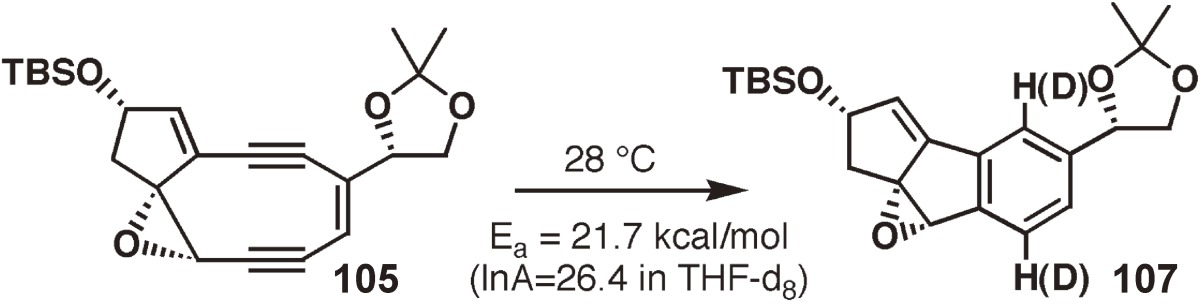
Abstract
Our chemical syntheses and related scientific investigations of natural products with complex architectures and powerful biological activities are described, focusing on the very large 3 nm-long polycyclic ethers called the ciguatoxins, highly strained and labile chromoprotein antitumor antibiotics featuring nine-membered enediyne cores, and extremely potent anthelmintic macrolides called the avermectins.
Keywords: natural products, total synthesis, ciguatoxin, monoclonal antibody, enediyne antibiotics, avermectin
1. Introduction
Nature has the incredible power to create new chemical molecules with remarkable structures and profound biological functions. These molecules or natural products often present a number of new challenges to researchers.1) One of the most simple and basic questions regarding natural products is if and how we can synthesize them chemically in the laboratory. The total synthesis of complex, large bioactive natural product molecules is one of the most difficult, exciting, and challenging endeavors in the chemical sciences. Such endeavors stimulate the development of powerful synthetic strategies, tactics, and methodologies, and constitute the basis for molecular science. Furthermore, we can help address public health problems and advance the biological, medicinal, and pharmaceutical studies of bioactive compounds by taking on these huge synthetic challenges.2,3)
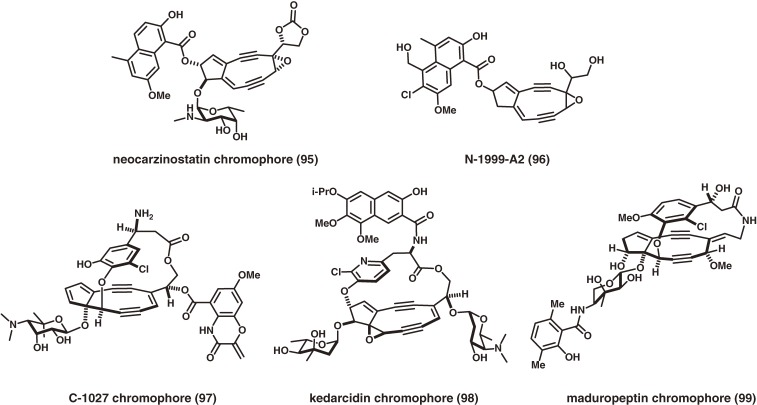
This review focuses on describing our synthetic studies and related studies of two families of bioactive natural products: the ciguatoxins, which are large 3 nm-long molecules that exhibit extremely potent neurotoxicity,4–12) and the nine-membered enediyne chromoprotein antitumor antibiotics, which have delicate architectures that include the chromophores of neocarzinostatin,13) N1999-A2,14) maduropeptin,15) C-1027,16) and kedarcidin.17) In addition, the innovative syntheses of other structurally complex bioactive natural products such as avermectin18) and milbemycin,19) which are potent anthelmintic macrolides, are outlined.
2. Determination of the absolute configuration of ciguatoxins and the first total synthesis of ciguatoxins and relevant associated studies
2.1. Initial stages of our synthetic study and the absolute configuration.
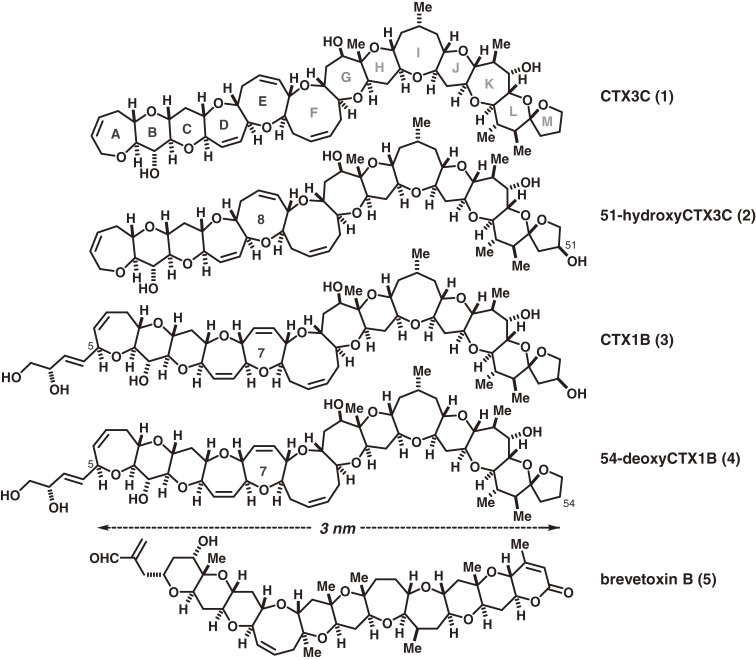
More than 50,000 people suffer annually from “ciguatera” fish poisoning (CFP), which is particularly common in subtropical and tropical regions. CFP is caused by the ingestion of a variety of reef fish that have accumulated trace amounts of the causative neurotoxins, designated as ciguatoxins (CTXs, Fig. 1).20–23) CTXs are synthesized by dinoflagellates and enter the food chain. These toxins cause gastrointestinal, cardiovascular, and neurological disorders, which may last for months or years. The lethal potency of CTX1B (LD50 = ∼0.25 µg/kg) by intraperitoneal injection into mice is much greater than that of the famous puffer fish toxin, tetrodotoxin (∼10 µg/kg). Difficulties in predicting sources of CTXs, and in detecting and treating ciguatera, have a significant economic and human health impact. The isolation and structural characterization of these toxins were long hampered by the extremely low concentrations of the toxins in fish and the complexity of their chemical structures. In 1989, Yasumoto and co-workers elucidated the structure of CTX1B (4), a huge ladder-like polycyclic ether with a molecular length of over 3 nm.24,25) To date, more than 20 congeners of CTXs have been structurally determined.26) This CTX family is far more toxic and dangerous than the related red-tide brevetoxins, such as brevetoxin B (5) (Fig. 1).20) Prof. Yasumoto asked me to collaborate with him in defining the structure and absolute stereochemistry of CTXs using synthetic strategies in 1988, just before their elucidation of the structure of CTX1B. The total synthesis of CTXs is a formidable challenge, yet is the sole realistic solution for obtaining sufficient quantities of CTXs for biological, medical, and pharmacological studies.
Figure 1.
Major Pacific ciguatoxins and brevetoxin B.
There was little prospect for our success in the total synthesis of CTXs, which possess 13 rings and over 30 stereogenic centers, when we launched our synthetic endeavor in 1989. Early on, we developed enantioselective routes to the medium (7-, 8-, and 9-membered) ring ethers of ciguatoxins27–31) and the circular dichroism (CD) studies of synthetic AB ring fragments implicated the absolute configuration of CTXs.28,32,33) Then, we quickly realized that convergent assembly of the structural fragments was the key for successful construction of the huge ladder-like polycyclic ether system.12,34)
In 1995, the formidable and pioneering total synthesis of brevetoxin B (5) was reported by Nicolaou and co-workers after a 12-year struggle.35) We had noticed the power of ring-closing metathesis (RCM) mediated by the Grubbs’ catalyst36) and thus completely revised our strategy for the total synthesis of ciguatoxins. Our 12-year effort culminated in the first total synthesis of CTX3C (1)37) in 2001 (Fig. 2).4) Our synthesis was appreciated as “The Art of Total Synthesis” by Science (2001, 294, 1842), “A Synthetic Tour de Force” by Chemical & Engineering News (USA) (2001, Dec. 3, p. 9), and “Organic Chemistry Takes on Tropical Seafood Poisoning” by The Lancet (2001, 358, 1278). Since then, our highly convergent and unified strategic approach featuring chemoselective RCM/radical cyclization reactions as key tactics has been improved,5,6,8) and enabled the total synthesis of three other important Pacific congeners, 51-hydroxyCTX3C,7) CTX1B,7,9) and 54-deoxyCTX1B,9) as well as F-ring modified analogs.38,39) The synthesis of these compounds has significantly impacted the biological and pharmacological studies of CTXs.
Figure 2.
Unified convergent [X + 2 + Y] synthetic strategy.
2.2. Unified convergent [X + 2 + Y] strategy.
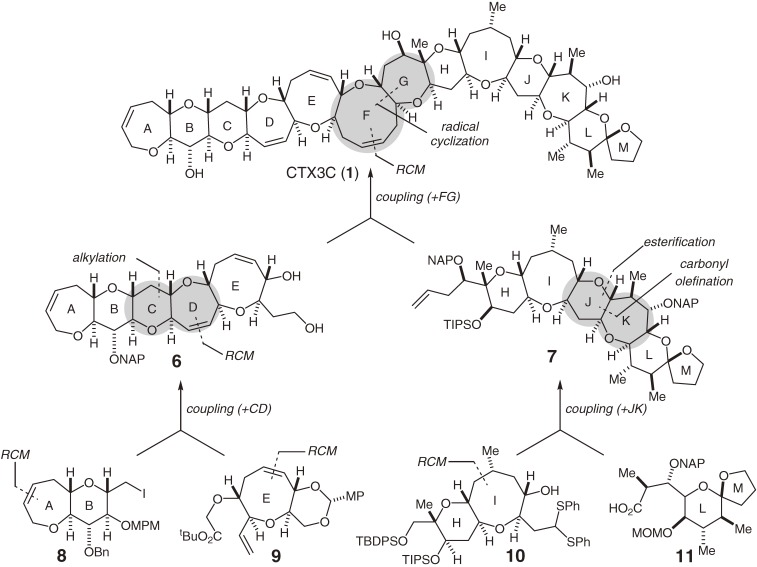
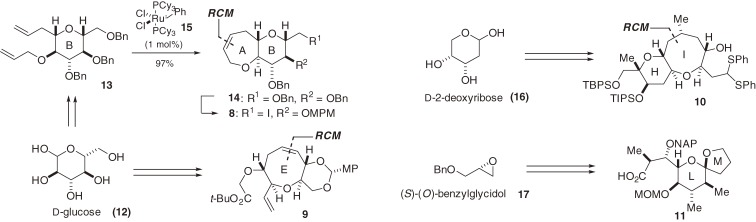
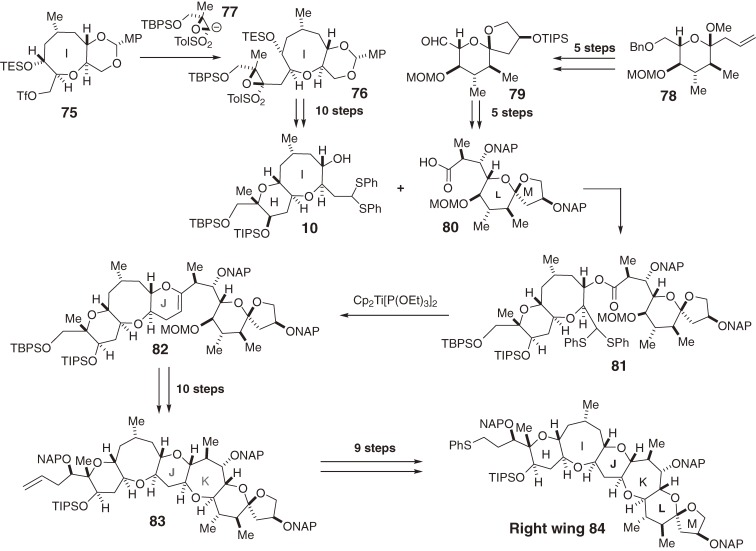
The synthetic strategy used for the first synthesis of ciguatoxin CTX3C (1), employing the RCM reaction and radical cyclization as key tactics, is illustrated in Fig. 2.4–6,8–12) The size and complexity of this fused ether array led us to use a unified convergent strategy called the [X + 2 + Y] strategy.12,40) This strategy involved the coupling of the synthetic fragments followed by the construction of the two rings and introduction of the two stereocenters. The challenge lay in developing a reaction sequence to construct the new ethers of the requisite ring sizes in a stereoselective manner without affecting the preexisting functionalities. Consequently, we improved the convergence of the assembly in which four simple fragments (8, 9, 10, and 11) were coupled and further modified to form the CD-, JK-, and FG-rings. The comparably complex ABCDE- and HIJLKLM-ring systems (6 and 7, respectively) would be synthesized prior to the final coupling at the central region of the molecule. The four fragments (8–11) were prepared from the starting materials D-glucose (12), D-2-deoxyribose (16), and (S)-(O)-benzylglycidol (17) (Scheme 1).10–12) The medium-sized ether rings (the A-, E-, and I-rings) were constructed using an RCM reaction36) (for example, 13 → 14), which greatly simplified the synthesis of the fragments.
Scheme 1.
Synthesis of the simple fragments.
2.3. Synthesis of the left wing of CTX3C.
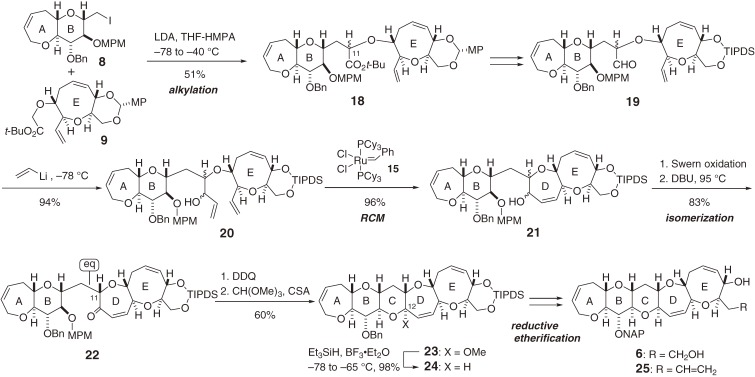
The left wing segment (6) of CTX3C (1) was synthesized41) from the AB- and E-rings (8 and 9) and subsequent construction of the CD-ring using an alkylation/metathesis sequence (Scheme 2).42) Tetraene 20 was smoothly cyclized using Grubbs’ catalyst 1536) to provide the seven-membered D-ring 21 without interfering with the olefins in the A and E rings. Removal of the p-methoxyphenylmethyl (MPM) group in 22 followed by methyl acetalization afforded pentacycle 23. The reductive etherification43) of 23 set the C12-stereocenter and provided the ABCDE-ring segment 24. Subsequent functional group manipulation of 24 yielded the 2-naphthylmethyl- (NAP-) protected left wing segments 6 and 25.
Scheme 2.
Synthesis of the left (A–E) wing of CTX3C.
2.4. Synthesis of the right wing of CTX3C.
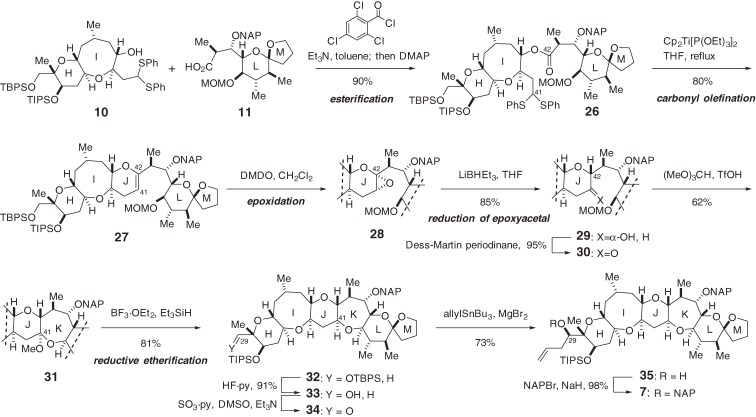
A different methodology was applied for the synthesis of the right wing segment (7) of CTX3C (1) (Scheme 3).44,45) Yamaguchi esterification46) between alcohol 10 and carboxylic acid 11 produced ester 26. Construction of the J-ring from 26 by C-C bond formation was challenging due to steric hindrance at C42.47) Intramolecular carbonyl olefination, however, using Cp2Ti[P(OEt)3]2 developed by Takeda and co-worker48) successfully closed the six-membered J-ring to afford pentacycle 27. The stereoselective introduction of hydrogen at C42 and the oxygen functionality at C41 was also problematic. Dihydropyran 27 has a strong conformational bias for accepting the reagent from the α-face, since the sterically demanding LM-ring portion projects toward the β-face. For example, hydroboration of 27 led predominantly to the undesired stereoisomer with an α-hydrogen at C42.
Scheme 3.
Synthesis of the right (H–M) wing of CTX3C.
To introduce the β-hydrogen at C42, it was necessary to develop a method with stereoselectivity complimentary to that of hydroboration. The new method employed was a two-step protocol based on the stereoselective reduction of an epoxyacetal.45) The α-epoxide 28 was synthesized from 27 as a sole product using dimethyldioxirane (DMDO). SN2-type hydride delivery to the C42-acetal epoxide of 28 was realized using LiBHEt3 to yield the desired isomer 29 exclusively. Alcohol 29 was oxidized to 30, which was then exposed to triflic acid and (MeO)3CH in hexane to produce the seven-membered methyl acetal 31 directly with concomitant loss of the MOM group. Reductive etherification of acetal 31 constructed the final ether ring with complete stereocontrol at C41, affording HIJKLM-ring system 32. The carbon chain corresponding to the G-ring was then introduced by chelation-controlled stereoselective allylation of aldehyde 34 and subsequent NAP protection of resultant alcohol 35 yielded the right wing segment 7.
2.5. The first total synthesis of CTX3C.
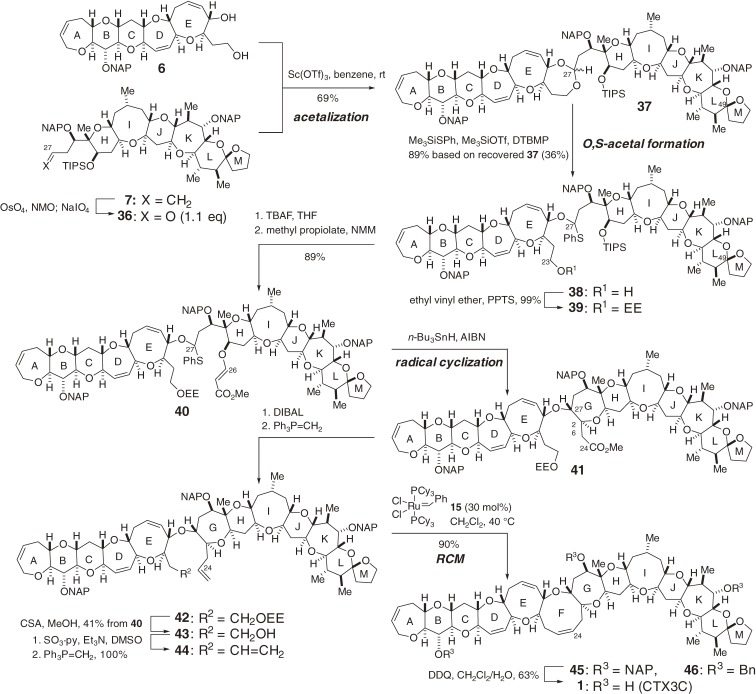
Coupling of the left and right wing segments of CTX3C (1) and construction of the central FG-ring is far more challenging than the previous two couplings because of the increased complexity of the substrates. After a considerable number of unsuccessful experiments with model systems, we found that the Sasaki protocol49–51) was adaptable for constructing the EFGH-ring system from the E- and H-ring fragments following several crucial modifications and refinements.52) The application of this modified Sasaki protocol to the synthesis of 1 was undertaken as shown in Scheme 4.4–6,8,10–12) Condensation of 1,4-diol 6 and aldehyde 36 using catalytic Sc(OTf)3 successfully delivered seven-membered acetal 37.50) The combination of Me3SiOTf and Me3SiSPh in the presence of 2,6-di-t-butyl-4-methyl pyridine (DTBMP)53) cleaved the acetal of 37 to form O,S-acetal 38.52) The C49-spiroacetal remained intact in this acetal cleavage reaction.
Scheme 4.
Total synthesis of CTX3C.
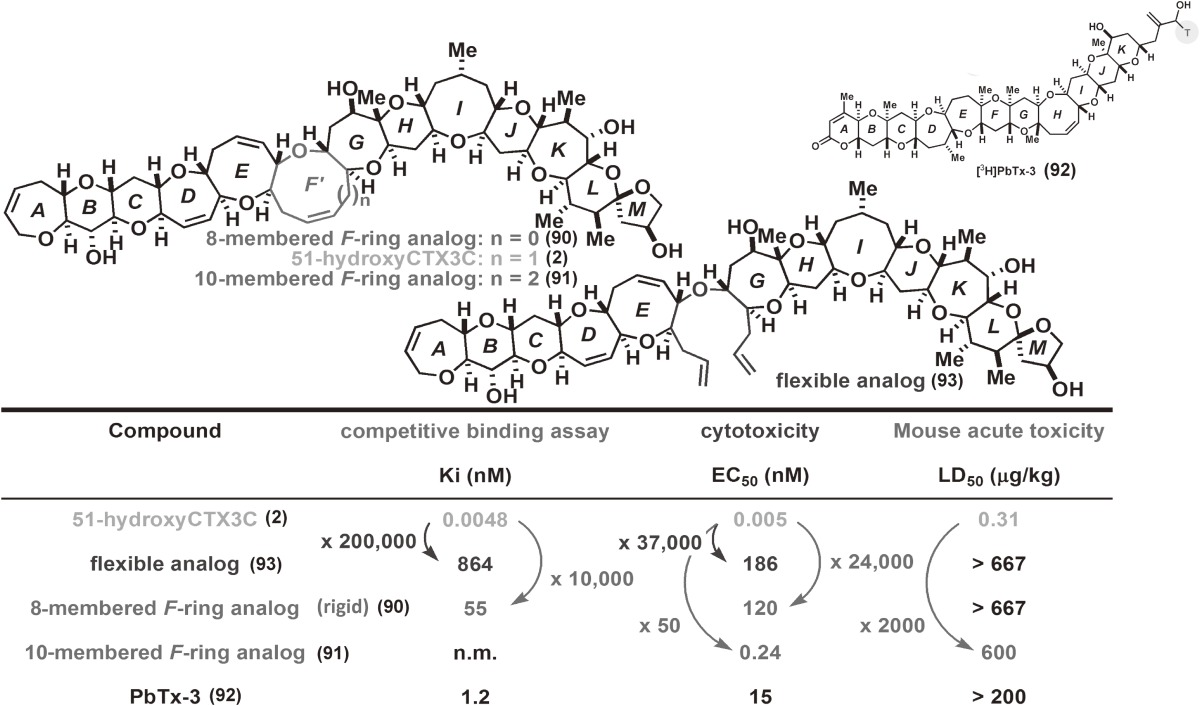
Stereoselective construction of the G-ring was then investigated. The primary alcohol of 38 was protected as the ethoxyethyl (EE) ether to give 39. Removal of the TIPS group from 39 followed by treatment with methyl propiolate and N-methylmorpholine (NMM) afforded β-(E)-alkoxyacrylate 40. Compound 40 was subjected to radical cyclization using n-Bu3SnH and 2,2′-azobisisobutyronitrile (AIBN), giving rise to the desired oxepane 41. The generated C27-radical added to the α,β-unsaturated ester in a completely stereo- and chemo-selective manner. Ester 41 was converted to pentaene 44 by conventional means. Grubbs’ catalyst 15 effectively induced RCM of the two terminal olefins of 44 to produce NAP-protected CTX3C 45 without touching the other olefins.52) The final global NAP-deprotection of 45 with DDQ successfully yielded the target CTX3C (1). However, the final deprotection of functional groups of a large complex polyether molecule is generally no easy task. In fact, the hydroxyl groups were originally protected as the benzyl ether and the deprotection of the tris-benzyl ether 46 was the most problematic step in our total synthesis performed in 2001.4–6,8,10–12) The synthetic CTX3C was determined to be identical to the naturally occurring form in every respect, including mouse acute toxicity, which unambiguously confirmed the absolute stereochemistry of ciguatoxins.28,32,33) Thus, the power of our unified convergent [X + 2 + Y] coupling strategy12,40) for constructing this large and complex ladder-like polyether system was clearly demonstrated. In addition, it should be noted that the synthetic fragments and penultimate intermediate, tris-benzyl ether 46, did not exhibit detectable toxicity (see Fig. 7 in Section 2.11). This suggests that the products of this synthetic route are nontoxic until the final deprotection step.
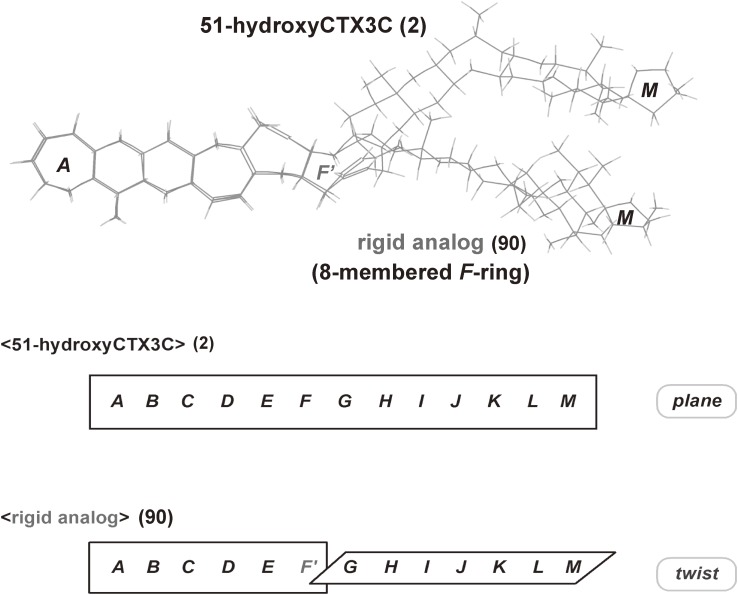
Figure 7.
Most stable molecular shapes of 51-hydroxyCTX3C and its 8-membered F-ring rigid analog, calculated by Macro Model ver. 8.6, MM2*.
2.6. The second-generation total synthesis of CTX3C.
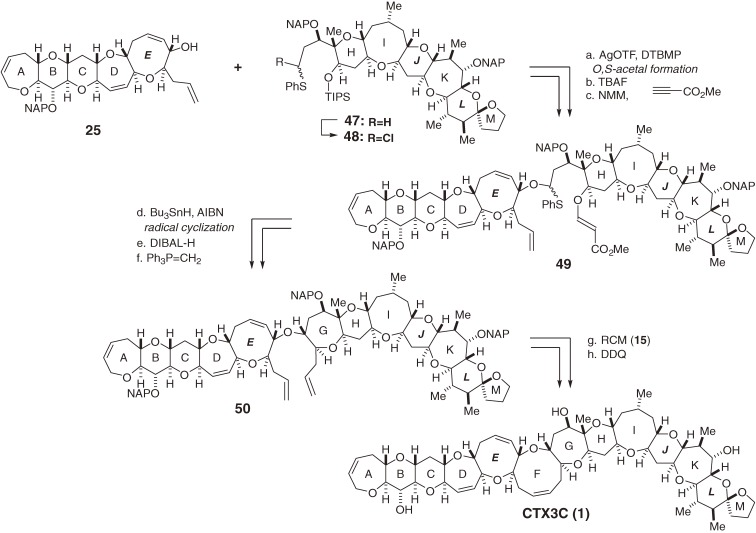
The first-generation total synthesis demonstrated the power of the O,S-acetal strategy to build complex polyether structures. In order to synthesize ciguatoxin congeners with acid-sensitive functionalities, such as CTX1B (4),24,25) we developed an alternative, direct, and milder route to the O,S-acetal without using highly acidic conditions (Scheme 5). Our new synthetic strategy relied on the direct construction of O,S-acetal 49 by coupling secondary alcohol 25, which possesses a terminal olefin, and α-halosulfide 48 using a halophilic activator, AgOTf in the presence of DTBMP and 4 Å molecular sieves.53–56) Then, similar to the first-generation synthesis, subjecting β-alkoxyacrylate 49 to radical cyclization allowed the stereoselective construction of the G-ring of 50. The RCM reaction of 50 and subsequent global deprotection provided the target CTX3C (1).6,8,11,12) This new streamlined assembly improved the delivery of 1.
Scheme 5.
The second-generation total synthesis of CTX3C.
2.7. Synthesis of the left wing of CTX1B.
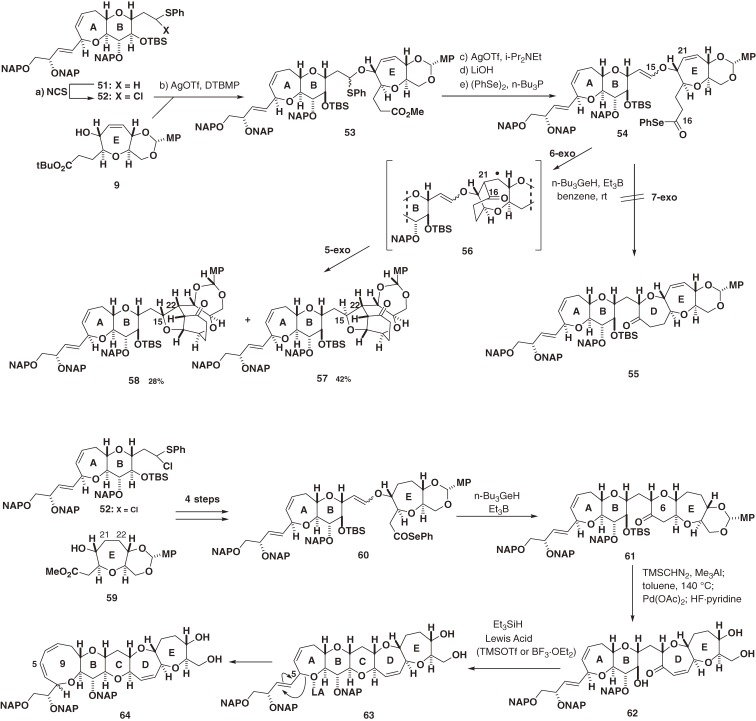
Ciguatoxin CTX1B (4) is biologically more potent and structurally more complex than CTX3C (1).21–25,37) CTX1B (4) not only contains an additional dihydroxybutenyl side chain embedded in the A-ring, but it also possesses a seven-membered E-ring rather than the eight-membered ring of CTX3C (1). The synthetic challenge presented by 4 is heightened by the presence of the acid/base/oxidant-sensitive bisallylic C5-ether.7,57) Indeed, the C-O bond at C5 was readily cleaved and rearrangement occurred, especially when Lewis acid was used (Scheme 6).9) Furthermore, the C21–C22 double bond in the E-ring presented unexpected additional complications upon radical cyclization. Thus, we were obliged to take a more reliable detour using the saturated E-ring during construction of the ABCDE ring system and then introducing the E-ring double bond at a later stage. Finally, the fully functionalized left wing segment 74 of 4 was synthesized as shown in Scheme 7 via C-ring formation through stereoselective radical cyclization of cis-vinyl sulfoxide as a key step.9)
Scheme 6.
Complications caused by the A-ring butenyl side chain and the double bond of the seven-membered E-ring.
Scheme 7.
Synthesis of the left (A–E) wing of CTX1B.
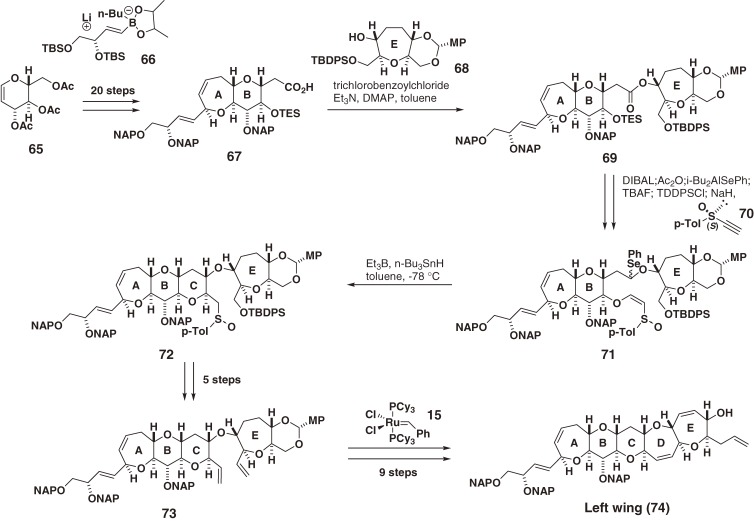
2.8. Synthesis of the right wing of CTX1B.
The right wing segment (84) of CTX1B (4) was synthesized from the HI ring fragment 10 and LM ring fragment 80 (Scheme 8) in a manner similar to 7 in the synthesis of CTX3C (1) (Scheme 3).44,45)
Scheme 8.
Synthesis of the right (H–M) wing of CTX1B.
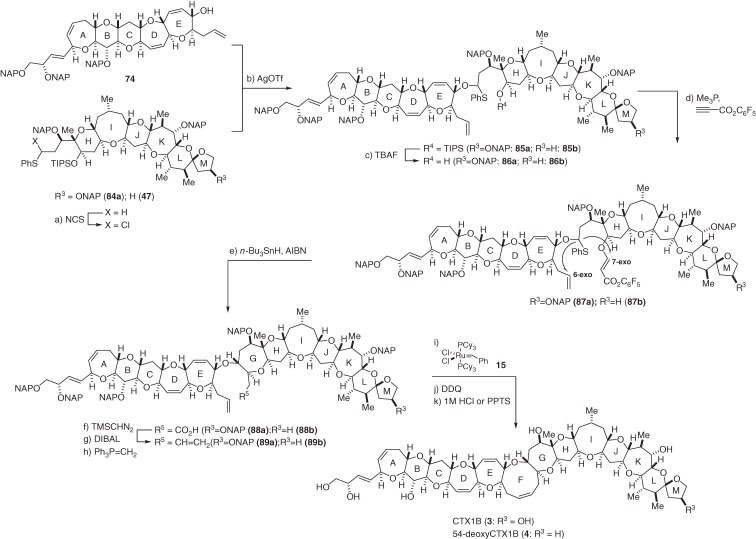
2.9. The first total syntheses of CTX1B and 54-deoxyCTX1B.
With a sufficient amount of the left wing 74 in hand, we embarked on the critical coupling of the left and right wings, 74 and 84a, to construct the two major Pacific ciguatoxins, CTX1B (3)24,25) and 54-deoxyCTX1B (4).26) This coupling reaction is described in Scheme 9. The right wing sulfide 84a44,45) was chlorinated using freshly recrystallized NCS and the resultant α-chlorosulfide was coupled without purification to the left wing alcohol 74 by the action of AgOTf to provide O,S-acetal 85a.7) Despite extensive efforts, the yield of 85a could not be improved (∼26%), possibly due to the presence of the A-ring dihydroxybutenyl substituent. Strongly electron-withdrawing pentafluorophenyl acrylate58) was attached to 86a instead of the methyl ester to improve chemoselective 7-exo radical cyclization. Formation of the 7-membered G-ring was achieved by radical reaction of 87a with n-Bu3SnH and AIBN, which provided 88a in 42% yield, along with the 6-exo product (20%).7–9) Use of the methyl ester significantly decreased the yield of the 7-exo product. The resulting carboxylic acid 88a was converted to the corresponding terminal olefin 89a, and RCM reaction promoted by Grubbs’ catalyst 15 constructed the nine-membered F-ring in 63% yield. Lastly, oxidative removal of the six 2-naphthylmethyl (NAP) groups21) with DDQ furnished CTX1B (3) in 20% overall yield.7,9) The synthesis of 54-deoxyCTX1B (4) was similarly accomplished from 74 and 47. Thus, a practical, reliable and stereoselective route to the Pacific ciguatoxins, CTX1B (3) and 54-deoxyCTX1B (4),7,9) as well as the left wing of Calibbean CTX (C-CTX)59,60) was established.
Scheme 9.
Total synthesis of CTX1B and 54-deoxyCTX1B.
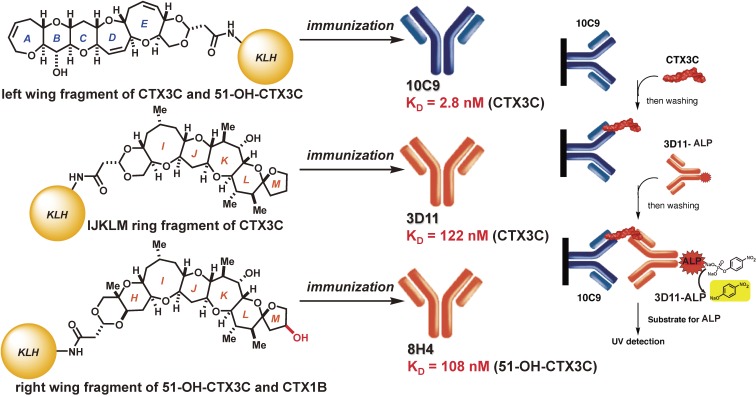
2.10. Rational design of specific monoclonal antibodies and direct sandwich immunoassay.
In addition to the traditional mouse bioassay using fish extracts, several other methods have been developed to detect ciguatoxins in contaminated fish.21,61–63) However, antibody-based immunoassays remain the most desirable method for accurate, sensitive, routine, and portable use. We therefore planned a synthesis-based approach using rationally designed synthetic haptens to address the problem of antibody development. Numerous immunization studies in collaboration with Profs. Tsumuraya and Fujii using synthetic hapten-keyhole limpet hemocyanin (KLH) conjugates showed that the polyether fragments, which possess more than five ether rings and have a surface area larger than 400 Å2, can be used as synthetic haptens to provide highly sensitive and specific anti-CTX monoclonal antibodies (mAbs) (Figs. 3 and 4).63–70) These mAbs (10C9, 3D11, 8H4, and 3G8) have been used to develop a direct sandwich enzyme-linked immunosorbent assay (ELISA) method for the reliable detection of CTXs.70) The protocol for this direct sandwich ELISA has been recently improved to provide a detection limit of 0.2 pg/mL (2 × 10−4 ppb) using an alkaline phosphatase (ALP)-fluorescent system (Fig. 5) (in preparation for publication). This detection limit is sufficient to detect the very small amount of CTX contaminants in fish, as stipulated by FDA regulations (0.01 ppb (=10 pg/g) CTX1B equivalents).61)
Figure 3.
Preparation of anti-ciguatoxin monoclonal antibodies and primary sandwich ELISA.
Figure 4.
New strategy for synthesizing the CTX1B left wing (hapten)-protein conjugate and successful preparation of anti-CTX1B monoclonal antibody.
Figure 5.
Detection limits of siguatoxins by advanced highly sensitive ELISA using an alkaline phosphatase (ALP)-fluorescent system.
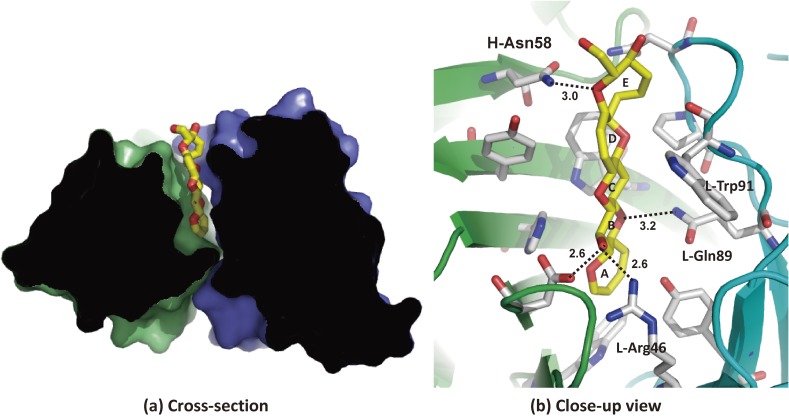
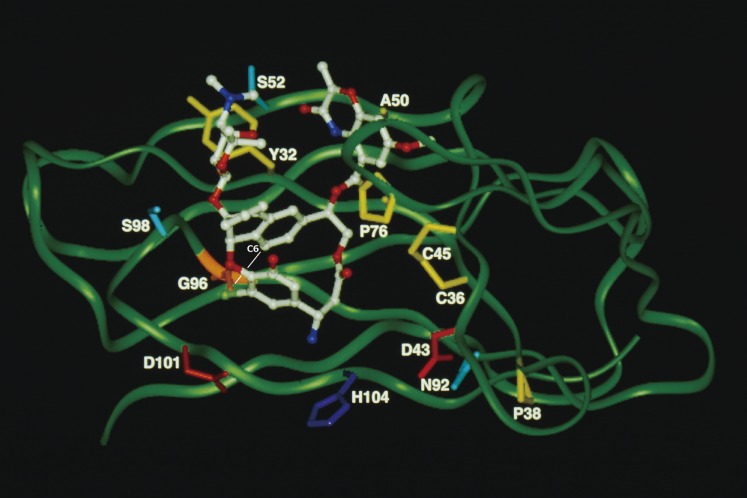
The molecular recognition and interactions between CTX3C fragments and its specific antibody 10C9 Fab were elucidated by X-ray crystal structure analysis to understand how protein recognizes ladder-like polycyclic ethers (Fig. 6).71,72) Antibody 10C9 Fab has an extraordinarily large and deep binding pocket at the center of the variable region, where CTX3C-ABCDE fragment binds longitudinally in the pocket via hydrogen bonds and van der Waals interactions. Upon antigen-antibody complexation, 10C9 Fab adjusts to the antigen fragment by means of rotational motion in the variable region, and furthermore its recognition requires molecular rearrangements over the entire antibody structure.
Figure 6.
The binding site of 10C9 Fab in complex with CTX3C-ABCDE fragment (antigen): (a) cross-section and (b) close-up view.
2.11. Structure-activity relationship (SAR) studies.
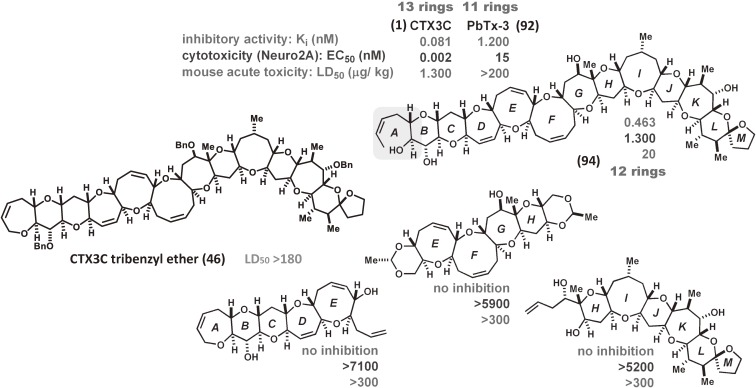
Our versatile synthetic strategy enabled the synthesis of F-ring modified analogs and their biological evaluation using three approaches: 1) competitive inhibition assays (Ki) using isotope-labeled dihydrobrevetoxin B ([3H]PbTx-3 (92)) against rat brain synaptosomes, 2) in vivo toxicity (cytotoxicity, EC50) tests using Neuro 2A, and 3) mouse acute toxicity (LD50) assays. Brevetoxins and CTXs bind to site 5 of the voltage-sensitive sodium channel (VSSC) of excitable membranes.20,61) We demonstrated that the nine-membered F ring plays a critical role in the binding of CTXs to VSSC and subsequent toxicity, and that the F ring drives the CTX molecule into a shape suitable for potent bioactivity (Table 1, Fig. 7). The rigid analog (90) which possesses an eight-membered F-ring, as well as the flexible analog (93) in which the F-ring is opened, showed almost no binding to VSSC and no toxicity,38) while the ten-membered F-ring analog (91) exhibited weak toxicity.39) These findings indicated that the planar molecular shape of CTXs (Fig. 7) and their limited conformational flexibility such as F-ring (up and down) flipping20,25,73) give rise to their biological activities. The synthetic fragments and protected CTX3C (46) exhibit no detectable toxicity, while the A-ring-opened CTX3C (94), which possesses 12 ether rings, exhibited toxicity intermediate between CTX3C (1) and the less toxic PbTx-3 (92), which possesses 11 rings (Fig. 8). These assays suggested that there is a significant relationship between the size of the polycyclic region (the number of fused rings) and biological activity.
Table 1.
Activity profiles of 51-hydroxyCTX3C analogs
Figure 8.
Activity profiles of synthetic compounds.
2.12. Related biological studies and remarks.
Since natural CTXs are not readily available, synthetic CTXs have been used as the standards for LC/MS analysis, and have led to confirmation of the causative CTXs in ciguatera fish worldwide.62,74,75) Synthetic CTXs have also accelerated studies on the mechanisms of CTX binding and the effects to voltage-sensitive sodium channels (VSSC) and other ion channels,76–82) the symptomatology of CTX poisoning, and the long-term neurological symptoms caused by CTX poisoning.83,84)
Before concluding this chapter, it should be noted that many synthetic studies have been reported by laboratories around the world since we completed the total synthesis of CTX3C (1) in 2001.4–9) However, only one total synthesis of CTX1B (3) aside from our synthesis has been completed, by the Isobe group in 2009.85,86) Neither the total synthesis of other ciguatoxin congeners nor the successful preparation of anti-CTX monoclonal antibodies has been reported to date.
3. Stereocontrolled syntheses of chromoprotein enediyne antitumor antibiotics and relevant mechanistic studies
Macromolecular chromoprotein antitumor antibiotics isolated from Actinomycete species, such as C-1027,87,88) neocarzinostatin,89,90) kedarcidin,91,92) and maduropeptin,93,94) are composed of a highly reactive enediyne chromophore (Fig. 9) complexed with an apoprotein. Their extremely potent cytotoxicities are believed to originate from their high DNA-binding affinity and the DNA-damaging reactivity of the chromophores. The apoproteins (>10 kDa) are single polypeptide chains of over 110 amino acid residues cross-linked by two disulfide bonds. The nine-membered enediyne chromophore is bound noncovalently in the cleft of its apoprotein and is stabilized.95–99) Chromophores are highly unstable at ambient temperature once released from the apoprotein and either undergo Masamune-Bergman aromatization spontaneously without an activator, or can be activated by external activators such as nucleophiles. Our project aimed to answer four questions:
Figure 9.
Proposed structures of nine-membered cyclic enediyne chromophores of chromoprotein antitumor antibiotics.
(1) How can we synthesize these highly strained, unstable, and functionally complex nine-membered enediyne chromophores?
(2) How does the apoprotein stabilize the chromophore?
(3) What is the exact mechanism of the Masamune-Bergman aromatization of enediyne chromophores?
(4) Can we design a more stable chromophore-apoprotein complex?
3.1. Synthesis of highly strained nine-membered enediyne system.
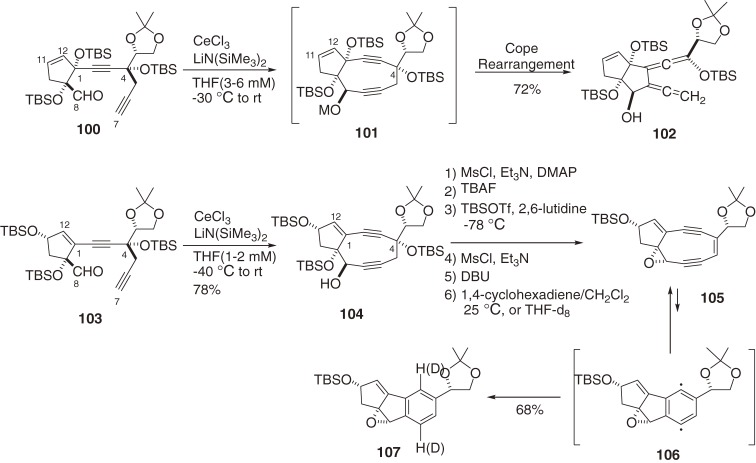
The highly strained, functionalized, and complex architecture of the unstable chromophores presents a daunting challenge to their chemical synthesis.13–17) After considerable effort, we developed a strategy to synthesize the highly strained cyclononadiyne system via intramolecular cerium acetylide addition to aldehyde, in which C7,C8-cyclization created a trans diol system suitable for generating epoxide functionality (Scheme 10).100–103) Interestingly, the cyclopentene (C1–C12) double bond exo to the nine-membered ring (104) is necessary to prevent extremely facile Cope rearrangement to bis-allene and ring opening of the strained cyclononadiyne system.100)
Scheme 10.
Synthetic studies of bicyclic nine-membered enediyne systems.
3.2. Equilibration of the bicyclic nine-membered enediyne with p-benzyne.
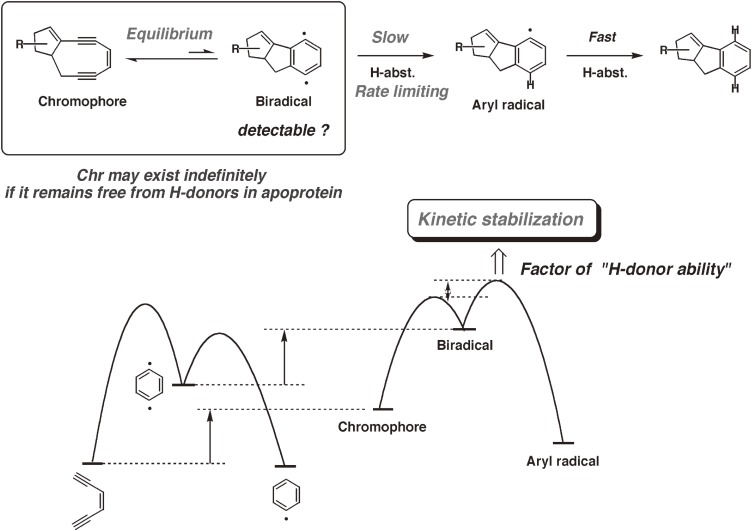
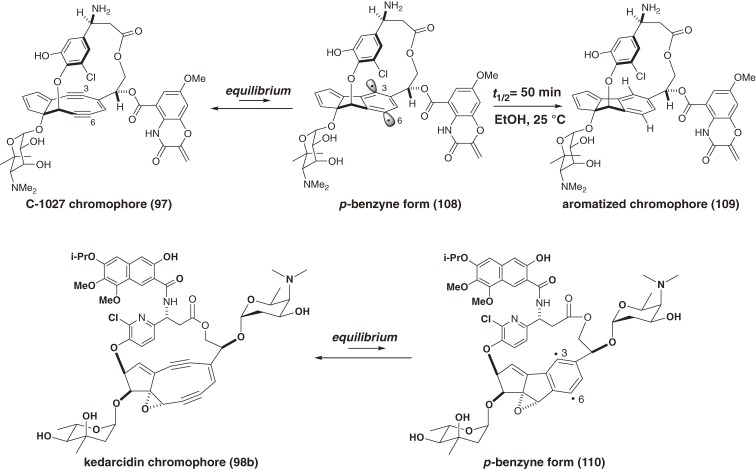
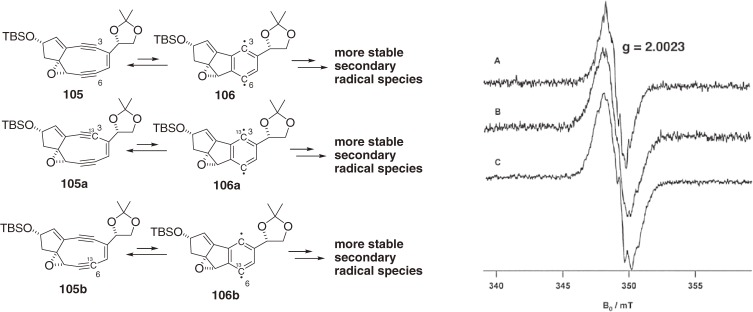
The cycloaromatization of noncyclic hex-3-ene-1,5-diyne was not affected by the reaction solvent and showed no kinetic isotope effects; thus, the cyclization step was concluded to be the rate-limiting (slowest) step.104) In contrast, the decay rate of the synthetic bicyclic nine-membered enediyne was dependent on the reaction solvent and exhibited kinetic isotope effects (Table 2).101,105) Thus, we found that the nine-membered enediynes are in equilibrium with the p-benzyne biradical intermediates and that hydrogen abstraction by the p-benzyne intermediates is the rate-limiting step (Fig. 10).101,102) This finding suggested that the chromophore could be kinetically stabilized and might exist indefinitely if it remains free from H-donors in apoprotein. Furthermore, the finding clarified how the nine-membered enediyne chromophores cut the DNA double strand.106–108) The natural nine-membered enediyne chromophores of C-1027 (97) and kedarcidin (98b) are also in equilibrium with their p-benzyne forms (108 and 110, respectively),101,102,105,107) which abstract hydrogen atoms from their surroundings (solvent, protein, or DNA, vide infra) to form stable aromatized chromophores such as 109 (Fig. 11).
Table 2.
Remarkable kinetic solvent isotope effect on the decay rate of the synthetic nine-membered enediyne system
| Entry | Solvent | t1/2 (min) | k (×10−5 s−1) | Relative Rate |
|---|---|---|---|---|
| 1 | CD2Cl2 | 680 | 1.7 | 0.035 |
| 2 | CH3CN | 610 | 1.9 | 0.039 |
| 3 | 1,4-dioxane-d8 | 310 | 3.7 | 0.076 |
| 4 | 1,4-dioxane | 110 | 11 | 0.22 |
| 5 | THF-d8a) | 220 | 5.4 | 0.11 |
| 6 | THF | 68 | 17 | 0.35 |
| 7 | CD3CD2OD | 130 | 8.8 | 0.18 |
| 8 | CH3CH2OH | 65 | 18 | 0.37 |
| 9 | 1,4-C6D8/CD2Cl2 | 28 | 41 | 0.84 |
| 10 | 1,4-C6H8/CH2Cl2 | 23 | 49 | 1.0 |
a) Measured by 1H-NMR.
Figure 10.
The kinetics and mechanism preventing spontaneous aromatization.
Figure 11.
The natural nine-membered enediyne chromophores of C-1027 and kedarcidin are in equilibrium with their p-benzyne form. Each p-benzyne form abstracts hydrogen atoms from the surroundings to form an aromatized chromophore.
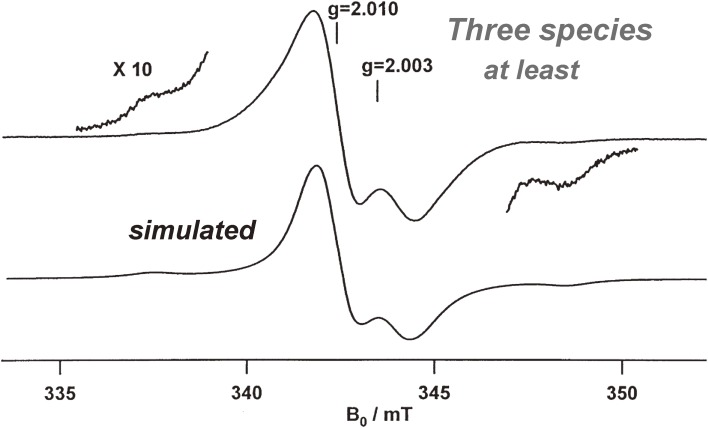
Based on the observed kinetics, we anticipated that the ground state of the intermediate p-benzyne biradical would be a triplet104) and thus detectable by ESR.109) We were delighted to find that the natural chromophore-apoprotein complex (holoprotein) of C-1027 and synthetic bicyclic nine-membered enediyne (105) are paramagnetic in the solid form110) and in solution,101) respectively, and exhibit steady ESR signals under deoxygenated conditions (Figs. 12 and 13). The spectra of 105 observed in CH2Cl2, CD2Cl2, and CD3CN were identical, demonstrating that the detected radical species did not arise from the solvents.111) The g values (2.0023) of 105 confirmed that the radical spectra were carbon-centered. Thus, to help determine the position of the observed radical species, C3- and C6-13C labeled isotopomers, 105a and 105b, respectively, were synthesized.112) However, their spectra showed no significant broadening compared to that of unlabeled 105 (Fig. 13).111) Based on the reported value of the 13C hyperfine splitting constant of phenyl radical (a13C-α = 12.25 mT), it was unlikely that the spin density is located at the 13C labeled C3 or C6 position. These results, including spin trapping experiments,113) indicated that the p-benzyne intermediate 106 was generated but the observed paramagnetic species should not be directly attributed to the equilibrated 106, but rather to more stable secondary radical species.111)
Figure 12.
The C-1027 antibiotic (holoprotein) powder is paramagnetic and exhibits an ESR signal.
Figure 13.
ESR spectra of synthetic nine-membered enediyne (105) in deoxygenated CD2Cl2 at rt: (A) unlabeled 105, (B) 13C3-labeled 105a, and (C) 13C6-labeled 105b.
3.3. Mechanism of self-degradation of C-1027 and design of a kinetically stabilized analog.
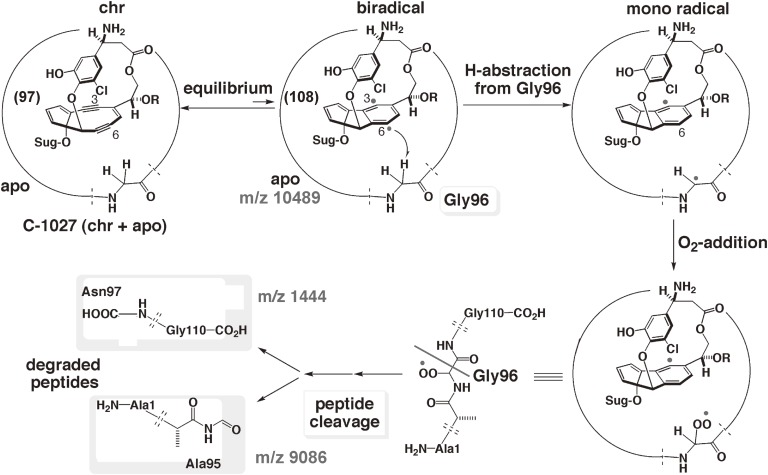
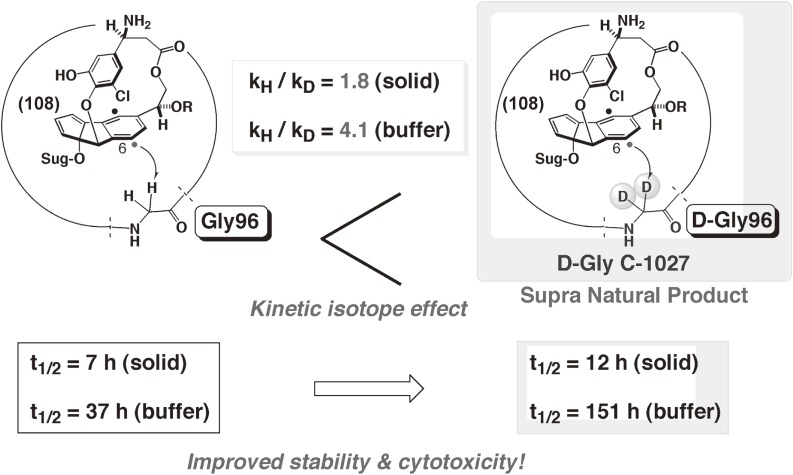
Chromoprotein antibiotics exemplified by C-1027 are remarkable because the apoprotein stabilizes the radical-generating chromophore by tight binding. Our NMR analysis of the structures of the C-1027 apoprotein and its complex with the aromatized chromophore (109) indicated that the apoprotein kinetically stabilizes the enediyne moiety of 97 by positioning the p-benzyne biradical of 108 in the cleft, thus limiting the accessibility of the biradical to hydrogen sources and preventing the chromophore from decomposing (Fig. 14).99,114) Once encapsulated stably in the apoprotein, the highly reactive chromophore (97) can be transported by the apoprotein through the cells to its target, double-strand DNA. Thus, the apoprotein appears to function both as a stabilizer and as an effective carrier, making it a potential drug delivery system (DDS). Despite these potentially ideal properties as a DDS for a reactive antitumor agent, C-1027 is known to undergo slow aging, resulting in chromophore-mediated self-decomposition. The apoprotein is presumably not able to completely inhibit the radical-mediated reactions of the chromophore (97), and C-1027 slowly decomposes to afford the aromatized chromophore. The NMR-analyzed 3D-structure of the complex (Fig. 14)99) indicated that the C6 (radical) position is in spatial proximity to the α-protons of Gly96 of the apoprotein (m/z 10489 Da) and suggested hydrogen-abstraction from Gly96. MALDI-TOFMS analysis of the aged C1027 complex showed new peaks at m/z 1444 and 9086 Da, which correspond to the peptide fragments oxidatively cleaved at the Gly96 residue (Fig. 15).114) We thus designed and prepared recombinant deuterated (D-Gly) apoprotein to improve the chromophore-stabilizing activity due to the kinetic isotope effect.115) The results demonstrated that kinetic stabilization of the reactive chromophore enhanced the overall stability of the small molecule-protein complex, thereby achieving more effective antitumor activities compared to that of natural C-1027 (Fig. 16).115)
Figure 14.
NMR analyzed solution structure of the complex of C-1027 apoprotein and the aromatized chromophore (109).
Figure 15.
Proposed self-degradation mechanism of C-1027.
Figure 16.
Rational design of more active C-1027.
3.4. The first total synthesis and elucidation of the stereochemistry of N1999-A2.
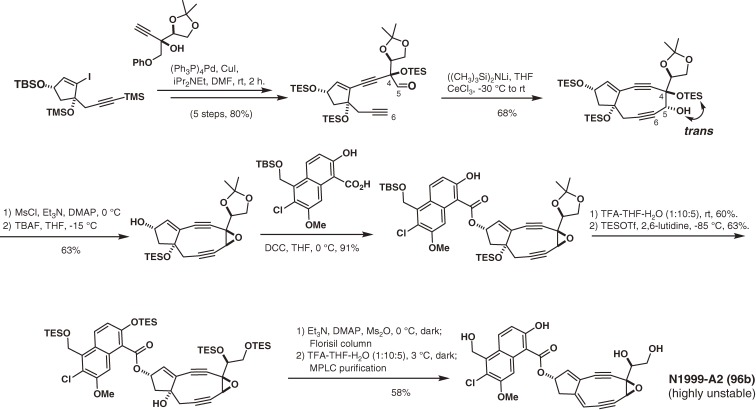
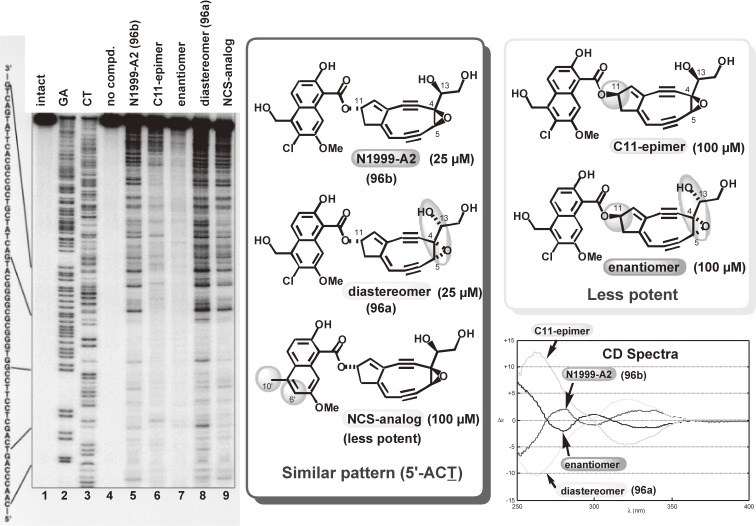
A novel and unstable nine-membered epoxyenediyne, N1999-A2 (96), was reported to exhibit extremely potent cytotoxicity toward cultured cancer cells in 1998 by Ando and coworkers at Ajinomoto Co. Ltd.116) The structure of 96 is very similar to that of the aglycon of neocarzinostatin chromophore (95) but lacks a stabilizing carrier apoprotein. Since the stereochemistry of 96 was unknown, we synthesized its stereoisomers through C7,C8- or C5,C6-cyclization using cerium acetylide (Scheme 11).14,117,118) Comparison of the NMR and CD spectra, and the base-selectivities of these stereoisomers during DNA cleavage (Fig. 17), resulted in the determination of the stereochemistry including the absolute configuration of 96.14,117)
Scheme 11.
Total synthesis of N1999-A2 via C5,C6-cyclization and determination of the stereochemistry of N1999-A2 including its absolute configuration.
Figure 17.
Thiol-triggered DNA-cleavage profiles and CD spectra of N1999-A2 stereoisomers.
3.5. Synthesis of the neocarzinostatin chromophore.
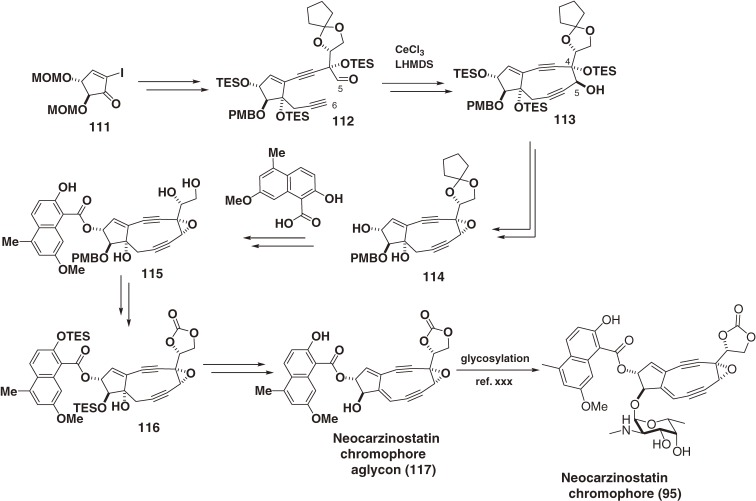
Neocarzinostatin (NCS), the first chromoprotein enediyne antibiotic, was isolated from a culture of Streptomyces carzinostatics in 1965 by Ishida and coworkers at Tohoku University.89, 90) Its potent antibacterial and antitumor activities derive from the inhibition of DNA synthesis and DNA degradation in cells by the labile chromophore (95). The chromophore-binding structure and the stabilization interactions in the NCS complex was elucidated by 2D-NMR method.95–98) Mechanistic studies using 95 and synthetic chromophore analogs clarified the various chemical mechanisms of triggering the aromatization, the carbon-radical formation, and DNA cleaving abilities.119–133) Then, an efficient route to the highly strained neocarzinostatin chromophore aglycon (117) was developed (Scheme 12).13,134–136) The present strategy involved a stereoselective intramolecular cerium acetylide-aldehyde cyclization to form the C4,C5-trans-diol system, which was adequate to form the α-epoxide. This aglycon (117) was extremely unstable, but was nonetheless glycosylated by the Myers group to complete the total synthesis of labile neocarzinostatin chromophore (95).137,138)
Scheme 12.
Total synthesis of neocarzinostatin chromophore.
3.6. Determination of the absolute configuration of the C-1027 chromophore and synthesis of its protected aglycon.
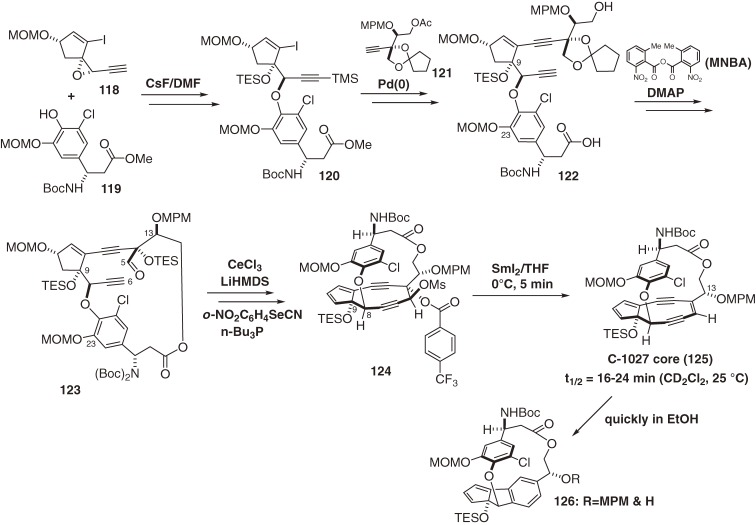
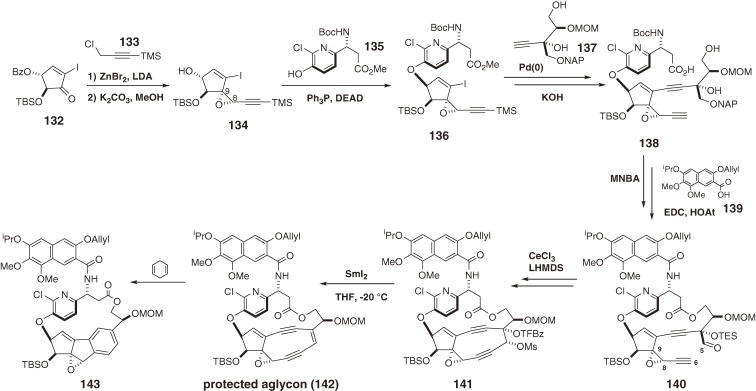
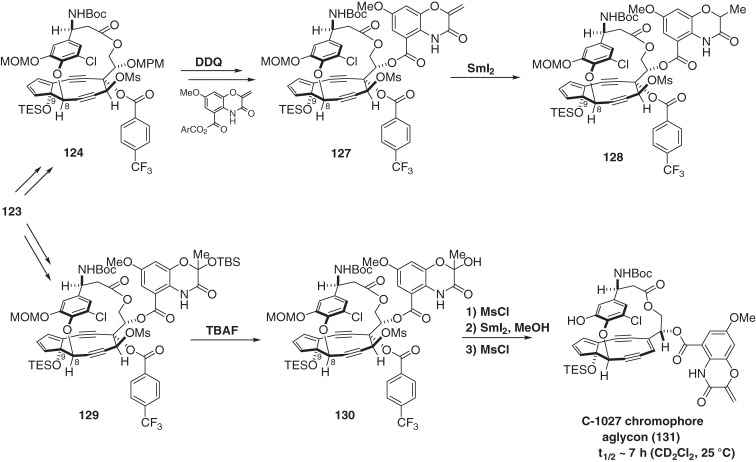
The antitumor antibiotic C-1027, which is a complex between the reactive chromophore 9788) and an apoprotein,99) was discovered by Otani and coworkers at Taiho Co. Ltd. in 1988.87) The chromophore 97 is responsible for DNA recognition and damage, and the apoprotein functions as an effective drug-delivery system (vide supra). The free chromophore (97) is the most labile enediyne studied to date. Chromophore 97 was transformed in ethanol at room temperature by Masamune-Bergman cyclization and subsequent hydrogen abstraction to provide an aromatized chromophore (109) with a half-life of 50 min and in 82% yield (Fig. 11).105) In a biological setting, the p-benzyne biradical 108 abstracts hydrogen atoms from DNA in a sequence-selective manner to cause oxidative double-strand cleavage. The structure of 9788) as well as kedarcidin chromophore (98b)92) is highly unusual. The complicated fused-ring system of 97 comprises a cyclopentadiene ring, a highly strained nine-membered enediyne ring, a functionalized benzoxazine ring, and a chlorocatechol-containing 17-membered macrolactone that displays nonbiaryl atropisomerism. These structural and functional complexities make the total synthesis of the chromophore (97) extremely challenging. We first determined the absolute configuration of 97,139,140) and then developed new and effective methodologies for the construction of the nine-membered enediyne structure.100,101) This approach enabled the first synthesis of the exceedingly unstable core structure (125) of the chromophore (97) (Scheme 13)141–149) and the labile protected aglycon (131) (Scheme 15; in preparation for publication).16)
Scheme 13.
Total synthesis of the core of the C-1027 chromophore and its rapid aromatization.
Scheme 15.
Total synthesis of the protected aglycon of the kedarcidin chromophore.
There are several key features of our syntheses. The first is stereoselective and efficient synthesis of three fragments (118, 119, and 121). The second is CsF- and Pd(0)-mediated convergent assembly of these three fragments. The third is an atropselective macrocyclization of 122 controlled by strategic protection of both the C9-OH and C23-OH groups.146) Without this protection, the atropselectivity was decreased or reversed; in addition, the C9-protection was also effective for preventing dimerization of the cyclopentadiene moiety introduced via deprotection of the MOM group followed by phenylselenenylation and H2O2 oxidation. The fourth key feature is a cerium amide promoted nine-membered diyne ring cyclization between C5 and C6 of 123,141) assisted by the ansa-macrolide linkage with a diBoc-protected amine. The final feature is an extremely facile SmI2-mediated reductive 1,2-elimination of 124 using p-trifluoromethylbenzoate as an electron acceptor for chemoselective olefination in the presence of potentially reactive functionalities such as the doubly allylic OTES group at C9 and the propargylic OAr moiety at C8.16) However, when the benzoxazine ester was attached, its α,β-unsaturated carbonyl group was reduced preferentially and rapidly to afford 128 (Scheme 14). Therefore, this functionality was masked in the hydrate form as 129 for the SmI2–reduction, then dehydrated to complete the total synthesis of the labile aglycon (131), a more stable compound than the core structure (125) (in preparation for publication). A stereocontrolled glycosylation method was developed,144,149) and further studies directed toward the total synthesis of 97 is under way.
Scheme 14.
Total synthesis of a protected aglycon of the C-1027 chromophore.
The powerful yet mild nature of this olefination methodology also enabled access to the aglycon of the kedarcidin chromophore (98b), as shown in Scheme 15.
3.7. Synthesis of the protected aglycon of the kedarcidin chromophore.
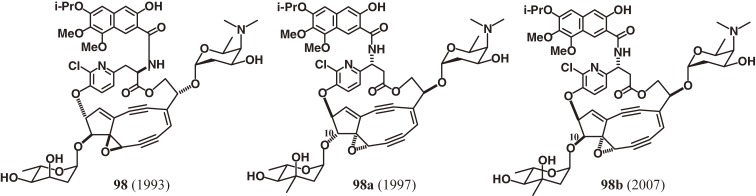
The structure of the chromophore of the chromoprotein enediyne antitumor antibiotic kedarcidine92,150,151) underwent several revisions because of its high instability and elusive, complex architecture. Its structure (98), possessing an α-azatyrosyl ansamacrolide linkage, was first assigned by scientists at Bristol-Myers Squibb in 1992.92) In 1997, we revised the α-amino acid to the corresponding β-amino acid derivative, and simultaneously the absolute structure of the whole molecule was updated as 98a based on the synthetic studies.150) In 2007, Myers and coworkers completed the formidable total synthesis of 98a, whose 1H NMR data led to an additional revision of the C10 stereochemistry, as shown in structure 98b (Fig. 18).151) Finally, we developed an enantioselective synthetic route152–156) to the unstable protected aglycon (142) of kedarcidin chromophore with the revised C10 stereochemistry (98b), which underwent spontaneous cycloaromatization in 1,4-cyclohexadiene/benzene to give an aromatized chromophore (143) (Scheme 15).17) Since the kedarcidin chromophore (98b) has also an additional ansa-macrolide linkage to the strained nine-membered enediyne core, similar to the C-1027 chromophore (97), their total syntheses were more difficult than those of neocarzinostatin (95)13,137,138) and N1999-A2 (96b).14,117) The key features of our synthesis of the aglycon (142) of the chromophore (98b) are: 1) the efficient convergent assembly of four fragments (134, 135, 137, and 139); 2) a novel strategy to synthesize the alkynyl epoxide (134) concisely from 132 and 133; 3) a cerium amide promoted nine-membered diyne ring cyclization between C5 and C6 of 140 in the presence of the ansa-bridge; and 4) a SmI2-mediated reductive 1,2-elimination for chemoselective olefination in the presence of the C8,C9 epoxide and the highly functionalized ansa-macrolide.17,156) The NMR data of 142,17) including chemical shifts, coupling constants, and NOE, were consistent with those of the natural chromophore,92) while synthetic 142 is a protected aglycon.157–159) The results of our spectroscopic studies17) strongly support the recently revised stereochemical structure (98b).151)
Figure 18.
Structure revisions of the kedarcidin chromophore.
3.8. The first total synthesis and structural revision of the maduropeptin chromophore.
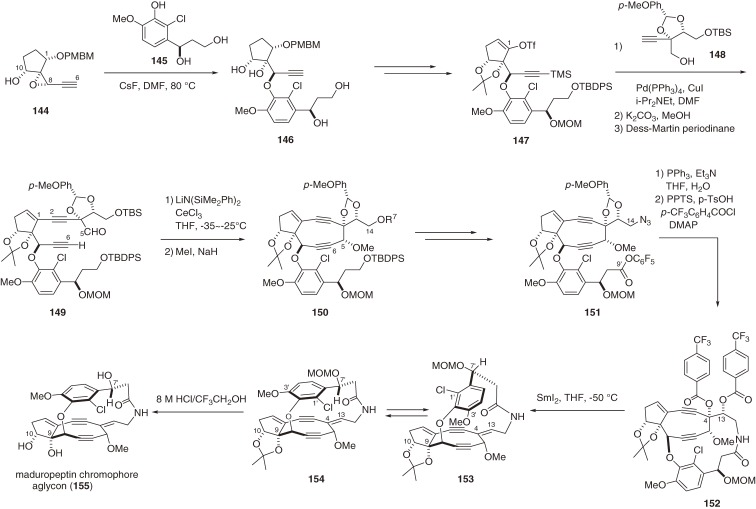
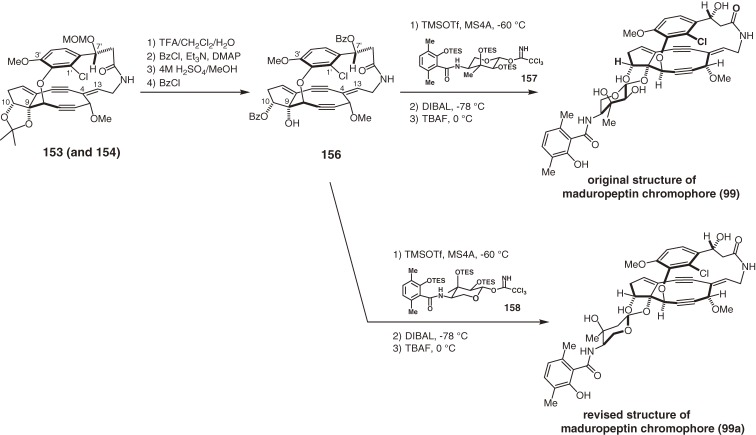
Maduropeptin is a novel member of the family of chromoprotein antitumor antibiotics.93) The isolated chromophore (99) is composed of a unique nine-membered diyne core and a glycoside side chain.94) Although 99 is the methanol adduct of a structurally undefined labile chromophore, it showed DNA cleavage site selectivity similar to that of the holoprotein. The complex, highly unsaturated, and functionalized molecular architecture of 99 differs from those of the other enediyne chromophores (95,90,138) 97,88,140) and 98b151)) of related chromoprotein antitumor antibiotics and clearly presented a daunting challenge for chemical synthesis. In particular, controlling the stereoselectivity of both the C4,13-Z-olefin and non-biaryl atropselectivity within the macrocycle necessitated the development and application of new strategies.160–163) The synthesis of aglycon 155 started with the convergent assembly of three fragments (144, 145, and 148) (Scheme 16). Our CsF-promoted coupling between epoxide 144 and the sterically hindered phenol 145 produced aryl ether 146,160) which was converted to enol trifrate 147 and coupled with acetylene moiety 148 under Sonogashira conditions. The two most characteristic rings, the highly strained 9-membered diyne and the 15-membered ansa-macrolactam, were then constructed. After screening various reaction conditions, we found that a mixture of LiN(SiMe2Ph)2 and CeCl3 in THF promoted the acetylide-aldehyde condensation to furnish diyne 150 with the C5-α-hydroxy group in a completely stereoselective fashion.163) The next lactamization was performed by slow addition of the isolated azido-pentafluorophenyl ester 151 to excess triphenylphosphine in THF-H2O (30:1) through the intermediacy of the corresponding C14 primary amine. It is noteworthy that those key ring formation reactions were performed under non-high-dilution conditions on a gram scale without decreasing the yield. The last phase of the aglycon synthesis was the introduction of the C4,13-Z-olefin through the SmI2-promoted facile 1,2-elimination of bis-p-(trifluoromethyl)benzoate 152. The stereoselective formation of the Z-olefin of the protected aglycon as a mixture of atropisomers (153 and 154) was realized by the ring strain of the 15-membered macrocycle; without the macrocycle, an E,Z-mixture was produced. The ratio of the atropisomers highly depends on the polarity of the solvent and the chromatographically separated isomers equilibrated at room temperature to provide the same mixture after several hours. Acid-promoted global deprotection of the mixture of 153 and 154 gave rise to the aglycon 155 as the sole atropisomer that corresponds to the natural chromophore 99.163)
Scheme 16.
Total synthesis of the aglycon of the maduropeptin chromophore.
The final manipulation for completing the total synthesis of maduropeptin chromophore 99 was a glycosylation (Scheme 17). The C9 tertiary alcohol 156 derived from a mixture of 153 and 154 was glycosylated smoothly with 157 using TMSOTf as a Lewis acid without the formation of the anomeric isomer or migration of the benzoyl group. Removal of two benzoyl groups and three TES groups completed the total synthesis of 99.15) However, the 1H and 13C NMR spectra of synthetic 99 were found to differ from those of the natural product. Upon closer inspection, the structure of the natural maduropeptin chromophore was suggested as the structure 99a, which possesses the antipodal madurosamine moiety, and was confirmed by its total synthesis using antipodal madurosamine derivative 158.15) The absolute structure of the chromophore remains to be determined.
Scheme 17.
Structural revision and total synthesis of the maduropeptin chromophore.
3.9. Biomimetic total syntheses of cyanosporasides and fijinolide from nine-membered cyclic enediyne precursors through site-selective p-benzyne hydrochlorination.
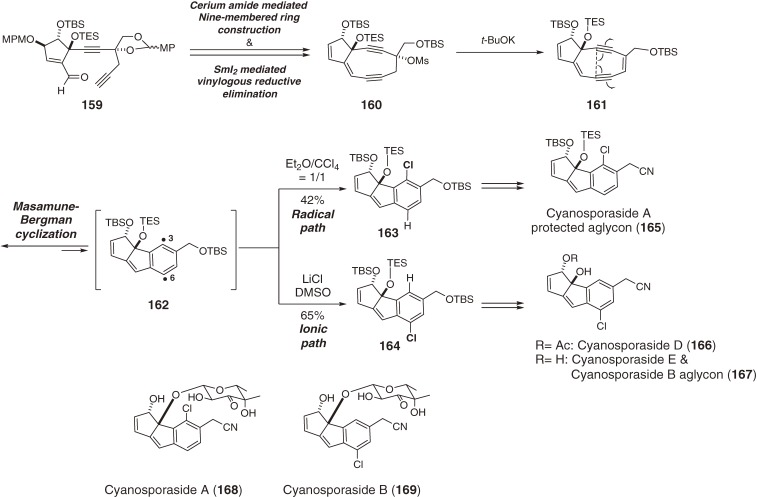
The cyanosporasides are a collection of monochlorinated benzenoid derivatives isolated from marine actinomycetes.164,165) All derivatives feature one of two types of cyanocyclopenta[a]indene frameworks which are regioisomeric in the position of a single chlorine atom. It is proposed that these chloro-substituted benzenoids are formed biosynthetically through the cycloaromatization of a bicyclic nine-membered enediyne precursor. We successfully synthesized unstable bicyclic precursor 161, which was spontaneously transannulated into the p-benzyne 162, and realized its differential 1,4-hydrochlorination to produce C3-chloro- (163) and C6-chloro-benzenoid (164) under either radical (organochlorine) or ionic (chloride-salt) conditions, respectively (Scheme 18). Our bio-inspired approach culminated in the first regiodivergent total syntheses of the aglycons 165 of type A (168), and 167 of type B (169), as well as of cyanosporasides D (166) and E (167).166) It is noteworthy that differential reactivity between C3 and C6 was observed in p-benzyne 162; thus, the sterically more accessible C6 position preferentially abstracted hydrogen over chlorine atoms in the radical pathway and reacted preferentially with a chloride anion in the ionic pathway.106,107,110,111,113)
Scheme 18.
Total synthesis of cyanosporasides via a single bicyclic nine-membered enediyne.
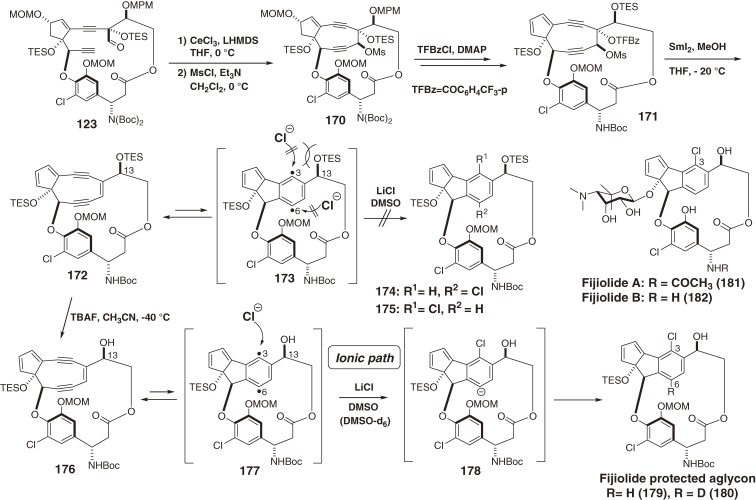
The above methodology was applied to a biomimetic synthesis of the aglycon of fijiolides A (181) and B (182).167) These 3-chlorocyclopenta[a]indene derivatives were isolated from marine Norcardiopsis species, whereas no C6-chlorinated fijiolides were isolated. New unstable 9-membered enediyne 172 with a TES group on C13-OH has the same structure as the core of C-1027 chromophore (97)16) and was synthesized from 123 (Scheme 13). Enediyne 172 was treated with LiCl in DMSO to determine if the C6-chlorinated product 174 (R1=H, R2=Cl) was formed as expected, given the results of 161 (Scheme 19). However, neither 174 nor C3-chlorinated 175 was produced, suggesting that the innately more reactive C6 position111,113) was covered by the benzene ring of the ansa-bridge, and that steric hindrance around the C3 due to the TES group might inhibit the reaction of p-benzyne 173. Therefore, the C13-TES group of 172 was selectively removed to afford 176. The reaction of 176 with LiCl in DMSO gave rise to the C3-chlorinated 179, which is a protected aglycon of fijiolides A (181) and B (182). Thus, the ansa-macrolide ring played a key role for controlling the regioselectivity of the reaction of p-benzyne intermediate 177. The intermediacy of carbanion 178 was confirmed by formation of C6-deuterated aglycon 180 in DMSO-d6 as a reaction solvent (in preparation for publication).
Scheme 19.
Total synthesis of fijiolide aglycon via a nine-membered enediyne.
4. Total syntheses of milbemycin α1 and avermectin B1a revisited
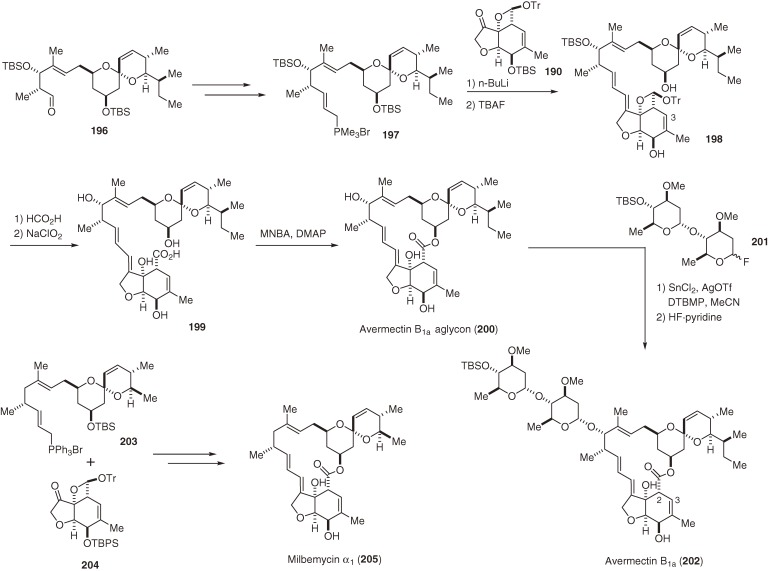
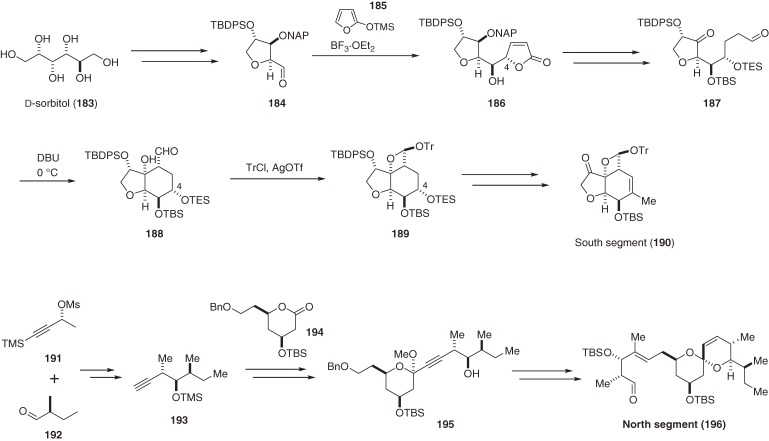
In the 1970s, Ōmura’s group at the Kitasato Institute and researchers at the Merck Sharp and Dohme Research Laboratories discovered potent antiparasitic agents, the avermectins, from the culture broth of Streptomyces avermitilis (S. avermectinius).168,169) Of these agents, avermectin B1a (202, Scheme 21) is the most potent anthelmintic congener. Avermectins are 16-membered macrolactones that consist of a 6,6-spiroacetal north segment attached to the disaccharide oleandrosyl-oleandrosyl, and a unique, highly sensitive hexahydrobenzofuran south segment, which is responsible for their biological activity. Avermectins and structurally related milbemycins170) attracted keen interest from synthetic organic chemists and the total syntheses of avermectin B1a (202) and milbemycins were achieved by several groups.171–178) These successful syntheses, however, used several indirect strategies, such as the deconjugation-epimerization strategy, to control the position of the C3–C4 double bond and C2-stereochemistry, and were less than satisfactory in terms of stereo- and regio-control. Previously, we developed a straightforward route to the hexahydrobenzofuran segment,179) which allowed us to complete a total synthesis of milbemycin α1 (205).19) We recently achieved an improved and efficient approach to the south (190) and north segments (196)180) (Scheme 20), as well as a stereocontrolled total synthesis of avermectin B1a (202) (Scheme 21).18) The highlight of our total synthesis of 202 was a unique but powerful strategy of protecting the β-hydroxy aldehyde moiety as trityl oxetane acetal (190, 198). This enabled us to synthesize and preserve the tetrahydrobenzofuran moiety without serious isomerization or decomposition during the entire synthetic sequence.18)
Scheme 21.
Total syntheses of avermectin B1a and milbemycin α1.
Scheme 20.
Syntheses of the south and north segments of avermectin B1a.
5. Other bioactive natural products
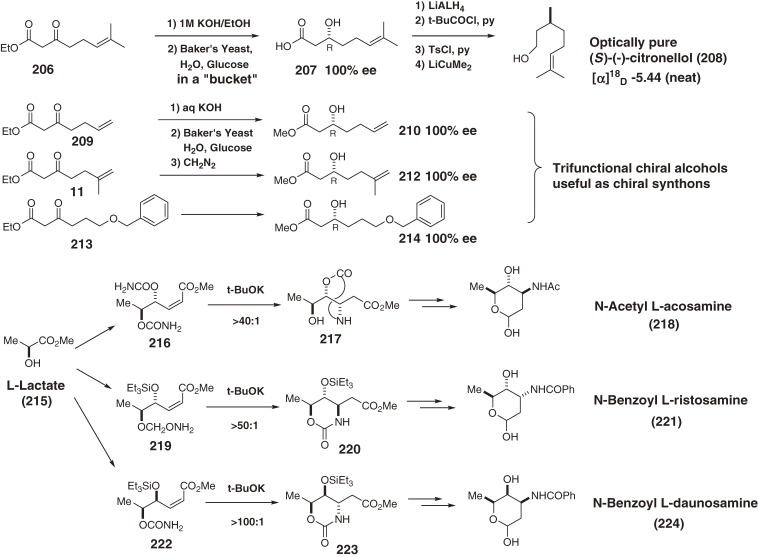
In addition to the above syntheses, we developed several convenient methodologies such as yeast-mediated enantiospecific reduction of potassium β-ketoalkanoates performed in a “bucket” (Scheme 22).181) This was applied to the large-scale preparation of optically pure (S)-citronellol, which is not readily available from natural sources.182–184) Another convenient methodologies185,186) are highly diastereoselective functionalizations of olefins mediated by iodocarbamation187,188) and conjugate addition189–195) of O-carbamates;196) these approaches are useful for the synthesis of important amino sugars of anthracycline antibiotics190–192) as well as 1,3-diols,187) amino alcohols,188) piperidines,193) and amino acids.194,195)
Scheme 22.
Convenient enantiospecific syntheses of trifunctional (R)-3-hydroxy esters by baker’s yeast reduction in a ‘bucket’, and L-amino sugars of anthracycline antibiotics mediated by a highly diastereoselective O-carbamate conjugate addition reaction.
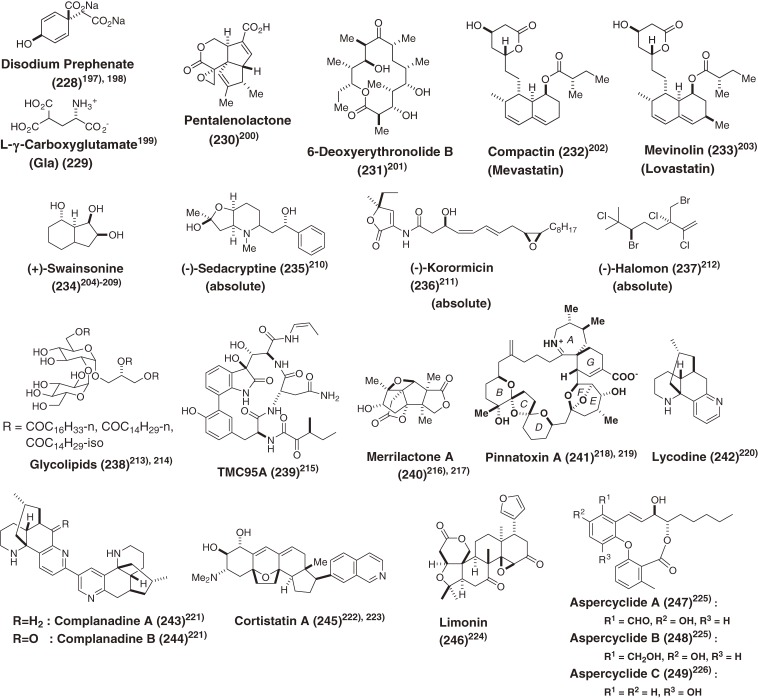
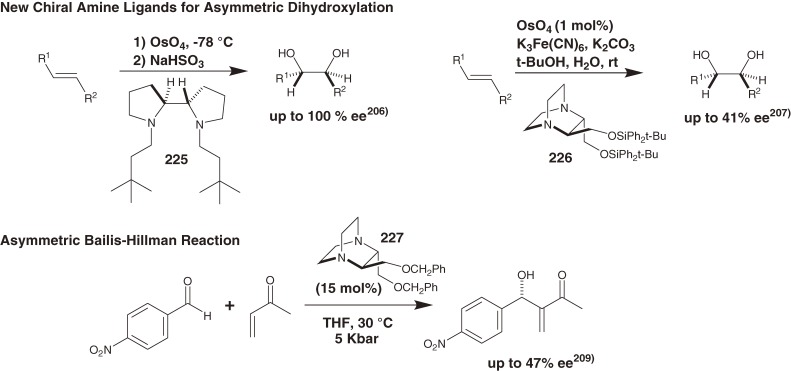
We also achieved total syntheses of the architecturally interesting bioactive natural products listed in Fig. 19,197–226) as well as development of asymmetric oxidation205–208) and asymmetric Bailis-Hillman reaction209) using newly developed chiral ligands (225–227) (Scheme 23) but do not discuss these in this account due to space limitations.
Figure 19.
Other bioactive natural products synthesized by our laboratory. Parentheses (absolute) mean that their absolute configurations were determined by our total syntheses.
Scheme 23.
Asymmetric reactions using newly developed chiral amines.
6. Conclusion
The total synthesis of natural products with complex architectures and potent bioactivities is a most rewarding and challenging endeavor in the chemical sciences. Our total syntheses and related innovative investigations have been reviewed, focusing on the 3 nm-long polycyclic ciguatoxins and the highly strained and labile nine-membered enediynes. Such endeavors have stimulated the development of a multitude of powerful synthetic strategies, tactics, and methodologies, and have not only advanced biological, medicinal, and pharmaceutical studies, but also helped to tackle real-life public health problems. As a consequence of these studies, the author has realized that the success in total synthesis is not merely the end of research, but rather the beginning of new scientific endeavors based on the power and versatility of chemical synthesis.
On the other hand, it should be emphasized that the discovery of new bioactive molecules from nature has long been the basis for developing the molecular sciences and addressing social welfare issues, as exemplified by the work of Prof. Satoshi Ōmura. However, the application of advanced powerful analytical tools, assay systems, and gene technologies, now means new bioactive natural products are being increasingly isolated and identified only in micro-, nano-, or pico-gram quantities. Thus, the discovery of new bioactive natural products will become increasingly more difficult and more challenging in the future.
Acknowledgments
I thank my co-workers listed in the references, in particular, Dr. S. Yamashita (currently at Harvard University), Profs. M. Inoue (currently at The University of Tokyo), T. Oishi (currently at Kyushu University), H. Oguri (currently at Tokyo University of Agriculture and Technology), Martin J. Lear (currently at The University of Lincoln, UK), S. Kobayashi (currently at Osaka Institute of Technology), T. Tanaka (currently at Tsukuba University), M. Yamaguchi (currently at Tohoku University), T. Noda (currently at Kanagawa Institute of Technology), K. Fujiwara (currently at Akita University), and I. Sato (currently at Ibaragi University). I also express my gratitude to Profs. I. Fujii and T. Tsumuraya (Osaka Prefecture University), and Dr. T. Sato (Cell Science & Technology, Inc.) for their collaboration in development of antibodies and immunoassays, and to Prof. K. Yamaoka (Hiroshima University) for electrophysiological studies. Profs. T. Yasumoto and Satoshi Ōmura are gratefully acknowledged for encouragements, valuable suggestions, and discussions. This work was financially supported by CREST and SORST, Japan Science and Technology Agency (JST), and a Grant-in-Aid for Specially Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Profile
Masahiro Hirama was born in 1948 in Tokyo. He studied for his Ph.D. at Tohoku University under Prof. Sho Ito in 1977 and completed postdoctoral studies at the University of Pittsburgh with Prof. S. J. Danishefsky and then at MIT with Prof. S. Masamune. In 1980, he returned to Japan and joined the Suntory Institute for Bioorganic Research (SUNBOR, Nakanishi’s Institute). In 1983, he moved to Tohoku University as an assistant professor in the research group of Prof. Sho Ito, and was promoted to associate professor and then to Professor of Chemistry in 1989. His research interests are natural product synthesis, development of new synthetic methods and strategies, and the design of bioactive molecules with a special emphasis on protein-ligand interactions. He retired from Tohoku University in 2012, and joined AcroScale, Inc. as a member of the board of directors, which focuses on the chemical analysis and quality control of glycoproteins such as chemically synthesized interferon β. He received the Incentive Award in Synthetic Organic Chemistry, Japan, in 1985, and was awarded the Inoue Prize for Science in 1998, Synthetic Organic Chemistry Award, Japan, in 2000, BCSJ Award in 2001, Chemical Society of Japan Award in 2003, Fujihara Award in 2010, and the National Medal with Purple Ribbon (Medals of Honor, Japan) in 2011.
This account is dedicated to Dr. Satoshi Ōmura, Distinguished Emeritus Professor at Kitasato University, on the occasion of his 2015 Nobel Prize in Physiology or Medicine.
References
- 1).Nicolaou, K.C. and Montagnon, T. (2008) Molecules that Changed the World. Wiley, Weinheim. [Google Scholar]
- 2).Nicolaou K.C. (2013) The emergence of the structure of the molecule and the art of its synthesis. Angew. Chem. Int. Ed. 52, 131–146. [DOI] [PubMed] [Google Scholar]
- 3).Tatsuta K. (2008) Total synthesis and development of bioactive natural products. Proc. Jpn. Acad., Ser. B 84, 87–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Hirama M., Oishi T., Uehara H., Inoue M., Maruyama M., Oguri H., Satake M. (2001) Total synthesis of ciguatoxin CTX3C. Science 294, 1904–1907. [DOI] [PubMed] [Google Scholar]
- 5).Inoue M., Uehara H., Maruyama M., Hirama M. (2002) Practical total synthesis of ciguatoxin CTX3C by improved protective group strategy. Org. Lett. 4, 4551–4554. [DOI] [PubMed] [Google Scholar]
- 6).Inoue M., Miyazaki K., Uehara H., Maruyama M., Hirama M. (2004) First- and second-generation total synthesis of ciguatoxin CTX3C. Proc. Natl. Acad. Sci. U.S.A. 101, 12013–12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Inoue M., Miyazaki K., Ishihara Y., Tatami A., Ohnuma Y., Kawada Y., Komano K., Yamashita S., Lee N., Hirama M. (2006) Total synthesis of ciguatoxin and 51-hydroxy CTX3C. J. Am. Chem. Soc. 128, 9352–9354. [DOI] [PubMed] [Google Scholar]
- 8).Yamashita S., Ishihara Y., Morita H., Uchiyama J., Takeuchi K., Inoue M., Hirama M. (2011) Stereoselective 6-exo radical cyclization using cis-vinyl sulfoxide: practical total synthesis of CTX3C. J. Nat. Prod. 74, 357–364. [DOI] [PubMed] [Google Scholar]
- 9).Yamashita S., Takeuchi K., Koyama T., Inoue M., Hayashi Y., Hirama M. (2015) Practical route to the left wing of CTX1B and total syntheses of CTX1B and 54-deoxyCTX1B. Chemistry 21, 2621–2628. [DOI] [PubMed] [Google Scholar]
- 10).Inoue M., Hirama M. (2004) Total synthesis of ciguatoxin CTX3C, a causative toxin of ciguatera seafood poisoning. Synlett, 577–595. [Google Scholar]
- 11).Inoue M., Hirama M. (2004) Evolution of a practical total synthesis of ciguatoxin CTX3C. Acc. Chem. Res. 37, 961–968. [DOI] [PubMed] [Google Scholar]
- 12).Hirama M. (2005) Total synthesis of ciguatoxin CTX3C: a venture into the problems of ciguatera seafood poisoning. Chem. Rec. 5, 240–250. [DOI] [PubMed] [Google Scholar]
- 13).Kobayashi S., Hori M., Wang G.X., Hirama M. (2006) Formal total synthesis of neocarzinostatin chromophore. J. Org. Chem. 71, 636–644. [DOI] [PubMed] [Google Scholar]
- 14).Kobayashi S., Ashizawa S., Takahashi Y., Sugiura Y., Nagaoka M., Lear M.J., Hirama M. (2001) The first total synthesis of N1999-A2: absolute stereochemistry and stereochemical implications into DNA cleavage. J. Am. Chem. Soc. 123, 11294–11295. [DOI] [PubMed] [Google Scholar]
- 15).Komano K., Shimamura S., Norizuki Y., Zhao D., Kabuto C., Sato I., Hirama M. (2009) Total synthesis and structure revision of the (−)-maduropeptin chromophore. J. Am. Chem. Soc. 131, 12072–12073. [DOI] [PubMed] [Google Scholar]
- 16).Inoue M., Ohashi I., Kawaguchi T., Hirama M. (2008) Total synthesis of the C-1027 chromophore core: extremely facile enediyne formation through SmI2-mediated 1,2-elimination. Angew. Chem. Int. Ed. 47, 1777–1779. [DOI] [PubMed] [Google Scholar]
- 17).Ogawa K., Koyama Y., Ohashi I., Sato I., Hirama M. (2009) Total synthesis of a protected aglycon of the kedarcidin chromophore. Angew. Chem. Int. Ed. 48, 1110–1113. [DOI] [PubMed] [Google Scholar]
- 18).Yamashita S., Hayashi D., Nakano A., Hayashi Y., Hirama M. (2016) Total synthesis of avermectin B1a revisited. J. Antibiot. 69, 31–50. [DOI] [PubMed] [Google Scholar]
- 19).Hirama M., Noda T., Yasuda S., Ito S. (1991) Simple strategy for the synthesis of the avermectin-milbemycin family. Total synthesis of milbemycin α1. J. Am. Chem. Soc. 113, 1830–1832. [Google Scholar]
- 20).Yasumoto T., Murata M. (1993) Marine toxins. Chem. Rev. 93, 1897–1909. [Google Scholar]
- 21).Scheuer P.J. (1994) Ciguatera and its off-shoots — Chance encounters en route to a molecular structure. Tetrahedron 50, 3–18. [Google Scholar]
- 22).Yasumoto T. (2001) The chemistry and biological function of natural marine toxins. Chem. Rec. 1, 228–242. [DOI] [PubMed] [Google Scholar]
- 23).Lewis R.J. (2001) The changing face of ciguatera. Toxicon 39, 97–106. [DOI] [PubMed] [Google Scholar]
- 24).Murata M., Legrand A.M., Ishibashi Y., Yasumoto T. (1989) Structures of ciguatoxin and its congener. J. Am. Chem. Soc. 111, 8929–8931. [Google Scholar]
- 25).Murata M., Legrand A.M., Ishibashi Y., Fukui M., Yasumoto T. (1990) Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 112, 4380–4386. [Google Scholar]
- 26).Yasumoto T., Igarashi T., Legrand A.M., Cruchet P., Chinain M., Fujita T., Naoki H. (2000) Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectroscopy. J. Am. Chem. Soc. 122, 4988–4989. [Google Scholar]
- 27).Suzuki T., Sato O., Hirama M. (1990) Palladium catalyzed stereospecific cyclization of hydroxyl epoxides. Stereocontrolled synthesis of cis- and trans-2-alkenyl-3-hydroxy-tetrahydropyrans. Tetrahedron Lett. 31, 4747–4750. [Google Scholar]
- 28).Suzuki T., Sato O., Hirama M., Yamamoto Y., Murata M., Yasumoto T., Harada N. (1991) Enantioselective synthesis of the AB ring fragment of Gambiertoxin 4B. Implication for the absolute configuration of gambiertoxin 4B and ciguatoxin. Tetrahedron Lett. 32, 4505–4508. [Google Scholar]
- 29).Sato O., Hirama M. (1992) Synthesis of the AB ring fragment of ciguatoxin: ligand controlled regioselective addition of osmium tetroxide to double bonds. Synlett, 705–707. [Google Scholar]
- 30).Oguri H., Hishiyama S., Oishi T., Hirama M. (1995) Enantio-controlled synthesis of the AB ring moiety of ciguatoxin. Synlett, 1252–1254. [Google Scholar]
- 31).Oishi T., Shoji M., Maeda K., Kumahara N., Hirama M. (1996) Extensive ring-expansion strategy for the enantioselective synthesis of the medium ring ethers, oxepene, oxocane, and oxonene, of ciguatoxin. Synlett, 1165–1167. [Google Scholar]
- 32).Oguri H., Hishiyama S., Sato O., Oishi T., Hirama M. (1997) Synthetic study of ciguatoxin. Absolute configuration of the C2 hydroxy group. Tetrahedron 53, 3057–3072. [Google Scholar]
- 33).Satake M., Morohashi A., Oguri H., Oishi T., Hirama M., Harada N., Yasumoto T. (1997) The absolute configuration of ciguatoxin. J. Am. Chem. Soc. 119, 11325–11326. [Google Scholar]
- 34).Hirama M., Oishi T. (2002) A journey to the total synthesis of ciguatoxin. Chemistry Today (Gendai kagaku) 373, 55–62 (in Japanese). [Google Scholar]
- 35).Nicolaou K.C., Rutjes F.P.J.T., Theodorakis J., Tiebes J., Sato M., Untersteller E. (1995) Total synthesis of brevetoxin B. 3. Final strategy and completion. J. Am. Chem. Soc. 117, 10252–10263. [Google Scholar]
- 36).Fu G.C., Nguyen S.T., Grubbs R.H. (1993) Catalytic ring-closing metathesis of functionalized dienes by a ruthenium carbine complex. J. Am. Chem. Soc. 115, 9856–9857. [Google Scholar]
- 37).Satake M., Murata M., Yasumoto T. (1993) The structure of CTX3C, a ciguatoxin congener isolated from cultured Gambierdiscus toxicus. Tetrahedron Lett. 34, 1975–1978. [Google Scholar]
- 38).Inoue M., Lee N., Miyazaki K., Usuki T., Matsuoka S., Hirama M. (2008) Critical importance of the 9-membered F-ring of ciguatoxin for potent bioactivity: total synthesis and biological evaluation of F-ring modified analogs. Angew. Chem. Int. Ed. 47, 8611–8614. [DOI] [PubMed] [Google Scholar]
- 39).Ishihara Y., Lee N., Oshiro N., Matsuoka S., Yamashita S., Inoue M., Hirama M. (2010) The first F-ring modified ciguatoxin analogue showing significant toxicity. Chem. Commun. 46, 2968–2970. [DOI] [PubMed] [Google Scholar]
- 40).Inoue M. (2005) Convergent strategies for syntheses of trans-fused polycyclic ethers. Chem. Rev. 105, 4379–4405. [DOI] [PubMed] [Google Scholar]
- 41).Maruyama M., Inoue M., Oishi T., Oguri H., Ogasawara Y., Shindo Y., Hirama M. (2002) Convergent synthesis of the ABCDE ring system of ciguatoxin CTX3C. Tetrahedron 58, 1835–1851. [Google Scholar]
- 42).Oishi T., Nagumo Y., Hirama M. (1998) Convergent synthesis of the trans-fused 6-n-6-6 (n = 7–10) tetracyclic ether system based on a ring-closing metathesis reaction. Chem. Commun., 1041–1042. [Google Scholar]
- 43).Lewis M.D., Cha J.K., Kishi Y. (1982) Highly stereoselective approaches to α- and β-C-glycopyranosides. J. Am. Chem. Soc. 104, 4976–4978. [Google Scholar]
- 44).Tatami A., Inoue M., Uehara H., Hirama M. (2003) A concise route to the right wing of ciguatoxin. Tetrahedron Lett. 44, 5229–5233. [Google Scholar]
- 45).Inoue M., Yamashita S., Tatami A., Miyazaki K., Hirama M. (2004) A new stereoselective synthesis of ciguatoxin right wing fragments. J. Org. Chem. 69, 2797–2804. [DOI] [PubMed] [Google Scholar]
- 46).Inanaga J., Hirata K., Saeki H., Katsuki T., Yamaguchi M. (1979) A rapid esterification by means of mixed anhydride and its application to large-ring lactonization. Bull. Chem. Soc. Jpn. 52, 1989–1993. [Google Scholar]
- 47).Uehara H., Oishi T., Inoue M., Shoji M., Nagumo Y., Kosaka M., Brazidec J.Y.L., Hirama M. (2002) Convergent synthesis of the HIJKLM ring fragment of ciguatoxin CTX3C. Tetrahedron 58, 6493–6512. [Google Scholar]
- 48).Rahim M.A., Sasaki H., Saito J., Fujiwara T., Takeda T. (2001) Intramolecular carbonyl olefination of esters. Regioselective preparation of enol ethers of cyclic ketones by the titanocene(II)-promoted reaction of alkyl ω,ω-bis(phenylthio)alkanoates. Chem. Commun., 625–626. [Google Scholar]
- 49).Sasaki M., Inoue M., Noguchi T., Takeichi A., Tachibana K. (1998) Convergent and stereoselective method for synthesis of O-linked oxepane ring system by intramolecular radical cyclization. Tetrahedron Lett. 39, 2783–2786. [Google Scholar]
- 50).Sasaki M., Noguchi T., Tachibana K. (1999) Synthesis of the FGH ring fragment of ciguatoxin. Tetrahedron Lett. 40, 1337–1340. [Google Scholar]
- 51).Inoue M., Sasaki M., Tachibana K. (1999) A convergent synthesis of the decacyclic ciguatoxin model containing the F-M ring framework. J. Org. Chem. 64, 9416–9429. [Google Scholar]
- 52).Imai H., Uehara H., Inoue M., Oguri, Oishi T., Hirama M. (2001) Convergent synthesis of the EFGH ring fragment of ciguatoxin CTX3C. Tetrahedron Lett. 42, 6219–6222. [Google Scholar]
- 53).Kim S., Do J.Y., Kim S.H., Kim D. (1994) Trimethylsilyl trifluoromethanesulfonate promoted chemoselective reactions of acyclic acetals in the presence of cyclic acetals. J. Chem. Soc., Perkin Trans. 1, 2357–2358. [Google Scholar]
- 54).Inoue M., Wang X.G., Wang J., Hirama M. (2002) Novel assembly of cyclic ethers by coupling α-chlorosulfides and alcohols. Org. Lett. 4, 3439–3442. [DOI] [PubMed] [Google Scholar]
- 55).Inoue M., Wang J., Wang G.X., Ogasawara Y., Hirama M. (2003) Divergent synthesis of the tetracyclic ethers of 6-X-7-6 ring systems. Tetrahedron 59, 5645–5659. [Google Scholar]
- 56).Inoue M., Yamashita S., Hirama M. (2004) A new synthesis of key intermediates for the assembly of polycyclic ethers: Yb(OTf)3-promoted formation of O,S-acetals from α-fluorosulfides and alcohols. Tetrahedron Lett. 45, 2053–2056. [Google Scholar]
- 57).Kobayashi S., Alizadeh B.H., Sasaki S., Oguri H., Hirama M. (2004) Synthesis of the fully functionalized ABCDE ring moiety of ciguatoxin. Org. Lett. 6, 751–754. [DOI] [PubMed] [Google Scholar]
- 58).Ohfune Y., Kan T., Nakajima T. (1998) Internal activation of acrylate-type dienophiles for the Diels-Alder reaction. Stereoselective synthesis of conformationally constrained glutamate analogs. Tetrahedron 54, 5207–5224. [Google Scholar]
- 59).Yamashita S., Uematsu R., Hirama M. (2011) Stereoselective synthesis of the left wing of Caribbean ciguatoxin. Tetrahedron 67, 6616–6626. [Google Scholar]
- 60).Yoshikawa K., Inoue M., Hirama M. (2007) Synthesis of the LMN-ring fragment of the Caribbean ciguatoxin C-CTX-1. Tetrahedron Lett. 48, 2177–2180. [Google Scholar]
- 61).Dickey R.W., Plakas S.M. (2010) Ciguatera: a public health perspective. Toxicon 56, 123–136. [DOI] [PubMed] [Google Scholar]
- 62).Oshiro N., Yogi K., Asato S., Sasaki T., Tamanaha K., Hirama M., Yasumoto T., Inafuku Y. (2010) Ciguatera incidence and fish toxicity in Okinawa, Japan. Toxicon 56, 656–661. [DOI] [PubMed] [Google Scholar]
- 63).Tsumuraya T., Fujii I., Hirama M. (2010) Production of monoclonal antibodies for sandwich immunoassay detection of Pacific ciguatoxins. Toxicon 56, 797–803. [DOI] [PubMed] [Google Scholar]
- 64).Oguri H., Tanaka S., Hishiyama S., Oishi T., Hirama M., Tsumuraya T., Tomioka Y., Mizugaki M. (1999) Designed hapten aimed at anti-ciguatoxin monoclonal antibody: synthesis, immunization and discrimination of the C2 configuration. Synthesis, 1431–1436. [Google Scholar]
- 65).Nagumo Y., Oguri H., Shindo Y., Sasaki S., Oishi T., Hirama M., Tomioka Y., Mizugaki M., Tsumuraya T. (2001) Concise synthesis of ciguatoxin ABC-ring fragments and surface plasmon resonance study of the interaction of their BSA conjugates with monoclonal antibodies. Bioorg. Med. Chem. Lett. 11, 2037–2040. [DOI] [PubMed] [Google Scholar]
- 66).Nagumo Y., Oguri H., Tsumoto K., Shindo Y., Hirama M., Tsumuraya T., Fujii I., Tomioka Y., Mizugaki M., Kumagai I. (2004) Phage-display selection of antibodies to the left end of CTX3C using synthetic fragments. J. Immunol. Methods 289, 137–146. [DOI] [PubMed] [Google Scholar]
- 67).Oguri H., Hirama M., Tsumuraya T., Fujii I., Maruyama M., Uehara H., Nagumo Y. (2003) Synthesis-based approach toward direct sandwich immunoassay for ciguatoxin CTX3C. J. Am. Chem. Soc. 125, 7608–7612. [DOI] [PubMed] [Google Scholar]
- 68).Tsumuraya T., Fujii I., Inoue M., Tatami A., Miyazaki K., Hirama M. (2006) Production of monoclonal antibodies for sandwich immunoassay detection of ciguatoxin 51-hydroxyCTX3C. Toxicon 48, 287–294. [DOI] [PubMed] [Google Scholar]
- 69).Tsumuraya T., Takeuchi K., Yamashita S., Fujii I., Hirama M. (2012) Development of a monoclonal antibody against the left wing of ciguatoxin CTX1B: Thiol strategy and detection using a sandwich ELISA. Toxicon 60, 348–357. [DOI] [PubMed] [Google Scholar]
- 70).Tsumuraya T., Fujii I., Hirama M. (2014) Preparation of anti-ciguatoxin monoclonal antibodies using synthetic haptens: sandwich ELISA detection of ciguatoxins. J. AOAC Int. 97, 373–379. [DOI] [PubMed] [Google Scholar]
- 71).Tsumoto K., Yokota A., Tanaka Y., Ui M., Tsumuraya T., Fujii I., Kumagai I., Nagumo Y., Oguri H., Inoue M., Hirama M. (2008) Critical contribution of aromatic rings to specific recognition of polyether rings. The case of ciguatoxin CTX3C-ABC and its specific antibody 1C49. J. Biol. Chem. 283, 12259–12266. [DOI] [PubMed] [Google Scholar]
- 72).Ui M., Tanaka Y., Tsumuraya T., Fujii I., Inoue M., Hirama M., Tsumoto K. (2008) How protein recognizes ladder-like polycyclic ethers. Interactions between ciguatoxin (CTX3C) fragments and its specific antibody 10C9. J. Biol. Chem. 283, 19440–19447. [DOI] [PubMed] [Google Scholar]
- 73).Lee N., Inoue M., Hirama M. (2009) Design and synthesis of a conformationally restricted analogue of the EFGH-ring system of ciguatoxin. Heterocycles 79, 325–330. [Google Scholar]
- 74).Otero P., Perez S., Alfonso A., Vale C., Rodriguez P., Gouveia N.N., Gouveia N., Delgado J., Vale P., Hirama M., Ishihara Y., Molgo J., Botana L.M. (2010) First toxin profile of ciguateric fish in Madeira Arquipelago (Europe). Anal. Chem. 82, 6032–6039. [DOI] [PubMed] [Google Scholar]
- 75).Yogi K., Oshiro N., Inafuku Y., Hirama M., Yasumoto T. (2011) Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics if fish and causative alga from the Pacific. Anal. Chem. 83, 8886–8891. [DOI] [PubMed] [Google Scholar]
- 76).Yamaoka K., Inoue M., Miyahara H., Miyazaki K., Hirama M. (2004) A quantitative and comparative study of the effects of a synthetic ciguatoxin CTX3C on the kinetic properties of voltage-dependent sodium channels. Br. J. Pharmacol. 142, 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Yamaoka K., Inoue M., Miyazaki K., Hirama M., Kondo C., Kinoshita E., Miyoshi H., Seyama I. (2009) Synthetic ciguatoxins selectively activate Nav 1.8-derived chimeric sodium channels expressed in HEK293 cells. J. Biol. Chem. 284, 7597–7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78).Ghiaroni V., Fuwa H., Inoue M., Sasaki M., Miyazaki K., Hirama M., Yasumoto T., Rossini G.P., Scalera G., Bigiani A. (2006) Effect of ciguatoxin 3C on voltage-gated Na+ and K+ currents in mouse taste cells. Chem. Senses 31, 673–680. [DOI] [PubMed] [Google Scholar]
- 79).Perez S., Vale C., Alonso E., Alfonso C., Rodriguez P., Otero P., Alfonso A., Vale P., Hirama M., Vieytes M.R., Botana L.M. (2011) A comparative study of the effect of ciguatoxins on voltage-dependent Na+ and K+ channels in cerebellar neurons. Chem. Res. Toxicol. 24, 587–596. [DOI] [PubMed] [Google Scholar]
- 80).Yamaoka K., Inoue M., Hirama M. (2011) A study on mechanisms of toxic actions of ciguatoxins: existence of functional relationship between CTX3C and charged residues of voltage sensors in Nav 1.4 sodium channel. Forensic Toxicol. 29, 125–131. [Google Scholar]
- 81).Martin V., Vale C., Antelo A., Hirama M., Yamashita S., Vieytes M.R., Botana L.M. (2014) Differential effects of ciguatoxin and maitotoxin in primary cultures of cortical neurons. Chem. Res. Toxicol. 27, 1387–1400. [DOI] [PubMed] [Google Scholar]
- 82).Martin V., Vale C., Hirama M., Yamashita S., Rubiolo J.A., Vieytes M.R., Botana L.M. (2015) Synthetic ciguatoxin CTX3C induces a rapid imbalance in neuronal excitability. Chem. Res. Toxicol. 28, 1095–1108. [DOI] [PubMed] [Google Scholar]
- 83).Martin V., Vale C., Rubiolo J.A., Roel M., Hirama M., Yamashita S., Vieytes M.R., Botana L.M. (2015) Chronic ciguatoxin treatment induces synaptic scaling through voltage gated sodium channels in cortical neurons. Chem. Res. Toxicol. 28, 1109–1119. [DOI] [PubMed] [Google Scholar]
- 84).Botana L.M. (2012) A perspective on the toxicology of marine toxins. Chem. Res. Toxicol. 25, 1800–1804. [DOI] [PubMed] [Google Scholar]
- 85).Hamajima A., Isobe M. (2009) Total synthesis of ciguatoxin. Angew. Chem. Int. Ed. 48, 2941–2945. [DOI] [PubMed] [Google Scholar]
- 86).Isobe M., Hamajima A. (2010) Ciguatoxin: developing the methodology for total synthesis. Nat. Prod. Rep. 27, 1204–1226. [DOI] [PubMed] [Google Scholar]
- 87).Sugimoto Y., Otani T., Oie S., Wierzba K., Yamada Y. (1990) Mechanism of action of a new macromolecular antitumor antibiotic, C-1027. J. Antibiot. 43, 417–421. [DOI] [PubMed] [Google Scholar]
- 88).Yoshida K., Minami Y., Azuma R., Saeki M., Otani T. (1993) Structure and cycloaromatization of a novel enediyne, C-1027 chromophore. Tetrahedron Lett. 34, 2637–2640. [Google Scholar]
- 89).Koide Y., Ishii F., Hasuda K., Koyama Y., Edo K., Katamine S., Kitame F., Ishida N. (1980) Isolation of a non-protein component and a protein component from neocarzinostatin (NCS) and their biological activities. J. Antibiot. 33, 342–346. [DOI] [PubMed] [Google Scholar]
- 90).Edo K., Mizugaki M., Koide Y., Seto H., Furihata K., Otake N., Ishida N. (1985) The structure of neocarzinostatin chromophore possessing a novel bicyclo[7,3,0]-dodecadiyne system. Tetrahedron Lett. 26, 331–334. [Google Scholar]
- 91).Hofstead S.J., Matson J.A., Malacko A.R., Marquardt H. (1992) Kedarcidin, a new chromoprotein antitumor antibiotic II. Isolation, purification and physico-chemical properties. J. Antibiot. 45, 1250–1254. [DOI] [PubMed] [Google Scholar]
- 92).Leet J.E., Schroeder D.R., Langley D.R., Colson K.L., Huang S., Klohr S.E., Lee M.S., Golik J., Hofstead S.J., Doyle T.W., Matson J.A. (1993) Chemistry and structure elucidation of the kedarcidin chromophore. J. Am. Chem. Soc. 115, 8432–8443. [Google Scholar]
- 93).Hanada M., Ohkuma H., Yonemoto T., Tomita K., Ohbayashi M., Kamei H., Miyaki T., Konishi M., Kawaguchi H., Forenza S. (1991) Maduropeptin, a complex of new macromolecular antitumor antibiotics. J. Antibiot. 44, 403–414. [DOI] [PubMed] [Google Scholar]
- 94).Schroeder D.R., Colson K.L., Klohr S.E., Zein N., Langley D.R., Lee M.S., Matson J.A., Doyle T.W. (1994) Isolation, structure determination, and proposed mechanism of action for artifacts of maduropeptin chromophore. J. Am. Chem. Soc. 116, 9351–9352. [Google Scholar]
- 95).Ishiguro M., Imajo S., Hirama M. (1991) Modeling study of the structure of the macromolecular antitumor antibiotic neocarzinostatin. Origin of the stabilization of the chromophore. J. Med. Chem. 34, 2366–2373. [DOI] [PubMed] [Google Scholar]
- 96).Tanaka T., Hirama M., Ueno M., Imajo S., Ishiguro M., Mizugaki M., Edo K., Komatsu H. (1991) Proton NMR studies on the chromophore binding structure in neocarzinostatin complex. Tetrahedron Lett. 32, 3175–3178. [Google Scholar]
- 97).Tanaka T., Hirama M., Fujita K., Imajo S., Ishiguro M. (1993) Solution structure of the antitumor antibiotic neocarzinostatin, a chromophore-protein complex. J. Chem. Soc., Chem. Commun., 1205–1207. [Google Scholar]
- 98).Hirama M., Tanaka T. (1994) Molecular recognition in neocarzinostatin complex: how does the apoprotein bind specifically and stabilize the chromophore? Pure Appl. Chem. 66, 791–796. [Google Scholar]
- 99).Tanaka T., Fukuda-Ishisaka S., Hirama M., Otani T. (2001) Solution structures of C-1027 apoprotein and its complex with the aromatized chromophore. J. Mol. Biol. 309, 267–283. [DOI] [PubMed] [Google Scholar]
- 100).Iida K., Hirama M. (1994) Efficient route to the nine-membered cyclic diyne system. Tuning of the extremely facile Cope rearrangement of 1,5-diyne. J. Am. Chem. Soc. 116, 10310–10311. [Google Scholar]
- 101).Iida K., Hirama M. (1995) Synthesis and characterization of nine-membered cyclic enediynes, models of C-1027 and kedarcidin chromophore: Equilibration with a p-benzyne biradical and kinetic stabilization. J. Am. Chem. Soc. 117, 8875–8876. [Google Scholar]
- 102).Hirama M. (1997) Synthesis and chemistry of chromoprotein antitumor antibiotics: Nine-membered enediynes are equilibrated with p-benzyne type biradicals. Pure Appl. Chem. 69, 525–530. [Google Scholar]
- 103).Mita T., Kawata S., Hirama M. (1998) Evidence for spontaneous cycloaromatization of nine-membered monocyclic enediyne. Chem. Lett. 27, 959–960. [Google Scholar]
- 104).Bergman R.G. (1973) Reactive 1,4-dehydroaromatics. Acc. Chem. Res. 6, 25–31. [Google Scholar]
- 105).Yoshida K., Minami Y., Otani T., Tada Y., Hirama M. (1994) Remarkable kinetic solvent isotope effect on the cycloaromatization of C-1027 chromophore, a 9-membered enediyne, and the thermochemistry. Tetrahedron Lett. 35, 5253–5256. [Google Scholar]
- 106).Usuki T., Inoue M., Akiyama K., Hirama M. (2002) Spin-trapping study of DNA cleavage induced by enediyne C-1027 chromophore. Chem. Lett. 31, 1148–1149. [DOI] [PubMed] [Google Scholar]
- 107).Usuki T., Inoue M., Akiyama K., Hirama M. (2005) ESR studies on DNA cleavage induced by enediyne C-1027 chromophore. Bioorg. Med. Chem. 13, 5218–5224. [DOI] [PubMed] [Google Scholar]
- 108).Chen P. (1996) Design of diradical-based hydrogen abstraction agents. Angew. Chem. Int. Ed. Engl. 35, 1478–1480. [Google Scholar]
- 109).Wenk H.H., Winkler M., Sander W. (2003) One century of aryne chemistry. Angew. Chem. Int. Ed. 42, 502–528. [DOI] [PubMed] [Google Scholar]
- 110).Hirama M., Akiyama K., Tanaka T., Noda T., Iida K., Sato I., Hanaishi R., Fukuda-Ishisaka S., Ishiguro M., Otani T., Leet J.E. (2000) Paramagnetic enediyne antibiotic C-1027: spin identification and characterization of radical species. J. Am. Chem. Soc. 122, 720–721. [Google Scholar]
- 111).Hirama M., Akiyama K., Das P., Mita T., Lear M.J., Iida K., Sato I., Yoshimura F., Usuki T., Tero-Kubota S. (2006) Direct observation of ESR spectra of bicyclic nine-membered enediynes at ambient temperature. Heterocycles 69, 83–89. [Google Scholar]
- 112).Das P., Mita T., Lear M.J., Hirama M. (2002) Synthesis of 13C-labelled, bicyclic mimetics of natural enediynes. Chem. Commun., 2624–2625. [DOI] [PubMed] [Google Scholar]
- 113).Usuki T., Mita T., Lear M.J., Das P., Yoshimura F., Inoue M., Hirama M., Akiyama K., Tero-Kubota S. (2004) Spin trapping of 13C-labeled p-benzynes generated by Masamune-Bergman cyclization of bicyclic nine-membered enediynes. Angew. Chem. Int. Ed. 43, 5249–5253. [DOI] [PubMed] [Google Scholar]
- 114).Usuki T., Inoue M., Hirama M., Tanaka T. (2004) Rational design of a supra C-1027: kinetically stabilized analogue of the antitumor enediyne chromoprotein. J. Am. Chem. Soc. 126, 3022–3023. [DOI] [PubMed] [Google Scholar]
- 115).Inoue M., Usuki T., Lee N., Hirama M., Tanaka T., Hosoi F., Ohie S., Otani T. (2006) Antitumor enediyne chromoprotein C-1027: mechanistic investigation of the chromophore-mediated self-decomposition pathway. J. Am. Chem. Soc. 128, 7896–7903. [DOI] [PubMed] [Google Scholar]
- 116).Ando T., Ishii M., Kajiura T., Kameyama T., Miwa K., Sugiura Y. (1998) A new non-protein enediyne antibiotic N19999A2: unique enediyne chromophore similar to neocarzinostatin and DNA cleavage feature. Tetrahedron Lett. 39, 6495–6498. [Google Scholar]
- 117).Kobayashi S., Reddy R.S., Sugiura Y., Sasaki D., Miyagawa N., Hirama M. (2001) Investigation of the total synthesis of N1999-A2: Implication of stereochemistry. J. Am. Chem. Soc. 123, 2887–2888. [DOI] [PubMed] [Google Scholar]
- 118).Takahashi K., Hagiwara M., Ashizawa S., Hirama M. (1999) Concise synthesis of the naphthoate component of N1999-A2. Synlett, 71–72. [Google Scholar]
- 119).Hirama M., Fujiwara K., Shigematu K., Fukazawa Y. (1989) The 10-membered ring analogues of neocarzinostatin chromophore: design, synthesis, and mode of decomposition. J. Am. Chem. Soc. 111, 4120–4122. [Google Scholar]
- 120).Fujiwara K., Kurisaki A., Hirama M. (1990) Two diverse modes of aromatization of a new 10-membered ring analogue of neocarzinostatin chromophore. Tetrahedron Lett. 31, 4329–4332. [Google Scholar]
- 121).Tanaka T., Fujiwara K., Hirama M. (1990) Oxidative triggering for aromatization of the neocarzinostatin chromophore. Tetrahedron Lett. 31, 5947–5950. [Google Scholar]
- 122).Hirama M., Gomibuchi T., Fujiwara K., Sugiura Y., Uesugi M. (1991) Synthesis and DNA-cleaving abilities of functional neocarzinostatin chromophore analogues. Base discrimination by a simple alcohol. J. Am. Chem. Soc. 113, 9851–9853. [Google Scholar]
- 123).Fujiwara K., Sakai H., Hirama M. (1991) Enyne[3]cumulene. Synthesis and mode of aromatization. J. Org. Chem. 56, 1688–1689. [Google Scholar]
- 124).Tokuda M., Fujiwara K., Gomibuchi T., Hirama M., Uesugi M., Sugiura Y. (1993) Synthesis of a hybrid molecule containing neocarzinostatin chromophore analogue and minor groove binder. Tetrahedron Lett. 34, 669–672. [Google Scholar]
- 125).Takahashi K., Suzuki T., Hirama M. (1992) A simple and efficient synthesis of the naphthoate moiety of neocarzinostatin chromophore. Tetrahedron Lett. 33, 4603–4604. [Google Scholar]
- 126).Takahashi K., Tanaka T., Suzuki T., Hirama M. (1994) Synthesis and binding of simple neocarzinostatin chromophore analogues to the apoprotein. Tetrahedron 50, 1327–1340. [Google Scholar]
- 127).Kawata S., Oishi T., Hirama M. (1994) Ten-membered neocarzinostatin chromophore analogs leading to s,s-biradical via a cumulene intermediate. Tetrahedron Lett. 35, 4595–4598. [Google Scholar]
- 128).Gomibuchi T., Hirama M. (1995) Photoactivation of neocarzinostatin chromophore: Photo-induced cycloaromatization via naphthoate participation. J. Antibiot. 48, 738–740. [DOI] [PubMed] [Google Scholar]
- 129).Kaneko T., Takahashi M., Hirama M. (1999) Photochemical cycloaromatization of non-benzenoid enediynes. Angew. Chem. Int. Ed. 38, 1267–1268. [DOI] [PubMed] [Google Scholar]
- 130).Kaneko T., Takahashi M., Hirama M. (1999) Benzannelation alters the rate limiting step in enediyne cycloaromatization. Tetrahedron Lett. 40, 2015–2018. [Google Scholar]
- 131).Koseki S., Fujimura Y., Hirama M. (1999) Benzannelation effect on enediyne cycloaromatization: an ab initio molecular orbital study. J. Phys. Chem. A 103, 7672–7675. [Google Scholar]
- 132).Hirama M. (1994) Neocarzinostatin complex: chromophore analogs, binding structure and stabilization mechanism. J. Synth. Org. Chem. Jpn. 52, 980–991. [Google Scholar]
- 133).Hirama M., Tokuda M., Fujiwara K. (1991) Synthesis and cycloaromatization of a neocarzinostatin chromophore analogue equipped with an intramolecular nucleophile. Synlett, 651–653. [Google Scholar]
- 134).Reddy R.S., Iguchi S., Kobayashi S., Hirama M. (1996) Palladium-catalyzed coupling of alkenyl iodides with ethynyl oxiranes: synthesis of epoxy enediyne core intermediates related to neocarzinostatin chromophore. Tetrahedron Lett. 37, 9335–9336. [Google Scholar]
- 135).Kaneko T., Takahashi K., Hirama M. (1996) Synthetic study of neocarzinostatin chromophore: stereoselective synthesis of N-methylfucosamine and its α-glycoside. Heterocycles 47, 91–96. [Google Scholar]
- 136).Toyama K., Iguchi S., Sakazaki H., Oishi T., Hirama M. (2001) Convenient route to both enantiomerically pure forms of trans-4,5-dihydroxy-2-cyclopenten-1-one: efficient synthesis of the neocarzinostatin chromophore core. Bull. Chem. Soc. Jpn. 74, 997–1008. [Google Scholar]
- 137).Myers A.G., Hammond M., Wu Y., Xiang J., Harrington P.M., Kuo E.Y. (1996) Enantioselective synthesis of neocarzinostatin chromophore aglycon. J. Am. Chem. Soc. 118, 10006–10007. [DOI] [PubMed] [Google Scholar]
- 138).Myers A.G., Glatthar R., Hammond M., Harrington P.M., Kuo E.Y., Liang J., Schaus S.E., Wu Y., Xiang J. (2002) Development of an enantioselective synthetic route to neocarzinostatin chromophore and its use for multiple radioisotopic incorporation. J. Am. Chem. Soc. 124, 5380–5401. [DOI] [PubMed] [Google Scholar]
- 139).Iida K., Ishii T., Hirama M., Otani T., Minami Y., Yoshida K. (1993) Synthesis and absolute stereochemistry of the aminosugar moiety of antibiotic C-1027 chromophore. Tetrahedron Lett. 34, 4079–4082. [Google Scholar]
- 140).Iida K., Fukuda S., Tanaka T., Hirama M. (1996) Absolute configuration of C-1027 chromophore. Tetrahedron Lett. 37, 4997–5000. [Google Scholar]
- 141).Sato I., Akahori Y., Iida K., Hirama M. (1996) Efficient synthesis of a carbocyclic core moiety with the stereochemistry of the C-1027 chromophore. Tetrahedron Lett. 37, 5135–5138. [Google Scholar]
- 142).Sato I., Toyama K., Kikuchi T., Hirama M. (1998) New synthetic route to the highly strained cores of C-1027 and neocarzinostatin chromophore. Synlett, 1308–1310. [Google Scholar]
- 143).Sato I., Kikuchi T., Hirama M. (1999) Synthetic study of C-1027 chromophore: enantioselective synthesis of β-tyrosine moiety and effective aryl ether formation. Chem. Lett., 511–512. [Google Scholar]
- 144).Sato I., Akahori Y., Sasaki T., Kikuchi T., Hirama M. (1999) Synthetic study of C-1027 chromophore. Highly stereoselective glycosylation. Chem. Lett., 867–868. [Google Scholar]
- 145).Wang G.X., Iguchi S., Hirama M. (2001) A very concise and stereoselective synthesis of 3-substituted cis-hex-3-ene-1,5-diyne and corresponding epoxydiyne. J. Org. Chem. 66, 2146–2148. [DOI] [PubMed] [Google Scholar]
- 146).Sasaki T., Inoue M., Hirama M. (2001) Synthetic studies toward C-1027 chromophore: construction of a highly unsaturated macrocycle. Tetrahedron Lett. 42, 5299–5303. [Google Scholar]
- 147).Inoue M., Kikuchi T., Hirama M. (2004) Synthesis of the fully functionalized nine-membered diyne core of the C-1027 chromophore. Tetrahedron Lett. 45, 6439–6442. [Google Scholar]
- 148).Inoue M., Hatano S., Kodama M., Sasaki T., Kikuchi T., Hirama M. (2004) Synthesis of the bicyclo[7.3.0]dodecatrienediyne core of the C-1027 chromophore. Org. Lett. 6, 3833–3836. [DOI] [PubMed] [Google Scholar]
- 149).Hirai K., Tamura Y., Sato I., Hirama M. (2010) Practical synthesis of the C-1027 aminosugar moiety. Synlett, 2156–2158. [Google Scholar]
- 150).Kawata S., Ashizawa S., Hirama M. (1997) Synthetic study of kedarcidin chromophore: revised structure. J. Am. Chem. Soc. 119, 12012–12013. [Google Scholar]
- 151).Ren F., Hogan P.C., Anderson A.J., Meyers A.G. (2007) Kedarcidin chromophore: synthesis of its proposed structure and evidence for a stereochemical revision. J. Am. Chem. Soc. 129, 5381–5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152).Kawata S., Hirama M. (1998) Synthetic study of the kedarcidin chromophore: efficient construction of the aryl alkyl ether linkage. Tetrahedron Lett. 39, 8707–8710. [Google Scholar]
- 153).Yoshimura F., Kawata S., Hirama M. (1999) Synthetic study of kedarcidin chromophore: atropselective construction of the ansamacrolide. Tetrahedron Lett. 40, 8281–8285. [Google Scholar]
- 154).Koyama Y., Lear M.J., Yoshimura F., Ohashi I., Mashimo T., Hirama M. (2005) Efficient construction of the kedarcidin chromophore ansamacrolide. Org. Lett. 7, 267–270. [DOI] [PubMed] [Google Scholar]
- 155).Yoshimura F., Lear M.J., Ohashi I., Koyama Y., Hirama M. (2007) Synthesis of the entire carbon framework of the kedarcidin chromophore aglycon. Chem. Commun., 3057–3059. [DOI] [PubMed] [Google Scholar]
- 156).Ogawa K., Koyama Y., Ohashi I., Sato I., Hirama M. (2008) Secure route to the epoxybicyclo[7.3.0]dodecadienediyne core of the kedarcidin chromophore. Chem. Commun., 6327–6329. [DOI] [PubMed] [Google Scholar]
- 157).Lear M.J., Hirama M. (1999) Convenient route to derivatives of the 2-deoxysugar subunits of the kedarcidin chromophore: L-mycarose and L-kedarosamine. Tetrahedron Lett. 40, 4897–4900. [Google Scholar]
- 158).Lear M.J., Yoshimura F., Hirama M. (2001) A direct and efficient α-selective glycosylation protocol for the kedarcidin sugar, L-mycarose: AgPF6 as a remarkable activator of 2-deoxythioglycosides. Angew. Chem. Int. Ed. 40, 946–949. [DOI] [PubMed] [Google Scholar]
- 159).Ohashi I., Lear M.J., Yoshimura F., Hirama M. (2004) Use of polystyrene-supported DBU in the synthesis and α-selective glycosylation study of the unstable Schmidt donor of L-kedarosamine. Org. Lett. 6, 719–722. [DOI] [PubMed] [Google Scholar]
- 160).Kato N., Shimamura S., Khan S., Takeda F., Kikai Y., Hirama M. (2004) Convergent approach to the maduropeptin chromophore: aryl ether formation of (R)-3-aryl-3-hydroxypropanamide and cyclization of macrolactam. Tetrahedron 60, 3161–3172. [Google Scholar]
- 161).Iso K., Inoue M., Kato N., Hirama M. (2008) Synthesis of the bicyclo[7.3.0]dodecadiyne core of the maduropeptin chromophore. Chem. Asian J. 3, 447–453. [DOI] [PubMed] [Google Scholar]
- 162).Khan S., Kato N., Hirama M. (2000) Stereoselective synthesis of functionalized (Z)-keto-enyne and its epoxide. Synlett, 1494–1496. [Google Scholar]
- 163).Komano K., Shimamura S., Inoue M., Hirama M. (2007) Total synthesis of the maduropeptin chromophore aglycon. J. Am. Chem. Soc. 129, 14184–14186. [DOI] [PubMed] [Google Scholar]
- 164).Oh D., Williams P.G., Kauffman C.A., Jensen P.R., Fenical W. (2006) Cyanosporasides A and B, chloro- and cyano-cyclopenta[a]indene glycosides from the marine actinomycete “Salinospora pacifica.”. Org. Lett. 8, 1021–1024. [DOI] [PubMed] [Google Scholar]
- 165).Lane A.L., Nam S., Fukuda T., Yamanaka K., Kauffman C.A., Jensen P.R., Fenical W., Moore B.S. (2013) Structures and comparative characterization of biosynthetic gene clusters for cyanosporasides, enediyne-derived natural products from marine actinomycetes. J. Am. Chem. Soc. 135, 4171–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166).Yamada K., Lear M.J., Yamaguchi T., Yamashita S., Gridnev I.D., Hayashi Y., Hirama M. (2014) Biomimetic total synthesis of cyanosporaside aglycons from a single enediyne precursor through site-selective p-benzyne hydrochlorination. Angew. Chem. Int. Ed. 53, 13902–13906. [DOI] [PubMed] [Google Scholar]
- 167).Nam S., Gaudencio S.P., Kauffman C.A., Jensen P.R., Kondratyuk T.P., Marler L.E., Pezzuto J.M., Fenical W. (2010) Fijiolides A and B, inhibitors of TNF-α-induced NFκB activation, from a marine-derived sediment bacterium of the genus Nocardiopsis. J. Nat. Prod. 73, 1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168).Springer J.P., Arison B.H., Hirshfield J.M., Hoogsteen K. (1981) The absolute stereochemistry and conformation of avermectin B2a aglycon and avermectin B1a. J. Am. Chem. Soc. 103, 4221–4224. [Google Scholar]
- 169).Ōmura S. (2011) Microbial metabolites: 45 years of wandering, wondering and discovering. Tetrahedron 67, 6420–6459. [Google Scholar]
- 170).Mishima H., Ide J., Muramatsu S., Ono M. (1983) Milbemycins, a new family of macrolide antibiotics. Structure determination of milbemycins D, E, F, G, H, J and K. J. Antibiot. 36, 980–990. [DOI] [PubMed] [Google Scholar]
- 171).Hanessian S., Ugolini A., Dube D., Hodges P.J., Andre C. (1986) Synthesis of (+)-avermectin B1a. J. Am. Chem. Soc. 108, 2776–2778. [Google Scholar]
- 172).Fraser-Reid B., Wolleb H., Faghih R., Barchi J., Jr. (1987) Avermectin chemistry: problems of conjugation, deconjugation, and epimerization. J. Am. Chem. Soc. 109, 933–935. [Google Scholar]
- 173).Hanessian S., Dube D., Hodges P.J. (1987) Studies on the deconjugation-epimerization strategy en route to avermectin B1a: problems and solutions. J. Am. Chem. Soc. 109, 7063–7067. [Google Scholar]
- 174).Danishefsky S.J., Armistead D.M., Wincott F.E., Selnick H.G., Hungate R. (1987) The total synthesis of avermectin A1a. The total synthesis of the aglycon of avermectin A1a. J. Am. Chem. Soc. 109, 8117–8119. [Google Scholar]
- 175).Danishefsky S.J., Armistead D.M., Wincott F.E., Selnick H.G., Hungate R. (1987) The total synthesis of avermectin A1a. J. Am. Chem. Soc. 111, 2967–2980. [Google Scholar]
- 176).White J.D., Bolton G.L. (1990) Synthesis of avermectin B1a aglycon. J. Am. Chem. Soc. 112, 1626–1628. [Google Scholar]
- 177).Ley S.V., Armstrong A., Diez-Martin D., Ford M.J., Grice P., Knight J.G., Kolb H.C., Madin A., Marby C.A., Mukherjee S., Shaw A.N., Slawin A.M.Z., Vile S., White A.D., Williams D.J., Woods M. (1991) Total synthesis of the anthelmintic macrolide avermectin B1a. J. Chem. Soc., Perkin Trans. 1, 667–692. [Google Scholar]
- 178).Crimmins M.T., Al-awar R.S., Vallin I.M., Hollis W.G., Jr., O’Mahony R., Lever J.G., Bankaitis-Davis D.M. (1996) Asymmetric total synthesis of (+)-milbemycin D. J. Am. Chem. Soc. 118, 7513–7528. [Google Scholar]
- 179).Hirama M., Noda T., Ito S., Kabuto C. (1988) Stereocontrolled construction of the hexahydrobenzofuran subunit of the avermectins and the milbemycins: The aldol strategy. J. Org. Chem. 53, 706–708. [Google Scholar]
- 180).Hirama M., Nakamine T., Ito S. (1986) Enantiospecific synthesis of the spiroacetal unit of avermectin B1a. Tetrahedron Lett. 27, 5281–5284. [Google Scholar]
- 181).Hirama M., Shimizu M., Iwashita M. (1983) Enantiospecific syntheses of trifunctional (R)-3-hydroxy esters by baker’s yeast reduction. J. Chem. Soc., Chem. Commun., 599–600. [Google Scholar]
- 182).Hirama M., Noda T., Ito S. (1985) Convenient synthesis of (S)-citronellol of high optical purity. J. Org. Chem. 50, 127–129. [Google Scholar]
- 183).Hirama M., Noda T., Ito S. (1985) Convenient synthesis of (S)-citronellol of high optical purity (Additions and Corrections). J. Org. Chem. 50, 5916. [Google Scholar]
- 184).Hirama M., Nakamine T., Ito S. (1986) Trifunctional chiral synthons via stereocontrolled yeast reduction. Preparation of chiral pentene-1,3,5-triol derivatives. Chem. Lett., 1381–1384. [Google Scholar]
- 185).Lilitkarntakul S., Hirama M., Ito S. (1987) Diastereoselective deoxymercuration in acyclic system. A remarkable assistance by the neighboring carbonate group. Tetrahedron Lett. 28, 1207–1210. [Google Scholar]
- 186).Hirama M., Shimizu M. (1983) Oxidation of benzyl ether to benzoate by ozone. Synth. Commun. 13, 781–786. [Google Scholar]
- 187).Hirama M., Uei M. (1982) Carbamate mediated 1,3-asymmetric induction. A stereoselective synthesis of acyclic 1,3-diol systems. Tetrahedron Lett. 23, 5307–5310. [Google Scholar]
- 188).Hirama M., Iwashita M., Yamazaki Y., Ito S. (1984) Carbamate mediated functionalization of unsaturated alcohols II. Regio- and stereo-selective synthesis of 1,3-syn and 1,2-anti amino alcohol derivatives via iodocarbamation. Tetrahedron Lett. 25, 4963–4964. [Google Scholar]
- 189).Hirama M., Shigemoto T., Yamazaki Y., Ito S. (1985) Intramolecular Michael addition of O-carbamates to α,β-unsaturated esters. A new diastereoselective amination in an acyclic system. J. Am. Chem. Soc. 107, 1797–1798. [Google Scholar]
- 190).Hirama M., Shigemoto T., Ito S. (1985) Reversal of diastereofacial selectivity in the intramolecular Michael addition of δ-carbamoyloxy-α,β-unsaturated esters. Synthesis of N-benzoyl-D,L-daunosamine. Tetrahedron Lett. 26, 4137–4140. [Google Scholar]
- 191).Hirama M., Nishizaki I., Shigemoto T., Ito S. (1986) New acyclic approach to 3-amino-2,3,6-trideoxy-L-hexoses: a stereocontrolled synthesis of N-benzoyl L-daunosamine. J. Chem. Soc., Chem. Commun., 393–394. [Google Scholar]
- 192).Hirama M., Shigemoto T., Ito S. (1987) Stereodivergent total synthesis of N-acetylacosamine and N-benzoylristosamine. J. Org. Chem. 52, 3342–3346. [Google Scholar]
- 193).Hirama M., Iwakuma T., Ito S. (1987) Diastereofacial selectivity in the intramolecular conjugate addition of a nitrogen nucleophile; stereocontrolled piperidine synthesis. J. Chem. Soc., Chem. Commun., 1523–1524. [Google Scholar]
- 194).Hirama M., Hioki H., Ito S., Kabuto C. (1988) Conjugate addition of internal nucleophile to chiral vinyl sulfoxides with stereogenic center at the allylic carbon. “Intramolecular” double asymmetric induction. Tetrahedron Lett. 29, 3121–3124. [Google Scholar]
- 195).Hirama M., Hioki H., Ito S. (1988) New entry to syn-β-hydroxy-α-amino acids. Tetrahedron Lett. 29, 3125–3128. [Google Scholar]
- 196).Hirama M., Ito S. (1989) Asymmetric induction in the intramolecular conjugate addition of γ- or δ-carbamoyloxy-α,β-unsaturated esters. A new method for diastereoselective amination and divergent syntheses of 3-amino-2,3,6-trideoxyhexoses. Heterocycles 28, 1229–1247. [Google Scholar]
- 197).Danishefsky S., Hirama M. (1977) Total synthesis of disodium prephenate. J. Am. Chem. Soc. 99, 7740–7741. [DOI] [PubMed] [Google Scholar]
- 198).Danishefsky S., Hirama M., Fritsch N., Clardy J. (1979) Synthesis of disodium prephenate and disodium epiprephenate. Stereochemistry of prephenic acid and an observation on the base-catalyzed rearrangement of prephenic acid to p-hydroxyphenyllactic acid. J. Am. Chem. Soc. 101, 7013–7018. [Google Scholar]
- 199).Danishefsky S., Berman E., Clizbe L.A., Hirama M. (1979) A simple synthesis of L-γ-carboxyglutamate and derivative thereof. J. Am. Chem. Soc. 101, 4385–4386. [Google Scholar]
- 200).Danishefsky S., Hirama M., Gombatz K., Harayama T., Berman E., Schuda P. (1978) Stereospecific total synthesis of dl-pentalenolactone. J. Am. Chem. Soc. 100, 6536–6538. [Google Scholar]
- 201).Masamune S., Hirama M., Mori S., Ali Sk.A., Garvey D.S. (1981) Total synthesis of 6-deoxyerythronolide B. J. Am. Chem. Soc. 103, 1568–1571. [Google Scholar]
- 202).Hirama M., Uei M. (1982) Chiral total synthesis of compactin. J. Am. Chem. Soc. 104, 4251–4253. [Google Scholar]
- 203).Hirama M., Iwashita M. (1983) Total synthesis of (+)-monacolin K (mevinolin). Tetrahedron Lett. 24, 1811–1812. [Google Scholar]
- 204).Oishi T., Iwakuma T., Hirama M., Ito S. (1995) Stereoselective synthesis of (+)-swainsonine and (−)-8,8a-di-epi-swainsonine. Synlett, 404–406. [Google Scholar]
- 205).Hirama M., Oishi T., Ito S. (1989) Asymmetric dihydroxylation of alkenes with osmium tetroxide: chiral N,N′-dialkyl-2,2′-bipyrrolidene complex. J. Chem. Soc., Chem. Commun., 665–666. [Google Scholar]
- 206).Oishi T., Hirama M. (1989) Highly enantioselective dihydroxylation of trans-disubstituted and monosubstituted olefins. J. Org. Chem. 54, 5834–5835. [Google Scholar]
- 207).Oishi T., Hirama M. (1992) Synthesis of chiral 2,3-disubstituted 1,4-diazabicyclo[2.2.2]octane. New ligand for the Osmium-catalyzed asymmetric dihydroxylation of Olefins. Tetrahedron Lett. 33, 639–642. [Google Scholar]
- 208).Oishi T., Iida K., Hirama M. (1993) Asymmetric dihydroxylation of chiral olefins. High control of diastereofacial selection. Tetrahedron Lett. 34, 3753–3756. [Google Scholar]
- 209).Oishi T., Oguri H., Hirama M. (1995) Asymmetric Baylis-Hillman reactions using chiral 2,3-disubstituted 1,4-diazabicyclo[2.2.2]octanes catalysts under high pressure conditions. Tetrahedron Asymmetry 6, 1241–1244. [Google Scholar]
- 210).Akiyama E., Hirama M. (1996) Total synthesis and absolute configuration of (−)-sedacryptine. Synlett, 100–102. [Google Scholar]
- 211).Uehara H., Oishi T., Yoshikawa K., Mochida K., Hirama M. (1999) The absolute configuration and total synthesis of korormicin. Tetrahedron Lett. 40, 8641–8645. [Google Scholar]
- 212).Sotokawa T., Noda T., Pi S., Hirama M. (2000) A three-step synthesis of halomon. Angew. Chem. Int. Ed. 39, 3430–3432. [DOI] [PubMed] [Google Scholar]
- 213).Imai H., Oishi T., Kikuchi T., Hirama M. (2000) Concise synthesis of 3-O-(2-O-α-D-glucopyranosyl-6-O-acyl-α-D-glucopyranosyl)-1,2-di-O-acyl-sn-glycerols. Tetrahedron 56, 8451–8459. [Google Scholar]
- 214).Shimizu T., Arai S., Imai H., Oishi T., Hirama M., Koito A., Kida Y., Kuwano K. (2004) Glycoglycerolipid from the membranes of Acholeplasma laidlawii binds to human immunodeficiency Virus-1 (HIV-1) and accelerates its entry into cells. Curr. Microbiol. 48, 182–188. [DOI] [PubMed] [Google Scholar]
- 215).Inoue M., Sakazaki H., Furuyama H., Hirama M. (2003) Total synthesis of TMC-95A. Angew. Chem. Int. Ed. 42, 2654–2657. [DOI] [PubMed] [Google Scholar]
- 216).Inoue M., Sato T., Hirama M. (2003) Total synthesis of merrilactone A. J. Am. Chem. Soc. 125, 10772–10773. [DOI] [PubMed] [Google Scholar]
- 217).Inoue M., Sato T., Hirama M. (2006) Asymmetric total synthesis of (−)-merrilactone A: Use of a bulky protecting group as long-range stereocontrolling element. Angew. Chem. Int. Ed. 45, 4843–4848. [DOI] [PubMed] [Google Scholar]
- 218).Nitta A., Ishiwata A., Noda T., Hirama M. (1999) Synthetic study of pinnnatoxin A: Intramolecular alkylation approach to the G-ring. Synlett, 695–696. [Google Scholar]
- 219).Sakamoto S., Sakazaki H., Hagiwara K., Kamada K., Ishii K., Noda T., Inoue M., Hirama M. (2004) A formal total synthesis of (+)-pinnatoxin A. Angew. Chem. Int. Ed. 43, 6505–6510. [DOI] [PubMed] [Google Scholar]
- 220).Tsukano C., Zhao L., Takemoto Y., Hirama M. (2010) Concise total synthesis of lycodine. Eur. J. Org. Chem., 4198–4200. [Google Scholar]
- 221).Zhao L., Tsukano C., Kwon E., Takemoto Y., Hirama M. (2013) Total syntheses of complanadines A and B. Angew. Chem. Int. Ed. 52, 1722–1725. [DOI] [PubMed] [Google Scholar]
- 222).Yamashita S., Iso K., Hirama M. (2008) A concise synthesis of the pentacyclic framework of cortistatins. Org. Lett. 10, 3413–3415. [DOI] [PubMed] [Google Scholar]
- 223).Yamashita S., Iso K., Kitajima K., Himuro M., Hirama M. (2011) Total synthesis of cortistatins A and J. J. Org. Chem. 76, 2408–2425. [DOI] [PubMed] [Google Scholar]
- 224).Yamashita S., Naruko A., Nakazawa Y., Zhao L., Hayashi Y., Hirama M. (2015) Total synthesis of limonin. Angew. Chem. Int. Ed. 54, 8538–8541. [DOI] [PubMed] [Google Scholar]
- 225).Yoshino T., Sato I., Hirama M. (2012) Total synthesis of aspercyclides A and B via intramolecular oxidative diaryl ether formation. Org. Lett. 14, 4290–4292. [DOI] [PubMed] [Google Scholar]
- 226).Yoshino T., Yamashita S., Sato I., Hayashi Y., Hirama M. (2013) Solvent-mediated tuning of the regioselectivity of intramolecular diaryl ether formation: Total synthesis of (+)-aspercyclide C. Chem. Lett. 43, 349–351. [Google Scholar]