Abstract
Free radicals may attack cells or tissue, leading to chronic diseases, and antioxidant consumption is potentially useful for removing free radicals. Egg proteins may be used as potential sources of antioxidant considering their ability of scavenging free radicals to apply for food or cosmetics industry. In this study, we obtained a natural antioxidant protein from fertilized eggs, which was a dietary supplement in some Asian countries. Meanwhile, antioxidant activities of these proteins were evaluated using different oxidation systems. With increasing incubation time, the antioxidant activity of these proteins increased during 15 d of incubation. The samples on day 15 were performed for isolation of antioxidant protein. The protein, named P4-1 (MW, 45 kDa), was isolated and purified by consecutive chromatographic methods. P4-1 contained 17 amino acids, which was determined by liquid chromatography-mass spectrometry and Amino Acid Analyzer. Moreover, the amino acid sequence was highly similar to that of ovalbumin. Differential scanning calorimetry showed that the denaturation temperature of P4-1 was 57.16℃. Furthermore, P4-1 suggested high oxygen radical-absorbance activity in ·OH assays, and its antioxidant activity was stable at 30-50℃ in acidic and neutral pH. Thus, these results revealed that P4-1 may be a potential resource as a natural antioxidant.
Keywords: fertilized eggs, protein, antioxidant, separation, purification
Introduction
Free radicals are defined as molecules with an unpaired electron in their outermost orbits. In general, they are unstable and reactive in nature (Kumar et al., 2012) and may attack proteins and DNA, leading to serious diseases such as hypertension, cancer, coronary heart disease, and senile dementia. Antioxidant consumption is potentially useful for removing free radicals from the body. Many evidences suggested that antioxidants played important roles in human health, as they could protect the human body against damage from free radicals (Chen et al., 2012). Synthetic antioxidants such as butylated hydroxyanisole (BHA), propyl gallate (PG), and butylated hydroxytoluene (BHT) were often used for daily consumption. However, these antioxidants were suspected to be responsible for liver damage and carcinogenesis (Finkel et al., 2000). Thus, many researchers had focused on identifying and developing natural, safe and edible antioxidants. Bioactive proteins from foods have received increasing attentions due to their safety and health benefits, such as a protein from the fruit of Terminalia chebula which exhibited significant radical scavenging in DPPH, NO, H2O2 and ABTS assays (Sriva et al., 2012). Meanwhile, a protein from pearl oyster named PMP (200 μg/mL) showed a high oxygen radical absorbance activity (ORAC) of 6.57 μmol Trolox/μmol protein (You et al., 2015), and the protein from Phyllantus nituri can enhance intracellular antioxidant power and protect the TBHP-induced alterations of the cytoprotective and antioxidant molecules (Sarkar et al., 2009). As natural dietary supplements, fertilized hen eggs are considered as a traditional food in some Asian countries, especially in China. Fresh fertilized eggs laid within 24 h from the farms, were used for the study or production. These eggs were incubated in an incubator with two turnings every day at 37.2±0.5℃ and 55±5% relative humidity with automatic ventilation. Considering the plentiful nutrients of fertilized eggs, numerous studies have reported the relative abundances of their component nutrients (Li et al., 2012). Previous data have indicated that fertilized eggs have high antioxidant activity and can prevent aging and skin wrinkling (Surai et al., 1996). Memarpoor et al. (2012) reported that chicken embryos can confer resistance to fatigue and senility in rats. Compared with the levels of vitamin (E, A, C), glutathione, peroxidase, and superoxide dismutase in the brain, the levels of these antioxidants in the liver were significantly higher for the newly hatched chicks (Gaal et al., 1995). Duan et al. (2014) found that peptides isolated from fertilized eggs on day 15 post-fertilization exhibited the highest antioxidant activity. Sriva et al. (2012) indicated that water-soluble proteins from fertilized eggs had strong antioxidant activities and a positive correlation between the antioxidant activity and the sample concentration. These studies showed that different tissues of fertilized eggs embryo displayed distinct antioxidant capacity. However, most previous studies focused on non-protein antioxidants, such as α-tocopherol, carotenoids, ascorbic acid, and superoxide dismutase, while the available information on antioxidant proteins remained limited. In this study, a novel, natural antioxidant protein was isolated from fertilized eggs, and the biological properties and antioxidant activities of this protein were simultaneously evaluated.
Materials and Methods
Materials
Fertilized hen eggs on days 15 of incubation were obtained from Yinkun Co. (China), which were incubated at 37.8℃ with the relative humidity of 60%. Eggs were automatically turned through with a 90° angle every hour. DEAE Cellulose FF was purchased from GE Healthcare Co. (USA). Sephadex G-100 was purchased from Sigma Co. (USA). The AKTA Purifier Plus chromatographic system (GE Healthcare Co.) was provided by the Hefei University of Technology. The compounds 2,2-diphenyl-1-picrylhydrazyl (abbreviated as DPPH), pyrogallic acid, and 1,10-phenanthroline were purchased from Biyuntian Biotechnology Co. (China). Other chemicals were of analytical grade and provided by the Laboratory of Biotechnology and Food Engineering at Hefei University of Technology.
Protein preparation
After mixing fertilized eggs with a high speed, we obtained homogenates, which were centrifuged (14,243 g) for 20 min at 4℃, using an ST-16R refrigerated centrifuge (Thermo Fisher Scientific, USA). The supernatants were freeze-dried at 50℃ for 24 h using an FD-1B-5D freeze drier (Boyikang Laboratory Instrument Co., Ltd., China). Protein was extracted from the powders using sodium phosphate buffer solution (0.2 M, pH 7) at a ratio of 1: 10 (w/v). The mixture was homogenized by stirring for 2 h at 4℃. Supernatants were collected after centrifugation at 14,243 g and 4℃ for 30 min. The supernatants were subjected to 80% ammonium sulfate saturation by adding solid ammonium sulfate with gentle stirring at 4℃ for 30 min, after which they were centrifuged at 1,281 g for 20 min at 4℃, desalted at 4℃, dialyzed against deionized water for 48 h, and concentrated subsequently. The powders were used as crude protein, which was stored at −20℃ until further analysis.
Determination of protein contents
The protein contents of crude extracts were determined using the Kjeldahl method after digestion, according to the national standard method (Ministry of Health of the People’s Republic of China, 2010).
DPPH radical-scavenging activity assay
DPPH radical-scavenging activity (RSA) assay was performed according to the method described by Je (2009). To test tube A, 2.5 mL of a DPPH-ethanol solution (6.5 × 10−5 M) and 0.5 mL ethanol were added and incubated in the dark at room temperature for 10 min. The absorbance of the solution A was monitored at 517 nm using a UV 752 spectrophotometer (Jinghua Science and Technology, Ltd., China) with distilled water serving as a control. Moreover, 2.5 mL DPPH-ethanol solution (6.5 × 10−5 M) and 0.5 mL sample solution (5 mg/mL) were added to tube B, with distilled water serving as the control. Tube C was prepared in the same manner as for tube B, except that 2.5 mL ethanol replaced the 2.5 mL of DPPH-ethanol solution (6.5 × 10−5 M). Here, determination of N-acetyl cysteine (NAC) was as a control. Each experiment was repeated 3 times to determine the average value. DPPH radical-scavenging activities in the samples were calculated as follows:
where Ao is the absorbance of tube A, Ai is the absorbance of tube B, and Aj is the absorbance of tube C.
Determination of · radical-scavenging activity
Tris-HCl buffer (4.5 mL; pH 8.2, 50 mM) and 4.2 mL distilled water were mixed and incubated in a 25℃ water bath for 20 min, after which the solution was further mixed with 0.5 mL pyrogallic acid solution (3 mM), forming solution A. Solution B (composed of 3.7 mL of distilled water) was mixed with 0.5 mL of sample solution [5 mg P4-1 powder dissolved in 1.0 mL of buffer (0.2 M PBS, pH 7)] and then added to Solution A. HCl solution (10 mM) was used as a blank control (Su et al., 2009). The absorbance of both solution A and solution B were measured at 320 nm using a UV 752 spectrophotometer. The absorbance was measured kinetically every 30 s and was calculated in the linear range. Determination of NAC was as a control. Each experiment was performed 3 times to determine the average value. ·radical-scavenging activity in the samples were calculated as follows:
where A1 is reaction rate of solution A, and A2 is the rate of solution B.
·OH radical-scavenging activity assay
The ·OH radical-scavenging ability was assayed according to the method described previously by Wang et al (2008). A reaction mixture (8 mL) was prepared, which contained 2 mL FeSO4 (9 mM), 2 mL hydroxylated salicylate complex (9 mM), 2 mL sample solution (5 mg/mL), and 2 mL H2O2 (8.8 mM). After incubation for 30 min in a 37℃ water bath, the absorbance of the hydroxylated salicylate complex was measured at 510 nm. Determination of NAC was as a control. The percentage scavenging activity was calculated as:
where K0 is the absorbance of the control (distilled water instead of sample), K1 is the absorbance of sample solution (5 mg/mL), and K2 is the absorbance without the hydroxylated salicylate complex (replaced by distilled water).
Ion-exchange chromatography
One gram of crude protein was dissolved in 50 mL buffer A (0.02 M, pH 6.8 phosphate buffer) and centrifuged at 3,000 rpm at 4℃ for 15 min. Subsequently, the supernatant was micro-filtered through a 0.22-μm membrane and loaded onto an anion-exchange column (16 × 500 mm) packed with DEAE Cellulose FF, followed by loading into the ∆KTA Purifier Plus chromatography system (GE Healthcare Co.), which was equilibrated with buffer A (2 column volumes) (Je et al., 2007). The column was then washed with 2 column volumes of buffer A at a flow rate of 1 mL/min. The crude proteins were eluted using a linear NaCl gradient (ranging from 0 to 1 M NaCl over 70 min) followed by 1 M NaCl for 50 min in buffer A at a flow rate of 1 mL/min. Elution peaks were detected at 280 nm. All fractions were collected and freeze-dried. The freeze-dried powders were stored for subsequent antioxidant assays.
Gel-filtration chromatography
Gel-filtration chromatography was performed according to the method described by Ahmed et al. (2013). Twenty-five millilitres of active fraction (20 mg/mL) was loaded onto a gel-chromatography column (10 × 300 mm) packed with Sephadex G-100 gel, using an ∆KTA Purifier Plus chromatography system for further purification. The gel-chromatography column was equilibrated and washed with 2 volumes of buffer B (20 M Tris-HCl, pH 8.0), for each step. The active fraction was eluted with a linear salt gradient (0 to 1.0 M NaCl over 70 min) at a flow rate of 1 mL/min. The fraction obtained was designated as P4-1 and was freeze-dried and stored for further analysis.
Relative molecular weight of antioxidant proteins
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis was performed using a 12% separating gel and a 5% stacking gel, and the gels were stained with Coomassie brilliant blue R-250 for 2 h. Then, the gels were de-stained for 24 h. The proteins were separated at 80 V for 30 min and then at 120 V for 120 min. The relative molecular masses and its abundances of the purified proteins were analysed using standard proteins with molecular masses ranging from 14.4-94.0 kDa.
Liquid-phase mass spectrometry
Protein bands were excised from SDS-PAGE gels and identified by analysis with a ProteomeX-LTQ 2D-LC-MS system (Thermo Fisher Scientific Company, USA). The working parameters of the instrument were as follows: separation mode of LC: The proteins were trapped on a C18 reversed phase capillary column ddH20 using the HPLC system; the mobile phases A was 0.1% FA/ddH20, and the mobile phases B was 100% acetonitrile; the mobile phase gradient was delivered over 10 min; ramping to solution B by 6 min, holding for one minute, and then transitioning back to mixtures over 1.5 min. The flow rate was 0.4 mL/min with an injection volume of 2 μL.
The mass spectrometer was performed in positive ion mode. MS/MS spectra were acquired on the three most abundant ions in each MS scan. The ionization parameters were as follows: capillary voltage of 3 kV, cone gas flow of 150 L/h, source temperature of 150℃, desolvation gas flow of 1000 L/h. All samples were analyzed at a cone voltage of 50 kV. Settings for CID are: default charge state 2, isolation width 2.0 Da, extra 57 Da for Cys and an extra 16 Da for Met, normalized collision energy 30.0% and Q 0.25 with activation time of 30 ms.
Amino acid composition analysis
The amino acid profile of P4-1 was determined using an L8900 Automatic Amino Acid Analyzer (Hitachi Co., Ltd., Japan) according to the Chinese National Standard Method (Ministry of Health, PRC, 2003). Proteins (0.1 g) were hydrolysed to amino acids in a vacuum with 10 ml HCl (6 M) at 110℃ for 24 h (Zhong et al., 2011). Impurities were filtered and removed and the samples were adjusted to a constant volume of 50 mL, after which 10 mL of sample was incubated in a vacuum at 65℃ for 4 h in a sealed tube. Twenty microliters of sample was then injected into the Hitachi Amino Acid Analyzer.
Differential scanning calorimetry (DSC)
The thermal transitions of P4-1 were determined using a TA Q200-DSC thermal analyser (TA Instruments, USA). Approximate 8.38 mg of the powdered P4-1 protein was transferred into aluminium liquid pans. The pans were hermetically sealed and heated from 20 to 100℃ at a rate of 10℃/min. A sealed empty pan was used as a reference. Nitrogen served as a protective gas and was flowed over the samples at a rate of 50.0 mL/min. The samples were equilibrated at 20℃ for 5 min (Splinter et al., 2012).
Effect of temperature
The effects of different temperatures on the antioxidant activity of P4-1 were measured by performing ·OH radical-scavenging activity assays.P4-1 powder (5 mg) was dissolved in 1.0 mL of buffer (0.2 M PBS, pH 7), using a water bath heated from 30℃ to 100℃. As the temperature increased, the sample antioxidant activity was measured for 30 min at 30, 40, 50, 60, 70, 80, 90 and 100℃ (Shukla and Bajwa, 2013). Other conditions were as described above for the ·OH radical-scavenging activity assay.
Effect of pH
The effects of different pH on the antioxidant activity of P4-1 were measured by performing the ·OH radical-scavenging activity assay. A solution of the P4-1 protein (5 mg/mL) was prepared. HCl and NaOH (1 M each) were used to adjust the pH value. After incubating replicate samples for 30 min in a 37℃ water bath, the samples were adjusted to a pH of 7.0 and a constant volume. Then, the pH of the sample solutions was adjusted to 3, 4, 5, 6, 7, 8, 9 or 10, after which the antioxidant activity in each solution was measured. Other conditions were as described above for the ·OH radical-scavenging activity assay.
Statistical analysis
Statistical analysis was performed in MS Excel (Microsoft Windows 7) using a 1-tailed Student’s t-test. Data were expressed as the mean ± standard error of the mean (n=3). All figures were prepared using origin 8.6 software (Ji et al., 2014).
Results
Protein content measurements
Using the Kjeldahl method, the sample proteins were found to contain an average 82.9% nitrogen, suggesting that the protein content of lyophilized samples was high. Thus, these samples can be used in the subsequent analysis.
Protein isolation
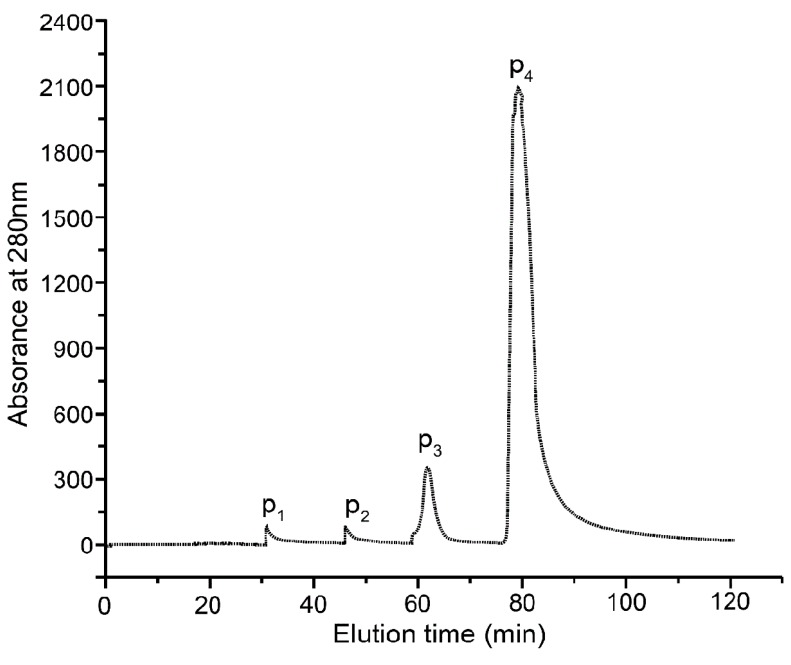
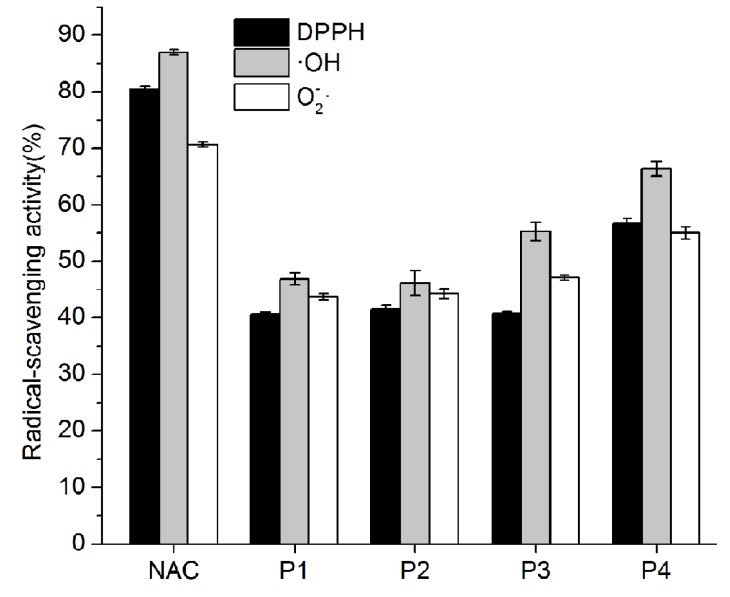
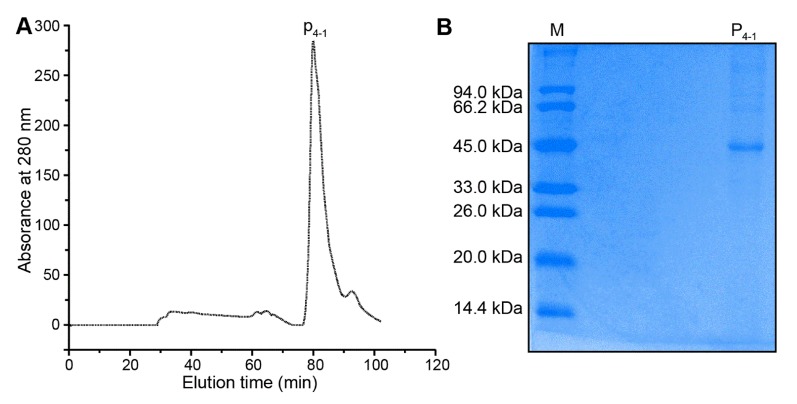
As shown in Fig. 1, crude protein was subjected to ion-exchange chromatography and separated into 4 peaks. The P4 fraction showed higher antioxidant activity in the oxidation-resistance test, and the percentages of scavenged DPPH·, ·OH, and were 56.77±0.86%, 66.37±1.33%, and 55±1.12%, respectively. Compared to other fractions, the radical-scavenging activities of fraction 4 were clearly the highest (Fig. 2). Furthermore, Fraction P4 was loaded onto a Sephadex G-100 column, and a single peak was obtained, as shown in Fig. 3A. The features of the eluted protein were analysed by the following tests.
Fig. 1. Elution profile of crude proteins separated by DEAE Cellulose FF on an ion-exchange column. Four fractions were collected (P1, P2, P3, and P4) from fertilized eggs on day 15; the protein concentration curves: crude protein was subjected to ion-exchange chromatography and separated into 4 peaks; the protein concentration of each tube was determined at a wavelength of 280 nm.
Fig. 2. Antioxidant activities of different fractions at the same concentration (5 mg/mL). The value of each fraction is the mean value (± SD) for three independent experiments, each of which was performed in triplicate; the values represent the DPPH·, ·OH, and of these fractions (P1, P2, P3, P4), respectively; NAC represents N-acetyl cysteine which was a standard antioxidant protein sample to compare the degree of antioxidant capacity with the purified samples.
Fig. 3. SDS-PAGE analysis of the purified protein. (a) Elution profile of P4-1 following Sephadexn G-100 gel chromatography, a wavelength of 280 nm represent the protein concentration of each tube respectively (b) SDS-PAGE analysis. Proteins in the SDS-PAGE gel were visualized using coomassie brilliant blue; the identified protein P4-1 is labeled with numbers that correspond to standard markers.
Relative molecular weight of the antioxidant protein
Fig. 3B exhibited the results of SDS-PAGE analysis. The lane left showed the molecular weight of markers, and the right lane showed the P4-1 protein. Fig. 3B showed the SDS-PAGE band pattern of the purified protein (P4-1) following gel-filtration chromatography, using an ∆KTA Purifier Plus chromatography system. The single protein band indicated that the molecular mass of the P4-1 protein was approximate 45 kDa. This observation of a single P4-1 band was in accordance with the single absorbance peak observed at 280 nm after gel-filtration chromatography.
Liquid-phase mass spectrometry analysis
The amino acid sequence identified by liquid chromatography-mass spectrometry (LC-MS) was shown in Table 1. The P4-1 protein contains 386 amino acids, with a molecular mass of 42,854.5 and a pl of 5.07. The obtained amino acid sequence was to analyse homology by NCBI protein database, and the results showed that the protein was highly similar to an ovalbumin protein designated as “Ovalbumin from Gallus gallus” in the UniProt Database (Accession Number P01012).
Table 1. The sequence of amino acids identified by LC-MS.
| 1–10 | 11–20 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | 71–80 | 81–90 | 91–100 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MGSIGAASME | FCFDVFKELK | VHHANENIFY | CPIAIMSALA | MVYLGAKDST | RTQINKVVRF | DKLPGFGDSI | EAQCGTSVNV | HSSLRDILNQ | ITKPNDVYSF |
| 101 | SLASRLYAEE | RYPILPEYLQ | CVKELYPGGC | EPINFQTAAD | QARELINSWV | ESQTNGIIRN | VLQPSSVDSQ | TAMVLVNAIV | FKGLWEKAFK | DEDTQAMPFR |
| 201 | VTEQESKPVQ | MMYQIGLFRV | ASMASELMKI | LELPFASGTM | SMLVLLPDEV | SGLEQLESII | NFEKLTEWTS | SNVMEERKIK | VYLPRMKMEE | KYNLTSVLMA |
| 301 | MGITDVFSSS | ANLSGISSAE | SLKISQAVHA | AHAEINEACR | EVVGSAEAGV | DAASVSEEFR | ADHPFLFCIK | HIATNAVLFF | GRCVSP |
Amino acid composition
The amino acid composition of the P4-1 protein was shown in Table 2. Essential amino acids required for maintaining good health were abundant in P4-1, which was rich in glutamic acid (24.17%), aspartate (17.19%), and tyrosine (15.69%). In general, the amino acid composition of proteins played a key role in their antioxidant activities. Tyrosine was known to act as a direct radical scavenger (Chalamaiah et al., 2012) and was found abundantly in the P4-1 protein. Phenylalanine, which was previously reported to promote radical-scavenging activity (Sarmadi and Ismail, 2010), was also found in the protein P4-1. Therefore, tyrosine and phenylalanine residues may significantly contribute to the antioxidant activity of P4-1.
Table 2. Amino acid composition of P4-1.
| Amino acid | (P4-1) g kg−1 |
|---|---|
| Asp | 17.19 |
| Thr | 8.19 |
| Ser | 11.16 |
| Glu | 24.17 |
| Gly | 6.06 |
| Ala | 10.18 |
| Cys | 3.69 |
| Val | 9.73 |
| Met | 4.41 |
| Ile | 8.73 |
| Leu | 15.69 |
| Tyr | 7.09 |
| Phe | 9.38 |
| Lys | 11.98 |
| His | 3.81 |
| Arg | 9.72 |
| Pro | 5.28 |
Data in this table indicated the amino acid content of Per kilogram of P4-1 protein.
Differential scanning calorimetry (DSC)
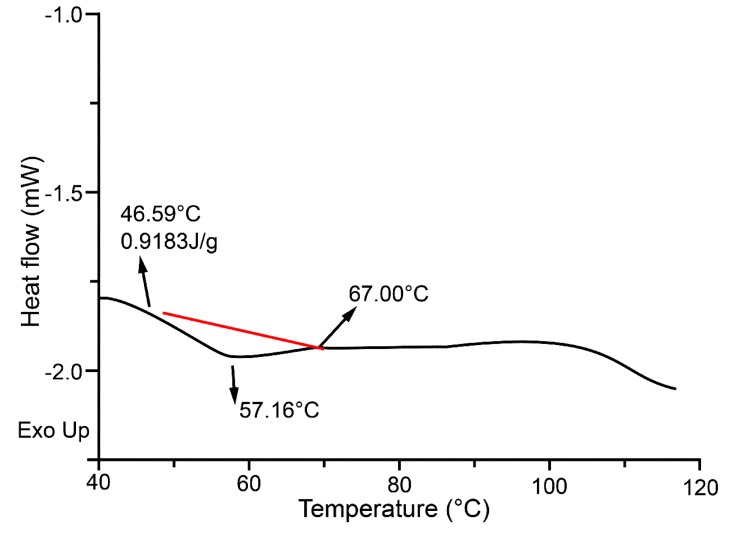
DSC can be used to monitor changes in the stability of proteins (Quinn et al., 1980). The thermo profiles of P4-1 were demonstrated in Fig. 4. The denaturation temperature was measured at 57.16℃ while raising the temperature from 46.59℃ to 67.00℃, and the thermal denaturation enthalpy (∆H) was 0.9183 J/g. Fig. 4 indicated that only one peak was obtained, which demonstrated the purity of protein obtained in this study.
Fig. 4. DSC analysis of P4-1. The denaturation temperature was measured at 57.16℃ while raising the temperature from 46.59℃ to 67.00℃. The red line represents the variation trend of heat flow for the protein P4-1 and only one peak demonstrates the purity of the protein P4-1.
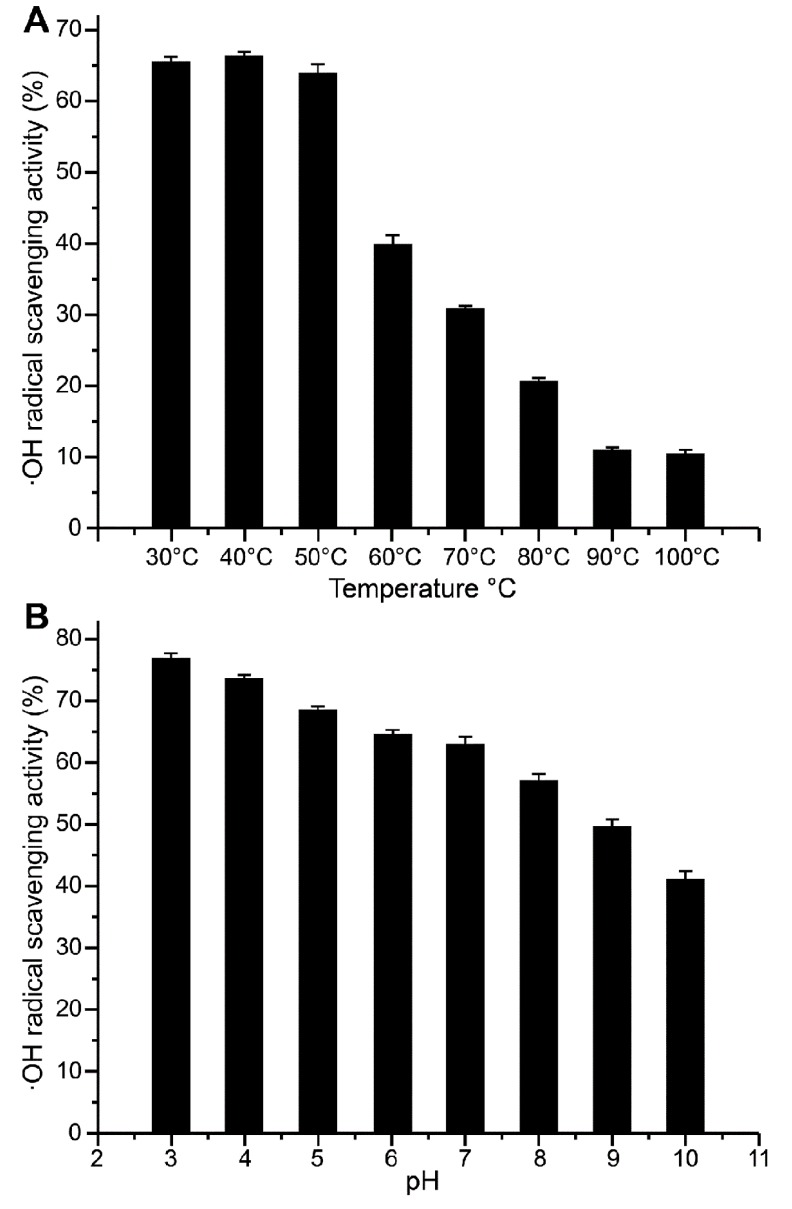
Effect of temperature
The effects of different temperatures on the antioxidant activity of P4-1 were shown in Fig. 5A. The antioxidant activity was comparatively stable when the temperature ranged from 30-50℃. When the temperature was increased to 60℃, the antioxidant activity decreased to approximately 40%. When the temperature was increased to 90 or 100℃, the effect of temperature on oxidation resistance was relatively stable, with the scavenging rate of the ·OH radical maintained at approximately 10%. This reason may be that high temperatures caused the protein to denature, resulting in activity loss. The results were in accordance with the DSC results, which showed that no significant changes in enthalpy occurred below 50℃.
Fig. 5. ·OH radical-scavenging activity. (a) ·OH radical-scavenging activity at different temperatures. The antioxidant activity reveals comparatively stable with the range of 30-50℃ and sustained decline with the rising of the temperature. (b) ·OH radical-scavenging activity at different pH values. The antioxidant activity reveals a sustained decline with the rising of the PH.
Effect of pH
Fig. 5B showed that the ·OH radical-scavenging activity of P4-1 tended to decrease with increasing pH values. This trend was apparent when the pH was greater than 7. We concluded that the antioxidant activity of P4-1 was comparatively high at acidic and neutral pHs. Thus, these findings indicated that P4-1 may be a useful antioxidant for both the stomach and intestine when consumed.
Discussion
Fertilized eggs are commonly consumed in China for their nutritional value and special flavour. During incubation, the antioxidant system was considerably advanced to protect the embryo from damaging effects of free radicals (Starrs et al, 2001). Some reported antioxidants, such as antioxidant vitamins and enzymes, dealt with oxidative stress at hatching time in different tissues (Chay et al., 2011; Mine et al, 2010; Wilson et al., 1992). In this study, proteins derived from fertilized eggs firstly exhibited antioxidant activity which increased during incubation significantly. It meant that these purified proteins may contribute to protect chick embryo from attack by free radicals during embryogenesis. Hence, fertilized eggs could be served as a new source of antioxidant proteins.
In this study, an antioxidant protein from fertilized eggs here was performed to purify and characterize, respectively. Estimation of the protein molecular weight based on SDS-PAGE analysis demonstrated that it had an approximate molecular weight of 45 kDa. Furthermore, LC-MS results showed that the amino acid sequence of the purified protein was highly similar to ovalbumin (Gallus gallus). Remarkably, the antioxidant activity of ovalbumin in fertilized eggs was improved compared with infertile eggs (Duan et al, 2010). Integrating SDS-PAGE and LC-MS was to further indicate that the active protein could be a functional ovalbumin with unique structure.
Additionally, a high thermal-denaturation temperature by DSC showed that the protein had a relatively stable characteristic. Amino acid composition analysis showed that the purified protein contained the essential amino acids proline, lysine, leucine, and alanine and well above hydrolysed whey protein (Powergrant et al., 2015). Pena (2004) had indicated that the antioxidant activity of some proteins was attributable to the presence of leucine, histidine and proline. Our results also suggested that the purified protein P4-1 had strong radical-scavenging activity, which was stable over a temperature range of 30-50℃. P4-1 was also demonstrated to be an effective antioxidant when the pH was less than 7. In conclusion, the antioxidant protein isolated from fertilized eggs exhibited antioxidant and free radical-scavenging activities. Further studies are necessary to understand the mechanism of its antioxidant activity, to evaluate its antioxidant activity in vivo, and to assess its potential for applications in model food systems.
Acknowledgments
This work was supported by Anhui Entry-exit Inspection and Quarantine Bureau, the special funds for central college of China (JZ2015HGBZ0522), Wanjiang Institute of Poultry Technology (XC2015AKKG002), the Science and Technology Bureau of Xuancheng (XC2015XKKJ02) and Science and Technology Bureau of AnHui (XC2014 AKKG002; XC2015AKKG003).
References
- 1.Ahmed H., Abu E., Zahab H., Alswiai G. Purification of antioxidant protein isolated from Peganumharmala and its protective effect against CCl4 toxicity in rats. Turk. J. Biol. 2013;37:39–48. [Google Scholar]
- 2.Chalamaiah M., Dinesh Kumar B., Hemalatha R., Jyothirmayi T. Fish protein hydrolysates: Proximate composition, amino acid composition, antioxidant activities and applications: A review. Food Chem. 2012;135:3020–3038. doi: 10.1016/j.foodchem.2012.06.100. [DOI] [PubMed] [Google Scholar]
- 3.Chay Pak Ting B. P., Mine Y., Juneja L. R., Okubo T., Gauthier S. F., Pouliot Y. Comparative composition and antioxidant activity of peptide fractions obtained by ultra filtration of egg yolk protein enzymatic hydrolysates. Membranes. 2011;1:149–161. doi: 10.3390/membranes1030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C., Chi Y. J., Xu W. Comparisons on the functional properties and antioxidant activity of spray-dried and freeze-dried egg white protein hydrolysate. Food Bioprocess Technol. 2012;5:2342–2352. doi: 10.1007/s11947-011-0606-7. [DOI] [Google Scholar]
- 5.Duan X., Ocen D., Wu F., Li M., Yang N., Xu J., Chen H., Huang L., Jin Z., Xu X. Purification and characterization of a natural antioxidant peptide from fertilized eggs. Food Res. Int. 2014;56:18–24. doi: 10.1016/j.foodres.2013.12.016. [DOI] [Google Scholar]
- 6.Finkel T., Holbrook N. J. Oxidants, oxidative stress and the biology of ageing. Nature. 2009;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 7.Gaal T., Mezes M., Noble R. C., Dixon J., Speake B. K. Development of antioxidant capacity in tissues of the chick embryo. Comp. Biochem. Physiol. 1995;4:711–716. [Google Scholar]
- 8.Je J. Y., Park P. J., Kim E. K., Park J. S., Yoon H. D. Antioxidant activity of enzymatic extracts from the brown seaweed Undariapinnatifida by electron spin resonance spectroscopy. LWT-Food Sci Technol. 2009;42:874–878. doi: 10.1016/j.lwt.2008.10.012. [DOI] [Google Scholar]
- 9.Je J. Y., Qian Z. J., Byun H. G., Kim S. K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007;42:840–846. doi: 10.1016/j.procbio.2007.02.006. [DOI] [Google Scholar]
- 10.Ji N., Sun C., Zhao Y., Xiong L., Sun Q. Purification and identification of antioxidant peptides from peanut protein isolate hydrolysates using UHR-Q-TOF mass spectrometer. Food Chem. 2014;161:148–154. doi: 10.1016/j.foodchem.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Li X., Su Y., Sun J., Yang Y. Chicken embryo extracts enhance spleen lymphocyte and peritoneal macrophages function. J. Ethnopharmacol. 2012;144:255–260. doi: 10.1016/j.jep.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Memarpoor-Yazdi M., Asoodeh A., Chamani J. A novel antioxidant and antimicrobial peptide from hen egg white lysozyme hydrolysates. J. Funct. Foods. 2012;4:278–286. doi: 10.1016/j.jff.2011.12.004. [DOI] [Google Scholar]
- 13.Pena-Ramos E. A., Xiong Y. L., Arteaga G. E. Fractionation and characterisation for antioxidant activity of hydrolysed whey protein. J. Sci. Food Agric. 2004;84:1908–1918. doi: 10.1002/jsfa.1886. [DOI] [Google Scholar]
- 14.Powergrant O., Bruen C., Brennan L., Giblin L., Jakeman P., Fitzgerald R. J. In vitro bioactive properties of intact and enzymatically hydrolysed whey protein: Targeting the enteroinsular axis. Food J. Funct. 2015;6:972–980. doi: 10.1039/C4FO00983E. [DOI] [PubMed] [Google Scholar]
- 15.Quinn J. R., Raymond D. P., Harwalkar V. R. Differential scanning calorimetry of meat proteins as affected by processing treatment. J. Food Sci. 1980;45:1146–1149. doi: 10.1111/j.1365-2621.1980.tb06507.x. [DOI] [Google Scholar]
- 16.Sampath Kumar N. S., Nazeer R. A., Jaiganesh R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspiscordyla) and croaker (Otolithesruber) Amino Acids. 2012;42:1641–1649. doi: 10.1007/s00726-011-0858-6. [DOI] [PubMed] [Google Scholar]
- 17.Sarkar M. K., Kinter M., Mazumder B., Sil P. C. Purification and characterisation of a novel antioxidant protein molecule from Phyllanthusniruri. Food Chem. 2009;114:1405–1412. doi: 10.1016/j.foodchem.2008.11.022. [DOI] [Google Scholar]
- 18.Sarmadi B. H., Ismail A. Antioxidative peptides from food proteins: A review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Shukla P., Bajwa U. Effect of heat treatments on antioxidant activity in sucrose milk protein model systems. Int. J. Comp. Eng. Res. 2013;3:20–24. [Google Scholar]
- 20.Splinter R., van Herwaarden A. W., Iervolino E., Vanden-Poel G., Istrate D., Sarro P. M. Analyzing protein denaturation using fast differential scanning calorimetry. Procedia Eng. 2012;47:140–143. doi: 10.1016/j.proeng.2012.09.104. [DOI] [Google Scholar]
- 21.Srivastava P., Raut H. N., Wagh R. S., Puntambekar H. M., Kulkarni M. J. Purification and characterization of an antioxidant protein (~16 kDa) from Terminaliachebula fruit. Food Chem. 2012;131:141–148. doi: 10.1016/j.foodchem.2011.08.048. [DOI] [Google Scholar]
- 22.Starrs A. P., Orgeig S., Daniels C. B., Davies M., Lopatko O. V. Antioxidant enzymesin the developing lungs of egg-laying and metamorphosing vertebrates. J. Exp. Biol. 2001;204:3973–3981. doi: 10.1242/jeb.204.22.3973. [DOI] [PubMed] [Google Scholar]
- 23.Su X. Y., Wang Z. Y., Liu J. R. In vitro and in vivo antioxidant activity of Pinuskoraiensis seed extract containing phenolic compounds. Food Chem. 2009;117:681–686. doi: 10.1016/j.foodchem.2009.04.076. [DOI] [Google Scholar]
- 24.Surai P. F., Noble R. C., Speake B. K. Tissuespecic differences in antioxidant distribution and susceptibility to lipid peroxidation during development of the chick embryo. Biochim. Biophys. Acta (BBA)-Lipids and Lipid Metabolism. 1996;1304:1–10. doi: 10.1016/S0005-2760(96)00099-9. [DOI] [PubMed] [Google Scholar]
- 25.Vasilescu J., Smith J. C., Ethier M., Figeys D. Proteomic analysis of ubiquitinated proteins from human MCF-7 breast cancer cells by immunoaffinity purification and mass spectrometry. J. Proteome Res. 2005;4:2192–2200. doi: 10.1021/pr050265i. [DOI] [PubMed] [Google Scholar]
- 26.Wang H., Gao X. D., Zhou G. C., Cai L., Yao W. B. In vitro and in vivo antioxidant activity of aqueous extract from Choerospondias axillaris fruit. Food Chem. 2008;106:888–895. doi: 10.1016/j.foodchem.2007.05.068. [DOI] [Google Scholar]
- 27.Wilson J. X., Lui E. M., Del Maestro R. F. Developmental profiles of antioxidant enzymes and trace metals in chick embryo. Mech. Ageing Dev. 1992;65:51–64. doi: 10.1016/0047-6374(92)90125-W. [DOI] [PubMed] [Google Scholar]
- 28.You L., Li Y., Zhao H., Regenstein J., Zhao M., Ren J. Purification and characterization of an antioxidant protein from pearl oyster (Pinctada fucata martensii) J. Aquat. Food Prod. Technol. 2015;24:661–671. doi: 10.1080/10498850.2013.804140. [DOI] [Google Scholar]
- 29.Zhong S., Ma C., Lin Y. C., Luo Y. Antioxidant properties of peptide fractions from silver carp (Hypophthalmichthysmolitrix) processing by-product protein hydrolysates evaluated by electron spin resonance spectrometry. Food Chem. 2011;126:1636–1642. doi: 10.1016/j.foodchem.2010.12.046. [DOI] [PubMed] [Google Scholar]