Abstract
Lipid mediators (LM) of inflammation are a class of compounds derived from ω-3 and ω-6 fatty acids that play a wide role in modulating inflammatory responses. Some LM possess pro-inflammatory properties, while others possess pro-resolving characteristics, and the class switch from pro-inflammatory to pro-resolving is crucial for tissue homeostasis. In this article, we review the major classes of LM, focusing on their biosynthesis and signaling pathways, and their role in systemic and, especially, oral health and disease. We discuss the detection of these LM in various body fluids, focusing on diagnostic and therapeutic applications. We also present data showing gender-related differences in salivary LM levels in healthy controls, leading to a hypothesis on the etiology of inflammatory diseases, particularly, Sjögren’s Syndrome. We conclude by enumerating open areas of research where further investigation of LM is likely to result in therapeutic and diagnostic advances.
Keywords: Resolvins, Lipoxins, Salivary Gland, Periodontal Disease, Oral Cancer
Introduction
Inflammation occurs following a challenge of host tissue by microorganisms, trauma, or other injuries (Gilroy and Lawrence, 2008). Following this challenge, pro-inflammatory signals are released to trigger key events such as recruitment of neutrophils from the blood to the site of the injury to clear microorganisms and cell debris through rapid phagocytosis and to release antimicrobial factors (Kolaczkowska and Kubes, 2013). Throughout this process, neutrophils undergo apoptosis and monocytes are recruited to clear the site of infection via lymphatic uptake (Ginhoux and Jung, 2014). This inflammatory stage bridges innate and adaptive immune responses, and leads to the recruitment of B- and T-cells (Lanier, 2013). All of these events result in the resolution of inflammation and restoration of tissue homeostasis (Serhan et al., 2008). Disruption of this process can lead to chronic inflammation, which is observed in autoimmune diseases (Tabas and Glass, 2013), and is thought to play a role in the development and tumor progression in cancer (Landskron et al., 2014). The onset and peak of inflammation is an active process controlled by several factors including cytokines and chemokines and recently, the resolution of inflammation has also been shown to be an active, rather than a passive, process (Buckley et al., 2013). For an in depth review of resolution, readers are encouraged to refer to the excellent reviews by Dr. Charles Serhan and colleagues (Serhan, 2014, Serhan et al., 2015a). Briefly, resolution of inflammation involves the cessation of polymorphonuclear leukocytes (PMNL) influx through the upregulation of PMNL apoptosis, restoration of normal cytokine gradients, and the clearance of cell debris by macrophages (Serhan, 2014, Serhan et al., 2015b, Basil and Levy, 2016).
One class of molecules that has been gaining prominence in relation to inflammation is the lipid mediators (LM) derived from polyunsaturated fatty acids (PUFA). PUFA are appreciated for their beneficial actions in the immune (Hubler and Kennedy, 2016), neural (Crupi et al., 2013, Bazinet and Layé, 2014, Hashimoto et al., 2014, Song et al., 2016) and cardiovascular systems (Dessì et al., 2013, Lorente-Cebrián et al., 2013, Nicholson et al., 2013, Colussi et al., 2014). Most notably, PUFA such as arachidonic acid (AA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) are precursors for the biosynthesis of a variety of LM (Tessaro et al., 2015). AA is an ω-6 PUFA that is generated from dietary linoleic acid through the successive actions of Δ6-desaturase, elongase and Δ5-desaturase (Astudillo et al., 2012) or from cellular phospholipids via cytosolic phospholipase 2 (cLPA2) (Serini et al., 2009). EPA and DHA are ω-3 PUFA that can be generated in the body from α-linoleic acid via elongase and Δ5-desaturase (Neff et al., 2011), but the conversion rate is minute (Goyens et al., 2005), necessitating dietary intake of EPA and DHA. The major dietary source of EPA and DHA are marine animal oils (Tur et al., 2012), though recently algae oil has been identified as a vegetarian source of DHA (Lane et al., 2014). EPA and DHA have been shown to have direct anti-inflammatory properties through the inhibition of NLRP3 pathway (Zhou et al., 2011, Calder, 2013, Yan et al., 2013), and are transported to the site of inflammation via lipoproteins coinciding with edema generation (Kasuga et al., 2008). Omega-3 dietary supplementation has been investigated for the treatment of cardiovascular disease (Rizos et al., 2012), metabolic syndrome (Lorente-Cebrián et al., 2013), and depression (Lorente-Cebrián et al., 2013), among others. Interestingly, Omega-3 dietary supplementation has been investigated for the treatment of periodontitis in a variety of preclinical and clinical models (El-Sharkawy et al., 2010, Deore et al., 2014, Chee et al., 2016). More importantly, EPA and DHA are important substrates to produce pro-resolving LM, such as resolvins and protectins (Hong and Lu, 2013, Buckley et al., 2014, Spite et al., 2014), whose functions will be discussed in detail in the following sections. LM are generated via two major biosynthetic pathways, specifically, cyclooxygenase and lipoxygenase pathways. These pathways have been widely described in systemic diseases for over 30 years (Gwebu, 1979, Samuelson and Paoletti, 1982, Pace-Asciak and Granström, 1983), and have been investigated in conjunction with oral diseases (El Attar and Lin, 1983, Porteder et al., 1984, Mendieta et al., 1985, Williams et al., 1988, Offenbacher et al., 1989). Interestingly, many inflammatory systemic diseases are associated with increased incidence of oral diseases. For example, there is strong evidence of a link between periodontitis and atherosclerosis and other cardiovascular diseases (Dietrich et al., 2013, Loos et al., 2016), and between periodontitis and diabetes (Taylor et al., 1996, Hasturk and Kantarci, 2016). It has been suggested that some LM complicit in oral diseases are potential risk factor markers of other systemic diseases (Bäck et al., 2007). This review seeks to provide an overview of the current state of the art regarding biosynthesis and functions of various LM. We also hope to provide clinical providers and researchers in the oral health field with a detailed account regarding the roles of pro-inflammatory and pro-resolving LM in both systemic and oral diseases. Additionally, we conclude by addressing some major questions, including the potential effects of gender differences on the progression of chronic inflammatory diseases, and future research directions regarding diagnostic and therapeutic potential of LM in oral health.
Lipid Mediators Involved In Inflammation
Several LM have been identified during a variety of physiological and pathological systemic conditions as well as in several oral diseases. This section will present the different LM, their synthetic pathways, receptors, and roles in both systemic and oral health and disease.
Leukotrienes
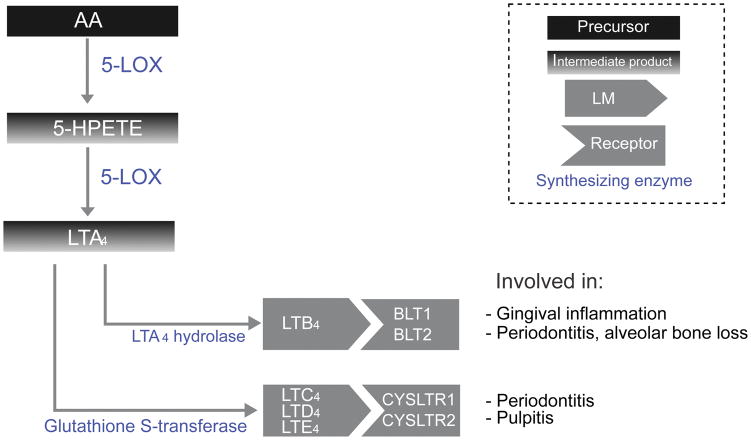
Leukotrienes are pro-inflammatory LM derived from AA, with a widespread role in the development of inflammation (Figure 1).
Figure 1. Leukotrienes biosynthetic pathways.
This diagram depicts biosynthesis of leukotrienes from arachidonic acid, including intermediate products, as well as various leukotriene receptors, and their role in oral disease.
Biosynthesis
During inflammatory responses, intracellular Ca2+ concentration is increased, leading to translocation of 5-LOX into the nuclear membrane (Wong et al., 1991, Capra et al., 2015). In the presence of 5-lipoxygenase activating protein (FLAP) (Dixon et al., 1990), 5-LOX catalyzes the transformation of AA into 5-HPETE which is then transformed into Leukotriene A4 (LTA4). LTA4 is an unstable intermediary, which is either hydrolyzed via leukotriene A4-hydrolase into Leukotriene B4 (LTB4) (Andberg et al., 2013) or transformed via glutathione S-transferase into leukotriene C4 (LTC4) which undergoes sequential cleavage of glutamyl and glycine residue to progressively produce the more stable leukotriene D4 (LTD4) and leukotriene E4 (LTE4) (Saino et al., 2011). LTC4, LTD4, and LTE4 will be collectively referred to as Cys-LT.
Signaling pathway
LTB4 binds and activates one of two leukotriene receptors (BLT1 and BLT2), leading to increased cytosolic calcium concentrations driving calcium-activated signaling molecules (Yokomizo, 2014). While the exact BLT signaling mechanism is not well understood, it is thought to involve regulation of COX-2 mRNA production (Zhai et al., 2010). Other studies suggests that BLT activation results in increases in metalloproteinase expression (Bäck et al., 2007).
The Cys-LTs (i.e., LTC4, LTD4 LTE4), act on one of two cysteinyl leukotriene receptors (CYSLTR1 and CYSLTR2) (Kanaoka and Boyce, 2014). The exact downstream pathways of Cys-LTs are also unclear, but it is known that LTD4 activates c-kit (Al Azzam et al., 2015), while LTE4 activates PPARγ (Paruchuri et al., 2008). Cys-LT activity can be regulated with Protein Kinase C (Kondeti et al., 2013).
Role in systemic health and disease
Recent studies on the involvement of leukotrienes in prominent systemic diseases suggest some potential therapeutic approaches. As an example, BLT1 activation with LTB4 inhibits TGF-β1 cell cycle arrest causing dysregulation of cell proliferation, suggesting a possible role for LTB4 in cancer (Jeon et al., 2015). Similarly, LTB4 inhibits L-type Ca2+ channels via p38 signaling in vascular smooth muscle cells, which may indicate a pathological role for LTB4 in atherosclerosis (Liu et al., 2015). In fact, a variety of promising BLT inhibitors are being tested in animal models of different diseases. For instance, treatment with the BLT1 antagonists BIIL284, CP105696, and U-75302 reduces atherosclerosis (Ketelhuth et al., 2015), insulin resistance (Li et al., 2015), and Chronic obstructive pulmonary disease (COPD) (Dong et al., 2016), respectively. Finally, a 5-LOX inhibitor, zileuton, which inhibits the synthesis of both LTB4 and Cys-LT, has been approved for the treatment of chronic asthma (Kubavat et al., 2013), and is being investigated as a treatment for colon polyps (Gounaris et al., 2015), sickle cell disease (Quarmyne et al., 2013), and stroke (Costa Silva et al., 2015).
Role in oral health and disease
Leukotrienes are well established as inflammatory mediators in periodontal disease (Yucel-Lindberg and Båge, 2013). Elevated LTB4 levels in gingival crevicular fluid are correlated with gingival inflammation (Tsai et al., 1998), and are strongly correlated with periodontal disease indices, clinical attachment loss, and alveolar bone loss (Pradeep et al., 2007). LTB4 levels are also elevated following periodontal surgery (O’Brien et al., 1996). Furthermore, elevated levels of CysLT have been detected in the submandibular glands from rats with experimental periodontitis (Busch et al., 2009). Higher LT levels are also associated with endodontic inflammation (pulpitis), having been observed in periapical and pulpal lesions (Okiji et al., 1991, Lim et al., 1996, Shon et al., 2000). However, treatments for oral diseases based on these promising findings have been limited to preclinical models with no translation into clinical therapies. For example, LT synthesis inhibitors, specifically phenidone and ketoconazole, were shown to reduce both the infiltration of PMNL within periodontal pockets and osteoclastic bone resorption in a hamster periodontitis model (Baroukh and Saffar, 1990, Baroukh and Saffar, 1991, Baroukh and Saffar, 1992). Zileuton was shown to reduce the incidence of oral squamous cell carcinoma in a hamster model (Li et al., 2005). In both models, reduction in disease severity was accompanied by lower LTB4 levels.
Prostaglandins
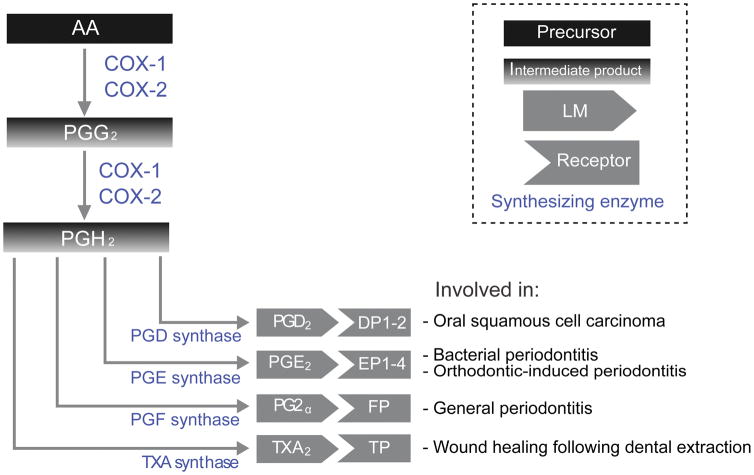
Prostaglandins are LM derived from AA that play an important regulatory role in physiological processes, but also have crucial pro-inflammatory effects (Figure 2).
Figure 2. Prostaglandins biosynthetic pathways.
This diagram depicts biosynthesis of prostaglandins from arachidonic acid, including intermediate products, as well as various prostaglandin receptors, and their role in oral disease.
Biosynthesis
All stable prostaglandins, including Prostaglandin D2 (PGD2), Prostaglandin E2 (PGE2), Prostaglandin F2α (PGF2α), and Thromboxane B2 (TXB2) are produced through the activity of cyclooxygenase-1 (COX-1) and -2 (COX-2) (Nugteren and Hazelhof, 1973, Kudalkar et al., 2015). COX-1 is constitutively expressed in most tissues and regulates a host of homeostatic functions, while COX-2 expression is typically induced in response to inflammatory assault (Islam et al., 2016). The two cyclooxygenases (COX) function similarly by catalyzing the conversion of AA into PGG2 via a first peroxidation step, then into PGH2 via a reduction step (Aronoff et al., 2006). PGH2 is converted into: 1) PGD2 via two forms of PGD synthase, 2) PGE2 via three forms of PGE synthase, 3) PGF2α via two forms of PGF synthase, and 4) TXA2 from TXA synthase (Chen et al., 2013a). The synthesis of PGG2 via COX activity is the rate limiting step, and therefore, any dysfunction will have profound downstream effects. Prostaglandin breakdown is mediated via 15-hydroxyl prostaglandin dehydrogenase, with a specialized prostaglandin transporter moving extracellular prostaglandins into the cytoplasm for enzymatic inactivation (Schuster et al., 2015).
Signaling pathway
PGD2 signaling involves a primary prostaglandin receptor, DP1 (Boie et al., 1995), and a secondary receptor, DP2, also termed CRTH2 (Nagata and Hirai, 2003). PGD2 binding to PD receptors leads to the activation of adenylyl cyclase resulting in cAMP formation (Hammad et al., 2007, Ayabe et al., 2013). Increased cAMP expression leads to activation of protein kinases, which regulate normal developmental processes such as sex determination via SOX9 phosphorylation (Malki et al., 2005). However, cAMP-dependent protein kinase activity induced by DP1 can also play pathological roles in inflammation. Particularly, cAMP inhibits IL-1β induced production of matrix metalloproteinases, leading to osteoarthritis (Zayed et al., 2008). Additionally, cAMP reduces IL-12 release by dendritic cells, leading to T-helper cell polarized responses (Theiner et al., 2006). Interestingly, PGD2 exhibits neuroprotective effects through DP1 signaling (Wu et al., 2007), but neurotoxic effects through its conversion to its cyclopentenone metabolites (Liu et al., 2013). DP1 cell signaling also mediates ERK1/2 signaling, which provides a cooperative mechanism to promote additional cell surface expression of DP1 (Binda et al., 2014). The DP2 receptor (also known as CRTH2) is less specific to PGD2, being capable of binding other prostaglandin synthesis metabolites that decrease cAMP expression while increasing intracellular Ca2+ (Hirai et al., 2001).
PGE2 exerts its effects through four receptors (EP1-EP4TP), EP1 and EP2 being low affinity, while EP3 and EP4 are high affinity PGE2 receptors (Kalinski, 2012). EP2 and EP4 are Gs-coupled receptors that function largely by activating the cAMP/PKA/CREB pathway (Fujino et al., 2005), but also activate the GSK3/β-catenin pathway (Fujino et al., 2002). EP4 also activates the PI3K-dependent ERK1/2 pathway (Fujino et al., 2003), and additionally controls integrin-mediated signaling pathways (Lee et al., 2013). EP1 and EP3 on the other hand, are Gi-coupled receptors that function primarily by inhibiting cAMP and increasing Ca2+ (Morimoto et al., 2014).
PGF2α has a single Gq-coupled receptor, FP, which activates the PI3/DAG/PKC signaling pathway, leading to elevated intracellular Ca2+ and MAP kinase activation (Woodward et al., 2011). Additionally, PGF2α/FP interaction regulates CXCR2 activation via a PKC/Ca2+/calcineurin pathway (Sales et al., 2009).
Finally, Thromboxane A2 exerts its effects through the Thromboxane Prostanoid receptor (TP), which has two isoforms, TPα, which is present in platelets (Habib et al., 1999), and TPβ, which is present in endothelial and smooth muscle cells (Kinsella, 2001) TP is a Gq- and G12-coupled receptor activating the PI3/DAG/PKC pathway (Ting et al., 2012, Capra et al., 2014). G12 activation via TP signaling also activates Rho kinase signaling, leading to myosin light chain phosphorylation (Klages et al., 1999) and mediating plate shape change during blood coagulation (Aburima et al., 2013). TXA2 is a transient molecule that is converted into the more stable, but biologically inactive, Thromboxane B2 (TXB2) (Hamberg et al., 1975, Halushka, 2016).
Role in Systemic Health and Disease
PGD2 has well known roles in female reproduction, specifically in the regulation of placental communication (Rossitto et al., 2015), and in male testis development (Moniot et al., 2014). It additionally regulates multiple neurological functions, such as sleep induction (Urade and Lazarus, 2013) and homeostasis (Porkka-Heiskanen, 2013), seizure suppression (Kaushik et al., 2014), and PNS myelination (Trimarco et al., 2014). PGD2 also has a potential role in bone anabolism following fracture (Gallant et al., 2010). Additionally, PGD2 has been shown to mitigate several inflammatory-mediated diseases, such as metabolic syndrome (Evans et al., 2013), cancer (Fukuoka et al., 2013, Tsai et al., 2013a), and acute lung injury (van den Brule et al., 2014). However, PGD2 is most known for its pro-inflammatory properties in a variety of pathologies. Notably, it exacerbates adjuvant induced joint inflammation (Tsubosaka et al., 2014), allergic rhinitis (Nakano et al., 2016), Crohn’s disease (Le Loupp et al., 2015, Radnai et al., 2016), and mast cell-induced vitreoretinopathy (Kuo et al., 2015). It also plays a role in depression-related behavior (Onaka et al., 2015) and in pain, being implicated in esophageal nociception (Zhang et al., 2013a), migraine (Antonova et al., 2013), and neuropathic spinal pain (Kanda et al., 2013). Additionally, PGD2 inhibits hair growth, being elevated in the bald scalp, and is an attractive therapeutic target for male-pattern baldness (Garza et al., 2012, Schmidt, 2016). PGD2-modulating therapeutics, such as DP antagonists have been shown to have anti-inflammatory effects. For example, the orally delivered DP2 antagonist QAW039 results in significant increase in bronchial epithelial integrity in asthmatics (Berair et al., 2015). DP1 antagonist ONO-4053 was more effective than LTR antagonists for the treatment of seasonal allergic rhinitis and congestion by potentially modulating histamine in addition to PGD2 (Okada et al., 2015, Hajime and Okubo, 2016). Specific inhibition of PGD synthase is another promising therapeutic approach, with multiple PGD synthase inhibitors being identified for the treatment of various inflammatory disorders (Edfeldt et al., 2015).
PGE2 has multiple normal physiological functions, most importantly labor induction (Goetzl, 2014). Additionally, the lung is a privileged site for beneficial actions of PGE2 (Vancheri et al., 2004). As an example, inhaled PGE2 improves pulmonary fibrosis (Ivanova et al., 2013). However, like PGD2, PGE2 is more known for its pathological effects. For instance, PGE2 signaling creates a carcinoma stem cell niche (Li et al., 2012), promotes intestinal cancer growth via DNA methylation (Xia et al., 2012). Tumor-derived PGE2 induces myeloid-derived suppressor cells and suppresses natural killer cell activity (Mao et al., 2014). EP1 is involved in cancer metastasis, its deletion suppressing metastasis in breast cancer cells (Reader et al., 2015) and colon cancer cells (O’Callaghan et al., 2013). Similarly, EP4 antagonist treatment suppresses angiogenesis, metastasis, and stem-like functions in murine breast cancer cells (Majumder et al., 2014, Majumder et al., 2015). A PGE2 neutralizing antibody suppresses cancer stem cell chemoresistance (Kurtova et al., 2015).
PGF2α plays an important reproductive role in pregnancy by regulating luteolysis (De Rensis et al., 2012), partially through upregulation of Slit/Robo expression (Zhang et al., 2013b). It has strong pro-contraction effects on the uterus (Ravanos et al., 2015). Elevated PGF2α levels are associated with endometriosis (Ahmad et al., 2015, Rakhila et al., 2015). Additionally, inhibiting PGF2α via a selective FP antagonist AS604872 exacerbates intracranial aneurysm and aortic dissection in a hypertensive rat model (Fukuda et al., 2014). The PGF2α isoform 8-iso-PGF2α is a reliable indicator of oxidative stress and can be used to assess the efficacy of treatments for cardiovascular disease (Rizos et al., 2013).
Thromboxane A2 plays an important role in thrombus formation through platelet activation and vascular reactivity (Ally and Horrobin, 1980, FitzGerald, 1991, Daniel et al., 1999), and some compounds have anti-thrombotic effects by binding to TP receptors (Guerrero et al., 2005). As an immune modulator, TXA2 regulates JAK3/STAT5 signaling to promote early B-cell development (Yang et al., 2014), and mediates neutrophil control of the magnitude and spread of the immune response (Yang and Unanue, 2013). TXA2 has been shown to promote cell proliferation in lung adenocarcinoma (Huang et al., 2014), implicating a potential role in cancer. Certain polymorphisms in the TXA2R gene were found to be correlated with an increase in the risk for acute cerebral infarction (Wang et al., 2014). Decreases in TXA2 formation via COX-2 inhibition improves glomerular filtration rate in endotoxemic mice (Mederle et al., 2015).
Role in Oral Health and Disease
While PGD2 has been implicated in a variety of general diseases; it has not been shown to play a major role in the progression of oral diseases. One report found that oral squamous cell carcinomas secrete PGD2 to attract eosinophils and that PGD synthase inhibitor HQL-79 reduced this migration (Davoine et al., 2013). Single nucleotide polymorphisms (SNP) in the PGD synthase gene appear with higher frequency in aggressive periodontitis patients (Suzuki et al., 2004). Additionally, SNP in the PGD synthase gene are linked to smoking and associated with head and neck cancer (Lee et al., 2015).
PGE2 has a better established role in oral disease. For example, human gingival fibroblasts exposed to LPS from periodontopathogenic bacteria produce PGE2 through COX-2 (Noguchi et al., 1996). Additionally, orthodontic tooth movement is known to increase the incidence of periodontitis. Research suggests that mechanical forces induce COX-2 overexpression in periodontal ligament cells, leading to a dramatic increase in PGE2 release (Shimizu et al., 1998, Yamaguchi and Garlet, 2015). PGE2 is associated with periodontal disease and bone loss, and COX inhibitors have been proposed as a treatment (Lohinai et al., 2001, Yamaguchi and Kasai, 2005, Noguchi et al., 2007). Gingival biopsies from periodontal patients reveal that COX-2 expression leading to PGE2 production correlates with connective tissue loss (Mesa et al., 2012, Mesa et al., 2014), and it is hypothesized that PGE2 inhibits in vitro mineral deposition by periodontal ligament cells via the modulation of TWIST1 & RUNX2 expression (Manokawinchoke et al., 2014). PGE2 production can be inhibited by IL-4 and IFNγ in periodontal ligament fibroblasts (Noguchi et al., 1999b), and by lidocaine irrigation in periodontal treatment (Camargo et al., 2015b).
PGF2α has a potential role in periodontal pathogenesis by upregulating multiple pro-inflammatory factors in gingival fibroblasts, such as ICAM-1 expression (Noguchi et al., 1999a), IL-6 (Noguchi et al., 2001a), and (Noguchi et al., 2001b). Non-surgical periodontal therapy which reduces a variety of canonical pro-inflammatory markers also reduces PGF2α levels (Koromantzos et al., 2011).
For dental applications, TXA2 activity is relevant for the care of dental extraction patients. Since TXA2 is a potent activator of platelet aggregation, special care needs to be taken with regards to prescribing aspirin (Nizarali and Rafique, 2013) or interrupting antiplatelet therapy (Lu et al.). Additionally, gingival TXB2 levels have long been known to be elevated in animal models of periodontal disease (Rifkin and Tai, 1981).
Lipoxins
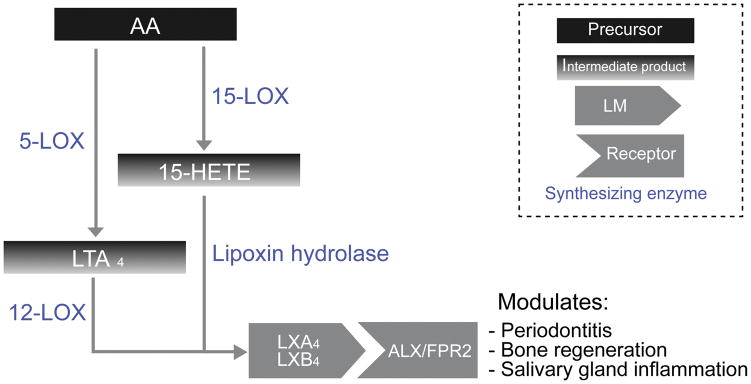
Lipoxins are LM derived either directly from AA, or from the conversion of LTA4. They possess a variety of immunomodulatory and anti-inflammatory actions (Figure 3).
Figure 3. Lipoxins biosynthetic pathways.
This diagram depicts biosynthesis of leukotrienes from arachidonic acid, including intermediate products, as well as various lipoxin receptors, and their role in oral disease.
Biosynthesis
Lipoxins are notable for possessing dual anti-inflammatory and pro-resolving roles in the body (Serhan, 1994, Serhan et al., 2008) and are synthesized through two major pathways. In mucosal membranes, leukocyte-epithelial interaction triggers the synthesis of lipoxin A4 (LXA4) and its isoform, lipoxin B4 (LXB4) through the conversion via lipoxin hydrolases of 15-HETE, an intermediary product of 15-LOX activity on AA (Serhan, 1997). In the blood, leukocyte-platelet interactions trigger the synthesis of lipoxins via lipoxins-synthase activity of 12-LOX on LTA4 (Serhan, 2005).
Signaling pathway
The primary action of LXA4 and its aspirin-triggered epimers (ATL) occurs via the specific binding to the LXA4 receptor (ALX/FPR2) to regulate gene expression, transmigration and chemotaxis in vitro and in vivo (Chiang et al., 2005, Chiang et al., 2006). ALX/FPR2 is a Gi-protein coupled receptor that is a member of the N-formyl peptide receptor family that, in humans, also includes FPR1 and FPR3. (Rabiet et al., 2011, Dorward et al., 2015). ALX/FPR2 expressed on leukocytes, neutrophils, monocytes, and lymphocytes (Fiore et al., 1994, Takano et al., 1997). FPR1 is the primary receptor for the pro-inflammatory formyl peptides (Boulay et al., 1990, Gao et al., 1994), while the function of FPR3 remains largely unknown, but is hypothesized to modulate the activity of FPR1 and ALX/FPR2 (Rabiet et al., 2011, Dorward et al., 2015).
Intracellular signaling pathways can either be activated or inhibited by LXA4 binding to ALX/FPR2 (Jozsef et al., 2002). While LXA4 increases intracellular Ca2+ levels, the chelation of calcium does not change the LXA4-mediated adherence response of monocytes, indicating that Ca2+ is not the second messenger for LXA4 signaling (Romano et al., 1996). The events triggered by the LXA4 or ATL binding can be very rapid, occurring within seconds or minutes, or can be late responses that occur many hours after exposure (Serhan, 2014). LXA4 and ATL trigger a variety of downstream pathways to achieve their anti-inflammatory and pro-resolving pathways. For example, LXA4 inhibits TNF-α secretion by blocking ERK activation (Ariel et al., 2003), and can exert protective effects against ischemia/reperfusion injury via p38 MAPK activation (Chen et al., 2013b). LXA4 has also been shown to down regulate pro-inflammatory genes by reducing NF-κB-mediated transcription activation (Bucci, 2014).
Role in systemic health and disease
As a LM, LXA4 is notable for possessing both anti-inflammatory and pro-resolving properties. Anti-inflammatory actions of LXA4 include the following: a) inhibition of tumor growth by targeting IL-10 production (Wang et al., 2015), b) attenuation of acute rejection following liver transplant through the modulation of cytokine balance (Liao et al., 2013), c) inhibition of neuroinflammation and neuropathic pain after spinal cord injury (Martini et al., 2016), d) reduction of Alzheimer-like pathology in mice (Dunn et al., 2015), e) inhibition of endometriosis development in mice (Xu et al., 2012), f) mitigation of cardiac ischemia-reperfusion injury (Chen et al., 2013c) and g) attenuation of adipose inflammation (Börgeson et al., 2012). Disruption of LXA4 signaling leads to an exacerbation of inflammation (Bozinovski et al., 2012). LXA4 has been suggested as a potential therapeutic target for neurodegenerative disease due to its allosteric enhancement of the CB1 cannabinoid receptor (Pamplona et al., 2012). Furthermore, LXA4 and ATL have been shown to enhance the action of dexamethasone towards the complete inhibition of neutrophil adhesion to endothelial cells both in vitro (Filep et al., 1999) and in vivo (Perretti et al., 2002). Synthetic LXA4 analogs, were shown to have antifibrotic effects in cystic fibrosis (Guilherme et al., 2013) and to modulate the interaction of epithelial and tumor cells (Vieira et al., 2014). Finally, a LXA4 receptor agonist, BML-111, promoted neurovascular protection in an ischemic stroke rat model (Hawkins et al., 2014).
As inflammation progresses, leukotriene generation by neutrophils decreases and 15-LOX-mediated synthesis of lipoxins commences prompting a lipid mediator class switch (Levy et al., 2001). Lipoxins act as a stop signal for neutrophil migration and a go signal for clearance of apoptotic neutrophils (Uddin and Levy, 2011). LXA4 exerts direct modulatory effects on multiple immune cells, including upregulating natural killer and type 2 innate lymphoid cell activation (Barnig et al., 2013) and decreasing memory B-cell responses (Ramon et al., 2014). It directly acts to stimulate macrophage clearance of apoptotic neutrophils and bacteria (Prescott and McKay, 2011).
Role in oral health and disease
LXA4 has been suggested as an immunomodulatory molecule in periodontal disease, inhibiting leukocyte recruitment to Porphyromonas gingivalis (Pouliot et al., 2000). More recently, LXA4 was shown to activate bone regeneration in a pig model of periodontitis (Van Dyke et al., 2015), and to induce proliferation and migration of human periodontal stem cells (Cianci et al., 2016). Additionally, LXA4 might play a role in modulating salivary gland inflammation by inhibiting immune cell binding to salivary epithelial cells (Chinthamani et al., 2012).
Resolvins
Resolvins are highly potent anti-inflammatory and pro-resolution agents derived from DHA and EPA that control the duration and magnitude of inflammation in animal models of complex diseases (Arita et al., 2007, Arita and Serhan, 2007, Bannenberg et al., 2007, Campbell et al., 2007) (Figure 4).
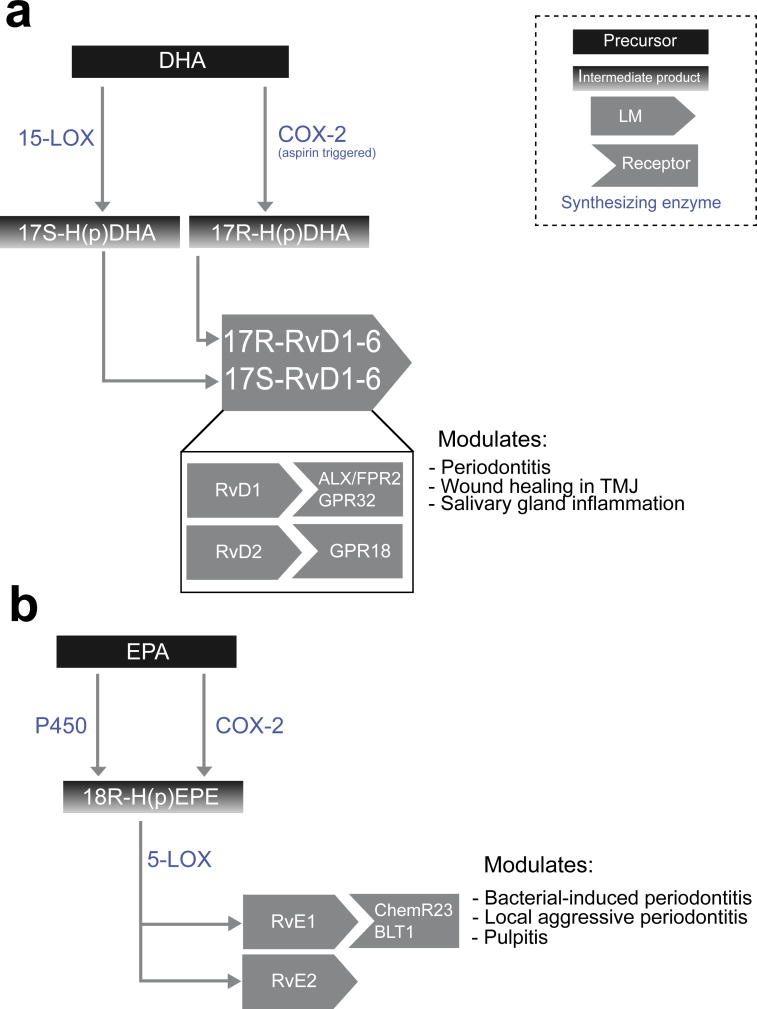
Figure 4. Resolvins biosynthetic pathways.
This diagram depicts biosynthesis of (a) D-series resolvins from DHA and (b) E-series resolvins from EPA, including intermediate products, as well as various resolvin receptors, and their role in oral disease.
Biosynthesis
D-series resolvins are derived by the hydroxylation of DHA, via two distinct biosynthesis pathways. The first involves multiple 15-LOX-catalyzed hydroxylation steps of DHA to produce 17S-H(p)DHA, which is then transformed into 17S-resolvins D1 through D6 (Serhan et al., 2004), whereas the second involves aspirin-dependent COX-2 mediated hydroxylation of DHA into 17R-H(p)DHA, which is then transformed into 17R-RvD1-4 (Sun et al., 2007). The 17S- and 17R- resolvins are diastereomers with extremely similar bioactivity (Weylandt et al., 2012). A 17R-epimer of RvD1, termed AT-RvD1, can also be generated in aspirin-treated monocytes and macrophages (Ariel and Serhan, 2007). E-series resolvins, on the other hand, are derived from EPA. Resolvin E1 (RvE1) is generated by an initial oxygenation step via aspirin-dependent COX-2 or cytochrome P450 monoxygenase activity to form 18R-H(p)EPE, which is then converted to RvE1 via 5-LOX activity (Arita et al., 2005b). RvE2 is produced through the hydroxylation of 18R-H(p)EPE generated from EPA (Tjonahen et al., 2006, Ogawa et al., 2009).
Signaling Pathway
RvD1 and AT-RvD1 exert their activity in a dose-dependent manner through binding to two distinct receptors, ALX/FPR2 and GPR32 (Krishnamoorthy et al., 2012). Notable effects of RvD1 signaling include blocking the upregulation of TNFα-induced IL-1β transcripts (Hong et al., 2003) and upregulating IL-10 and miR-208a, a key regulator of immune responses (Krishnamoorthy et al., 2012). Both RvD1 receptors are PTX-sensitive and do not employ the classical Ca2+ or cAMP second messengers in PMN cells (Krishnamoorthy et al., 2010). More recently however, RvD1 was found to elicit a Ca2+ response and increase Akt phosphorylation in salivary epithelial cells, suggesting that ALX/FPR2 could activate PI3K pathways in some salivary cells (Leigh et al., 2014, Nelson et al., 2014). RvD2 binds to GPR18, also known as DRV2, to activate cAMP signaling (Chiang et al., 2015).
RvE1 interacts with two distinct G protein-coupled receptors binds to control inflammation and promote resolution. The first is ChemR23, a G protein coupled receptor, and causes Gi/o activation and phosphorylation of ERK, while the second is the leukotriene B4 receptor 1 (BLT1) on human PMNL (Arita et al., 2007).
Role in systemic health and disease
Both RvD1 and its 17(R) epimer modulate innate immune responses such as reducing PMNL transendothelial migration (Serhan et al., 2002), and exhibiting a dose-dependent reduction in leukocyte infiltration (Serhan et al., 2002). They also exert effects of multiple lymphocyte type; for example, increasing IgM and IgG production from activated B cells (Ramon et al., 2012). RvD1 produced beneficial effects in multiple inflammatory murine models. Examples include: 1) reducing functional and morphological kidney injury following bilateral ischemia/reperfusion injury (Marcheselli et al., 2003, Duffield et al., 2006), 2) decreasing adipose tissue macrophage accumulation to improve insulin sensitivity in obese-diabetic mice (Hellmann et al., 2011), 3) attenuating arthritis severity and enhancing collagen repair in arthritic mice (Norling et al., 2016), 4) alleviating inflammation associated with endometriosis (Dmitrieva et al., 2014), 5) reducing mucosal inflammation following acute lung injury (Eickmeier et al., 2013), and alleviating allergic airway responses (Rogerio et al., 2012) and chronic emphysema (Hsiao et al., 2015). RvD2 appears to be a potent regulator of leukocytes, controlling microbial sepsis (Spite et al., 2009) and responding to E. coli peritonitis (Gao et al., 2013). RvD2 also appears to modulate variety of vascular injuries, including venous thrombosis (Diaz et al., 2015), aortic aneurysm (Pope et al., 2014), and peripheral vascular ischemia and subsequent revascularization (Zhang et al., 2014). The effects of RvD3 and RvD4 have not yet been established in great detail. Thus far, RvD3 is known to exert potent antineutrophil activity and strongly upregulate apoptotic PMN phagocytosis (Dalli et al., 2013, Norris et al., 2016), while RvD4 plays a similar role in sterile peritonitis and Staphylococcus aureus skin infection (Winkler et al., 2016).
RvE1 displays potent, stereoselective actions in vitro and in vivo (Hasturk et al., 2007, Haworth et al., 2008). RvE1 is anti-inflammatory, pro-resolving, and tissue-protective. RvE1 stops neutrophil infiltration in vivo and retards their transendothelial migration (Arita et al., 2007), attenuates IL-12 production by dendritic cells and reduces their mobility toward pathogens, and has protective actions in a mouse model of colitis (Arita et al., 2005b). RvE1 may attenuate TNFα-mediated signals; for example, RvE1 reduces TNFα-mediated activation of NFκB in the human embryonic kidney 293 cell line (HEK293) (Arita et al., 2005a) and in a TNFα-induced mouse model of dermal inflammation (Arita et al., 2007). RvE1 also acts on epithelial cells, having been shown to reduce proinflammatory mediator release from corneal epithelial cells (Yuan et al., 2012) and promote intestinal mucosal wound repair (Quiros et al., 2016). Preliminary studies show that RvE1 is effective at attenuating atherosclerosis and augmenting the actions of anti-atherogenic drugs (Salic et al., 2014, Hasturk et al., 2015, Salic et al., 2016).
Since resolvins are rapidly hydrolyzable, more stable analogs have been developed that exhibit similar bioactivity, for example, sulfido-conjugates (e.g. 8-glutathionyl, 7,17-dihydroxy-4Z,9,11,13Z,15E,19Z-docosahexaenoic acid and 8-cysteinylglycinyl, 7,17-dihydroxy-4Z,9,11,13Z,15E,19Z-docosahexaenoic acid) (Dalli et al., 2015) and the benzo-diacetylenic-conjugate benzo-diacetylenic-17R-RvD1-methyl ester (BDA-RvD1) (Orr et al., 2015). Another approach to improve the stability of resolvins is the use of controlled release technology. For example, RvD1-containing nanoparticles were shown to limit PMN infiltration and improve wound healing in mouse models of peritonitis (Norling et al., 2011).
Role in oral health and disease
RvD1 plays a protective role in periodontitis, enhancing periodontal ligament fibroblast proliferation and wound closure (Mustafa et al., 2013), and is secreted by human periodontal stem cells (Cianci et al., 2016). It has been shown to promote wound healing in a mouse model of TMD (Norling et al., 2011). RvD1 has recently been of interest for the mitigation of salivary gland inflammation. For example, RvD1 biosynthetic pathways have been discovered in the salivary gland epithelium (Leigh et al., 2014), and RvD1 blocks cytokine signaling in salivary epithelium to promote cell survival and tissue integrity in rat parotid Par-C10 cells (Odusanwo et al., 2012). This was also shown to be true in mouse submandibular gland (SMG) cells coupled with the confirmation of the presence and functionality of the ALX/FPR2 (Nelson et al., 2014). Loss of ALX/FPR2 results in unresolved acute inflammation and SMG dysfunction (xerostomia) in vivo (Wang et al., 2016). AT-RvD1 can also reduce the expression of inflammatory genes and reduce apoptosis in a mouse model of Sjögren’s Syndrome (SS) (Easley et al., 2015).
RvE1 reduces oral inflammation and alveolar bone loss (Hasturk et al., 2007) and generation of reactive oxygen species (Damgaard et al., 2016) in Porphyromonas gingivalis–induced periodontitis. In localized aggressive periodontitis, RvE1 rescues impaired phagocytosis activity (Fredman et al., 2011), reduces the expression of CXCL1 and osteoclast activity in ligature-induced periodontitis (Lee, 2015), and has been shown to directly act on bone cells to promote bone preservation (Van Dyke, 2011, Gyurko and Van Dyke, 2014). Additionally, RvE1 has a protective effect against pulp inflammation in a rat model (Dondoni et al., 2014).
Lipid mediators in body fluids
The ubiquity of LM in physiological and pathological processes has led to an increased interest in identifying LM in various body fluids. Particularly, as they represent a simple and noninvasive approach for research and diagnostic purposes.
Serum & plasma
Serum levels of various LM have been investigated to study whether they correlated with certain diseases. High serum LTB4 levels have been shown to be correlated with an elevated risk of acute coronary syndrome in adults (He et al., 2014). Additionally, high LTB4 levels may predispose children to tonsillar hypertrophy (Alexopoulos et al., 2015). Further, serum levels of Cys-LTs are elevated during anaphylaxis and have been suggested to be a reliable marker of this condition (Nassiri et al., 2016). Finally, elevated serum levels of LTB4 and Cys-LTs are correlated with higher levels of circulating neutrophils in children with sleep-disordered breathing (Shen et al., 2014).
Increased PGE2 serum levels indicate overexpression of aromatase (Subbaramaiah et al., 2012), which is linked to hormone-related breast cancer (Subbaramaiah et al., 2008). Additionally, PGE2 serum levels, along with 8-Isoprostane, C-reactive protein, and amyloid A, have been suggested as inflammatory markers for antiphospholipid syndrome (Sciascia et al., 2012). Similarly, serum thromboxane levels are an indicator of platelet inhibition by aspirin, and can thus be used for epidemiological studies on the effects of aspirin (Reny et al., 2012, Zantek et al., 2014). However, measurement methods for serum thromboxane need to be standardized to produce consistent results across studies (Brun et al., 2016).
In contrast to the pro-inflammatory leukotrienes and prostaglandins, decreased lipoxin serum levels are typically observed in several pathological conditions. For example, lowered LXA4 serum levels were found in patients with sepsis (Tsai et al., 2013b) and in wheezy infants (Eke Gungor et al., 2014). Serum LXA4 levels were also found to have an inverse relationship to the risk of metabolic syndrome (Yu et al., 2015). Of note to dental professionals, periodontitis patients might have elevated LXA4 serum levels compared with healthy controls (Doğan et al., 2015).
RvD1 and RvE1 serum levels have been investigated as markers for the efficacy of diclofenac in acute pancreatitis treatment, with increased resolvin and lipoxin serum levels observed in the drug receiving group (Zhao et al., 2014). Additionally, higher RvD1 serum levels have been described as a potential biomarker for Familial Mediterranean Fever (Taylan et al., 2015).
Urine
Similar to serum, urine levels of various LM have been used to investigate multiple diseases. High levels of urinary leukotriene have been observed in pediatric patients with wheezing and recurrent asthma (Morales et al., 2016). High levels of urinary leukotrienes have also been detected in adult individuals with systemic mastocytosis (Lueke et al., 2016), as well as in aspirin-intolerant asthmatic individuals (Yamaguchi et al., 2015, Hagan et al., 2016). Urinary leukotriene levels are directly correlated to the severity of arterial occlusive disease following transluminal angioplasty (Maga et al., 2016). Additionally, they are inversely correlated with renal function in Type 2 diabetes (Rafnsson and Bäck, 2013). Therefore, they have been suggested as prognostic markers for these diseases.
While levels of total unmodified prostaglandin in urine have been correlated with only a few diseases (e.g., cardiovascular disease (Raatz et al., 2012) and anaphylaxis (Lieberman, 2013)), levels of prostaglandin metabolites in urine have attracted more scrutiny. High levels of the PGD2 metabolite tetranor PGDM in urine, have been correlated with Duchenne muscular dystrophy, systemic mastocytosis, rheumatoid arthritis, colitis, and colon cancer (Nakagawa et al., 2013b, Nakagawa et al., 2013a, Iwanaga et al., 2014, Cho et al., 2015). High levels of urinary PGE2 metabolite levels appear to be associated with colorectal adenoma (Shrubsole et al., 2012, Davenport et al., 2015), pancreatic cancer (Zhao et al., 2015), and breast cancer in postmenopausal women (Cui et al., 2014). Additionally, elevated levels of urinary thromboxane have been suggested as a predictor of atherthrombotic risk (Neath et al., 2013).
In contrast to the pro-inflammatory LM, there is a dearth of studies examining urinary levels of pro-resolving LM. Decreased urine levels of lipoxins and resolvins have been hypothesized to be related to inflammatory bowel disease (Das, 2016), while elevated urinary LXA4/creatinine ratio has been observed in systematic lupus erythematosus (Abdou et al., 2015).
Sputum and Exhaled breath condensate
Lung fluids are an obvious target for analysis of LM involved in inflammatory lung diseases. Asthmatic patients had elevated sputum levels of Cys-LTs and PGE2 compared to healthy subjects (Papadaki et al., 2013), with smoking asthmatics exhibiting even higher sputum levels of these LM (Kontogianni et al., 2013). COPD patients, on the other hand, only exhibited elevated PGE2 sputum levels, but no differences in Cys-LT levels, compared to healthy controls (Drozdovszky et al., 2013). An analysis of sputum from adult cystic fibrosis patients found elevated levels of LTB4 and PGE2 (Yang et al., 2012). Pediatric cystic fibrosis patients, however, did not have elevated LTB4 sputum levels, but the ratio of LXA4 to LTB4 was depressed (Ringholz et al., 2014). Accurate measurements of LTB4 using UPLC-MS/MS require a refinement of sputum processing due to the instability of LTB4 in the presence of standard preparation reagents (Jian et al., 2013). In exhaled breath condensate (EBC), similar findings with regards to higher leukotriene levels were observed as well. For example, smoking asthmatic patients displayed elevated EBC Cys-LT levels (Celik et al., 2013). Cys-LT EBC levels are correlated with the severity of asthma (Kazani et al., 2013). Cys-LT levels in exhaled breath condensate were found to be elevated in children with asthma and allergic rhinitis (Wan et al., 2013). 8-iso-PGE2 levels in exhaled breath condensate were found to be elevated in aspirin-hypersensitive asthmatics (Mastalerz et al., 2015). Lipoxin/Leukotriene imbalance was also detected in exhaled breath condensate of severe refractory asthma patients (Sedlák et al., 2014). Exhaled breath condensate has recently emerged as a simple and accurate medium to aid the diagnosis and evaluation of inflammatory severity in asthmatic patients (Thomas et al., 2013).
LXA4 sputum levels in pediatric severe asthmatics were found to be depressed, along with reduced ALX/FPR2 expression in induced sputum cells (Gagliardo et al., 2016). Conversely, LXA4 levels in exhaled breath condensate were found to be elevated in asthmatic children with exercise-induced bronchoconstriction (Tahan et al., 2016). Such investigations are eminently useful for understanding the pathologies of different respiratory disorders. As for resolvins, there is currently a lack of rigorous analysis of the levels of resolvins in sputum and exhaled breath condensate. More research into this area would contribute greatly to the understanding of the development and resolution of various respiratory conditions.
Breast milk
Breast milk is extremely important for developing innate immunity and healthy intestinal homeostasis in babies (Cederlund et al., 2013, Jakaitis and Denning, 2014). Elevated PUFA levels in breast milk decrease during the first month after birth, but lipoxin and resolvin levels remain stable, indicating an important role in neonatal immunity (Weiss et al., 2013). LM profile in breast milk could be associated with conditions that affect neonatal health. For example, maternal smoking can negatively affect the LM profile in breast milk (Szlagatys-Sidorkiewicz et al., 2013). Furthermore, mastitis results in elevated LTB4 and decreased lipoxin and resolvin levels (Arnardottir et al., 2015)
Synovial fluid
LM profiling of synovial fluid from rheumatoid arthritis reveals the presence of both pro-inflammatory and pro-resolving LM (Giera et al., 2012). Elevated levels of PGD2 and PGE2 in synovial fluid from arthritic patients have been linked to dendritic cells in the synovial fluid (Moghaddami et al., 2013). Of note for dental clinicians, synovial fluid from painful, dysfunctional temporomandibular joints (TMJ) was found to contain elevated levels of LTB4 and PGE2 (Quinn and Bazan, 1990). LTB4 synovial fluids were found to be prognostic markers for the success of arthrocentesis of the TMJ, with higher LTB4 levels indicating unsuccessful treatment (Kaneyama et al., 2007). Similarly, glucosamine-chondroitin sulfate treatment for TMJ derangement results in lower PGE2 synovial fluid levels (Damlar et al., 2015).
Gingival crevicular fluid
Gingival crevicular fluid (GCF) is an important periodontal diagnostic marker (Gupta, 2013). LTB4 levels in GCF are higher in chronic periodontitis patients (Emingil et al., 2001, Pradeep et al., 2007). Similarly, PGE2 levels in GCF are a strong marker of gingivitis and periodontitis (Heasman et al., 1998, Camargo et al., 2015a), and are correlated with the severity of periodontal disease (Kumar et al., 2013). GCF from orthodontic patients displaying gingival inflammation who received a chlorhexidine/thymol-containing dental varnish administered exhibited a reduction in LTB4 and PGE2 levels (Sköld et al., 1998, Yucel-Lindberg et al., 1999).
GCF levels of pro-resolving LM are generally decreased in periodontal patients. For example, LXA4 levels were found to be significantly lower in periodontal patients compared to healthy controls, combined with a negative correlation between LXA4 levels and clinical attachment loss (Tarannum and Faizuddin, 2016). GCF LXA4 levels were similarly found to be lowest in smokers with aggressive periodontitis (Heasman et al., 1998). The ration of pro-resolving and pro-inflammatory LM has been suggested as a better marker for aggressive periodontitis (Elabdeen et al., 2013).
Saliva
Saliva diagnostics are an intuitive and comprehensive approach for oral diseases (Zhang et al., 2015), and they have recently emerged as a method for determining general health status without the invasiveness of traditional methods (Punyadeera and Slowey, 2013, Schafer et al., 2014). Metabolomic and bioinformatic approaches are bringing these applications even closer (Ai et al., 2012, Zhang et al., 2012). The development of higher sensitivity assays should accelerate the adoption of salivary diagnostics over serum (Khaitan et al., 2015). In addition to the traditional inflammatory biomarkers, salivary levels of LM can also be used for diagnostic and prognostic purposes, though they lag behind other body fluids. For example, elevated salivary LTB4 and PGE2 are correlated with arterial stiffness (Labat et al., 2013). Aspirin-intolerant asthma patients exhibit elevated Cys-LT levels in saliva (Gaber et al., 2008, Ono et al., 2011). Salivary prostaglandin levels have long been suggested to be indicators of major depression (Ohishi et al., 1988, Nishino et al., 1989).
Saliva levels of various LM are more widely used for studying oral diseases than for systemic diseases. For example, patients with radiotherapy-caused oral mucositis displayed higher saliva levels of PGD2, PGE2, and PGF2 (Grunberg et al., 2013). Similarly, a different study found elevated levels of LTA4 hydrolase in oral mucositis patients (Jehmlich et al., 2015). Elevated salivary levels of PGE2 were found to be correlated with gingivitis (Syndergaard et al., 2014, Gümüş et al., 2016) and periodontitis (Sánchez et al., 2013b). In chronic periodontitis patients, salivary LTB4 levels were to found to correlate with the severity of alveolar bone loss (Sánchez et al., 2013a). Ratios of pro-resolving LM to pro-inflammatory LM that were found to be correlated with aggressive periodontitis in GCF were not found to be similarly correlated in saliva (Elabdeen et al., 2013). No studies to date have examined salivary levels of pro-resolving LM, such as lipoxins and resolvins, for diagnostic studies of oral disease.
One interesting aspect of saliva diagnostics is the potential to explain the etiology of various diseases. Some diseases display marked gender differences, notably SS, which affects older females primarily. Differences in LM levels have been observed between males and females in multiple diseases, such as diabetes (Tessaro et al., 2015), COPD (Balgoma et al., 2016), Alzheimer’s (Pomponi et al., 2011), and metabolic syndrome (Yu et al., 2015).
To test this hypothesis in saliva, we investigated differences in salivary LM levels between healthy male and female donors. For this previously unpublished study, we recruited patients and students at the University of Utah School of Dentistry student clinics, who were between 18 and 40 years old and had no history or visible signs of gingivitis or periodontitis (11 females and 19 males). All human specimen usage was conducted under the strict guidelines and approval of the University of Utah Health Sciences Institutional Review Board, and informed consent was obtained for each patient (IRB approval: IRB_00081821 on October 29th 2015). Donor demographic information is shown in Table 1. Unstimulated whole saliva was collected by having subjects spit into a sterile 50 mL conical tube for five minutes after a brief mouth rinse with water, then transported to the laboratory on ice. We opted for the spitting method over cotton-based methods due to the potential of interference with immunoassay results (Shirtcliff et al., 2001). Samples were centrifuged at 5000 × g for 10 minutes at 4°C to pellet out debris, and the supernatant was collected and broken up via trituration with a 19G hypodermic needle to reduce its viscosity. Saliva was consecutively filtered through a 40 μm and a 0.2 μm filter to remove debris and bacteria, respectively. Enzyme-linked immunosorbent assays (ELISA) were performed immediately for the following LM: LTB4, PGD2, PGE2, PGF2α, TXB2, RvD1, and RvD2 (Cayman Chemical, Ann Arbor, MI). Measured LM concentrations (mean ± standard deviation) are shown in Table 2.
Table 1.
Saliva donor demographics
| Male | Female | |
|---|---|---|
| N | 19 | 11 |
| Age (years, mean ± standard deviation) | 27.6 ± 3.3 | 27.8 ± 3.1 |
Table 2.
LM concentrations pg/mL (mean ± standard deviation)
| Male | Female | Results | |
|---|---|---|---|
| LTB4 | 786 ± 654.7 | 666 ± 745.2 | n.s. |
| PGD2 | 1393 ± 696.1 | 1505 ± 866.9 | n.s. |
| PGE2 | 843.2 ± 901.7 | 433.9 ± 448.3 | n.s. |
| PGF2α | 306.2 ± 235.6 | 174.1 ± 116 | P = 0.026 |
| TXB2 | 557 ± 502.9 | 471.6 ± 296.2 | n.s. |
| RvD1 | 297.9 ± 189.3 | 409 ± 286.3 | n.s. |
| RvD2 | 93.98 ± 45.37 | 179.8 ± 92.15 | P = 0.041 |
n.s.= non-significant
Using this method, we show that we can detect salivary levels of LTB4, PGD2, PGE2, PGF2α, TXB2, RvD1, and RvD2. We observed significantly lower PGF2α in female saliva as compared to male saliva. As indicated above, PGF2α plays a role in many female functions such as reproduction and menstrual cycle (Wilks et al., 1973, Downie et al., 1974); therefore, we could speculate that females demand and use this lipid mediator for more functions than males, thereby levels in saliva are decreased. Additionally, we observed that saliva levels of RvD2 from male subjects were significantly lower than female subjects. This finding raises interesting questions regarding the etiology of some inflammatory diseases that skew females, such as SS. One hypothesis that needs further investigation is that higher levels of pro-resolving LM, such as RvD2, might indicate the presence of inflammatory assaults that the body is attempting to resolve over time, leading to persistent chronic inflammation. More research needs to be conducted to confirm such a hypothesis, including comparing saliva LM levels of SS patients to healthy subjects, and investigating whether gender-related differences that are observed in healthy subjects disappear in SS patients.
Summary
The diverse role of LM in the development of various systemic and oral inflammatory diseases presents a challenge and an opportunity to oral health clinicians and researchers. In this review, we have presented an overview of the most important LM that control inflammation, a brief summary of biosynthesis and signaling pathways, and roles in systemic and oral health. Understanding the distinct signaling pathways and functions of LM will be crucial for designing treatment approaches and therapeutics (i.e., a clear picture of how LM profile shifts from pro-inflammatory to pro-resolving). LM influence both systemic and oral diseases, which are often interrelated, making it important to understand the impact of systemic diseases in oral health and vice versa. Additionally, lipid mediators will be useful as diagnostic tools. Particularly, lipidomic profiling should be included in the current repertoire of diagnostic and prognostic indicators of both systemic and oral health. Finally, saliva LM mediator levels could be an additional tool to study the etiology, development, and treatment for a variety oral diseases, including periodontitis, SS, and oral cancer.
Acknowledgments
This work was supported by the NIH-NIDCR grants R01DE022971, R01DE021697 to OJB. The authors would like to thank Mr. Hani Sommakia for assistance with figures.
Footnotes
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- ABDOU MMS, EFFAT DA, MANSOUR LA, EL BAKY NMA, SALAM MMA. Urinary lipoxin A4 as a biomarker for systemic lupus erythematosus. Egyptian Rheumatology and Rehabilitation. 2015;42:55. [Google Scholar]
- ABURIMA A, WRAITH KS, RASLAN Z, LAW R, MAGWENZI S, NASEEM KM. cAMP signaling regulates platelet myosin light chain (MLC) phosphorylation and shape change through targeting the RhoA-Rho kinase-MLC phosphatase signaling pathway. Blood. 2013;122:3533–3545. doi: 10.1182/blood-2013-03-487850. [DOI] [PubMed] [Google Scholar]
- AHMAD SF, AKOUM A, HORNE AW. Selective modulation of the prostaglandin F2α pathway markedly impacts on endometriosis progression in a xenograft mouse model. Molecular human reproduction. 2015;21:905–916. doi: 10.1093/molehr/gav056. [DOI] [PubMed] [Google Scholar]
- AI JY, SMITH B, WONG DTW. Bioinformatics advances in saliva diagnostics. In J Oral Sci. 2012;4:85–87. doi: 10.1038/ijos.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL AZZAM N, KONDETI V, DUAH E, GOMBEDZA F, THODETI CK, PARUCHURI S. Modulation of mast cell proliferative and inflammatory responses by leukotriene d4 and stem cell factor signaling interactions. Journal of cellular physiology. 2015;230:595–602. doi: 10.1002/jcp.24777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXOPOULOS E, HARITOS G, BEFANI C, MOUSAILIDIS G, LACHANAS V, LIAKOS P, GOURGOULIANIS K, KADITIS A. Serum leukotriene B4 levels, tonsillar hypertrophy and obstructive sleep-disordered breathing in childhood. European Respiratory Journal. 2015:46. doi: 10.1016/j.ijporl.2018.08.007. [DOI] [PubMed] [Google Scholar]
- ALLY AI, HORROBIN DF. Thromboxane A2 in blood vessel walls and its physiological significance: Relevance to thrombosis and hypertension. Prostaglandins and Medicine. 1980;4:431–438. doi: 10.1016/0161-4630(80)90051-8. [DOI] [PubMed] [Google Scholar]
- ANDBERG M, HAMBERG M, HAEGGSTRÖM JZ. Evidence for a Carbocation Intermediate in the Enzymatic Transformation of Leukotriene A4 into Leukotrine B4. In: HONN KENNETHV, LJM, NIGAM SANTOSH, DENNIS EDWARDA, editors. Eicosanoids and Other Bioactive Lipids in Cancer, Inflammation, and Radiation Injury. 1. Vol. 4. Springer Science & Business Media; 2013. [Google Scholar]
- ANTONOVA M, WIENECKE T, OLESEN J, ASHINA M. Prostaglandins in migraine: update. Current opinion in neurology. 2013;26:269–275. doi: 10.1097/WCO.0b013e328360864b. [DOI] [PubMed] [Google Scholar]
- ARIEL A, CHIANG N, ARITA M, PETASIS NA, SERHAN CN. Aspirin-Triggered Lipoxin A4 and B4 Analogs Block Extracellular Signal-Regulated Kinase-Dependent TNF-α Secretion from Human T Cells. The Journal of Immunology. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- ARIEL A, SERHAN CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–83. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- ARITA M, BIANCHINI F, ALIBERTI J, SHER A, CHIANG N, HONG S, YANG R, PETASIS NA, SERHAN CN. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. The Journal of Experimental Medicine. 2005a;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARITA M, OHIRA T, SUN YP, ELANGOVAN S, CHIANG N, SERHAN CN. Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol. 2007;178:3912–7. doi: 10.4049/jimmunol.178.6.3912. [DOI] [PubMed] [Google Scholar]
- ARITA M, SERHAN CN. Novel chemical mediators in the resolution of inflammation. Tanpakushitsu Kakusan Koso. 2007;52:348–54. [PubMed] [Google Scholar]
- ARITA M, YOSHIDA M, HONG S, TJONAHEN E, GLICKMAN JN, PETASIS NA, BLUMBERG RS, SERHAN CN. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005b;102:7671–6. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARNARDOTTIR H, ORR S, DALLI J, SERHAN C. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal immunology. 2015 doi: 10.1038/mi.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARONOFF DM, OATES JA, BOUTAUD O. New insights into the mechanism of action of acetaminophen: Its clinical pharmacologic characteristics reflect its inhibition of the two prostaglandin H2 synthases. Clinical Pharmacology & Therapeutics. 2006;79:9–19. doi: 10.1016/j.clpt.2005.09.009. [DOI] [PubMed] [Google Scholar]
- ASTUDILLO AM, BALGOMA D, BALBOA MA, BALSINDE J. Dynamics of arachidonic acid mobilization by inflammatory cells. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2012;1821:249–256. doi: 10.1016/j.bbalip.2011.11.006. [DOI] [PubMed] [Google Scholar]
- AYABE S, KIDA T, HORI M, OZAKI H, MURATA T. Prostaglandin D2 Inhibits Collagen Secretion From Lung Fibroblasts by Activating the DP Receptor. Journal of Pharmacological Sciences. 2013;121:312–317. doi: 10.1254/jphs.12275fp. [DOI] [PubMed] [Google Scholar]
- BÄCK M, AIRILA-MÅNSSON S, JOGESTRAND T, SÖDER B, SÖDER P-Ö. Increased leukotriene concentrations in gingival crevicular fluid from subjects with periodontal disease and atherosclerosis. Atherosclerosis. 2007;193:389–394. doi: 10.1016/j.atherosclerosis.2006.07.003. [DOI] [PubMed] [Google Scholar]
- BALGOMA D, YANG M, SJÖDIN M, SNOWDEN S, KARIMI R, LEVÄNEN B, MERIKALLIO H, KAARTEENAHO R, PALMBERG L, LARSSON K, ERLE DJ, DAHLÉN S-E, DAHLÉN B, SKÖLD CM, WHEELOCK ÅM, WHEELOCK CE. Linoleic acid-derived lipid mediators increase in a female-dominated subphenotype of COPD. European Respiratory Journal. 2016 doi: 10.1183/13993003.01080-2015. [DOI] [PubMed] [Google Scholar]
- BANNENBERG G, ARITA M, SERHAN CN. Endogenous receptor agonists: resolving inflammation. ScientificWorldJournal. 2007;7:1440–62. doi: 10.1100/tsw.2007.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNIG C, CERNADAS M, DUTILE S, LIU X, PERRELLA MA, KAZANI S, WECHSLER ME, ISRAEL E, LEVY BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Science translational medicine. 2013;5:174ra26–174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAROUKH B, SAFFAR J. The effect of leukotriene synthesis inhibitors on hamster periodontitis. Archives of oral biology. 1990;35:S189–S192. doi: 10.1016/0003-9969(90)90155-4. [DOI] [PubMed] [Google Scholar]
- BAROUKH B, SAFFAR J. Identification of osteoclasts and their mononuclear precursors. A comparative histological and histochemical study in hamster periodontitis. Journal of periodontal research. 1991;26:161–166. doi: 10.1111/j.1600-0765.1991.tb01640.x. [DOI] [PubMed] [Google Scholar]
- BAROUKH B, SAFFAR J. Leukotriene inhibition in hamster periodontitis. A histochemical and morphometric study. Mediators of inflammation. 1992;1:335–339. doi: 10.1155/S0962935192000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASIL MC, LEVY BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nature Reviews Immunology. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAZINET RP, LAYÉ S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nature Reviews Neuroscience. 2014;15:771–785. doi: 10.1038/nrn3820. [DOI] [PubMed] [Google Scholar]
- BERAIR R, GONEM S, SINGAPURI A, HARTLEY R, LAURENCIN M, BACHER G, HOLZHAUER B, BOURNE M, MISTRY V, PAVORD I. LATE-BREAKING ABSTRACT: Effect of QAW039, an oral prostaglandin D2 receptor (DP2/CRTh2) antagonist, upon bronchial epithelial integrity in treatment-resistant asthma in a randomized, placebo controlled study. European Respiratory Journal. 2015;46:OA290. [Google Scholar]
- BINDA C, GÉNIER S, CARTIER A, LARRIVÉE JF, STANKOVA J, YOUNG JC, PARENT JL. AG protein–coupled receptor and the intracellular synthase of its agonist functionally cooperate. The Journal of cell biology. 2014;204:377–393. doi: 10.1083/jcb.201304015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOIE Y, SAWYER N, SLIPETZ DM, METTERS KM, ABRAMOVITZ M. Molecular cloning and characterization of the human prostanoid DP receptor. Journal of Biological Chemistry. 1995;270:18910–18916. doi: 10.1074/jbc.270.32.18910. [DOI] [PubMed] [Google Scholar]
- BÖRGESON E, MCGILLICUDDY FC, HARFORD KA, CORRIGAN N, HIGGINS DF, MADERNA P, ROCHE HM, GODSON C. Lipoxin A4 attenuates adipose inflammation. The FASEB Journal. 2012;26:4287–4294. doi: 10.1096/fj.12-208249. [DOI] [PubMed] [Google Scholar]
- BOULAY F, TARDIF M, BROUCHON L, VIGNAIS P. Synthesis and use of a novel N-formyl peptide derivative to isolate a human N-formyl peptide receptor cDNA. Biochemical and Biophysical Research Communications. 1990;168:1103–1109. doi: 10.1016/0006-291x(90)91143-g. [DOI] [PubMed] [Google Scholar]
- BOZINOVSKI S, UDDIN M, VLAHOS R, THOMPSON M, MCQUALTER JL, MERRITT AS, WARK PA, HUTCHINSON A, IRVING LB, LEVY BD. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proceedings of the National Academy of Sciences. 2012;109:935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRUN C, DAALI Y, COMBESCURE C, ZUFFEREY A, MICHELSON AD, FONTANA P, RENY JL, FRELINGER AL. Aspirin response: Differences in serum thromboxane B2 levels between clinical studies. Platelets. 2016;27:196–202. doi: 10.3109/09537104.2015.1072147. [DOI] [PubMed] [Google Scholar]
- BUCCI M. Channels: Dual eicosanoid activity. Nat Chem Biol. 2014;10:87–87. [Google Scholar]
- BUCKLEY CD, GILROY DW, SERHAN CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUCKLEY CD, GILROY DW, SERHAN CN, STOCKINGER B, TAK PP. The resolution of inflammation. Nature Reviews Immunology. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- BUSCH L, MIOZZA V, STERIN-BORDA L, BORDA E. Increased leukotriene concentration× in submandibular glands from rats with experimental periodontitis. Inflammation Research. 2009;58:423–430. doi: 10.1007/s00011-009-0008-8. [DOI] [PubMed] [Google Scholar]
- CALDER PC. Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? British journal of clinical pharmacology. 2013;75:645–662. doi: 10.1111/j.1365-2125.2012.04374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMARGO GADCG, DOS SANTOS MP, SILVA NLC, DE MIRANDA ALP, TRIBUTINO JLM. Levels of prostaglandin E2 (PGE2) in gingival crevicular fluid from smokers and non-smokers with gingivitis and chronic periodontal disease. Journal of Dentistry and Oral Hygiene. 2015a;7:54–59. [Google Scholar]
- CAMARGO GADCG, FELLOWS JEM, POIATE IA, POLA EV, DE AGUIAR RIBEIRO A, DIP EC, SILVA NLC, DE MIRANDA AL, PALHARES I, TRIBUTINO JLM. Lidocaine subgingival irrigation modulates the levels of prostaglandin E2 levels in gingival crevicular fluid after periodontal therapy. Journal of Dentistry and Oral Hygiene. 2015b;7:190–196. [Google Scholar]
- CAMPBELL EL, LOUIS NA, TOMASSETTI SE, CANNY GO, ARITA M, SERHAN CN, COLGAN SP. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–70. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- CAPRA V, BÄCK M, ANGIOLILLO D, CATTANEO M, SAKARIASSEN K. Impact of vascular thromboxane prostanoid receptor activation on hemostasis, thrombosis, oxidative stress, and inflammation. Journal of Thrombosis and Haemostasis. 2014;12:126–137. doi: 10.1111/jth.12472. [DOI] [PubMed] [Google Scholar]
- CAPRA V, ROVATI GE, MANGANO P, BUCCELLATI C, MURPHY RC, SALA A. Transcellular biosynthesis of eicosanoid lipid mediators. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2015;1851:377–382. doi: 10.1016/j.bbalip.2014.09.002. [DOI] [PubMed] [Google Scholar]
- CEDERLUND A, KAI-LARSEN Y, PRINTZ G, YOSHIO H, ALVELIUS G, LAGERCRANTZ H, STRÖMBERG R, JÖRNVALL H, GUDMUNDSSON GH, AGERBERTH B. Lactose in human breast milk an inducer of innate immunity with implications for a role in intestinal homeostasis. PLoS One. 2013;8:e53876. doi: 10.1371/journal.pone.0053876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CELIK D, DORUK S, INONU KOSEOGLU H, SAHIN S, CELIKEL S, ERKORKMAZ U. Cysteinyl leukotrienes in exhaled breath condensate of smoking asthmatics. Clinical Chemistry and Laboratory Medicine. 2013 doi: 10.1515/cclm-2012-0479. [DOI] [PubMed] [Google Scholar]
- CHEE B, PARK B, FITZSIMMONS T, COATES A, BARTOLD P. Omega-3 fatty acids as an adjunct for periodontal therapy—a review. Clinical oral investigations. 2016:1–16. doi: 10.1007/s00784-016-1750-2. [DOI] [PubMed] [Google Scholar]
- CHEN L, YANG G, GROSSER T. Prostanoids and inflammatory pain. Prostaglandins & other lipid mediators. 2013a;104:58–66. doi: 10.1016/j.prostaglandins.2012.08.006. [DOI] [PubMed] [Google Scholar]
- CHEN XQ, WU SH, ZHOU Y, TANG YR. Lipoxin A 4-induced heme oxygenase-1 protects cardiomyocytes against hypoxia/reoxygenation injury via p38 MAPK activation and Nrf2/ARE complex. PloS one. 2013b;8:e67120. doi: 10.1371/journal.pone.0067120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN Z, WU Z, HUANG C, ZHAO Y, ZHOU Y, ZHOU X, LU X, MAO L, LI S. Effect of Lipoxin A4 on Myocardial Ischemia Reperfusion Injury Following Cardiac Arrest in a Rabbit Model. Inflammation. 2013c;36:468–475. doi: 10.1007/s10753-012-9567-x. [DOI] [PubMed] [Google Scholar]
- CHIANG N, ARITA M, SERHAN CN. Anti-inflammatory circuitry: lipoxin, aspirin-triggered lipoxins and their receptor ALX. Prostaglandins Leukot Essent Fatty Acids. 2005:73. doi: 10.1016/j.plefa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- CHIANG N, DALLI J, COLAS RA, SERHAN CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. The Journal of Experimental Medicine. 2015;212:1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIANG N, SERHAN CN, DAHLEN SE, DRAZEN JM, HAY DW, ROVATI GE, SHIMIZU T, YOKOMIZO T, BRINK C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–87. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- CHINTHAMANI S, ODUSANWO O, MONDAL N, NELSON J, NEELAMEGHAM S, BAKER OJ. Lipoxin A4 inhibits immune cell binding to salivary epithelium and vascular endothelium. American Journal of Physiology - Cell Physiology. 2012;302:C968–C978. doi: 10.1152/ajpcell.00259.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHO C, NGUYEN A, BRYANT KJ, O’NEILL SG, MCNEIL HP. Prostaglandin D2 metabolites as a biomarker of in vivo mast cell activation in systemic mastocytosis and rheumatoid arthritis. Immunity, Inflammation and Disease. 2015 doi: 10.1002/iid3.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIANCI E, RECCHIUTI A, TRUBIANI O, DIOMEDE F, MARCHISIO M, MISCIA S, COLAS RA, DALLI J, SERHAN CN, ROMANO M. Human Periodontal Stem Cells Release Specialized Proresolving Mediators and Carry Immunomodulatory and Prohealing Properties Regulated by Lipoxins. Stem Cells Translational Medicine. 2016;5:20–32. doi: 10.5966/sctm.2015-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLUSSI G, CATENA C, SECHI L. ω-3 Polyunsaturated Fatty Acids Effects on the Cardiometabolic Syndrome and their Role in Cardiovascular Disease Prevention: An Update from the Recent Literature. Recent patents on cardiovascular drug discovery. 2014;9:78–96. doi: 10.2174/1574890110666150724115111. [DOI] [PubMed] [Google Scholar]
- COSTA SILVA B, SILVA DE MIRANDA A, GUIMARAES RODRIGUES F, LETICIA MALHEIROS SILVEIRA A, HENRIQUE DE SOUZA RESENDE G, FLAVIO DUTRA MORAES M, CARLOS PINHEIRO DE OLIVEIRA A, MARTINS PARREIRAS P, DA SILVA BARCELOS L, MARTINS TEIXEIRA M. The 5-lipoxygenase (5-LOX) inhibitor zileuton reduces inflammation and infarct size with improvement in neurological outcome following cerebral ischemia. Current neurovascular research. 2015;12:398–403. doi: 10.2174/1567202612666150812150606. [DOI] [PubMed] [Google Scholar]
- CRUPI R, MARINO A, CUZZOCREA S. n−3 fatty acids: role in neurogenesis and neuroplasticity. Current medicinal chemistry. 2013;20:2953–2963. doi: 10.2174/09298673113209990140. [DOI] [PubMed] [Google Scholar]
- CUI Y, SHU XO, GAO YT, CAI Q, JI BT, LI HL, ROTHMAN N, WU J, YANG G, XIANG YB. Urinary prostaglandin e2 metabolite and breast cancer risk. Cancer Epidemiology Biomarkers & Prevention. 2014;23:2866–2873. doi: 10.1158/1055-9965.EPI-14-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLI J, RAMON S, NORRIS PC, COLAS RA, SERHAN CN. Novel proresolving and tissue-regenerative resolvin and protectin sulfido-conjugated pathways. The FASEB Journal. 2015;29:2120–2136. doi: 10.1096/fj.14-268441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLI J, WINKLER JEREMYW, COLAS ROMAINA, ARNARDOTTIR H, CHENG C-YEEC, CHIANG N, PETASIS NICOSA, SERHAN CHARLESN. Resolvin D3 and Aspirin-Triggered Resolvin D3 Are Potent Immunoresolvents. Chemistry & Biology. 2013;20:188–201. doi: 10.1016/j.chembiol.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMGAARD C, KANTARCI A, HOLMSTRUP P, HASTURK H, NIELSEN CH, VAN DYKE TE. Porphyromonas gingivalis-induced production of reactive oxygen species, tumor necrosis factor-α, interleukin-6, CXCL8 and CCL2 by neutrophils from localized aggressive periodontitis and healthy donors: modulating actions of red blood cells and resolvin E1. Journal of Periodontal Research. 2016 doi: 10.1111/jre.12388. n/a-n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAMLAR İ, ESEN E, TATLI U. Effects of glucosamine-chondroitin combination on synovial fluid IL-1β, IL-6, TNF-α and PGE2 levels in internal derangements of temporomandibular joint. Medicina Oral, Patologia Oral y Cirugia Bucal. 2015;20:e278–e283. doi: 10.4317/medoral.20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL TO, LIU H, MORROW JD, CREWS BC, MARNETT LJ. Thromboxane A2 Is a Mediator of Cyclooxygenase-2-dependent Endothelial Migration and Angiogenesis. Cancer Research. 1999;59:4574–4577. [PubMed] [Google Scholar]
- DAS UN. Inflammatory bowel disease as a disorder of an imbalance between pro-and anti-inflammatory molecules and deficiency of resolution bioactive lipids. Lipids in health and disease. 2016;15:1. doi: 10.1186/s12944-015-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVENPORT JR, CAI Q, NESS RM, MILNE G, ZHAO Z, SMALLEY WE, ZHENG W, SHRUBSOLE MJ. Evaluation of pro-inflammatory markers plasma C-reactive protein and urinary prostaglandin-E2 metabolite in colorectal adenoma risk. Molecular carcinogenesis. 2015 doi: 10.1002/mc.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVOINE F, SIM A, TANG C, FISHER S, ETHIER C, PUTTAGUNTA L, WU Y, MCGAW WT, YU D, CAMERON L. Eosinophils in human oral squamous carcinoma; role of prostaglandin D2. Journal of Inflammation. 2013;10:1. doi: 10.1186/1476-9255-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE RENSIS F, SALERI R, TUMMARUK P, TECHAKUMPHU M, KIRKWOOD R. Prostaglandin F2α and control of reproduction in female swine: a review. Theriogenology. 2012;77:1–11. doi: 10.1016/j.theriogenology.2011.07.035. [DOI] [PubMed] [Google Scholar]
- DEORE GD, GURAV AN, PATIL R, SHETE AR, NAIKTARI RS, INAMDAR SP. Omega 3 fatty acids as a host modulator in chronic periodontitis patients: a randomised, double-blind, palcebo-controlled, clinical trial. Journal of periodontal & implant science. 2014;44:25–32. doi: 10.5051/jpis.2014.44.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DESSÌ M, NOCE A, BERTUCCI P, MANCA DI VILLAHERMOSA S, ZENOBI R, CASTAGNOLA V, ADDESSI E, DI DANIELE N. Atherosclerosis, dyslipidemia, and inflammation: the significant role of polyunsaturated fatty acids. ISRN inflammation. 2013;2013 doi: 10.1155/2013/191823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAZ JA, SHAYDAKOV ME, CHATTERJEE A, FARRIS DM, BALLARD-LIPKA NE, HAWLEY AE, SIGLER RE, HENKE PK, MYERS DD, WAKEFIELD TW. Resolvin D2 Reduces Thrombus Burden and Attenuates Inflammatory Signaling Pathways in a Murine Model of Venous Thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2015;35:A24–A24. [Google Scholar]
- DIETRICH T, SHARMA P, WALTER C, WESTON P, BECK J. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. Journal of clinical periodontology. 2013:40. doi: 10.1111/jcpe.12062. [DOI] [PubMed] [Google Scholar]
- DIXON RAF, DIEHL RE, OPAS E, RANDS E, VICKERS PJ, EVANS JF, GILLARD JW, MILLER DK. Requirement of a 5-lipoxygenase-activating protein for leukotriene synthesis. Nature. 1990;343:282–284. doi: 10.1038/343282a0. [DOI] [PubMed] [Google Scholar]
- DMITRIEVA N, SUESS G, SHIRLEY R. Resolvins RvD1 and 17(R)-RvD1 alleviate signs of inflammation in a rat model of endometriosis. Fertility and Sterility. 2014;102:1191–1196. doi: 10.1016/j.fertnstert.2014.06.046. [DOI] [PubMed] [Google Scholar]
- DOĞAN B, FENTOĞLU Ö, KıRZıOĞLU FY, KEMER ES, KÖROĞLU BK, AKSU O, ÇARSANCAKLı SA, ORHAN H. Lipoxin A(4) and Neutrophil/Lymphocyte Ratio: A Possible Indicator in Achieved Systemic Risk Factors for Periodontitis. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research. 2015;21:2485–2493. doi: 10.12659/MSM.895115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONDONI L, SCARPARO RK, KANTARCI A, VAN DYKE TE, FIGUEIREDO JAP, BATISTA EL. Effect of the pro-resolution lipid mediator Resolvin E1 (RvE1) on pulp tissues exposed to the oral environment. International Endodontic Journal. 2014;47:827–834. doi: 10.1111/iej.12224. [DOI] [PubMed] [Google Scholar]
- DONG R, XIE L, ZHAO K, ZHANG Q, ZHOU M, HE P. Cigarette smoke-induced lung inflammation in COPD mediated via LTB4/BLT1/SOCS1 pathway. International Journal of Chronic Obstructive Pulmonary Disease. 2016;11:31–41. doi: 10.2147/COPD.S96412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DORWARD DA, LUCAS CD, CHAPMAN GB, HASLETT C, DHALIWAL K, ROSSI AG. The Role of Formylated Peptides and Formyl Peptide Receptor 1 in Governing Neutrophil Function during Acute Inflammation. The American Journal of Pathology. 2015;185:1172–1184. doi: 10.1016/j.ajpath.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWNIE J, POYSER N, WUNDERLICH M. Levels of prostaglandins in human endometrium during the normal menstrual cycle. The Journal of physiology. 1974;236:465–472. doi: 10.1113/jphysiol.1974.sp010446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DROZDOVSZKY O, BARTA I, ANTUS B. Assessment of sputum prostaglandins and leukotrienes in COPD exacerbations. European Respiratory Journal. 2013:42. [Google Scholar]
- DUFFIELD JS, HONG S, VAIDYA VS, LU Y, FREDMAN G, SERHAN CN, BONVENTRE JV. Resolvin D Series and Protectin D1 Mitigate Acute Kidney Injury. The Journal of Immunology. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- DUNN HC, AGER RR, BAGLIETTO-VARGAS D, CHENG D, KITAZAWA M, CRIBBS DH, MEDEIROS R. Restoration of lipoxin A4 signaling reduces Alzheimer’s disease-like pathology in the 3xTg-AD mouse model. Journal of Alzheimer’s Disease. 2015;43:893–903. doi: 10.3233/JAD-141335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EASLEY JT, NELSON JW, MELLAS RE, SOMMAKIA S, WU C, TRUMP B, BAKER OJ. Aspirin-Triggered Resolvin D1 Versus Dexamethasone in the Treatment of Sjogren’s Syndrome-Like NOD/ShiLtJ Mice - A Pilot Study. J Rheum Dis Treat. 2015:1. doi: 10.23937/2469-5726/1510027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDFELDT F, EVENÄS J, LEPISTÖ M, WARD A, PETERSEN J, WISSLER L, ROHMAN M, SIVARS U, SVENSSON K, PERRY M. Identification of indole inhibitors of human hematopoietic prostaglandin D 2 synthase (hH-PGDS) Bioorganic & medicinal chemistry letters. 2015;25:2496–2500. doi: 10.1016/j.bmcl.2015.04.065. [DOI] [PubMed] [Google Scholar]
- EICKMEIER O, SEKI H, HAWORTH O, HILBERATH JN, GAO F, UDDIN M, CROZE RH, CARLO T, PFEFFER MA, LEVY BD. Aspirin-triggered resolvin D1 reduces mucosal inflammation and promotes resolution in a murine model of acute lung injury. Mucosal Immunol. 2013;6:256–266. doi: 10.1038/mi.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EKE GUNGOR H, TAHAN F, GOKAHMETOGLU S, SARAYMEN B. Decreased Levels of Lipoxin A4 and Annexin A1 in Wheezy Infants. International Archives of Allergy and Immunology. 2014;163:193–197. doi: 10.1159/000358490. [DOI] [PubMed] [Google Scholar]
- EL-SHARKAWY H, ABOELSAAD N, ELIWA M, DARWEESH M, ALSHAHAT M, KANTARCI A, HASTURK H, VAN DYKE TE. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. Journal of periodontology. 2010;81:1635–1643. doi: 10.1902/jop.2010.090628. [DOI] [PubMed] [Google Scholar]
- EL ATTAR TMA, LIN HS. Relative conversion of arachidonic acid through lipoxygenase and cyclooxygenase pathways by homogenates of diseased periodontal tissues. Journal of Oral Pathology & Medicine. 1983;12:7–10. doi: 10.1111/j.1600-0714.1983.tb00311.x. [DOI] [PubMed] [Google Scholar]
- ELABDEEN HRZ, MUSTAFA M, SZKLENAR M, RÜHL R, ALI R, BOLSTAD AI. Ratio of pro-resolving and pro-inflammatory lipid mediator precursors as potential markers for aggressive periodontitis. PloS one. 2013;8:e70838. doi: 10.1371/journal.pone.0070838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMINGIL G, ÇINARCIK S, BAYLAS H, ÇOKER I, HÜSEYINOV A. Levels of leukotriene B4 in gingival crevicular fluid and gingival tissue in specific periodontal diseases. Journal of periodontology. 2001;72:1025–1031. doi: 10.1902/jop.2001.72.8.1025. [DOI] [PubMed] [Google Scholar]
- EVANS JF, ISLAM S, URADE Y, EGUCHI N, RAGOLIA L. The lipocalin-type prostaglandin D2 synthase knockout mouse model of insulin resistance and obesity demonstrates early hypothalamic–pituitary–adrenal axis hyperactivity. Journal of Endocrinology. 2013;216:169–180. doi: 10.1530/JOE-12-0275. [DOI] [PubMed] [Google Scholar]
- FILEP JG, ZOUKI C, PETASIS NA, HACHICHA M, SERHAN CN. Anti-inflammatory actions of lipoxin A(4) stable analogs are demonstrable in human whole blood: modulation of leukocyte adhesion molecules and inhibition of neutrophil-endothelial interactions. Blood. 1999;94:4132–42. [PubMed] [Google Scholar]
- FIORE S, MADDOX JF, PEREZ HD, SERHAN CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180:253–60. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZGERALD GA. Mechanisms of platelet activation: thromboxane A 2 as an amplifying signal for other agonists. The American journal of cardiology. 1991;68:B11–B15. doi: 10.1016/0002-9149(91)90379-y. [DOI] [PubMed] [Google Scholar]
- FREDMAN G, OH SF, AYILAVARAPU S, HASTURK H, SERHAN CN, VAN DYKE TE. Impaired Phagocytosis in Localized Aggressive Periodontitis: Rescue by Resolvin E1. PLoS ONE. 2011;6:e24422. doi: 10.1371/journal.pone.0024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJINO H, SALVI S, REGAN JW. Differential regulation of phosphorylation of the cAMP response element-binding protein after activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Molecular pharmacology. 2005;68:251–259. doi: 10.1124/mol.105.011833. [DOI] [PubMed] [Google Scholar]
- FUJINO H, WEST KA, REGAN JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. Journal of Biological Chemistry. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- FUJINO H, XU W, REGAN JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. Journal of Biological Chemistry. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- FUKUDA M, AOKI T, MANABE T, MAEKAWA A, SHIRAKAWA T, KATAOKA H, TAKAGI Y, MIYAMOTO S, NARUMIYA S. Exacerbation of Intracranial Aneurysm and Aortic Dissection in Hypertensive Rat Treated With the Prostaglandin F–Receptor Antagonist AS604872. Journal of Pharmacological Sciences. 2014;126:230–242. doi: 10.1254/jphs.14148fp. [DOI] [PubMed] [Google Scholar]
- FUKUOKA T, YASHIRO M, TAKEDA H, MARUYAMA T, KINOSHITA H, MORISAKI T, HASEGAWA T, HIRAKAWA T, AOMATSU N, SAKURAI K. Antitumor effect of prostaglandin D2 by peroxisome proliferator-activated receptor gamma (PPARγ)-dependent pathway in gastric carcinoma. Cancer Research. 2013;73:3995–3995. [Google Scholar]
- GABER F, DAHAM K, HIGASHI A, HIGASHI N, GÜLICH A, DELIN I, JAMES A, SKEDINGER M, GYLLFORS P, NORD M. Increased levels of cysteinyl-leukotrienes in saliva, induced sputum, urine and blood from patients with aspirin-intolerant asthma. Thorax. 2008;63:1076–1082. doi: 10.1136/thx.2008.101196. [DOI] [PubMed] [Google Scholar]
- GAGLIARDO R, GRAS D, LA GRUTTA S, CHANEZ P, DI SANO C, ALBANO GD, VACHIER I, MONTALBANO AM, ANZALONE G, BONANNO A, RICCOBONO L, GJOMARKAJ M, PROFITA M. Airway lipoxin A4/formyl peptide receptor 2–lipoxin receptor levels in pediatric patients with severe asthma. Journal of Allergy and Clinical Immunology. 2016 doi: 10.1016/j.jaci.2015.11.045. [DOI] [PubMed] [Google Scholar]
- GALLANT MA, CHAMOUX E, BISSON M, WOLSEN C, PARENT JL, ROUX S, DE BRUM-FERNANDES AJ. Increased concentrations of prostaglandin D2 during post-fracture bone remodeling. The Journal of rheumatology. 2010;37:644–649. doi: 10.3899/jrheum.090622. [DOI] [PubMed] [Google Scholar]
- GAO F, DALLI J, CLARIA J, SERHAN CN. E. coli peritonitis initiates D-series Resolvin-metabolome. The FASEB Journal. 2013;27:822–9. [Google Scholar]
- GAO JL, BECKER EL, FREER RJ, MUTHUKUMARASWAMY N, MURPHY PM. A high potency nonformylated peptide agonist for the phagocyte N-formylpeptide chemotactic receptor. The Journal of Experimental Medicine. 1994;180:2191–2197. doi: 10.1084/jem.180.6.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARZA LA, LIU Y, YANG Z, ALAGESAN B, LAWSON JA, NORBERG SM, LOY DE, ZHAO T, BLATT HB, STANTON DC. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Science translational medicine. 2012;4:126ra34–126ra34. doi: 10.1126/scitranslmed.3003122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIERA M, IOAN-FACSINAY A, TOES R, GAO F, DALLI J, DEELDER AM, SERHAN CN, MAYBORODA OA. Lipid and lipid mediator profiling of human synovial fluid in rheumatoid arthritis patients by means of LC–MS/MS. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids. 2012;1821:1415–1424. doi: 10.1016/j.bbalip.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILROY D, LAWRENCE T. The resolution of acute inflammation: A ‘tipping point’ in the development of chronic inflammatory diseases. In: ROSSI ADRIANOG, DAS, editors. The resolution of inflammation. Springer; 2008. [Google Scholar]
- GINHOUX F, JUNG S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nature Reviews Immunology. 2014;14:392–404. doi: 10.1038/nri3671. [DOI] [PubMed] [Google Scholar]
- GOETZL L. Methods of cervical ripening and labor induction: pharmacologic. Clinical obstetrics and gynecology. 2014;57:377–390. doi: 10.1097/GRF.0000000000000024. [DOI] [PubMed] [Google Scholar]
- GOUNARIS E, HEIFERMAN MJ, HEIFERMAN JR, SHRIVASTAV M, VITELLO D, BLATNER NR, KNAB LM, PHILLIPS JD, CHEON EC, GRIPPO PJ. Zileuton, 5-lipoxygenase inhibitor, acts as a chemopreventive agent in intestinal polyposis, by modulating polyp and systemic inflammation. PloS one. 2015;10:e0121402. doi: 10.1371/journal.pone.0121402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOYENS PL, SPILKER ME, ZOCK PL, KATAN MB, MENSINK RP. Compartmental modeling to quantify α-linolenic acid conversion after longer term intake of multiple tracer boluses. Journal of lipid research. 2005;46:1474–1483. doi: 10.1194/jlr.M400514-JLR200. [DOI] [PubMed] [Google Scholar]
- GRUNBERG SM, VITHALA MV, VIZZARD M, ADAMS NB, GAGNE HM, BRUNDAGE W, ASHIKAGA T. Role of prostaglandin pathways in radiotherapy-induced mucositis. ASCO Annual Meeting Proceedings. 2013:e20509. [Google Scholar]
- GUERRERO JA, LOZANO ML, CASTILLO J, BENAVENTE-GARCÍA O, VICENTE V, RIVERA J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. Journal of Thrombosis and Haemostasis. 2005;3:369–376. doi: 10.1111/j.1538-7836.2004.01099.x. [DOI] [PubMed] [Google Scholar]
- GUILHERME RF, XISTO DG, KUNKEL SL, FREIRE-DE-LIMA CG, ROCCO PRM, NEVES JS, FIERRO IM, CANETTI C, BENJAMIM CF. Pulmonary Antifibrotic Mechanisms Aspirin-Triggered Lipoxin A4 Synthetic Analog. American Journal of Respiratory Cell and Molecular Biology. 2013;49:1029–1037. doi: 10.1165/rcmb.2012-0462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GÜMÜŞ P, ÖZTÜRK VÖ, BOZKURT E, EMINGIL G. Evaluation of the gingival inflammation in pregnancy and postpartum via 25-hydroxy-vitamin D3, prostaglandin E2 and TNF-α levels in saliva. Archives of Oral Biology. 2016;63:1–6. doi: 10.1016/j.archoralbio.2015.11.018. [DOI] [PubMed] [Google Scholar]
- GUPTA G. Gingival crevicular fluid as a periodontal diagnostic indicator-II: inflammatory mediators, host-response modifiers and chair side diagnostic aids. Journal of medicine and life. 2013;6:7. [PMC free article] [PubMed] [Google Scholar]
- GWEBU ET. Antioxidants and the inhibition of lypoxygenase and cyclooxygenase enzyme systems 1979 [Google Scholar]
- GYURKO R, VAN DYKE TE. The Role of Polyunsaturated ω-3 Fatty Acid Eicosapentaenoic Acid−Derived Resolvin E1 (RvE1) in Bone Preservation. 2014;34:347–357. doi: 10.1615/critrevimmunol.2014009982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABIB AD, FITZGERALD GA, MACLOUF J. Phosphorylation of the thromboxane receptor α, the predominant isoform expressed in human platelets. Journal of Biological Chemistry. 1999;274:2645–2651. doi: 10.1074/jbc.274.5.2645. [DOI] [PubMed] [Google Scholar]
- HAGAN JB, LAIDLAW TM, DIVEKAR RD, O’BRIEN E, KITA H, VOLCHECK GW, HAGAN CR, LAL D, TEAFORD HG, ERWIN PJ. The Diagnostic Testing Accuracy of Urinary Leukotriene E4 in Determining Aspirin Intolerance in Asthma: A Systematic Review and Meta-Analysis. Journal of Allergy and Clinical Immunology. 2016;137:AB390. doi: 10.1016/j.jaip.2016.11.004. [DOI] [PubMed] [Google Scholar]
- HAJIME Y, OKUBO K. Phase II Clinical Trial of ONO-4053, a Novel DP1 Antagonist, in Patients with Seasonal Allergic Rhinitis. Journal of Allergy and Clinical Immunology. 2016;137:AB160. doi: 10.1111/all.13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALUSHKA PV. Thromboxane A2 and TP receptors: A trail of research, well traveled. Journal of the South Carolina Academy of Science. 2016;14:3. [Google Scholar]
- HAMBERG M, SVENSSON J, SAMUELSSON B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proceedings of the National Academy of Sciences. 1975;72:2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMAD H, KOOL M, SOULLIÉ T, NARUMIYA S, TROTTEIN F, HOOGSTEDEN HC, LAMBRECHT BN. Activation of the D prostanoid 1 receptor suppresses asthma by modulation of lung dendritic cell function and induction of regulatory T cells. The Journal of experimental medicine. 2007;204:357–367. doi: 10.1084/jem.20061196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO M, MAEKAWA M, KATAKURA M, HAMAZAKI K, MATSUOKA Y. Possibility of polyunsaturated fatty acids for the prevention and treatment of neuropsychiatric illnesses. Journal of pharmacological sciences. 2014;124:294–300. doi: 10.1254/jphs.13r14cp. [DOI] [PubMed] [Google Scholar]
- HASTURK H, ABDALLAH R, KANTARCI A, NGUYEN D, GIORDANO N, HAMILTON J, VAN DYKE TE. Resolvin E1 (RvE1) attenuates atherosclerotic plaque formation in diet and inflammation-induced atherogenesis. Arteriosclerosis, thrombosis, and vascular biology. 2015;35:1123–1133. doi: 10.1161/ATVBAHA.115.305324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTURK H, KANTARCI A. Biological Mechanisms Relating Periodontitis and Diabetes. Current Oral Health Reports. 2016:1–11. [Google Scholar]
- HASTURK H, KANTARCI A, GOGUET-SURMENIAN E, BLACKWOOD A, ANDRY C, SERHAN CN, VAN DYKE TE. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–9. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- HAWKINS KE, DEMARS KM, SINGH J, YANG C, CHO HS, FRANKOWSKI JC, DORÉ S, CANDELARIO-JALIL E. Neurovascular protection by post-ischemic intravenous injections of the lipoxin A4 receptor agonist, BML-111, in a rat model of ischemic stroke. Journal of Neurochemistry. 2014;129:130–142. doi: 10.1111/jnc.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWORTH O, CERNADAS M, YANG R, SERHAN CN, LEVY BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–9. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HE G, YE S, HUI J, SHEN D, QI C, XU L, QIAN Y. Interrelationships between ALOX5AP Polymorphisms, Serum Leukotriene B4 Level and Risk of Acute Coronary Syndrome. PLoS ONE. 2014;9:e106596. doi: 10.1371/journal.pone.0106596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEASMAN PA, LAUFFART BL, PRESHAW PM. Crevicular fluid prostaglandin E2 levels in periodontitis-resistant and periodontitis-susceptible adults. Journal of Clinical Periodontology. 1998;25:1003–1007. doi: 10.1111/j.1600-051x.1998.tb02405.x. [DOI] [PubMed] [Google Scholar]
- HELLMANN J, TANG Y, KOSURI M, BHATNAGAR A, SPITE M. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. The FASEB Journal. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRAI H, TANAKA K, YOSHIE O, OGAWA K, KENMOTSU K, TAKAMORI Y, ICHIMASA M, SUGAMURA K, NAKAMURA M, TAKANO S. Prostaglandin D2 selectively induces chemotaxis in T helper type 2 cells, eosinophils, and basophils via seven-transmembrane receptor CRTH2. The Journal of experimental medicine. 2001;193:255–262. doi: 10.1084/jem.193.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HONG S, GRONERT K, DEVCHAND PR, MOUSSIGNAC RL, SERHAN CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–87. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- HONG S, LU Y. Omega-3 fatty acid-derived resolvins and protectins in inflammation resolution and leukocyte functions: targeting novel lipid mediator pathways in mitigation of acute kidney injury. Front Immunol. 2013;4:13. doi: 10.3389/fimmu.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSIAO HM, THATCHER TH, COLAS RA, SERHAN CN, PHIPPS RP, SIME PJ. Resolvin D1 Reduces Emphysema and Chronic Inflammation. The American Journal of Pathology. 2015;185:3189–3201. doi: 10.1016/j.ajpath.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG RY, LI SS, GUO HZ, HUANG Y, ZHANG X, LI MY, CHEN GG, ZENG X. Thromboxane A2 exerts promoting effects on cell proliferation through mediating cyclooxygenase-2 signal in lung adenocarcinoma cells. Journal of Cancer Research and Clinical Oncology. 2014;140:375–386. doi: 10.1007/s00432-013-1573-3. [DOI] [PubMed] [Google Scholar]
- HUBLER MJ, KENNEDY AJ. Role of lipids in the metabolism and activation of immune cells. The Journal of Nutritional Biochemistry. 2016;34:1–7. doi: 10.1016/j.jnutbio.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISLAM AB, DAVE M, AMIN S, JENSEN RV, AMIN AR. Genomic, Lipidomic and Metabolomic Analysis of Cyclooxygenase-null Cells: Eicosanoid Storm, Cross Talk, and Compensation by COX-1. Genomics, Proteomics & Bioinformatics. 2016 doi: 10.1016/j.gpb.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOVA V, GARBUZENKO OB, REUHL KR, REIMER DC, POZHAROV VP, MINKO T. Inhalation treatment of pulmonary fibrosis by liposomal prostaglandin E2. European Journal of Pharmaceutics and Biopharmaceutics. 2013;84:335–344. doi: 10.1016/j.ejpb.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWANAGA K, NAKAMURA T, MAEDA S, ARITAKE K, HORI M, URADE Y, OZAKI H, MURATA T. Mast Cell–Derived Prostaglandin D2 Inhibits Colitis and Colitis-Associated Colon Cancer in Mice. Cancer research. 2014;74:3011–3019. doi: 10.1158/0008-5472.CAN-13-2792. [DOI] [PubMed] [Google Scholar]
- JAKAITIS BM, DENNING PW. Human breast milk and the gastrointestinal innate immune system. Clinics in perinatology. 2014;41:423–435. doi: 10.1016/j.clp.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEHMLICH N, STEGMAIER P, GOLATOWSKI C, SALAZAR MG, RISCHKE C, HENKE M, VÖLKER U. Differences in the whole saliva baseline proteome profile associated with development of oral mucositis in head and neck cancer patients undergoing radiotherapy. Journal of Proteomics. 2015;125:98–103. doi: 10.1016/j.jprot.2015.04.030. [DOI] [PubMed] [Google Scholar]
- JEON WK, CHOI J, PARK SJ, JO EJ, LEE YK, LIM S, KIM JH, LETTERIO JJ, LIU F, KIM SJ, KIM BC. The proinflammatory LTB(4)/BLT1 signal axis confers resistance to TGF-β1-induced growth inhibition by targeting Smad3 linker region. Oncotarget. 2015;6:41650–41666. doi: 10.18632/oncotarget.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIAN W, EDOM RW, XUE X, HUANG MQ, FOURIE A, WENG N. Quantitation of leukotriene B4 in human sputum as a biomarker using UPLC–MS/MS. Journal of Chromatography B. 2013;932:59–65. doi: 10.1016/j.jchromb.2013.06.010. [DOI] [PubMed] [Google Scholar]
- JOZSEF L, ZOUKI C, PETASIS NA, SERHAN CN, FILEP JG. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit peroxynitrite formation, NF-kappa B and AP-1 activation, and IL-8 gene expression in human leukocytes. Proc Natl Acad Sci U S A. 2002;99:13266–71. doi: 10.1073/pnas.202296999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALINSKI P. Regulation of immune responses by prostaglandin E2. The Journal of Immunology. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANAOKA Y, BOYCE JA. Cysteinyl leukotrienes and their receptors; emerging concepts. Allergy, asthma & immunology research. 2014;6:288–295. doi: 10.4168/aair.2014.6.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDA H, KOBAYASHI K, YAMANAKA H, NOGUCHI K. COX-1-dependent prostaglandin D2 in microglia contributes to neuropathic pain via DP2 receptor in spinal neurons. Glia. 2013;61:943–956. doi: 10.1002/glia.22487. [DOI] [PubMed] [Google Scholar]
- KANEYAMA K, SEGAMI N, SATO J, FUJIMURA K, NAGAO T, YOSHIMURA H. Prognostic Factors in Arthrocentesis of the Temporomandibular Joint: Comparison of Bradykinin, Leukotriene B4, Prostaglandin E2, and Substance P Level in Synovial Fluid Between Successful and Unsuccessful Cases. Journal of Oral and Maxillofacial Surgery. 2007;65:242–247. doi: 10.1016/j.joms.2005.10.068. [DOI] [PubMed] [Google Scholar]
- KASUGA K, YANG R, PORTER TF, AGRAWAL N, PETASIS NA, IRIMIA D, TONER M, SERHAN CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. The Journal of Immunology. 2008;181(12):8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUSHIK MK, ARITAKE K, KAMAUCHI S, HAYAISHI O, HUANG ZL, LAZARUS M, URADE Y. Prostaglandin D 2 is crucial for seizure suppression and postictal sleep. Experimental neurology. 2014;253:82–90. doi: 10.1016/j.expneurol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- KAZANI S, PLANAGUMA A, ONO E, BONINI M, ZAHID M, MARIGOWDA G, WECHSLER ME, LEVY BD, ISRAEL E. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. Journal of Allergy and Clinical Immunology. 2013;132:547–553. doi: 10.1016/j.jaci.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KETELHUTH DFJ, HERMANSSON A, HLAWATY H, LETOURNEUR D, YAN Z-Q, BÄCK M. The leukotriene B4 receptor (BLT) antagonist BIIL284 decreases atherosclerosis in ApoE−/− mice. Prostaglandins & Other Lipid Mediators. 2015;121(Part A):105–109. doi: 10.1016/j.prostaglandins.2015.05.007. [DOI] [PubMed] [Google Scholar]
- KHAITAN T, KABIRAJ A, BHATTACHARYA TP, GINJUPALLY U, JHA H. Diagnostic efficacy of saliva in oral and systemic health. international journal of stomatology & occlusion medicine. 2015;8:83–86. [Google Scholar]
- KINSELLA BT. Thromboxane A2 signalling in humans: a ‘Tail’ of two receptors. Biochemical Society Transactions. 2001;29:641–654. doi: 10.1042/0300-5127:0290641. [DOI] [PubMed] [Google Scholar]
- KLAGES B, BRANDT U, SIMON MI, SCHULTZ G, OFFERMANNS S. Activation of G12/G13 Results in Shape Change and Rho/Rho-Kinase–mediated Myosin Light Chain Phosphorylation in Mouse Platelets. The Journal of Cell Biology. 1999;144:745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLACZKOWSKA E, KUBES P. Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- KONDETI V, DUAH E, AL-AZZAM N, THODETI CK, BOYCE JA, PARUCHURI S. Differential regulation of cysteinyl leukotriene receptor signaling by protein kinase C in human mast cells. PloS one. 2013;8:e71536. doi: 10.1371/journal.pone.0071536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONTOGIANNI K, BAKAKOS P, KOSTIKAS K, HILLAS G, PAPAPORFYRIOU A, PAPIRIS S, KOULOURIS NG, LOUKIDES S. Levels of prostaglandin E2 and Cysteinyl-leukotrienes in sputum supernatant of patients with asthma: the effect of smoking. Clinical & Experimental Allergy. 2013;43:616–624. doi: 10.1111/cea.12077. [DOI] [PubMed] [Google Scholar]
- KOROMANTZOS PA, MAKRILAKIS K, DEREKA X, OFFENBACHER S, KATSILAMBROS N, VROTSOS IA, MADIANOS PN. Effect of Non-Surgical Periodontal Therapy on C-Reactive Protein, Oxidative Stress, and Matrix Metalloproteinase (MMP)-9 and MMP-2 Levels in Patients With Type 2 Diabetes: A Randomized Controlled Study. Journal of Periodontology. 2011;83:3–10. doi: 10.1902/jop.2011.110148. [DOI] [PubMed] [Google Scholar]
- KRISHNAMOORTHY S, RECCHIUTI A, CHIANG N, FREDMAN G, SERHAN CN. Resolvin D1 Receptor Stereoselectivity and Regulation of Inflammation and Proresolving MicroRNAs. The American Journal of Pathology. 2012;180:2018–2027. doi: 10.1016/j.ajpath.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRISHNAMOORTHY S, RECCHIUTI A, CHIANG N, YACOUBIAN S, LEE CH, YANG R, PETASIS NA, SERHAN CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBAVAT AH, KHIPPAL N, TAK S, RIJHWANI P, BHARGAVA S, PATEL T, SHAH N, KSHATRIYA RR, MITTAL R. A Randomized, Comparative, Multicentric Clinical Trial to Assess the Efficacy and Safety of Zileuton Extended-Release Tablets With Montelukast Sodium Tablets in Patients Suffering From Chronic Persistent Asthma. American Journal of Therapeutics. 2013;20:154–162. doi: 10.1097/MJT.0b013e318254259b. [DOI] [PubMed] [Google Scholar]
- KUDALKAR SN, ROUZER CA, MARNETT LJ. The Peroxidase and Cyclooxygenase Activity of Prostaglandin H Synthase. In: RAVEN EMMA, DUNFORD B, editors. Heme Peroxidases. Cambridge, UK: Royal Society of Chemistry; 2015. [Google Scholar]
- KUMAR AK, REDDY NR, BABU M, KUMAR PM, REDDY VS, CHAVAN CV. Estimation of prostaglandin E2 levels in gingival crevicular fluid in periodontal health, disease and after treatment. Contemporary clinical dentistry. 2013;4:303. doi: 10.4103/0976-237X.118354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUO H-K, CHEN Y-H, HUANG F, WU Y-C, SHIEA J, WU P-C. The upregulation of zinc finger protein 670 and prostaglandin D2 synthase in proliferative vitreoretinopathy. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2015:1–9. doi: 10.1007/s00417-015-3022-2. [DOI] [PubMed] [Google Scholar]
- KURTOVA AV, XIAO J, MO Q, PAZHANISAMY S, KRASNOW R, LERNER SP, CHEN F, ROH TT, LAY E, HO PL. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517:209–213. doi: 10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LABAT C, TEMMAR M, NAGY E, BEAN K, BRINK C, BENETOS A, BÄCK M. Inflammatory mediators in saliva associated with arterial stiffness and subclinical atherosclerosis. Journal of hypertension. 2013;31:2251–2258. doi: 10.1097/HJH.0b013e328363dccc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANDSKRON G, DE LA FUENTE M, THUWAJIT P, THUWAJIT C, HERMOSO MA. Chronic inflammation and cytokines in the tumor microenvironment. Journal of immunology research. 2014;2014 doi: 10.1155/2014/149185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANE K, DERBYSHIRE E, LI W, BRENNAN C. Bioavailability and potential uses of vegetarian sources of omega-3 fatty acids: a review of the literature. Critical reviews in food science and nutrition. 2014;54:572–579. doi: 10.1080/10408398.2011.596292. [DOI] [PubMed] [Google Scholar]
- LANIER LL. Shades of grey—the blurring view of innate and adaptive immunity. Nature Reviews Immunology. 2013;13:73–74. doi: 10.1038/nri3389. [DOI] [PubMed] [Google Scholar]
- LE LOUPP AG, BACH-NGOHOU K, BOURREILLE A, BOUDIN H, ROLLI-DERKINDEREN M, DENIS MG, NEUNLIST M, MASSON D. Activation of the prostaglandin D2 metabolic pathway in Crohn’s disease: involvement of the enteric nervous system. BMC gastroenterology. 2015;15:112. doi: 10.1186/s12876-015-0338-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE C-T. Impact of Resolvin E1 on Experimental Periodontitis and Periodontal Biofilm 2015 [Google Scholar]
- LEE J, BANU SK, BURGHARDT RC, STARZINSKI-POWITZ A, AROSH JA. Selective Inhibition of Prostaglandin E2 Receptors EP2 and EP4 Inhibits Adhesion of Human Endometriotic Epithelial and Stromal Cells Through Suppression of Integrin-Mediated Mechanisms. Biology of Reproduction. 2013;88:77, 1–11. doi: 10.1095/biolreprod.112.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE WT, HUANG CC, CHEN KC, WONG TY, OU CY, TSAI ST, YEN CJ, FANG SY, LO HI, WU YH, HSUEH WT, YANG MW, LIN FC, HSIAO JR, HUANG JS, CHANG JY, CHANG KY, WU SY, LIN CL, WANG YH, WENG YL, YANG HC, CHANG JS. Genetic polymorphisms in the prostaglandin pathway genes and risk of head and neck cancer. Oral Diseases. 2015;21:207–215. doi: 10.1111/odi.12244. [DOI] [PubMed] [Google Scholar]
- LEIGH NJ, NELSON JW, MELLAS RE, AGUIRRE A, BAKER OJ. Expression of resolvin D1 biosynthetic pathways in salivary epithelium. J Dent Res. 2014;93:300–5. doi: 10.1177/0022034513519108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY BD, CLISH CB, SCHMIDT B, GRONERT K, SERHAN CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- LI HJ, REINHARDT F, HERSCHMAN HR, WEINBERG RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E2 signaling. Cancer discovery. 2012;2:840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI N, SOOD S, WANG S, FANG M, WANG P, SUN Z, YANG CS, CHEN X. Overexpression of 5-lipoxygenase and cyclooxygenase 2 in hamster and human oral cancer and chemopreventive effects of zileuton and celecoxib. Clinical cancer research. 2005;11:2089–2096. doi: 10.1158/1078-0432.CCR-04-1684. [DOI] [PubMed] [Google Scholar]
- LI P, OH DY, BANDYOPADHYAY G, LAGAKOS WS, TALUKDAR S, OSBORN O, JOHNSON A, CHUNG H, MAYORAL R, MARIS M, OFRECIO JM, TAGUCHI S, LU M, OLEFSKY JM. LTB4 promotes insulin resistance in obese mice by acting on macrophages, hepatocytes and myocytes. Nat Med. 2015;21:239–247. doi: 10.1038/nm.3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIAO W, ZENG F, KANG K, QI Y, YAO L, YANG H, LING L, WU N, WU D. Lipoxin A4 Attenuates Acute Rejection Via Shifting Th1/Th2 Cytokine Balance in Rat Liver Transplantation. Transplantation Proceedings. 2013;45:2451–2454. doi: 10.1016/j.transproceed.2013.01.069. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN P. Repeated episodes of anaphylaxis with normal serum tryptase but elevated levels of urinary prostaglandin D2. The journal of allergy and clinical immunology In practice. 2013;1:539. doi: 10.1016/j.jaip.2013.03.008. [DOI] [PubMed] [Google Scholar]
- LIM SS, KWON HC, YOON SH. A STUDY ON THE CONCENTRATIONS OF LEUKOTRIENE B4 IN RELATION TO THE CLINICAL SYMPTOM OF PULPITIS IN HUMAN DENTAL PULP. Restorative Dentistry and Endodontics. 1996;21:353–359. [Google Scholar]
- LIU H, LI W, ROSE ME, PASCOE JL, MILLER TM, AHMAD M, POLOYAC SM, HICKEY RW, GRAHAM SH. Prostaglandin D 2 toxicity in primary neurons is mediated through its bioactive cyclopentenone metabolites. Neurotoxicology. 2013;39:35–44. doi: 10.1016/j.neuro.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU X, YANG T, MIAO L, MEI YA, HU C. Leukotriene B4 Inhibits L-Type Calcium Channels via p38 Signaling Pathway in Vascular Smooth Muscle Cells. Cellular Physiology and Biochemistry. 2015;37:1903–1913. doi: 10.1159/000438551. [DOI] [PubMed] [Google Scholar]
- LOHINAI Z, STACHLEWITZ R, SZEKELY A, FEHER E, DEZSI L, SZABO C. Evidence for the expression of cyclooxygenase-2 enzyme in periodontitis. Life sciences. 2001;70:279–290. doi: 10.1016/s0024-3205(01)01391-1. [DOI] [PubMed] [Google Scholar]
- LOOS BG, TEEUW WJ, NICU EA. Oral Infections and General Health. Springer; 2016. Plausible Mechanisms Explaining the Association of Periodontitis with Cardiovascular Diseases. [Google Scholar]
- LORENTE-CEBRIÁN S, COSTA AG, NAVAS-CARRETERO S, ZABALA M, MARTÍNEZ JA, MORENO-ALIAGA MJ. Role of omega-3 fatty acids in obesity, metabolic syndrome, and cardiovascular diseases: a review of the evidence. Journal of physiology and biochemistry. 2013;69:633–651. doi: 10.1007/s13105-013-0265-4. [DOI] [PubMed] [Google Scholar]
- LU SY, TSAI CY, LIN LH, LU SN. Dental extraction without stopping single or dual antiplatelet therapy: results of a retrospective cohort study. International Journal of Oral and Maxillofacial Surgery. doi: 10.1016/j.ijom.2016.02.010. [DOI] [PubMed] [Google Scholar]
- LUEKE AJ, MEEUSEN JW, DONATO LJ, GRAY AV, BUTTERFIELD JH, SAENGER AK. Analytical and clinical validation of an LC–MS/MS method for urine leukotriene E 4: A marker of systemic mastocytosis. Clinical biochemistry. 2016 doi: 10.1016/j.clinbiochem.2016.02.007. [DOI] [PubMed] [Google Scholar]
- MAGA P, SANAK M, REWERSKA B, MAGA M, JAWIEN J, WACHSMANN A, REWERSKI P, SZCZEKLIK W. Urinary cysteinyl leukotrienes in one-year follow-up of percutaneous transluminal angioplasty for peripheral arterial occlusive disease. Atherosclerosis. 2016;249:174–180. doi: 10.1016/j.atherosclerosis.2016.04.013. [DOI] [PubMed] [Google Scholar]
- MAJUMDER M, LANDMAN E, LIU L, HESS D, LALA PK. COX-2 Elevates oncogenic miR-526b in breast cancer by EP4 activation. Molecular Cancer Research. 2015;13:1022–1033. doi: 10.1158/1541-7786.MCR-14-0543. [DOI] [PubMed] [Google Scholar]
- MAJUMDER M, XIN X, LIU L, BELL G, LANDMAN E, RODRIGUEZ-TORRES M, POSTOVIT LM, HESS D, LALA PK. Stem like cells in human breast cancer: EP4 as a therapeutic target. Cancer Research. 2014;74:3905–3905. [Google Scholar]
- MALKI S, NEF S, NOTARNICOLA C, THEVENET L, GASCA S, MEJEAN C, BERTA P, POULAT F, BOIZET-BONHOURE B. Prostaglandin D2 induces nuclear import of the sex-determining factor SOX9 via its cAMP-PKA phosphorylation. The EMBO journal. 2005;24:1798–1809. doi: 10.1038/sj.emboj.7600660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANOKAWINCHOKE J, PIMKHAOKHUM A, EVERTS V, PAVASANT P. Prostaglandin E2 inhibits in-vitro mineral deposition by human periodontal ligament cells via modulating the expression of TWIST1 and RUNX2. Journal of periodontal research. 2014;49:777–784. doi: 10.1111/jre.12162. [DOI] [PubMed] [Google Scholar]
- MAO Y, SARHAN D, STEVEN A, SELIGER B, KIESSLING R, LUNDQVIST A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clinical cancer research. 2014;20:4096–4106. doi: 10.1158/1078-0432.CCR-14-0635. [DOI] [PubMed] [Google Scholar]
- MARCHESELLI VL, HONG S, LUKIW WJ, TIAN XH, GRONERT K, MUSTO A, HARDY M, GIMENEZ JM, CHIANG N, SERHAN CN, BAZAN NG. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–17. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- MARTINI AC, BERTA T, FORNER S, CHEN G, BENTO AF, JI RR, RAE GA. Lipoxin A4 inhibits microglial activation and reduces neuroinflammation and neuropathic pain after spinal cord hemisection. Journal of Neuroinflammation. 2016;13:1–11. doi: 10.1186/s12974-016-0540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTALERZ L, JANUSZEK R, KASZUBA M, WÓJCIK K, CELEJEWSKA-WÓJCIK N, GIELICZ A, PLUTECKA H, OLEŚ K, STRĘK P, SANAK M. Aspirin provocation increases 8-iso-PGE2 in exhaled breath condensate of aspirin-hypersensitive asthmatics. Prostaglandins & Other Lipid Mediators. 2015;121(Part B):163–169. doi: 10.1016/j.prostaglandins.2015.07.001. [DOI] [PubMed] [Google Scholar]
- MEDERLE K, MEURER M, CASTROP H, HÖCHERL K. Inhibition of COX-1 attenuates the formation of thromboxane A2 and ameliorates the acute decrease in glomerular filtration rate in endotoxemic mice. American Journal of Physiology - Renal Physiology. 2015;309:F332–F340. doi: 10.1152/ajprenal.00567.2014. [DOI] [PubMed] [Google Scholar]
- MENDIETA CF, REEVE CM, ROMERO JC. Biosynthesis of Prostaglandins in Gingiva of Patients with Chronic Periodontitis. Journal of Periodontology. 1985;56:44–47. doi: 10.1902/jop.1985.56.1.44. [DOI] [PubMed] [Google Scholar]
- MESA F, AGUILAR M, GALINDO-MORENO P, BRAVO M, O’VALLE F. Cyclooxygenase-2 expression in gingival biopsies from periodontal patients is correlated with connective tissue loss. Journal of periodontology. 2012;83:1538–1545. doi: 10.1902/jop.2012.110561. [DOI] [PubMed] [Google Scholar]
- MESA F, O’VALLE F, RIZZO M, CAPPELLO F, DONOS N, PARKAR M, CHAUDHARY N, CARINI F, MUNOZ R, NIBALI L. Association between COX-2 rs 6681231 genotype and Interleukin-6 in periodontal connective tissue. A pilot study. PloS one. 2014;9:e87023. doi: 10.1371/journal.pone.0087023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOGHADDAMI M, RANIERI E, JAMES M, FLETCHER J, CLELAND LG. Prostaglandin D 2 in Inflammatory Arthritis and Its Relation with Synovial Fluid Dendritic Cells. Mediators of inflammation. 2013;2013 doi: 10.1155/2013/329494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONIOT B, UJJAN S, CHAMPAGNE J, HIRAI H, ARITAKE K, NAGATA K, DUBOIS E, NIDELET S, NAKAMURA M, URADE Y. Prostaglandin D2 acts through the Dp2 receptor to influence male germ cell differentiation in the foetal mouse testis. Development. 2014;141:3561–3571. doi: 10.1242/dev.103408. [DOI] [PubMed] [Google Scholar]
- MORALES M, FLORES C, PINO K, ANGULO J, LÓPEZ-LASTRA M, CASTRO-RODRIGUEZ J. Urinary leukotriene and Bcl I polymorphism of glucocorticoid receptor gene in preschoolers with recurrent wheezing and high risk of asthma. Allergologia et immunopathologia. 2016;44:59–65. doi: 10.1016/j.aller.2015.02.003. [DOI] [PubMed] [Google Scholar]
- MORIMOTO K, SHIRATA N, TAKETOMI Y, TSUCHIYA S, SEGI-NISHIDA E, INAZUMI T, KABASHIMA K, TANAKA S, MURAKAMI M, NARUMIYA S, SUGIMOTO Y. Prostaglandin E2–EP3 Signaling Induces Inflammatory Swelling by Mast Cell Activation. The Journal of Immunology. 2014;192:1130–1137. doi: 10.4049/jimmunol.1300290. [DOI] [PubMed] [Google Scholar]
- MUSTAFA M, ZARROUGH A, BOLSTAD AI, LYGRE H, MUSTAFA K, HASTURK H, SERHAN C, KANTARCI A, VAN DYKE TE. Resolvin D1 protects periodontal ligament. American Journal of Physiology - Cell Physiology. 2013;305:C673–C679. doi: 10.1152/ajpcell.00242.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATA K, HIRAI H. The second PGD 2 receptor CRTH2: structure, properties, and functions in leukocytes. Prostaglandins, leukotrienes and essential fatty acids. 2003;69:169–177. doi: 10.1016/s0952-3278(03)00078-4. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA T, TAKEUCHI A, KAKIUCHI R, LEE T, YAGI M, AWANO H, II, JIMA K, TAKESHIMA Y, URADE Y, MATSUO M. P. 13.2 A prostaglandin D2 metabolite is elevated in the urine samples of patients with Duchenne muscular dystrophy. Neuromuscular Disorders. 2013a;23:809. doi: 10.1016/j.cca.2013.03.031. [DOI] [PubMed] [Google Scholar]
- NAKAGAWA T, TAKEUCHI A, KAKIUCHI R, LEE T, YAGI M, AWANO H, II, JIMA K, TAKESHIMA Y, URADE Y, MATSUO M. A prostaglandin D 2 metabolite is elevated in the urine of Duchenne muscular dystrophy patients and increases further from 8years old. Clinica chimica acta. 2013b;423:10–14. doi: 10.1016/j.cca.2013.03.031. [DOI] [PubMed] [Google Scholar]
- NAKANO Y, KIDANI Y, GOTO K, FURUE S, TOMITA Y, INAGAKI N, TANAKA H, SHICHIJO M. Role of prostaglandin D2 and DP1 receptor on Japanese cedar pollen-induced allergic rhinitis in mice. Journal of Pharmacology and Experimental Therapeutics. 2016;357:258–263. doi: 10.1124/jpet.115.229799. [DOI] [PubMed] [Google Scholar]
- NASSIRI M, ECKERMANN O, BABINA M, EDENHARTER G, WORM M. Serum levels of 9α,11β-PGF2 and cysteinyl leukotrienes are useful biomarkers of anaphylaxis. Journal of Allergy and Clinical Immunology. 2016;137:312–314. e7. doi: 10.1016/j.jaci.2015.07.001. [DOI] [PubMed] [Google Scholar]
- NEATH SX, JEFFERIES JL, BERGER JS, WU A, MCCONNELL JP, BOONE JL, MCCULLOUGH PA, JESSE RL, MAISEL AS. The current and future landscape of urinary thromboxane testing to evaluate atherothrombotic risk. Reviews in cardiovascular medicine. 2013;15:119–130. doi: 10.3909/ricm0739. [DOI] [PubMed] [Google Scholar]
- NEFF LM, CULINER J, CUNNINGHAM-RUNDLES S, SEIDMAN C, MEEHAN D, MATURI J, WITTKOWSKI KM, LEVINE B, BRESLOW JL. Algal docosahexaenoic acid affects plasma lipoprotein particle size distribution in overweight and obese adults. The Journal of nutrition. 2011;141:207–213. doi: 10.3945/jn.110.130021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NELSON JW, LEIGH NJ, MELLAS RE, MCCALL AD, AGUIRRE A, BAKER OJ. ALX/FPR2 receptor for RvD1 is expressed and functional in salivary glands. Am J Physiol Cell Physiol. 2014;306:C178–85. doi: 10.1152/ajpcell.00284.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLSON T, KHADEMI H, MOGHADASIAN MH. The role of marine n−3 fatty acids in improving cardiovascular health: a review. Food & function. 2013;4:357–365. doi: 10.1039/c2fo30235g. [DOI] [PubMed] [Google Scholar]
- NISHINO S, UENO R, OHISHI K, SAKAI T, HAYAISHI O. Salivary prostaglandin concentrations: possible state indicators for major depression. Am J Psychiatry. 1989;146:365–368. doi: 10.1176/ajp.146.3.365. [DOI] [PubMed] [Google Scholar]
- NIZARALI N, RAFIQUE S. Special Care Dentistry: Part 2. dental management of patients with drug-related acquired bleeding disorders. Dent Update. 2013;40:711–718. doi: 10.12968/denu.2013.40.9.711. [DOI] [PubMed] [Google Scholar]
- NOGUCHI K, ENDO H, KONDO H, ISHIKAWA I. Prostaglandin F2α upregulates interleukin-6 production in human gingival fibroblasts. Journal of Periodontal Research. 2001a;36:80–87. doi: 10.1034/j.1600-0765.2001.360203.x. [DOI] [PubMed] [Google Scholar]
- NOGUCHI K, IWASAKI K, ISHIKAWA I. Prostaglandin F2α upregulates intercellular adhesion molecule-1 expression in human gingival fibroblasts. Journal of Periodontal Research. 1999a;34:277–281. doi: 10.1111/j.1600-0765.1999.tb02254.x. [DOI] [PubMed] [Google Scholar]
- NOGUCHI K, MIYAUCHI M, OKA H, KOMAKI M, SOMERMAN MJ, TAKATA T. Cyclooxygenase-2-Dependent Prostaglandin E2 Upregulates Interleukin (IL)-1α-Induced IL-6 Generation in Mouse Cementoblasts. Journal of periodontology. 2007;78:135–140. doi: 10.1902/jop.2007.060257. [DOI] [PubMed] [Google Scholar]
- NOGUCHI K, SHITASHIGE M, WATANABE H, MUROTA S, ISHIKAWA I. Interleukin-4 and interferon-γ inhibit prostaglandin production by interleukin-1β-stimulated human periodontal ligament fibroblasts. Inflammation. 1999b;23:1–13. doi: 10.1023/a:1020231331932. [DOI] [PubMed] [Google Scholar]
- NOGUCHI K, SHITASHIGE M, YANAI M, MORITA I, NISHIHARA T, MUROTA S, ISHIKAWA I. Prostaglandin production via induction of cyclooxygenase-2 by human gingival fibroblasts stimulated with lipopolysaccharides. Inflammation. 1996;20:555–568. doi: 10.1007/BF01487046. [DOI] [PubMed] [Google Scholar]
- NOGUCHI K, TOMINAGA Y, MATSUSHITA K, IZUMI Y, ENDO H, KONDO H, ISHIKAWA I. Upregulation of matrix metalloproteinase-1 production by prostaglandin F2α in human gingival fibroblasts. Journal of periodontal research. 2001b;36:334–339. doi: 10.1034/j.1600-0765.2001.360510.x. [DOI] [PubMed] [Google Scholar]
- NORLING LV, HEADLAND SE, DALLI J, ARNARDOTTIR HH, HAWORTH O, JONES HR, IRIMIA D, SERHAN CN, PERRETTI M. Proresolving and cartilage-protective actions of resolvin D1 in inflammatory arthritis. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORLING LV, SPITE M, YANG R, FLOWER RJ, PERRETTI M, SERHAN CN. Cutting Edge: Humanized Nano-Proresolving Medicines Mimic Inflammation-Resolution and Enhance Wound Healing. The Journal of Immunology. 2011;186:5543–5547. doi: 10.4049/jimmunol.1003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORRIS PC, ARNARDOTTIR H, SANGER JM, FICHTNER D, KEYES GS, SERHAN CN. Resolvin D3 multi-level proresolving actions are host protective during infection. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA) 2016 doi: 10.1016/j.plefa.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NUGTEREN D, HAZELHOF E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism. 1973;326:448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- O’BRIEN TP, ROSZKOWSKI MT, WOLFF LF, HINRICHS JE, HARGREAVES KM. Effect of a non-steroidal anti-inflammatory drug on tissue levels of immunoreactive prostaglandin E2, immunoreactive leukotriene, and pain after periodontal surgery. Journal of periodontology. 1996;67:1307–1316. doi: 10.1902/jop.1996.67.12.1307. [DOI] [PubMed] [Google Scholar]
- O’CALLAGHAN G, RYAN A, NEARY P, O’MAHONY C, SHANAHAN F, HOUSTON A. Targeting the EP1 receptor reduces Fas ligand expression and increases the antitumor immune response in an in vivo model of colon cancer. International Journal of Cancer. 2013;133:825–834. doi: 10.1002/ijc.28076. [DOI] [PubMed] [Google Scholar]
- ODUSANWO O, CHINTHAMANI S, MCCALL A, DUFFEY ME, BAKER OJ. Resolvin D1 prevents TNF-alpha-mediated disruption of salivary epithelial formation. Am J Physiol, Cell Physiol. 2012 doi: 10.1152/ajpcell.00207.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OFFENBACHER S, ODLE BM, BRASWELL LD, JOHNSON HG, HALL CM, MCCLURE H, ORKIN JL, STROBERT EA, GREEN MD. Changes in cyclooxygenase metabolities in experimental periodontitis in Macaca mulatta. Journal of Periodontal Research. 1989;24:63–74. doi: 10.1111/j.1600-0765.1989.tb00859.x. [DOI] [PubMed] [Google Scholar]
- OGAWA S, URABE D, YOKOKURA Y, ARAI H, ARITA M, INOUE M. Total Synthesis and Bioactivity of Resolvin E2. Organic Letters. 2009;11:3602–3605. doi: 10.1021/ol901350g. [DOI] [PubMed] [Google Scholar]
- OHISHI K, UENO R, NISHINO S, SAKAI T, HAYAISHI O. Increased level of salivary prostaglandins in patients with major depression. Biological psychiatry. 1988;23:326–334. doi: 10.1016/0006-3223(88)90283-1. [DOI] [PubMed] [Google Scholar]
- OKADA Y, YAMAGUCHI S, MATSUNAGA Y, NAMBU F. Effect of ONO-4053, a DP1 (prostaglandin D2 receptor) Antagonist, on Prostaglandin D2-Induced Nasal Congestion. Journal of Allergy and Clinical Immunology. 2015;135:AB219. [Google Scholar]
- OKIJI T, MORITA I, SUNADA I, MUROTA S. The role of leukotriene B4 in neutrophil infiltration in experimentally-induced inflammation of rat tooth pulp. Journal of dental research. 1991;70:34–37. doi: 10.1177/00220345910700010501. [DOI] [PubMed] [Google Scholar]
- ONAKA Y, SHINTANI N, NAKAZAWA T, HABA R, AGO Y, WANG H, KANOH T, HAYATA-TAKANO A, HIRAI H, NAGATA KY. CRTH2, a prostaglandin D 2 receptor, mediates depression-related behavior in mice. Behavioural brain research. 2015;284:131–137. doi: 10.1016/j.bbr.2015.02.013. [DOI] [PubMed] [Google Scholar]
- ONO E, TANIGUCHI M, HIGASHI N, MITA H, YAMAGUCHI H, TATSUNO S, FUKUTOMI Y, TANIMOTO H, SEKIYA K, OSHIKATA C. Increase in salivary cysteinyl-leukotriene concentration in patients with aspirin-intolerant asthma. Allergology International. 2011;60:37–43. doi: 10.2332/allergolint.09-OA-0166. [DOI] [PubMed] [Google Scholar]
- ORR SK, COLAS RA, DALLI J, CHIANG N, SERHAN CN. Proresolving actions of a new resolvin D1 analog mimetic qualifies as an immunoresolvent. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2015;308:L904–L911. doi: 10.1152/ajplung.00370.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PACE-ASCIAK C, GRANSTRÖM E. Prostaglandins and related substances. Elsevier Publishing Company; 1983. [Google Scholar]
- PAMPLONA FA, FERREIRA J, MENEZES DE LIMA O, DUARTE FS, BENTO AF, FORNER S, VILLARINHO JG, BELLOCCHIO L, WOTJAK CT, LERNER R, MONORY K, LUTZ B, CANETTI C, MATIAS I, CALIXTO JB, MARSICANO G, GUIMARÃES MZP, TAKAHASHI RN. Anti-inflammatory lipoxin A4 is an endogenous allosteric enhancer of CB1 cannabinoid receptor. Proceedings of the National Academy of Sciences. 2012;109:21134–21139. doi: 10.1073/pnas.1202906109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPADAKI G, BAKAKOS P, KOSTIKAS K, HILLAS G, TSILOGIANNI Z, KOULOURIS NG, PAPIRIS S, LOUKIDES S. Vascular endothelial growth factor and cysteinyl leukotrienes in sputum supernatant of patients with asthma. Respiratory Medicine. 2013;107:1339–1345. doi: 10.1016/j.rmed.2013.06.014. [DOI] [PubMed] [Google Scholar]
- PARUCHURI S, JIANG Y, FENG C, FRANCIS SA, PLUTZKY J, BOYCE JA. Leukotriene E4 activates peroxisome proliferator-activated receptor γ and induces prostaglandin D2 generation by human mast cells. Journal of Biological Chemistry. 2008;283:16477–16487. doi: 10.1074/jbc.M705822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRETTI M, CHIANG N, LA M, FIERRO IM, MARULLO S, GETTING SJ, SOLITO E, SERHAN CN. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8:1296–302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POMPONI MFL, GAMBASSI G, POMPONI M, DI GIOIA A, MASULLO C. Why docosahexaenoic acid and aspirin supplementation could be useful in women as a primary prevention therapy against Alzheimer’s disease? Ageing Research Reviews. 2011;10:124–131. doi: 10.1016/j.arr.2010.09.003. [DOI] [PubMed] [Google Scholar]
- POPE NH, SALMON M, CONTE MS, AILAWADI G, UPCHURCH GR. Resolvin D2 Inhibits Murine Abdominal Aortic Aneurysm and Increases M2 Macrophage Differentiation. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:A492–A492. [Google Scholar]
- PORKKA-HEISKANEN T. Sleep homeostasis. Current opinion in neurobiology. 2013;23:799–805. doi: 10.1016/j.conb.2013.02.010. [DOI] [PubMed] [Google Scholar]
- PORTEDER H, MATEJKA M, ULRICH W, SINZINGER H. The cyclo-oxygenase and lipoxygenase pathways in human oral cancer tissue. Journal of Maxillofacial Surgery. 1984;12:145–147. doi: 10.1016/s0301-0503(84)80234-9. [DOI] [PubMed] [Google Scholar]
- POULIOT M, CLISH CB, PETASIS NA, VAN DYKE TE, SERHAN CN. Lipoxin A4 Analogues Inhibit Leukocyte Recruitment to Porphyromonas gingivalis: A Role for Cyclooxygenase-2 and Lipoxins in Periodontal Disease. Biochemistry. 2000;39:4761–4768. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- PRADEEP A, MANJUNATH S, SWATI PP, SHIKHA C, SUJATHA PB. Gingival crevicular fluid levels of leukotriene B4 in periodontal health and disease. Journal of periodontology. 2007;78:2325–2330. doi: 10.1902/jop.2007.070135. [DOI] [PubMed] [Google Scholar]
- PRESCOTT D, MCKAY DM. Aspirin-triggered lipoxin enhances macrophage phagocytosis of bacteria while inhibiting inflammatory cytokine production. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2011;301:G487–G497. doi: 10.1152/ajpgi.00042.2011. [DOI] [PubMed] [Google Scholar]
- PUNYADEERA C, SLOWEY PD. Chapter 22 - Saliva as an Emerging Biofluid for Clinical Diagnosis and Applications of MEMS/NEMS in Salivary Diagnostics A2 - Subramani, Karthikeyan. In: AHMED W, HARTSFIELD JK, editors. Nanobiomaterials in Clinical Dentistry. William Andrew Publishing; 2013. [Google Scholar]
- QUARMYNE MO, RAYES O, GONSALVES CS, VANDERAH L, CANTER C, HAYNES J, CLAVIJIO CF, UWE C, KALINYAK K, JOINER CH. A phase I trial of zileuton in sickle cell disease. Blood. 2013;122:993–993. [Google Scholar]
- QUINN JH, BAZAN NG. Identification of prostaglandin E 2 and leukotriene B 4 in the synovial fluid of painful, dysfunctional temporomandibular joints. Journal of Oral and Maxillofacial Surgery. 1990;48:968–971. doi: 10.1016/0278-2391(90)90011-p. [DOI] [PubMed] [Google Scholar]
- QUIROS M, NISHIO H, LEONI G, AGARWAL R, BERNAL G, GERNER-SMITH C, COLAS R, GRAHAM K, SERHAN C, GARCIA A, PARKOS C, NUSRAT A. The Specialized Pro-resolving Lipid Mediator Resolvin E1 Promotes Intestinal Mucosal Wound Repair. The FASEB Journal. 2016;30:305–7. [Google Scholar]
- RAATZ SK, YOUNG LR, PICKLO MJ, SAUTER ER, QIN W, KURZER MS. Total dietary fat and fatty acid content modifies plasma phospholipid fatty acids, desaturase activity indices, and urinary prostaglandin E in women. Nutrition Research. 2012;32:1–7. doi: 10.1016/j.nutres.2011.12.006. [DOI] [PubMed] [Google Scholar]
- RABIET MJ, MACARI L, DAHLGREN C, BOULAY F. N-Formyl Peptide Receptor 3 (FPR3) Departs from the Homologous FPR2/ALX Receptor with Regard to the Major Processes Governing Chemoattractant Receptor Regulation, Expression at the Cell Surface, and Phosphorylation. Journal of Biological Chemistry. 2011;286:26718–26731. doi: 10.1074/jbc.M111.244590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADNAI B, STURM EM, STANČIĆ A, JANDL K, LABOCHA S, FERREIRÓS N, GRILL M, HASENOEHRL C, GORKIEWICZ G, MARSCHE G. Eosinophils contribute to intestinal inflammation via chemoattractant receptor-homologous molecule expressed on Th2 cells, CRTH2, in experimental Crohn’s disease. Journal of Crohn’s and Colitis. 2016:jjw061. doi: 10.1093/ecco-jcc/jjw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAFNSSON A, BÄCK M. Urinary leukotriene E4 is associated with renal function but not with endothelial function in type 2 diabetes. Disease markers. 2013;35:475–480. doi: 10.1155/2013/370461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAKHILA H, BOURCIER N, AKOUM A, POULIOT M. Abnormal Expression of Prostaglandins E2 and F2α Receptors and Transporters in Patients with Endometriosis. BioMed research international. 2015;2015 doi: 10.1155/2015/808146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMON S, BANCOS S, SERHAN CN, PHIPPS RP. Lipoxin A4 modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. European journal of immunology. 2014;44:357–369. doi: 10.1002/eji.201343316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMON S, GAO F, SERHAN CN, PHIPPS RP. Specialized Proresolving Mediators Enhance Human B Cell Differentiation to Antibody-Secreting Cells. The Journal of Immunology. 2012;189:1036–1042. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVANOS K, DAGKLIS T, PETOUSIS S, MARGIOULA-SIARKOU C, PRAPAS Y, PRAPAS N. Factors implicated in the initiation of human parturition in term and preterm labor: a review. Gynecological Endocrinology. 2015;31:679–683. doi: 10.3109/09513590.2015.1076783. [DOI] [PubMed] [Google Scholar]
- READER JC, MA X, KUNDU N, GOLOUBEVA O, FULTON A. Prostaglandin E2 receptor EP1 suppresses breast cancer metastasis. Cancer Research. 2015;75:3250–3250. doi: 10.1158/1541-7786.MCR-10-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENY J-L, BERDAGUÉ P, PONCET A, BARAZER I, NOLLI S, FABBRO-PERAY P, SCHVED J-F, BOUNAMEAUX H, MACH F, DE MOERLOOSE P, FONTANA P. Antiplatelet Drug Response Status Does Not Predict Recurrent Ischemic Events in Stable Cardiovascular Patients: Results of the ADRIE Study. Circulation. 2012 doi: 10.1161/CIRCULATIONAHA.111.085464. [DOI] [PubMed] [Google Scholar]
- RIFKIN BR, TAI HH. Elevated thromboxane B2 levels in periodontal disease. Journal of Periodontal Research. 1981;16:194–198. doi: 10.1111/j.1600-0765.1981.tb00966.x. [DOI] [PubMed] [Google Scholar]
- RINGHOLZ FC, BUCHANAN PJ, CLARKE DT, MILLAR RG, MCDERMOTT M, LINNANE B, HARVEY BJ, MCNALLY P, URBACH V. Reduced 15-lipoxygenase 2 and lipoxin A4/leukotriene B4 ratio in children with cystic fibrosis. European Respiratory Journal. 2014;44:394–404. doi: 10.1183/09031936.00106013. [DOI] [PubMed] [Google Scholar]
- RIZOS CV, LIBEROPOULOS EN, TELLIS CC, TSELEPIS AD, ELISAF MS. The effect of combining rosuvastatin with sartans of different peroxisome proliferator receptor-γ activating capacity on plasma 8-isoprostane prostaglandin F(2a) levels. Archives of Medical Science : AMS. 2013;9:172–176. doi: 10.5114/aoms.2013.33357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIZOS EC, NTZANI EE, BIKA E, KOSTAPANOS MS, ELISAF MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. Jama. 2012;308:1024–1033. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- ROGERIO AP, HAWORTH O, CROZE R, OH SF, UDDIN M, CARLO T, PFEFFER MA, PRILUCK R, SERHAN CN, LEVY BD. Resolvin D1 and Aspirin-Triggered Resolvin D1 Promote Resolution of Allergic Airways Responses. The Journal of Immunology. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMANO M, MADDOX JF, SERHAN CN. Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+ mobilization. The Journal of Immunology. 1996;157:2149–54. [PubMed] [Google Scholar]
- ROSSITTO M, UJJAN S, POULAT F, BOIZET-BONHOURE B. Multiple roles of the prostaglandin D2 signaling pathway in reproduction. Reproduction. 2015;149:R49–R58. doi: 10.1530/REP-14-0381. [DOI] [PubMed] [Google Scholar]
- SAINO H, UKITA Y, AGO H, IRIKURA D, NISAWA A, UENO G, YAMAMOTO M, KANAOKA Y, LAM BK, AUSTEN KF. The catalytic architecture of leukotriene C4 synthase with two arginine residues. Journal of Biological Chemistry. 2011;286:16392–16401. doi: 10.1074/jbc.M110.150177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALES KJ, MALDONADO-PÉREZ D, GRANT V, CATALANO RD, WILSON MR, BROWN P, WILLIAMS ARW, ANDERSON RA, THOMPSON EA, JABBOUR HN. Prostaglandin F2α-F-prostanoid receptor regulates CXCL8 expression in endometrial adenocarcinoma cells via the calcium–calcineurin–NFAT pathway. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793:1917–1928. doi: 10.1016/j.bbamcr.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALIC K, MORRISON MC, VERSCHUREN L, WIELINGA PY, WU L, KLEEMANN R, GJORSTRUP P, KOOISTRA T. Resolvin E1 attenuates atherosclerosis in absence of cholesterol-lowering effects and on top of atorvastatin. Atherosclerosis. 2016 doi: 10.1016/j.atherosclerosis.2016.05.001. [DOI] [PubMed] [Google Scholar]
- SALIC K, WIELINGA PW, VAN DER HOORN JW, VERSCHUREN L, KLEEMANN R, GJORSTRUP P, KOOISTRA T. Intervention With Anti-Inflammatory Resolvin E1 Attenuates Atherosclerosis Without Decreasing Plasma Cholesterol and Adds to the Antiatherogenic Effect of Atorvastatin. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:A637–A637. [Google Scholar]
- SAMUELSON B, PAOLETTI R. Leukotrienes and other lipoxygenase products. Raven Press; 1982. [Google Scholar]
- SÁNCHEZ G, MIOZZA V, DELGADO A, BUSCH L. Relationship between salivary leukotriene B4 levels and salivary mucin or alveolar bone resorption, in subjects with periodontal health and disease. Journal of periodontal research. 2013a;48:810–814. doi: 10.1111/jre.12070. [DOI] [PubMed] [Google Scholar]
- SÁNCHEZ GA, MIOZZA VA, DELGADO A, BUSCH L. Salivary IL-1β and PGE2 as biomarkers of periodontal status, before and after periodontal treatment. Journal of Clinical Periodontology. 2013b;40:1112–1117. doi: 10.1111/jcpe.12164. [DOI] [PubMed] [Google Scholar]
- SCHAFER C, SCHAFER J, YAKOB M, LIMA P, CAMARGO P, WONG D. Saliva diagnostics: utilizing oral fluids to determine health status. 2014 doi: 10.1159/000358791. [DOI] [PubMed] [Google Scholar]
- SCHMIDT C. Biotechs target stagnant baldness market. Nature biotechnology. 2016;34:120–121. doi: 10.1038/nbt0216-120. [DOI] [PubMed] [Google Scholar]
- SCHUSTER VL, CHI Y, LU R. The Prostaglandin Transporter: Eicosanoid Reuptake, Control of Signaling, and Development of High-Affinity Inhibitors as Drug Candidates. Transactions of the American Clinical and Climatological Association. 2015;126:248. [PMC free article] [PubMed] [Google Scholar]
- SCIASCIA S, ROCCATELLO D, BERTERO MT, DI SIMONE D, COSSEDDU D, VACCARINO A, BAZZAN M, ROSSI D, GARCIA-FERNANDEZ C, CEBERIO L, STELLA S, MENEGATTI E, BALDOVINO S. 8-Isoprostane, prostaglandin E2, C-reactive protein and serum amyloid A as markers of inflammation and oxidative stress in antiphospholipid syndrome: a pilot study. Inflammation Research. 2012;61:809–816. doi: 10.1007/s00011-012-0468-0. [DOI] [PubMed] [Google Scholar]
- SEDLÁK V, CÁP P, KACER P, RUZICKOVÁ O, TERL M, SYSLOVÁ K, KOBLÍZEK V, PELCLOVÁ D. Imbalance of anti-inflammatory (lipoxin A4) and pro-inflammatory (leukotrien B4) arachidonic acid products in exhaled breath condensate of severe refractory asthma. European Respiratory Journal. 2014:44. [Google Scholar]
- SERHAN CN. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- SERHAN CN. Lipoxins and novel aspirin-triggered 15-epi-lipoxins (ATL): A jungle of cell-cell interactions or a therapeutic opportunity? Prostaglandins. 1997;53:107–137. doi: 10.1016/s0090-6980(97)00001-4. [DOI] [PubMed] [Google Scholar]
- SERHAN CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- SERHAN CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERHAN CN, CHIANG N, DALLI J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Seminars in Immunology. 2015a;27:200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERHAN CN, CHIANG N, DALLI J, LEVY BD. Lipid mediators in the resolution of inflammation. Cold Spring Harbor perspectives in biology. 2015b;7:a016311. doi: 10.1101/cshperspect.a016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERHAN CN, CHIANG N, VAN DYKE TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERHAN CN, GOTLINGER K, HONG S, ARITA M. Resolvins, docosatrienes, and neuroprotectins, novel omega-3-derived mediators, and their aspirin-triggered endogenous epimers: an overview of their protective roles in catabasis. Prostaglandins & other lipid mediators. 2004;73:155–172. doi: 10.1016/j.prostaglandins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- SERHAN CN, HONG S, GRONERT K, COLGAN SP, DEVCHAND PR, MIRICK G, MOUSSIGNAC RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SERINI S, PICCIONI E, MERENDINO N, CALVIELLO G. Dietary polyunsaturated fatty acids as inducers of apoptosis: implications for cancer. Apoptosis. 2009;14:135–152. doi: 10.1007/s10495-008-0298-2. [DOI] [PubMed] [Google Scholar]
- SHEN Y, QIN Q, XU Z, SHEN K. Serum leukotrienes, circulating neutrophils, and high sensitivity C-reactive protein in Chinese children with sleep-disordered breathing. International Immunopharmacology. 2014;22:120–125. doi: 10.1016/j.intimp.2014.05.029. [DOI] [PubMed] [Google Scholar]
- SHIMIZU N, OZAWA Y, YAMAGUCHI M, GOSEKI T, OHZEKI K, ABIKO Y. Induction of COX-2 Expression by Mechanical Tension Force in Human Periodontal Ligament Cells. Journal of Periodontology. 1998;69:670–677. doi: 10.1902/jop.1998.69.6.670. [DOI] [PubMed] [Google Scholar]
- SHIRTCLIFF EA, GRANGER DA, SCHWARTZ E, CURRAN MJ. Use of salivary biomarkers in biobehavioral research: cotton-based sample collection methods can interfere with salivary immunoassay results. Psychoneuroendocrinology. 2001;26:165–173. doi: 10.1016/s0306-4530(00)00042-1. [DOI] [PubMed] [Google Scholar]
- SHON WJ, BAEK SH, LIM SS. THE CONCENTRATIONS OF PROSTAGLANDIN E 2, 6-KETO-PROSTAGLANDIN F 1 α, AND LEUKOTRIENE B 4 IN PULPAL AND PERIAPICAL LESIONS. Restorative Dentistry and Endodontics. 2000;25:193–201. [Google Scholar]
- SHRUBSOLE MJ, CAI Q, WEN W, MILNE G, SMALLEY WE, CHEN Z, NESS RM, ZHENG W. Urinary prostaglandin E2 metabolite and risk for colorectal adenoma. Cancer Prevention Research. 2012;5:336–342. doi: 10.1158/1940-6207.CAPR-11-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKÖLD K, TWETMAN S, HALLGREN A, YUCEL-LINDBERG T, MODÉER T. Effect of a chlorhexidine/thymol-containing varnish on prostaglandin E2 levels in gingival crevicular fluid. European Journal of Oral Sciences. 1998;106:571–575. doi: 10.1046/j.0909-8836.1998.eos106106.x. [DOI] [PubMed] [Google Scholar]
- SONG C, SHIEH CH, WU YS, KALUEFF A, GAIKWAD S, SU KP. The role of omega-3 polyunsaturated fatty acids eicosapentaenoic and docosahexaenoic acids in the treatment of major depression and Alzheimer’s disease: Acting separately or synergistically? Progress in lipid research. 2016;62:41–54. doi: 10.1016/j.plipres.2015.12.003. [DOI] [PubMed] [Google Scholar]
- SPITE M, CLÀRIA J, SERHAN CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell metabolism. 2014;19:21–36. doi: 10.1016/j.cmet.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPITE M, NORLING LV, SUMMERS L, YANG R, COOPER D, PETASIS NA, FLOWER RJ, PERRETTI M, SERHAN CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBBARAMAIAH K, HUDIS C, CHANG SH, HLA T, DANNENBERG AJ. EP2 and EP4 Receptors Regulate Aromatase Expression in Human Adipocytes and Breast Cancer Cells: EVIDENCE OF A BRCA1 AND p300 EXCHANGE. Journal of Biological Chemistry. 2008;283:3433–3444. doi: 10.1074/jbc.M705409200. [DOI] [PubMed] [Google Scholar]
- SUBBARAMAIAH K, MORRIS PG, ZHOU XK, MORROW M, DU B, GIRI D, KOPELOVICH L, HUDIS CA, DANNENBERG AJ. Increased Levels of COX-2 and Prostaglandin E2 Contribute to Elevated Aromatase Expression in Inflamed Breast Tissue of Obese Women. Cancer Discovery. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- SUN YP, OH SF, UDDIN J, YANG R, GOTLINGER K, CAMPBELL E, COLGAN SP, PETASIS NA, SERHAN CN. Resolvin D1 and its aspirin-triggered 17R epimer stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. Journal of Biological Chemistry. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- SUZUKI A, JI G, NUMABE Y, MURAMATSU M, GOMI K, KANAZASHI M, OGATA Y, SHIMIZU E, SHIBUKAWA Y, ITO A. Single nucleotide polymorphisms associated with aggressive periodontitis and severe chronic periodontitis in Japanese. Biochemical and biophysical research communications. 2004;317:887–892. doi: 10.1016/j.bbrc.2004.03.126. [DOI] [PubMed] [Google Scholar]
- SYNDERGAARD B, AL-SABBAGH M, KRYSCIO RJ, XI J, DING X, EBERSOLE JL, MILLER CS. Salivary Biomarkers Associated With Gingivitis and Response to Therapy. Journal of Periodontology. 2014;85:e295–e303. doi: 10.1902/jop.2014.130696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZLAGATYS-SIDORKIEWICZ A, MARTYSIAK-ŻUROWSKA D, KRZYKOWSKI G, ZAGIERSKI M, KAMIŃSKA B. Maternal smoking modulates fatty acid profile of breast milk. Acta Paediatrica. 2013;102:e353–e359. doi: 10.1111/apa.12276. [DOI] [PubMed] [Google Scholar]
- TABAS I, GLASS CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–172. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAHAN F, EKE G, BICICI E, SARAYMEN B, AKAR H. Increased Postexercise Lipoxin A4 Levels in Exhaled Breath Condensate in Asthmatic Children With Exercise-Induced Bronchoconstriction. Journal of investigational allergology & clinical immunology. 2016;26:19. doi: 10.18176/jiaci.0003. [DOI] [PubMed] [Google Scholar]
- TAKANO T, FIORE S, MADDOX JF, BRADY HR, PETASIS NA, SERHAN CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TARANNUM F, FAIZUDDIN M. Association between lipoxin A4 and interleukin-12 in gingival crevicular fluid: a preliminary investigation. Journal of Periodontal Research. 2016 doi: 10.1111/jre.12383. [DOI] [PubMed] [Google Scholar]
- TAYLAN A, GURLER O, TOPRAK B, SISMAN AR, YALCIN H, COLAK A, SARI I. S1000A12, Chitotriosidase, and Resolvin D1 as Potential Biomarkers of Familial Mediterranean Fever. J Korean Med Sci. 2015;30:1241–1245. doi: 10.3346/jkms.2015.30.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR GW, BURT BA, BECKER MP, GENCO RJ, SHLOSSMAN M, KNOWLER WC, PETTITT DJ. Severe Periodontitis and Risk for Poor Glycemic Control in Patients with Non-Insulin-Dependent Diabetes Mellitus. Journal of Periodontology. 1996;67:1085–1093. doi: 10.1902/jop.1996.67.10s.1085. [DOI] [PubMed] [Google Scholar]
- TESSARO FH, AYALA TS, MARTINS JO. Lipid mediators are critical in resolving inflammation: a review of the emerging roles of eicosanoids in diabetes mellitus. BioMed research international. 2015;2015 doi: 10.1155/2015/568408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEINER G, GESSNER A, LUTZ MB. The mast cell mediator PGD 2 suppresses IL-12 release by dendritic cells leading to Th2 polarized immune responses in vivo. Immunobiology. 2006;211:463–472. doi: 10.1016/j.imbio.2006.05.020. [DOI] [PubMed] [Google Scholar]
- THOMAS P, LOWE A, SAMARASINGHE P, LODGE C, HUANG Y, ABRAMSON M, DHARMAGE S, JAFFE A. Exhaled breath condensate in pediatric asthma: promising new advance or pouring cold water on a lot of hot air? A systematic review. Pediatric pulmonology. 2013;48:419–442. doi: 10.1002/ppul.22776. [DOI] [PubMed] [Google Scholar]
- TING HJ, MURAD JP, ESPINOSA EV, KHASAWNEH FT. Thromboxane A2 Receptor Biology and Function of a Peculiar Receptor that Remains Resistant for Therapeutic Targeting. Journal of cardiovascular pharmacology and therapeutics. 2012;17:248–259. doi: 10.1177/1074248411424145. [DOI] [PubMed] [Google Scholar]
- TJONAHEN E, OH SF, SIEGELMAN J, ELANGOVAN S, PERCARPIO KB, HONG S, ARITA M, SERHAN CN. Resolvin E2: Identification and Anti-Inflammatory Actions: Pivotal Role of Human 5-Lipoxygenase in Resolvin E Series Biosynthesis. Chemistry & Biology. 2006;13:1193–1202. doi: 10.1016/j.chembiol.2006.09.011. [DOI] [PubMed] [Google Scholar]
- TRIMARCO A, FORESE MG, ALFIERI V, LUCENTE A, BRAMBILLA P, DINA G, PIERAGOSTINO D, SACCHETTA P, URADE Y, BOIZET-BONHOURE B. Prostaglandin D2 synthase/GPR44: a signaling axis in PNS myelination. Nature neuroscience. 2014;17:1682–1692. doi: 10.1038/nn.3857. [DOI] [PubMed] [Google Scholar]
- TSAI CC, HONG Y, CHEN C, WU Y. Measurement of prostaglandin E 2 and leukotriene B 4 in the gingival crevicular fluid. Journal of dentistry. 1998;26:97–103. doi: 10.1016/s0300-5712(96)00084-x. [DOI] [PubMed] [Google Scholar]
- TSAI FM, WU CC, SHYU RY, JIANG SY. Hrev107 enhances prostaglandin D2 synthase-mediated cancer cells suppression in the testis. Cancer Research. 2013a;73:5384–5384. [Google Scholar]
- TSAI WH, SHIH CH, YU YB, HSU HC. Plasma levels in sepsis patients of annexin A1, lipoxin A4, macrophage inflammatory protein-3a, and neutrophil gelatinase-associated lipocalin. Journal of the Chinese Medical Association. 2013b;76:486–490. doi: 10.1016/j.jcma.2013.05.004. [DOI] [PubMed] [Google Scholar]
- TSUBOSAKA Y, NAKAMURA T, HIRAI H, HORI M, NAKAMURA M, OZAKI H, MURATA T. A Deficiency in the Prostaglandin D2 Receptor CRTH2 Exacerbates Adjuvant-Induced Joint Inflammation. The Journal of Immunology. 2014;193:5835–5840. doi: 10.4049/jimmunol.1303478. [DOI] [PubMed] [Google Scholar]
- TUR J, BIBILONI M, SUREDA A, PONS A. Dietary sources of omega 3 fatty acids: public health risks and benefits. British Journal of Nutrition. 2012;107:S23–S52. doi: 10.1017/S0007114512001456. [DOI] [PubMed] [Google Scholar]
- UDDIN M, LEVY BD. Resolvins: Natural agonists for resolution of pulmonary inflammation. Progress in Lipid Research. 2011;50:75–88. doi: 10.1016/j.plipres.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URADE Y, LAZARUS M. The genetic basis of sleep and sleep disorders. Cambridge University; New York: 2013. Prostaglandin D2 in the regulation of sleep; pp. 73–83. [Google Scholar]
- VAN DEN BRULE S, HUAUX F, UWAMBAYINEMA F, IBOURAADATEN S, YAKOUB Y, PALMAI-PALLAG M, TROTTEIN F, RENAULD JC, LISON D. Lung Inflammation and Thymic Atrophy after Bleomycin Are Controlled by the Prostaglandin D2 Receptor DP1. American journal of respiratory cell and molecular biology. 2014;50:212–222. doi: 10.1165/rcmb.2012-0520OC. [DOI] [PubMed] [Google Scholar]
- VAN DYKE TE. Proresolving lipid mediators: potential for prevention and treatment of periodontitis. Journal of Clinical Periodontology. 2011;38:119–125. doi: 10.1111/j.1600-051X.2010.01662.x. [DOI] [PubMed] [Google Scholar]
- VAN DYKE TE, HASTURK H, KANTARCI A, FREIRE MO, NGUYEN D, DALLI J, SERHAN CN. Proresolving Nanomedicines Activate Bone Regeneration in Periodontitis. Journal of Dental Research. 2015;94:148–156. doi: 10.1177/0022034514557331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANCHERI C, MASTRUZZO C, SORTINO MA, CRIMI N. The lung as a privileged site for the beneficial actions of PGE 2. Trends in immunology. 2004;25:40–46. doi: 10.1016/j.it.2003.11.001. [DOI] [PubMed] [Google Scholar]
- VIEIRA AM, NETO EH, FIGUEIREDO CC, BARJA FIDALGO C, FIERRO IM, MORANDI V. ATL-1, a synthetic analog of lipoxin, modulates endothelial permeability and interaction with tumor cells through a VEGF-dependent mechanism. Biochemical Pharmacology. 2014;90:388–396. doi: 10.1016/j.bcp.2014.05.019. [DOI] [PubMed] [Google Scholar]
- WAN GH, YAN DC, TSENG HY, TUNG TH, LIN SJ, LIN YW. Cysteinyl leukotriene levels correlate with 8-isoprostane levels in exhaled breath condensates of atopic and healthy children. Pediatr Res. 2013;74:584–591. doi: 10.1038/pr.2013.142. [DOI] [PubMed] [Google Scholar]
- WANG CS, WEE Y, YANG CH, MELVIN JE, BAKER OJ. ALX/FPR2 Modulates Anti-Inflammatory Responses in Mouse Submandibular Gland. Sci Rep. 2016;6:24244. doi: 10.1038/srep24244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Y, MAO L, LIN B, ZHANG Y, TAO T, QI Y, LIN X, JIN J, HUANG R, YAN Z, WEN S, ZHANG D. The role of thromboxane A2 receptor gene promoter polymorphism in acute cerebral infarction. Zhonghua wei zhong bing ji jiu yi xue. 2014;26:309–314. doi: 10.3760/cma.j.issn.2095-4352.2014.05.005. [DOI] [PubMed] [Google Scholar]
- WANG Z, CHENG Q, TANG K, SUN Y, ZHANG K, ZHANG Y, LUO S, ZHANG H, YE D, HUANG B. Lipid mediator lipoxin A4 inhibits tumor growth by targeting IL-10-producing regulatory B (Breg) cells. Cancer Letters. 2015;364:118–124. doi: 10.1016/j.canlet.2015.04.030. [DOI] [PubMed] [Google Scholar]
- WEISS GA, TROXLER H, KLINKE G, ROGLER D, BRAEGGER C, HERSBERGER M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids in health and disease. 2013;12:1. doi: 10.1186/1476-511X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEYLANDT KH, CHIU CY, GOMOLKA B, WAECHTER SF, WIEDENMANN B. Omega-3 fatty acids and their lipid mediators: Towards an understanding of resolvin and protectin formation. Prostaglandins & Other Lipid Mediators. 2012;97:73–82. doi: 10.1016/j.prostaglandins.2012.01.005. [DOI] [PubMed] [Google Scholar]
- WHELAN J, GOWDY KM, SHAIKH SR. N-3 polyunsaturated fatty acids modulate B cell activity in pre-clinical models: Implications for the immune response to infections. European journal of pharmacology. 2015 doi: 10.1016/j.ejphar.2015.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKS JW, FORBES KK, NORLAND JF. Prostaglandins and in vitro ovarian progestin biosynthesis. Prostaglandins. 1973;3:427–437. doi: 10.1016/0090-6980(73)90150-0. [DOI] [PubMed] [Google Scholar]
- WILLIAMS RC, OFFENBACHER S, JEFTCOAT MK, HOWELL TH, JOHNSON HG, HALL CM, WECHTER WJ, GOTDHABER P. Indomethacin or flurbiprofen treatment of periodontitis in beagles: Effect on crevicular fluid arachidonic acid metabolites compared with effect on alveolar bone loss. Journal of Periodontal Research. 1988;23:134–138. doi: 10.1111/j.1600-0765.1988.tb01346.x. [DOI] [PubMed] [Google Scholar]
- WINKLER JW, ORR SK, DALLI J, CHENG CYC, SANGER JM, CHIANG N, PETASIS NA, SERHAN CN. Resolvin D4 stereoassignment and its novel actions in host protection and bacterial clearance. Scientific Reports. 2016;6:18972. doi: 10.1038/srep18972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG A, COOK MN, FOLEY JJ, SARAU HM, MARSHALL P, HWANG SM. Influx of extracellular calcium is required for the membrane translocation of 5-lipoxygenase and leukotriene synthesis. Biochemistry. 1991;30:9346–9354. doi: 10.1021/bi00102a030. [DOI] [PubMed] [Google Scholar]
- WOODWARD DF, JONES RL, NARUMIYA S. International Union of Basic and Clinical Pharmacology. LXXXIII: Classification of Prostanoid Receptors, Updating 15 Years of Progress. Pharmacological Reviews. 2011;63:471–538. doi: 10.1124/pr.110.003517. [DOI] [PubMed] [Google Scholar]
- WU L, WANG Q, LIANG X, ANDREASSON K. Divergent effects of prostaglandin receptor signaling on neuronal survival. Neuroscience letters. 2007;421:253–258. doi: 10.1016/j.neulet.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIA D, WANG D, KIM SH, KATOH H, DUBOIS RN. Prostaglandin E2 promotes intestinal tumor growth via DNA methylation. Nature medicine. 2012;18:224–226. doi: 10.1038/nm.2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU Z, ZHAO F, LIN F, CHEN J, HUANG Y. Lipoxin A4 Inhibits the Development of Endometriosis in Mice: The Role of Anti-Inflammation and Anti-Angiogenesis. American Journal of Reproductive Immunology. 2012;67:491–497. doi: 10.1111/j.1600-0897.2011.01101.x. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI M, GARLET GP. The role of inflammation in defining the type and pattern of tissue response in orthodontic tooth movement. In: KRISHNAN V, DAVIDOVICH Z, editors. Biological Mechanisms of Tooth Movement. UK: Wiley Blackwell; 2015. [Google Scholar]
- YAMAGUCHI M, KASAI K. Inflammation in periodontal tissues in response to mechanical forces. ARCHIVUM IMMUNOLOGIAE ET THERAPIAE EXPERIMENTALIS-ENGLISH EDITION- 2005;53:388. [PubMed] [Google Scholar]
- YAMAGUCHI T, ISHII T, YAMAMOTO K, HIGASHI N, TANIGUCHI M, OKAMOTO M. Differences in urinary leukotriene E4 levels and distribution of eosinophils between chronic rhinosinusitis patients with aspirin-intolerant and-tolerant asthma. Auris Nasus Larynx. 2015 doi: 10.1016/j.anl.2015.09.016. [DOI] [PubMed] [Google Scholar]
- YAN Y, JIANG W, SPINETTI T, TARDIVEL A, CASTILLO R, BOURQUIN C, GUARDA G, TIAN Z, TSCHOPP J, ZHOU R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- YANG CW, UNANUE ER. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. The Journal of Experimental Medicine. 2013;210:375–387. doi: 10.1084/jem.20122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG J, EISERICH JP, CROSS CE, MORRISSEY BM, HAMMOCK BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radical Biology and Medicine. 2012;53:160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Q, SHI M, SHEN Y, CAO Y, ZUO S, ZUO C, ZHANG H, GABRILOVICH DI, YU Y, ZHOU J. COX-1–derived thromboxane A2 plays an essential role in early B-cell development via regulation of JAK/STAT5 signaling in mouse. Blood. 2014;124:1610–1621. doi: 10.1182/blood-2014-03-559658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOKOMIZO T. Two distinct leukotriene B4 receptors, BLT1 and BLT2. Journal of biochemistry. 2014:mvu078. doi: 10.1093/jb/mvu078. [DOI] [PubMed] [Google Scholar]
- YU D, XU Z, YIN X, ZHENG F, LIN X, PAN Q, LI H. Inverse Relationship between Serum Lipoxin A4 Level and the Risk of Metabolic Syndrome in a Middle-Aged Chinese Population. PLoS ONE. 2015;10:e0142848. doi: 10.1371/journal.pone.0142848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUAN J, NAUMOVITZ S, YANG H, REINACH PS, GJORSTRUP P. Resolvin E1 ( RvE1) Suppresses Hypertonic-induced Inflammatory Release Through Chemr23 By Human Corneal Epithelial Cells. Investigative Ophthalmology & Visual Science. 2012;53:5242–5242. [Google Scholar]
- YUCEL-LINDBERG T, BÅGE T. Inflammatory mediators in the pathogenesis of periodontitis. Expert reviews in molecular medicine. 2013;15:e7. doi: 10.1017/erm.2013.8. [DOI] [PubMed] [Google Scholar]
- YUCEL-LINDBERG T, TWETMAN S, SKÖLD-LARSSON K, MODÉER T. Effect of an antibacterial dental varnish on the levels of prostanoids, leukotriene B4, and interleukin-1 β in gingival crevicular fluid. Acta Odontologica Scandinavica. 1999;57:23–27. doi: 10.1080/000163599429066. [DOI] [PubMed] [Google Scholar]
- ZANTEK ND, LUEPKER RV, DUVAL S, MILLER K, OLDENBURG N, HIRSCH AT. Confirmation of Reported Aspirin Use in Community Studies: Utility of Serum Thromboxane B2 Measurement. Clinical and Applied Thrombosis/Hemostasis. 2014;20:385–392. doi: 10.1177/1076029613486537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAYED N, AFIF H, CHABANE N, MFUNA-ENDAM L, BENDERDOUR M, MARTEL-PELLETIER J, PELLETIER JP, MOTIANI RK, TREBAK M, DUVAL N. Inhibition of interleukin-1β–induced matrix metalloproteinases 1 and 13 production in human osteoarthritic chondrocytes by prostaglandin D2. Arthritis & Rheumatism. 2008;58:3530–3540. doi: 10.1002/art.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAI B, YANG H, MANCINI A, HE Q, ANTONIOU J, DI BATTISTA JA. Leukotriene B4 BLT receptor signaling regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of Ras/Raf/ERK/p42 AUF1 pathway. Journal of Biological Chemistry. 2010;285:23568–23580. doi: 10.1074/jbc.M110.107623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG A, SUN H, WANG X. Saliva Metabolomics Opens Door to Biomarker Discovery, Disease Diagnosis, and Treatment. Applied Biochemistry and Biotechnology. 2012;168:1718–1727. doi: 10.1007/s12010-012-9891-5. [DOI] [PubMed] [Google Scholar]
- ZHANG MJ, HELLMANN J, BAKER JF, GUO L, CONKLIN DJ, BHATNAGAR A, SPITE M. Inflammation-resolving Lipid Mediator Resolvin D2 Enhances Revascularization in Hind Limb Ischemia. Circulation. 2014;130:A17194–A17194. doi: 10.1161/CIRCULATIONAHA.116.021894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG S, GRABAUSKAS G, WU X, JOO MK, HELDSINGER A, SONG I, OWYANG C, YU S. Role of prostaglandin D2 in mast cell activation-induced sensitization of esophageal vagal afferents. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2013a;304:G908–G916. doi: 10.1152/ajpgi.00448.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG X, KULASINGHE A, KARIM RS, PUNYADEERA C. Saliva Diagnostics for Oral Diseases. In: STRECKFUS FC, editor. Advances in Salivary Diagnostics. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015. [Google Scholar]
- ZHANG X, LI J, LIU J, LUO H, GOU K, CUI S. Prostaglandin F2 α upregulates Slit/Robo expression in mouse corpus luteum during luteolysis. Journal of Endocrinology. 2013b;218:299–310. doi: 10.1530/JOE-13-0088. [DOI] [PubMed] [Google Scholar]
- ZHAO J, WANG J, DU J, XU H, ZHANG W, NI Q-X, YU H, RISCH HA, GAO Y-T, GAO Y. Urinary Prostaglandin E2 Metabolite and Pancreatic Cancer Risk: Case-Control Study in Urban Shanghai. PloS one. 2015:10. doi: 10.1371/journal.pone.0118004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO XW, BAO JJ, HU C, DING H, LIU XC, MEI Q, XU JM. Effect of Diclofenac on the Levels of Lipoxin A4 and Resolvin D1 and E1 in the Post-ERCP Pancreatitis. Digestive Diseases and Sciences. 2014;59:2992–2996. doi: 10.1007/s10620-014-3280-6. [DOI] [PubMed] [Google Scholar]
- ZHOU R, YAZDI AS, MENU P, TSCHOPP J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]