Abstract
A concise asymmetric synthesis of an 11β-HSD-1 inhibitor has been achieved using inexpensive starting materials with excellent step-economy at low catalyst loadings. The catalytic enantioselective total synthesis of 1 was accomplished in 7 steps and 38% overall yield aided by the development of an innovative, sequential strategy involving Pd-catalyzed pyridinium C–H arylation and Ir-catalyzed asymmetric hydrogenation of the resulting fused tricyclic indenopyridinium salt highlighted by the use of a unique P,N-ligand (MeO-BoQPhos) with 1000 ppm of [Ir(COD)Cl]2.
Graphical abstract
INTRODUCTION
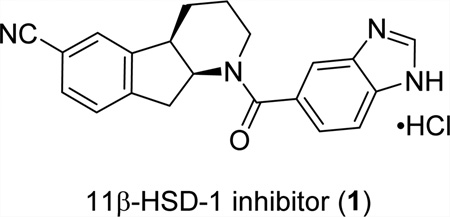
Type 2 diabetes is a metabolic disorder characterized by hyperglycemia, insulin resistance, and relative insulin deficiency.1 The global prevalence of diabetes was estimated to be 9% in 2014 among adults and is projected to increase annually.2 In the search for an effective therapeutic agent to curb this global epidemic, inhibition of the sodium-dependent glucose cotransporter enzyme, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD-1), has been investigated as a clinically relevant tactic for reduction of cortisol production in various tissues believed to be responsible for obesity and insulin resistance in children and adults. To this end, compound 1 emerged from our discovery program as a potent, metabolically stable 11β-HSD-1 inhibitor3 and has quickly advanced in clinical trials.
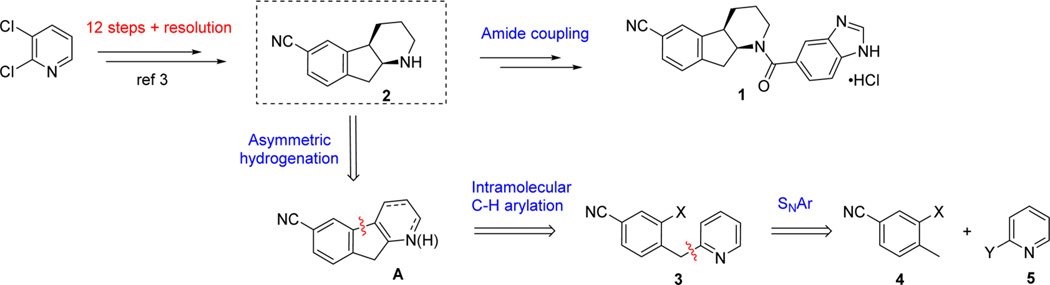
Compound 1 contains an intriguing tricyclic chiral indenopiperidine core that has also been observed in several other biologically active molecules and alkaloid natural products.4 Despite its relatively small molecular size and the deceptively simple structure, the architecturally unusual tricyclic indenopiperidine nucleus containing two embedded contiguous stereogenic centers presented a formidable synthetic challenge. Indeed, the first-generation route toward 1 required 12 linear steps to construct tricyclic core 2 starting from 2,3-dichloropyridine.3 The lengthy, racemic synthesis relies on a Pd-catalyzed cyanation with Zn(CN)2 and a late-stage resolution to supply drug candidate for early toxicological studies. The overall yield for the synthesis of 1 was less than 3% over a total of 15 steps with poor atom efficiency. In order to provide large quantities of compound for accelerated clinical studies (>3 tons for Phase III), a more efficient and economical synthesis was urgently required.
Herein, we describe our design and development of a concise asymmetric route to 11β-HSD-1 inhibitor 1 based on the successful, sequential implementation of an intramolecular C–H pyridinium arylation and enantioselective hydrogenation of the resulting fused indenopyridinium salt enabled by Boehringer Ingelheim’s P,N-ligand BoQPhos.5,6
RESULTS AND DISCUSSION
Retrosynthetic Analysis
Chiral piperidines are common structural motifs exhibited in natural products and highly pursued in drug discovery; in this vein, numerous synthetic methodologies have been reported.7 In designing an ideal synthesis8 for compound 1, a synthetic strategy was crafted in order to provide the shortest possible synthetic route to the target molecule by taking full advantage of catalytic technologies to drive down cost and effectively increase throughput of the synthesis.
Tactically, synthetic approaches to piperidines utilizing pyridines as starting materials became attractive due to their high abundance and low cost. We envisioned that the two stereogenic centers at the piperidine ring juncture could be introduced via a catalytic asymmetric hydrogenation of a tetrasubstituted double bond as part of a piperidine or pyridine ring (Scheme 1, synthon A). Fully aware of the challenges associated with the asymmetric hydrogenation of tetrasubstituted olefins,9 we aspired to engineer a new catalytic system for this important transformation by leveraging our recently developed dihydrobenzooxaphosphole (BOP)-based ligand series (vide infra).10,11 To access requisite hydrogenation precursor A, a conceptually concise sequence was devised involving an SNAr coupling of fragments 4 and 5 followed by an intramolecular C–H arylation. At the outset, the proposed cyclization to the C-3 position of pyridine was expected to be challenging, with few related examples found in the literature.12,13 However, if successful, then an expedient route to the key intermediate 2 could be developed from the simple and inexpensive starting materials.
Scheme 1.
Fifteen-Step Discovery Approach and New Streamlined Retrosynthesis toward 1
Synthesis of Pyridine Precursors for Cyclization
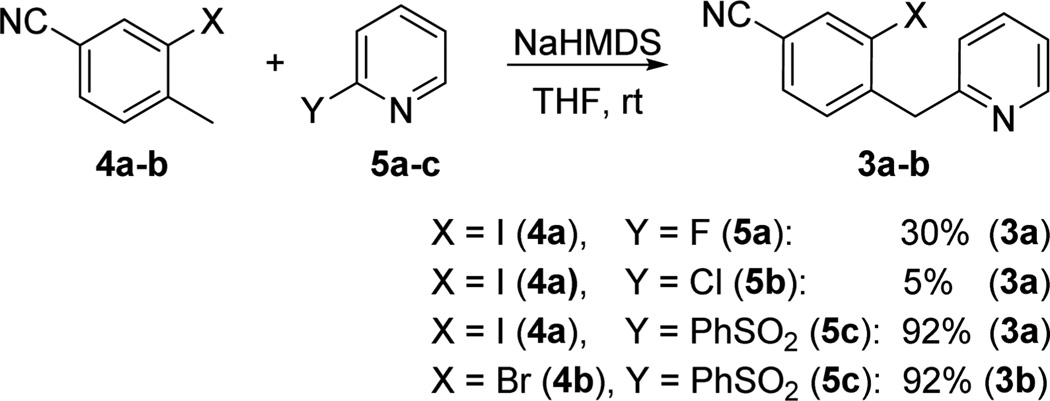
2-Benzyl-substituted pyridines are highly valuable compounds in organic synthesis. Existing synthetic approaches employ elaborate starting materials and require either high reaction temperatures or the use of Pd catalysts at high loadings,14 both of which negatively impact development of a cost-effective process. The SNAr coupling reaction between 3-iodo-4-methylbenzonitrile 4a and 2-fluoropyridine 5a was first examined in order to prepare the requisite benzylpyridine precursor 3 for cyclization; however, 30% yield was observed with significant amount of self-condensation side products. Switching to 2-chloropyridine 5b provided again trace product formation (Scheme 2).
Scheme 2.
SNAr Reaction of Nitrile 4 with 2-Substituted Pyridine Electrophiles
Prior experience from our laboratories15 has shown that the reaction between a carbanion and a sulfonylpyridine is an efficient method for construction of pyridine derivatives; this guided our investigations toward the coupling of 4a with 5c. To our delight, the reaction occurred smoothly in the presence of NaHMDS at room temperature in high yield after 2 h, allowing facile access to the key intermediates 3a–b in a straightforward and economical fashion.
Pd-Catalyzed C–H Arylative Cyclization
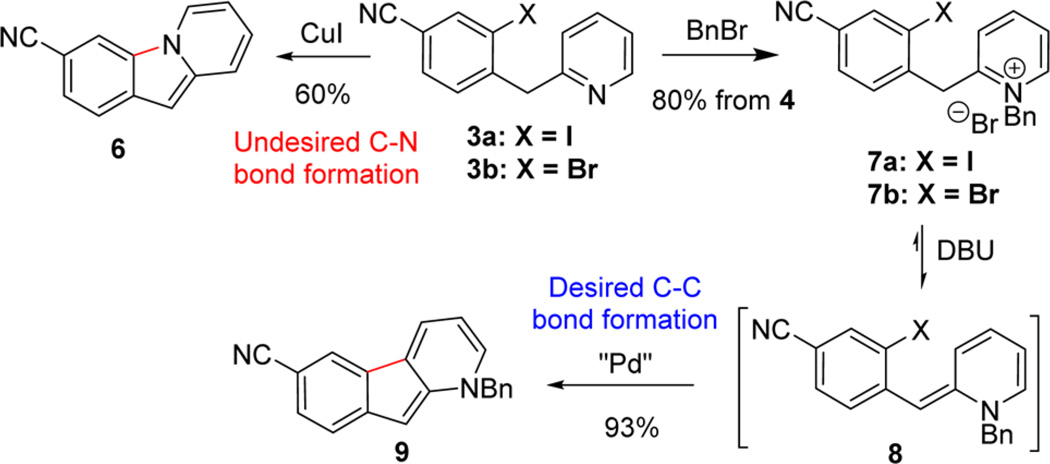
With compound 3 in hand, the key intramolecular cyclization was investigated next. Direct intramolecular C–H arylation of heteroarenes has become an increasingly popular approach for the construction of fused aromatic structures due to the inherent cost advantages.12,13 However, C–H arylation of pyridines has remained an outstanding challenge, especially in an intramolecular fashion. To the best of our knowledge, literature precedent for this type of cyclization to forge the indenopyridine scaffold is nonexistent. Indeed, initial attempts to achieve this cyclization employing 3a as substrate using Pd or Cu catalysts failed to deliver the desired indenopyridine product; undesired C–N bond formation generated 6 as the sole product (Scheme 3).
Scheme 3.
Pd-Catalyzed C–H Arylative Cyclization
To circumvent this undesired pathway, pyridine N-alkylation and acylation were pursued as a means to block C–N bond formation and potentially facilitate formation of benzylpyridinylidene benzonitrile 8 via deprotonation, which, in turn, allows access to a cyclization via a Heck type manifold. N-Acylation of pyridine 3 with phenyl chloroformate, however, was unsuccessful due to nonregioselective C-acylation at the benzylic position. Fortunately, crystalline pyridinium salts 7a and 7b could be directly isolated after benzylation with BnBr at 75 °C in high yields, providing a convenient means for purification following the SNAr coupling. An overall yield of >80% was readily achieved on metric ton scale by performing the SNAr coupling and benzylation in a single batch to obtain pyridinium salts 7a and 7b as white crystalline solids with >99% purity.
The pyridinium salts 7a,b were then subjected to cyclization with Pd catalysts. In the presence of Pd(OAc)2, weaker organic bases (Et3N or i-Pr2NEt) afforded low conversion even under forcing conditions. However, stronger base DBU provided desired tricyclic structure 9 with complete consumption of 7a,b. The highly conjugated neutral species 816 was observed as an intensely red-colored compound upon treatment of 7a,b with DBU. A detailed screening of Pd catalysts and reaction conditions for the economically preferred aryl bromide 7b identified Pd(dppf)Cl2 as the best catalyst system. With 1.25 mol % Pd(dppf)Cl2 and 3 equiv of DBU in DMF at 110 °C for 2–5 h, highly conjugated indenopyridine 9 was obtained as a deep-red crystalline solid in 93% yield and >99% purity after direct crystallization from the crude reaction mixture. This novel and direct C–H arylation enabled the construction of the requisite tricyclic indenopyridine structure in a concise and highly efficient manner, and was successfully implemented for production of compound 9 in >1 t quantities. Moreover, this work further serves as the basis for development of a nickel catalyzed intramolecular arylation as a general method for the synthesis of 1-azafluorenes.17,18
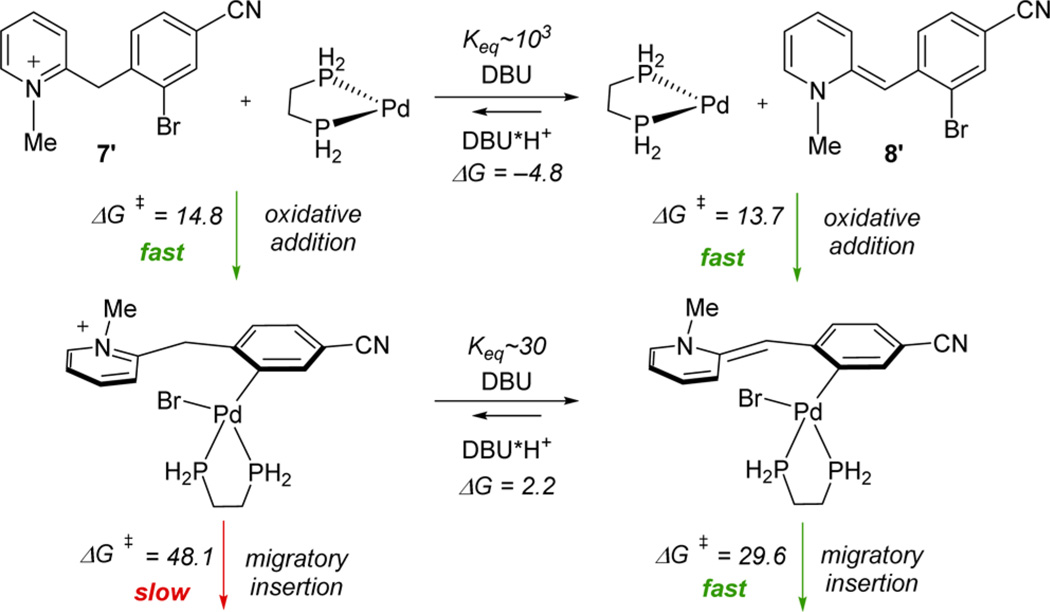
Mechanistically, the intramolecular cyclization can occur through either neutral intermediate 8 or cationic pyridinium salt 7b (Scheme 4). To fully understand the mechanism while optimizing for process robustness, DFT calculations were performed with Gaussian 0919 at the B3LYP/6-31G(d)-LANL2DZ(for Pd) level in the gas phase.20 With DBU, free base form 8/DBU·H+ was computed to be 4.8 kcal/mol lower in energy than 7b/DBU, consistent with 8 being the major species in solution (see below, benzyl replaced with methyl for all intermediates). Notably, computations (see the Supporting Information) revealed that this situation is dependent on both the nitrile substitution acidifying the pyridinium and the use of a stronger amine base DBU in accord with experimental findings.
Scheme 4.
Equilibria and Key Reaction Barriers for Pd-Catalyzed Cyclizationa
aComputed using the basis set M06/6-311+G(d,p)-LANL2DZ(Pd)-SMD-nitromethane//B3LYP/6-31G(d)/LANL2DZ(Pd)-gas; values in kcal/mol.
Both 7b and 8 were found to undergo facile oxidative addition to the corresponding aryl palladium species, in contrast to the related nickel system where oxidative addition to the pyridinium species is markedly lower in energy.17 Although significant amounts of both aryl palladium species are expected based on the computed equilibrium for the acid–base interchange, the subsequent migratory insertion onto a double bond of the charged species is untenable (48.1 kcal/mol) due to the aromatic stabilization of the pyridinium. However, the energetic barrier (29.6 kcal/mol) for migratory insertion of the neutral species is readily accessible under the reaction conditions of this process.
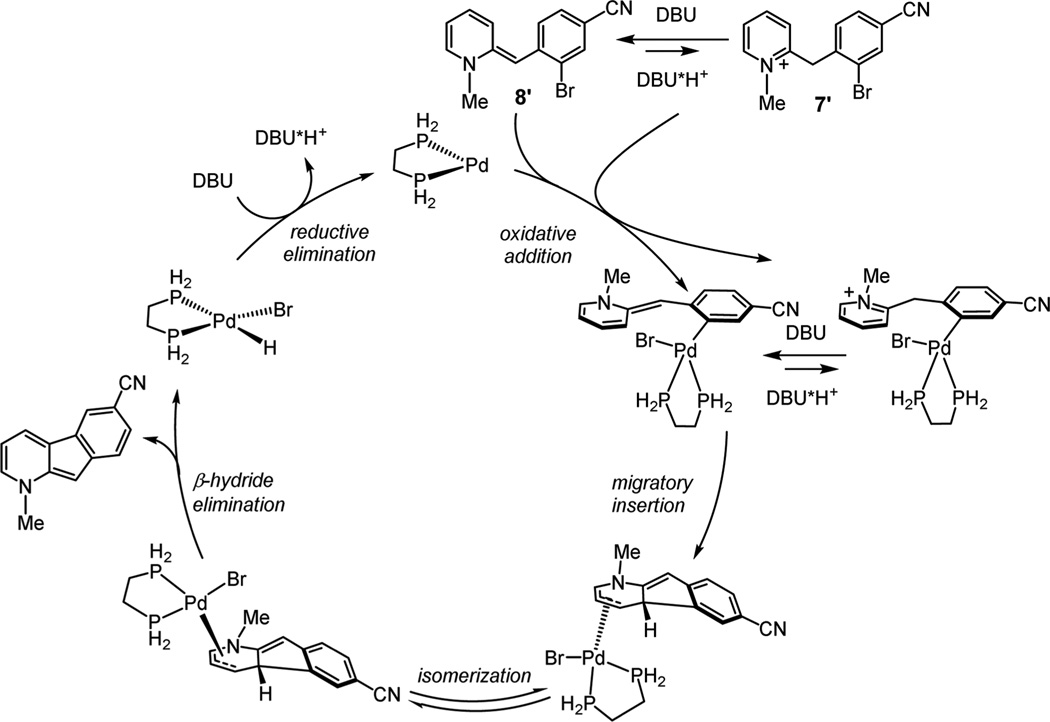
The set of equilibria prior to migratory insertion is consistent with the reaction kinetics, and the reaction is expected to be first-order in Pd catalyst and zero-order in substrate (see the Supporting Information). Moreover, since the deprotonation equilibria control the concentration of the preinsertion intermediates, a fractional order in base is expected. The catalytic cycle proposed based on the reaction kinetics and computational results is shown in Scheme 5. Base-mediated deprotonation sets up an equilibrium favoring neutral species 8′, which undergoes oxidative addition of the catalyst. Alternately, oxidative addition to pyridinium form 7′ followed by base mediated-deprotonation generates the same neutral precursor required for the migratory insertion to occur. Isomerization to a syn configuration then facilitates β-hydride elimination to yield the cyclization product and generate a PdII species that reforms the Pd0 catalyst closing the catalytic cycle. Although a lower energy pathway is calculated for free base 8, we speculate that the higher yields obtained from pyridinium salt 7b are due to its greater stability, mitigating byproduct formation.16
Scheme 5.
Proposed Catalytic Cycle for Pd-Catalyzed Cyclization
Nonenantioselective Hydrogenation and Chiral Resolution
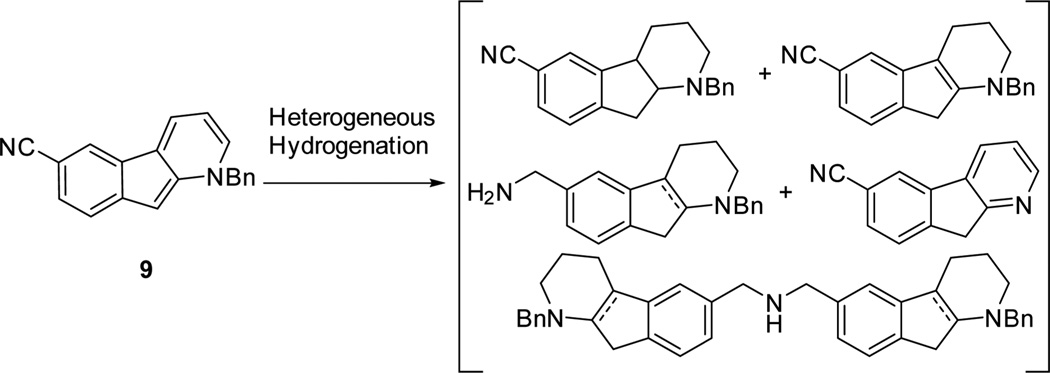
With a concise approach to the highly conjugated tricyclic indenopyridine 9 in hand, we next focused our attention on the desired ring reduction (Scheme 6). Unfortunately, extensive evaluation of the reduction of 9 in the presence of a range of heterogeneous catalysts (Pd/C, Pt/C, Raney Ni, Rh/C, and Ru/C) clearly indicated that the cyano moiety is incompatible under these conditions. A complex reaction mixture was always obtained as a combination of partial ring reduction, debenzylation, and nitrile reduction.
Scheme 6.
Challenging Hydrogenation of Indenopyridine 9
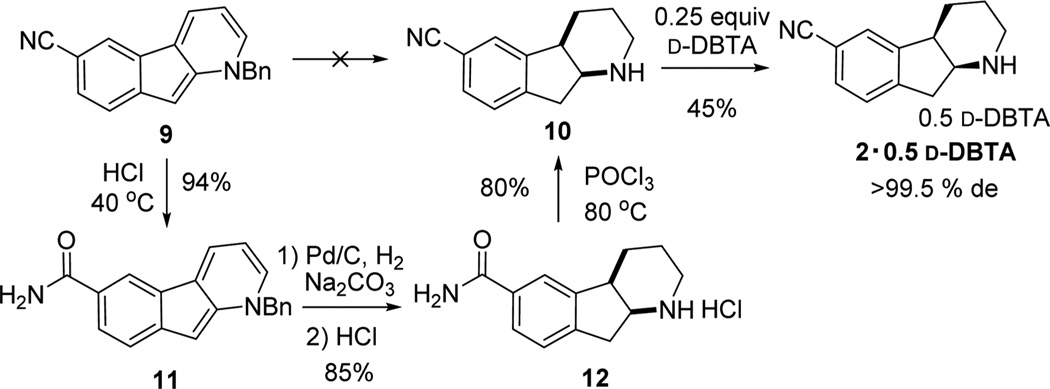
In view of these significant challenges and urgent material requirements during early stages of development, we pursued a temporary solution in which the nitrile group was selectively hydrolyzed to the corresponding amide as a protection step, followed by ring reduction/debenzylation and subsequent restoration of the nitrile group by amide dehydration (Scheme 7). Even though no nitrile hydrolysis occurs under forcing basic conditions (NaOH or KOH), strongly acidic conditions were found to facilitate the desired hydration. The corresponding amide was cleanly generated in 24 h by using HCl in dioxane. Highly conjugated amide 11 was isolated as a deep red crystalline solid in 94% yield.
Scheme 7.
Resolution Route via Nitrile “Protection”
Amide 11 was then subjected to hydrogenation conditions for a concomitant pyridine ring reduction and debenzylation. Screening of different types of heterogeneous catalysts identified the optimal conditions with 3 mol % Pd/C in methanol at 80 °C and 200 psi H2. It was found that addition of Na2CO3 induced a mildly basic reaction medium that favored first reduction of the ring and then hydrogenolysis. In this manner, piperidine 1221 was obtained as its hydrochloride salt in ~85% isolated yield. Dehydration with POCl3 subsequently afforded racemic nitrile 10 as a white crystalline solid in 80% yield. Resolution of piperidine 10 was successfully achieved using 0.25 equiv of d-dibenzoyl-tartaric acid (d-DBTA). Enantiomerically pure amine salt 2·0.5 d-DBTA (>99.5% de) was obtained in an excellent isolated yield of 45% (theoretical yield 50%).
Enantioselective Reduction via Known Ligands
In searching for a direct method to convert compound 9 to the reduced product, we postulated that homogeneous hydrogenation could potentially provide better control over chemoselectivity and lead to an enantioselective reduction if the proper chiral ligand was employed. Pioneering work over the past few years on enantioselective reduction of substituted pyridines and heteroarenes was highly encouraging.22,23 Nevertheless, most of the strategies are not applicable to the enantioselective hydrogenation of this indenopyridine system, highlighting the challenges associated with 2,3-disubtituted and fused pyridines. Additionally, industrial applications have not been reported. Indenopyridine 9 was initially tested in iridium catalyzed asymmetric hydrogenation; reactivity was expected due to the partial aromaticity of the dihydropyridine fragment. Yet it was quickly recognized that compound 9 is not a suitable substrate due to the low reactivity of the highly conjugated system and formation of the dimeric impurities derived from the nonchemoselective reduction of the nitrile moiety.
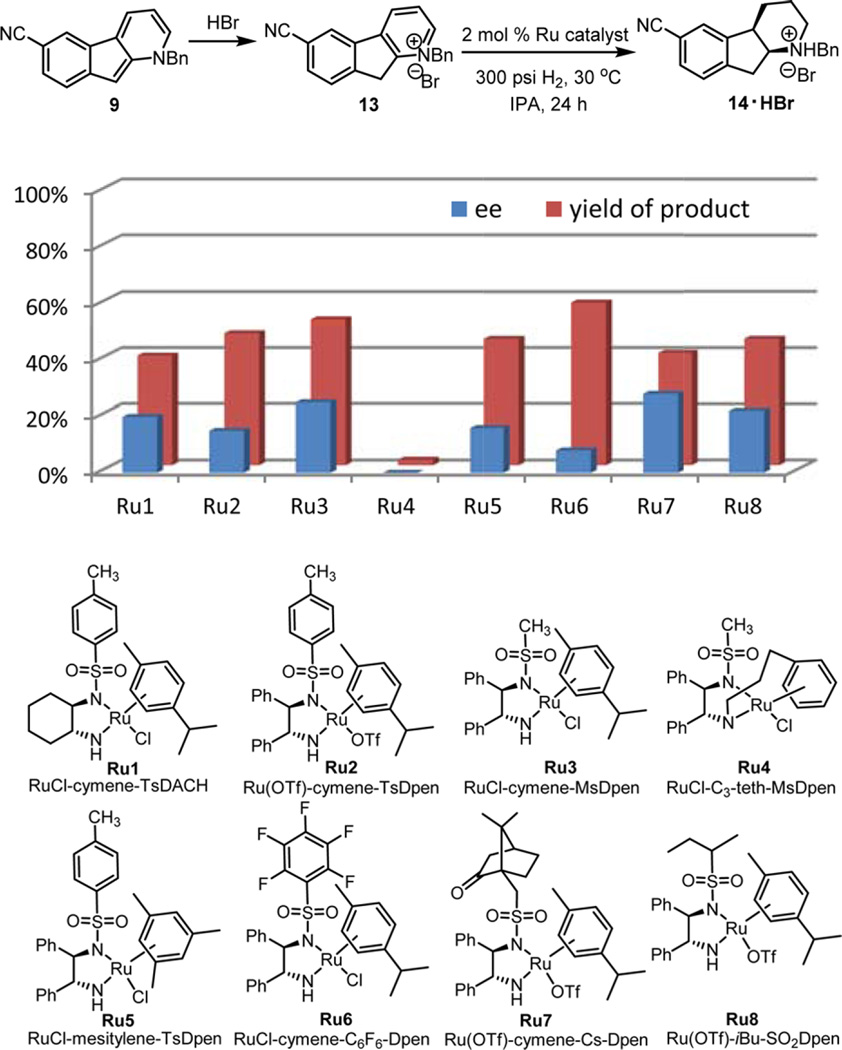
N-Benzylation of pyridines has recently been shown to activate the substrates for heterocycle reduction and suppress catalyst deactivation via coordination of the nitrogen atom.23c,d In addition, the activated pyridinium salts should, in principle, undergo chemoselective ring reduction even in the presence of a nitrile group. Therefore, we investigated the reduction of pyridinium salt 13. Different transition metals have been applied in asymmetric reduction of heteroarenes including ruthenium, rhodium, and iridium catalysts. We first tested the cationic ruthenium diamine catalysts, which have been reported to reduce substituted quinolines with high enantioselectivity.24 Even though highly reactive, large amounts of nitrile reduction were observed and the highest enantioselectivity was at 27% ee (Figure 1).
Figure 1.
Ruthenium catalysts examined for asymmetric hydrogenation of 13.
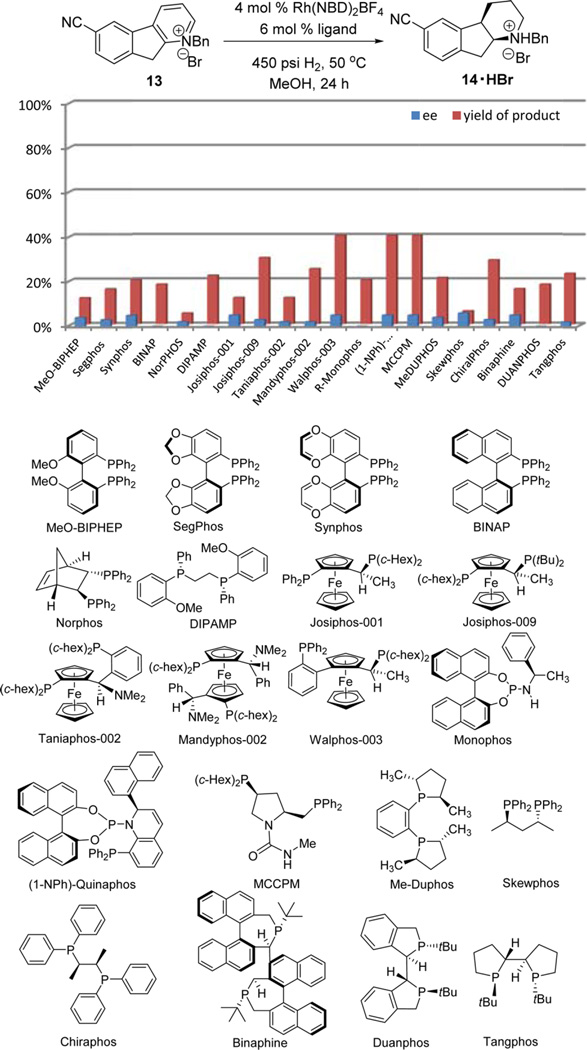
Rhodium bisphoshine catalysts are highly effective for tetrasubstituted alkene reduction25 and have been applied to the reduction of tetrahydropyridine enamine derivative to produce chiral piperidines.26 We thus pursued the asymmetric reduction of pyridinium salt 13 with rhodium catalysts in the presence of different families of chiral phosphine ligands including bisphosphines with axial chirality, Solvias’ ferrocene-based ligands, Ferringa’s monophosphoramidite ligands, and other reported chiral ligands. Disappointingly, both low conversion and low enantioselectivities were observed for all the ligands evaluated (Figure 2). Reactions stalled at different stages of reduction (dihydropiperidine, tetrahydropiperidine, etc.).
Figure 2.
Rhodium catalysts investigated for asymmetric hydrogenation of 13.
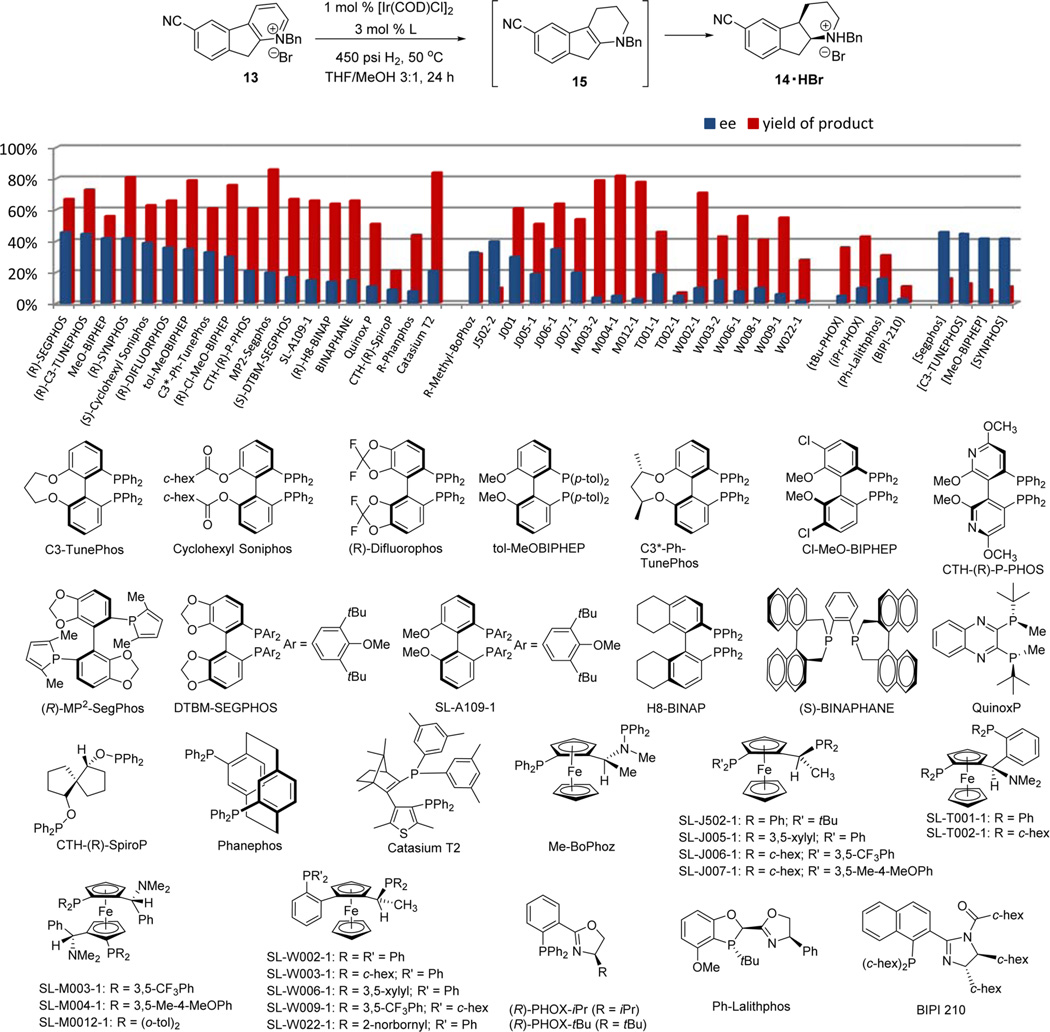
Iridium catalysts are known to reduce alkenes without the assistance of any coordinating group on the substrate.27 To facilitate the asymmetric reduction of pyridines, Charette and Legault first demonstrated an activation process for pyridine derivatives by formation of N-acyliminopyridinium ylides; effective reduction was achieved applying Pfaltz’s PHOX type P,N-ligands.23b Recently Zhou’s23c and Zhang’s23d groups demonstrated efficient asymmetric pyridine reduction by forming activated N-benzylpyridinium salts. A number of monosubstituted 2-aryl derived pyridines were reduced successfully using biaryl ligands with axial chirality including Synphos and MP2–Segphos. With N-benzylpyridinium salt 13 in hand, we first tested the biaryl ligands. Indeed, promising results were obtained compared to the Ru and Rh catalysts. About 40% ee was observed with Segphos, C3-Tunephos, MeO-biphep, and Synphos ligands. However, comprehensive screening of related ligands did not further improve the enantioselectivity (Figure 3). The best ligand MP2–Segphos reported for 2-arylpyridine reduction23d provided only 20% ee. The Solvias’ ligands are equally ineffective. In addition to the low enantioselectivity, a major issue was the inability of the catalyst system to fully reduce substrate 13. When the catalyst loading was decreased to an economically feasible level of 0.1 mol % [Ir(COD)Cl]2, less than 15% product formation was observed (last four ligands in bracket). Existing P,N-ligands including PHOX ligands,23b Ph-LalithPhos,28 and BIPI 21029 produced unsatisfactory reactivity and enantioselectivity even with 1 mol % of [Ir(COD)Cl]2.
Figure 3.
Evaluation of iridium catalysts with known ligands in the asymmetric hydrogenation of 13. Reaction conditions: 30 mg of 13, 1.0 mol % [Ir(COD)Cl]2, and 3 mol % ligand at 450 psi H2 and 50 °C in 0.9 mL of THF/MeOH (3:1) for 24 h; I2 (5 mol %) was added to the mixture of the four P,N-ligands listed in parentheses. The last four ligands in bracket were run at low catalyst load: 0.3 g of 13, 0.1 mol % [Ir(COD)Cl]2, and 0.3 mol % ligand in 5 mL of solvent under the same conditions. Product yield was calculated from quantitative HPLC solution assay of the product 14 upon Et2NH treatment; ee was obtained on HPLC with a chiral stationary phase.
Enantioselective Hydrogenation with BoQPhos Ligands
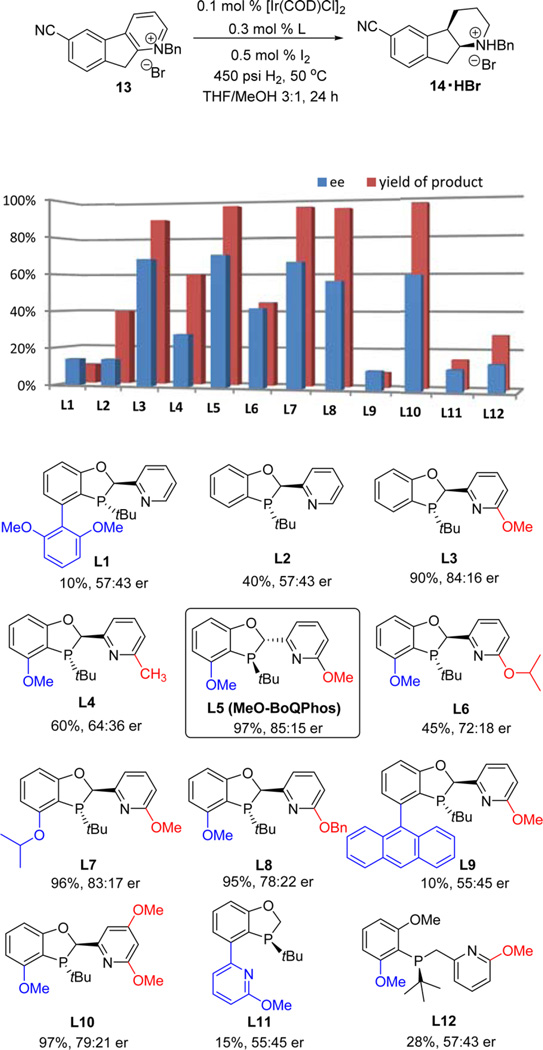
To overcome the tremendous challenges associated with the desired pyridinium hydrogenation, our efforts shifted toward the development of a new chiral ligand system. Recently, our group has reported a highly modular dihydrobenzooxaphosphole (BOP) core for ligand design.10 Many effective ligands have been derived from this motif and have been found to mediate a variety of different catalytic transformations.11 In particular, the rigid bidentate BoQPhos P,N-ligands, which were constructed from the BOP core by anchoring a less basic nitrogen-containing pyridine fragment onto the carbon atom of the O,P ring system (see Figure 4), have been successfully demonstrated for asymmetric hydrogenation of nonfunctionalized tri- and tetrasubstituted olefins.6 Since the presumed final intermediate in the reduction of tricyclic substrate 13 contains a tetrasubstituted double bond (see compound 15 in Figure 3), we anticipated that the BoQPhos series would be applicable to the asymmetric reduction of 2,3-disubstituted pyridinium salts (Figure 4).
Figure 4.
Evaluation of BI’s pyridine-containing P,N-ligands for asymmetric hydrogenation of 13. Reaction conditions: 300 mg of 13, 0.1 mol % [Ir(COD)Cl]2, 0.3 mol % ligand, and 0.5 mol % I2, at 450 psi H2 and 50 °C in 5 mL of THF/MeOH (3:1) for 24 h. Product yield was calculated from quantitative HPLC solution assay of the product 14 upon Et2NH treatment; ee is measured on HPLC with a chiral stationary phase.
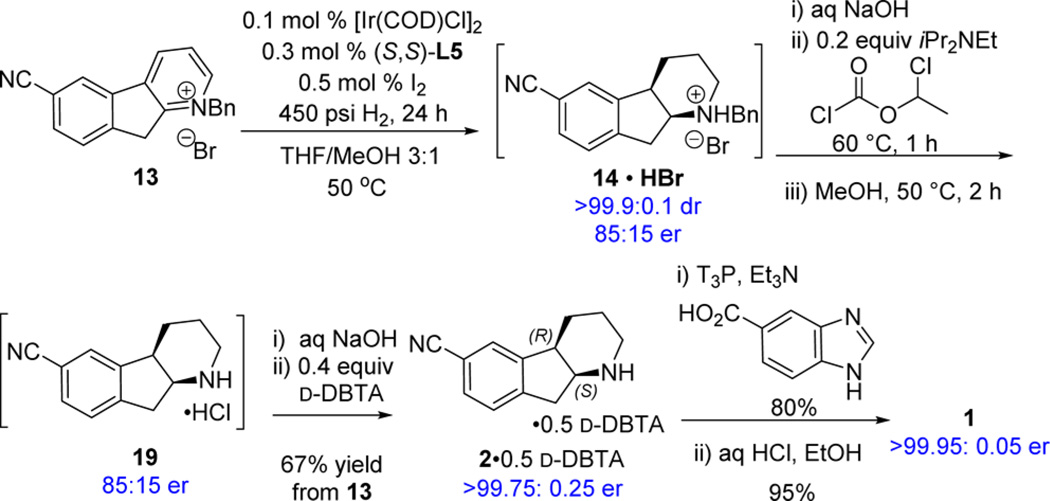
In our first approach, pyridinium salt 13 was subjected to hydrogenation conditions in the presence of [Ir(COD)Cl]2 and L1, which was the best ligand previously found for olefin hydrogenation.6 However, limited product formation (10%) and low enantioselectivity (57:43 er) were observed. We postulated that the low reactivity was caused by the steric hindrance from the dimethoxyphenyl moiety on the western hemisphere of the ligand. Indeed, removal of this group (L2) increased the desired product to 40% with the same enantioselectivity. To identify the key interactions of the ligand, structural modifications were pursued on both the western aryl and eastern pyridine rings. The substitution on the pyridine ring affects the enantioselectivity significantly. An ortho-MeO substitution on pyridine (L3) increased the er to 84:16 with 90% yield. The highest enantiomeric ratio of 85:15 was obtained with MeO-BoQPhos (L5) containing methoxy substituents on both the aryl and pyridine rings and produced 97% yield of the reduced product. Changing one of the methoxy groups to isopropoxy (L6 or L7) or adding an additional methoxy group on the pyridine ring (L10) resulted in slightly decrease in enantioselectivity. As expected, use of nonrigid open-chain ligand L12 also decreased the enantioselectivity. Gratifyingly, the P,N-ligand is completely chemoselective; the dimeric impurity derived from nitrile reduction was not observed. Furthermore, MeO-BoQPhos is highly reactive in this challenging catalytic asymmetric hydrogenation process, allowing catalyst load to be decreased as low as 0.1 mol % [Ir(COD)Cl]2 while still providing 93% isolated yield and 85:15 er, clearly demonstrating the superb activity and robustness of the BOP-pyridine P,N-ligands for the effective asymmetric pyridine reduction. In contrast to the bisphosphine ligands,23c,d the use of iodine as an additive improves both the enantioselectivity and the reactivity of the hydrogenation reaction using the P,N-ligands.
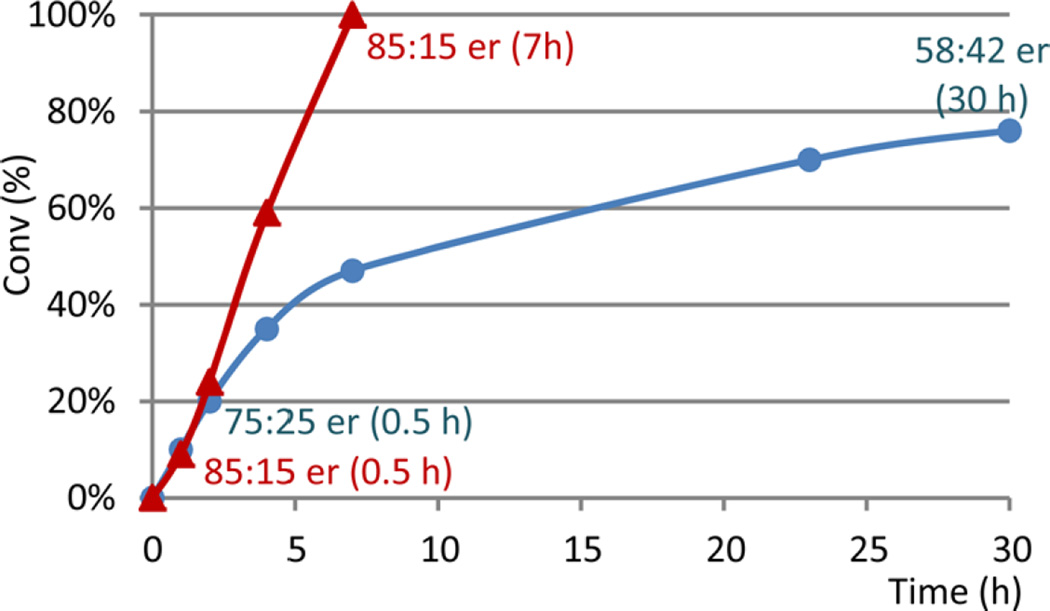
We have also evaluated the pyridinium salts with different counterions including Cl−, I−, HSO4−, BF4−, and PF6−. All these salts demonstrated similar enantioselectivity of 85:15 er at high catalyst load of more than 3 mol % iridium.30 For a cost-effective process, low catalyst load at ≤1 mol % iridium was further investigated. The chloride salt is highly hydroscopic and was isolated as a monohydrate (containing about 5% water); the unproductive pathways of enamine hydrolysis and condensation become more competitive at low catalyst load. BF4− and PF6− salts were found to be much more soluble. However, reaction monitoring revealed that lower enantioselectivity was obtained at low catalyst loads, and the enantioselectivity continued to decrease over the course of the reaction (Figure 5). The bromide salt of 13 was eventually selected for the process. The optimized conditions with 0.1 mol % Ir(COD)Cl]2 and 0.3 mol % L5 were successfully applied to deliver the desired product 14·HBr on multikilogram scale.
Figure 5.
Comparison of asymmetric hydrogenation reaction profiles with low catalyst load. Red: Br− salt with 0.2 mol % Ir(COD)Cl]2/0.5 mol % L5/1 mol % I2 at 50 °C and 450 psi H2; blue: PF6− salt with 0.5 mol % Ir(COD)Cl]2/1.2 mol % L5/2.5 mol % I2 at 50 °C and 450 psi H2.
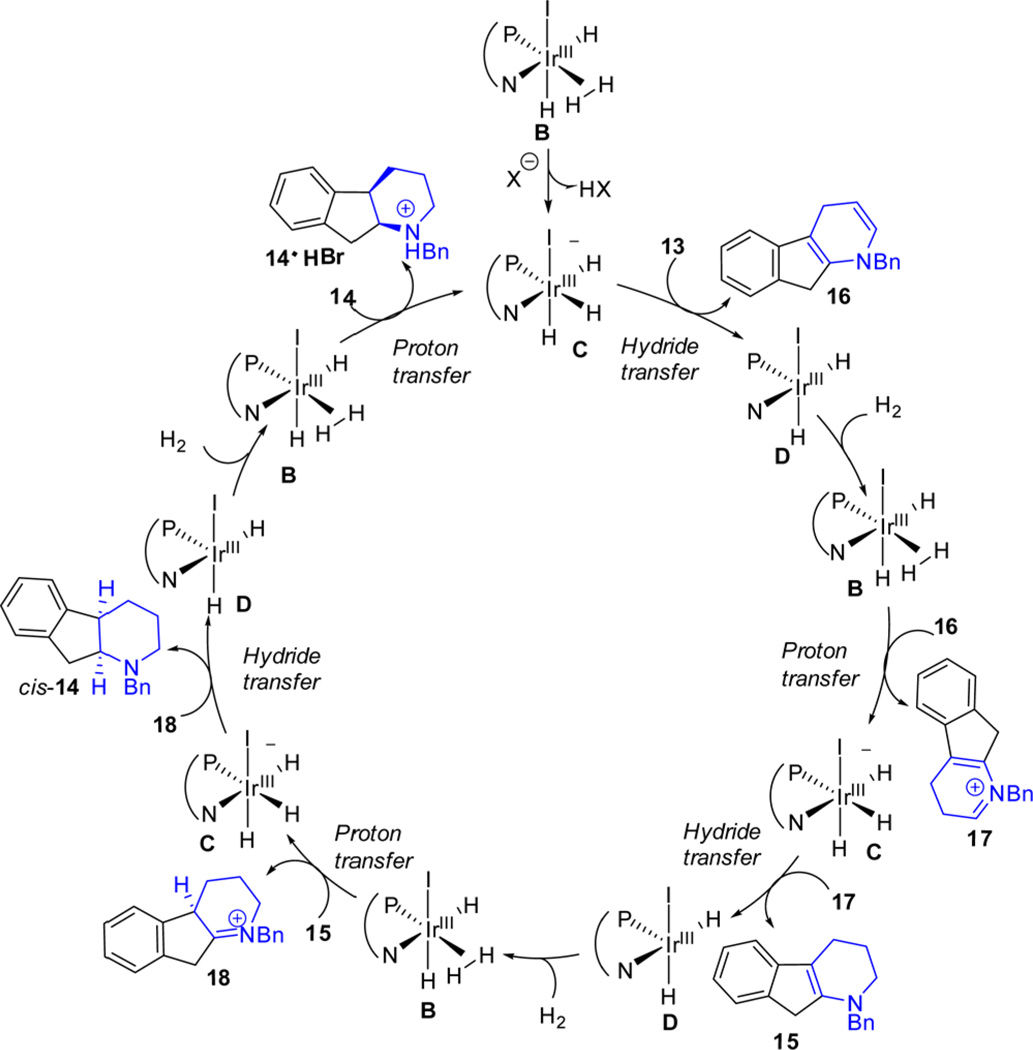
The catalytic cycle of this asymmetric hydrogenation process was proposed as an outer-sphere reaction pathway (Figure 6) based on the previously described mechanism of quinoline reduction31 and computational studies on the enantiodetermining step of key enamine intermediate 15 (Figure 7). The anionic iodo-Ir(III) hydride complex C first delivers one hydride to cationic pyridinium salt 13 to afford 1,4-hydride addition product 16 and a catalytic neutral species D which then coordinates one hydrogen molecule to generate B. Continuous proton transfer from B to 16 and hydride transfer from C to 17 produces key enamine intermediate 15, which undergoes enantio-determining proton transfer and syn hydride delivery to produce cis-14. The catalyst is regenerated by coordinating hydrogen and proton transfer to yield the protonated amine salt 14·HBr.
Figure 6.
Proposed catalytic cycle of outer-sphere dissociative pathway for asymmetric hydrogenation of 13 (CN group omitted).
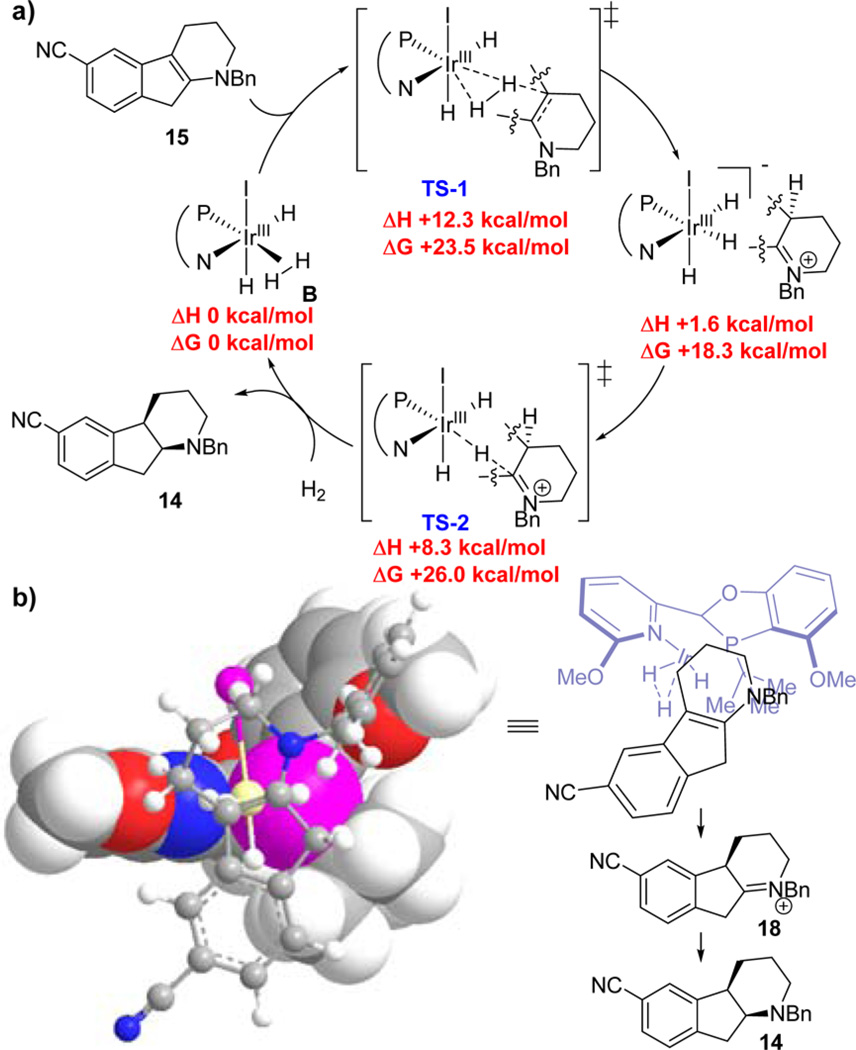
Figure 7.
(a) Proposed asymmetric outer-sphere dissociative catalytic cycle of the Ir-Hydrogenation enamine intermediate 15. DFT calculations: B3LYP/LANL2DZ, CPCM solvation model with THF. Thermal corrections at: 323.15 K at 1 atm. (b) 3D-Model of TS-1 and stereochemical model via syn-Ir-hydride reduction of iminium intermediate 18.
Calculations were conducted with the Gaussian 0919 program at the DFT level of theory employing B3LYP32 and the LANL2DZ basis set with ECP for Ir20 and D95v for the remaining atoms.33 An outer-sphere dissociated mechanism34 similar to the DFT-computed Ir-catalyzed hydrogenation mechanism of imines35 and quinolines31 was used as the basis for the computational studies of the asymmetric Ir-(S,S-L5)-catalyzed hydrogenation of enamine intermediate 15. Iodo-Ir(III) complex B was found to be a viable catalyst for the sequential protonation and hydride delivery pathway for enamine reduction. Although the rate limiting step is hydride delivery (TS-2), the initial hydrogen transfer (protonation) dictates the stereochemical outcome of the transformation (TS-1) as the diastereoselective iridium-hydride delivery would preferentially occur from the less sterically demanding convex face (syn) directed by the stereocenter of iminium intermediate 18. This result is consistent with the experimental observation that the addition of the two hydrogens across the double bond in enamine 15 occurs in an exclusively cis fashion, with no trans isomer detected in the reaction mixture.21
Completion of the Synthesis
With enantioenriched indenopiperidine 14·HBr in hand, selective debenzylation was pursued en route to the final API (Scheme 8). As previously described, incompatibility of the nitrile to hydrogenolysis conditions thwarted our initial efforts. Fortunately, α-chloroethyl chloroformate has been shown to cleave N-alkyl bonds under mild conditions while tolerating a variety of sensitive functionalities.36 Following in situ salt break and extraction, application of this method effects debenzylation to afford amine 19 as its hydrochloride salt. Without isolation, chirality upgrade of the amine with d-DBTA furnished salt 2 in >99.75:0.25 er and 67% overall isolated yield for the telescopic process starting from pyridinium salt 13. Propane phosphonic acid anhydride (T3P)-mediated amide coupling with benzimidazole acid followed by the subsequent hydrochloride salt formation yielded target compound 1 in 76% yield over two steps.
Scheme 8.
Completion of the Synthesis
CONCLUSIONS
An efficient enantioselective synthesis of 11β-HSD-1 inhibitor 1 was developed starting from inexpensive starting materials utilizing low catalyst loadings and excellent step-economy. The total synthesis of the target molecule was achieved in 7 steps with 38% overall yield starting from 3-bromo-4-methylbenzonitrile (4b). The key feature of the synthesis is a sequence involving intramolecular direct C–H arylation to construct the tricyclic indenopyridine and subsequent asymmetric hydrogenation of the resulting fused indenopyridinium salt. The key asymmetric hydrogenation process was achieved by designing a unique, rigid P,N-ligand MeO-BoQPhos. With a low catalyst loading of 1000 ppm [Ir(COD)Cl]2, the enantio-, diastero-, and chemoselective reduction of three contiguous double bonds of compound 13, including a most challenging tetrasubstituted alkene intermediate, was accomplished. This concise and innovative synthetic approach avoided functional group manipulation of the labile nitrile moiety and accelerated the production of 1 on multikilogram scale for clinical studies of this diabetes drug candidate.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health (GM087605 to M.C.K.) for financial support. Computational support for the intramolecular cyclization was provided by XSEDE on SDSC Gordon (TG-CHE120052).
Footnotes
ASSOCIATED CONTENT
Supporting Information
- Experimental procedures, NMR spectra, chromatograms, calculation data, kinetics data (PDF)
The authors declare no competing financial interest.
REFERENCES
- 1.World Health Organization. Geneva, Switzerland: World Health Organization; 2014. Global status report on noncommunicable diseases 2014. [Google Scholar]
- 2.(a) World Health Organization. Geneva, Switzerland: World Health Organization; 2014. Global Health Estimates: Deaths by Cause, Age, Sex and Country, 2000–2012. [Google Scholar]; (b) Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 3.Eckhardt M, Peters S, Mar H, Himmelsbach F. 2011/057054 A1. Patent WO. 2011
- 4.(a) Burns NZ, Krylova IN, Hannoush RN, Baran PS. J. Am. Chem. Soc. 2009;131:9172–9173. doi: 10.1021/ja903745s. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cook CE, Jump JM, Zhang P, Stephens JR, Fail PA, Lee Y-W, Wani MC. 005952336A. U.S. Patent US. 1999
- 5.(a) Qu B, Saha A, Savoie J, Wei X, Yee NK. 2013/25664. Patent WO. 2013; (b) Desrosiers J-N, Li Z, Qu B, Savoie J, Senanayake CH, Wei X, Zeng X. 2015/141651. U.S. Patent US. 2015
- 6.Qu B, Samankumara LP, Savoie J, Fandrick DR, Haddad N, Wei X, Ma S, Lee H, Rodriguez S, Busacca CA, Yee NK, Song JJ, Senanayake CH. J. Org. Chem. 2014;79:993–1000. doi: 10.1021/jo4024864. [DOI] [PubMed] [Google Scholar]
- 7.(a) Bailey PD, Millwood PA, Smith PD. Chem. Commun. 1998:633–640. [Google Scholar]; (b) Hong S, Kawaoka AM, Marks TJ. J. Am. Chem. Soc. 2003;125:15878–15892. doi: 10.1021/ja036266y. [DOI] [PubMed] [Google Scholar]; (c) Leighty MW, Georg GI. ACS Med. Chem. Lett. 2011;2:313–315. doi: 10.1021/ml1003074. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Rueping M, Hubener L. Synlett. 2011;2011:1243–1246. [Google Scholar]; (e) Subba Reddy BV, Ghanty S, Reddy NSS, Reddy YJ, Yadav JS. Synth. Commun. 2014;44:1658–1663. [Google Scholar]; (f) Hamilton JY, Sarlah D, Carreira EM. Angew. Chem. Int. Ed. 2015;54:7644–7647. doi: 10.1002/anie.201501851. [DOI] [PubMed] [Google Scholar]; (g) Tseng C-C, Greig IR, Harrison WTA, Zanda M. Synthesis. 2015;48:73–78. [Google Scholar]
- 8.(a) Gaich T, Baran PS. J. Org. Chem. 2010;75:4657–4673. doi: 10.1021/jo1006812. [DOI] [PubMed] [Google Scholar]; (b) Wender PA. Nat. Prod. Rep. 2014;31:433–440. doi: 10.1039/c4np00013g. [DOI] [PubMed] [Google Scholar]
- 9.(a) Wang Q, Huang W, Yuan H, Cai Q, Chen L, Lv H, Zhang X. J. Am. Chem. Soc. 2014;136:16120–16123. doi: 10.1021/ja509005e. [DOI] [PubMed] [Google Scholar]; (b) Molinaro C, Scott JP, Shevlin M, Wise C, Ménard A, Gibb A, Junker EM, Lieberman D. J. Am. Chem. Soc. 2015;137:999–1006. doi: 10.1021/ja511872a. [DOI] [PubMed] [Google Scholar]
- 10.Tang W, Qu B, Capacci AG, Rodriguez S, Wei X, Haddad N, Narayanan B, Ma S, Grinberg N, Yee NK, Krishnamurthy D, Senanayake CH. Org. Lett. 2010;12:176–179. doi: 10.1021/ol9025815. [DOI] [PubMed] [Google Scholar]
- 11.(a) Tang W, Capacci AG, Wei X, Li W, White A, Patel ND, Savoie J, Gao JJ, Rodriguez S, Qu B, Haddad N, Lu BZ, Krishnamurthy D, Yee NK, Senanayake CH. Angew. Chem. Int. Ed. 2010;49:5879–5883. doi: 10.1002/anie.201002404. [DOI] [PubMed] [Google Scholar]; (b) Fandrick DR, Fandrick KR, Reeves JT, Tan Z, Tang W, Capacci AG, Rodriguez S, Song JJ, Lee H, Yee NK, Senanayake CH. J. Am. Chem. Soc. 2010;132:7600–7601. doi: 10.1021/ja103312x. [DOI] [PubMed] [Google Scholar]; (c) Tang W, Capacci AG, White A, Ma S, Rodriguez S, Qu B, Savoie J, Patel ND, Wei X, Haddad N, Grinberg N, Yee NK, Krishnamurthy D, Senanayake CH. Org. Lett. 2010;12:1104–1107. doi: 10.1021/ol1000999. [DOI] [PubMed] [Google Scholar]; (d) Rodriguez S, Qu B, Haddad N, Reeves DC, Tang W, Lee H, Krishnamurthy D, Senanayake CH. Adv. Synth. Catal. 2011;353:533–537. [Google Scholar]; (e) Xu G, Fu W, Liu G, Senanayake CH, Tang W. J. Am. Chem. Soc. 2014;136:570–573. doi: 10.1021/ja409669r. [DOI] [PubMed] [Google Scholar]; (f) Qu B, Mangunuru HPR, Wei X, Fandrick KR, Desrosiers J-N, Sieber JD, Kurouski D, Haddad N, Samankumara LP, Lee H, Savoie J, Ma S, Grinberg N, Sarvestani M, Yee NK, Song JJ, Senanayake CH. Org. Lett. 2016;18:4920–4923. doi: 10.1021/acs.orglett.6b02401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.For reviews, see Alberico D, Scott ME, Lautens M. Chem. Rev. 2007;107:174–238. doi: 10.1021/cr0509760. Ackermann L, Vicente R, Kapdi AR. Angew. Chem. Int. Ed. 2009;48:9792–9826. doi: 10.1002/anie.200902996.
- 13.For the examples on intramolecular quinoline directed C-3 arylation, see Singh SK, Ruchelman AL, Li T-K, Liu A, Liu LF, LaVoie EJ. J. Med. Chem. 2003;46:2254–2257. doi: 10.1021/jm020498a. Meyers C, Rombouts G, Loones KTJ, Coelho A, Maes BUW. Adv. Synth. Catal. 2008;350:465–470.
- 14.(a) Furukawa N, Tsuruoka M, Fujihara H. Heterocycles. 1986;24:3337–3340. [Google Scholar]; (b) Niwa T, Yorimitsu H, Oshima K. Angew. Chem. Int. Ed. 2007;46:2643–2645. doi: 10.1002/anie.200604472. [DOI] [PubMed] [Google Scholar]; (c) Shang R, Yang Z-W, Wang Y, Zhang S-L, Liu L. J. Am. Chem. Soc. 2010;132:14391–14393. doi: 10.1021/ja107103b. [DOI] [PubMed] [Google Scholar]; (d) Zhu F, Wang Z-X. J. Org. Chem. 2014;79:4285–4292. doi: 10.1021/jo500619f. [DOI] [PubMed] [Google Scholar]; (e) Kianmehr E, Faghih N, Khan KM. Org. Lett. 2015;17:414–417. doi: 10.1021/ol503238a. [DOI] [PubMed] [Google Scholar]
- 15.(a) Busacca CA, Wei X, Haddad N, Kapadia S, Lorenz JC, Saha AK, Varsolona RJ, Berkenbusch T, Campbell SC, Farina V, Feng X, Gonnella NC, Grinberg N, Jones P-J, Lee H, Li Z, Niemeier O, Samstag W, Sarvestani M, Schroeder J, Smoliga J, Spinelli EM, Vitous J, Senanayake CH. Asian J. Org. Chem. 2012;1:80–89. [Google Scholar]; (b) Wei X, Shu C, Haddad N, Zeng X, Patel ND, Tan Z, Liu J, Lee H, Shen S, Campbell S, Varsolona RJ, Busacca CA, Hossain A, Yee NK, Senanayake CH. Org. Lett. 2013;15:1016–1019. doi: 10.1021/ol303498m. [DOI] [PubMed] [Google Scholar]
- 16.E-configuration of the free base 8 was confirmed by NMR analyses (See the Supporting Information). Compound 8 decomposes (polymerization) gradually in air at rt.
- 17.Desrosiers J-N, Wei X, Gutierrez O, Savoie J, Qu B, Zeng X, Lee H, Grinberg N, Haddad N, Yee NK, Roschangar F, Song JJ, Kozlowski MC, Senanayake CH. Chem. Sci. 2016;7:5581–5586. doi: 10.1039/c6sc01457g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Ni-catalyzed conditions for substrate 7b led to lower yields than those of the Pd-catalyzed system.
- 19.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, revision C.01. Wallingford, CT: Gaussian, Inc.; 2009. [Google Scholar]
- 20.(a) Hay PJ, Wadt WR. J. Chem. Phys. 1985;82:270–283. [Google Scholar]; (b) Hay PJ, Wadt WR. J. Chem. Phys. 1985;82:299–310. [Google Scholar]
- 21.About 5% of the trans-isomer was generated during heterogeneous reduction. It was isolated from mother liquor and characterized (see the Supporting Information).
- 22.(a) Wang D-S, Chen Q-A, Li W, Yu C-B, Zhou Y-G, Zhang X. J. Am. Chem. Soc. 2010;132:8909–8911. doi: 10.1021/ja103668q. [DOI] [PubMed] [Google Scholar]; (b) Dobereiner GE, Nova A, Schley ND, Hazari N, Miller SJ, Eisenstein O, Crabtree RH. J. Am. Chem. Soc. 2011;133:7547–7562. doi: 10.1021/ja2014983. [DOI] [PubMed] [Google Scholar]; (c) Woodmansee DH, Pfaltz A. Top. Organomet. Chem. 2011;34:31–76. [Google Scholar]; (d) Wang D-S, Chen Q-A, Lu S-M, Zhou Y-G. Chem. Rev. 2012;112:2557–2590. doi: 10.1021/cr200328h. [DOI] [PubMed] [Google Scholar]; (e) Liu Y, Du H. J. Am. Chem. Soc. 2013;135:12968–12971. doi: 10.1021/ja406761j. [DOI] [PubMed] [Google Scholar]; (f) Zhao D, Glorius F. Angew. Chem. Int. Ed. 2013;52:9616–9618. doi: 10.1002/anie.201304756. [DOI] [PubMed] [Google Scholar]; (g) Huang W-X, Liu L-J, Wu B, Feng G-S, Wang B, Zhou Y-G. Org. Lett. 2016;18:3082–3085. doi: 10.1021/acs.orglett.6b01190. [DOI] [PubMed] [Google Scholar]; (h) Gualandi A, Savoia D. RSC Adv. 2016;6:18419–18451. [Google Scholar]
- 23.(a) Glorius F, Spielkamp N, Holle S, Goddard R, Lehmann CW. Angew. Chem. Int. Ed. 2004;43:2850–2852. doi: 10.1002/anie.200453942. [DOI] [PubMed] [Google Scholar]; (b) Legault CY, Charette AB. J. Am. Chem. Soc. 2005;127:8966–8967. doi: 10.1021/ja0525298. [DOI] [PubMed] [Google Scholar]; (c) Ye Z-S, Chen M-W, Chen Q-A, Shi L, Duan Y, Zhou Y-G. Angew. Chem. Int. Ed. 2012;51:10181–10184. doi: 10.1002/anie.201205187. [DOI] [PubMed] [Google Scholar]; (d) Chang M, Huang Y, Liu S, Chen Y, Krska SW, Davies IW, Zhang X. Angew. Chem. Int. Ed. 2014;53:12761–12764. doi: 10.1002/anie.201406762. [DOI] [PubMed] [Google Scholar]; (e) Kita Y, Iimuro A, Hida S, Mashima K. Chem. Lett. 2014;43:284–286. [Google Scholar]; (f) Chen M-W, Ye Z-S, Chen Z-P, Wu B, Zhou Y-G. Org. Chem. Front. 2015;2:586–589. [Google Scholar]; (g) Zhou Q, Zhang L, Meng W, Feng X, Yang J, Du H. Org. Lett. 2016;18:5189–5191. doi: 10.1021/acs.orglett.6b02610. [DOI] [PubMed] [Google Scholar]
- 24.Li Z-W, Wang T-L, He Y-M, Wang Z-J, Fan Q-H, Pan J, Xu L-J. Org. Lett. 2008;10:5265–5268. doi: 10.1021/ol802016w. [DOI] [PubMed] [Google Scholar]
- 25.(a) Wang Q, Huang W, Yuan H, Cai Q, Chen L, Lv H, Zhang X. J. Am. Chem. Soc. 2014;136:16120–16123. doi: 10.1021/ja509005e. [DOI] [PubMed] [Google Scholar]; (b) Molinaro C, Scott JP, Shevlin M, Wise C, Ménard A, Gibb A, Junker EM, Lieberman D. J. Am. Chem. Soc. 2015;137:999–1006. doi: 10.1021/ja511872a. [DOI] [PubMed] [Google Scholar]
- 26.Lei A, Chen M, He M, Zhang X. Eur. J. Org. Chem. 2006;2006:4343–4347. [Google Scholar]
- 27.Crabtree RH. Acc. Chem. Res. 1979;12:331–337. [Google Scholar]
- 28.Qu B, Samankumara LP, Ma S, Fandrick KR, Desrosiers J-N, Rodriguez S, Li Z, Haddad N, Han ZS, McKellop K, Pennino S, Grinberg N, Gonnella NC, Song JJ, Senanayake CH. Angew. Chem. Int. Ed. 2014;53:14428–14432. doi: 10.1002/anie.201408929. [DOI] [PubMed] [Google Scholar]
- 29.Busacca CA, Qu B, Grĕt N, Fandrick KR, Saha AK, Marsini M, Reeves D, Haddad N, Eriksson M, Wu J-P, Grinberg N, Lee H, Li Z, Lu B, Chen D, Hong Y, Ma S, Senanayake CH. Adv. Synth. Catal. 2013;355:1455–1463. [Google Scholar]
- 30.Free base indenopyridine 9 has demonstrated similar reactivity at high catalyst load and the same enantioselectivity at 85:15 er, but with formation of about 10% dimeric impurity resulted from nonchemoselective nitrile reduction.
- 31.Dobereiner GE, Nova A, Schley ND, Hazari N, Miller SJ, Eisenstein O, Crabtree RH. J. Am. Chem. Soc. 2011;133:7547–7562. doi: 10.1021/ja2014983. [DOI] [PubMed] [Google Scholar]
- 32.(a) Becke AD. J. Chem. Phys. 1993;98:5648–5652. [Google Scholar]; (b) Lee C, Yang W, Parr RG. Phys. Rev. B: Condens. Matter Mater. Phys. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 33.Dunning TH, Jr, Hay PJ. Modern Theoretical Chemistry. In: Schaefer HF III, editor. Methods of Electronic Structure Theory series. Vol. 3. New York: Plenum; 1977. pp. 1–28. [Google Scholar]
- 34.(a) Martín M, Sola E, Tejero S, Andrés JL, Oro LA. Chem. - Eur. J. 2006;12:4043–4056. doi: 10.1002/chem.200501230. [DOI] [PubMed] [Google Scholar]; (b) Martín M, Sola E, Tejero S, López JA, Oro LA. Chem. - Eur. J. 2006;12:4057–4068. doi: 10.1002/chem.200501231. [DOI] [PubMed] [Google Scholar]
- 35.Hopmann KH, Bayer A. Organometallics. 2011;30:2483–2497. [Google Scholar]
- 36.(a) Olofson RA, Martz JT, Senet J-P, Piteau M, Malfroot T. J. Org. Chem. 1984;49:2081–2082. [Google Scholar]; (b) Yang BV, O’Rourke D, Li J. Synlett. 1993;1993:195–196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.