Abstract
Ferula persica, is the well-known species of the genus Ferula in Iran and has two varieties: persica and latisecta. They have both been extensively used in traditional medicine for a wide range of ailments. A great number of chemical compounds including sesquiterpene coumarins and polysulfides have been isolated from this plant. Fresh plant materials, crude extracts and isolated components of F. persica have shown a wide spectrum of pharmacological properties including anti-pigmentation in Serratia marcescens, cytotoxic, antibacterial, anti-fungal, anti-leishmanial, cancer chemopreventive, reversal of multi-drug resistance, anti-inflammatory and lipoxygenase inhibitory activity. The present review summarizes the data available regarding the chemical constituents and biological activities of F. persica.
Keywords: Apiaceae, Ferula persica Sesquiterpene coumarins, Sulfur-containing – compounds, Umbelliprenin
Introduction
The genus Ferula belongs to the family Apiaceae. The family Apiaceae consists of flowering and usually aromatic plants that mostly grow in temperate areas and are distributed throughout the Mediterranean region and central Asia (1). 300 Genera and 3000 species of this family have been explored worldwide. In Iranian flora, the family Apiaceae consists of 112 genera, 316 species and 75 endemic plant species. In Iran, the genus Ferula contains 30 species including 15 endemic plants (2). Several species of this genus have been used in traditional medicine for the treatment of various organ disorders. In Iran, Ferula species are typically called koma or kema (2). Ferula persica (Figure 1) is the well-known species of the genus Ferula that is traditionally used as laxative, carminative, anti-hysteric, and for the treatment of diabetes, rheumatism and backache (3). The plant with stout, hollow, somewhat succulent stems reaching up to 1 m in height and yellow flowers, is endemic to Iran, Turkey and Afghanistan. The chemical constituents from F. persica include volatile compounds (3, 4), sesquiterpene coumarins (5, 6), sulfur-containing compounds (7-10), and sesquiterpene coumarin glycosides (Figure 2) (11). F. persica contains biologically active sesquiterpene coumarins including umbelliprenin (1), farnesiferols A (2) and B (3). Umbelliprenin has shown anti-inflammatory (12), apoptotic (13, 14) and anti-pigmentation (15) properties. Farnesiferols A and B indicated reversal of multi-drug resistance (16, 17) and cytotoxic properties (13).
Figure 1.

The plant Ferula persica
Figure 2.
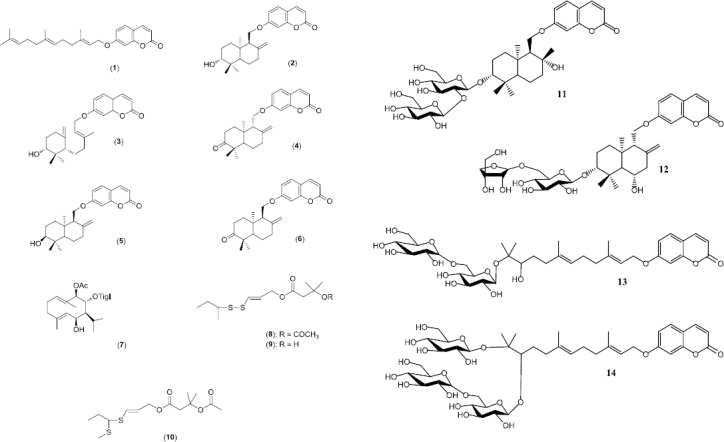
Chemical constituents from Ferula persica
In this paper, we aimed to highlight phytochemicals and pharmacological effects of F. persica and discussed about the potential effects of the plant and its constituents that deserve future research.
All relevant databases were searched for the terms “F. persica” and the names of its chemical constituents including “umbelliprenin”, “auraptene”, “farnesiferols A, B and C” and “sesquiterpene coumarins” without limitation up to April 2016. Information on the mentioned terms was collected via electronic search using Pubmed, Scopus, Web of Science and SID (for articles in Persian language), and local books on ethnopharmacology.
Phytochemistry
Ferula species are rich sources of sesquiterpene coumarins (5, 18), sesquiterpenes (19), sesquiter-pene coumarin glycosides (5, 11) and sulfur containing compounds (7, 9). To date, promising bioactive compounds such as auraptene (20-23) (antihypertensive, anti-inflammatory and cancer chemopreventive), umbelliprenin (12, 23-25) (anti-inflammatory and cancer chemopreventive), and galbanic acid (26) (anti-tumor and anti-angiogenic) have been reported from Ferula species.
Sesquiterpenes and sesquiterpene coumarins
Tsukevanik et al was the first group to begin to investigate plants of the genus Ferula in 1935 (5). Ferula is a genus rich of coumarins, particularly sesquiterpene coumarins (5). Sesquiterpene deriva-tives, especially sesquiterpene coumarins, are stored in the roots of the plant, therefore the roots are a better source for isolating sesquiterpene coumarins compared to the aerial parts. The chemical constituents of plants in the genus Ferula have been studied by many research groups. Studies showed that F. persica contains sesquiterpene coumarins and sulfur compounds (volatile and non-volatile) as main constituents.
In Figure 1, the chemical constituents from F. persica that have been reported to date (18) are depicted. Sesquiterpene coumarins isolated from the roots of F. persica include umbelliprenin (1), farnesiferol A (2), farnesiferol B (3), badrakemone (4), gummosin (5) and farnesiferone A (Mogoltadone, 6).
In addition, badrakemone (4), farnesiferone A (6) (18) farnesiferol A (2) and a sesquiterpene with a germacrene structure (9-0-acetyl-8-0-tigloyltovarol, 7) were isolated from the aerial parts (19). Recently, Iranshahi et al have also reported sesquiterpene coumarin glycosides including persicaosides A-D (11-14) from the roots of F. persica (11).
Sulfur-containing compounds and volatile constituents of F. persica compounds
Sulfur- containing compounds play an important role in the odor and taste of F. persica. Three major sulfur constituents that have been identified in F. persica (Figure 1) include persicasulfide A (8), persicasulfide B (9) and persicasulfide C (10) (9). To date, two studies have focused on the chemical composition of the volatile oil of F. persica which is also known as the synonym name of Peucedanum persicum (4). In one study on the aerial parts of this plant from Iran, sixty-one components amounting to 93.7% of the total oil were identified (Table 1) with the essential oil yield being 0.2% v/w (27). In another study on the root oil of F. persica from Iran, thirty-nine compounds comprising 82.0% of the oil were characterized (Table 2) (28). Unlike the oil from aerial parts, sulfur compounds (28.6%) were the major group of compounds present in the root oil.
Table 1.
The chemical composition of Ferula persica aerial parts and its main components
| Chemical composition | % | Main components | Reference |
|---|---|---|---|
| Essential oil | 0.2% v/w | Dill-apiole, elemicin, limonene, 6-camphenol acetate | (27) |
| Phenylpropanoids | 64.7% | ||
| Oxygenated monoterpenes | 13.0% | ||
| Monoterpene hydrocarbons | 6.7% | ||
| Sesquiterpene hydrocarbons | 3.6% | ||
| Oxygenated sesquiterpenes | 0.8% |
Table 2.
The chemical composition of Ferula persica root and its main components
| Chemical composition | % | Main components | Reference |
|---|---|---|---|
| Essential oil | 0.15%v/w | Dimethyl trisulphide, dimethyl tetrasulphide, α-barbatene, lavandulyl 2-methyl butanoate, α-terpinyl isopentanoate, α-terpinyl n-pentanoate | (28) |
| Sulfur compounds | 28.6% | ||
| Oxygenated monoterpenes | 23.2% | ||
| Sesquiterpene hydrocarbons | 11.1% |
The essential oil has been studied in only a few species of the genus Ferula and some of them include sulfur-containing compounds. Javidnia et al (27) investigated the chemical composition of F. persica essential oil that was obtained from the aerial parts (Table 1). The main components of the oil were dill-apiole (57.3%) and elemicine (5.6%). Iranshahi et al in 2005 (28) also reported volatile sulfur-containing compounds in the essential oil of the root of F. persica (Table 2). Dimethyl trisulphide (18.2%), myristicin (8.9%) and dimethyl tetrasulphide (7.6%) were the main volatile sulfur-containing components.
Pharmacological and biological effects
F. persica traditionally used as laxative, carmina-tive, anti-hysteric, and for the treatment of diabetes, rheumatism and backache (3). The other biological properties reported from this plant include anti-inflammatory, antimicrobial and cytotoxic properties. In Table 3, we summarized biological properties of F. persica that have been studied yet in details. In the following, we have reported the most important biological activities from F. persica and its constituents to date.
Cytotoxic properties
In 2010, Bagheri et al (29) evaluated cytotoxicity and anticonvulsant activity of methanol extracts from several Ferula species. Their results revealed all tested methanol Ferula extracts, especially F. latisecta roots, to possess cytotoxic activity. Hajimehdipoor et al in 2012 investigated the cytotoxic effects of F. persica against tumor cell lines MCF7, HepG2, A549, HT29, and a normal cell line MDBK using the MTT method (30). Their results showed that hexane and chloroform fractions of the plants have cytotoxic activities up to the concentration of 100 µg/ml. They showed that the extracts of F. persica are more cytotoxic on the cancer cell lines than F. hezarlalezarica (IC50 values, 22.3-71.8 µg/ml for F. persica and 76.7-105.3 µg/ml for F. hezarlalezarica) (30).
Metastatic malignant melanoma has a bad prognosis mainly due to the development of lung, hepatic and brain metastases. Barthomeuf et al in 2008 (14) used the resazurin reduction test and FACS analysis to evaluate the cytotoxic effects of umbelliprenin (1) on cancer cells and primary fibroblasts. They observed that cell susceptibility to umbelliprenin (1) decreases in the order M4Beu >A549 ≈ PC3 (androgen-resistant prostate carcinoma) >PA1 > human primary fibroblasts ≈ MCF7 >DLD1. The finding suggested that the cytotoxic effect of umbelliprenin (1) is markedly more pronounced in M4Beu cells than in primary fibroblasts and M4Beu cell proliferation is potently inhibited by umbelliprenin (1) (IC50= 12.3 μM).
Recent studies showed that matrix metallopro-teinases (MMPs) are critical enzymes in tumor growth invasion, metastasis, and neovascularization. In 2006, Iranshahi and coworkers (13) reported umbelliprenin (1) from F. persica as a potent MMPs inhibitor [IC50 = 14 µg/ml comparable with IC50 value of diclofenac sodium as the reference drug (46 µg/ml)] together with farnesiferol A (2), gummosin (5) and badrakemone (4) as weak inhibitors of metalloproteinase production by the fibrosarcoma cell line. Furthermore, they described umbelliprenin (1), farnesiferol A (2), gummosin (5) and badrakemone (4) as weak cytotoxic agents with IC50 values of 51, 37, 53 and 40 µg/ml, respectively. They concluded that umbelliprenin (1) is not a potent cytotoxic agent, however, it inhibits MMPs activity (MMP-9) compared to diclofenac sodium.
To reduce the side effects of these drugs many attempts have been conducted for the improvement of drug delivery systems of active compounds. One of the novel drug delivery system is nanoparticles. Khorramizadeh in 2010 (31) prepared umbelliprenin (1)-coated Fe3O4 magnetite nanoparticles (MNPs) and evaluated the anti-proliferative effect of this combination in vitro. They demonstrated that umbelliprenin (1) has moderate anti-proliferative effects with an IC50 value of 50 µg/ml. However, the combination of umbelliprenin (1) and Fe3O4 MNPs showed an IC50 value of 9 µg/ml. This means that the antiproliferative effect of umbelliprenin (1) enhances up to 350%, after coated on Fe3O4 MNPs. It is possible that Fe3O4 MNPs may be efficient carriers for natural compounds, by increasing their water-solubility properties (31).
Chronic lymphocytic leukemia (CLL) is a kind of cancer that requires innovative new approaches to improve its therapeutic result. Ziai et al in 2011 (32) showed umbelliprenin (1) induces apoptosis in leukemic cells in a dose- and time-dependent manner and that CLL cells are more susceptible to umbelliprenin (1), inducing more cell death than normal peripheral blood mononuclear cells. They also studied the induction of apoptosis in Jurkat cells by umbelliprenin (1) in the presence of Interleukin 4 (IL-4), which causes resistance to apoptosis in CLL cells. They reported that IL-4 was not able to reduce the apoptotic effect of umbelliprenin (1).
In this regard, Gholami et al in 2013 (33) studied the effect of umbelliprenin (1) on Jurkat T-CLL and Raji B-CLL cell lines. They showed that umbelliprenin (1) activates intrinsic and extrinsic pathways of apoptosis by the activation of caspases -8 and -9, respectively.
Myeloid cell leukemia 1 (Mcl-1) is one member of the Bcl-2 family proteins that is expressed in various cancer tissues such as CLL (34), where its expression is significantly associated with a failure to achieve complete remission following cytotoxic therapy. Gholami and coworkers (35) reported that umbelliprenin could inhibit Mcl-1 protein. They concluded that umbelliprenin (1) treatment modulates Mcl-1 expression at both the transcriptional and post translational levels.
Antimicrobial properties
In 2005, Shahverdi et al evaluated the antibacterial activities of chloroform and water extracts of F. persica roots. While the chloroform extract showed antibacterial activity, the water extract showed no activity. In continuing and completing their research, they isolated and characterized the active component umbelliprenin (1) (36). This coumarin at a concentration of 500 µg/ml showed its highest activity against Bacillus subtillis, Bacillus cereus, Escherichia coli, Klebsiella ponumoniae, Salmonella typhy, Staphylococcus aureus, and Staphylococcus epidermilis. Umbelliprenin also showed an anti-pigmentation effect on Serratia marcescens. S. marcescens is a Gram-negative bacterium that causes diseases in plants and animal hosts. Environmental S. marcescens strains are often red, due to the presence of prodigiosin. It is well known that there are some antibiotics affecting pigmentation in bacteria. In 2004, Iranshahi et al (15) showed umbelliprenin (1) to be effective on depigmentation of S. marcesens. The bleaching effect of umbelliprenin (1) was concentration dependent for S. marcescens. The highest concentration tested was 1.5 µmol, whereas a bleaching effect was observed at 0.6 µmol concentration of umbelliprenin (1). In that study, umbelliprenin (1) showed no antibacterial activity against the test strain at concentrations tested (15). In another study, Shahverdi et al (37) investigated the effect of the other coumarins extracted from F. persica roots for depigmentation of S. marcescens. None of these compounds appeared to have a bleaching effect against a test strain at concentrations tested. They concluded that the linear umbelliprenin structure may be essential for the bleaching effect in S. marcescens.
The antifungal activities of chloroform extracts of F. persica roots were also studied using conventional disk diffusion method by Mirjani R. et al in 2005 (38). They identified persicasulfide A (8) and persicasulfide B (9) as the most potent antifungal activity with the minimum inhibitory concentrations (MICs) of ≤ 62.5 mg/ml against filamentous fungi.
The first antileishmanial tests on sesquiterpene coumarins were performed in 2007, by Iranshahi et al (33). They found that umbelliprenin (1) and galbanic acid have inhibitory activities against promastigotes of Leishmania major after an incubation interval of 48 hr. Their results revealed that umbelliprenin (1) inhibit the growth of L. major promastigotes with an IC50 value of 17.1 µM (39).
Cancer chemopreventive effect
It should be noted, however, sesquiterpene coumarins and the other constituents of F. persica also possess cancer chemopreventive and antigeno-toxic properties. For example, Soltani et al (40) tested the protective properties of umbelliprenin (1), on the human lymphocytes DNA lesions in 2008. Umbelliprenin (1) exhibited a concentration-dependent increase in the protective activity against DNA damage induced by 25 μM H2O2 (from 67.28% to 39.17%). They also showed the anti-genotoxic activity of ascorbic acid, in the range 0–50 μM, to be greater than that of umbelliprenin (1). However, no significant difference (P>0.05) in the protective activity was found between umbelliprenin (1) and ascorbic acid at concentrations higher than 50 μM. In addtition, Noroozi et al (41) reported that the antigenotoxic effect of persicasulfide A (8) from F. persica on DNA damage that is induced by hydrogen peroxide (H2O2). In this report, the degree of damage to DNA after exposure to persicasulfide A (8) and ascorbic acid in the presence of H2O2 was calculated based on the amount of DNA present in the tail compared to the total amounts of lymphocyte DNA. They stated that PSA (8) does not show genotoxicity causing a 50% reduction in DNA damage induced by H2O2 (EC50:476.47 µM). Compared to the EC50 of ascorbic acid (1399.23 µM), persicasulfide A (8) was more effective than ascorbic acid in the prevention of oxidative damage to DNA (41).
Iranshahi et al in 2008 (23) carried out a primary screening test of ten terpenoid coumarins isolated from plants of the Ferula species, examining their possible inhibitory effects on Epstein-Barr virus early antigen (EBV-EA) activation induced by 12-O-tetradecanoylphorbol 13-acetate (TPA) in Raji cells. Umbelliprenin (1) significantly inhibited EBV-EA activation along with preserving the high viability of Raji cells, suggesting that it is a valuable anti-tumor-promoting agents (IC50 9.1 nM). They concluded that the presence of a prenyl moiety in the terpenoid coumarins plays an important role in their anti-tumor promoting activity.
In an in vivo study by Iranshahi et al (25) umbelliprenin (1) was reported to be a valuable cancer chemopreventive agent. Their findings showed a reduction in the number of skin tumors per mouse by 45% by umbelliprenin after 20 weeks of promotion compared to the control group. Interestingly, this was equal to the corresponding value (45%) for curcumin, used as a reference standard compound in their study. In addition, the pattern of tumor promotion was slower in mice treated with umbelliprenin (1) compared with the curcumin.
Reversal of multi-drug resistance
One of the main molecular mechanisms involved in failure of chemotherapy is multi-drug resistance (MDR). The inhibition of the function of p-glycoprotein (P-gp) in the MDR tumor cells by co-administration of transporter inhibitors and the anticancer agents is a useful strategy to reverse the transporter-mediated MDR (42).
Hanafi-Bojd et al in 2010 (17) investigated the effects of farnesiferol A (2) (from the roots of F. persica) on the functionality of the drug transporter P-gp using a rhodamine 123 efflux assay in a doxorubicin resistant breast cancer cell line (MCF7/Adr). The inhibition of the P-gp transporter by farnesiferol A (2) (0.5 µg/ml) was more potent than that of verapamil (the well-known inhibitor of P-gp) at 15 min exposure.
Recently Kasaian et al (16) investigated fifteen sesquiterpene coumarins which were isolated and purified from different Ferula species, and were tested for their MDR reversal properties. They showed enhancement of doxorubicin cytotoxicity in MCF-7/Adr cells (doxorubicin resistant derivatives of MCF-7 cells overexpressing P-gp) when combined with very non-toxic concentrations of the sesquiterpene coumarins (50 μM) including umbelliprenin (1), farnesiferol B (3), farnesiferol C and lehmferin, proving the significant MDR reversal activity of these coumarins.
Anti-inflammatory and lipoxygenase inhibitory activity
Inhibition of lipoxygenase is beneficial in the treatment of various diseases including asthma, chronic obstructive pulmonary disease (COPD), osteoporosis and atherosclerosis. Agents that block lipoxygenase catalyzed activity may be effective in preventing cancer by interfering in signaling events needed for tumor growth (12). In 2009, anti-inflammatory and lipoxygenase inhibitory activity of umbelliprenin (1) in its synthetic form was reported by Iranshahi et al (12). Preliminarily, umbelliprenin (1) was tested for its lipoxygenase inhibitory activity (lipoxygenases are a family of iron containing enzymes that convert arachidonic acid in membrane phospholipids into leukotriene pro-inflammatory mediators) (43). Iranshahi and coworkers reported that umbelliprenin (1) has a significant inhibitory activity against soybean lipoxygenase with an IC50 value of 0.0725 µM. In the second round of the test, the anti-inflammatory activity of umbelliprenin (1) was evaluated in in vivo on carrageenan mouse paw edema as a clinical model for inflammation. Again, umbelliprenin (1) inhibited the inflammation process up to 39% (compared to 47% in indomethacin as a reference control) (24). Iranshahi et al in 2012 (44) synthesized all the mono isopentenyloxy, -geranyloxy and -farnesyloxy derivatives of coumarin and determined their inhibitory potency against soybean 15-lipoxygenase (SLO) and human 15-lipoxygenase-1 (HLO-1). Amongst the synthetic derivatives, 5-farnesyloxycoumarin exhibited the most potent inhibitory activity against SLO (IC50 = 0.8 μM) while 6-farnesyloxycoumarin was the strongest HLO-1 inhibitor (IC50 = 1.3 μM). The IC50 variations of the farnesyl analogs for HLO-1 (1.3 to ∼75 μM) were much higher than those observed for SLO (0.8–5.8 μM).
It may be concluded that the coumarin umbelliprenin and some flavonoids including luteolin 7-O-glucoside that are present in F. persica play antioxidant and anti-inflammatory roles.
Miscellaneous activities
Inhibition of acetyl cholinesterase (AChE) is currently regarded as the leading strategy against Alzheimer’s disease. In 2010, Karimi et al (45) reported the inhibitory activities of 10 naturally occurring terpenoid and coumarin derivatives against human erythrocyte AChE for determining the rate of hydrolysis of acetyl thiochoine in comparison to the reference compound galanthamine, using the modified method of Ellman et al (46). In this report, farnesiferol A (2) and umbelliprenin (1) inhibits AChE 20.6% and 17.5%, respectively. However, it should be pointed out that these activities are not comparable to that of the reference inhibitor compound (Galantamine 86.4%).
F. persica has been used in traditional medicine for treatment of high blood pressure. Ghanbari et al (47) investigated the acute and chronic effect of aqueous F. persica extract on blood pressure (BP) of hypertensive rats. They showed the intravenous administration of F. persica reduces BP of hypertensive rats (P<0.001), but chronic administration of F. persica has no effect on BP.
Neuropathic Pain (NP) is caused by cancer, diabetes mellitus, Parkinson’s disease and Alzheimer’s disease. About 10-30% of patients suffering from syndromes of NP are drug resistant. There is remarkable need for novel analgesic being more effective or safer. Hashemzaei et al recently (48) evaluated acute and neuropathic pain, using hot-plate, formalin and morphine tests. Their results indicated that the administration of a single dose of umbelliprenin (0.01 Mm) significantly reduces neuropathic pain (P<0.05) compared to the negative control while not changing acute pain against diclofenac. Their research indicates that umbelliprenin (1) alone reduces neuropathic pain while its combination with morphine potentiates morphine effects (48).
Discussion and concluding remarks
F. persica is native to central Asia, particularly Eastern Iran and Afghanistan. The plant has been used in traditional medicine for various purposes. New pharmacological studies have almost confirmed the traditional uses of F. persica as an antispasmodic, antibacterial and anti-hypertensive. In addition, there is a correlation between some traditional uses of F. persica and those of new studies. F. persica is a strong background in traditional medicine for the treatment of diabetes, however, this activity has not been evaluated to date. The authors strongly recommend that the future research is focused on anti-diabetic effects of F. persica.
As reported in this paper. umbelliprenin (1) is one of the most interesting bioactive constituents from the genus Ferula. It is the first sesquiterpene coumarin that is synthesized in the plant F. persica. The most interesting properties of umbelliprenin (1) were its anti-inflammatory and cancer chemopre-ventive activities (24). Different mechanisms seem to play in this activity including lipoxygenase inhibitory property. The lipoxygenase inhibitory effect by umbelliprenin (1) and its derivatives increase in the presence of endogenous antioxidants and by reduction of oxidative parameters. Blocking the 5-lipoxygenase enzyme may be a plausible mechanism accounting for at least part of the observed chemo- preventive activity of umbelliprenin (1). It reduces the formation of lipoxygenase-carcinogenic products. We have also found that the cancer chemoprevention of umbelliprenin (1) is comparable with curcumin, a well-known cancer chemopreventive agent (25). In a study, Shahverdi et al (13) showed umbelliprenin (1) as a potent matrix metalloproteinase (MMP) inhibitor. The cytotoxicity of umbelliprenin (1) has been found to be related to the presence of the aliphatic sesquiterpenoid group linked at C7-OH. Another reported activity of umbelliprenin is its anti-proliferative effect (44). This effect of umbelliprenin (1) enhances up to 350% when coated on the surface of Fe3O4 MNPs. This compound by altering their water-solubility properties, be an efficient carrier for natural compounds and lower amounts of these substances may be needed therefore reduce the potential hazards of using metallic nanoparticles. As noted above, umbelliprenin is not cytotoxic on all cell lines. Jurkat T-CLL, Raji B-CLL and M4Beu cell lines are more sensitive to this compound. Gholami et al also demonstrated that umbelliprenin (1) activates the intrinsic and extrinsic pathways of apoptosis by the activation of caspase-8 and -9, respectively. In addition, umbelliprenin (1) inhibits the Mcl-1 protein. They concluded that umbelliprenin treatment modulates Mcl-1 expression at both the transcriptional and post-translational levels (49).
One of the main molecular mechanisms involved in the failure of chemotherapy is multi-drug resistance (MDR). Kasaian et al (16) showed the enhancement of doxorubicin cytotoxicity in MCF-7/Adr cells, when combined with non-toxic concentrations of the sesquiterpene coumarins (50 μM) including umbelliprenin (1), farnesiferol B (3), farnesiferol C that exhibited significant MDR reversal activity.
Noroozi et al reported the anti-genotoxic effect of persicasulfide A (8) on DNA damage induced by H2O2, and persicasulfide A (8) was more effective than ascorbic acid in the prevention of oxidative damage to DNA. It is probable that persicasulfide A (8) interacts with thiol-containing proteins and alters the activation and metabolism of some genotoxins (41).
It is strongly believed that detailed information on the phytochemical and biological activities of F. persica, as presented in this review, provides certain evidence for the use of this plant in different medicines. Furthermore, it seems that umbelliprenin (1), as a prenylated coumarin, showed various biological activities and it seems that umbelliprenin (1) might be a leading compound for designing and synthesizing new derivatives with higher potency and more safety. On the basis of 12 year work on the genus Ferula and the plant F. persica, the authors strongly recommend to focus future research on anti-diabetic and anti-viral activities of the plant and its sesquiterpene coumarins.
Acknowledgment
This study was partially supported by the Research Deputy of Mashhad University of Medical Sciences. Mashhad, Iran.
References
- 1.Mahran G, El Alfy T, Ansari S. A phytochemical study of volatile oil of Afghanian Asafoetida. Bull Fac Pharm Cairo Univ. 1973;12:101–107. [Google Scholar]
- 2.Mozaffarian V. A dictionary of Iranian plant names: Latin, English. Persian: Farhang Mo’aser; 1996. [Google Scholar]
- 3.Sahebkar A, Iranshahi M. Biological activities of essential oils from the genus Ferula (Apiaceae) Asian Biomed. 2010;4:835–847. [Google Scholar]
- 4.Sahebkar A, Iranshahi M. Volatile constituents of the genus Ferula (Apiaceae): A review. J Essent Oil Bear Pl. 2011;14:504–531. [Google Scholar]
- 5.El-Razek MHA, Ohta S, Hirata T. Terpenoid coumarins of the genus Ferula. Heterocycles. 2003;60:689–716. [Google Scholar]
- 6.El-Razek MHA, Ohta S, Ahmed AA, Hirata T. Sesquiterpene coumarins from the roots of Ferula assa-foetida. Phytochemistry. 2001;58:1289–1295. doi: 10.1016/s0031-9422(01)00324-7. [DOI] [PubMed] [Google Scholar]
- 7.Iranshahi M. A review of volatile sulfur-containing compounds from terrestrial plants: Biosynthesis, distribution and analytical methods. J Essent Oil Res. 2012;24:393–434. [Google Scholar]
- 8.Iranshahi M, Amin GR, Amini M, Shafiee A. Sulfur containing derivatives from Ferula persica var. latisecta. Phytochemistry. 2003;63:965–966. doi: 10.1016/s0031-9422(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 9.Iranshahi M, Noroozi S, Behravan J, Karimi G, Schneider B. Persicasulphide C, a new sulphur-containing derivative from Ferula persica. Nat Prod Res. 2009;23:1584–1588. doi: 10.1080/14786410802393571. [DOI] [PubMed] [Google Scholar]
- 10.Iranshahi M, Yazdi MC, Hassanzadeh-Khayyat M, Sahebkar A. Sulfur containing compounds in the volatile oil of Ferula latisecta Rech. f. & Aell. Leaves. J Essent Oil-Bearing Pl. 2009;12:64–648. [Google Scholar]
- 11.Iranshahi M, Mojarab M, Sadeghian H, Hanafi-Bojd MY, Schneider B. Polar secondary metabolites of Ferula persica roots. Phytochemistry. 2008;69:473–478. doi: 10.1016/j.phytochem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Iranshahi M, Askari M, Sahebkar A, Adjipavlou-Litina D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. Daru. 2009;17:99–103. [Google Scholar]
- 13.Shahverdi A, Saadat F, Khorramizadeh M, Iranshahi M, Khoshayand M. Two matrix metalloproteinases inhibitors from Ferula persica var persica. Phytomedicine. 2006;13:712–717. doi: 10.1016/j.phymed.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Barthomeuf C, Lim S, Iranshahi M, Chollet P. Umbelliprenin from Ferula szowitsiana inhibits the growth of human M4Beu metastatic pigmented malignant melanoma cells through cell-cycle arrest in G1 and induction of caspase-dependent apoptosis. Phytomedicine. 2008;15:103–111. doi: 10.1016/j.phymed.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Iranshahi M, Shahverdi AR, Mirjani R, Amin G, Shafiee A. Umbelliprenin from Ferula persica roots inhibits the red pigment production in Serratia marcescens. Z Naturforsch C. 2004;59:506–508. doi: 10.1515/znc-2004-7-809. [DOI] [PubMed] [Google Scholar]
- 16.Kasaian J, Mosaffa F, Behravan J, Masullo M, Piacente S, Ghandadi M, et al. Reversal of P-glycoprotein-mediated multidrug resistance in MCF-7/Adr cancer cells by sesquiterpene coumarins. Fitoterapia. 2015;103:149–154. doi: 10.1016/j.fitote.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Hanafi-Bojd MY, Iranshahi M, Mosaffa F, Tehrani SO, Kalalinia F, Behravan J. Farnesiferol A from Ferula persica and galbanic acid from Ferula szowitsiana inhibit P-glycoprotein-mediated rhodamine efflux in breast cancer cell lines. Planta Med. 2011;77:1590–1593. doi: 10.1055/s-0030-1270987. [DOI] [PubMed] [Google Scholar]
- 18.Iranshahi M, Amin G, Shafiee A. A new coumarin from Ferula persica. Pharm Biol. 2004;42:440–442. [Google Scholar]
- 19.Iranshahi M, Amin G-R, Jalalizadeh H, Shafiee A. New germacrane derivative from Ferula persica. Pharm Biol. 2003;41:431–433. [Google Scholar]
- 20.Razavi BM, Arasteh E, Imenshahidi M, Iranshahi M. Antihypertensive effect of auraptene, a monoterpene coumarin from the genus Citrus upon chronic administration. Iran J Basic Med Sci. 2015;18:153–158. [PMC free article] [PubMed] [Google Scholar]
- 21.Soltani F, Mosaffa F, Iranshahi M, Karimi G, Malekaneh M, Haghighi F, Behravan J. Auraptene from Ferula szowitsiana protects human peripheral lymphocytes against oxidative stress. Phythoter Res. 2010;24:85–89. doi: 10.1002/ptr.2874. [DOI] [PubMed] [Google Scholar]
- 22.Imenshahidi M, Eghbal M, Sahebkar A, Iranshahi M. Hypotensive activity of auraptene, a monoterpene coumarin from Citrus spp. Pharm Biol. 2013;51:545–549. doi: 10.3109/13880209.2012.747546. [DOI] [PubMed] [Google Scholar]
- 23.Iranshahi M, Kalategi F, Rezaee R, Shahverdi AR, Ito C, Furukawa H, et al. Cancer chemopreventive activity of terpenoid coumarins from Ferula species. Planta med. 2008;74:147. doi: 10.1055/s-2008-1034293. [DOI] [PubMed] [Google Scholar]
- 24.Shakeri A, Iranshahy M, Iranshahi M. Biological properties and molecular targets of umbelliprenin – a mini-review. J Asian Nat Prod Res. 2014;16:884–889. doi: 10.1080/10286020.2014.917630. [DOI] [PubMed] [Google Scholar]
- 25.Iranshahi M, Sahebkar A, Takasaki M, Konoshima T, Tokuda H. Cancer chemopreventive activity of the prenylated coumarin, umbelliprenin in vivo. Eur J Cancer Prev. 2009;18:412–415. doi: 10.1097/CEJ.0b013e32832c389e. [DOI] [PubMed] [Google Scholar]
- 26.Kasaian J, Iranshahy M, Iranshahi M. Synthesis, biosynthesis and biological acitivities of galbanic acid - a review. Pharm Biol. 2014;52:524–531. doi: 10.3109/13880209.2013.846916. [DOI] [PubMed] [Google Scholar]
- 27.Javidnia K, Miri R, Kamalinejad M, Edraki N. Chemical composition of Ferula persica Wild. essential oil from Iran. Flavour Frag J. 2005;20:605–606. [Google Scholar]
- 28.Iranshahi M, Amin G, Sourmaghi MS, Shafiee A, Hadjiakhoondi A. Sulphur-containing compounds in the essential oil of the root of Ferula persica Willd. var persica. Flavour Frag J. 2006;21:260–261. [Google Scholar]
- 29.Bagheri SM, Sahebkar A, Gohari AR, Saeidnia S, Malmir M, Iranshahi M. Evaluation of cytotoxicity and anticonvulsant activity of some Iranian medicinal Ferula species. Pharm Biol. 2010;48:242–246. doi: 10.3109/13880200903081796. [DOI] [PubMed] [Google Scholar]
- 30.Hajimehdipoor H, Esmaeili S, Ramezani R, Jafari Anaraki M, Mosaddegh M. The cytotoxic effects of Ferula persica var. persica and Ferula hezarlalehzarica against HepG2, A549, HT29, MCF7 and MDBK cell lines. Iran J Pharm Sci. 2012;8:113–117. [Google Scholar]
- 31.Khorramizadeh M, Esmail-Nazari Z, Zarei-Ghaane Z, Shakibaie M, Mollazadeh-Moghaddam K, Iranshahi M, et al. Umbelliprenin-coated Fe3O4 magnetite nanoparticles: Antiproliferation evaluation on human Fibrosarcoma cell line (HT-1080) Mater Sci Eng C. 2010;30:1038–1042. [Google Scholar]
- 32.Ziai SA, Iranshahi M, Zarnani AH, Jeddi-Tehrani M. Umbelliprenin induces apoptosis in CLL cell lines. Iran J Pharm Res. 2012;11:653–659. [PMC free article] [PubMed] [Google Scholar]
- 33.Gholami O, Jeddi-Tehrani M, Iranshahi M, Zarnani AH, Ziai SA. Umbelliprenin from Ferula szowitsiana Activates both Intrinsic and Extrinsic Pathways of Apoptosis in Jurkat T-CLL cell line. Iran J Pharm Res. 2013;12:371–376. [PMC free article] [PubMed] [Google Scholar]
- 34.Gandhi V, Balakrishnan K, Chen LS. Mcl-1: the 1 in CLL. Blood. 2008;112:3538–3540. doi: 10.1182/blood-2008-07-170241. [DOI] [PubMed] [Google Scholar]
- 35.Gholami O, Jeddi-Tehrani M, Iranshahi M, Zarnani AH, Ziai SA. Mcl-1 Is Up regulated by prenylated coumarin, Umbelliprenin in jurkat cells. Iran J Pharm Res. 2014;13:1387. [PMC free article] [PubMed] [Google Scholar]
- 36.Shahverdi A-R, Iranshahi M, Mirjani R, Jamalifar H, Amin G, Shafiee A. Bioassay-guided isolation and identification of an antibacterial compound from Ferula persica var. persica roots. DARU J Pharm Sci. 2005;13:17–19. [Google Scholar]
- 37.Shahverdi AR, Mirjani R, Amin G, Shafiee A, Iranshahi M. Bleaching of Serratia marcescens by some coumarins: a spectrophotometric study. J Basic Microbiol. 2005;45:470–474. doi: 10.1002/jobm.200410509. [DOI] [PubMed] [Google Scholar]
- 38.Mirjani R, Shahverdi A-R, Iranshahi M, Amin G, Shafiee A. Identification of Antifungal Compounds from Ferula persica. var persica. Pharm Biol. 2005;43:293–295. doi: 10.1080/13880200590951658. [DOI] [PubMed] [Google Scholar]
- 39.Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, et al. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry. 2007;68:554–561. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Soltani F, Mosaffa F, Iranshahi M, Karimi G, Malekaneh M, Haghighi F, et al. Evaluation of antigenotoxicity effects of umbelliprenin on human peripheral lymphocytes exposed to oxidative stress. Cell Biol Toxicol. 2009;25:291–296. doi: 10.1007/s10565-008-9083-9. [DOI] [PubMed] [Google Scholar]
- 41.Noroozi S, Mosaffa F, Soltani F, Iranshahi M, Karimi G, Malekaneh M, et al. Antigenotoxic effects of the disulfide compound persicasulfide A (PSA) on rat lymphocytes exposed to oxidative stress. Planta Med. 2009;75:32–36. doi: 10.1055/s-0028-1088360. [DOI] [PubMed] [Google Scholar]
- 42.Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 2004;65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 43.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 44.Iranshahi M, Jabbari A, Orafaie A, Mehri R, Zeraatkar S, Ahmadi T, et al. Synthesis and SAR studies of mono O-prenylated coumarins as potent 15-lipoxygenase inhibitors. Eur J Med Chem. 2012;57:134–142. doi: 10.1016/j.ejmech.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 45.Karimi G, Iranshahi M, Hosseinalizadeh F, Riahi B, Sahebkar A. Screening of acetylcholinesterase inhibitory activity of terpenoid and coumarin derivatives from the genus Ferula. Pharmacologyonline. 2010;1:566–574. [Google Scholar]
- 46.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 47.Ghanbari M, Zahedi KM, Vakili A. Acute and chronic effects of Ferula persica on blood pressure of hypertensive rats and its possible mechanism of action. J Med Plants. 2012;43:62–68. [Google Scholar]
- 48.Hashemzaei M, SadeghiBonjar MA, Tabrizian K, Iranshahi M, Iranshahy M, Rezaee R. Evaluation of the analgesic effect of Umbelliprenin and Umbelliprenin-morphine co-administration on the acute, chronic and neuropathic pain. Indian J Pharm Educ. 2015;49:121–125. [Google Scholar]
- 49.Gholami O, Jeddi-Tehrani M, Iranshahi M, Zarnani AH, Ziai SA. Umbelliprenin from Ferula szowitsiana Activates both Intrinsic and Extrinsic Pathways of Apoptosis in Jurkat T-CLL cell line. Iran J Pharm Res. 2013;12:371. [PMC free article] [PubMed] [Google Scholar]


