Abstract
Objective(s):
Global cerebral ischemia-reperfusion (GCIR) causes disturbances in brain functions as well as other organs such as kidney. Our aim was to evaluate the protective effects of ellagic acid (EA) on certain renal disfunction after GCIR.
Materials and Methods:
Adult male Wistar rats (n=32, 250-300 g) were used. GCIR was induced by bilateral vertebral and common carotid arteries occlusion (4-VO). Animal groups were: 1) received DMSO/saline (10%) as solvent of EA, 2) solvent + GCIR, 3) EA + GCIR, and 4) EA. Under anesthesia with ketamine/xylazine, GCIR was induced (20 and 30 min respectively) in related groups. EA (100 mg/kg, dissolved in DMSO/saline (10%) or solvent was administered (1.5 ml/kg) orally for 10 consecutive days to the related groups. EEG was recorded from NTS in GCIR treated groups.
Results:
Our data showed that: a) EEG in GCIR treated groups was flattened. b) GCIR reduced GFR (P<0.01) and pretreatment with EA attenuated this reduction. c) BUN was increased by GCIR (P<0.001) and pretreatment with EA improved the BUN to normal level. d) Serum creatinine concentration was elevated by GCIR but not significantly, however, in EA+GCIR group serum creatinine was reduced (P<0.05). e) GCIR induced proteinuria (P<0.05) but, EA was unable to reduced proteinuria.
Conclusion:
Results indicate that GCIR impairs certain renal functions and EA as an antioxidant can improve these functions. Our results suggest the possible usefulness of ellagic acid in patients with brain stroke.
Keywords: BUN, Creatinine, Ellagic acid, GFR, Global cerebral ischemia, Proteinuria
Introduction
Based on the World Health Organization, 15 million people suffer from stroke in worldwide per year (1). Normally, cardiac arrest and shock may lead to global cerebral ischemia (2). The severity of brain injury is dependent to the magnitude and duration of the interruption in the blood supply, and followed damage induced by reperfusion (3). The role of nucleus tractus solitarius (NTS) in cerebral ischemia has been recognized in clinical and experimental animal models (4, 5). Studies have shown that NTS is more susceptible to destroying on middle cerebral arteries occlusion (6). On the other hand, NTS play crucial role for adjusting respiratory, cardiovascular, renal and digestive functions (7). Therefore, cerebral ischemia not only impairs brain functions, but rather the disturbances will occurred in other organs such as heart (8), intestine (9) and as well as kidney (10). After cerebral ischemia-reperfusion injury, the generation of reactive oxygen species (ROS) and reactive nitrogen species may contribute to the neurodegenerative disease process through alterations in the structure of DNA, RNA, proteins, and lipids (11).
Flavonoids are polyphenolic antioxidants occur obviously in vegetables and fruits. Ellagic acid (EA) as 2,3,7,8-Tetrahydroxy-chromeno[5,4,3-cde]chromene 5,10-dione, exists in sure fruits and nuts, such as raspberry, strawberry, walnut and longan seed (12). EA has a numerous of biological activities including potent antioxidant properties (13). As mentioned above, brain ischemia induces renal disfunction therefore the main aim of the present study was to evaluate the effect of EA with potent antioxidant property on certain renal disfunction followed by the experimental induction of global cerebral ischemia-reperfusion.
Materials and Methods
Chemicals and kits
EA, and dimethyl sulfoxide (DMSO), were purchased from Sigma-Aldrich Co (St Louis, USA). EA dissolved in DMSO/normal saline (10%) as the solvent or vehicle, buffered to a pH of 7 and prepared daily. For assessment of serum and urine BUN, creatinine (Cr), and protein, the related kits from Pars azmoon Co (Iran) were used. The used methods were enzymatic, calorimetric without deletion of protein on JAFFE assay for Cr, enzymatic on urease-GLDH assay for BUN, and photometric on pyrogallol red assay for protein. According to instruction of Pars azmoon Company, the sensitivity range of the used kits was as follow: urea= 2 mg/dl, Cr= 0.2 mg/dl and protein = 0.5 g/dl.
In this study, proteinuria is presented as ratio of urinary concentrations of protein to creatinine [(mg/dl)] / [(mg/dl)] for 50 min of the experiment duration as reported (14).
Animals
A total of 32 male Wistar rats weighing 250-300 g were purchased from Animal House of Ahvaz Jundishapur University of Medical Sciences. Rats were housed in cages under a 12 hr light/dark cycle at 20±2 °C with 50% humidity. Animals had free access to water and standard pellet diet. The experiments were approved by the Animal Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (No. ajums. B-9348).
Experimental protocols
Rats were divided randomly into four equal groups as follow: (1) sham-operated (SO), received only solvent, (2) global cerebral ischemia-reperfusion (GCIR) group: received solvent and treated with GCIR (20 min and 30 min respectively) (15), (3) ellagic acid + global cerebral ischemia-reperfusion (EA+GCIR) group: received EA (100 mg/kg) and treated with GCIR (20 and 30 min respectively), and (4) ellagic acid group (EA): received only ellagic acid (100 mg/kg). EA (dissolved in solvent) or solvent was gavaged orally in the same volume (1.5 ml/kg) to the related groups for 10 consecutive days before experiments. Induction of ischemia-reperfusion in the related groups was carried out 24 hr after last gavage. The dose of EA (100 mg/kg) was selected based on previous reports (16).
Before the commencement of testing, all animals were slightly handled for 5 days (10 min daily). All efforts were made to minimize animal suffering and reduce the number of animals used.
Surgery for recording EEG
All rats were anesthetized with ketamine/xylazine (50/5 mg/kg, IP). The head was shaved from the area just above the eyes to the back of the head and from ear to ear. The skin retracted, revealing the skull. Head was mounted in a stereotaxic device and holes were drilled to allow stereotaxic embedding of the EEG recording. A coated stainless steel bipolar metal wire electrode (stainless steel Teflon, 0.005” bare, 0.008” coated, A-M systems, Inc. WA) was implanted in the NTS (SolM) with stereotaxic coordination of AP= -14.04 mm; ML= 0.4 mm to bregma, and DV= 8 mm from the dura, correspondingly (17). All implants were fixed to the skull by dental acrylic cement and two glass anchor small bolts. The animals were allowed to recover for ten days before the commencement of experiments. The location of implanted electrodes in NTS was confirmed microscopically at the end of each experiment.
Local EEG recording
Electrical field potentials (local EEG) from the NTS of rats were fed to a ML135 bio-amplifier (AD Instruments, 4-Channels Power Lab, LabChart software version 7, Australia) with 1 mV amplification, sample recording 400 Hz, and 0.3–70 Hz band pass filtration for 5 min.
Surgery for induction of global cerebral ischemia-reperfusion
Rats underwent transient forebrain global ischemia as described by others (18). Temporarily, on the first day, rats were anesthetized by ketamine/xylazine (50/5 mg/kg, IP). A neck ventral midline incision was made and the common carotid arteries were exposed and marginally separated from the vagus nerves. Then, a sterile string was lightly placed around each common carotid artery (CCA) without interrupting carotid blood flow and the incision was sutured. At the same time, a second incision, 1 cm in length, was made behind the occipital bone directly overlying the first two cervical vertebrae. The paraspinal muscles were separated from the midline, the right and left alar foramina of the first cervical vertebrae were exposed. A 0.5 mm diameter electrocautery needle (Bowie Monopolar Electrocautery, Cincinnati, Ohio) was inserted through each alar foramen and both vertebral arteries electrocauterized and permanently occlud-ed. On the second day, under ketamine/xylazine anesthesia both CCA were occluded by micro clamps for 20 min (19) to produce 4-vessel occlusion (18). Therefore, in this study the method of 4-vessel occlusion (4-VO) has been carried out. Reperfusion (30 min) was started by opening the carotid clamps after 20 min of ischemia. Those rats with 4-VO were only involved for tests if their EEG was flattened during ischemia period. Similar procedures were carried out in SO and EA groups without vertebral arteries electro-coagulation and carotid arteries occlusion. Urinary bladder was drained before experiment by inserting a poly ethylene tube in the body of urinary bladder and at the end of reperfusion period, blood was collected from the heart right ventricle and rats were sacrificed. Urine in bladder was also collected at the same time. Blood samples after coagulation (at room temperature) were centrifuged at 4000 rpm (10 min at ambient temperature). The collected serum and urine samples were stored at - 80 °C until analysis. Rectal temperature was monitored and body temperature was kept at 36.5±0.5 °C by surface heating and cooling throughout the experiment. For computing of glomerular filtration rate (GFR), the following formula was used:
GFR= ([Cr] urine/[Cr] plasma)×(urine output/50 min)/body weight (kg)
In this study, therefore, GFR will be reported as ml/min/kg of animal body weight
To assure that recording electrodes were placed in NTS correctly, in all rats that treated with GCIR, at the end of experiments, NTS was lesioned by passing a direct current (3 mA, 3 seconds) through recording electrodes. Brain was then removed from skull and placed in formalin (10%) for 7 days. Then, slices of the brain was prepared (50 µm) from coronal part and location of lesion was checked with rat brain atlas. If the lesioned point was not at NTS, the other obtained data of that rat was discarded from the results.
Statistical analysis
Data expressed as mean±SEM and analyzed by a one-way analysis of variance (ANOVA) followed by LSD (Least Significant Difference) as post hoc test for multiple comparisons, using SPSS (version 16.0). P-value<0.05 was considered a statistically significant level.
Results
Histological verification
The prepared brain slices verified that the electrode was implanted in NTS correctly as Figure 1 shows.
Figure 1.
Verification of NTS lesioned induced by direct current (3 mA, 3 sec) throw recording electrode to show that the implanted electrode is located in NTS correctly
EEG results
Figure 2 shows that, the induction of global cerebral ischemia flattened the EEG recorded from NTS. Therefore, this flattening indicates that the global cerebral ischemia has been done successfully. However, the reopening of the carotid arteries (reperfusion) did not return EEG records to normal state.
Figure 2.

A typical recording of EEG from NTS in animal before and after global cerebral ischemia and reperfusion periods. As this figure shows, EEG from NTS has been flattened by global cerebral ischemia but reperfusion did not return EEG to normal state
Effect of EA on impaired GFR induced by GCIR
As indicated in Figure 3, GFR in the GCIR group is significantly lower than SO group (P<0.05). GFR in the pretreated group (EA+GCIR) was not signifi-cantly different from SO group but significantly higher than GCIR group (P<0.01). GFR in the EA group which only pretreated with EA (100 mg/kg) for ten days was identical to SO group.
Figure 3.
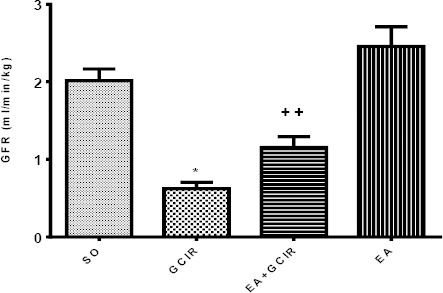
Comparison of GFR after GCIR in different rat groups [SO (Sham), GCIR (global cerebral ischemia reperfusion), EA +GCIR (ellagic acid 100 mg/kg, 10 days +global cerebral ischemia reperfusion), EA (ellagic acid 100 mg/kg, 10 days)].*P<0.05 Significant compared to so group,++ P<0.01, Significant compared to GCIR group. Results are expressed as mean±SEM with eight rats per group; one way ANOVA followed by post-hoc LSD test was used
Effect of EA pretreatment on elevated BUN induced by GCIR
As Figure 4 indicates, blood urea nitrogen was elevated significantly (P<0.001) by inducing GCIR nearly twice as compare to sham operated (SO) group. EA pretreatment for ten days in EA+GCIR group, prevented this elevation in BUN significantly (P<0.001) on the other word, BUN in EA+GCIR group was similar to the SO group. However, as it is seen in this Figure, the BUN level was not altered by pretreatment with EA alone.
Figure 4.
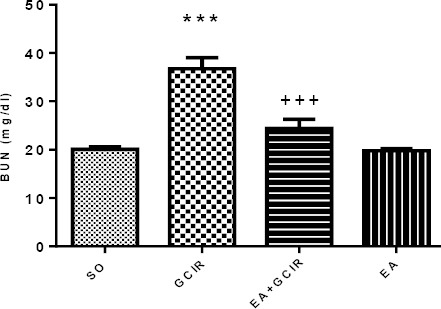
Comparison of BUN after GCIR in different rat groups [SO (Sham), GCIR (global cerebral ischemia reperfusion), EA+GCIR (ellagic acid 100 mg/kg, 10 days +global cerebral ischemia reperfusion), EA (ellagic acid 100 mg/kg, 10 days)].***P<0.001 Significant compared to so group, +++P<0.001, Significant compared to GCIR group. Results are expressed as mean±SEM with eight rats per group; one way ANOVAfollowed by post-hoc LSD test was used
Effect of EA pretreatment on changes of serum creatinine induced by GCIR
Figure 5 indicates that serum creatinine concentration was higher in GCIR group as compared to SO group but this elevation was not significant (P>0.05). However, this figure shows that in the EA+GCIR group, ten days pretreatment with EA (100 mg/kg) prevented the serum creatinine concentration significantly (P<0.05) as compare to GCIR group. Serum creatinine level in EA group was not significantly different from the SO group.
Figure 5.
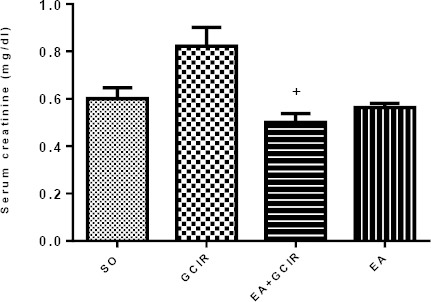
Comparison of Cr after GCIR in different rat groups [SO (Sham), GCIR (global cerebral ischemia reperfusion), EA+GCIR (ellagic acid 100 mg/kg, 10 days +global cerebral ischemia reperfusion), EA (ellagic acid 100 mg/kg, 10 days)]. +P<0.05, Significant compared to GCIR group. Results are expressed as mean±SEM with eight rats per group; one way ANOVA followed by post-hoc LSD test was used
Effect of EA pretreatment on protein/Cr ratio induced by GCIR
As Figure 6 shows, the urine protein/urine creatinine ratio as an index of proteinuria is elevated by GCIR as compared to SO group (P<0.05). However, ten days pretreatment with EA (100 mg/kg) was unable to attenuate proteinuria in EA + GCIR group. Proteinuria in EA group was not significantly different from the SO group.
Figure 6.
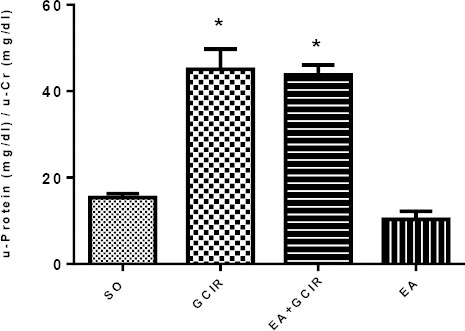
Comparison of U-protein/U-Cr after GCIR in different rat groups [SO (Sham), GCIR (global cerebral ischemia reperfusion), EA+GCIR (ellagic acid 100 mg/kg, 10 days +global cerebral ischemia reperfusion), EA (ellagic acid 100 mg/kg, 10 days)]. * P<0.05 Significant compared to so group. Results are expressed as mean±SEM with eight rats per group; one way ANOVA followed by post-hoc LSD test was used
Discussion
The data of the present study indicated that, ten days orally pretreatment of EA prevent certain disturbances in renal functions caused by global cerebral ischemia-reperfusion (GCIR). First of all, as shown in results section, inducing GCIR abolished the EEG recorded from NTS which indicates the successfulness of our method and in addition starting reperfusion was unable to restore EEG to normal state. Our method to induce GCIR as presented in method section (as 4-VO) is one the best method to induce brain acute blood supply reduction. Model of 4-VO is a method for making bilateral hemispheric ischemia which decreased cerebral blood flow to 12% - 14% of baseline levels and this method is a minimally invasive, speedy, and consistent procedure for ischemia induction (20).
Cerebral ischemia is narrowly associated with inflammatory responses especially infiltration of circulating neutrophils to focal ischemic tissue (21). Neutrophils are potential source of reactive oxygen species (ROS) when activated during inflammatory responses (22). Cytokines produced by inflammatory target cells can trigger firm adhesion of circulating neutrophils to endothelial cells and generation of ROS that increases neutrophil infiltration (23). Enormous ROS produced by these primed/activated neutrophils not only damages neighboring tissue but also signals the inflammatory responses (24). Therefore inhibiting neutrophil adhesion (25), calcium mobilization (26) and ROS production (27) can reduce neutrophil infiltration or recruitment to cerebral ischemia–reperfusion injured tissue. It has been shown that incomplete global cerebral ischemia activates platelets (28) and this activation is related to kidney disease (29). On the other hand, ROS causes platelet activation (30). In addition, it has been shown that impaired kidney function, as measured by reduction in GFR, is related to subclinical markers of cerebral small vessel disease (31).
The vascular beds of both the kidney and the brain have very low resistance and are passively perfused at high flow throughout cardiac systole and diastole periods (32). Indeed, it has been shown that vascular risk factors may lead to glomerular lipohyalinosis and endothelium dysfunction (33). Recent studies have shown that ROS may increase renal vascular tone, sensitizing to vasoconstrictors, and damaging endothelium-dependent vasodilation. Oxygen radicals directly limit the renal microcir-culation and indirectly affect renal vascular tone by mediating the effects of other vasoconstrictors, stimulating the production of vasoconstrictors, and modulating the actions of vasodilators such as NO (34).
Although cerebral ischemia itself causes brain injury but in some animal stroke models reperfusion can cause a larger infarct size than that associated with permanent vessel occlusion. Thus, while reper-fusion may reduce infarct size but it may exacerbate the brain injury and produce a so-called “cerebral reperfusion injury” (35).
Our results showed that GCIR causes reduction in GFR as reported (36) in which GFR was attenuated by focal cerebral ischemia and also it has been showed that brain ischemia induces renal dysfunc-tion (37). The GFR reduction can be explained by low renal blood flow (RBF) as reported (10). On the other hand, reduction in RBF induces renal ischemia (38). Reduced RBF even may cause by renal artery stenosis (39).
Development of therapies to prevent the progre-ssion of the oxidative damage induced by ischemia-reperfusion injury is important and for this purpose, several natural antioxidant agents such as melatonin and vitamin E (40) and garlic oil (41) have been used. It has been reported that EA is responsible for the antioxidant activity of pomegranate juice (42). EA is a naturally occurring plant polyphenol which exhibits antioxidative properties both in vivo (13) and in vitro (42). In fact, it has been shown that EA exerts a potent scavenging action on ROS, as well as lipid peroxidation (43). In addition, EA treatment provides a protection against cisplatin-induced nephrotoxicity and reduces plasma creatinine and urea levels (44).
As mentioned previously, our results showed that GFR was reduced in GCIR group. However pretreatment with EA prevented this reduction. It may conclude that GFR reduction was induced by reduction in renal blood supplied caused by released ROS through GCIR and on the other hand, EA as a potent antioxidant, abolished the ROS actions. Therefore, GFR in EA+GCIR group was not significantly different from SO group. In addition as mentioned previously, cerebral ischemia causes platelet activation (28) and EA inactivates platelet as reported (45). It was reported that EA improved antioxidant associated enzymes such as superoxide dismutase (SOD), malondialdehyde (MDA), and glutathione (GPX) level in ischemic brain (46). We have also shown in another report that MDA was increased in CGIR group and ten days pretreatment with EA (100 mg/kg) attenuated this elevation significantly. Also, superoxidase (SOD) was reduced in GCIR group and in the EA pretreated group this index was elevated significantly (47).
BUN in this study was raised by GCIR. This elevation can be the result of reduction in GFR as reported by other investigators (48). In group which received EA as the preventive pretreatment agent, GCIR was unable to elevate BUN. This preventing effect of EA can be through its antioxidant effect as mentioned above and also improving RBF and GFR as mentioned above. The same results have been reported in which, EA at dose of 30 mg/kg mitigated the elevated BUN in cisplatin-induced nephrotoxicity (49).
Serum creatinine concentration was elevated by induction of CGIR although it was not significant. Elevated the creatinine concentration in serum is one of the best known index of disturbance in GFR as reported by other investigators (50). Since urinary creatinine excretion is mainly related to glomerular filtration and non tubular reabsorption in part and also by proximal tubular secretion, therefore, the higher serum creatinine can be explain by reducing both in GFR and secretion induced by GCIR which both related to RBF. However, the results of EA + GCIR group shows that pretreatment with EA caused a significant prevention in serum creatinine concentration alteration. This EA beneficial effect could be explained by improving of RBF and GFR induced by antioxidant properties of EA as a ROS scavenger. In this regard, it has been shown that EA attenuates creatinine level in cisplatin-induced nephrotoxicity (51). Furthermore, serum creatinine concentration was not affected by EA pretreatment alone.
Most plasma proteins are too large to pass through the kidney plasma/filtrate barrier into the urine unless the kidney is damaged. Our results showed that proteinuria was occurred in GCIR group. Furthermore, it has been shown that there is a strong association between proteinuria with the frequency and number of cerebral microbleeds in patients with recent cerebral ischemia (52). It seems that this barrier has been damaged by high ROS production induced by GCIR as reported (53). It has been suggested that increasing ROS production reduces the glomerular barrier charges which increase the barrier permeability and therefore proteinuria will occurred (54). However, in our study, pretreatment with EA was unable to protect kidney against proteinuria which can be related to the dose and pretreatment period that we have used. Possibly, the disturbance in filtration barrier charges induced by GCIR was more permanent which could not prevented by EA in compare to other measured variables in this study. In addition, proteinuria could be caused by impaired reabsorption of filtered proteins by the epithelial cells of proximal tubule as well. Furthermore it is reported that cerebral ischemia-reperfusion causes inflammation (55) and EA has anti-inflammatry effect (56). While the anti-inflammatroy and reducing permeability effects of EA in acute lung injury have been reported (57) but EA in our study was unable to protect kidney against GCIR- induced proteinuria. The reason could be explained of wide disturbances induced by ROS production from brain and as well kidney and liver and other organs.
Conclusion
In summary, based on the results of this study, we have concluded that oral pretreatment of EA has a renal protective effect. Our results indicated that global cerebral ischemia-reperfusion impairs certain renal functions and EA as an antioxidant, improved most of these functions. Our results may suggest the possible usefulness of EA in patients with brain stroke.
Acknowledgment
The source of data used in this paper was from PhD thesis of Mrs Khojasteh Hoseiny Nejad, from Abadan Arvand International Division, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran. Authors gratefully acknowledge the help and financial support of Physiology Research Center of Ahvaz Jundishapur University of Medical Sciences (grant No. B-9348).
Conflict of interest
There is no conflict of interest.
References
- 1.Mendis S, Norrving B. Organizational update: World Health Organization. Stroke. 2014;45:e22–e23. doi: 10.1161/STROKEAHA.113.003377. [DOI] [PubMed] [Google Scholar]
- 2.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 3.Sanada S, Komuro I, Kitakaze M. Pathophysiology of myocardial reperfusion injury: preconditioning, postconditioning and translational aspects of protective measures. Am J Physiol Heart Circ Physiol. 2011;301:H1723–H1741. doi: 10.1152/ajpheart.00553.2011. [DOI] [PubMed] [Google Scholar]
- 4.Sottiurai VS, Herbert L, Hatter D. Revascularization of cerebral ischemia after previous bilateral extracranial-intracranial bypass procedures. J Vasc Surg. 1997;26:160–163. doi: 10.1016/s0741-5214(97)70163-0. [DOI] [PubMed] [Google Scholar]
- 5.Klein KU, Stadie A, Fukui K, Schramm P, Werner C, Oertel J, et al. Measurement of cortical microcirculation during intracranial aneurysm surgery by combined laser-Doppler flowmetry and photospectrometry. Neurosurgery. 2011;69:391–398. doi: 10.1227/NEU.0b013e3182178bc9. [DOI] [PubMed] [Google Scholar]
- 6.Liu AJ, Ling G, Wu J, Shen FM, Wang DS, Lin LL, et al. Arterial baroreflex function is an important determinant of acute cerebral ischemia in rats with middle cerebral artery occlusion. Life Sci. 2008;83:388–393. doi: 10.1016/j.lfs.2008.06.021. [DOI] [PubMed] [Google Scholar]
- 7.Tracey KJ. Physiology and immunology of the cholinergic anti-inflammatory pathway. J Clin Invest. 2007;117:289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Min J, Farooq MU, Greenberg E, Aloka F, Bhatt A, Kassab M, et al. Cardiac dysfunction after left permanent cerebral focal ischemia: the brain and heart connection. Stroke. 2009;40:2560–2563. doi: 10.1161/STROKEAHA.108.536086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Donnell MJ, Kapral MK, Fang J, Saposnik G, Eikelboom JW, Oczkowski W, et al. Gastrointestinal bleeding after acute ischemic stroke. Neurology. 2008;71:650–655. doi: 10.1212/01.wnl.0000319689.48946.25. [DOI] [PubMed] [Google Scholar]
- 10.Yao H, Sadoshima S, Shiokawa O, Fujii K, Fujishima M. Renal blood flow in acute cerebral ischemia in spontaneously hypertensive rats: effects of alpha- and beta-adrenergic blockade. Stroke. 1987;18:629–633. doi: 10.1161/01.str.18.3.629. [DOI] [PubMed] [Google Scholar]
- 11.Yun X, Maximov VD, Yu J, Zhu H, Vertegel AA, Kindy MS. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33:583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soong YY, Barlow PJ. Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chem. 2006;97:524–530. [Google Scholar]
- 13.Hassoun EA, Vodhanel J, Abushaban A. The modulatory effects of ellagic acid and vitamin E succinate on TCDD-induced oxidative stress in different brain regions of rats after subchronic exposure. J Biochem Mol Toxicol. 2004;18:196–203. doi: 10.1002/jbt.20030. [DOI] [PubMed] [Google Scholar]
- 14.Matsui I, Hamano T, Tomida K, Inoue K, Takabatake Y, Nagasawa Y, et al. Active vitamin D and its analogue, 22-oxacalcitriol, ameliorate puromycin aminonucleoside-induced nephrosis in rats. Nephrol Dial Transplant. 2009;24:2354–2361. doi: 10.1093/ndt/gfp117. [DOI] [PubMed] [Google Scholar]
- 15.Yonekura I, Kawahara N, Nakatomi H, Furuya K, Kirino T. A model of global cerebral ischemia in C57 BL/6 mice. J Cereb Blood Flow Metab. 2004;24:151–158. doi: 10.1097/01.WCB.0000096063.84070.C1. [DOI] [PubMed] [Google Scholar]
- 16.Girish C, Koner BC, Jayanthi S, Ramachandra RK, Rajesh B, Pradhan SC. Hepatoprotective activity of picroliv, curcumin and ellagic acid compared to silymarin on paracetamol induced liver toxicity in mice. Fundam Clin Pharmacol. 2009;23:735–745. doi: 10.1111/j.1472-8206.2009.00722.x. [DOI] [PubMed] [Google Scholar]
- 17.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. Amsterdam. Boston: Academic Press/Elsevier; 2007. [Google Scholar]
- 18.Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–272. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- 19.Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Göbel K, Schuhmann MK, et al. Regulatory T. cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–691. doi: 10.1182/blood-2012-04-426734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamaguchi M, Calvert JW, Kusaka G, Zhang JH. One-stage anterior approach for four-vessel occlusion in rat. Stroke. 2005;36:2212–2214. doi: 10.1161/01.STR.0000182238.08510.c5. [DOI] [PubMed] [Google Scholar]
- 21.Heinel LA, Rubin S, Rosenwasser RH, Vasthare US, Tuma RF. Leukocyte involvement in cerebral infarct generation after ischemia and reperfusion. Brain Res Bull. 1994;34:137–141. doi: 10.1016/0361-9230(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 22.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 23.Williams FM. Role of neutrophils in reperfusion injury. In: Hellewell PG, Williams TJ, editors. Immunopharmacology of Neutrophils. London: Academic Press; 1994. pp. 245–257. [Google Scholar]
- 24.Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 25.Matsuo Y, Onodera H, Shiga Y, Shozuhara H, Ninomiya M, Kihara T, et al. Role of cell adhesion molecules in brain injury after transient middle cerebral artery occlusion in the rat. Brain Res. 1994;656:344–352. doi: 10.1016/0006-8993(94)91478-8. [DOI] [PubMed] [Google Scholar]
- 26.Lew PD, Wolheim CB, Waldvogel FA, Pozzan T. Modulation of cytosolic free calcium transients by changes in intra-cellular calcium buffering capacity;correlation with exocytosis and O2 production inhuman neutrophils. J Cell Biol. 1984;99:1212–1220. doi: 10.1083/jcb.99.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fabian RH, Kent TA. Superoxide anion production during reperfusion is reduced by an anti-neutrophil antibody after prolonged cerebral ischemia. Free Radic Biol Med. 1999;26:355–361. doi: 10.1016/s0891-5849(98)00215-9. [DOI] [PubMed] [Google Scholar]
- 28.Littleton-Kearney MT, Hurn PD, Kickler TS, Traystman RJ. Incomplete global cerebral ischemia alters platelet biology in neonatal and adult sheep. Am J Physiol. 1998;274:1293–1300. doi: 10.1152/ajpheart.1998.274.4.H1293. [DOI] [PubMed] [Google Scholar]
- 29.Gremmel T, Müller M, Steiner S, Seidinger D, Koppensteiner R, Kopp CW, et al. Chronic kidney disease is associated with increased platelet activation and poor response to antiplatelet therapy. Nephrol Dial Transplant. 2013;28:2116–2122. doi: 10.1093/ndt/gft103. [DOI] [PubMed] [Google Scholar]
- 30.Vlădăreanu AM, Popov V, Radeşi S, Onisâi M, Bumbea H. Role of reactive oxygen species in platelet function in normal states and chronic myeloproliferative disorders. Rom J Bioch. 2008;45:221–232. [Google Scholar]
- 31.Au R, Massaro JM, Wolf PA, Young ME, Beiser A, Seshadri S, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 32.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 33.Wong TY, Coresh J, Klein R, Muntner P, Couper DJ, Sharrett AR, et al. Retinal microvascular abnormalities and renal dysfunction: the atherosclerosis risk in communities study. J Am Soc Nephrol. 2004;15:2469–2476. doi: 10.1097/01.ASN.0000136133.28194.E4. [DOI] [PubMed] [Google Scholar]
- 34.Schnackenberg CG. Physiological and patho-physiological roles of oxygen radicals in the renal microvasculature. Am J Physiol Regul Integr Comp Physiol. 2002;282:R335–R342. doi: 10.1152/ajpregu.00605.2001. [DOI] [PubMed] [Google Scholar]
- 35.Aronowski J, Strong R, Grotta JC. Reperfusion injury: demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Saad MA, Abbas AM, Boshra V, Elkhateeb M, El Aal IA. Effect of angiotensin II type 1 receptor blocker, candesartan, and beta 1 adrenoceptor blocker, atenolol, on brain damage in ischemic stroke. Acta Physiol Hung. 2010;97:159–171. doi: 10.1556/APhysiol.97.2010.2.2. [DOI] [PubMed] [Google Scholar]
- 37.Hojs Fabjan T, Penko M, Hojs R. Cystatin C, creatinine, estimated glomerular filtration, and long-term mortality in stroke patients. Ren Fail. 2014;36:81–86. doi: 10.3109/0886022X.2013.832314. [DOI] [PubMed] [Google Scholar]
- 38.Long DA, Price KL, Herrera-Acosta J, Johnson RJ. How does angiotensin II cause renal injury? Hypertension. 2004;43:722–723. doi: 10.1161/01.HYP.0000120964.22281.3e. [DOI] [PubMed] [Google Scholar]
- 39.Radermacher J, Haller H. The right diagnostic work-up: investigating renal and renovascular disorders. J Hypertens Suppl. 2003;21:S19–S24. doi: 10.1097/00004872-200305002-00004. [DOI] [PubMed] [Google Scholar]
- 40.Aktoz T, Aydogdu N, Alagol B, Yalcin O, Huseyinova G, Atakan IH. The protective effects of melatonin and vitamin E against renal ischemia-reperfusion injury in rats. Ren Fail. 2007;29:535–542. doi: 10.1080/08860220701391738. [DOI] [PubMed] [Google Scholar]
- 41.Savas M, Yeni E, Ciftci H, Yildiz F, Gulum M, Keser BS, et al. The antioxidant role of oral administration of garlic oil on renal ischemia-reperfusion injury. Ren Fail. 2010;32:362–367. doi: 10.3109/08860221003611711. [DOI] [PubMed] [Google Scholar]
- 42.Seeram NP, Adams LS, Henning SM, Niu Y, Zhang Y, Nair MG, et al. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J Nutr Biochem. 2005;16:360–367. doi: 10.1016/j.jnutbio.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Iino T, Nakahara K, Miki W, Kiso Y, Ogawa Y, Kato S, et al. Less damaging effect of whisky in rat stomachs in comparison with pure ethanol. Role of ellagic acid, the nonalcoholic component. Digestion. 2001;64:214–221. doi: 10.1159/000048864. [DOI] [PubMed] [Google Scholar]
- 44.Tian L, Shi MM, Forman HJ. Increased transcription of the regulatory subunit of γ-glutamylcysteine synthase in rat lung epithelial L2 cells exposed to oxidative stress or glutathione depletion. Arch Biochem Biophys. 1997;342:126–133. doi: 10.1006/abbi.1997.9997. [DOI] [PubMed] [Google Scholar]
- 45.Attilio P, Merritt C, Sims J, Kane N, O’Sullivan J. The effect of ellagic acid on platelet activation as measured by the quantification of P-selectin using flow cytometry. AANA J. 2010;78:453–459. [PubMed] [Google Scholar]
- 46.Pang X, Li T, Feng L, Zhao J, Zhang X, Liu J. Ellagic acid-induced thrombotic focal cerebral ischemic model in rats. J Pharmacol Toxicol Methods. 2014;69:217–222. doi: 10.1016/j.vascn.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 47.Hoseiny Nejad K, Dianat M, Sarkaki AR, Gharib Naseri MK, Badavi M, Farbood Y. Ellagic acid improves electrocardiogram waves and blood pressure against global cerebral ischemia rat experimental models. Electron Phys. 2015;7:1153–1162. doi: 10.14661/2015.1153-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sasaki Y, Iwama R, Sato T, Heishima K, Shimamura S, Ichijo T, et al. Estimation of glomerular filtration rate in conscious mice using a simplified equation. Physiol Rep. 2014;2:e12135. doi: 10.14814/phy2.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Al-Kharusi N, Babiker HA, Al-Salam S, Waly MI, Nemmar A, Al-Lawati I, et al. Ellagic acid protects against cisplatin-induced nephrotoxicity in rats: a dose-dependent study. Eur Rev Med Pharmacol Sci. 2013;17:299–310. [PubMed] [Google Scholar]
- 50.Sadick M, Attenberger U, Kraenzlin B, Kayed H, Schoenberg SO, Gretz N, et al. Two non-invasive GFR-estimation methods in rat models of polycystic kidney disease: 3.0 Tesla dynamic contrast-enhanced MRI and optical imaging. Nephrol Dial Transplant. 2011;26:3101–3108. doi: 10.1093/ndt/gfr148. [DOI] [PubMed] [Google Scholar]
- 51.Ateşşahín A, Ceríbaşi AO, Yuce A, Bulmus O, Cikim G. Role of ellagic acid against cisplatin-induced nephrotoxicity and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007;100:121–126. doi: 10.1111/j.1742-7843.2006.00015.x. [DOI] [PubMed] [Google Scholar]
- 52.Ovbiagele B, Liebeskind DS, Pineda S, Saver JL. Strong independent correlation of proteinuria with cerebral microbleeds in patients with stroke and transient ischemic attack. Arch Neurol. 2010;67:45–50. doi: 10.1001/archneurol.2009.310. [DOI] [PubMed] [Google Scholar]
- 53.Singh A, Ramnath RD, Foster RR, Wylie EC, Fridén V, Dasgupta I, et al. Reactive oxygen species modulate the barrier function of the human glomerular endothelial glycocalyx. PLoS One. 2013;8:e55852. doi: 10.1371/journal.pone.0055852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maezawa Y, Takemoto M, Yokote K. Cell biology of diabetic nephropathy: roles of endothelial cells, tubulointerstitial cells and podocytes. J Diabetes Investig. 2015;6:3–15. doi: 10.1111/jdi.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu YG, Li FJ, Wang J, Wang XD. Effects of ginkgolide B on inflammation induced by cerebral ischemia-reperfusion in rats. Zhong Yao Cai. 2010;33:578–580. [PubMed] [Google Scholar]
- 56.El-Garhy AM, Abd El-Raouf OM, El-Sayeh BM, Fawzy HM, Abdallah DM. Ellagic acid antiinflammatory and antiapoptotic potential mediate renoprotection in cisplatin nephrotoxic rats. J Biochem Mol Toxicol. 2014;28:472–479. doi: 10.1002/jbt.21587. [DOI] [PubMed] [Google Scholar]
- 57.Cornélio Favarin D, Martins Teixeira M, Lemos de Andrade E, de Freitas Alves C, Lazo Chica JE, ArtérioSorgi C, et al. Anti-inflammatory effects of ellagic acid on acute lung injury induced by acid in mice. Mediators Inflamm. 2013:164202. doi: 10.1155/2013/164202. [DOI] [PMC free article] [PubMed] [Google Scholar]


