Abstract
The generation of COPII vesicles from synthetic liposome membranes requires the minimum coat components Sar1p, Sec23/24p, Sec13/31p, and a nonhydrolyzable GTP analog such as GMP-PNP. However, in the presence of GTP and the full complement of coat subunits, nucleotide hydrolysis by Sar1p renders the coat insufficiently stable to sustain vesicle budding. In order to recapitulate a more authentic, GTP-dependent budding event, we introduced the Sar1p nucleotide exchange catalyst, Sec12p, and evaluated the dynamics of coat assembly and disassembly by light scattering and tryptophan fluorescence measurements. The catalytic, cytoplasmic domain of Sec12p (Sec12ΔCp) activated Sar1p with a turnover 10-fold higher than the GAP activity of Sec23p stimulated by the full coat. COPII assembly was stabilized on liposomes incubated with Sec12ΔCp and GTP. Numerous COPII budding profiles were visualized on membranes, whereas a parallel reaction conducted in the absence of Sec12ΔCp produced no such profiles. We suggest that Sec12p participates actively in the growth of COPII vesicles by charging new Sar1p-GTP molecules that insert at the boundary between a bud and the surrounding endoplasmic reticulum membrane.
Keywords: COPII, GAP, GEF, vesicle transport
Introduction
In eukaryotic cells, transport between the compartments of the secretory pathway is mediated by vesicles, which are produced by budding from donor membranes and fuse with acceptor membranes. The mechanisms of vesicle formation and fusion have been investigated in great detail in recent years, largely through the in vitro reconstitution of transport steps with pure components (Bonifacino and Glick, 2004). Cytoplasmic coat complexes drive vesicle biogenesis and act during different transport steps: clathrin and its assembly proteins function in the late secretory pathway, the COPI coat acts in intra-Golgi and retrograde Golgi–endoplasmic reticulum (ER) transport, and the COPII coat acts in ER–Golgi transport. These coat proteins interact directly with cargo proteins (Cosson and Letourneur, 1994; Hoflack, 1998; Springer and Schekman, 1998) and also deform the membrane to form a bud (Matsuoka et al, 1998b; Spang et al, 1998; Peter et al, 2004), thereby performing the essential aspects of transport: cargo concentration and membrane deformation. The Ras family G-proteins, ARF and Sar1p, appear to be the main regulators of vesicle formation. ARF and Sar1p are activated by binding GTP, which stimulates coat assembly. Small GTPases have very low intrinsic nucleotide exchange or GTPase activity; thus, these events are regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), which are required for activation and inactivation of the GTPase, respectively (Vetter and Wittinghofer, 2001).
Genetic studies in yeast revealed that Sec12p, Sec13p, Sec16p, Sec23p, Sec24p, Sec31p, and Sar1p are essential for vesicle formation at the ER. These proteins contribute to the formation of the COPII coat, which is composed of heteromeric protein complexes, comprising Sec23p/Sec24p (heterodimer) and Sec13p/Sec31p (heterotetramer), and the Sar1p GTPase. Sec23p acts as a GAP specific for Sar1p (Yoshihisa et al, 1993). Sec23p/Sec24p is responsible for the selection of cargo proteins for packaging, providing binding sites for multiple cargo proteins (Miller et al, 2003; Mossessova et al, 2003). Sec13/31p bridges Sec23/24p molecules bound to cargo proteins to create a coat that envelops the membrane into a bud (Matsuoka et al, 2001). The entire process is localized and initiated by Sec12p, an ER-resident membrane GEF that recruits and activates Sar1p (Barlowe and Schekman, 1993). An additional organizing factor, Sec16p, associates with activated Sar1p to provide binding sites for Sec23p, Sec24p, and Sec31p (Espenshade et al, 1995; Gimeno et al, 1996; Shaywitz et al, 1997; Supek et al, 2002).
The mechanism of COPII coat and vesicle formation has been probed with liposomes formulated with defined, synthetic phospholipids. However, an authentic budding reaction that reproduces the normal physiologic event has yet to be recapitulated with the full set of Sec proteins known to operate in vivo. The minimum requirements for synthetic COPII vesicle formation are Sar1p, the core coat subunits, and a nonhydrolyzable analog of GTP, GMP-PNP (Matsuoka et al, 1998b). In conditions of low Mg2+, Sar1p is activated by spontaneous nucleotide exchange and recruited to the membrane. The next two coat complexes are recruited sequentially. Sec23/24p interacts with both Sar1p-GMP-PNP and the charged head groups of acidic phospholipids. Sec13/31p is then recruited, presumably through interactions with both Sar1p and Sec23/24p. During the process of coat recruitment, the membrane is deformed to generate vesicles (Matsuoka et al, 1998b). Even in the absence of liposomes, high concentrations of Sec23/24p and Sec13/31p polymerize into a cage structure, suggesting that these complexes have an intrinsic ability to assemble (Antonny et al, 2003).
COPII coats formed on liposomes with GTP are very unstable and dissociate rapidly from the membrane due to the high GAP activity of the assembled coat (Antonny et al, 2001). The GAP activity of Sec23/24p is stimulated ∼10-fold by Sec13/31p, resulting in a built-in coat disassembly mechanism (Antonny et al, 2001). Thus, although coated vesicles form in a synthetic budding reaction conducted in the presence of GMP-PNP, no evidence of budding vesicles is seen in a reaction containing GTP. Our goal has been to reconstitute the salient features of this process and thereby resolve the essential mechanistic questions.
How can the intrinsic instability of the COPII coat be reconciled with the efficient budding of COPII vesicles from microsomes in the presence of GTP (Barlowe et al, 1994), and indeed with the use of GTP by living cells? One hypothesis is that proteins within the ER membrane inhibit the GAP activity or stabilize the coat after GTP hydrolysis. Cargo proteins may fulfill this role; peptides of p24a protein inhibit Arf1p-GAP activity during COPI coat formation (Goldberg, 2000). Other candidates are the essential genes for COPII formation, Sec16p and Sec12p. Sec16p has binding sites for individual coat subunits and is thought to nucleate the COPII budding sites. However, Sec16p showed no effect on Sec23/24p GAP activity and only partial coat stabilization after GTP hydrolysis (Supek et al, 2002). In this study, we show that the GEF activity of Sec12p surpasses the GAP activity of Sec23/24p and promotes coat stability and vesicle bud formation on liposomes in reactions containing GTP.
Results
Sec12p keeps COPII coats with Sar1p activated by GTP
The real-time assembly of the COPII coat on liposomes can be monitored and quantified by light scattering (Antonny et al, 2001). The intensity of scattered light depends on a mass increase associated with coat polymerization on the liposome surface (Antonny et al, 2001). Using the light scattering assay, we directly assessed the effect of the cytoplasmic domain of Sec12p (Sec12ΔCp) on coat formation on liposomes (major–minor mix of lipids) previously established as optimal for COPII recruitment (Matsuoka et al, 1998b). Coat assembly was monitored by the sequential recruitment of each coat component. In the absence of Sec12ΔCp, Sar1p exchanged GDP for GTP slowly and bound to liposomes to produce a gradual increase in the light scattering signal. Addition of Sec23/24p yielded a sharp increase in the light scattering followed by a slow decay with a half-life of ∼2 min (Figure 1A). Addition of Sec13/31p induced a rapid assembly/disassembly response as previously reported (Figure 1A; Antonny et al, 2001).
Figure 1.
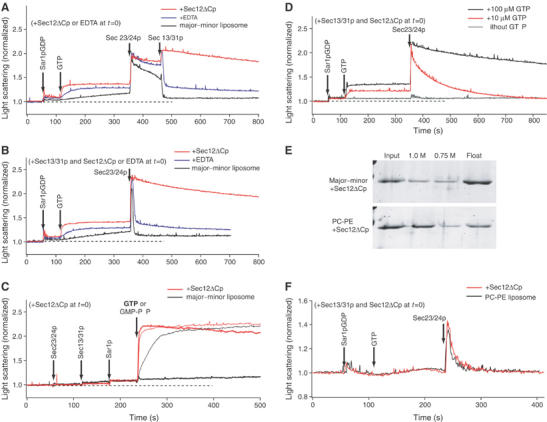
Sec12ΔCp stabilizes GTP-driven COPII coat assembly on liposomes. (A–D) The light scattering of a suspension of major–minor mix liposomes (100 μg ml−1) was continuously monitored upon the addition of 950 nM Sar1p GDP, 160 nM Sec23/24p, 260 nM Sec13/31p, and 100 μM GTP or GMP-PNP, at specific time points as indicated by arrows. (A, B) The initial mixture contained 50 nM Sec12ΔCp (red trace), or 2 mM EDTA (blue traces) in HKM buffer (20 mM Hepes-K, pH 6.8, 160 mM KOAc, 1 mM MgCl2), or buffer alone (black trace). EDTA decreases the concentration of free Mg2+ from 1 mM to 1 μM, and accelerates GDP–GTP exchange by Sar1p. Sec13/31p was added to the initial mixture in (B). (C) Sar1p-GDP was activated by addition of GTP (bold trace) or GMP-PNP (thin trace). The initial mixture contained 50 nM Sec12ΔCp in HKM buffer (red trace), or buffer alone (black trace). (D) Different levels of GTP were added to the reaction mixture: 100 μM GTP (black trace), 10 μM GTP (red trace), or without GTP (gray trace). The initial mixture contained Sec13/31p and 50 nM Sec12ΔCp. (E) Sec12ΔCp binding to liposomes. Major–minor or PC/PE liposomes (400 μg ml−1) were incubated with Sec12ΔCp (500 nM) and separated by flotation centrifugation through sucrose step gradients (1.0, 0.75, and 0 M sucrose in HKM buffer). Proteins recovered in fractions (input (1.0 M sucrose), 1.0 or 0.75 M sucrose, and float fractions (without sucrose)) were analyzed by SDS–PAGE. (F) Light scattering of a suspension of PC/PE liposomes was monitored upon addition of Sar1p-GDP, GTP, and Sec23/24p. The initial mixture contained Sec13/31p and 50 nM Sec12ΔCp (red trace) or HKM buffer alone (black trace).
The spontaneous exchange of nucleotide by Sar1p is facilitated in incubations containing a low concentration of Mg2+. Addition of EDTA to a standard incubation accelerated the rise in the Sar1p-GTP-liposome light scattering signal and stabilized the Sec23/24p, Sar1p-GTP-liposome complex. However, subsequent addition of Sec13/31p produced the usual unstable coat complex.
Addition of Sec12ΔCp produced a more rapid rise in the light scattering signal, consistent with faster nucleotide exchange on Sar1p and enhanced recruitment of Sar1p-GTP by the membrane. The coat intermediate formed on addition of Sec23/24p, and the completion of coat assembly by Sec13/31p were significantly stabilized by Sec12ΔCp (Figure 1A). This stabilization was more obvious in incubations containing Sec13/31p from the outset (Figure 1B). In such a reaction, the coat formed and disassembled rapidly on addition of Sec23/24p in the absence of Sec12ΔCp (t1/2∼2 s), but depolymerized slowly (t1/2∼15 min) in the presence of Sec12ΔCp (Figure 1B). As before, EDTA did not promote stabilization of the fully assembled coat. These results suggest that the continuous generation of Sar1p-GTP promotes COPII coat stability.
In the absence of Sec12ΔCp, the order of addition of coat proteins with respect to the nucleotide was critical, because coat formation was limited by the slow Sar1p activation by spontaneous nucleotide exchange. When we added GTP to a mixture containing all three coat components, no binding signal was observed (Figure 1C). However, in the presence of Sec12ΔCp, the addition of GTP induced an instant increase in the signal followed by a slow decay (Figure 1C). Thus, the potent exchange of nucleotide catalyzed by Sec12ΔCp more than compensated for the high GAP activity of the full coat. Under these conditions, coat assembly and stability were similar in incubations containing Sec12ΔCp with GTP or GMP-PNP (Figure 1C). However, at lower concentrations of GTP (10 μM), we observed a decrease in coat stability (t1/2∼45 s) (Figure 1D), suggesting that GTP turnover enhanced by continuous nucleotide exchange and hydrolysis limited coat longevity.
We examined the activity of Sec12ΔCp on liposomes composed of neutral phospholipids (PC/PE), a formulation that does not permit the stable recruitment of coat subunits. Whereas Sec12ΔCp bound well to major–minor mix liposomes, it was not recruited significantly to PC/PE liposomes in a liposome flotation assay (Figure 1E) (Matsuoka et al, 1998b). Although coat subunits induced a transient light scattering signal on PC/PE liposomes, Sec12ΔCp was unable to stabilize the coat on these membranes (Figure 1F). These results suggest that Sec12ΔCp acts from a membrane surface, in this case most likely bound electrostatically, rather than from solution to promote coat assembly and stability.
Relative rates of Sec12pGEF and Sec23pGAP activity
To better quantify the effect of Sec12p on coat assembly, we employed a tryptophan fluorescence assay that detects a fluorescence change in Sar1p upon nucleotide exchange or hydrolysis (Antonny et al, 2001). The experiment in Figure 2A shows the relative rates of nucleotide exchange in incubations of Sar1p with GTP, GTP+EDTA, and GTP+Sec12ΔCp. In addition to a substantial rate enhancement, Sec12ΔCp increased the amplitude of Sar1p-GTP exchange; Sec12ΔCp promoted reasonably stable retention of Sar1p-GTP in these incubations, which contained the full set of COPII proteins. The retention of Sar1-GTP declined in incubations with coat proteins and lower concentrations of GTP (data not shown). Sec12ΔCp did not support exchange activity in incubations containing neutral (PC/PE) liposomes, consistent with the diminished binding of the protein to such membranes (data not shown).
Figure 2.

Exchange activity of Sec12ΔCp on Sar1p is ∼10-fold higher than GAP activity of Sec23/24p complex with saturating Sec13/31p. (A) The GEF activity of Sec12ΔCp and the GAP activity of Sec23/24p were measured by tryptophan fluorescence of Sar1p. A large tryptophan fluorescence change accompanies a conformational change by Sar1p from the GDP-bound to the GTP-bound state. Sar1p-GDP (2 μM) was added to major–minor liposomes (300 μg ml−1) and activated by the addition of GTP. After 6 min, Sec23/24p (55 nM) was added to activate GTP hydrolysis. The initial mixture contained 50 nM Sec12ΔCp (red trace), 2 mM EDTA (blue traces) in HKM buffer, or buffer alone (black trace) and 90 nM Sec13/31p, which stimulates GAP activity at saturation. EDTA decreases the concentration of free Mg2+ from 1 mM to 1 μM, and accelerates GDP–GTP exchange by Sar1p. (B) The rate constants for Sar1p activation were plotted against Sec12ΔCp concentration (left) to determine the nucleotide exchange activity (kactivation=kspontaneous+kexchange [Sec12ΔCp]). The specific nucleotide exchange activity (kexchange/[Sec12ΔCp]) corresponds to the rate constant of the activation of Sar1p normalized to the concentration of Sec12ΔCp (see Materials and methods). The rate constant for Sar1p inactivation by Sec23/24p with saturating Sec13/31p was plotted against Sec23/24p concentration (right) to determine catalytic activity (kinactivation=kcatalyze [Sec23/24p]). (C) Light scattering assays with the indicated concentration of Sec12ΔCp (0–200 nM). The experimental conditions were as in Figure 1. (D) In the experiments (C), light scattering signal decays instantly after Sec23/24p addition. The intensity of signals after instant decay was measured at 400 s. Percentage of the maximal amplitude was calculated, and plotted against Sec12ΔCp concentration.
We determined the GEF and GAP activities from the rates of Sar1p activation and inactivation (Figure 2B). The exchange rate catalyzed by Sec12ΔCp was 1.57±0.06 (106 s−1 M−1), whereas the spontaneous exchange rate was 5.03±0.65 (10−3 s−1). The activation rate saturated at 300 nM Sec12ΔCp (data not shown). Sec12ΔCp is remarkably active as an exchange factor, comparable to the activities of the ARNO-Sec7 domain on [Δ17]ARF GTPase (kex=7.6±0.2 (105 s−1 M−1)) (Beraud-Dufour et al, 1998) and RCC1 on Ran GTPase (kex=2.2 (106 s−1 M−1)) (Klebe et al, 1995). The catalytic rate of Sec23GAP, measured in the presence of the full coat ensemble, was 1.44±0.01 (105 s−1 M−1) (Figure 2B). Thus the catalytic rate of Sec12GEF is 10-fold higher than that of Sec23GAP. A 1:10 relative rate was in good agreement with light scattering experiments measuring coat stability in a range of Sec12ΔCp concentrations. At reduced levels of Sec12ΔCp, coat dissociation was biphasic (Figure 2C). This result suggested that at limiting Sec12ΔCp, coat disassembly progressed rapidly to a new equilibrium characterized by slow dissociation linked to depletion of the GTP pool. Quantification of COPII retained on membranes at the end of the first phase (t=400 s) indicated that ∼50% of maximum binding was obtained at ∼11.1±1.05 nM Sec12ΔCp in the presence of 160 nM Sec23/24p and saturating Sec13/31p (Figure 2D). This stoichiometry is roughly consistent with the measured catalytic rates of Sec12ΔGEF and Sec23GAP.
Morphology of COPII vesicle formed in the presence of Sec12ΔCp and GTP
To visualize coat assembly and vesicle formation in the presence of Sec12ΔCp, we examined the morphology of liposomes by thin-section electron microscopy (EM). When the liposomes were incubated with GTP and COPII proteins, we observed virtually no coats on membranes (Figure 3A). In the liposome mixture with Sec12ΔCp, GTP, and COPII proteins, numerous coated surfaces and coated buds but few coated vesicles were observed (Figure 3B). Incubations containing GMP-PNP with and without Sec12ΔCp produced broadly coated surfaces, coated buds, and a size range of coated vesicles. The ratio of buds/completed vesicles in three separate experiments was quantified by thin-section EM (Table I), confirming the preponderance of COPII buds in incubation with Sec12ΔCp and GTP. We conclude that Sec12ΔCp promotes coat stability and coated bud formation in incubations containing GTP, but it appears that such reactions do not progress efficiently through coated vesicle fission.
Figure 3.
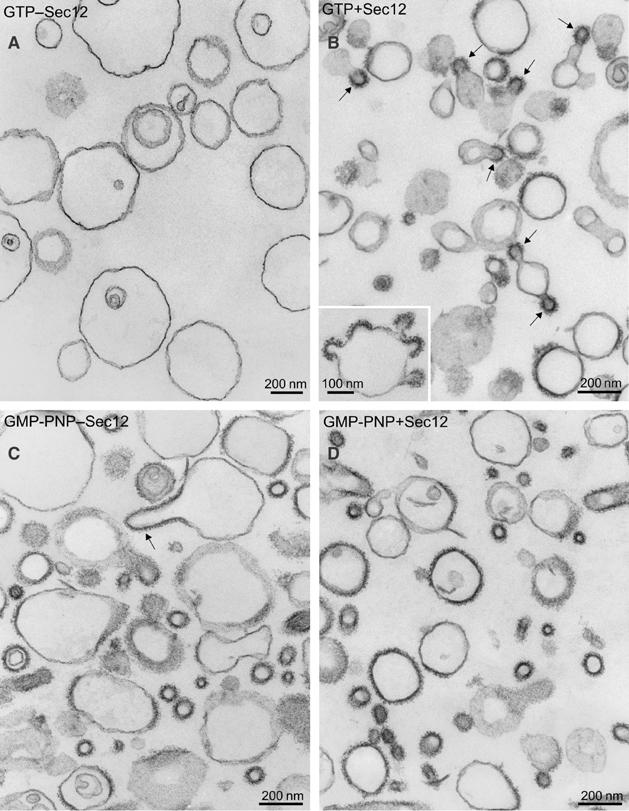
Morphology of major–minor liposomes incubated with COPII proteins and Sec12ΔCp. (A–D) The reaction contains major–minor liposomes (100 μg ml−1), 950 nM Sar1p, 160 nM Sec23/24p, and 260 nM Sec13/31p. The reaction was initiated by the addition of 300 μM GTP and incubated at 27°C. After 15 min, the samples were processed for EM. On thin-section electron micrographs, characteristic profiles observed without (A) or with (B) 50 nM Sec12ΔCp are shown. The inset in (B) depicts liposome with multiple coated buds. Comparison of COPII-like vesicles formed with GMP-PNP in the absence (C) or presence (D) of Sec12ΔCp is shown. Scale bars, 200 nm. The arrows point to coated buds (B, C).
Table 1. Morphogenesis of COPII coat maintained with Sec12ΔC.
| Experimenta/incubation | Number of buds+vesicles | Ratio of buds/vesicles |
|---|---|---|
| 1 GMP-PNP+sec12 | 379 | 0.46 |
| GTP+sec12 | 256 | 4.95 |
| 2 GMP-PNP+sec12 | 238 | 0.44 |
| GTP+sec12 | 148 | 6.4 |
| GMP-PNP−sec12 | 134 | 0.26 |
| 3 GMP-PNP+sec12 | 294 | 0.47 |
| GTP+sec12 | 273 | 6.2 |
| GMP-PNP−sec12 | 223 | 0.23 |
| >In all, 100 μm2 of pellet sections was evaluated in each sample. Pictures (15 for each sample) were printed at a final magnification of × 76 000. |
N40 as a potential catalytic site
We performed a structure–function analysis to test the notion that the nucleotide exchange activity of Sec12ΔCp is responsible for COPII assembly and stability. The cytosolic region of Sec12p is predicted to form a β-propeller structure with seven WD repeats (Chardin and Callebaut, 2002). The crystal structure of another GEF with WD repeats, RCC1, was solved in complex with the Ran GTPase (Renault et al, 2001). In the case of RCC1, loop regions between β-strands interact with Ran. In particular, the asparagine-rich β-wedge region is in contact with the P-loop of Ran and is thought to facilitate nucleotide removal. In the predicted seven-bladed β-propeller structure of Sec12p, four residues (P13, N40, H308, and T313) on loops of one face are completely conserved among species (Chardin and Callebaut, 2002). The original temperature-sensitive mutant sec12-1/4 contains a P73L mutation (d'Enfert et al, 1991). P73 is also in a loop region on the same face, but it is not well conserved. Mutational analysis of these residues was performed and we found that the N40A mutant had the most severe defect in exchange activity, ∼2% of wild type (Figure 4A). Other mutants also showed substantial defects, with the exception of H308A, which reproducibly stimulated nucleotide exchange approximately 10% above wild type but had a marginally lower COPII coat stabilizing activity. P73L showed less activity (13%) at 37°C than at 30°C (39.3%), consistent with the temperature-sensitive character of sec12-1 mutant cells. These results support a conclusion that the nucleotide exchange activity of Sec12 is directly responsible for stabilizing COPII coat assembly.
Figure 4.

Exchange activity of Sec12ΔCp mutants on Sar1p. (A) For each mutant, experiments similar to that shown in Figure 2A were performed to determine value ±s.e. of the specific exchange activity (kexchange/[Sec12ΔCp]). Except for [N40A] Sec12ΔCp, the mutants were assayed at a concentration up to 20 nM. A very weak stimulation of Sar1p activation as detected by varying concentrations of [N40A] Sec12ΔCp up to 600 nM. (B) Sar1p binding to GSTSec12ΔCp wild type or mutants. GSTSec12ΔCp (3 μM) was incubated with Sar1p-GDP (3 μM) and GMP-PNP (300 μM) (left), GDP (300 μM) (middle), or EDTA (2 mM) with alkaline phosphatase (calf intestine, Promega) (right) in HKM buffer containing 0.2% octylglucoside. The incubation with EDTA and alkaline phosphatase renders Sar1p nucleotide free. GSTSec12ΔCp was recovered with glutathione agarose and washed extensively with HKM buffer (0.2% octylglucoside) containing GMP-PNP (left), GDP (middle), or EDTA (right). Sar1p bound to the beads was analyzed by SDS–PAGE and quantified by Sypro-Ruby staining. (C) Light scattering assays with Sec12ΔCp wild type or mutants. The experimental conditions were as in Figure 1. Sec12ΔCp (50 nM) wild-type or mutant proteins were added to the initial mixture.
We analyzed the interaction of Sar1p with wild-type and mutant Sec12ΔCp in the presence and absence of nucleotide. For this purpose, GST-Sec12ΔCp hybrid proteins were employed and the recruitment of Sar1p was evaluated using GSH beads. Wild-type Sec12ΔCp bound best to nucleotide-free Sar1p, with background binding (compared to GST alone) in the presence of nucleotide (GMP-PNP or GDP) (Figure 4B). Sec12ΔCp mutants with exchange defects showed reduced binding to Sar1p, and the binding correlated well with the defective exchange activity. Control experiments showed that the mutant proteins were not defective in binding to the major–minor mix liposomes used in the nucleotide exchange assays (not shown). Exchange factors operate by binding to the nucleotide-free form of GTP-binding proteins. Thus the mutants are substrate binding and catalytic mutants. N40, P73, T313, and P13 are important for the interaction of Sec12p with Sar1p.
Light scattering assays were performed to evaluate the influence of Sec12ΔCp mutations on coat assembly and stabilization. The N40A mutant was inert and other mutants reduced coat stability in relation to a defect in Sar1p interaction (Figure 4C). These results suggest that one face of Sec12ΔCp contains a binding site for Sar1p, and the asparagine-rich loop of the first blade, containing N40, is critically important, similar to the β-wedge region in RCC1.
COPII vesicle formation from Sec12p proteoliposomes
In order to examine the incorporation of Sec12p into COPII vesicles, we employed a COPII vesicle budding assay (Matsuoka et al, 1998b) using proteoliposomes. Although Sec12p is not enriched in COPII vesicles formed from ER membranes (Barlowe et al, 1994), it was important to document this property with synthetic membranes. We reconstituted proteoliposomes with Sec12TMp (1–373), which contains the transmembrane domain, with the SNARE proteins, Bet1p, Bos1p, and Ufe1p, and with Texas red-phosphatidylethanolamine (Texas red-PE) to permit quantification of phospholipid distribution. The proteoliposomes were reconstituted by a detergent removal method (Parlati et al, 2000), which produced vesicles with an average diameter of 290±150 nm as determined by dynamic light scattering measurements. Carbonate extraction of the proteoliposomes showed that reconstituted proteins remained membrane bound (data not shown), suggesting that the proteins were incorporated into the lipid bilayler.
We probed the orientation of two proteins integrated into the proteoliposomes: Bet1p and Sec12p. An N-terminal GST-Bet1p fusion protein, membrane integrated through the C-terminal membrane anchor domain of Bet1p, was accessible to thrombin, which cleaved 70% of the GST at a thrombin linker sequence to Bet1p. Reconstituted Sec12TM liposomes were assayed in Sar1p nucleotide exchange reactions to measure enzyme latency. Comparing the specific activity of Sec12TM liposomes to soluble Sec12ΔCp showed that 62% of the Sec12 active site was accessible to Sar1p. Furthermore, incubations containing liposomes with 50 nM Sec12TMp and GTP produced COPII light scattering signal stability comparable to that promoted by a similar level of Sec12ΔCp (Supplementary Figure S1).
Reaction mixtures with COPII protein, proteoliposomes, and nucleotide were subjected to centrifugation through sucrose gradients (Figure 5A), which separate partially coated liposomes and coated COPII vesicles (Matsuoka et al, 1998b). For nucleotide, we compared GMP-PNP and GDP because reactions driven by GTP failed to retain membrane-bound coat proteins during sedimentation to equilibrium on a sucrose density gradient. The separated fractions were evaluated by EM to confirm the recovery of partially coated liposomes or COPII vesicles (data not shown). Qualitative and quantitative protein detection by dot blot analysis showed that Bet1p and Bos1p were enriched in the COPII vesicle fraction (∼70% recovery) (Figure 5B and C), indicating that the selection of cargo proteins was reproduced with the proteoliposomes. However, Sec12TMp and Ufe1p, ER-resident proteins, and phospholipids were neither enriched nor excluded in vesicle fractions (Figure 5B and C). These results suggest that the proteoliposome reaction reconstitutes a faithful protein sorting reaction and that the integral membrane-bound form of Sec12p is not enriched in COPII-coated vesicles during the synthetic budding reaction.
Figure 5.

Sorting of Sec12TMp and cargo proteins into COPII vesicles. (A) Proteoliposomes (100 μg ml−1) with Sec12TMp, Bet1p, Bos1p, and GST-Ufe1p were incubated for 30 min at 27°C with COPII proteins (130 μg ml−1 Sec23/24p, 150 μg ml−1 sec13/31p, and 80 mg ml−1 Sar1p) and nucleotide (GMP-PNP or GDP). COPII vesicles were separated from donor proteoliposomes by sucrose density gradient sedimentation (2.2–0.2 M sucrose). After separation, lipid recovery was monitored by fluorescence of Texas red-PE. (B) Bet1p, Bos1p, Sec12TMp, and GST-Ufe1p in each fraction were detected by immunoblot. (C) From the blot in (B), recovery of Bet1p and Sec12TMp was quantified using 35S-labeled anti-IgG and a phosphorimager. The recovery in the fractions for liposomes (Fr 1–9) or COPII vesicles (Fr 10–15) was calculated. Bos1 and Bet1 were enriched in the COPII vesicles, but Sec12TM and Ufe1 were neither enriched nor excluded in the vesicle fractions.
Discussion
SEC12 was identified as an essential gene required for protein transport from the ER to the Golgi apparatus in yeast (Nakano et al, 1988). Sec12p is an ER-resident transmembrane protein (Sato et al, 1996) and its cytosolic domain catalyzes guanine nucleotide exchange (GEF) specifically on Sar1p (Barlowe and Schekman, 1993). Recently, a mammalian Sec12 homolog (PREB) was also identified and shown to have GEF activity on Sar1 and was required for COPII vesicle formation in vitro (Weissman et al, 2001). Sec12p is thought to initiate coat polymerization and to ensure that coat proteins are recruited specifically to the ER. This hypothesis extends to the localization of coating reactions initiated by ARF, which employs Sec7 domain GEF proteins that are peripherally associated with specific membranes through interaction with lipids or protein (Jackson et al, 2000; Chantalat et al, 2004). Here, using liposome reconstitution, we directly show that the high nucleotide exchange activity of Sec12p is required to keep Sar1p activated at membranes engaged in COPII vesicle budding.
In an effort to probe the details of cargo sorting and vesicle budding, we developed a reconstituted liposome reaction that reproduces some but not all aspects of COPII vesicle formation (Matsuoka et al, 1998b). One obvious limitation of our reaction is the rapid rate of coat disassembly observed when the coat polymerizes in the presence of GTP (Antonny et al, 2001). Although coats and coated vesicles form in the presence of GMP-PNP, no evidence of vesicles is seen in incubations with GTP (Matsuoka et al, 1998b). In an effort to mitigate the effect of rapid GTP hydrolysis mediated by coat assembly, we explored the effect of membrane cargo proteins and Sec16p, a peripheral membrane protein that organizes COPII vesicle budding. SNAREs and other cargo molecules do not dampen the rate of GTP hydrolysis (unpublished observations). Although Sec16p and Sar1p-GTP stabilizes the recruitment of Sec23/24p to liposomes, no diminished rate of GTP hydrolysis or stable recruitment of the full coat is detected (Supek et al, 2002).
In contrast, addition of a soluble cytoplasmic domain fragment of Sec12 (Sec12ΔCp) or proteoliposome reconstitution of an integral membrane form of Sec12 (Sec12TMp) promoted the stable formation of coated membranes and coated buds. We used light scattering and tryptophan fluorescence assays to document the catalysis of nucleotide exchange and its effect on the recruitment of Sar1p to liposome membranes. Light scattering and thin-section EM revealed a stable coat and coated buds, most likely due to a continuous recharging of Sar1p in the area of a nascent vesicle.
The exchange activity of Sec12p is much higher than we previously estimated and instead is comparable to the activity reported for other GEFs (1.57±0.06 (106 s−1 M−1)). Our previous assays were conducted in the presence of 0.1% Triton X-100, whereas here we used liposomes. Under these conditions, the specific GEF activity was approximately 10-fold greater than the Sar1p GTPase activity stimulated by Sec23 GAP and the rest of the COPII coat. A ratio of 1:15 of Sec12 to COPII components produced half-maximal coat stabilization in an incubation conducted in the presence of GTP. As a consequence of the greatly enhanced cycle of GTP hydrolysis stimulated by these conditions, coat stability is limited at lower concentrations of nucleotide (e.g. 10 μM).
In previous work, we showed that coated buds and coated vesicles are observed in thin sections of liposome budding reactions conducted in the presence of GMP-PNP. We now find that similar profiles of coated membranes and coated buds are visible in preparations incubated with Sec12ΔCp and GTP. Interestingly, few clear profiles of separate coated vesicles are observed (Figure 3B). It may be that these conditions are insufficient to reproduce the fission reaction that completes vesicle budding. Unfortunately, we were unable to preserve and fractionate coated membranes in these preparations and thus we have no means of quantifying the fraction of membrane included in separate coated vesicles. Resolution of this issue awaits the development of a real-time vesicle budding reaction suitable for assays of liposome and proteoliposome populations.
Sec12p is largely excluded from COPII vesicles formed from microsomes incubated with GTP or GMP-PNP (Barlowe et al, 1994). However, some low level, perhaps representing the concentration prevailing in the ER membrane, is packaged because Sec12p is known to cycle between the ER and Golgi and requires a membrane protein, Rer1p, to facilitate recycling (Sato et al, 1996). Similarly, Sec12TMp and Ufe1p, an ER membrane t-SNARE, were neither enriched nor depleted in a proteoliposome budding reaction. In contrast, membrane cargo proteins, such as the SNAREs Bet1p and Bos1p, are enriched in COPII vesicles by virtue of direct interactions with binding sites on the membrane proximal surface of Sec24p (Miller et al, 2003). We reproduced this sorting event with GST hybrid proteins linked to the surface of a liposome through glutathione-derivatized PE (Matsuoka et al, 1998a), and now using integral membrane forms of these proteins (Figure 5).
The enrichment of cargo proteins and of clustered COPII subunits on the surface of a bud results in a diminished ratio of Sec12GEF/Sec23GAP. As a result, GTP hydrolysis would tend to destabilize the coat except at the growing boundary between a bud and the surrounding ER membrane where the ratio of GEF/GAP is higher than elsewhere (Figure 6A and B). Coat subunits remain transiently bound to completed COPII vesicles even after GTP hydrolysis has rendered the vesicles devoid of Sar1p (Barlowe et al, 1994). This simple consideration may explain how a coat remains relatively intact until the moment of fission separates a budded vesicle (Figure 6B and C).
Figure 6.

Coordination of COPII coating and uncoating by localized action of Sec12 GEF.
Other factors may facilitate the exclusion of Sec12p from budded vesicles. In Pichia pastoris and in mammalian cells, Sec12p is organized in a transitional ER membrane segregated from the bulk ER (Rossanese et al, 1999; Ellgaard and Helenius, 2003). Although this segregation is not apparent in Saccharomyces cerevisiae, small domains of Sec12p may be organized by the recruitment of Sar1p and Sec16p to the ER at the onset of a budding event (Supek et al, 2002; Bonifacino and Glick, 2004).
We probed the GEF activity of Sec12p using a structural model based on the interaction of the RCC1 GEF and its cognate GTPase, Ran (Renault et al, 2001). Sec12ΔCp binds well to nucleotide-free Sar1p and less well to Sar1p-GTP or -GDP just as has been shown for binding of RCC1 to Ran (Klebe et al, 1995) and ARNO to Δ17ARF (Beraud-Dufour et al, 1998) (Figure 4). We mutated residues conserved in a variety of Sec12s (P13, N40, and T313, H380A), which are located to putative loop regions between blades of the proposed Sec12 β-propeller structure (Chardin and Callebaut, 2002). Several of these, as well as a residue identified in the initial selection for temperature-sensitive sec mutants (P73L), reduced or eliminated Sar1p binding, GEF activity, and stable COPII polymerization on liposomes (Figure 5A). The most dramatic effect was produced by the N40A mutation, which is predicted to reside in a β-wedge region similar to RCC1, a position known to be associated with catalysis. A structure of Sec12p-Sar1p would allow this prediction to be tested. We conclude that the nucleotide exchange activity of Sec12p, rather than some other structural feature of the protein, is actively involved in building the COPII coat, most likely by stabilizing the growing boundary of the coat polymer.
Materials and methods
Proteins and liposomes
Sar1p, Sec23/24 (His6)p, and Sec13/31 (His6)p were prepared as described (Barlowe et al, 1994; Salama et al, 1997). Sar1p was purified in the presence of 5 μM GDP. Sec12ΔC(1–354)p, Sec12TM(1–373)p, Bet1p, Bos1p, and Ufe1p were produced as N-terminal GST fusion proteins in Escherichia coli from the vectors pGEX-2T (for GST-Sec12ΔC, GST-Sec12TM, GST-Bos1, GST-Ufe1) (Pharmacia) and pETGEX (for GST-Bet1) (Sharrocks, 1994) and purified as described (Parlati et al, 2000). GST tags were removed by thrombin cleavage for Sec12ΔCp, Sec12TMp, Bet1p, and Bos1p. Ufe1p was purified as a GST fusion (GST-Ufe1p).
The composition of phospholipids in the major–minor or PC/PE mix liposomes is essentially the same as previously described (Matsuoka et al, 1998b) with some changes: we incorporated 1% (mol%) Texas red-PE instead of NBD-phospholipids and 10% (w/w) cholesterol instead of ergosterol. Texas red-PE was used to quantify lipid recovery. Liposomes were prepared as described (Antonny et al, 2001).
Light scattering measurement and tryptophan fluorescence
The light scattering and tryptophan fluorescence assays were performed as described previously (Antonny et al, 2001). For better temporal resolution, all experiments were performed in a cylindrical cuvette (sample volume 550 μl) in which the reaction mixtures were mixed by a magnetic stir bar, and injection of reactants (COPII proteins and nucleotides) was performed through a Hamilton syringe. With this device, the recording was not interrupted by the injections and the mixing time was <2 s, allowing kinetic measurement in the range of few seconds.
Kinetic measurements
Activation of Sar1p upon GDP/GTP exchange was measured by tryptophan fluorescence. The apparent rate constant of Sar1p activation (kact) was determined by fitting the fluorescence change with a single exponential and plotted as a function of [Sec12ΔCp]. kact increases linearly with the concentration of Sec12ΔCp according to the equation kact=a+b [Sec12ΔCp], where a is the rate constant of spontaneous nucleotide exchange, which was measured in the absence of Sec12ΔCp, and b is the specific exchange activity of Sec12ΔCp, that is, the rate constant of catalyzed GDP/GTP exchange normalized to the concentration of Sec12ΔCp (kexchange/[Sec12ΔCp]). Inactivation of Sar1p upon GAP-catalyzed GTP hydrolysis was also measured by tryptophan fluorescence. The apparent rate constant for Sar1p inactivation was difficult to determine by fitting to single exponential and was determined by the value τinact (kinact=1/τinact), which is obtained graphically, and plotted as a function of [Sec23/24p]. Assuming kinact is 0 in the absence of Sec23/24p, kinact increases linearly with the concentration of Sec23/24p according to the equation kinact=c [Sec23/24p], where c is the specific catalytic activity of Sec23/24p, that is, the rate constant of catalyzed GTP hydrolysis normalized to the concentration of Sec23/24p saturated with Sec13/31p (kcatalyze/[Sec23/24p]). These parameters (kexchange, kcatalyze) correspond to the apparent second-order rate constant kcat/Km of a Michaelis–Menten mechanism. Note that we did not determine the individual kcat and Km values.
Liposome binding and budding assays
Recruitment of Sec12ΔCp proteins to synthetic liposomes was performed as described previously (Matsuoka et al, 1998b). Sec12ΔCp (500 nM) was incubated for 30 min at 27°C with liposomes (400 μg ml−1) in HKM buffer (170 μl) (20 mM Hepes-K, pH 6.8, 160 mM KOAc, 1 mM MgCl2) and mixed with 2.5 M sucrose (110 μl) in HKM to make a 1.0 M sucrose solution. The mixture was aliquoted into 11 × 34 mm polycarbonate centrifuge tubes (Beckman) and overlaid with 0.75 M sucrose in HKM (200 μl) and HKM (50 μl). The samples were centrifuged in a TLS55 rotor in an Optima TLX table top ultracentrifuge (Beckman) at 55 000 r.p.m. for 2 h at 4°C. After centrifugation, fractions floated to the 0.75 and 1.0 M sucrose interfaces were collected and proteins were analyzed by SDS–PAGE. Budding of COPII vesicles from synthetic liposomes was performed as described previously (Matsuoka et al, 1998b).
Protein reconstitution into liposomes
Proteoliposomes were prepared by detergent depletion as described previously (Parlati et al, 2000) with modifications. A lipid film was dissolved with buffer A (25 mM Tris–HCl, pH 8.0, 400 mM KCl, 10% glycerol, 1% octyl-β-D-glucopyranoside (octylglucoside), 2 mM 2-mercaptoethanol) and mixed with proteins to make a solution of 1 mM phospholipids and 0.33 μM proteins. The detergent–lipid–protein mixture was incubated for 15 min at room temperature. Octylglucoside was removed by extensive dialysis to buffer B (25 mM Hepes-K, pH 7.4, 400 mM KCl, 10% glycerol, 1 mM DTT) at 4°C for 30 h with four buffer changes, using 10 000 MWCO dialysis cassettes (Pierce). Each ∼500 μl dialysate was mixed with 330 μl of 2.5 M sucrose in HK (20 mM Hepes-K, pH 6.8, 250 mM KOAc) and aliquoted into 13 × 51 mm polycarbonate centrifuge tubes (Beckman). Each was overlaid with 660 μl 0.75 M sucrose in HK and 200 μl HK. The samples were centrifuged in an SW55 rotor in the Optima table top ultracentrifuge (Beckman) at 55 000 r.p.m. for 4 h at 4°C. Proteoliposomes were harvested from the top fraction and extruded through a 400 nm polycarbonate filter to remove lipid aggregates. The lipid concentration was determined by the fluorescence of Texas red.
Electron microscopy
Liposomes were made from a mixture of phospholipids and cholesterol by extrusion through 400 nm polycarbonate filters. The experimental conditions were the same as those used for the light scattering experiments. After incubations, samples were processed for conventional EM, as described (Matsuoka et al, 1998b).
Supplementary Material
Supplementary Figure S1
Acknowledgments
We thank Crystal Chan and Robert Lesch for COPII proteins and Matthew Welsh and David G Drubin for sharing their equipment. We thank Elizabeth Miller and Marcus Lee for improving the manuscript and Bruno Antonny for kinetic calculations of real-time assays. We thank members of the Schekman lab for discussions and encouragement. This work was supported by the HHMI (RS), the Swiss National Science Foundation (LO), and postdoctoral research fellowships from JSPS and ACS (EF).
References
- Antonny B, Gounon P, Schekman R, Orci L (2003) Self-assembly of minimal COPII cages. EMBO Reports 4: 419–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonny B, Madden D, Hamamoto S, Orci L, Schekman R (2001) Dynamics of the COPII coat with GTP and stable analogues. Nat Cell Biol 3: 531–537 [DOI] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R (1994) COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77: 895–907 [DOI] [PubMed] [Google Scholar]
- Barlowe C, Schekman R (1993) SEC12 encodes a guanine nucleotide exchange factor essential for transport vesicle budding from ER. Nature 365: 347–349 [DOI] [PubMed] [Google Scholar]
- Beraud-Dufour S, Robineau S, Chardin P, Paris S, Chabre M, Cherfils J, Antonny B (1998) A glutamic finger in the guanine nucleotide exchange factor ARNO displaces Mg2+ and the beta-phosphate to destabilize GDP on ARF1. EMBO J 17: 3651–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Glick BS (2004) The mechanism of vesicle budding and fusion. Cell 116: 153–166 [DOI] [PubMed] [Google Scholar]
- Chantalat S, Park SK, Hua Z, Liu K, Gobin R, Peyroche A, Rambourg A, Graham TR, Jackson CL (2004) TheArf activator Gea2p and the P-type ATPase Drs2p interact at the Golgi in Saccharomyces cerevisiae. J Cell Sci 117: 711–722 [DOI] [PubMed] [Google Scholar]
- Chardin P, Callebaut I (2002) The yeast Sar1 exchange factor Sec12, and its higher organism orthologs, fold as β-propellers. FEBS Lett 525: 171–173 [DOI] [PubMed] [Google Scholar]
- Cosson P, Letourneur F (1994) Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 263: 1629–1631 [DOI] [PubMed] [Google Scholar]
- d'Enfert C, Wuestehube LJ, Lila T, Schekman R (1991) Sec12p-dependent membrane binding of the small GTP-binding protein Sar1p promotes formation of transport vesicles from the ER. J Cell Biol 114: 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard L, Helenius A (2003) Quality control in the endoplasmic reticulum. Nat Rev Mol Cell Biol 4: 181–191 [DOI] [PubMed] [Google Scholar]
- Espenshade P, Gimeno RE, Holzmacher E, Teung P, Kaiser CA (1995) Yeast Sec16 gene encodes a multidomain vesicle coat protein that interact with Sec23p. J Cell Biol 131: 311–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno RE, Espenshade P, Kaiser CA (1996) COPII coat subunit interactions: Sec24p and Sec23p bind to adjacent regions of Sec16p. Mol Biol Cell 7: 1815–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J (2000) Decoding of sorting signals by coatomer through a GTPase switch in the COPI coat complex. Cell 100: 671–679 [DOI] [PubMed] [Google Scholar]
- Hoflack B (1998) Mechanism of protein sorting and coat assembly; clathrin coated vesicle pathways. Curr Opin Cell Biol 10: 499–503 [DOI] [PubMed] [Google Scholar]
- Jackson TR, Kearns BG, Theibert AB (2000) Cytohesins and centaurins: mediators of PI 3-kinase regulated Arf signaling. Trends Biochem Sci 25: 489–495 [DOI] [PubMed] [Google Scholar]
- Klebe C, Bischoff FR, Ponstingl H, Wittinghofer A (1995) Interaction of the nuclear GTP-binding protein Ran with its regulatory proteins RCC1 and RanGAP1. Biochemistry 34: 639–647 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Morimitsu Y, Uchida K, Schekman R (1998a) Coat assembly directs v-SNARE concentration into synthetic COPII vesicles. Mol Cell 2: 703–708 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Orci L, Amherdt M, Bednarek SY, Hamamoto S, Schekman R, Yeung T (1998b) COPII-coated vesicle formation reconstituted with purified coat proteins and chemically defined liposomes. Cell 93: 263–275 [DOI] [PubMed] [Google Scholar]
- Matsuoka K, Schekman R, Orci L, Heuser JE (2001) Surface structure of the COPII-coated vesicle. Proc Natl Acad Sci USA 98: 13705–13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EA, Beilharz TH, Malkus PN, Lee MC, Hamamoto S, Orci L, Schekman R (2003) Multiple cargo binding sites on the COPII subunit Sec24p ensure capture of diverse membrane proteins into transport vesicles. Cell 114: 497–509 [DOI] [PubMed] [Google Scholar]
- Mossessova E, Bickford LC, Goldberg J (2003) SNARE selectivity of COPII coat. Cell 114: 483–495 [DOI] [PubMed] [Google Scholar]
- Nakano A, Brada D, Schekman R (1988) A membrane glycoprotein, Sec12p, required for protein transport from the endoplasmic reticulum to the Golgi apparatus in yeast. J Cell Biol 107: 851–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F, McNew JA, Fukuda R, Miller R, Sollner TH, Rothman JE (2000) Topological restriction of SNARE dependent membrane fusion. Nature 407: 194–198 [DOI] [PubMed] [Google Scholar]
- Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT (2004) BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499 [DOI] [PubMed] [Google Scholar]
- Renault L, Kuhlmann J, Henkel A, Wittinghofer A (2001) Structural basis for guanine nucleotide exchange on Ran by the regulator of chromosome condensation (RCC1). Cell 105: 245–255 [DOI] [PubMed] [Google Scholar]
- Rossanese OW, Soderholm J, Bevis BJ, Sears IB, O'Conner J, Williamson EK, Glick BS (1999) Golgi structure correlates with traditional endoplasmic reticulum organization in Pichia pastoris and Saccharomyces cerevisiae. J Cell Biol 145: 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama NR, Chuang JS, Schekman RW (1997) Sec31 encodes an essential component of the COPII coat required for transport vesicle budding from the endoplasmic reticulum. Mol Biol Cell 8: 205–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sato K, Nakano A (1996) Endoplasmic reticulum localization of Sec12p is activated by two mechanisms: Rer1p-depenedent retrieval that requires the transmembrane domain and Rer1p-independent retrieval that involves the cytoplasmic domain. J Cell Biol 134: 279–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrocks AD (1994) A T7 expression vector for providing N- and C-terminal fusion proteins with glutathione S-transferase. Gene 138: 105–108 [DOI] [PubMed] [Google Scholar]
- Shaywitz DA, Espenshade PJ, Gimeno RE, Kaiser CA (1997) COPII subunit interactions in the assembly of the vesicle coat. J Biol Chem 272: 25413–25416 [DOI] [PubMed] [Google Scholar]
- Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L (1998) Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc Natl Acad Sci USA 95: 11199–11204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Schekman R (1998) Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science 281: 698–700 [DOI] [PubMed] [Google Scholar]
- Supek F, Madden DT, Hamamoto S, Orci L, Schekman R (2002) Sec16p potentiate the action of COPII proteins to bud transport vesicles. J Cell Biol 158: 1029–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter IR, Wittinghofer A (2001) The guanine nucleotide-binding switch in three dimensions. Science 294: 1299–1304 [DOI] [PubMed] [Google Scholar]
- Weissman JT, Plutner H, Balch WE (2001) The mammalian guanine nucleotide exchange factor mSec12 is essential for activation of the Sar1 GTPase directing endoplasmic reticulum export. Traffic 2: 465–475 [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R (1993) Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Science 259: 1466–1468 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1


