IP) were separated by SDS–PAGE, along with 10 μg of crude extracts, and revealed by Western blotting with monoclonal anti-Myc or anti-HA antibodies. The band noted IgG corresponds to the heavy chains of the mouse anti-Myc antibodies used for the immunopurification. (B) Co-purification of Med13∷3HA with immunoprecipitated TFIIS∷13Myc. Strain YPH499 was transformed with plasmids encoding the TFIIS∷13Myc fusion (pVV234) and/or the Med13∷3HA fusion (pVV229). Protein extraction, immunopurification and detection were as above. (C) Co-purification of Cdk8∷13Myc with immunoprecipitated TFIIS∷3HA. Strain YPH499 was transformed with plasmids encoding the TFIIS∷3HA fusion (pVV228) and/or the Cdk8∷13Myc (pVV233). TFIIS∷3HA was immunoprecipitated by mouse monoclonal anti-HA antibodies. Protein extraction and detection were as above. (D) Co-purification of TFIIS∷3HA with immunoprecipitated Cdk8∷13Myc. Strains YPH499 (MED13+) and YBV39 (med13Δ) were transformed with plasmids encoding the Cdk8∷13Myc (pVV233) and/or the TFIIS∷3HA fusion (pVV228). Protein extraction, immunopurification and detection were as in (A, B). No co-purification was observed when Cdk8∷13Myc and TFIIS∷3HA were expressed from their respective chromosomal locus (data not shown).
Since Med13 belongs to the Cdk8 module of the Mediator, we also examined whether Cdk8 itself might co-purify with TFIIS. Indeed, Cdk8∷13Myc co-purified with the immunoprecipitated TFIIS∷3HA and TFIIS∷3HA co-purified with Cdk8∷13Myc (Figure 2C and D). Moreover, this co-purification was substantially reduced in a med13Δ context (Figure 2D). Along with our two-hybrid data, this indicates that, in vivo, TFIIS may associate with the Cdk8 module and that this association is, to a large extent, dependent on Med13. We note that med13Δ also had a minor effect on the electrophoretic mobility of TFIIS, suggesting a change in its phosphorylation pattern.
dst1Δ and med13Δ mutants have different phenotypes
TFIIS is encoded by the DST1 gene. dst1Δ null mutants have no detectable defect except their sensitivity to 6-azauracil and mycophenolate, two nucleotide-depleting drugs that are thought to impair elongation (Exinger and Lacroute, 1992). med13Δ and cdk8Δ are not sensitive to mycophenolate and do not aggravate the sensitivity of dst1Δ (data not shown). Moreover, the med13Δ dst1Δ spt8Δ triple mutant grew like its dst1Δ spt8Δ parent, and with the same sensitivity to mycophenolate.
The only known phenotype that relates Med13 to TFIIS is their opposite effect on rpb1 mutants with partial deletion of the Pol II CTD, such as the rpb1Δ104 mutant used in this study (Allison and Ingles, 1989). Indeed, med13Δ and cdk8Δ were isolated as suppressors of such rpb1 mutants (Liao et al, 1995), while dst1Δ is lethal in this context (Lindstrom and Hartzog, 2001). The triple mutant dst1Δ med13Δ rpb1Δ104 is also lethal (data not shown), suggesting that the integrity of TFIIS may be required for the suppressor effect of med13Δ on Pol II CTD mutants.
TFIIS co-purifies with Spt8 and other SAGA subunits in cell-free extracts
SAGA is a Pol II co-activator bearing the Gcn5 histone acetyl-transferase (Grant et al, 1997). It was recently shown to exist in two subforms that essentially differ by the presence or absence of the Spt8 subunit (Belotserkovskaya et al, 2000; Pray-Grant et al, 2002; Sterner et al, 2002; Wu and Winston, 2002). Since our two-hybrid data suggested that TFIIS may directly interact with Spt8, we constructed a double-mutant strain where Spt8∷13Myc and TFIIS∷3HA fusions were expressed from the chromosomal locus. Under these conditions, Spt8∷13Myc only pulled down a barely detectable amount of TFIIS∷3HA (data not shown). The expression of the two proteins from replicative vectors significantly enhanced the co-immunopurification signal, and this occurred no matter whether cells expressed Med13 (MED13+) or not (med13Δ) (Figure 3A). In a reciprocal experiment, Spt8∷3HA co-purified with the immunoprecipitated TFIIS∷13Myc (Figure 3B). Thus, Spt8 has affinity for TFIIS in terms of two-hybrid interactions and co-purification from a yeast cell-free extract.
Figure 3.
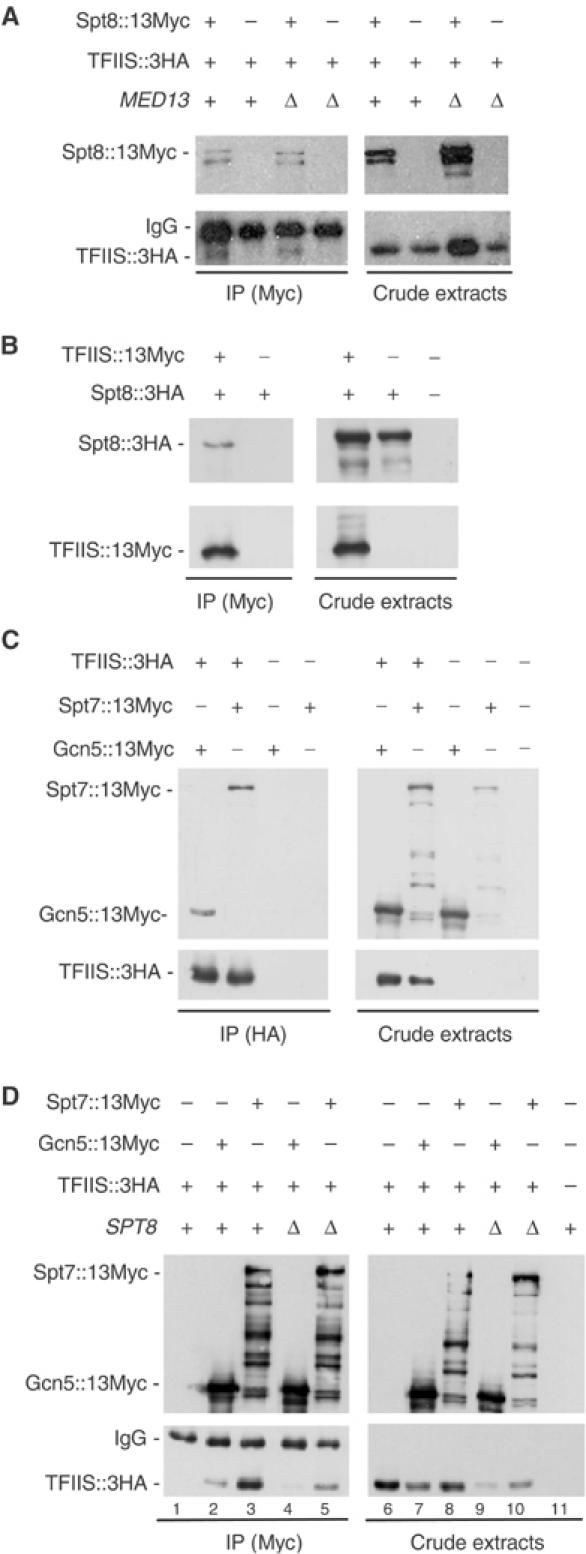
Co-purification of TFIIS with components of SAGA in cell-free extracts. (A) Co-purification of TFIIS∷3HA with immunoprecipitated Spt8∷13Myc. Strains YPH499 (MED13+) and YBV39 (med13Δ) were transformed with plasmids encoding the Spt8∷13Myc fusion (pVV225) and/or the TFIIS∷3HA fusion (pVV227). Protein extraction, immunopurification and detection were as for Figure 2A. (B) Co-purification of Spt8∷3HA with immunoprecipitated TFIIS∷13Myc. Strain YPH499 was transformed with plasmids encoding the TFIIS∷13Myc fusion (pVV234) and/or the Spt8∷3HA fusion (pVV230). Protein extraction, immunopurification and detection were as in Figure 2A. (C) Co-purification of Gcn5∷13Myc and Spt7∷13Myc with immunoprecipitated TFIIS∷3HA. Strain YPH499 was transformed with plasmids encoding the TFIIS∷3HA fusion (pVV228) and/or the Gcn5∷13Myc (pVV231) or the Spt7∷13Myc (pVV232) fusions. Protein extraction, immunopurification and detection were as for Figure 2C. (D) Co-purification of TFIIS∷3HA with immunoprecipitated Gcn5∷13Myc and Spt7∷13Myc. Strains YPH499 (SPT8+) and YMW220 (spt8Δ) were transformed with plasmids encoding the Gcn5∷13Myc (pVV231) or Spt7∷13Myc (pVV232) fusions and/or the TFIIS∷3HA fusion (pVV228). Protein extraction, immunopurification and detection were as for Figure 2A. No co-purification was observed when Gcn5∷13Myc and TFIIS∷3HA were expressed from their respective chromosomal locus (data not shown).
These data raise the question of whether Spt8 binds TFIIS in its own right or as part of the Spt8-containing form of SAGA. Figure 3C and D tentatively suggest that the latter may be true since TFIIS∷3HA pulled down Gcn5∷13Myc and Spt7∷13Myc. Conversely, Gcn5∷13Myc and Spt7∷13Myc pulled down TFIIS∷3HA. However, there was no clear indication that this co-purification depended on Spt8: the genetic inactivation of Spt8 reduced the co-purification, but this might be an indirect effect as there was also less TFIIS∷3HA in the corresponding crude extracts (Figure 3D, compare lanes 7–8 and 9–10).
dst1Δ and spt8Δ mutants have related phenotypes
As already mentioned, dst1Δ has little or no growth defect but is sensitive to nucleotide-depleting drugs such as mycophenolate (Exinger and Lacroute, 1992; see also Figure 4A). Null mutants of several other nonessential components of the Pol II transcription machinery are also sensitive to these inhibitors (Desmoucelles et al, 2002). They include the spt3Δ, spt7Δ and spt8Δ mutants of the SAGA co-activator and the rpb4Δ and rpb9Δ mutants lacking the nonessential Pol II subunits Rpb4 or Rpb9. Figure 4A illustrates the different levels of sensitivity to mycophenolate displayed by these mutants. This drug sensitivity is not a general property of SAGA, since null mutants of Gcn5 or of the Ada2 and Ada3 subunits of SAGA (ada2Δ, ada3Δ and gcn5Δ) are not sensitive.
Figure 4.
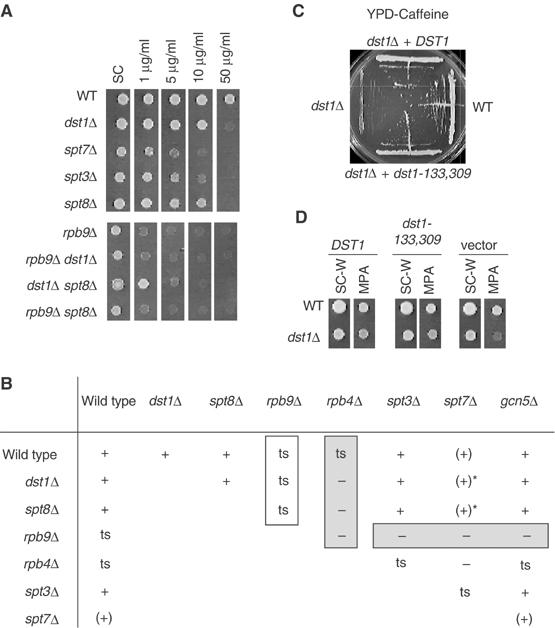
Phenotypic effects of dst1Δ and spt8Δ mutants. (A) Mycophenolate sensitivity and epistasis with rpb9Δ. Wild type (YPH499), dst1Δ (CMKy1), spt7Δ (Y03218), spt3Δ (Y04228), spt8Δ (Y12666), rpb9Δ (YVV9), rpb9Δ dst1Δ (YVV62), dst1Δ spt8Δ (YBV55) and rpb9Δ spt8Δ (YMW238) cells were spotted on SC medium without or with mycophenolate (1, 5, 10 or 50 μg/ml). Plates were incubated at 25°C for 5 days. (B) Recapitulation of synthetic phenotypes involving dst1Δ and spt8Δ. Symbols: +, wild type; (+), partial growth defect at 30°C; −, lethality; ts, temperature sensitivity. In each case, synthetic lethal patterns were based on the analysis of at least 15 meiotic tetrads. Stars indicate that spt7Δ dst1Δ or spt7Δ spt8Δ mutants can be temperature sensitive depending on the genetic background. This phenotype probably reflects the intervention of a third mutation present in some genetic backgrounds, but the corresponding gene has not been identified. (C) Caffeine sensitivity of dst1 mutants. CMKy1 (dst1Δ) was transformed with pCM-DST1 (DST1), pCM-ΔN (dst1-133,309) and the empty vector pCM185. Transformants were streaked on YPD with 10 mM caffeine and incubated for 7 days at 30°C, using YPH500 (WT) as a wild-type control. (D) Mycophenolate sensitivity of dst1 mutants. Wild type (DY236-6B) and CMKy1 (dst1Δ) were transformed with pVV80 (DST1) and pVV81 (dst1-133,309), using the empty vector (pGEN) as a control. Transformants were spotted on SC-W and SC-W with 50 μg/ml of mycophenolate. Plates were incubated at 25°C for 3 days.
dst1Δ and rpb9Δ are epistatic, that is, the double mutant is indistinguishable from rpb9Δ alone in terms of growth and drug sensitivity (Figure 4A; Van Mullem et al, 2002b). This also holds for the spt8Δ rpb9Δ double mutant (Figure 4A and B). Remarkably, null mutants of all the other nonessential subunits of SAGA (Ada2, Ada3, Gcn5, Spt3 and Spt7) are lethal in a rpb9Δ context, disregarding whether they are sensitive to mycophenolate (spt3Δ and spt7Δ) or not (ada2Δ, ada3Δ and gcn5Δ) (Figure 4B; Van Mullem et al, 2002b). Thus, epistasis with rpb9Δ is a unique property of spt8Δ among SAGA null mutants.
Crosses between rpb4Δ and rpb9Δ yield no double mutants, suggesting that these mutations are synthetic lethal (Li and Smerdon, 2002). We confirmed this interpretation by complementation tests showing that lethality is relieved in the presence of a plasmid bearing the wild-type RPB4 or RPB9 genes. dst1Δ and spt8Δ were also synthetic lethal with rpb4Δ. In both cases, double mutants grew as microcolonies but did not form viable clones when further streaked on YPD. Moreover, the double mutants were rescued by complementation with the RPB4 and DST1/SPT8 wild-type genes (Figure 5). Again, this was not a general property of SAGA since spt3Δ and gcn5Δ are epistatic with rpb4Δ (Figure 4B). This is consistent with the fact that SAGA complexes purified from spt3Δ and gcn5Δ contain Spt8 (Sterner et al, 1999). In contrast, a spt7Δ deletion that leads to the disruption of SAGA (Sterner et al, 2002) is lethal in both rpb9Δ and rpb4Δ contexts.
Figure 5.
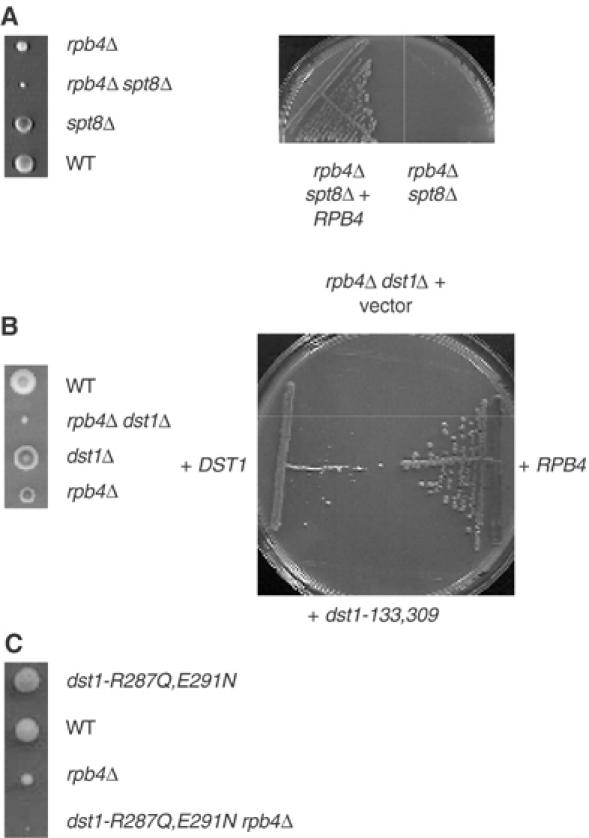
Spt8 and the N-terminal domain of TFIIS are critical in an rpb4Δcontext. (A) Synthetic lethality between spt8Δ and rpb4Δ. Left panel: Strains ESH13-8D (spt8Δ) and ESH27-15C (rpb4Δ) were crossed and submitted to tetrad analysis. Plates were incubated on YPD. Minute colonies corresponding to spt8Δ rpb4Δ double mutants were obtained but could not be further propagated on YPD. One tetratype is shown after 5 days at 30°C. Right panel: The cross above was repeated but diploid strains were transformed with pYX212-RPB4 (2 μURA3 RPB4) prior to sporulation. Strain ESH28-2A (spt8Δ rpb4Δ/2 μ URA3 RPB4) was isolated in the meiotic offspring and then transferred on FOA to counterselect the pYX212-RPB4 plasmid. This material was then transferred to YPD and incubated for 5 days at 30°C and compared to the strain ESH28-2A as a control. (B) Synthetic lethality between rpb4Δ and mutants lacking the N-terminal domain of TFIIS. Left panel: Strain ESH1 (dst1Δ) was crossed to SL21-3A (rpb4Δ) and submitted to tetrad analysis. Plates were incubated on YPD. Minute colonies corresponding to dst1Δ rpb4Δ double mutants were obtained but have an extremely poor viability when further propagated on YPD. One tetratype is shown after 5 days at 30°C. Right panel: Strain ESH29-1B (dst1Δ rpb4Δ with the 2 μ URA3 RPB4 plasmid pYX212-RPB4) was transformed by the TRP1 centromeric plasmids pCM185 (vector), pCM-DST1 (DST1) or pCM-ΔN (dst1-133,309). These transformants were transferred on FOA to counterselect the pYX212-RPB4 plasmid, transferred to YPD and incubated for 5 days at 30°C, using ESH29-1B as a control (RPB4). Control experiments showed that pCM-ΔN complements the mycophenolate sensitivity of dst1Δ and is thus functional (data not shown). (C) Synthetic lethality between rpb4D and the dst1-R287Q,E291N mutant defective in the RNA cleavage activity. Strain SL21-3A (rpb4D) was crossed with D495-4D (dst1-R287Q,E291N) and 20 tetrads were analysed. The dst1 rpb4Δ double mutants invariably produced minute colonies that failed to propagate when further restreaked on YPD. One tetratype is shown after 3 days at 30°C.
From the data above, dst1Δ and spt8Δ behave very similarly in terms of lethality with rpb4Δ, epistasis to rpb9Δ and mycophenolate sensitivity. Yet, their physiological effects do not fully overlap. spt8 mutants suppress his4917δ and are protrophic for lysine in a LYS2–173R2 context, which reflects the less efficient transcription of solo δ and Ty1 elements in the his4-917δ and LYS2-173R2 alleles (Eisenmann et al, 1994; Wu and Winston, 2002). These spt phenotypes (suppression of Ty1) are not found in dst1Δ and are not aggravated in a dst1Δ spt8Δ double mutant (data not shown). As shown in Figure 4C, dst1Δ is sensitive to caffeine (trimethylxanthine). This phenotype is shared by rpb4Δ and rpb9Δ but not by spt8Δ (data not shown; Sterner et al, 1999). Finally, dst1Δ aggravates mycophenolate sensitivity in spt8Δ (Figure 4A), indicating that the drug sensitivity of Spt8 cannot be directly mediated by its binding to TFIIS.
The N-terminal domain of TFIIS is critical in an rpb4Δ context
The N-terminal domain of TFIIS is one of the most conserved components of that polypeptide (see Figure 1D) but does not participate in Pol II binding and is not required for transcript cleavage (Agarwal et al, 1991; Awrey et al, 1998). This is consistent with the observation that a dst1-133,309 mutant lacking this domain is not sensitive to 6-azauracil (Nakanishi et al, 1995; Ubukata et al, 2003) to mycophenolate (Figure 4D) or to caffeine (Figure 4C), and is in fact indistinguishable from wild type by all criteria examined so far. On the other hand, our two-hybrid data suggest that this N-terminal domain specifically interacts with Spt8. A deletion of that domain is therefore expected to disrupt the Spt8–TFIIS interaction. As shown in Figure 5B, a dst1-133,309 mutant lacking this N-terminal domain is lethal in an rpb4Δ context and behaves like dst1Δ and spt8Δ themselves. The same colethality was observed in rpb4Δ dst1-R287Q,E291N double mutants affecting the invariant RSADE domain of TFIIS (Figure 5C). dst1-R287Q,E291N is specifically defective in the transcript cleavage activity, but is not affected for Pol II binding (Ubukata et al, 2003). Taken together, these data show that, in the absence of Rpb4, transcription is strictly dependent on transcript cleavage and also requires a functional interaction between Spt8 and TFIIS.
Discussion
Elongating RNA polymerases that meet obstacles on the DNA template become arrested and probably displace the 3′ end of the transcript relatively to the catalytic site of polymerisation. Escape from arrest requires an RNA cleavage process that, in the case of Pol II, is strongly stimulated by the elongation factor TFIIS (Fish and Kane, 2002). Elegant structural studies by Kettenberger et al (2003) have shown that TFIIS binds Pol II on its Rpb9/Rpb1 jaw and that its C-terminal part reaches the internal active site of the enzyme, where the factor induces a conformational change switching Pol II to its RNA cleavage mode. However, they provide no function for the N-terminal domain of TFIIS.
This N-terminal domain forms a bulky four-helix bundle (Booth et al, 2000) on the periphery of the Pol II structure, approximately facing the downstream DNA (Kettenberger et al, 2003), and is thus available for interaction with other Pol II factors. Its physiological role has remained a mystery, but studies on the human and yeast factor have shown that it is not needed for transcript cleavage, for the binding of TFIIS to Pol II or for the stimulation of elongation in vitro (Agarwal et al, 1991; Nakanishi et al, 1995; Awrey et al, 1998). Indeed, a deletion of this domain could not so far be associated to any growth phenotype in S. cerevisiae. In mammals, this domain has a clear homology to the N-terminal region of the transcription factors MED26 and Elongin A (Aso et al, 1995; Ryu et al, 1999), and it is hard to believe that this evolutionary conservation is not associated with some specific function.
We report here that the N-terminal domain of TFIIS interacts with Spt8 and Med13. Both are well-defined components of the Pol II transcription machinery, but were so far not connected to each other or to TFIIS. Med13 belongs to the Cdk8 kinase module of the Mediator. The two-hybrid interaction between Med13 and TFIIS is further supported by co-immunoprecipitation data showing that TFIIS co-purified with Med13 and its associated Cdk8 kinase. Moreover, co-purification with Cdk8 was substantially reduced in a med13Δ mutant. In yeast and human cells, GST∷TFIIS fusions pull down a Pol II holoenzyme that contains Cdk8 (Pan et al, 1997; Hirst et al, 1999). Furthermore, this only requires the N-terminal part of the human TFIIS (positions 1–103). Taken together, these data support the idea that TFIIS binds the Mediator at the level of its Cdk8 module, by a specific interaction between Med13 and the N-terminal part of TFIIS. Since Cdk8 inhibits transcription via its CTD kinase activity (Hengartner et al, 1998), it may facilitate the TFIIS-dependent cleaving process by temporarily holding back elongation (Figure 6A). Alternatively, the kinase could phosphorylate the N-end of TFIIS, as the latter is phosphorylated in human cells (Horikoshi et al, 1985; Agarwal et al, 1991).
Figure 6.
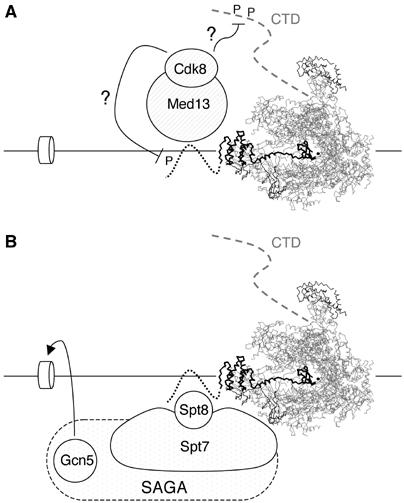
Model of the interactions relating arrested Pol II, TFIIS, SAGA and the Cdk8 module. (A) Interaction between arrested Pol II, TFIIS and the Cdk8 module. The schematic representation of the Pol II/TFIIS complex is as in Figure 1A. The TFIIS and Med13 interaction recruits the Cdk8 module on an arrested Pol II/TFIIS complex. The Cdk8 kinase may facilitate the TFIIS-dependent cleaving process by temporarily holding back elongation via inhibitory phosphorylation (marked by ‘P') on the CTD. Cdk8 may also phosphorylate TFIIS on its N-terminal domain. (B) Interaction between arrested Pol II, TFIIS and the Spt8-containing form of SAGA. Pol II may become frequently arrested during elongation. Transcript cleavage is then required to resume transcription, in a way that depends on TFIIS and Rpb9. The N-terminal domain of TFIIS (dashed line) binds Spt8 and recruits the Spt8-containing form of SAGA. Based on genetic evidence, this is proposed to be essential in the absence of Rpb4, but does not depend on the Gcn5 histone acetylase. However, Gcn5 may optimise elongation, as suggested by the synthetic lethality between rpb9Δ and gcn5Δ.
Spt8 defines a recently discovered subform of the SAGA Pol II co-activator (Belotserkovskaya et al, 2000; Pray-Grant et al, 2002; Sterner et al, 2002; Wu and Winston, 2002). Along with our two-hybrid data, the co-immunopurification of TFIIS with Spt8, Gcn5 and Spt7 argues for a direct interaction between TFIIS and the Spt8-containing form of SAGA. However, the genetic inactivation of Spt8 did not impair the co-purification of TFIIS with Gcn5 and Spt7. One possibility is that SAGA binds the Pol II/TFIIS transcription complex by additional contact points. In this context, we note that Spt7 is a two-hybrid partner of the Rpb9 subunit of Pol II (M Werner and P Thuriaux, unpublished observation).
Spt8 and TFIIS are not normally required for transcription in S. cerevisiae, since spt8Δ and dst1Δ null mutants have little or no effect on growth, and since a deletion of the Spt8-interacting N-terminal domain of TFIIS (dst1-133,309) is indistinguishable from wild type. Remarkably, both factors are essential in rpb4Δcells lacking the Rpb4 subunit of Pol II. This is not a general property of SAGA mutants since gcn5Δ and spt3Δ mutants, for example, are viable in an rpb4Δ context. gcn5Δ lacks the histone acetylase of SAGA but, like spt3Δ, still produces an Spt8-containing form of SAGA (Sterner et al, 2002; Wu and Winston, 2002). In the case of TFIIS, the lethality of rpb4Δ dst1-R287Q,E291N (where dst1-R287Q,E291N affects the invariant RSADE motif and specifically lacks the cleavage activity, see Ubukata et al, 2003) proves that transcript cleavage is critical in the absence of Rpb4. The fact that this lethality extends to the dst1-133,309 mutant deleted for the N-terminal domain of TFIIS strongly suggests that the interaction with Spt8 is also needed in an Rpb4-less context.
spt8Δ and dst1Δ are epistatic with rpb9Δ mutants lacking Rpb9, the other nonessential Pol II subunit (Van Mullem et al (2002b) and this study). Again, this is not a general property of SAGA mutants since gcn5Δ rpb9Δ double mutants are lethal, suggesting that the histone acetylase activity of SAGA is critical in an Rpb9-less context. Thus, Rpb4 and Rpb9 are not directly involved in RNA polymerisation but may stabilise two distinct conformations of Pol II. The absence of Rpb9 makes Pol II incompetent for transcript cleavage (as shown by Awrey et al, 1998), and we speculate that the absence of Rpb4 would instead make Pol II strictly dependent on transcript cleavage. Both conditions would lead to slow growth and to a strong sensitivity to nucleotide-depleting drug, but for different reasons. For example, Pol II molecules lacking Rpb4 could be prone to transcription accidents, obliging them to resort to cleavage. This would account for the strict dependency of rpb4Δ on Rpb9 and TFIIS, since both are required for RNA cleavage (Awrey et al, 1997). As sketched out in Figure 6B, the ability to recruit the Spt8-containing form of SAGA via the N-terminal of TFIIS may also be essential under these conditions. This may not be restricted to the elongating Pol II, as TFIIS binds the promoter and coding part of GAL1 (Pokholok et al, 2002; D Prather, E Larschan and F Winston, personal communication). SAGA itself is recruited to the promoter (Cosma et al, 1999; Bhaumik and Green, 2001; Larschan and Winston, 2001). This, however, need not apply to the Spt8-containing form, and an elongation role of SAGA is indeed suggested by the sensitivity of spt3Δ, spt7Δ and spt8Δ to nucleotide-depleting drugs (Desmoucelles et al, 2002).
Materials and methods
Strains
Yeast strains were constructed by standard meiotic crosses and transformations (Table I). Most of the mutant strains were full deletions with a KanMX4 insertion (Euroscarf: http://www.uni-frankfurt.de/fb15/mikro/euroscarf/). In crosses involving the KanMX4 marker in the two parental strains, double mutant segregants were isolated from a nonparental ditype tetrad, and confirmed by PCR. Y14279 (med13Δ) and Y12666 (spt8Δ) derive from the BY4742 strain (MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0). Y04411 (dst1Δ), Y04228 (spt3Δ), Y03218 (spt7Δ) and Y02666 (spt8Δ) derive from BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0). The other parental strains used were CMKy1 (Davie and Kane, 2000), FY1093 (spt7Δ; Wu and Winston, 2002) RPO21-Δ104 (Allison and Ingles, 1989), MC11-1 (Choder and Young, 1993), OG30-4C (gcn5Δ∷HIS3) and YVV9 (rpb9Δ; Van Mullem et al, 2002b), SL21 (Shpakovski et al, 2000), YPH499 and YPH500 (Sikorski and Hieter, 1989). Mt8 is a dst1-R287Q, E291N double-mutant defective in the RNA cleavage activity (Ubukata et al, 2003). DY236-6B is a MATa trp1Δ63 strain obtained by crossing FY67 (Winston et al, 1995) to YPH500. Strain Y190 (MATa gal4 gal80 his3 trp1-901 ade2-1 ura3-52 leu2-2,112 CYH1RURA3∷GAL1∷lacZ LYS2∷GAL4(UAS)∷HIS3) was used as host in two-hybrid tests (Flores et al, 1999). In this strain, HIS3 and lacZ are used as reporter genes of the two-hybrid interaction.
Table 1.
Yeast strains
| Strain | Genotype | Origin |
|---|---|---|
| D485-4D | MATa ade2-1 leu2-Δ1 lys2-801 ura3-52 dst1-R287Q,E291N | Mt8 × YPH500 |
| ESH1 | MATa ade2-1 his3Δ200 leu2Δ1 lys2-801 trp1Δ63 ura3-52 dst1∷hisG | Spontaneous subclone of CMKy1 |
| ESH9-2A | MATα his3 leu2 met15Δ0 trp1Δ63 ura3 dst1Δ∷KanMX4 | YPH500 × Y04411 |
| ESH13-8D | MATα ade2-1 his3 leu2 met15Δ0 trp1Δ63 ura3 spt8Δ∷KanMX4 | SL21-3A × Y02666 |
| ESH27-15C | MATa ade2-1 his3 leu2 trp1 ura3 rpb4Δ∷HIS3 | RPO21-Δ104 × MC11-1 |
| ESH28-2A | MATα ade2-1 his3 leu2 trp1 ura3 spt8Δ∷KanMX4 rpb4Δ∷HIS3 /2 μ URA3 RPB4 | ESH13-8D × ESH27-15C with pYX-RPB4 |
| ESH29-1B | MATa ade2-1 his3 leu2 trp1 ura3 dst1Δ∷KanMX4 rpb4Δ∷HIS3 /2 μ URA3 RPB4 | ESH9-2A × ESH27-15C with pYX-RPB4 |
| SL21-3A | MATα ade2-1 his3Δ200 leu2Δ1 lys2-801 trp1Δ63 rpb4Δ∷URA3(Kl) | Segregant of SL21 |
| YBV39 | MATα ade2-1 his3 leu2 lys2 trp1Δ63 ura3 med13Δ∷KanMX4 | CMKy1 × Y14279 |
| YBV55 | MATα ade2-1 his3Δ leu2Δ lys2 ura3 spt8Δ∷KanMX4 dst1Δ∷hisG-URA3-hisG | CMKy1 × Y12666 |
| YBV61 | MATα ade2-1 his3Δ leu2Δ lys2 trp1Δ63 ura3 spt8Δ∷KanMX4 | CMKy1 × Y12666 |
| YMW135 | MATα his3Δ1 leu2Δ0 lys2Δ0 trp1Δ63 spt8Δ∷KanMX4 | Y12666 × DY236-6b |
| YMW220 | MATa his3Δ1 leu2Δ0 lys2Δ0 met15Δ0 trp1Δ63 ura3Δ0 spt8Δ∷KanMX4 | Y03218 × YMW135 |
| YMW238 | MATa ade2-1 his3 leu2 lys2 ura3 rpb9Δ∷HIS3 spt8Δ∷KanMX4 | YVV9 × Y12666 |
| YVV62 | MATα ade2-1 his3Δ200 leu2 lys2 trp1Δ63 ura3-52 rpb9Δ∷HIS3 dst1Δ∷hisG-URA3-hisG | YVV9 × CMKy1 |
Growth media
YPD and SC are standard complete or synthetic growth media with 2% glucose. YPD-Caffeine contains caffeine at 10 mM. SC-W is tryptophan omission media. MPA contains mycophenolate (Sigma) freshly dissolved in methanol (10 mg/ml), added to SC or SC-W. FOA (Boeke et al, 1984) is SC with 0.1% of 5-fluoro orotic acid (Toronto Research Chemicals). Spt phenotypes were assessed by their ability to restore histidine prototrophy in his4-917δ and to generate lysine auxotrophy in a LYS2-173R2 context (Wu and Winston, 2002).
Plasmids
Plasmids (Table II) were prepared by standard subcloning or by the GATEWAY™ technique (Walhout et al, 2000), except for pSPT8-84 and pMED13-111, which were isolated from a random library of genomic fragments (about 0.7 kb) fused to the Gal4AD(768–881). Constructs based on polymerase chain reaction (PCR) were sequenced to avoid spurious mutations generated by the amplification process. pVV70–pVV75 are derivatives of pGBT9 with full-length or partly deleted forms of DST1 fused to the Gal4BD(1–147) domain, constructed by directional cloning between SmaI and BamHI. Their expression was tested by Western blotting assay using anti-Gal4BD(1–147) antibodies (Clontech). pVV80 (2 μ TRP1 DST1) was constructed by cloning the DST1 SmaI–SalI insert from pVV70 into the multicopy expression vector pGEN (Shpakovski et al, 1995). pVV81 (2 μ TRP1 dst1-133,309) was obtained by directional cloning of a PCR-amplified BamHI–ClaI dst1-133,309 insert into the pGEN. pCM-DST1 and pCM-ΔN were obtained by subcloning the BamHI–MluI DST1 and dst1-133,309 inserts of pVV80 and pVV81 into pCM185 (Gari et al, 1997). PYX212-RPB4 was constructed by cloning a PCR-amplified RPB4 coding sequence between the NcoI and SalI sites of the multicopy expression vector pYX212 (Yeast R&D Systems). pVV225–pVV234 were constructed using the GATEWAY™ technique (Walhout et al, 2000). Briefly, the SPT8, GCN5, SPT7, MED13, CDK8 and DST1 coding sequences were PCR-amplified without their termination codon from oligonucleotides ending with attB1 and attB2 sites. Entry clones were generated by in vitro recombination with the attP1 and attP2 sites of the pDONR™201 vector (Invitrogen). Inserts were sequenced and then subcloned using LR reactions into appropriate pVV201, pVV203, pVV215 or pVV217 pGEN-derived vectors (Van Mullem et al, 2003).
Table 2.
Plasmids
| Plasmid | Yeast genes | Backbone vector |
|---|---|---|
| pCM-DST1 | CEN TRP1 ptetO7∷DST1 | pCM185 |
| pCM-ΔN | CEN TRP1 ptetO7∷dst1-133,309 | pCM185 |
| pSPT8-84 | 2 μ LEU2 Gal4AD(768–881)∷spt8-334,520 | pACT2 |
| PMED13-111 | 2 μ LEU2 Gal4AD(768–881)∷med13-106,669 | pACT2 |
| pVV70 | 2 μ TRP1 Gal4BD(1–147)∷DST1 | pGBT9 |
| pVV71 | 2 μ TRP1 Gal4BD(1–147)∷dst1-1,132 | pGBT9 |
| pVV72 | 2 μ TRP1 Gal4BD(1–147)∷dst1-1,242 | pGBT9 |
| pVV73 | 2 μ TRP1 Gal4BD(1–147)∷dst1-75,309 | pGBT9 |
| pVV74 | 2 μ TRP1 Gal4BD(1–147)∷dst1-133,309 | pGBT9 |
| pVV75 | 2 μ TRP1 Gal4BD(1–147)∷dst1-243,309 | pGBT9 |
| pVV80 | 2 μ TRP1 pPGK∷DST1 | pGEN |
| pVV81 | 2 μ TRP1 pPGK∷dst1-133,309 | pGEN |
| pVV225 | 2 μ TRP1 pPGK∷SPT8∷13Myc | pVV203 |
| pVV226 | 2 μ TRP1 pPGK∷MED13∷13Myc | pVV203 |
| pVV227 | 2 μ URA3 pPGK∷DST1∷3HA | pVV215 |
| pVV228 | 2 μ TRP1 pPGK∷DST1∷3HA | pVV201 |
| pVV229 | 2 μ TRP1 pPGK∷MED13∷3HA | pVV201 |
| pVV230 | 2 μ TRP1 pPGK∷SPT8∷3HA | pVV201 |
| pVV231 | 2 μ URA3 pPGK∷GCN5∷13Myc | pVV217 |
| pVV232 | 2 μ URA3 pPGK∷SPT7∷13Myc | pVV217 |
| pVV233 | 2 μ URA3 pPGK∷CDK8∷13Myc | pVV217 |
| pVV234 | 2 μ URA3 pPGK∷DST1∷13Myc | pVV217 |
| pYX212-RPB4 | 2 μ URA3 pTPI1∷RPB4 | pYX212 |
Immunopurification
Immunopurification was carried out as previously described (Van Mullem et al, 2002b), starting from 1 mg of yeast crude extract and using about 0.8 μg of mouse monoclonal anti-Myc (9E10 from Babco) or anti-HA (12CA5) antibody. Beads were washed three times for 5 min in a modified IP buffer (20 mM HEPES, pH 7.5, 0.5 mM EDTA, 500 mM NaCl, 1 mM dithiothreitol, 20% glycerol, 0.1% Triton X-100). Immunoprecipitated proteins were eluted, heated for 10 min at 95°C, separated by SDS–PAGE and revealed by monoclonal anti-HA or anti-Myc antibodies (Babco) using the ECL™ Western Blotting Detection kit (Amersham).
Acknowledgments
We thank André Sentenac for his kind support and Michel Werner for helpful comments during this work. Drs Fred Winston, Caroline Kane and Toshiyuki Nakanishi provided useful mutant strains, Audrey Suleau constructed plasmid pYX212-RPB4 and Séverine Coddens helped in immunopurification experiments. We also thank an anonymous referee for illuminating critics of an earlier version of this paper. This work was partly supported by the Direction des Relations Internationales (CEA). MW was supported by short-term fellowships of EMBO and FEBS. ES was supported by a Young Scientist Fellowship from INTAS, by the Russian Federation of Young Scientists and by research programmes from the Russian Academy of Sciences and the Russian Foundation of Basic Research. BVD and VVM were supported by the Belgian Fonds pour la Recherche dans l'Industrie et l'Agriculture and Fonds National de la Recherche Scientifique, respectively.
References
- Agarwal K, Baek KH, Jeon CJ, Miyamoto K, Ueno A, Yoon HS (1991) Stimulation of transcript elongation requires both the zinc finger and RNA polymerase II binding domains of human TFIIS. Biochemistry 30: 7842–7851 [DOI] [PubMed] [Google Scholar]
- Allison LA, Ingles CJ (1989) Mutations in RNA polymerase II enhance or suppress mutations in gal4. Proc Natl Acad Sci USA 86: 2794–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archambault J, Lacroute F, Ruet A, Friesen JD (1992) Genetic interaction between transcription elongation factor TFIIS and RNA polymerase II. Mol Cell Biol 12: 4142–4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso T, Lane WS, Conaway JC, Conaway RC (1995) Elongin (SIII): a multisubunit regulator of elongation by RNA polymerase II. Science 269: 1439–1443 [DOI] [PubMed] [Google Scholar]
- Awrey DE, Shimasaki N, Koth C, Weilbaecher R, Olmsted V, Kazanis S, Shan X, Arellano J, Arrowsmith CH, Kane CM, Edwards AM (1998) Yeast transcript elongation factor (TFIIS), structure and function. II: RNA polymerase binding, transcript cleavage, and read-through. J Biol Chem 273: 22595–22605 [DOI] [PubMed] [Google Scholar]
- Awrey DE, Weilbaecher RG, Hemming SA, Orlicky SM, Kane CM, Edwards AM (1997) Transcription elongation through DNA arrest sites. A multistep process involving both RNA polymerase II subunit RPB9 and TFIIS. J Biol Chem 272: 14747–14754 [DOI] [PubMed] [Google Scholar]
- Belotserkovskaya R, Sterner DE, Deng M, Sayre MH, Lieberman PM, Berger SL (2000) Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters. Mol Cell Biol 20: 634–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik SR, Green MR (2001) SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev 15: 1935–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke JD, Lacroute F, Fink GR (1984) A positive selection for mutants lacking orotidine-5′-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet 197: 345–346 [DOI] [PubMed] [Google Scholar]
- Booth V, Koth CM, Edwards AM, Arrowsmith CH (2000) Structure of a conserved domain common to the transcription factors TFIIS, elongin A, and CRSP70. J Biol Chem 275: 31266–31268 [DOI] [PubMed] [Google Scholar]
- Borggrefe T, Davis R, Erdjument-Bromage H, Tempst P, Kornberg RD (2002) A complex of the srb8, -9, -10, and -11 transcriptional regulatory proteins from yeast. J Biol Chem 277: 44202–44207 [DOI] [PubMed] [Google Scholar]
- Borukhov S, Sagitov V, Goldfarb A (1993) Transcript cleavage factors from E. coli. Cell 72: 459–466 [DOI] [PubMed] [Google Scholar]
- Boube M, Joulia L, Cribbs DL, Bourbon HM (2002) Evidence for a Mediator of RNA polymerase II transcriptional regulation conserved from yeast to man. Cell 110: 143–151 [DOI] [PubMed] [Google Scholar]
- Bourbon HM, Aguilera A, Ansari AZ, Asturias FJ, Berk AJ, Bjorklund S, Blackwell TK, Borggrefe T, Carey M, Carlson M, Conaway JW, Conaway RC, Emmons SW, Fondell JD, Freedman LP, Fukasawa T, Gustafsson CM, Han M, He X, Herman PK, Hinnebusch AG, Holmberg S, Holstege FC, Jaehning JA, Kim YJ, Kuras L, Leutz A, Lis JT, Meisterernest M, Naar AM, Nasmyth K, Parvin JD, Ptashne M, Reinberg D, Ronne H, Sadowski I, Sakurai H, Sipiczki M, Sternberg PW, Stillman DJ, Strich R, Struhl K, Svejstrup JQ, Tuck S, Winston F, Roeder RG, Kornberg RD (2004) A Unified Nomenclature for Protein Subunits of Mediator Complexes Linking Transcriptional Regulators to RNA Polymerase II. Mol Cell 14: 553–557 [DOI] [PubMed] [Google Scholar]
- Chédin S, Riva M, Schultz P, Sentenac A, Carles C (1998) The RNA cleavage activity of RNA polymerase III is mediated by an essential TFIIS-like subunit and is important for transcription termination. Genes Dev 12: 3857–3871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choder M, Young RA (1993) A portion of RNA polymerase II molecules has a component essential for stress responses and stress survival. Mol Cell Biol 13: 6984–6991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosma MP, Tanaka T, Nasmyth K (1999) Ordered recruitment of transcription and chromatin remodeling factors to a cell cycle- and developmentally regulated promoter. Cell 97: 299–311 [DOI] [PubMed] [Google Scholar]
- Davie JK, Kane CM (2000) Genetic interactions between TFIIS and the Swi–Snf chromatin-remodeling complex. Mol Cell Biol 20: 5960–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmoucelles C, Pinson B, Saint-Marc C, Daignan-Fornier B (2002) Screening the yeast ‘disruptome' for mutants affecting resistance to the immunosuppressive drug, mycophenolic acid. J Biol Chem 277: 27036–27044 [DOI] [PubMed] [Google Scholar]
- Eisenmann DM, Chapon C, Roberts SM, Dollard C, Winston F (1994) The Saccharomyces cerevisiae SPT8 gene encodes a very acidic protein that is functionally related to SPT3 and TATA-binding protein. Genetics 137: 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exinger F, Lacroute F (1992) 6-azauracil inhibition of GTP biosynthesis in Saccharomyces cerevisiae. Curr Genet 22: 9–11 [DOI] [PubMed] [Google Scholar]
- Fish R, Kane C (2002) Promoting elongation with transcript cleavage stimulatory factors. Biochim Biophys Acta 1577: 287–307 [DOI] [PubMed] [Google Scholar]
- Flores A, Briand JF, Boschiero C, Gadal O, Andrau JC, Rubbi L, Van Mullem V, Goussot M, Marck C, Carles C, Thuriaux P, Sentenac A, Werner M (1999) A protein–protein interaction map of yeast RNA polymerase III. Proc Natl Acad Sci USA 96: 7815–7820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromont-Racine M, Rain JC, Legrain P (1997) Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat Genet 16: 277–282 [DOI] [PubMed] [Google Scholar]
- Gari E, Piedrafita L, Aldea M, Herrero E (1997) A set of vectors with tetracycline-regulatable promoter system for modulated gene expression in Saccharomyces cerevisiae. Yeast 13: 837–848 [DOI] [PubMed] [Google Scholar]
- Grant PA, Duggan L, Cote J, Roberts SM, Brownell JE, Candau R, Ohba R, Owen-Hughes T, Allis CD, Winston F, Berger SL, Workman JL (1997) Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev 11: 1640–1650 [DOI] [PubMed] [Google Scholar]
- Hengartner CJ, Myer VE, Liao SM, Wilson CJ, Koh SS, Young RA (1998) Temporal regulation of RNA polymerase II by Srb10 and Kin28 cyclin-dependent kinases. Mol Cell 2: 43–53 [DOI] [PubMed] [Google Scholar]
- Hirst M, Kobor MS, Kuriakose N, Greenblatt J, Sadowski I (1999) GAL4 is regulated by the RNA polymerase II holoenzyme-associated cyclin-dependent protein kinase SRB10/CDK8. Mol Cell 3: 673–678 [DOI] [PubMed] [Google Scholar]
- Horikoshi M, Sekimizu K, Hirashima S, Mitsuhashi Y, Natori S (1985) Structural relationships of the three stimulatory factors of RNA polymerase II from Ehrlich ascites tumor cells. J Biol Chem 260: 5739–5744 [PubMed] [Google Scholar]
- Izban MG, Luse DS (1992) The RNA polymerase II ternary complex cleaves the nascent transcript in a 3′–5′ direction in the presence of elongation factor SII. Genes Dev 6: 1342–1356 [DOI] [PubMed] [Google Scholar]
- Kettenberger H, Armache KJ, Cramer P (2003) Architecture of the RNA polymerase II–TFIIS complex and implications for mRNA cleavage. Cell 114: 347–357 [DOI] [PubMed] [Google Scholar]
- Labhart P, Morgan GT (1998) Identification of novel genes encoding transcription elongation factor TFIIS (TCEA) in vertebrates: conservation of three distinct TFIIS isoforms in frog, mouse, and human. Genomics 52: 278–288 [DOI] [PubMed] [Google Scholar]
- Larschan E, Winston F (2001) The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev 15: 1946–1956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao SM, Zhang J, Jeffery DA, Koleske AJ, Thompson CM, Chao DM, Viljoen M, Van Vuuren H, Young RA (1995) A kinase–cyclin pair in the RNA polymerase II holoenzyme. Nature 374: 193–196 [DOI] [PubMed] [Google Scholar]
- Lindstrom DL, Hartzog GA (2001) Genetic interactions of Spt4–Spt5 and TFIIS with the RNA polymerase II CTD and CTD modifying enzymes in Saccharomyces cerevisiae. Genetics 159: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Smerdon MJ (2002) Rpb4 and Rpb9 mediate subpathways of transcription-coupled DNA repair in Saccharomyces cerevisiae. EMBO J 21: 5921–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH (1972) Experiments in Molecular Genetics. Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory [Google Scholar]
- Nakanishi T, Nakano A, Nomura K, Sekimizu K, Natori S (1992) Purification, gene cloning, and gene disruption of the transcription elongation factor S-II in Saccharomyces cerevisiae. J Biol Chem 267: 13200–13204 [PubMed] [Google Scholar]
- Nakanishi T, Shimoaraiso M, Kubo T, Natori S (1995) Structure–function relationship of yeast S-II in terms of stimulation of RNA polymerase II, arrest relief, and suppression of 6-azauracil sensitivity. J Biol Chem 270: 8991–8995 [DOI] [PubMed] [Google Scholar]
- Natori S, Takeuchi K, Takahashi K, Mizuno D (1973) DNA dependent RNA polymerase from Ehrlich ascites tumor cells. II. Factors stimulating the activity of RNA polymerase II. J Biochem (Tokyo) 73: 879–888 [DOI] [PubMed] [Google Scholar]
- Nogi Y, Yano R, Dodd J, Carles C, Nomura M (1993) Gene RRN4 in Saccharomyces cerevisiae encodes the 12.2 subunit of RNA polymerase I and is essential only at high temperature. Mol Cell Biol 13: 114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan G, Aso T, Greenblatt J (1997) Interaction of elongation factors TFIIS and elongin A with a human RNA polymerase II holoenzyme capable of promoter-specific initiation and responsive to transcriptional activators. J Biol Chem 272: 24563–24571 [DOI] [PubMed] [Google Scholar]
- Pokholok DK, Hannett NM, Young RA (2002) Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol Cell 9: 799–809 [DOI] [PubMed] [Google Scholar]
- Pray-Grant MG, Schieltz D, McMahon SJ, Wood JM, Kennedy EL, Cook RG, Workman JL, Yates JR III, Grant PA (2002) The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol Cell Biol 22: 8774–8786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reines D (1992) Elongation factor-dependent transcript shortening by template-engaged RNA polymerase II. J Biol Chem 267: 3795–3800 [PMC free article] [PubMed] [Google Scholar]
- Ryu S, Zhou S, Ladurner AG, Tjian R (1999) The transcriptional cofactor complex CRSP is required for activity of the enhancer-binding protein Sp1. Nature 397: 446–450 [DOI] [PubMed] [Google Scholar]
- Sawadogo M, Sentenac A, Fromageot P (1980) Interaction of a new polypeptide with yeast RNA polymerase B. J Biol Chem 255: 12–15 [PubMed] [Google Scholar]
- Shpakovski GV, Acker J, Wintzerith M, Lacroix JF, Thuriaux P, Vigneron M (1995) Four subunits shared by the three classes of RNA polymerases are functionally interchangeable between Homo sapiens and Saccharomyces cerevisiae. Mol Cell Biol 15: 4702–4710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpakovski GV, Gadal O, Labarre-Mariotte S, Lebedenko EN, Miklos I, Sakurai H, Proshkin SA, Van Mullem V, Ishihama A, Thuriaux P (2000) Functional conservation of RNA polymerase II in fission and budding yeasts. J Mol Biol 295: 1119–1127 [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P (1989) A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122: 19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner DE, Belotserkovskaya R, Berger SL (2002) SALSA, a variant of yeast SAGA, contains truncated Spt7, which correlates with activated transcription. Proc Natl Acad Sci USA 99: 11622–11627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner DE, Grant PA, Roberts SM, Duggan LJ, Belotserkovskaya R, Pacella LA, Winston F, Workman JL, Berger SL (1999) Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol Cell Biol 19: 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschochner H (1996) A novel RNA polymerase I-dependent RNase activity that shortens nascent transcripts from the 3′ end. Proc Natl Acad Sci USA 93: 12914–12919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata T, Shimizu T, Adachi N, Sekimizu K, Nakanishi T (2003) Cleavage, but not read-through, stimulation activity is responsible for three biologic functions of transcription elongation factor S-II. J Biol Chem 278: 8580–8585 [DOI] [PubMed] [Google Scholar]
- Van Mullem V, Landrieux E, Vandenhaute J, Thuriaux P (2002a) Rpa12p, a conserved RNA polymerase I subunit with two functional domains. Mol Microbiol 43: 1105–1113 [DOI] [PubMed] [Google Scholar]
- Van Mullem V, Wery M, De Bolle X, Vandenhaute J (2003) Construction of a set of Saccharomyces cerevisiae vectors designed for recombinational cloning. Yeast 20: 739–746 [DOI] [PubMed] [Google Scholar]
- Van Mullem V, Wery M, Werner M, Vandenhaute J, Thuriaux P (2002b) The Rpb9 subunit of RNA polymerase II binds TFIIE and functionally interacts with the SAGA and Elongator factors. J Biol Chem 277: 10220–10225 [DOI] [PubMed] [Google Scholar]
- Walhout AJ, Temple GF, Brasch MA, Hartley JL, Lorson MA, van den Heuvel S, Vidal M (2000) GATEWAY recombinational cloning: application to the cloning of large numbers of open reading frames or ORFeomes. Methods Enzymol 328: 575–592 [DOI] [PubMed] [Google Scholar]
- Williams LA, Kane CM (1996) Isolation and characterization of Schizosaccharomyces pombe gene encoding transcript elongation factor TFIIS. Yeast 12: 227–236 [DOI] [PubMed] [Google Scholar]
- Winston F, Dollard C, Ricuperco-Hovasse S (1995) Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11: 53–55 [DOI] [PubMed] [Google Scholar]
- Wu PY, Winston F (2002) Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex. Mol Cell Biol 22: 5367–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki K, Guo L, Sugahara K, Zhang C, Enzan H, Nakabeppu Y, Kitajima S, Aso T (2002) Identification and biochemical characterization of a novel transcription elongation factor, Elongin A3. J Biol Chem 277: 26444–26451 [DOI] [PubMed] [Google Scholar]


