Abstract
Although birth weight is a potential causal risk factor for attention-deficit/hyperactivity disorder (ADHD) symptoms, both the specificity of this association and its mediating pathways are largely unknown. We carefully assessed youth with and without ADHD (i.e., Wave 1), and followed them prospectively for 2 years (i.e., Wave 2). We (a) tested the association of birth weight with Wave 2 ADHD symptoms, and (b) evaluated biologically plausible neurocognitive functions from Wave 1 as temporally ordered mediators of birth weight and Wave 2 ADHD symptoms in a multiple mediation framework. At Wave 1, 222 ethnically diverse youth (30% female; ages 5–10) completed the Digit Span, Vocabulary, Symbol Search, and Arithmetic subtests of the Wechsler Intelligence Scale for Children–IV. At both Wave 1 and Wave 2 (ages 7–13), multiple informants (i.e., parents, teachers) rated youth ADHD symptoms and co-occurring psychopathology using multiple methods (i.e., structured interview, rating scale). Controlling for demographic factors, gestational age, and co-occurring externalizing and internalizing psychopathology, birth weight inversely predicted Wave 2 ADHD symptoms across multiple methods and informants. Additionally, controlling for Wave 1 ADHD symptoms and relevant covariates, Wave 1 Arithmetic uniquely mediated the association of birth weight with multi-method/informant Wave 2 ADHD symptoms. These findings suggest that birth weight is a relatively specific risk factor for youth ADHD symptoms and they implicate individual differences in fluid reasoning as a preliminary causal mediator of this association. We discuss implications for future research evaluating causal mechanisms underlying risk factors for ADHD.
Individual differences in attention-deficit/hyperactivity disorder (ADHD) are sensitive to multiple causal influences (i.e., equifinality), including substantial heritability as well as pre-natal and perinatal factors (Nigg, Willcutt, Doyle, & Sonuga-Barke, 2005; Thapar, Cooper, Eyre, & Langley, 2013). Meta-analytic and prospective longitudinal evidence converge to suggest that low birth weight (i.e., ≤ 88 oz/2,500 g) predicts ADHD diagnosis and symptoms in both youth and adults (Aarnoudse-Moens, Weisglas-Kuperus, Van Goudoever, & Oosterlaan, 2009; Bhutta, Cleves, Casey, Cradock, & Anand, 2002; Halmøy, Klungsøyr, Skjærven, & Haavik, 2012; Martel, Lucia, Nigg, & Breslau, 2007; Nigg & Breslau, 2007). Even co-twin control designs, which provide quasi-experimental evidence for causal effects independent of genetic and environmental confounds, suggest that birth weight predicts youth inattention and hyperactivity/impulsivity (H/I) symptoms (Groen-Blokhuis, Middeldorp, Van Beijsterveldt, & Boomsma, 2011; Pettersson et al., 2015). That is, birth weight is unlikely to correlate with ADHD symptoms due to its association with other correlates of poor fetal development (e.g., prenatal exposure to maternal stress, substance use, nutrition); rather, it appears to be a preliminary independent causal risk factor for ADHD symptoms.
Although low birth weight reliably predicts ADHD diagnostic status and symptoms, it may also constitute a non-specific risk for multiple poor outcomes. Meta-analytic evidence suggests a significant, albeit weaker, association of low birth weight with internalizing problems (i.e., depression and anxiety), as well as oppositional defiant disorder (ODD) and conduct disorder (CD) symptoms (Aarnoudse-Moens et al., 2009; Bhutta et al., 2002). This is consistent with evidence that ODD/CD and, to a lesser extent, internalizing symptoms exhibit etiologic and phenotypic overlap with ADHD (Cosgrove et al., 2011; Lahey, Van Hulle, Singh, Waldman, & Rathouz, 2011). Despite these trans-diagnostic associations, surprisingly few studies account for co-occurring mental health problems in predictions of ADHD symptoms from birth weight. Although low birth weight predicted ADHD symptoms, but not disruptive behavior, concurrently within the same sample (Martel et al., 2007; Nigg & Breslau, 2007), we know of no study that has simultaneously controlled for multiple dimensions of co-occurring psychopathology (i.e., ODD, CD, and internalizing problems). Thus, it remains unclear if birth weight predicts ADHD symptoms specifically, or is sensitive to ADHD symptoms via shared variance with other disorders or even general psychopathology (i.e., p factor; Caspi et al., 2014). The present study addresses this important gap directly.
Beyond predictions of ADHD symptoms from birth weight, perhaps more importantly, the pathways mediating this association are largely unknown. That is, if low birth weight is a causal risk factor, elucidating plausible risk processes is necessary to develop effective prevention and intervention strategies (Sonuga-Barke & Halperin, 2010). In particular, given their biological plausibility as causal mediators, we prioritized higher-order neurocognitive factors. Accumulating evidence suggests that birth weight positively predicts IQ and related constructs including working memory, fluid reasoning, verbal comprehension, and processing speed (Aarnoudse-Moens et al., 2009; Bhutta et al., 2002; Hutchinson, De Luca, Doyle, Roberts, & Anderson, 2013; Lahat, Van Lieshout, Saigal, Boyle, & Schmidt, 2014). In turn, working memory deficits feature prominently in causal theories of ADHD (Nigg, 2006; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005), and deficits in fluid reasoning, processing speed, and verbal comprehension were observed in youth and adults with ADHD (Doyle, Biederman, Seidman, Reske-Nielsen, & Faraone, 2005; Tamm & Juranek, 2012; Willcutt et al., 2010). Crucially, mediation by these higher-order domains is biologically plausible, given that they are correlated with neural abnormalities (e.g., reduced cortical surface area, thickness, volume; Martinussen et al., 2005; Skranes et al., 2013) that are sequelae of low birth weight (Martinussen et al., 2005; Skranes et al., 2013; Walhovd et al., 2012) and central to ADHD etiology (Narr et al., 2009; Shaw et al., 2012). For example, compared to normal birth weight controls, young adult survivors of low birth weight exhibited reduced cortical surface area that correlated with working memory and processing speed specifically in regions where underdeveloped surface area has been observed in youth with ADHD (e.g., superior frontal and medial temporal gyri; Shaw et al., 2012; Skranes et al., 2013). However, no study has evaluated separable higher-order neurocognitive functions as pathways from birth weight to subsequent ADHD symptoms.
Several methodological considerations may facilitate identification of causal mediators. First, although there is preliminary evidence that low birth weight predicts ADHD symptoms independent of gestational age, gestational age is associated with both birth weight (Valero De Bernabé et al., 2004) and ADHD (Halmøy et al., 2012); some studies even contend that gestational age is a stronger predictor of ADHD than birth weight (Linnet et al., 2006; Oerlemans et al., 2016). Thus, gestational age must be evaluated as a potential confound to adequately specify birth weight predictions of ADHD symptoms. Second, continuous measures of birth weight and ADHD parallel pathophysiology and improve statistical power. Whereas most studies have dichotomized low birth weight (i.e., ≤ 88 oz) versus normal birth weight, birth weight is monotonically associated with ADHD symptoms (Groen-Blokhuis et al., 2011; Pettersson et al., 2015). Likewise, there is strong evidence that ADHD is best characterized continuously rather than dichotomously (Haslam, Holland, & Kuppens, 2012; Lubke et al., 2007). Third, hypothesized mediators should be temporally ordered relative to key constructs (i.e., ADHD symptoms). Preliminary research suggests that early-developing primary neurocognitive functions (e.g., sensorimotor, visuospatial) partially mediated the association of birth weight and ADHD symptoms in young children (Hatch, Healey, & Halperin, 2014; Martel et al., 2007). However, neurocognition and ADHD symptoms were assessed concurrently, whereas temporally ordered predictors, mediators, and outcomes are necessary to infer causal mediation (Kraemer, Stice, Kazdin, Offord, & Kupfer, 2001). Fourth, given the centrality of equifinality to ADHD, including the likelihood of multiple causal pathways (Nigg et al., 2005), simultaneous evaluation of multiple candidate mediators is heuristic. Thus, a strong design would implement a multiple mediation framework consisting of temporally ordered constructs to disentangle the cumulative and unique effects of neurocognitive mediators.
To review, whereas birth weight may constitute a causal risk factor for ADHD symptoms, the specificity of birth weight to ADHD symptoms (given their overlap with most major psychopathology dimensions) and the mechanisms underlying this association are unknown. We examined biologically plausible higher-order neurocognitive functions (i.e., working memory, processing speed, verbal comprehension, fluid reasoning) within the context of a prospective longitudinal study with temporally ordered constructs. To clarify potential causal processes underlying birth weight and ADHD symptoms, the present study had two key aims: (a) to test the prospective association of individual differences in birth weight with multi-method/informant measures of youth ADHD symptoms, with stringent control of demographic characteristics, gestational age, and multi-method/informant measures of co-occurring internalizing and externalizing symptoms, and (b) to test separable higher-order neurocognitive functions as collective and unique mediators of predictions of ADHD symptoms from birth weight in a multiple mediation framework.
METHODS
Participants
Participants were 222 children with (n = 115) and without (n = 107) ADHD who were intensively evaluated at ages 5–10 (i.e., Wave 1), and followed prospectively for 2 years (i.e., Wave 2; complete demographic data and descriptive statistics are presented in Table 1). Families were recruited from a large metropolitan city in California via advertisements at local schools, pediatric offices, and self-help groups, as well as referrals from mental health providers. Recruitment materials encouraged parents of children with and without ADHD to contact the study staff to determine eligibility. Participants were required to be fluent in English and living with at least one biological parent at least half the time. Exclusion criteria consisted of an IQ below 70 or a diagnosis of an autism spectrum or neurological disorder that prevented full study participation. Children meeting diagnostic criteria for other psychiatric disorders (e.g., depression) were included in the non-ADHD group to enhance external validity.
TABLE 1.
Sample Demographics and Descriptive Statistics
| % of Sample or M (SD), Range | M (SD), Range | ||
|---|---|---|---|
| Sex (Female) | 30.63 | ADHD Outcomes | |
| Ethnicity | DISC Symptoms | 7.05 (5.58), 0–18 | |
| Caucasian | 53.15 | CBCL/TRF Attention | 60.33 (9.65), 50–93 |
| African American | 8.56 | Externalizing Outcomes | |
| Hispanic/Latino | 11.26 | DISC ODD Symptoms | 1.88 (1.97), 0–8 |
| Asian | 3.60 | DISC CD Symptoms | 0.32 (0.66), 0–4 |
| Mixed | 23.42 | CBCL/TRF Externalizing | 52.61 (9.25), 33–77 |
| Mother Has College-Level Degree or Higher | 75.11 | Internalizing Outcomes | |
| Father Has College-level Degree or Higher | 65.66 | DISC Symptoms | 0.72 (1.47), 0–7 |
| CBCL/TRF Internalizing | 53.22 (9.91), 33–80 | ||
| Family Income | 7.68 (2.17), 1–9 | WISC Digit Span | 10.22 (2.62), 2–17 |
| Age | 10.21 (1.32), 7–13 | WISC Vocabulary | 11.50 (3.32), 3–19 |
| Gestational Age | 38.56 (2.71), 28–42 | WISC Symbol Search | 10.54 (2.75), 1–17 |
| Birth Weight in Ounces | 117.83 (19.00), 42–159 | WISC Arithmetic | 11.08 (3.17), 3–19 |
Note: Values are from Wave 2, excluding Wave 1 Wechsler Intelligence Scale for Children (WISC) subtests. Family income was assessed on an ordinal scale from 1 (less than $10,000) to 9 (greater than $75,000) annually. ADHD = attention-deficit/hyperactivity disorder; DISC = Diagnostic Interview Schedule for Children; CBCL/TRF = mean composite of parent and teacher ratings on the Child Behavior Checklist and Teacher Report Form; ODD = oppositional defiant disorder; CD = conduct disorder.
Procedures
Initial study eligibility was determined during a telephone screening. Eligible families (n = 230) were mailed rating scales and invited to complete a laboratory-based assessment (i.e., Wave 1); rating scales were also mailed to children’s teachers. After parents and children gave consent and assent, respectively, parents completed multi-method measures of child psychopathology while children completed neurocognitive and socioemotional assessments in a separate room. At the time of the laboratory-based assessment, 80.45% of children were not regularly taking psychotropic medication of any kind. For the 19.55% of children who were normally medicated, parents and teachers were asked to provide ratings based on the child’s unmedicated behavior, if possible. Additionally, parents were asked to have their child abstain from medication on the day of the assessment; however, this was not a requirement for study participation if the parent objected or had reason to believe that missing 1 day of medication was unsafe for the child. Thus, 7.48% of children completed the Wave 1 neurocognitive assessment with psychotropic medication. Two years later (i.e., Wave 2), families were invited for a laboratory follow-up consisting of assessment procedures highly parallel to Wave 1. Two hundred twenty-two families completed the laboratory-based assessment and returned completed or partially completed rating scales at Wave 1, of which 200 were retained at Wave 2. Missing Wave 2 data were non-randomly distributed by race-ethnicity, with African American youth underrepresented at Wave 2, χ2(4) = 12.18, p = .01, but unrelated to age, sex, family income, and psychopathology symptoms (p > .08 for all tests). We employed multiple imputation procedures (described next) so that analyses were conducted on the full sample of 222 youth. All study procedures were approved by the Institutional Review Board.
Measures
Perinatal Factors
Parents retrospectively reported children’s birth weights in pounds and ounces, which were converted to ounces for all analyses (M = 117.83, SD = 19.00, range = 42–159), on a questionnaire at Wave 1. Notably, parental recall of birth weight is highly correlated with medical record data up to 15 years postpartum (Intraclass Correlation Coefficient = .99; Yawn, Suman, & Jacobsen, 1998). Parents also retrospectively reported children’s gestational age in weeks. Birth weight and gestational age were correlated in this sample (r = .36, p < .001).
Neurocognitive Functioning
Neurocognitive functioning was assessed at Wave 1 using the Digit Span (combined Forward/Backward), Vocabulary, Symbol Search, and Arithmetic subtests of the Wechsler Intelligence Scale for Children–IV (WISC), which demonstrates excellent psychometric properties (Wechsler, 2003). Whereas Digit Span Forward measures short-term auditory memory, Digit Span Backward assesses verbal working memory; their combination reflects both (Wechsler, 2003). Vocabulary likely involves crystallized knowledge but primarily reflects verbal comprehension, and Symbol Search primarily assesses processing speed (Keith, Fine, Taub, Reynolds, & Kranzler, 2006; Wechsler, 2003; Weiss, Keith, Zhu, & Chen, 2013). Although Arithmetic is sensitive to working memory, verbal comprehension, and quantitative reasoning, factor analyses suggest that it reflects fluid reasoning, which may subsume working memory and quantitative reasoning (Keith et al., 2006; Weiss et al., 2013). Fluid reasoning consists of logical thinking and problem solving under novel circumstances, and is factorially separate from crystallized knowledge (Cattell, 1987). We used scaled scores for each subtest.
Youth Psychopathology
Diagnostic Interview Schedule for Children–IV. (DISC; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone 2000)
At Wave 1 and Wave 2, youth symptom counts as well as ADHD diagnostic status were determined with the DISC, a fully structured computer-assisted diagnostic interview that is conducted with the parent and keyed to Diagnostic and Statistical Manual of Mental Disorders (4th ed. [DSM-IV]; American Psychiatric Association, 1994) criteria. The DISC has been extensively validated and demonstrates excellent psychometric properties (Shaffer et al., 2000). For the current study, we extracted the total ADHD, ODD, and CD symptom counts. To estimate internalizing symptoms, we calculated the total number of symptoms from the major depression, generalized anxiety, and social phobia modules, which were identically assessed at Wave 1 and Wave 2.
Child Behavior Checklist/Teacher Report Form (CBCL/TRF; Achenbach & Rescorla 2001)
At both waves, parents completed the CBCL, a normed 113-item rating scale yielding eight narrowband syndrome scales and broadband internalizing and externalizing scales. Each item was rated from 0 (not true) to 2 (very true/often true). Teachers also completed the TRF at both waves, yielding parallel scales to the CBCL. Although the CBCL and TRF use different items than the DISC to assess ADHD, they (a) are highly correlated with DSM-based symptom measures, (b) are extensively validated and demonstrate excellent reliability and validity, and (c) can be easily combined as a single multi-informant measure to conservatively reduce the number of statistical tests (Achenbach & Rescorla, 2001). Thus, we used a mean composite of parent and teacher reported T scores from the Attention Problems scale (Wave 1 rs = .50, p < .001; Wave 2 rs = .52, p < .001), which includes inattention and H/I items. Although the CBCL/TRF Attention Problems T scores do not reflect ADHD diagnostic symptoms per se, we refer to both DSM-based DISC ADHD symptom counts and mean composite CBCL/TRF Attention Problems as “ADHD symptoms” henceforth. Mean CBCL/TRF composite scores for the Externalizing Problems and Internalizing Problems scales were calculated for use as covariates in models predicting CBCL/TRF Attention Problems.
Statistical Analysis
Given that the ADHD symptom data were overdispersed, we fit general linear models specifying a negative binomial distribution in separate predictions of (a) parent-reported ADHD symptoms from the DISC and (b) mean composite parent- and teacher-rated Attention Problems T scores (CBCL/TRF Attention Problems). Age and sex were controlled in the model predicting DISC ADHD symptoms but not in the model predicting CBCL/TRF Attention Problems given that the T scores are already adjusted for age and sex. In both models, race-ethnicity, family income, and gestational age were controlled, as well as measure-consistent Wave 2 psychopathology symptoms: specifically, DISC internalizing, ODD, and CD symptoms were controlled in prediction of DISC ADHD symptoms, and CBCL/TRF Internalizing Problems and Externalizing Problems were controlled in prediction of CBCL/TRF Attention Problems.
Next, we used the multiple mediation PROCESS macro (Hayes, 2013; http://www.processmacro.org) to evaluate Wave 1 Digit Span, Vocabulary, Symbol Search, and Arithmetic as mediators of birth weight and Wave 2 ADHD symptoms (i.e., DISC, CBCL/TRF). Multiple mediation employs bootstrapping, a nonparametric resampling procedure that evaluates total mediation and unique mediation by individual constructs; it is statistically more powerful than traditional mediation techniques (Zhao, Lynch, & Chen, 2010) and robust to non-normal data (Preacher & Hayes, 2008). Each mediation model simultaneously calculated (a) regression-based path coefficients and (b) point estimates and 95% bias-corrected confidence intervals for the total and specific indirect effects of the mediators using 5,000 bootstrap simulations (statistical significance is assumed when the interval excludes zero). Measure-consistent Wave 1 ADHD symptoms were included as covariates in both mediation models. Additionally, because psychotropic medication may impact neurocognitive performance and given that 7.48% of youth were medicated on the day of Wave 1 neurocognitive testing, we controlled for medication status on the day of testing in both mediation models. Finally, we also included all covariates that were at least marginally associated with ADHD symptoms in the respective negative binomial regression models (Table 2 and Table 3), except age as it is already accounted for in the scaled WISC scores. Per recommendations by Preacher and Kelley (2011), effect sizes were calculated using the completely standardized indirect effect, which can be interpreted on a scale of .01 = small, .09 = medium, and .25 = large.
TABLE 2.
Negative Binomial Regression Model Predicting Wave 2 DISC ADHD Symptoms
| Independent Variables | DISC ADHD Symptoms
|
|||
|---|---|---|---|---|
| B | SE | p | 95% CI | |
| Age | −.107 | .05 | .01* | [−.196, −.018] |
| Sex (Female) | −.254 | .14 | .06 | — |
| Ethnicity (African American) | .362 | .19 | .06 | — |
| Ethnicity (Hispanic/Latino) | .404 | .17 | .01* | [.076, .732] |
| Ethnicity (Asian) | .239 | .29 | .40 | — |
| Ethnicity (Mixed) | −.032 | .15 | .82 | — |
| Income | .022 | .03 | .42 | — |
| Gestational Age | .007 | .03 | .83 | — |
| Internalizing Symptoms | .045 | .04 | .21 | — |
| ODD Symptoms | .171 | .03 | < .01*** | [.107, .235] |
| CD Symptoms | .077 | .08 | .31 | — |
| Birth Weight | −.008 | < .01 | .03* | [−.015, −.001] |
Note: Reference group for Ethnicity = “Caucasian.” DISC = parent reports on the Diagnostic Interview Schedule for Children; ADHD = attention-deficit/hyperactivity disorder; B = unstandardized logit parameter; CI = confidence interval; ODD = oppositional defiant disorder; CD = conduct disorder.
p < .05.
p < .01.
p < .001.
TABLE 3.
Negative Binomial Regression Model Predicting Wave 2 CBCL/TRF Attention Problems
| Independent Variables | CBCL/TRF Attention Problems
|
|||
|---|---|---|---|---|
| B | SE | p | 95% CI | |
| Ethnicity (African American) | .021 | .04 | .57 | — |
| Ethnicity (Hispanic/Latino) | .050 | .03 | .05 | — |
| Ethnicity (Asian) | .027 | .04 | .48 | — |
| Ethnicity (Mixed) | −.016 | .02 | .42 | — |
| Income | −.001 | < .01 | .85 | — |
| Gestational Age. | < .001 | < .01 | .93 | — |
| Internalizing Problems | .003 | < .01 | .02* | [.001, .005] |
| Externalizing Problems | .009 | < .01 | < .01*** | [.006, .011] |
| Birth Weight | −.001 | < .01 | < .01** | [−.002, > −.001] |
Note: Reference group for Ethnicity = “Caucasian.” Attention Problems T-scores are adjusted for age and sex. CBCL/TRF = mean composite of parent and teacher ratings on the Child Behavior Checklist and Teacher Report Form; B = unstandardized logit parameter; CI = confidence interval.
p < .05.
p < .01.
p < .001.
Because Wave 2 data were available for 200 of the original 222 youth with data at Wave 1, of which only 172 youth had complete data on key study variables, we used 50 iterations of multiple imputation by chained equations (MICE) in Stata 13.1. Per Seaman and colleagues (2012), we calculated the mean composite CBCL/TRF Attention Problems, Externalizing Problems, and Internalizing Problems T scores prior to conducting MICE to avoid statistical issues when passive variables are created from imputed data (e.g., misspecification of the imputation model, biased parameter estimates; Seaman et al., 2012; Von Hippel, 2009). For the CBCL/TRF calculations, parent ratings were used exclusively when teacher data were missing (n = 123), given that youth with teacher data were similar to youth without teacher data with respect to age, sex, race-ethnicity, income, birth weight, gestational age, neurocognitive functioning, and psychopathology symptoms (p > .10 for all tests suggesting that teacher data were missing at random). Imputed data (n = 222) were used in both negative binomial regression models evaluating the specificity of birth weight to DISC ADHD symptoms and CBCL/TRF Attention Problems. However, given that the PROCESS macro does not accommodate multiple imputation files, the mediation models evaluating the indirect effects of birth weight on DISC ADHD symptoms and CBCL/TRF Attention Problems through the Wave 1 WISC subtests were conducted on the subset of 172 youth with complete data using listwise deletion. Notably, a sample size of 172 significantly exceeds the required sample size (n = 148) to adequately power product-of-coefficients tests of mediation using bootstrap methods for path coefficients halfway between the values for small and medium effects (Fritz & Mackinnon, 2007).
RESULTS
Specificity of Birth Weight to Wave 2 ADHD Symptoms
We first evaluated the specificity of birth weight to multi-method/informant measures of Wave 2 ADHD symptoms. B values in this section are unstandardized logits. To facilitate interpretation, B values have also been exponentiated to provide the incidence rate ratio (IRR). First, controlling for youth age, sex, race-ethnicity, family income, and gestational age, as well as Wave 2 DISC internalizing, ODD, and CD symptoms, birth weight inversely predicted the total number of DISC ADHD symptoms (B = −.008, SE < .01, p = .03; Table 2) with an associated IRR of 0.99. That is, for every 1-oz increase in birth weight, DISC ADHD symptoms decrease by 1% (i.e., are multiplied by 0.99). Second, controlling for race-ethnicity, family income, and gestational age, as well as Wave 2 CBCL/TRF Internalizing Problems and Externalizing Problems, birth weight inversely predicted CBCL/TRF Attention Problems T scores (B = −.001, SE < .01, p < .01; IRR = .998; Table 3; T scores are adjusted for age and sex). Thus, birth weight inversely predicted Wave 2 ADHD symptoms across all methods and informants.
Wave 1 WISC Subtests as Mediators of Birth Weight and Wave 2 ADHD Symptoms
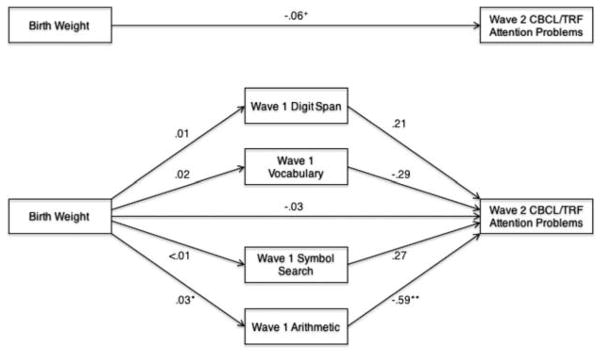
Correlations among the neurocognitive mediators are presented in Table 4. We evaluated whether Wave 1 WISC subtests mediated the association of birth weight with Wave 2 CBCL/TRF Attention Problems, controlling for Wave 1 CBCL/TRF Attention Problems, Wave 2 CBCL/TRF Internalizing Problems and Externalizing Problems, race-ethnicity, and medication status on the day of neurocognitive testing (CBCL/TRF Attention Problems T scores are also adjusted for age and sex). Gestational age and family income were unrelated to CBCL/TRF Attention Problems (Table 3) and thus not controlled. Regression-based path coefficients generated by the PROCESS macro for this multiple mediation model are presented in Figure 1. The total indirect effect of birth weight on Wave 2 CBCL/TRF Attention Problems through the mediators (i.e., the difference between the total effect and direct effect) differed significantly from zero, such that Wave 1 Arithmetic mediated the association of birth weight with Wave 2 CBCL/TRF Attention Problems (Table 5); the indirect effects of Digit Span, Vocabulary, and Symbol Search were not significant. The effect sizes (i.e., the completely standardized indirect effect) for the total indirect effect as well as the specific indirect effect of Arithmetic were −.06 and −.05, respectively, indicating small to medium effects.
TABLE 4.
Bivariate Associations Among the Wechsler Intelligence Scale for Children Neurocognitive Mediators
| Digit Span | Vocabulary | Symbol Search | |
|---|---|---|---|
| Digit Span | — | ||
| Vocabulary | .45 | — | |
| Symbol Search | .25 | .35 | — |
| Arithmetic | .51 | .52 | .41 |
Note: p < .001 for all correlations.
FIGURE 1.
Multiple mediation of birth weight and Wave 2 Child Behavior Checklist/Teacher Report Form (CBCL/TRF) Attention Problems by Wave 1 neurocognitive functions, controlling for race-ethnicity, psychotropic medication status on the day of neurocognitive testing, Wave 1 CBCL/TRF Attention Problems, and Wave 2 CBCL/TRF Internalizing and Externalizing Problems (T scores are adjusted for age and sex). Note: Numbers shown reflect unstandardized beta coefficients. +p < .10. *p < .05. **p < .01.
TABLE 5.
Indirect Effects of Birth Weight on Wave 2 ADHD Symptoms Through the Wave 1 Neurocognitive Functions
| Point Est. | SE | 95% BC Bootstrap CI
|
||
|---|---|---|---|---|
| Lower | Upper | |||
| CBCL/TRF Attention Problems | ||||
| Digit Span | .003 | .005 | −.003 | .018 |
| Vocabulary | −.006 | .007 | −.028 | .001 |
| Symbol Search | < .001 | .004 | −.008 | .009 |
| Arithmetic | −.017 | .010 | −.047 | −.003 |
| Total | −.020 | .011 | −.049 | −.003 |
| DISC ADHD symptoms | ||||
| Digit Span | .002 | .003 | −.001 | .012 |
| Vocabulary | < .001 | .003 | −.005 | .006 |
| Symbol Search | < .001 | .002 | −.002 | .006 |
| Arithmetic | −.007 | .005 | −.021 | −.001 |
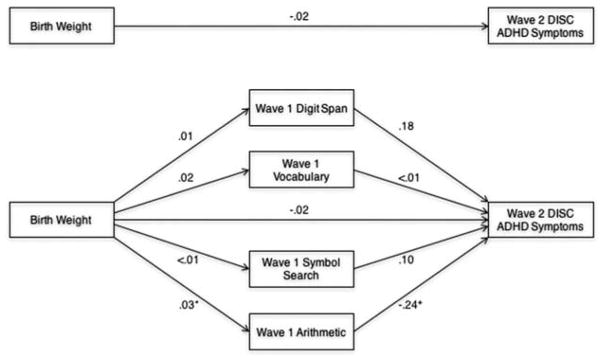
| Total | −.004 | .005 | −.015 | .004 |
Note: Boldface indicates significant mediation. ADHD = attention-deficit/hyperactivity disorder; Point est. = point estimate of the indirect effect; BC Bootstrap CI = bias corrected confidence intervals; CBCL/TRF = mean composite parent and teacher ratings on the Child Behavior Checklist and Teacher Report Form; DISC = parent reports on the Diagnostic Interview Schedule for Children.
Next, we evaluated whether the Wave 1 WISC subtests mediated the association of birth weight with Wave 2 DISC ADHD symptoms, controlling for Wave 1 DISC ADHD symptoms, Wave 2 DISC ODD symptoms, race-ethnicity, sex, and medication status on the day of neurocognitive testing (scaled WISC scores are also adjusted for age). Gestational age, family income, and DISC internalizing and CD symptoms were not controlled given that they were unrelated to DISC ADHD symptoms (Table 2). Regression-based path coefficients generated by the PROCESS macro for this model are presented in Figure 2. Although the total indirect effect on Wave 2 DISC ADHD symptoms was not significant, a specific indirect effect was observed such that Wave 1 Arithmetic uniquely mediated the association of birth weight with Wave 2 DISC ADHD symptoms (Table 5); the indirect effects of Digit Span, Vocabulary, and Symbol Search were not significant. The effect size for the specific indirect effect of Arithmetic was −.03, indicating a small effect.
FIGURE 2.
Multiple mediation of birth weight and Wave 2 Diagnostic Interview Schedule for Children (DISC) attention-deficit/hyperactivity disorder (ADHD) symptoms by Wave 1 neurocognitive functions, controlling for race-ethnicity, sex, psychotropic medication status on the day of neurocognitive testing, Wave 1 DISC ADHD symptoms, and Wave 2 DISC ODD symptoms (scaled neurocognitive function scores are adjusted for age). Note: Numbers shown reflect unstandardized beta coefficients. *p < .05. +p < .10.
DISCUSSION
We tested the specificity of predictions of ADHD symptoms from birth weight and their mediation by biologically plausible higher-order neurocognitive functions in a prospective longitudinal sample. Birth weight inversely predicted ADHD symptoms consistently across multiple methods and informants at 7–13 years postpartum (i.e., Wave 2), even with stringent control of age, sex, race-ethnicity, family income, and gestational age, as well as concurrent internalizing and externalizing symptoms. Next, based on temporally ordered multiple mediation, fluid reasoning (i.e., WISC Arithmetic) at Wave 1 mediated the association of birth weight with multi-method/informant Wave 2 ADHD symptoms, controlling for Wave 1 ADHD symptoms, medication status on the day of neurocognitive testing, key demographic factors, and relevant co-occurring Wave 2 psychopathology; WISC Digit Span, Vocabulary, and Symbol Search were not significant mediators. These findings (a) suggest that birth weight specifically and uniquely predicts the development of ADHD symptoms, even with control of gestational age and co-occurring psycho-pathology, and (b) provide preliminary evidence that fluid reasoning is part of a causal pathway from birth weight to individual differences in youth ADHD.
Although Arithmetic involves multiple neurocognitive functions (Wechsler, 2003), recent factor analyses indicate that Arithmetic principally reflects fluid reasoning. That is, whereas Arithmetic loads moderately onto working memory and modestly onto verbal reasoning domains in traditional four-factor WISC-IV models, there is replicated evidence that it loads strongly onto fluid reasoning in better fitting five-factor models (Keith et al., 2006; Weiss et al., 2013). Fluid reasoning broadly predicts diverse neurocognitive domains (Ferrer, O’Hare, & Bunge, 2009) and may be central to, or even subsume, executive function facets (Cho et al., 2010; Conway, Cowan, Bunting, Therriault, & Minkoff, 2002). Moreover, fluid reasoning strongly predicts general intelligence and has the highest g factor loading of all the WISC subtests (Keith et al., 2006; Weiss et al., 2013). That fluid reasoning mediated the pathogenesis of ADHD symptoms from birth weight converges with prior evidence of fluid reasoning deficits in low birth weight survivors (e.g., Lahat et al., 2014), and fluid reasoning deficits as well as hypoactivation in brain regions relevant to fluid reasoning in youth with ADHD (Tamm & Juranek, 2012). However, the current study is the first to implicate fluid reasoning as a potential mediator of birth weight and ADHD symptoms.
Individual differences within youth with ADHD have been well characterized (e.g., inattention versus H/I, comorbidity, stability), including across multiple levels of putative causal influences (e.g., genetic, neural, cognitive; Nigg et al., 2005; Sonuga-Barke & Halperin, 2010). Thus, whereas the present study suggests that fluid reasoning may reflect part of a causal pathway from birth weight to ADHD symptoms, substantial variance remained unexplained, especially given the only small to medium effect sizes observed in this study. That is, additional neurocognitive functions (e.g., executive functions) may mediate parallel pathways from other risk factors (Nigg et al., 2005), or even from birth weight. For example, working memory was implicated as a potential endophenotype for youth ADHD, especially from dopaminergic genes (Loo et al., 2008). Crucially, prospective longitudinal designs that test heterogeneous pathways to ADHD symptoms are necessary to characterize these multiple complex mechanisms and inform effective prevention strategies (Sonuga-Barke & Halperin, 2010). Thus, evaluation of diverse biologically plausible causal mediators for ADHD symptoms must be a continued priority.
Several key limitations should be noted. First, birth weight was assessed retrospectively, although parental recall of birth weight is highly correlated with medical record data up to 15 years postpartum (Intraclass Correlation Coefficient = .99; Yawn et al., 1998). Second, whereas youth internalizing symptoms were assessed via parent and teacher reports, there is evidence that youth-reported internalizing symptoms may be more reliable (Mesman & Koot, 2000). Third, given that the featured WISC subtests may also tap other domains of functioning in addition to working memory, verbal comprehension, processing speed, and fluid reasoning, replication with more specific measures of these constructs will be helpful in determining their relevance, or lack thereof, to birth weight and ADHD symptoms; this is especially true for working memory given that Digit Span may reflect short-term memory rather than working memory (Colom, Abad, Rebollo, & Shih, 2005). Finally, whereas our study examined mediated main effects, subgroups may exist within neurocognitive pathways (i.e., moderated mediation). For example, mediation of birth weight and ADHD symptoms by motor coordination was stronger for boys than girls (Martel et al., 2007). We await additional studies examining moderators of mediation by fluid reasoning and other neurocognitive functions.
We observed individual differences in birth weight as a specific predictor of youth ADHD symptoms and found that fluid reasoning uniquely mediates this association. Notably, if replicated, fluid reasoning will reflect a single step in a complex, multilevel pathway from birth weight to ADHD. For example, deficient in utero nourishment preceding birth weight and/or postnatal complications arising from birth weight (e.g., neonatal malnutrition; De Curtis & Rigo, 2004) are plausible mechanisms underlying neurodevelopmental impairments that trigger fluid reasoning deficits and ADHD (Georgieff, 2007; Groen-Blokhuis et al., 2011). Therefore, future studies must aim to characterize the proximal mechanisms that mediate the association of birth weight with fluid reasoning and the association of fluid reasoning with ADHD symptoms. To this end, deep phenotyping approaches across multiple levels of analysis (e.g., cellular, neural, behavioral) are promising (Bilder, Howe, Howe, & Sabb, 2013; Calkins et al., 2015), and should be prioritized. Crucially, elucidation of the causal risk processes underlying ADHD symptoms will highlight precise targets for prevention and intervention efforts.
Acknowledgments
FUNDING
This work was supported by the National Institute of Health (1R03AA020186-01 to Steve Lee).
Contributor Information
Julia E. Morgan, Department of Psychology, University of California, Los Angeles
Sandra K. Loo, Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles
Steve S. Lee, Department of Psychology, University of California, Los Angeles
References
- Aarnoudse-Moens CSH, Weisglas-Kuperus N, Van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728. doi: 10.1542/peds.2008-2816. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA School-Age: Forms & profiles. Burlington, VT: ASEBA; 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. Journal of the American Medical Association. 2002;288(6):728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Howe AG, Howe AS, Sabb FW. Multilevel models from biology to psychology: Mission impossible? Journal of Abnormal Psychology. 2013;122(3):917–927. doi: 10.1037/a0032263. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Merikangas KR, Moore TM, Burstein M, Behr MA, Satterthwaite TD, … Gur RE. The Philadelphia Neurodevelopmental Cohort: Constructing a deep phenotyping collaborative. Journal of Child Psychology and Psychiatry. 2015;56:1356–1369. doi: 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, … Moffitt TE. The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB. Intelligence: Its structure, growth and action. Amsterdam, the Netherlands: Elsevier; 1987. [Google Scholar]
- Cho S, Moody TD, Fernandino L, Mumford JA, Poldrack RA, Cannon TD, … Holyoak KJ. Common and dissociable prefrontal loci associated with component mechanisms of analogical reasoning. Cerebral Cortex. 2010;20(3):524–533. doi: 10.1093/cercor/bhp121. [DOI] [PubMed] [Google Scholar]
- Colom R, Abad FJ, Rebollo I, Shih PC. Memory span and general intelligence: A latent-variable approach. Intelligence. 2005;33(6):623–642. doi: 10.1016/j.intell.2005.05.006. [DOI] [Google Scholar]
- Conway ARA, Cowan N, Bunting MF, Therriault DJ, Minkoff SRB. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002;30(2):163–183. doi: 10.1016/S0160-2896(01)00096-4. [DOI] [Google Scholar]
- Cosgrove VE, Rhee SH, Gelhorn HL, Boeldt D, Corley RC, Ehringer MA, … Hewitt JK. Structure and etiology of co-occurring internalizing and externalizing disorders in adolescents. Journal of Abnormal Child Psychology. 2011;39(1):109–123. doi: 10.1007/s10802-010-9444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Curtis M, Rigo J. Extrauterine growth restriction in very-low-birthweight infants. Acta Paediatrica. 2004;93(12):1563–1568. doi: 10.1111/j.1651-2227.2004.tb00844.x. [DOI] [PubMed] [Google Scholar]
- Doyle AE, Biederman J, Seidman LJ, Reske-Nielsen JJ, Faraone SV. Neuropsychological functioning in relatives of girls with and without ADHD. Psychological Medicine. 2005;35(8):1121–1132. doi: 10.1017/S0033291705004496. [DOI] [PubMed] [Google Scholar]
- Ferrer E, O’Hare ED, Bunge SA. Fluid reasoning and the developing brain. Frontiers in Neuroscience. 2009;3(1):46–51. doi: 10.3389/neuro.01.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychological Science. 2007;18(3):233–239. doi: 10.1111/j.1467-9280.2007.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgieff MK. Nutrition and the developing brain: Nutrient priorities and measurement. American Journal of Clinical Nutrition. 2007;85(2):614–620. doi: 10.1093/ajcn/85.2.614S. [DOI] [PubMed] [Google Scholar]
- Groen-Blokhuis MM, Middeldorp CM, Van Beijsterveldt CEM, Boomsma DI. Evidence for a causal association of low birth weight and attention problems. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50(12):1247–1254. doi: 10.1016/j.jaac.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Halmøy A, Klungsøyr K, Skjærven R, Haavik J. Pre- and perinatal risk factors in adults with attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;71(5):474–481. doi: 10.1016/j.biopsych.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Haslam N, Holland E, Kuppens P. Categories versus dimensions in personality and psychopathology: A quantitative review of taxometric research. Psychological Medicine. 2012;42(5):903–920. doi: 10.1017/S0033291711001966. [DOI] [PubMed] [Google Scholar]
- Hatch B, Healey DM, Halperin JM. Associations between birth weight and attention-deficit/hyperactivity disorder symptom severity: Indirect effects via primary neuropsychological functions. Journal of Child Psychology and Psychiatry. 2014;55(4):384–392. doi: 10.1111/jcpp.2014.55.issue-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131(4):e1053–e1061. doi: 10.1542/peds.2012-2311. [DOI] [PubMed] [Google Scholar]
- Keith TZ, Fine JG, Taub GE, Reynolds MR, Kranzler JH. Higher order, multisample, confirmatory factor analysis of the Wechsler Intelligence Scale for Children–Fourth Edition: What does it measure? School Psychology Review. 2006;35(1):108–127. [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158(6):848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Lahat A, Van Lieshout RJ, Saigal S, Boyle MH, Schmidt LA. ADHD among young adults born at extremely low birth weight: The role of fluid intelligence in childhood. Frontiers in Psychology. 2014;5:1–7. doi: 10.3389/fpsyg.2014.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Van Hulle CA, Singh AL, Waldman ID, Rathouz PJ. Higher-order genetic and environmental structure of prevalent forms of child and adolescent psychopathology. Archives of General Psychiatry. 2011;68(2):181–189. doi: 10.1001/archgenpsychiatry.2010.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnet KM, Wisborg K, Agerbo E, Secher NJ, Thomsen PH, Henriksen TB. Gestational age, birth weight, and the risk of hyperkinetic disorder. Archives of Disease in Childhood. 2006;91(8):655–660. doi: 10.1136/adc.2005.088872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo SK, Rich EC, Ishii J, McGough J, McCracken J, Nelson S, Smalley SL. Cognitive functioning in affected sibling pairs with ADHD: Familial clustering and dopamine genes. Journal of Child Psychology and Psychiatry. 2008;49(9):950–957. doi: 10.1111/jcpp.2008.49.issue-9. [DOI] [PubMed] [Google Scholar]
- Lubke GH, Muthén B, Moilanen IK, McGough JJ, Loo SK, Swanson JM, Smalley SL. Subtypes versus severity differences in attention-deficit/hyperactivity disorder in the Northern Finnish Birth Cohort. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(12):1584–1593. doi: 10.1097/chi.0b013e31815750dd. [DOI] [PubMed] [Google Scholar]
- Martel MM, Lucia VC, Nigg JT, Breslau N. Sex differences in the pathway from low birth weight to inattention/hyper-activity. Journal of Abnormal Child Psychology. 2007;35(1):87–96. doi: 10.1007/s10802-006-9089-9. [DOI] [PubMed] [Google Scholar]
- Martinussen M, Fischl B, Larsson HB, Skranes J, Kulseng S, Vangberg TR, Dale AM. Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain. 2005;128:2588–2596. doi: 10.1093/brain/awh610. [DOI] [PubMed] [Google Scholar]
- Mesman J, Koot HM. Child-reported depression and anxiety in preadolescence: I. Associations with parent- and teacher-reported problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(11):1371–1378. doi: 10.1097/00004583-200011000-00011. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del’Homme M, Levitt JG. Widespread cortical thinning is a robust anatomical marker for attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(10):1014– 1022. doi: 10.3758/BRM.40.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT. What causes ADHD? New York, NY: Guilford Press; 2006. [Google Scholar]
- Nigg JT, Breslau N. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46(3):362–369. doi: 10.1097/01.chi.0000246054.76167.44. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Willcutt EG, Doyle AE, Sonuga-Barke EJS. Causal heterogeneity in attention-deficit/hyperactivity disorder: Do we need neuropsychologically impaired subtypes? Biological Psychiatry. 2005;57(11):1224–1230. doi: 10.1016/j.biopsych.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Oerlemans AM, Burmanje MJ, Franke B, Buitelaar JK, Hartman CA, Rommelse NNJ. Identifying unique versus shared pre-and perinatal risk factors for ASD and ADHD using a simplex-multiplex stratification. Journal of Abnormal Child Psychology. 2016;44(5):923–935. doi: 10.1007/s10802-015-0081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson E, Sjölander A, Almqvist C, Anckarsäter H, D’Onofrio BM, Lichtenstein P, Larsson H. Birth weight as an independent predictor of ADHD symptoms: A within-twin pair analysis. Journal of Child Psychology and Psychiatry. 2015;56(4):453–459. doi: 10.1111/jcpp.12299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/BRM.40.3.879. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Kelley K. Effect size measures for mediation models: Quantitative strategies for communicating indirect effects. Psychological Methods. 2011;16(2):93–115. doi: 10.1037/a0022658. [DOI] [PubMed] [Google Scholar]
- Seaman SR, Bartlett JW, White IR. Multiple imputation of missing covariates with non-linear effects and interactions: An evaluation of statistical methods. BMC Medical Research Methodology. 2012;12(1):1–13. doi: 10.1186/1471-2288-12-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;72(3):191–197. doi: 10.1016/j.biopsych.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skranes J, Løhaugen GCC, Martinussen M, Håberg A, Brubakk AM, Dale AM. Cortical surface area and IQ in very-low-birth-weight (VLBW) young adults. Cortex. 2013;49(8):2264–2271. doi: 10.1016/j.cortex.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Halperin JM. Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: Potential targets for early intervention? Journal of Child Psychology and Psychiatry. 2010;51(4):368–389. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Tamm L, Juranek J. Fluid reasoning deficits in children with ADHD: Evidence from fMRI. Brain Research. 2012;1465:48–56. doi: 10.1016/j.brainres.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Cooper M, Eyre O, Langley K. Practitioner review: What have we learnt about the causes of ADHD? Journal of Child Psychology and Psychiatry. 2013;54(1):3–16. doi: 10.1111/jcpp.2012.54.issue-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero De Bernabé J, Soriano T, Albaladejo R, Juarranz M, Calle ME, Martínez D, Domínguez-Rojas V. Risk factors for low birth weight: A review. European Journal of Obstetrics Gynecology and Reproductive Biology. 2004;116:3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Von Hippel PT. How to impute interactions, squares, and other transformed variables. Sociological Methodology. 2009;39(1):265–291. doi: 10.1111/some.2009.39.issue-1. [DOI] [Google Scholar]
- Walhovd KB, Fjell AM, Brown TT, Kuperman JM, Chung Y, Hagler DJ, … Dale AM. Long-term influence of normal variation in neonatal characteristics on human brain development. Proceedings of the National Academy of Sciences. 2012;109:20089–20094. doi: 10.1073/pnas.1208180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children–Fourth Edition (WISC-IV) San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Weiss LG, Keith TZ, Zhu J, Chen H. WISC-IV and clinical validation of the four- and five-factor interpretative approaches. Journal of Psychoeducational Assessment. 2013;31(2):114–131. doi: 10.1177/0734282913478032. [DOI] [Google Scholar]
- Willcutt EG, Betjemann RS, McGrath LM, Chhabildas NA, Olson RK, DeFries JC, Pennington BF. Etiology and neuropsychology of comorbidity between RD and ADHD: The case for multiple-deficit models. Cortex. 2010;46(10):1345–1361. doi: 10.1016/j.cortex.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyper-activity disorder: A meta-analytic review. Biological Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Suman VJ, Jacobsen SJ. Maternal recall of distant pregnancy events. Journal of Clinical Epidemiology. 1998;51(5):399–405. doi: 10.1016/S0895-4356(97)00304-1. [DOI] [PubMed] [Google Scholar]
- Zhao X, Lynch JG, Chen Q. Reconsidering Baron and Kenny: Myths and truths about mediation analysis. Journal of Consumer Research. 2010;37(2):197–206. doi: 10.1086/651257. [DOI] [Google Scholar]