Abstract
Background
Teduglutide is a GLP-2 analogue indicated for treatment of adults with short bowel syndrome (SBS). Due to the rarity of SBS, real-world safety or efficacy data are not available in patients with Crohn’s disease (CD) and SBS treated with teduglutide.
Aim
To evaluate teduglutide’s safety and efficacy in CD patients with SBS.
Methods
We conducted a retrospective cohort study at three tertiary centers in the United States between 2012 and 2014. Demographic, clinical and therapeutic data were retrieved from medical record systems.
Results
Thirteen CD patients were included, eight (62%) of whom were on concomitant immunosuppression. Median duration of teduglutide therapy was 365 days (interquartile range (IQR) 122–482 days) and 9/13 patients (69%) remain on therapy. At teduglutide initiation, 69% were on parenteral nutrition (PN). At conclusion of follow-up, 1 patient was on PN. All patients were on intravenous fluids (IVF) prior to teduglutide; median IVF were 9000ml/week (IQR 7000–14000 ml/week). IVF requirements decreased by a median of 3100 ml/week (IQR 2400–8400 ml/week). Six patients (46%) ceased IVF. Adverse events attributed to teduglutide were obstructive symptoms (n=1), pancreatitis (n=1), asymptomatic lipase and amylase elevation (n=1), nausea (n=1) and abdominal pain (n=1). Catheter-related sepsis occurred in four patients.
Conclusion
This is the first report evaluating the safety and efficacy of teduglutide in a cohort of CD patients with SBS requiring parenteral support. More of half the cohort was on concomitant immunosuppression. Teduglutide appeared to be safe and the majority of patients were weaned off parenteral support.
Keywords: Crohn’s Disease, Short Bowel Syndrome, Parenteral Nutrition, Teduglutide, Immunosuppression
Introduction
Patients with Crohn’s disease (CD) often require surgery. As many as 55% of patients with Crohn’s disease undergo surgery in the first ten years after diagnosis and up to 35% require a second surgery in the subsequent 10–15 years [1]. Overall 9–13% of CD patients require two or more surgeries [2, 3]. In the age of biological therapy, stricturing disease is one of the more common indications for intestinal resections in CD. Most primary intestinal resections involve the ileocecal region and require only a short segment resection. A small group of patients require multiple resections resulting in short bowel syndrome (SBS) associated intestinal failure and require parenteral support [4, 5].
Glucagon-like peptide 2 (GLP-2) is a 33-amino acid peptide, involved in the normal growth and maintenance of the intestinal epithelium. The secretion of GLP-2 by enteroendocrine cells, located primarily in the terminal ileum and colon, is stimulated by the presence of nutrients [6]. GLP-2 has been shown to slow gastric emptying, decrease gastric secretions and increase the intestinal blood flow, stimulating growth of the large and small intestine [7–13]. The half-life of GLP-2 is only seven minutes. Substituting glycine for alanine extends the half-life to three hours, which makes the GLP-2 analogue, teduglutide, an attractive therapeutic agent [14, 15]. Teduglutide was given orphan drug status for short bowel syndrome (SBS) by the Food and Drug Administration (FDA) in 2000. It was approved in 2012 for the treatment of adult patients with SBS who are dependent on parenteral support. This approval was based on two placebo-controlled trials over 24 weeks of 169 patients treated with teduglutide (either 0.05 mg/kg or 0.1 mg/kg) or placebo [16, 17]. Additionally, data are available from a 28 week double blind extension study of one of the previous 24 week studies, in which 52 patients were either treated with 0.05 or 0.1 mg/kg bodyweight or placebo [18]. In these studies, 36% and 21% of patients had Crohn’s disease. However, in all of these studies, concomitant therapy with biological agents or immunomodulators was an exclusion criterion. As clinical trials, especially in the field of inflammatory bowel diseases, are not representative of clinical practice [19], IBD providers are cautious in the use of this novel medication in Crohn’s disease patients.
To date, there are no real-world safety and efficacy reports available of teduglutide in CD patients with SBS requiring parenteral support, especially those who are treated with biological agents. Therefore, we retrospectively evaluated a cohort of patients with Crohn’s disease treated with teduglutide in three tertiary care centers in the United States to evaluate the safety and efficacy of teduglutide.
Methods
We conducted a retrospective cohort study at the University of North Carolina, Massachusetts General Hospital and Boston Medical Center. The institutional review boards of all three institutions approved the study protocol. Chart reviews identified all patients between 2007 and 2014 with CD and SBS treated with teduglutide therapy. Demographic, clinical, surgical and therapeutic data were retrieved from the electronic medical record systems. Past and current medications (defined in relation to teduglutide therapy) for Crohn’s disease were evaluated. Additionally, the need for parenteral support before and during therapy with teduglutide was calculated.
Statistical Analysis
We used descriptive statistics to summarize the characteristics of all patients. Continuous variables are reported as median with interquartile range (IQR), and categorical variables are reported as percentages. Analyses were performed using Stata 10.0 (College Station, TX).
Results
We identified 13 CD patients on teduglutide therapy (table 1). All patients were started on the recommended dose of 0.05mg/kg/day. Eight patients (61.5%) were treated with concomitant immunosuppressive therapy. The median duration of teduglutide therapy was 365 days (IQR: 122–482 days) and nine patients (69.2%) remain on teduglutide therapy at the conclusion of follow up. Duration of follow up ranged from 3 months to 22 months with a median of 14 months. The median body weight increase was 3 kilograms (kg) (IQR: 0–6 kg), which was a median increase of 3.8% of total body weight. The median increase in the body mass index (BMI) was 1.9 (IQR: −0.6 – 2.4).
Table 1.
Crohn’s Disease patients on teduglutide
| Gender | Age | Disease Duration (years) |
Disease Location |
Disease Phenotype |
# of bowel resections |
Ostomy | Medications |
|---|---|---|---|---|---|---|---|
| Female | 46 | 25 | Ileo-colonic | Penetrating | 3 | Ileostomy | Certolizumab, budesonide, prednisone |
| Female | 37 | 14 | Ileo-colonic | Penetrating | 2 | Ileostomy | Methotrexate, budesonide, Ustekinumab |
| Male | 56 | 38 | Ileo-colonic | Penetrating | 3 | None | None |
| Female | 38 | 17 | Ileo-colonic | Penetrating | 6 | Ileostomy | Adalimumab |
| Female | 41 | 23 | Ileo-colonic | Penetrating | 3 | Ileostomy | None |
| Male | 45 | 26 | Ileo-colonic | Penetrating | 4 | Colostomy | Infliximab, thiopurine |
| Female | 60 | 19 | Ileal | Stricturing | 3 | None | Narcotics |
| Female | 68 | 46 | Ileo-colonic | Stricturing | 8 | Ileostomy | Mesalamine, narcotics |
| Male | 59 | 45 | Ileo-colonic | Stricturing | 6 | Ileostomy | None |
| Female | 59 | 35 | Ileal | Stricturing | 5 | None | Thiopurine, prednisone |
| Female | 49 | 19 | Ileo-colonic | Stricturing | 1 | None | Ustekinumab, narcotics |
| Female | 72 | 48 | Ileo-colonic | Stricturing | 4 | Ileostomy | Thiopurine |
| Male | 51 | 31 | Ileo-colonic | Stricturing | 5 | None | Vedolizumab, thiopurine |
Parenteral Support
Nine of the thirteen patients (69.2%) were on parenteral nutrition (PN) at the initiation of teduglutide therapy. Only one patient (7.7%) was on PN at the conclusion of follow up (figure 1). All patients were on intravenous fluid (IVF) support at the start of therapy, with a median of 9000 ml/week (IQR: 7000–14000 ml). Fluid volume requirements decreased during the course of observation by a median of 3100 ml/week (IQR: 2400–8400 ml/week). Six patients (46%) did not require any intravenous fluids at the conclusion of follow up. Two patients stopped teduglutide due to stable nutritional status without the need for parenteral support after 14 and 23 months of therapy.
Figure 1.
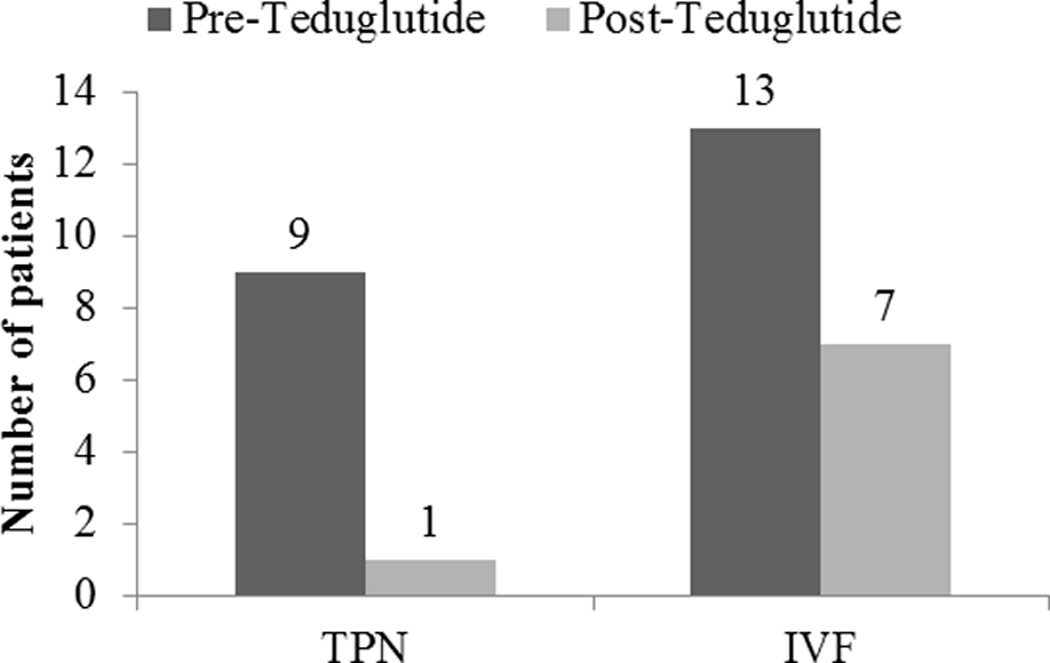
Patients on parenteral nutrition (PN) and intravenous fluid (IVF) support before and after the start of teduglutide therapy
Adverse events
Adverse events that may have been associated with the teduglutide included pancreatitis (n=1), asymptomatic elevation of lipase and amylase (n=1) and abdominal pain (n=2); these were all in patients on concomitant immunosuppression (table 2). One patient, not on immunosuppression, reported nausea. Another patient, not on immunosuppression, developed intermittent obstructive symptoms, necessitating teduglutide cessation. Three patients on concomitant immunosuppression experienced infectious line complications compared to one patient on no concomitant immunosuppression.
Table 2.
Adverse event profile of Crohn’s disease patients on teduglutide
| Adverse Event | On Immunosuppression (n=8) |
Not on Immunosuppression (n=5) |
|---|---|---|
| Pancreatitis | 1 | 0 |
| Asymptomatic elevation of lipase and amylase | 1 | 0 |
| Abdominal Pain | 2 | 0 |
| Nausea | 0 | 1 |
| Intermittent obstructive symptoms | 0 | 1 |
| Catheter related sepsis | 3 | 1 |
Discussion
This is the first real-world experience describing the safety and effectiveness of teduglutide in Crohn’s disease patients, including those on immunosuppression, with SBS associated intestinal failure requiring parenteral support. Other than two placebo-controlled trials, which only included a small number of CD patients, there are no published reports about the use of teduglutide in patients with CD associated SBS. Furthermore, in all prospective studies of teduglutide, patients were not treated with concomitant immunosuppressive therapies. Thus our cohort presents new and relevant information on the efficacy and safety of teduglutide in practice beyond clinical trials.
The strict pathophysiological definition of short bowel associated intestinal failure, small bowel length less than 200 centimeters, is no longer clinically relevant because it does not reflect the absorptive capacities of the remaining bowel [20]. The colon, which conserves fluid, electrolytes and salvages mal-absorbed carbohydrate and protein, at least partially compensates for resected small bowel [21]. Thus even limited resections with a consecutive ileostomy, can result in short bowel syndrome, requiring parenteral support.
In our cohort, teduglutide was highly effective in reducing the need for parenteral support. Our results are consistent with the only other report of treatment outcomes in teduglutide. However, that was in a case series of six patients without Crohn’s disease who have short bowel syndrome [22]. In this series, parenteral support could be reduced by at least 20% in all patients and completely discontinued in 4/6 patients (67%). In our cohort, parenteral support was reduced in 100% of patients and completely discontinued in 6/13 (46%) of patients. This case series and our cohort found the ability to decrease and discontinue parenteral support to be higher than reported in the 52 week prospective trial [18].
Overall teduglutide appeared to be safe and well tolerated in our cohort. The most common adverse events observed in the prospective trials of teduglutide were headache, abdominal pain, nausea, nasopharyngitis, vomiting and catheter sepsis [16–18, 23]. Nausea and/or vomiting appeared to be dose related, occurring in approximately 20% of CD patients treated with 0.05 mg/kg daily and increased to nearly 40% with the highest dose of 0.2 mg/kg daily [24]. This is likely due to the effects of teduglutide on gastric emptying and motility [25, 26]. Abdominal pain and nausea were the most common side effects observed in our study. However, none of the patients had to stop therapy due to these side effects. Catheter associated sepsis, which we observed in four patients, is most likely because all these patients have permanent intravenous catheters, which by itself is a risk for catheter sepsis [27, 28]. Teduglutide can cause congestive heart failure due to improved absorption of fluids, however, this was not observed in our small cohort [29].
Another adverse event of concern is bowel obstruction. In patients with stricturing Crohn’s disease, bowel obstruction is due to the trophic effects of teduglutide with the increase in size and density of the intestinal villi. One patient in the 52-week teduglutide treatment trial experienced acute intestinal obstruction, which resolved after teduglutide discontinuation. In our cohort, over 50% of patients had a history of stricturing Crohn’s disease. One patient, not on concomitant immunosuppression, developed obstructive episodes, which resolved after teduglutide was reduced to every other day therapy. An increase in the size of ileostomies was also reported. Given the retrospective nature of the study, it was not possible to assess the increase in ostomy size in our cohort.
There is also a risk of neoplastic proliferation in the intestine with teduglutide, which, as in the clinical trials, was not observed in our small cohort [23, 30]. However, the average follow-up time of one year, both in our cohort and the clinical trials, is short. Long term observations, such in the prospective, multi-center registry for patients with SBS, are necessary to adequately assess the safety and efficacy of teduglutide [31].
Teduglutide was also evaluated in a prospective placebo controlled study for the treatment of moderate to severe Crohn’s disease. Three different doses of teduglutide, 0.05 mg/kg daily, 0.10 mg/kg daily and 0.2 mg/kg daily were compared to placebo in an 8 week trial. This was followed by an open label 12 week extension trial [24]. There were no significant differences in response and remission for all three teduglutide doses compared to placebo after 8 weeks. Circulating citrulline, an end product of the glutamine metabolism is a solely product of enterocytes and a proposed biomarker for the small bowel absorptive area and function [32]. Plasma citrulline, increased significantly at week 2, 4 and 8 in all three teduglutide groups, supporting the biological activity of teduglutide.
Given the need for long-term therapy with teduglutide, it will become important to determine if there is a risk of developing neutralizing antibodies, which might be decreased by immunosuppressive therapy. Currently, there are no commercial assays to measure antibodies to teduglutide. In the prospective trials 6/43 (14%) and 14/52 (27%) of patients had detectable non-neutralizing antibodies after 24 and 52 weeks of therapy, respectively. Similarly it is not known if a drug holiday or cessation in patients with successful teduglutide therapy is warranted or if this would result in the need to restart parenteral support. In our cohort, two patients had teduglutide therapy stopped after remaining completely off parenteral support for several months. Both had some part of their colon in place; this may be a positive predictor for stability off teduglutide once the parenteral support is weaned off [33].
There are limitations to our study. The small number of patients in our cohort is reflective of how rarely either CD and SBS coincide or how infrequently teduglutide is used in CD patients even in tertiary care institutions across the United States. Its use may be significantly hampered by the expense of therapy ($295,000 per year) and difficulty of insurance approval. Other reasons for the small number of patients may be due to provider concerns of adverse events especially in combination with immunosuppression. The retrospective nature of the study resulted in limitations to the information available for the subjects. Due to the small number of patients and short duration of follow-up, we are also not able to address the long-term durability or effects of teduglutide therapy. It also remains unknown whether concomitant immunotherapy has a protective effect on the clinical efficacy of teduglutide. We were also unable to determine the length of remaining small bowel in the individual patients because patients underwent multiple surgeries at various institutions. However, as discussed, the current classification of intestinal failure in the setting of SBS focuses on bowel adsorptive function rather than length [20].
In summary we present the first real-world report of the efficacy and safety of teduglutide in Crohn’s disease patients with short bowel syndrome. Due to the scarcity of this patient population, long-term follow-up in a multi-center registry is necessary for further evaluation of the safety and efficacy of teduglutide in this patient population.
Acknowledgments
This research was supported, in part, by grants from the National Institutes of Health T32 DK07634, 5U01DK092239 and P30DK34987.
Footnotes
Disclosures:
Bharati Kochar – no disclosures
Millie D. Long – consulting fees for NPS Pharma/Shire
Edward Shelton – no disclosures
Lorraine Young – no disclosures
Francis A. Farraye – consulting fees for NPS Pharma/Shire
Vijay Yajnik – consulting fees for NPS Pharma/Shire
Hans Herfarth – consulting fees for NPS Pharma/Shire
References
- 1.Peyrin-Biroulet L, Loftus EV, Jr, Colombel JF, et al. The natural history of adult Crohn's disease in population-based cohorts. Am J Gastroenterol. 2010;105:289–297. doi: 10.1038/ajg.2009.579. [DOI] [PubMed] [Google Scholar]
- 2.Solberg IC, Vatn MH, Hoie O, et al. Clinical course in Crohn's disease: results of a Norwegian population-based ten-year follow-up study. Clin Gastroenterol Hepatol. 2007;5:1430–1438. doi: 10.1016/j.cgh.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Binder V, Hendriksen C, Kreiner S. Prognosis in Crohn's disease--based on results from a regional patient group from the county of Copenhagen. Gut. 1985;26:146–150. doi: 10.1136/gut.26.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Messing B, Lémann M, Landais P, et al. Prognosis of patients with nonmalignant chronic intestinal failure receiving long-term home parenteral nutrition. Gastroenterology. 1995;108:1005–1010. doi: 10.1016/0016-5085(95)90196-5. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe K, Sasaki I, Fukushima K, et al. Long-term incidence and characteristics of intestinal failure in Crohn's disease: a multicenter study. J Gastroenterol. 2014;49:231–238. doi: 10.1007/s00535-013-0797-y. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ. Gut adaptation and the glucagon-like peptides. Gut. 2002;50:428–435. doi: 10.1136/gut.50.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagell CF, Wettergren A Fau, Pedersen JF, Pedersen Jf Fau, Mortensen D, et al. Glucagon-like peptide-2 inhibits antral emptying in man, but is not as potent as glucagon-like peptide-1. doi: 10.1080/00365520410004424. [DOI] [PubMed] [Google Scholar]
- 8.Wojdemann M, Wettergren A, Hartmann B, et al. Inhibition of sham feeding-stimulated human gastric acid secretion by glucagon-like peptide-2. J Clin Endocrinol Metab. 1999;84:2513–2517. doi: 10.1210/jcem.84.7.5840. [DOI] [PubMed] [Google Scholar]
- 9.Guan X, Karpen HE, Stephens J, et al. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Washizawa N, Gu LH, Gu L, et al. Comparative effects of glucagon-like peptide-2 (GLP-2), growth hormone (GH), and keratinocyte growth factor (KGF) on markers of gut adaptation after massive small bowel resection in rats. JPEN J Parenter Enteral Nutr. 2004;28:399–409. doi: 10.1177/0148607104028006399. [DOI] [PubMed] [Google Scholar]
- 11.Litvak DA, Hellmich MR, Evers BM, et al. Glucagon-like peptide 2 is a potent growth factor for small intestine and colon. J Gastrointest Surg. 1998;2:146–150. doi: 10.1016/s1091-255x(98)80005-x. [DOI] [PubMed] [Google Scholar]
- 12.Tsai CH, Hill M, Asa SL, et al. Intestinal growth-promoting properties of glucagon-like peptide-2 in mice. Am J Physiol. 1997;273:E77–E84. doi: 10.1152/ajpendo.1997.273.1.E77. [DOI] [PubMed] [Google Scholar]
- 13.Drucker DJ, Erlich P, Asa SL, et al. Induction of intestinal epithelial proliferation by glucagon-like peptide 2. Proc Natl Acad Sci U S A. 1996;93:7911–7916. doi: 10.1073/pnas.93.15.7911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marier JF, Mouksassi MS, Gosselin NH, et al. Population pharmacokinetics of teduglutide following repeated subcutaneous administrations in healthy participants and in patients with short bowel syndrome and Crohn's disease. J Clin Pharmacol. 2010;50:36–49. doi: 10.1177/0091270009342252. [DOI] [PubMed] [Google Scholar]
- 15.Jeppesen PB, Sanguinetti EL, Buchman A, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54:1224–1231. doi: 10.1136/gut.2004.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeppesen PB, Pertkiewicz M, Messing B, et al. Teduglutide reduces need for parenteral support among patients with short bowel syndrome with intestinal failure. Gastroenterology. 2012;143:1473–1481. e1473. doi: 10.1053/j.gastro.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Jeppesen PB, Gilroy R, Pertkiewicz M, et al. Randomised placebo-controlled trial of teduglutide in reducing parenteral nutrition and/or intravenous fluid requirements in patients with short bowel syndrome. Gut. 2011;60:902–914. doi: 10.1136/gut.2010.218271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Keefe SJ, Jeppesen PB, Gilroy R, et al. Safety and efficacy of teduglutide after 52 weeks of treatment in patients with short bowel intestinal failure. Clin Gastroenterol Hepatol. 2013;11:815–823. e811–e813. doi: 10.1016/j.cgh.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 19.Ha C, Ullman TA, Siegel CA, et al. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002–1007. doi: 10.1016/j.cgh.2012.02.004. quiz e1078. [DOI] [PubMed] [Google Scholar]
- 20.O'Keefe SJ, Buchman AL, Fishbein TM, et al. Short bowel syndrome and intestinal failure: consensus definitions and overview. Clin Gastroenterol Hepatol. 2006;4:6–10. doi: 10.1016/j.cgh.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171–180. doi: 10.1016/j.clnu.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Ukleja AAA, Alvarez K, et al. Teduglutide for patients with short bowel syndrome-intestinal failure: a single center experience. United European Gastroenterology. 2014 [Google Scholar]
- 23.Schwartz LK, O'Keefe SJ, Fujioka K, et al. Long-Term Teduglutide for the Treatment of Patients With Intestinal Failure Associated With Short Bowel Syndrome. Clin Transl Gastroenterol. 2016;7:e142. doi: 10.1038/ctg.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buchman AL, Katz S, Fang JC, et al. Teduglutide, a novel mucosally active analog of glucagon-like peptide-2 (GLP-2) for the treatment of moderate to severe Crohn's disease. Inflamm Bowel Dis. 2010;16:962–973. doi: 10.1002/ibd.21117. [DOI] [PubMed] [Google Scholar]
- 25.Berg JK, Kim EH, Li B, et al. A randomized, double-blind, placebo-controlled, multiple-dose, parallel-group clinical trial to assess the effects of teduglutide on gastric emptying of liquids in healthy subjects. BMC Gastroenterol. 2014;14:25. doi: 10.1186/1471-230X-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeppesen PB, Hartmann B, Thulesen J, et al. Glucagon-like peptide 2 improves nutrient absorption and nutritional status in short-bowel patients with no colon. Gastroenterology. 2001;120:806–815. doi: 10.1053/gast.2001.22555. [DOI] [PubMed] [Google Scholar]
- 27.Gillanders L, Angstmann K, Ball P, et al. A prospective study of catheter-related complications in HPN patients. Clin Nutr. 2012;31:30–34. doi: 10.1016/j.clnu.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Santarpia L, Alfonsi L, Tiseo D, et al. Central venous catheter infections and antibiotic therapy during long-term home parenteral nutrition: an 11-year follow-up study. JPEN J Parenter Enteral Nutr. 2010;34:254–262. doi: 10.1177/0148607110362900. [DOI] [PubMed] [Google Scholar]
- 29.Naberhuis JK, Tappenden KA. Teduglutide for Safe Reduction of Parenteral Nutrient and/or Fluid Requirements in Adults: A Systematic Review. JPEN J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115582063. [DOI] [PubMed] [Google Scholar]
- 30.Tappenden KA, Edelman J, Joelsson B. Teduglutide enhances structural adaptation of the small intestinal mucosa in patients with short bowel syndrome. J Clin Gastroenterol. 2013;47:602–607. doi: 10.1097/MCG.0b013e3182828f57. [DOI] [PubMed] [Google Scholar]
- 31.Bowlin S. ClinicalTrials.gov[Internet] Bethesda (MD): National Library of Medicine (US); 2000. A Prospective, Multi-center Registry for Patients With Short Bowel Syndrome. [January 2016]. Identifier: NCT01990040. [Google Scholar]
- 32.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Compher C, Gilroy R, Pertkiewicz M, et al. Maintenance of parenteral nutrition volume reduction, without weight loss, after stopping teduglutide in a subset of patients with short bowel syndrome. JPEN J Parenter Enteral Nutr. 2011;35:603–609. doi: 10.1177/0148607111414431. [DOI] [PubMed] [Google Scholar]


