Abstract
Target selection is a key feature in cancer immunotherapy, a promising field in cancer research. In this respect, gangliosides, a broad family of structurally related glycolipids, were suggested as potential targets for cancer immunotherapy based on their higher abundance in tumors when compared with the matched normal tissues. GD2 is the first ganglioside proven to be an effective target antigen for cancer immunotherapy with the regulatory approval of dinutuximab, a chimeric anti-GD2 therapeutic antibody. Although the therapeutic efficacy of anti-GD2 monoclonal antibodies is well documented, neuropathic pain may limit its application. O-Acetyl-GD2, the O-acetylated-derivative of GD2, has recently received attention as novel antigen to target GD2-positive cancers. The present paper examines the role of O-acetyl-GD2 in tumor biology as well as the available preclinical data of anti-O-acetyl-GD2 monoclonal antibodies. A discussion on the relevance of O-acetyl-GD2 in chimeric antigen receptor T cell therapy development is also included.
1. Introduction
Cancer immunotherapy comprises different strategies that use distinct effector mechanisms of the immune system to specifically target and eliminate tumor cells. Such strategies include specific monoclonal antibodies (mAbs), checkpoint inhibitors, cytokines, cancer vaccines, dendritic cell vaccines, tumor-infiltrating lymphocytes, and more recently genetically engineered T cells. Each one of these approaches holds promise, but their generalized success has been impaired by the paucity of specific tumor antigens resulting in suboptimal tumor efficacy and unpredictable side effects. Remarkably, the vast majority of these strategies have focused on protein antigens and recently mAbs recognizing cell surface gangliosides have recently proven to be effective for cancer therapeutic targets [1]. Gangliosides are sialic acid-enriched glycosphingolipids that contain at least one monosaccharide residue associated with a ceramide chain [2]. They are ubiquitously expressed in vertebrate tissues and are most abundant in the nervous system (for review see [3]). They exhibit a huge diversity due to the structural variations in both their oligosaccharide chain and ceramide moiety.
Almost 200 gangliosides species have been described, showing differences in the number, the order, and the linkage of the glycosyl and sialyl residues [4]. Sialic acid is a generic term for a member of a family of molecules presented by over 25 members. In addition, sialic acids can be further O-acetylated [5], de-N-acetylated [6], sulfated [7], or modified by lactonization [8]. The structural complexity and diversity further increase when variations of their ceramide anchor are taken into account. Such variations include the length, the saturation, and the hydroxylation of both the fatty acid chain and the long chain base. Remarkably, the diversity of gangliosides is generated by only a few glycosyltransferases and sialyltransferases that act within a combinatorial biosynthetic pathway [9]. After synthesis in the endoplasmic reticulum, the ceramide tail is transported to the Golgi apparatus. Then, glycosylation of ceramide occurs by membrane-bound glycosyltransferases and sialyltransferases that interact with their membrane-bound substrates [9]. Glycosylation and sialylation are coupled to exocytosis through the Golgi apparatus and transport vesicles to the plasma membrane [10]. After synthesis, gangliosides are present on the outer leaflet of the plasma membrane with their hydrophobic ceramide backbone anchored in the membrane and their hydrophilic carbohydrate residue projected into the extracellular environment. They are believed to be concentrated in ordered microdomains referred to as lipid rafts [11] where they can interact with different functional membrane proteins involved in cell adhesion and cell signaling [12]. Through these interactions, they can regulate crucial cell functions such as cell proliferation and apoptosis, adhesion, migration, and differentiation [13].
In addition, ganglioside concentration and distribution vary according to tissue, cell type, differentiation, and development stage [13]. It is therefore possible to define, for each cell type, a specific ganglioside profile, which is modified during embryogenesis, and ontogenesis [13]. Some ganglioside species further demonstrate very restricted expression in normal tissues and markedly enhanced expression in particular malignant tumor [14]. For example, ganglioside GD2 (Figure 1) is expressed at low concentration in the central nervous system [15], on peripheral nerves [15], skin melanocytes [15], and mesenchymal stem cells [16] in healthy adults. On the other hand, GD2 is overexpressed in tumors including neuroblastoma [15, 17], melanoma [18], small cell lung carcinoma [19], brain tumors [20], retinoblastoma [21], Ewing's sarcoma [22], and osteosarcoma [23]. Of note, the presence of GD2 was detected recently on breast cancer stem cells [24]. Cancer stem cells (CSC) represent a small population of tumor cells that are endowed with self-renewal and tumor-initiating capabilities [25]. Due to their inherent resilience, cancer stem cells are believed to underpin tumor recurrence and therapy resistance [25]. Thus, GD2 may also provide an effective target antigen for CSC immunotherapy. In fact, the National Cancer Institute pilot program for the prioritization of the most important cancer antigens ranks GD2 as number 12 out of 75 selected tumor antigens based on therapeutic function, immunogenicity, oncogenicity, specificity, expression level and percent of antigen-positive cells, stem cell expression, number of patients with antigen-positive cancers, number of epitopes, and cellular location of antigen expression [26] (Table 1). Of note, 3 other gangliosides (GD3, fucosyl-GM1, and GM3) were also selected, ranking between the positions 12 and 48 [26].
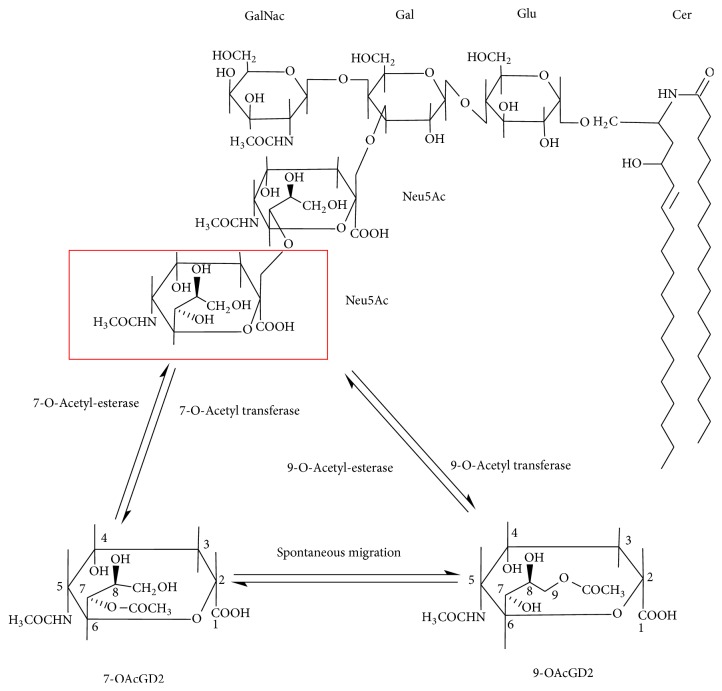
Figure 1.
Structure of 9-O-acetyl-GD2. O-acetylated GD2 is constituted by a ceramide chain, which is anchored into plasma membrane, and a hydrophile chain. Oligosaccharide chain and O-acetylation are oriented to extracellular matrix. O-acetyl-GD2 is formed by the addition of an O-acetyl ester to the external sialic acid residue by a 9(7)-O-acetyl transferase. O-Acetyl esters located at the C7 position are mobile and can spontaneously migrate to the C9 position. They can be removed by 9(7)-acetyl esterases. Cer, ceramide; Gal, galactose; GalNAc, N-acetylgalactosamine; Neu5Ac, N-acetylneuraminic acid.
Table 1.
Relevant characteristic of O-acetyl-GD2 ganglioside as a cancer antigen according to Cheever et al. [26].
| Criteria | Data on O-acetyl-GD2 |
|---|---|
| Therapeutic function | Preclinical data showing that anti-O-acetyl-GD2 mAbs induce tumor cell death by immunological and nonimmunological mechanisms [39, 80, 87, 104]. |
| Immunogenicity | Poorly immunogenic [95]. |
| Oncogenicity | Increased expression in adult and pediatric solid tumors, to be determined with a clear association with oncogenic process [39, 40, 65, 87]. |
| Expression level and positive cell | Overexpressed in cancer with little or no expression in normal tissues [39]. |
| Stem cell expression | Expression on cancer with cancer stem cell issue such as glioblastoma, but without information about putative stem cells [87]. |
| Number of patients with antigen-positive cancers | High level of expression in >70% of patients with a particular cancer type [39, 87]. |
| Number of epitopes | Short antigenic segment with one or few epitopes [95]. |
| Cellular location of expression | Expressed on the cell surface [39, 87] with little or no circulating antigen [104]. |
The clinical development of anti-GD2 mAbs for neuroblastoma patients originated from the discovery of two distinct murine anti-GD2 antibodies designated as 3F8 [27] and 14.18 [19], respectively. Dinutuximab, a.k.a. ch14.18, is a chimeric mouse/human IgG1 antibody obtained from the parental mouse IgG2a mAb 14G2a [28, 29] and named after the original mouse 14.18 IgG3 isotype [30]. In Europe, the cell line used for the production of ch14.18 antibody was changed. The plasmid encoding for ch14.18 antibody was recloned into CHO cells (ch14.18/CHO), and ch14.18/CHO antibody was designated as dinutuximab ß [31]. This antibody is currently under review by the EMA. Antibodies ch14.18 and 3F8 were further humanized to form hu14.18 and hu3F8, respectively [32, 33].
In 2010, Yu et al. reported a breakthrough randomized Phase 3 study in which the combination of cytokines IL-2 and GM-CSF with the anti-GD2 mAb ch14.18 (dinutuximab) showed a significant improvement in the event-free survival of high-risk neuroblastoma patients at the 2-year median follow-up [1]. These results led to the regulatory approval of dinutuximab by the FDA and the EMA in 2015 for the treatment of patients with high-risk neuroblastoma [34]. It is important to note that patients treated with anti-GD2 mAbs, such as dinutuximab, display dose-limiting acute toxicities, including hypotension, neuropathic pain, and fever upon antibody infusion [1]. These side effects are related to dinutuximab binding on GD2-positive sensitive nerve fibers followed by complement activation [32, 35]. Thus, improving the tolerance to anti-GD2 antibodies remains a key objective for GD2-targeted immunotherapies and different strategies are currently developed. A novel delivery method of ch14.18/CHO by continuous long-term infusion over 10 days is currently investigated in patients [36]. The binding to C1q can be abolished by incubation of the antibody at 56°C for 30 minutes [37] or by Fc-molecular engineering [32]. The most recent data challenge the use of IL-2 on the basis of a randomized trial using long-term infusion of dinutuximab ß combined with or without IL-2 [38]. In this trial, the addition of IL-2 increased pain and did not enhance anti-GD2 cytotoxicity, which therefore questioned the use of IL-2 with dinutuximab ß. Another strategy consists of targeting O-acetyl-GD2 that is not expressed on peripheral nerves [39]. In this review, we will present the O-acetyl-GD2 tumor antigen and its potential interest for antibody-based immunotherapy and chimeric antigen receptor cellular immunotherapy.
2. Structure and Physicochemical Properties of O-Acetyl-GD2 Ganglioside
O-Acetyl-GD2 is the O-acetyl derivative of GD2 ganglioside, in which the outer sialic acid residue is modified by an O-acetyl ester [40] (Figure 1). O-acetylated gangliosides arise by enzymatic transfer of O-acetyl group to the C7 and/or C9 hydroxyl groups on the glycerol-like side chain of a specific terminal α2-8 linked sialic acid residue. The commonest sialic acids found in ganglioside are N-acetyl-neuraminic acid and N-glycolyl-neuraminic acid [41]. N-Glycolyl-neuraminic acid cannot be synthesized in man but can be expressed by human malignant tissue as a result of the metabolic incorporation of dietary N-glycolyl-neuraminic acid [42]. The addition of an O-acetyl group modifies several chemical properties of the ganglioside acceptor. For example, O-acetylation decreases the polarity and the hydrophobicity of the ganglioside but does not appear to affect its overall conformation [43]. The decrease in the polarity and the hydrophobicity is observable on thin-layer chromatography and high-performance liquid chromatography and can be used for separation of the O-acetylated ganglioside species. Of note, this group is very sensitive towards high temperature, alkaline pH, and naturally occurring esterases [44, 45]. The most striking chemical property of O-acetyl groups is the spontaneous migration from position 7 to position 9 observed in free O-acetylated sialic acids exposed to mild alkaline conditions [45]. Therefore, temperature and pH of the buffer should critically be maintained during sample collection and preparation, to avoid the loss and the migration of the O-acetyl group. This might explain the differences between authors describing the O-acetylation site in ganglioside. Thurin et al. [46] and Ostrander et al. [47] found that the O-acetyl group was located at the C9 position of the outer sialic acid of O-acetyl-GD3. Sjoberg et al. also found the O-acetyl group located at the C9 position of O-acetyl-GD2 [40]. In a later study, Ren et al. found that the O-acetyl ester was located at the C7 position of O-acetyl-GD3 [48]. However, some of these previous experiments have relied extensively on poorly resolved resonances in the 1H MNR spectra, which would be difficult in solving the resonance assignments for these compounds. In addition, in a follow-up paper, Manzi et al. found that the O-acetyl group located at both 7 and 9 positions [49]. This discrepancy might be related to the neuraminidase treatment and analysis of free sialic acid in their experimental procedure [49], which could facilitate the spontaneous migration of the O-acetyl group from the position C7 to C9 [45].
3. Metabolism of O-Acetyl-GD2 Ganglioside
The modification of ganglioside expression in oncogenesis is mainly associated with altered glycosyltransferase and sialyltransferase activities [50]. The level of N-acetylgalactosaminyltransferase I (GM2/GD2 synthase) activity is generally high in neuroectoderm-derived tumor cells, such as neuroblastomas and melanomas [50]. Although there is some evidence that posttranslational factors may influence enzyme activity [51] transcriptional regulation probably plays the major role [52]. Furukawa et al. evidenced that the GM2/GD2 synthase gene has three transcription initiation sites and further revealed that the regulatory mechanisms for each transcription of this gene were more complex than expected [52]. Not much information is now available on this point. The high expression of GM2/GD2 synthase leads to the accumulation of GD2 ganglioside in neuroblastoma cells [50]. Accumulation of tumor-associated gangliosides is further associated with aberrant sialylation of their sialic acid content. As mentioned above, human tumor cells can incorporate dietary N-glycolyl-neuraminic acid in their gangliosides [42], but also can O-acetylate their outmost sialic acid residue [5].
Current models suggest that two types of enzymes are involved in the metabolism of O-acetylated sialic acids: O-acetyl transferase [53] and O-acetyl esterase [54]. As mentioned above, the initial site of the O-acetylation remains debated [45]. Apparently, the O-acetyl transferase activity transfers the O-acetyl group to carbon C7 [55]. Once located at cell surface and exposed to higher pH, 7-O-acetyl group may migrate to position 9, explaining part of the above discrepancies observed between authors. Nevertheless, the O-acetyl transferase reaction appears to occur concertedly with sialyltransferases in the Golgi apparatus, which synthesizes the ganglioside acceptor, and to be acetyl-coenzyme A dependent [56]. Singularly, the relevant O-acetyl transferase responsible for ganglioside O-acetylation has not yet been isolated or identified. No gangliosidosis due to a defect of the O-acetyl transferase has been found so far. The direct purification of the O-acetyl transferase activity seems to be affected by an inherent sensitivity to membrane solubilization and remains difficult [56]. Different attempts for cloning the cDNA of the O-acetyl transferase by heterologous cell-cDNA library expression cloning resulted in the identification of proteins inducing O-acetylation but that are not O-acetyl transferases specific for ganglioside [57–61]. Of note, Vandamme-Feldhaus and Schauer found the O-acetyl transferase activity in fraction associated with membranes [62]. Thus, in the most recent attempt to isolate a true O-acetyl transferase Arming et al. screened the human genome database targeting the gene candidates that would fit the proposed model of a membrane-bound O-acetyltransferase located in the Golgi apparatus [63]. Their search resolved the candidate gene CASD1 (capsule structure 1 domain containing 1) [63]. Baumann et al., in the follow-up paper, gathered stronger evidence of the involvement of CAS 1 in sialic acid O-acetylation using CRISPR/Cas 9 gene edition and recombinant CAS 1 protein [64]. The authors were further able to O-acetylate free sialic acid molecule using a soluble recombinant CAS 1 protein [64]. However, the authors did not provide evidence that the recombinant form of CAS1 protein they used in their study was also able to transfer the O-acetyl group directly to the terminal sialic acid of GD3 ganglioside. An alternative explanation is that O-acetylation of sialic acid actually takes place at the sugar nucleotide level. CMP-O-acetyl-sialate could then act as a donor for synthesis of O-acetyl GD3 from GM3, by the action of GD3 synthase.
Surprisingly, O-acetyl-GD2 is concomitantly expressed with GD2 at the tumor cell surface. The ratio between the amount of O-acetyl-GD2 and the amount of GD2 varies from 10% up to 50% [39, 65]. This observation suggests another point of control in O-acetyl-GD2 biosynthesis. In fact, any given step in ganglioside biosynthesis requires the colocalization of the appropriate acceptor, sugar nucleotide transporter, and glycosyl-transferase activity. Thus, the synthesis of O-acetyl-GD2 can be regulated by the amount of acetyl-CoA concentrations within the Golgi apparatus [56]. The use of biochemical compounds that selectively block the transport from the endoplasmic reticulum to the Golgi apparatus further suggested that O-acetyl-GD2 can be synthesized from either O-acetyl-GD3 or GD2, in respect to either the nature or the localization of the glycosyltransferases expressed by the cells [40, 66]. In addition, the above mechanisms do not further exclude a possible turnover of O-acetyl esters bound to sialic acids of gangliosides controlled by sialate-O-acetylesterases [67]. Hence, the expression of O-acetyl-GD2 in a cell type may be the result of the conjunction of, at least, four parameters (Figure 2): the balance between two enzymatic systems, O-acetyl transferase and O-acetyl esterase; the activity of the N-acetyl-galactosaminyltransferase I that synthesizes O-acetyl-GD2 from O-acetyl-GD3; the activity of the α2-8 sialyltransferase II that forms GD2 from GM2; and the activity of the galactosyl-transferase II that forms GD1b from GD2 (Figure 2). Given the complexity of this biosynthetic model, the clarification of the mechanisms that regulate the expression of O-acetyl-GD2 remains challenging. This complexity further delays the elucidation of O-acetyl-GD2 functional role in tumorigenesis through gene edition approaches and its potential interest as a prognostic marker. Given that, mAbs specific for O-acetyl-GD2 remain the best reagents available to study O-acetyl-GD2 functions in tumor progression.
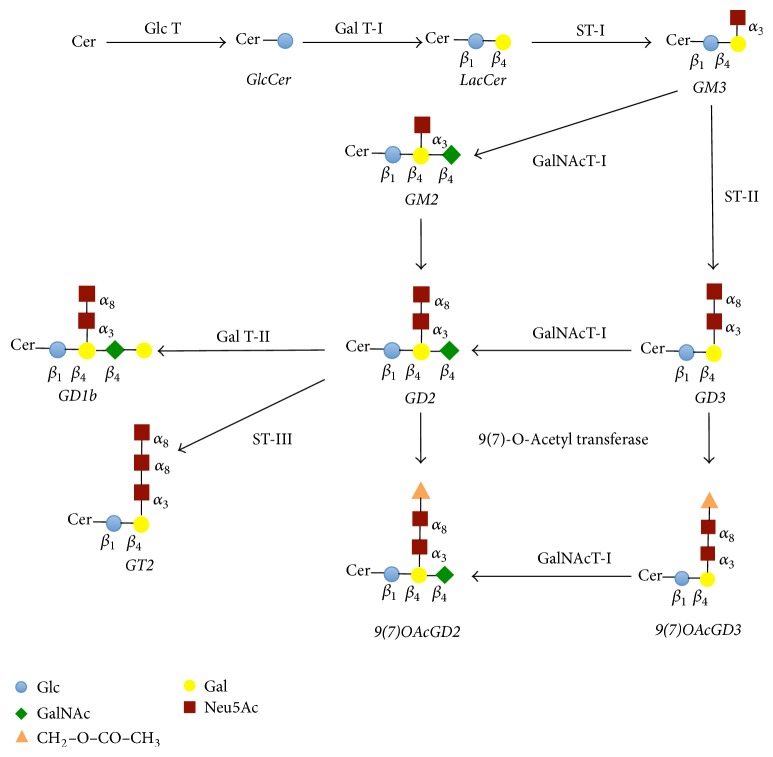
Figure 2.
Schematic representation of the main pathway of O-acetyl-GD2 ganglioside biosynthesis. GD2 ganglioside is synthesized by the action of N-acetyl-galactosyltransferase I, which transfers N-acetyl-galactosaminyl residue from UDP-N-acetyl-galactosamine to GD3 [66]. GD2 can be also formed by the action of alpha 2–8 sialyltransferase II, which transfers a sialic acid residue from CMP-sialic acid to GM2 [66]. After synthesis, GD2 can be converted into either GD1b or GT2. GD1b is formed by the action of galactosyltransferase II that transfers a galactose residue from UDP-galactose to GD2. GT2 is synthesized by the action of sialyltransferase III that transfers a sialic acid residue from CMP-sialic acid to GD2. Then, the O-acetyl group addition occurs in a postsynthetic fashion [66]. Thus, O-acetyl-GD2 can be synthesized either by the action of sialate-O-acetyltransferase, which transfers the O-acetyl group to GD2, or by the action of N-acetyl-galactosyltransferase I, which transfers N-acetyl-galactosaminyl residue from UDP-N-acetyl-galactosamine to O-acetyl-GD3. Cer, ceramide; GlcCer, glucosylceramide; LacCer, lactosylceramide; Gal, galactose; Glc, glucose; GalNAc, N-acetylgalactosamine; Neu5Ac, N-acetylneuraminic acid; Glc T, glucosyltransferase; Gal T, galactosyltransferase; ST, sialyltransferase; GalNacT, N-acetyl-galactosaminyltransferase; CASD 1, Cas 1 domain containing 9(7)-O-acetyl transferase.
4. Functional Aspects of O-Acetyl-GD2
In the absence of the characterization of a specific ganglioside O-acetyl transferase, the elucidation of the functional implications of O-acetylated gangliosides remains, somehow, a conundrum. The most elaborately studied O-acetylated ganglioside remains O-acetyl-GD3 because it was the first identified member of this family [5]. In the central nervous system, O-acetyl-GD3 appears to be involved in the extension of neuronal growth cone and neurite extension since these phenomena can be inhibited in vitro by anti-O-acetyl-GD3 mAbs [68]. Elimination of O-acetyl-GD3 expression in the retina and adrenal of transgenic mice gave variable abnormalities in development [69]. Postnatally, O-acetyl-GD3 species defines the epitope for anti-CDw60 antibodies in human lymphocytes [70], although similar structure on glycoprotein glycans also contributes [71]. Additional evidence indicates that O-acetyl-GD3 is involved as cell surface structures in lymphocyte activation [72, 73] and as intracellular substances in the regulation of apoptosis [74, 75]. In tumor cells, such as melanoma, O-acetyl-GD3 seems to contribute to tumor cell proliferation [76]. In glioblastoma, it may also promote tumor cell survival [77]. Similar observations were reported in acute lymphoblastic leukemia cells [74, 78] in which O-acetyl-GD3 seems further to contribute to their drug resistance capacity [79]. Importantly, the evidence for the antiapoptotic functions of O-acetyl-GD3 in the above studies remains indirect. In addition to the effects of mAbs specific for O-acetyl-GD3 [68, 72, 73, 80], they consist of the effects induced by the addition of O-acetyl-GD3 into cells [74, 75] and the effect of the viral 9-O-acetylesterase [69, 76, 77, 79].
In contrast to O-acetyl-GD3, very little is known about the biological role of O-acetyl-GD2. We showed that O-acetyl-GD2 is a proapoptotic constituent in tumor cells activated on binding with hostile antibodies [80]. While O-acetyl-GD2 can transmit signals resulting in apoptosis, the precise mechanisms induced by the binding of O-acetyl-GD2 antibody to O-acetyl-GD2-expressing tumor cells leading to apoptosis require further investigation. In the case of GD2, initial indications suggest that anti-GD2 mAbs induce apoptosis of SCLC cells by interfering with the association of GD2 ganglioside to ß1-integrin and focal adhesion kinase, which triggers the p38-dependent apoptotic pathway [81]. Data obtained with antibody 14G2a in neuroblastoma cells further suggested that apoptosis induced by anti-GD2 mAbs resulted in activation of both extrinsic and intrinsic caspase-dependent and caspase-independent apoptotic pathways [82]. In melanoma cells binding of antibody 3F8 resulted in the activation of caspases 3, 7, and 8, the release of cytochrome c and AIF, and the downregulation of both survivin and Apaf-1 without the activation of caspase 9 [83]. Of note, both mAbs 14G2a and 3F8 cross-react with O-acetyl-GD2 [40, 65]. Therefore, it is not excluded that the mechanisms evidenced in these studies also involved O-acetyl-GD2. Hence, O-acetyl-GD2 may behave very similarly to GD2 in mediating apoptosis in the GD2/OAcGD2-expressing tumor cells [81, 83–85], in disagreement with the antiapoptotic role evidenced for O-acetyl-GD3 [74, 77–79].
5. Distribution of Ganglioside O-Acetyl-GD2 in Normal Tissues
Beside many unknowns regarding its biosynthesis and biological roles, O-acetyl-GD2 provides an opportunity to develop new immunotherapeutic strategies based on therapeutic antibodies, cancer vaccines, and adoptive transfer of lymphocytes, genetically engineered to acquire tumor cell specificity. Immunotherapies of cancers exploit the fact that tumor cells often expressed antigen molecules on their surface that can be detected by specific antibodies. Such molecules are known as tumor-associated antigens (TAA). When the same or related antigenic determinant is expressed on human cells or tissues other than the intended target tissue, binding of the antibody to this tissue may be observed. Nontarget tissue binding may have serious consequences, leading to on-target off-tumor toxicities. Accordingly, anti-TAA mAbs may cross-react with other antigens expressed by normal tissues. It is therefore advisable to establish the TAA and the cross-reactivity profile of the anti-TAA antibody before initiating any experiment. The most common approach to assess antibody cross-reactivity is immunohistochemistry or immunofluorescence using animal and human tissues to allow comparison of the results. Within the U.S. Food and Drug Administration (FDA), the Center for Biologics Evaluation and Research (CBER) provides, and regularly updates, “points to consider (PTC) in the manufacture and testing of mAb products for human use” (http://www.fda.gov/cber/gdlns/ptc mab.pdf) [86].
Thus, our group studied the O-acetyl-GD2 distribution in healthy tissues by immunohistochemistry using the anti-O-acetyl-GD2 mAb 8B6 [39] according to the FDA guidelines [86]. We found that, in contrast to GD2, O-acetyl-GD2 was not detected on peripheral nerves [39]. As mentioned earlier, the therapeutic use of anti-GD2 mAbs is associated with important neurotoxic effects in patients, due to the cross-reactivity of anti-GD2 mAbs normal nerve fibers [32, 35]. Hence, our results suggest that mAbs specific for O-acetyl-GD2 should be less toxic because they do not bind to peripheral nerves [39]. Some other side effects observed in patients after anti-GD2 mAb infusions include hematopoietic suppression [16] and a syndrome of inappropriate antidiuretic hormone [35]. These side effects may be related to the possible immune recognition of GD2 on mesenchymal stromal cells in the marrow microenvironment and the anti-GD2 mAb cross-reactivity with the posterior lobe of the pituitary gland [35]. There was also slight reactivity with Purkinje cells, the Bergmann glia in the cerebellum, and the dorsal horns in the spinal cord. Furthermore, mAb 8B6 did not show any binding either to mesenchymal stromal cells in the bone marrow or to the posterior lobe of the pituitary gland. These data indicate that mAb 8B6 presents a very interesting safety reactivity profile for its clinical use.
6. Distribution of Ganglioside O-Acetyl-GD2 in Malignant Tissues
We examined the immunohistochemical O-acetyl-GD2 expression in a number of malignant tissues and found that mAb 8B6 showed strong reactivity with neuroectodermic tumor biopsy tissues, such as melanoma and neuroblastoma [39] similar to previous investigations [40, 65]. In vitro data further demonstrated a high expression of O-acetyl-GD2 at the tumor cell surface by Scatchard analysis, with an average of sites/cell ranging from 50,000 sites/cell up to 5 × 106 sites/cell [39]. Importantly, we showed that the amount of O-acetyl-GD2 molecules present at the cell surface was comparable, though lower, to that of GD2 epitope [39]. Taken together, these data suggest that GD2 is differentially acetylated in normal and tumor tissue and that normal tissues expressing GD2 may not express O-acetyl-GD2. This prompted us to investigate the expression of O-acetyl-GD2 in glioblastoma multiforme (GBM) [87], a cancer that is known to express GD2 [20]. We confirmed the presence of O-acetyl-GD2 on GBM biopsies obtained after surgical resection. We also demonstrated high O-acetyl-GD2 expression on GBM cell lines and patient-derived tumor cells [87]. Of note, GBM is a lethal and therapy-resistant brain cancer comprised of several tumor cell subpopulations, including glioblastoma stem cells (GSCs) [88]. As mentioned above, these cells demonstrate resistance to current chemoradiotherapeutic options and are believed to reinitiate malignancies after initial responses to therapies [25, 89]. Therefore, new therapeutic approaches must consider eliminating both GSCs and the entire bulk of the tumor. The markers used to define GSCs have been however in constant evolution since the evidence of a CD133-positive subpopulation in glioblastoma that retained stem-like properties and were capable of establishing glioblastoma tumors in mice with similar phenotype to those of the patients [88]. Interestingly, Battula et al. [24] reported that in breast carcinomas ganglioside GD2 is a marker of breast carcinoma stem cells capable of initiating tumors at a higher frequency than GD2-negative cells. Of note, they used in their study the anti-GD2 mouse mAb 14G2a that, as mentioned earlier, cross-reacts with O-acetyl-GD2 ganglioside [40]. Thus, O-acetyl-GD2 may be also expressed by GD2-positive cancer stem cells, if not involved in cancer cell stemness. However, over the past decade, multiple other cancer stem cell markers have been identified challenging a reliable identification of tumor-specific antigen to be used for anti-GSC immunotherapies [90]. This question is currently studied in our laboratory.
7. Immunogenicity of O-Acetyl-GD2
Being a carbohydrate antigen, O-acetyl-GD2 possesses identical biochemical structures between species, even in very distant species. Thus, specific antibody specific for O-acetyl-GD2 expressed by human tumor cells can easily cross-react with the antigen in animals. Moreover, it is a T cell-independent antigen, an interesting characteristic in tumors that are either poorly immunogenic for T cells or that evade T cells mainly by downregulating or losing human leukocyte antigen (HLA) expression. However, one of the most challenges to generate mAb against such antigen remains the low efficiency of the ganglioside immunization [91, 92]. This is due to poor immunogenicity of carbohydrate antigens. Repeated injection of gangliosides alone in human or in mouse is not enough for inducing the synthesis of anti-ganglioside antibodies in vaccinated host. The reason for their poor immunogenicity may be due, in part, to the phylogenetic conservation of ganglioside structures resulting in tolerance. In addition, carbohydrate antigens generally invoke a T cell-independent immune response, during which IgM can be typically driven into IgG3 in naive mice. For this reason, gangliosides are generally classified as T-independent type 2 antigen. Activation of specific B cells to these antigens in the absence of MHC class II-restricted T cell help requires antigen receptor cross-linking. However, this cannot be achieved with small antigens such as gangliosides. Thus, optimization of the immunization protocols against O-acetylated gangliosides is required to generate high-titers of both affinity-matured and class-switched antibodies for the production of mAbs as tool for research, diagnosis, and therapy. Another approach to circumvent the limitation imposed by the intrinsic immunogenicity of ganglioside consists in the in vitro isolation of anti-ganglioside antibody fragments based on the screening of a large library followed by refined mutagenesis [93, 94].
Given all these limitations, our group used whole-cell immunization protocol to generate the mouse mAb 8B6 specific for O-acetylated-GD2 [95]. The antibody 8B6 (IgG3, kappa) was derived from A/J mice immunized with LAN-1 neuroblastoma cells [95]. The specificity of antibody 8B6 for O-acetyl-GD2 was confirmed by immunostaining on thin-layer chromatography. When tested on total neuroblastoma ganglioside, antibody 8B6 stained exclusively O-acetyl-GD2 [95]. We also calculated the affinity of antibody 8B6 for O-acetylated GD2 to be 32 nM [39]. In addition, we evidenced the structure of the variable VH and VL gene encoding 8B6 hybridoma [95]. Surprisingly, the VH segment of hybridoma 8B6 revealed the presence of somatic mutations, suggesting the occurrence of an affinity maturation process [95].
As mentioned above, targeting GD2 has been a matter of concern due to the possibility of inducing autoimmune responses against peripheral nerves. Indeed, in most neuropathies of immunological origin, endogenous gangliosides have been shown to be the target of the autoimmune reactions [96]. Thus, the tumor-specific expression of O-acetyl-GD2 in some human tumors suggests that the induction of an effective immune response against these antigens may be useful for patients with antigen-positive tumors. The absence of expression in normal tissue allows for increased immune responses to immunization while precluding self-targeted reactions.
An original strategy to elicit an immune response against ganglioside antigen relies on the development of anti-idiotypic mAbs as antigen surrogates. Such an antibody represents the internal image of the antigen. Thus, anti-idiotype antibodies can act as antigens, inducing a response against the original antigen. A remarkable advantage of anti-idiotype mAbs is the fact that the constant regions of the anti-idiotypic antibody can serve to boost antitumor immune responses [97]. This is a useful strategy to induce an antibody response towards a ganglioside, which is a weak immunogenic molecule in itself. Furthermore, anti-idiotypic antibody provides important tools for the immune monitoring of clinical trial with anti-gangliosides antibodies. An example of a vaccine development using this strategy is given by racotumomab, a murine anti-idiotypic antibody raised from the anti-de-N-glycolyl-GM3 mAb P3 [98]. Racotumomab had been used in several clinical trials and its safety and efficacy were assessed in different tumor localizations: melanoma, breast, and lung cancers. More recently, there was a specific interest in pediatric tumors expressing N-glycosylated gangliosides. Racotumomab has now reached the Phase III clinical trials with possible indication in lung cancer [99] and a possible extent to pediatric tumors [100]. Anti-idiotypic mAbs are conceptually easy to generate after immunization of mouse with the parental mAb. However, generating one remains challenging since it requires tight selection, as most of the epitopes on the parental mAb will be irrelevant.
8. Passive Immunotherapy with Anti-O-acetyl-GD2 mAbs
MAbs have demonstrated their potential as anticancer therapies. Since the first approval of rituximab—a chimeric mAb targeting CD20—for the treatment of patients with lymphoma in 1994, more than 10 therapeutic antibodies have been approved for passive immunotherapy of cancer; all of them are directed against protein antigens. Although a long list of tumor-associated carbohydrate antigens has been identified over the past two decades, many of the clinical trials did not proceed beyond Phase I/II studies. In this regard, with the approval of dinutuximab in 2015, GD2 became the first glycan antigen proven to be effective target antigen for cancer immunotherapy [1, 34]. Three other immunotherapeutic strategies are currently developed to enhance the potency of anti-GD2 immunotherapy such as immunocytokines, bispecific antibodies, GD2-specific chimeric antigen receptor T cells, and GD2 vaccines. However, given the tissue distribution pattern of O-acetyl-GD2, the O-acetyl derivative of GD2 provides a potential opportunity to develop safer immunotherapeutic strategies.
Our group analyzed the antitumor activity and the preclinical toxicity of the mAb 8B6 using different animal models, according to the putative mechanisms of action of dinutuximab. Dinutuximab (ch14.18) and other anti-GD2 antibodies have been shown to induce ADCC as well as CDC in GD2-expressing cell lines [31, 101]. However, ADCC is considered as an important clinical mechanism for anti-GD2 immunotherapy [102, 103]. In this respect, anti-O-acetyl-GD2 mAbs seem to be particularly effective as compared to anti-GD2 antibodies in mediating ADCC against O-acetyl-GD2-expressing tumor cells [39, 87, 104]. We also found that anti-O-acetyl-GD2 mAb antibodies induce significant CDC in addition to ADCC in vitro [39]. Since anti-GD2 antibody CDC activity is believed to be responsible for the pain side effects [32] an overdrive of CDC may also be desirable to further enhance anti-O-acetyl-GD2 mAbs antitumor activity.
As mentioned earlier, our group reported a possible role of O-acetyl-GD2 in tumor cell death with the mAb 8B6, in addition to its immunological cytotoxicity [80]. The cell death induced by the binding of antibody 8B6 on the target cancer cells triggered the p38-signaling pathway. This was correlated with a cycle arrest of the cell, an increase in p21 protein levels, and the expression of apoptosis-associated proteins such as phospho-p38, BAX, cytochrome c in cytoplasm, and cleaved caspase 3 [80]. Peculiarly, this mechanism of killing requires a far higher concentration of antibody than is typically required for ADCC and CDC but not one that is compatible with serum concentration commonly observed in patients with melanoma and neuroblastoma following the infusion of anti-GD2 mAbs [105]. The precise mechanisms by which antibodies specific for O-acetyl-GD2 trigger tumor cell death still require clarification. While they seem to be influenced by O-acetyl-GD2 density at the tumor cell surface, they are also influenced by the antibody isotype [104] similarly to anti-GD2 mAbs [83]. Thus, the proapoptotic activity of anti-O-acetyl-GD2 mAbs cannot be fully explained by their sole antigen recognition activity. Finally, we demonstrated the implication of this proapoptotic activity on the antitumor efficacy of antibody 8B6 in vivo in human neuroblastoma IMR5 tumor-bearing NOD-SCID mice, which lack NK cell-cytotoxic activity and circulating complement [80]. From a clinical stand point, the apoptosis inducing activity of anti-O-acetyl-GD2 mAb 8B6 seems, however, very promising when applied to cancer therapy. This property may be important in the treatment of tumors that have evolved complex mechanisms to protect themselves from ADCC and CDC.
To facilitate clinical development of therapeutic antibodies targeting O-acetyl-GD2, our group also developed a mouse/human IgG1 chimeric version of antibody 8B6 [104]. The chimeric IgG1 c.8B6 antibody was obtained from the mouse mAb 8B6 to O-acetyl-GD2 and expressed in CHO-S cells. It retains the same antigen binding affinity and specificity as its parental mouse mAb [104]. We further showed that chimeric 8B6 antibody was able to inhibit NXS2 liver metastasis as efficiently as dinutuximab (ch14.18) [104]. More importantly, we performed studies in rat demonstrating that intravenous c.8B6 treatment did not induce allodynia as compared to ch14.18 [104]. In addition to the absence of O-acetyl-GD2 expression on nerve fibers, the lack of allodynic properties of anti-GD2 antibodies provided an important rationale for the clinical application of chimeric 8B6 antibody in patients with O-acetyl-GD2-expressing tumors. Clinical trial of mAb c.8B6 is eagerly awaited.
9. Adoptive Immunotherapy with O-Acetyl-GD2-Specific CARs
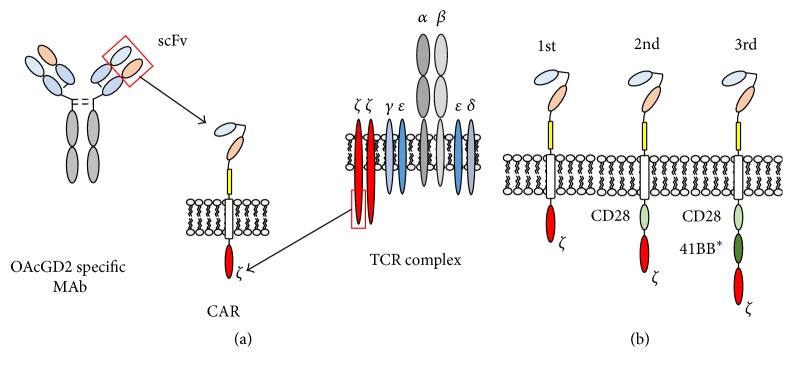
Chimeric antigen receptor (CAR) based adoptive immunotherapy is an attractive approach to treat patients with cancer as this strategy can combine the specificity of mAb with the active biodistribution, expansion potential, long-term persistence, and cytotoxic function of effector immune-cells [106, 107]. The technology holds great promise for cancer therapy and has generated breakthrough responses in recent years including 91% complete remission rates in patients with CD19-positive B cell acute lymphoblastic leukemia and complete tumor regression in patients with bulky CD19-positive B cell lymphoma [108–111]. CAR expressing effector cells can recognize tumor cells in a Major Histocompatibility Complex/Human Leukocyte Antigen (MHC-HLA) independent manner; thus the recognition of tumor cells is not affected by two key tumor escape mechanisms: downregulation of MHC Class 1 or Class 2 molecules and altered antigen processing [106, 112, 113]. CARs are derived from a single chain fragment variable (scFv) of mAb linked through a spacer and transmembrane domains to intracellular signaling domains forming a unique fusion protein (Figure 3(a)) [114]. While the scFv binds the tumor antigen, the signaling domains are responsible for initiating the activating signals in effector cells leading to tumor elimination and expansion of CAR expressing cells resulting in long-term tumor surveillance. Given the promising potential of CAR based therapies, the development of O-acetyl-GD2-specific CARs may provide an effective therapeutic approach for patients with several solid malignancies.
Figure 3.
Structure of O-acetyl-GD2 CAR examples. (a) An O-acetyl-GD2-specific mAb derived scFv is linked through a spacer and a transmembrane domain to the T cell receptor (TCR) complex CD3ζ chain intracellular signaling domain. (b) Additional costimulatory endodomains are shown in 2nd- and 3rd-generation CARs derived from CD28 or CD28 and 41BB, respectively. ∗41BB may be exchanged to other domains such as OX40 or ICOS.
Results from early phase clinical studies showed that the incorporation of endodomains from costimulatory receptors (Figure 3(b)) is critical for potent antitumor effect [106, 115–117]. The first GD2-CAR was developed by Rossig et al. based on scFv derived from the GD2-specific mAb 14G2a [118, 119] and was tested in Phase 1 clinical study [120, 121]. This trial evaluated a 1st-generation GD2-specific CAR in children with relapsing/refractory neuroblastoma and compared the safety, persistence, and antitumor efficacy of two effector cell populations: activated T cells (ATC) and Epstein-Barr virus specific cytotoxic lymphocytes (EBV-CTLs) [120]. No dose-limiting toxicity was found, the therapy was well tolerated, and some patients achieved long-term complete tumor regression. Interestingly, the GD2-CAR expressing EBV-CTLs persisted significantly longer and not surprisingly long-term effector cell persistence was also associated with better antitumor efficacy [121]. Given the limited persistence of adoptively transferred GD2-CAR T cells, a 3rd-generation GD2-CAR incorporating CD28 and OX40 costimulatory endodomains was developed by Pulè et al. [122]. A Phase 1 clinical trial recently completed accrual testing this 3rd-generation CAR in children with relapsed/refractory neuroblastoma and the results of this study are expected in 2016 (NCT01822652). Another Phase 1 study (NCT01953900) is open for accrual using the same 3rd-generation CAR construct expressed in varicella zoster virus specific CTLs (VZV-CTLs) testing the approach in patients with osteosarcoma. This strategy will assess the effect of VZV vaccination on the in vivo expansion and persistence of transgenic VZV-CTLs.
Given the restricted expression of O-acetyl-GD2 on cancer cell and its presence on certain cancer stem cells, developing CARs to target O-acetyl-GD2 is a promising approach to help patients. To find the CAR constructs with the antitumor potential resulting in complete regression of O-acetyl-GD2-expressing solid tumors, the construct and the effector cell type will have to be carefully selected for the specific tumor type and several variables must be carefully evaluated.
The scFvs, particularly those targeting GD2, can induce clustering of CARs resulting in chronic tonic signaling which can exhaust effector cells leading to decreased cytotoxic capacity, proliferation, and persistence after adoptive transfer [123]. Therefore, O-acetyl-GD2-CAR should derive from scFv with minimal tonic signaling induction. Several clinical trials have detected B and T cell responses against mouse-derived scFvs, ultimately leading to the elimination of CAR T cells and therefore limiting their ability to effectively eliminate tumors and provide long-term tumor surveillance. Thus, the use of full mouse-scFv can negatively affect the efficacy of adoptive cell therapies by inducing immune responses [124–126]. Fully human or at least humanized scFvs are likely better candidates for CAR development as these scFvs are the least likely to induce an adoptive immune response.
In addition, to find the optimal scFv, the spacer and transmembrane domains need attention as well. Accumulating evidence suggests that the spacer region of the CAR is not a simple bridge to the transmembrane domain; rather it plays an important role in building and maintaining the immunologic synapse and therefore providing the optimal activating signals for the effector cells. Studies have shown that the optimal length of the spacer region is target antigen-dependent and different tumor targets require different space lengths to optimize the CAR function [127, 128]. The spacer length may need to be adjusted specific antigens to resemble the distance between effector and target cells similarly to the physiologic immune synapse. A recent publication confirmed the importance of CAR design by showing that, for GD2, the IgG Fc region appeared to be the optimal spacer [129]. However, with this type of spacer there is a theoretical risk of engagement of CAR expressing T cells with FcR-expressing cells resulting in both off-target toxicity of myeloid cells and diversion of the CAR T cells from their intended effector function [130, 131].
The transmembrane domain is not only responsible for keeping the CAR membrane bound, but also important for stable CAR expression. Transmembrane domains can be derived from several transmembrane proteins including CD3ζ, CD4, CD8, or CD28 molecules [113]. The initial CAR design incorporated CD3ζ derived transmembrane sequences. On one hand this domain allows incorporation of the CAR into the T cell receptor complex and improves signaling [132, 133]. However, CAR cell surface expression is less stable compared to CD28 transmembrane domain [117]. It is not clear which transmembrane domain is optimal for CAR based therapies and testing distinct versions of this domain in the context of a specific target antigen and various effector cell population may be necessary. In addition, the incorporation of costimulatory endodomains into the CAR structure can significantly improve the antitumor potential of effector cells [106]. These endodomains can derive, for example, from CD28, OX40, ICOS, or 4-1BB [110, 115, 117, 122, 134–137] and the type and combination of these endodomains may require further testing depending on the effector cell used to express the O-acetyl-GD2-specific CAR.
The role and difference of specific effector cell population have gained more attention in recent years as another important aspect for effective adoptive immunotherapy. In the context of 1st-generation CARs, virus specific T cells (VSTs) can provide additional costimulatory signals and improve CAR T cell persistence and antitumor functions [120, 121, 138]. Manufacturing of CAR-VSTs is significantly more complex compared to polyclonal activated T cells (i.e., T cells stimulated with CD3-CD28 beads and IL-2) and it is still to be elucidated whether VSTs provide any advantage over activated T cells when 2nd- or 3rd-generation CARs are used.
To date, polyclonal activated T cells have been the most common CAR expressing effectors, but other cell populations may become promising platforms for CAR based immunotherapy. Effector T cells (CD45RO-pos, CD62L-neg) are a logical choice for immunotherapy due to their high cytotoxic potential; however, these cells have limited persistence which is essential for adoptive immunotherapy. Long-lived, central memory or stem cell memory T cells have significantly better expansion potential, persist longer, and have been shown to induce superior antitumor effects in preclinical studies making this population particularly attractive for adoptive immunotherapy [121, 139–141]. Other cell populations such as natural killer T cells (NKTs) hold a great promise for the treatment of neuroblastoma. NKTs are innate lymphocytes that recognize glycolipids expressed by the MHC Class I-like CD1d molecule [142]. NKTs actively traffic to neuroblastoma tissues and destroy CD1d-positive, cancer supporting tumor-associated macrophages (TAMs), and the presence of NKTs in neuroblastoma is associated with improved outcomes [142–146]. GD2-CARs can be expressed in NKTs. The generated CAR-NKTs can destroy both GD2-positive neuroblasts and CD1d-positive TAMs resulting in a potent antitumor effect in an aggressive metastatic neuroblastoma model in humanized mice [147].
Taken into account the above parameter, our group is currently developing and testing the 8B6 mAb based CARs expressed against neuroblastoma to find a potent and safe immunotherapeutic approach for patients. As trafficking may improve at the tumor sites, O-acetyl-GD2-CAR expressing effector cells may reach areas in the central nervous system (CNS) with O-acetyl-GD2-expressing resulting in on-target/off-tumor side effects. The two clinical studies previously testing GD2-CARs (NCT00085930; NCT01822652) have not shown thus far significant CNS-specific side effects and the final confirmation of safety for O-acetyl-GD2-CARs will also have to come from Phase 1 studies testing distinct effector lymphocyte subsets.
10. Conclusion
To date, passive immunotherapy with anti-GD2 therapeutic antibody in patients with neuroblastoma is the first successful glycan-targeted immunotherapy. Thus, targeting glycan antigens is a feasible therapeutic option for cancer immunotherapy, beside many unknown regarding their biological functions. The absence of O-acetyl-GD2 expression on nerve fibers and the lack of allodynic properties of anti-GD2 antibodies, which are believed to play a major role in mediating anti-GD2 therapeutic antibodies dose-limiting side effects, provide an important rationale for the clinical application of immunotherapeutic strategies in patients with O-acetyl-GD2-expressing tumors. Better tolerance shall allow the development of next generation of targeted immunotherapies. For example, the therapeutic antibodies can be engineered into more potent molecules. In this regard, constant efforts are needed to assess the functions of this particular antigen in tumor cells, since this information should provide a mechanistic basis for the optimization of the rational design of anti-O-acetyl-GD2 therapeutic antibodies. Other applications include CAR cell therapy. Here, several parameters remain to be defined given that there is a fine interplay between the scFv, spacer, transmembrane, signaling domains, and the effector cell type. The current strategies widely used, however, may not be sufficient to account for all of these variables in the quest of finding the best O-acetyl-GD2-CAR, and large-scale approaches are necessary to find the construct in a specific effector cell type with the most potent antitumor efficacy. Lastly, for vaccine strategies, it remains necessary to design immunization protocols that allow a high-affinity IgG response for O-acetyl-GD2 because of their weak immunogenicity. To this end, anti-idiotypic mAbs represent an attractive approach.
Acknowledgments
The authors thank La Ligue Contre le Cancer for supporting Julien Fleurence's and Meriem Bahri's Ph. D. scholarship. This paper was written as a part of research project which received funding from the association Hubert Gouin—Enfance et Cancer.
Competing Interests
Stéphane Birklé is coinventor on pending patent applications that relate to the use of ganglioside O-acetyl-GD2 as therapeutic target for cancer immunotherapy. The work that led to the characterization of O-acetyl-GD2 as therapeutic target was conducted at the University of Nantes and was licensed by the spin-off company OGD2 Pharma SAS that Stéphane Birklé is a cofunder. At present, Denis Cochonneau is an employee at OGD2 Pharma SAS.
References
- 1.Yu A. L., Gilman A. L., Ozkaynak M. F., et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. New England Journal of Medicine. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Svennerholm L. Composition of gangliosides from human brain. Nature. 1956;177(4507):524–525. doi: 10.1038/177524b0. [DOI] [PubMed] [Google Scholar]
- 3.Yu R. K., Tsai Y.-T., Ariga T., Yanagisawa M. Structures, biosynthesis, and functions of gangliosides-an overview. Journal of Oleo Science. 2011;60(10):537–544. doi: 10.5650/jos.60.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu R. K., Ynagisawa M., Ariga T. Comprehensive Glycoscience. Oxford, UK: Elsevier; 2007. Glycosphingolipid structures; pp. 73–122. [Google Scholar]
- 5.Cheresh D. A., Reisfeld R. A., Varki A. P. O-Acetylation of disialoganglioside GD3 by human melanoma cells creates a unique antigenic determinant. Science. 1984;225(4664):844–846. doi: 10.1126/science.6206564. [DOI] [PubMed] [Google Scholar]
- 6.Chammas R., Sonnenburg J. L., Watson N. E., et al. De-N-acetyl-gangliosides in humans: unusual subcellular distribution of a novel tumor antigen. Cancer Research. 1999;59(6):1337–1346. [PubMed] [Google Scholar]
- 7.Slomiany B. L., Kojima K., Banas‐Gruszka Z., Murty V. L. N., Galicki N. I., Slomiany A. Characterization of the sulfated monosialosyltriglycosylceramide from bovine gastric muscosa. European Journal of Biochemistry. 1981;119(3):647–650. doi: 10.1111/j.1432-1033.1981.tb05656.x. [DOI] [PubMed] [Google Scholar]
- 8.Ando S., Yu R. K., Scarsdale J. N., Kusunoki S., Prestegard J. H. High resolution proton NMR studies of gangliosides. Structure of two types of GD3 lactones and their reactivity with monoclonal antibody R24. The Journal of Biological Chemistry. 1989;264(6):3478–3483. [PubMed] [Google Scholar]
- 9.Iber H., Zacharias C., Sandhoff K. The c-series gangliosides GT3, GT2 and GP1C are formed in rat liver golgi by the same set of glycosyltransferases that catalyse the biosynthesis of asialo-, a- and b-series gangliosides. Glycobiology. 1992;2(2):137–142. doi: 10.1093/glycob/2.2.137. [DOI] [PubMed] [Google Scholar]
- 10.Crespo P. M., Iglesias-Bartolomé R., Daniotti J. L. Ganglioside GD3 traffics from the trans-Golgi network to plasma membrane by a Rab11-independent and brefeldin A-insensitive exocytic pathway. The Journal of Biological Chemistry. 2004;279(46):47610–47618. doi: 10.1074/jbc.m407181200. [DOI] [PubMed] [Google Scholar]
- 11.Sonnino S., Mauri L., Chigorno V., Prinetti A. Gangliosides as components of lipid membrane domains. Glycobiology. 2007;17(1):1R–13R. doi: 10.1093/glycob/cwl052. [DOI] [PubMed] [Google Scholar]
- 12.Hakomori S.-I. The glycosynapse. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birklé S., Zeng G., Gao L., Yu R. K., Aubry J. Role of tumor-associated gangliosides in cancer progression. Biochimie. 2003;85(3-4):455–463. doi: 10.1016/S0300-9084(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 14.Feizi T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature. 1985;314(6006):53–57. doi: 10.1038/314053a0. [DOI] [PubMed] [Google Scholar]
- 15.Lammie G. A., Cheung N.-K. V., Gerald W., Rosenblum M., Cordon-Cardo C. Ganglioside GD2 expression in the human nervous system and in neuroblastomas—an immunohistochemical study. International Journal of Oncology. 1993;3(5):909–915. doi: 10.3892/ijo.3.5.909. [DOI] [PubMed] [Google Scholar]
- 16.Martinez C., Hofmann T. J., Marino R., Dominici M., Horwitz E. M. Human bone marrow mesenchymal stromal cells express the neural ganglioside GD2: a novel surface marker for the identification of MSCs. Blood. 2007;109(10):4245–4248. doi: 10.1182/blood-2006-08-039347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z.-L., Schwartz E., Seeger R., Ladisch S. Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Research. 1986;46(1):440–443. [PubMed] [Google Scholar]
- 18.Watanabe T., Pukel C. S., Takeyama H., et al. Human melanoma antigen AH is an autoantigenic ganglioside related to GD2. Journal of Experimental Medicine. 1982;156(6):1884–1889. doi: 10.1084/jem.156.6.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheresh D. A., Rosenberg J., Mujoo K., Hirschowitz L., Reisfeld R. A. Biosynthesis and expression of the disialoganglioside GD2, a relevant target antigen on small cell lung carcinoma for monoclonal antibody-mediated cytolysis. Cancer Research. 1986;46(10):5112–5118. [PubMed] [Google Scholar]
- 20.Mennel H. D., Bosslet K., Wiegandt H., Sedlacek H. H., Bauer B. L., Rodden A. F. Expression of GD2-epitopes in human intracranial tumors and normal brain. Experimental and Toxicologic Pathology. 1992;44(6):317–324. doi: 10.1016/S0940-2993(11)80218-6. [DOI] [PubMed] [Google Scholar]
- 21.Portoukalian J., David M., Richard M., Gain P. Shedding of GD2 ganglioside in patients with retinoblastoma. International Journal of Cancer. 1993;53(6):948–951. doi: 10.1002/ijc.2910530614. [DOI] [PubMed] [Google Scholar]
- 22.Kailayangiri S., Altvater B., Meltzer J., et al. The ganglioside antigen GD2 is surface-expressed in Ewing sarcoma and allows for MHC-independent immune targeting. British Journal of Cancer. 2012;106(6):1123–1133. doi: 10.1038/bjc.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibuya H., Hamamura K., Hotta H., et al. Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Science. 2012;103(9):1656–1664. doi: 10.1111/j.1349-7006.2012.02344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Battula V. L., Shi Y., Evans K. W., et al. Ganglioside GD2 identifies breast cancer stem cells and promotes tumorigenesis. The Journal of Clinical Investigation. 2012;122(6):2066–2078. doi: 10.1172/jci59735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips T. M., McBride W. H., Pajonk F. The response of CD24-/low/CD44+ breast cancer-initiating cells to radiation. Journal of the National Cancer Institute. 2006;98(24):1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 26.Cheever M. A., Allison J. P., Ferris A. S., et al. The prioritization of cancer antigens: A National Cancer Institute pilot project for the acceleration of translational research. Clinical Cancer Research. 2009;15(17):5323–5337. doi: 10.1158/1078-0432.ccr-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito M., Yu R. K., Cheung N.-K. V. Ganglioside GD2 specificity of monoclonal antibodies to human neuroblastoma cell. Biochemical and Biophysical Research Communications. 1985;127(1):1–7. doi: 10.1016/S0006-291X(85)80117-0. [DOI] [PubMed] [Google Scholar]
- 28.Gillies S. D., Lo K.-M., Wesolowski J. High-level expression of chimeric antibodies using adapted cDNA variable region cassettes. Journal of Immunological Methods. 1989;125(1-2):191–202. doi: 10.1016/0022-1759(89)90093-8. [DOI] [PubMed] [Google Scholar]
- 29.Mujoo K., Kipps T. J., Yang H. M., et al. Functional properties and effect on growth suppression of human neuroblastoma tumors by isotype switch variants of monoclonal antiganglioside GD2 antibody 14.18. Cancer Research. 1989;49(11):2857–2861. [PubMed] [Google Scholar]
- 30.Mujoo K., Cheresh D. A., Yang H. M., Reisfeld R. A. Disialoganglioside GD2 on human neuroblastoma cells: target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Research. 1987;47(4):1098–1104. [PubMed] [Google Scholar]
- 31.Zeng Y., Fest S., Kunert R., et al. Anti-neuroblastoma effect of ch14.18 antibody produced in CHO cells is mediated by NK-cells in mice. Molecular Immunology. 2005;42(11):1311–1319. doi: 10.1016/j.molimm.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Sorkin L. S., Otto M., Baldwin W. M., III, et al. Anti-GD2 with an FC point mutation reduces complement fixation and decreases antibody-induced allodynia. Pain. 2010;149(1):135–142. doi: 10.1016/j.pain.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung N.-K. V., Guo H., Hu J., Tassev D. V., Cheung I. Y. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. OncoImmunology. 2012;1(4):477–486. doi: 10.4161/onci.19864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhillon S. Dinutuximab: first global approval. Drugs. 2015;75(8):923–927. doi: 10.1007/s40265-015-0399-5. [DOI] [PubMed] [Google Scholar]
- 35.Yuki N., Yamada M., Tagawa Y., Takahashi H., Handa S. Pathogenesis of the neurotoxicity caused by anti-GD2 antibody therapy. Journal of the Neurological Sciences. 1997;149(2):127–130. doi: 10.1016/S0022-510X(97)05390-2. [DOI] [PubMed] [Google Scholar]
- 36.Siebert N., Eger C., Seidel D., et al. Pharmacokinetics and pharmacodynamics of ch14.18/CHO in relapsed/refractory high-risk neuroblastoma patients treated by long-term infusion in combination with IL-2. mAbs. 2016;8(3):604–616. doi: 10.1080/19420862.2015.1130196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kushner B. H., Kramer K., Modak S., Cheung N.-K. V. Successful multifold dose escalation of anti-GD2 monoclonal antibody 3F8 in patients with neuroblastoma: a phase I study. Journal of Clinical Oncology. 2011;29(9):1168–1174. doi: 10.1200/jco.2010.28.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lode H. N., Sibert N., Eger C. Interleukin-2 adds toxicity but not measurable activity in relapsed/refractory neuroblastoma patients treated with long term infusion of anti-GD2 antibody ch14.18/CHO. Pediatric Blood Cancer. 2015;62, Abstract PD-057 [Google Scholar]
- 39.Alvarez-Rueda N., Desselle A., Cochonneau D., et al. A monoclonal antibody to O-Acetyl-GD2 ganglioside and not to GD2 shows potent anti-tumor activity without peripheral nervous system cross-reactivity. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025220.e25220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sjoberg E. R., Manzi A. E., Khoo K.-H., Dell A., Varki A. Structural and immunological characterization of O-acetylated GD2: evidence that GD2 is an acceptor for ganglioside O-acetyltransferase in human melanoma cells. Journal of Biological Chemistry. 1992;267(23):16200–16211. [PubMed] [Google Scholar]
- 41.Varki A. Diversity in the sialic acids. Glycobiology. 1992;2(1):25–40. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bardor M., Nguyen D. H., Diaz S., Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. Journal of Biological Chemistry. 2005;280(6):4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 43.Siebert H. C., von der Lieth C. W., Dong X., et al. Molecular dynamics-derived conformation and intramolecular interaction analysis of the N-acetyl-9-O-acetylneuraminic acid-containing ganglioside GD1a and NMR-based analysis of its binding to a human polyclonal immunoglobulin G fraction with selectivity for O-acetylated sialic acids. Glycobiology. 1996;6(6):561–572. doi: 10.1093/glycob/6.6.561-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varki A., Diaz S. The release and purification of sialic acids from glycoconjugates: methods to minimize the loss and migration of O-acetyl groups. Analytical Biochemistry. 1984;137(1):236–247. doi: 10.1016/0003-2697(84)90377-4. [DOI] [PubMed] [Google Scholar]
- 45.Kamerling J. P., Schauer R., Shukla A. K., Stoll S., Van Halbeek H., Vliegenthart J. F. Migration of O-acetyl groups in N,O-acetylneuraminic acids. European Journal of Biochemistry. 1987;162(3):601–607. doi: 10.1111/j.1432-1033.1987.tb10681.x. [DOI] [PubMed] [Google Scholar]
- 46.Thurin J., Herlyn M., Hindsgaul O., et al. Proton NMR and fast-atom bombardment mass spectrometry analysis of the melanoma-associated ganglioside 9-O-acetyl-G(D3) Journal of Biological Chemistry. 1985;260(27):14556–14563. [PubMed] [Google Scholar]
- 47.Ostrander G. K., Bozlee M., Fukuda M., et al. Isolation and characterization of the major glycosphingolipids from the liver of the rainbow trout (Oncorhynchus mykiss): identification of an abundant source of 9-O-acetyl GD3. Archives of Biochemistry and Biophysics. 1991;284(2):413–421. doi: 10.1016/0003-9861(91)90317-c. [DOI] [PubMed] [Google Scholar]
- 48.Ren S., Ariga T., Scarsdale J. N., et al. Characterization of a hamster melanoma-associated ganglioside antigen as 7-O-acetylated disialoganglioside GD3. Journal of Lipid Research. 1993;34(9):1565–1572. [PubMed] [Google Scholar]
- 49.Manzi A. E., Sjoberg E. R., Diaz S., Varki A. Biosynthesis and turnover of O-acetyl and N-acetyl groups in the gangliosides of human melanoma cells. The Journal of Biological Chemistry. 1990;265(22):13091–13103. [PubMed] [Google Scholar]
- 50.Ruan S., Lloyd K. O. Glycosylation pathways in the biosynthesis of gangliosides in melanoma and neuroblastoma cells: relative glycosyltransferase levels determine ganglioside patterns. Cancer Research. 1992;52(20):5725–5731. [PubMed] [Google Scholar]
- 51.Yamashiro S., Ruan S., Furukawa K., et al. Genetic and enzymatic basis for the differential expression of GM2 and GD2 gangliosides in human cancer cell lines. Cancer Research. 1993;53(22):5395–5400. [PubMed] [Google Scholar]
- 52.Furukawa K., Soejima H., Niikawa N., Shiku H., Furukawa K. Genomic organization and chromosomal assignment of the human β1,4-N- acetylgalactosaminyltransferase gene. Identification of multiple transcription units. Journal of Biological Chemistry. 1996;271(34):20836–20844. doi: 10.1074/jbc.271.34.20836. [DOI] [PubMed] [Google Scholar]
- 53.Stoddart A., Zhang Y., Paige C. J. Molecular cloning of the cDNA encoding a murine sialic acid-specific 9-O-acetylesterase and RNA expression in cells of hematopoietic and non-hematopoietic origin. Nucleic Acids Research. 1996;24(20):4003–4008. doi: 10.1093/nar/24.20.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guimarães M. J., Bazan J. F., Castagnola J., et al. Molecular cloning and characterization of lysosomal sialic acid O-acetylesterase. The Journal of Biological Chemistry. 1996;271(23):13697–13705. doi: 10.1074/jbc.271.23.13697. [DOI] [PubMed] [Google Scholar]
- 55.Schauer R., Wember M., Do Amaral C. F. Synthesis of CMP-glycosides of radioactive N-acetyl-,N-glycoloyl-,N-acetyl-7-O-acetyl- and N-acetyl-8-O-acetylneuraminic acids by CMP-sialate synthase from bovine submaxillary glands. Hoppe-Seyler's Zeitschrift fur Physiologische Chemie. 1972;353(1):883–886. doi: 10.1515/bchm2.1972.353.1.883. [DOI] [PubMed] [Google Scholar]
- 56.Higa H. H., Butor C., Diaz S., Varki A. O-Acetylation and de-O-acetylation of sialic acids. O-Acetylation of sialic acids in the rat liver Golgi apparatus involves an acetyl intermediate and essential histidine and lysine residues—a transmembrane reaction? Journal of Biological Chemistry. 1989;264(32):19427–19434. [PubMed] [Google Scholar]
- 57.Ogura K., Nara K., Watanabe Y., Kohno K., Tai T., Sanai Y. Cloning and expression of cDNA for O-acetylation of GD3 ganglioside. Biochemical and Biophysical Research Communications. 1996;225(3):932–938. doi: 10.1006/bbrc.1996.1274. [DOI] [PubMed] [Google Scholar]
- 58.Kanamori A., Nakayama J., Fukuda M. N., et al. Expression cloning and characterization of a cDNA encoding a novel membrane protein required for the formation of O-acetylated ganglioside: a putative acetyl-CoA transporter. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(7):2897–2902. doi: 10.1073/pnas.94.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi W.-X., Chammas R., Varki A. Induction of sialic acid 9-O-acetylation by diverse gene products: implications for the expression cloning of sialic acid O-acetyltransferases. Glycobiology. 1998;8(2):199–205. doi: 10.1093/glycob/8.2.199. [DOI] [PubMed] [Google Scholar]
- 60.Satake H., Chen H. Y., Varki A. Genes modulated by expression of GD3 synthase in Chinese hamster ovary cells: evidence that the Tis21 gene is involved in the induction of GD3 9-O-acetylation. Journal of Biological Chemistry. 2003;278(10):7942–7948. doi: 10.1074/jbc.m210565200. [DOI] [PubMed] [Google Scholar]
- 61.Furukawa K., Aixinjueluo W., Kasama T., et al. Disruption of GM2/GD2 synthase gene resulted in overt expression of 9-O-acetyl GD3 irrespective of Tis21. Journal of Neurochemistry. 2008;105(3):1057–1066. doi: 10.1111/j.1471-4159.2008.05232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandamme-Feldhaus V., Schauer R. Characterization of the enzymatic 7-O-acetylation of sialic acids and evidence for enzymatic O-acetyl migration from C-7 to C-9 in bovine submandibular gland. Journal of Biochemistry. 1998;124(1):111–121. doi: 10.1093/oxfordjournals.jbchem.a022069. [DOI] [PubMed] [Google Scholar]
- 63.Arming S., Wipfler D., Mayr J., et al. The human Cas1 protein: a sialic acid-specific O-acetyltransferase? Glycobiology. 2011;21(5):553–564. doi: 10.1093/glycob/cwq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baumann A.-M. T., Bakkers M. J. G., Buettner F. F. R., et al. 9-O-Acetylation of sialic acids is catalysed by CASD1 via a covalent acetyl-enzyme intermediate. Nature Communications. 2015;6, article 7673 doi: 10.1038/ncomms8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye J. N., Cheung N.-K. V. A novel O-acetylated ganglioside detected by anti-GD2 monoclonal antibodies. International Journal of Cancer. 1992;50(2):197–201. doi: 10.1002/ijc.2910500207. [DOI] [PubMed] [Google Scholar]
- 66.Sjoberg E. R., Varki A. Kinetic and spatial interrelationships between ganglioside glycosyltransferases and O-acetyltransferase(s) in human melanoma cells. Journal of Biological Chemistry. 1993;268(14):10185–10196. [PubMed] [Google Scholar]
- 67.Butor C., Diaz S., Varki A. High level O-acetylation of sialic acids on N-linked oligosaccharides of rat liver membranes: differential subcellular distribution of 7- and 9-O-acetyl groups and of enzymes involved in their regulation. Journal of Biological Chemistry. 1993;268(14):10197–10206. [PubMed] [Google Scholar]
- 68.Araujo H., Menezes M., Mendez-Otero R. Blockage of 9-O-acetyl gangliosides induces microtubule depolymerization in growth cones and neurites. European Journal of Cell Biology. 1997;72(3):202–213. [PubMed] [Google Scholar]
- 69.Varki A., Hooshmand F., Diaz S., Varki N. M., Hedrick S. M. Developmental abnormalities in transgenic mice expressing a sialic acid-specific 9-O-acetylesterase. Cell. 1991;65(1):65–74. doi: 10.1016/0092-8674(91)90408-q. [DOI] [PubMed] [Google Scholar]
- 70.Kniep B., Peter-Katalinić J., Flegel W., Northoff H., Rieber E. P. CDw 60 antibodies bind to acetylated forms of ganglioside GD3. Biochemical and Biophysical Research Communications. 1992;187(3):1343–1349. doi: 10.1016/0006-291X(92)90450-Y. [DOI] [PubMed] [Google Scholar]
- 71.Fox D. A., He X., Abe A., et al. The T lymphocyte structure CD60 contains a sialylated carbohydrate epitope that is expressed on both gangliosides and glycoproteins. Immunological Investigations. 2001;30(2):67–85. doi: 10.1081/IMM-100104017. [DOI] [PubMed] [Google Scholar]
- 72.Vater M., Kniep B., Groß H.-J., Claus C., Dippold W., Schwartz-Albiez R. The 9-O-acetylated disialosyl carbohydrate sequence of CDw60 is a marker on activated human B lymphocytes. Immunology Letters. 1997;59(3):151–157. doi: 10.1016/S0165-2478(97)00116-8. [DOI] [PubMed] [Google Scholar]
- 73.Higgs J. B., Zeldes W., Kozarsky K., et al. A novel pathway of human T lymphocyte activation: identification by a monoclonal antibody generated against a rheumatoid synovial T cell line. Journal of Immunology. 1988;140(11):3758–3765. [PubMed] [Google Scholar]
- 74.Mukherjee K., Chava A. K., Mandal C., et al. O-acetylation of GD3 prevents its apoptotic effect and promotes survival of lymphoblasts in childhood acute lymphoblastic leukaemia. Journal of Cellular Biochemistry. 2008;105(3):724–734. doi: 10.1002/jcb.21867. [DOI] [PubMed] [Google Scholar]
- 75.Malisan F., Franchi L., Tomassini B., et al. Acetylation suppresses the proapoptotic activity of GD3 ganglioside. Journal of Experimental Medicine. 2002;196(12):1535–1541. doi: 10.1084/jem.20020960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Birklé S., Ren S., Slominski A., Zeng G., Gao L., Yu R. K. Down-regulation of the expression of O-acetyl-GD3 by the O- acetylesterase cDNA in hamster melanoma cells: effects on cellular proliferation, differentiation, and melanogenesis. Journal of Neurochemistry. 1999;72(3):954–961. doi: 10.1046/j.1471-4159.1999.0720954.x. [DOI] [PubMed] [Google Scholar]
- 77.Birks S. M., Danquah J. O., King L., Vlasak R., Gorecki D. C., Pilkington G. J. Targeting the GD3 acetylation pathway selectively induces apoptosis in glioblastoma. Neuro-Oncology. 2011;13(9):950–960. doi: 10.1093/neuonc/nor108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kniep B., Kniep E., Özkucur N., et al. 9-O-acetyl GD3 protects tumor cells from apoptosis. International Journal of Cancer. 2006;119(1):67–73. doi: 10.1002/ijc.21788. [DOI] [PubMed] [Google Scholar]
- 79.Parameswaran R., Lim M., Arutyunyan A., et al. O-acetylated N-acetylneuraminic acid as a novel target for therapy in human pre-B acute lymphoblastic leukemia. Journal of Experimental Medicine. 2013;210(4):805–819. doi: 10.1084/jem.20121482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cochonneau D., Terme M., Michaud A., et al. Cell cycle arrest and apoptosis induced by O-acetyl-GD2-specific monoclonal antibody 8B6 inhibits tumor growth in vitro and in vivo. Cancer Letters. 2013;333(2):194–204. doi: 10.1016/j.canlet.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 81.Aixinjueluo W., Furukawa K., Zhang Q., et al. Mechanisms for the apoptosis of small cell lung cancer cells induced by anti-GD2 monoclonal antibodies: roles of anoikis. The Journal of Biological Chemistry. 2005;280(33):29828–29836. doi: 10.1074/jbc.m414041200. [DOI] [PubMed] [Google Scholar]
- 82.Kowalczyk A., Gil M., Horwacik I., Odrowaz Z., Kozbor D., Rokita H. The GD2-specific 14G2a monoclonal antibody induces apoptosis and enhances cytotoxicity of chemotherapeutic drugs in IMR-32 human neuroblastoma cells. Cancer Letters. 2009;281(2):171–182. doi: 10.1016/j.canlet.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 83.Tsao C.-Y., Sabbatino F., Cheung N.-K. V., et al. Anti-proliferative and pro-apoptotic activity of GD2 ganglioside-specific monoclonal antibody 3F8 in human melanoma cells. OncoImmunology. 2015;4(8) doi: 10.1080/2162402x.2015.1023975.e1023975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoshida S., Kawaguchi H., Sato S., Ueda R., Furukawa K. An anti-GD2 monoclonal antibody enhances apoptotic effects of anti-cancer drugs against small cell lung cancer cells via JNK (c-Jun Terminal Kinase) activation. Japanese Journal of Cancer Research. 2002;93(7):816–824. doi: 10.1111/j.1349-7006.2002.tb01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yoshida S., Fukumoto S., Kawaguchi H., Sato S., Ueda R., Furukawa K. Ganglioside G(D2) in small cell lung cancer cell lines: enhancement of cell proliferation and mediation of apoptosis. Cancer Research. 2001;61:4244–4252. [PubMed] [Google Scholar]
- 86.Lynch C. M., Hart B. W., Grewal I. S. Practical considerations for nonclinical safety evaluation of therapeutic monoclonal antibodies. mAbs. 2009;1(1):2–11. doi: 10.4161/mabs.1.1.7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fleurence J., Cochonneau D., Fougeray S., et al. Targeting and killing glioblastoma with monoclonal antibody to O-acetyl GD2 ganglioside. Oncotarget. 2016 doi: 10.18632/oncotarget.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Singh S. K., Clarke I. D., Terasaki M., et al. Identification of a cancer stem cell in human brain tumors. Cancer Research. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 89.Tanei T., Morimoto K., Shimazu K., et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential paclitaxel and epirubicin-based chemotherapy for breast cancers. Clinical Cancer Research. 2009;15(12):4234–4241. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 90.Esparza R., Azad T. D., Feroze A. H., Mitra S. S., Cheshier S. H. Glioblastoma stem cells and stem cell-targeting immunotherapies. Journal of Neuro-Oncology. 2015;123(3):449–457. doi: 10.1007/s11060-015-1729-x. [DOI] [PubMed] [Google Scholar]
- 91.Ozawa H., Kotani M., Kawashima I., Tai T. Generation of one set of monoclonal antibodies specific for b-pathway ganglio-series gangliosides. Biochimica et Biophysica Acta. 1992;1123(2):184–190. doi: 10.1016/0005-2760(92)90110-h. [DOI] [PubMed] [Google Scholar]
- 92.Kotani M., Ozawa H., Kawashima I., Ando S., Tai T. Generation of one set of monoclonal antibodies specific for a-pathway ganglio-series gangliosides. Biochimica et Biophysica Acta. 1992;1117(1):97–103. doi: 10.1016/0304-4165(92)90168-t. [DOI] [PubMed] [Google Scholar]
- 93.Lee K. J., Mao S., Sun C., et al. Phage-display selection of a human single-chain Fv antibody highly specific for melanoma and breast cancer cells using a chemoenzymatically synthesized GM3-carbohydrate antigen. Journal of the American Chemical Society. 2002;124(42):12439–12446. doi: 10.1021/ja020737j. [DOI] [PubMed] [Google Scholar]
- 94.Rojas G., Pupo A., Gómez S., Krengel U., Moreno E. Engineering the binding site of an antibody against N-glycolyl GM3: from functional mapping to novel anti-ganglioside specificities. ACS Chemical Biology. 2013;8(2):376–386. doi: 10.1021/cb3003754. [DOI] [PubMed] [Google Scholar]
- 95.Cerato E., Birkle S., Portoukalian J., Mezazigh A., Chatal J.-F., Aubry J. Variable region gene segments of nine monoclonal antibodies specific to disialogangliosides (GD2, GD3) and their O-acetylated derivatives. Hybridoma. 1997;16(4):307–316. doi: 10.1089/hyb.1997.16.307. [DOI] [PubMed] [Google Scholar]
- 96.Yuki N., Miyatake T., Ichihashi Y., Sato S., Katagiri T. IgM anti-(GalNAc β1-4 Gal[3-2α NeuAc]β1-) antibody-mediated cytotoxicity in a patient with amyotrophic lateral sclerosis-like disorder. Muscle and Nerve. 1992;15(12):1371–1373. [PubMed] [Google Scholar]
- 97.Ladjemi M. Z. Anti-idiotypic antibodies as cancer vaccines: achievements and future improvements. Frontiers in Oncology. 2012;2, article 158 doi: 10.3389/fonc.2012.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez A., Mier E. S., Vispo N. S., Vazquez A. M., Rodríguez R. P. A monoclonal antibody against NeuGc-containing gangliosides contains a regulatory idiotope involved in the interaction with B and T cells. Molecular Immunology. 2002;39(1-2):103–112. doi: 10.1016/S0161-5890(02)00041-X. [DOI] [PubMed] [Google Scholar]
- 99.Alfonso S., Valdés-Zayas A., Santiesteban E. R., et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clinical Cancer Research. 2014;20(14):3660–3671. doi: 10.1158/1078-0432.CCR-13-1674. [DOI] [PubMed] [Google Scholar]
- 100.Cacciavillano W., Sampor C., Venier C., et al. A phase I study of the anti-idiotype vaccine racotumomab in neuroblastoma and other pediatric refractory malignancies. Pediatric Blood and Cancer. 2015;62(12):2120–2124. doi: 10.1002/pbc.25631. [DOI] [PubMed] [Google Scholar]
- 101.Imai M., Landen C., Ohta R., Cheung N.-K. V., Tomlinson S. Complement-mediated mechanisms in anti-GD2 monoclonal antibody therapy of murine metastatic cancer. Cancer Research. 2005;65(22):10562–10568. doi: 10.1158/0008-5472.CAN-05-1894. [DOI] [PubMed] [Google Scholar]
- 102.Cheung N.-K. V., Sowers R., Vickers A. J., Cheung I. Y., Kushner B. H., Gorlick R. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. Journal of Clinical Oncology. 2006;24(18):2885–2890. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 103.Tarek N., Luduec J.-B. L., Gallagher M. M., et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. Journal of Clinical Investigation. 2012;122(9):3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Terme M., Dorvillius M., Cochonneau D., et al. Chimeric antibody c.8B6 to O-acetyl-GD2 mediates the same efficient anti-neuroblastoma effects as therapeutic ch14.18 antibody to GD2 without antibody induced allodynia. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0087210.e87210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cheung N.-K. V., Lazarus H., Miraldi F. D., et al. Ganglioside GD2 specific monoclonal antibody 3F8: a phase I study in patients with neuroblastoma and malignant melanoma. Journal of Clinical Oncology. 1987;5(9):1430–1440. doi: 10.1200/JCO.1987.5.9.1430. [DOI] [PubMed] [Google Scholar]
- 106.Curran K. J., Pegram H. J., Brentjens R. J. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. Journal of Gene Medicine. 2012;14(6):405–415. doi: 10.1002/jgm.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kershaw M. H., Westwood J. A., Darcy P. K. Gene-engineered T cells for cancer therapy. Nature Reviews Cancer. 2013;13(8):525–541. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 108.Maude S. L., Frey N., Shaw P. A., et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. New England Journal of Medicine. 2014;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Grupp S. A., Kalos M., Barrett D., et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. New England Journal of Medicine. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davila M. L., Riviere I., Wang X., et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science Translational Medicine. 2014;6(224) doi: 10.1126/scitranslmed.3008226.224ra25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kochenderfer J. N., Dudley M. E., Kassim S. H., et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. Journal of Clinical Oncology. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dotti G., Gottschalk S., Savoldo B., Brenner M. K. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunological Reviews. 2014;257(1):107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dotti G., Savoldo B., Brenner M. Fifteen years of gene therapy based on chimeric antigen receptors: ‘are we nearly there yet?’. Human Gene Therapy. 2009;20(11):1229–1239. doi: 10.1089/hum.2009.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Savoldo B., Dotti G. Chimeric antigen receptors (CARs) from bench-to-bedside. Immunology Letters. 2013;155(1-2):40–42. doi: 10.1016/j.imlet.2013.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brentjens R. J., Latouche J.-B., Santos E., et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nature Medicine. 2003;9(3):279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 116.Brentjens R. J., Santos E., Nikhamin Y., et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clinical Cancer Research. 2007;13(18, part 1):5426–5435. doi: 10.1158/1078-0432.ccr-07-0674. [DOI] [PubMed] [Google Scholar]
- 117.Savoldo B., Ramos C. A., Liu E., et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. Journal of Clinical Investigation. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rossig C., Nuchtern J. G., Brenner M. K. Selection of human antitumor single-chain Fv antibodies from the B-cell repertoire of patients immunized against autologous neuroblastoma. Medical and Pediatric Oncology. 2000;35(6):692–695. doi: 10.1002/1096-911x(20001201)35:6<692::aid-mpo45>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 119.Rossig C., Bollard C. M., Nuchtern J. G., Merchant D. A., Brenner M. K. Targeting of GD2-positive tumor cells by human T lymphocytes engineered to express chimeric T-cell receptor genes. International Journal of Cancer. 2001;94(2):228–236. doi: 10.1002/ijc.1457. [DOI] [PubMed] [Google Scholar]
- 120.Pule M. A., Savoldo B., Myers G. D., et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Medicine. 2008;14(11):1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Louis C. U., Savoldo B., Dotti G., et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6056. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pulè M. A., Straathof K. C., Dotti G., Heslop H. E., Rooney C. M., Brenner M. K. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Molecular Therapy. 2005;12(5):933–941. doi: 10.1016/j.ymthe.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 123.Long A. H., Haso W. M., Shern J. F., et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nature Medicine. 2015;21(6):581–590. doi: 10.1038/nm.3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Davis J. L., Theoret M. R., Zheng Z., Lamers C. H. J., Rosenberg S. A., Morgan R. A. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clinical Cancer Research. 2010;16(23):5852–5861. doi: 10.1158/1078-0432.CCR-10-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jensen M. C., Popplewell L., Cooper L. J., et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biology of Blood and Marrow Transplantation. 2010;16(9):1245–1256. doi: 10.1016/j.bbmt.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lamers C. H. J., Willemsen R., Van Elzakker P., et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood. 2011;117(1):72–82. doi: 10.1182/blood-2010-07-294520. [DOI] [PubMed] [Google Scholar]
- 127.Hudecek M., Lupo-Stanghellini M.-T., Kosasih P. L., et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clinical Cancer Research. 2013;19(12):3153–3164. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kuünkele A., Johnson A. J., Rolczynski L. S., et al. Functional tuning of CARs reveals signaling threshold above which CD8+ CTL antitumor potency is attenuated due to cell fas-FasL-dependent AICD. Cancer Immunology Research. 2015;3(4):368–379. doi: 10.1158/2326-6066.cir-14-0200. [DOI] [PubMed] [Google Scholar]
- 129.Thomas S., Straathof K., Himoudi N., Anderson J., Pule M. An optimized GD2-targeting retroviral cassette for more potent and safer cellular therapy of neuroblastoma and other cancers. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0152196.e0152196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clémenceau B., Valsesia-Wittmann S., Jallas A.-C., et al. In vitro and in vivo comparison of lymphocytes transduced with a human CD16 or with a chimeric antigen receptor reveals potential off-target interactions due to the IgG2 CH2-CH3 CAR-spacer. Journal of Immunology Research. 2015;2015:13. doi: 10.1155/2015/482089.482089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hombach A., Hombach A. A., Abken H. Adoptive immunotherapy with genetically engineered T cells: modification of the IgG1 Fc ‘spacer’ domain in the extracellular moiety of chimeric antigen receptors avoids ‘off-target’ activation and unintended initiation of an innate immune response. Gene Therapy. 2010;17(10):1206–1213. doi: 10.1038/gt.2010.91. [DOI] [PubMed] [Google Scholar]
- 132.Eshhar Z., Waks T., Gross G., Schindler D. G. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bridgeman J. S., Hawkins R. E., Bagley S., Blaylock M., Holland M., Gilham D. E. The optimal antigen response of chimeric antigen receptors harboring the CD3ζ transmembrane domain is dependent upon incorporation of the receptor into the endogenous TCR/CD3 complex. The Journal of Immunology. 2010;184(12):6938–6949. doi: 10.4049/jimmunol.0901766. [DOI] [PubMed] [Google Scholar]
- 134.Milone M. C., Fish J. D., Carpenito C., et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Molecular Therapy. 2009;17(8):1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carpenito C., Milone M. C., Hassan R., et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(9):3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tammana S., Huang X., Wong M., et al. 4-1BB and CD28 signaling plays a synergistic role in redirecting umbilical cord blood T cells against B-cell malignancies. Human Gene Therapy. 2010;21(1):75–86. doi: 10.1089/hum.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guedan S., Chen X., Madar A., et al. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124(7):1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cruz C. R. Y., Micklethwaite K. P., Savoldo B., et al. Infusion of donor-derived CD19-redirected virus-specific T cells for B-cell malignancies relapsed after allogeneic stem cell transplant: a phase 1 study. Blood. 2013;122(17):2965–2973. doi: 10.1182/blood-2013-06-506741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gattinoni L., Finkelstein S. E., Klebanoff C. A., et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. The Journal of Experimental Medicine. 2005;202(7):907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Klebanoff C. A., Gattinoni L., Restifo N. P. Sorting through subsets: which T-cell populations mediate highly effective adoptive immunotherapy? Journal of Immunotherapy. 2012;35(9):651–660. doi: 10.1097/cji.0b013e31827806e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klebanoff C. A., Gattinoni L., Torabi-Parizi P., et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Metelitsa L. S. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clinical Immunology. 2011;140(2):119–129. doi: 10.1016/j.clim.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heczey A., Liu D., Courtney A., et al. NKT cells as a novel platform for cancer immunotherapy with chimeric antigen receptors. Proceedings of the American Society of Gell and Cell Therapy Annual Meeting; 2013; Salt Lake City, Utah, USA. [Google Scholar]
- 144.Liu D., Song L., Wei J., et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. Journal of Clinical Investigation. 2012;122(6):2221–2233. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Metelitsa L. S., Wu H.-W., Wang H., et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. Journal of Experimental Medicine. 2004;199(9):1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Song L., Asgharzadeh S., Salo J., et al. Vα24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. Journal of Clinical Investigation. 2009;119(6):1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Heczey A., Liu D., Tian G., et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124(18):2824–2833. doi: 10.1182/blood-2013-11-541235. [DOI] [PMC free article] [PubMed] [Google Scholar]