Abstract
Background:
The invasive tumour front may provide prognostic information. We examined the relationship between the presence of cancer stem cells (CSCs) at the invasive tumour front and prognosis in gastric cancer (GC).
Methods:
CD44 is a CSC marker; accordingly, CD44 standard (CD44s), CD44 variant-6 (CD44v6), and CD44 variant-9 (CD44v9) expression were examined in 123 resected primary GCs and the clinical significance of CSCs at the invasive tumour front was analysed.
Results:
Thirteen (10.6%), 79 (64.2%), and 47 (38.2%) GCs were CD44s-, CD44v6-, and CD44v9-positive, respectively. Patients with CD44-positive expression at the invasive tumour front had significantly poorer disease-specific survival than those with negative expression (CD44s: P<0.00001, CD44v6: P=0.013, CD44v9: P=0.0002). CD44s expression at the invasive tumour front was an independent prognostic factor in resectable GC patients (hazard ratio=3.13; 95% confidence interval, 1.09–9.01; P=0.035) and was significantly associated with peritoneal (P<0.001), lymphatic (P<0.001), and haematogenous recurrences (P=0.008). In addition, the number of CD44 isoforms expressed in cancer cells at the invasive tumour front was associated with patient prognosis. No conventional clinicopathological factors were independently associated with CD44 expression at the invasive tumour front.
Conclusions:
CD44-positive cancer stem-like cells at the invasive tumour front indicate poor survival and can be a unique biological prognostic factor for GC.
Keywords: CD44, cancer stem cells, prognosis, gastrectomy, recurrence, survival
Cancer stem cells (CSCs) are a subpopulation of tumour cells with the ability to initiate tumours as well as reconstitute the cellular heterogeneity typical of their tumours of origin (Schwitalla, 2014). CD44-positive fractions of gastric cancers (GCs) can generate spheroid colonies under non-adherent conditions, and small numbers of CD44-positive cells can generate tumours in severe combined immunodeficiency (SCID) mice (Takaishi et al, 2009), indicating that CD44-expressing cancer cells include CSCs. Several reports have suggested that CSCs can initiate and facilitate cancer progression by inducing cancer metastasis and therapeutic resistance in GC, resulting in poor survival (Klonisch et al, 2008; Ishigami et al, 2010; Wang et al, 2011).
CD44 is a family of transmembrane glycoprotein receptors that bind to hyaluronic acid, and the resultant intracellular signalling is linked to diverse cellular functions, including cell adhesion, migration, and invasion (Jackson et al, 1992; Sneath and Mangham, 1998). The gene encoding the CD44 standard isoform (CD44s) consists of 20 exons, wherein the distal extracellular domain is responsible for binding to hyaluronic acid and the proximal extracellular domain is the site of alternative mRNA splicing, which results in multiple variant isoforms (CD44v2–v10) (Sneath and Mangham, 1998). Among the variant isoforms, the roles of CD44 variant-6 (CD44v6) and CD44 variant-9 (CD44v9) are well-studied in GC. CD44v6-positive advanced GCs are associated with haematogenous metastasis, and the survival of CD44v6-positive patients is significantly poorer than that of CD44v6-negative patients (Yamaguchi et al, 2002). CD44v9 interacts with and stabilises xCT, a glutamate-cystine transporter, resulting in increased intracellular levels of reduced glutathione. CD44v9-positive cells have an enhanced ability to suppress reactive oxygen species (ROS) production, resulting in the therapeutic resistance, recurrence, and metastasis of tumours (Ishimoto et al, 2011; Tsugawa et al, 2012; Yae et al, 2012).
The invasive front of tumours has been proposed to contain important prognostic information owing to the lack of cohesiveness, secretion of proteolytic enzymes, reorganisation of the extracellular matrix, and increased cell proliferation (Rowe and Weiss, 2009; Sharma et al, 2013).
However, no studies have evaluated CD44 expression at the invasive tumour front (ITF). Here, we examined the expression and localisation of CD44 isoforms in GC tissues to determine the association between the presence of cancer stem-like cells at the ITF and the prognosis of patients with resectable GC. In order to evaluate the prognostic significance of CD44 isoforms based on immunohistochemical analyses of whole-tumour sections and the ITF, the primary end points were disease-specific survival and patterns of relapse in resectable GC patients, and the secondary end point was to stratify the survival risk of GC cases according to the prognostic analysis.
Materials and methods
Patients and study materials
One hundred and twenty-three primary gastric adenocarcinoma specimens were obtained from patients who underwent gastrectomy between January 2007 and December 2009 at the Shiga University of Medical Science Hospital, Japan. Specimens from patients with distant metastasis, peritoneal dissemination, or mucosal infiltration of the tumour (pT1(M)) were excluded. The histological type was determined as differentiated or undifferentiated; specifically, highly and moderately differentiated tubular or papillary adenocarcinomas were classified as the differentiated type, and poorly differentiated adenocarcinomas and signet-ring cell carcinomas were classified as the undifferentiated type. Mucinous adenocarcinomas were classified depending on other predominant elements. Tumour stage and pathological classification were defined according to the Japanese Gastric Cancer Association, 2011. Postoperative adjuvant chemotherapy was given to 51 patients with pathological T2–T4 tumours or lymph node metastasis. Among these 51 patients, 25 were treated with tegafur, 5-chloro-2,4-dihydroxypyridine, and oxonic acid (S1), 11 were treated with tegafur and uracil (UFT), 8 were treated with S1+docetaxel, 6 were treated with S1+irinotecan, and 1 was treated with S1+paclitaxel. The median follow-up time was 68 months (range: 1–97). A total of 22 patients died of GC-related causes during the study period. Peritoneal, lymphatic, and haematogenous recurrences occurred in 17, 8, and 9 cases, respectively. Written informed consent was obtained from all patients in accordance with the local ethical guidelines. The study was approved by the Institutional Review Board of the Shiga University of Medical Science.
Pathological examination
Formalin-fixed, paraffin-embedded tissue blocks of the resected GC specimens were cut into 3-μm-thick sections, deparaffinised, and rehydrated. Each section was stained with haematoxylin and eosin and then used for immunostaining. Immunohistochemical analyses were performed using an autostainer (Benchmark XT System; Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer's instructions. The following primary antibodies were used: primary antibodies against CD44s (H-CAM, 1 : 200 dilution; Novocastra Laboratories Ltd, Newcastle upon Tyne, UK), CD44v6 (VFF-18, 1 : 500 dilution; Abcam, Cambridge, UK), and CD44v9 (RV3, 1 : 5000 dilution; Cosmo Bio, Tokyo, Japan). Lymph nodes were used as the positive control for CD44s expression, and skin tissue was used as the positive control for CD44v6 and CD44v9 expression (Sneath and Mangham, 1998). The negative control was evaluated by substituting the primary antibodies with similarly diluted non-immunised mouse serum. Immunostaining examinations were assessed independently by two pathologists blinded to the clinical or pathological features. When there were discrepancies, a final decision was established by reassessment using a double-headed microscope.
The ITF was evaluated at the deepest invasive section of the tumour (Sentani et al, 2014). CD44s, CD44v6, and CD44v9 expression was semiquantitatively analysed in whole-tumour tissue sections or at the ITF as the percentage of positive tumour cells. The percentage of positive tumour cells was evaluated in low-power fields (× 40) in the whole-tumour section; subsequently, positive membrane staining of the tumour cells was confirmed at × 100 magnification. A result was defined as positive when >5% of cancer cells were positively stained and negative when <5% of the cancer cells were positively stained. This cut-off value of 5% was consistent with that used in the previous reports (Yasui et al, 1998; Okayama et al, 2009; Ryu et al, 2012; Wu et al, 2015). Tumour and stromal cells were identified based on the morphological features of the positive cells. When it was not clear whether positive cells were tumour cells or stromal cells using morphological methods, especially for poorly differentiated adenocarcinomas, immunostaining for cytokeratin (AE1/AE3, 1:10 dilution; Abcam, Cambridge, UK) was combined with morphological methods.
CD44 expression, initial recurrence pattern, and survival
Initial recurrence patterns, based on the first recurrent lesion detected during postoperative follow-up, were determined using computed tomography at intervals of at least 6 months. Recurrence patterns were classified into three types; peritoneal, lymphatic, and haematogenous (i.e., liver, lung, brain, and bone metastasis). Disease-specific survival was calculated from the date of gastric surgery to either the date of death from GC or the date of the last follow-up.
Statistical analysis
Univariate and multivariate analyses were performed using the Cox proportional hazards model to evaluate the associations between clinical covariates and disease-specific survival. Variables associated with survival with P<0.05 in a univariate analysis were used for a multivariate analysis. Kaplan–Meier survival curves were constructed to compare patients with positive and negative CD44 expression. Significant differences were determined using the log-rank test. Associations of clinicopathological variables with CD44s, CD44v6, or CD44v9 expression were evaluated using the χ2-test or Fisher's exact test, as appropriate. Variables that showed differences at P<0.10 were included in the multivariate logistic regression analyses. Cumulative incidence rates were calculated to evaluate the associations between initial recurrence patterns and CD44s, CD44v6, or CD44v9 expression. The cumulative incidence of each recurrence type was estimated using the competing-risk method, in which death from other causes was considered a competing risk. Comparisons between patients with positive and negative CD44 expression were performed using the Gray test. All P-values were two-sided, and the significance level was set at P<0.05. Statistical analyses, except the Gray test, were performed using SPSS 22.0 (SPSS Inc., Chicago, IL, USA). Gray tests were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Expression of CD44 isoforms in GC
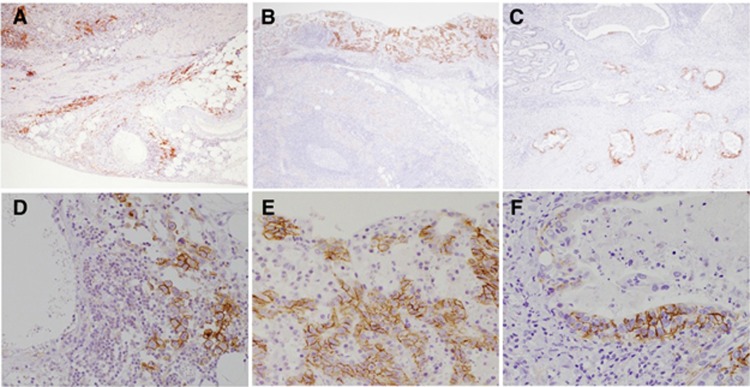
Patient and tumour characteristics are summarised in Table 1. Positive staining was observed for CD44s in 27 (22.0%) or 13 (10.6%) of the GC patients, positive staining for CD44v6 in 108 (87.8%) or 79 (64.2%) GC patients, and positive for CD44v9 in 72 (58.5%) or 47 (38.2%) GC patients in whole-tumour tissue sections or at the ITF, respectively. The immunohistochemical staining patterns of CD44s, CD44v6, and CD44v9 in whole-tumour sections and at the ITF of GC patients are shown in Figure 1. Membranous immunoreactivity was observed for CD44s, CD44v6, and CD44v9.
Table 1. Characteristics of patients with gastric cancer.
| Patients | (n=123) |
|---|---|
| Age(median, range) (years) | 70 (38–91) |
| Gender | |
| Male | 83 (67.5%) |
| Female | 40 (32.5%) |
| Tumour invasion | |
| pT1 (SM) | 53 (43.1%) |
| pT2 (MP) | 12 (9.8%) |
| pT3 (SS) | 26 (21.1%) |
| pT4a (SE) | 31 (25.2%) |
| pT4b (SI) | 1 (0.8%) |
| LN metastasis | |
| pN0 | 70 (56.9%) |
| pN1 | 19 (15.4%) |
| pN2 | 18 (14.6%) |
| pN3 | 16 (13.0%) |
| pStage | |
| I | 60 (48.8%) |
| II | 23 (18.7%) |
| III | 29 (23.6%) |
| IV | 11 (8.9%) |
| Lymphatic invasion | |
| Absent | 32 (26.0%) |
| Present | 91 (74.0%) |
| Blood vessel invasion | |
| Absent | 41 (33.3%) |
| Present | 82 (66.7%) |
| CD44s in whole-tumour section | |
| Negative | 96 (78.0%) |
| Positive | 27 (22.0%) |
| CD44v6 in whole-tumour section | |
| Negative | 15 (12.2%) |
| Positive | 108 (87.8%) |
| CD44v9 in whole-tumour section | |
| Negative | 51 (41.5%) |
| Positive | 72 (58.5%) |
| CD44s at invasive tumour front | |
| Negative | 110 (89.4%) |
| Positive | 13 (10.6%) |
| CD44v6 at invasive tumour front | |
| Negative | 44 (35.8%) |
| Positive | 79 (64.2%) |
| CD44v9 at invasive tumour front | |
| Negative | 76 (61.8%) |
| Positive | 47 (38.2%) |
Abbreviations: CD44s=CD44 standard isoform; CD44v6=CD44 variant isoform 6; CD44v9=CD44 variant isoform 9; LN=lymph node.
pT1 (SM): tumour invades the submucosa.
pT2 (MP): tumour invades the muscularis propria.
pT3 (SS): tumour invades the subserosa.
pT4a (SE): tumour invasion is contiguous with or extends beyond the serosa.
pT4b (SI): tumour invades adjacent structures.
pN0: no regional lymph node metastasis.
pN1: metastasis in 1–2 regional lymph nodes.
pN2: metastasis in 3–6 regional lymph nodes.
pN3: metastasis in 7 or more regional lymph nodes.
Eleven patients showed CY1, accordingly were classified as Stage IV.
Figure 1.
CD44s, CD44v6, and CD44v9 expression in primary GC by IHC staining. Representative images from three cases for the expression of each CD44 isoform are shown. (A and D), (B and E), and (C and F) are from the same patient. (A) Positive staining for CD44s both in whole tumour sections and at the ITF in GC with serosal exposure. (original magnification: × 40); (B) Positive staining for CD44v6 at the mucosal layer, but negative staining at the ITF of submucosal invasive GC. (C) Negative staining for CD44v9 in the surface layer, but positive staining at the ITF of muscularis propria invasive GC. (original magnification: × 40). In the high-power field, the expression of each CD44 isoform was identified along the membrane of cancer cells (D–F). (original magnification: × 200). Abbreviations: CD44s=CD44 standard isoform; CD44v6=CD44 variant isoform 6; CD44v9=CD44 variant isoform 9; GC=gastric cancer; IHC=immunohistochemistry; ITF=invasive tumour front.
CD44 isoform expression and patient outcome
To evaluate the prognostic potential of the presence of cancer stem-like cells at the ITF, we examined the relationship between CD44 isoform expression in whole-tissue sections or at the ITF and disease-specific survival (Table 2). A univariate Cox analysis showed that stage (hazard ratio (HR)=6.84; 95% confidence interval (CI), 2.67–17.54; P<0.0001), blood vessel invasion (HR=12.5; 95% CI, 1.67–90.90; P=0.014), tumour size >5 cm (HR=4.18; 95% CI, 1.63–10.75; P=0.003), CD44v9 expression in whole-tissue sections (HR=2.87; 95% CI, 1.06–7.75; P=0.039), CD44s expression at the ITF (HR=6.80; 95% CI, 2.73–16.95; P<0.0001), CD44v6 expression at the ITF (HR=4.15; 95% CI, 1.23–14.08; P=0.022), and CD44v9 expression at the ITF (HR=4.65; 95% CI, 1.89–11.49; P=0.001) were associated with disease-specific survival. These results suggest that CD44 isoform expression at the ITF was a strong prognostic factor for GC. Furthermore, a multivariate Cox analysis revealed that CD44s expression at the ITF was a poor independent prognostic factor (HR=3.13; 95% CI, 1.09–9.01; P=0.035).
Table 2. Relationship between clinicopathological variables and disease-specific survival.
|
Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|
Gender | ||||
| Male | 1 (reference) | |||
| Female | 0.711 (0.413–1.222) | 0.216 | ||
|
Age | ||||
| <65 years | 1 (reference) | |||
| ⩾65 years | 1.404 (0.600–3.29) | 0.433 | ||
|
pStage | ||||
| StageI/StageII | 1 (reference) | 1 (reference) | ||
| StageIII/StageIV | 6.84 (2.67–17.54) | <0.0001a | 3.12 (0.75–12.9) | 0.118 |
|
Lympatic invasion | ||||
| Negative | 1 (reference) | |||
| Positive | 37.18 (0.875–1611) | 0.06 | ||
|
Blood vessel invasion | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 12.5 (1.67–90.90) | 0.014a | 6.02 (0.72–50.0) | 0.098 |
|
Tumour size | ||||
| ⩽5 cm | 1 (reference) | 1 (reference) | ||
| >5 cm | 4.18(1.63–10.75) | 0.003a | 1.13 (0.28–4.63) | 0.291 |
|
Histological type | ||||
| Differentiated | 1 (reference) | |||
| Undifferentiated | 2.13(0.917–4.923) | 0.079 | ||
|
CD44s expression in whole-tumour section | ||||
| Negative | 1 (reference) | |||
| Positive | 2.28 (0.96–5.45) | 0.063 | ||
|
CD44v6 expression in whole-tumour section | ||||
| Negative | 1 (reference) | |||
| Positive | 24.49 (0.09–6591) | 0.263 | ||
|
CD44v9 expression in whole-tumour section | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 2.87 (1.06–7.75) | 0.039a | 1.10 (0.21–5.78) | 0.912 |
|
CD44s expression at invasive tumour front | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 6.80 (2.73–16.95) | <0.0001a | 3.13 (1.09–9.01) | 0.035a |
|
CD44v6 expression at invasive tumour front | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 4.15 (1.23–14.08) | 0.022a | 1.35 (0.35–5.26) | 0.667 |
|
CD44v9 expression at invasive tumour front | ||||
| Negative | 1 (reference) | 1 (reference) | ||
| Positive | 4.65 (1.89–11.49) | 0.001a | 2.51 (0.49–12.8) | 0.292 |
Abbreviations: CD44s=CD44 standard isoform; CD44v6=CD44 variant isoform 6; CD44v9=CD44 variant isoform 9; CI=confidence interval; HR=hazard ratio.
Eleven patients showed CY1, accordingly were classified as Stage IV.
Statistically significant.
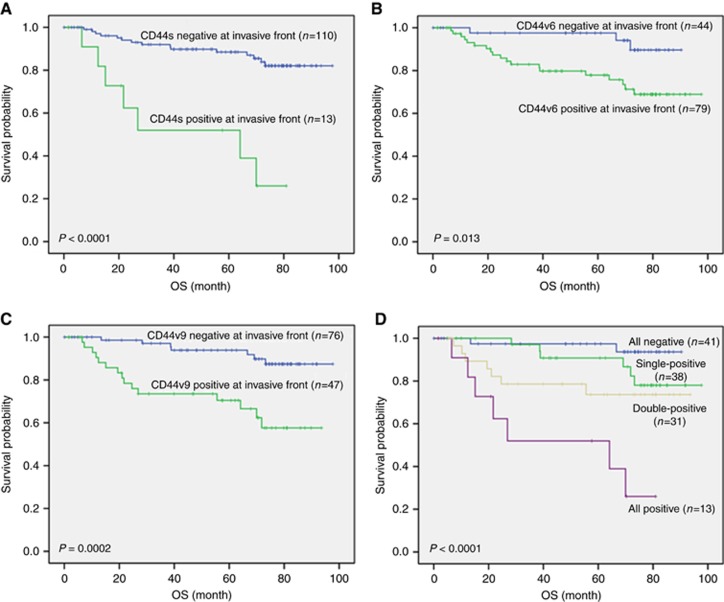
Kaplan–Meier survival curves according to the expression of each CD44 isoform at the ITF are shown in Figure 2. Patients with CD44s-, CD44v6-, or CD44v9-positive expression at the ITF showed significantly poorer survival than those with negative expression (P<0.0001, P=0.013, and P=0.0002, respectively) (Figure 2A–C).
Figure 2.
Disease-specific survival calculated by the Kaplan—Meier method in patients who underwent gastrectomy for resectable gastric cancer (GC). (A) disease-specific survival in patients with or without CD44s expression at the invasive tumour front (ITF); (B) disease-specific survival in patients with or without CD44v6 expression at the ITF; (C) disease-specific survival in patients with or without CD44v9 expression at the ITF; (D) disease-specific survival in patients with the expression of more than one CD44 isoforms at the ITF; all-negative: patients who stained negative for all the CD44 isoforms at the ITF; single-positive: patients showing positive staining for one CD44 isoform at the ITF; double-positive: patients showing positive staining for two CD44 isoforms at the ITF; all-positive: patients showing positive staining for all the CD44 isoforms at the ITF.
Predictive model for survival
To generate a detailed predictive model for patients with GC surgery, we stratified patients based on the expression of each CD44 isoform at the ITF (Figure 2D). There were significant differences in survival among the four groups of patients: patients with negative expression for all CD44 isoforms (all-negative) (n=41), patients with positive expression for one CD44 isoform (single-positive) (n=38), patients with positive expression for two CD44 isoforms (double-positive) (n=31), and patients with positive expression for all CD44 isoforms (all-positive) (n=13) (P<0.0001). The all-positive group showed the worst survival, that is, a 51.9% 5-year survival rate; the double-positive and single-positive groups showed 73.7% and 90.8% 5-year survival rates, respectively, and the all-negative group showed the best 5-year survival rate, that is, 97.4%. This stratified model based on the number of the CD44 isoforms detected predicts GC patient survival.
CD44 expression at the ITF and clinicopathological features
To clarify the factors affecting the presence of cancer stem-like cells at the ITF, we examined the correlation between conventional clinicopathological variables and CD44 isoform expression at the ITF. Univariate analyses revealed that CD44s expression at the ITF was not correlated with any clinicopathological variables (Table 3a). However, CD44v6 expression at the ITF was significantly correlated with the depth of tumour invasion (P=0.004), lymphatic invasion (P=0.005), and blood vessel invasion (P=0.011) (Table 3b). CD44v9 expression at the ITF was significantly correlated with tumour size (P=0.036) (Table 3c). However, according to a subsequent multivariate logistic regression analysis, no independent clinicopathological factors were associated with the expression of any CD44 isoform at the ITF.
Table 3. Relationship between CD44 isoform expression at the invasive tumour front and clinicopathological variables.
|
(a)
CD44s expression at invasive tumour front | ||||||
|---|---|---|---|---|---|---|
| Positive | Negative |
Multivariate analysis |
||||
| Variables | n=13 (10.6%) | n=110 (89.4%) | P-value | Odds ratio | 95% CI | P-value |
|
Gender | ||||||
| Male | 10(12.0%) | 73(88.0%) | ||||
| Female | 3(7.5%) | 37(92.5%) | 0.442 | |||
|
Age | ||||||
| <65 years | 5(12.8%) | 34(87.2%) | ||||
| ⩾65 years | 8(9.5%) | 76(90.5%) | 0.58 | |||
|
Depth of invasion | ||||||
| pT1/pT2 | 6(9.2%) | 59(90.8%) | ||||
| pT3/pT4 | 7(12.1%) | 51(87.9%) | 0.609 | |||
|
LN metastasis | ||||||
| pN0 | 5(7.1%) | 65(92.9%) | ||||
| pN1/pN2/pN3 | 8(15.1%) | 45(84.9%) | 0.155 | |||
|
Lympatic invasion | ||||||
| Negative | 3(9.4%) | 29(90.6%) | ||||
| Positive | 10(11.0%) | 81(89.0%) | 0.798 | |||
|
Blood vessel invasion | ||||||
| Negative | 2(4.9%) | 39(95.1%) | ||||
| Positive | 11(13.4%) | 71(866%) | 0.147 | |||
|
Tumour size | ||||||
| <5 cm | 6(8.7%) | 63(91.3%) | ||||
| ⩾5 cm | 7(13.0%) | 47(87.0%) | 0.423 | |||
|
Histological type | ||||||
| Differentiated | 6(8.2%) | 67(91.8%) | ||||
| Undifferentiated | 7(14.0%) | 43(86.0%) | 0.306 | |||
|
(b)
CD44v6 expression at invasive tumour front | ||||||
|---|---|---|---|---|---|---|
| Positive | Negative |
Multivariate analysis |
||||
| Variables | n=79 (64.2%) | n=44 (35.8%) | P-value | Odds ratio | 95% CI | P-value |
|
Gender | ||||||
| Male | 51(61.4%) | 32(38.6%) | ||||
| Female | 28(70.0%) | 12(30.0%) | 0.354 | |||
|
Age | ||||||
| <65 years | 25(64.1%) | 14(35.9%) | ||||
| ⩾65 years | 54(64.3%) | 30(35.7%) | 0.984 | |||
|
Depth of invasion | ||||||
| pT1/pT2 | 34(52.3%) | 31(47.7%) | ||||
| pT3/pT4 | 45(77.6%) | 13(22.4%) | 0.004a | 2.4 | 0.77–7.52 | 0.131 |
|
LN metastasis | ||||||
| pN0 | 40(57.1%) | 30(42.9%) | ||||
| pN1/pN2/pN3 | 39(73.6%) | 14(26.4%) | 0.06 | 1.34 | 0.431–4.15 | 0.614 |
|
Lympatic invasion | ||||||
| Negative | 14(43.8%) | 18(56.3%) | ||||
| Positive | 65(71.4%) | 26(28.6%) | 0.005a | 1.81 | 0.59–5.52 | 0.3 |
|
Blood vessel invasion | ||||||
| Negative | 20(48.8%) | 21(51.2%) | ||||
| Positive | 59(72.0%) | 23(28.0%) | 0.011a | 1.41 | 0.51–3.94 | 0.508 |
|
Tumour size | ||||||
| <5 cm | 40(58.8%) | 29(42.0%) | ||||
| ⩾5 cm | 39(72.2%) | 15(27.8%) | 0.118 | |||
|
Histological type | ||||||
| Differentiated | 44(60.3%) | 29(39.7%) | ||||
| Undifferentiated | 35(70.0%) | 15(30.0%) | 0.269 | |||
|
(c)
CD44v9 expression at invasive tumour front | ||||||
|---|---|---|---|---|---|---|
| Positive | Negative |
Multivariate analysis |
||||
| Variables | n=47 (38.2%) | n=76 (61.8%) | P-value | Odds ratio | 95% CI | P-value |
|
Gender | ||||||
| Male | 30(36.1%) | 53(63.9%) | ||||
| Female | 17(42.5%) | 23(57.5%) | 0.497 | |||
|
Age | ||||||
| <65 years | 10(25.6%) | 29(74.4%) | ||||
| ⩾65 years | 37(44.0%) | 47(56.0%) | 0.051 | 2.07 | 0.87–4.89 | 0.099 |
|
Depth of invasion | ||||||
| pT1/pT2 | 20(30.8%) | 45(69.2%) | ||||
| pT3/pT4 | 27(46.6%) | 31(53.4%) | 0.072 | 1.23 | 0.43–3.48 | 0.701 |
|
LN metastasis | ||||||
| pN0 | 23(32.9%) | 47(67.1%) | ||||
| pN1/pN2/pN3 | 24(45.3%) | 29(54.7%) | 0.16 | |||
|
Lympatic invasion | ||||||
| Negative | 8(25.0%) | 24(75.0%) | ||||
| Positive | 39(42.9%) | 52(57.1%) | 0.074 | 1.38 | 0.46–4.17 | 0.569 |
|
Blood vessel invasion | ||||||
| Negative | 14(34.1%) | 27(65.9%) | ||||
| Positive | 33(40.2%) | 49(59.8%) | 0.512 | |||
|
Tumour size | ||||||
| <5 cm | 21(30.4%) | 48(69.6%) | ||||
| ⩾5 cm | 26(48.1%) | 28(51.9%) | 0.036a | 1.57 | 0.59–4.15 | 0.365 |
|
Histological type | ||||||
| Differentiated | 26(35.6%) | 47(64.4%) | ||||
| Undifferentiated | 21(42.0%) | 29(58.0%) | 0.474 | |||
Abbreviations: CD44s=CD44 standard isoform; CD44v6=CD44 variant isoform 6; CD44v9=CD44 variant isoform 9; CI=confidence interval; LN=lymph node.
Statistically significant.
CD44 expression at the ITF and recurrence
We examined whether the expression of each CD44 isoform at the ITF was associated with recurrence after surgery for resectable GC. As summarised in Table 4, the cumulative recurrence rate at 5 years for each recurrence type was significantly higher in patients with CD44s-positive expression at the ITF than in patients with CD44s-negative expression at the ITF (peritoneal recurrence: 43.4%, 95% CI, 14.1–70.0% vs 5.8% 95% CI, 2.4–11.5%, P<0.001; lymphatic recurrence: 51.3%, 95% CI, 12.1–80.9% vs 4.3%, 95% CI, 1.4–10.0%, P<0.001; haematogenous recurrence: 29.4%, 95% CI, 5.4–59.7% vs 6.2% 95% CI, 2.5–12.2%, P=0.008). The cumulative peritoneal recurrence rate at 5 years was significantly higher in patients with CD44v6- or CD44v9-positive expression at the ITF than in those with negative expression (CD44v6 expression: 13.8%, 95% CI, 7.0–22.8% vs 2.4%, 95% CI, 0.2–11.2%, P=0.007; CD44v9 expression: 21.2%, 95% CI, 10.3–34.6% vs 2.9% 95% CI, 0.5–9.0%, P=0.041).
Table 4. 5-year cumulative incidence of each recurrence pattern for GC patients with and without CD44 isoform expression at the invasive tumour front.
|
CD44s expression at invasive tumour front |
CD44v6 expression at invasive tumour front |
CD44v9 expression at invasive tumour front |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Positive | Negative | ||||
| Recurrence pattern | % (95% CI) | % (95% CI) | P-value | % (95% CI) | % (95% CI) | P-value | % (95% CI) | % (95% CI) | P-value |
| Peritoneal recurrence rate | 43.4% (14.1–70.0%) | 5.8% (2.4–11.5%) | <0.001a | 13.8% (7.0–22.8%) | 2.4% (0.2–11.2%) | 0.007a | 21.2% (10.3–34.6%) | 2.9% (0.5–9.0%) | 0.041a |
| Lymphatic recurrence rate | 51.3% (12.1–80.9%) | 4.3% (1.4–10.0%) | <0.001a | 11.0% (4.8–20.1%) | 2.8% (0.2–12.8%) | 0.108 | 14.6% (5.2–28.6%) | 4.6% (1.2–11.7%) | 0.062 |
| Haematogenous recurrence rate | 29.4% (5.4–59.7%) | 6.2% (2.5–12.2%) | 0.008a | 8.7% (3.5–16.9%) | 7.3% (1.8–18.1%) | 0.719 | 12.9% (4.6–25.6%) | 5.7% (1.8–13.0%) | 0.156 |
Abbreviations: CD44s=CD44 standard isoform; CD44v6=CD44 variant isoform 6; CD44v9=CD44 variant isoform 9; CI=confidence interval; GC=gastric cancer.
Statistically significant.
Discussion
The results of this study showed that the presence of CD44-positive cancer stem-like cells at the ITF, independent of any conventional clinicopathological factors, is strongly correlated with poor survival of patients with resectable GC.
Cancer stem cells are a malignant subset found in hierarchically organised tumours and are capable of tumour initiation, self-renewal, and differentiation to the bulk of non-tumorigenic cancer cells. CD44 is a CSC surface marker in several cancers, including GC (Al-Hajj et al, 2003; Collins et al, 2005; Dalerba et al, 2007; Takaishi et al, 2009). Furthermore, in GC, CD44 variant isoforms have recently been reported to affect tumour initiation and cancer cell maintenance (Ishimoto et al, 2010), and may play a functional role in the protection of CSCs from high levels of ROS in the tumour microenvironment (Ishimoto et al, 2011). Several studies have reported that CD44 isoform expression in GC suggests a poor prognosis (Wakamatsu et al, 2012; Cao et al, 2014; Xie et al, 2015); however, most studies of CSC marker expression in tumours assess the intensity of expression or proportion of cells expressing CSC markers using whole-tumour sections or tissue microarrays. The ITF, the deepest and most invasive area of the tumour, has been reported to contain useful prognostic information. In addition, various critical molecular events that regulate tumour proliferation, adhesion/migration, and angiogenesis occur in the ITF (Bankfalvi and Piffko, 2000; Rowe and Weiss, 2009; Sharma et al, 2013). Accordingly, the ITF is mainly responsible for the clinical behaviour of the tumour (Khanh do et al, 2011; Tanaka et al, 2014). However, it is not clear whether the presence of CSCs at the ITF has clinical significance. In our study, CD44v9 expression in the whole-tumour sections was associated with survival, and the expression of all tested CD44 isoforms at the ITF was strongly associated with survival (CD44s: P<0.00001, CD44v6: P=0.013, CD44v9: P=0.0002). These findings show that CD44-positive cancer stem-like cells at the ITF are more important prognostic factors than those in whole-tumour sections. Moreover, a multivariate logistic regression analysis showed that the expression of each CD44 isoform at the ITF was independent of conventional clinicopathological factors, suggesting that the expression of CD44 isoforms at the ITF is a distinctive prognostic factor. Additional studies are needed to reveal the association between the expression of CD44 isoforms and molecular or morphological changes in tumours at the ITF.
Epithelial-mesenchymal transition (EMT) is an important step in cancer invasion and metastasis (Wu and Zhou, 2008; Voulgari and Pintzas, 2009). A switch from CD44v to CD44s is essential for EMT and tumour progression in breast cancer (Brown et al, 2011). Furthermore, the ratio of vimentin/E-cadherin mRNA expression is significantly correlated with the CD44s/CD44v9 ratio in colorectal cancer, indicating that CD44 switching might induce EMT (Mashita et al, 2014). These findings suggest that CD44s confers mesenchymal properties, unlike other CD44-variants, and is associated with metastasis. Our data also showed that CD44s expression at the ITF is associated with peritoneal, lymphatic, and haematogenous recurrences, suggesting that CD44s-expressing cancer stem-like cells at the ITF are likely to metastasise in various directions. Moreover, CD44s expression was also recognised an independent prognostic factor. These findings support the hypothesis that CD44s switching affects EMT and metastasis in GC; however, experimental verification is still needed to conclusively evaluate the hypothesis.
We recently observed cancer cells that disseminated into the peritoneal cavity during curative gastrectomy in GC; moreover, these cancer cells have proliferative and tumorigenic capacity, which could result in peritoneal metastasis (Takebayashi et al, 2014). We also recently confirmed the presence of disseminated CD44s-expressing cancer stem-like cells during gastrectomy (Murata et al, unpublished data). In addition, we detected viable, proliferative, and clustered cancer cells, including CD44s-expressing cancer stem-like cells, in remnant gastric lumens immediately before gastrointestinal reconstruction (Murata et al, 2016). In this study, the presence of CD44s-expressing tumour cells at the invasive front of the gastric wall was significantly correlated with peritoneal recurrences and poor survival. Cancer stem-like cells may spill out easily during resection when they are present at the ITF; CD44s-positive cancer cells that have mesenchymal properties may easily leave the ITF of the gastric wall and disseminate into the peritoneal cavity.
When patients were classified based on the number of CD44 isoforms expressed at the ITF, we found that the prognosis was worse in patients expressing more CD44 isoforms. This result suggests that each CD44 isoform in GC may have a distinct function. GC frequently exhibits marked histological heterogeneity with various differentiated cancer cells, and these might be produced by different undifferentiated cells with the properties of each CSC. Therefore, the functional role of each CD44 isoform in the tumour differentiation pathway, heterogeneity, and therapeutic resistance should be elucidated in the future. All patients with CD44s-positive cells in this study also had CD44v6- and CD44v9-positive cells at the ITF, and GC patients with CD44s-positive cells at the ITF had the worst prognosis in the predictive model for survival. The number of CD44 isoforms expressed was an indicator of patient outcome. In particular, the all-negative group showed the best survival among all predictive model groups. These findings suggest that both the expression of CD44s itself and the number of CD44 isoforms expressed have prognostic value in GC patients. From a clinical perspective, patients with positive expression for all CD44 isoforms at the ITF have a very poor prognosis, suggesting the need for more intensive adjuvant chemotherapy. Patients with negative expression of all CD44 isoforms at the ITF have extremely good outcomes, suggesting that adjuvant chemotherapy is not necessary after gastrectomy. To verify the utility of these biomarkers, that is, CD44 isoforms, at the ITF for making treatment decisions, clinical studies are needed.
In conclusion, the presence of CD44-expressing cancer stem-like cells at the ITF was significantly associated with poor survival in GC patients, indicating that CD44 isoforms are promising biomarkers for GC.
Acknowledgments
We thank Mrs Ikuko Arikawa and Mrs Miho Yamamoto for their excellent technical assistance. We also thank Mrs Ryoko Tanaka and Mrs Masami Yamato for their help with clinical data preparation. We are also grateful to Mr Yoshimitsu Miyahira at the Department of Clinical Laboratory Medicine for his valuable assistance with cell staining. We would like to thank Editage (www.editage.jp) for English language editing. This work was supported by JSPS KAKENHI Grant Number JP15K10096.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF (2003) Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA 100(7): 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankfalvi A, Piffko J (2000) Prognostic and predictive factors in oral cancer: the role of the invasive tumour front. J Oral Pathol Med 29(7): 291–298. [DOI] [PubMed] [Google Scholar]
- Brown RL, Reinke LM, Damerow MS, Perez D, Chodosh LA, Yang J, Cheng C (2011) CD44 splice isoform switching in human and mouse epithelium is essential for epithelial-mesenchymal transition and breast cancer progression. J Clin Invest 121(3): 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Cao D, Jin M, Jia Z, Kong F, Ma H, Wang Y, Jiang J (2014) CD44 but not CD24 expression is related to poor prognosis in non-cardia adenocarcinoma of the stomach. BMC Gastroenterol 14: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ (2005) Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 65(23): 10946–10951. [DOI] [PubMed] [Google Scholar]
- Dalerba P, Dylla SJ, Park IK, Liu R, Wang X, Cho RW, Hoey T, Gurney A, Huang EH, Simeone DM, Shelton AA, Parmiani G, Castelli C, Clarke MF (2007) Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA 104(24): 10158–10163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami S, Ueno S, Arigami T, Uchikado Y, Setoyama T, Arima H, Kita Y, Kurahara H, Okumura H, Matsumoto M, Kijima Y, Natsugoe S (2010) Prognostic impact of CD133 expression in gastric carcinoma. Anticancer Res 30(6): 2453–2457. [PubMed] [Google Scholar]
- Ishimoto T, Nagano O, Yae T, Tamada M, Motohara T, Oshima H, Oshima M, Ikeda T, Asaba R, Yagi H, Masuko T, Shimizu T, Ishikawa T, Kai K, Takahashi E, Imamura Y, Baba Y, Ohmura M, Suematsu M, Baba H, Saya H (2011) CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell 19(3): 387–400. [DOI] [PubMed] [Google Scholar]
- Ishimoto T, Oshima H, Oshima M, Kai K, Torii R, Masuko T, Baba H, Saya H, Nagano O (2010) CD44+ slow-cycling tumor cell expansion is triggered by cooperative actions of Wnt and prostaglandin E2 in gastric tumorigenesis. Cancer Sci 101(3): 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DG, Buckley J, Bell JI (1992) Multiple variants of the human lymphocyte homing receptor CD44 generated by insertions at a single site in the extracellular domain. J Biol Chem 267(7): 4732–4739. [PubMed] [Google Scholar]
- Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2): 101–112. [DOI] [PubMed] [Google Scholar]
- Khanh do T, Mekata E, Mukaisho K, Sugihara H, Shimizu T, Shiomi H, Murata S, Naka S, Yamamoto H, Endo Y, Tani T (2011) Prognostic role of CD10(+) myeloid cells in association with tumor budding at the invasion front of colorectal cancer. Cancer Sci 102(9): 1724–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonisch T, Wiechec E, Hombach-Klonisch S, Ande SR, Wesselborg S, Schulze-Osthoff K, Los M (2008) Cancer stem cell markers in common cancers - therapeutic implications. Trends Mol Med 14(10): 450–460. [DOI] [PubMed] [Google Scholar]
- Mashita N, Yamada S, Nakayama G, Tanaka C, Iwata N, Kanda M, Kobayashi D, Fujii T, Sugimoto H, Koike M, Nomoto S, Fujiwara M, Kodera Y (2014) Epithelial to mesenchymal transition might be induced via CD44 isoform switching in colorectal cancer. J Surg Oncol 110(6): 745–751. [DOI] [PubMed] [Google Scholar]
- Murata S, Yamamoto H, Yamaguchi T, Kaida S, Ishida M, Kodama H, Takebayashi K, Shimizu T, Miyake T, Tani T, Kushima R, Tani M (2016) Viable cancer cells in the remnant stomach are a potential source of peritoneal metastasis after curative distal gastrectomy for gastric cancer. Ann Surg Oncol 23(9): 2920–2927. [DOI] [PubMed] [Google Scholar]
- Okayama H, Kumamoto K, Saitou K, Hayase S, Kofunato Y, Sato Y, Miyamoto K, Nakamura I, Ohki S, Sekikawa K, Takenoshita S (2009) CD44v6, MMP-7 and nuclear Cdx2 are significant biomarkers for prediction of lymph node metastasis in primary gastric cancer. Oncol Rep 22(4): 745–755. [DOI] [PubMed] [Google Scholar]
- Rowe RG, Weiss SJ (2009) Navigating ECM barriers at the invasive front: the cancer cell-stroma interface. Annu Rev Cell Dev Biol 25: 567–595. [DOI] [PubMed] [Google Scholar]
- Ryu HS, Park DJ, Kim HH, Kim WH, Lee HS (2012) Combination of epithelial-mesenchymal transition and cancer stem cell-like phenotypes has independent prognostic value in gastric cancer. Hum Pathol 43(4): 520–528. [DOI] [PubMed] [Google Scholar]
- Schwitalla S (2014) Tumor cell plasticity: the challenge to catch a moving target. J Gastroenterol 49(4): 618–627. [DOI] [PubMed] [Google Scholar]
- Sentani K, Matsuda M, Oue N, Uraoka N, Naito Y, Sakamoto N, Yasui W (2014) Clinicopathological significance of MMP-7, laminin gamma2 and EGFR expression at the invasive front of gastric carcinoma. Gastric Cancer 17(3): 412–422. [DOI] [PubMed] [Google Scholar]
- Sharma M, Sah P, Sharma SS, Radhakrishnan R (2013) Molecular changes in invasive front of oral cancer. J Oral Maxillofac Pathol 17(2): 240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneath RJ, Mangham DC (1998) The normal structure and function of CD44 and its role in neoplasia. Mol Pathol 51(4): 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi S, Okumura T, Tu S, Wang SS, Shibata W, Vigneshwaran R, Gordon SA, Shimada Y, Wang TC (2009) Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells 27(5): 1006–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi K, Murata S, Yamamoto H, Ishida M, Yamaguchi T, Kojima M, Shimizu T, Shiomi H, Sonoda H, Naka S, Mekata E, Okabe H, Tani T (2014) Surgery-induced peritoneal cancer cells in patients who have undergone curative gastrectomy for gastric cancer. Ann Surg Oncol 21(6): 1991–1997. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Shimura T, Kitajima T, Kondo S, Ide S, Okugawa Y, Saigusa S, Toiyama Y, Inoue Y, Araki T, Uchida K, Mohri Y, Kusunoki M (2014) Tropomyosin-related receptor kinase B at the invasive front and tumour cell dedifferentiation in gastric cancer. Br J Cancer 110(12): 2923–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, Nagano O, Matsuzaki J, Hibi T (2012) Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe 12(6): 764–777. [DOI] [PubMed] [Google Scholar]
- Voulgari A, Pintzas A (2009) Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 1796(2): 75–90. [DOI] [PubMed] [Google Scholar]
- Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y, Uraoka N, Anami K, Sentani K, Oue N, Yasui W (2012) Expression of cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and lymph node metastasis of gastric cancer. Pathol Int 62(2): 112–119. [DOI] [PubMed] [Google Scholar]
- Wang T, Ong CW, Shi J, Srivastava S, Yan B, Cheng CL, Yong WP, Chan SL, Yeoh KG, Iacopetta B, Salto-Tellez M (2011) Sequential expression of putative stem cell markers in gastric carcinogenesis. Br J Cancer 105(5): 658–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Li Z, Zhang C, Yu K, Teng Z, Zheng G, Wang S, Liu Y, Cui L, Yu X (2015) CD44 family proteins in gastric cancer: a meta-analysis and narrative review. Int J Clin Exp Med 8(3): 3595–3606. [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhou BP (2008) New insights of epithelial-mesenchymal transition in cancer metastasis. Acta Biochim Biophys Sin 40(7): 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JW, Chen PC, Zheng CH, Li P, Wang JB, Lin JX, Lu J, Chen QY, Cao LL, Lin M, Lin Y, Huang CM (2015) Evaluation of the prognostic value and functional roles of CD44v6 in gastric cancer. J Cancer Res Clin Oncol 141(10): 1809–1817. [DOI] [PubMed] [Google Scholar]
- Yae T, Tsuchihashi K, Ishimoto T, Motohara T, Yoshikawa M, Yoshida GJ, Wada T, Masuko T, Mogushi K, Tanaka H, Osawa T, Kanki Y, Minami T, Aburatani H, Ohmura M, Kubo A, Suematsu M, Takahashi K, Saya H, Nagano O (2012) Alternative splicing of CD44 mRNA by ESRP1 enhances lung colonization of metastatic cancer cell. Nat Commun 3: 883. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K (2002) Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol 79(4): 230–235. [DOI] [PubMed] [Google Scholar]
- Yasui W, Kudo Y, Naka K, Fujimoto J, Ue T, Yokozaki H, Tahara E (1998) Expression of CD44 containing variant exon 9 (CD44v9) in gastric adenomas and adenocarcinomas: relation to the proliferation and progression. Int J Oncol 12(6): 1253–1258. [DOI] [PubMed] [Google Scholar]