Abstract
One of the main challenges for laser-scanning microscopy of biological tissues with refractive heterogeneities is the degradation in spatial resolution that occurs as a result of beam steering and distortion. This challenge is particularly significant for dual-axis confocal (DAC) microscopy, which achieves improved spatial-filtering and optical-sectioning performance over traditional confocal microscopy through off-axis illumination and collection of light with low-numerical aperture (NA) beams that must intersect precisely at their foci within tissues. DAC microscope image quality is sensitive to positional changes and distortions of these illumination- and collection-beam foci. Previous studies have shown that Bessel beams display improved positional stability and beam quality than Gaussian beams when propagating through tissues with refractive heterogeneities, which suggests that Bessel-beam illumination may enhance DAC microscopy of such tissues. Here, we utilize both Gaussian and Bessel illumination in a point-scanned DAC microscope and quantify the resultant degradation in resolution when imaging within heterogeneous optical phantoms and fresh tissues. Results indicate that DAC microscopy with Bessel illumination exhibits reduced resolution degradation from microscopic tissue heterogeneities compared to DAC microscopy with conventional Gaussian illumination.
Keywords: confocal microscopy, beam-steering and distortion, resolution degradation, Bessel beams
Graphical abstract
The propagation-invariant and self-reconstructing features of Bessel beams have been shown to benefit volumetric microscopy of tissues with refractive heterogeneities. This study examined the resolution degradation that occurs when performing dual-axis confocal (DAC) microscopy within tissues using Gaussian or Bessel illumination. Results indicate Bessel beams are useful for preserving resolution in DAC microscopy, in which tissue-imaging performance is sensitive to positional changes and distortions of off-axis illumination and collection beams.
1. Introduction
Over the past few decades, confocal microscopes have been widely used for bench-top biological investigations [1, 2], as well as for clinical applications such as in vivo microendoscopy and point-of-care pathology [3-15]. By means of point illumination and pinhole detection, confocal microscopy effectively rejects out-of-focus and multiply scattered light, providing users with high-contrast images of three-dimensional tissue microstructure [1]. Typically, a confocal microscope utilizes a single high-numerical-aperture (NA) objective for the illumination and collection of light in order to resolve subcellular features within tissues. As a result of this common-path arrangement for incoming and outgoing photons, background from out-of-focus and multiply scattered light is significant and reduces the imaging contrast and therefore the maximum-achievable imaging depth [16, 17]. With dual-axis confocal (DAC) microscopy [17-22], two off-axis low-NA beams, which only intersect at their foci, are used for illumination and collection of light (Figure 1). As demonstrated through diffraction theory calculations [10, 23], Monte Carlo scattering simulations [22, 24], tissue-phantom measurements [21, 22], as well as ex vivo imaging studies [21, 22], DAC microscopy exhibits improved optical-sectioning performance (i.e. contrast) over conventional single-axis confocal microscopy while providing comparable spatial resolution.
Figure 1.
(a) A schematic of a point-scanned DAC microscope. The focusing element along the illumination path, L2, is either a standard aspheric focusing lens, or an axicon, which generates a Bessel beam. (b) Zoomed-in view of the DAC microscope near the sample. A galvanometric scanning mirror is used to scan the dual-axis beams along the y axis (1-kHz fast axis) while the sample holder is scanned along the z dimension for vertical imaging at 2 frames/sec. The sample holder is also scanned in the x dimension for 3D imaging.
One of the challenges for laser-scanning microscopy of biological tissues is that refractive heterogeneities due to micro-architectural structures - such as nuclei, organelles, glands, and vasculature - can cause spatial variations of optical beam foci in terms of shape (aberrations / distortions) and position (beam steering) [25-28]. In particular, the tissue-imaging performance of a DAC microscope is highly sensitive to the effects of refractive heterogeneities because of its reliance on two beam paths (illumination and collection) that must intersect at their foci [29-31]. For example, it has been shown that the in-focus signal collected by a DAC microscope is significantly decreased when the alignment of the illumination and collection beams is spatially modulated at the micron scale [29, 30]. In addition, as shown in previous studies with tissue phantoms and through in vivo imaging experiments of human epidermis, refractive heterogeneities in tissues lead to a degradation in spatial resolution for DAC microscopy [31, 32], with a concomitant loss in sensitivity (signal-to-noise ratio, SNR) and contrast (signal-to-background ratio, SBR).
The use of Bessel-beam illumination has recently been investigated for a variety of volumetric microscopy approaches [25-28, 33, 34, 35]. Specifically, for light sheet microscopy, the propagation-invariant (“diffraction-free”) property of the main lobe of a Bessel beam is valuable to generate a large depth of focus. Others have investigated the “self-healing” property of Bessel beams that allow for their efficient propagation in tissues in spite of the presence of obstacles that may partially disturb or obstruct the main lobe [25, 26]. It is reasoned that this self-healing behavior is due to the fact that the side lobes of a Bessel beam act to continuously reconstruct the main lobe as the beam propagates [36-40]. However, these side lobes also contribute out-of-focus background that can reduce image contrast, which is a major trade-off when implementing Bessel beams in differnt optical microscopy applications [25-28, 33, 34, 35]. Our preliminary studies have shown that in media with refractive heterogeneities, the irradiance profile of a focused Bessel beam exhibits superior positional stability and beam quality (in the main lobe of the Bessel beam) compared with a focused Gaussian beam, which suggests that Bessel beams may be useful for preserving resolution in DAC microscopy of tissues [41]. In order to validate this hypothesis, this study utilized both Gaussian and Bessel illumination in a point-scanned DAC microscope in order to quantify the resultant degradation of resolution when imaging in optical phantoms with tissue-like heterogeneities, as well as when imaging fresh biological tissues. Experimental results are in agreement with realistic simulations employing a novel fractal propagation method (FPM) that we have recently developed [42]. Results indicate that the use of Bessel beam illumination in DAC microscopy of tissues results in less degradation in resolution compared to conventional Gaussian beam illumination.
2. Methods
Figure 1 provides a schematic of the optical setup of a point-scanned fluorescence DAC microscope [21, 22]. The light source is a singlemode fiber-coupled diode laser (OBIS FP 660LX, Coherent, CA, USA) at a wavelength of 660 nm. For illumination, the Gaussian point source of light emanating from the tip of a singlemode fiber (1/e2 focusing NA (α) of 0.12) is collimated and focused into the sample without magnification at an angle, θ = 30 deg, with respect to the tissue’s normal direction, through a pair of matched aspheric lenses, L1 and L2 (18.4-mm focal lengths, KGA280-B-MT, Newport Corporation, CA, USA). An axicon with a 20-degree base angle (AX2520-A, Thorlabs, NJ, USA), as shown in the dash box in Figure 1(a), is utilized instead of the second aspheric lens (L2) in the illumination path to create a Bessel beam with nearly identical spatial resolutions as the Gaussian beam. Specimens are positioned on top of a hemispherical solid immersion lens (SIL, QU-HS-6, ISP Optics, NY, USA), which provides index matching to allow the focused beams to enter into the samples with minimal aberration and increases the NA of the beams by a factor of ~1.4 (index of fused silica) [23]. Fluorescence signal from the specimen is collected off-axis (θ = 30 deg with respect to the tissue’s normal direction), transmitted through a 664-nm long-pass fluorescence filter (LP02-664RU, Semrock, NY, USA) and coupled into a single-mode fiber, which serves as the pinhole for spatially filtering (rejecting) the out-of-focus and multiply scattered light. The collection beam path utilizes the same pair of aspheric lenses (L1 = L3 = L4) as the Gaussian illumination optics. A PMT detector (H7422-40, Hamamatsu, NJ, USA) in conjunction with a transimpedence amplifier (DHPCA-100, FEMTO, Berlin, Germany) converts the detected photons into a voltage signal. This voltage single is digitized (PCI-6115, National Instruments, TX, USA) and assembled into an image by a custom framegrabber written in LabVIEW (National Instruments, TX, USA) [21].
The DAC microscope performs vertical sectioning by utilizing a galvanometric mirror (6210H, Cambridge Technology, MA, USA) to scan both the illumination and collection beams in the y-direction at a rate of 1 kHz while a linear piezoelectric actuator (P-601.4SL, Physik Instrumente LP) is used to scan the specimens in the vertical direction (z) at a rate of 2 Hz, which defines the frame rate of the microscope (2 frames/sec in the y-z plane). For volumetric microscopy, a motorized actuator (LTA-HL, Newport Corporation, CA, USA) is utilized to translate the sample holder in the third dimension (x direction). With the focusing angle (α = 0.12) and half beam-crossing angle (θ = 30 deg) utilized for this DAC microscope, the theoretical full-width at half-maximum (FWHM) spatial resolutions [10, 23, 43] are: Δx= 1.42 μm; Δy= 1.23 μm; Δz= 2.45 μm.
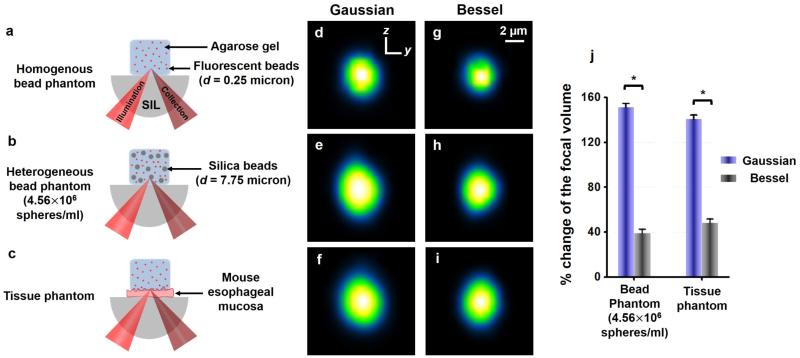
Sub-resolution (0.25-μm diameter) fluorescent beads (λEx/Em = 660/680, F-8807, 2% solids, Invitrogen, MA, USA) were evenly distributed into a 2% w/v agarose solution to create a homogenous optical phantom (Figure 2(a)) for the measurement of an unperturbed point-spread function (PSF). To quantify the effect of refractive heterogeneities on resolution, we also fabricated silica-bead phantoms with a heterogeneous refractive-index distribution (Figure S1). These silica-bead phantoms (Figure 2(b) and Figure S1) were prepared by mixing both the sub-micron fluorescent beads (targets for PSF measurements) at a concentration of 0.50% v/v, and monodisperse silica microspheres (7.75-µm diameter, 2.28×109 spheres per gram, Cospheric, CA, USA) at various concentrations within the agarose gel. The silica-bead concentration depicted in Figure 2(b) (~4.56×106 spheres/ml) was the optimal concentration for mimicking the aberrating properties of fresh tissues (Figure 2(j)). Finally, a tissue phantom was also fabricated (Figure 2(c)) by placing a thin layer of fresh biological tissue (mouse esophageal mucosa, ~100 μm-thick) before the homogeneous agarose phantom that contained sub-micron fluorescent beads for PSF measurements.
Figure 2.
Panels (a)-(c) illustrate three different types of optical phantoms (homogenous fluorescent-bead phantom, heterogeneous silica-bead phantom, and fresh-tissue phantom) utilized for experimental PSF measurements. Panels (d)-(f) are self-normalized y-z cross sections of the average PSFs for a DAC microscope when imaging within these three phantoms using Gaussian illumination. Panels (g)-(i) are the corresponding average PSFs when using Bessel illumination. Panel (j) shows a plot of the degradation in the size of the focal volume for a DAC microscope when imaging within the heterogeneous silica-bead phantom and tissue phantom (compared to the unperturbed focal volume in the homogeneous phantom). * P-value < 0.001. SIL : solid immersion lens.
All phantoms (Figure 2(a)-2(c)) were imaged volumetrically, using a DAC microscope with Gaussian or Bessel illumination. Vertical sections (y-z) were acquired and stored at 2 frames/sec, while the specimen was translated in the third dimension (x direction) at a constant velocity to obtain serial sections separated by 0.7 μm. We sampled 25 discrete sub-resolution fluorescence beads in each volumetric imaging dataset to determine an average 3D PSF of the microscope (as shown in Figure S1). Figures 2(d) to 2(i) display the vertical (y-z) cross-sections of the average 3D PSF of a DAC microscope when imaging within the fluorescent-bead phantom, silica-bead phantom, and fresh-tissue phantom, respectively. The overall resolution degradation was quantified as the percentage change in the volume of the measured PSFs when imaging within heterogeneous phantoms (Figure 2(b) or 2(c)) compared to an unperturbed PSF when imaging within a homogenous phantom (Figure 2(a)), where , and are the FWHM spatial dimensions of each average 3D PSF. In addition, we performed ex vivo fluorescence DAC microscopy of mouse liver vasculature to compare the imaging resolution when using Gaussian vs. Bessel illumination.
Additional details about the development and optimization of optical phantoms, quantification of resolution degradation, and ex vivo imaging experiments, are provided in the supporting materials online.
3. Results and discussion
Figure 2(j) shows the degradation of the focal volume when performing DAC microscopy within various heterogeneous phantoms using Gaussian or Bessel illumination. Refractive heterogeneities in both the silica-bead and fresh-tissue phantoms cause beam steering and distortion artifacts, which deteriorate the spatial resolution in all three dimensions (Figure S1 and S2). However, this degradation in the PSF is reduced significantly when using Bessel illumination compared to Gaussian illumination.
Figure 3 displays the average-intensity projections of a small z-stack of images (thickness = 5 μm) vs. a large z-stack of images (thickness = 50 μm) of mouse liver vasculature, acquired at a moderate depth of ~100 μm. The average-intensity projection (Figure 3 and S3) displays the mean intensity from each vertical column of pixels in a 3D dataset, and shows it as a 2D (x-y) image (the projection is in the z-direction). Even though the diffraction side lobes of a Bessel beam contribute out-of-focus background that deteriorates image contrast (signal-to-background ratio), the image projections obtained with Bessel illumination (Figure 3(b) and 3(d)) exhibit better resolution than the images obtained with Gaussian illumination (Figure 3(a) and 3(c)), especially for the 50-μm thick image stack. Our hypothesis is that due to the superior propagation stability of a Bessel illumination beam, there is less random motion and uncertainty in the position of the focal volume as it is raster scanned in 3D within a thick tissue (to collect a volumetric image). As a result, the average-intensity depth projections are less blurred when using Bessel illumination compared to Gaussian illumination, in which the position of each image voxel is less stable (with respect to the position of other voxels) due to refractive beam steering. This hypothesis was further validated through optical simulations using a novel fractal propagation method (FPM) that was recently developed in our group [42]. It has been shown that the FPM can accurately and efficiently model scattering, diffraction and refractive aberrations in three dimensions, as is relevant for optical microscopy of tissues. The FPM was used to simulate a focused Gaussian or Bessel beam propagating in a realistic tissue with refractive heterogeneities. These simulations were performed using the same parameters as the ex vivo imaging experiments, where λ = 661 nm, and the diffraction-limited FWHM resolution of the focused Gaussian and Bessel beams was 1.42 μm (Figure 4(a) and 4(d)). Individual irradiance profiles of focused Gaussian and Bessel beams were simulated over a range of focal depths within a fractal medium (a realistic model of mouse liver tissue). We then calculated the average Gaussian and Bessel beam irradiance profiles over a range of focal depths, either from approximately 100 ± 2.5 μm (Figure 4(b) and 4(e)), or from approximately 100 ± 25 μm (Figure 4(c) and 4(f)). Details about the simulation method are provided in the online supporting materials. Figure 4 displays the self-normalized irradiance profile of a diffraction-limited beam focus (in a homogeneous medium), as well as the average-intensity projections for a single focused Gaussian or Bessel beam propagating through simulated mouse liver tissue. The focus of a Gaussian beam in tissue exhibits more severe resolution degradation (Figure 4(b)) and beam steering (Figure 4(c)) than the main lobe of a focused Bessel beam (Figure 4(e) and 4(f)). Note that the simulations are similar to the experimental DAC images in showing that averaging the irradiance profiles of a Gaussian beam focus over a large range of focal depths (50 μm) causes significant broadening of the profile due to both distortion and accumulated beam steering effects. However, the main lobe of a Bessel beam is less sensitive to such effects.
Figure 3.
Panels (a) and (b) are average-intensity projections of a stack of DAC microscopy images of mouse liver vasculature, collected over a small range of depths: z = 100 ± 2.5 μm. Panels (c) and (d) are the average-intensity projections of a stack of images collected over a moderate range of depths: z = 100 ± 25.0 μm. Panels (a) and (c) were obtained with Gaussian illumination whereas panels (b) and (d) were obtained with Bessel illumination. The zoomed-in views of the regions outlined in (a)–(d) are presented in panels (e)–(h), respectively. Average line profiles from the highlighted region in (e)–(h) are shown at the bottom of each panel.
Figure 4.
The self-normalized irradiance profiles of simulated Gaussian and Bessel beam foci. Panels (a) and (d) are profiles of diffraction-limited beam foci (Gaussian and Bessel, respectively). Panels (b) and (e) are average-intensity projections, over a limited range of depths (z = 100 ± 2.5-μm deep), of the irradiance profiles of a focused Gaussian and Bessel beam, respectively. Panels (c) and (f) are average-intensity projections, over a larger range of depths (z = 100 ± 25.0-μm deep), of the irradiance profiles of a focused Gaussian and Bessel beam, respectively. Due to greater beam steering and distortion of the Gaussian beam as it propagates in tissue, the Gaussian beam focus is significantly enlarged when viewing a 50-μm thick average-intensity projection vs. a 5-μm thick average-intensity projection of the irradiance profiles (see text for details).
4. Conclusions
Previous studies have shown that the DAC microscope configuration provides superior optical-sectioning contrast (SBR) compared to conventional single-axis confocal microscopes when imaging within homogeneous scattering media. However, biological tissues often contain refractive heterogeneities at sub-micron to mesoscopic scales, which cause beam steering and aberrations that can reduce the resolution and sensitivity of all laser-scanning microscopes, and DAC microscopes in particular [31, 32, 44]. This study focused on quantifying the degradation in resolution that occurs when performing DAC microscopy within realistic phantoms and fresh tissues using either Gaussian or Bessel beam illumination. We previously performed experiments to demonstrate that Bessel illumination is superior for maintaining pointing accuracy and beam quality in realistic tissues. In this study, we further demonstrate that these characteristics of Bessel beams make them particularly beneficial for DAC microscopy, in which the positional stability and high beam quality are critical for such an off-axis illumination and collection architecture that requires two beams to intersect precisely at their foci. A trade-off that must be addressed, as shown in our ex vivo images, is that the diffraction side lobes of a Bessel beam contribute out-of-focus background that reduces image contrast (signal-to-background ratio). Various approaches have been investigated by others to mitigate this background (e.g. two-photon excitation, structured illumination, deconvolution, sectioned Bessel beams, energy-efficient low-Fresnel-number Bessel beams, etc.) [34-35, 40, 44-46]. Similar approaches will need to be explored in the future to obtain an optimal balance between image contrast and resolution for various biomedical applications of DAC microscopy. For example, for in vivo and ex vivo clinical applications of DAC microscopy, the ability to image with high resolution and contrast at depths of hundreds of microns within tissues could be valuable for early disease detection, surgical guidance, post-operative pathology, and biopsy guidance [32, 47-51].
Supplementary Material
Acknowledgements
The authors acknowledge funding support from the NIH/NIDCR R01 DE023497, and the NIH/NCI R01 CA175391.
Footnotes
Supporting Information Additional supporting information may be found in the online version of this article at the publisher’s website.
Reference
- [1].Pawley J. Handbook of Biological Confocal Microscopy. Springer; NY, USA: 2010. [Google Scholar]
- [2].Diaspro A. Confocal and Two-Photon Microscopy: Foundations, Applications and Advances. Wiley-VCH; Germany: 2001. p. 576. [Google Scholar]
- [3].Sabharwal YS, Rouse AR, Donaldson L, Hopkins MF, Gmitro AF. Appl. Opt. 1999;38:7133–7144. doi: 10.1364/ao.38.007133. [DOI] [PubMed] [Google Scholar]
- [4].Rajadhyaksha M, Anderson RR, Webb RH. Appl. Opt. 1999;38:2105–2115. doi: 10.1364/ao.38.002105. [DOI] [PubMed] [Google Scholar]
- [5].Sung KB, Liang C, Descour M, Collier T, Follen M, Malpica A, Richards-Kortum R. J. Microsc. 2002;207:137–145. doi: 10.1046/j.1365-2818.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- [6].Kiesslich R, Burg J, Vieth M, Gnaendiger J, Enders M, Delaney P, Polglase A, McLaren W, Janell D, Thomas S, Nafe B, Galle PR, Neurath MF. Gastroenterology. 2004;127:706–713. doi: 10.1053/j.gastro.2004.06.050. [DOI] [PubMed] [Google Scholar]
- [7].Wang TD, Friedland S, Sahbaie P, Soetikno R, Hsiung PL, Liu JT, Crawford JM, Contag CH. Clin. Gastroenterol Hepatol. 2007;5:1300–1305. doi: 10.1016/j.cgh.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kim P, Puoris'haag M, Cote D, Lin CP, Yun SH. J. Biomed. Opt. 2008;13:010501. doi: 10.1117/1.2839043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thiberville L, Salaun M, Lachkar S, Dominique S, Moreno-Swirc S, Vever-Bizet C, Bourg-Heckly G. Proc. Am. Thorac. Soc. 2009;6:444–449. doi: 10.1513/pats.200902-009AW. [DOI] [PubMed] [Google Scholar]
- [10].Liu JT, Mandella MJ, Loewke NO, Haeberle H, Ra H, Piyawattanametha W, Solgaard O, Kino GS, Contag CH. J. Biomed. Opt. 2010;15:026029. doi: 10.1117/1.3386055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leigh SY, Liu JT. Opt. Lett. 2012;37:2430–2432. doi: 10.1364/OL.37.002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jabbour JM, Bentley JL, Malik BH, Nemechek J, Warda J, Cuenca R, Cheng S, Jo JA, Maitland KC. Biomed. Opt. Express. 2014;5:3781–3791. doi: 10.1364/BOE.5.003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qiu Z, Khondee S, Duan X, Li H, Mandella MJ, Joshi BP, Zhou Q, Owens SR, Kurabayashi K, Oldham KR, Wang TD. Gastroenterology. 2014;146:615–617. doi: 10.1053/j.gastro.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Flores ES, Cordova M, Kose K, Phillips W, Rossi A, Nehal K, Rajadhyaksha M. J. Biomed. Opt. 2015;20:61103. doi: 10.1117/1.JBO.20.6.061103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yin C, Glaser AK, Leigh SY, Chen Y, Wei L, Pillai PC, Rosenberg MC, Abeytunge S, Peterson G, Glazowski C, Sanai N, Mandella MJ, Rajadhyaksha M, Liu JT. Biomed. Opt. Express. 2016;7:251–263. doi: 10.1364/BOE.7.000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Patel YG, Rajadhyaksha M, Dimarzio CA. Biomed. Opt. Express. 2011;2:2231–2242. doi: 10.1364/BOE.2.002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stelzer EHK, Lindek S. Opt. Commun. 1994;111:536–547. [Google Scholar]
- [18].Wang TD, Mandella MJ, Contag CH, Kino GS. Opt. Lett. 2003;28:414–416. doi: 10.1364/ol.28.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stelzer EHK, Lindek S, Albrecht S, Pick R, Ritter G, Salmon NJ, Stricker R. J. Microsc. 1995;179:1–10. [Google Scholar]
- [20].Webb RH, Rogomentich F. Appl. Opt. 1999;38:4870–4875. doi: 10.1364/ao.38.004870. [DOI] [PubMed] [Google Scholar]
- [21].Liu JT, Mandella MJ, Crawford JM, Contag CH, Wang TD, Kino GS. J. Biomed. Opt. 2008;13:034020. doi: 10.1117/1.2939428. [DOI] [PubMed] [Google Scholar]
- [22].Wang D, Chen Y, Wang Y, Liu JT. Opt, Lett. 2013;38:5280–5283. doi: 10.1364/OL.38.005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu JT, Mandella MJ, Friedland S, Soetikno R, Crawford JM, Contag CH, Kino GS, Wang TD. J. Biomed. Opt. 2006;11:054019. doi: 10.1117/1.2363363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen Y, Wang D, Liu JT. Opt. Lett. 2012;37:4495–4497. doi: 10.1364/OL.37.004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Fahrbach FO, Rohrbach A. Nat. Commun. 2012;3:632. doi: 10.1038/ncomms1646. [DOI] [PubMed] [Google Scholar]
- [26].Fahrbach FO, Simon P, Rohrbach A. Nat. Photon. 2010;4:780–785. [Google Scholar]
- [27].Moosavi SH, Gohn-Kreuz C, Rohrbach A. Appl. Opt. 2013;52:5835–5842. doi: 10.1364/AO.52.005835. [DOI] [PubMed] [Google Scholar]
- [28].Gao L, Shao L, Chen B-C, Betzig E. Nat. Protocols. 2014;9:1083–1101. doi: 10.1038/nprot.2014.087. [DOI] [PubMed] [Google Scholar]
- [29].Leigh SY, Chen Y, Liu JT. Biomed. Opt. Express. 2014;5:1709–1720. doi: 10.1364/BOE.5.001709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Leigh S, Chen Y, Liu J. IEEE Trans. Biomed. Eng. 2015:1–1. [Google Scholar]
- [31].Wang D, Chen Y, Liu JT. Biomed. Opt. Express. 2012;3:3153–3160. doi: 10.1364/BOE.3.003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dwyer PJ, DiMarzio CA, Zavislan JM, Fox WJ, Rajadhyaksha M. Opt. Lett. 2006;31:942–944. doi: 10.1364/ol.31.000942. [DOI] [PubMed] [Google Scholar]
- [33].Curatolo A, Munro PR, Lorenser D, Sreekumar P, Singe CC, Kennedy BF, Sampson DD. Sci. Rep. 2016;6:23483. doi: 10.1038/srep23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lorenser D, Singe CC, Curatolo A, Sampson DD. Opt. Lett. 2014;39:548–551. doi: 10.1364/OL.39.000548. [DOI] [PubMed] [Google Scholar]
- [35].Zhao M, Zhang H, Li Y, Ashok A, Liang R, Zhou W, Peng L. Biomed. Opt. Express. 2014;5:1296–1308. doi: 10.1364/BOE.5.001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Durnin J, Miceli JJ, Eberly JH. Phys. Rev. Lett. 1987;58:1499–1501. doi: 10.1103/PhysRevLett.59.2612. [DOI] [PubMed] [Google Scholar]
- [37].Lin Y, Seka W, Eberly JH, Huang H, Brown DL. Appl. Opt. 1992;31:2708–2713. doi: 10.1364/AO.31.002708. [DOI] [PubMed] [Google Scholar]
- [38].Durnin J, Miceli JJ, Jr., Eberly JH. Opt. Lett. 1988;13:79. doi: 10.1364/ol.13.000079. [DOI] [PubMed] [Google Scholar]
- [39].Arimoto R, Saloma C, Tanaka T, Kawata S. Appl. Opt. 1992;31:6653–6657. doi: 10.1364/AO.31.006653. [DOI] [PubMed] [Google Scholar]
- [40].Fahrbach FO, Rohrbach A. Opt. Express. 2010;18:24229–24244. doi: 10.1364/OE.18.024229. [DOI] [PubMed] [Google Scholar]
- [41].Chen Y, Liu JT. Biomed. Opt. Express. 2015;6:1318–1330. doi: 10.1364/BOE.6.001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Glaser AK, Chen Y, Liu JT. Optica. 2016;3:861–869. doi: 10.1364/OPTICA.3.000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen Y, Liu JT. J. Biomed. Opt. 2013;18:066006. doi: 10.1117/1.JBO.18.6.066006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fahrbach FO, Gurchenkov V, Alessandri K, Nassoy P, Rohrbach A. Opt. Express. 2013;21:11425–11440. doi: 10.1364/OE.21.011425. [DOI] [PubMed] [Google Scholar]
- [45].Fahrbach FO, Gurchenkov V, Alessandri K, Nassoy P, Rohrbach A. Opt. Express. 2013;21:13824–13839. doi: 10.1364/OE.21.013824. [DOI] [PubMed] [Google Scholar]
- [46].Planchon TA, Gao L, Milkie DE, Davidson MW, Galbraith JA, Galbraith CG, Betzig E. Nat. Meth. 2011;8:417–423. doi: 10.1038/nmeth.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Maslov K, Zhang HF, Hu S, Wang LV. Opt Lett. 2008;33:929–931. doi: 10.1364/ol.33.000929. [DOI] [PubMed] [Google Scholar]
- [48].Tao YK, Shen D, Sheikine Y, Ahsen OO, Wang HH, Schmolze DB, Johnson NB, Brooker JS, Cable AE, Connolly JL, Fujimoto JG. Proc. Natl. Acad. Sci. U.S.A. 2014;111:15304–15309. doi: 10.1073/pnas.1416955111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Curatolo A, Villiger M, Lorenser D, Wijesinghe P, Fritz A, Kennedy BF, Sampson DD. Opt. Lett. 2016;41:21–24. doi: 10.1364/OL.41.000021. [DOI] [PubMed] [Google Scholar]
- [50].Wang K, Sun W, Richie CT, Harvey BK, Betzig E, Ji N. Nat. Commun. 2015;6:7276. doi: 10.1038/ncomms8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Park J-H, Sun W, Cui M. Proc. Natl. Acad. Sci. U.S.A. 2015;112:9236–9241. doi: 10.1073/pnas.1505939112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.