Abstract
Background:
PDZ-binding kinase/T-LAK cell-originated protein kinase (PBK/TOPK) is a serine–threonine kinase and overexpressed in various types of cancer by inhibiting the transactivation activities of p53 and PTEN. We tested whether PBK/TOPK acts as a cancer-promoting gene through its activation/overexpression in gastric cancer (GC).
Methods:
We analysed five GC cell lines and 144 primary tumours, which were curatively resected in our hospital between 2001 and 2003.
Results:
Overexpression of the PBK/TOPK protein was frequently detected in GC cell lines (4 out of 5 lines, 80.0%) was detected in primary tumour samples of GC (24 out of 144 cases, 16.6%) and was significantly correlated with venous invasion, tumour depth and recurrence rate. PDZ-binding kinase/T-LAK cell-originated protein kinase-overexpressing tumours had a worse survival rate than those with non-expressing tumours (P=0.0009, log-rank test). PDZ-binding kinase/T-LAK cell-originated protein kinase positivity was independently associated with a worse outcome in multivariate analysis (P<0.0001, hazard ratio 6.40 (2.71–14.49)). In PBK/TOPK-overexpressing GC cells, knockdown of PBK/TOPK inhibited the cell proliferation through the p53 activation in a TP53 mutation-dependent manner and inhibited the migration/invasion through the PTEN upregulation in a TP53 mutation-independent manner.
Conclusions:
These findings suggest PBK/TOPK plays a crucial role in tumour malignant potential through its overexpression and highlight its usefulness as a prognostic factor and potential therapeutic target in GC.
Keywords: PBK/TOPK, TP53, PTEN, gastric cancer, prognosis, oncogene
Gastric cancer (GC) is one of the most common causes of death from cancer worldwide (Siegel et al, 2013). Although recent progress in diagnostic and treatment techniques have contributed to early detection, less invasive treatments and decreased mortality rate, patients with advanced disease still frequently develop recurrent disease, despite extended radical resections and consequently present extremely poor survival rates (Martin et al, 2002).
Because understanding the molecular mechanisms of carcinogenesis and identifying the molecular targets for treatment may contribute to the improvement of survival of patients with GC, only a few molecular targets with frequent targets have been identified (Ushijima and Sasako, 2004), such as gene amplifications of MET and ERBB2; hypermethylation of p16 (Oue et al, 2002; Ding et al, 2003); mutations of TP53, APC and E-cadherin (Becker et al, 1994; Maesawa et al, 1995; Lee et al, 2002); oncogenic activation of β-catenin and K-ras (Park et al, 1999); and inactivation of the mismatch repair gene hMLH1, which is associated with microsatellite instability (Fang et al, 2003). However, in clinical settings, only a few genes have been used as diagnostic biomarkers and/or molecular therapeutic targets (Bang et al, 2010; Wilke et al, 2014). These findings prompted us to identify novel genes associated with the progression of GC.
PDZ-binding kinase/T-LAK cell-originated protein kinase (PBK/TOPK) encodes a serine/threonine protein kinase and is highly expressed in various types of human cancer, such as breast and lung cancers (Park et al, 2006; Shih et al, 2012; O Leary et al, 2013). Physiologically, PBK/TOPK plays a positive regulatory role in proper chromosomal separation and cytokinesis through phosphorylation of various targets (Matsumoto et al, 2004; Park et al, 2010). PDZ-binding kinase/T-LAK cell-originated protein kinase gene expression is regulated by the cell cycle-specific transcription factors E2F and CREB/ATF (Nandi and Rapoport, 2006). PBK/TOPK prevents cancer cell death by impairing processes in the DNA damage-induced apoptosis pathway (Ayllon and O'Connor, 2007; Nandi et al, 2007; Hu et al, 2010), such as tumour suppressor p53 and p38-MAPK activities. Also, PBK/TOPK promotes cell migration by modulating PI3K/PTEN/AKT-dependent signalling (Shih et al, 2012). Although more recently, the prognostic significance and some functional analyses of PBK/TOPK in GC were reported (Kwon et al, 2016), to date, there has been no report on the prognostic significance of PBK/TOPK as an independent prognostic factor and its molecular mechanisms through the p53 and PI3K/AKT pathways that contributes to the tumour development of GC. Therefore, we closely wished to investigate the effects of PBK/TOPK overexpression and activation in GC.
Consequently, we demonstrated that PBK/TOPK was frequently overexpressed in GC cell lines and primary GCs. Overexpression of PBK/TOPK was a poor prognosticator independent of other prognostic factors. We also demonstrated that knockdown of PBK/TOPK expression in PBK/TOPK-overexpressing GC cells suppressed the cell proliferation through the activation of the p53 pathway in a TP53 mutation-dependent manner, and suppressed migration and invasion through the upregulation of PTEN in a TP53 mutation-independent manner. Our results provided evidence that PBK/TOPK could be an important molecular marker for determining the malignant properties and a target for molecular therapy in patients with GC.
Materials and methods
Cell lines and primary tissue samples
A total of five GC cell lines (Kato III, NUGC4, HGC27, MKN45 and MKN74 cells), a TP53-null osteosarcoma cell line (SaOS2), a wild-type TP53 osteosarcoma cell line (U2OS) and the fibroblast cell line (WI-38) were used in this study. All cell lines were purchased from the RIKEN Cell Bank (Tsukuba, Japan) and the Japanese Collection of Research Bioresources Cell Bank (Osaka, Japan), and all cell lines were authenticated using STR genotyping. HGC27 and U2OS cells were cultured in Dulbecco's Minimum Essential Medium. SaOS2 cells were cultured in HyClone McCOY'S 5A Minimum (GE Healthcare Life Sciences, Amersham, UK). The other cells were cultured in Roswell Park Memorial Institute-1640 medium (Sigma, St Louis, MO, USA). All mediums were purchased from Sigma and supplemented with 100 ml l−1 FBS (Trace Scientific, Melbourne, Australia). All cell lines were cultured in 50 ml l−1 carbon dioxide at 37 °C in a humidified chamber. Primary tumour samples of GC were obtained from 144 consecutive GC patients, who has undergone curative gastrectomy at the Division of Digestive Surgery, Department of Surgery, Kyoto Prefectural University of Medicine (Kyoto, Japan) between 2001 and 2003. Each sample was embedded in paraffin after 24 h of formalin fixation. Relevant clinical and survival data were available for all patients. Written consent was always obtained in the formal style and after approval by the local ethics committee. None of these patients had undergone endoscopic mucosal resection, palliative resection, preoperative chemotherapy or radiotherapy, and none of them had synchronous or metachronous multiple cancers in other organs. Disease clinical and pathological stage was defined in accordance with the International Union Against Cancer tumour-lymph node-metastases (TNM) classification (Sobin and Compton, 2010). The median follow-up period for surviving patients was 80.0 months (ranging from 0.5 to 165.9 months).
Quantitative real-time RT–PCR
Single-stranded complementary DNA generated from total RNA was amplified with primers specific for each gene, as described below. The abundance of messenger RNA was measured with a quantitative real-time fluorescence detection method (ABI StepOnePlus Sequence Detection System; Applied Biosystems, Foster City, CA, USA) using TaqMan Gene Expression Assays (Hs00902992_m1 for PBK/TOPK, Hs00355782_m1 for p21 and Hs02621230_s1 for PTEN; Applied Biosystems) according to the manufacturer's instructions. The total RNA of normal organs was purchased from Takara Bio Inc., Shiga, Japan (Human Total RNA Master Panel II (Cat. No. 636643) and Human Liver Total RNA (Cat. No. 636531)) and BioChain Institute Inc., CA, USA (Human OEsophagus Total RNA (Cat. No. R1234106-50)). The results of gene expression were calculated as a ratio between PBK/TOPK and expression of an internal reference gene (Hs01060665_g1 for β-actin; Applied Biosystems) that provides a normalisation factor for the amount of RNA isolated from a specimen. This assay was performed in duplicate for each sample.
Western blotting
Anti-PBK/TPOK mouse polyclonal antibody (sc-293028) and anti-ACTB antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). PDZ-binding kinase/T-LAK cell-originated protein kinase is an affinity purified mouse polyclonal antibody raised against a recombinant protein of PBK/TOPK. Anti-p53 (DO7) antibodies were from Novocastra Laboratories (Newcastle upon Tyne, UK), and anti-p53 (DO1) and anti-p21 were from Santa Cruz Biotechnology. Anti-PTEN, anti-AKT and anti-phospho-AKT (pAKT) were from Cell Signaling Technology (Cell Signaling, Massachusetts, USA). Cells were lysed and their proteins were extracted by M-PER Mammalian Protein Extraction Reagent (Thermo Scientific, Waltham, MA, USA).
Loss of function by small interfering RNA and cell growth analysis
For loss-of-function analysis by knocking down endogenous gene expression, each of the small interfering RNAs (siRNAs) targeting PBK/TOPK (Stealth RNAi siRNA #HSS183331; Invitrogen Corporation, Carlsbad, CA, USA), p53 (Stealth RNAi siRNA #HSS186390; Invitrogen Corporation) a negative control (Stealth RNAi siRNA Cat. No.12935-112; Invitrogen Corporation) or Luciferase (Luc) 5′-CGUACGCGGAAUACUUCGA-3′ (Sigma, Tokyo, Japan) was transfected into cells (10 nmol l−1) using Lipofectamine RNAiMAX (Invitrogen Corporation) according to the manufacturer's instructions. The knockdown of a target gene was confirmed by western blotting. For measurements of cell growth, the number of viable cells at various time points after transfection was assessed by the colorimetric water-soluble tetrazolium salt assay (Cell counting kit-8; Dojindo Laboratories, Kumamoto, Japan). Cell cycle phase was investigated 72 h after transfection by fluorescence-activated cell sorting (FACS), as described elsewhere (Komatsu et al, 2009).
Transwell migration and invasion assays
Transwell migration and invasion assays were carried out in 24-well-modified Boyden chambers (transwell-chamber, Becton Dickinson and Company, Franklin Lakes, NJ, USA). The upper surface of 6.4 mm diameter filters with 8-μm pores was precoated with (invasion assay) or without (migration assay) Matrigel (Becton Dickinson and Company). The siRNA transfectants (8 × 105 cells per well) were transferred into the upper chamber. Following 22 h of incubation, the migrated or invasive cells on the lower surface of the filters were fixed and stained with Diff-Quik stain (Sysmex, Kobe, Japan), and stained cell nuclei were counted directly in triplicate. We assessed the invasive potential according to the protocol of Corning BioCoat Matrigel Invasion Chamber (Catalogue No. 354480) by calculating the number of cells, which is the ratio of the percentage invasion through the Matrigel-coated filters relative to the migration through the uncoated filters of test cells over that in the control counterparts as described elsewhere (Kashimoto et al, 2012; Nishimura et al, 2013; Komatsu et al, 2015).
Immunohistochemistry
Primary tumour samples were fixed with 10% formaldehyde in phosphate buffered saline and routinely embedded in paraffin. The horseradish peroxidase (HRP) staining method was performed. In brief, after deparaffinization, antigen retrieval was performed by heating the samples in 10 mmol l−1 citrate buffer (pH 6.0) at 95 °C for 1 h. Endogenous peroxidases were quenched by incubating the sections for 20 min in 3% H2O2. After treatment with Block Ace (Dainippon Sumitomo Pharmaceutical, Osaka, Japan) for 30 min at room temperature, sections were incubated at room temperature for 1 h with an anti-PBK/TOPK (1 : 500) or at 4 °C overnight with an anti-p53 (DO7, Novocastra Laboratories; 1 : 200). Phosphate buffered saline was used for all dilutions and washings. The bound primary antibody was detected with EnVision+ Horse Radish Peroxidase Systems (EnVision+dual link System-HRP; Dako North America, Inc., Carpinteria, CA, USA). Horseradish peroxidase labelling was visualised using colour development with diaminobenzidine tetrahydrochloride. Slides were counterstained with Mayer's haematoxylin. A formalin-fixed GC cell line-overexpressing PBK/TOPK (HGC27 cells), in which >50% of cells showed staining of PBK/TOPK protein, was used as a positive control, whereas HGC27 cells incubated without the PBK/TOPK antibody were used as a negative control.
For scoring PBK/TOPK expression, the intensity (intensity score: 0=negative, 1=weak, 2=moderate, 3=strong) and percentage of the total cell population (proportion score: 0<10%, 10%⩽1<30%, 30%⩽2<60%, 60%⩽3⩽100%) that expressed PBK/TOPK was evaluated for each case. Expression of PBK/TOPK was graded as high expression (intensity plus proportion scores ⩾5 of tumour cells showing immunopositivity) or low expression (intensity plus proportion scores ⩽4 of tumour cells showing immunopositivity) using high-power (× 200) microscopy (Tsuda, 2008).
For scoring p53 expression, the intensity (intensity score: 0=negative, 1=weak, 2=moderate, 3=strong) and percentage of the total cell population (proportion score: 0%⩽0<30%, 30%⩽1⩽100%) that expressed p53 was evaluated for each case. A distinct nuclear immunoreaction in both 3+ and ⩾30% of the cancer cells was judged positive. The remaining cases were judged negative.
Statistical analysis
Clinicopathological categorical variables pertaining to the corresponding patients were analysed for significance by the χ2-test or Fisher's exact test. Differences of non-categorical variables between subgroups were tested with the non-parametric Mann–Whitney U test. For the analysis of survival, Kaplan–Meier survival curves were constructed for groups based on univariate predictors, and differences between the groups were tested with the log-rank test. Univariate and multivariate survival analyses were performed using the likelihood ratio test of the stratified Cox proportional hazards model. Differences were assessed with a two-sided test and considered significant at the P<0.05 level.
Results
Overexpression of PBK/TOPK in GC cell lines
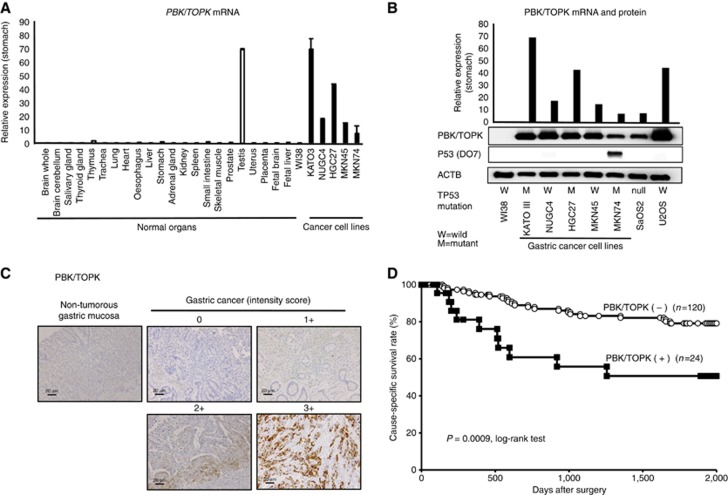
Quantitative RT–PCR analysis was performed to test whether PBK/TPOPK is overexpressed in GC cell lines compared with the normal organs and the fibroblast cell line WI-38 (Figure 1A). PBK/TPOPK mRNA overexpression was observed in almost all GC cell lines (4 out of 5 lines, 80.0%) and testis compared with the other organs and the fibroblast cell line WI-38, suggesting this gene to be a cancer–testis antigen and a target for activation in GC. Expression of PBK/TOPK protein detected by the PBK/TOPK-specific antibody (4 out of 5 lines, 80.0%) correlated with that of PBK/TOPK mRNA in GC cell lines (Figure 1B). Because suppression of p53 activity through the DNA-binding domain (DBD) by PBK/TOPK was reported previously (Hu et al, 2010), we used representative osteosarcoma cell lines (SaOS2; TP53-null, U2OS; wild-type TP53) and confirmed the expression of p53-DO7 associated with the status of TP53 mutation in the GC cell lines, including these osteosarcoma cell lines by western blotting. These statuses of TP53 mutation in various cell lines are positively associated with their reported status of TP53 mutation in the database (http://p53.free.fr/index.html; W: wild-type TP53, M: mutant TP53). Although wild-type TP53 may be expected to be a more suitable substrate than mutant TP53 for PBK/TOPK, expression of PBK/TOPK mRNA and protein was not correlated with the mutation status and expression of p53 (Figure 1B).
Figure 1.
Expression of PBK/TOPK was correlated with poor prognosis in gastric cancer patients. (A) Expression of PBK/TOPK mRNA in five GC cell lines compared with that of the normal organs and the fibroblast cell line WI-38. (B) Expression of PBK/TOPK mRNA and protein in five GC cell lines and osteosarcoma cell lines, such as TP53-null SaOS2 and TP53 wild-type U2OS, compared with that in the fibroblast cell line WI-38. The status of TP53 mutation in each cancer cell line was evaluated by western blotting. The status of TP53 mutation was positively associated with the reported status of TP53 mutation in the database (http://p53.free.fr/index.html, W: wild-type TP53, M: mutant TP53). (C) Specific immunostaining of PBK/TOPK in a representative primary tumour sample. On the basis of this result, the intensity scores for PBK/TOPK staining were determined as follows: 0=negative, 1=weak, 2=moderate, 3=strong. Magnification: × 200; Scale bar, 20 μm. (D) Cause-specific survival rates of GC patients (as determined by Kaplan–Meier plots) depending on the combination scores of intensity and proportion in PBK/TOPK expression.
Immunohistochemical analysis of PBK/TOPK expression in primary tumours of GC
Because the PBK/TOPK protein was overexpressed in almost all GC cell lines, it was hypothesised that PBK/TOPK was also highly expressed in GC tissues and would be associated with carcinogenesis and malignant outcomes. We examined the prognostic and clinicopathological significance of PBK/TOPK expression in primary tumour samples of GC based on the immunohistochemical staining pattern of this protein. PDZ-binding kinase/T-LAK cell-originated protein kinase protein was observed in both the cytoplasm and nucleus of cancer cells (Figure 1C). We classified 144 GC samples into positive and negative groups according to the intensity and proportion of PBK/TOPK staining among tumour cells, as described in Materials and Methods. In primary cases, PBK/TOPK protein expression was negative in the non-tumorous gastric mucosal cell population (Figure 1C), and it was weakly detected in the lymphoid follicles. Supplementary Table 1 shows the distribution of patients based on the intensity and proportion scores of PBK/TOPK immunoreactivity of tumour samples. Kaplan–Meier survival estimates showed that PBK/TOPK immunoreactivity in tumour cells was associated with a worse cause-specific survival according to the extent in each intensity and proportion scoring (Supplementary Figure S1A and B).
In the total scores of intensity plus proportion (Supplementary Table 1), the high-expression group of PBK/TOPK presenting scores ⩾5 tumour cells showing immunopositivity presented significantly poorer prognosis than the low-expression group (P=0.0009, log-rank test; Figure 1D). Five-year survival rates of patients with PBK/TOPK high-and low-expression cancer in each stage were 98.4% vs 100% (P=0.7077) in stage I, 9.2% vs 34.7% (P=0.0013) in stage II+III, respectively.
Association between PBK/TOPK protein abundance and clinicopathological characteristics in primary cases of GC
The relationship between the expression of the PBK/TOPK protein and clinicopathological characteristics is summarised in Table 1. Protein expression of PBK/TOPK was significantly associated with venous invasion, depth of invasion and higher recurrence rate, and tended to be associated with pStage in the TNM classification, whereas other characteristics, including histological grade were not.
Table 1. Association between clinicopathological characteristics and PBK/TOPK expression.
|
PBK/TOPK immunoreactivity |
||||
|---|---|---|---|---|
| n | Positive (%) | Negative (%) | P-valuea | |
| Total | 144 | 24 | 120 | |
| Gender | ||||
| Male | 97 | 15 (62%) | 82 (68%) | 0.5995 |
| Female | 47 | 9 (38%) | 38 (32%) | |
| Age (years) | ||||
| <60 | 55 | 9 (38%) | 46 (38%) | 0.9388 |
| ⩾60 | 89 | 15 (63%) | 74 (62%) | |
| Location | ||||
| Upper | 19 | 4 (17%) | 15 (13%) | 0.5740 |
| Middle | 74 | 10 (42%) | 64 (44%) | |
| Lower | 51 | 10 (42%) | 41 (34%) | |
| Histological grade | ||||
| Differentiated | 69 | 15 (62%) | 54 (45%) | 0.1162 |
| Undifferentiated | 75 | 9 (38%) | 66 (55%) | |
| Macroscopic appearance | ||||
| Type 0 | 80 | 13 (54%) | 69 (57%) | 0.2952 |
| Type 1/2/3/4 | 64 | 11 (46%) | 51 (43%) | |
| Tumour size (mm) | ||||
| <60 | 103 | 16 (67%) | 87 (73%) | 0.5680 |
| ⩾60 | 41 | 8 (33%) | 33 (27%) | |
| Venous invasion | ||||
| Negative | 102 | 10 (42%) | 92 (77%) | 0.0010 |
| Positive | 42 | 14 (58%) | 28 (23%) | |
| Lymphatic invasion | ||||
| Negative | 77 | 11 (46%) | 66 (55%) | 0.4117 |
| Positive | 67 | 13 (54%) | 54 (45%) | |
| TNM classification | ||||
| pT categories | ||||
| T1 | 80 | 11 (45%) | 69 (58%) | 0.0062 |
| T2 | 12 | 0 (0%) | 12 (10%) | |
| T3 | 22 | 9 (38%) | 13 (11%) | |
| T4 | 30 | 4 (17%) | 26 (21%) | |
| pN categories | ||||
| N0 | 93 | 13 (54%) | 80 (67%) | 0.4481 |
| N1 | 18 | 5 (21%) | 13 (11%) | |
| N2 | 10 | 1 (4%) | 9 (8%) | |
| N3 | 23 | 5 (21%) | 18 (15%) | |
| pStage | ||||
| I | 87 | 11 (46%) | 76 (63%) | 0.0792 |
| II | 16 | 6 (25%) | 10 (8%) | |
| III | 41 | 7 (29%) | 34 (28%) | |
| Rucurrence | ||||
| Absent | 112 | 14 (58%) | 98 (82%) | 0.0178 |
| Present | 32 | 10 (42%) | 22 (18%) | |
| p53 (DO7) | ||||
| Positive | 38 | 10 (42%) | 27 (23%) | 0.1047 |
| Negative | 106 | 14 (58%) | 82 (77%) | |
Abbreviations: PBK/TOPK=PDZ-binding kinase/T-LAK cell-originated protein kinase; TNM=tumour-lymph node-metastases.
NOTE: statistically significant values are in bold type.
P values are from χ2 or Fisher's exact test and were statistically significant at <0.05.
In the Cox proportional hazard regression model (Table 2), univariate analyses demonstrated that PBK/TOPK protein expression, age, location, macroscopic appearance, tumour size, venous invasion, lymphatic invasion, pT category and pN category were significantly correlated with cause-specific survival. When data were stratified for multivariate analysis using both the forward and backward stepwise Cox regression procedures, PBK/TOPK immunoreactivity in tumour cells remained significant at P<0.0001 (hazard ratio, 6.40 (2.71–14.49)) for cause-specific survival in all patients, suggesting that immunoreactivity may be an independent predictor of cause-specific survival.
Table 2. Cox proportional hazard regression analysis for overall survival.
|
Univariatea |
Multivariateb |
|||
|---|---|---|---|---|
| Factor | P-valuec | HR | 95%CI | P-valuec |
| Gender | ||||
| Male vs female | 0.6793 | — | ||
| Age (years) | ||||
| ⩾60 vs <60 | 0.0294 | — | ||
| Location | ||||
| Upper vs middle/lower | 0.0296 | 2.776 | 1.125–6.27 | 0.0282 |
| Histological grade | ||||
| Undifferentiated vs differentiated | 0.8722 | — | ||
| Macroscopic appearance | ||||
| Type 3/4/5 vs type 0/1/2 | <0.0001 | |||
| Tumour size (mm) | ||||
| ⩾60 vs <60 | <0.0001 | 2.842 | 1.336–6.44 | 0.0063 |
| Venous invasion | ||||
| Positive vs negative | <0.0001 | — | ||
| Lymphatic invasion | ||||
| Positive vs negative | <0.0001 | — | ||
| pT-stage | ||||
| pT 2/3/4 vs pT 1 | <0.0001 | 14.48 | 1.611–310.70 | 0.0164 |
| pN-stage | ||||
| pN 1/2/3 vs pN 0 | <0.0001 | 4.14 | 1.242–23.31 | 0.0174 |
| PBK/TOPK expressiond | ||||
| High vs low | 0.0009 | 6.403 | 2.712–14.49 | <0.0001 |
| p53 (DO7) | ||||
| Positive vs negative | 0.5273 | |||
Abbreviations: CI=confidence interval; HR=hazard ratio.
Statistically significant values are in boldface type.
Kaplan and Meier method, and the statistical significance was determined by log-rank test.
Multivariate survival analysis was performed using Cox's proportional hazard model.
P-values were from two-sided tests and were statistically significant at <0.05.
PBK/TOPK expression was evaluated by immunohistochemical analysis as described in Materials and Methods.
Suppression of cell proliferation by knockdown of PBK/TOPK and the knockdown effect according to TP53 mutation status
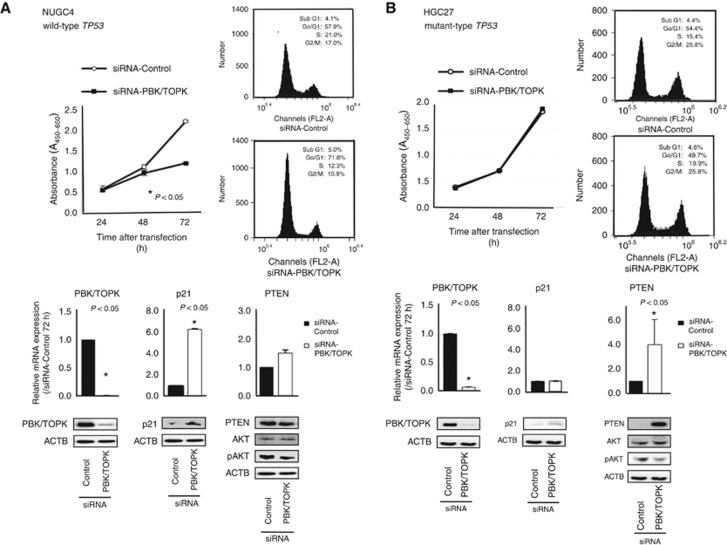
To gain an insight into the potential role of PBK/TOPK as an oncogene whose overexpression could be associated with gastric tumorigenesis, we first performed a cell proliferation assay using several siRNAs specific to PBK/TOPK to investigate whether knockdown of PBK/TOPK would suppress proliferation of GC cells that overexpress PBK/TOPK. In all TP53 wild-type cell lines, such as NUGC4 (Figure 2A), MKN45 (Supplementary Figure S2A) and U2OS (Supplementary Figure S2B), expression of the PBK/TOPK protein was more efficiently knocked down 24–72 h after the transient introduction of a PBK/TOPK-specific siRNA (siRNA-PBK/TOPK) than with the control siRNA (siRNA-Control; Figure 2A). The proliferation of all these cell lines was significantly lower than with controls after the knockdown of endogenous PBK/TOPK expression. Whereas in all TP53 mutant cell lines, such as HGC27 (Figure 2B) and SaOS2 (Supplementary Figure S2C), the suppression of the cell proliferation was not detected despite the complete knockdown of the PBK/TOPK protein.
Figure 2.
Effects of PBK/TOPK knockdown by siRNA-PBK/TOPK compared with those of control siRNA in NUGC4 and HGC27 cell lines. (A) shows that the knockdown of PBK/TOPK overexpression in TP53 wild-type NUGC4 cells downregulated expression of pAKT and upregulated that of p21, which resulted mainly in G0–G1 arrest. The proliferation of NUGC4 cell was significantly lower than with controls after the knockdown of endogenous PBK/TOPK expression. However, (B) shows that there was no difference of cell proliferation and FACS results between siRNA-PBK/TOPK and control siRNA cells in TP53 mutant HGC27 cells. The p21 level was not changed, but pAKT level was decreased and PTEN level was increased at 72 h in siRNA-PBK/TOPK-transfected cells. Including the results of Supplementary Figure S2, these results suggested that PBK/TOPK might be related to the cell proliferation in a TP53 mutation-dependent manner.
Cell cycle analysis by downregulation of PBK/TOPK expression using FACS
Fluorescence-activated cell sorting analysis demonstrated that transfection of TP53 wild-type NUGC4 cells with siRNA-PBK/TOPK resulted in an accumulation of cells in the G0–G1 phase compared with transfection with control siRNA (Figure 2A). In TP53 mutant HGC27 cells, however, there was no difference of FACS results between siRNA-PBK/TOPK and control siRNA cells (Figure 2B). Specifically, in NUGC4 cells, p21 and PTEN level was increased, and pAKT was decreased at 72 h protein level in siRNA-PBK/TOPK transfected cells (Figure 2A). However, in TP53 mutant HGC27 cells, p21 level was not changed, but PTEN level was increased and pAKT was decreased at 72 h in siRNA-PBK/TOPK transfected cells (Figure 2B). These findings suggested that PBK/TOPK might be related to the cell proliferation in a TP53 mutation-dependent manner.
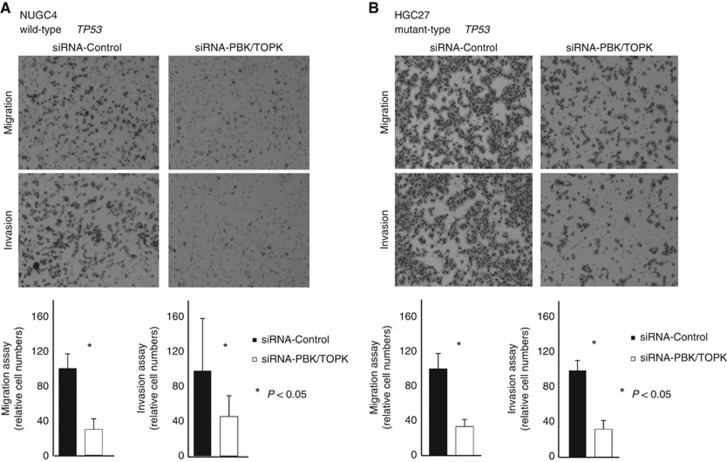
Suppression of cell migration and invasion by downregulation of PBK/TOPK expression and the knockdown effect according to TP53 mutation status
Next, transwell migration and invasion assays were performed to examine the ability of TP53 wild-type NUGC4 and TP53 mutant HGC27 cells transfected with siRNA-PBK/TOPK to move through pores under different conditions. Uncoated membrane was used for the migration assays, whereas Matrigel-coated membrane was used for invasion assays. In Figure 3, the number of both NUGC4 (Figure 3A) and HGC27 (Figure 3B) cells that migrated into the lower chamber was significantly lower for siRNA-PBK/TOPK-transfected cells than for siRNA-control-transfected cells under both conditions, suggesting that PBK/TOPK may increase the ability of GC cells to migrate and invade in a TP53 mutation-independent manner.
Figure 3.
Regarding the cell migration and invasion, the results of the increase of PTEN and/or the decrease of pAKT in siRNA-PBK/TOPK-transfected cells suggested that PBK/TOPK may increase the ability of GC cells to migrate and invade via the PI3K/Akt pathway activation in a TP53 mutation-independent manner. (A) NUGC wild-type TP53 and (B) mutant-type TP53.
Relationship between protein expression of PBK/TOPK and p53 in primary cases of GC
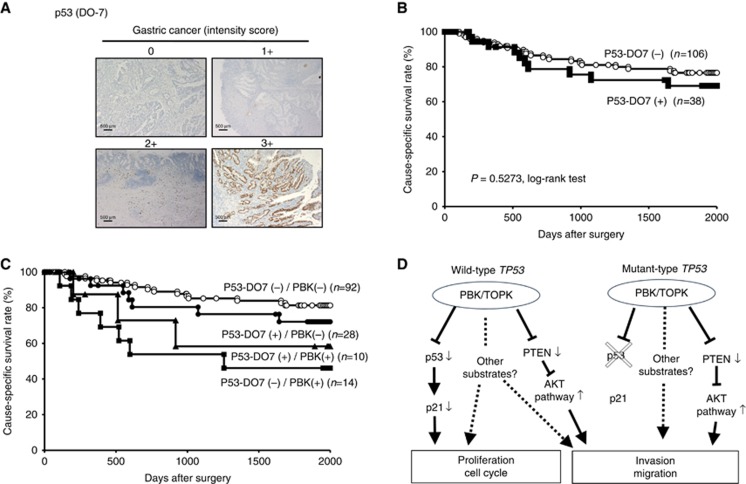
A previous report indicated that PBK/TOPK physically interacts with the tumour suppressor p53 through its DBD (Hu et al, 2010). Therefore, we examined the relationship between the protein expression of PBK/TOPK and p53 in primary tumour cells (Figure 4A). Because a good correlation is known to exist between p53-DO7 protein accumulation and the presence of TP53 gene mutations, especially missense mutations (Bennett et al, 1991; Wagata et al, 1993), we used immunohistochemistry to evaluate TP53 mutation status. As a result, of 144 cases of GC, tumours showing positive p53-DO7 immunoreactivity were detected in 38 cases (26.4%, Table 1). Expression of p53 was not observed in non-tumorous gastric mucosal cells. No significant correlations were found between PBK/TOPK and p53-DO7 immunohistochemical staining patterns (P=0.1407; Table 1). Kaplan–Meier survival estimates showed that the expression of p53-DO7 was not associated with cause-specific survival (P=0.5273, log-rank test; Figure 4B). However, in both p53-DO7-negative and p53-DO7-positive groups, the cause-specific survival rate was significantly poorer in patients with PBK/TOPK expression than in those without (P=0.0228, log-rank test in four groups; Figure 4C). Five-year survival rates of PBK/TOPK high expression vs low expression were: 46.2% vs 81.3% in p53-DO7-negative patients and 58.3% vs 72.1% in p53-DO7-positive patients. Notably, a strong significant difference of the cause-specific survival was found between patients positive and negative for PBK/TOPK in the p53-DO7-negative group (P=0.0027, log-rank test). However, no significant difference was found between patients positive and negative for PBK/TOPK in the p53-DO7-positive group (P=0.4519, log-rank test), although the PBK/TOPK positive group presented poorer prognosis than the negative group.
Figure 4.
Relationship between protein expression of PBK/TOPK and p53 in primary cases of gastric cancer. (A) Specific immunostaining of PBK/TOPK in a representative primary tumour sample. On the basis of this result, the intensity scores for p53 (DO7) staining were determined as follows: 0=negative, 1=weak, 2=moderate, 3=strong. Magnification: × 40; Scale bar, 500 μm. (B) Kaplan–Meier curves for cause-specific survival rates of patients at all stages according to the expression of p53. There was no difference of survival between the patients positive and negative for p53-DO7. (C) Postoperative overall survival curves according to a combination of the expression of PBK/TOPK and p53. A significant difference in survival was observed among the patients positive (+) or negative (−) for p53-DO7 and PBK/TOPK (P=0.0228, log-rank test). Notably, in the p53-DO7-negative group, the patients with PBK/TOPK expression had a markedly worse outcome than those with no PBK/TOPK expression. (D) Hypothetical model of the overexpression/activation of PBK/TOPK in GC cells.
Discussion
The PBK/TOPK gene is upregulated in various types of cancer and tumours such as bladder cancer, brain tumour, breast cancer, liver cancer, lung cancer and sarcoma (Park et al, 2006; Herrero-Martin et al, 2009; He et al, 2010; Shih et al, 2012; O Leary et al, 2013; Singh et al, 2014; Joel et al, 2015). PDZ-binding kinase/T-LAK cell-originated protein kinase was initially identified as a mitotic protein kinase (Gaudet et al, 2000), assisting the recruitment of cdk1/cyclin B to mitotic spindles, where PBK/TOPK plays a role in the formation of the spindle midzone and cytokinesis (Matsumoto et al, 2004). Previous studies have identified various oncogenic functions of PBK/TOPK related to multiple signalling pathways, such as the mitogen-activated protein kinase (MAPK) pathway (Ayllon and O'Connor, 2007), PI3K/AKT pathway (Abe et al, 2000; Zhu et al, 2007; Shih et al, 2012) and p53 pathway (Nandi et al, 2007; Hu et al, 2010). Through the MAPK pathway, PBK/TOPK functions as mediator of growth factor activation of p38 with a role in motility and as part of the DNA damage-sensing machinery and is necessary for the phosphorylation of histone H2AX (Ayllon and O'Connor, 2007). Regarding the PI3K/AKT pathway, PBK/TOPK decreases the PTEN level and regulates PI3K/AKT-stimulated migration (Shih et al, 2012). Most importantly, by impairing the DNA damage-induced apoptotic pathway, p53 pathway, PBK/TOPK prevents cancer cell death (Nandi et al, 2007). PDZ-binding kinase/T-LAK cell-originated protein kinase physically interacts with p53, specifically through its DBD and promotes tumour cell survival and resistance to chemotherapy-induced apoptosis through suppression of p21 (Hu et al, 2010). Thus, PBK/TOPK has promising oncogenic functions, and these findings prompted us to clarify the clinicopathological and independent prognostic significance of PBK/TOPK overexpression/activation through molecular mechanisms associated with the p53 and PI3K/AKT pathways in primary GC.
Here we hypothesised that overexpression/activation of PBK/TOPK may promote tumour cell proliferation and/or poor survival of GC patients. To verify this hypothesis, we firstly examined the expression status of PBK/TOPK in cell lines and primary tumours of GC, and its clinicopathological and prognostic significance were evaluated. As a result, we demonstrated that PBK/TOPK was overexpressed in 16.7% (24 out of 144) of primary GCs, as well as in 80.0% (4 out of 5) of GC cell lines, and this overexpression was an indicator of poor prognosis independent of other prognostic factors in both an intensity and proportion-dependent manner (Supplementary Figure S1A and B). In combination with p53, there was no relationship between the expression levels of PBK/TOPK and p53 in primary tumours and cell lines, although one previous report suggested these positive correlations in lung cancer (Lei et al, 2015). These results suggest that immunoreactivity to PBK/TOPK may be useful as an independent prognosticator in patients with GC.
In our in vitro analyses, knockdown of PBK/TOPK overexpression in wild-type TP53 cells induced cell cycle G0–G1 arrest, but knockdown of PBK/TOPK overexpression in mutant TP53 cells did not (Figure 2; Supplementary Figure S2). This suggests that, in tumours with wild-type TP53, p53 may be an important substrate for PBK/TOPK. Indeed, particular in the p53-DO7-negative group, patients with tumours positive for PBK/TOPK expression showed significantly worse survival than those with tumours lacking PBK/TOPK expression (Figure 4C), suggesting that a combination of p53 and PBK/TOPK immunoreactivity might be a more useful predictor for survival of patients with GC, because p53 and its target molecules, which regulate the cell cycle and trigger apoptosis after DNA damage, play a key role in a wide range of human cancers, including GC (Caelles et al, 1994; Fenoglio-Preiser et al, 2003; Bellini et al, 2012).
Next, we investigated further the molecular mechanism affecting the malignant potential in tumour cells with both overexpression of PBK/TOPK and mutant TP53. As a result, knockdown of PBK/TOPK suppressed invasion and migration through PTEN upregulation in TP53 mutant GC cells, as well as wild-type TP53 cells (Figure 3A and B). Indeed, as shown in our immunohistochemical analysis, overexpression of PBK/TOP is related to poor survival even in patients with putative mutant TP53 GC. Thus, in vitro findings strongly supported our in vivo results of immunohistochemical analysis and strongly suggested that PBK/TOPK plays a pivotal role in the malignant potential of GC. We presented our hypothetical model of the overexpression/activation of PBK/TOPK in GC (Figure 4D).
Recently, PBK/TOPK-specific inhibitors such as OTS514, OTS964 (OncoTherapy Science Inc., Japan; Matsuo et al, 2014; Ikeda et al, 2016) and HI-TOPK-032 (Kim et al, 2012; Joel et al, 2015) have been developed. Particularly, the PBK/TOPK-specific inhibitor OTS964 was proved to be extremely effective in treating lung cancer in a xenograft mouse model without showing any adverse reactions (Matsuo et al, 2014). Regarding p53 status, Kim et al (2012) reported that the administration of PBK/TOPK-specific inhibitor, HI-TOPK-032 suppressed tumour growth in TP53 wild-type HCT116 colon cancer xenograft model. Whereas, Ikeda et al (2016) more recently reported that oral administration of PBK/TOPK-specific inhibitor, OTS514 significantly elongated overall survival in TP53 mutant-type ES-2 abdominal dissemination xenograft model, compared with vehicle control in ovarian cancer. These data indicated that the molecular targeting to PBK/TOPK may work for tumours of both TP53 wild type and mutant type in various cancers. Therefore, the PBK/TOPK-specific inhibitors may be a key molecule for the treatment of patients with GC-developed PBK/TOPK overexpression. These inhibitors are currently being evaluated in vitro and in vivo at our institute.
In conclusion, this is the first report to show that PBK/TOPK has a pivotal oncogenic role through molecular mechanisms associated with the p53 and PI3K/AKT pathways and is a potential therapeutic target in GC. We clearly demonstrated the frequent overexpression of PBK/TOPK protein and its prognostic value in patients with GC. Although studies of larger cohorts are needed to validate these findings before moving to clinical settings, our results provide evidence that PBK/TOPK could be a crucial molecular marker to determine the malignant properties of GC cells and that it could be a target for molecular therapy in patients with GC.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Abe Y, Matsumoto S, Kito K, Ueda N (2000) Cloning and expression of a novel MAPKK-like protein kinase, lymphokine-activated killer T-cell-originated protein kinase, specifically expressed in the testis and activated lymphoid cells. J Biol Chem 275(28): 21525–21531. [DOI] [PubMed] [Google Scholar]
- Ayllon V, O'Connor R (2007) PBK/TOPK promotes tumour cell proliferation through p38 MAPK activity and regulation of the DNA damage response. Oncogene 26(24): 3451–3461. [DOI] [PubMed] [Google Scholar]
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Ruschoff J, Kang YK (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376(9742): 687–697. [DOI] [PubMed] [Google Scholar]
- Becker KF, Atkinson MJ, Reich U, Becker I, Nekarda H, Siewert JR, Hofler H (1994) E-cadherin gene mutations provide clues to diffuse type gastric carcinomas. Cancer Res 54(14): 3845–3852. [PubMed] [Google Scholar]
- Bellini MF, Cadamuro AC, Succi M, Proenca MA, Silva AE (2012) Alterations of the TP53 gene in gastric and esophageal carcinogenesis. J Biomed Biotechnol 2012: 891961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett WP, Hollstein MC, He A, Zhu SM, Resau JH, Trump BF, Metcalf RA, Welsh JA, Midgley C, Lane DP, Harris CC (1991) Archival analysis of p53 genetic and protein alterations in Chinese esophageal cancer. Oncogene 6(10): 1779–1784. [PubMed] [Google Scholar]
- Caelles C, Helmberg A, Karin M (1994) p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature 370(6486): 220–223. [DOI] [PubMed] [Google Scholar]
- Ding Y, Le XP, Zhang QX, Du P (2003) Methylation and mutation analysis of p16 gene in gastric cancer. World J Gastroenterol 9(3): 423–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang DC, Wang RQ, Yang SM, Yang JM, Liu HF, Peng GY, Xiao TL, Luo YH (2003) Mutation and methylation of hMLH1 in gastric carcinomas with microsatellite instability. World J Gastroenterol 9(4): 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio-Preiser CM, Wang J, Stemmermann GN, Noffsinger A (2003) TP53 and gastric carcinoma: a review. Hum Mut 21(3): 258–270. [DOI] [PubMed] [Google Scholar]
- Gaudet S, Branton D, Lue RA (2000) Characterization of PDZ-binding kinase, a mitotic kinase. Proc Natl Acad Sci USA 97(10): 5167–5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Yan Q, Fan L, Liu Y, Cui J, Wang J, Wang L, Wang Y, Wang Z, Guo Y, Huang G (2010) PBK/TOPK in the differential diagnosis of cholangiocarcinoma from hepatocellular carcinoma and its involvement in prognosis of human cholangiocarcinoma. Hum Pathol 41(3): 415–424. [DOI] [PubMed] [Google Scholar]
- Herrero-Martin D, Osuna D, Ordonez JL, Sevillano V, Martins AS, Mackintosh C, Campos M, Madoz-Gurpide J, Otero-Motta AP, Caballero G, Amaral AT, Wai DH, Braun Y, Eisenacher M, Schaefer KL, Poremba C, de Alava E (2009) Stable interference of EWS-FLI1 in an Ewing sarcoma cell line impairs IGF-1/IGF-1R signalling and reveals TOPK as a new target. Br J Cancer 101(1): 80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F, Gartenhaus RB, Eichberg D, Liu Z, Fang HB, Rapoport AP (2010) PBK/TOPK interacts with the DBD domain of tumor suppressor p53 and modulates expression of transcriptional targets including p21. Oncogene 29(40): 5464–5474. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Park JH, Miyamoto T, Takamatsu N, Kato T, Iwasa A, Okabe S, Imai Y, Fujiwara K, Nakamura Y, Hasegawa K (2016) T-LAK cell-originated protein kinase (TOPK) as a prognostic factor and a potential therapeutic target in ovarian cancer. Clin Cancer Res e-pub ahead of print 22 June 2016; pii:clincanres.0207.2016. [DOI] [PubMed]
- Joel M, Mughal AA, Grieg Z, Murrell W, Palmero S, Mikkelsen B, Fjerdingstad HB, Sandberg CJ, Behnan J, Glover JC, Langmoen IA, Stangeland B (2015) Targeting PBK/TOPK decreases growth and survival of glioma initiating cells in vitro and attenuates tumor growth in vivo. Mol Cancer 14(1): 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimoto K, Komatsu S, Ichikawa D, Arita T, Konishi H, Nagata H, Takeshita H, Nishimura Y, Hirajima S, Kawaguchi T, Shiozaki A, Fujiwara H, Okamoto K, Tsuda H, Otsuji E (2012) Overexpression of TRIM44 contributes to malignant outcome in gastric carcinoma. Cancer Sci 103(11): 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Li Y, Reddy K, Lee MH, Kim MO, Cho YY, Lee SY, Kim JE, Bode AM, Dong Z (2012) Novel TOPK inhibitor HI-TOPK-032 effectively suppresses colon cancer growth. Cancer Res 72(12): 3060–3068. [DOI] [PubMed] [Google Scholar]
- Komatsu S, Ichikawa D, Hirajima S, Nagata H, Nishimura Y, Kawaguchi T, Miyamae M, Okajima W, Ohashi T, Konishi H, Shiozaki A, Fujiwara H, Okamoto K, Tsuda H, Imoto I, Inazawa J, Otsuji E (2015) Overexpression of SMYD2 contributes to malignant outcome in gastric cancer. Br J Cancer 112(2): 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu S, Imoto I, Tsuda H, Kozaki KI, Muramatsu T, Shimada Y, Aiko S, Yoshizumi Y, Ichikawa D, Otsuji E, Inazawa J (2009) Overexpression of SMYD2 relates to tumor cell proliferation and malignant outcome of esophageal squamous cell carcinoma. Carcinogenesis 30(7): 1139–1146. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Park HJ, Choi YR, Kim A, Kim HW, Choi JH, Hwang CS, Lee SJ, Choi CI, Jeon TY, Kim DH, Kim GH, Park do Y (2016) PSMB8 and PBK as potential gastric cancer subtype-specific biomarkers associated with prognosis. Oncotarget 7(16): 21454–21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Abraham SC, Kim HS, Nam JH, Choi C, Lee MC, Park CS, Juhng SW, Rashid A, Hamilton SR, Wu TT (2002) Inverse relationship between APC gene mutation in gastric adenomas and development of adenocarcinoma. Am J Pathol 161(2): 611–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei B, Qi W, Zhao Y, Li Y, Liu S, Xu X, Zhi C, Wan L, Shen H (2015) PBK/TOPK expression correlates with mutant p53 and affects patients' prognosis and cell proliferation and viability in lung adenocarcinoma. Hum Pathol 46(2): 217–224. [DOI] [PubMed] [Google Scholar]
- Maesawa C, Tamura G, Suzuki Y, Ogasawara S, Sakata K, Kashiwaba M, Satodate R (1995) The sequential accumulation of genetic alterations characteristic of the colorectal adenoma-carcinoma sequence does not occur between gastric adenoma and adenocarcinoma. J Pathol 176(3): 249–258. [DOI] [PubMed] [Google Scholar]
- Martin RC 2nd, Jaques DP, Brennan MF, Karpeh M (2002) Extended local resection for advanced gastric cancer: increased survival versus increased morbidity. Ann Surg 236(2): 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto S, Abe Y, Fujibuchi T, Takeuchi T, Kito K, Ueda N, Shigemoto K, Gyo K (2004) Characterization of a MAPKK-like protein kinase TOPK. Biochem Biophys Res Commun 325(3): 997–1004. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Park JH, Miyamoto T, Yamamoto S, Hisada S, Alachkar H, Nakamura Y (2014) TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci Trans Med 6(259): 259ra145. [DOI] [PubMed] [Google Scholar]
- Nandi AK, Ford T, Fleksher D, Neuman B, Rapoport AP (2007) Attenuation of DNA damage checkpoint by PBK, a novel mitotic kinase, involves protein-protein interaction with tumor suppressor p53. Biochem Biophys Res Commun 358(1): 181–188. [DOI] [PubMed] [Google Scholar]
- Nandi AK, Rapoport AP (2006) Expression of PDZ-binding kinase (PBK) is regulated by cell cycle-specific transcription factors E2F and CREB/ATF. Leuk Res 30(4): 437–447. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Komatsu S, Ichikawa D, Nagata H, Hirajima S, Takeshita H, Kawaguchi T, Arita T, Konishi H, Kashimoto K, Shiozaki A, Fujiwara H, Okamoto K, Tsuda H, Otsuji E (2013) Overexpression of YWHAZ relates to tumor cell proliferation and malignant outcome of gastric carcinoma. Br J Cancer 108(6): 1324–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O Leary PC, Penny SA, Dolan RT, Kelly CM, Madden SF, Rexhepaj E, Brennan DJ, McCann AH, Ponten F, Uhlen M, Zagozdzon R, Duffy MJ, Kell MR, Jirstrom K, Gallagher WM (2013) Systematic antibody generation and validation via tissue microarray technology leading to identification of a novel protein prognostic panel in breast cancer. BMC Cancer 13: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oue N, Motoshita J, Yokozaki H, Hayashi K, Tahara E, Taniyama K, Matsusaki K, Yasui W (2002) Distinct promoter hypermethylation of p16INK4a, CDH1, and RAR-beta in intestinal, diffuse-adherent, and diffuse-scattered type gastric carcinomas. J Pathol 198(1): 55–59. [DOI] [PubMed] [Google Scholar]
- Park JH, Lin ML, Nishidate T, Nakamura Y, Katagiri T (2006) PDZ-binding kinase/T-LAK cell-originated protein kinase, a putative cancer/testis antigen with an oncogenic activity in breast cancer. Cancer Res 66(18): 9186–9195. [DOI] [PubMed] [Google Scholar]
- Park JH, Nishidate T, Nakamura Y, Katagiri T (2010) Critical roles of T-LAK cell-originated protein kinase in cytokinesis. Cancer Sci 101(2): 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WS, Oh RR, Park JY, Lee SH, Shin MS, Kim YS, Kim SY, Lee HK, Kim PJ, Oh ST, Yoo NJ, Lee JY (1999) Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res 59(17): 4257–4260. [PubMed] [Google Scholar]
- Shih MC, Chen JY, Wu YC, Jan YH, Yang BM, Lu PJ, Cheng HC, Huang MS, Yang CJ, Hsiao M, Lai JM (2012) TOPK/PBK promotes cell migration via modulation of the PI3K/PTEN/AKT pathway and is associated with poor prognosis in lung cancer. Oncogene 31(19): 2389–2400. [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63(1): 11–30. [DOI] [PubMed] [Google Scholar]
- Singh PK, Srivastava AK, Dalela D, Rath SK, Goel MM, Bhatt ML (2014) Expression of PDZ-binding kinase/T-LAK cell-originated protein kinase (PBK/TOPK) in human urinary bladder transitional cell carcinoma. Immunobiology 219(6): 469–474. [DOI] [PubMed] [Google Scholar]
- Sobin LH, Compton CC (2010) TNM seventh edition: what's new, what's changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer 116(22): 5336–5339. [DOI] [PubMed] [Google Scholar]
- Tsuda H (2008) Individualization of breast cancer based on histopathological features and molecular alterations. Breast Cancer 15(2): 121–132. [DOI] [PubMed] [Google Scholar]
- Ushijima T, Sasako M (2004) Focus on gastric cancer. Cancer Cell 5(2): 121–125. [DOI] [PubMed] [Google Scholar]
- Wagata T, Shibagaki I, Imamura M, Shimada Y, Toguchida J, Yandell DW, Ikenaga M, Tobe T, Ishizaki K (1993) Loss of 17p, mutation of the p53 gene, and overexpression of p53 protein in esophageal squamous cell carcinomas. Cancer Res 53(4): 846–850. [PubMed] [Google Scholar]
- Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY, Cunningham D, Rougier P, Komatsu Y, Ajani J, Emig M, Carlesi R, Ferry D, Chandrawansa K, Schwartz JD, Ohtsu A (2014) Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol 15(11): 1224–1235. [DOI] [PubMed] [Google Scholar]
- Zhu F, Zykova TA, Kang BS, Wang Z, Ebeling MC, Abe Y, Ma WY, Bode AM, Dong Z (2007) Bidirectional signals transduced by TOPK-ERK interaction increase tumorigenesis of HCT116 colorectal cancer cells. Gastroenterology 133(1): 219–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.