Abstract
Phagocytosis relies on extension of plasmalemmal pseudopods generated by focal actin polymerisation and delivery of membranes from intracellular pools. Here we show that compartments of the late endocytic pathway, bearing the tetanus neurotoxin-insensitive vesicle-associated membrane protein (TI-VAMP/VAMP7), are recruited upon particle binding and undergo exocytosis before phagosome sealing in macrophages during Fc receptor (FcR)-mediated phagocytosis. Expression of the dominant-negative amino-terminal domain of TI-VAMP or depletion of TI-VAMP with small interfering RNAs inhibited phagocytosis mediated by Fc or complement receptors. In addition, inhibition of TI-VAMP activity led to a reduced exocytosis of late endocytic vesicles and this resulted in an early blockade of pseudopod extension, as observed by scanning electron microscopy. Therefore, TI-VAMP defines a new pathway of membrane delivery required for optimal FcR-mediated phagocytosis.
Keywords: endosomes, exocytosis, phagocytosis, recycling, SNARE
Introduction
In mammals, phagocytosis is a hallmark of specialised cells such as macrophages, dendritic cells and polymorphonuclear neutrophils, while it can also be accomplished by other cells, such as thyroid and bladder epithelial cells or mesangial cells in the kidney. It is induced by the recognition of the particle by a large variety of cell surface receptors, among which are receptors for components of microorganisms surface or receptors specific for opsonins, like immunoglobulins (Fc receptors, FcRs) or complement proteins (complement receptors, CRs). Entry is followed by the maturation of the phagosome in a phagolysosome that becomes competent for the degradation of the internalised particle and eventually the presentation of derived peptidic antigens (Aderem and Underhill, 1999; Underhill and Ozinsky, 2002).
Upon receptor ligation, signalling cascades (Greenberg and Grinstein, 2002) lead to actin polymerisation, which is considered to be the driving force allowing the plasma membrane to be extended around the particle. Concomitant with actin polymerisation, and to accommodate the extension of the plasma membrane, focalised exocytosis of membrane from internal pools takes place at the site of the phagocytic cup formation (Holevinsky and Nelson, 1998; Booth et al, 2001). The nature and origin of the membranes contributing to phagocytosis is still an open question. Recycling vesicles containing the transferrin receptor (TfR) or cellubrevin/vesicle-associated membrane protein 3 (VAMP3) are exocytosed at the site of phagocytosis (Bajno et al, 2000; Niedergang et al, 2003). More recently, the endoplasmic reticulum (ER) has been observed in association with forming phagosomes in J774.1 macrophages (Gagnon et al, 2002). In addition, calreticulin and calnexin, two resident proteins of the ER, are required for phagocytosis in Dictyostelium discoideum (Muller-Taubenberger et al, 2001).
More specifically, the role of vesicle-associated, soluble N-ethylmaleimide-sensitive factor attachment protein receptors (v-SNAREs) in focal exocytosis during phagocytosis was revealed in studies where phagocytosis was inhibited by tetanus neurotoxin (TeNT), which cleaves and inactivates synaptobrevin/VAMP2 and cellubrevin/VAMP3 (Hackam et al, 1998). However, macrophages of mice lacking VAMP3 were still able to phagocytose opsonised particles or latex beads, although a delay was observed in the uptake of zymosan (Allen et al, 2002). These observations suggested that other SNAREs, presumably TeNT insensitive, could be implicated in phagocytosis. Here we show that the TeNT-insensitive v-SNARE protein TI-VAMP/VAMP7 plays a crucial role in the onset of phagocytosis in macrophages.
Results
TI-VAMP is associated to a late endosomal compartment in macrophages
In order to gain insight into the function of TI-VAMP in macrophages, we first analysed its subcellular localisation. Immunofluorescence analysis in RAW264.7 macrophages showed a high degree of colocalisation of endogenous TI-VAMP with Lamp1-positive late endosomes/lysosomes, and often the TI-VAMP/VAMP7 vesicles were found on the periphery of the Lamp1 compartment (Figure 1A, panels e–h). By contrast, TI-VAMP is only poorly colocalised with the TfR, a marker of early and recycling endosomes (Figure 1A). The same observations were made in primary bone marrow-derived macrophages and in RAW264.7 cells transiently expressing a GFP-tagged TI-VAMP (data not shown). In addition, immunogold labelling of ultrathin cryosections of RAW264.7 macrophages with an antibody against TI-VAMP detected multivesicular compartments (Figure 4). All these results indicate that TI-VAMP/VAMP7 is associated with a late endocytic compartment in macrophages.
Figure 1.
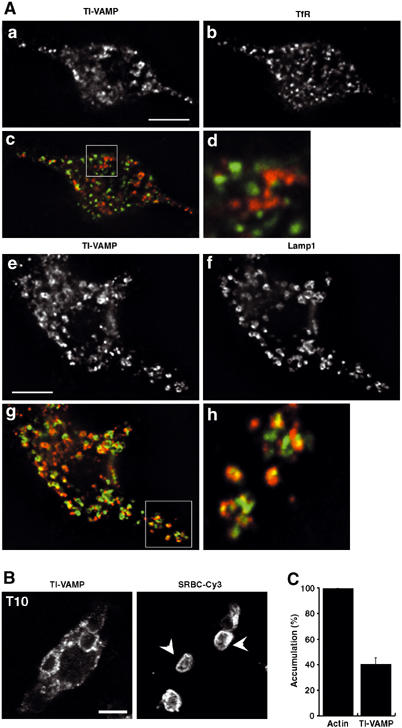
Endogenous TI-VAMP/VAMP7, colocalised with the lysosomal marker Lamp1, is recruited early and accumulates at the site of phagocytosis. (A) RAW264.7 cells were fixed, permeabilised and stained with anti-TI-VAMP and Cy3-anti-mouse IgG (a, c, e, g) and either anti-TfR (b, d) or anti-Lamp1 (f, h) followed by Cy2-anti-rat IgG. (c, g) Combined images; (d, h) insets in (c, g). Cells were analysed by wide-field fluorescence microscopy with deconvolution. Medial optical sections are shown. Bar, 5 μm. An average of 3.7±0.5 (n=10) TI-VAMP-positive and TfR-positive structures were colocalised per cell representing 8.7±1.1% of total TfR-positive structures while an average of 12±1.6 (n=10) TI-VAMP-positive structures were found to overlap with Lamp1-positive structures per cell which corresponded to 41±5.8% of total Lamp1-positive structures. (B) RAW264.7 cells were incubated for 10 min at 37°C with IgG-SRBCs, and then fixed and stained with Cy3-anti-rabbit IgG (right panel). This staining reveals particles that are still accessible to antibodies and therefore in nonclosed phagosomes, whereas internal particles were observed by phase contrast. The cells were then permeabilised, labelled with anti-TI-VAMP followed by Cy2-anti-mouse IgG (left panel) and analysed by confocal microscopy. One optical section is shown. The arrowheads point to external particles positive for TI-VAMP. Bar, 5 μm. (C) GFP-TI-VAMP is recruited early to phagosomes. RAW264.7 cells transiently transfected to express GFP-TI-VAMP were incubated for 10 min at 37°C with IgG-SRBCs, then placed on ice and, without fixation, stained with Cy3-anti-rabbit IgG to detect external particles. Then, cells were fixed, permeabilised and polymerised actin was labelled with phalloïdine-Alexa350. The number of accumulations of polymerised actin and GFP-TI-VAMP were scored for 50 GFP-TI-VAMP-expressing cells and expressed as an index of accumulation per cell. Then, the index obtained for TI-VAMP recruitment was expressed as a percentage of the index of actin cups. Data are the mean±s.e.m. of three independent experiments.
Figure 4.
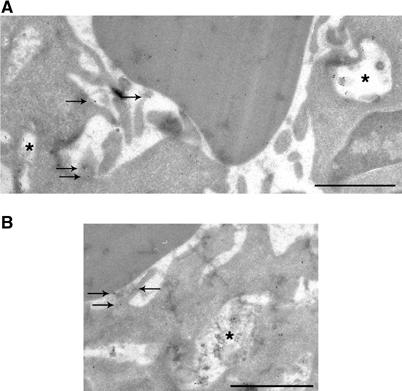
Exocytosis of Lamp1- and TI-VAMP-positive compartments. (A) RAW264.7 macrophages were allowed to phagocytose IgG-SRBCs for 10 min at 37°C and then fixed and processed for ultrathin cryosectioning. Cryosections were immunogold labelled with anti-Lamp1 antibody followed by Protein A-Gold. Bar, 1 μm. Staining of SRBCs was due to a nonspecific reaction. The asterisks indicate Lamp1-positive compartments. The arrows show Lamp1 that was found on membrane ruffles under particles, facing the extracellular medium. (B) Cells were treated as described in (A). Cryosections were immunogold labelled with anti-TI-VAMP antibody followed by Protein A-Gold. Bar, 1 μm. A TI-VAMP-positive compartment is indicated by an asterisk and the arrows show TI-VAMP facing the extracellular medium.
TI-VAMP is recruited to phagosomes
We next analysed TI-VAMP/VAMP7 dynamics during phagocytosis in macrophages allowed to phagocytose IgG-opsonised sheep red blood cells (IgG-SRBCs) at 37°C. Endogenous TI-VAMP, as well as the GFP fusion chimaera, accumulated around particles internalised for 60 min, as described for late endosomal/lysosomal markers during phagosome maturation (data not shown; Desjardins et al, 1994; Oh and Swanson, 1996; Bajno et al, 2000; Henry et al, 2004). This recruitment was specific, as endobrevin/VAMP8, which labels vesicles along the endocytic pathway (Antonin et al, 2000; Mullock et al, 2000 and our unpublished observations), was not accumulated around SRBCs in the same conditions (data not shown). When we analysed early time points, such as 10 min, we noticed that TI-VAMP/VAMP7 was also present around the particles that were still at the beginning of the phagocytic process and engaged in a nonclosed phagosome, defined by their accessibility to fluorescent antibodies directed against the opsonised SRBCs (arrowhead in Figure 1B at 10 min, pointing to external particles accessible to extracellular labelling). To make sure that only external SRBCs were stained, we labelled the cells with the antibodies against SRBCs prior to fixation and staining with phalloidin. We then scored the presence of TI-VAMP recruitment and polymerised actin. Actin cups form in the very first minutes of phagocytosis and then depolymerise (Swanson et al, 1999; Coppolino et al, 2002; Araki et al, 2003; Henry et al, 2004). We observed that TI-VAMP was present in 40% of phagocytic cups defined by F-actin (Figure 1C). The accumulation of TI-VAMP in actin cups was also observed in primary macrophages after 5 min of phagocytosis (data not shown). Therefore, TI-VAMP/VAMP7 recruitment can be detected as early as filamentous actin during the process of phagosome formation.
To further analyse the kinetics of recruitment of TI-VAMP during phagocytosis, the internalisation of IgG-SRBCs was followed by video microscopy in RAW264.7 cells expressing GFP-TI-VAMP (Figure 2 and Movie 1 in Supplementary data). TI-VAMP-positive vesicles migrated towards the site of phagocytosis during the first minutes of phagocytosis and thereafter, and were progressively incorporated around the particles as the phagosome was formed.
Figure 2.
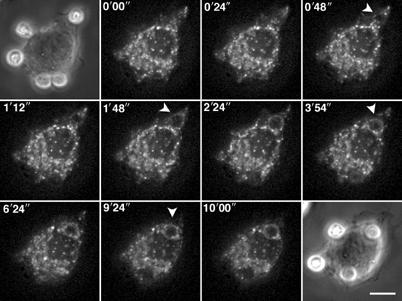
TI-VAMP/VAMP7-positive compartments are recruited early during phagocytosis. RAW264.7 cells transfected to express GFP-TI-VAMP were put into contact with IgG-SRBCs and recorded at 37°C using 4D deconvolution video microscopy. A time-stack was built with maximum intensity projections of deconvolved image stacks (see Movie 1, Supplementary data). Selected images are shown (top left corner, time in s).
Together, these results indicate that TI-VAMP/VAMP7 translocates to the phagosomal membrane early during phagosome formation in macrophages.
TI-VAMP-positive vesicles are exocytosed during phagosome formation
The early recruitment of TI-VAMP at the onset of phagocytosis prompted us to assess whether TI-VAMP/Lamp1-positive vesicles were exocytosed before phagosome sealing. Exocytosis was analysed by electron microscopy, after loading of the TI-VAMP-positive compartments with a fluid-phase marker, horseradish peroxidase (HRP), and detection of the enzymatic activity by conventional diaminobenzidine tetrahydrochloride (DAB) cytochemistry. Fluorescence microscopy confirmed that internalised HRP accumulated in the Lamp1-positive compartment after a 30 min pulse and 1 h chase (data not shown). Electron microscopy showed that DAB was present in multivesicular late endosomes (Figure 3Aa). After 10 min of contact between RAW264.7 cells and IgG-SRBCs, we observed DAB-positive compartments recruited near the cell surface in 40% of the cells (n=22 cells and 342 structures counted in total) (arrowheads in Figure 3Ab), whereas only 25% of the DAB-positive structures were close to the cell surface in control nonphagocytosing cells (n=41 cells and 591 structures counted in total) (Figure 3Aa). In addition, DAB-positive membranes (thin arrows in Figure 3Ac and d) reminiscent of an exocytic event could be found in the extracellular space under or very close to bound particles.
Figure 3.
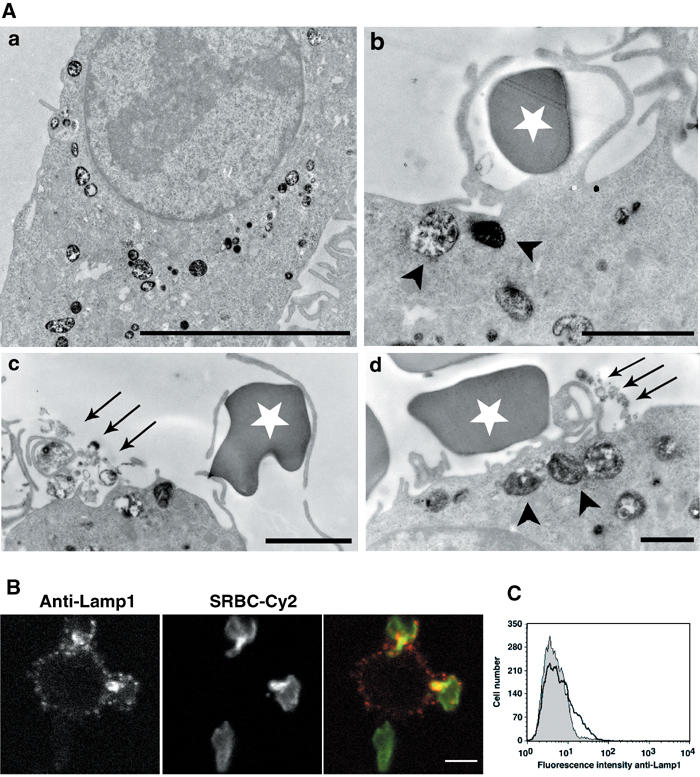
TI-VAMP/Lamp1-positive compartments are exocytosed early during phagocytosis. (A) RAW264.7 cells were allowed to internalise HRP (50 mg/ml) for 30 min at 37°C. After a 1 h chase, phagocytosis was performed for 10 min. The cells were then fixed and processed for DAB cytochemistry and conventional electron microscopy. The arrowheads point to HRP-loaded vesicles recruited to sites of red blood cell attachment and the arrows to exocytosed DAB-positive membranes. The white stars label external SRBCs. Bar, 2 μm. (B) RAW264.7 cells were incubated with IgG-SRBCs for 10 min at 37°C, then placed on ice and, without fixation, stained with anti-Lamp1 antibodies followed by Cy3-anti-rat IgG. External red blood cells were detected with Cy2-anti-rabbit IgG antibodies. Finally, the cells were fixed and analysed by confocal microscopy. One medial optical section is shown. Bar, 5 μm. (C) RAW264.7 cells were treated as in (B), except that secondary antibodies were RPE-coupled. Live cells were then analysed by flow cytometry (bold line). As a control, the cells were incubated with IgG-SRBCs on ice before labelling (dotted histogram).
In order to quantify exocytosis of the TI-VAMP compartment during phagocytosis, we monitored the phenomenon by fluorescence microscopy and flow cytometry. We set up an assay to follow the exposure to the cell surface of a luminal epitope of Lamp1. For this purpose, RAW264.7 macrophages were incubated with IgG-SRBCs for 10 min at 37°C, then placed on ice and labelled to detect only surface-exposed Lamp1. The microscopic analysis revealed that Lamp1 was detected under opsonised particles bound to the macrophages (Figure 3B). To make sure that this staining was not due to clustering of Lamp1 molecules already present at the plasma membrane, cells were analysed by flow cytometry (Figure 3C). Compared to cells incubated with IgG-SRBCs at 4°C, a population of phagocytosing macrophages (around 15% of the total cell population) showed an increase in surface Lamp1 levels, further demonstrating exocytosis of Lamp1-positive membranes from internal stores.
We then sought to detect this exocytotic phenomenon by direct opening of the TI-VAMP/VAMP7 compartment at the site of attachment of particles after immunogold labelling of ultrathin cryosections (Figure 4A and B). Antibodies directed against TI-VAMP as well as Lamp1 decorated multivesicular compartments (asterisks in Figure 4A and B). Direct contacts between the Lamp1-positive vesicles and the plasma membrane were observed at sites of particle attachment (see arrows in Figure 4A). Importantly, similar observations were made for TI-VAMP-positive compartments (arrows in Figure 4B).
Taken together, all these results demonstrate that exocytosis of TI-VAMP/Lamp1-positive compartments occurs during phagosome formation.
TI-VAMP fusion activity is required for optimal phagocytosis via FcR and CR
TI-VAMP belongs to the Longin family of v-SNAREs characterised by the presence of a long amino-terminal autoinhibitory domain (Martinez-Arca et al, 2003), called the Longin domain (Filippini et al, 2001). The Longin domain negatively regulates the ability of TI-VAMP to participate in SNARE complexes (Martinez-Arca et al, 2003) and has a dominant-negative effect in neuronal cells, where its overexpression leads to an inhibition of neurite outgrowth (Martinez-Arca et al, 2000). In RAW264.7 cells, while full-length GFP-TI-VAMP had no effect on phagocytosis of IgG-SRBCs, expression of GFP-Longin led to a diminution of phagocytosis efficiency that was comparable to the inhibition observed with dominant-negative Cdc42, known to inhibit phagocytosis (Figure 5A) (Caron and Hall, 1998; Massol et al, 1998). In contrast, the association of IgG-opsonised particles was not strongly affected. The same results were obtained after 10 min (data not shown) or 60 min of phagocytosis (Figure 5). These results show that the fusion activity of TI-VAMP is important for FcR-mediated phagocytosis.
Figure 5.
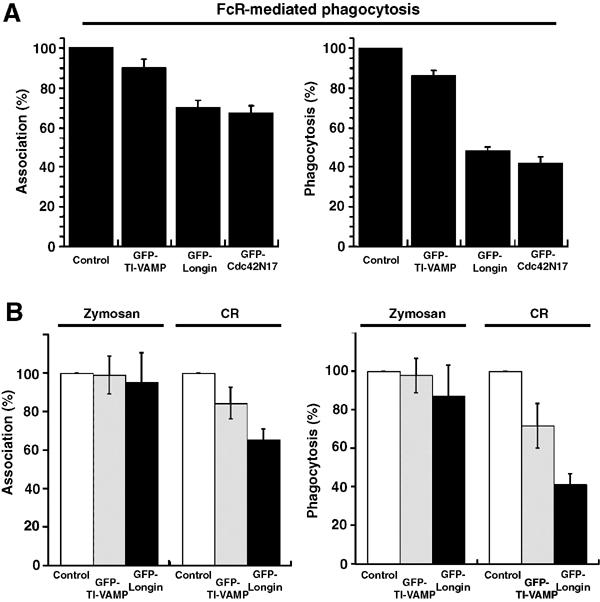
The amino-terminal domain of TI-VAMP inhibits FcR and CR-mediated phagocytosis. (A) RAW264.7 macrophages transiently expressing GFP-TI-VAMP, GFP-Longin or GFP-Cdc42N17 were incubated with IgG-SRBCs for 60 min at 37°C. The cells were then fixed and external SRBCs stained with Cy3-anti-rabbit IgG antibodies. The efficiencies of association (left) and phagocytosis (right) were calculated on 50 transfected and 50 control cells. The mean±s.e.m. of nine independent experiments is plotted. Control, GFP-negative cells. (B) RAW264.7 macrophages transiently expressing GFP-TI-VAMP (grey bars) or GFP-Longin (black bars) were allowed to phagocytose zymosan or C3bi-SRBCs for 60 min at 37°C. The samples were processed as in (A). The mean±s.e.m. of three independent experiments is plotted. Control, GFP-negative cells.
We next analysed whether the role of TI-VAMP could be generalised to other types of phagocytosis. As shown in Figure 5B, CR-mediated phagocytosis was inhibited in cells expressing the dominant-negative Longin domain, whereas the uptake of zymosan was not affected. This result indicates that TI-VAMP activity is required in both type I FcR-mediated and type II CR-mediated phagocytosis that rely on different signalling cascades (Caron and Hall, 1998). By contrast, it is not implicated in the entry of zymosan, which involves various surface receptors, including mannose receptors, CR3 and the recently described Dectin1 (Brown and Gordon, 2001).
TI-VAMP siRNA depletion leads to a defect in FcR-mediated phagocytosis
In order to impair the function of TI-VAMP by another approach, we designed siRNA targeted to the sequence of murine TI-VAMP and analysed the consequences of RNAi depletion of this protein on FcR-mediated phagocytosis. With two different TI-VAMP siRNA, we observed a significant decrease in total level of the protein after 24 h of treatment in RAW264.7 cells (Figure 6B). By immunofluorescence, the depletion appeared to affect clusters of cells, while in some cells the fluorescence was still bright (Figure 6A). Therefore, phagocytosis of IgG-opsonised SRBCs was performed and the efficiency of phagocytosis was scored in cells exhibiting a clear decrease of TI-VAMP fluorescent signal and compared to control cells (treated either with a control siRNA or with oligofectamine alone) (Figure 6). The depletion of TI-VAMP led to an inhibition of phagocytosis comparable to that observed in cells expressing the inhibitory Longin domain (Figure 6C). Therefore, blocking or depleting TI-VAMP inhibited FcR-mediated phagocytosis by approximately 50% and this further shows that TI-VAMP activity is crucial for receptor-mediated phagocytosis.
Figure 6.
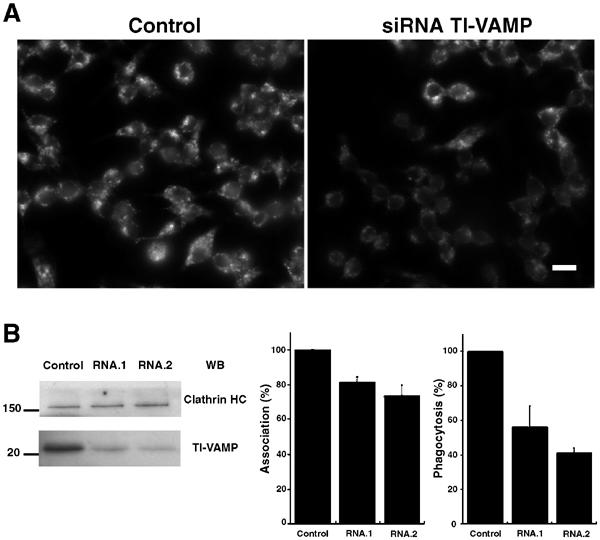
TI-VAMP siRNA treatment inhibits phagocytosis. RAW264.7 macrophages were transfected with siRNA directed against TI-VAMP (RNA.1 or RNA.2). After 24 and 48 h of treatment, cells were analysed by immunofluorescence (A), Western blot (B) or allowed to phagocytose IgG-SRBCs (C). (A) Cells were fixed, permeabilised, then labelled with anti-TI-VAMP followed by Cy3-anti-mouse IgG and analysed by wild-field fluorescence microscopy. Control cells were mock transfected. Bar, 10 μm. (B) Lysates were prepared and Western blotting was performed with anti-TI-VAMP (lower panel) and, after stripping of the membrane, with anti-clathrin HC (upper panel). Control cells were mock-transfected cells. (C) Transfected cells were incubated for 60 min at 37°C with IgG-SRBCs, then fixed and stained with Cy2-anti-rabbit IgG. The cells were then permeabilised and labelled with anti-TI-VAMP followed by Cy3-anti-mouse IgG. The efficiencies of association (left) and phagocytosis (right) were calculated on 50 cells that presented a decrease of TI-VAMP signal and 50 control cells (mock-transfected cells or cells transfected with siRNA GFP). The mean±s.e.m. of three independent experiments is plotted.
Inhibition of both TeNT-sensitive VAMPs and TI-VAMP
The implication of TeNT-sensitive SNAREs in phagocytosis has been reported earlier (Hackam et al, 1998). Therefore, in order to further analyse both the TeNT-sensitive-VAMP- and TI-VAMP-dependent pathways during phagocytosis, we sought to inhibit both sets of proteins simultaneously. For this purpose, macrophages were transfected to express TeNT, which cleaves cellubrevin/VAMP3 (McMahon et al, 1993 and our unpublished results), together with the Longin domain of TI-VAMP. None of the treatment led to a strong inhibition of particle association to the cells (Figure 7A). As reported earlier (Hackam et al, 1998), expression of the TeNT inhibited phagocytosis by 50%, whereas an inactivated toxin did not show any effect (Figure 7B). The simultaneous expression of TeNT and GFP-Longin did not lead to a higher inhibition. This was not due to an inability to further block phagocytosis in RAW264.7 cells, because in cells treated with cytochalasin D, phagocytosis was completely abolished (Figure 7B). Therefore, in contrast to inhibiting actin polymerisation, blocking endomembrane delivery does not completely prevent phagocytosis, and this most probably reflects the fact that the plasma membrane might account for particle engulfment without the need for internal membrane recruitment. These experiments could also suggest that TeNT-sensitive VAMPs, like VAMP3, and TI-VAMP function in a coordinated manner. To address this possibility, we followed simultaneously cellubrevin/VAMP3 and TI-VAMP/VAMP7 during the first steps of IgG-SRBC internalisation in living cells (Figure 7C and Movie 2 in Supplementary data). The dynamics of CFP-cellubrevin and YFP-TI-VAMP indicated that both proteins relocalised to the same phagosome (see arrowheads in Figure 7C, indicating the presence of the probes in extending pseudopods). It appeared that the recruitment of TI-VAMP immediately followed the accumulation of cellubrevin/VAMP3 (arrowheads in Figure 7C) and that the kinetics was slightly shifted in some cases (see arrows for cellubrevin/VAMP3 in Figure 7C). In a parallel set of experiments, Cy5-coupled secondary antibodies were used to detect external particles on cells prior to fixation. At 5, 10 and 20 min, VAMP3 is accumulated on 51, 45 and 51% of the phagosomes still accessible to Cy5-anti-rabbit antibodies, respectively, and TI-VAMP on 13, 20 and 20% of these phagosomes (data not shown). Therefore, there was no strong variation with time of the results for nascent phagosomes. It is worth noting, however, that the presence of a pool of VAMP3 already at the plasma membrane accounts for the signal detected very early around the phagosomes. Interestingly, there is a certain degree of colocalisation of CFP-cellubrevin and YFP-TI-VAMP in phagocytosing macrophages, suggesting that the two pathways of focal exocytosis might cooperate in time and space during phagocytosis.
Figure 7.
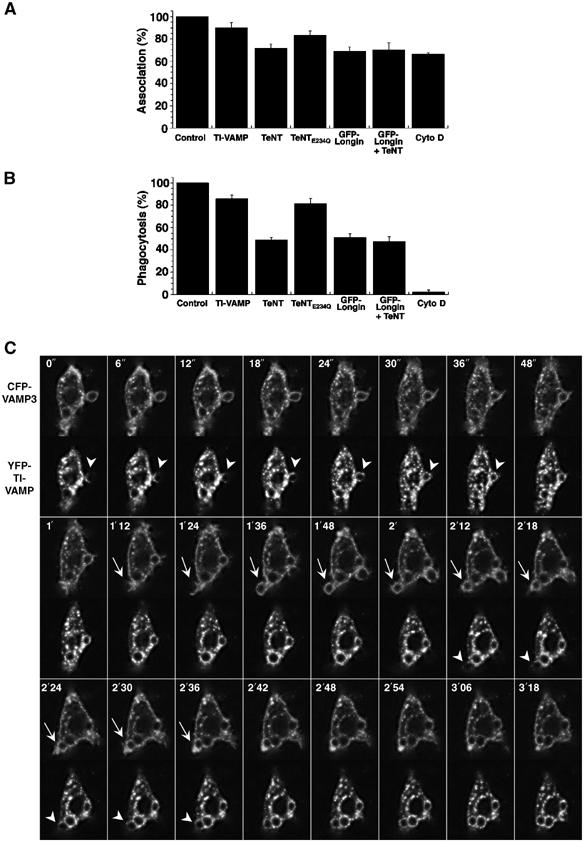
VAMP3- and TI-VAMP-mediated membrane delivery. (A) RAW264.7 macrophages transiently expressing GFP-TI-VAMP, GFP-Longin, TeNTE234Q, TeNT or both GFP-Longin and TeNT, or nontransfected cells pretreated for 30 min at 37°C with cytochalasin D (Cyto D) were processed for phagocytosis as described in Figure 5. Controls for transfection were negative cells and for Cytochalasin D, DMSO-treated cells. The mean efficiency of association±s.e.m. of four independent transfection experiments is plotted. (B) RAW264.7 cells were treated as in (A). The mean efficiency of phagocytosis±s.e.m. of four independent transfection experiments is represented. (C) RAW264.7 cells cotransfected to express YFP-TI-VAMP and CFP-VAMP3 were put into contact with IgG-SRBCs and followed at 37°C under a Zeiss LSM510 Meta confocal microscope. Images were recorded simultaneously in the two channels with a line average of eight every 3 s (see Movie 2, Supplementary data). Selected images are shown (top left corner, time in s). The arrowheads point to accumulation of YFP-TI-VAMP and the arrows to CFP-VAMP3.
TI-VAMP controls exocytosis and membrane extension during phagocytosis
In order to further dissect the role of TI-VAMP in focal exocytosis, we analysed the effect of the GFP-Longin inhibitory construct on the early exposure of Lamp1 on the surface of phagocytosing cells (Figure 8A). The surface level of Lamp1 was measured by flow cytometry after gating on live, GFP-positive cells, expressing either the full-length GFP-TI-VAMP protein or the GFP-Longin domain and placed into contact with IgG-SRBCs for 10 min at 37°C. Whereas 18% of the phagocytosing cells expressing GFP-TI-VAMP showed an increase in the surface expression of Lamp1 (see marker M1 in histogram in bold, Figure 8A), only 6% of the cells expressing GFP-Longin exhibited an increased surface level of Lamp1 (marker M1, filled histogram in Figure 8A). Therefore, exocytosis of Lamp1 is inhibited when the fusion activity of TI-VAMP is impaired.
Figure 8.
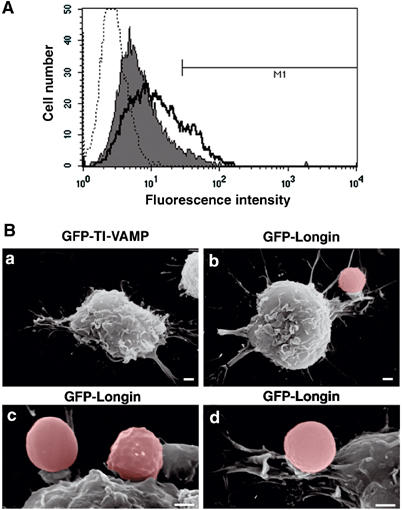
TI-VAMP controls exocytosis and membrane extension during phagocytosis. (A) RAW264.7 cells transfected to express GFP-TI-VAMP or GFP-Longin were incubated with IgG-SRBCs for 10 min at 37°C, then placed on ice and, without fixation, stained with anti-Lamp1 antibodies followed by RPE-anti-rat IgG. At least 5000 live, GFP-positive cells were gated and analysed by flow cytometry. Control cells incubated with secondary antibodies alone (dotted histogram), GFP-Longin-expressing cells (filled histogram), GFP-TI-VAMP-expressing cells (bold line) are shown. M1: Lamp1-positive cells, 18% for GFP-TI-VAMP- and 6% for GFP-Longin-expressing cells. (B) RAW264.7 cells expressing GFP-TI-VAMP (a) or GFP-Longin (b–d) were grown on CeLLocate coverslips, incubated with IgG-SRBCs for 60 min at 37°C, fixed, located on coverslips by fluorescence microscopy and then imaged by SEM. Red blood cells were artificially coloured under Adobe Photoshop 7.0. Bar, 1 μm.
To gain further insight into the stage of FcR-mediated phagocytosis that is affected by the inhibition of TI-VAMP, RAW264.7 cells were transfected to express the GFP-Longin construct and analysed by scanning electron microscopy (SEM) after identification by immunofluorescence of the transfected positive cells on coverslips. After 60 min at 37°C, macrophages expressing GFP-Longin showed particles still associated to the surface (Figure 8Bb), whereas IgG-SRBCs were all internalised in cells expressing GFP-TI-VAMP (Figure 8Ba and observation by fluorescence microscopy not shown). Very small extensions of membrane and pedestal-like structures were observed (Figure 8Bc and d). Therefore, expression of the dominant-negative form of TI-VAMP blocked phagocytosis at an early stage, before extension of the pseudopods. Together, these results show that TI-VAMP fusion activity is crucial for focal exocytosis of late endocytic vesicles and phagosome formation.
Discussion
In this study, we demonstrate that the delivery of TI-VAMP/Lamp1-positive late endosomal membranes occurs early upon particle binding, is controlled by the v-SNARE protein TI-VAMP/VAMP7 and is necessary to complete phagocytosis via FcR.
The intracellular compartment defined by TI-VAMP in macrophages corresponds to late endosomes/multivesicular bodies, and does not differ much from what was described in fibroblastic and neuronal cells (Advani et al, 1998; 1999; Coco et al, 1999; Martinez-Arca et al, 2003; Pryor et al, 2004). In phagocytosing cells, the association of TI-VAMP with phagosomes increased with time. Indeed, accumulation of late endosomal and lysosomal markers was previously reported to occur only 10–20 min after the beginning of phagocytosis of latex beads or opsonised red blood cells (Desjardins et al, 1994; Oh and Swanson, 1996; Bajno et al, 2000; Henry et al, 2004). These observations suggested that the lysosomal markers accumulated as a consequence of the fusion of late endosomes/lysosomes with the maturing phagolysosome and indicated that lysosomes were unlikely to contribute to phagosome formation, although this possibility had never been formally ruled out. Here we report that TI-VAMP/Lamp1-positive compartments fuse with the cell surface already before phagosome closure, and that surface expression of Lamp1 is under the control of active TI-VAMP. Furthermore, the inhibition of phagocytosis observed when the fusion activity of TI-VAMP was blocked with an inhibitory construct, or when the protein was depleted from cells by RNAi, clearly supports a role of TI-VAMP-positive vesicles in the beginning of phagocytosis mediated by FcR or CR. In addition, it is very unlikely that the implication of TI-VAMP at the onset of phagocytosis reflects a general perturbation of the endocytic pathway, since inhibition of Rab5 (on early endosomes), Rab7 (on late endosomes), Syntaxin 7 (on late endosomes) or Syntaxin 13 (on recycling endosomes) does not interfere with the efficiency of internalisation (Collins et al, 2002; Vieira et al, 2003). Therefore, as for VAMP3-positive recycling endosomes, the fusion of TI-VAMP/VAMP7-positive late endocytic compartments with the phagosome occurs early and contributes to phagosome formation. Based on the SNARE model, syntaxins present on the plasma membrane should be cognate partners for cellubrevin/VAMP3 and TI-VAMP/VAMP7 in order to target fusion of internal endocytic membranes with the plasma membrane. Syntaxins 2, 3 or 4, which are known to be plasmalemmal in macrophages, could play this role (Hackam et al, 1996). TI-VAMP was indeed reported to interact with Syntaxin 3 and Syntaxin 4 (Martinez-Arca et al, 2003; Rao et al, 2004). By contrast, Syntaxin 13 and Syntaxin 7 would be involved in fusions with endocytic compartments during maturation of the phagosome (Collins et al, 2002).
There are now potentially three compartments contributing to focal exocytosis during phagocytosis in macrophages, the VAMP3-positive recycling endosomes (Bajno et al, 2000; Niedergang et al, 2003), the TI-VAMP-positive late endosomes (this study) and the ER (Gagnon et al, 2002). In addition, particles may also enter the cells after stretching of the plasma membrane pushed by actin, without any contribution of internal membranes. Some pathways may be able to compensate for others when they are blocked. Indeed, inhibition of both TeNT-sensitive and -insensitive pathways leads to a 50% reduction in phagocytosis (Hackam et al, 1998 and this study), but inhibiting both pathways at the same time did not result in an increased inhibition of phagocytosis. The fact that TI-VAMP/VAMP7 and cellubrevin/VAMP3 are recruited sequentially to the phagosome before sealing highlights a potential link between the two pathways. Vesicles of the endocytic and exocytic pathways are specifically defined by sets of SNARE proteins, which allow fusion between two compartments, as well as the Rab GTPases, which control vesicle targeting and tethering (Chen and Scheller, 2001; Zerial and McBride, 2001). The endocytic pathway is highly dynamic and has been proposed to be organised in domains that share partners of the docking/fusion machinery (Gruenberg, 2001), and the phagosome provides a unique occasion to reveal the dynamic mosaicism of the endosomal system (Niedergang and Chavrier, 2004).
The involvement of ER in phagocytosis is supported by the ultrastructural observation of a recruitment of the ER in J774.1 macrophages around phagosomes containing latex beads, or IgG- or complement-opsonised SRBCs, as well as microorganisms like Leishmania (Gagnon et al, 2002) and by the phagocytic defect of calreticulin and calnexin mutants in D. discoideum (Muller-Taubenberger et al, 2001). However, fluorescent ER markers could not be detected around phagosomes containing IgG-SRBCs in RAW264.7 macrophages (Henry et al, 2004 and our unpublished results). Accumulation of ER markers around the phagosomes was nevertheless reported by Henry et al when RAW264.7 cells phagocytosed latex beads. It is noteworthy that, although it was necessary for the internalisation of opsonised particles via FcR and CR, TI-VAMP was dispensable for uptake of zymosan, which relies on several receptors including CR3, mannose receptors and Dectin1 (Herre et al, 2004; Taylor et al, 2004). Therefore, differences in cell lines as well as receptors engaged on their surface may explain the differences in the nature of the intracellular compartments mobilised. Apart from compensating for membrane loss during engulfment, the fusion of phagosomes with intracellular compartments may deliver specific components required later for specialised phagosomal functions (Underhill and Ozinsky, 2002). Interestingly, the fusion between the phagosome and the ER membrane has recently been shown to efficiently provide phagocytosed antigens to the antigen crosspresentation machinery (Ackerman et al, 2003; Guermonprez et al, 2003; Houde et al, 2003).
The fusion of TI-VAMP-positive vesicles with the plasma membrane during phagocytosis in macrophages is reminiscent of the TI-VAMP-dependent neurite outgrowth in primary and cultured neurons (Martinez-Arca et al, 2000). This process is also related to the regulated exocytosis of lysosome-related organelles (‘secretory lysosomes'), like specialised granules in activated neutrophils or basophils (Hibi et al, 2000; Tapper et al, 2002) or lytic granules of cytotoxic T lymphocytes (Stinchcombe et al, 2001). The exocytosis of lysosomes also promotes cell invasion by Trypanosoma cruzi and this process is also of physiological relevance during plasma membrane repair in nonsecretory cells such as endothelial cells and fibroblasts (Andrews, 2002; Blott and Griffiths, 2002). In this context, the exocytosis of lysosomal vesicles, which is triggered by calcium and regulated by synaptotagmin VII (SytVII), has recently been shown to involve TI-VAMP/VAMP7 (Rao et al, 2004). Therefore, to some extent, all these events may be related, and the TI-VAMP-positive compartment appears as a major membrane donor in various physiological situations.
Materials and methods
Plasmids and reagents
pCMV-TeNT, pCMV-TeNT(E234Q) and pEGFPC3 encoding GFP-VAMP3, GFP-TI-VAMP or GFP-Longin have been described previously (Martinez-Arca et al, 2000). The pEGFPL11-Cdc42N17 plasmid was a kind gift from Dr P Fort (UPR 1086, Montpellier, France). The pYFP-TI-VAMP plasmid was a generous gift from Dr E Bertrand (IGH, Montpellier, France). The pECFP-C3 plasmid was constructed by replacing the cDNA of EGFP from the pEGFP-C3 plasmid (Clontech) by the cDNA of ECFP from the pECFP-C1 plasmid (Clontech), using the Age1–BsrG1 sites. The cDNA of VAMP3 was cloned into the pECFP-C3 plasmid by using the KpnI–XbaI sites.
Cytochalasin D, HRP, DAB, human C5-deficient serum and PMA were from Sigma. Zymosan was from Molecular Probes. Anti-TI-VAMP antibodies have been described (Muzerelle et al, 2003). The anti-TeNT antibodies were from the late Dr H Niemann and Dr T Binz (Hannover School of Medicine, Hannover, Germany). The monoclonal anti-mTfR (clone R17.217.1.3) was kindly provided by Dr A Dautry-Varsat (URA CNRS 2582, Institut Pasteur, Paris, France). The following antibodies were used: anti-clathrin heavy chain (Becton Dickinson/Transduction Laboratories), anti-Lamp1 (Becton Dickinson/Pharmingen), rabbit IgG anti-SRBCs (ICN), rabbit IgM anti-SRBCs (Accurate) and R-phycoerythrin (RPE)-, Cy2-, Cy3- or Cy5-labelled F(ab′)2 anti-mouse, anti-rat and anti-rabbit IgG (Jackson Immunoresearch). Protein A-Gold was from Utrecht University.
Cell culture and transfection
RAW264.7 macrophages were grown and transfected as described (Niedergang et al, 2003).
Small interfering double-stranded RNA treatment of RAW264.7
RAW264.7 macrophages were transfected with small interfering RNA (siRNA) duplex (Proligo) specific for mouse TI-VAMP or for GFP with Oligofectamine® according to the manufacturer's instructions (Invitrogen; Elbashir et al, 2001). TI-VAMP siRNA duplex was as follows: (RNA.1) 5′-AACCUUGUGGAUUCAUCUGUC-3′; (RNA.2) 5′-AGCACAAGUGGAUGAACUGTT-3′. SiRNA duplex targeting GFP (unpublished results) (5′-GAACGGCAUCAAGGUGAACTT-3′) was kindly provided by Dr Franck Perez (UMR144, Institut Curie, Paris, France). After 24 and 48 h, cells were lysed in RIPA buffer (50 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS, 1 mM EDTA, supplemented with protease inhibitors (Roche)). After a 15 min centrifugation at 13 000 g, the amount of proteins in the postnuclear supernatants was quantitated with a BCA Protein Assay kit (Pierce). Proteins were separated on 13% polyacrylamide gel, transferred to PVDF membrane and detected by Western blotting and the ECL procedure (Amersham Biosciences). Stripping was performed in 0.2 M glycine buffer (pH 2.2) (three 10 min incubations at room temperature (RT)). The membrane was then blocked in 10% milk and further detected with antibodies. Control conditions (either a mock transfection or a transfection with a GFP siRNA) gave the same results.
Phagocytosis assays
When indicated, cells were preincubated with 1 μg/ml of cytochalasin D or with the equivalent amount of DMSO for 30 min at 37°C in phagocytosis medium (serum-free complete RPMI) and then phagocytosis was performed. SRBCs were washed two times with PBS1 × and then incubated with rabbit IgG or IgM anti-SRBCs at RT for 30 min. After washing in PBS1 ×, SRBCs opsonised with IgG (IgG-SRBCs) were resuspended in prewarmed phagocytosis medium and IgM-opsonised SRBCs were further incubated for 20 min at 37°C in phagocytosis medium containing 10% C5-deficient complement. Opsonised SRBCs were then resuspended in prewarmed phagocytosis medium and distributed on the cells grown on coverslips (SRBC:macrophage ratio 10). For CR3-mediated phagocytosis, macrophages were incubated with 150 ng/ml PMA in phagocytosis medium for 15 min at 37°C before the start of phagocytosis assay to activate the CR3 receptors.
Phagocytosis was synchronised by centrifugation for 2 min at 400 g and the kinetics was started by incubating the plates at 37°C, 7% CO2. At different time points, the cells were placed on ice, washed once with cold phagocytosis medium and processed for immunofluorescence or electron microscopy.
To quantitate phagocytosis, macrophages were stained with labelled F(ab′)2 anti-rabbit IgG and external SRBCs are defined as particles positive for this labelling and not detectable by phase contrast. Internal particles appear negative for staining with the labelled F(ab′)2 anti-rabbit IgG, or weekly stained if the cells are fixed prior to labelling. They are detectable by phase contrast (Patel et al, 2000). The number of internalised SRBCs was counted in 50 cells randomly chosen on the coverslips, and the phagocytic index, that is, the mean number of phagocytosed SRBCs per cell, was calculated. The index obtained for transfected cells was divided by the index obtained for control nontransfected cells and expressed as a percentage of control cells. We also counted the number of cell-associated (bound+internalised) SRBCs, and calculated the association index (mean number of associated SRBCs per cell) and expressed it as a per cent of control nontransfected cells. To quantitate actin and membrane recruitments, we scored the accumulations of GFP-VAMP7 and polymerised F-actin in 50 cells randomly chosen on the coverslips and calculated the accumulation index, that is, the mean number of accumulations per cell.
Immunofluorescence
Cells were fixed in 4% PFA–PBS and labelled (Niedergang et al, 2003). To stain the exocytosed luminal domain of Lamp1, the cells were first placed on ice to stop phagocytosis and then incubated with anti-Lamp1 for 40 min at 4°C. The cells were then fixed in 4% PFA–PBS for 45 min at 4°C, incubated for 10 min with 50 mM NH4Cl–PBS, washed and then incubated with Cy2-labelled F(ab′)2 anti-rabbit IgG and Cy3-labelled F(ab′)2 anti-rat IgG antibodies in 2% FCS–PBS for 45 min at 4°C to detect external IgG-SRBCs and exocytosed Lamp1, respectively. The cells were then washed twice each in PBS–FCS and PBS, and mounted on microscope slides in 100 mg/ml Mowiol, 25% (v/v) glycerol and 100 mM Tris (pH 8). Alternatively, cells were analysed by flow cytometry on a FACScan cytometer (Becton Dickinson) after labelling with RPE-conjugated (Fab′)2 anti-rat antibodies.
The samples were examined under a confocal microscope (Leica SP2) as described (Niedergang et al, 2003). Alternatively, the cells were examined under a motorised upright wide-field microscope (Leica DMRA2) equipped for image deconvolution. Acquisition was performed using an oil immersion objective (× 100 PL APO HCX, 1.4 NA) and a high-sensitive cooled interlined CCD camera (Roper CoolSnap HQ). The Z-positioning was accomplished by a piezo-electric motor (LVDT, Physik Instrument) mounted underneath the objective lens. The system was steered by Metamorph Software (Universal Imaging Corporation). Z-series of images were taken at 0.2 μm increments. Deconvolution was performed by the new 3D deconvolution module from Metamorph, using the fast Iterative Constrained PSF-based algorithm (Sibarita et al, 2002). Acquisitions were also performed using a motorised upright wide-field microscope (Leica DM RXA2) equipped with oil immersion objectives (× 63 PL APO HCX, 1.32 NA and × 100 PL APO HCX, 1.4 NA) and a high-sensitive cooled interlined CCD camera (Roper CoolSnap HQ).
Electron microscopy
Macrophages were prepared for SEM as described (Niedergang et al, 2003), except that transiently transfected RAW264.7 cells were plated onto CeLLocate (Eppendorf) coverslips to allow the localisation of the GFP-expressing cells under a fluorescence microscope, and then the observation of the same cells by SEM.
For transmission electron microscopy, RAW264.7 cells were serum-starved for 30 min at 37°C in phagocytosis medium, then incubated with 50 mg/ml of HRP for 30 min at 37°C and chased in the same medium for 1 h before starting the phagocytosis assay. Cells were then fixed with a mixture of 2% PFA and 0.5% glutaraldehyde in 0.2 M phosphate buffer (pH 7.4) for 90 min. After several washes with 50 mM Tris–HCl (pH 7.6), the DAB reaction proceeded for 20 min with 0.03% DAB in the presence of 1 μl/ml H2O2 (30 vol). Cells were fixed with 2.5% glutaraldehyde in 0.1 M cacodylate buffer for 90 min, postfixed with 2% OsO4, dehydrated in ethanol and embedded in Epon. Ultrathin sections were counterstained with uranyl acetate before observation under a Philips CM120 Electron Microscope (FEI Company, Eindoven, The Netherlands).
For immunogold labelling on ultrathin cryosections, macrophages were fixed with a mixture of 2% PFA and 0.125% glutaraldehyde in 0.2 M phosphate buffer (pH 7.4) for 2 h at room temperature. Fixed cells were processed for ultrathin cryosectioning as described previously (Raposo et al, 1997). Ultrathin cryosections were immunogold labelled with anti-Lamp1 or anti-TI-VAMP antibody and Protein A-Gold conjugates (10 nm). After embedding in a mixture of methylcellulose and uranyl acetate, they were viewed and photographed as above.
Supplementary Material
Supplementary Material
Supplementary Movie 1
Supplementary Movie 2
Acknowledgments
We thank Dr Emma Colucci-Guyon for her contribution to initial experiments, Michèle Grasset (CIME, Université Paris 6) for assistance with SEM and Jean-François Alkombre (INRA, Centre de Jouy-en Josas) for collecting samples of sheep blood. We are grateful to Drs Alice Dautry-Varsat and Andrés Alcover (Institut Pasteur, Paris) for critical reading of the manuscript. This work was supported by institutional funding from the CNRS and grants from the Institut Curie, the PRFMMIP (Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires) and the Fondation BNP Paribas to PC, and grants from the Human Frontier Science Program (RGY0027_2001-B101), Association pour la Recherche sur le Cancer (ARC) the AFM and EC 6th FP (Signalling and Trafficking STREP 503229) to TG. VB is supported by a doctoral Allocation de Recherche du Ministère de l'Enseignement Supérieur et de la Recherche.
References
- Ackerman AL, Kyritsis C, Tampe R, Cresswell P (2003) Early phagosomes in dendritic cells form a cellular compartment sufficient for cross presentation of exogenous antigens. Proc Natl Acad Sci USA 100: 12889–12894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17: 593–623 [DOI] [PubMed] [Google Scholar]
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH (1998) Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem 273: 10317–10324 [DOI] [PubMed] [Google Scholar]
- Advani RJ, Yang B, Prekeris R, Lee KC, Klumperman J, Scheller RH (1999) VAMP-7 mediates vesicular transport from endosomes to lysosomes. J Cell Biol 146: 765–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LA, Yang C, Pessin JE (2002) Rate and extent of phagocytosis in macrophages lacking VAMP3. J Leukoc Biol 72: 217–221 [PMC free article] [PubMed] [Google Scholar]
- Andrews NW (2002) Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J Cell Biol 158: 389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonin W, Holroyd C, Tikkanen R, Honing S, Jahn R (2000) The R-SNARE endobrevin/VAMP-8 mediates homotypic fusion of early endosomes and late endosomes. Mol Biol Cell 11: 3289–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki N, Hatae T, Furukawa A, Swanson JA (2003) Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci 116: 247–257 [DOI] [PubMed] [Google Scholar]
- Bajno L, Peng X-R, Schreiber AD, Moore H-P, Trimble WS, Grinstein S (2000) Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J Cell Biol 149: 697–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blott EJ, Griffiths GM (2002) Secretory lysosomes. Nat Rev Mol Cell Biol 3: 122–131 [DOI] [PubMed] [Google Scholar]
- Booth JW, Trimble WS, Grinstein S (2001) Membrane dynamics in phagocytosis. Semin Immunol 13: 357–364 [DOI] [PubMed] [Google Scholar]
- Brown GD, Gordon S (2001) Immune recognition. A new receptor for beta-glucans. Nature 413: 36–37 [DOI] [PubMed] [Google Scholar]
- Caron E, Hall A (1998) Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282: 1717–1721 [DOI] [PubMed] [Google Scholar]
- Chen YA, Scheller RH (2001) SNARE-mediated membrane fusion. Nat Rev Mol Cell Biol 2: 98–106 [DOI] [PubMed] [Google Scholar]
- Coco S, Raposo G, Martinez S, Fontaine J-J, Takamori S, Zahraoui A, Jahn R, Matteoli M, Louvard D, Galli T (1999) Subcellular localization of tetanus neurotoxin-insensitive vesicle-associated membrane protein (VAMP)/VAMP7 in neuronal cells: evidence for a novel membrane compartment. J Cell Sci 19: 9803–9812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins RF, Schreiber AD, Grinstein S, Trimble WS (2002) Syntaxins 13 and 7 function at distinct steps during phagocytosis. J Immunol 169: 3250–3256 [DOI] [PubMed] [Google Scholar]
- Coppolino MG, Dierckman R, Loijens J, Collins RF, Pouladi M, Jongstra-Bilen J, Schreiber AD, Trimble WS, Anderson R, Grinstein S (2002) Inhibition of phosphatidylinositol-4-phosphate 5-kinase Iα impairs localized actin remodeling and suppresses phagocytosis. J Biol Chem 277: 43849–43857 [DOI] [PubMed] [Google Scholar]
- Desjardins M, Huber LA, Parton RG, Griffiths G (1994) Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J Cell Biol 124: 677–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir SM, Lendeckel W, Tuschl T (2001) RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev 15: 188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini F, Rossi V, Galli T, Budillon A, D'Urso M, D'Esposito M (2001) Longins: a new evolutionary conserved VAMP family sharing a novel SNARE domain. Trends Biochem Sci 26: 407–409 [DOI] [PubMed] [Google Scholar]
- Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, Bergeron JJ, Desjardins M (2002) Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110: 119–131 [DOI] [PubMed] [Google Scholar]
- Greenberg S, Grinstein S (2002) Phagocytosis and innate immunity. Curr Opin Immunol 14: 136–145 [DOI] [PubMed] [Google Scholar]
- Gruenberg J (2001) The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol 2: 721–730 [DOI] [PubMed] [Google Scholar]
- Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, van Endert P, Amigorena S (2003) ER–phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425: 397–402 [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Bennett MK, Klip A, Grinstein S, Manolson MF (1996) Characterization and subcellular localization of target membrane soluble NSF attachment protein receptors (t-SNAREs) in macrophages. Syntaxins 2, 3, and 4 are present on phagosomal membranes. J Immunol 156: 4377–4383 [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Sjolin C, Schreiber AD, Trimble WS, Grinstein S (1998) v-SNARE-dependent secretion is required for phagocytosis. Proc Natl Acad Sci USA 95: 11691–11696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RM, Hoppe AD, Joshi N, Swanson JA (2004) The uniformity of phagosome maturation in macrophages. J Cell Biol 164: 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herre J, Gordon S, Brown GD (2004) Dectin-1 and its role in the recognition of beta-glucans by macrophages. Mol Immunol 40: 869–876 [DOI] [PubMed] [Google Scholar]
- Hibi T, Hirashima N, Nakanishi M (2000) Rat basophilic leukemia cells express syntaxin-3 and VAMP7 in granule membranes. Biochem Biophys Res Commun 271: 36–41 [DOI] [PubMed] [Google Scholar]
- Holevinsky KO, Nelson DJ (1998) Membrane capacitance changes associated with particle uptake during phagocytosis in macrophages. Biophys J 75: 2577–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M (2003) Phagosomes are competent organelles for antigen cross-presentation. Nature 425: 402–406 [DOI] [PubMed] [Google Scholar]
- Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T (2000) Role of neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol 149: 889–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Arca S, Rudge R, Vacca M, Raposo G, Camonis J, Proux-Gillardeaux V, Daviet L, Formstecher E, Hamburger A, Filippini F, D'Esposito M, Galli T (2003) A dual mechanism controlling the localization and function of exocytic v-SNAREs. Proc Natl Acad Sci USA 100: 9011–9016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massol P, Montcourrier P, Guillemot J-C, Chavrier P (1998) Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J 17: 6219–6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Ushkaryov YA, Edelman L, Link E, Binz T, Niemann H, Jahn R, Südhof TC (1993) Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature 364: 346–349 [DOI] [PubMed] [Google Scholar]
- Muller-Taubenberger A, Lupas AN, Li H, Ecke M, Simmeth E, Gerisch G (2001) Calreticulin and calnexin in the endoplasmic reticulum are important for phagocytosis. EMBO J 20: 6772–6782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock BM, Smith CW, Ihrke G, Bright NA, Lindsay M, Parkinson EJ, Brooks DA, Parton RG, James DE, Luzio JP, Piper RC (2000) Syntaxin 7 is localized to late endosome compartments, associates with Vamp 8, and is required for late endosome–lysosome fusion. Mol Biol Cell 11: 3137–3153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzerelle A, Alberts P, Martinez-Arca S, Jeannequin O, Lafaye P, Mazie JC, Galli T, Gaspar P (2003) Tetanus neurotoxin-insensitive vesicle-associated membrane protein localizes to a presynaptic membrane compartment in selected terminal subsets of the rat brain. Neuroscience 122: 59–75 [DOI] [PubMed] [Google Scholar]
- Niedergang F, Chavrier P (2004) Signaling and membrane dynamics during phagocytosis: many roads lead to the phagos(R)ome. Curr Opin Cell Biol 16: 422–428 [DOI] [PubMed] [Google Scholar]
- Niedergang F, Colucci-Guyon E, Dubois T, Raposo G, Chavrier P (2003) ADP ribosylation factor 6 is activated and controls membrane delivery during phagocytosis in macrophages. J Cell Biol 161: 1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YK, Swanson JA (1996) Different fates of phagocytosed particles after delivery into macrophage lysosomes. J Cell Biol 132: 585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel JC, Hall A, Caron E (2000) Rho GTPases and macrophage phagocytosis. Methods Enzymol 325: 462–473 [DOI] [PubMed] [Google Scholar]
- Pryor PR, Mullock BM, Bright NA, Lindsay M, Gray SR, Richardson SCW, Stewart A, James DE, Piper RC, Luzio JP (2004) Combinatorial SNARE complexes with VAMP7 or VAMP8 define different late endocytic fusion events. EMBO Reports 5: 590–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao SK, Huynh C, Proux-Gillardeaux V, Galli T, Andrews NW (2004) Identification of SNAREs involved in synaptotagmin VII-regulated lysosomal exocytosis. J Biol Chem 279: 20471–20479 [DOI] [PubMed] [Google Scholar]
- Raposo G, Kleijmeer MJ, Posthuma G, Slot JW, Geuze HJ (1997) Immunogold labeling of ultrathin cryosections: application in immunology. In Handbook of Experimental Immunology, Science B (ed) Vol. 4, 5th edn, pp 1–11. Cambridge, MA: Blackwell Science Inc. [Google Scholar]
- Sibarita J-B, Magnin H, De Mey J (2002) Proceedings of the 2002 IEEE International Symposium on Biomedical Imaging, pp 769–772 [Google Scholar]
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM (2001) The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15: 751–761 [DOI] [PubMed] [Google Scholar]
- Swanson JA, Johnson MT, Beningo K, Post P, Mooseker M, Araki N (1999) A contractile activity that closes phagosomes in macrophages. J Cell Sci 112 (Part 3): 307–316 [DOI] [PubMed] [Google Scholar]
- Tapper H, Furuya W, Grinstein S (2002) Localized exocytosis of primary (lysosomal) granules during phagocytosis: role of Ca(2+)-dependent tyrosine phosphorylation and microtubules. J Immunol 168: 5287–5296 [DOI] [PubMed] [Google Scholar]
- Taylor PR, Brown GD, Herre J, Williams DL, Willment JA, Gordon S (2004) The role of SIGNR1 and the beta-glucan receptor (dectin-1) in the nonopsonic recognition of yeast by specific macrophages. J Immunol 172: 1157–1162 [DOI] [PubMed] [Google Scholar]
- Underhill DM, Ozinsky A (2002) Phagocytosis of microbes: complexity in action. Annu Rev Immunol 20: 825–852 [DOI] [PubMed] [Google Scholar]
- Vieira OV, Bucci C, Harrison RE, Trimble WS, Lanzetti L, Gruenberg J, Schreiber AD, Stahl PD, Grinstein S (2003) Modulation of Rab5 and Rab7 recruitment to phagosomes by phosphatidylinositol 3-kinase. Mol Cell Biol 23: 2501–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H (2001) Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Movie 1
Supplementary Movie 2


