Abstract
Background:
Organ preservation has been proposed as an alternative to radical surgery for rectal cancer to reduce morbidity and mortality, and to improve functional outcome.
Methods:
Locally advanced non-metastatic rectal cancers were identified from a prospective database. Patients staged ⩾T3 or any stage N+ were referred for neoadjuvant chemoradiotherapy (CRT) (50–54 Gy and 5-fluorouracil), and were reassessed 6–8 weeks post treatment. An active surveillance programme (‘watch and wait') was offered to patients who were found to have a complete endoluminal response. Transanal excision was performed in patients who were found to have an objective clinical response and in whom a residual ulcer measured ⩽3 cm. Patients were followed up clinically, endoscopically and radiologically to assess for local recurrence or disease progression.
Results:
Of 785 patients with rectal cancer between 2005 and 2015, 362 had non-metastatic locally advanced tumours treated with neoadjuvant CRT. Sixty out of three hundred and sixty-two (16.5%) patients were treated with organ-preserving strategies – 10 with ‘watch and wait' and 50 by transanal excision. Fifteen patients were referred for salvage total mesorectal excision post local excision owing to adverse pathological findings. There was no significant difference in overall survival (85.6% vs 93.3%, P=0.414) or disease-free survival rate (78.3% vs 80%, P=0.846) when the outcomes of radical surgery were compared with organ preservation. Tumour regrowth occurred in 4 out of 45 (8.9%) patients who had organ preservation.
Conclusions:
Organ preservation for locally advanced rectal cancer is feasible for selected patients who achieve an objective endoluminal response to neoadjuvant CRT. Transanal excision defines the pathological response and refines decision-making.
Keywords: rectal cancer, neoadjuvant therapy, chemotherapy, radiotherapy, local excision, organ preservation, watch and wait
Surgery to remove the mesorectum is the standard of care for patients with locally advanced rectal cancer (Heald et al, 1982). In locally advanced disease, when neoadjuvant chemoradiotherapy (CRT) is combined with radical surgery, very low rates of local recurrence are reported (Sauer et al, 2004; van Gijn et al, 2011; Bosset et al, 2014). However, radical surgery combined with neoadjuvant therapy is associated with considerable perioperative morbidity with many patients experiencing diminished quality of life owing to bladder/sexual dysfunction, low anterior resection syndrome in addition to the potential for a permanent stoma (Kim et al, 2002; Paun et al, 2010; Smith et al, 2010; Juul et al, 2014).
The ultimate goal of organ preservation is to deliver equivalent oncological outcomes with reduced surgical risk and improved functional outcome leading to ‘better' quality of life. Previously only used for palliative purposes and in the local treatment of early (T1) rectal cancer, organ (rectum)-preservation strategies have been proposed as a curative approach for more advanced rectal cancer, where an objective response to neoadjuvant CRT has been observed (Kennelly et al, 2012; Lezoche et al, 2012; Shaikh et al, 2015; Verseveld et al, 2015; Garcia-Aguilar et al, 2015b). Complete response (no residual tumour) to treatment is seen in 10–30% of patients in whom active surveillance or local excision may be a viable option (Beddy et al, 2008; Martin et al, 2012; Smith et al, 2012b). Increasing the interval to assessment beyond 6–8 weeks following the completion of neoadjuvant treatment, radiotherapy boost via external-beam or contact therapy, and induction/consolidation chemotherapy are strategies that may increase complete response rates (Sun Myint et al, 2007; Gerard et al, 2008; Fernandez-Martos et al, 2010; Sloothaak et al, 2013; Garcia-Aguilar et al, 2015a).
Implementation of organ-preservation strategies has been hindered by the inability to accurately define a complete clinical response using imaging modalities. A complete endoluminal response on sigmoidoscopy is suggestive of complete pathological response, however, neither magnetic resonance imaging (MRI) or positron emission tomography (PET)–computed tomography (CT) are sufficiently sensitive at current resolutions to be definitive (Patel et al, 2011; Smith et al, 2012a, 2014; Hanly et al, 2014). Mucosal biopsies of a scar or residual ulcer may also not be definitive, owing to tumour scatter and submucosal persistence resulting in false-negative results (Guillem et al, 2005; Hayden et al, 2012; Perez et al, 2012; Duldulao et al, 2013). Patients downstaged to ypT0 or ypT1 have a low risk of positive lymph nodes (<5%), however, tumours staged ypT2 carry a risk of positive nodes closer to 20%, thus a decision concerning definitive surgery must incorporate methods to stratify patients according to ypT stage. One option is to perform transanal excision of any residual scar or ulcer (excision biopsy) to establish the ypT stage (Smith et al, 2010; Martin et al, 2012). The results presented here represent an observational study of organ-preservation strategies in patients with locally advanced rectal cancer found to have objective endoluminal response to neoadjuvant CRT.
Materials and methods
Study design and participants
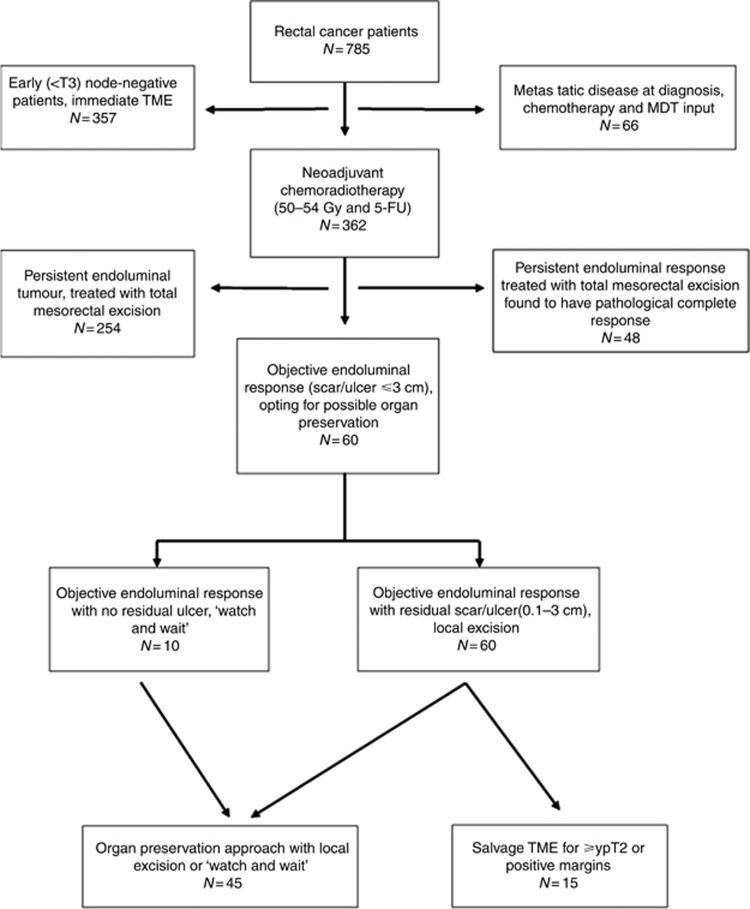
Institutional ethical approval was granted for the prospective accrual and follow-up of patients with adenocarcinoma of the rectum (<15 cm from the anal verge) (Figure 1). All patients underwent full colonoscopy and complete staging with digital rectal exam, serum carcinoembryonic antigen, CT of thorax, abdomen and pelvis (CT–TAP), MRI of the pelvis and PET–CT if indicated. Endoscopic ultrasound was used to differentiate T1 from T2 tumours that could not be adequately staged by MRI. Patients were discussed at a multidisciplinary team meeting (MDT) and categorised into receiving neoadjuvant CRT, or to proceed directly to surgical excision with total mesorectal excision. Locally advanced rectal cancer was defined as tumours ⩾T3 or any T stage with nodal involvement evident on MRI imaging, defined as both short-axis dimension >5 mm and abnormal morphological features.
Figure 1.
Overview of patient care.
Procedures
Patients received standard long-course CRT (50–54 Gy for 5 weeks with 5-fluorouracil). After a 6–8 weeks interval, they underwent clinical, endoscopic and radiological (CT thorax and abdomen, and MRI pelvis) restaging and MDT review. Patients with complete endoluminal response to treatment (visible scar only) or a residual scar/ulcer ⩽3 cm in diameter were defined as having an objective clinical response if this correlated with radiological downstaging in the absence of distant disease. Written informed consent was obtained from all patients who were fully appraised of the treatment options including standard surgery with total mesorectal excision. Patients with a complete endoluminal response to therapy were offered an active surveillance programme (‘watch and wait'). In the case of objective endoluminal response but residual ulcer, transanal excision was offered and performed 10–12 weeks after completion of neoadjuvant CRT. This was performed via transanal excision, transanal endoscopic microsurgery (TEM) or transanal minimally invasive surgery (TAMIS) under general anaesthetic. A 1 cm macroscopic margin was considered desirable. A predetermined strategy was salvage total mesorectal excision for ⩾ypT2 tumours or those with positive margins. Local regrowth was treated with salvage surgery. Adjuvant chemotherapy was given to patients who were clinically node-positive prior to neoadjuvant CRT.
Resected specimens were examined by two consultant pathologists. Tumour staging was performed using the TNM classification and response to therapy was assessed using a three-point tumour regression grade system (TRG 1–3; Ryan et al, 2005). Clinical follow-up was at 6 weeks and 3–6 monthly intervals thereafter including endoscopic assessment. All patients had six monthly CT–TAP scans (for 3 years), whereas organ-preserved patients had MRI pelvis every 3–6 months. A tumour regrowth was defined as a luminal recurrence at the site of the original tumour, whereas a local recurrence was defined as extra luminal tumour regrowth.
Outcomes
The primary study end points were overall and disease-free survival. Secondary end points were complete pathological response rates and local regrowth/recurrence rates.
Statistics
Continuous data are presented as medians with interquartile ranges (IQRs) or means if normally distributed (s.d.). Overall and disease-free survival was calculated using Kaplan–Meier analysis with a 95% confidence interval and log-rank test. Statistical analysis was performed using IBM SPSS Statistics (version 20, Armonk, NY, USA).
Results
A total of 785 patients were treated for rectal cancer (2005–2015), of whom 362 patients with non-metastatic, locally advanced rectal cancer were treated with neoadjuvant CRT (Figure 1). Sixty out of three hundred and sixty-two (16.5%) patients had an objective clinical response and chose to be treated by an organ-preservation approach. The remaining 302 patients with persistent endoluminal tumour underwent total mesorectal excision, of whom 48 (15.9%) were subsequently found to have a complete pathological response (Table 1).
Table 1. Patient characteristics.
| Patient characteristics | Radical surgery (n=302) | Organ preservation (n=60) |
|---|---|---|
| Median age (range) | 64 (27–90) | 67 (46–87) |
| Gender | ||
| Male: female | 189 : 113 (3 : 1) | 45 : 15 (2 : 1) |
| Clinical T stage (%) | ||
| T1 | 0 | 2 (3.3%) |
| T2 | 36 (12%) | 20 (33.3%) |
| T3 | 230 (76%) | 38 (63.4%) |
| T4 | 36 (12%) | 0 |
| Clinical nodal stage (%) | ||
| Positive | 220 (72.8%) | 35 (58.3%) |
| Negative | 82 (27.2%) | 25 (41.7%) |
| Pathological differentiation (%) | ||
| Well/moderate | 271 (89.7%) | 60 (100%) |
| Poor | 31 (10.3%) | 0 |
| Pathological T stage (%) | ||
| ypT0 | 48 (15.9%) | 26 (43.33%)a |
| ypT1 | 21 (7%) | 4 (6.67%) |
| ypT2 | 72 (23.8%) | 17 (28.33%) |
| ypT3 | 136 (45%) | 3 (5%) |
| ypT4 | 25 (8.3%) | 0 |
| Pathological nodal stage (%) | ||
| ypN0 | 198 (65.5%) | — |
| ypN1/ypN2 | 104 (34.5%) | — |
| Pathological tumour regression grade (TRG) | ||
| TRG 1 | 48 (15.9%) | 26 (52%)a |
| TRG 2 | 182 (60.3%) | 24 (48%) |
| TRG 3 | 72 (23.8%) | 0 |
Ten patients entered into watch and wait programme – clinical complete responders.
Ten patients with complete endoluminal response were treated with a ‘watch and wait' (active surveillance) approach. Fifty patients with an objective endoluminal response underwent local excision at 10–12 weeks post CRT. Twenty-eight patients (56%) were treated with transanal excision, 16 (32%) had TEMs, whereas 6 (12%) had TAMIS. Fifteen patients had ⩾ypT2 tumours or margins <1 cm post local excision and were subsequently treated with total mesorectal excision. Forty-five patients were maintained in an organ-preservation programme, 10 in active surveillance (cT0 tumours) and 35 in local excision group (26 ypT0, 4 ypT1 and 5 patients with ypT2 tumours who declined radical surgery/stoma).
After a median follow-up of 29 months (IQR 12–49) for the local excision group (n=35), overall survival was 94.3%. One death occurred in the ypT0 cohort owing to respiratory sepsis, while one patient staged ypT1 died of disease progression (Table 2). Disease-free survival was 80%, five patients developed a distant recurrence after a median of 20 months (range 5–45) and 3 patients developed tumour regrowth (2 ypT0) after a median of 21 months (range 17–31). One patient, staged ypT2, developed both distant recurrence and local regrowth (Table 2). All tumour regrowths were treated with salvage total mesorectal excision (two abdominoperineal resections) achieving negative resection margins with no post-operative mortality.
Table 2. Patient outcomes.
| TME (n=302) | Watch and wait (n=10) | Local excision (n=35) | Salvage TME post local excision (n=15) | |
|---|---|---|---|---|
| Overall survival | 85.6% | 90% | 94.3% | 86.6% |
| Disease-free survival | 78.2% | 80% | 80% | 60% |
| Tumour regrowth/recurrence | 19 | 1 | 3 | 1 |
| Distant recurrence | 47 | 1 | 5 | 5 |
Abbreviation: TME=total mesorectal excision.
Ten patients underwent active surveillance (‘watch and wait') with a median follow-up of 42 months (IQR 22.5–55.2 months). Disease-free survival was 80%. Tumour regrowth occurred in one patient at 19 months that was treated with total mesorectal excision achieving clear margins. One distant recurrence occurred at 50 months with one death occurring owing to disease progression at 51 months.
Fifteen patients were referred directly for radical surgery post local excision owing to adverse pathological findings (⩾ypT2 tumours or positive margins). Pathological assessment of resected specimens showed 2 ypT0 tumours, 10 ypT2 tumours and 3 ypT3 tumours. Nodal positivity occurred in four patients with negative resection margins achieved in all (five abdominoperineal resections). One death occurred owing to respiratory sepsis after a median of 70 months follow-up, whereas disease progression accounted for the other death after 16 months. Overall survival was 86.7% (Table 2). Adjuvant chemotherapy was given to 39 out of 60 patients treated initially with organ preservation, 23 ypT0, 2 ypT1, 11 ypT2 and 3 ypT3.
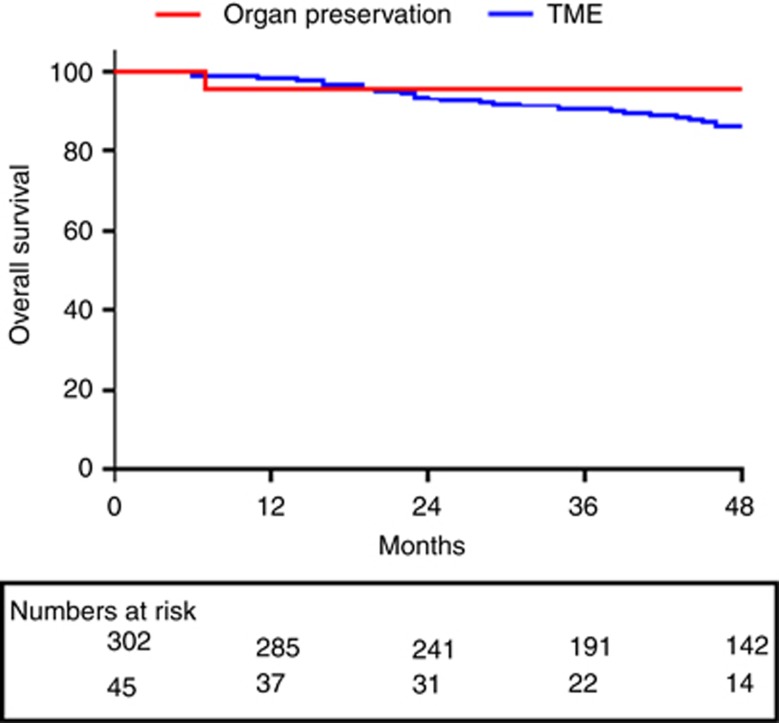
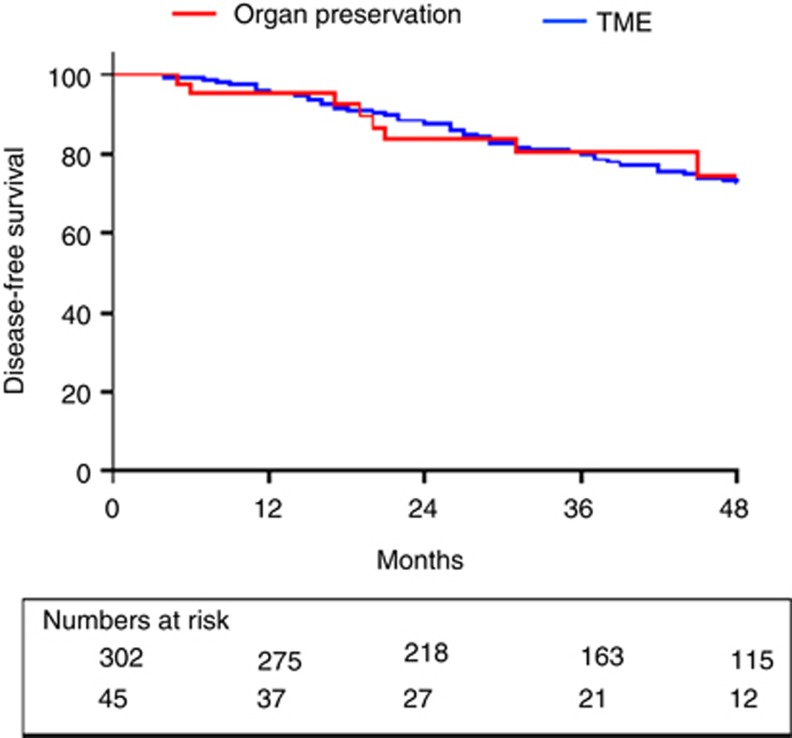
After a median follow-up of 45 months (range 5–120 months), overall survival was 85.6% in the radical resection group with disease-free survival of 78.3%. There was no significant difference in overall survival (85.6% vs 93.3%, P=0.414) or disease-free survival rate (78.3% vs 80%, P=0.846) when the outcomes of radical surgery were compared with organ preservation (active surveillance and local excision, n=45; Figures 2 and 3).
Figure 2.
Kaplan–Meier estimates of overall survival for patients maintained by organ-preservation techniques (n=45) vs total mesorectal excision (n=302), P=0.414.
Figure 3.
Kaplan–Meier estimates of disease-free survival for patients maintained by organ-preservation techniques (n=45) vs total mesorectal excision (n=302), P=0.846.
Discussion
Proctectomy with total mesorectal excision is the standard of care for locally advanced rectal cancer (Heald et al, 1982). In locally advanced disease, when neoadjuvant CRT is combined with radical surgery, very low rates of local recurrence are reported (Sauer et al, 2004; van Gijn et al, 2011; Bosset et al, 2014). The present study shows that organ-preservation techniques are suitable for selected patients with locally advanced rectal cancer in whom an objective clinical response is found after neoadjuvant therapy. This approach can achieve equivalent oncological outcomes by means of either active surveillance (‘watch and wait') or local excision followed by active surveillance.
The multicentre ACOSOG Z6041 trial evaluated organ preservation for T2N0 distal rectal tumours treated with neoadjuvant CRT and local excision. High toxicity rates were encountered when oxaliplatin was included, however, long-term results were impressive with 3-year overall survival of 94.8% and excellent local recurrence rates of 4% (Garcia-Aguilar et al, 2015b). Functional outcomes and quality of life were assessed in this trial, with a return to normal bowel function and good quality of life observed 1 year after surgery (Garcia-Aguilar et al, 2015b). Radical surgery combined with neoadjuvant therapy is associated with considerable perioperative morbidity with many patients experiencing diminished quality of life owing to bladder/sexual dysfunction, low anterior resection syndrome in addition to the potential for a permanent stoma (Kim et al, 2002; Paun et al, 2010). The purpose of organ preservation is to achieve similar oncological outcomes to those of radical surgery while maintaining quality of life and functional ability (Allaix et al, 2011; Garcia-Aguilar et al, 2015b; Pucciarelli et al, 2016).
Lezoche et al (2012) randomised patients with T2N0M0 distal rectal cancers <3 cm in diameter to receive neoadjuvant CRT followed by local excision or radical surgery. The local recurrence rates in the local excision group were 8% compared with 6% in the radical resection group after 5 years of follow-up. Similar recurrence rates were seen in the present study, even with inclusion of patients with more locally advanced (>T3) tumours. A meta-analysis of local excision post neoadjuvant therapy vs radical surgery showed that local recurrences were not significantly greater (10.1% local excision vs 8% radical resection), and there was no difference in overall or disease-free survival, even in T3 and node-positive patients (Shaikh et al, 2015).The Dutch CARTS trial assessed organ sparing surgery by TEM for patients clinically staged with distal T1-3N0 cancers. They achieved organ-preservation surgery in 55% of patients. Complete pathological rates were seen in >30% with no local recurrences occurring in this group (Verseveld et al, 2015).
Organ-preservation strategies are dependent on tumour regression following neoadjuvant CRT. Complete response is seen in 10–30% of patients undergoing neoadjuvant CRT (Martin et al, 2012; Smith et al, 2012a). Short-course radiotherapy has been shown to achieve similar oncological outcomes when compared with long course, however, without a delay to surgery, pathological downstaging is more frequently seen with long-course therapy (van Gijn et al, 2011; Zhou et al, 2014; Pettersson et al, 2015). Increasing the interval to surgery >8 weeks following neoadjuvant therapy may improve response rates (Kalady et al, 2009; Sloothaak et al, 2013; Petrelli et al, 2015). This has been questioned recently, as the Greccar 6 trial showed little benefit (on complete response rates) if the interval to surgery was longer than 7 weeks (Lefevre et al, 2016). Some centres have omitted radiotherapy from their regimes, with complete response rates of 25% being reported with chemotherapy alone (Schrag et al, 2014). However, when contact or external-beam radiotherapy are used, better local control and pathological outcomes are seen (Sun Myint et al, 2007; Gerard et al, 2008).
Adjuvant chemotherapy is used to improve overall and disease-free survival in node-positive patients, although the true benefit of adjuvant chemotherapy after neoadjuvant CRT has been questioned (Breugom et al, 2015). Giving systemic chemotherapy in the neoadjuvant setting (induction/consolidation chemotherapy) has the added benefit of managing micrometastasis earlier, while delivering treatment to well-vascularised tissues and improving compliance rates. Increased pathological responses are seen when this technique has been used, making it an attractive approach for patients entered into an organ-preservation approach (Fernandez-Martos et al, 2010; Bujko et al, 2013; Garcia-Aguilar et al, 2015a).
One pitfall to implementing an organ-preservation approach is the difficulty in accurately defining a complete clinical response. Local excision allows full-thickness assessment of the tumour site and accurate ypT staging, an advantage over a ‘watch and wait' approach. Full-thickness excision may lead to better local control compared with a non-operative approach, however, this was not assessed here. This must be balanced against the potential morbidity of local excision in a radiated operative field (Morino et al, 2013; Habr-Gama et al, 2016).
Patients in the present study who were found not to have an objective clinical response underwent timely radical surgery. Total mesorectal excision allows accurate lymph-node staging, something organ-preservation strategies do not. Patients with ypT0-1 have a lymph-node positivity rate of ∼3% (range 1–10), but this increases to >20% with ypT2 tumours and >33% with ypT3 tumours (Smith et al, 2010, 2012a; Martin et al, 2012). Therefore, total mesorectal excision should be considered for ⩾ypT2 tumours.
Recurrence rates in patients treated with local excision of rectal cancer can be high. Depth of tumour invasion, presence of lymphovascular or perineural invasion, poor differentiation or positive margins, all contribute to increased risk of local recurrence. In the present study, all local recurrences and locally excised tumours found to have adverse pathological features were treated with salvage total mesorectal excision, however, anatomical distortion post local excision can lead to more challenging resections with higher rates of positive margins and abdominoperineal resections reported (Levic et al, 2013; Morino et al, 2013).
There are a number of limitations to this study. It is a single-centre experience with a relatively small sample size and short follow-up. No randomisation occurred that could have contributed to selection bias. However, this centre is participating in the international watch and wait programme, a registry incorporating multiple worldwide institutes, which will further help to validate this approach (Beets et al, 2015).
In conclusion, this study shows that this organ-preservation strategy can achieve equivalent oncological outcomes in selected patients with locally advanced rectal cancer achieving an objective response to neoadjuvant CRT.
Acknowledgments
We wish to express their gratitude to the following colleagues: Professor John Hyland, Ms Ann White, Ms Michelle Loughrey, Professor John Armstrong, Dr Gerard McVey, Dr David Fennelly, Professor Raymond McDermott, Dr Stephen Skehan, Dr David Brophy, Professor Colm McMahon, Dr Robin Gibney, Dr David Gibbons, Mr Robert Geraghty, Dr Elizabeth Ryan, Professor Hugh Mulcahy, Professor Glen Doherty, Dr Garret Cullen, Dr Gareth Horgan, Dr Juliette Sheridan and Dr Marie Buckley.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Allaix ME, Rebecchi F, Giaccone C, Mistrangelo M, Morino M (2011) Long-term functional results and quality of life after transanal endoscopic microsurgery. Br J Surg 98(11): 1635–1643. [DOI] [PubMed] [Google Scholar]
- Beddy D, Hyland JM, Winter DC, Lim C, White A, Moriarty M, Armstrong J, Fennelly D, Gibbons D, Sheahan K (2008) A simplified tumor regression grade correlates with survival in locally advanced rectal carcinoma treated with neoadjuvant chemoradiotherapy. Ann Surg Oncol 15(12): 3471–3477. [DOI] [PubMed] [Google Scholar]
- Beets GL, Figueiredo NL, Habr-Gama A, van de Velde CJ (2015) A new paradigm for rectal cancer: organ preservation: introducing the international watch and wait database (IWWD). Eur J Surg Oncol 41(12): 1562–1564. [DOI] [PubMed] [Google Scholar]
- Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M, Marchal D, Van Laethem JL, Klein V, Giralt J, Clavere P, Glanzmann C, Cellier P, Collette L (2014) Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 15(2): 184–190. [DOI] [PubMed] [Google Scholar]
- Breugom AJ, Swets M, Bosset JF, Collette L, Sainato A, Cionini L, Glynne-Jones R, Counsell N, Bastiaannet E, van den Broek CB, Liefers GJ, Putter H, van de Velde CJ (2015) Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 16(2): 200–207. [DOI] [PubMed] [Google Scholar]
- Bujko K, Nasierowska-Guttmejer A, Wyrwicz L, Malinowska M, Krynski J, Kosakowska E, Rutkowski A, Pietrzak L, Kepka L, Radziszewski J, Olszyna-Serementa M, Bujko M, Danek A, Kryj M, Wydmanski J, Zegarski W, Markiewicz W, Lesniak T, Zygulski I, Porzuczek-Zuziak D, Bebenek M, Maciejczyk A, Polkowski W, Czeremszynska B, Cieslak-Zeranska E, Toczko Z, Radkowski A, Kolodziejski L, Szczepkowski M, Majewski A, Jankowski M (2013) Neoadjuvant treatment for unresectable rectal cancer: an interim analysis of a multicentre randomized study. Radiother Oncol 107(2): 171–177. [DOI] [PubMed] [Google Scholar]
- Duldulao MP, Lee W, Streja L, Chu P, Li W, Chen Z, Kim J, Garcia-Aguilar J (2013) Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer. Dis Colon Rectum 56(2): 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E, Mengual JL, Saigi E, Estevan R, Mira M, Polo S, Hernandez A, Gallen M, Arias F, Serra J, Alonso V (2010) Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol 28(5): 859–865. [DOI] [PubMed] [Google Scholar]
- Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG, Kumar AS, Oommen S, Coutsoftides T, Hunt SR, Stamos MJ, Ternent CA, Herzig DO, Fichera A, Polite BN, Dietz DW, Patil S, Avila K (2015. a) Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 16(8): 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR Jr, Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R (2015. b) Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol 16(15): 1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard JP, Ortholan C, Benezery K, Ginot A, Hannoun-Levi JM, Chamorey E, Benchimol D, Francois E (2008) Contact X-ray therapy for rectal cancer: experience in Centre Antoine-Lacassagne, Nice, 2002–2006. Int J Radiat Oncol Biol Phys 72(3): 665–670. [DOI] [PubMed] [Google Scholar]
- Guillem JG, Chessin DB, Shia J, Moore HG, Mazumdar M, Bernard B, Paty PB, Saltz L, Minsky BD, Weiser MR, Temple LK, Cohen AM, Wong WD (2005) Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol 23(15): 3475–3479. [DOI] [PubMed] [Google Scholar]
- Habr-Gama A, Lynn PB, Jorge JM, Sao Juliao GP, Proscurshim I, Gama-Rodrigues J, Fernandez LM, Perez RO (2016) Impact of organ-preserving strategies on anorectal function in patients with distal rectal cancer following neoadjuvant chemoradiation. Dis Colon Rectum 59(4): 264–269. [DOI] [PubMed] [Google Scholar]
- Hanly AM, Ryan EM, Rogers AC, McNamara DA, Madoff RD, Winter DC (2014) Multicenter evaluation of rectal cancer reimaging post neoadjuvant (MERRION) therapy. Ann Surg 259(4): 723–727. [DOI] [PubMed] [Google Scholar]
- Hayden DM, Jakate S, Pinzon MC, Giusto D, Francescatti AB, Brand MI, Saclarides TJ (2012) Tumor scatter after neoadjuvant therapy for rectal cancer: are we dealing with an invisible margin? Dis Colon Rectum 55(12): 1206–1212. [DOI] [PubMed] [Google Scholar]
- Heald RJ, Husband EM, Ryall RD (1982) The mesorectum in rectal cancer surgery—the clue to pelvic recurrence? Br J Surg 69(10): 613–616. [DOI] [PubMed] [Google Scholar]
- Juul T, Ahlberg M, Biondo S, Espin E, Jimenez LM, Matzel KE, Palmer GJ, Sauermann A, Trenti L, Zhang W, Laurberg S, Christensen P (2014) Low anterior resection syndrome and quality of life: an international multicenter study. Dis Colon Rectum 57(5): 585–591. [DOI] [PubMed] [Google Scholar]
- Kalady MF, de Campos-Lobato LF, Stocchi L, Geisler DP, Dietz D, Lavery IC, Fazio VW (2009) Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg 250(4): 582–589. [DOI] [PubMed] [Google Scholar]
- Kennelly RP, Heeney A, White A, Fennelly D, Sheahan K, Hyland JM, O'Connell PR, Winter DC (2012) A prospective analysis of patient outcome following treatment of T3 rectal cancer with neo-adjuvant chemoradiotherapy and transanal excision. Int J Colorectal Dis 27(6): 759–764. [DOI] [PubMed] [Google Scholar]
- Kim NK, Aahn TW, Park JK, Lee KY, Lee WH, Sohn SK, Min JS (2002) Assessment of sexual and voiding function after total mesorectal excision with pelvic autonomic nerve preservation in males with rectal cancer. Dis Colon Rectum 45(9): 1178–1185. [DOI] [PubMed] [Google Scholar]
- Lefevre JH, Mineur L, Kotti S, Rullier E, Rouanet P, de Chaisemartin C, Meunier B, Mehrdad J, Cotte E, Desrame J, Karoui M, Benoist S, Kirzin S, Berger A, Panis Y, Piessen G, Saudemont A, Prudhomme M, Peschaud F, Dubois A, Loriau J, Tuech JJ, Meurette G, Lupinacci R, Goasgen N, Parc Y, Simon T, Tiret E (2016) Effect of interval (7 or 11 weeks) between neoadjuvant radiochemotherapy and surgery on complete pathologic response in rectal cancer: a multicenter, randomized, controlled trial (GRECCAR-6). J Clin Oncol 34: 3773–3780. [DOI] [PubMed] [Google Scholar]
- Levic K, Bulut O, Hesselfeldt P, Bulow S (2013) The outcome of rectal cancer after early salvage TME following TEM compared with primary TME: a case-matched study. Tech Coloproctol 17(4): 397–403. [DOI] [PubMed] [Google Scholar]
- Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M (2012) Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg 99(9): 1211–1218. [DOI] [PubMed] [Google Scholar]
- Martin ST, Heneghan HM, Winter DC (2012) Systematic review and meta-analysis of outcomes following pathological complete response to neoadjuvant chemoradiotherapy for rectal cancer. Br J Surg 99(7): 918–928. [DOI] [PubMed] [Google Scholar]
- Morino M, Allaix ME, Arolfo S, Arezzo A (2013) Previous transanal endoscopic microsurgery for rectal cancer represents a risk factor for an increased abdominoperineal resection rate. Surg Endosc 27(9): 3315–3321. [DOI] [PubMed] [Google Scholar]
- Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R, Guthrie A, Bees N, Swift I, Pennert K, Brown G (2011) Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 29(28): 3753–3760. [DOI] [PubMed] [Google Scholar]
- Paun BC, Cassie S, MacLean AR, Dixon E, Buie WD (2010) Postoperative complications following surgery for rectal cancer. Ann Surg 251(5): 807–818. [DOI] [PubMed] [Google Scholar]
- Perez RO, Habr-Gama A, Pereira GV, Lynn PB, Alves PA, Proscurshim I, Rawet V, Gama-Rodrigues J (2012) Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Colorectal Dis 14(6): 714–720. [DOI] [PubMed] [Google Scholar]
- Petrelli F, Sgroi G, Sarti E, Barni S (2015) Increasing the interval between neoadjuvant chemoradiotherapy and surgery in rectal cancer: a meta-analysis of published studies. Ann Surg 263: 458–464. [DOI] [PubMed] [Google Scholar]
- Pettersson D, Lorinc E, Holm T, Iversen H, Cedermark B, Glimelius B, Martling A (2015) Tumour regression in the randomized Stockholm III Trial of radiotherapy regimens for rectal cancer. Br J Surg 102(8): 972–978, discussion 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucciarelli S, Giandomenico F, De Paoli A, Gavaruzzi T, Lotto L, Mantello G, Barba C, Zotti P, Flora S, Del Bianco P (2016) Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. Br J Surg; e-pub ahead of print 5 October 2016; doi:10.1002/bjs.10318. [DOI] [PubMed]
- Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, O'Donoghue DP, Moriarty M, Fennelly D, Sheahan K (2005) Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology 47(2): 141–146. [DOI] [PubMed] [Google Scholar]
- Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R (2004) Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 351(17): 1731–1740. [DOI] [PubMed] [Google Scholar]
- Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG, Temple LK, Paty PB, Saltz LB (2014) Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol 32(6): 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh I, Askari A, Ouru S, Warusavitarne J, Athanasiou T, Faiz O (2015) Oncological outcomes of local excision compared with radical surgery after neoadjuvant chemoradiotherapy for rectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis 30(1): 19–29. [DOI] [PubMed] [Google Scholar]
- Sloothaak DA, Geijsen DE, van Leersum NJ, Punt CJ, Buskens CJ, Bemelman WA, Tanis PJ (2013) Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 100(7): 933–939. [DOI] [PubMed] [Google Scholar]
- Smith FM, Chang KH, Sheahan K, Hyland J, O'Connell PR, Winter DC (2012. a) The surgical significance of residual mucosal abnormalities in rectal cancer following neoadjuvant chemoradiotherapy. Br J Surg 99(7): 993–1001. [DOI] [PubMed] [Google Scholar]
- Smith FM, Waldron D, Winter DC (2010) Rectum-conserving surgery in the era of chemoradiotherapy. Br J Surg 97(12): 1752–1764. [DOI] [PubMed] [Google Scholar]
- Smith FM, Wiland H, Mace A, Pai RK, Kalady MF (2014) Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum 57(3): 311–315. [DOI] [PubMed] [Google Scholar]
- Smith JD, Ruby JA, Goodman KA, Saltz LB, Guillem JG, Weiser MR, Temple LK, Nash GM, Paty PB (2012. b) Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg 256(6): 965–972. [DOI] [PubMed] [Google Scholar]
- Sun Myint A, Grieve RJ, McDonald AC, Levine EL, Ramani S, Perkins K, Wong H, Makin CA, Hershman MJ (2007) Combined modality treatment of early rectal cancer: the UK experience. Clin Oncol (R Coll Radiol) 19(9): 674–681. [DOI] [PubMed] [Google Scholar]
- van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van de Velde CJ (2011) Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 12(6): 575–582. [DOI] [PubMed] [Google Scholar]
- Verseveld M, de Graaf EJ, Verhoef C, van Meerten E, Punt CJ, de Hingh IH, Nagtegaal ID, Nuyttens JJ, Marijnen CA, de Wilt JH (2015) Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg 102(7): 853–860. [DOI] [PubMed] [Google Scholar]
- Zhou ZR, Liu SX, Zhang TS, Chen LX, Xia J, Hu ZD, Li B (2014) Short-course preoperative radiotherapy with immediate surgery versus long-course chemoradiation with delayed surgery in the treatment of rectal cancer: a systematic review and meta-analysis. Surg Oncol 23(4): 211–221. [DOI] [PubMed] [Google Scholar]