Abstract
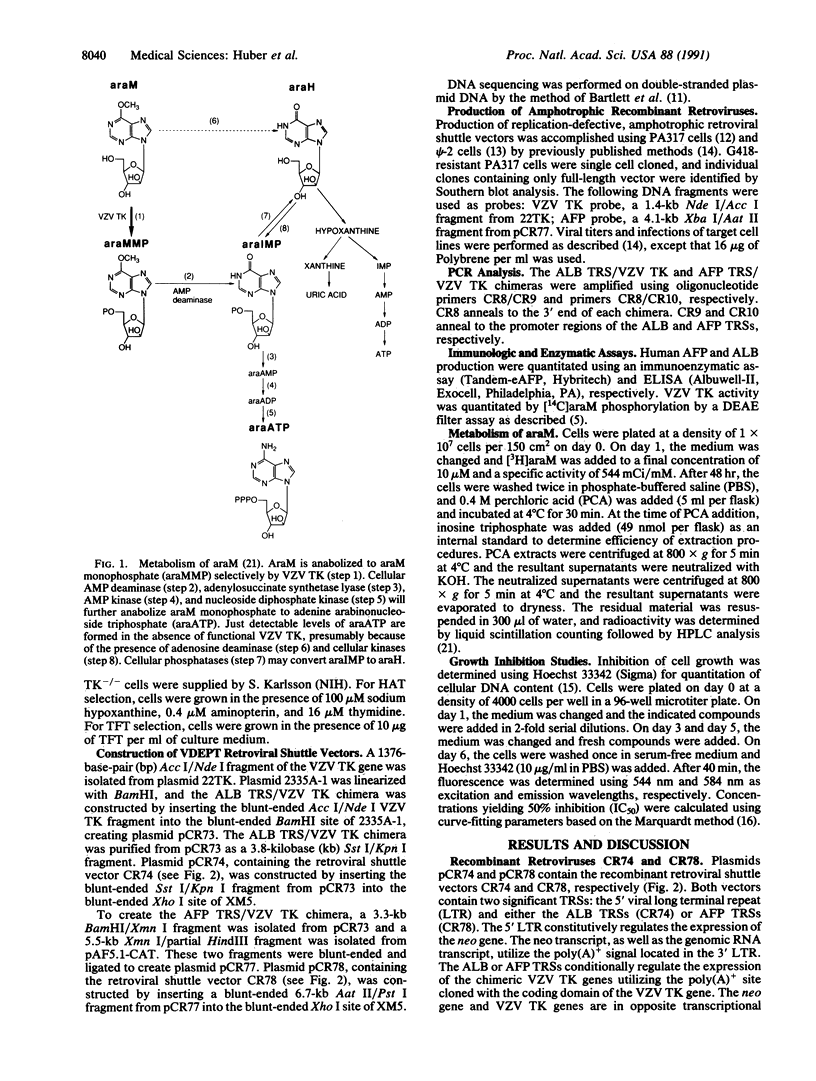
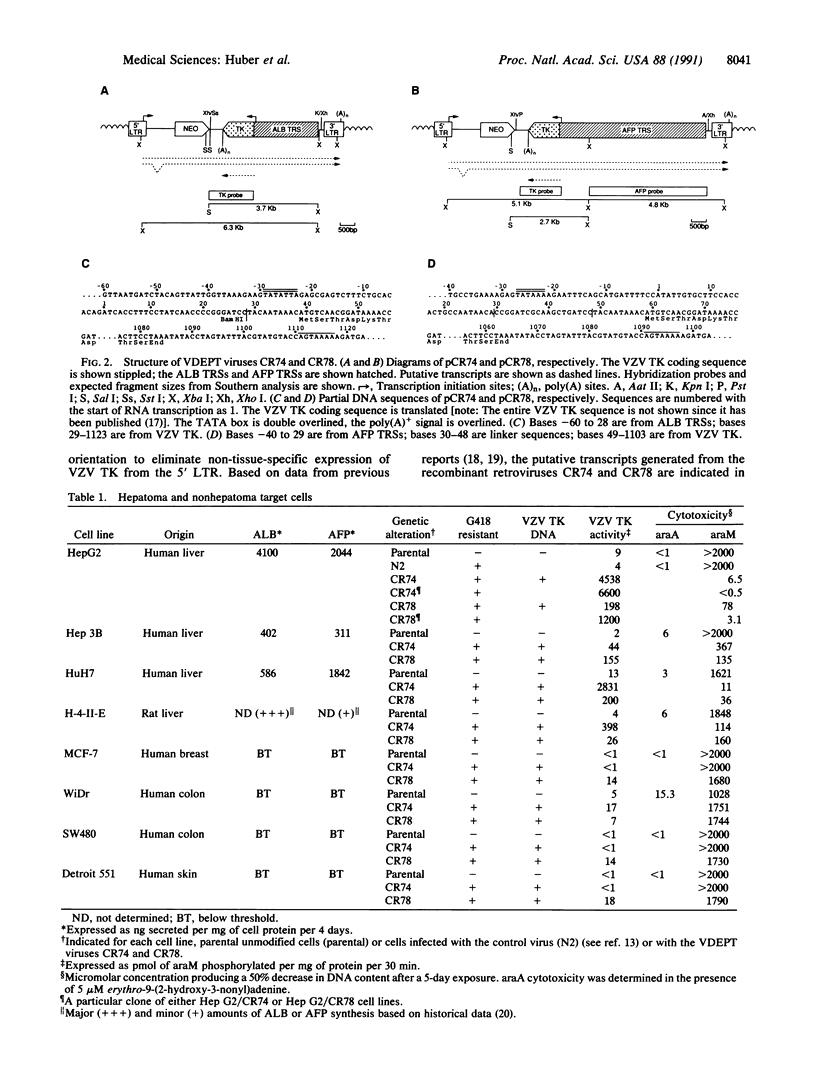
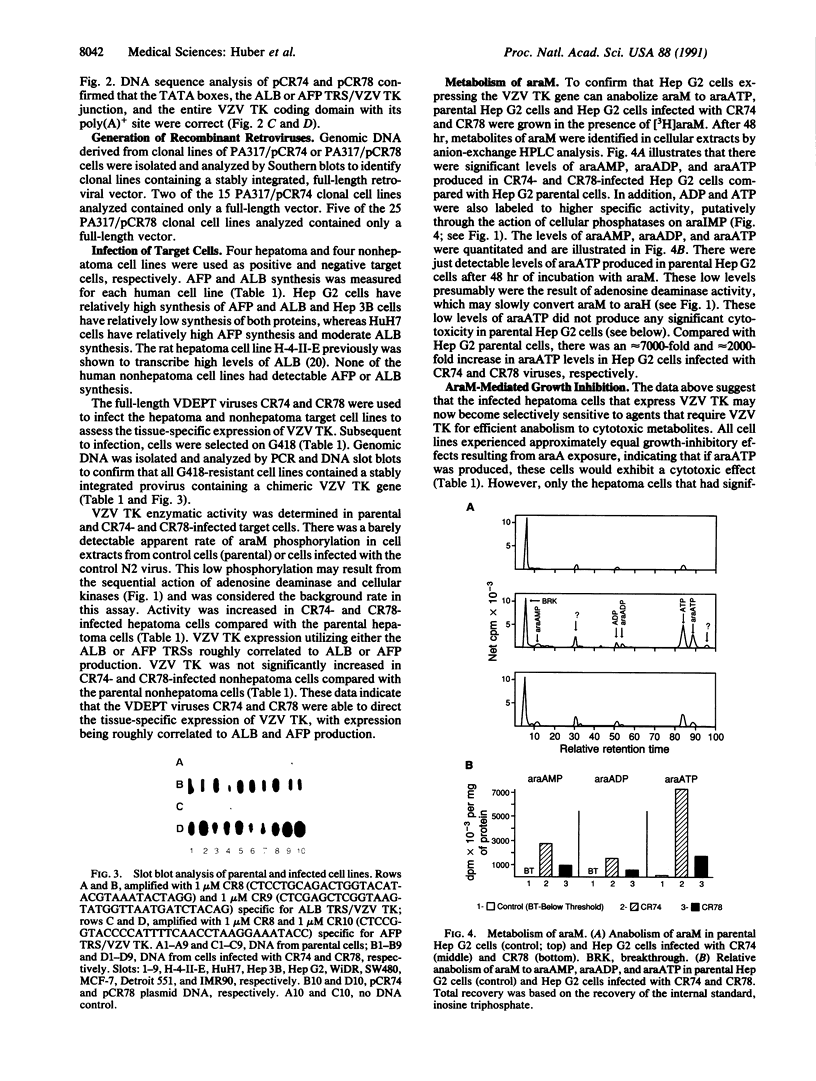
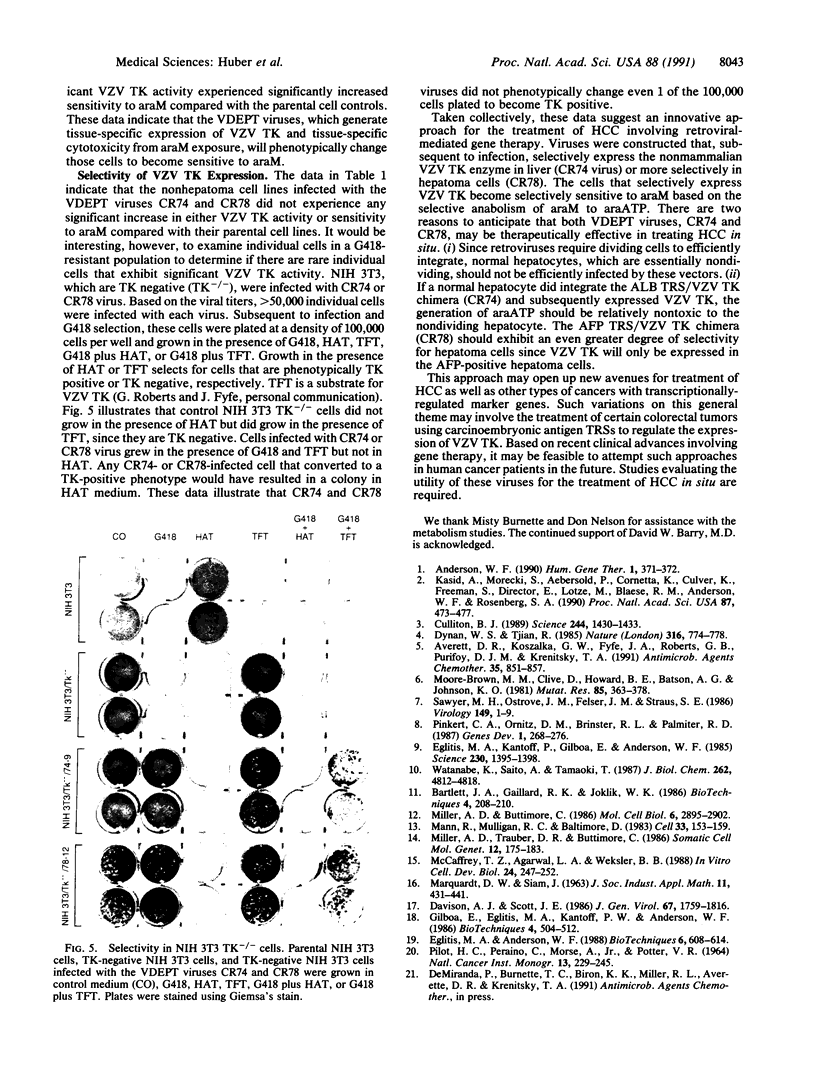
An approach involving retroviral-mediated gene therapy for the treatment of neoplastic disease is described. This therapeutic approach is called "virus-directed enzyme/prodrug therapy" (VDEPT). The VDEPT approach exploits the transcriptional differences between normal and neoplastic cells to achieve selective killing of neoplastic cells. We now describe development of the VDEPT approach for the treatment of hepatocellular carcinoma. Replication-defective, amphotrophic retroviruses were constructed containing a chimeric varicella-zoster virus thymidine kinase (VZV TK) gene that is transcriptionally regulated by either the hepatoma-associated alpha-fetoprotein or liver-associated albumin transcriptional regulatory sequences. Subsequent to retroviral infection, expression of VZV TK was limited to either alpha-fetoprotein- or albumin-positive cells, respectively. VZV TK metabolically activated the nontoxic prodrug 6-methoxypurine arabinonucleoside (araM), ultimately leading to the formation of the cytotoxic anabolite adenine arabinonucleoside triphosphate (araATP). Cells that selectively expressed VZV TK became selectively sensitive to araM due to the VZV TK-dependent anabolism of araM to araATP. Hence, these retroviral-delivered chimeric genes generated tissue-specific expression of VZV TK, tissue-specific anabolism of araM to araATP, and tissue-specific cytotoxicity due to araM exposure. By utilizing such retroviral vectors, araM was anabolized to araATP in hepatoma cells, producing a selective cytotoxic effect.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. September 14, 1990: the beginning. Hum Gene Ther. 1990 Winter;1(4):371–372. doi: 10.1089/hum.1990.1.4-371. [DOI] [PubMed] [Google Scholar]
- Averett D. R., Koszalka G. W., Fyfe J. A., Roberts G. B., Purifoy D. J., Krenitsky T. A. 6-Methoxypurine arabinoside as a selective and potent inhibitor of varicella-zoster virus. Antimicrob Agents Chemother. 1991 May;35(5):851–857. doi: 10.1128/aac.35.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culliton B. J. Fighting cancer with designer cells. Science. 1989 Jun 23;244(4911):1430–1433. doi: 10.1126/science.2660264. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Scott J. E. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986 Sep;67(Pt 9):1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Eglitis M. A., Anderson W. F. Retroviral vectors for introduction of genes into mammalian cells. Biotechniques. 1988 Jul-Aug;6(7):608–614. [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P., Gilboa E., Anderson W. F. Gene expression in mice after high efficiency retroviral-mediated gene transfer. Science. 1985 Dec 20;230(4732):1395–1398. doi: 10.1126/science.2999985. [DOI] [PubMed] [Google Scholar]
- Kasid A., Morecki S., Aebersold P., Cornetta K., Culver K., Freeman S., Director E., Lotze M. T., Blaese R. M., Anderson W. F. Human gene transfer: characterization of human tumor-infiltrating lymphocytes as vehicles for retroviral-mediated gene transfer in man. Proc Natl Acad Sci U S A. 1990 Jan;87(1):473–477. doi: 10.1073/pnas.87.1.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Agarwal L. A., Weksler B. B. A rapid fluorometric DNA assay for the measurement of cell density and proliferation in vitro. In Vitro Cell Dev Biol. 1988 Mar;24(3):247–252. doi: 10.1007/BF02623555. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Moore-Brown M. M., Clive D., Howard B. E., Batson A. G., Johnson K. O. The utilization of trifluorothymidine (TFT) to select for thymidine kinase-deficient (TK-/-) mutants from L5178Y/TK+/- mouse lymphoma cells. Mutat Res. 1981 Oct;85(5):363–378. doi: 10.1016/0165-1161(81)90227-2. [DOI] [PubMed] [Google Scholar]
- PITOT H. C., PERAINO C., MORSE P. A., Jr, POTTER V. R. HEPATOMAS IN TISSUE CULTURE COMPARED WITH ADAPTING LIVER IN VIVO. Natl Cancer Inst Monogr. 1964 Apr;13:229–245. [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Sawyer M. H., Ostrove J. M., Felser J. M., Straus S. E. Mapping of the varicella zoster virus deoxypyrimidine kinase gene and preliminary identification of its transcript. Virology. 1986 Feb;149(1):1–9. doi: 10.1016/0042-6822(86)90081-4. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Saito A., Tamaoki T. Cell-specific enhancer activity in a far upstream region of the human alpha-fetoprotein gene. J Biol Chem. 1987 Apr 5;262(10):4812–4818. [PubMed] [Google Scholar]